Abstract
The antifungal substances SH-1 and SH-2 were isolated from Streptomyces humidus strain S5-55 cultures by various purification procedures and identified as phenylacetic acid and sodium phenylacetate, respectively, based on the nuclear magnetic resonance, electron ionization mass spectral, and inductively coupled plasma mass spectral data. SH-1 and SH-2 completely inhibited the growth of Pythium ultimum, Phytophthora capsici, Rhizoctonia solani, Saccharomyces cerevisiae, and Pseudomonas syringae pv. syringae at concentrations from 10 to 50 μg/ml. The two compounds were as effective as the commercial fungicide metalaxyl in inhibiting spore germination and hyphal growth of P. capsici. However, the in vivo control efficacies of the two antifungal compounds against P. capsici infection on pepper plants were similar to those of H3PO3 and fosetyl-AI but less than that of metalaxyl.
Streptomyces spp. are capable of producing microbial antibiotics with a wide variety of chemical structures. In particular, approximately 60% of antibiotics developed for agricultural use were isolated from Streptomyces spp. (32). It is interesting that Streptomyces strains continue to provide a larger number and wider variety of new antibiotics than any other actinomycete genus, suggesting that substantial numbers of Streptomyces species or strains with novel antibiotic productivity exist in nature (27). In searches for bioactive antibiotics, Streptomyces strains have been isolated from various types of soils, including rice paddy, lake mud and water, deciduous forest, tropical forest, wasteland, and cave soils (9, 16, 19, 30, 31, 34).
So far, various antifungal antibiotics active against the oomycete plant pathogen Phytophthora have been isolated and characterized from actinomycetes (2, 12, 13, 15, 20–23). In our previous search program for microorganisms producing antifungal antibiotics useful for the control of plant diseases, Streptomyces humidus strain S5-55 was isolated from soils in Korea, which showed substantial antagonistic activity against plant pathogens (25). The antifungal substances active against Phytophthora capsici and Magnaporthe grisea were partially purified from the culture filtrates of S. humidus strain S5-55. In the present study, the antifungal substances SH-1 and SH-2, active against some plant-pathogenic fungi, were purified from the culture broth of S. humidus strain S5-55 by various purification procedures. By analyzing various spectral and other physicochemical data, their chemical structures were elucidated and the two compounds were identified as phenylacetic acid (SH-1) and sodium phenylacetic acid (SH-2). In addition to an in vitro bioassay for antifungal activity, we also evaluated the control efficacy of SH-1 and SH-2 against phytophthora blight of pepper plants compared to those of commercial fungicides.
Isolation of antifungal substances from S. humidus cultures.
S. humidus strain S5-55 antagonistic to various plant-pathogenic fungi was isolated from soil from Kwangwon Province in Korea (25). The culture broth (100 liters) of strain S5-55, which was incubated in soluble starch broth (5 g of soluble starch, 10 g of glycerol, 4 g of yeast extract, 0.3 g of K2HPO4, 0.2 g of KH2PO4, 0.5 g of MgSO4 · 7H2O [all in 1 liter of H2O]) at 28°C on a rotary shaker at 150 rpm for 14 days, was centrifuged at 1,250 × g for 30 min and filtered through Whatman no. 2 filter paper. The culture filtrate was extracted with n-butanol (100 liters). The butanol phase was concentrated in vacuo by using a rotary evaporator (Büchi, Switzerland). The crude extracts were purified by C18 reversed-phase flash column chromatography. The open glass column (150 by 200 mm) was packed with C18 resin (Lichroprep RP-18, 40-63 μm; Merck, Darmstadt, Germany). The column loaded with crude extracts was eluted with stepwise gradients of methanol and water (0:100, 20:80, 40:60, 60:40, 80:20, and 100:0 [vol/vol]). Each fraction (2.5 liters) of the eluate was concentrated in vacuo. The antifungal activity of each fraction against P. capsici, M. grisea, and Rhizoctonia solani was measured by a paper disk method (21). The 40% methanol fraction (7.5 ml), which showed a high antifungal activity, was further purified by preparative thin-layer chromatography (TLC) (silica gel 60 F254 [0.2 mm thick]; Merck). TLC plates loaded with crude extracts were developed with a chloroform-methanol (8:2 [vol/vol]) solvent system. After the plate was air dried, a silica gel band which showed antifungal activity against P. capsici and M. grisea at the position of Rf 0.7 was collected by scraping off the band and then extracting it with methanol. The inhibition zones produced on TLC plates were visualized by the bioautographic technique (11).
The antifungal extract was concentrated to dryness and dissolved in 4 ml of methanol. The crude substances were purified on a Sephadex LH-20 column (26 by 950 mm column packed with Sephadex LH-20 resin; Pharmacia, Uppsala, Sweden). Each fraction (2 ml) was collected using a fraction collector (RediFrac; Pharmacia). The antifungal activity of the fractions against P. capsici and M. grisea was examined by the paper disk method. Fractions 71 to 79 (SH-1) and 89 to 97 (SH-2) showed antifungal activity against P. capsici. The antifungal substances SH-1 and SH-2 were further purified by a preparative high-performance liquid chromatographic system (Gilson, Middleton, Wis.) with a C18 reversed-phase column (SymmetryPrep C18, 7 μm, 7.8 by 300 mm, Waters). The antifungal substances SH-1 and SH-2 were eluted using a linear gradient solvent system from 10% acetonitrile in water to 100% acetonitrile at a flow rate of 2 ml/min under the UV absorbance of 210 nm. The pure antifungal substance SH-1 was obtained from a single peak with the retention time of 22.06 min at 210 nm. The pure antifungal substance SH-2 was also obtained from a peak at the retention time of 5.50 min at 210 nm. Finally, 150 and 100 mg of the antifungal substances SH-1 and SH-2, respectively, were produced from 100 liters of the culture extracts.
Structure elucidation of SH-1 (phenylacetic acid) and SH-2 (sodium phenylacetate) within S. humidus cultures.
The UV absorption spectra of SH-1 and SH-2 were measured with a Beckman DU 650 spectrometer (Beckman Instruments Inc., Fullerton, Calif.). Nuclear magnetic resonance (NMR) spectra of the purified antifungal substances SH-1 and SH-2 were recorded on a Bruker AMX 500 NMR spectrometer (Billerica, Mass.). 1H NMR and 13C NMR spectra were measured in CD3OD. Low-resolution electron ionization (EI) mass spectra were recorded with a VG70-VSEQ mass spectrometer (VG ANALYTICAL, Manchester, United Kingdom) to elucidate the structures of antifungal substances SH-1 and SH-2. Inductively coupled plasma (ICP) mass spectra were recorded with an Elan 6100 mass spectrometer (Perkin-Elmer, Norwalk, Conn.) to elucidate the structure of antifungal substance SH-2.
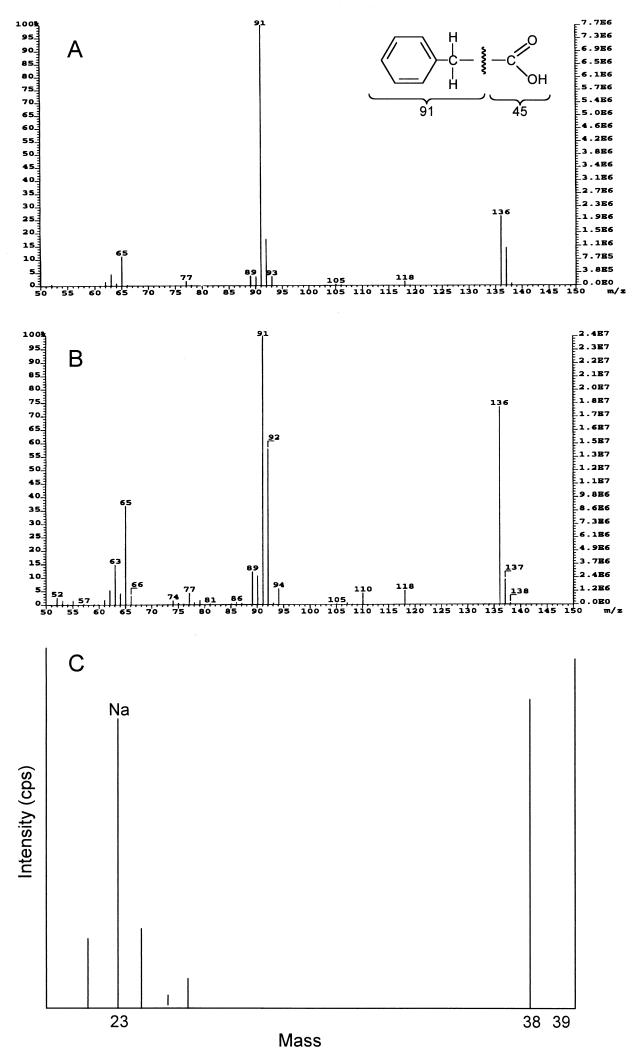
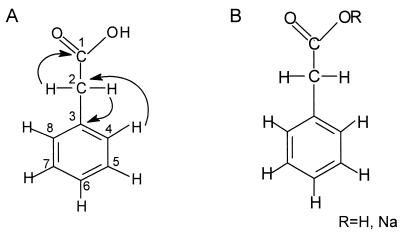
The structure of antifungal substance SH-1 was elucidated by EI mass spectral, 1H, 13C, and two-dimensional NMR (COSY, HMQC, and HMBC) spectral analyses. Based on the EI mass spectral data, the molecular formula of SH-1 was deduced to be C8H8O2. The antifungal substance SH-1 gave molecular ion at m/z 136 (M+): EI MS m/z 65 (10%), 91 (97%), 92 (19%), and 136 (26%) (Fig. 1A). NMR data indicated a hydrogen count of eight, including one exchangeable proton, and a carbon count of eight in CD3OD. 1H NMR (CD3OD): δ 3.58 (2H, s) and 7.32-7.20 (5H, m) ppm; 13C NMR δ 175.56 (C-1), 136.07 (C-3), 130.34 (C-4, C-8), 129.45 (C-5, C-7), 127.89 (C-6), and 41.94 (C-2) ppm. The COSY and HMQC spectral data revealed that SH-1 has three partial structures (Fig. 2A). With the HMBC spectral data, the substructure aromatic ring could be connected to methylene protons (δ 3.58). Methylene protons were connected to the carbonyl carbon (δ 175.56) and C-3 (δ 139.41) in aromatic ring. With all the spectral data, the structure of antifungal substance SH-1 was determined to be phenylacetic acid (Fig. 2B). The structure of antifungal substance SH-2 was elucidated by EI mass spectral, NMR, and ICP mass spectral analyses. Based on the EI mass spectral data, the antifungal substance SH-2 gave molecular ion at m/z 136 (M+): 136 (70%), 92 (60%), 91 (99%), 65 (35%), and 63 (15%) (Fig. 1B). NMR data indicated a hydrogen count of seven and a carbon count of eight in CD3OD. 1H NMR: δ 3.46 (2H, s) and 7.14 (1H, tt, J = 7.3, 1.9 Hz), 7.24 (2H, brt, J = 7.4 Hz), 7.31 (2H, brd, J = 7.5 Hz) ppm; 13C NMR: δ 180.55 (C-1), 139.41 (C-3), 130.28 (C-4, C-8), 129.15 (C-5, C-7), 126.92 (C-6), and 46.43 (C-2) ppm. Analyses of two-dimensional NMR spectrum indicated that the organic portion of the structure of SH-2 was identical to that of SH-1. However, the ICP mass spectral data confirmed that Na ion exists in the structure of SH-2 (Fig. 1C). Based on all the spectral data, the antifungal substance SH-2 was determined to be sodium phenylacetate (Fig. 2B). When the SH-2 powder (1 mg) was hydrolyzed with a small amount of 0.01 N HCl in methanol, the active peak appeared at the same retention time of SH-1 as the original SH-2 peak by high-performance liquid chromatography. The melting point of SH-1 was dramatically higher than that of SH-2 (data not shown). The only difference in the NMR spectral data was that there was no proton at carbonyl residue in SH-2. ICP mass spectral data were further examined to confirm whether SH-2 is a salt form of SH-1. The intensity level (in counts per second) of Na was higher than that of standard Na level in ICP mass spectral data.
FIG. 1.
EI mass spectrum (A) of the antifungal substances SH-1 (phenylacetic acid), and EI (B) and ICP (C) mass spectra of SH-2 (sodium phenylacetate).
FIG. 2.
(A) Correlations of partial structures of the antifungal substance SH-1 from HMBC spectra and (B) structures of the antifungal substances SH-1 (R = H) and SH-2 (R = Na) isolated from S. humidus strain S5-55.
In vitro antimicrobial activity of SH-1 (phenylacetate acid) and SH-2 (sodium phenylacetate).
Microorganisms such as Alternaria mali, Colletotrichum orbiculare, Cylindrocarpon destructans, Fusarium moniliforme, Fusarium oxysporum f. sp. cucumerinum, M. grisea, Didymella bryoniae, R. solani, P. capsici, Pythium ultimum, Bacillus subtilis, Pseudomonas syringae pv. syringae, Saccharomyces cerevisiae, and Candida albicans were used to determine the MICs of SH-1 (phenylacetic acid) and SH-2 (sodium phenylacetate) using a modified version of the antimicrobial bioassay method of Nair et al. (26). Potato dextrose broth (1 ml) supplemented with SH-1 and SH-2 at concentrations from 0 to 1,000 μg/ml was pipetted into each well of a 24-well microtiter dish (Cell Wells; Corning Glass Works, Corning, N.Y.) to ascertain the MICs against fungi. Nutrient broth was also used for to ascertain the MICs against bacteria and yeasts. Germ suspension (10 μl) was added to each well. The concentration of fungal spores or zoospores tested was 104 spores/ml. Bacteria and yeasts were adjusted to 104 CFU/ml. The inoculated plates were incubated at 28°C on a rotary shaker at 120 rpm. The inhibition of microbial growth was evaluated after incubation for 3 or 4 days. The lowest concentrations of SH-1 and SH-2 that completely inhibited microbial growth were considered to be MICs. SH-1 and SH-2 completely inhibited the growth of P. capsici, R. solani, S. cerevisiae, and P. syringae pv. syringae at the concentration of 50 μg/ml (Table 1). The growth of P. ultimum was also completely inhibited at 10 μg/ml, whereas A. mali, C. destructans, F. moniliforme, and F. oxysporum f. sp. cucumerinum showed little inhibition even at 500 or 1,000 μg/ml. The antifungal substances SH-1 and SH-2 exhibited an intermediate level of inhibitory activity against C. orbiculare, C. albicans, and B. subtilis, with MICs ranging from 50 to 100 μg/ml.
TABLE 1.
MICs of antifungal substances SH-1 (phenylacetic acid) and SH-2 (sodium phenylacetate) from S. humidus strain S5-55 against various microorganisms
| Test organism | MIC (μg/ml)a
|
|
|---|---|---|
| SH-1 | SH-2 | |
| A. mali | 500 | >1,000b |
| C. orbiculare | 100 | 50 |
| C. destructans | 500 | >1,000 |
| F. moniliforme | >1,000 | >1,000 |
| F. oxysporum f. sp. cucumerinum | >1,000 | >1,000 |
| M. grisea | 100 | >1,000 |
| P. capsici | 50 | 50 |
| P. ultimum | 10 | 10 |
| R. solani | 50 | 50 |
| C. albicans | 100 | 100 |
| S. cerevisiae | 50 | 50 |
| B. subtilis | 100 | 100 |
| P. syringae pv. syringae | 50 | 50 |
The lowest concentration that completely inhibited the growth of microorganisms was examined after incubation for 3 or 4 days.
>1,000 means that the growth of microorganisms was not inhibited at a concentration of 1,000 μg/ml.
Zoospore suspension of P. capsici was prepared by the method of Kim et al. (24) using the culture plates grown on oatmeal agar for 10 days at 28°C. The zoospore suspensions were mixed with SH-1, SH-2, metalaxyl, fosetyl-AI, and H3PO3 to give the concentrations of 0, 1, 10, 50, 100, and 500 μg/ml. After incubation for 4 h at 28°C, zoospore germination was microscopically examined in two experiments with five replicates. SH-1 and SH-2 completely inhibited zoospore germination of P. capsici at 50 μg/ml, whereas the commercial fungicide metalaxyl was not effective against zoospore germination at concentrations up to 100 μg/ml (data not shown). There was no difference between SH-1 and SH-2 in inhibiting zoospore germination. Treatment with H3PO3 began to inhibit zoospore germination at 1 μg/ml and the inhibitory effect was maximum at 100 μg/ml.
To examine the inhibitory effects of the chemicals on hyphal growth of P. capsici, SH-1, SH-2, metalaxyl, fosetyl-AI, and H3PO3 were added to a suspension of germinated zoospores with an average length of 30 μm. After further incubation of the mixtures for 3 h at 28°C, hyphal growth of P. capsici was measured under a light microscope, as previously described (15). The effects of these chemicals on the hyphal growth of P. capsici were determined by comparing the hyphal length of the oomycete pathogen in each of the chemicals with that of a control preparation. The experiments were repeated twice with three replicates. Hyphal growth of P. capsici was strongly inhibited by treatment with SH-1, metalaxyl, and H3PO3. However, the inhibitory effect of SH-2 against hyphal growth was not as great as those of other compounds tested (data not shown).
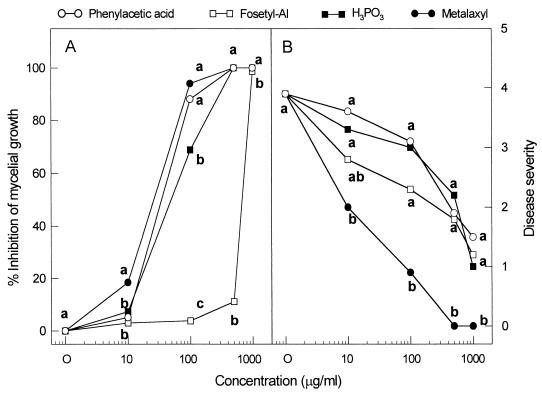
Mycelial disks (7-mm diameter) of P. capsici were placed in the center of the V8 agar plates supplemented with phenylacetic acid (Sigma), metalaxyl, fosetyl-AI, or H3PO3. The mycelial growth of P. capsici was rated after incubation for 7 days at 28°C. The percentage inhibition of mycelial growth by the chemical was calculated by the following formula: [1 − (diameter of mycelial growth in the chemical-treated plate/diameter of mycelial growth in the untreated control)] × 100. Treatment with phenylacetic acid strongly inhibited mycelial growth of P. capsici in potato dextrose agar (PDA) plates supplemented with the compound at various concentrations (see Fig. 4A). Compared to phenylacetic acid, metalaxyl was highly active against P. capsici. However, H3PO3 was less effective than phenylacetic acid in inhibiting the growth of the oomycete pathogen. The commercial fungicide fosetyl-AI did not show any antifungal activity against P. capsici, even at 500 μg/ml.
FIG. 4.
In vitro and in vivo efficacy of the authentic phenylacetic acid, H3PO3, fosetyl-AI, and metalaxyl against mycelial growth of P. capsici (A) and the disease development in pepper plants (B). Mycelial growth was measured on potato dextrose agar containing different concentrations of the chemicals when the control plates (9 cm in diameter) were completely covered by the fungus. Each chemical was sprayed on the foliage of plants 1 day before inoculation. Disease severity was rated 7 days after inoculation on pepper plants at the first-branch stage. Means at each concentration followed by the same letter are not significantly different (P = 0.05) according to the least significant difference test.
Taken together, the in vitro data obtained by the microtiter broth dilution, zoospore germination, and mycelial growth inhibition tests strongly suggested that phenylacetic acid (SH-1) and sodium phenylacetate (SH-2) have antifungal activity against the plant-pathogenic oomycete P. capsici. In zoospore germination tests, both compounds inhibited zoospore germination of P. capsici. The phosphonate fungicide fosetyl-AI, which is being used for control of oomycetes, was highly inhibitory to some Phytophthora spp. (6). Phenylacetic acid strongly inhibited the hyphal growth of P. capsici at 100 μg/ml, whereas sodium phenylacetate did not show inhibitory activity against hyphal growth at the same concentration without lysis of the zoospores. However, both compounds were shown to be inhibitory to zoospore germination of P. capsici compared to fosetyl-AI (or H3PO3) and phenylacetic acid (or sodium phenylacetate) (data not shown). Some compounds which showed in vitro antifungal activity were often found to have negligible in vivo control efficacy against plant diseases (7). Because phenylacetic acid and sodium phenylacetate exhibited a high antifungal activity against P. capsici in vitro, their in vivo control efficacy of plant diseases should be further examined.
In vivo antifungal activity of SH-1 (phenylacetic acid) and SH-2 (sodium phenylacetate).
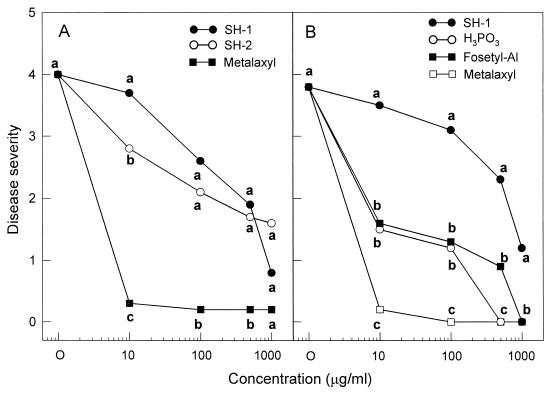
The antifungal substance SH-1 was evaluated for the ability to suppress phytophthora blight on pepper plants in a growth room. Seeds of pepper (Capsicum annuum L.) cv. Hanbyul were sown in a plastic tray (55 by 15 by 10 cm) containing steam-sterilized soil mix (peat moss, perlite, and vermiculite [5:3:2, vol/vol/vol]), sand, and loam soil (1:1:1, vol/vol/vol). Six seedlings at the four-leaf stage were transplanted into a plastic pot (5 by 15 by 10 cm). Pepper plants were raised to the first-branch stage in a growth chamber at 28°C (±2°C) for 16 h a day. Antifungal substances SH-1 and SH-2 and the commercial fungicide metalaxyl dissolved in methanol and acetone, respectively, were diluted to give concentrations of 0, 10, 100, 500, and 1,000 μg/ml. The 30-ml chemical solution was soil drenched into each pot 1 day before inoculation of P. capsici on pepper plants. A zoospore suspension was prepared by the method of Kim et al. (24) using the culture plates grown on oatmeal agar for 11 days at 28°C. The pepper plants were inoculated with a zoospore suspension (105 zoospores/ml) by the stem wound inoculation method. Antifungal substance SH-1, SH-2, phenylacetic acid, metalaxyl, fosetyl-AI, and H3PO3 dissolved in water were diluted to give concentrations of 0, 10, 100, 500, and 1,000 μg/ml. The chemical solutions were sprayed to the pepper plants until they ran off 1 day before inoculation of P. capsici. The pepper plants were also inoculated with a zoospore suspension (105 zoospores/ml) by the soil drench method. Disease severity on pepper plants was rated daily after inoculation based on a scale from 0 to 5 as follows: 0 for no visible disease symptoms, 1 for slightly wilted leaves, with brownish lesions beginning to appear on the stems, 2 for 30 to 50% of the entire plant diseased, 3 for 50 to 70% of the entire plant diseased, 4 for 70 to 90% of the entire plant diseased, and 5 for a dead plant. Data are the means of 10 plants per treatment. Statistical analyses were conducted with the Statistical Analysis System for personal computers (SAS Institute, Cary, N.C.). Percent data were subjected to an angular transformation (arcsine square root) to normalize the variance prior to analysis. Fisher's protected least significant difference with a P of 0.05 was used to separate the means. In vivo efficacy of SH-1 and SH-2 for the control of phytophthora blight in pepper plants was examined after inoculation of P. capsici using stem wound and soil drench methods under controlled environmental conditions (Fig. 3). The symptoms of phytophthora blight began to appear on pepper plants 4 days after inoculation. When inoculated with P. capsici, brownish lesions occurred on the pepper stem and extended rapidly to the upper part of plants, accompanied by wilting of the entire plant, leaf defoliation, and damping-off. Treatment with the antifungal substances SH-1 and SH-2 greatly inhibited the phytophthora disease in pepper plants. The suppressing effect of both compounds against phytophthora blight was pronounced at 1,000 μg/ml (Fig. 3A). In contrast, the commercial fungicide metalaxyl completely inhibited the development of phytophthora blight in pepper plants at the concentration of 10 μg/ml, irrespective of stem wound or soil drench inoculation methods. When soil drenched 1 day before inoculation of P. capsici, the control efficacy of SH-1 was less than that of H3PO3 or fosetyl-AI (Fig. 3B).
FIG. 3.
In vivo control efficacy of SH-1, SH-2, H3PO3, fosetyl-AI, and metalaxyl against P. capsici infection on pepper plants at the first-branch stage. (A) Foliar spray treatment on pepper plants just before stem wound inoculation. (B) Soil drench treatment 1 day before soil drench inoculation. The disease severity rating is based on a scale of 0 to 5 scale, with a score of 0 for no visible symptoms and a score of 5 for a dead plant. Means at each concentration followed by the same letter are not significantly different (P = 0.05) according to the least significant difference test.
In vivo efficacy of phenylacetic acid, metalaxyl, fosetyl-AI, and H3PO3 for the control of phytophthora blight in pepper plants was evaluated under greenhouse conditions (Fig. 4B). As the concentration of phenylacetic acid and other compounds increased, the phytophthora disease was gradually inhibited on the pepper plants at the first-branch stage. Treatments with 500 or 1,000 μg of phenylacetic acid, fosetyl-AI, and H3PO3 per ml showed a relatively high level of protective activity against P. capsici infection. The control efficacy of phenylacetic acid against phytophthora blight was in general similar to those of fosetyl-AI and H3PO3 but less than that of metalaxyl, which showed complete control at 500 and 1,000 μg/ml.
Concluding remarks.
To our knowledge, this is the first study to demonstrate the in vivo efficacy of phenylacetic acid and sodium phenylacetate for the control of phytophthora blight in pepper plants, although it should be noted that Burkhead et al. (3) previously provided preliminary evidence for antifungal activity against Gibberella pulicaris. In the present study, phenylacetic acid and sodium phenylacetate were found to be very effective not only in inhibiting zoospore germination and mycelial growth of P. capsici but also in controlling phytophthora blight in pepper plants. The synthetic fungicides such as prothiocarb, propamocarb, phosphate, and acyanilide including metalaxyl have practically been used to control the plant diseases caused by oomycetes (6). Among the oomycetes, P. capsici, which causes root and crown rot and blight of pepper (Capsicum annum L.) plants, is one of the limiting factors in production of pepper in pepper-growing fields worldwide (14).
Phenylacetic acid, a deamination product of phenylalanine, has been known to possess a positive effect on the growth and development of maize (29). The plants and microorganisms which produce phenylalanine ammonia lyase can derive phenylacetic acid from phenylalanine in nature (29). Wightman and Lighty (33) found that phenylacetic acid acts as a natural auxin in the shoots of higher plants, such as barley, corn, tobacco, and tomato. Some microorganisms can utilize phenylacetic acid during their metabolic process. Penicillium chrysogenum takes up phenylacetic acid as a precursor of penicillin G (10). The transport system of phenylacetic acid in P. crysogenum is well understood (8). Ralstonia solanacearum was shown to utilize phenylalanine and phenylacetic acid as the sole carbon and nitrogen source (1). Kawazu et al. (17, 18) have also demonstrated that phenylacetic acid produced by Bacillus subtilis strain HY-16, Bacillus cereus strain HY-3, and Bacillus megaterium strain HY-17 has in vitro toxic effect against the pine wood nematode Barsaphelenchus xylophilusi.
Phenylacetic acid suppressed phytophthora blight at the concentration of 1,000 μg/ml, but sodium phenylacetate showed less efficacy at 1,000 μg/ml. Fosetyl-AI, which breaks down rapidly in soil and plant tissues to phosphorous acid (H3PO3) and CO2 (5), was known to have low activity against Phytophthora and Pythium spp. in vitro (6). Phosphorous acid was highly inhibitory to mycelial growth, sporangium development, and zoospore release of several Phytophthora spp. even at low concentrations (4, 5). Because it seems likely that P. capsici could not utilize phenylacetic acid, phenylacetic acid and sodium phenylacetate may successfully inhibit the growth of P. capsici in vitro. Papavizas and Bowers (28) demonstrated that metalaxyl was effective in inhibiting zoospore germination of P. capsici at 100 μg/ml. Phenylacetic acid and sodium phenylacetate were more effective than metalaxyl in reducing zoospore germination of P. capsici. However, in vivo modes of actions of phenylacetic acid against phytophthora blight in pepper plants remain to be elucidated in detail. In an earlier study, phenylacetic acid was found to act as a natural auxin in some higher plants (33), which suggests that treatment with phenylacetic acid may enhance the growth rate of plants. Phenylacetic acid may induce some resistance in pepper plants against infection by P. capsici. However, it will be difficult to determine whether phenylacetic acid can trigger systematic acquired resistance in pepper plants, because the chemical has direct antifungal activity in vitro against P. capsici. Application of phenylacetic acid may also result in the reduction of the primary inoculum density of P. capsici in soils of pepper-growing fields.
Acknowledgments
This research was financially supported from 1999 to 2002 by the special research fund of the Ministry of Agriculture and Forestry of Korea.
We thank E. J. Bang and J. J. Seo (Korea Basic Science Institute, Seoul, Korea) for NMR, El mass spectroscopy, and ICP mass spectroscopy. We also thank D. A. Holte critically for reading our manuscript.
REFERENCES
- 1.Agrawal P, Latha S, Mahadevan A. Utilization of phenylalanine and phenylacetic acid by Pseudomonas solanacearum. Appl Biochem Biotechnol. 1996;61:379–391. [Google Scholar]
- 2.Aizawa S, Akutsu H, Seto H, Otaka N. Capsimycin, an antibiotic active against phytopathogenic fungi. Kyoto, Japan: The Fifth International Congress of Pesticide Chemistry (IUPAC).; 1982. [Google Scholar]
- 3.Burkhead K D, Slininger P J, Schisler D A. Biological control bacterium Enterobacter cloacae S11:T:07 (NRRL B-21050) produces the antifungal compound phenylacetic acid. Soil Biol Biochem. 1998;30:665–667. [Google Scholar]
- 4.Coffey M D, Bower L A. In vitro variability among isolates of eight Phytophthora species in response to phosphorous acid. Phytopathology. 1984;74:738–742. [Google Scholar]
- 5.Coffey M D, Joseph M C. Effects of phosphorous acid and fosetyl-AI on the life cycle of Phytophthora cinnamomi and P. citricola. Phytopathology. 1985;75:1042–1046. [Google Scholar]
- 6.Cohen Y, Coffey M D. Systemic fungicides and the control of oomycetes. Annu Rev Phytopathol. 1986;24:311–338. [Google Scholar]
- 7.Fawcett C H, Spencer D M. Plant chemotherapy with natural products. Annu Rev Phytopathol. 1970;8:403–418. [Google Scholar]
- 8.Fernandez-Canon J M, Reglero A, Martinez-Blanco H, Luengo J M. Uptake of phenylacetic acid by Penicillium chrysogenum Wis 54-1255: a critical regulatory point in benzylpenicillin biosynthesis. J Antibiot. 1989;42:1398–1409. doi: 10.7164/antibiotics.42.1398. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa M, Ishizawa K, Nonomura H. Distribution of rare actinomycetes in Japanese soils. J Ferment Technol. 1988;66:367–373. [Google Scholar]
- 10.Hillenga D J, Hanneke J M, Versantvoort S, van der Molen A, Driessen J M, Konings W N. Penicillium chrysogenum takes up the penicillin G precursor phenylacetic acid by passive diffusion. Appl Environ Microbiol. 1995;61:2589–2595. doi: 10.1128/aem.61.7.2589-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homans A L, Fuchs A. Direct bioautography on thin-layer chromatograms as a method for detecting fungitoxic substances. J Chromatogr. 1970;51:327–329. doi: 10.1016/s0021-9673(01)96877-3. [DOI] [PubMed] [Google Scholar]
- 12.Hwang B K, Ahn S J, Moon S S. Production, purification, and antifungal activity of the antibiotic nucleoside, tubercidin, produced by Streptomyces violaceoniger. Can J Bot. 1994;72:480–485. [Google Scholar]
- 13.Hwang B K, Kim B S. In-vivo efficacy and in-vitro activity of tubercidin, an antibiotic nucleoside, for control of Phytophthora capsici blight in Capsicum annuum. Pestic Sci. 1995;44:255–260. [Google Scholar]
- 14.Hwang B K, Kim C H. Phytophthora blight of pepper and its control in Korea. Plant Dis. 1995;79:221–227. [Google Scholar]
- 15.Hwang B K, Lee J Y, Kim B S, Moon S S. Isolation, structure elucidation, and antifungal activity of a manumycin-type antibiotic from Streptomyces flaveus. J Agric Food Chem. 1996;44:3653–3657. [Google Scholar]
- 16.Jiang C L, Xu L H. Diversity of aquatic actinomycetes in lakes of the middle plateau, Yunnan, China. Appl Environ Microbiol. 1996;62:249–253. doi: 10.1128/aem.62.1.249-253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawazu K, Zhang H, Kanzaki H. Accumulation of benzoic acid in suspensions of cultured cells of Pinus thunbergii Parl. in response to phenylacetic acid administration. Biosci Biotechnol Biochem. 1996;60:1410–1412. doi: 10.1271/bbb.60.1410. [DOI] [PubMed] [Google Scholar]
- 18.Kawazu K, Zhang H, Yamashita H, Kanzaki H. Relationship between the pathogenicity of the pine wood nematode, Bursaphelenchus xylophilus, and phenylacetic acid. Biosci Biotechnol Biochem. 1996;60:1413–1415. doi: 10.1271/bbb.60.1413. [DOI] [PubMed] [Google Scholar]
- 19.Kim B S, Lee J Y, Hwang B K. Diversity of actinomycetes antagonistic to plant pathogenic fungi in cave and sea-mud soils of Korea. J Microbiol. 1998;36:86–92. [Google Scholar]
- 20.Kim B S, Moon S S, Hwang B K. Isolation, identification, and antifungal activity of a macrolide antibiotic, oligomycin A, produced by Streptomyces libani. Can J Bot. 1999;77:850–858. [Google Scholar]
- 21.Kim B S, Moon S S, Hwang B K. Isolation, antifungal activity, and structure elucidation of the glutarimide antibiotic, streptimidone, produced by Micromonospora coerulea. J Agric Food Chem. 1999;47:3372–3380. doi: 10.1021/jf981259s. [DOI] [PubMed] [Google Scholar]
- 22.Kim B S, Moon S S, Hwang B K. Structure elucidation and antifungal activity of an anthracycline antibiotic, daunomycin, isolated from Actinomadura roseola. J Agric Food Chem. 2000;48:1875–1881. doi: 10.1021/jf990402u. [DOI] [PubMed] [Google Scholar]
- 23.Kim C J, Lee I K, Yun B S, Yoo I D. Concanamycin B, active substance against Phytophthora capsici produced by Streptomyces neyagawaensis 38D10 strain. Korean J Microbiol Biotechnol. 1993;21:322–328. [Google Scholar]
- 24.Kim Y J, Hwang B K, Park K W. Expression of age-related resistance in pepper plants infected with Phytophthora capsici. Plant Dis. 1989;73:745–747. [Google Scholar]
- 25.Lim S W, Kim J D, Kim B S, Hwang B K. Isolation and numerical identification of Streptomyces humidus strain S5–55 antagonistic to plant pathogenic fungi. Plant Pathol J. 2000;16:189–199. [Google Scholar]
- 26.Nair M G, Mishra S K, Putnam A R. Antifungal anthracycline antibiotics, spartanamicins A and B from Micromonospora spp. J Antibiot. 1992;45:1738–1745. doi: 10.7164/antibiotics.45.1738. [DOI] [PubMed] [Google Scholar]
- 27.Okami Y, Hotta K. Search and discovery of new antibiotics. In: Goodfellow M, Williams S T, Mordaski M, editors. Actinomycetes in biotechnology. London, United Kingdom: Academic Press; 1988. pp. 33–67. [Google Scholar]
- 28.Papavizas G C, Bowers J H. Comparative fungitoxicity of captafol and metalaxyl to Phytophthora capsici. Phytopathology. 1981;71:123–128. [Google Scholar]
- 29.Sarwar M, Frankenberger W T., Jr Fate of L-phenylalanine in soil and its effect on plant growth. Soil Sci. 1995;59:1625–1630. [Google Scholar]
- 30.Shomura T. Screening for new products of new species of Dactylosporangium and other actinomycetes. Actinomycetology. 1993;7:88–98. [Google Scholar]
- 31.Suzuki K, Nagai K, Shimizu Y, Suzuki Y. Search for actinomycetes in screening for new bioactive compounds. Actinomycetology. 1994;8:122–127. [Google Scholar]
- 32.Tanaka Y T, Ōmura S. Agroactive compounds of microbial origin. Annu Rev Microbiol. 1993;47:57–87. doi: 10.1146/annurev.mi.47.100193.000421. [DOI] [PubMed] [Google Scholar]
- 33.Wightman F, Lighty D L. Identification of phenylacetic acid as a natural auxin in the shoot of higher plants. Physiol Plant. 1982;55:17–24. [Google Scholar]
- 34.Xu L H, Li Q R, Jiang C L. Diversity of soil actinomycetes in Yunnan, China. Appl Environ Microbiol. 1996;62:244–248. doi: 10.1128/aem.62.1.244-248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]