Abstract
Objectives
Pure membranous (class V) LN is considered a less aggressive phenotype, but tissue fibrosis and chronic kidney disease may still develop. This study aimed to elucidate the prognostic value of a history of class switch in pure membranous LN.
Methods
We included LN patients with at least two clinically indicated kidney biopsies. New onset of end stage kidney disease (ESKD) was defined as estimated glomerular filtration rate <15 ml/min/1.73 m2, initiation of dialysis or kidney transplantation.
Results
Among 220 patients (542 biopsies), 199 (90%) were female, and 118 (54%) were African American, 59 (27%) Caucasian, with median age of 28 years at the first kidney biopsy. Patients with pure class V in a first biopsy converted to proliferative LN in 41% of cases. Pure class V in a repeat biopsy was preceded by proliferative LN in 52%. Trajectory analysis of up to four repeat biopsies revealed that ISN class switch may happen at any time, even after multiple biopsies with the same class. New onset ESKD was observed within 2 years in 5/56 (9%) patients with pure class V in a repeat biopsy. All five patients had proliferative LN in the first biopsy (log rank P = 0.024).
Conclusions
The conversion from proliferative to membranous (and vice-versa) is frequent in SLE. It can occur at any time in the course of disease, limiting the prognostic value of the first biopsy. Evidence of prior proliferative LN is key as it is associated with higher risk of ESKD in non-proliferative LN.
Keywords: lupus nephritis, SLE, repeat biopsy, membranous, ESKD
Rheumatology key messages.
Class switch in lupus nephritis (LN) is frequent and can occur at any time in the course of disease.
Pure membranous LN in repeat biopsies is not benign as it is associated with 9% progression to end-stage renal disease (ESKD).
ESKD in patients with pure membranous LN developed only in patients with previous history of proliferative LN (class III or IV ± V).
Introduction
LN is a severe manifestation of SLE [1] leading to end-stage kidney disease (ESKD) in 10% of cases and carrying an 8-fold increase in mortality [2–8]. The diagnosis of LN relies on kidney biopsy in patients with proteinuria, as LN is often asymptomatic. Kidney biopsies are critical to guide treatment. International Society of Nephrology (ISN)/Renal Pathology Society (RPS) class III and IV identify a more aggressive phenotype characterized by immune cell infiltration, endocapillary hypercellularity/proliferation (hence called ‘proliferative LN’) and worse prognosis [9]. Proliferative LN is thus treated more aggressively with higher degrees of immunosuppression. Response to treatment in clinical practice and in clinical trials is defined by reduction of proteinuria, stabilization of creatinine levels and ability to taper corticosteroids [10]. However, patients who achieved remission based on clinical parameters may still have histologically active proliferative LN leading to relapses [11–15], highlighting the indispensable role of repeat kidney biopsies in LN.
LN is a very dynamic disease. Studies of repeat biopsies obtained during proteinuria flares revealed that change from one histological class to another occurs in >50% of cases [16–25], without specific predictors in clinical parameters [16]. Yet, the clinical necessity of repeat biopsies still remains controversial [26]. Repeat biopsies during proteinuric flares revealed that up to 78% (∼50% on average) of patients with a previous non-proliferative class (I, II and V) converted to proliferative LN and required escalation of treatment [27]. In contrast, proliferative LN tended to reoccur on repeat biopsies (73%) such that empirical treatment (without a repeat biopsy) was sometimes considered [27, 28]. The conversion from proliferative to non-proliferative LN is not negligible as it was observed in up to 31% of cases, indicating that a repeat biopsy might avoid unnecessarily aggressive treatment [17, 21, 27–29]. This is based on the assumption that the histological findings on the last most recent biopsy are the most accurate assessment to inform prognosis and treatment [12]. Whether evidence of class conversion from the previous biopsy is an indicator of risk of progression in patients with non-proliferative LN has not been adequately established.
Here, we studied LN class conversion in 220 unique patients with repeat biopsies to establish the risk of poor kidney outcomes in patients with conversion to pure class V on the second biopsy. We discovered that, compared with patients with the same class in both biopsies, a history of proliferative LN in a preceding biopsy predicted future ESKD. These findings have prognostic implications and need to be considered in treatment decisions.
Methods
Patients and data
The Hopkins Lupus Cohort is a prospective longitudinal single‐centre cohort of SLE patients ongoing since 1987. Patients met either the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria [30] or the classification criteria as defined by the ACR [31] as updated in 1997 [32]. Patients were seen by a rheumatologist at least every 3 months. Spot urine protein to creatinine ratio (pr/cr), C3, C4, anti-dsDNA antibody titre and serum creatinine were assessed at each visit. LN class from biopsies obtained before enrolment in the Hopkins Lupus Cohort was recorded based on review of previous records at the time of enrolment. Clinical features including proteinuria, renal function or biopsy indication were not available for patients with biopsies obtained before cohort enrolment. All other patients had abnormal proteinuria preceding the renal biopsy. All patients with two or more renal biopsies were included without exclusions. In our clinic, kidney biopsies are obtained in patients with abnormal proteinuria or unexplained decline of renal function. Proliferative LN was defined as ISN/RPS class III ± V or IV ± V. Membranous LN was defined as pure ISN/RPS class V. New onset of EKSD was defined as estimated glomerular filtration rate (eGFR) <15 ml/min/1.73 m2, initiation of dialysis or kidney transplantation. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease (MDRD) equation [33]. The NIH chronicity indices in class V LN were scored by two kidney pathologists and adjudicated if discordant. In these patients, pure class V LN was confirmed in accordance with the 2018 ISN/RPS guidelines [34]. All patients provided informed written consent, and the study was approved yearly by the Johns Hopkins University School of Medicine Institutional Review Board.
Statistical analysis
Circle plots were used to show LN class changes between biopsies. Mann–Whitney test for continuous variables or Fisher’s exact test for categorical variables (where appropriate) were used to determine whether there was a significant difference between patient characteristics and biopsy class categories.
To evaluate whether proliferative LN in the first biopsy was associated with time to ESKD among patients with pure class V LN, the Kaplan–Meier approach was used to estimate the probability of ESKD after the date of second biopsy, censoring patients who had not had ESKD at their last recorded visit in the cohort.
Data are presented as mean (s.d.), median and range, or count and percentage unless otherwise indicated.
All analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). The survival package was used for the Kaplan–Meier analysis [35] and the glm function for logistic regression.
Results
Non-proliferative LN is often preceded by proliferative disease
In the Hopkins Lupus Cohort, there were 220 patients who had at least two kidney biopsies with LN between 1993 and 2019. Patients were mostly female (90%), the median age was 28 years (range 9–60), and the distribution of race/ethnicity was 54% Black/African American, 27% White, 11% Asian and 8% other (Table 1). Kidney biopsies were obtained for clinical purposes such as proteinuria (urine protein/creatinine >0.5) or unexplained worsening kidney function.
Table 1.
Clinical and demographic characteristics
| Variable | LN patients with two or more renal biopsies (n = 220) |
|---|---|
| Female, n (%) | 199 (90) |
| Age at first biopsy, median (range), years | 28 (9–60) |
| Race/ethnicity, n (%) | |
| Black/African American | 118 (54) |
| White | 59 (27) |
| Asian | 25 (11) |
| Other | 18 (8) |
| Time between first and second biopsy, mean (range), years | 3.9 (0.3–28) |
| Treatment after first biopsy (n = 63), n (%) | |
| HCQ | 51 (81) |
| Mycophenolate | 31 (49) |
| AZA | 12 (19) |
| CYC | 4 (6) |
| Prednisone | 52 (82) |
| ACE inhibitors or ARBs | 24 (38) |
| Vitamin D (n = 21) | 16 (76) |
| Treatment at the time of second biopsy (n = 64), n (%) | |
| HCQ | 57 (89) |
| Mycophenolate | 38 (59) |
| AZA | 14 (22) |
| CYC | 1 (1.6) |
| Prednisone (n = 54) | 40 (63) |
| ACE inhibitors or ARBs | 37 (58) |
| Vitamin D (n = 39) | 33 (52) |
| Tacrolimus | 1 (1.6) |
All LN patients in the Hopkins Lupus Cohort with two or more renal biopsies were included (n = 220, 542 biopsies).
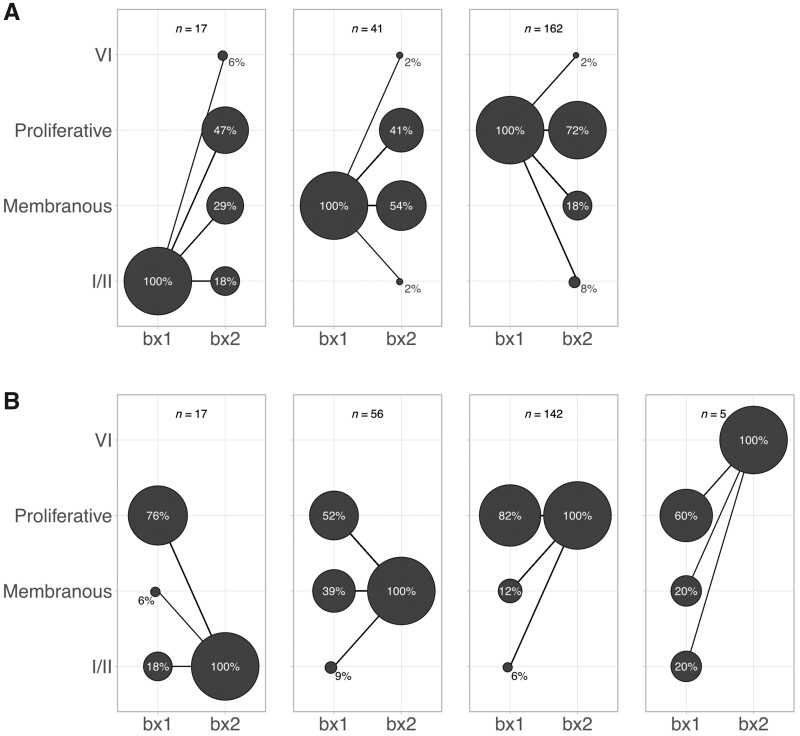
On the first biopsy, 73.5% of patients had proliferative LN (ISN/RPS class III or IV ± V, n = 162), 18.5% had pure membranous LN (n = 41) and 8% had ISN/RPS class I or II LN (Fig. 1A). On the second biopsy, proliferative LN was slightly less common (n = 142, 64.5%), there were more patients with pure membranous LN (n = 56, 25.5%), 17/220 (8%) had ISN/RPS class I or II LN, and 5/220 (2%) developed advanced sclerosis (ISN/RPS class VI) (Fig. 1B).
Fig. 1.
LN class conversion from the first to the second kidney biopsy
Count plot illustrating the percentage of biopsies with each LN class at biopsy 1 (bx1) and biopsy 2 (bx2). The area of the circle is proportional to the total biopsies in each plot. (A) Distribution of classes according to the class at biopsy 1. (B) Distribution of classes according to biopsy 2. Proliferative LN was defined as ISN/RPS class III or IV ± V. Membranous LN was defined as pure ISN/RPS class V.
Overall conversion to a different class group was observed in 35% of patients who required a second biopsy (Fig. 1A). Class conversion was more common in patients with non-proliferative LN on the first biopsy. In particular, 82% of patients with class I/II converted to a higher ISN class, including one patient who progressed to class VI, advanced sclerosis. Patients with pure class V converted to proliferative LN in 41% of cases. Conversely, 72% of patients with proliferative LN had a similar phenotype on the second biopsy, but 18% converted to pure class V and 8% to class I or II. Patients with mixed LN (III/IV + V) equally relapsed as proliferative, membranous or mixed LN. Altogether, these findings demonstrate that changes from non-proliferative to proliferative classes were common and could impact treatment decisions and prognosis.
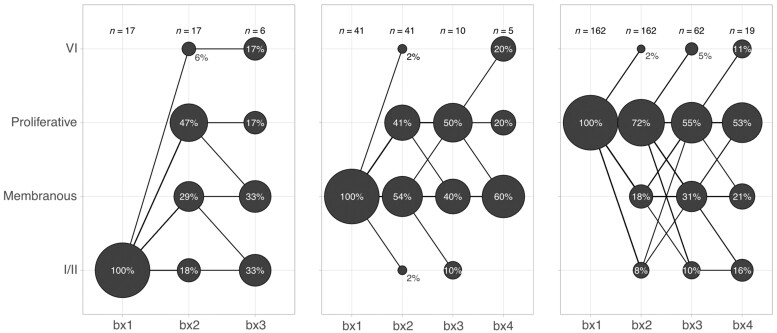
Class conversion can happen at any time during the course of LN
We analysed the change in LN classes in up to four repeat biopsies. There were 79 patients with three and 24 with four kidney biopsies. Almost all possible class conversions were observed at any time point as summarized by Fig. 2. These included conversion to class VI, advanced sclerosis, directly from class I/II or V. Overall, our findings indicate that class changes were very common, even after more than one biopsy showing the same class.
Fig. 2.
LN class conversion in up to four kidney biopsies
Count plot illustrating the percentage of biopsies with each LN class at biopsy 1–4 (bx1–4). The area of the circle is scaled within each biopsy episode within each plot. Almost all possible class switches were observed at any time point. Proliferative LN was defined as ISN/RPS class III or IV ± V. Membranous LN was defined as pure ISN/RPS class V.
Background treatment did not influence LN class at relapse
We tested whether background medications at the time of the second biopsy influenced LN class. More specifically, we asked whether background immunosuppression could prevent proliferative LN in a repeat biopsy. Treatment data for the year preceding biopsy 2 were available for 64 patients (Table 1). We did not observe any impact from underlying treatment on LN class (Supplementary Table S1, available at Rheumatology online).
Evidence of proliferative LN in the first biopsy is associated with progression to ESKD in patients with pure class V LN
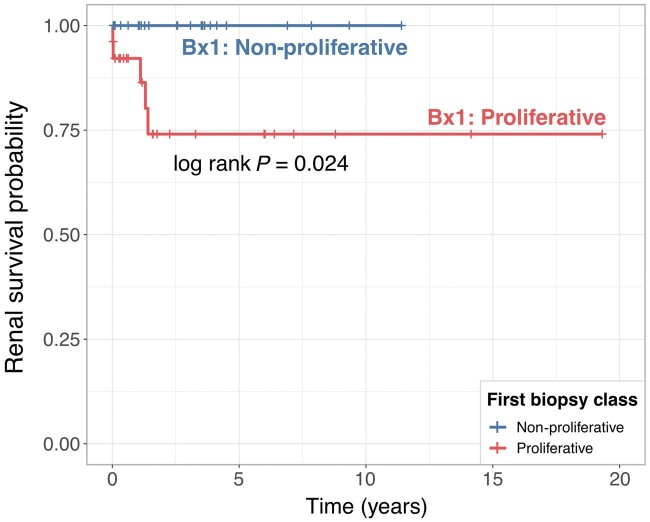
We noted that a large proportion of patients (57.5%) with non-proliferative LN (class I, II or V) on the second biopsy had proliferative LN on the first biopsy (Fig. 1B). We hypothesized that patients with class V LN with a documented preceding episode of proliferative LN would have worse kidney outcomes. To test this hypothesis, we focused on the 56 patients with pure class V LN on the second biopsy (Fig. 1B). Of these, 29 had proliferative LN and 27 had non-proliferative (pure class V) LN on the first biopsy (Table 2). We quantified the rate of progression to ESKD after the second biopsy (the one showing pure class V LN). Five patients progressed to ESKD within 1.5 year (median follow-up was 7.5 years, range 1–20 years). All five patients had proliferative LN in the first biopsy, thus demonstrating that a history of proliferative LN is associated with worse kidney survival in patients with pure class V LN (log rank P = 0.024) (Fig. 3).
Table 2.
Clinical and demographic characteristics of the patient subset with class V LN on biopsy 2
| Biopsy 1 |
||||
|---|---|---|---|---|
| Variable | Total | Non-proliferative | Proliferative | P-value |
| (n = 56) | (n = 27) | (n = 29) | ||
| Age at biopsy 2, mean (s.d.), years | 37.7 (10.7) | 32.7 (9.5) | 0.04 | |
| Sex, n (%) | 0.462 | |||
| Female | 48 (85.7) | 22 (81.5) | 26 (89.7) | |
| Male | 8 (14.3) | 5 (18.5) | 3 (10.3) | |
| Race, n (%) | 0.25 | |||
| Asian | 5 (8.9) | 3 (11.1) | 2 (6.9) | |
| Black | 41 (73.2) | 21 (77.8) | 20 (69) | |
| White | 6 (10.7) | 3 (11.1) | 3 (10.3) | |
| Other | 4 (7.1) | 0 (0) | 4 (14.8) | |
| New eGFR < 15 after biopsy 2, n (%) | 0.052 | |||
| No | 51 (91) | 27 (100) | 24 (82.8) | |
| Yes | 5 (9) | 0 (0) | 5 (17.2) | |
| Follow up from biopsy 1, mean (s.d.), years | 12.5 (6.3) | 12.1 (7.0) | 13.0 (6.0) | 0.597 |
| Follow up from biopsy 2, mean (s.d.), years | 8.0 (5.1) | 7.9 (5.3) | 8.5 (5.1) | 0.679 |
| Time between biopsy 1 and 2, mean (s.d.), years | 4.5 (4.4) | 4.6 (4.3) | 4.5 (4.6) | 0.927 |
| eGFR 2–12 months before biopsy 2. mean (s.d.), mL/min/1.73m2 | 91.7 (32.4) | 95.2 (27.4) | 87.5 (36.7) | 0.396 |
| Urine pr/cr before biopsy 2, mean (s.d.) | 1.1 (1.3) | 1.0 (1.0) | 1.3 (1.5) | 0.361 |
| Nephrotic range, n (%) | 0.908 | |||
| No | 36 (64.3) | 18 (66.7) | 18 (62.1) | |
| Yes | 16 (28.6) | 7 (25.9) | 9 (31) | |
| Class at biopsy 1, n (%) | <0.001 | |||
| I | 1 (1.8) | 1 (3.7) | 0 (0) | |
| II | 4 (7.1) | 4 (14.8) | 0 (0) | |
| III | 3 (5.4) | 0 (0) | 3 (10.3) | |
| IV | 10 (17.9) | 0 (0) | 10 (34.5) | |
| Mixed | 16 (28.6) | 0 (0) | 16 (55.2) | |
| V | 22 (39.3) | 22 (81.5) | 0 (0) | |
| Anti-dsDNA, n (%) | 0.322 | |||
| No | 28 (50) | 15 (55.6) | 13 (44.8) | |
| Yes | 24 (42.9) | 9 (33.3) | 15 (51.7) | |
| C3, mean (s.d.), mg/dl | 95.1 (26.7) | 99.8 (31.1) | 90.8 (21.5) | 0.223 |
| C4, mean (s.d.), mg/dl | 19.9 (8.8) | 20.2 (9.6) | 19.7 (8.2) | 0.82 |
| HCQa, n (%) | 0.605 | |||
| No | 30 (53.6) | 13 (48.1) | 17 (58.6) | |
| Yes | 26 (46.4) | 14 (51.9) | 12 (41.4) | |
| Mycophenolatea, n (%) | 0.263 | |||
| No | 24 (42.9) | 9 (33.3) | 15 (51.7) | |
| Yes | 32 (57.1) | 18 (66.7) | 14 (48.3) | |
| CYCa, n (%) | 1 | |||
| No | 56 (100) | 27 (100) | 29 (100) | |
| Yes | 0 (0) | 0 (0) | 0 (0) | |
| AZAa, n (%) | 0.503 | |||
| No | 46 (82.1) | 24 (88.9) | 22 (75.9) | |
| Yes | 9 (16.1) | 3 (11.1) | 6 (20.7) | |
| Prednisonea, n (%) | 0.117 | |||
| No | 17 (30.4) | 5 (18.5) | 12 (41.4) | |
| Yes | 39 (69.6) | 22 (81.5) | 17 (58.6) | |
| ACE inhibitors or ARBsa, n (%) | 0.81 | |||
| No | 25 (44.6) | 13 (48.1) | 12 (41.4) | |
| Yes | 31 (55.4) | 14 (51.9) | 17 (58.6) | |
Only if confirmed treatment adherence. P-values were calculated using Fisher’s exact or Kruskal–Wallis test as appropriate.
Fig. 3.
Kidney survival of patients with pure class V lupus nephritis in a repeat kidney biopsy according to the histological class of the initial biopsy
Kaplan–Meier survival curves for observed ESKD after a repeat biopsy (biopsy 2) showing class V LN according to the class of the preceding biopsy (biopsy 1). P-value was calculated using the log rank test.
Because no patients with non-proliferative LN on biopsy 1 developed ESKD after biopsy 2, a hazard ratio to quantify the risk of ESKD in this group could not be calculated. For the same reason, we could not adjust for confounders in the multivariable model with 0 events in one group. Nonetheless, we did not identify clinico-demographic features at the time of the second biopsy that were associated with future ESKD based on the univariate analysis, except for HCQ use (Table 3). All five patients who developed ESKD were not taking HCQ (P = 0.055). The eGFR preceding the second biopsy was not available for 4/5 patients and so this potential confounder could not be analysed.
Table 3.
Clinical and demographic characteristics of the patient subset with class V LN on biopsy 2, according to renal outcome
| ESKD onset after biopsy 2 |
|||
|---|---|---|---|
| Variable | No | Yes | P-value |
| (n = 51) | (n = 5) | ||
| Age at biopsy 2, mean (s.d.), years | 34.7 (10.3) | 38.8 (10.6) | 0.481 |
| Sex, n (%) | 1 | ||
| Female | 44 (86.3) | 4 (80) | |
| Male | 7 (13.7) | 1 (20) | |
| Race, n (%) | 0.497 | ||
| Asian | 5 (9.8) | 0 (0) | |
| Black | 37 (72.5) | 4 (80) | |
| White | 6 (11.8) | 0 (0) | |
| Other | 3 (5.9) | 1 (20) | |
| Biopsy 1, n (%) | 0.052 | ||
| Non-proliferative | 27 (52.9) | 0 (0) | |
| Proliferative | 24 (47.1) | 5 (100) | |
| Class of biopsy 1, n (%) | 0.064 | ||
| I | 1 (2) | 0 (0) | |
| II | 4 (7.8) | 0 (0) | |
| III | 2 (3.9) | 1 (20) | |
| IV | 7 (13.7) | 3 (60) | |
| Mixed | 15 (29.4) | 1 (20) | |
| V | 22 (43.1) | 0 (0) | |
| Follow up from first biopsy, mean (s.d.), years | 12.3 (6.4) | 14.4 (5.4) | 0.412 |
| Follow up from second biopsy, mean (s.d.), years | 7.7 (5.2) | 11.2 (2.4) | 0.095 |
| Time between biopsy 1 and 2, mean (s.d.), years | 4.7 (4.5) | 3.2 (3.8) | 0.25 |
| Urine pr/cr before biopsy 2, mean (s.d.) | 1.2 (1.4) | 1.5 (2.0) | 0.97 |
| Nephrotic range, n (%) | 0.893 | ||
| No | 31 (60.8) | 4 (80) | |
| Yes | 16 (31.4) | 1 (20) | |
| NA | 4 (7.8) | 0 (0) | |
| Anti-dsDNA, n (%) | 0.495 | ||
| No | 27 (52.9) | 1 (20) | |
| Yes | 21 (41.2) | 3 (60) | |
| NA | 3 (5.9) | 1 (20) | |
| C3, mean (s.d.), mg/dl | 95.8 (27.8) | 84.5 (3.9) | 0.552 |
| C4, mean (s.d.), mg/dl | 19.8 (8.8) | 21.2 (10.5) | 0.869 |
| HCQ, n (%) | 0.087 | ||
| No | 25 (49) | 5 (100) | |
| Yes | 26 (51) | 0 (0) | |
| Mycophenolate, n (%) | 0.735 | ||
| No | 21 (41.2) | 3 (60) | |
| Yes | 30 (58.8) | 2 (40) | |
| AZA, n (%) | 1 | ||
| No | 42 (82.4) | 4 (80) | |
| Yes | 8 (15.7) | 1 (20) | |
| Prednisone, n (%) | 0.3 | ||
| No | 17 (33.3) | 0 (0) | |
| Yes | 34 (66.7) | 5 (100) | |
| ACE inhibitors or ARBs, n (%) | 0.801 | ||
| No | 22 (43.1) | 3 (60) | |
| Yes | 29 (56.9) | 2 (40) | |
P-values were calculated using Fisher’s exact or Kruskal–Wallis test as appropriate. NA: not available.
History of previous proliferative LN is associated with future proliferative LN in patients with non-proliferative LN
We hypothesized that patients who converted from proliferative to non-proliferative LN were more likely to re-convert to proliferative LN. There were 21 patients with non-proliferative LN on biopsy 2 who also had a third biopsy (Table 4). On biopsy 1, 13/21 had proliferative LN and 8/21 had non-proliferative LN. Six patients with proliferative LN on biopsy 1 re-converted to proliferative LN on biopsy 3 (46%). In contrast, only 1/8 (12.5%) of those with non-proliferative LN on both biopsy 1 and 2 converted to proliferative LN on biopsy 3 (odds ratio [OR]: 6, 95% CI: 0.6, 64). Even though the difference did not reach statistical significance (likely because of small sample size), the large effect size (OR: 6) and biological plausibility suggest that a history of proliferative LN carries a high risk of redeveloping proliferative LN. Conversion from non-proliferative LN in the first biopsy to proliferative LN was associated with poor outcome (Supplementary Fig. S1, available at Rheumatology online).
Table 4.
Risk of developing future proliferative LN in patients with non-proliferative LN on biopsy 2
| Biopsy 3 |
||
|---|---|---|
| Biopsy 1 | Non-proliferative | Proliferative |
| Non-proliferative, n (%) | 7 (87.5) | 1 (12.5) |
| Proliferative, n (%) | 7 (54) | 6 (46) |
Patients with non-proliferative (class I, II or V) on biopsy 2 who underwent a third biopsy (n = 21) were stratified according to the class of biopsy 1. Patients with proliferative LN on biopsy 1 who converted to non-proliferative LN on biopsy 2 were numerically more likely to reconvert to a proliferative class on the third biopsy as compared with those who had non-proliferative LN in both biopsy 1 and 2 (46% vs 12.5%; odds ratio: 6.0, 95% CI: 0.6, 64).
Histological chronicity is associated with a history of proliferative LN
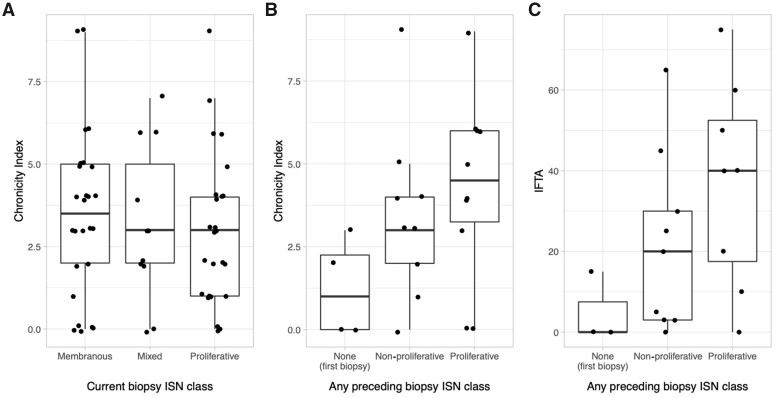
Next, we explored whether the degree of chronic kidney damage and scarring could be used to infer previous episodes of proliferative LN, thus identifying patients at higher risk of poor kidney outcomes. Although expected for all classes, the NIH chronicity index is usually reported only in biopsies with proliferative LN (class III or IV ± V) [34, 36, 37]. The impact of LN class on chronicity has not been adequately studied [18]. We evaluated a subset of patients of the Hopkins Lupus Cohort who were more recently enrolled in the Accelerating Medicines Partnership cohort and whose kidney biopsy was collected at Johns Hopkins University (n = 62). Surprisingly, we noted that the NIH chronicity index did not differ between class V vs class III and IV (P = 0.31) and it was actually numerically higher (Fig. 4A).
Fig. 4.
Chronic damage in non-proliferative lupus nephritis
(A) Box-plots showing the NIH chronicity index in n = 62 LN kidney biopsies according to ISN class. (B, C) NIH chronicity indices (B) and interstitial fibrosis and tubular atrophy (IFTA) percentage (C) in n = 21 ISN class V biopsies according the ISN class of the preceding biopsy. None of the differences across groups reached statistical significance using the Kruskal–Wallis test.
In clinical practice as in this study, kidney biopsies are mostly triggered by an increase in or persistence of proteinuria or an unexplained kidney function decline in asymptomatic patients. However, patients with proteinuria <0.5 g/day may have histologically active proliferative LN [11–15]. It is thus conceivable that even patients with pure class V in repeat kidney biopsies may have interbiopsy episodes of proliferative LN. To test this hypothesis, we quantified the NIH chronicity index with pure class V LN in the repeat biopsy. Since necrotizing lesions in patients with active proliferative LN lead to higher chronicity [18], we hypothesized that a higher chronicity index is expected in patients with proliferative LN in previous biopsies. Among patients with pure class V in the second biopsy, those with proliferative LN in the first biopsy had numerically higher NIH chronicity index as compared with those with pure class V in both biopsies, but the difference was not statistically significant (median [interquartile range, IQR] 4.5 [3.25–6] vs 3 [2–4], P = 0.32) (Fig. 4B). Similarly, the degree of interstitial fibrosis and tubular atrophy was substantially numerically higher in those whose pure class V LN was preceded by proliferative LN (median [IQR] 40 [17.5–52.5] vs 20 [3–30], P = 0.25) (Fig. 4C). Notably, there was a patient with both biopsies with pure class V with a very high chronicity index, 9/12. Review of her medical records revealed that her first episode of class V LN was not treated with immunosuppression and she had untreated symptomatic SLE for several years before re-establishing care due to proteinuria and having the second biopsy. The second biopsy showed a severely sclerosed kidney. It is tempting to speculate that if she had regular clinical care during the time after her first biopsy, she might have shown findings prompting an additional kidney biopsy that would clarify the course of her disease, perhaps with interim proliferative LN.
As expected, patients at their first biopsy showing pure class V had lower chronicity (median [IQR] 1 [0–2.25] vs 4 [2.5–5], P = 0.048) (Fig. 4B). In fact, in patients requiring multiple biopsies, the chronicity index tended to increase with the number of biopsies (Spearman’s r: 0.44, P = 0.04) (Supplementary Fig. S2, available at Rheumatology online) suggesting that episodes of refractory or relapsing LN that lead to more biopsies may result in more permanent kidney damage.
Discussion
LN is a very dynamic disease. Diagnosis and treatment decisions should account for this ever-changing nature. Here, we have shown that (i) >50% of patients with pure class V may have had previous proliferative LN; (ii) a history of class conversion in patients with pure class V LN portended a significant risk of poor kidney survival and higher risk of future proliferative LN; and (iii) formal assessment of chronicity in non-proliferative LN may help identify patients at high risk of poor kidney outcomes, as recommended by the ISN/RPS [34].
It is well established that proliferative LN is associated with poor kidney outcomes leading to consensus that treatment should be prompt and aggressive. However, pure membranous LN (class V) is sometimes treated without immunosuppression as it is considered to have better long-term outcomes. Thus, a patient with a history of LN presenting with a proteinuric flare who is found to have class V LN in a repeat biopsy might not receive aggressive treatment. We have shown that 9% of these patients may progress to ESKD in the 2 years following the repeat biopsy. All patients who progressed had a history of proliferative LN in a preceding biopsy. This outcome may have several explanations. We showed that previous episodes of proliferative LN may lead to chronic kidney damage, which is itself a risk for poor kidney survival [24, 38]. We posit that LN exists in a continuum and, therefore, the disease state inferred from kidney biopsies at one time point may not capture the full extent of the disease process. This latter theory better responds to a principle of parsimony (LN is one disease with proliferative LN representing its most aggressive form), in contrast to regarding proliferative and non-proliferative LN as biologically separate entities [39] (but this remains an open question as no definitive evidence is available). This hypothesis is best illustrated by the frequent finding of mixed LN in which INS/RPS class III or IV coexist with the membranous findings of class V. As such, a history of class change may identify a subset of patients at higher risk of continuing to switch between proliferative and non-proliferative LN. This group should thus be functionally considered to have proliferative LN, as the kidney biopsy may have captured the moment in time with just the membranous phenotype, without acknowledging the risk of developing proliferative LN in the future. In fact, our data revealed that 46% of patients that converted from proliferative to non-proliferative LN eventually re-developed proliferative LN as documented by their third or fourth biopsy.
Renal biopsies have an indispensable role in that they can distinguish active nephritis from chronic damage, both of which manifest with proteinuria. Our findings highlighted the importance of repeat biopsies. We have shown that the class of a previous biopsy influences the outcome after the second one. Therefore, the absence of a repeat biopsy in patients with proteinuric flares may miss important histological findings that not only affect the immediate treatment decision, but also inform long-term prognosis. It follows that, until a better non-invasive biomarker is developed [40], our findings suggest that repeat biopsies should be frequently considered in treated LN patients with reoccurring or refractory proteinuria, or with worsening renal function.
We showed that patients with pure class V LN may have high chronicity scores that were comparable, if not worse, to those of patients with proliferative LN. A similar trend was observed using the NIH chronicity score and IFTA. Although not meeting statistical significance, our findings suggested that patients with class V LN with high chronicity scores were more likely to have had a previous episode of proliferative LN suggesting that chronicity, representing the ‘scar’ from previous insults, might be helpful to identify patients with previous undiagnosed episodes of proliferative LN. Tubulointerstitial damage, captured by both the NIH chronicity index and IFTA, has been linked to tubulointerstitial immune complex deposition [41] and proteinuria [41]. The degree of tubulointerstitial lesions has been shown to be more severe in patients with proliferative LN than pure membranous LN and correlate with kidney outcome [42]. Altogether, our findings support reporting the chronicity scores in all classes, including non-proliferative LN, as recommended by ISN/RPS [34].
It is not fully clear whether certain treatments affect membranous or proliferative LN differently. In this study, there was no influence of background treatment at the time of relapse on the class of the repeat biopsy. Although this may suggest that immunosuppression affects proliferative and non-proliferative LN equally, larger studies are needed to clarify this point. Further, patients with a history of proliferative LN who are found to have non-proliferative LN on a repeat biopsy might benefit from immunosuppression regardless of class or nephrotic range proteinuria given the higher risk of ESKD and relapse of proliferative LN.
We acknowledge the limitations of our study. Although we described the largest cohort of pure class V LN on repeat biopsy to date, our study did not have adequate sample size for all the analyses, which limited statistical power [43, 44]. However, the large effect sizes in the setting of biological plausibility suggest that our findings are clinically important [43, 44]. The histological slides from some of the historical repeat biopsies recorded by Hopkins Lupus Cohort were not available to score the NIH chronicity index. The assessment of the impact of previous LN class on the chronicity in repeat biopsies with class V LN was thus performed on a limited sample size. We did not have information on the urinary sediment analysis. The eGFR preceding biopsy 2 was not available for all patients and therefore we could not establish its effect as a confounder.
We note that selecting patients with multiple clinically indicated biopsies may introduce a selection bias. Because this analysis was within patients with multiple biopsies and from a single centre, the effects of such bias should be mitigated. We observed a correlation between the NIH chronicity scores and the number of biopsies. Although this association could be driven by the two cases with more than four biopsies, this is consistent with what has been previously described [45]. Finally, we note that according to the 2018 ISN/RPS guidelines [34], patients with membranous LN and non-wire loop subendothelial deposits are ascribed to class III/IV+V. Because we could not verify the presence of subendothelial deposits in historical biopsies, there is the possibility that a few patients with pure membranous LN could have been misclassified. However, this would not affect our results because all the patients who developed new ESKD after a repeat biopsy with confirmed pure membranous LN had clear evidence of proliferative LN in the preceding biopsy. It might change the Kaplan–Meier estimator. Inter-observer and intra-observer variation could also not be evaluated.
In summary, this study provides evidence that class V LN is often preceded by proliferative LN and carries a significant risk of progression to ESKD. Repeat biopsies provide critical information to manage and prognosticate LN and should be pursued for kidney flares unless contraindicated. Evidence of non-proliferative LN in repeat biopsies should not automatically lead to reassurance, inasmuch as prognosis and treatment decisions should be informed by patient specific LN history and the degree of chronicity. The NIH chronicity score should be quantified in all LN classes as it provides an immediate measure of chronic damage and might help in identifying patients with previously undetected episodes of proliferative LN.
Supplementary Material
Acknowledgements
A.F. is supported by the Jerome L. Greene Foundation and the Cupid Foundation.
Funding: The Hopkins Lupus Cohort is funded by NIH AR 69572.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Fava A, Petri M.. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun 2019;96:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alarcón GS, Roseman J, Bartolucci AA. et al. ; Lumina Study Group. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. Arthritis Rheum 1998;41:1173–80. [DOI] [PubMed] [Google Scholar]
- 3. Mok CC, Kwok RCL, Yip PSF.. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 2013;65:2154–60. [DOI] [PubMed] [Google Scholar]
- 4. Lim SS, Bayakly AR, Helmick CG. et al. The incidence and prevalence of systemic lupus erythematosus, 2002-2004: the Georgia Lupus Registry. Arthritis Rheumatol 2014;66:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferucci ED, Johnston JM, Gaddy JR. et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska Native people, 2007-2009. Arthritis Rheumatol 2014;66:2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dall'Era M, Cisternas MG, Snipes K. et al. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: the California Lupus Surveillance Project. Arthritis Rheumatol 2017;69:1996–2005. [DOI] [PubMed] [Google Scholar]
- 7. Izmirly PM, Wan I, Sahl S. et al. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York: the Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017;69:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Somers EC, Marder W, Cagnoli P. et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan lupus epidemiology and surveillance program. Arthritis Rheumatol 2014;66:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sloan RP, Schwartz MM, Korbet SM, Borok RZ.. Long-term outcome in systemic lupus erythematosus membranous glomerulonephritis. Lupus Nephritis Collaborative Study Group. J Am Soc Nephrol 1996;7:299–305. [DOI] [PubMed] [Google Scholar]
- 10. Wofsy D, Hillson JL, Diamond B.. Comparison of alternative primary outcome measures for use in lupus nephritis clinical trials. Arthritis Rheum 2013;65:1586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malvar A, Pirruccio P, Alberton V. et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant 2017;32:1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Rosa M, Azzato F, Toblli JE. et al. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int 2018;94:788–94. [DOI] [PubMed] [Google Scholar]
- 13. Olson SW, Lee JJ, Prince LK. et al. Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin J Am Soc Nephrol 2013;8:1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wakasugi D, Gono T, Kawaguchi Y. et al. Frequency of class III and IV nephritis in systemic lupus erythematosus without clinical renal involvement: an analysis of predictive measures. J Rheumatol 2012;39:79–85. [DOI] [PubMed] [Google Scholar]
- 15. De Rosa M, Rocha AS, De Rosa G. et al. Low-grade proteinuria does not exclude significant kidney injury in lupus nephritis. Kidney Int Rep 2020;5:1066–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moroni G, Depetri F, Ponticelli C.. Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmun 2016;74:27–40. [DOI] [PubMed] [Google Scholar]
- 17. Greloni G, Scolnik M, Marin J. et al. Value of repeat biopsy in lupus nephritis flares. Lupus Sci Med 2014;1:e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alsuwaida A, Husain S, Al Ghonaim M. et al. Glomerular necrotic lesions and long-term outcomes among patients with proliferative lupus nephritis. Int J Clin Exp Pathol 2015;8:5787–92. [PMC free article] [PubMed] [Google Scholar]
- 19. Pagni F, Galimberti S, Goffredo P. et al. The value of repeat biopsy in the management of lupus nephritis: an international multicentre study in a large cohort of patients. Nephrol Dial Transplant 2013;28:3014–23. [DOI] [PubMed] [Google Scholar]
- 20. Bao WG, Jin XZ, Fa LH. et al. Changes in pathological pattern and treatment regimens based on repeat renal biopsy in lupus nephritis. Chin Med J (Engl) 2012;125:2890–4. [PubMed] [Google Scholar]
- 21. Lu J, Tam LS, Lai FMM. et al. Repeat renal biopsy in lupus nephritis: a change in histological pattern is common. Am J Nephrol 2011;34:220–5. [DOI] [PubMed] [Google Scholar]
- 22. Sun HO, Hu WX, Xie HL. et al. Long-term outcome of Chinese patients with membranous lupus nephropathy. Lupus 2008;17:56–61. [DOI] [PubMed] [Google Scholar]
- 23. Bajaj S, Albert L, Gladman DD. et al. Serial renal biopsy in systemic lupus erythematosus. J Rheumatol 2000;27:2822–6. [PubMed] [Google Scholar]
- 24. Moroni G, Pasquali S, Quaglini S. et al. Clinical and prognostic value of serial renal biopsies in lupus nephritis. Am J Kidney Dis 1999;34:530–9. [DOI] [PubMed] [Google Scholar]
- 25. Esdaile JM, Joseph L, MacKenzie T, Kashgarian M, Hayslett JP.. The pathogenesis and prognosis of lupus nephritis: information from repeat renal biopsy. Semin Arthritis Rheum 1993;23:135–48. [DOI] [PubMed] [Google Scholar]
- 26. Daleboudt GMN, Bajema IM, Goemaere NNT. et al. The clinical relevance of a repeat biopsy in lupus nephritis flares. Nephrol Dial Transplant 2009;24:3712–7. [DOI] [PubMed] [Google Scholar]
- 27. Narváez J, Ricse M, Gomà M. et al. The value of repeat biopsy in lupus nephritis flares. Medicine (Baltimore) 2017;96:e7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilhelmus S, Bajema IM, Bertsias GK. et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant 2016;31:904–13. [DOI] [PubMed] [Google Scholar]
- 29. Pakozdi A, Pyne D, Sheaff M, Rajakariar R.. Utility of a repeat renal biopsy in lupus nephritis: a single centre experience. Nephrol Dial Transplant 2018;33:507–13. [DOI] [PubMed] [Google Scholar]
- 30. Petri M, Orbai AM, Alarcõn GS. et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan EM, Cohen AS, Fries JF. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 32. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 33. Levey AS, Coresh J, Greene T. et al. ; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- 34. Bajema IM, Wilhelmus S, Alpers CE. et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93:789–96. [DOI] [PubMed] [Google Scholar]
- 35. Feldman CH, Hiraki LT, Liu J. et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum 2013;65:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weening JJ, D'agati VD, Schwartz MM. et al. ; International Society of Nephrology and Renal Pathology Society Working Group on the Classification of Lupus Nephritis. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004;65:521–30. [DOI] [PubMed] [Google Scholar]
- 37. Austin HA, Muenz LR, Joyce KM, Antonovych TT, Balow JE.. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int 1984;25:689–95. [DOI] [PubMed] [Google Scholar]
- 38. Austin HA, Muenz LR, Joyce KM. et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med 1983;75:382–91. [DOI] [PubMed] [Google Scholar]
- 39. Farinha F, Pepper RJ, Oliveira DG. et al. Outcomes of membranous and proliferative lupus nephritis—analysis of a single-centre cohort with more than 30 years of follow-up. Rheumatology 2020;59:3314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fava A, Buyon J, Mohan C. et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight 2020;5:e138345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hill GS, Delahousse M, Nochy D, Mandet C, Bariéty J.. Proteinuria and tubulointerstitial lesions in lupus nephritis. Kidney Int 2001;60:1893–903. [DOI] [PubMed] [Google Scholar]
- 42. Yu F, Wu L, Tan Y. et al. Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int 2010;77:820–9. [DOI] [PubMed] [Google Scholar]
- 43. Sullivan GM, Feinn R.. Using effect size—or why the P value is not enough. J Grad Med Educ 2012;4:279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thompson B. Effect sizes, confidence intervals, and confidence intervals for effect sizes. Psychol Sch 2007;44:423–32. [Google Scholar]
- 45. Marinaki S, Kapsia E, Liapis G. et al. Clinical impact of repeat renal biopsies in patients with lupus nephritis: renal biopsy is essential especially later in the course of the disease. Eur J Rheumatol 2020;7:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.