Abstract
Background
Data regarding humoral and cellular response against SARS-CoV-2 in children are scarce. We analysed seroconversion rate, decrease of anti-RBD IgG antibodies over time and T-cell response in paediatric patients who suffered COVID-19.
Methods
Longitudinal study of paediatric patients COVID-19 diagnosed by positive molecular assay in nasopharyngeal swabs. Blood samples were drawn 1–2 months and 6–7 months after acute infection. Anti-RBD IgG were determined using the Alinity® SARS-CoV-2 IgG II Quant assay (Abbott). Cellular immune response was analysed by T-SPOT® SARS-CoV-2 assay kit (Oxford Immunotec Ltd.).
Results
27/39 (69,2%) patients seroconverted. Despite a significant decrease in antibody levels over time (p < 0,01), no children seroreverted between first and second visits. Only 6/16 (37,2%) children under 6 years-old were seropositive compared to 21/23 (91,3%) over 6 years-old (p < 0,01). Highest antibody levels were found in seropositive younger children (p = 0,036). Thirteen (33,3%) children showed T-cell response. Among participants showing humoral response, no cellular response was detected in 14 (51,9%).
Conclusions
Anti-RBD IgG antibodies persistence at 6–7-months after SARS-CoV-2 infection was observed. A different IgG response was found depending on age. As measured by T-SPOT, most patients did not display cellular response 6–7 months after infection.
Keywords: SARS-CoV-2, IgG antibodies, Children, Humoral response, Cellular response
1. Introduction
It is well known that Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection presents a milder form in children than in adults (Dong et al., 2020) which results in common underdiagnosis in this population (Di Nardo et al., 2021). However, children can also develop severe respiratory disease, especially those who have comorbidities and underlying conditions (Shekerdemian et al., 2020). In addition, severe cases of multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection have been reported (Davies et al., 2020, Moraleda et al., 2021, Remppis et al., 2021, Feldstein et al., 2020).
At the present time, there are many aspects that remain unknown about pathophysiology, immune response or antibody kinetics, among others, related to SARS-CoV-2 infection. Although much information is being published about SARS-CoV-2, knowledge in some population such as children, is still limited. Understanding the kinetics of humoral response in the paediatric population could help to properly develop vaccination programs in children and a better knowledge of the response to vaccination in both patients with and without previous SARS-CoV-2 infection (Ali et al., 2021, Renk et al., 2021).
SARS-CoV-2 has a spike (S) protein which contains a receptor-binding domain (RBD). The RBD of the S-protein mediates the entry into human cells by binding the angiotensin-converting enzyme 2 (ACE-2) receptor (Di Nardo et al., 2021). Positive correlation between anti-RBD immunoglobulin G (IgG) antibody levels and the inhibition of ACE2 binding and neutralising antibodies has been described (Ni et al., 2020, Suhandynata et al., 2021, Dwyer et al., 2021). It is suggested that the determination of anti-RBD IgG antibodies could be a good marker of neutralising activity (Dwyer et al., 2021, Han et al., 2021, Beaudoin-Bussières et al., 2020). However, data in children are still uncertain and great variability has been reported across the few available studies. Some of them have described a persistence in neutralising antibodies over a period of 2–8 months, as well as an inverse correlation between neutralising antibodies and age, with younger children having the highest titre (Bonfante et al., 2021, Yang et al., 2021). In contrast, other authors have described higher anti-S IgG antibody levels in children, but with a lower overall level of neutralising activity compared to adults (Weisberg et al., 2021).
Since antibody levels are known to be variable in the population, cellular immune response to SARS-CoV-2 is also important in detecting and monitoring SARS-CoV-2 infection. Several publications have demonstrated that T-cell response to different human coronaviruses could remain active for a long time (Zuo et al., 2021, Le Bert et al., 2020) maintaining T-cell immunity for 6–9 months after infection (Zuo et al., 2021, Dan et al., 2021). Moreover, specific T-cell response could protect against reinfection (McMahan et al., 2021, Sauer and Harris, 2020). Therefore, there is an interest in measuring adaptative cellular response to SARS-CoV-2 in order to obtain a more detailed information about the immune response.
The objectives of this work were to study the seroconversion rate in paediatric patients at 1–2 months after confirmed non-severe SARS-CoV-2 infection and to analyse the decrease of anti-RBD IgG antibody levels up to 7 months after the acute infection, as well as assessing cellular immune response as measured by T-SPOT. In addition, possible related factors associated with seroconversion were described.
2. Patients and methods
2.1. Overview
This longitudinal study was conducted from August 2020 to May 2021 at Hospital Clínico San Carlos and Hospital Universitario de Getafe, Madrid, Spain. Ethics approval was obtained from the Institutional Review Board. Informed consent signed by parents or guardians was required in all patients and informed assent in the mature minor (over 12 years old), following current regulations (Declaration of Helsinki, Law 14/2007 of July 3 on Biomedical Research).
2.2. Patients and clinical features
The inclusion criteria were: children and adolescents under 18 years of age and to have a confirmed diagnosis by the SARS-CoV-2 molecular technique available in the microbiology laboratory for routine diagnosis in a nasopharyngeal swab (“COVID-19 + test”, from now on). Exclusion criteria was having any condition that could be associate with immunosuppression.
All subjects who fulfilled the inclusion criteria were offered to participate in the study, which required two appointments at the hospital for clinical follow-up and three blood samples collection.
In parallel, a cross-sectional assessment of antibody levels in control children without known SARS-CoV-2 contacts nor compatible symptoms of infection from the beginning of the pandemic was carried out. Healthy asymptomatic children and adolescents attending the hospital for routine blood draws, preoperative studies, sexually transmitted infection consultation, or the endocrinology department were offered to participate in the study as controls. Only children with negative IgG test, were selected as negative control participants (NCPs) of T-SPOT assay.
Clinical and demographical data of each participant were collected, including sex, age, signs and symptoms and duration of symptoms. None of the children was vaccinated before or during the study period.
The severity of SARS-CoV-2 infection was established as asymptomatic-mild or moderate. The asymptomatic-mild infection group included those patients with a COVID-19 + test without symptoms or signs of infection and those subjects with myalgia, anosmia, dysgeusia, fatigue, fever, symptoms of upper respiratory tract infection or digestive symptoms without pneumonia. The patients with short-term and mild shortness of breath or pneumonia were included in the moderate disease group.
The patients were also classified as short-duration (<7 days) and long-duration symptoms (≥7 days). Finally, the participants were grouped by age as young children (<6 years) and school age children (≥ 6 years).
2.3. Samples, serological and cellular assays
Two blood samples were collected from each patient at different time points. One in the first visit (1stV), established 1–2 months after the COVID-19 + test and other in the second visit (2ndV), 6–7 months after the COVID-19 + test. The samples were immediately sent to the microbiology laboratory to determinate anti-RBD IgG antibody levels. In the 2ndV, one blood sample was also collected to study the T-cell response.
Regarding to NCPs, after having a negative IgG test result, an additional blood sample was collected to analyse the T-cell response.
Detection of SARS-CoV-2 antibodies from serum samples was performed according with the manufacturer’s instructions by the Alinity® SARS-CoV-2 IgG II Quant assay (Abbott). This is a chemiluminescent microparticle immunoassay (CMIA) designed to detect IgG class immunoglobulins against the RBD region of the spike protein of SARS-CoV-2. A result of ≥ 50 AU/mL was interpreted as reactive and a result of < 50 AU/mL was interpreted as nonreactive.
Cellular immunity response was measured using the T-SPOT® SARS-CoV-2 assay kit (Oxford Immunotec Ltd., Oxford, UK), according to the manufacturer´s protocol. It is a standardised ELISPOT (Enzyme Linked ImmunoSpot) based technique intended for qualitative detection of a cell mediated (T-cell) immune response to SARS-CoV-2 in human whole blood. Peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood samples by density gradient centrifugation method (Ficoll-Paque®). Then, the PBMCs were counted by flow cytometry to be standardised in order to ensure an adequate number in all patient´s samples. The PBMCs were incubated with a Nil control to identify the non-specific cell activation, a positive control and two specific SARS-CoV-2 antigens derived from Spike and Nucleocapsid proteins to allow stimulation of any antigen specific T cells present. These cells produce IFN-gamma which is then captured by specific antibodies on the well membrane. After, a second conjugated antibody is added and binds the IFN-gamma. Finally, a substrate is added and cleaved to form a spot in the site of the reaction, which provides a measurement of the abundance of antigen specific effector T cells in the peripheral blood. Results were interpreted according to the manufacturer´s instructions. Invalid result: if the Nil control spots count is > 10; if spots in positive control panel is < 20 or if the highest count of Spike minus Nil or Nucleocapsid minus Nil is 5–7 spots. Positive result: if spots number in Spike or Nucleocapsid panels minus spots in Nil panel is ≥ 8 and negative when both counts are ≤ 4 spots.
2.4. Statistics
Qualitative variables were presented with their frequency distribution. Quantitative variables were showed with its mean value and standard deviation (SD), and those showing asymmetric distribution were summarised with the median and interquartile range (IQR).
The association between qualitative variables was evaluated with the Chi-square (χ2) test or with the Fisher's exact test when more of 25% of the expected values were less than 5.
In the study of the antibody level decrease, being a quantitative variable, the Wilcoxon non-parametric test was used, due to the small sample size of the study groups and because there were only two comparison times.
For each independent variable (sex, age, symptoms and days with symptoms), the individual association with the dependent variable “IgG result” was studied through Odds Ratio (OR) calculated using simple binary logistic regressions.
A significance value of 5% was accepted for all tests. Data analysis and processing were performed with IBM SPSS Statistics v.26 software.
3. Results
A total of 40 patients with SARS-CoV-2 infection were included in the study. One patient did not return for the second scheduled visit and was excluded. As a result, a total of 39 children were included. The mean age of the patients was 8,9 ± 6,0 years (range: 0,2–17,9) and 23 (59%) were female. Regarding to severity disease, there were 33 (84,6%) patients with asymptomatic-mild disease (5 asymptomatic, 12,8% of the total) and 6 (15,4%) with moderate disease. There were no patients with severe or critical disease. Among those subjects with mild and moderate disease, median duration of the symptoms was 6 (IQR:3–10) days.
Additionally, we selected 20 children with negative anti-RBD IgG serology results as NCPs of T-SPOT assay, as explained in methods section. T-cell response was measured in these participants, being negative all T-cell determinations. The mean age of control patients was 7,9 ± 4,4 years (range: 1,5–16,3). Seven (35%) control patients were young children (<6 years), 14 (70%) were female and there were not significant differences related to age (p = 0,65) nor sex (p = 0,41) among NCPs and convalescent children.
Seventy-eight samples were collected to determine anti-RBD IgG antibody levels at the two scheduled time points. The first antibody determination was performed a mean of 39,8 ± 9,6 days after the COVID-19 + test and the second one a mean of 195,5 ± 14,4 days after the COVID-19 + test. At the 2ndV, 39 additional blood samples were also collected to study the T-cell response.
A total of 27 (69,2%) patients had a positive result for IgG determination on both first and second visits. No seroconversion occurred between both study visits and all subjects remained positive for SARS-CoV-2 IgG antibodies in the 2ndV.
We analysed the differences in seroconversion rates among all participants. Age group was the only one showing differences in seroconversion, younger children being the group with the lowest percentage of seroconversion (37,5%) compared with a 91,3% in school age children (OR:17, 5; 95% CI: 3–102,6; p = 0,002) ( Table 1 ). Among young children showing IgG response, 2 were asymptomatic, 2 presented mild disease and 2 showed moderate disease. Regarding seronegative young children, one presented moderate disease and 9 mild symptoms.
Table 1.
IgG results.
| Patient´s characteristics | n (%) |
Anti-RBD IgG result n (%) |
OD (CI 95%) | p | ||
|---|---|---|---|---|---|---|
| Positive 27 (69,2) | Negative 12 (30,8) | |||||
| Sex | Female | 23 (59) | 18 (78,3) | 5 (21,7) | 2,8 (0,7–11,3) | 0,149 |
| Male | 16 (41) | 9 (56,2) | 7 (43,8) | |||
| Age | < 6 years | 16 (41) | 6 (37,5) | 10 (62,5) | 17,5 (3,0–102,6) | 0,002 |
| >6 years | 23 (59) | 21 (91,3) | 2 (8,7) | |||
| Symptoms | Asymptomatic-Mild | 33 (84,6) | 23 (69,7) | 10 (30,3) | 0,870 (0,136–5,545) | 0,882 |
| Moderate | 6 (15,4) | 4 (66,7) | 2 (33,3) | |||
| Symptoms-duration | < 7a days | 23 (59) | 16 (69,6) | 7 (30,4) | 1,0 (0,2–3,8) | 0,957 |
| ≥ 7 days | 16 (41) | 11 (68,8) | 5 (31,2) | |||
Five asymptomatic patients included.
The median IgG antibody levels in seropositive cases was 1455,0 UA/mL (728,2–2874,3) at the 1stV. A significant decrease (p < 0,01) was observed in the 2ndV with a median of 451,1 UA/mL (366,0–904,7). The antibody levels decreased a mean of 58% ± 34% between both visits. Table 2 shows the median antibody levels at both visits and the mean number of days on which serologies were obtained after the COVID-19 + test stratified by the different study groups.
Table 2.
Comparison of IgG antibody levels between first and second visits.
| Groups (n) | 1stV |
2ndV |
|||
|---|---|---|---|---|---|
| Days after COVID-19 + Mean ± SD | 1st IgG Median (IQR) AU/mL | Days after COVID-19 + Mean ± SD | 2nd IgG Median (IQR) AU/mL | Percentage differencea Mean ± SD (%) | |
| Seropositive patients (27) | 41,7 ± 10,1 | 1455,0 (728,2–2874,3) | 200,2 ± 14,4 | 451,1 (366,0–904,7) | -58 ± 34 |
| Male (9) | 43,0 ± 8,2 | 2057,7 (1414,7–2568,4) | 203,8 ± 14,5 | 460,7 (402,6–775,1) | -69 ± 16 |
| Female (18) | 41,0 ± 11,1 | 1222,6 (670,7–3326,6) | 201,0 ± 13,9 | 400,4 (298,9–1121,4) | -52 ± 39 |
| < 6 years (6) | 38,5 ± 10,8 | 3309,8 (2234,9–4968,6) | 205,7 ± 15,5 | 958,4 (518,7–1367,8) | -66 ± 20 |
| ≥ 6 years (21) | 42,6 ± 10,0 | 1360,0 (728,3–2076,1) | 200,1 ± 13,6 | 407,8 (345,2–716,1) | -55 ± 37 |
| Asymptomatic-Mild (23) | 41,6 ± 10,8 | 1455,0 (881,7–2786,3) | 200,1 ± 14,1 | 451,1 (366,1–904,7) | -54 ± 36 |
| Moderate (4) | 42,0 ± 5,6 | 1948,4 (664,6–5815,5) | 212,0 ± 7,0 | 433,9 (176,2–991,1) | -77 ± 6 |
| < 7 days of symptoms (16)b | 40,7 ± 10,2 | 1756,4 (943,5–3182,4) | 202,3 ± 13,2 | 483,8 (384,8–1167,2) | -59 ± 23 |
| ≥ 7 days of symptoms (11) | 42,7 ± 10,7 | 1316,5 (343,0–2786,3) | 201,5 ± 15,4 | 369,1 (221,4–645,5) | -56 ± 47 |
The antibody decrease is statistically significant (p < 0,01) in all groups, except in patients with moderate disease, which was not calculated due to the small sample size.
Relative percentage difference in IgG levels from 1st to 2nd visit.
Five asymptomatic patients included.
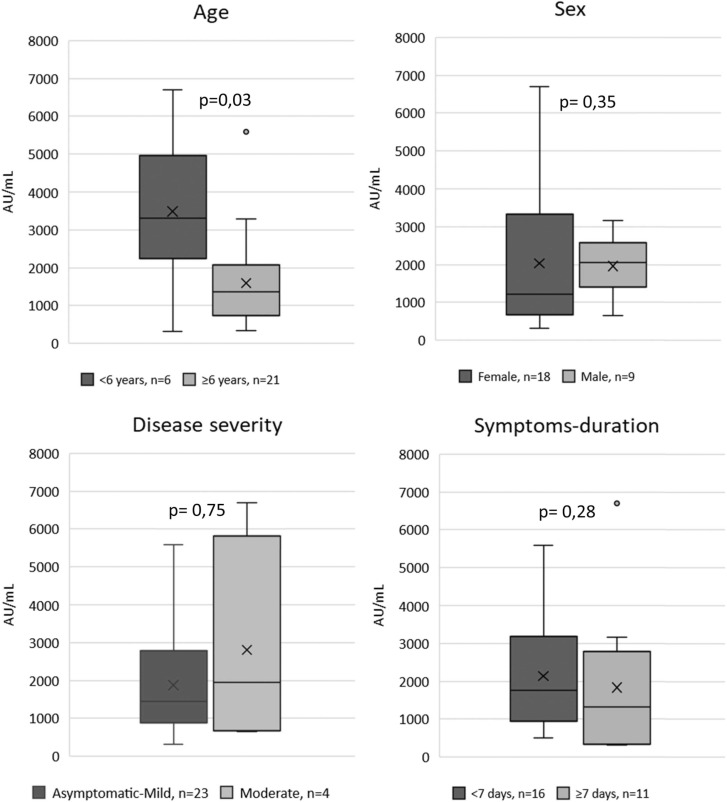
Regarding the antibody levels among seropositive cases, the younger children, showed the highest values (p = 0,036). No differences were observed in antibody levels among the other groups ( Fig. 1). A slightly negative correlation was observed between antibody levels and age, although this correlation was less pronounced at the second visit ( Fig. 2).
Fig. 1.
Antibody levels in 1st visit.
Fig. 2.
Antibody levels by age.
T-SPOT assay was positive in 13 (33,3%) patients. Statistically significant differences (p = 0,021) for cellular response were only observed in the age group, being the young children group the one that showed the lower proportion of cellular immune response 2/16 (12,5%) compared to 11/23 (47,8%) in school age children. All subjects having a negative IgG test (12 patients), also had a negative result for the detection of cellular immune response by the T-SPOT assay. Among those participants showing humoral immune response (27 patients), 14 (51,9%) of them did not show cellular immune response ( Table 3). No differences in antibody levels were observed among patients with a positive or negative T-cell result at first (p = 0,48) nor second visit (p = 0,59). When we analysed the relationship between IgG and T-cell results, we observed an association (p < 0,01), but a low concordance (kappa= 0,36) between the results of both tests.
Table 3.
Relationship between IgG positive results and the T-SPOT results classified by the different study groups.
| Patients with positive IgG result (n) |
T-SPOT result n (%) |
||
|---|---|---|---|
| Positive 13 (48,1) | Negative 14 (51,9) | ||
| Sex | Female (18) | 7 (38,9) | 11 (61,1) |
| Male (9) | 6 (66,7) | 3 (33,3) | |
| Age | <6 years (6) | 2 (33,3) | 4 (66,7) |
| ≥6 years (21) | 11 (52,4) | 10 (47,6) | |
| Symptoms | Asymptomatic-Mild (23) | 9 (39,1) | 14 (60,9) |
| Moderate (4) | 4 (100) | 0 (0) | |
| Symptoms-duration | <7 days (16)a | 8 (50) | 8 (50) |
| ≥ 7 days (11) | 5 (45,5) | 6 (54,5) | |
Five asymptomatic patients included.
4. Discussion
In our work, almost a third of SARS-CoV-2 confirmed infected children were seronegative, which is in concordance with other studies in children that showed a seroconversion rate around 70% (Breuer et al., 2021). However, between 1stV and 2ndV none of the seropositive subjects became seronegative. Regarding seroreversion rate, there is a great variability among different studies (Bonfante et al., 2021, Van Elslande et al., 2021, Méndez-Echevarría et al., 2021). It could be due to differences in methodology, as well as to the different techniques performed to analyse the humoral response (Muecksch et al., 2021).
Although younger children showed an extremely low seroconversion rate (37,5%), those seroconverting, showed higher IgG levels than school-age children. As mentioned above, higher IgG antibody levels in young children have been described in previous works (Yang et al., 2021, Breuer et al., 2021). A recent preprint study also has reported that children showed higher antibody levels and were more persistent over time than in adults (Renk et al., 2021).
In adults, several studies have reported the highest anti-RBD IgG response in those with a severe disease course (Weisberg et al., 2021, Marklund et al., 2020). In our cohort, all asymptomatic children (5 patients) showed IgG response and no differences in seroconversion were observed among children with asymptomatic-mild disease and moderate disease. This fact has been described in other studies, showing different IgG responses depending on the disease severity in adults but not in children (Weisberg et al., 2021, Breuer et al., 2021, Oygar et al., 2021). Instead, Van Elslande et al. reported lower antibody levels in asymptomatic and mild SARS-CoV-2 infected children. However, in this study, they measured anti-nucleocapsid IgG response (Van Elslande et al., 2021).
Some authors have described a more pronounced antibody decline in those patients with asymptomatic and mild disease than in patients with moderate disease (Méndez-Echevarría et al., 2021, Oygar et al., 2021). Oygar et al. also reported similar results, being the antibody decline statistically significant in asymptomatic and mild-moderate cases, but not in severe-critical cases (Oygar et al., 2021). However, in our study, we could not analyse the significance of the antibody decrease in the moderate disease group due to the small sample size of patients in this group. More studies including a larger sample of children with moderate-severe disease are needed to determine if there are differences in antibody decline in this group.
The anti-RBD IgG antibodies are the most correlated with neutralising activity and are of great utility in daily clinical setting. Vaccination of the paediatric population has now started, but there are still no long-term studies of the immune response. Several studies in adults have demonstrated that anti-RBD IgG levels were significantly higher (Lo Sasso et al., 2021, Narasimhan et al., 2021) and showed greater IgG avidity (Neumann et al., 2021) in vaccinated subjects than in recovered COVID-19 patients. Although 30.8% of our cohort were seronegative, these children would be expected to generate higher antibody levels when vaccinated than recovered COVID-19 patients.
Our work has several limitations as well as several strengths. As this is a longitudinal study including all children with confirmed COVID-19, the sample size in the different groups is heterogeneous. However, at the same time, this is a strength because it describes our real children population and its antibody response. Therefore, we have been able to observe a low seroconversion rate in young children, which could not have been detected with other study design. Most of the antibody response studies in children are carried out by selecting patients with a positive antibody test or, on the contrary, they are seroprevalence studies, which does not allow detecting those children who have been infected by SARS-CoV-2 and who have not shown antibody response.
Regarding cellular immunity, we only had the opportunity to measure the T-cell response on the second visit, due to the lack of availability in our centre of T-SPOT kits at the beginning of the study. This makes cellular immunity data difficult to interpret, as we cannot discern whether the low T-cell response in our cohort is due to an initial lack of response, because cellular immunity has been lost over time, or if it could be due to a low sensitivity of the T-SPOT test in children population. Some authors have described a more robust T-cell response in adults compared to paediatric subjects (Pierce et al., 2020). However, there are not large studies measuring T-cell response using the T-SPOT assay in children.
Our findings show a long-term persistence of anti-RBD IgG antibodies in paediatric population and no seroreversion during the study period. Our results suggest a different IgG response depending on the age, showing a low seroconversion rate among young children and a negative correlation between antibody levels and age. However, more studies in this population are needed in order to elucidate the impact of IgG antibodies in protective immunity.
Long-term and well-defined studies of the immune response, both cellular and humoral are needed to better understand its relationship with clinical features, disease outcome, protection against SARS-CoV-2 infection as well the differences in immune response between adults and children.
Author statement
AR and JTR conceptualized and designed the study. ABS, MI, IC, SG, ABP, SR, OP, RV, DLL and JTR enroled participants and participated in collection data. AR, IR, and PM performed microbiological tests. AR and RS performed data management and the statistical analysis. AR, ABP and JTR drafted the manuscript. All co-authors AR, ABS, MI, IC, SG, JTR, ABP, RS, SR, OP, RV, DLL, IR, PM participated and were involved in the preparation and critical review of the final manuscript. All authors have read and approved the revised version of the manuscript.
Declaration of interests
The authors have declared that no competing interests exist.
Acknowledgements
Manuel Enrique Fuentes Ferrer and Irene Serrano García, Instituto de Investigación Sanitaria (IdISSC) - Unidad de Apoyo Metodológico a la Investigación and Preventive Department, Madrid, Spain. Esther Culebras López, Clinical Microbiology and Parasitology Department, Hospital Clínico San Carlos, Madrid, Spain.
Funding source
This work was supported by the Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), research project [SUBV.COV.2020. JRA].
Data availability statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient confidentiality.
References
- Ali K., Berman G., Zhou H., Deng W., Faughnan V., Coronado-Voges M., et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N. Engl. J. Med. 2021;385:2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin-Bussières G., Laumaea A., Anand S.P., Prévost J., Gasser R., Goyette G., et al. Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. mBio. 2020;11 doi: 10.1128/mBio.02590-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante F., Costenaro P., Cantarutti A., Di Chiara C., Bortolami A., Petrara M.R., et al. Mild SARS-CoV-2 infections and neutralizing antibody titers. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052173. [DOI] [PubMed] [Google Scholar]
- Breuer A., Raphael A., Stern H., Odeh M., Fiszlinski J., Algur N., et al. SARS-CoV-2 antibodies started to decline just four months after COVID-19 infection in a paediatric population. Acta Paediatr. 2021;110:3054–3062. doi: 10.1111/apa.16031. Epub 2021 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc. Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo M., van Leeuwen G., Loreti A., Barbieri M.A., Guner Y., Locatelli F. A literature review of 2019 novel coronavirus (SARS-CoV2) infection in neonates and children. Pediatr. Res. 2021;89:1101–1108. doi: 10.1038/s41390-020-1065-5. [DOI] [PubMed] [Google Scholar]
- Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020:145. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- Dwyer C.J., Cloud C.A., Wang C., Heidt P., Chakraborty P., Duke T.F., et al. Comparative analysis of antibodies to SARS-CoV-2 between asymptomatic and convalescent patients. iScience. 2021;24 doi: 10.1016/j.isci.2021.102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. 10,1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Liu P., Qiu Y., Zhou J., Liu Y., Hu X., et al. Effective virus-neutralizing activities in antisera from the first wave of survivors of severe COVID-19. JCI Insight. 2021;6 doi: 10.1172/jci.insight.146267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- Lo Sasso B., Giglio R.V., Vidali M., Scazzone C., Bivona G., Gambino C.M., et al. Evaluation of anti-SARS-Cov-2 S-RBD IgG antibodies after COVID-19 mRNA BNT162b2 vaccine. Diagnostics. 2021;11:1135. doi: 10.3390/diagnostics11071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund E., Leach S., Axelsson H., Nyström K., Norder H., Bemark M., et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Echevarría A., Sainz T., Falces-Romero I., de Felipe B., Escolano L., Alcolea S., et al. Long-term persistence of anti-SARS-CoV-2 antibodies in a pediatric population. Pathogens. 2021;10:700. doi: 10.3390/pathogens10060700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraleda C., Serna-Pascual M., Soriano-Arandes A., Simó S., Epalza C., Santos M., et al. Multi-inflammatory syndrome in children related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Spain. Clin. Infect. Dis. 2021;72:e397–e401. doi: 10.1093/cid/ciaa1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F., Wise H., Batchelor B., Squires M., Semple E., Richardson C., et al. Longitudinal serological analysis and neutralizing antibody levels in coronavirus disease 2019 convalescent patients. J. Infect. Dis. 2021;223:389–398. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan M., Mahimainathan L., Araj E., Clark A.E., Markantonis J., Green A., et al. Clinical evaluation of the abbott alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J. Clin. Microbiol. 2021;59 doi: 10.1128/JCM.00388-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann F., Rose R., Römpke J., Grobe O., Lorentz T., Fickenscher H., et al. Development of SARS-CoV-2 Specific IgG and virus-neutralizing antibodies after infection with variants of concern or vaccination. Vaccines. 2021;9:700. doi: 10.3390/vaccines9070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Ye F., Cheng M.L., Feng Y., Deng Y.Q., Zhao H., et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oygar P.D., Ozsurekci Y., Gurlevik S.L., Aykac K., Kukul M.G., Cura Yayla B.C., et al. Longitudinal follow-up of antibody responses in pediatric patients with COVID-19 up to 9 months after infection. Pediatr. Infect. Dis. J. 2021;40:e294–e299. doi: 10.1097/INF.0000000000003199. 10.1097/INF.0000000000003199. [DOI] [PubMed] [Google Scholar]
- Pierce C.A., Preston-Hurlburt P., Dai Y., Aschner C.B., Cheshenko N., Galen B., et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020;12:eabd5487. doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remppis J., Ganzenmueller T., Kohns Vasconcelos M., Heinzel O., Handgretinger R., Renk H. A case series of children and young people admitted to a tertiary care hospital in Germany with COVID-19. BMC Infect. Dis. 2021;21:133. doi: 10.1186/s12879-021-05791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renk H., Dulovic A., Becker M., Fabricius D., Zernickel M., Junker D., et al. Typically asymptomatic but with robust antibody formation: children’s unique humoral immune response to SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.07.20.21260863. [DOI] [Google Scholar]
- Sauer K., Harris T. An effective COVID-19 vaccine needs to engage T cells. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.581807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhandynata R.T., Hoffman M.A., Huang D., Tran J.T., Kelner M.J., Reed S.L., et al. Commercial serology assays predict neutralization activity against SARS-CoV-2. Clin. Chem. 2021;67:404–414. doi: 10.1093/clinchem/hvaa262. 10.1093/clinchem/hvaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elslande J., Oyaert M., Ailliet S., Van Ranst M., Lorent N., Vande Weygaerde Y. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 2021;136 doi: 10.1016/j.jcv.2021.104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P., Connors T.J., Zhu Y., Baldwin M.R., Lin W.H., Wontakal S., et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.S., Costa V., Racine-Brzostek S.E., Acker K.P., Yee J., Chen Z., et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Dowell A.C., Pearce H., Verma K., Long H.M., Begum J., et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021;22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient confidentiality.