Abstract
Objectives
We evaluated the safety and efficacy of oral treprostinil in preventing progression of SSc-associated calcinosis.
Methods
This prospective open-label study enrolled 12 SSc patients meeting 2013 ACR/EULAR classification criteria with confirmed clinical and radiographic evidence of one or more calcinosis deposit in the hands. Patients received oral treprostinil for 1 year. Primary endpoints were safety/tolerability and percentage of patients without radiographic progression of calcinosis at 1 year (<25% increase in Scleroderma Clinical Trials Consortium radiographic score). Secondary endpoints included 1-year changes in Scleroderma HAQ (SHAQ), Cochin Hand Functional Scale, Medical Outcomes Survey Short Form 36 (SF-36), Raynaud Condition Score and patient/physician assessment of calcinosis severity.
Results
Twelve female patients were enrolled, half with diffuse cutaneous disease; median age was 55 years (range 35–68 years). Five patients completed the study. Seven patients withdrew due to intolerable adverse effects (n = 3), intercurrent unrelated illness (n = 2, cirrhosis, cancer), progressive SSc (n = 1) and personal reasons (n = 1). Most patients developed headaches and gastrointestinal adverse effects. Four of 11 (36%) patients with 1-year follow-up hand radiographs experienced progression of calcinosis. Of five who completed treatment, calcinosis was stable in four (80%) with progression in one. Based on SF-36 Physical and Mental Component and Domain scores, transition question and SF-6D utility score, all patients who finished the trial reported overall improvement or no change compared with baseline.
Conclusion
Oral treprostinil was poorly tolerated in SSc patients with calcinosis. Of five patients who completed treatment, most (80%) had documented stability of calcinosis on hand radiographs at 1 year.
ClinicalTrials.gov identifier
Keywords: SSc, scleroderma, calcinosis, cutis, treprostinil, prostacyclin, clinical trial
Rheumatology key messages.
We evaluated the safety and efficacy of oral treprostinil in preventing progression of SSc-associated calcinosis.
Oral treprostinil was poorly tolerated due to headaches and gastrointestinal side effects.
Of patients who completed the treatment period, most (80%) had stability of calcinosis on hand radiographs at 1 year.
Introduction
Calcinosis cutis is characterized by deposition of calcium in the skin and subcutaneous tissues [1], affecting 18–49% of patients with SSc [2]. Calcinosis is a late complication of the disease and tends to progress over time [3]. Although the underlying pathogenesis is unknown, there is growing evidence supporting vascular hypoxia as a contributing factor [4, 5], including several observational studies that have shown an association between calcinosis and ischaemic manifestations of SSc, such as the presence or history of digital ulcers (DU) [6, 7] and acro-osteolysis [8].
Calcinosis is responsible for a high burden of hand dysfunction and disability in patients with SSc [9], and progresses over 1 year in >40% of SSc patients [3]. Surgical removal of calcium deposits can be useful for isolated lesions, but is not feasible for multiple small deposits, extensive deposits, those fixed to bone and/or deposits that are not easily accessible; in addition, lesions may recur and surgery can result in infectious complications. Lack of effective therapeutic options makes treatment of calcinosis a major clinical challenge. Several pharmacologic therapies aimed to reduce calcinosis, such as calcium channel blockers, minocycline and sodium thiosulphate, have been successful in small observational studies, but none is consistently effective [1], nor has been evaluated in a prospective clinical trial using validated outcome measures.
Treprostinil is a prostacyclin analogue that causes direct vasodilation of pulmonary and systemic arterial vascular beds and inhibition of platelet aggregation, and is approved in the USA for treatment of pulmonary arterial hypertension (PAH) [10]. Treprostinil has been used for the treatment of digital ulcers with varying results. Treprostinil delivered by continuous s.c. infusion was effective both for healing and preventing DU in SSc patients in an open-label, single-centre clinical trial of five patients able to tolerate the medication [11]. In a multicentre retrospective study of 51 SSc patients with RP, total DU burden increased significantly after discontinuation of oral treprostinil [12]. In a multicentre randomized controlled trial of 148 SSc subjects with DU, administration of oral treprostinil over 20 weeks did not meet the desired endpoint (change in net ulcer burden at 20 weeks), but there was a significant improvement in many secondary endpoints that measured RP severity [13].
Since calcinosis has been shown to be associated with DU and vascular ischaemia, we hypothesized that oral treprostinil would prevent progression of calcinosis associated with SSc. We performed a pilot open-label clinical trial at Stanford University to evaluate the safety and efficacy of oral treprostinil in preventing progression of calcinosis in patients with SSc.
Methods
Study design and eligibility
This was a prospective, single-arm, open-label, dose-escalation trial using oral treprostinil over 1 year in 12 adult SSc patients with calcinosis affecting their hands (ClinicalTrials.gov identifier: NCT02663895). Written informed consent was obtained from all enrolled patients in accordance with the declaration of Helskinski, and the study was approved by the Stanford Institutional Review Board.
All patients were >18 years of age, fulfilled the 2013 ACR/EULAR classification criteria for diffuse or limited cutaneous SSc, and were required to have both radiographic and physical examination evidence of at least one subcutaneous calcium deposit affecting the hands. Calcium channel blockers, alpha-1-antagonists, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and proton-pump inhibitors were permitted if doses were stable ≥4 weeks prior to screening and throughout the study. Oral CS (≤10 mg/day of prednisone or equivalent) and NSAIDs were permitted if the patient was receiving a stable dose regimen for ≥2 weeks prior to screening and throughout the study. Women of childbearing potential agreed to use adequate contraception when sexually active with any combination of at least two effective methods of birth control.
Patients with rheumatic diseases other than SSc were excluded. Additional exclusion criteria included patients with PAH determined by right heart catheterization who were New York Heart Association (NYHA) Class III or IV or receiving PAH-approved medications. Patients were not allowed to receive phosphodiesterase inhibitors (including sildenafil, tadalafil, dipyridamole or theophylline), endothelin receptor antagonists, prostanoids, riociguat, nitrates, bisphosphonates, warfarin, colchicine, minocycline, IVIG or biologic agents within 4 weeks of screening. Patients were not allowed to receive local treatments for calcinosis of the hands including surgical removal or intralesional steroid injections within 12 weeks of screening or throughout the study. Although not a strict exclusion criterion, no patients enrolled in the study received topical, intralesional or i.v. sodium thiosulphate. Other exclusion criteria included: diverticulosis; anaemia (haemoglobin <75% of lower limits of normal); moderate or severe hepatic impairment (Child Pugh Class C) or transaminase elevations (ALT or AST) >3× upper limits of normal at the screening visit; hypotension (systolic blood pressure <95 mmHg or diastolic blood pressure <50 mmHg); haemodynamic instability; concurrent malignancy except non-melanoma skin cancers; pregnant or breastfeeding; participation in another clinical trial of an investigative agent within 30 days of screening; acute renal, cardiac or pulmonary failure; or any life-threatening condition.
Treatment
All subjects were to receive active therapy with oral treprostinil three times daily (TID) for 52 weeks, followed by a 4-week observation period off therapy. Treprostinil was initiated at a dose of 0.125 mg TID and increased by an additional 0.125 mg TID every 3–4 days as tolerated until the maximum tolerable dose was reached; oral treprostinil has no labelled maximum dose and is dependent on patient tolerability. Pharmacokinetic data suggest that TID dosing reduces peak-to-trough plasma treprostinil fluctuations compared with twice daily (BID) and may improve overall tolerability [14]. When needed, the titration rate was slowed at the investigator’s discretion based upon individual subject tolerability.
Primary endpoints: assessment of response and safety
The primary endpoints were safety and tolerability of oral treprostinil in SSc patients with calcinosis affecting the hands, and percentage of patients without radiographic progression of calcinosis over 1 year (at baseline prior to treprostinil initiation and at the 12-month visit) assessed by the Scleroderma Clinical Trials Consortium (SCTC) radiographic score [2]. The SCTC radiographic score for calcinosis is a validated scoring system for severity of calcinosis affecting the hands of SSc patients according to area coverage, density and anatomic location of lesions. This scoring system is feasible with excellent intra- and inter-rater reliability. Progression of calcinosis was defined as >25% increase in score, improvement as >25% decrease and stabilization as values in-between. These cut-offs were pre-defined from an independent cohort of SSc patients with calcinosis, in which the Kappa coefficient of agreement between SCTC radiographic scores and Likert scores were maximized (publication in submission) [3]. At study conclusion, hand radiographs were randomized and scored using the SCTC radiographic score by a radiologist (K.S.), who was blinded to the treatment history and sequence of images. After a delay of 6 months, K.S. was then unblinded to compare overall changes in calcinosis burden at baseline and the 12-month visit for each patient using a Likert scale (1 = a lot better, 2 = a little better, 3 = no change, 4 = a little worse, 5 = a lot worse), without knowledge of the corresponding SCTC radiographic score.
The incidence and severity of treatment emergent adverse events (AEs) (TEAEs) were assessed in all patients who received at least one dose of treprostinil. Safety assessments included TEAEs, serious adverse events, laboratory values and vital signs. AEs were summarized based on the number and percentage of patients reporting events, as well as the severity and median time to resolution. Patients were assessed in person every 3 months.
Secondary endpoints
Secondary endpoints included changes in the following patient-reported outcomes over 1 year: Scleroderma HAQ (SHAQ); Cochin Hand Functional Scale; Medical Outcomes Survey Short Form 36 (SF-36) version 1 Physical (PCS) and Mental (MCS) Component Summary and 8 Domain scores, including SF-6D utility score and transition question; Raynaud Condition Score; and patient and physician assessment of calcinosis severity using a visual analogue scale. The total number of DU and modified Rodnan Skin Score over the study period were also assessed.
SF-36 analysis
We performed further assessments of health-related quality of life (HRQOL) according to patient-reported SF-36 scores. The minimum clinically important differences in PCS and MCS were defined as ≥2.5 for improvement and ≤−0.8 for deterioration, and for the eight domains: ≥5.0 and ≤–2.5, respectively [15, 16]. For a simplified means to visualize complex results across all domains of SF-36 in a single figure, we used ‘spydergrams’ to present changes in SF-36 domains from baseline to last treatment visit, compared with US age- and gender-matched norms for the entire protocol population as a benchmark comparison [17, 18].
Since the SF-36 alone does not evaluate trade-offs between different dimensions of health (e.g. such as between pain and physical functioning), we also calculated the SF-6D health utility score that generates one continuous index based on all SF-36 domain scores (physical function, role physical, bodily pain, general health perceptions, vitality, social functioning, role emotional and mental health) [19, 20]. Changes in SF-6D were calculated from last visit vs baseline, with the minimum important difference defined as ≥0.041 [17, 18].
Using four different measures for each patient (changes in PCS and MCS scores from last visit vs baseline; changes in SF-6D as quantification of the changes in domain scores from last visit vs baseline, illustrated by the spydergrams; and changes in SF-36 transition question from last visit vs baseline, which asks respondents to rate their general health compared with 1 year ago), we categorized each respective measure as ‘improved’, ‘worsened’ or ‘no change’ based on the minimum clinically important differences/minimum important difference cut-offs cited above. We defined ‘overall improvement’ as patients who reported improvements in three or more of four outcomes, ‘overall worsening’ as patients with worsening in two or more of four measures or lacked improvements in any, and ‘no change’ as patients who either reported equal numbers of improvements/deteriorations or whose measures did not meet criteria for overall improvement/worsening.
Statistical analysis
We estimated that 11 patients (with paired radiographs at 1 year) would need to be enrolled for the trial to have at least 80% power to detect a mean change in SCTC radiologic score of 12.0 (equivalent to a minimally significant change in score of 25%) with an s.d. of 8.2 and moderate correlation r = 0.5 using a two-sided test at alpha 0.05 level, assuming a 15% loss to follow-up rate. All continuous measurements were summarized by means (or medians for non-normal data) and s.d. (or minimum and maximum values). Categorical data were summarized by frequency counts and percentages. Simple descriptive statistics were used to summarize the AE profile of treprostinil based on the number and percentage of patients reporting system organ class events. Changes in SCTC radiographic score and secondary endpoints from baseline to last study visit were assessed according to changes from 0 using the Wilcoxon signed rank test. Associations between the radiologist’s final Likert score and SCTC % changes were determined using the Cochran–Mantel–Haenszel test, Cohen’s kappa coefficients (when classifying the SCTC radiographic score as categorical variables into worsening vs improvement/stability) and Spearman correlation coefficients (when classifying the SCTC radiographic score as a continuous variable). All tests were two-sided and P < 0.05 was considered significant. Statistical software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used.
Results
Study population demographics
Twelve SSc patients with calcinosis affecting the hands were enrolled at Stanford University between December 2016 and May 2019, with the last patient completing the trial in May 2020. All were female and half had diffuse cutaneous disease. Median age was 55 years (range 35–68 years) and median disease duration was 12 years (range 5–20 years) from first non-Raynaud’s symptom to screening (Table 1). Five of 12 patients completed the study. Seven patients withdrew early from the trial at a median follow-up of 5 months (range 0.25–10 months) due to the following: intolerable adverse effects (n = 3, diarrhoea, headaches); withdrawn by the investigator for intercurrent illness unrelated to study drug administration (n = 2, cirrhosis, cancer); progressive SSc (n = 1); and personal reasons (time constraints) limiting further participation in the trial (n = 1).
Table 1.
Baseline patient characteristics
| (n = 12) | |
|---|---|
| Age, median (range) | 55 (35–68) |
| Female, n (%) | 12 (100) |
| Race, n (%) | |
| White | 9 (75) |
| Hispanic | 2 (16.7) |
| Black | 1 (8.3) |
| Diffuse cutaneous disease, n (%) | 6 (50) |
| Years from 1st symptom, median (range) | |
| RP | 15.1 (4.8–32.9) |
| Non-RP | 12.3 (5.6–20.5) |
| Ever smoker, n (%) | 4 (33.3) |
| Autoantibodies, n (%) | |
| Anticentromere | 6 (50) |
| Anti-Scl-70 | 1 (8.3) |
| Anti-RNA-polymerase III | 3 (25) |
| Anti-PM-Scl | 1 (8.3) |
| ANA | 9 (75) |
| aPL | 2 (16.7) |
| Disease features, current or prior, n (%) | |
| Digital ulcers | 9 (75) |
| Gangrene | 1 (8.3) |
| Gastrointestinal involvement | 12 (100) |
| Gastroesophageal reflux disease | 12 (100) |
| Esophageal/small bowel dysmotility | 3 (25) |
| Malabsorption syndrome | 1 (8.3) |
| Gastric antral vascular ectasia | 1 (8.3) |
| Small intestinal bacterial overgrowth | 0 |
| Parenteral nutrition | 0 |
| Interstitial lung disease | 6 (50) |
| Arthritis | 6 (50) |
| Myositis | 3 (25) |
| Scleroderma renal crisis | 0 |
| Baseline therapy for RP, n (%)a | |
| Calcium channel blocker | 5 (41.7) |
| Aspirin | 3 (25) |
| SSRI/SNRI | 2 (16.7) |
| ACE inhibitor | 1 (8.3) |
| No treatment | 4 (33.3) |
| Baseline immunosuppressive therapy, n (%)a | |
| MMF | 4 (33.3) |
| MTX | 2 (16.7) |
| CYC | 0 |
| Prednisone | 1 (8.3) |
| Tocilizumab | 1 (8.3) |
| No treatment | 6 (50) |
At time of study enrolment (patients could be on multiple medications simultaneously). Patients with pulmonary arterial hypertension excluded from trial. anti-Scl-70: anti-topoisomerase antibody; anti-PM-Scl: anti-PM-scleroderma antibody; SSRI/SNRI: selective serotonin reuptake inhibitor/selective norepinephrine reuptake inhibitor; ACE: angiotensin-converting enzyme.
Safety and tolerability of study drug
Of the 12 patients enrolled, all received at least one dose of study treatment and were evaluated for safety. Most patients (92%, n = 11) reported at least one TEAE. Most patients developed headaches (n = 7) and gastrointestinal adverse effects (n = 8, comprised of abdominal pain, diarrhoea and nausea) of mild or moderate severity, consistent with the known AE profile of treprostinil (Table 2). There were no serious AEs, hospitalizations or deaths. Most patients (75%) reported a dose-dependent increase in the incidence of gastrointestinal toxicity limiting further dose escalation. Of five patients who completed the study, the median tolerated dose was 1.0 mg TID (range 0.25–7.625 mg TID) at study end. Of seven patients who withdrew from the trial, the median tolerated dosage was 1.3 mg TID (range 0–5.0 mg TID).
Table 2.
Adverse events with oral treprostinil in SSc patients (n = 12)
| Event | No. of patients (%) | Mild | Moderate | Severe | Median (range) time to resolution, days |
|---|---|---|---|---|---|
| Headache | 7 (58) | 5 | 2 | 0 | 12 (1–90) |
| Abdominal pain | 6 (50) | 4 | 2 | 0 | 74 (3–134) |
| Diarrhoea | 5 (42) | 3 | 2 | 0 | 76 (3–143) |
| Nausea | 3 (25) | 2 | 1 | 0 | 38 (20–56) |
| Jaw pain | 2 (16) | 1 | 1 | 0 | 11 (1–20) |
| Dizziness | 1 (8) | 0 | 1 | 0 | 20 |
| Hypotensiona | 1 (8) | 1 | 0 | 0 | 2 |
| Epistaxis | 1 (8) | 1 | 0 | 0 | 2 |
| Weight loss | 1 (8) | 1 | 0 | 0 | 30 |
| Flushing | 1 (8) | 1 | 0 | 0 | 1 |
Defined as systolic blood pressure <90 mmHg or diastolic blood pressure <60 mmHg.
Changes in calcinosis
One patient lacked follow-up radiographs due to early withdrawal and declined repeat imaging. Four of the remaining 11 (36%) patients experienced progression of calcinosis at 1 year based on the SCTC radiographic score. Of the five patients who completed 12 months of treatment, calcinosis was stable in four and progressed in one based on the SCTC radiographic score [overall 1-year median % SCTC radiographic score change of 22.2% (range 5.3–85.5%); P = 0.06] (Table 3). Of six patients who withdrew but had 1-year follow-up hand radiographs, calcinosis was stable/improved in three and progressed in three based on the SCTC radiographic score [overall 1-year median % SCTC radiographic score change of 38.4% (range –38.9 to 111%); P = 0.16]. Fig. 1 demonstrates an example of interval decrease in calcinosis burden. There was a significant association between the final Likert score and changes in SCTC radiographic score, when categorized as worsened vs improved/stable, with a Kappa coefficient of 0.56 (95% CI 0.06–1; P = 0.049), and when categorized as a continuous variable, with a Spearman correlation coefficient of 0.66 (P = 0.027).
Table 3.
Changes in calcinosis at 1 year compared with baseline based on SCTC radiographic scores and Likert scores
| Patient ID | Disease subtype | Autoantibody | Baseline SCTC radiographic score | Post-treatment SCTC radiographic score | % Change in SCTC radiographic score | Overall change in SCTC radiographic scoreb | Likert score | Length of treprostinil treatment |
|---|---|---|---|---|---|---|---|---|
| 1a | Diffuse | RNA polymerase III | 261.0 | 296.1 | 13.5 | Stabilization | A little better | 12 months |
| 2 | Limited | Anti-centromere | 171.1 | 189.0 | 10.4 | Stabilization | A little better | 1 week |
| 3 | Diffuse | RNA polymerase III | 38.0 | 46.5 | 22.4 | Stabilization | A little better | 12 months |
| 5 | Diffuse | Anti-Scl-70 | 2327.5 | 2844.0 | 22.2 | Stabilization | A little better | 12 months |
| 7 | Diffuse | Anti-centromere | 45.5 | 96.0 | 111.0 | Progression | A little worse | 6 months |
| 8 | Diffuse | Negative | 241.4 | 282.1 | 16.9 | Stabilization | No change | 10 months |
| 9 | Limited | Anti-centromere | 25.0 | 46.9 | 87.5 | Progression | A little worse | 8 months |
| 10 | Limited | Anti-centromere | 14.0 | 22.4 | 59.8 | Progression | No change | 5 months |
| 11 | Limited | Anti-centromere | 126.8 | 232.6 | 83.5 | Progression | No change | 12 months |
| 12 | Diffuse | RNA polymerase III | 241.6 | 147.8 | −38.9 | Improvement | A little better | 5 months |
| 14 | Limited | Anti-centromere | 1314.8 | 1384.5 | 5.3 | Stabilization | No change | 12 months |
Patient ID numbers are not all sequential as some patients failed screening and were excluded from the study. Five patients completed the study (patients 1, 3, 5, 11, 14). Patient 6 did not have follow-up radiographs thus was excluded from this table. bProgression (i.e. worsening) of calcinosis was defined as >25% increase in score, improvement as >25% decrease and stabilization (i.e. no change) as values in-between. SCTC: Scleroderma Clinical Trials Consortium.
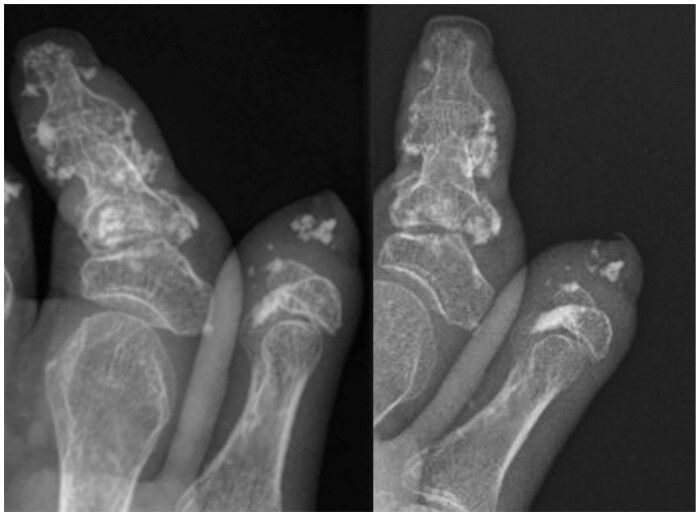
Fig. 1.
Forty-six-year-old female with SSc and severe acro-osteolysis (patient 12)
Radiographs of the right thumb and index fingers at baseline (left) and 1-year follow-up (right) demonstrating interval decrease in calcinosis burden.
Although the sample sizes are small, the patient whose calcinosis improved on the SCTC radiographic score tested positive for RNA polymerase III antibodies. In the four patients whose calcinosis progressed, all had anti-centromere antibodies. In the six patients whose calcinosis remained stable, there was a mix of autoantibodies (two had anti-centromere antibodies, two had RNA polymerase III antibodies, one had anti-Scl-70 antibodies and one was autoantibody negative) (Table 3).
Secondary endpoints
Including 11 enrolled patients with longitudinal data, significant worsening of the SHAQ-VAS-Gastrointestinal (GI) score occurred [median change per month 0.03 (range 0–0.25); P = 0.016] (Table 4), consistent with the large number of gastrointestinal AEs reported with treprostinil. There was slight improvement on the transition question of the SF-36 [median change per month 2.08 (range 0–8.33); P = 0.03]. There were no statistically significant changes in other patient-reported outcomes, including the remaining components of HAQ or SHAQ (including pain, breathing, RP, digital ulcers, disease severity and disability index), Cochin Hand Functional Scale, Raynaud Condition Score and patient as well as physician assessment of calcinosis severity. There were no statistically significant changes in the number of DUs or modified Rodnan Skin Score from baseline to last follow-up. None of the patients with improvement in calcinosis had draining ulcers due to calcinosis as a reason for their improvement.
Table 4.
Changes in secondary endpoints after treatment with oral treprostinil
| Variable (range) | Baseline score, median (range) | Post-treatment score, median (range) | Change per montha, median (range) | P-value |
|---|---|---|---|---|
| HAQ-Disability Index (0–3) | 1.4 (0.1, 2.6) | 1.6 (0.3, 2.5) | 0 (−0.08, 0.1) | 0.734 |
| HAQ-Pain (0–3) | 1.2 (0.3, 3) | 1.2 (0, 2.7) | −0.03 (−0.1, 0.13) | 0.461 |
| SHAQ (0–3) | ||||
| SHAQ-VAS-Gastrointestinal | 0.8 (0, 3) | 1.2 (0, 3) | 0.03 (0, 0.25) | 0.016 |
| SHAQ-VAS-Breathing | 0.5 (0, 2.1) | 0.3 (0, 3) | 0 (−0.15, 0.3) | 0.438 |
| SHAQ-VAS-RP | 0.9 (0, 2.4) | 1.2 (0, 3) | 0 (−0.1, 0.2) | 0.172 |
| SHAQ-VAS-Digital Ulcers | 1.5 (0, 2.4) | 1.5 (0, 3) | 0 (−0.15, 0.2) | 0.820 |
| SHAQ-VAS-Disease Severity | 1.4 (0.6, 2.7) | 1.8 (0.9, 3) | 0 (−0.05, 0.1) | 0.328 |
| Cochin Hand Functional Score (0–90) | 21 (0, 72) | 19 (2, 65) | −0.17 (–1, 4.33) | 0.966 |
| SF-36 (0–100) | ||||
| Physical Function | 32.5 (5, 90) | 30 (0, 95) | 0 (−3.33, 8.33) | 0.766 |
| Role Physical | 0 (0, 75) | 0 (0, 100) | 0 (0, 4.17) | 1.000 |
| Role Emotional | 100 (0, 100) | 100 (0, 100) | 0 (−33.33, 5.58) | 0.625 |
| Vitality | 30 (0, 65) | 30 (10, 65) | 0 (−0.83, 6.67) | 0.602 |
| Mental Health | 76 (8, 84) | 76 (48, 92) | 1 (−1.33, 6.67) | 0.055 |
| Social Functioning | 63 (13, 88) | 50 (25, 100) | 0 (−8.33, 6.17) | 0.797 |
| Bodily Pain | 45 (0, 68) | 43 (13, 80) | 0 (−4, 7.67) | 0.359 |
| General Health Perceptions | 30 (5, 75) | 30 (15, 80) | 0.42 (−1.67, 3.33) | 0.059 |
| Transition Question | 25 (0, 75) | 25 (25, 100) | 2.08 (0, 8.33) | 0.031 |
| Raynaud Condition Score (0–10) | 3 (0, 10) | 4 (1, 10) | 0 (−0.67, 0.33) | 1.000 |
| Patient Global Assessment (0–10) | 4 (1, 10) | 4 (2, 10) | 0 (−0.83, 0.33) | 0.398 |
| Physician Global Assessment (0–10) | 5 (1, 10) | 4 (2, 9) | −0.08 (−0.33, 0.33) | 0.399 |
| Total number of digital ulcers | 1 (0, 5) | 1 (0, 7) | 0 (−1, 0.67) | 0.719 |
| Modified Rodnan skin score (0–51) | 13.5 (3, 34) | 14.5 (3, 29) | −0.13 (−0.92, 0.17) | 0.172 |
Change from baseline to last visit assessed using Wilcoxon signed rank test. Bold text indicates significance. SHAQ: Scleroderma HAQ; SHAQ-VAS: Scleroderma HAQ-Visual Analogue Score; SF-36: 36-Item Short Form Survey.
SF-36 analysis
Using SF-36 responses from the last visit vs baseline, we compared changes in SF-36 PCS and MCS and domain scores, SF-6D and transition question, in each of the 11 patients who received treprostinil (supplementary Table S1, available at Rheumatology online). Spydergrams depicting all eight domains of SF-36 for each patient are included in supplementary Fig. S1, available at Rheumatology online. Based on changes in PCS scores from last visit vs baseline, four patients reported improvement, four worsening and three no change. Changes in MCS scores from last visit vs baseline indicated improvement in four patients, deterioration in five and two with no change. According to the SF-36 transition question, four patients reported improvement, one deterioration and six no change. Using the change in SF-6D utility from last visit vs baseline, four patients improved, two worsened and five had no change. Across all four measures, four patients reported overall improvement, three worsening overall and four no change. Of the five patients who completed the study, three reported overall improvement and two no change. Of the six patients who withdrew from the study, one reported overall improvement, three overall worsening and two no change.
Discussion
The primary objective of this study was to determine the safety and efficacy of oral treprostinil in SSc patients with calcinosis. Of the 5 out of 12 patients who completed the treatment period, most (80%) had stability of calcinosis on hand radiographs at 1 year based on the SCTC radiographic score, with a median tolerated dosage of 1.0 mg TID (range 0.25–7.625 mg TID). Based on our analysis of patient-reported outcomes (SF-36 transition question and changes in PCS, MCS and SF-6D scores), all five patients with 12 months of treprostinil treatment experienced overall improvement (n = 3) or no change (n = 2) compared with their baseline visit, with no overall worsening throughout the trial.
We do not have extensive data about the natural history of calcinosis in patients with SSc; however, it has long been recognized as a late complication of the disease. In a large prospective study of 568 SSc patients, those with calcinosis had significantly longer disease duration than patients without (17.1 ± 11.4 years vs 14.0 ± 12.1 years; P < 0.001) [9]. A recent study designed to establish changes in calcinosis over time evaluated baseline and 1-year antero-posterior hand radiographs from 39 SSc patients with calcinosis [3]. Using the same outcome measure and a similar definition for progression of calcinosis involving the SCTC radiographic score, 16 patients (41%) experienced progression over 1 year, 18 (46%) remained stable and 5 (13%) had improvement in calcinosis. In the present study, although not directly comparable, a larger proportion of patients had calcinosis that remained stable at 1 year, but whether this is directly attributable to the study drug is unclear.
Unfortunately, oral treprostinil was poorly tolerated in our cohort of SSc patients with calcinosis, with most patients developing headaches and gastrointestinal adverse effects (abdominal pain, diarrhoea and nausea), consistent with treprostinil’s known AE profile. Although we initiated the drug at very low doses consistent with prior studies, AEs were reported at doses as low as 0.125 mg TID. Headaches were the most common side effect, generally considered to be mild and tolerable. In contrast, 75% of patients reported a dose-dependent increase in gastrointestinal toxicity limiting further dose escalation, and two patients withdrew from the trial due to intolerable gastrointestinal AEs. Gastrointestinal AEs from treprostinil were impactful enough to negatively influence HRQOL, as evidenced by a statistically significant worsening of the SHAQ-VAS-GI score. All patients in our study had underlying gastrointestinal involvement and half were prescribed concurrent medications such as MTX and MMF, which may have impacted the tolerability of oral treprostinil. Despite this, all patients who finished the trial experienced overall improvement or no change in HRQOL compared with their baseline visit based off the SF-36 transition question and changes in PCS, MCS and SF-6D scores.
Randomized controlled trials supporting the approval of oral treprostinil for PAH [21, 22] and those studying oral treprostinil for DU in patients with SSc [11, 13] were based on a BID dosing schedule. However, evidence suggests that TID regimens provide a sustained plasma exposure in normal volunteers [23], and this dose regimen has been used in later randomized controlled trials of PAH [24]. It is possible that a therapeutic dose of treprostinil was not reached in patients enrolled in our study given the poor tolerability of treprostinil. The median tolerated dosage of treprostinil was 1.0 mg TID at 12 months, whereas in a recent randomized controlled trial of 690 participants with PAH treated with oral treprostinil (25.8% of whom had PAH secondary to CTD), the mean dose of oral treprostinil achieved at week 24 was 3.56 mg TID. Only 18.8% of patients discontinued the drug because of adverse events, and this occurred at a median oral treprostinil dose of 1.4 mg TID [24]. Similarly, in the study of oral treprostinil for DU in patients with SSc [13], the mean (s.d.) maximum dose of oral treprostinil at week 20 was 3.25 (2.8) mg BID, approximately twice the maximum median dose achieved in the present study. Contrary to a few prior studies [11–13], we found no significant improvement in the Raynaud Condition Score and in the number of DU after treatment with treprostinil. The lack of efficacy of treprostinil for RP and DU may be due to the lower therapeutic dosages achieved. The high prevalence of SSc-related gastrointestinal disease in our cohort may in part explain why higher doses of the study medication were not tolerated.
To our knowledge, this is the first prospective clinical trial evaluating the safety and efficacy of a therapeutic agent for the treatment of calcinosis using a validated objective radiographic scoring system to assess severity of calcinosis. This study had some limitations, as this was an open-label, single-arm, single-institution study with a small sample size. There was a high withdrawal rate mostly due to intolerability of the study drug, although the majority still participated in safety follow-up and had repeat radiographs at the end of the study. The presence of calcinosis was evaluated only in the hands and not in other regions of the body, thus the effects of treprostinil in other sites are not known and require future study. Furthermore, although patients were followed for up to 1 year, some calcinosis lesions may continue to change over a longer period, whether spontaneously or while on therapy. Of note, in the study by Valenzuela et al. [3] ∼40% of patients in two independent cohorts experienced progression of calcinosis (defined as an increase in their radiological score >25%) over 1 year, while only a minority (13%) experienced improvement. There were no men enrolled in this trial, but this was due to chance as SSc is more common in women.
In conclusion, our data show that oral treprostinil was poorly tolerated in SSc patients with calcinosis, as most patients developed headaches and gastrointestinal AEs. Of patients who were able to complete the treatment period, 80% had stability of calcinosis on hand radiographs at 1 year. Further research is needed to determine whether alternative formulations of prostacyclins, such as s.c. or i.v. infusions, are better tolerated and efficacious in treating calcinosis in SSc patients. Placebo-controlled studies should also be performed as spontaneous improvement of calcinosis can occasionally occur in untreated patients.
Supplementary Material
Acknowledgements
Authors had full editorial control and provided final approval of all content. M.P.C. received funding from the Training Program in Adult and Pediatric Rheumatology 5T32AR050942-12. L.C. receives funding from the Scleroderma Research Foundation. A.V. received support from Development (ANID) Grant Fondecyt de Iniciación en Investigación No. 11190426.
Funding: This work was supported by the study sponsor, United Therapeutics Corporation (Research Triangle Park, NC, USA). United Therapeutics provided study drug and funding to conduct the study. United Therapeutics was not involved in the study design; the collection, analysis, or interpretation of data; or in writing the manuscript. The sponsors provided approval to submit the manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data collected for this study (including deidentified participant data and a data dictionary), as well as the study protocol, statistical analysis plan and informed consent form, will be made available to others after publication for up to 10 years upon request from the corresponding author. Prior to data transfer, a signed data access agreement will need to be completed.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Valenzuela A, Song P, Chung L.. Calcinosis in scleroderma. Curr Opin Rheumatol 2018;30:554–61. [DOI] [PubMed] [Google Scholar]
- 2. Valenzuela A, Baron M, Herrick AL. et al. Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: a Scleroderma Clinical Trials Consortium study. Semin Arthritis Rheum 2016;46:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valenzuela AC, Rodríguez-Reyna T, Proudman S. et al. Change in calcinosis over 1 year using the SCTC radiologic scoring system for calcinosis of the hands in patients with systemic sclerosis [abstract]. Arthritis Rheumatol 2019;71(Suppl 10). [DOI] [PubMed] [Google Scholar]
- 4. Chander S, Gordon P.. Soft tissue and subcutaneous calcification in connective tissue diseases. Curr Opin Rheumatol 2012;24:158–64. [DOI] [PubMed] [Google Scholar]
- 5. Daoussis D, Antonopoulos I, Liossis SN, Yiannopoulos G, Andonopoulos AP.. Treatment of systemic sclerosis-associated calcinosis: a case report of rituximab-induced regression of CREST-related calcinosis and review of the literature. Semin Arthritis Rheum 2012;41:822–9. [DOI] [PubMed] [Google Scholar]
- 6. Avouac J, Mogavero G, Guerini H. et al. Predictive factors of hand radiographic lesions in systemic sclerosis: a prospective study. Ann Rheum Dis 2011;70:630–3. [DOI] [PubMed] [Google Scholar]
- 7. Koutaissoff S, Vanthuyne M, Smith V. et al. Hand radiological damage in systemic sclerosis: comparison with a control group and clinical and functional correlations. Semin Arthritis Rheum 2011;40:455–60. [DOI] [PubMed] [Google Scholar]
- 8. Johnstone EM, Hutchinson CE, Vail A, Chevance A, Herrick AL.. Acro-osteolysis in systemic sclerosis is associated with digital ischaemia and severe calcinosis. Rheumatology (Oxford) 2012;51:2234–8. [DOI] [PubMed] [Google Scholar]
- 9. Valenzuela A, Baron M, Rodriguez-Reyna TS. et al. Calcinosis is associated with ischemic manifestations and increased disability in patients with systemic sclerosis. Semin Arthritis Rheum 2020;50:891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar P, Thudium E, Laliberte K, Zaccardelli D, Nelsen A.. A comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clin Pharmacokinet 2016;55:1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung L, Fiorentino D.. A pilot trial of treprostinil for the treatment and prevention of digital ulcers in patients with systemic sclerosis. J Am Acad Dermatol 2006;54:880–2. [DOI] [PubMed] [Google Scholar]
- 12. Shah AA, Schiopu E, Chatterjee S. et al. The recurrence of digital ulcers in patients with systemic sclerosis after discontinuation of oral treprostinil. J Rheumatol 2016;43:1665–71. [DOI] [PubMed] [Google Scholar]
- 13. Seibold JR, Wigley FM, Schiopu E. et al. Digital ulcers in Ssc treated with oral treprostinil: a randomized, double-blind, placebo-controlled study with open-label follow-up. J Scleroderma Relat Disord 2017;2:42–9. [Google Scholar]
- 14. Balasubramanian VP, Messick CR, Broderick M, Nelsen AC.. Dosing characteristics of oral treprostinil in real-world clinical practice. Pulm Circ 2018;8:2045894018770654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strand V, Crawford B.. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res 2005;5:317–26. [DOI] [PubMed] [Google Scholar]
- 16. Strand V, Boers M, Idzerda L. et al. It’s good to feel better but it’s better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol 2011;38:1720–7. [DOI] [PubMed] [Google Scholar]
- 17. Strand V, Sharp V, Koenig AS. et al. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Ann Rheum Dis 2012;71:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strand V, Crawford B, Singh J. et al. Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis 2009;68:1800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ara R, Brazier J.. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 2008;11:1131–43. [DOI] [PubMed] [Google Scholar]
- 20. Ara R, Brazier J.. Predicting the short form-6D preference-based index using the eight mean short form-36 health dimension scores: estimating preference-based health-related utilities when patient level data are not available. Value Health 2009;12:346–53. [DOI] [PubMed] [Google Scholar]
- 21. Tapson VF, Jing ZC, Xu KF. et al. ; FREEDOM-C2 Study Team. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013;144:952–8. [DOI] [PubMed] [Google Scholar]
- 22. Jing ZC, Parikh K, Pulido T. et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013;127:624–33. [DOI] [PubMed] [Google Scholar]
- 23. Jones A, Wang-Smith L, Pham T, Laliberte K.. Pharmacokinetics of 3 times a day dosing of oral treprostinil in healthy volunteers. J Cardiovasc Pharmacol 2014;63:227–32. [DOI] [PubMed] [Google Scholar]
- 24. White RJ, Jerjes-Sanchez C, Bohns Meyer GM. et al. ; FREEDOM-EV Investigators. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med 2020;201:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this study (including deidentified participant data and a data dictionary), as well as the study protocol, statistical analysis plan and informed consent form, will be made available to others after publication for up to 10 years upon request from the corresponding author. Prior to data transfer, a signed data access agreement will need to be completed.