Abstract
Background
Hypercalcemia is associated with chronic kidney disease (CKD) in cats, but studies assessing the physiologically relevant ionized calcium fraction are lacking.
Objectives
To describe the prevalence and incidence rate of ionized hypercalcemia, and to explore predictor variables to identify cats at risk of ionized hypercalcemia in a cohort of cats diagnosed with azotemic CKD.
Animals
One hundred sixty‐four client‐owned cats with azotemic CKD.
Methods
Variables independently associated with ionized hypercalcemia at diagnosis of azotemic CKD were explored by binary logistic regression. Cats that were normocalcemic at diagnosis of azotemic CKD were followed over a 12‐month period or until ionized hypercalcemia occurred and baseline predictor variables for ionized hypercalcemia explored using Cox proportional hazards and receiver operating characteristic curve analysis.
Results
Ionized hypercalcemia (median, 1.41 mmol/L; range, 1.38‐1.68) was observed in 33/164 (20%) cats at diagnosis of azotemic CKD and was associated with male sex, higher plasma total calcium and potassium concentrations, and lower plasma parathyroid hormone concentrations. Twenty‐five of 96 initially normocalcemic (26%) cats followed for minimum 90 days developed ionized hypercalcemia (median, 1.46 mmol/L; range, 1.38‐1.80) at a median of 140 days after diagnosis of azotemic CKD (incidence rate, 0.48 per feline patient‐year). Only body condition score was independently associated with incident ionized hypercalcemia.
Conclusions and Clinical Importance
The occurrence of ionized hypercalcemia is high in cats with CKD. Continued monitoring of blood ionized calcium concentrations is advised.
Keywords: calcium, CKD‐MBD, diet, feline, renal
Abbreviations
- 95% CI
95% confidence interval
- ALP
alkaline phosphatase activity
- BCS
body condition score
- CKD
chronic kidney disease
- FGF23
fibroblast growth factor 23
- HR
hazard ratio
- IRIS
International Renal Interest Society
- ln
logarithmus naturalis
- MMS
muscle mass score
- NCa‐HCa
baseline normocalcemic cats that become hypercalcemic
- NCa‐NCa
baseline normocalcemic cats that remain normocalcemic
- OR
odds ratio
- PTH
parathyroid hormone
- SBP
systolic blood pressure
- SD
standard deviation
- UPC
urine protein‐to‐creatinine ratio
- USG
urine specific gravity
1. INTRODUCTION
Hypercalcemia is commonly observed with chronic kidney disease (CKD) in cats. 1 , 2 , 3 The pathophysiology of hypercalcemia in CKD is incompletely understood, but several mechanisms have been proposed in humans, such as decreased glomerular filtration and increased renal tubular reabsorption of calcium, increased intestinal calcium absorption, and increased release of calcium from bone as part of the mineral bone disorder. 4 The clinical consequences of hypercalcemia in cats with CKD are unclear, but high serum ionized calcium concentration could decrease glomerular filtration rate by decreasing medullary tonicity and renal blood flow, 5 , 6 has been associated with calcium oxalate nephrolithiasis, 7 and could stimulate soft‐tissue calcification, especially in conjunction with increased serum phosphate concentrations. 8 , 9 , 10
The percentage of cats with CKD diagnosed with hypercalcemia based on total calcium concentration varies between 10% and 20%, 2 , 3 , 11 , 12 while ionized hypercalcemia is observed in 10% to 30% of cats with CKD. 2 , 3 In previous studies of cats with azotemic CKD, the development of ionized hypercalcemia was observed in 13% to 76%, 13 , 14 and the development of hypercalcemia based on plasma total calcium concentration in 60 of 191 (31%) cats. 1 The majority of cats in the latter study lacked information on ionized calcium concentration, which is the physiologically relevant fraction of the plasma total calcium concentration. 15 Although serum total calcium concentration has a high specificity for the identification of ionized hypercalcemia (94%‐100%), its sensitivity is low (28%‐52%) in cats with CKD. 1 , 2 Therefore, cats with total hypercalcemia were likely to have ionized hypercalcemia but use of total calcium concentration as a screening test will have underestimated the number of cats with ionized hypercalcemia. Furthermore, the identification of risk factors for hypercalcemia was obscured, because cats with true ionized hypercalcemia were likely categorized as normocalcemic based on their plasma total calcium concentration. 1
The aims of this study were to describe the prevalence and incidence rate of ionized hypercalcemia and to explore predictor variables to identify cats at risk of ionized hypercalcemia in a cohort of cats diagnosed with azotemic CKD.
2. METHODS
2.1. Case selection
Cats recruited to this study formed part of a large prospective observational cohort for which owner consent was obtained and approval of the Ethics and Welfare Committee of the Royal Veterinary College had been granted (URN 2013 1258E). Inclusion and exclusion criteria and statistical methods were derived after the event of interest for this retrospective analysis. The clinical records of 2 companion animal clinics in central London (Beaumont Sainsbury Animal Hospital, Camden, and People's Dispensary for Sick Animals, Bow) were searched for cats newly diagnosed with azotemic CKD between January 1, 2012 and January 1, 2018. A diagnosis of azotemic CKD had been made based on plasma creatinine concentration ≥2 mg/dL (the upper limit of the laboratory reference interval) in conjunction with a urine specific gravity (USG) <1.035, or plasma creatinine concentration ≥2 mg/dL on 2 consecutive visits 2 to 4 weeks apart. To be included, cats had to have blood ionized calcium concentration available from the date of diagnosis of azotemic CKD. Cats with a diagnosis of hyperthyroidism or diabetes mellitus, treated with corticosteroids, those already being fed a clinical renal diet, or cats with plasma total thyroxine concentration >40 nmol/L were excluded. Cats receiving amlodipine besylate for treatment of systemic hypertension, and cats that had been euthyroid for at least 6 months following thyroidectomy or radioactive iodine‐treatment were eligible for inclusion.
2.2. Data collection
The date of diagnosis of azotemic CKD was designated as baseline. Clinical information was extracted from the electronic records for all cats where follow‐up blood ionized calcium was available within the first 12 months after diagnosis of azotemic CKD. Routine re‐check examinations with blood sampling were advised at 4 to 6, 20 to 22, 36 to 38, 52 to 54 weeks after diagnosis of azotemic CKD. Each clinic visit consisted of clinical history taking, systolic blood pressure (SBP) measurement by Doppler method, physical examination, and jugular venipuncture for blood sampling and urine collected by cystocentesis. The information retrieved from the clinical records included age, breed, sex, body weight, body condition score (BCS; 9‐point system), muscle mass score (MMS; 4‐point system), SBP, and owner reported dietary information. Laboratory data retrieved included plasma total protein, albumin, globulin, creatinine, sodium, potassium, chloride, cholesterol, phosphate, total calcium, calcidiol, calcitriol, intact fibroblast growth factor 23 (FGF23), intact parathyroid hormone (PTH), and total thyroxine concentrations, and alkaline phosphatase activity (ALP), PCV, blood ionized calcium concentration, venous bicarbonate (HCO3 −), and pH. The urinalysis results accessed included USG, urine culture result, urine protein‐to‐creatinine ratio (UPC). Staging of CKD and phosphate status were performed according to International Renal Interest Society (IRIS) guidelines (International Renal Interest Society Guidelines: IRIS Staging of CKD. http://iris-kidney.com/guidelines/staging.html). Progression of CKD was defined as >25% increase in plasma creatinine concentration between the date of diagnosis of azotaemic CKD and the visit ionized hypercalcemia was identified. The cut‐point of 25% was based on the presumption that smaller changes could be caused by poor measurement precision rather than actual progression of azotemia. 16 , 17
Ionized calcium concentration was measured in nonheparinized whole blood immediately after jugular venipuncture (iSTAT 1 point‐of‐care analyzer, Abbott Point of Care Inc., Princeton, New Jersey). Ionized calcium concentration was measured at initial diagnosis of azotemic CKD and at follow‐up visits as part of the standard clinic protocol. Heparinized plasma was sent to an external laboratory (IDEXX laboratories, Wetherby, UK) on the day venipuncture was performed for routine biochemistry, including total calcium concentration (laboratory reference interval, 8.2 to 11.8 mg/dL). Aliquots of EDTA plasma were stored at −80°C for batch‐analysis of FGF23 and PTH concentrations using validated 18 , 19 assays (FGF‐23 ELISA Kit, Kainos Laboratories, Tokyo, Japan; Total intact PTH immunoradiometric assay‐coated bead version, 3KG600, Scantibodies, Santee, California). The PTH‐assay had a lower limit of detection of 5.2 pg/mL, 19 and a PTH concentration of 2.6 pg/mL was assigned to samples with a concentration <5.2 pg/mL. Plasma calcidiol and calcitriol concentrations had been measured at the Michigan State University Diagnostic Center for Population and Animal Health (Lansing, Michigan).
2.3. Statistical analyses
Statistical analyses were performed using R Foundation for Statistical Computing, Vienna, Austria. For all reported analyses P ≤ .05 defined statistical significance. The distribution of numerical variables was assessed for normality by Shapiro‐Wilk test and visual inspection of quantile‐quantile plots. Continuous clinical data are presented as median [25th, 75th percentiles or range]. Plasma FGF23 and PTH values were logarithmically transformed prior to analysis (logarithmus naturalis [ln]).
2.4. Comparison of hypercalcemic and normocalcemic cats at diagnosis of azotemic CKD
Ionized hypercalcemia was suspected based on a blood ionized calcium measurement greater than a 95% reference interval for whole blood ionized calcium in older cats which had previously been derived using an identical analyzer (1.19‐1.37 mmol/L). 20 Cats were categorized into 2 groups: cats with ionized hypercalcemia (ie, blood ionized calcium ≥1.38 mmol/L) and normocalcemic cats (ie, blood ionized calcium concentration <1.38 mmol/L) at diagnosis of azotemic CKD; this division was based on a single time point. Baseline characteristics of these 2 groups were compared using independent t‐tests for continuous variables with a normal distribution, or with Mann‐Whitney U tests for skewed variables. Proportions were compared with Chi square test. Binary logistic regression was performed to explore risk factors for ionized hypercalcemia with normocalcemic cats as controls. Variables significantly associated with ionized hypercalcemia were entered into a multivariable binary logistic regression model. The final model was derived by manual backward elimination. Results are reported as odds ratio (OR) and 95% confidence interval (95% CI).
2.5. Evaluation of risk factors for incident ionized hypercalcemia in cats with CKD
The cats that were normocalcemic at diagnosis of azotemic CKD were offered a clinical renal diet (Veterinary Diet Renal, Royal Canin SAS, Aimargues, France [dry and wet formulations]), and were followed over time to explore cat characteristics associated with incident ionized hypercalcemia within 12 months of diagnosis of azotemic CKD. Cats that developed ionized hypercalcemia were followed until the first visit where this was observed. Cats that did not develop ionized hypercalcemia had to have at least 90 days of follow‐up information available to be included in this part of the study. A diagnosis of hypercalcemia was based on blood ionized calcium concentration ≥1.38 mmol/L on at least 2 occasions or >1.50 mmol/L on a single occasion with subsequent dietary intervention. 21 Baseline characteristics of the groups of cats that remained normocalcemic (NCa‐NCa) and cats that developed ionized hypercalcemia (NCa‐HCa) were compared using independent t‐tests for continuous variables with a normal distribution, Mann‐Whitney U tests for skewed continuous variables, and Chi square tests for proportions.
Predictors of incident ionized hypercalcemia were explored using time‐invariant Cox regression. The date of diagnosis of azotemic CKD was designated as baseline, ionized hypercalcemia (as specified above) was the event of interest, and censoring occurred for cats that remained normocalcemic at the last available visit within the 12‐month interval. Model assumptions for each predictor variable were evaluated by inspection of Kaplan‐Meier curves and assessment of statistical interaction of each variable with time (Figure 1). Variables associated with ionized hypercalcemia with P < .2 entered multivariable analysis. The final multivariable model was derived by manual backward elimination. Blood ionized calcium and venous pH values were multiplied by 10, prior to analysis. Plasma calcidiol and calcitriol concentrations and USG were only available in a limited number of cats and were therefore excluded from the model. Results are reported as hazard ratio (HR; 95% CI).
FIGURE 1.
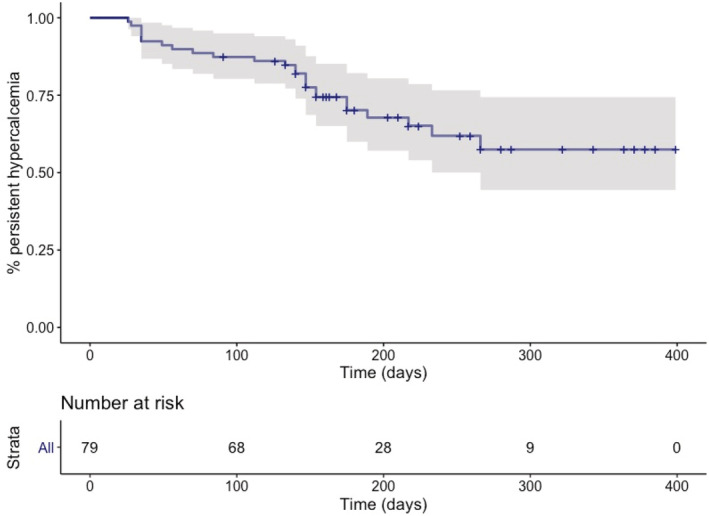
Kaplan‐Meier curve depicting the development of ionized hypercalcemia after the diagnosis of azotemic chronic kidney disease in 79 initially normocalcemic cats. Twenty‐five of 79 initially normocalcemic cats developed persistent ionized hypercalcemia (≥1.38 mmol/L on 2 occasions) or started with substantive clinical ionized hypercalcemia (>1.50 mmol/L).
Receiver operating characteristic curves (ROC) were constructed to explore cut‐off values for 95% sensitivity and 95% specificity for potential baseline predictors of ionized hypercalcemia that were identified by multivariable time‐invariant Cox regression.
3. RESULTS
Between January 1, 2012 and January 1, 2018, a diagnosis of azotemic CKD was made in 269 cats, of which 62 were excluded from analysis for hyperthyroidism and 43 for missing information on blood ionized calcium concentration. The 164 remaining cats included 88 females (1 entire) and 76 males (2 entire) of the following breeds: domestic shorthair (n = 128), domestic longhair (n = 20), Siamese (n = 6), Burmese (n = 5), Persian (n = 2), and 1 each of Abyssinian, British shorthair, and Maine coon. Based on the IRIS staging system, 130 cats had Stage 2, 30 cats Stage 3, and 4 cats Stage 4 CKD. Plasma phosphate concentration exceeded the upper limit of the IRIS stage‐specific target range in 50 cats (31%). Thirty‐five cats (21%) had been diagnosed with systemic hypertension and were administered PO amlodipine besylate. The median blood ionized calcium concentration at diagnosis of azotemic CKD for the study population was 1.32 [1.28, 1.36] mmol/L (range, 1.08‐1.68).
3.1. Ionized hypercalcemia at diagnosis of azotemic CKD
Ionized hypercalcemia defined as a single blood ionized calcium ≥1.38 mmol/L was noted in 33 of 164 cats at diagnosis of azotemic CKD (prevalence, 0.20; 95% CI, 0.15‐0.27). These 33 cats with initial ionized hypercalcemia had a median blood ionized calcium concentration of 1.41 [1.39, 1.51] mmol/L (range, 1.38‐1.68), and included 12 females and 21 males (all neutered), 26 domestic shorthairs, 2 domestic longhairs, 3 Siamese, 1 Burmese, and 1 Persian. Twenty‐eight initial hypercalcemic cats had IRIS Stage 2 CKD, of which 10 were hyperphosphatemic, and 5 cats IRIS Stage 3 CKD, of which 2 were hyperphosphatemic. No statistically significant associations were found between the proportions of cats with initial ionized hypercalcemia and IRIS stage (P = .7) or phosphate status (P = .5).
Plasma total calcium concentration exceeded the upper limit of the reference interval (ie, >11.8 mg/dL) in 7 of 164 cats (prevalence, 0.04; 95% CI, 0.02‐0.09). These 7 cats had a median plasma total calcium concentration of 12.6 mg/dL (range, 12.2‐14.0), and all 7 cats had ionized hypercalcemia (median blood ionized calcium concentration, 1.60 mmol/L; range, 1.50‐1.68).
The proportion of male cats (P = .042) and the plasma concentrations of total calcium (P < .001), FGF23 (P = .001), and potassium (P = .022) were significantly higher in the initial hypercalcemic group (Table 1). Plasma PTH concentration was available for 27 initial hypercalcemic cats and 118 normocalcemic cats, and was numerically lower in the group with ionized hypercalcemia, but this difference did not reach statistical significance (median, 10.4 [2.6, 36.6] pg/mL versus 19.6 [10.7, 37.5] pg/mL; P = .053). In the 27 initial hypercalcemic cats with information on plasma PTH available, plasma PTH concentration was below the reference interval (ie, <2.6 pg/mL) in 8 cats, within the reference interval in 7 cats, and above the reference interval (ie, >17.6 pg/mL) in 12 cats (maximum, 106.8 pg/mL). Eight cats with hyperparathyroidism at baseline had follow‐up information available and plasma PTH decreased over time in these cats. Information on vitamin D‐metabolites was only available for 1 hypercalcemic cat and were within reference interval.
TABLE 1.
Selected clinicopathological variables of cats with single measurement ionized hypercalcemia and normocalcemic cats at diagnosis of azotemic chronic kidney disease.
| Variables | Normocalcemic (n = 131) | n | Hypercalcemic (n = 33) | n | P |
|---|---|---|---|---|---|
| Median [25th, 75th percentile] | Median [25th, 75th percentile] | ||||
| Ionized calcium (mmol/L) | 1.31 [1.28, 1.34] | 131 | 1.41 [1.39, 1.50] | 33 | |
| Age (years) | 15.2 [13.0, 16.9] | 128 | 15.2 [11.8, 17.1] | 31 | .95 |
| Albumin (g/dL) | 3.1 [2.9, 3.3] | 131 | 3.1 [3.0, 3.3] | 33 | .65 |
| ALP (U/L) | 30 [23, 39] | 131 | 31 [23, 37] | 33 | .38 |
| BCS (1‐9) | 4 [3, 5] | 131 | 4 [3, 4] | 33 | .27 |
| Calcidiol (pmol/L) | 137 [109, 177] | 34 | 134 | 1 | |
| Calcitriol (pmol/L) | 424 [341, 456] | 34 | 146 | 1 | |
| Chloride (mEq/L) | 118 [116, 120] | 129 | 118 [117, 122] | 29 | .15 |
| Creatinine (mg/dL) | 2.39 [2.13, 2.70] | 131 | 2.38 [2.20, 2.74] | 33 | .91 |
| FGF23 (pg/mL) | 443 [265, 1015] | 123 | 1608 [643, 2527] | 29 | .001 |
| Globulin (g/dL) | 4.6 [4.3, 5.1] | 131 | 4.4 [4.1, 5.3] | 33 | .35 |
| HCO3 − (mEq/L) | 20 [19, 22] | 131 | 20 [18, 21] | 33 | .23 |
| MMS (0‐2) | 1 [1, 1] | 127 | 1 [1, 1] | 32 | 1 |
| PCV (%) | 35 [31, 38] | 131 | 32 [30, 35] | 33 | .24 |
| Venous pH | 7.35 [7.32, 7.38] | 131 | 7.34 [7.30, 7.37] | 33 | .68 |
| Phosphate (mg/dL) | 3.87 [3.50, 4.80] | 131 | 4.12 [3.78, 4.86] | 33 | .38 |
| Potassium (mEq/L) | 4.0 [3.7, 4.3] | 129 | 4.2 [3.9, 4.6] | 29 | .022 |
| PTH (pg/mL) | 19.6 [10.7, 37.5] | 118 | 10.4 [2.6, 36.6] | 27 | .053 |
| SBP (mmHg) | 135 [124, 146] | 131 | 137 [126, 151] | 32 | .53 |
| Sex (n [%] male) | 55 (42) | 131 | 21 (64) | 33 | .042 |
| Sodium (mEq/L) | 153 [151, 155] | 129 | 153 [150, 156] | 29 | .49 |
| Total calcium (mg/dL) | 10.0 [9.6, 10.3] | 131 | 10.7 [10.3, 11.4] | 33 | <.001 |
| Total protein (g/dL) | 7.8 [7.4, 8.1] | 131 | 7.6 [7.1, 8.3] | 33 | .31 |
| USG | 1.017 [1.015, 1.020] | 81 | 1.018 [1.016, 1.020] | 21 | .25 |
| Weight (kg) | 3.70 [3.15, 4.45] | 127 | 3.81 [3.18, 4.28] | 31 | .98 |
Abbreviations: ALP, alkaline phosphatase; BCS, body condition score; FGF23, fibroblast growth factor 23; MMS, muscle mass score; PTH, parathyroid hormone; SBP, systolic blood pressure; USG, urine specific gravity.
Univariable binary logistic regression identified higher plasma total calcium (OR, 6.70; 95% CI, 3.16‐14.22; P < .001), potassium (OR, 2.83; 95% CI, 1.22‐6.55; P = .015) and FGF23 (OR, 1.43; 95% CI, 1.06‐1.94; P = .020) concentrations, lower plasma PTH concentration (OR, 0.62; 95% CI, 0.41‐0.94; P = .024), and male sex (OR, 2.42; 95% CI, 1.10‐5.33; P = .028) as risk factors for ionized hypercalcemia at diagnosis of azotemic CKD. Plasma FGF23 was eliminated from the multivariable binary logistic regression model and plasma total calcium (OR, 9.05; 95% CI, 2.99‐27.43; P < .001), potassium (OR, 3.73; 95% CI, 1.03‐13.61; P = .046), and PTH (OR, 0.43; 95% CI, 0.23‐0.81; P = .009) concentrations, and male sex (OR, 12.29; 95% CI, 2.67‐56.61; P = .001) remained independently associated with ionized hypercalcemia at diagnosis of azotemic CKD in cats (Nagelkerke R 2, .48).
3.2. The development of ionized hypercalcemia in initially normocalcemic cats with a diagnosis of azotemic CKD
Thirty‐five of the 131 cats with normal blood ionized calcium concentrations at diagnosis of azotemic CKD had <90 days follow‐up information available and were excluded from the analysis. Forty‐two of 96 (44%) initially normocalcemic cats were reported to reach a blood ionized calcium concentration of ≥1.38 mmol/L at a median of 147 [46, 184] days after diagnosis of azotemic CKD. Of these 42 cats, 20 were documented to have persistent ionized hypercalcemia. Five cats had a single blood ionized calcium concentration >1.50 mmol/L which normalized following diet change. In 10 cats, hypercalcemia was transient and 7 cats were lost to follow‐up. Of note, 3 cats developed persistent ionized hypercalcemia at >12 months after baseline but were not included in the hypercalcemic analysis. Therefore, 25 cats were considered to fulfill the criteria for ionized hypercalcemia (26%; incidence rate 0.48 per patient‐year). The median blood ionized calcium concentration in this group was 1.46 [1.40, 1.58] mmol/L (range, 1.38‐1.80 mmol/L). Cats that demonstrated a transient blood ionized hypercalcemia and those that were lost to follow up without the ability to confirm ionized hypercalcemia were excluded from further analysis (Table 2).
TABLE 2.
Selected clinicopathological baseline variables of initially normocalcemic cats that transiently reached an ionized calcium concentration of ≥1.38 but excluded from further analysis.
| Variables | Transiently hypercalcemic (n = 23) | n |
|---|---|---|
| Median [25th, 75th percentile] | ||
| Ionized calcium (mmol/L) | 1.32 [1.28, 1.33] | 17 |
| Age (years) | 14.4 [12.6, 16.2] | 17 |
| Albumin (g/dL) | 3.2 [2.9, 3.3] | 17 |
| ALP (U/L) | 35 [26, 51] | 17 |
| BCS (1‐9) | 4 [3, 5.5] | 17 |
| Chloride (mEq/L) | 118 [116, 120] | 16 |
| Creatinine (mg/dL) | 2.49 [2.22, 2.80] | 17 |
| FGF23 (pg/mL) | 447 [247, 1730] | 16 |
| Globulin (g/dL) | 4.7 [4.4, 4.9] | 17 |
| HCO3 − (mEq/L) | 21 [20, 22] | 17 |
| MMS (0‐2) | 1 [1, 1.5] | 17 |
| PCV (%) | 34 [31, 38] | 17 |
| Venous pH | 7.35 [7.32, 7.38] | 17 |
| Phosphate (mg/dL) | 3.72 [3.58, 4.58] | 17 |
| Potassium (mEq/L) | 4.1 [3.7, 4.3] | 16 |
| PTH (pg/mL) | 20.2 [10.4, 115.6] | 16 |
| SBP (mmHg) | 136 [125, 144] | 17 |
| Sex (n [%] male) | 7 (41) | 17 |
| Sodium (mEq/L) | 152 [150, 153] | 16 |
| Total calcium (mg/dL) | 10.2 [9.6, 10.7] | 17 |
| Total protein (g/dL) | 7.9 [7.1, 8.0] | 17 |
| Weight (kg) | 3.58 [3.11, 4.62] | 16 |
Abbreviations: ALP, alkaline phosphatase; BCS, body condition score; FGF23, fibroblast growth factor 23; MMS, muscle mass score; PTH, parathyroid hormone; SBP, systolic blood pressure; USG, urine specific gravity.
The NCa‐NCa group (n = 54) consisted of 32 females (1 entire) and 22 males (all neutered), 42 domestic shorthairs, 8 domestic longhairs, 2 Siamese, 1 Burmese, and 1 Persian. Cats in this group had a median of 2 re‐check visits (range, 1‐5) and a median follow‐up time of 175 [154, 259] days. The median of the maximum ionized calcium concentration observed in each cat during follow‐up in the NCa‐NCa group was 1.33 [1.30, 1.35] mmol/L.
The NCa‐HCa group consisted of 15 females and 10 males all neutered, 20 domestic shorthairs, 2 domestic longhairs, 1 domestic medium hair, 1 Abyssinian, and 1 Burmese. Cats in this group had a median of 3 re‐check visits (range, 1‐4) until the first ionized hypercalcemia measurement was observed. At the hypercalcemic visit, median blood ionized calcium concentration was not different from the blood ionized calcium concentration of the 33 cats with ionized hypercalcemia at diagnosis of azotemic CKD (P = .31).
In cats fulfilling the criteria for ionized hypercalcemia, nonspecific clinical signs which could potentially be attributed to hypercalcemia were noted in 8/24 cats at the first hypercalcemic visit (no historical data available for 1 cat). Decreased appetite was noted in 1 cat (4%), signs of constipation in 1 cat (4%), polyuria and polydipsia in 6 cats (25%), and decreased activity in 1 (4%) of 24 hypercalcemic cats.
There was no significant difference in either ionized or total calcium between cats that remained normocalcemic and those that developed an ionized hypercalcemia during follow up (Table 3). Baseline packed cell volume was significantly higher in those cats that went on to develop ionized hypercalcemia (NCa‐NCa 35 [30, 36] %, NCa‐HCa 35 [32, 42] %). No other significant differences in baseline variables were identified (Table 3).
TABLE 3.
Selected clinicopathological baseline variables of initially normocalcemic cats that remain normocalcemic and those that develop persistent ionized hypercalcemia after diagnosis of azotemic chronic kidney disease.
| Variables | NCa‐NCa (n = 54) | n | NCa‐HCa (n = 25) | n | P |
|---|---|---|---|---|---|
| Median [25th, 75th percentile] | Median [25th, 75th percentile] | ||||
| Ionized calcium (mmol/L) | 1.31 [1.28, 1.33] | 54 | 1.32 [1.27, 1.35] | 25 | .29 |
| Age (years) | 15.4 [13.8, 16.7] | 53 | 14.3 [11.3, 17.2] | 24 | .31 |
| Albumin (g/dL) | 3.1 [2.9, 3.3] | 54 | 3.1 [2.9, 3.3] | 25 | .59 |
| ALP (U/L) | 30 [24, 40] | 54 | 27 [22, 39] | 25 | .63 |
| BCS (1‐9) | 4 [3, 5] | 54 | 5 [3, 6] | 25 | .17 |
| Chloride (mEq/L) | 119 [116, 120] | 54 | 117 [115, 119] | 25 | .22 |
| Creatinine (mg/dL) | 2.38 [2.10, 2.89] | 54 | 2.30 [2.05, 2.62] | 25 | .32 |
| FGF23 (pg/mL) | 523 [317, 874] | 49 | 322 [218, 812] | 25 | .38 |
| Globulin (g/dL) | 4.6 [4.3, 5.2] | 54 | 4.5 [4.2, 4.7] | 25 | .12 |
| HCO3 − (mEq/L) | 20 [18, 23] | 53 | 20 [19, 22] | 25 | .65 |
| MMS (0‐2) | 1 [1, 1] | 52 | 1 [1, 1] | 25 | .64 |
| PCV (%) | 35 [30, 36] | 54 | 35 [32, 42] | 25 | .031 |
| Venous pH | 7.35 [7.30, 7.38] | 54 | 7.35 [7.31, 7.38] | 24 | .48 |
| Phosphate (mg/dL) | 3.92 [3.51, 4.76] | 54 | 3.53 [3.09, 4.49] | 25 | .09 |
| Potassium (mEq/L) | 3.9 [3.6, 4.2] | 54 | 4.0 [3.5, 4.3] | 25 | .84 |
| PTH (pg/mL) | 20.6 [13.6, 39.7] | 54 | 11.4 [7.9, 34.0] | 22 | .10 |
| SBP (mmHg) | 133 [118, 142] | 54 | 132 [119, 147] | 24 | .69 |
| Sex (n [%] male) | 22 (41) | 54 | 10 (40) | 25 | 1 |
| Sodium (mEq/L) | 153 [151, 155] | 54 | 152 [149, 154] | 25 | .35 |
| Total calcium (mg/dL) | 9.9 [9.4, 10.2] | 54 | 10.0 [9.8, 10.4] | 25 | .08 |
| Total protein (g/dL) | 7.7 [7.4, 8.2] | 54 | 7.5 [7.2, 7.9] | 25 | .11 |
| Weight (kg) | 3.58 [3.06, 4.25] | 52 | 4.04 [3.06, 4.72] | 25 | .25 |
Abbreviations: ALP, alkaline phosphatase; BCS, body condition score; FGF23, fibroblast growth factor 23; MMS, muscle mass score; PTH, parathyroid hormone; SBP, systolic blood pressure; USG, urine specific gravity.
Median plasma total calcium concentration was 11.5 [10.7, 12.3] mg/dL (range, 9.4‐13.8) at the visit on which ionized hypercalcemia was identified and exceeded the upper limit of the reference interval (ie, >11.8 mg/dL) in 8 of 25 (32%) cats with ionized hypercalcemia. Total hypercalcemia was not observed prior to or independent from ionized hypercalcemia. The cats with total hypercalcemia had a median ionized calcium concentration of 1.61 mmol/L (range, 1.39‐1.80).
Information on plasma PTH concentration was available for 17 cats from the NCa‐HCa group at the visit ionized hypercalcemia was first observed, and was below the lower limit of detection of the assay in 5 cats (assigned value 2.6 pg/mL). The median plasma PTH concentration at the hypercalcemic visit was 4.9 [2.6, 11.0] (range, 2.6‐94.8 pg/mL). Looking at individual cats in the NCa‐HCa group, 2/17 cats showed a mild increase in plasma PTH concentration at the hypercalcemic visit compared to previous visits, 12/17 cats a decrease in plasma PTH, and plasma PTH remained below the lower limit of detection in 3 cats (7 cats lacked sequential information on plasma PTH). In the NCa‐NCa group, information on plasma PTH concentration on the last follow‐up visit was available for 35 cats, which had a median plasma PTH concentration of 14.6 [8.4, 27.2] pg/mL (range, 2.6‐193.5). Plasma PTH was below the lower limit of detection in 3 cats. Compared to baseline, plasma PTH had decreased in 22 cats, was stable in 7 cats, and had increased in 6 cats.
Information on plasma FGF23 concentration was available for 16 cats from the NCa‐HCa group at the visit ionized hypercalcemia was observed. The median plasma FGF23 concentration at the hypercalcemic visit was 610.2 [254.7, 1436.5] pg/mL (range, 210.0‐3779.0). Plasma FGF23 had decreased compared to previous visits in 3 cats, was stable in 5 cats and had increased in 8 cats. Information on plasma FGF23 concentration on the last follow‐up visit was available for 36 cats in the NCa‐NCa group. The median plasma FGF23 concentration on the last visit was 404 [232, 1421] pg/mL (range, 74‐28 210). Plasma FGF23 had decreased compared to baseline in 15 cats, was stable in 6 cats, and had increased in 15 cats.
Information on plasma concentrations of vitamin D‐metabolites were available for 6 cats at the visit ionized hypercalcemia was observed (median plasma calcidiol, 1.2 nmol/L; range, 0.9‐10.1, and median plasma calcitriol 124 pmol/L; range, 46‐204). Plasma concentrations of both vitamin D‐metabolites decreased in 5 of 6 cats from the NCa‐HCa group that had sequential information available. Plasma calcidiol and calcitriol increased in 1 cat, although both plasma analytes remained below the lower limit of the reference interval in this individual animal (60 nmol/L and 46 pmol/L, respectively).
Plasma creatinine concentration had increased by >25% compared to baseline concentration in 3/25 (12%) cats in the NCa‐HCa group and 8/54 (15%) cats in the NCa‐NCa group. Six hypercalcemic cats had a plasma phosphate concentration above the upper limit of the IRIS‐stage specific target range at the visit hypercalcemia was identified (median plasma phosphate 4.2 mg/dL; range, 2.8‐5.2 [n = 25]).
Owner reported information on diet during follow‐up was available for 13/25 cats in the NCa‐HCa group and 31/54 cats in the NCa‐NCa group. In the NCa‐HCa group, the median proportion of daily food intake consisting of a clinical renal diet was 100 [70, 100] % (range, 20‐100). The clinical renal diet fed was Royal Canin Veterinary Diet Renal in all cats. In the NCa‐NCa group, the median proportion of daily food intake consisting of a clinical renal diet was 100 [50, 100] % (range, 10‐100), all Royal Canin Veterinary Diet Renal. The cats that were not fed solely a clinical renal diet on a daily basis, were fed a wide variety of commercial cat food brands as part of their daily food intake. The proportion of daily food intake consisting of a clinical renal diet was not significantly different between groups (P = .23). Other treatments that were started at baseline or during follow‐up in the NCa‐HCa group was amlodipine besylate for systemic hypertension in 5 cats, meloxicam for suspected osteoarthrosis in 1 cat and liquid paraffin for constipation in 1 cat. In the NCa‐NCa group, newly instigated treatments were amlodipine besylate in 3 cats, aluminium hydroxide in cat, and amoxicillin in 1 cat.
Univariable time‐invariant Cox regression identified higher PCV and BCS as predictors associated with incident ionized hypercalcemia with P < .2 (Table 4). Only BCS remained as a significant independent predictor of incident ionized hypercalcemia in the final multivariable model. Based on ROC analysis the diagnostic accuracy of BCS for the prediction of incident ionized hypercalcemia was insufficient, with an area under the curve of 0.60 (95% CI, 0.45‐0.74; P = .17).
TABLE 4.
Univariable and multivariable time‐invariant Cox regression results identifying risk factors for incident persistent ionized hypercalcaemia in cats with azotaemic chronic kidney disease (n = 79).
| Predictors | HR (95% CI) | P |
|---|---|---|
| Univariable results | ||
| Ionized calcium (mmol/L × 10) | 1.37 (0.59‐3.15) | .46 |
| Total calcium (mg/dL) | 1.75 (0.96‐3.18) | .06 |
| Age (years) | 0.89 (0.76‐1.03) | .13 |
| BCS (1‐9) | 1.44 (1.11‐1.86) | .006 |
| Chloride (mEq/L) | 0.93 (0.83‐1.03) | .17 |
| Creatinine (mg/dL) | 0.49 (0.19‐1.24) | .13 |
| Phosphate (mg/dL) | 0.78 (0.53‐1.14) | .20 |
| Albumin (g/dL) | 1.82 (0.51‐6.54) | .36 |
| Globulin (g/dL) | 0.60 (0.31‐1.17) | .13 |
| Total protein (g/dL) | 0.67 (0.36‐1.26) | .22 |
| Sodium (mEq/L) | 0.97 (0.88‐1.07) | .53 |
| Potassium (mEq/L) | 0.99 (0.46‐2.16) | .99 |
| ln[FGF23 (pg/mL)] | 0.86 (0.60‐1.24) | .43 |
| ln[PTH (pg/mL)] | 0.68 (0.43‐1.06) | .09 |
| HCO3 − (mEq/L) | 1.03 (0.91‐1.17) | .67 |
| pH × 10 | 1.28 (0.57‐2.87) | .55 |
| Weight (kg) | 1.30 (0.92‐1.85) | .14 |
| ALP (U/L) | 1.00 (0.98‐1.02) | .88 |
| MMS (0‐2) | 1.75 (0.64‐4.77) | .27 |
| PCV (%) | 1.08 (1.01‐1.16) | .035 |
| SBP (mmHg) | 1.01 (0.98‐1.03) | .58 |
| Sex (male vs female) | 1.17 (0.52‐2.61) | .71 |
| Multivariable results | ||
| BCS (1‐9) | 1.44 (1.11‐1.86) | .006 |
Abbreviations: ALP, alkaline phosphatase; BCS, body condition score; FGF23, fibroblast growth factor 23; MMS, muscle mass score; SBP, systolic blood pressure.
4. DISCUSSION
In the presented study population, ionized hypercalcemia on a single occasion was identified in 20% of cats at diagnosis of azotemic CKD. Persistent and clinically significant ionized hypercalcemia was observed in 26% of initially normocalcemic cats within the first 12 months after diagnosis of azotemic CKD. Transient hypercalcemia where blood ionized calcium reached or exceeded 1.38 mmol/L occurred in 16.7% (16/96) cats with ionized calcium normalizing on a subsequent visit highlighting the importance of serial assessment of ionized calcium to document persistence. Only higher BCS was significantly associated with incident hypercalcemia but the clinical significance of this finding remains uncertain.
CKD is commonly associated with hypercalcemia in cats: it was reported in 11/85 cats with ionized hypercalcemia 22 and 18/71 cats with total hypercalcemia. 23 The prevalence of ionized hypercalcemia at diagnosis of azotemic CKD found in the present study sits within the range of 10% to 30% previously reported. 2 , 3 The proportion of cats that developed ionized hypercalcemia during follow‐up might be overestimated, because cats that did not develop ionized hypercalcemia had to have at least 90 days of follow‐up information available to be included in the analysis. The severity of ionized hypercalcemia observed in the present study population was generally mild (median, 1.42 mmol/L; range, 1.38‐1.84 for all 75 cats). This is comparable to the findings in a recent publication on hypercalcemia in cats presented to the Cornell University Hospital for Animals, in which the severity of ionized hypercalcemia was <1.6 mmol/L in 13/16 cats with renal dysfunction. 22 Total hypercalcemia was found in 4% of cats at diagnosis of azotemic CKD and in 32% of cats with persistent ionized hypercalcemia during follow‐up. It was only observed concurrently with ionized hypercalcemia and those cats with total hypercalcemia generally had more severe ionized hypercalcemia. These findings are likely explained by the narrower width of the reference interval of blood ionized calcium concentration compared to that of plasma total calcium, enabling blood ionized calcium to exceed the upper limit of the reference interval before plasma total calcium concentration will. 24 Furthermore, this finding supports the previously reported low sensitivity of plasma total calcium as a screening test for ionized hypercalcemia 1 , 2 and endorses measurement of ionized calcium for adequate assessment of calcium status in cats with CKD.
CKD has a high prevalence in older cats with up to 80% of the population affected. 25 Therefore, it is a likely comorbidity with other causes of hypercalcemia in cats, such as neoplasia. The study performed at the Cornell University Hospital for Animals 22 found ionized hypercalcemia in 119 cats, of which 34 cases were inconsequential hypercalcemia and in 11 cases the cause was not determined. Neoplasia (mostly lymphoma) was the most common pathologic cause of ionized hypercalcemia in that study (27/119 cats), followed by idiopathic hypercalcemia (15/119 cats), CKD (11/119 cats), and parathyroid‐dependent hypercalcemia (4/119). 22 A major limitation of the present study is that diagnostic imaging was not performed in hypercalcemic cats and therefore causes of hypercalcemia other than CKD cannot be reliably ruled out. Cats with hypercalcemia of malignancy tend to have moderate‐to‐severe ionized hypercalcemia and severe clinical signs, 22 , 24 which were generally not recognized in the present study population.
Primary hyperparathyroidism is an uncommon cause of hypercalcemia in cats and, like the situation in CKD, the severity of hypercalcemia and clinical signs are generally mild. 22 , 23 , 24 Unlike primary hyperparathyroidism, secondary renal hyperparathyroidism does not appear to cause tubular reabsorptive hypercalcemia in humans with CKD, 26 and its role in the development of hypercalcemia in cats could therefore be questioned. Information on plasma PTH was available for most hypercalcemic cats in this study. Although increased above the upper limit of the reference interval in 12/27 cats with ionized hypercalcemia at diagnosis of azotemic CKD, plasma PTH decreased over time following diagnosis of CKD in most of these cats, and significant increases were also not observed in cats that developed ionized hypercalcemia during follow‐up. Therefore, parathyroid‐dependent hypercalcemia is considered unlikely in most cats presented herein.
Hypervitaminosis D appeared an unlikely cause of hypercalcemia, because none of the cats were treated with vitamin D‐analogues. Information on plasma concentrations of vitamin D metabolites was available for only a small number of cats in this study. When available, these were generally seen to decrease with the development of ionized hypercalcemia. Plasma FGF23 was significantly higher in cats with ionized hypercalcemia at diagnosis of azotemic CKD. Plasma total calcium was previously identified as an independent predictor of plasma FGF23 in cats. 18 Plasma samples for PTH and FGF23 analysis were stored at −80°C for more than 12 months, which could have resulted in degradation of these hormones. 27 , 28 This would have affected the baseline samples the most, as these had the longest storage time for each individual cat. However, the different groups of cats would have been equally affected as cats that were or became hypercalcemic and cats that were and remained normocalcemic were randomly distributed over time.
It cannot be ruled out that idiopathic hypercalcemia preceded the development of renal azotemia in cats that were hypercalcemic at diagnosis of CKD. Cats with idiopathic hypercalcemia tend to have more severe ionized hypercalcemia compared to cats with CKD, 22 but are known to show no to mild clinical signs, which are similar to those observed in cats with azotemic CKD (ie, anorexia, vomiting, lethargy, and constipation). 24 , 29 Male sex was identified as an independent risk factor for ionized hypercalcemia at diagnosis of azotemic CKD, with 21/33 (64%) males in the group of cats with ionized hypercalcemia. This is in agreement with a report on feline idiopathic hypercalcemia, which found 12/20 (60%) hypercalcemic cats to be male. 29 However, others report no sex predilection in cats with hypercalcemia 23 , 24 and both sexes were equally distributed between cats that developed ionized hypercalcemia and cats that remained normocalcemic in the present study, as well as in a previous study on incident total hypercalcemia. 1 Therefore, this result could be because of a type I error.
It could be hypothesized that the pathophysiology is different between cats with ionized hypercalcemia at diagnosis of azotemic CKD and cats that developed ionized hypercalcemia during follow‐up. Some studies suggest an association with feeding a clinical renal diet, as it was reported that ionized hypercalcemia developed in 2/15 cats on 84 and 149 days after starting a phosphate and protein‐restricted clinical renal diet, 13 total calcium increased in cats with IRIS stage 1 and 2 CKD fed different clinical renal diets for 6 months, 30 and ionized hypercalcemia developed after 17 months of feeding a phosphate‐restricted diet in 76% of cats with IRIS CKD stage 1 and 2. 14 Moreover, hypercalcemia was reported to resolve after discontinuation of stringent dietary phosphate restriction. 13 , 21 Cats in the present study were also offered a phosphate restricted clinical renal diet following the diagnosis of azotemic CKD, and most cats were fed this diet >50% of their daily food intake. Although the cause‐and‐effect relationship cannot be inferred from these observational studies, a role for dietary phosphate restriction in the development of hypercalcemia in some cats with CKD is implied. The present study, however, found no difference in the proportion of renal diet fed on a daily basis between cats that developed ionized hypercalcemia and those that remained normocalcemic. These results were based on owner self‐reporting of compliance, which might have overestimated true compliance, although this would have equally affected both groups. To adequately assess the effect of feeding a phosphate‐restricted clinical renal diet on the development of hypercalcemia, a randomized controlled clinical trial should be performed, in which cats that are normocalcemic at diagnosis of CKD are offered either a clinical renal diet or a less phosphate restricted control diet. However, phosphate restricted diets are currently considered the mainstay of management for CKD in cats, with multiple studies supporting beneficial effects. 13 , 31 , 32 Therefore, such a clinical trial would need to be carefully designed as withholding the benefits of a clinical renal diet may not be considered ethical.
Identification of baseline characteristics of cats that develop hypercalcemia would allow close monitoring and early intervention, but no clear differences between CKD cats that developed ionized hypercalcemia and cats that remained normocalcemic were identified. 1 Although BCS was identified as a predictor of incident ionized hypercalcemia, no clear clinical association can be drawn here.
A limitation of the current study was the reliance on a single ionized calcium measurement to define hypercalcaemia at the diagnosis of azotemic CKD. It is possible that some cats classified as hypercalcemic at this point in fact only had transient hypercalcemia; a phenomenon which is subsequently seen in those cats that were normocalcemic and monitored for the development of incident hypercalcemia. Lack of a clear predictor further emphasizes the importance of monitoring for the development of ionized hypercalcemia as part of standard protocols for all cats diagnosed with CKD and in particular in association with any dietary change. Where ionized calcium concentrations ≥1.38 mmol/L are identified, repeat evaluation is recommended to ensure persistence prior to any consideration for therapeutic or dietary intervention. Based on the high incident rate of ionized hypercalcemia in cats with azotemic CKD reported in the present study, continued monitoring of blood ionized calcium concentration is strongly recommended.
CONFLICT OF INTEREST DECLARATION
Henk van den Broek and Rebecca Geddes received PhD studentship grant funding from Royal Canin. Jonathan Elliott has received funding from consultancies with Elanco Ltd, CEVA Animal Health Ltd, Boehringer Ingelheim Ltd, Orion Incorp, Idexx Ltd, Waltham Centre for Pet Nutrition, Kindred Biosciences Inc, Invetx Inc; Merck Animal Health, has received grant funding from Elanco Ltd, Waltham Centre for Pet Nutrition, Royal Canin SAS, Idexx Ltd, Zoetis Ltd and CEVA Animal Health; is a member of the International Renal Interest Society which receives a grant from Elanco Ltd. Rosanne Jepson, Nicola Lötter, and Ruby Chang declare no conflicts of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
The authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Cats recruited to this study formed part of an observational cohort for which owner consent was obtained and approval of the Ethics and Welfare Committee of the Royal Veterinary College had been granted (2013 1258E).
HUMAN ETHICS APPROVAL DECLARATION
The authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This work was supported by a grant from Royal Canin SAS, Aimargues, France. The Renal Research Clinic at the Royal Veterinary College acknowledges support from Royal Canin for its research on chronic kidney disease‐mineral and bone disorder in cats.
van den Broek DHN, Geddes RF, Lötter NS, Chang Y‐M, Elliott J, Jepson RE. Ionized hypercalcemia in cats with azotemic chronic kidney disease (2012‐2018). J Vet Intern Med. 2022;36(4):1312‐1321. doi: 10.1111/jvim.16430
Funding information Royal Canin
REFERENCES
- 1. van den Broek DHN, Chang Y, Elliott J, Jepson RE. Chronic kidney disease in cats and the risk of total hypercalcemia. J Vet Intern Med. 2017;31(2):465‐475. doi: 10.1111/jvim.14643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res. 2010;74(3):209‐213. [PMC free article] [PubMed] [Google Scholar]
- 3. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39(3):108‐116. doi: 10.1111/j.1748-5827.1998.tb03613.x [DOI] [PubMed] [Google Scholar]
- 4. Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(Suppl 1):23. doi: 10.2215/CJN.05910809 [DOI] [PubMed] [Google Scholar]
- 5. Levi M, Peterson L, Berl T. Mechanism of concentrating defect in hypercalcemia. Role of polydipsia and prostaglandins. Kidney Int. 1983;23(3):489‐497. [DOI] [PubMed] [Google Scholar]
- 6. Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin‐angiotensin system, and calcium. J Clin Invest. 1983;71(6):1624‐1632. doi: 10.1172/jci110918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of five cases. J Am Anim Hosp Assoc. 1999;35(4):297‐301. doi: 10.5326/15473317-35-4-297 [DOI] [PubMed] [Google Scholar]
- 8. McLeland SM, Lunn KF, Duncan CG, Refsal KR, Quimby JM. Relationship among serum creatinine, serum gastrin, calcium‐phosphorus product, and uremic gastropathy in cats with chronic kidney disease. J Vet Intern Med. 2014;28(3):827‐837. doi: 10.1111/jvim.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21(1):103‐112. doi: 10.1681/ASN.2009060640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertazzolo W, Toscani L, Calcaterra S, Crippa L, Caniatti M, Bonfanti U. Clinicopathological findings in five cats with paw calcification. J Feline Med Surg. 2003;5(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973‐1984). J Am Vet Med Assoc. 1987;190(9):1196‐1202. [PubMed] [Google Scholar]
- 12. Lulich JP, Osborne CA, O'brien TD, Polzin DJ. Feline renal failure: Questions, answers, questions. The Compendium on continuing education for the practicing veterinarian (USA). 1992.
- 13. Barber PJ, Rawlings JM, Markwell PJ, Elliott J. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract. 1999;40(2):62‐70. doi: 10.1111/j.1748-5827.1999.tb03039.x [DOI] [PubMed] [Google Scholar]
- 14. Schauf S, Coltherd JC, Atwal J, et al. Clinical progression of cats with early‐stage chronic kidney disease fed diets with varying protein and phosphorus contents and calcium to phosphorus ratios. J Vet Intern Med. 2021;35:2797‐2811. doi: 10.1111/jvim.16263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schenck PA, Chew DJ. Calcium: Total or ionized? Vet Clin North Am Small Anim Pract. 2008;38(3):497‐502. doi: 10.1016/j.cvsm.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 16. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26(2):275‐281. doi: 10.1111/j.1939-1676.2011.00874.x [DOI] [PubMed] [Google Scholar]
- 17. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29(6):1494‐1501. doi: 10.1111/jvim.13625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27(2):234‐241. doi: 10.1111/jvim.12044 [DOI] [PubMed] [Google Scholar]
- 19. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats: associations with development of azotaemia and survival time. J Small Anim Pract. 2012;53(10):561‐571. doi: 10.1111/j.1748-5827.2012.01253.x [DOI] [PubMed] [Google Scholar]
- 20. Geddes RF, Jepson RE, Forcada Y, Elliott J, Syme HM. Associations between single nucleotide polymorphisms in the calcium sensing receptor and chronic kidney disease‐mineral and bone disorder in cats. Vet J. 2018;235:34‐41. [DOI] [PubMed] [Google Scholar]
- 21. Geddes RF, van den Broek DHN, Chang YM, Biourge V, Elliott J, Jepson RE. The effect of attenuating dietary phosphate restriction on blood ionized calcium concentrations in cats with chronic kidney disease and ionized hypercalcemia. J Vet Intern Med. 2021;35(2):997‐1007. doi: 10.1111/jvim.16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coady M, Fletcher DJ, Goggs R. Severity of ionized hypercalcemia and hypocalcemia is associated with etiology in dogs and cats. Front Vet Sci. 2019;6:276. doi: 10.3389/fvets.2019.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savary KC, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991‐1997). J Vet Intern Med. 2000;14(2):184‐189. doi: 10.1892/0891-6640(2000)0142.3.co;2 [DOI] [PubMed] [Google Scholar]
- 24. de Galvão B, Felipe J, Parker V, Schenck PA, Chew DJ. Update on feline ionized hypercalcemia. Vet Clin N Am: Small Anim Pract. 2017;47(2):273‐292. doi: 10.1016/j.cvsm.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 25. Marino CL, Lascelles BD, Vaden SL, Gruen ME, Marks SL. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg. 2014;16(6):465‐472. doi: 10.1177/1098612X13511446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phelps KR, Stote KS, Mason D. Tubular calcium reabsorption and other aspects of calcium homeostasis in primary and secondary hyperparathyroidism. Clin Nephrol. 2014;82(2):83‐91. doi: 10.5414/CN108223 [DOI] [PubMed] [Google Scholar]
- 27. Hanon EA, Sturgeon CM, Lamb EJ. Sampling and storage conditions influencing the measurement of parathyroid hormone in blood samples: a systematic review. Clin Chem Lab Med. 2013;51(10):1925‐1941. doi: 10.1515/cclm-2013-0315 [DOI] [PubMed] [Google Scholar]
- 28. El‐Maouche D, Dumitrescu CE, Andreopoulou P, et al. Stability and degradation of fibroblast growth factor 23 (FGF23): the effect of time and temperature and assay type. Osteoporos Int. 2016;27(7):2345‐2353. doi: 10.1007/s00198-016-3543-5 [DOI] [PubMed] [Google Scholar]
- 29. Midkiff AM, Chew DJ, Randolph JF, Center SA, DiBartola SP. Idiopathic hypercalcemia in cats. J Vet Intern Med. 2000;14(6):619‐626. doi: 10.1892/0891-6640(2000)0142.3.co;2 [DOI] [PubMed] [Google Scholar]
- 30. Hall JA, Fritsch DA, Jewell DE, Burris PA, Gross KL. Cats with IRIS stage 1 and 2 chronic kidney disease maintain body weight and lean muscle mass when fed food having increased caloric density, and enhanced concentrations of carnitine and essential amino acids. Vet Rec. 2019;184(6):190. doi: 10.1136/vr.104865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plantinga EA, Everts H, Kastelein AM, Beynen AC. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec. 2005;157(7):185‐187. [DOI] [PubMed] [Google Scholar]
- 32. Ross SJ, Osborne CA, Kirk CA, Lowry SR, Koehler LA, Polzin DJ. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc. 2006;229(6):949‐957. doi: 10.2460/javma.229.6.949 [DOI] [PubMed] [Google Scholar]


