Abstract
A dimethoate-degrading enzyme from Aspergillus niger ZHY256 was purified to homogeneity with a specific activity of 227.6 U/mg of protein. The molecular mass of the purified enzyme was estimated to be 66 kDa by gel filtration and 67 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The isoelectric point was found to be 5.4, and the enzyme activity was optimal at 50°C and pH 7.0. The activity was inhibited by most of the metal ions and reagents, while it was induced by Cu2+. The Michaelis constant (Km) and Vmax for dimethoate were 1.25 mM and 292 μmol min−1 mg of protein−1, respectively.
Organophosphorus pesticides have been used in large quantities throughout the world since the first introduction of a synthetic insecticide, parathion, for use in crop protection in 1944 (16). Problems of contamination resulting from surplus pesticides and wastewater from pesticide factories have become obvious. The transformation of pesticides in the environment results from physicochemical reactions as well as from the activity of cellular or extracellular components of the biota (microorganisms, plants, and animals), but the principal biological pathway is microbial degradation (7): microorganisms can metabolize various pesticides both in soil and in water. The earlier metabolic studies on pesticides helped to develop a new approach to the detoxification of pesticides using cell-free enzymes from adapted microorganisms to resolve problems related to whole-cell metabolism of pesticides. Munnecke purified an enzyme from a mixed bacterial culture grown on parathion and found that it hydrolyzed parathion and related organophosphorus insecticides (13). Mulbry and Karns purified three unique parathion hydrolases from gram-negative bacterial isolates. These enzymes with different characteristics were exploited in different types of waste disposal (12). Although there have been a number of reports on the metabolism of parathion by enzyme preparations from bacteria or by mixed-culture bacteria (12–14), there have been no successful cell-free studies with fungi capable of degrading organophosphorus insecticides. To our knowledge, this is the first dimethoate-degrading enzyme purified to homogeneity from a fungus. Characterization and comparison of the degrading enzymes from different microorganisms may help us to understand fully how these enzymatic activities may evolve and how organophosphorus insecticides are degraded in the ecosystem.
Microorganism and enzyme production.
All experiments were performed in triplicate, and results, where appropriate, are presented as means. Aspergillus niger ZHY256 was isolated from sewage and the soil of cotton fields where pesticides were heavily used and maintained on potato dextrose agar. For enzyme production, the fungus was grown on basal salt medium (0.2% NaNO3, 0.05% KCl, 0.05% MgSO4 · 7H2O, 0.02% BaCl2, 0.01% MnSO4, 0.005% CaCl2, pH 6.8, supplemented with dimethoate at a concentration of 0.25% [wt/vol]). Dimethoate-degrading enzyme activity was detected at the late log phase and reached the maximum level 5 days after the start of cultivation. It was found that Ba2+ greatly stimulated the enzyme production with an optimum concentration of 1 mM in the medium. The addition of Ba2+ to the cell extract cultured without Ba2+ did not accomplish any acceleration of the enzyme activity, showing that Ba2+ is needed to produce the enzyme or to stabilize the enzyme produced. The effects of various carbon sources on dimethoate-degrading enzyme production by A. niger ZHY256 were investigated (Table 1). The microorganism grew on every substrate tested, although the mycelial mass yield was about four times lower on dimethoate- or malathion-containing media than on dextrose- or sucrose-containing media. Differences in growth yield were, however, not related to differences in total or relative enzyme production. The greatest amount of dimethoate-degrading enzyme activity was from cell extract grown in dimethoate-containing basal salt medium.
TABLE 1.
Effects of various carbohydrates on dimethoate-degrading enzyme production
| Carbon source | Total dimethoate-degrading enzyme activity (U) | Total mycelial dry wt (mg) |
|---|---|---|
| Dextrose | 116 ± 3 | 287 ± 6 |
| Sucrose | 159 ± 5 | 312 ± 7 |
| Maltose | 187 ± 6 | 249 ± 5 |
| Dimethoate | 1,008 ± 24 | 81 ± 3 |
| Malathion | 903 ± 18 | 76 ± 2 |
Enzyme purification.
All experiments described below were carried out between 0 and 4°C unless otherwise specified. A. niger ZHY256 was incubated at 30°C for 5 days in 500-ml Erlenmeyer flasks containing 150 ml of medium on a rotary shaker at 150 rpm and harvested by centrifugation at 12,000 × g for 20 min. The collected mycelia were washed twice with cold 50 mM Tris-HCl buffer (pH 7.0) and disrupted (8 ml of buffer/g of wet mycelia) in a vibration homogenizer (Vibrogen vi4; Edmund, Tuebingen, Germany) with glass beads (0.1-mm diameter). After standing at 4°C overnight, the suspension was centrifuged (12,000 × g for 20 min at 4°C) to remove the unbroken cells and cellular debris. The supernatant was filtered through a 0.22-μm-pore-size membrane (Millipore), the filtrate being the crude extract of the enzyme. The protein of the crude extract was precipitated overnight with 80% (NH4)2SO4. The resulting precipitate was collected by centrifugation at 12,000 × g for 20 min, dissolved in the smallest possible volume of 50 mM Tris-HCl buffer (pH 7.0), dialyzed 1,000-fold against the same buffer, and concentrated by ultrafiltration. The concentrated enzyme solution was loaded onto a Sephadex G-100 column (1.8 by 100 cm) preequilibrated with 50 mM Tris-HCl buffer (pH 7.0). The column was washed at a flow rate of 24 ml/h with 400 ml of the same buffer, and 5-ml fractions were collected. The molecular weight of the native enzyme was determined by the method of Andrew by using blue dextran and molecular weight markers from Sigma as standards (2). Proteins were eluted in fractions 18 to 80, whereas the enzyme was confined to fractions 26 to 38. The fractions with high specific activity were then pooled and concentrated for further purification. Fractions with dimethoate-degrading enzyme activity were loaded on a DEAE-Sepharose CL-6B ion-exchange column (1.2 by 30 cm) preequilibrated with 50 mM Tris-HCl buffer (pH 7.0). The column was washed at a flow rate of 20 ml/h with 500 ml of the same buffer, and proteins were eluted with a linear gradient of NaCl from 0 to 1.0 M. Five-milliliter fractions were collected. This enzyme solution was the purified dimethoate-degrading enzyme preparation used for subsequent characteristic studies. The data for the purification are summarized in Table 2. The enzyme was purified 36.1-fold to a specific activity of 227.6 U/mg of protein from the mycelia with a yield of 33.48%.
TABLE 2.
Purification of dimethoate-degrading enzyme from A. niger ZHY256
| Purification step | Total protein (mg) | Total activity (U) | Sp act (U/mg of protein) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude extract | 146 ± 3.3 | 919.8 ± 17 | 6.3 ± 0.1 | 1.0 | 100 |
| Ammonium sulfate | 72.9 ± 2.1 | 839 ± 14 | 11.5 ± 0.3 | 1.83 ± 0.04 | 91.2 ± 3.1 |
| Sephadex G-100 | 16.6 ± 0.51 | 656 ± 11 | 39.4 ± 1.1 | 6.25 ± 0.15 | 71.3 ± 1.8 |
| DEAE-Sepharose CL-6B | 1.35 ± 0.02 | 308 ± 8 | 227.6 ± 6.2 | 36.1 ± 0.7 | 33.48 ± 1.1 |
Enzyme assay.
Dimethoate-degrading enzyme activity was assayed with 50 mM dimethoate as a substrate. The reaction was carried out at 50°C in a final volume of 1 ml of 50 mM Tris-HCl buffer (pH 7.0) and was stopped by adding 1 ml of 2 M trichloroacetic acid, and the amounts of all organophosphorus insecticides tested in the experiments were determined by a gas chromatograph (model PE Sigma 2000) with a glass column (1-m length, 2-mm inner diameter) packed with 5% OV-17 on Chromosorb WDMCS, operated at 220°C, with the detector at 250°C and the injector at 230°C (3, 5, 18). One unit of activity was defined as the amount of enzyme that catalyzes the degradation of 1 μmol of dimethoate per min under these conditions. The protein concentration was determined according to the method of Lowry et al., with bovine serum albumin as the standard (11).
Enzyme characterization.
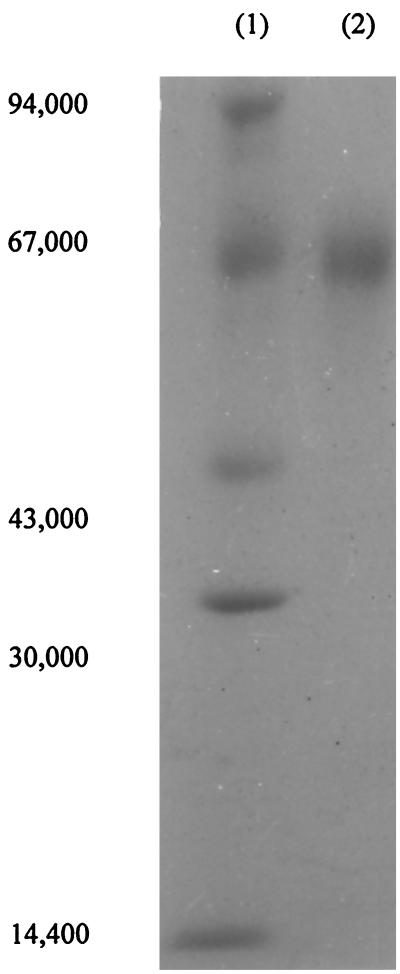
The purified enzyme gave a single band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by the method of Laemmli (8). This indicated that the purified sample was electrophoretically homogeneous under the dissociating conditions (Fig. 1). The molecular mass of the purified enzyme was estimated to be approximately 66 kDa by gel filtration on a calibrated column of Sephadex G-100, which was quite close to the value determined for parathion hydrolase of strain SC (12). The value was confirmed by SDS-PAGE (Fig. 1). Hence, it is assumed that the native dimethoate-degrading enzyme is a monomer.
FIG. 1.
SDS-PAGE of the purified dimethoate-degrading enzyme from A. niger ZHY256. Lane 1, marker proteins (from top to bottom) phosphorylase b (Mr, 94,000), bovine serum albumin (Mr, 67,000), ovalbumin (Mr, 43,000), carbonic anhydrase (Mr, 30,000), and α-lactalbumin (Mr, 14,400), respectively; lane 2, purified enzyme. The gel was stained for protein with Coomassie brilliant blue R-250 and destained in methanol-acetic acid-water (7:6:47).
The isoelectric point (pI) was estimated by PAGE with 6.25% Ampholine (pH 3.5 to 10) in a gel rod (0.5 by 10 cm) using a kit for isoelectric focusing calibration (Pharmacia LKB) according to the recommendations of the supplier. The pI value was estimated to be 5.4.
The N-terminal amino acid sequence of the protein was determined in samples of purified dimethoate-degrading enzyme after running them on an SDS-polyacrylamide gel; the protein was transferred to a polyvinylidene fluoride membrane (Millipore Corp.) by electroblotting and then stained (9). The polyvinylidene fluoride membrane slice containing the purified enzyme was excised, and the sequence was determined in a Beckman LF300 sequencer. The results revealed the following sequence: MKTLELEEREV.
For determination of the pH optimum, the activity was determined by incubating purified enzyme (0.3 μg/ml) with 50 mM dimethoate and at pH values between 5.5 and 10.0 at 50°C for 30 min. For the pH stability determination, samples were incubated in buffers from pH 5.5 to 10.0 and at 40°C for 2 h, and the relative residual activity was assayed under standard conditions as described above. The temperature optimum was determined analogously with a constant pH of 7.0 and different temperatures. For determination of the thermostability, purified enzyme (0.3 μg/ml) was incubated in reaction buffer for 4 h at temperatures ranging from 20 to 75°C, and the remaining dimethoate-degrading enzyme activity was measured as before. As shown in Fig. 2, the optimal temperature and pH were 50°C and 7.0, respectively. The enzyme was found to be stable in the pH range between 6.0 and 9.5. The enzyme was active over a wide temperature range around 40°C, similarly to the enzyme produced by the three gram-negative bacterial strains described above (12).
FIG. 2.
Effects of temperature and pH on activity (▪) and stability (●) of the purified enzyme.
Effects of reagents and metal ions on the enzyme activity were examined by preincubating the enzyme with 2 mM chemicals in 50 mM Tris-HCl buffer (pH 7.0) for 30 min at 40°C and then measuring the residual activities of the enzyme by using dimethoate as a substrate. The results are presented in Table 3. The purified enzyme was strongly inhibited by Hg2+, Ag+, and Fe3+; this may indicate that thiol groups are involved in the active catalytic site. The enzyme was completely inhibited by sulfhydryl reagents (such as 2-mercaptoethanol, dithiothreitol, and glutathione), well-known thiol group inhibitors, therefore suggesting again that sulfhydryl groups may be involved in the catalytic center of the enzyme (1, 4, 15, 19–21). The enzyme was not sensitive to phenylmethanesulfonyl fluoride, which suggests that serine may not be involved in the active site of the enzyme. The metal-chelating agent EDTA and 1,10-phenanthroline did not inhibit the purified enzyme activity, indicating that divalent cations are not required for enzyme activation. However, Cu2+ did evidently activate the enzyme activity, which was the same as that of parathion hydrolase of strain SC described above (12). The optimum concentration was 6 mM. A further increase of the concentration of copper ion above the optimum value, however, resulted in a decrease in enzyme activity. No inhibition was observed with certain organic solvents at a low (3% [vol/vol]) concentration such as acetone, methanol, and ethanol.
TABLE 3.
Effects of chemicals on dimethoate-degrading enzyme from A. niger ZHY256
| Chemical | Relative activity (%) |
|---|---|
| Control | 100 |
| AgCl | 5.8 ± 0.17 |
| HgCl2 | 2.8 ± 0.06 |
| FeCl3 | 16 ± 0.46 |
| CuCl2 | 159 ± 5.5 |
| 2-Mercaptoethanol | 3.4 ± 0.06 |
| Dithiothreitol | 0 |
| Glutathione | 2.1 ± 0.04 |
Organophosphorus pesticides such as parathion, dichlorovos, dimethoate, formothion, and malathion were tested for substrate specificity of the enzyme, which was determined by measuring the decrease of substrate concentration. The results revealed that dimethoate, formothion, and malathion were hydrolyzed by the enzyme while other pesticides were not (Table 4). The first two compounds possess a P—O—C bond, while formothion and malathion are characterized by a P—S—C bond identical to that of dimethoate. Products of the degradation assay were determined by previously reported methods (6). According to colorimetric results and thin-layer chromatography, O,O-dimethyl phosphorothioate exists in the supernatant of the degradation assay mixture but not in that of the control assay mixture. Therefore, we may primarily conclude that the purified enzyme can degrade the P—S linkage of dimethoate, formothion, and malathion, which is different from parathion hydrolases, which attack the P—O bond in gram-negative bacterial strains (12) and Flavobacterium sp. (17). Products of degradation of dimethoate are (CH3)2P(S)OH and HSCH2C(O)NHCH3.
TABLE 4.
Substrate specificity of dimethoate-degrading enzyme from A. niger ZHY256
| Organophosphorus insecticide |
Extent of degradation (%) |
|---|---|
| Dimethoate | 87 ± 2.8 |
| Formothion | 81 ± 2.6 |
| Malathion | 78 ± 2.1 |
| Parathion | 0 |
| Dichlorovos | 0 |
The kinetic properties of the purified enzyme were studied by measuring the initial velocity of the reaction in 50 mM Tris-HCl buffer (pH 7.0) at 40°C over the substrate concentration range of 0.1 to 5 mM. The kinetic constants of the purified enzyme were determined by the Lineweaver-Burk equation (10) by using the Microcal Origin software program. The regression of experimental data showed a linear response. The Km value of dimethoate was estimated to be 1.25 mM. The maximum initial velocity for the conversion of the substrate was 292 μmol min−1 mg of protein−1.
In conclusion, there are some difficulties in comparing the inherent properties of various organophosphorus pesticide-degrading enzymes because of the differences in experimental conditions, the purity of the enzyme preparations, and the origin of organophosphorus pesticide-degrading enzymes. All these results suggest that A. niger ZHY256 produces a novel organophosphorus pesticide-degrading enzyme.
REFERENCES
- 1.Ait N, Creuzet N, Cattaneo J. Properties of β-glucosidase purified from Clostridium thermocellum. J Gen Microbiol. 1982;128:569–577. [Google Scholar]
- 2.Andrew P. Estimation of the molecular weights of proteins by Sephadex gel filtration. Biochem J. 1965;91:595–606. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AOAC International. Official methods of analysis, 15th ed., section 970.52 K (a)(1). Arlington, Va: AOAC International; 1990. [Google Scholar]
- 4.Bruinenberg P G, de Roo G, Limsowtin G K Y. Purification and characterization of cystathionine γ-lyase from Lactococcus lactis subsp. cremoris SK11: possible role in flavor compound formation during cheese maturation. Appl Environ Microbiol. 1997;63:561–566. doi: 10.1128/aem.63.2.561-566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulson D M. Gas chromatography of pesticides. Adv Pest Control Res. 1962;V:153–158. [Google Scholar]
- 6.Kanagawa T, Dazai M, Takahara Y. Degradation of O,O-dimethyl phosphorothioate by activated sludge. Agric Biol Chem. 1980;44:2631–2635. [Google Scholar]
- 7.Karns J S, Kilbane J J, Duttagupta S, Chakrabarty A M. Metabolism of halophenols by 2,4,5-trichlorophenoxyacetic acid-degrading Pseudomonas cepacia. Appl Environ Microbiol. 1983;46:1176–1184. doi: 10.1128/aem.46.5.1176-1181.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Legendre N, Mansfield M, Weiss A, Matsudaira P. Purification of proteins and peptides by SDS-PAGE. In: Matsudaira P, editor. A practical guide to protein and peptide purification for microsequencing. San Diego, Calif: Academic Press, Inc.; 1993. pp. 71–101. [Google Scholar]
- 10.Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. [Google Scholar]
- 11.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Mulbry W W, Karns J S. Purification and characterization of three parathion hydrolases from gram-negative bacterial strains. Appl Environ Microbiol. 1989;55:289–293. doi: 10.1128/aem.55.2.289-293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munnecke D M. Enzymatic hydrolysis of organophosphate insecticides, a possible pesticide disposal method. Appl Environ Microbiol. 1976;32:7–13. doi: 10.1128/aem.32.1.7-13.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munnecke D M, Hsieh D P H. Pathways of microbial metabolism of parathion. Appl Environ Microbiol. 1976;31:63–69. doi: 10.1128/aem.31.1.63-69.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh K-B, Hamada K, Saito M, Lee H-J. Isolation and properties of an extracellular β-glucosidase from a filamentous fungus, Cladosporium resinae, isolated from kerosene. Biosci Biotechnol Biochem. 1999;63:759–763. doi: 10.1271/bbb.63.281. [DOI] [PubMed] [Google Scholar]
- 16.Saunders B C. Some aspects of the chemistry and toxic action of organic compounds containing phosphorus and fluorine. London, United Kingdom: Cambridge University Press; 1957. [Google Scholar]
- 17.Sethunatnan N, Yoshida T. A Flavobacterium that degrades diazinon and parathion. Can J Microbiol. 1973;19:873–875. doi: 10.1139/m73-138. [DOI] [PubMed] [Google Scholar]
- 18.Sherma J, Zweig G. Gas chromatography. In: Zweig G, editor. Analytical methods for pesticides and plant growth regulators. II. New York, N.Y: Academic Press; 1972. pp. 377–384. [Google Scholar]
- 19.Toida J, Arikawa Y, Kondou K, Fukuzawa M, Sekiguchi J. Purification and characterization of triacylglycerol lipase from Aspergillus oryzae. Biosci Biotechnol Biochem. 1998;62:759–763. doi: 10.1271/bbb.62.759. [DOI] [PubMed] [Google Scholar]
- 20.Wang S L, Chang W T. Purification and characterization of two bifunctional chitinases/lysozymes extracellularly produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Appl Environ Microbiol. 1997;63:380–386. doi: 10.1128/aem.63.2.380-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanker E, Huber A, Schwab H. Purification and characterization of the Bacillus subtilis levanase produced in Escherichia coli. Appl Environ Microbiol. 1995;61:1953–1958. doi: 10.1128/aem.61.5.1953-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]