Abstract
Oral anticoagulants (OAC) are medications commonly used in patients with atrial fibrillation and other cardiovascular conditions. Both warfarin and direct oral anticoagulants (DOAC) are susceptible to drug-drug interactions (DDI). DDI are an important cause of adverse drug reactions and exact a large toll on the healthcare system. DDI for warfarin mainly involve moderate to strong inhibitors / inducers of cytochrome P450 (CYP) 2C9, which is responsible for the elimination of the more potent S-isomer of warfarin. However, inhibitor / inducers of CYP3A4 and CYP1A2 may also cause DDI with warfarin. Recognition of these precipitating agents along with increased frequency of monitoring when these agents are initiated or discontinued will minimize the impact of warfarin DDI. DOAC DDI are mainly affected by medications strongly affecting the permeability glycoprotein (P-gp), and to a lesser extent, strong CYP3A4 inhibitors / inducers. Dabigatran and edoxaban are affected by P-gp modulation. Strong inducers of CYP3A4 or P-gp should be avoided in all patients taking DOAC unless previously proven to be otherwise safe. Simultaneous strong CYP3A4 and P-gp inhibitors should be avoided in patients taking apixaban and rivaroxaban. Concomitant antiplatelet / anticoagulant use confers additive risk for bleeding, but their combination is unavoidable in many cases. Minimizing duration of concomitant anticoagulant/antiplatelet therapy as indicated by evidence-based clinical guidelines is the best way to reduce the risk of bleeding.
Keywords: drug interactions, anticoagulant drugs, direct oral anticoagulants, vitamin K antagonists, pharmacokinetics
Introduction
Oral anticoagulants (OACs) are now commonly prescribed for atrial fibrillation (AF) and other conditions associated with risk for thromboembolism.1–4 These conditions occur more frequently in older patients who are also at higher risk for the bleeding complications of OACs.5, 6 As of 2017, over 37 million people worldwide have AF with the highest risk among developed countries.5 While vitamin K antagonists (VKA) have long been associated with a narrow therapeutic index, clinically relevant changes in the serum concentration of direct acting oral anticoagulants (DOACs) can also alter their efficacy and safety.7 It is estimated that up to 80% of AF patients will receive a medication that interacts with their OAC over their lifetime.8 Thus, it is important for clinicians to be aware of the common medications that interact with OACs.
In this state-of-the-art review, we summarize drug-drug interactions (DDI) and drug-food interactions affecting VKAs and DOACs and provide recommendations to minimize adverse interactions. Recommendations in this paper will generally apply to patients without significant genetic polymorphisms that precipitate increased or decreased drug clearance. The benefits of pharmacogenetic testing prior to initiation of both VKA and DOAC have been studied, however, there is currently not enough evidence to recommend routine pharmacogenetic testing in clinical practice.9–12 Throughout the paper, we refer to specific drugs as strong, moderate or weak inhibitors of cytochrome P450 (CYP) enzymes, according to the following definitions: a strong inhibitor causes a > 5-fold increase in the plasma area under the plasma concentration versus time curve (AUC) or ≥80% decrease in clearance; a moderate inhibitor causes a > 2-fold increase in the AUC or ≥50% to <80% decrease in clearance; a weak inhibitor causes a > 1.25-fold increase in the AUC or ≥20% to <50% decrease in clearance.13 A strong CYP inducer will decrease the AUC ≥80%; a moderate inducer will decrease the AUC by ≥50% to <80%; and a weak inducer will decrease AUC by ≥20% to <50%.
Vitamin K Antagonist: Warfarin
Pharmacodynamics and pharmacokinetics of warfarin
Warfarin, a vitamin K epoxide reductase inhibitor, exerts its anticoagulation effects by inhibiting synthesis of vitamin K, which is required to activate key components of the coagulation cascade (Figure 1).14 Commercially available warfarin is a racemic mix of 2 active stereoisomers: the S-enantiomer, metabolized by the CYP2C9 enzyme, is approximately 5 times more potent than the R-enantiomer, which is primarily metabolized by CYP3A4, but with contributions from CYP1A1, CYP1A2, CYP2C8, CYP2C18, and CYP2C19. 13, 15, 16 Warfarin is almost completely eliminated via hepatic metabolism with an elimination half-life of 35 hours.15 It is highly bound to plasma proteins with anticoagulant effects stabilizing approximately 3 days after warfarin plasma concentrations reach steady state.15, 16
Figure 1:
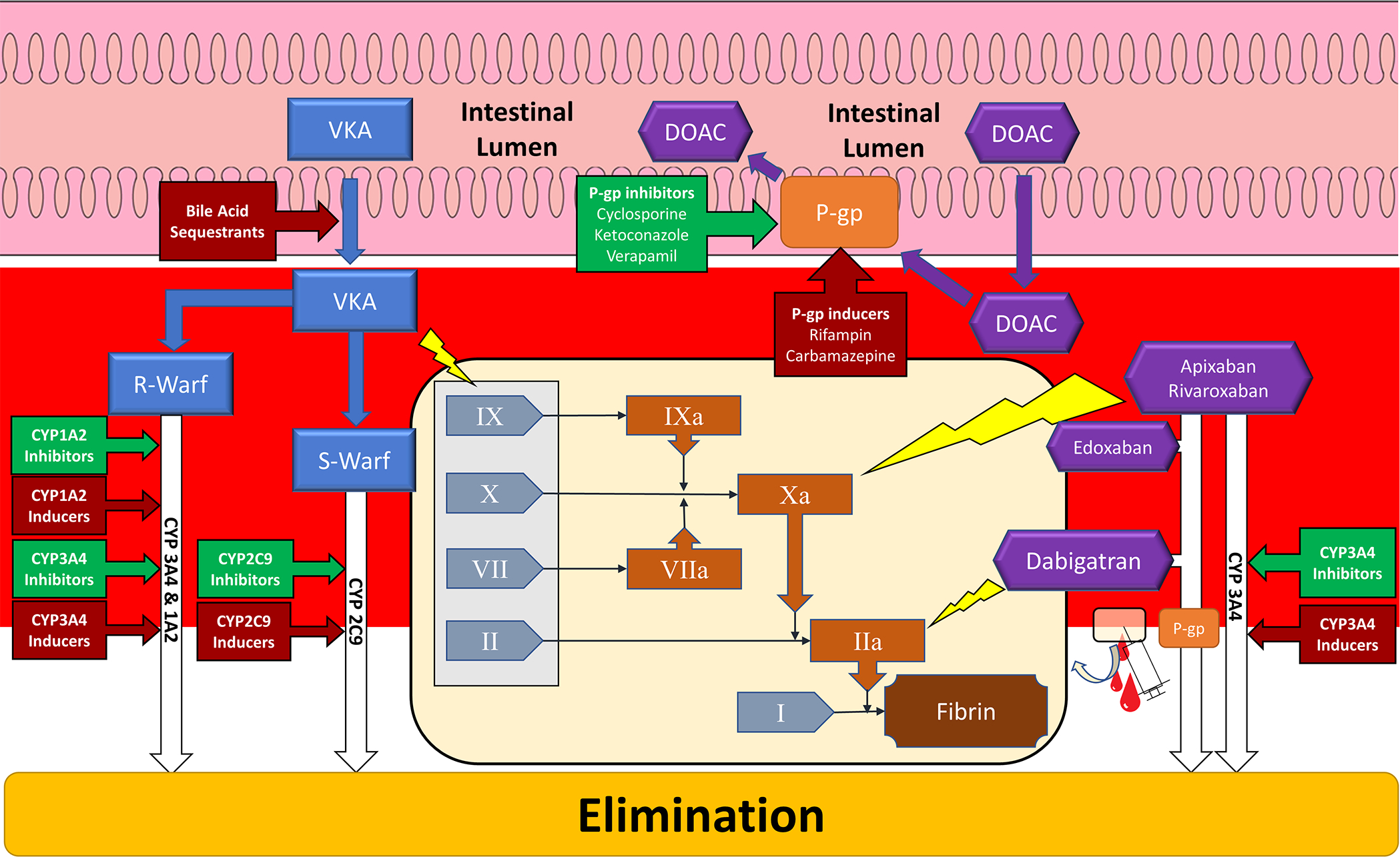
Schematic of major vitamin K antagonist and direct oral anticoagulant sites of drug interactions. Tan panel depicts simplified coagulation cascade and yellow lightning bolts indicate sites of inhibition by anticoagulants (blue rectangles – vitamin K antagonist; purple hexagons – direct oral anticoagulant). Gray rectangle encompass coagulation factors affected by vitamin K antagonism. Red arrowed boxes indicate interactions that inhibit anticoagulation. Green arrowed boxes indicate interactions that potentiate anticoagulation. CYP – Cytochrome P-450; DOAC – Direct oral anticoagulant; R-Warf – R-warfarin; S-Warf – S-warfarin; P-gp – P-glycoprotein; VKA – Vitamin K Antagonist; I – Fibrinogen; II – Prothrombin; IIa – Thrombin; VII – Factor 7; VIIa – Activated Factor 7; X – Factor 10; Xa – Activated Factor 10; IX – Factor 9; IXa – Activated Factor 9
Drug Interactions
There are over 500 distinct warfarin drug interactions reported in the literature.17 For the majority of these interactions, there is a lack of consensus regarding their clinical significance.17 Moreover, certain warfarin drug interactions may only be clinically relevant under certain circumstances, such as, in patients with certain genetic polymorphisms of CYP2C9 substrates.18
The order of initiation of a precipitant drug versus warfarin can also affect the impact of a warfarin-drug interaction. When warfarin is initiated in the setting of a CYP2C9 inhibitor, as is estimated to occur 20% of the time, a patient may not develop a supratherapeutic INR because monitoring is more frequent during the initiation phase of warfarin.19, 20 However, if the same precipitant drug is initiated in the setting of chronic and stable warfarin therapy, then the patient may develop an elevated INR if the interaction goes unrecognized and more frequent INR monitoring is not performed. Figure 2 depicts a simple algorithm clinicians can use to screen and manage drug interactions with warfarin use. Given the plethora of warfarin-drug interactions, it is helpful to categorize the interactions into certain high-risk drug classes.14
Figure 2:
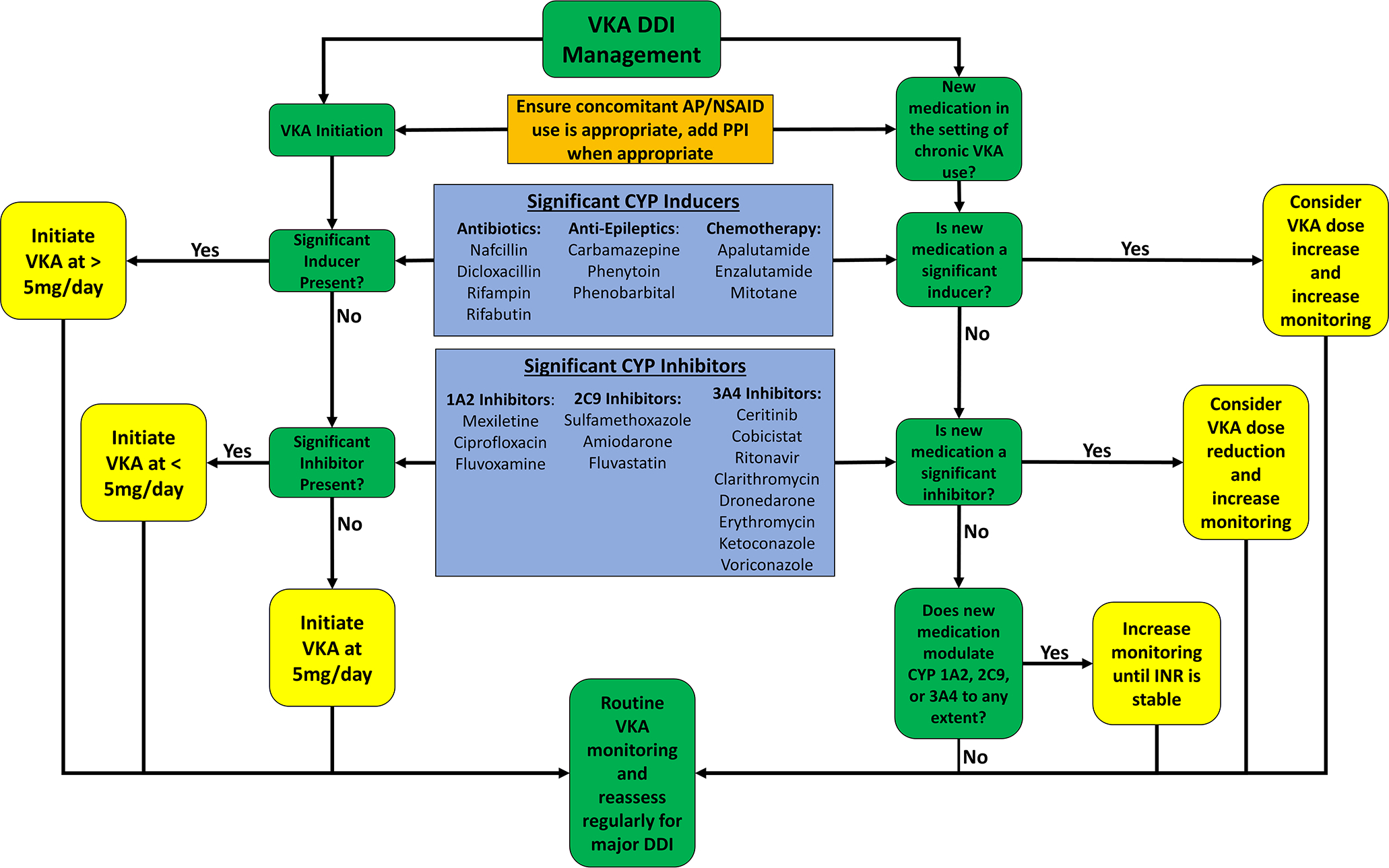
Algorithm to manage warfarin drug-drug interactions during warfarin initiation as well as adding a potentially interacting drug on chronic warfarin therapy. AP – Anti-platelet; CYP – cytochrome P450; DDI – drug-drug interaction; INR – International normalized ratio; NSAID – Non-steroidal anti-inflammatory drugs; VKA - warfarin
Antibiotics
All antibiotics can alter the gut microbiome, which is a rich source of vitamin K, and thereby potentiate anticoagulant effects of warfarin.16, 21 Thus, it is important to monitor INRs closely whenever antibiotics are initiated in the setting of chronic warfarin use.14 However, antibiotics known to inhibit the CYP2C9 isoenzyme (Table 1), such as sulfonamides, including sulfaphenazole and sulfamethoxazole (commonly administered in combination with trimethoprim as trimethoprim/sulfamethoxazole), and metronidazole, can further exacerbate this interaction.22–24 The interaction between sulfamethoxazole and warfarin has not only been described in multiple case reports, but large national insurance database analyses have confirmed the risk of serious bleeding that nearly doubles compared to patients receiving warfarin alone.25, 26 Pre-emptive warfarin dose reductions of 25% and 33% for sulfamethoxazole and metronidazole respectively, are recommended when co-administered with warfarin.24, 27
Table 1:
| CYP450 2C9 Modulator | |||
|---|---|---|---|
| Inhibitor | Inducer | ||
| Strong Inhibitor | Sulfaphenazole | Strong Inducer | |
| Moderate Inhibitor | Amiodarone | Moderate Inducer | Enzalutamide |
| Fluconazole | Rifampin | ||
| Metronidazole | |||
| Miconazole | |||
| Weak Inhibitor | Capecitabine | Weak Inducer | Apalutamide |
| Certinib | Aprepitant | ||
| Disulfirim | Carbamazepine | ||
| Efavirenz | Ritonavir | ||
| Fluvastatin | |||
| Fluvoxamine | |||
| Isoniazid | |||
| Quercetin | |||
| Sulfamethoxazole | |||
| Voriconazole | |||
| In-vitro or undetermined | Alcohol | In-vitro or undetermined | Aminoglutethimide |
| Azapropazone | Bosentan | ||
| Berberine | Carbamazepine | ||
| Co-trimoxazole | Dabrafenib | ||
| Delavirdine | Fosphenytoin | ||
| Doxifluridine | Griseofulvin | ||
| Etravirine | Letermovir | ||
| Fenofibrate | Nafcillin | ||
| Fluorouracil | Nelfinavir | ||
| Gemfibrozil | Phenobarbital | ||
| Leflunomide | Phenytoin | ||
| Mifepristone | Primidone | ||
| Rucaparib | Rifapentine | ||
| Sulfamethizole | Secobarbital | ||
| Sulfinpyrazone | St. John’s wort | ||
| Tamoxifen | |||
| Valproic Acid | |||
| Zafirlukast | |||
CYP1A2 (Table 2) and 3A4 inhibitors decrease clearance of the less potent R-isomer of warfarin. Ciprofloxacin, a strong CYP1A2 inhibitor, can increase serum R-warfarin concentrations.28 Other fluoroquinolones in combination with warfarin can elevate the INR and increase the risk of adverse bleeding versus those taking warfarin alone, both in case reports as well as large national database registries.25, 26, 29 Similarly, the macrolide antibiotics have also been widely reported to potentiate warfarin’s effects.25, 26
Table 2:
| CYP450 1A2 Modulator | |||
|---|---|---|---|
| Inhibitor | Inducer | ||
| Strong Inhibitor | Ciprofloxacin | Strong Inducer | |
| Fluvoxamine | |||
| Moderate Inducer | Phenytoin | ||
| Moderate Inhibitor | Rifampin | ||
| Ritonavir | |||
| Weak Inhibitor | Citalopram | Teriflunomide | |
| Efavirenz | Tobacco | ||
| Quercetin | |||
| Ribociclib | Weak Inducer | ||
| Simeprevir | |||
| Undetermined | Broccoli | ||
| Undetermined | Amiodarone | Brussels sprouts | |
| Carbamazepine | |||
| Insulin | |||
| Methylcholanthrene | |||
| Modafinil | |||
| Nafcillin | |||
| Omeprazole | |||
| Rucaparib | |||
Clarithromycin and erythromycin are strong and moderate inhibitors, respectively, of CYP3A4.30 (Table 3) The intravenous formulation of azithromycin was cited by the United States (US) Food and Drug Administration (FDA) in 2009 as a drug that may significantly increase the risk of bleeding when co-administered with warfarin.16 The antibiotic dose will also contribute to the severity of this interaction. In a prospective study of 120 patients who received a combination of amoxicillin/clavulanate, patients who received the higher maintenance dose (10–12 g/day) versus the usual dose (3.6 g/day) developed a higher proportion of INR values ≥ 4.31
Table 3:
| CYP450 3A4 Modulator | |||
|---|---|---|---|
| Inhibitor | Inducer | ||
| Strong Inhibitor | Boceprevir | Strong Inducer | Apalutamide |
| Ceritinib | Carbamazepine | ||
| Clarithromycin* although a strong inhibitor of both 3A4 and P-gp, pharmacokinetic data suggests concomitant use with a DOAC is safe (labels) | Enzalutamide | ||
| Cobicistat | Mitotane | ||
| Grapefruit Juice | Phenytoin | ||
| Idelalisib | Rifampin | ||
| Indinavir | St. John's wort | ||
| Itraconazole | |||
| Ketoconazole | Moderate Inducer | Bosentan | |
| Nefazodone | Efavirenz | ||
| Nelfinavir | Etravirine | ||
| Posaconazole | Phenobarbital | ||
| Ribociclib | Primidone | ||
| Ritonavir | |||
| Saquinavir | Weak Inducer | Armodafinil | |
| Telaprevir | Modafinil | ||
| Tucatinib | Rufinamide | ||
| Voriconzole | |||
| Undetermined | Aminoglutethimide | ||
| Bexarotene | |||
| Moderate Inhibitor | Aprepitant | Brigatinib | |
| Ciprofloxacin | Dabrafenib | ||
| Conivaptan | Dexamethasone | ||
| Crizotinib | Dicloxacillin | ||
| Cyclosporine | Eslicarbazepine | ||
| Diltiazem | Fosphenytoin | ||
| Dronedarone | Griseofulvin | ||
| Erythromycin | Lesinurad | ||
| Fluconazole | Lumacaftor | ||
| Fluvoxamine | Nafcillin | ||
| Grapefruit | Nevirapine | ||
| Imatinib | Rifabutin | ||
| Netupitant and Palonostrone (Akynzeo®) | Rifapentine | ||
| Verapamil | Sarilumab | ||
| Telotristat | |||
| Weak Inhibitor | Amiodarone | Tocilizumab | |
| Atomoxetine | Troglitazone | ||
| Chlorzoxazone | Vemurafenib | ||
| Cilostazol | Vinblastine | ||
| Clotrimazole | |||
| Entrectinib | |||
| Esomeprazole | |||
| Fosaprepitant | |||
| Istradefylline | |||
| Ivcaftor | |||
| Lomitapide | |||
| Omeprazole | |||
| Quercetin | |||
| Ranitidine | |||
| Ranolazine | |||
| Simeprevir | |||
| Ticagrelor | |||
| Undetermined | Amprenavir | ||
| Atazanavir | |||
| Berberine | |||
| Chloramphenicol | |||
| Dalfoprestin | |||
| Danazol | |||
| Darunavir | |||
| Dasatinib | |||
| Delavirdine | |||
| Dexmedetomidine | |||
| Ethinyl Estradiol | |||
| Fosaprenavir | |||
| Isavuconazonium | |||
| Isoniazid | |||
| Lapatinib | |||
| Larotrectinib | |||
| Letermovir | |||
| Miconazole | |||
| Mifepristone | |||
| Netupitant | |||
| Palbociclib | |||
| Quinupristin (Synercid) | |||
| Simeprevir | |||
| Stiripentol | |||
| Tamoxifen | |||
| Voxilaprevir | |||
Several antibiotics have also been identified as major CYP450 enzyme inducers. Nafcillin is a CYP3A4 and CYP2C9 inducer and significantly higher maintenance doses of warfarin were required after initiating long-term (> 6 weeks) nafcillin treatment.13, 32, 33 Several case reports for other anti-staphylococcal penicillins, including flucloxacillin and cloxacillin, show a similar effect.34, 35 Flucloxacillin has been shown to induce CYP3A4.36 In two patients, the dose of warfarin needed to be doubled from 5 mg/day to 10 mg/day after initiation of cloxacillin to maintain a therapeutic INR.35 Another antibiotic well-known to induce CYP450 enzymes is rifampin.37
These CYP450 inducing antibiotics are particularly important because they are indicated for conditions such as endocarditis or tuberculosis which require a protracted course of treatment. After increasing the warfarin dose to account for these interactions, it is important to remember to decrease the dose of warfarin once the course of treatment with these antibiotics is completed. The full induction of involved CYP450 enzymes appears to take 2–4 weeks after nafcillin initiation, and the effects persist for up to 2–4 weeks after nafcillin discontinuation.32, 33 It is therefore important to more frequently monitor the INR both during the initiation and discontinuation of these particular antibiotics.14 A list of clinically significant antibiotic-warfarin interactions is provided in Table 4.
Table 4:
Important warfarin-drug interactions and management recommendations
| Precipitant Drug | Nature of Interaction | Recommendations | |
|---|---|---|---|
| Antibiotics | All antibiotics | Alteration in gut flora production of vitamin K21 | Closer monitoring of INR levels |
| Sulfonamides | CYP450 2C9 inhibition24 | Reduce dose of warfarin by 25%24 | |
| Metronidazole | CYP450 2C9 inhibition27 | Reduce dose of warfarin by 33%27 | |
| Ciprofloxacin | CYP450 1A2 inhibition28 | Closer monitoring of INR levels | |
| Clarithromycin | CYP450 3A4 inhibition30 | Closer monitoring of INR levels | |
| Erythromycin | CYP450 3A4 inhibition30 | Closer monitoring of INR levels | |
| Azithromycin | CYP450 3A4 inhibition16 | Closer monitoring of INR levels | |
| Nafcillin | CYP450 3A4 induction32, 33 | Closer monitoring of INR levels; full induction of CYP450 enzymes occurs in 2–4 weeks after initiation and persists up to 2–4 weeks after discontinuation of nafcillin | |
| Flucloxacillin | CYP450 3A4 induction34 | Closer monitoring of INR levels | |
| Cloxacillin | CYP450 3A4 induction35 | Closer monitoring of INR levels | |
| Rifampin | CYP450 3A4 induction166 | Closer monitoring of INR levels | |
| Antifungals | Fluconazole | CYP450 2C9 inhibition 38 | Use alternative if possible, otherwise closer monitoring is warranted |
| Voriconazole | CYP450 2C9 inhibition 40 | Use alternative if possible, otherwise closer monitoring is warranted | |
| Miconazole | CYP450 2C9 inhibition 38 | Consider alternative such as nystatin, otherwise closer monitoring is warranted | |
| Antiretrovirals | Ritonavir | CYP450 3A446 | Closer monitoring of INR levels |
| Antiarrhythmic drugs | Amiodarone | CYP450 2C9 and 3A4 inhibition150 | Decrease dose of warfarin by 25%152 |
| Dronedarone | CYP450 3A4 inhibition148 | Closer monitoring of INR levels | |
| Anti-lipidemic agents | Atorvastatin | CYP450 3A4 inhibition 51 | Closer monitoring of INR levels |
| Rosuvastatin | CYP450 3A4 inhibition51 | Closer monitoring of INR levels | |
| Simvastatin | CYP450 3A4 inhibition 51 | Closer monitoring of INR levels | |
| Fluvastatin | CYP450 2C9 inhibition 51 | Decrease dose of warfarin by 25% | |
| Gemfibrozil | CYP450 2C9 inhibition56 | Decrease dose of warfarin by 20% 58 | |
| Antiepileptic drugs | Carbamazepine | CYP450 enzyme induction 69 | Increase dose of warfarin by 50% with close follow-up69 |
| Phenytoin | Biphasic interaction with initial displacement from protein binding and then enzyme induction72 | Closer monitoring of INR levels | |
| Antidepressants | Fluvoxamine | CYP450 2C9 and 3A4 inhibition (68, 71 | Consider alternatives such as sertraline, citalopram, or escitalopram; otherwise closer monitoring of INR levels is warranted71 |
| Fluoxetine | CYP450 2C9 and 3A4 inhibition 71, 74 | Consider alternatives such as sertraline, citalopram, or escitalopram; otherwise closer monitoring of INR levels is warranted71 | |
| Anti-platelet agents | Aspirin Clopidogrel Prasugrel Ticagrelor |
Potentiation of bleeding | Minimize overlap to shortest duration indicated and consider starting a proton pump inhibitor 155 |
| Chemotherapy | Fluorouracil | Multiple mechanisms 78 | Decrease dose of warfarin by 20% 78 |
| Capecitabine | Possible enzyme induction | Closer monitoring of INR levels | |
| Paclitaxel | Displacement from protein binding or CYP450 3A4 inhibition 84 | Closer monitoring of INR levels | |
| Enzalutamide | CYP450 enzyme induction79 | Closer monitoring of INR levels | |
| Apreptiant | CYP450 enzyme induction 86 | Closer monitoring of INR levels | |
| Non-steroidal anti-inflammatory drugs | Both selective and non-selective cyclooxygenase inhibitors | Cyclooxygenase inhibition88 | Avoid unless benefits clearly outweigh risk 14 |
| Miscellaneous agents | Ethanol | CYP450 2C9 inhibition 56 | Avoid excessive alcoholic consumption (< 60 gm/day) 93 |
| Acetaminophen | CYP450 enzyme inhibition92 | When doses of acetaminophen exceed 2g/day, closer monitoring of INR levels is warranted | |
| Danazol | Inhibition of metabolism 96 | Closer monitoring of INR levels | |
| Herbal Agents | St. John’s Wort | CYP450 enzyme induction 66, 71, 102 | Closer monitoring of INR levels |
| Grapefruit Juice | CYP450 3A4 enzyme inhibition 100 | Consume no more than 200 mL per day107 | |
| Cranberry Juice | CYP450 enzyme inhibition 100 | Consume no more than 24 ounces per day100 |
Antifungals
The triazole antifungals fluconazole and voriconazole inhibit CYP2C9 and can increase the risk of serious bleeding in patients taking warfarin.38–40 Fluconazole is also a moderate inhibitor of CYP3A4, while voriconazole is also a moderate inhibitor of CYP2C19.13 Analysis of a large national prescription database of treatment for oral candidiasis demonstrated that systemic fluconazole administration was associated with increased INR levels in warfarin users.39 Additionally, voriconazole is a moderate inhibitor of CYP2C19 and a weak inhibitor of CYP2C9.13 Ketoconazole is a strong inhibitor of CYP3A4 and a moderate inhibitor of CYP2C19, and itraconazole is a strong inhibitor of CYP3A4.13 Due to these CYP450 interactions, triazole antifungals should be initiated carefully and increased monitoring of the INR is warranted 14
Topical administration of antifungals has been associated with supratherapeutic INR and bleeding events.39, 41, 42 A large national prescription database study of oral candidiasis therapy demonstrated that miconazole oral gel use was associated with increased INRs in warfarin users. However, the same study demonstrated that nystatin oral solution use did not appreciably affect the INR, making this a good alternative for oral candidiasis.39 Vaginal administration of miconazole cream has also been reported to potentiate bleeding among warfarin users.42 Griseofulvin decreases the anticoagulation effect of warfarin. Patients on chronic warfarin therapy starting griseofulvin generally will require higher than baseline doses of warfarin to maintain therapeutic INR.43
Antivirals
Antiretroviral agents are the most common antivirals that interact with warfarin; ritonavir is the most-commonly cited drug.44, 45 Ritonavir, a protease inhibitor capable of strong CYP3A4 inhibition, is commonly used to “boost” the potency of other protease inhibitors that are metabolized by CYP3A4.46 Paradoxically, however, antiretroviral regimens that involve ritonavir require higher doses of warfarin to maintain INR values in the therapeutic range. The mechanism is thought to be concomitant induction of CYP2C9 and CYP1A2, which will increase clearance of the S-isomer of warfarin.44, 45, 47 Nevirapine has also been found to increase warfarin maintenance dose requirements.45 On the contrary, efavirenz and saquinavir potentiated effects of warfarin.45
With the exception of antiretrovirals, other antivirals tend to have little interaction with warfarin. A 5-day course of prophylactic oseltamivir for influenza prophylaxis maximally elevated the INR at day 4 by a mean of 0.13, not considered clinically significant.48
Cardiovascular medications
Statins, specifically fluvastatin, lovastatin, rosuvastatin, and simvastatin may interact with warfarin.49–54 An analysis of a large national database registry demonstrated that atorvastatin, rosuvastatin, and simvastatin all increased the mean INR by approximately 0.3, with a peak at around 4 weeks.55 This degree of INR elevation was not thought to be clinically significant to warrant a preemptive dose reduction of warfarin. The authors of this study recommended close monitoring after initiation of these statins, as patients with certain CYP450 genotypes may be more vulnerable.49, 55
The mechanism of this interaction for atorvastatin, rosuvastatin, and simvastatin is likely a combination of displacement of warfarin from plasma protein binding in addition to inhibition of CYP3A4, which will increase the unbound plasma concentration of warfarin.51, 54 Fluvastatin, on the other hand, inhibits CYP450 2C9, and likely potentiates warfarin to a greater extent given its effect on the more potent S-isomer of warfarin.51, 52 Pitavastatin, at a dose of 4 mg, did not appear to increase INR levels among healthy controls taking warfarin.53 An American Heart Association (AHA) Scientific Statement recommends close monitoring of the INR after the initiation of a statin or a change in statin dose and reports that pitavastatin and atorvastatin exert the lowest impact on the INR..54
Other anti-hyperlipidemic mediations may interact with warfarin. Cholestyramine and other bile acid sequestrants interfere with intestinal absorption of warfarin, and while spacing warfarin administration 2 hours before or 6 hours after bile acid sequestrant administration will mitigate this interaction, this inhibition is unavoidable as warfarin undergoes enterohepatic circulation.14, 56, 57 Case reports describe clinically significant increases in INR for patients taking gemfibrozil.58, 59 The mechanism is unclear but the authors recommend a preemptive 20% dose reduction in warfarin maintenance doses.58 Case reports also suggest fenofibrate may potentiate warfarin’s effects60. However, a large retrospective study of 321patients showed no effect on the INR nor warfarin maintenance dosages after initiating fenofibrate.61 Also, fish oil (1–2 g/day) can increase INR and has antiplatelet effects.62 Antiplatelet and antiarrhythmic medications will be discussed in a separate section.
Psychotropics
Many psychotropic drugs can interact with warfarin.19, 63–75 Two older antiepileptic medications, carbamazepine and phenytoin, are known inducers of multiple CYP 450; carbamazepine decreased mean INR levels by 0.63 and, in a large retrospective cohort study, patients already taking warfarin required a 50% increase in warfarin maintenance dose after initiation of carbamazepine.69, 70 Phenytoin exerts a biphasic interaction with warfarin.14 Upon initiation, phenytoin will displace warfarin from protein binding sites, transiently potentiating bleeding effects.72 However, phenytoin will ultimately induce CYP 450 enzymes so that higher doses of warfarin are required to maintain therapeutic INR.72
Selective serotonin reuptake inhibitors (SSRI) can affect warfarin dosing.71 In a retrospective, single-center study, the concomitant use of SSRI with warfarin more than doubled the risk of bleeding compared to warfarin alone.76 A large national insurance database analysis also demonstrated increased risk of hospitalization for GI bleeding following SSRI initiation.77 The mechanism of action of these interactions is believed to be mediated by inhibition of CYP450 enzymes.71 Fluvoxamine and fluoxetine deserve special attention as they inhibit CYP2C9 and CYP 3A4.68, 71, 74 In these cases, sertraline and citalopram/escitalopram are better alternatives if an SSRI is required.
Other miscellaneous psychotropics that potentiate warfarin via inhibition of CYP450 enzymes include quetiapine,75 valproic acid,67 entacapone,64 and tramadol.65, 73 In contrast, Small et al. published a case series on an interaction between warfarin and trazodone possibly related to the induction of CYP450 enzymes in which high doses of warfarin were required to maintain therapeutic INR after initiation of trazodone.73
Cancer Chemotherapy
Several chemotherapeutic agents interact with warfarin.78–84 Two fluoropyrimidines in particular have been widely reported to interact with warfarin: fluorouracil and capecitabine.78, 80 Fluorouracil increases INR and the risk of bleeding via many mechanisms, including inhibition of CYP2C9, direct injury to the gastrointestinal (GI) tract, or alteration of GI flora; experts recommend decreasing the dose of warfarin prophylactically by 20–70%.78 Capecitabine increases INR in patients taking warfarin, and decreased warfarin requirements may continue for up to two weeks after discontinuing capecitabine.80 Gemcitabine also interacts with warfarin, and even intra-bladder instillation of gemicitabine can cause an elevated INR in patients taking warfarin.81, 82
Paclitaxel can potentiate warfarin anticoagulation by displacing it from protein binding sites.84 Enzalutamide, a strong inducer of CYP2C9, enhances clearance of warfarin and the INR should be monitored closely during its initiation and following discontinuation.79 Two cases of warfarin potentiation with concomitant trastuzumab administration have been reported.85
Aprepitant, a weak inducer of CYP2C9, can reduce serum plasma concentrations of warfarin.86 Increased warfarin requirements during co-administration of aprepitant have been reported.83
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAID) potentiate the risk of bleeding with warfarin.14, 19, 87, 88 This increased risk of bleeding is present for non-selective cyclooxygenase (COX) as well as COX-2 selective inhibitor NSAIDs.14, 88 The nature of this interaction extends beyond purely a pharmacological interaction, but comprises a pharmacokinetic component as well, likely involving displacement of warfarin from plasma proteins.89 Zapata et al demonstrated that a combination of NSAID and warfarin doubles the risk of bleeding versus warfarin alone.88 The results of a retrospective, single center study demonstrated that the odds of bleeding with concomitant NSAID and warfarin administration are similar to concomitant administration of a CYP2C9 inhibitor and warfarin.19 Therefore, the combination of any NSAID, with the exception of aspirin in certain circumstances, and warfarin should be discouraged.14 However, if an NSAID must be co-administered, a large retrospective national insurance database analysis suggests that using a proton pump inhibitor may reduce the risk of GI bleeding.90 Interactions with aspirin and other antiplatelet agents are discussed below.
Acetaminophen
Acetaminophen co-administration increases INR in a dose dependent manner, with the risk of developing an INR > 6 increasing 10-fold once acetaminophen intake exceeds 9.1 gram per week.91 In a prospective, placebo-controlled study of patients on stable warfarin therapy, concomitant acetaminophen administration at doses >2 g/day significantly increased INR by day 3 by an average by 0.7. As a result, the authors recommend close monitoring of INR during the initiation of acetaminophen.92
Miscellaneous agents
Alcohol ingestion inhibits hepatic enzymes, impairs warfarin clearance and can significantly increase INR levels. However, modest consumption of alcohol (~60gm or 2 ounces/day) has been shown to be safe in patients taking warfarin.93 A meta-analysis of smoking and warfarin demonstrated that increased warfarin dosages were required among smokers, suggesting that warfarin clearance is enhanced in smokers.94 In a small, prospective, cohort study, omeprazole was found to interact with warfarin via inhibition of CYP450 2C19.95 Uno et al concluded this was not a clinically significant interaction unless patients were extensive CYP2C19 metabolizers.95 Danazol potentiates warfarin by inhibiting its metabolism, and case reports of serious bleeding have been reported.96
Herbal supplements
Determining risks of interactions with herbal supplements is challenging due to lack of standardization and quality control.14 Most reported data come from case reports or small case series.97–101 However, St. John’s wort, a popular herbal antidepressant, is well-documented to enhance clearance of warfarin via induction of CY2C9, 2C19, and 3A4.66, 71, 102 In a small, prospective cohort study amongst healthy controls, St. John’s Wort increased warfarin clearance and reduced the INR by 20%.102 In this same study, ginseng did not affect warfarin clearance or INR.102 Ginkgo and ginger do not appear to interact with warfarin at modest doses.103 Green tea also does not affect INR at modest doses although a case of decreased INR following excessive consumption of green tea (up to 1 gallon / day) has been reported. 104, 105 While there are multiple reports of cranberry potentiating warfarin effects, sometimes fatally, in a small, randomized, placebo-controlled trial, modest cranberry consumption did not affect INR.99, 106 There are several case reports of various fruits (including pomegranate, avocado, grapefruit, mango, and papaya) interacting with warfarin.100 However, these reports are limited by the lack of standardization and quantification of fruit consumed. For now, it is recommended that in patients who prefer cranberry juice, no more than 24 ounces / day be consumed.100 In patients who prefer grapefruit juice, no more than 200cc/day should be consumed.107 Clinicians should also inquire about consumption of mangos, pomegranate, and avocados.100
Direct acting Oral Anticoagulants (DOACs)
Pharmacodynamics and pharmacokinetics
Apixaban, edoxaban, and rivaroxaban exert their anticoagulant activity by selectively inhibiting factor Xa and prothrombinase, thereby preventing the conversion of prothrombin into thrombin. Dabigatran is a direct thrombin inhibitor. These drugs reduce thrombus formation and thrombin-induced platelet aggregation. Apixaban and rivaroxaban undergo some metabolism via CYP3A4 and this can create clinically relevant drug interactions.1–4 DOACs are also substrates for the permeability glycoprotein (P-gp), a drug effluxer in the cell membranes of many barriers, with the intestinal lining and renal tubules being the sites most clinically relevant for DOAC interactions. A strong P-gp inhibitor will increase the AUC of a substrate drug by more than 2-fold.108 A P-gp inducer that will decrease the AUC of a substrate drug by more than 50% will be referred to as a strong inducer. Figure 3 provides a useful and convenient algorithm to quickly screen for clinically significant DOAC interactions, which are listed in detail on Table 5. In certain clinical scenarios, when an combination is unavoidable, plasma level assessment of the DOAC is an option, but this should only be done at specialized centers with expertise in this area.109
Figure 3:
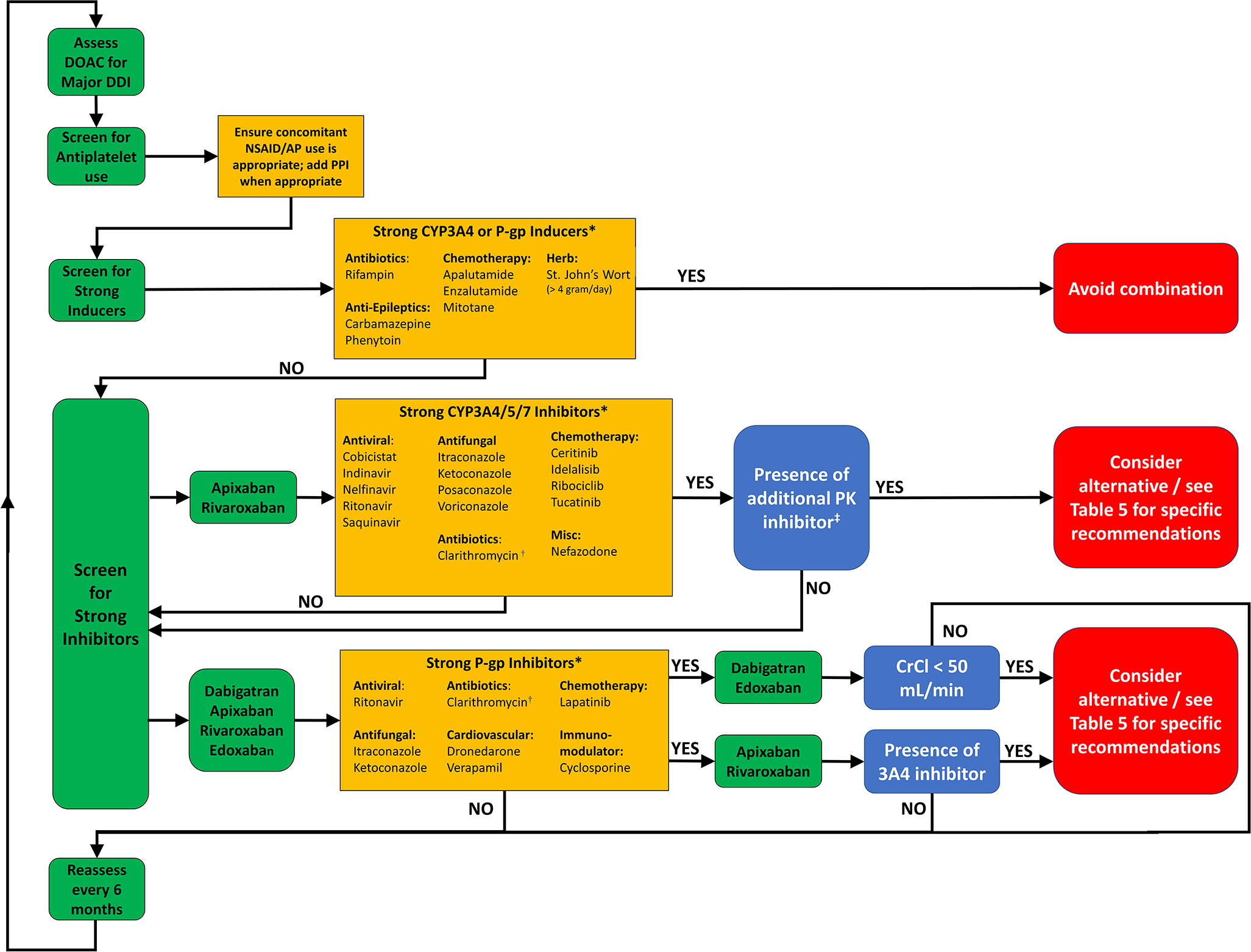
Algorithm to screen for significant direct oral anticoagulant drug-drug interactions. AP – Anti-platelet; DDI – drug-drug interaction; DOAC – direct oral anticoagulant drug-drug; CYP – Cytochrome P450; NSAID – Non-steroidal anti-inflammatory drugs; P-gp – P-glycoprotein; PK – Pharmacokinetic *List is not exhaustive †Although clarithromycin is both a strong CYP3A4 and P-gp inhibitor, pharmacokinetic analyses show that it is safe to use with apixaban and rivaroxaban ‡Refers to additive P-gp inhibition from the same interacting agent, or another agent that the patient is taking with either CYP3A4 or P-gp inhibition
Table 5:
Important DOAC-drug interactions and management recommendations
| DOAC | Precipitant Drug | Nature of Interaction | Recommendations | Clinical Significance |
|---|---|---|---|---|
| All DOACs | Apalutamide | Strong CYP3A4 induction164 | Avoid Combination1–4 | Major |
| Carbamazepine | Strong CYP3A4 induction1–4 | Avoid Combination1–4 | Major | |
| Enzalutamide | Strong CYP3A4 induction79 | Avoid Combination1–4 | Major | |
| Phenytoin | Strong CYP3A4 induction1–4 | Avoid Combination1–4 | Major | |
| Rifampin | Strong CYP3A4 induction123 | Avoid Combination1–4 | Major | |
| Ritonavir | Strong P-gp inhibition and Strong CYP3A4 inhibition163 | Avoid Combination1–4 | Major | |
| St John's Wort | Strong CYP3A4 induction1–4 | Limit St. John's Wort to less than 4g/day167 | Minor | |
| Grapefruit Juice | Strong CYP3A4 inhibition | Minimize grapefruit juice intake107 to < 200cc | Minor | |
| Apixaban | Dronedarone | Strong P-gp inhibition and moderate CYP3A4 inhibition2 | Monitor for bleeding | Minor |
| Cyclosporine | Strong P-gp inhibition and CYP3A4 inhibition2 | Combination is ok, monitor for bleeding168 | Minor | |
| Itraconazole | Strong P-gp inhibition and moderate CYP3A4 inhibition2 | Consider alternative, decrease dose by 50% if unavoidable2 | Major | |
| Ketoconazole | Strong P-gp inhibition and moderate CYP3A4 inhibition2 | Consider alternative, decrease dose by 50% if unavoidable2 | Major | |
| Nefazodone | Strong CYP3A4 inhibition13 | Ok if no other PK inhibitor is present, otherwise requires 50% apixaban dose reduction.2 | Minor | |
| Posaconazole | Strong CYP3A4 inhibition163 | Ok if no other PK inhibitor is present, otherwise requires 50% apixaban dose reduction 2 | Minor | |
| Protease inhibitors : Cobicistat Indinavir Nelfinavir Saquinavir |
Strong CYP3A4 inhibition163 | Ok if no other PK inhibitor is present, otherwise requires 50% apixaban dose reduction 2 | Minor | |
| Tyrosine kinase inhibitors | Strong CYP3A4 inhibition | Ok if no other PK inhibitor is present, otherwise requires 50% apixaban dose reduction.2 | Minor | |
| Verapamil | Strong P-gp inhibition and moderate CYP3A4 inhibition | Ok if no other PK inhibitor | Minor | |
| Voriconazole | Strong CYP3A4 inhibition2, 13 | Ok if no other PK inhibitor is present, otherwise requires 50% apixaban dose reduction.2 | Minor | |
| Dabigatran | Amiodarone | P-gp inhibition129 | Administer amiodarone 2 hours after dabigatran1 | Minor |
| Cyclosporine | Strong P-gp inhibition108 | Avoid use169 | Major | |
| Dronedarone | P-gp inhibition129 | Administer dronedarone 2 hours after dabigatran if CrCl is ≥ 50 mL/min1, decrease dose to 75mg PO BID in patients with CrCl < 50mL/min if unavoidable1, avoid if < 30 mL/min | Major | |
| Ketoconazole | Strong P-gp inhibition108 | Consider alternative, decrease dose to 75mg PO BID in patients with CrCl < 50mL/min if unavoidable1, avoid if < 30 mL/min | Major | |
| Lapatanib | Strong P-gp inhibition108 | Not studied, avoid combination1 | Major | |
| Ticagrelor | P-gp inhibition1 | Administer ticagrelor 2 hours after dabigatran1 | Minor | |
| Verapamil | P-gp inhibition129 | Administer dabigatran 2 hours before verapamil.170 | Minor | |
| Edoxaban | Cyclosporine | Strong P-gp inhibition108 | Ok unless another P-gp inhibitor is present. Reduce dose of edoxaban by 50% if anticoagulation is for venous thromboembolism or CrCl is < 50 mL/min 3, 135 | Major |
| Dronedarone | Strong P-gp inhibition108 | Administer dronedarone 2 hours after edoxaban. Reduce dose of edoxaban by 50% if anticoagulation is for venous thromboembolism or CrCl is < 50 mL/min 3, 135 | Major | |
| Itraconazole | Strong P-gp inhibition108 | Ok unless another P-gp inhibitor is present. Reduce dose of edoxaban by 50% if anticoagulation is for venous thromboembolism or CrCl is < 50 mL/min 3, 135 | Major | |
| Ketoconazole | Strong P-gp inhibition108 | Ok unless another P-gp inhibitor is present. Reduce dose of edoxaban by 50% if anticoagulation is for venous thromboembolism or CrCl is < 50 mL/min 3, 135 | Major | |
| Lapatinib | Strong P-gp inhibition108 | Ok unless another P-gp inhibitor is present. Reduce dose of edoxaban by 50% if anticoagulation is for venous thromboembolism or CrCl is < 50 mL/min3, 135 | Major | |
| Quinidine | Strong P-gp inhibition108 | Reduce dose of edoxaban by 50% if anticoagulation is for venous thromboembolism or CrCl is < 50 mL/min3, 134, 135 | Major | |
| Verapamil | Strong P-gp inhibition108 | Reduce dose of edoxaban by 50% if anticoagulation is for venous thromboembolism or CrCl is < 50 mL/min 3, 132, 135 | Minor | |
| Rivaroxaban | Amiodarone | Weak CYP3A4 and P-gp inhibition (PI) | Monitor for bleeding | Minor |
| Cyclosporine | Strong P-gp inhibition and CYP3A4 inhibition4 | Ok unless CrCl is < 50 mL/min4, 171 | Minor | |
| Dronedarone | Strong P-gp inhibition and moderate CYP3A4 inhibition4 | Ok unless CrCl is < 80 mL/min, otherwise consider alternative | Major | |
| Itraconazole | Strong P-gp inhibition and moderate CYP3A4 inhibition4 | Avoid combination4 | Major | |
| Ketoconazole | Strong P-gp inhibition and moderate CYP3A4 inhibition4 | Avoid combination4 | Major | |
| Lapatanib | Strong P-gp inhibition108 | Not studied, avoid combination4 | Major | |
| Nefazodone | Strong CYP3A4 inhibition13 | Ok if no other PK inhibitor is present, otherwise avoid combination.4 | Minor | |
| Posaconazole | Strong CYP3A4 inhibition163 | Ok if no other PK inhibitor is present, otherwise avoid combination.4 | Minor | |
| Protease inhibitors : Cobicistat Indinavir Nelfinavir Saquinavir |
Strong CYP3A4 inhibition163 | Ok if no other PK inhibitor is present, otherwise avoid combination4 | Major | |
| Tyrosine kinase inhibitors | Strong CYP3A4 inhibition4 | Ok if no other PK inhibitor is present, otherwise consider alternative4 | Major | |
| Verapamil | Strong P-gp inhibition and moderate CYP3A4 inhibition4 | Ok unless CrCl is < 80mL/min, otherwise use diltiazem4 | Minor | |
| Voriconazole | Strong CYP3A4 inhibition13 | Ok if no other PK inhibitor is present, otherwise avoid combination.4 | Minor |
Apixaban is 25% hepatically metabolized and a substrate for P-gp.2 The enzyme mainly responsible for this metabolism is CYP3A4 with CYP1A2, 2C8, 2C9, 2C19, and 2J2 playing minor roles.2 Apixaban achieves peak plasma concentration between 1–3 hours after oral administration.110, 111 With a half-life of ~12 hours, apixaban reaches steady state levels within 3 days; it is highly bound to plasma proteins.2, 110
Edoxaban is minimally metabolized by CYP3A4 oxidation. While it is a substrate of P-gp, it is not affected by other transport proteins.3 After oral administration, edoxaban plasma concentrations peak at 1.3 hours.112 The elimination half-life is 9–11 hours, so steady state anticoagulant effects are achieved within 3 days.112, 113 Edoxaban is 55% plasma protein bound.3
Rivaroxaban is metabolized by the liver more than other DOACs; 51% of the drug is recovered in the urine and feces as inactive metabolites. CYP3A4, 3A5, and 2J2 are the primary sites for oxidative degradation. Like the other DOACs, it is also a substrate for P-gp.4 With an elimination half-life of 5–9 hours in healthy adults, steady state concentrations are expected within 2–3 days.114 Rivaroxaban levels peak just under 2 hours after oral administration.114 Rivaroxaban is extensively bound to plasma proteins. Of note, rivaroxaban absorption is significantly increased when administered with fatty food, so the manufacturer recommends that doses of 15–20 mg be taken with food, and, preferably, the largest meal of the day, to increase bioavailability.4
Dabigatran etexilate is a pro-drug, undergoing rapid ester hydrolysis to its active moiety, dabigatran, with no significant CYP 450 metabolism.1, 115 After oral administration, plasma concentrations peak within 1.5 hours.116 The drug is then eliminated renally with a half-life of 12–14 hours.116, 117 Dabigatran levels are very sensitive to P-gp interactions and renal function, and it is the only DOAC that is substantially removed by dialysis.1, 118 Dabigatran is so sensitive to P-gp modulation that it is commonly used as a probe drug to help quantify a precipitant drug’s effect on the P-gp transporter.108 A list of P-gp modulators can be found on Table 6.
Table 6:
| P-gp modulators (Lund, Terrier, FDA website) | |||
|---|---|---|---|
| Inhibitor | Inducer | ||
| Strong Inhibitor | Cyclosporine | Strong Inducer | Carbamazepine |
| Dronedarone | Rifampin | ||
| Ketoconazole | St. John’s Wort167* | ||
| Itraconazole | |||
| Lapatinib | |||
| Ritonavir | |||
| Verapamil | |||
| Weak Inhibitor | Amiodarone | Weak Inducer | Phenytoin |
| Carvedilol | |||
| Clarithromycin | |||
| Diltiazem | |||
| Eliglustat | |||
| Fluvoxamine | |||
| Maraviroc | |||
| Mirabegron | |||
| Paroxetine | |||
| Propafenone | |||
| Quinidine | |||
| Ranolazine | |||
| Simeprevir | |||
| Saquinavir | |||
| Ticagrelor | |||
| Tipranavir | |||
| Uncertain Inhibitor | Carbozantinib | ||
| Enzlutamide | |||
| Erythromycin | |||
| Ibrutinib | |||
| Imatinib | |||
| Irbesartan | |||
| Ivermectin | |||
| Ledipasvir | |||
| Lomitapide | |||
| Loperamide | |||
| Meflokine | |||
| Ponatinib | |||
| Regorafenib | |||
| Simvastatin | |||
| Tacrolimus | |||
All DOACs must be dose-adjusted in the setting of renal impairment due to reduced clearance, but, in the setting of mild hepatic impairment, drug clearance was not significantly affected.118 In patients with moderate hepatic impairment (Child-Pugh B), dabigatran and apixaban exposure was not significantly increased while manufacturers for edoxaban and rivaroxaban specifically do not recommend use in this patient population.1–4, 119, 120
Apixaban drug interactions
Apixaban interacts with drugs that induce or inhibit CYP3A4 or P-gp.2, 121–123 Pharmacokinetic analysis reports significant decreases in (AUC∞) with rifampin, a combined P-gp and strong CYP3A4 inducer.123 Reduced drug exposure could lead to an increased risk of thrombotic outcomes and lower efficacy. Conversely, combined P-gp and strong CYP3A4 inhibitors increase maximum concentration (Cmax) and AUC∞, increasing the risk of bleeding.121, 122 However, this effect is less pronounced with P-gp inhibitors that only moderately inhibit CYP3A4.121 This has led researchers to question the significance of P-gp transporter interactions and assert CYP3A4 interactions may play a greater role than P-gp interactions in affecting apixaban metabolism.121, 124, 125
Extensive pharmacokinetic studies detailing the effect of strong inhibitors and inducers on DOAC plasma concentrations outside of healthy controls are not available. One retrospective study examining the safety of clarithromycin (a strong CYP3A4 and P-gp inhibitor) versus azithromycin (a P-gp inhibitor) for patients taking DOACs, including apixaban, found that clarithromycin use was associated with a higher incidence of hospitalization for major bleeding.126 A post-hoc analysis of the ARISTOTLE trial evaluated the effect of drug interactions on safety and efficacy.127 Combined moderate CYP3A4 and P-gp inhibitors comprised most drug interactions with apixaban and no differences in outcome was found compared to those patients not taking potentially interacting medications.127
Experts recommend avoiding the combination of apixaban with a strong CYP3A4 and P-gp inhibitor. If the combination of apixaban and strong CYP3A4 inhibitors is unavoidable, reduce the dose of apixaban by 50% if on a regimen of 5–10 mg by mouth twice daily.2 Concomitant use is not recommended for dosing regimens of 2.5 mg by mouth twice daily.2 Empiric reduction of standard apixaban doses is not recommended when co-administrated with moderate CYP3A4 inhibitors. Strong inducers of CYP3A4 or P-gp should be avoided with apixaban use.2, 128
Dabigatran drug interactions
The majority of drug interactions involve P-gp inhibitors and inducers. Co-administration of rifampin, a P-gp inducer, for 6 days decreased total AUC of dabigatran by 66%. This effect was no longer present 7 days after daily rifampin administration.1. Therefore, strong inducers of P-gp should be avoided with dabigatran.1, 128 The co-administration of P-gp inhibitors likewise increases dabigatran effects by enhancing absorption.1, 129, 130 The administration of ketoconazole more than doubles the plasma dabigatran concentrations, and dosing adjustments should be made if there is renal impairment. (Table 5) 1 Since dabigatran is ~80% eliminated renally, drug accumulation due to strong P-gp inhibition in the setting of renal impairment (CrCl < 50 mL/min) is significantly exacerbated, likely more than tripling the AUC, and should therefore be avoided. (Figure 3) 1, 116, 128, 131 However, administration of amiodarone, dronedarone, and verapamil only moderately increased dabigatran AUC0–∞ by 12%, 10%, 23% respectively in real-world, pharmacokinetic studies.129, 130 Furthermore, this effect, potentiated by P-gp, can be mitigated when the drugs are administered 2 hours after dabigatran.1, 130 A good example of this is seen with a loading dose of ticagrelor, which increases the peak concentration of dabigatran by 65% if given together; however, when the dose is staggered 2 hours apart, the peak concentration is only increased by 24%.1
Edoxaban drug interactions
As edoxaban is minimally metabolized by CYP3A4, drug interactions with this CYP enzyme are less relevant than those of P-gp inhibitors.122, 132, 133 One study evaluated the pharmacokinetic effect of strong CYP3A4 and/or P-gp inhibitor coadministration on edoxaban plasma concentrations in healthy volunteers.122 CYP3A4 inhibition alone did not significantly affect edoxaban exposure and serum concentration; however, P-gp inhibition with or without CYP3A4 inhibition increased edoxaban Cmax and AUC0–∞ by greater than 2.3-fold and 1.7-fold, respectively.132, 133 P-gp inhibition occurs primarily through intestinal P-gp efflux transporters based on bioavailability data which demonstrated significantly greater increases in edoxaban AUC0–∞ with oral compared to intravenous administration.134 While data from clinical trials do not support dose reduction of edoxaban in the setting of a strong P-gp inhibitor, some experts recommend switching edoxaban to warfarin instead, especially in the setting of renal impairment (CrCl < 50mL/min).3, 128, 135–137 Strong CYP3A4 and P-gp inducers should be avoided with edoxaban.3, 128
Rivaroxaban drug interactions
Rivaroxaban has a similar drug interaction profile as apixaban because it is metabolized by enzymes CYP3A4 and 2J2 and is also a substrate of P-gp.2, 138 Pharmacokinetic studies show that rivaroxaban Cmax and AUC∞ are significantly increased and total body clearance is decreased when given with combined strong CYP3A4, CYP2J2, and P-gp inhibitors such as ketoconazole, ritonavir, and cobicistat.122, 138 When rivaroxaban is given to patients taking a strong CYP3A4 and P-gp inducer, Cmax and AUC∞ are decreased.4
Rivaroxaban drug-drug interactions can be clinically relevant. Multiple studies have evaluated the clinical effect of combined strong CYP3A4 and P-gp inhibitor use on patients taking rivaroxaban and found increased risk of major and minor bleeding.126, 139 Patients taking rivaroxaban, in combination with strong CYP3A4 and P-gp inducers, have reduced effectiveness as evidenced by recurrent thromboembolism.140, 141 Therefore, concomitant use of strong CYP3A4 and P-gp inhibitors or inducers with rivaroxaban should be avoided.128 Moderate CYP3A4 and P-gp inhibitors increase rivaroxaban plasma concentrations, though this does not correlate to an increased risk of bleeding in patients with normal kidney function.138, 142–144. In a pharmacokinetic study evaluating the effect of renal dysfunction on the concentration of rivaroxaban in subjects taking erythromycin, a moderate CYP3A4 and P-gp inhibitor, AUC∞ was significantly increased by 76% and 99% in mild and moderate renal impairment, respectively.4 Cmax was also increased in both groups.4 P-gp is primarily responsible for active renal secretion of rivaroxaban which could explain why these inhibitors have a greater effect in patients with renal dysfunction.122, 124, 138 However, a post hoc analysis of the ROCKET-AF trial demonstrated that there was not a clinically significant difference in terms of efficacy and safety outcomes between those taking a moderate inhibitor of CYP3A4 randomized to receive rivaroxaban vs. warfarin.145 Therefore, as long as renal function is preserved, moderate CYP3A4 and P-gp inhibitors are safe to use with rivaroxaban.146
Oral anticoagulants and anti-arrhythmic drugs
Quinidine is a moderate inhibitor of P-gp and modestly increases the plasma concentration of edoxaban and dabigatran.3, 108, 136 In patients who are at high risk for edoxaban or dabigatran accumulation, such as those with impaired renal function or taking another P-gp inhibitor, consider using warfarin instead.128 Quinidine is also a weak inhibitor of CYP3A4 and INR should be monitored more frequently when quinidine is initiated in the setting of chronic warfarin use.14, 147
Dronedarone is a strong inhibitor of P-gp and simultaneous oral administration with dabigatran can double plasma concentrations.1 This increase in plasma concentration can be tempered by administering dronedarone 2 hours after DOAC administration, but this combination should generally be avoided with all DOACs. 128, 147 While a study reported no clinically significant elevation in INR in patients taking warfarin who were initiated on dronedarone, a moderate CYP3A4 inhibitor, cases of dronedarone-associated increases in INR have been reported.148, 149
Amiodarone is a less potent inhibitor of P-gp, can be co-administered with DOACs as long as other risk factors for DOAC accumulation, such as impaired renal function or administration of another P-gp inhibitor, are not present.128 Amiodarone is a moderate inhibitor of CYP2C9 which increases warfarin levels in proportion to the dose of amiodarone administered.150, 151 In a national database analysis of 754 patients, the mean INR increased from 2.6 to 3.1 following initiation of amiodarone therapy, necessitating an average 25% decrease in warfarin dose.152
Verapamil is a potent inhibitor of P-gp.108 Like dronedarone, simultaneous administration with dabigatran significantly increased dabigatran plasma concentrations and can be avoided by spacing verapamil 2 hours after dabigatran is given.1 However, verapamil should be avoided if risk factors for DOAC accumulation are present.128 In contrast, diltiazem did not significantly increase plasma concentrations of apixaban, and may be a more suitable combination in some circumstances.2 Diltiazem, also a moderate CYP3A4 inhibitor, also does not appear to impair warfarin clearance to an appreciable extent.153
Oral anticoagulants with antiplatelet therapy
Perhaps one of the most important and common anticoagulant drug-drug interactions cardiologists encounter is concomitant antiplatelet therapy. As both of these classes of drugs increase the risk of bleeding, it is not surprising that adverse bleeding is compounded when taken together.154 However, there are many scenarios when these classes of drugs have to be taken together, such as AF patients undergoing angioplasty or in the immediate post-procedural setting after valve implantation.155, 156 In these situations, clinicians must be familiar with the indications for concomitant anticoagulant/antiplatelet administration and minimize the amount of overlap between these drug classes per current guidelines and recommendations to optimize risk/benefit ratio of this combination therapy.155, 156
Anticoagulants and antiplatelet drugs are commonly given together in the immediate postoperative setting after valve implantation. Patients with a transcatheter aortic valve replacement should receive anticoagulation during the first 3 months after valve implantation, whereas those with other bioprosthetic valves should receive anticoagulation for the first 6 months and warfarin should be used during this period, targeting an INR of 2.5.156 The addition of aspirin to warfarin during this period was shown to decrease the incidence of thrombotic events (1.0% vs 0.6%) at the expense of increased bleeding (1.4% vs 2.8%) at 3 months in a registry cohort of 25,656 patients undergoing bioprosthetic aortic valve implantation.157 Therefore, it is reasonable to add aspirin to warfarin in patients at high risk for thrombotic complications during the initial 3 months after valve implantation along with a PPI, but the dose of aspirin should not exceed 100 mg.90, 156 For patients with AF or another long-term indication for anticoagulation, anticoagulation can be continued alone, either with warfarin or a DOAC, as there is no evidence to support continued antiplatelet use beyond the initial 3–6 months unless a patient is at an exceptionally high risk for stroke or has another indication for antiplatelet use.156, 158 This is in contrast to patients with mechanical valves, who require lifelong aspirin, 75–100mg/day, in addition to anticoagulation with warfarin only and not a DOAC.156 The addition of clopidogrel after TAVR to aspirin and warfarin can be considered in certain high-risk patients, but the risk of bleeding needs to be carefully weighed against the potential benefits, and should not exceed 6 months.156
For patients with AF or an indication for anticoagulation, acute coronary syndrome (ACS) is the most common scenario when dual antiplatelet therapy must be initiated on top of anticoagulation. Prospective randomized studies have demonstrated that “triple therapy”, or aspirin + a P2Y12 inhibitor + an OAC, is no better than a P2Y12 inhibitor + anticoagulant at preventing thrombotic events and triple therapy caused more bleeding events.159 The initial antiplatelet of choice in patients receiving anticoagulation during ACS is clopidogrel due to higher risk of bleeding associated with ticagrelor versus clopidogrel.155 However, in patients who are at an exceptional high-risk for stent thrombosis, the risks of bleeding may not outweigh the benefits of ticagrelor use, and ticagrelor may be a better P2Y12 inhibitor to use in these circumstances.155 Currently, triple therapy should only be reserved to patients at the highest risk for thrombotic complications, and triple therapy should ideally not exceed 30 days.155, 160 The dose of aspirin should not exceed 100 mg in these cases.155 Apixaban was superior to warfarin in reducing bleeding events in the setting of ACS with no difference in thrombotic events, and guidelines currently recommend a DOAC over warfarin.155, 160 In an effort reduce GI bleeding, a proton pump inhibitor should be initiated prophylactically in patients on simultaneous antiplatelet and anticoagulant therapy.90, 155
In patients with new onset AF with an indication for anticoagulation who are already on aspirin for ACS, aspirin should be discontinued after anticoagulation is initiated. However, for patients with stable coronary artery disease, antiplatelet medications should be stopped no later than 12 months after the last percutaneous coronary intervention. Within 6 months of drug eluting stent placement, the recommended antiplatelet medication is clopidogrel, and between 6–12 months, either aspirin or clopidogrel can be given concomitantly with anticoagulation.155 As with ACS, DOAC are also preferred over warfarin in patients with stable coronary artery disease when an indication for anticoagulation is present.155
It is essential that cardiologists be aware of indications for concomitant anticoagulants / antiplatelet administration and adhere to current recommendations whenever possible to reduce the risk of adverse bleeding. Though these 2 classes of drugs should predominantly be prescribed together for the minimal amount of time recommended by guidelines, clinicians must also carefully assess risk and benefit for each patient and individualize therapy in cases when patients have an extraordinary history of bleeding or thrombosis.
Discussion/Recommendation
Warfarin has many drug interactions, but most of these can be addressed with a basic understanding of warfarin pharmacokinetics and frequent drug monitoring. On the other hand, DOACs have a very wide therapeutic window, and there are only a handful of medications that cannot be administered concomitantly with DOACs. These medications include strong inducers of CYP3A4, strong inducers of P-gp, and strong inhibitors of either or both CYP3A4 and P-gp, depending on the DOAC. These DDI interactions are particularly important in certain clinical settings, such as renal impairment or the presence of additional pharmacokinetic inhibitors.
Conclusions
Drug-drug-associated adverse effects from OACs represent a major problem for electrophysiologists. AF patients, in particular, have a high incidence of polypharmacy.161 Unsurprisingly, polypharmacy is a risk factor for DDI related adverse drug reaction.162 Strong inhibitors and inducers of CYP2C9 and CYP3A4 tend to be problematic for VKA while P-gp and CYP450 3A4 modulators affect DOAC. Dietary interactions, although substantially less important with DOAC use, is another source of interactions for VKA. As renal function is a major determinant of DOAC clearance, impaired renal function can exacerbate DOAC DDI to a greater extent than it can VKA DDI. Increasing providers’ awareness about drug-drug interactions coupled with strengthening pharmacists’ oversight will reduce drug interaction related adverse drug events.
Nonstandard Abbreviations and Acronyms:
- AF
atrial fibrillation
- AP
anti-platelet
- AUC
area under the curve
- Cmax
peak plasma concentrations
- COX
cyclooxygenase
- CRCL
creatinine clearance
- CYP
cytochrome P-450
- DDI
drug-drug interactions
- DOAC
direct oral anticoagulant
- FDA
Food and Drug Administration
- GI
gastrointestinal
- INR
international normalized ratio
- NSAID
non-steroidal anti-inflammatory drug
- OAC
oral anticoagulants
- P-gp
permeability glycoprotein
- PM
poor metabolizers
- SSRI
selective serotonin reuptake inhibitor
- TdP
torsade de pointes
- VKA
vitamin K antagonists
Footnotes
Disclosures: R.G. is a speaker and consultant for the following companies: Abbott Medical, Boston Scientific, Biotronik, Zoll Medical. R.G. serves on the advisory board for Altathera, and PaceMate (no compensation). M.K.C. receives research funding from the NIH NHLBI R01 HL111314, the American Heart Association Atrial Fibrillation Strategically Focused Research Network Grants 18SFRN34110067 and 18SFRN34170013; the NIH National Center for Research Resources for Case Western Reserve University and Cleveland Clinic Clinical and Translational Science Award UL1-RR024989, the Cleveland Clinic Department of Cardiovascular Medicine philanthropy research funds, and the Tomsich Atrial Fibrillation Research Fund. J.W.D. is a speaker/consultant and receives research grants from the following companies: Pfizer/BMS, Biosense Webster, Medtronic, Biotronik. M.D.E. is a consultant for Alta Thera, Anthos Therapuetics, Biogen Idec, Boston Scientific, Sanofi-Aventis, Daiichi Sankyo, Pfizer. M.D.E. receives grants from Pfizer, Boehringer Ingelheim, D.L. is a speaker/consultant and receives honoraria/research grants from the following companies: Abbott, Janssen, Boston Scientific, Johnson and Johnson, Biotronik, Bristol Myers Squibb, Pfizer, Northeast scientific, Acutus. G.Y.H.L. is a speaker and consultant for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. P.A.N. receives research funding (outside to the submitted work) from National Institutes of Health (NIH, including the National Heart, Lung, and Blood Institute [NHLBI, R21AG 62580–1, R01HL 131535–4, R01HL 143070–2] the National Institute on Aging [NIA, R01AG 062436–1]), Agency for Healthcare Research and Quality (AHRQ, R01HS 25402–3), Food and Drug Administration (FDA, FD 06292), and the American Heart Association (18SFRN34230146, AHA). PAN is a study investigator in an ablation trial sponsored by Medtronic (no personal compensation). PAN and Mayo Clinic are involved in potential equity/royalty relationship with AliveCor. PAN and Mayo Clinic have filed patents related to the application of AI to the ECG for diagnosis and risk stratification. PAN has served on an expert advisory panel for Optum. J.A.R has served as an investigator for Medtronic, Janssen/J&J, Amarin, and Sanofi, and as a consultant for Medtronic, Sanofi, InCardia Therapeutics, Daiichi Sankyo, and Acesion. J.E.T receives research grants from the following entities: NHLBI, AHRQ, American Heart Association, Indiana Clinical & Translational Sciences Institute. J.E.T is a volunteer member of the Scientific Advisory Board for the QT drugs list at www.crediblemeds.org. B.O - DSMB Amarin REDUCE-IT, US Co-coordinator GLORIA AF Boehringer Ingelheim, Consultant Sanofi Aventis, Consultant Speaker Lundbeck. All others report none.
References:
- 1.Boehringer Ingelheim Pharmaceuticals. Pradaxa prescribing information. 2015;2015, [Google Scholar]
- 2.Squibb Bristol-Myers. Eliquis prescribing information. 2014;2015, [Google Scholar]
- 3.Sankyo Daiichi. Savaysa prescribing information. 2015;2015, [Google Scholar]
- 4.Janssen Pharmaceuticals. Xarelto prescribing information. 2015;2015,
- 5.Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, Adawi S, Lu Y, Bragazzi NL, Wu J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the global burden of disease study 2017. Eur Heart J Qual Care Clin Outcomes. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kniffin WD Jr., Baron JA, Barrett J, Birkmeyer JD, Anderson FA Jr. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med. 1994;154:861–866 [PubMed] [Google Scholar]
- 7.Chang JB, Quinnies KM, Realubit R, Karan C, Rand JH, Tatonetti NP. A novel, rapid method to compare the therapeutic windows of oral anticoagulants using the hill coefficient. Sci Rep. 2016;6:29387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suh DC, Nelson WW, Choi JC, Choi I. Risk of hemorrhage and treatment costs associated with warfarin drug interactions in patients with atrial fibrillation. Clin Ther. 2012;34:1569–1582 [DOI] [PubMed] [Google Scholar]
- 9.Flockhart DA, O’Kane D, Williams MS, Watson MS, Flockhart DA, Gage B, Gandolfi R, King R, Lyon E, Nussbaum R, et al. Pharmacogenetic testing of cyp2c9 and vkorc1 alleles for warfarin. Genet Med. 2008;10:139–150 [DOI] [PubMed] [Google Scholar]
- 10.Grossniklaus D Testing of vkorc1 and cyp2c9 alleles to guide warfarin dosing. Test category: Pharmacogenomic (treatment). PLoS Curr. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng AS, Patel RD, Quist HE, Kekic A, Maddux JT, Grilli CB, Shamoun FE. Clinical review of the pharmacogenomics of direct oral anticoagulants. Cardiovasc Drugs Ther. 2018;32:121–126 [DOI] [PubMed] [Google Scholar]
- 12.Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH. Evidence-based management of anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e152S–e184S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flockhart DA. Drug interactions flockhart table. 2015;https://drug-interactions.medicine.iu.edu/MainTable.aspx
- 14.Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, Wells PS. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095–1106 [DOI] [PubMed] [Google Scholar]
- 15.Holford NH. Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokinet. 1986;11:483–504 [DOI] [PubMed] [Google Scholar]
- 16.Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: An update. J Thromb Thrombolysis. 2011;31:326–343 [DOI] [PubMed] [Google Scholar]
- 17.Martins MA, Carlos PP, Ribeiro DD, Nobre VA, César CC, Rocha MO, Ribeiro AL. Warfarin drug interactions: A comparative evaluation of the lists provided by five information sources. Eur J Clin Pharmacol. 2011;67:1301–1308 [DOI] [PubMed] [Google Scholar]
- 18.Huupponen R, Agrawal S, Heiss MS, Fenter RB, Abramova TV, Perera MA, Pacheco JA, Smith ME, Rasmussen-Torvik LJ, George AL, Jr. Impact of cyp2c9-interacting drugs on warfarin pharmacogenomics. Drugs Aging. 2020;13:941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauta-Aho M, Teperi S, Korhonen MJ, Bell JS, Farinola N, Johns S, Shakib S, Huupponen R. Frailty and co-prescribing of potentially interacting drugs in new users of warfarin. 2020;37:373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–e88S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Udall JA. Human sources and absorption of vitamin k in relation to anticoagulation stability. Jama. 1965;194:127–129 [PubMed] [Google Scholar]
- 22.Kudo T, Endo Y, Taguchi R, Yatsu M, Ito K. Metronidazole reduces the expression of cytochrome p450 enzymes in heparg cells and cryopreserved human hepatocytes. Xenobiotica. 2015;45:413–419 [DOI] [PubMed] [Google Scholar]
- 23.Miners JO, Birkett DJ. Cytochrome p4502c9: An enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers A, Loesch EB, Weiland A, Fioravanti N, Lucius D. Preemptive warfarin dose reduction after initiation of sulfamethoxazole-trimethoprim or metronidazole. J Thromb Thrombolysis. 2017;44:88–93 [DOI] [PubMed] [Google Scholar]
- 25.Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657–663.e652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, Hennessy S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: Interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther. 2008;84:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt RK, Anderson EA, Cantrell MA, Shaw RF, Egge JA. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35–39 [DOI] [PubMed] [Google Scholar]
- 28.Israel DS, Stotka J, Rock W, Sintek CD, Kamada AK, Klein C, Swaim WR, Pluhar RE, Toscano JP, Lettieri JT, et al. Effect of ciprofloxacin on the pharmacokinetics and pharmacodynamics of warfarin. Clin Infect Dis. 1996;22:251–256 [DOI] [PubMed] [Google Scholar]
- 29.Chao CM, Lin SH, Lai CC. Abdominal wall hematoma and hemoperitoneum in an individual with concomitant use of warfarin and moxifloxacin. J Am Geriatr Soc. 2013;61:1432–1433 [DOI] [PubMed] [Google Scholar]
- 30.Zhou SF, Xue CC, Yu XQ, Li C, Wang G. Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome p450 3a4 and the role of therapeutic drug monitoring. Ther Drug Monit. 2007;29:687–710 [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Aziz MI, Ali MA, Hassan AK, Elfaham TH. Warfarin-drug interactions: An emphasis on influence of polypharmacy and high doses of amoxicillin/clavulanate. J Clin Pharmacol. 2016;56:39–46 [DOI] [PubMed] [Google Scholar]
- 32.Kim KY, Frey RJ, Epplen K, Foruhari F. Interaction between warfarin and nafcillin: Case report and review of the literature. Pharmacotherapy. 2007;27:1467–1470 [DOI] [PubMed] [Google Scholar]
- 33.King CA, Babcock KM, Godios RJ, King BS. Significant drug-drug interaction between warfarin and nafcillin. Ther Adv Drug Saf. 2018;9:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri A, Wade SL. Flucloxacillin-warfarin interaction: An under-appreciated phenomenon. Intern Med J. 2018;48:860–863 [DOI] [PubMed] [Google Scholar]
- 35.Khalili H, Nikvarz N, Najmeddin F, Dashti-Khavidaki S. A probable clinically significant interaction between warfarin and cloxacillin: Three case reports. Eur J Clin Pharmacol. 2013;69:721–724 [DOI] [PubMed] [Google Scholar]
- 36.Huwyler J, Wright MB, Gutmann H, Drewe J. Induction of cytochrome p450 3a4 and p-glycoprotein by the isoxazolyl-penicillin antibiotic flucloxacillin. Curr Drug Metab. 2006;7:119–126 [DOI] [PubMed] [Google Scholar]
- 37.O’Reilly RA. Interaction of sodium warfarin and rifampin. Studies in man. Ann Intern Med. 1974;81:337–340 [DOI] [PubMed] [Google Scholar]
- 38.de Filette J, Michiels V. Bleeding interaction between fluconazole and warfarin. Lancet. 2018;392:e9. [DOI] [PubMed] [Google Scholar]
- 39.Iversen DB, Hellfritzsch M, Stage TB, Aabenhus RM, Lind BS, Pottegård A. Antimycotic treatment of oral candidiasis in warfarin users. Am J Med. 2020 [DOI] [PubMed] [Google Scholar]
- 40.Purkins L, Wood N, Kleinermans D, Nichols D. Voriconazole potentiates warfarin-induced prothrombin time prolongation. Br J Clin Pharmacol. 2003;56 Suppl 1:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans J, Orme DS, Sedgwick ML, Youngs GR. Treating oral candidiasis: Potentially fatal. Br Dent J. 1997;182:452. [DOI] [PubMed] [Google Scholar]
- 42.Thirion DJ, Zanetti LA. Potentiation of warfarin’s hypoprothrombinemic effect with miconazole vaginal suppositories. Pharmacotherapy. 2000;20:98–99 [DOI] [PubMed] [Google Scholar]
- 43.Cullen SI, Catalano PM. Griseofulvin-warfarin antagonism. Jama. 1967;199:582–583 [PubMed] [Google Scholar]
- 44.Esterly JS, Darin KM, Gerzenshtein L, Othman F, Postelnick MJ, Scarsi KK. Clinical implications of antiretroviral drug interactions with warfarin: A case-control study. J Antimicrob Chemother. 2013;68:1360–1363 [DOI] [PubMed] [Google Scholar]
- 45.Liedtke MD, Rathbun RC. Warfarin-antiretroviral interactions. Ann Pharmacother. 2009;43:322–328 [DOI] [PubMed] [Google Scholar]
- 46.Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in hiv therapy. Ann Med. 2011;43:375–388 [DOI] [PubMed] [Google Scholar]
- 47.Kirby BJ, Collier AC, Kharasch ED, Dixit V, Desai P, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of hiv protease inhibitors 2: In vivo induction and in vitro to in vivo correlation of induction of cytochrome p450 1a2, 2b6, and 2c9 by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39:2329–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith KR, Bryan WE 3rd, Townsend ML, Randolph AE, Vanderman AJ, Woodard CL, Brown JN. Impact of prophylactic oseltamivir on inr in patients on stable warfarin therapy. J Thromb Thrombolysis. 2020;50:452–456 [DOI] [PubMed] [Google Scholar]
- 49.Andersson ML, Eliasson E, Lindh JD. A clinically significant interaction between warfarin and simvastatin is unique to carriers of the cyp2c9*3 allele. Pharmacogenomics. 2012;13:757–762 [DOI] [PubMed] [Google Scholar]
- 50.Andersson ML, Mannheimer B. The effect of simvastatin on warfarin anticoagulation: A swedish register-based nationwide cohort study. 2019;75:1387–1392 [DOI] [PubMed] [Google Scholar]
- 51.Shaik AN, Bohnert T, Williams DA, Gan LL, LeDuc BW. Mechanism of drug-drug interactions between warfarin and statins. J Pharm Sci. 2016;105:1976–1986 [DOI] [PubMed] [Google Scholar]
- 52.Trilli LE, Kelley CL, Aspinall SL, Kroner BA. Potential interaction between warfarin and fluvastatin. Ann Pharmacother. 1996;30:1399–1402 [DOI] [PubMed] [Google Scholar]
- 53.Yu CY, Campbell SE, Zhu B, Knadler MP, Small DS, Sponseller CA, Hunt TL, Morgan RE. Effect of pitavastatin vs. Rosuvastatin on international normalized ratio in healthy volunteers on steady-state warfarin. Curr Med Res Opin. 2012;28:187–194 [DOI] [PubMed] [Google Scholar]
- 54.Wiggins BS, Saseen JJ, Page RL 2nd, Reed BN, Sneed K, Kostis JB, Lanfear D, Virani S, Morris PB. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: A scientific statement from the american heart association. Circulation. 2016;134:e468–e495 [DOI] [PubMed] [Google Scholar]
- 55.Engell AE, Svendsen ALOL, B. S., Andersen CL, Andersen JS, Willadsen TG, Persson F, Pottegård A. Drug-drug interaction between warfarin and statins: A danish cohort study. 2021;87:694–699 [DOI] [PubMed] [Google Scholar]
- 56.Hansten PD, Horn JR. The top 100 drug interactions: A guide to patient management. Freeland, WA: H&H Publications, LLP; 2019. [Google Scholar]
- 57.Jähnchen E, Meinertz T, Gilfrich HJ, Kersting F, Groth U. Enhanced elimination of warfarin during treatment with cholestyramine. Br J Clin Pharmacol. 1978;5:437–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixon DL, Williams VG. Interaction between gemfibrozil and warfarin: Case report and review of the literature. Pharmacotherapy. 2009;29:744–748 [DOI] [PubMed] [Google Scholar]
- 59.Rindone JP, Keng HC. Gemfibrozil-warfarin drug interaction resulting in profound hypoprothrombinemia. Chest. 1998;114:641–642 [DOI] [PubMed] [Google Scholar]
- 60.Ascah KJ, Rock GA, Wells PS. Interaction between fenofibrate and warfarin. Ann Pharmacother. 1998;32:765–768 [DOI] [PubMed] [Google Scholar]
- 61.Polnak JF, Delate T, Clark NP. The influence of fibrate initiation on inr and warfarin dose in patients receiving chronic warfarin therapy. J Thromb Thrombolysis. 2018;46:264–270 [DOI] [PubMed] [Google Scholar]
- 62.Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Ann Pharmacother. 2004;38:50–52 [DOI] [PubMed] [Google Scholar]
- 63.Apseloff G, Wilner KD, Gerber N, Tremaine LM. Effect of sertraline on protein binding of warfarin. Clin Pharmacokinet. 1997;32 Suppl 1:37–42 [DOI] [PubMed] [Google Scholar]
- 64.Dingemanse J, Meyerhoff C, Schadrack J. Effect of the catechol-o-methyltransferase inhibitor entacapone on the steady-state pharmacokinetics and pharmacodynamics of warfarin. Br J Clin Pharmacol. 2002;53:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dumo PA, Kielbasa LA. Successful anticoagulation and continuation of tramadol therapy in the setting of a tramadol-warfarin interaction. Pharmacotherapy. 2006;26:1654–1657 [DOI] [PubMed] [Google Scholar]
- 66.Henderson L, Yue QY, Bergquist C, Gerden B, Arlett P. St john’s wort (hypericum perforatum): Drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiang TK, Ho PC, Anari MR, Tong V, Abbott FS, Chang TK. Contribution of cyp2c9, cyp2a6, and cyp2b6 to valproic acid metabolism in hepatic microsomes from individuals with the cyp2c9*1/*1 genotype. Toxicol Sci. 2006;94:261–271 [DOI] [PubMed] [Google Scholar]
- 68.Limke KK, Shelton AR, Elliott ES. Fluvoxamine interaction with warfarin. Ann Pharmacother. 2002;36:1890–1892 [DOI] [PubMed] [Google Scholar]
- 69.Mannheimer B, Andersson ML, Järnbert-Pettersson H, Lindh JD. The effect of carbamazepine on warfarin anticoagulation: A register-based nationwide cohort study involving the swedish population. J Thromb Haemost. 2016;14:765–771 [DOI] [PubMed] [Google Scholar]
- 70.Martín-Pérez M, Gaist D, de Abajo FJ, Rodríguez LAG. Population impact of drug interactions with warfarin: A real-world data approach. Thromb Haemost. 2018;118:461–470 [DOI] [PubMed] [Google Scholar]
- 71.Nadkarni A, Oldham MA, Howard M, Berenbaum I. Drug-drug interactions between warfarin and psychotropics: Updated review of the literature. Pharmacotherapy. 2012;32:932–942 [DOI] [PubMed] [Google Scholar]
- 72.Panegyres PK, Rischbieth RH. Fatal phenytoin warfarin interaction. Postgrad Med J. 1991;67:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Small NL, Giamonna KA. Interaction between warfarin and trazodone. Ann Pharmacother. 2000;34:734–736 [DOI] [PubMed] [Google Scholar]
- 74.Woolfrey S, Gammack NS, Dewar MS, Brown PJ. Fluoxetine-warfarin interaction. Bmj. 1993;307:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang FW, Liang CS. Multiple intracerebral hemorrhages in an elderly patient after adding quetiapine to a stable warfarin regimen. Gen Hosp Psychiatry. 2011;33:302.e301–302 [DOI] [PubMed] [Google Scholar]
- 76.Hauta-Aho M, Tirkkonen T, Vahlberg T, Laine K. The effect of drug interactions on bleeding risk associated with warfarin therapy in hospitalized patients. Ann Med. 2009;41:619–628 [DOI] [PubMed] [Google Scholar]
- 77.Schelleman H, Brensinger CM, Bilker WB, Hennessy S. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study. PLoS One. 2011;6:e21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carabino J, Wang F. International normalized ratio fluctuation with warfarin-fluorouracil therapy. Am J Health Syst Pharm. 2002;59:875. [DOI] [PubMed] [Google Scholar]
- 79.Gibbons JA, de Vries M, Krauwinkel W, Ohtsu Y, Noukens J, van der Walt JS, Mol R, Mordenti J, Ouatas T. Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015;54:1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikenishi M, Ueda M, Kuroda A, Tsukazaki H, Nakao M, Takeuchi M, Konishi Y, Matsuda T, Figoni W, Ohtori T, et al. A study on drug interaction between warfarin and capecitabine with special reference to the co-administered term or the discontinuation term of capecitabine. Gan To Kagaku Ryoho. 2015;42:833–839 [PubMed] [Google Scholar]
- 81.Kinikar SA, Kolesar JM. Identification of a gemcitabine-warfarin interaction. Pharmacotherapy. 1999;19:1331–1333 [DOI] [PubMed] [Google Scholar]
- 82.Kurtzhalts K, Gee ME, Feuz L, Krajewski KC. Evidence of a clinically significant interaction between warfarin and intravesical gemcitabine. Am J Health Syst Pharm. 2016;73:1508–1511 [DOI] [PubMed] [Google Scholar]
- 83.Ohno Y, Yamada M, Yamaguchi R, Hisaka A, Suzuki H. Persistent drug interaction between aprepitant and warfarin in patients receiving anticancer chemotherapy. Int J Clin Pharm. 2014;36:1134–1137 [DOI] [PubMed] [Google Scholar]
- 84.Thompson ME, Highley MS. Interaction between paclitaxel and warfarin. Ann Oncol. 2003;14:500. [DOI] [PubMed] [Google Scholar]
- 85.Nissenblatt MJ, Karp GI. Bleeding risk with trastuzumab (herceptin) treatment. Jama. 1999;282:2299–2301 [DOI] [PubMed] [Google Scholar]
- 86.Depré M, Van Hecken A, Oeyen M, De Lepeleire I, Laethem T, Rothenberg P, Petty KJ, Majumdar A, Crumley T, Panebianco D, et al. Effect of aprepitant on the pharmacokinetics and pharmacodynamics of warfarin. Eur J Clin Pharmacol. 2005;61:341–346 [DOI] [PubMed] [Google Scholar]
- 87.Chan TY, Lui SF, Chung SY, Luk S, Critchley JA. Adverse interaction between warfarin and indomethacin. Drug Saf. 1994;10:267–269 [DOI] [PubMed] [Google Scholar]
- 88.Villa Zapata L, Hansten PD, Panic J, Horn JR, Boyce RD, Gephart S, Subbian V, Romero A, Malone DC. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: A systematic review and meta-analysis. Thromb Haemost. 2020;120:1066–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi KH, Kim AJ, Son IJ, Kim KH, Kim KB, Ahn H, Lee EB. Risk factors of drug interaction between warfarin and nonsteroidal anti-inflammatory drugs in practical setting. J Korean Med Sci. 2010;25:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ray WA, Chung CP, Murray KT, Smalley WE, Daugherty JR, Dupont WD, Stein CM. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105–1112.e1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE. Acetaminophen and other risk factors for excessive warfarin anticoagulation. Jama. 1998;279:657–662 [DOI] [PubMed] [Google Scholar]
- 92.Zhang Q, Bal-dit-Sollier C, Drouet L, Simoneau G, Alvarez JC, Pruvot S, Aubourg R, Berge N, Bergmann JF, Mouly S, et al. Interaction between acetaminophen and warfarin in adults receiving long-term oral anticoagulants: A randomized controlled trial. Eur J Clin Pharmacol. 2011;67:309–314 [DOI] [PubMed] [Google Scholar]
- 93.O’Reilly RA. Lack of effect of fortified wine ingested during fasting and anticoagulant therapy. Arch Intern Med. 1981;141:458–459 [PubMed] [Google Scholar]
- 94.Nathisuwan S, Dilokthornsakul P, Chaiyakunapruk N, Morarai T, Yodting T, Piriyachananusorn N. Assessing evidence of interaction between smoking and warfarin: A systematic review and meta-analysis. Chest. 2011;139:1130–1139 [DOI] [PubMed] [Google Scholar]
- 95.Uno T, Sugimoto K, Sugawara K, Tateishi T. The role of cytochrome p2c19 in r-warfarin pharmacokinetics and its interaction with omeprazole. Ther Drug Monit. 2008;30:276–281 [DOI] [PubMed] [Google Scholar]
- 96.Meeks ML, Mahaffey KW, Katz MD. Danazol increases the anticoagulant effect of warfarin. Ann Pharmacother. 1992;26:641–642 [DOI] [PubMed] [Google Scholar]
- 97.Açıkgöz SK, Açıkgöz E. Gastrointestinal bleeding secondary to interaction of artemisia absinthium with warfarin. Drug Metabol Drug Interact. 2013;28:187–189 [DOI] [PubMed] [Google Scholar]
- 98.Lam AY, Elmer GW, Mohutsky MA. Possible interaction between warfarin and lycium barbarum l. Ann Pharmacother. 2001;35:1199–1201 [DOI] [PubMed] [Google Scholar]
- 99.Li Z, Seeram NP, Carpenter CL, Thames G, Minutti C, Bowerman S. Cranberry does not affect prothrombin time in male subjects on warfarin. J Am Diet Assoc. 2006;106:2057–2061 [DOI] [PubMed] [Google Scholar]
- 100.Norwood DA, Parke CK, Rappa LR. A comprehensive review of potential warfarin-fruit interactions. J Pharm Pract. 2015;28:561–571 [DOI] [PubMed] [Google Scholar]
- 101.Yu CM, Chan JC, Sanderson JE. Chinese herbs and warfarin potentiation by ‘danshen’. J Intern Med. 1997;241:337–339 [DOI] [PubMed] [Google Scholar]
- 102.Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, Day RO, McLachlan AJ. Effect of st john’s wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;57:592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, Day RO, McLachlan AJ. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan CSS, Lee SWH. Warfarin and food, herbal or dietary supplement interactions: A systematic review. Br J Clin Pharmacol. 2021;87:352–374 [DOI] [PubMed] [Google Scholar]
- 105.Taylor JR, Wilt VM. Probable antagonism of warfarin by green tea. Ann Pharmacother. 1999;33:426–428 [DOI] [PubMed] [Google Scholar]
- 106.Suvarna R, Pirmohamed M, Henderson L. Possible interaction between warfarin and cranberry juice. Bmj. 2003;327:1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? Cmaj. 2013;185:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lund M, Petersen TS, Dalhoff KP. Clinical implications of p-glycoprotein modulation in drug-drug interactions. Drugs. 2017;77:859–883 [DOI] [PubMed] [Google Scholar]
- 109.Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, et al. 2021 european heart rhythm association practical guide on the use of non-vitamin k antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23:1612–1676 [DOI] [PubMed] [Google Scholar]
- 110.Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, Reeves RA, LaCreta F. Apixaban, an oral, direct factor xa inhibitor: Single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75:476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81 [DOI] [PubMed] [Google Scholar]
- 112.Ogata K, Mendell-Harary J, Tachibana M, Masumoto H, Oguma T, Kojima M, Kunitada S. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50:743–753 [DOI] [PubMed] [Google Scholar]
- 113.Yin OQ, Miller R. Population pharmacokinetics and dose-exposure proportionality of edoxaban in healthy volunteers. Clin Drug Investig. 2014;34:743–752 [DOI] [PubMed] [Google Scholar]
- 114.Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of bay 59–7939, an oral, direct factor xa inhibitor. Clin Pharmacol Ther. 2005;78:412–421 [DOI] [PubMed] [Google Scholar]
- 115.Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stahle H, Rathgen K, Svard R. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:555–563 [DOI] [PubMed] [Google Scholar]
- 116.Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47–59 [DOI] [PubMed] [Google Scholar]
- 118.Mar PL, Familtsev D, Ezekowitz MD, Lakkireddy D, Gopinathannair R. Periprocedural management of anticoagulation in patients taking novel oral anticoagulants: Review of the literature and recommendations for specific populations and procedures. Int J Cardiol. 2016;202:578–585 [DOI] [PubMed] [Google Scholar]
- 119.Frost C YZ, Wang J, Li C, Zeigler C, Schuster A, Ly V, Zhang D, LaCreta F. Single-dose safety and pharmacokinetics of apixaban in subjects with mild or moderate hepatic impairment. Clin Pharmacol Ther 2009. 2013;85:S34–PI-84 [Google Scholar]
- 120.Stangier J, Stahle H, Rathgen K, Roth W, Shakeri-Nejad K. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol. 2008;48:1411–1419 [DOI] [PubMed] [Google Scholar]
- 121.Frost CE, Byon W, Song Y, Wang J, Schuster AE, Boyd RA, Zhang D, Yu Z, Dias C, Shenker A, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor xa inhibitor. Br J Clin Pharmacol. 2015;79:838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mikus G, Foerster KI, Schaumaeker M, Lehmann ML, Burhenne J, Haefeli WE. Application of a microdosed cocktail of 3 oral factor xa inhibitors to study drug-drug interactions with different perpetrator drugs. Br J Clin Pharmacol. 2020;86:1632–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vakkalagadda B, Frost C, Byon W, Boyd RA, Wang J, Zhang D, Yu Z, Dias C, Shenker A, LaCreta F. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor xa. Am J Cardiovasc Drugs. 2016;16:119–127 [DOI] [PubMed] [Google Scholar]
- 124.Hodin S, Basset T, Jacqueroux E, Delezay O, Clotagatide A, Perek N, Mismetti P, Delavenne X. In vitro comparison of the role of p-glycoprotein and breast cancer resistance protein on direct oral anticoagulants disposition. 2018;43:183–191 [DOI] [PubMed] [Google Scholar]
- 125.Sodhi JK, Liu S, Benet LZ. Intestinal efflux transporters p-gp and bcrp are not clinically relevant in apixaban disposition. 2020;37:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hill K, Sucha E, Rhodes E, Carrier M, Garg AX, Harel Z, Hundemer GL, Clark EG, Knoll G, McArthur E, et al. Risk of hospitalization with hemorrhage among older adults taking clarithromycin vs azithromycin and direct oral anticoagulants. JAMA Intern Med. 2020;180:1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Washam JB. Interacting medication use and the treatment effects of apixaban versus warfarin: Results from the aristotle trial. Br J Clin Pharmacol. 2019;47:345–352 [DOI] [PubMed] [Google Scholar]
- 128.Terrier J, Gaspar F, Fontana P, Youssef D, Reny JL, Csajka C, Samer CF. Drug-drug interactions with direct oral anticoagulants: Practical recommendations for clinicians. Am J Med. 2021;134:939–942 [DOI] [PubMed] [Google Scholar]
- 129.Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, Yusuf S, Wallentin L, Haertter S, Staab A. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the re-ly trial. J Thromb Haemost. 2011;9:2168–2175 [DOI] [PubMed] [Google Scholar]
- 130.Mochalina N, Juhlin T, Platonov PG, Svensson PJ, Wieloch M. Concomitant use of dronedarone with dabigatran in patients with atrial fibrillation in clinical practice. Thromb Res. 2015;135:1070–1074 [DOI] [PubMed] [Google Scholar]
- 131.Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: An open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–268 [DOI] [PubMed] [Google Scholar]
- 132.Mendell J, Zahir H, Matsushima N, Noveck R, Lee F, Chen S, Zhang G, Shi M. Drug-drug interaction studies of cardiovascular drugs involving p-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor xa inhibitor. Am J Cardiovasc Drugs. 2013;13:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parasrampuria DA, Mendell J, Shi M, Matsushima N, Zahir H, Truitt K. Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol. 2016;82:1591–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matsushima N, Lee F, Sato T, Weiss D, Mendell J. Bioavailability and safety of the factor xa inhibitor edoxaban and the effects of quinidine in healthy subjects. Clin Pharmacol Drug Dev. 2013;2:358–366 [DOI] [PubMed] [Google Scholar]
- 135.Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, Segers A, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415 [DOI] [PubMed] [Google Scholar]
- 136.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104 [DOI] [PubMed] [Google Scholar]
- 137.Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, Deenadayalu N, Jarolim P, Betcher J, Shi M, et al. Association between edoxaban dose, concentration, anti-factor xa activity, and outcomes: An analysis of data from the randomised, double-blind engage af-timi 48 trial. Lancet. 2015;385:2288–2295 [DOI] [PubMed] [Google Scholar]
- 138.Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: Pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoong D, Naccarato M, Gough K. Extensive bruising and elevated rivaroxaban plasma concentration in a patient receiving cobicistat-boosted elvitegravir. Ann Pharmacother. 2017;51:713–714 [DOI] [PubMed] [Google Scholar]
- 140.Altena R, van Roon E, Folkeringa R, de Wit H, Hoogendoorn M. Clinical challenges related to novel oral anticoagulants: Drug-drug interactions and monitoring. Haematologica. 2014;99:e26–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stöllberger C, Finsterer J. Recurrent venous thrombosis under rivaroxaban and carbamazepine for symptomatic epilepsy. Neurol Neurochir Pol. 2017;51:194–196 [DOI] [PubMed] [Google Scholar]
- 142.Bartlett JW, Renner E, Mouland E, Barnes GD, Kuo L, Ha NB. Clinical safety outcomes in patients with nonvalvular atrial fibrillation on rivaroxaban and diltiazem. Ann Pharmacother. 2019;53:21–27 [DOI] [PubMed] [Google Scholar]
- 143.Brings A, Lehmann ML, Foerster KI, Burhenne J, Weiss J, Haefeli WE, Czock D. Perpetrator effects of ciclosporin (p-glycoprotein inhibitor) and its combination with fluconazole (cyp3a inhibitor) on the pharmacokinetics of rivaroxaban in healthy volunteers. Eur J Clin Pharmacol. 2019;85:1528–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pham P, Schmidt S, Lesko L, Lip GYH, Brown JD. Association of oral anticoagulants and verapamil or diltiazem with adverse bleeding events in patients with nonvalvular atrial fibrillation and normal kidney function. JAMA Netw Open. 2020;3:e203593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Washam JB, Hellkamp AS, Lokhnygina Y, Piccini JP, Berkowitz SD, Nessel CC, Becker RC, Breithardt G, Fox KAA, Halperin JL, et al. Efficacy and safety of rivaroxaban versus warfarin in patients taking nondihydropyridine calcium channel blockers for atrial fibrillation (from the rocket af trial). Am J Cardiol. 2017;120:588–594 [DOI] [PubMed] [Google Scholar]
- 146.Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, Halperin JL, Hankey GJ, Hacke W, Mahaffey KW, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation. 2016;133:352–360 [DOI] [PubMed] [Google Scholar]
- 147.Konieczny KM, Dorian P. Clinically important drug-drug interactions between antiarrhythmic drugs and anticoagulants. J Innov Card Rhythm Manag. 2019;10:3552–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pogge EK, Haber SL. Elevated international normalized ratio associated with use of dronedarone and warfarin. Ann Pharmacother. 2011;45:e46. [DOI] [PubMed] [Google Scholar]
- 149.Sanofi aventis. Product labeling - multaq® (dronedarone) tablets. 2009
- 150.Heimark LD, Wienkers L, Kunze K, Gibaldi M, Eddy AC, Trager WF, O’Reilly RA, Goulart DA. The mechanism of the interaction between amiodarone and warfarin in humans. Clin Pharmacol Ther. 1992;51:398–407 [DOI] [PubMed] [Google Scholar]
- 151.Sanoski CA, Bauman JL. Clinical observations with the amiodarone/warfarin interaction: Dosing relationships with long-term therapy. Chest. 2002;121:19–23 [DOI] [PubMed] [Google Scholar]
- 152.Holm J, Lindh JD, Andersson ML, Mannheimer B. The effect of amiodarone on warfarin anticoagulation: A register-based nationwide cohort study involving the swedish population. J Thromb Haemost. 2017;15:446–453 [DOI] [PubMed] [Google Scholar]
- 153.Stoysich AM, Lucas BD, Mohiuddin SM, Hilleman DE. Further elucidation of pharmacokinetic interaction between diltiazem and warfarin. Int J Clin Pharmacol Ther. 1996;34:56–60 [PubMed] [Google Scholar]
- 154.Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: A meta-analysis of randomized trials. Arch Intern Med. 2007;167:117–124 [DOI] [PubMed] [Google Scholar]
- 155.Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, Marine JE, Mehran R, Messe SR, Patel NS, et al. 2020 acc expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: A report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. 2021;77:629–658 [DOI] [PubMed] [Google Scholar]
- 156.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 aha/acc focused update of the 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–289 [DOI] [PubMed] [Google Scholar]
- 157.Brennan JM, Edwards FH, Zhao Y, O’Brien S, Booth ME, Dokholyan RS, Douglas PS, Peterson ED. Early anticoagulation of bioprosthetic aortic valves in older patients: Results from the society of thoracic surgeons adult cardiac surgery national database. J Am Coll Cardiol. 2012;60:971–977 [DOI] [PubMed] [Google Scholar]
- 158.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 esc/eacts guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791 [DOI] [PubMed] [Google Scholar]
- 159.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115 [DOI] [PubMed] [Google Scholar]
- 160.Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, et al. Antithrombotic therapy after acute coronary syndrome or pci in atrial fibrillation. N Engl J Med. 2019;380:1509–1524 [DOI] [PubMed] [Google Scholar]
- 161.Proietti M, Raparelli V, Olshansky B, Lip GY. Polypharmacy and major adverse events in atrial fibrillation: Observations from the affirm trial. Clin Res Cardiol. 2016;105:412–420 [DOI] [PubMed] [Google Scholar]
- 162.Obreli Neto PR, Nobili A, de Lyra DP Jr., Pilger D, Guidoni CM, de Oliveira Baldoni A, Cruciol-Souza JM, de Carvalho Freitas AL, Tettamanti M, Gaeti WP, et al. Incidence and predictors of adverse drug reactions caused by drug-drug interactions in elderly outpatients: A prospective cohort study. J Pharm Pharm Sci. 2012;15:332–343 [DOI] [PubMed] [Google Scholar]
- 163.Us food and drug administration. Drug development and drug interactions -- table of substrates, inhibitors and inducers.
- 164.Duran I, Carles J, Bulat I, Hellemans P, Mitselos A, Ward P, Jiao J, Armas D, Chien C. Pharmacokinetic drug-drug interaction of apalutamide, part 1: Clinical studies in healthy men and patients with castration-resistant prostate cancer. Clin Pharmacokinet. 2020;59:1135–1148 [DOI] [PubMed] [Google Scholar]
- 165.Shatzel JJ, Daughety MM, Olson SR, Beer TM, DeLoughery TG. Management of anticoagulation in patients with prostate cancer receiving enzalutamide. J Oncol Pract. 2017;13:720–727 [DOI] [PubMed] [Google Scholar]
- 166.O’Reilly RA. Interaction of chronic daily warfarin therapy and rifampin. Ann Intern Med. 1975;83:506–508 [DOI] [PubMed] [Google Scholar]
- 167.Mueller SC, Uehleke B, Woehling H, Petzsch M, Majcher-Peszynska J, Hehl EM, Sievers H, Frank B, Riethling AK, Drewelow B. Effect of st john’s wort dose and preparations on the pharmacokinetics of digoxin. Clin Pharmacol Ther. 2004;75:546–557 [DOI] [PubMed] [Google Scholar]
- 168.Bashir B, Stickle DF, Chervoneva I, Kraft WK. Drug-drug interaction study of apixaban with cyclosporine and tacrolimus in healthy volunteers. Clin Transl Sci. 2018;11:590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abbvie inc. Product labeling - gengraf® (cyclosporine capsules). 2021
- 170.Härtter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (pradaxa(®) ) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Salerno DM, Tsapepas D, Papachristos A, Chang JH, Martin S, Hardy MA, McKeen J. Direct oral anticoagulant considerations in solid organ transplantation: A review. Clin Transplant. 2017;31 [DOI] [PMC free article] [PubMed] [Google Scholar]


