Abstract
Aims: Interleukin-1β (IL-1β) contributes to the development of bronchopulmonary dysplasia (BPD). Thioredoxin reductase-1 (Txnrd1) inhibition activates nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent responses. Txnrd1 activity is selenium (Se) dependent and Se deficiency is common in prematurity. Auranofin (AFN), a Txnrd1 inhibitor, decreases IL-1β levels and increases Nrf2 activation in lipopolysaccharide (LPS) treated alveolar macrophages. In lung epithelia, AFN-induced Nrf2 activation is Se dependent. We tested the hypothesis that the effects of Txnrd1 inhibition in alveolar macrophages are Se dependent. Main Methods: To establish Se sufficient (Se+) and deficient (Se-) conditions, alveolar (MH-S) macrophages were cultured in 2.5% fetal bovine serum (FBS) ± 25 nM Na2SeO3. Se- (2.5% FBS) and Se+ (2.5% FBS + 25 nM Na2SeO3) cells were cultured in the presence or absence of 0.05 μg/mL LPS and/or 0.5 μM AFN. Nrf2 activation was determined by measuring NADPH quinone oxidoreductase-1 (Nqo1) and glutathione levels. IL-1β mRNA (Il1b) and protein levels were measured using qRT-PCR and ELISA. Data were analyzed by ANOVA followed by Tukey’s post-hoc. Key Findings: We detected an independent effect of AFN, but not LPS, on Nqo1 expression and GSH levels in Se+ and Se- cells. LPS significantly increased Il1b and IL-1β levels in both groups. AFN-mediated attenuation of this effect was not impacted by Se status. Significance: The beneficial effects of Txnrd1 inhibition in alveolar macrophages are Se-independent and therefore unlikely to be diminished by clinical Se deficiency.
Keywords: auranofin, thioredoxin, thioredoxin reductase, selenium, prematurity, macrophage, interleukin-1β
Introduction
Bronchopulmonary dysplasia (BPD) is a heterogeneous chronic lung disease in preterm infants. The proinflammatory cytokine interleukin-1β (IL-1β) is involved in the initiation and propagation of inflammation [1]. IL-1β levels are increased in amniotic fluid of expectant mothers with chorioamnionitis and preterm labor [2] and in tracheal aspirates collected from infants who develop bronchopulmonary dysplasia (BPD) [3]. Alveolar macrophages regulate inflammatory responses via production of reactive oxygen species and cytokines including IL-1β [4].
In the lung, thioredoxin reductase-1 (Txnrd1) is most abundantly expressed in epithelial cells and alveolar macrophages cells [5]. Txnrd1 contributes to the regulation of antioxidant responses via nuclear factor erythroid 2-related factor 2 (Nrf2) [6]. Mechanistically, pharmacologic Txnrd1 inhibition with auranofin (AFN) enhances Nrf2 activation. This results in increased activation of antioxidant responses as evidenced by enhanced NADPH quinone oxidoreductase-1 (Nqo1) expression and glutathione levels [5–7]. AFN-mediated Txnrd1 inhibition also attenuates lipopolysaccharide (LPS)-induced IL-1β production in vitro [8].
Txnrd1 is a selenoenzyme due to the presence of a selenocysteine residue in the active site. Selenoenzymes require selenium (Se) for optimal catalytic activity [9]. In the fetus, Se is primarily acquired during the third trimester via placental transfer. A 2003 meta-analysis revealed an inverse relationship between low plasma Se in neonates <2 kg and respiratory complications of prematurity, including BPD [10].
We have previously established that Txnrd1 inhibition activates Nrf2-dependent antioxidant responses in lung epithelia via Se-dependent mechanisms. Given that Txnrd1 inhibition attenuates LPS-induced IL-1β production in vitro, the present studies were designed to test the hypothesis that the effects of Txnrd1 inhibition in alveolar macrophages are Se dependent.
Materials and methods
Cell culture and treatments
Murine alveolar macrophage (MH-S) cells, were first cultured in Roswell Park Memorial Institute (RPMI) media supplemented with 5% fetal bovine serum (FBS; Mediatech) and 1% penicillin-streptomycin. Cells were then plated at equal densities in media containing 5% FBS alone or 2.5% FBS supplemented with 0, 10, or 25 nM sodium selenite (Na2SeO3) for 72 h. Each group was then treated with 0.1% dimethyl sulfoxide (DMSO, Fisher), or 0.5 μM Auranofin (AFN, Sigma) in DMSO for 1, 4 or 6 h in the presence or absence of 0.05 μg/mL LPS (Sigma) in Dulbecco’s phosphate-buffered saline (PBS, Sigma). At experiment termination, cells were washed with PBS and collected.
Plate coverage or cell proliferation assessment
After culturing cells were acclimated to the FBS containing media for 72 hr. Cells were plated in 6-well plates at equal density, allowed to attach for 2 hr, assessed using the SpectraMax MiniMax 300 Imaging Cytometer using the StainFree Cell Detection Algorithm. Percent coverage was averaged from 4 separate readings per well at each timepoint.
TXNRD1 activity
TXNRD1 activity in cell lysates was determined by insulin disulfide reduction assay [5]. Assays were performed in duplicate.
Quantitative real-time PCR
RNA was isolated from cell lysates (RNeasy Plus Mini Kit, Qiagen). DNA was synthesized using a High Capacity cDNA Reverse Transcription Kit and quantitative real time PCR was performed using TaqMan Fast Advanced Master Mix (Thermo) on an iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). Primers (Thermo Fisher) were: 18S (431089E), murine Il1b (Mm00434228_m1), and Nqo1 (Mm01253561_m1). Quantification of mRNA was calculated by the comparative CT method and is given as fold change of expression (2-ΔΔCT) and normalized to 18S mRNA levels.
Glutathione contents
Total glutathione (GSH) was determined using high pressure liquid chromatography followed by electrochemical detection (HPLC-ED). Briefly, thawed cells were resuspended in 200 μL PBS, sonicated three times for 10 seconds then centrifuged at 20,000x gravity at 4°C for 10 minutes. Supernatant was collected and 50 μL set aside for protein measurement (BCA assay, Pierce). Supernatant (150 μL) was mixed 1:1 with 0.4 N perchloric acid (HPLC grade) then centrifuged again. Supernatant was filtered by 0.2 μm Millipore-LCR (PTFE) membrane (SLLGC13NL). Sample (5 μL) was loaded into the HPLC-ECD and run with 65% Buffer A [1ml o-Phosphoric Acid (HPLC grad, Fisher A260–500) + 1000ml ddH2O] and 35% Buffer B [Test Mobile Phase (75–05-8)] for 13 min on an Acclaim RSLC PA2 column (074814) at 40°C. The GSH peak was observed at 4 min.
IL-1β protein expression
Frozen cells were re-suspended in 50 μL lysis buffer and centrifuged at 15,000 x gravity for 10 min at 4°C. Enzyme-linked immunosorbent assay (ELISA; R&D Systems) was used to quantify IL-1β protein concentration in cell supernatant.
Statistical analyses
Data are representative of at least 2 replicate experiments and were analyzed using GraphPad Prism 8.4. All data were normalized vs respective controls, combined, tested for homogeneity of variances, and log-transformed when indicated. Data were analyzed by one-way or two-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison tests with significance accepted at p<0.05.
Results
Establishing Selenium Conditions in MH-S Cells
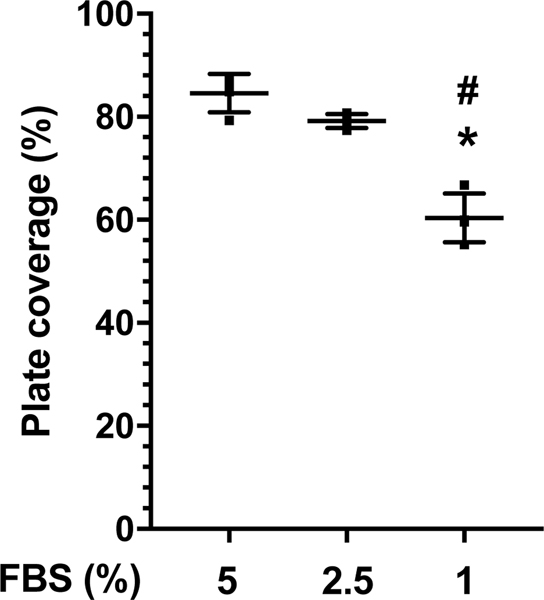
Standard culture of MH-S cells in media containing 10% FBS resulted in optimal Txnrd1 activity without additional Se supplementation (not shown). To establish Se deficient conditions, MH-S cells were cultured in RPMI media containing 5%, 2.5% or 1% FBS and cellular proliferation was monitored (Figure 1). When compared to 5% FBS and 2.5% FBS, our data revealed a significantly lower percent plate coverage in MH-S cells cultured in media supplemented with 1% FBS.
Figure 1. Cell growth at 72 h.
Cells were cultured for 72 h in RPMI media supplemented with 5%, 2.5%, or 1% FBS. Data (Mean ± SD, n=4) were analyzed by ANOVA. (*p<0.0001 vs 5% FBS; #p=0.0001 vs 2.5% FBS).
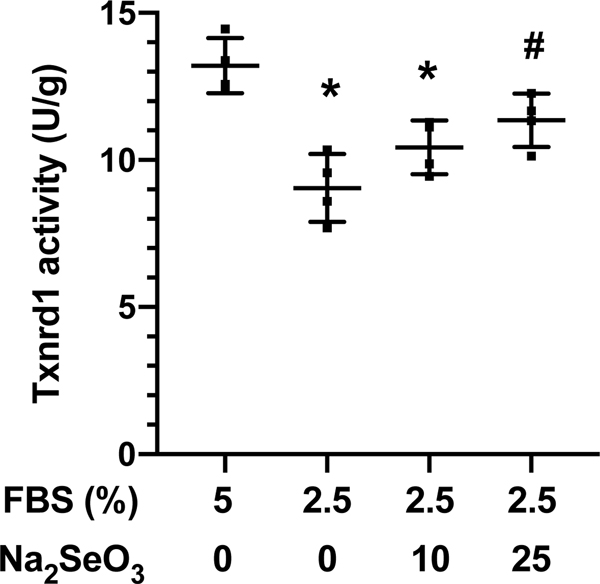
Txnrd1 activity was determined in cell lysates as an index of Se status [5]. Txnrd1 activity was significantly lower in cells cultured in 2.5% FBS alone or 2.5% FBS + 10 Na2SeO3 when compared to cells cultured in media containing unsupplemented 5% FBS (Figure 2). Txnrd1 activity was significantly greater in cells cultured in 2.5% + 25 nM Na2SeO3 when compared to 2.5% FBS alone. There was no significant difference in Txnrd1 activity between cells cultured in 5% FBS and 2.5% FBS + 25 nM Na2SeO3. Thus, subsequent experiments were conducted under Se deficient (Se-; 2.5% FBS alone) or Se sufficient (Se+; 2.5% FBS + 25 nM Na2SeO3) conditions.
Figure 2.
Txnrd1 activity. MH-S cells were cultured for 72 h in RPMI media supplemented with 5% or 2.5% FBS and 0, 10, or 25 nM Na2SeO3. Txnrd1 activity was determined by insulin-disulfide reductase assay. Data (mean ± SD, n=4) were analyzed by ANOVA. (*p<0.009 vs 5% FBS; #p=0.028 vs 2.5% FBS).
AFN enhances Nqo1 expression
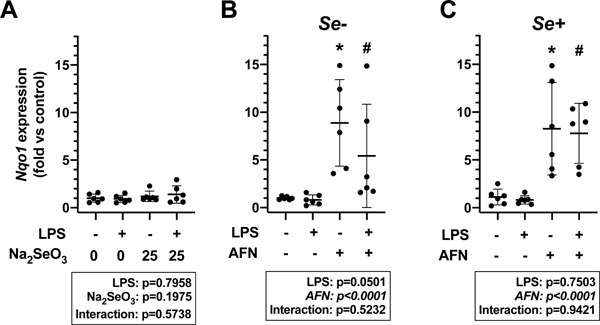
Nqo1 mRNA levels more specifically represent AFN-mediated Nrf2 activation when compared to other antioxidant genes [11]. To evaluate the independent effects of LPS, AFN, and Se levels on Nrf2 activation in MH-S cells, Nqo1 expression was determined. Neither LPS treatment nor Na2SeO3 supplementation impacted Nqo1 levels (Figure 3A). In contrast, AFN treatment significantly increased Nqo1 expression in both Se- (Figure 3B) and Se+ (Figure 3C) groups when compared to respective no LPS and LPS controls. The magnitude AFN-induced increase in Nqo1 expression did not appear to be different between Se- and Se+ groups.
Figure 3. Nqo1 expression.
A) MH-S cells were cultured for 72h in RPMI media containing 2.5% FBS supplemented with 0 or 25 nM Na2SeO3 and treated with 0.05 μg/mL LPS or vehicle for 4h. MH-S cells were cultured in RPMI media containing 2.5% FBS supplemented with (B) 0 (Se-) or (C) 25 nM Na2SeO3 (Se+) for 4 h in the presence or absence of 0.05 μg/mL LPS and/or 0.5 μM AFN. Data (mean ± SD, n=6) were analyzed by two-way ANOVA followed by Tukey’s post hoc analysis (*p<0.003 vs control; #p<0.003 vs LPS alone).
AFN enhances GSH levels
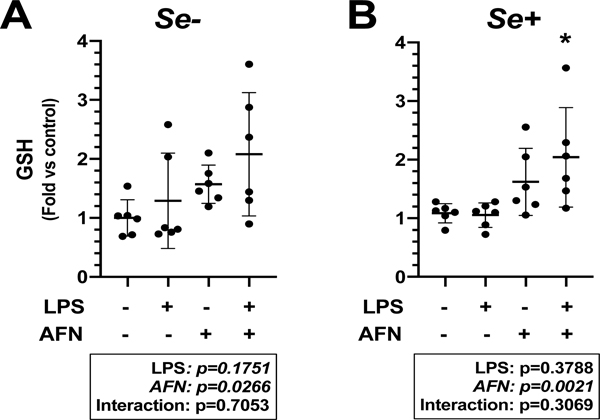
We have previously shown that treatment of cells with 0.5 μM AFN for 1 h significantly inhibits Txnrd1 activity, elicits Nrf2 activation, and increases intracellular glutathione (GSH) [5]. For the present studies, GSH concentrations were evaluated after MH-S cells were cultured for 72 h in Se+ or Se- media and treated with 0.5 μM AFN or control for 6h in the presence or absence of 0.05 μg/mL LPS. Data demonstrated an independent effect of AFN on GSH contents in both Se- (Figure 4A) and Se+ conditions (Figure 4B). There was no significant effect of LPS on GSH levels under Se- or Se+ conditions. Under Se+ conditions, GSH contents were greater in AFN+LPS-treated cells than in cells treated with LPS alone (Figure 4B).
Figure 4. GSH contents.
MH-S cells were cultured in RPMI media containing 2.5% FBS supplemented with (A) 0 (Se-) or (B) 25 nM Na2SeO3 (Se+) for 6 h in the presence or absence of 0.05 μg/mL LPS and/or 0.5 μM AFN. Data (mean ± SD, n=6) were analyzed by two-way ANOVA followed by Tukey’s post hoc analysis. (*p=0.0198 vs LPS alone).
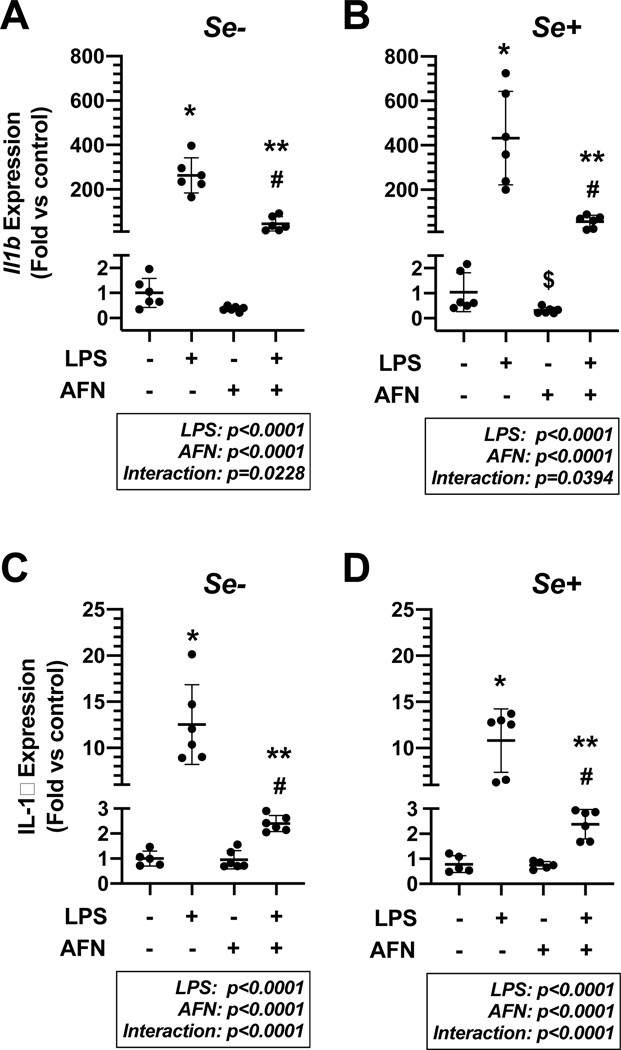
IL-1β mRNA concentration attenuated by AFN
We previously identified that AFN attenuates LPS-induced increases in Il1b mRNA and IL-1β protein levels [8]. To determine the impact of Se on these findings, MH-S cells were cultured for 72 h in Se+ or Se- media and then treated with 0.5 μM AFN or control in the presence or absence of 0.05 μg/mL LPS for 4 h (Figure 5A, B) or 6 h (Figure 5C, D). Our analyses indicated independent effects of AFN and LPS and an interaction between AFN and LPS on Il1b and IL-1β expression under both Se- and Se+ conditions. Specifically, LPS significantly increased Il1b levels under Se- conditions (Figure 5A) and this effect was significantly attenuated by AFN treatment (263±78-fold vs 45±32-fold, p<0.0001). Under Se+ conditions (Figure 5B), LPS treatment significantly increased Il1b levels; an effect that was also attenuated by AFN (432±211-fold vs 56±28-fold, p<0.0001).
Figure 5. IL-1β mRNA and protein levels.
MH-S cells were cultured in RPMI media containing 2.5% FBS supplemented with 0 (Se-) or 25 nM Na2SeO3 (Se+) for 4 h (A, B) or 6 h (C, D) in the presence or absence of 0.05 μg/mL LPS and/or 0.5 μM AFN. Data (mean ± SD, n=5–6) were log-transformed and analyzed by 2-way ANOVA followed by Tukey’s analysis post hoc. (*p<0.0001 vs control; #p<0.0001 vs AFN alone; **p<0.0001 for LPS alone).
Similar to effects on IL1b, IL-1β expression was also significantly increased in LPS-treated MH-S cells under Se- conditions (Figure 5C). IL-1β expression was significantly lower in LPS + AFN cells when compared to cells treated with LPS alone (2.4±0.3-fold vs 13±4.3-fold, p<0.0001). Under Se+ conditions, IL-1β levels were significantly greater in LPS-treated cells than in vehicle-treated cells (Figure 5D). AFN treatment significantly attenuated the effect of LPS (2.4±0.6 vs 11±3.4, p<0.0001).
Discussion
The present studies build upon our overall body of work that has identified Txnrd1 inhibition as a novel strategy to reduce lung injury via Nrf2-dependent mechanisms [5, 7, 8, 11–14]. In lung epithelia, AFN-induced Nrf2 activation is Se-dependent [14]. Our findings in alveolar macrophages indicate that: 1) Se supplementation of 2.5% FBS-containing culture media normalizes Txnrd1 activity enabling evaluation of Se-deficient and Se-sufficient conditions; 2) AFN enhances Nrf2 activation independent of Se status; 3) AFN, but not LPS, enhances GSH contents of MH-S cells; 4) LPS-induced increases in the pro-inflammatory cytokine IL-1β are attenuated by AFN treatment; and, 5) AFN-mediated decreases in IL-1β mRNA and protein levels occur independent of Se status.
Lung epithelial Txnrd1 activity is sensitive to media Se content [5, 14]. It is important to note that we used the same source and lot of FBS in the present studies as we used in our previous studies in lung epithelia [14]. Se content in FBS correlates with the Se status of the donor. Our data indicated that 5% FBS provided sufficient Se for MH-S cell growth (Figure 1) and Txnrd1 activity (Figure 2). In MH-S cells, Se deficiency was achieved by culturing cells in required culture in 2.5% FBS-containing media (Figure 2). We interpret these data to suggest that lung macrophages require less Se or are more efficient at Se uptake and utilization when compared to lung epithelia. Cell growth was significantly decreased in cells cultured in 1% FBS. We are unable to determine if this decrease in growth is due to Se deficiency or is due to deficiency of other growth factors limited by this dilution of FBS (Figure 1). Future studies will investigate the mechanisms by which Se uptake and processing differs in lung epithelia and macrophages but such studies are beyond the scope of the present report.
We have consistently demonstrated consistent Nrf2-mediated Nqo1 induction following Txnrd1 inhibition with AFN [5, 8, 11, 14]. Our data confirmed that Txnrd1 inhibition elicits Nrf2 activation in lung macrophages (Figure 3). Se deficiency is common in preterm infants though the impact of this deficiency on endogenous or inducible Nrf2 responses is not clear. In the present studies, our data revealed that AFN-induced Nrf2 activation in MH-S cells is similar regardless of Se status. These data are in contrast to our findings in lung epithelia [14]. The lung epithelia used by our group originated in FvB mice while MH-S cells are of BALB/c origin [15, 16]. We cannot exclude the possibility that differences in murine strain contributed to differences in Se need and Nrf2 activation by AFN, as is the case in vivo [12, 17].
The protective effects of Txnrd1 inhibition in vivo are GSH-dependent and GSH-dependent responses are enhanced by Txnrd1 inhibition [7, 8, 18, 19]. This suggests that clinical Se deficiency could influence the therapeutic efficacy of Txnrd1 inhibition. Thus, the influence of Se status on AFN-mediated GSH induction was also investigated (Figure 4). Similar to our Nqo1 data, AFN-mediated increases in cellular GSH levels were unaffected by Se status. In an adult lung injury model in which we observed improved survival and decreased lung injury following Txnrd1 inhibition, we found that bronchoalveolar cell GSH contents were increased and that inflammation was decreased [18]. Collectively, our data suggest that Se deficiency is unlikely to alter lung macrophage responses to Txnrd1 inhibition. Studies are ongoing to formally test this speculation in vivo.
Txnrd1 inhibition decreases lung inflammation [18, 19]. IL-1β is a potent proinflammatory molecule that contributes to lung injury. AFN attenuates LPS-induced IL-1β protein expression in immortalized neoplastic cells of BALB/c origin, though the contribution of Se was not investigated [20]. AFN robustly attenuated LPS-induced increases in IL-1β mRNA and protein levels (Figure 5), which is consistent with our previous preliminary report [8]. Similar to other effects of Txnrd1 inhibition in MH-S cells, Se status did not appear to influence AFN-mediated decreases in IL-1β expression.
Conclusions
We hypothesized, based upon our previous findings in lung epithelia, that the anti-inflammatory and antioxidant effects of Txnrd1 inhibition in lung macrophages are Se-dependent. Contrary to our hypothesis, our findings reveal that the effects of Txnrd1 inhibition are Se-independent in alveolar macrophages. We conclude that the beneficial effects of Txnrd1 inhibition are unlikely to be diminished in settings of clinical Se deficiency.
Highlights.
Txnrd1 inhibition elicits antioxidant and anti-inflammatory effects in vivo and in vitro.
Txnrd1 inhibition using AFN elicits increases in Nrf2 activation and increases GSH contents in murine lung macrophages.
AFN attenuates LPS-induced increases in IL-1β mRNA and protein expression in murine lung macrophages.
The anti-inflammatory and antioxidant effects of AFN-mediated Txnrd1 inhibition in lung macrophages are not altered by Se deficiency.
Acknowledgements
This work is supported the National Institutes of Health [RO1HL119280 (T.E.T.)].
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Palomo J, et al. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine, 2015, 76(1): 25–37 [DOI] [PubMed] [Google Scholar]
- [2].Yoon BH, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol, 1997, 177(4): 825–30 [DOI] [PubMed] [Google Scholar]
- [3].Kotecha S, et al. Increase in the concentration of transforming growth factor beta-1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity. J Pediatr, 1996, 128(4): 464–9 [DOI] [PubMed] [Google Scholar]
- [4].Brune B, et al. Redox control of inflammation in macrophages. Antioxid Redox Signal, 2013, 19(6): 595–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Locy ML, et al. Thioredoxin Reductase Inhibition Elicits Nrf2-Mediated Responses in Clara Cells: Implications for Oxidant-Induced Lung Injury. Antioxid Redox Signal, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tipple TE. The thioredoxin system in neonatal lung disease. Antioxid Redox Signal, 2014, 21(13): 1916–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wall SB, et al. Thioredoxin Reductase-1 Inhibition Augments Endogenous Glutathione-Dependent Antioxidant Responses in Experimental Bronchopulmonary Dysplasia. Oxid Med Cell Longev, 2019, 2019: 7945983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wall SB, Li R, Li Q, Casey J, Dunigan K, Carter AB, Tipple TE Suppression of IL-1β production in alveolar macrophages by the thioredoxin reductase inhibitor auranofin. Free Radic Biol Med, 2018, 128: S112 [Google Scholar]
- [9].Tindell R and Tipple T. Selenium: implications for outcomes in extremely preterm infants. J Perinatol, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Darlow BA and Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev, 2003(4): CD003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dunigan K, et al. The Thioredoxin Reductase Inhibitor Auranofin Induces Heme Oxygenase-1 in Lung Epithelial Cells Via Nrf2-dependent Mechanisms. Am J Physiol Lung Cell Mol Physiol, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li Q, et al. Aurothioglucose does not improve alveolarization or elicit sustained Nrf2 activation in C57BL/6 models of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol, 2018, 314(5): L736–L42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Britt RD, Jr., et al. Lipopolysaccharide-induced cyclooxygenase-2 expression in mouse transformed Clara cells. Cell Physiol Biochem, 2012, 29(1–2): 213–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tindell R, et al. Selenium supplementation of lung epithelial cells enhances nuclear factor E2-related factor 2 (Nrf2) activation following thioredoxin reductase inhibition. Redox Biol, 2018, 19: 331–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mbawuike IN and Herscowitz HB. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J Leukoc Biol, 1989, 46(2): 119–27 [DOI] [PubMed] [Google Scholar]
- [16].Magdaleno SM, et al. Interferon-gamma regulation of Clara cell gene expression: in vivo and in vitro. Am J Physiol, 1997, 272(6 Pt 1): L1142–51. [DOI] [PubMed] [Google Scholar]
- [17].Li Q, et al. Thioredoxin Reductase Inhibition Attenuates Neonatal Hyperoxic Lung Injury and Enhances Nrf2 Activation. Am J Respir Cell Mol Biol, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Britt RD Jr., et al. The thioredoxin reductase-1 inhibitor aurothioglucose attenuates lung injury and improves survival in a murine model of acute respiratory distress syndrome. Antioxid Redox Signal, 2014, 20(17): 2681–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tipple TE, et al. Thioredoxin-related mechanisms in hyperoxic lung injury in mice. Am J Respir Cell Mol Biol, 2007, 37(4): 405–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Isakov E, et al. Suppression of the pro-inflammatory NLRP3/interleukin-1beta pathway in macrophages by the thioredoxin reductase inhibitor auranofin. Biochim Biophys Acta, 2014, 1840(10): 3153–61 [DOI] [PubMed] [Google Scholar]