Abstract
Background
Acute phase proteins (APP) may guide treatment of pneumonia in dogs but correlations with radiographic abnormalities are poorly characterized.
Objectives
Develop a thoracic radiographic severity scoring system (TRSS), assess correlation of radiographic changes with APP concentrations, and compare time to APP and radiograph normalization with duration of antimicrobials treatment.
Animals
Sixteen client‐owned dogs, 12 with aspiration pneumonia, and 4 with community‐acquired pneumonia.
Methods
Concentrations of C‐reactive protein (CRP), serum amyloid A (SAA), and haptoglobin were measured on days 1, 3, 7, 14, 28, and 60 and orthogonal 2‐view thoracic radiographs were obtained on days 1, 7, 14, 28, and 60. Treatment was clinician‐guided and blinded to APP concentrations. Radiographic severity scores were assigned by blinded, randomized retrospective review by 2 board‐certified radiologists with arbitration by a third radiologist.
Results
Median (interquartile range [IQR]) time to normalization of CRP (7 days [7‐14]) and SAA concentrations (7 days [7‐14]) were shorter than antimicrobial treatment duration (17.5 days [14.5‐33.5]; P = .001 and .002, respectively) and TRSS normalization (14 days [8.8‐52], P = .02 and .02, respectively). The CRP and SAA concentrations were positively correlated with TRSS (CRP r s , 0.643; SAA r s , 0.634; both P < .0001). Both CRP and SAA identified normal thoracic radiographs area under the curve (AUC) 0.873 and 0.817, respectively, both P < .0001. Interobserver agreement for TRSS assignment was moderate (κ, .499; P < .0001).
Conclusion and Clinical Importance
Concentrations of CRP and SAA normalized before radiographic resolution and before clinicians discontinued antimicrobial treatment. The CRP and SAA concentrations may guide duration of antimicrobial treatment for dogs with pneumonia.
Keywords: antimicrobial treatment, CRP, haptoglobin, respiratory disease, SAA, scoring system
Abbreviations
- AMD
antimicrobial drug
- APP
acute phase proteins
- APPLEfast
acute patient physiologic and laboratory evaluation
- AUC
area under the curve
- AUROC
area under the receiver operating characteristic
- bpm
breath per minute
- CLSI
Clinical and Laboratory Standard Institute
- CRP
C‐reactive protein
- HPT
haptoglobin
- IQR
interquartile range
- ROC
receiver operating characteristic
- RR
respiratory rate
- SAA
serum amyloid A
- TRSS
thoracic radiographic severity scoring system
1. INTRODUCTION
Pneumonia is common in dogs, with aspiration pneumonia and community‐acquired pneumonia most frequently encountered. Diagnosis typically is based on history, physical examination findings, and thoracic radiography. Airway cytology and bacterial culture and susceptibility testing are recommended, but uncommonly performed, and hence antimicrobial drug (AMD) treatment is often empirical. 1 The optimal duration of AMD treatment for pneumonia in dogs is unknown, 2 but extended courses of treatment beyond clinical or radiographic resolution are widely recommended. 3 , 4 , 5 However, recent guidelines from the International Society for Companion Animal Infectious Diseases (ISCAID) recommend re‐evaluating animals 10 to 14 days after initiating treatment and suggest basing decisions to discontinue AMDs on clinical, hematological, and radiographic findings. 2 Successful treatment of presumptive bacterial pneumonia with <14 days of AMD is reported. 6 , 7
The World Health Organization has developed standardized criteria for diagnosis of pneumonia in human pediatrics patients based on radiographic pulmonary consolidation. 8 The addition of plasma biomarker measurements improves pneumonia diagnosis, 9 enhances discrimination of pulmonary infiltrate causes, 10 , 11 and improves severity assessments. 12 Additionally, radiographic resolution of pneumonia in humans can lag behind clinical resolution. 13 , 14 In humans, duration of AMD treatment for pneumonia is commonly 5 to 10 days, 15 and serial biomarker measurement enables shorter durations of AMD treatment without apparent harm. 16 , 17 , 18 , 19 Similarly, in dogs with pneumonia, serial measurement of C‐reactive protein (CRP) and serum amyloid A (SAA) can enable significant decreases in the duration of AMD treatment without adverse effects, suggesting clinical resolution coincided with acute phase protein (APP) concentration normalization. 20 In that study, most dogs showed radiographic resolution of alveolar infiltrates within 15 days. However, no standardized radiographic score for pneumonia in dogs is available, and the clinical and radiographic severity of pneumonia has not been compared with contemporaneous biomarker concentrations. Assessing the correlation between radiographic severity and biomarker concentrations and determining whether radiographic resolution and biomarker normalization are contemporaneous would help clinicians use radiographs and biomarkers in combination to better monitor and manage dogs with pneumonia.
Our aims were to develop a thoracic radiographic severity scoring system (TRSS) for pneumonia, to assess correlation of radiographic changes with APP concentrations in dogs with pneumonia, and to compare the time to normalization of APP concentrations and radiographic changes with the duration of AMD prescribing in dogs with pneumonia. We hypothesized that radiographic severity of pneumonia would positively correlate with APP concentrations, that biomarker concentrations would normalize before radiographic changes resolve, and that biomarker concentrations would normalize before clinicians discontinue AMDs.
2. MATERIALS AND METHODS
2.1. Animals
Ours was a prospective observational study of client‐owned dogs diagnosed with pneumonia based on the following inclusion criteria: acute onset of respiratory distress, cough or tachypnea (respiratory rate [RR] >30 breath per minute [bpm] or PaCO2 < 35 mm Hg), and a clinical diagnosis of aspiration pneumonia or community‐acquired pneumonia. Dogs were diagnosed with aspiration pneumonia if they had a known risk factor (recent anesthesia or sedation, regurgitation or vomiting, laryngeal or pharyngeal dysfunction, esophageal disease or neurologic disease), and a cranioventral distribution of interstitial or alveolar pattern on thoracic radiographs. 21 Dogs were diagnosed with community‐acquired pneumonia if they had a known risk factor (communal housing, exposure to a contagious respiratory pathogen, upper respiratory tract disease), and interstitial or alveolar pattern on thoracic radiographs consistent with bacterial pneumonia. 22 Exclusion criteria included body weight <5 kg (to prevent excessive blood volume collection), dogs for which owners declined medical management of pneumonia, and AMD prescription for >24 hours before study enrollment. Signalment, previous medical history, and physical examination findings at presentation were recorded. Blood pressure was measured by oscillometric (Cardell 9401, Midmark, Dayton, Ohio) or Doppler methods. Arterial hemoglobin oxygen saturation was measured by pulse oximetry (Rad‐87, Masimo, Irvine, California). Mentation and body cavities fluid scores in the pleural, pericardial, and abdominal cavities (score 1 if fluid detected by point‐of‐care ultrasound) were assessed at admission to enable calculation of the Acute Patient Physiologic and Laboratory Evaluation (APPLEfast) score. 23 The study was approved by the Institutional Animal Care and Use Committee (Protocol #2014‐0053). Dogs were enrolled with written informed client consent. Clients received a financial incentive to attend all scheduled re‐evaluation appointments. Relevant attending clinicians directed all aspects of clinical care, including airway sampling by transtracheal wash or endotracheal wash for cytology and bacterial culture, 24 choice of AMD, and duration of treatment. Details of the type and duration of AMD treatment were extracted from medical records. Prescription of parenteral and enteral formulations of the same drug were counted as 1 drug (eg, IV and PO enrofloxacin), whereas prescriptions with distinct chemical composition were considered as 2 separate prescriptions, even if they shared antimicrobial properties (eg, amoxicillin‐clavulanate and ampicillin‐sulbactam). Attending clinicians were provided with the results of CBC, serum biochemistry profiles, and radiologist interpretations of thoracic radiographs at each time point. Biomarker concentrations were not disclosed to attending clinicians.
2.2. Blood sampling protocol
At hospital admission (day 1) blood samples were obtained from IV catheters or by direct venipuncture into evacuated tubes (Vacutainer, BD, Franklin Lakes, New Jersey) containing no additive, lithium heparin and K2‐EDTA for serum biochemistry profiles, biomarker measurements and venous blood gas analyses, and CBC, respectively. Aerobic and anaerobic blood cultures were performed by aseptically drawing between 1 and 5 mL of venous blood into commercial blood culture bottles (VersaTrek EZ Draw REDOX 2, Thermo Scientific, Oakwood Village, Ohio) from a peripheral blood vessel. Blood culture bottles were transferred to a 37°C incubator using an automated system (VersaTREK 240, Thermo Scientific) and incubated for up to 5 days or until positive. Gram stain was performed on culture bottles samples and, if organisms were observed, samples were plated to trypticase soy agar with 5% sheep blood and chocolate agar, and incubated at 33°C to 37°C with 6% CO2 for 48 hours. Plates were inspected daily, and bacterial identification of colonies performed by matrix‐assisted laser desorption ionization‐time of flight mass spectrometry (MALDI‐TOF; Bruker Microflex LT/SH, Real‐Time Classification v3.1, reference library 7854), conventional biochemical methods or 16s RNA sequencing as needed. Automated AMD susceptibility testing using a range of drugs (Figure 1A) was performed after 18 to 24 hours of incubation using broth microdilution according to Clinical and Laboratory Standard Institute (CLSI) guidelines, using commercial plates (Sensititre, COMPGN1F Vet AST plate, Thermo Fisher Scientific, Waltham, Massachusetts). Minimum inhibitory concentration and interpretations (sensitive, intermediate, resistant) were made using proprietary software (Sensititre SWIN Software System, Thermo Fisher Scientific) with recent CLSI (Vet01‐S2, 2013/Vet 08, 2018) breakpoints. On days 3, 7, 14, 28, and 60, blood samples were obtained for serum biochemistry profiles, biomarker measurements, and CBC.
FIGURE 1.
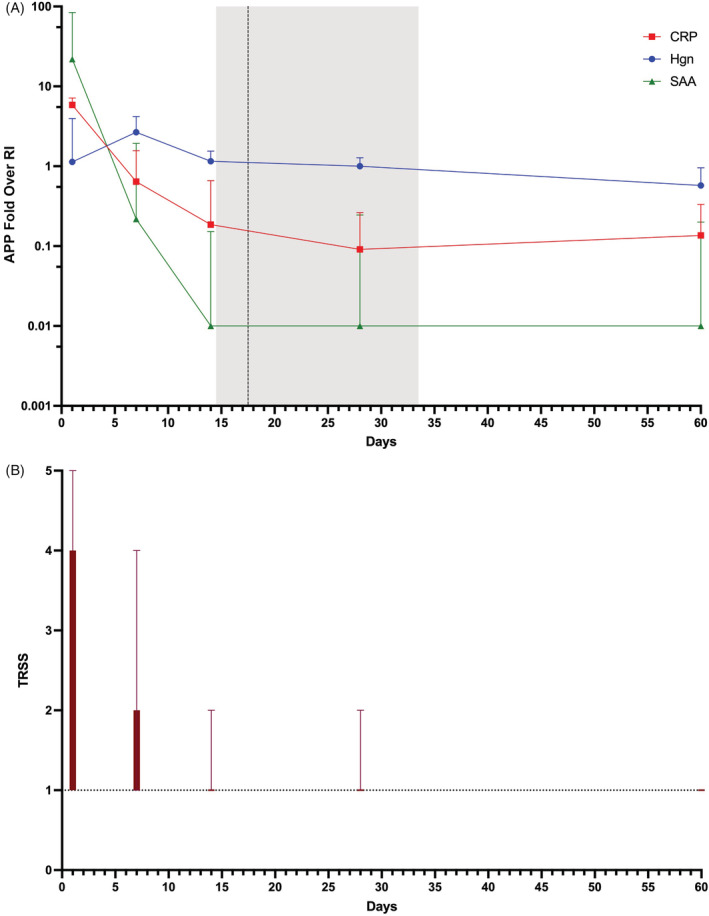
(A) Median ± interquartile ranges of concentrations of acute phase proteins (APP) over time (days) represented as fold over reference interval (RI). C‐reactive protein (CRP) is represented by the red squares, serum amyloid A (SAA) by the green triangles, and haptoglobin (Hgn) by the blue solid circles. Duration of antimicrobial drug (AMD) administration is represented by the vertical dotted line (mean) ± SD (shaded gray area). (B) Median ± interquartile ranges of the thoracic radiograph severity score (TRSS) over time, wherein a score of 1 is normal (represented by the horizontal dotted line), score 2 indicates equivocal radiographic changes, score 3 indicates mild disease, score 4 represents moderate disease, and score 5 represents severe disease
2.3. Clinicopathologic and biomarker analyses
Venous blood gas analyses (RapidPoint 500, Siemens Healthcare, Malvern, Pennsylvania) were performed immediately after sample collection. Complete blood counts (ADVIA 2120, Siemens Healthcare, Malvern, Pennsylvania) and serum biochemistry profiles (Cobas C501, Roche Diagnostics, Indianapolis, Indiana) were performed immediately whenever possible, but always within 48 hours of collection. Heparin plasma samples were prepared from whole blood by centrifugation and frozen at −80°C for batch biomarker analysis. Samples for biomarker analyses were shipped overnight on dry ice in 3 batches for analysis at a reference laboratory (Acute Phase Protein Laboratory, University of Miami, Miami, Florida) using assays validated for use in dogs. 25 , 26 , 27 , 28 , 29 The maximum storage time before analysis was 12 months (median, 7). 29 , 30 Plasma CRP concentrations were determined using an anti‐human CRP reagent (Randox Laboratories, Kearneysville, West Virginia) on a Daytona RX analyzer (Randox). Concentrations of SAA were determined using Vet‐SAA (Eiken Chemical Co, Tokyo, Japan). Haptoglobin (HPT) concentrations were determined using a phase colorimetric assay (Tri‐DD, Boonton, New Jersey). Quality controls were performed, and analyzers maintained, according to manufacturer recommendations. Reference intervals for the APPs were: CRP (0‐20 μg/mL), SAA (0‐10 μg/mL), and HPT (0‐2 μg/mL), established by the reference laboratory according to the American Society for Veterinary Clinical Pathology guidelines. 31
2.4. Clinician‐guided diagnostic tests
Respiratory panel PCRs designed for dogs were performed on samples collected from the external nares and upper airways swabbed with sterile cotton applicators. Swabs were placed in sterile glass vials with a few drops of saline and submitted for detection of respiratory betacoronavirus, distemper virus, adenovirus, parainfluenza virus, pneumovirus, influenza virus (A‐subtypes), Bordetella bronchoseptica, and Mycoplasma cynos. Extractions were performed using the MagMAX CORE Nucleic Acid Purification Kit (Thermo Fisher Cat#A3202) in an automated KingFisher Flex nucleic acid extractor following the manufacturer's instructions. Reverse transcription‐PCR reactions were performed using the Path‐ID qPCR Master Mix and Applied Biosystems 7500 Fast Real‐Time PCR Systems or the TaqMan Fast Virus 1‐Step Master Mix and the QuantStudio 12K Flex Real‐Time PCR Open Array System, using locally developed and verified primers and probes (Animal Health Diagnostic Center, Cornell University).
Aerobic bacterial culture: Respiratory samples for bacterial isolation were plated on trypticase soy agar with 5% sheep blood agar, chocolate agar, MacConkey agar, Levine EMB agar, and Columbia CNA agar. MacConkey plates were incubated in ambient air, whereas other culture plates were incubated at 33°C to 37°C. At 18 and 24 hours, culture plates were evaluated for growth that was described using a semi‐quantitative scale: few referring to a sparse number of colonies in the initial plate quadrant; moderate to increased number of colonies in the initial quadrant or some extension into the second quadrant; many referring to a lawn of colonies with extension into other quadrants. Bacterial identification, susceptibility testing and breakpoint interpretation were as described above for blood cultures.
2.5. Thoracic radiography, interpretation, and scoring
Three‐view thoracic radiographs were obtained on day 1, and 2 view (lateral plus dorsoventral projection) thoracic radiographs were obtained on days 7, 14, 28, and 60. The lateral view for these follow‐up time points was chosen based on the lateral view showing the most severe abnormalities on day 1. Dogs were lightly sedated using butorphanol (Torbugesic, Zoetis, Kalamazoo, Michigan) if necessary for restraint. Contemporaneous radiologist review was performed for all thoracic radiographs, informed by the medical history and clinical assessment and with access to previous radiographic studies for comparison. After completion of enrollment, all radiology reports were retrospectively reviewed and a single author (Julie Menard) assigned each study as disease absent or present. All thoracic radiographs then were imported into an image archiving system, anonymized and randomized using an online random number generator (https://numbergenerator.org). Two board‐certified radiologists (Ian Porter and Assaf Lerer) then reviewed the radiographs in a blinded fashion and assigned a TRSS value (Table 1). If these scorers disagreed, a third board‐certified radiologist (Philipa J. Johnson), blinded to the other reviews, also assigned a score, and the modal score was used for subsequent analyses. If all 3 scorers disagreed, the median score was assigned and used for subsequent analyses.
TABLE 1.
Thoracic radiographic severity scoring system (TRSS)
| Score | Interpretation | Description |
|---|---|---|
| 1 | Normal | No pulmonary radiographic abnormalities detected |
| 2 | Equivocal | Very mild pulmonary opacification where differentiation between mild pathological consolidation, atelectasis, or scar tissue is not possible. Mild bronchial thickening with a cranioventral distribution in the absence of pulmonary consolidation may also be included in this category |
| 3 | Mild | Pulmonary consolidation involving less than 50% of 1 lobe |
| 4 | Moderate | Pulmonary consolidation involving greater than 50% consolidation of 1 lung lobe or less than 50% consolidation of multiple lung lobes |
| 5 | Severe | Greater than 50% consolidation of multiple lung lobes. |
2.6. Statistical analyses
Continuous variables were assessed using the D'Agostino Pearson test. Numerical categorical variables such as APPLEfast and the TRSS were assumed to be nonparametric. Parametric data are reported as mean ± SD, whereas nonparametric data are reported as median (interquartile range, IQR). Continuous demographic and clinicopathologic data were compared between diagnoses using Student's t‐tests or the Mann Whitney U‐test, based on distribution. Frequencies of categorical variables (eg, sex) were compared using χ 2 or Fisher's exact test where appropriate. The resulting P‐values were corrected for multiple comparisons using the Benjamini‐Hochberg procedure (5% false discovery rate). 32 Duration of AMD administration was compared with time to normalization of APP concentrations and with time to normalization of TRSS using the Friedman test with Dunn's post‐hoc multiple comparisons adjustment. Interobserver agreement among radiologists was assessed by calculation of Cohen's kappa value (κ), 33 with interpretation as previously described, 34 (κ, .0‐.20 slight, .21‐.40 fair, .41‐.60 moderate, .61‐.80 substantial, .81‐.1 nearly perfect). Sensitivity and specificity values of the TRSS using the radiographic report as reference method were calculated from 2 × 2 contingency tables for the detection of abnormal thoracic radiographs based on the thoracic radiography score (1 vs >1). Accuracy of TRSS in identification of disease (score 1 vs >1) compared to the reference method also was calculated as a percentage. The APP concentrations were plotted over time and against corresponding TRSS. Comparisons of APPs over time and with TRSS were performed using the Kruskal‐Wallis test with Dunn's correction for multiple comparisons. The APP concentrations also were dichotomized based on interpretation of thoracic radiographs (normal vs abnormal) and their values compared using box‐whisker plots and the Mann‐Whitney U test. Receiver operating characteristic (ROC) curves were constructed to evaluate discriminatory ability and associated P‐values calculated using a previously described method. 35 Optimal values for discrimination of abnormal from normal thoracic radiographs were identified by calculation of the Youden index. 36 Multiple logistic regression analysis then was used to model identification of abnormal thoracic radiograph status using APP concentrations that were associated with thoracic radiograph interpretation in univariate analyses. Model accuracy was determined using 2 × 2 classification tables. Model discrimination was determined by calculating the area under the receiver operating characteristic (AUROC) curve. Model calibration was assessed by Hosmer‐Lemeshow goodness‐of‐fit (model rejected if P < .05) and visual inspection of contingency tables. Model utility was assessed using Nagelkerke's R2. Alpha was set at .05 and all analyses were 2‐sided and conducted using commercial software (Prism 9.1.2, GraphPad, La Jolla, California; SPSS 27, IBM Corp, Armonk, New York).
3. RESULTS
3.1. Animals
Nineteen dogs with pneumonia were enrolled. Of these, 2 were excluded because of euthanasia for disease severity on days 7 and 16. One additional dog was excluded because of lack client compliance and lack of radiographic assessment on days 7 and 28. Two dogs completed the study, but no day 7 radiographs were available for review; these dogs were included in the final analyses. Dogs displayed clinical signs for a median of 0 day (IQR, 3). Demographic characteristics are described in Table 2. Final diagnosis was aspiration pneumonia in 12 dogs and community‐acquired pneumonia in 4. Dogs with community‐acquired disease were younger than dogs with aspiration pneumonia (P = .02), but no other differences were observed between dogs with distinct diagnoses. Molecular diagnostics using PCR also were performed on 3/16 (18%) dogs, all of which were positive for Mycoplasma spp, whereas 1 dog was also positive for respiratory coronavirus. Airway sample bacterial cultures were performed in 3/16 (18%) dogs with isolation of Bordetella bronchiseptica and Escherichia coli (n = 1), Klebsiella pneumoniae (n = 1), and no growth (n = 1) that had a positive PCR test results for Mycoplasma spp. Blood cultures were positive in 2/16 (12.5%), with 1 dog identified with a gram positive bacteria and the second with Staphylococcus capitis and Streptococcus sp. Mean duration of antimicrobial treatment was 22.9 ± 10.2 days. Dogs were prescribed a median of 3 (IQR, 3‐4) AMDs (Table 3). Dogs received >3 AMDs because of suspected treatment failure, susceptibility results, and transition from parenteral ceftazidime to PO cefpodoxime (n = 1), poor owner compliance resulting in use of additional medication (n = 1), and prescription of AMDs for unrelated infections (skin infection near skin incision in 1 dog) or immunomodulatory purposes where such treatment would have contributed to antibiosis for the primary disease. The rationale for addition of a fourth AMD was not documented in 1 dog. Complete blood count and serum biochemistry profiles performed at admission are summarized in Table 2.
TABLE 2.
Summary of population characteristics including complete blood count and serum biochemistry data from study entry
| Parameter | All dogs (n = 16) | Aspiration pneumonia (n = 12) | Bacterial pneumonia (n = 4) | P‐value | Adjusted P‐value |
|---|---|---|---|---|---|
| Age (years) | 4.3 ± 3.8 | 5.5 ± 3.7 | 0.8 ± 0.2 | <.01 | .02 |
| Bodyweight (kg) | 25.9 ± 16.3 | 24.0 ± 17.2 | 31.5 ± 13.6 | .4 | .56 |
| Sex (FI/FS/MI/MC) | 1/5/6/4 | 1/3/4/4 | 0/2/2/0 | .38 | .56 |
| APPLEfast score | 14 (11‐20) | 14 (11‐22) | 16 (12‐20) | .58 | .75 |
| Length of hospitalization (days) | 3 (2‐4) | 3 (2‐4) | 3.3 (1.3‐13.0) | 1.0 | 1.0 |
| Duration of antimicrobials (days) | 22.9 ± 10.2 | 20.7 ± 8.7 | 29.8 ± 12.7 | .25 | .47 |
| Antimicrobial drugs prescribed (n) | 3 (3‐4) | 3 (3‐4) | 4 (3‐6) | .06 | .25 |
| HCT (%) | 46.8 ± 7.9 | 48.9 ± 7.7 | 40.3 ± 4.3 | .02 | .15 |
| Hgb (g/dL) | 15.6 ± 2.6 | 16.4 ± 2.5 | 13.3 ± 1.4 | .01 | .13 |
| Leukocytes (×103/μL) | 13.5 ± 7.1 | 11.5 ± 6.3 | 19.7 ± 6.5 | .08 | .25 |
| Neutrophils (×103/μL) | 10.3 ± 6.1 | 9.0 ± 5.4 | 14.3 ± 7.2 | .24 | .47 |
| Bands (×103/μL) | 1.3 ± 1.4 | 1.1 ± 1.4 | 2.0 ± 1.4 | .31 | .51 |
| Lymphocytes (×103/μL) | 0.9 (0.4‐1.2) | 0.7 (0.3‐1.5) | 1.6 (0.8‐2.7) | .05 | .25 |
| Monocytes (×103/μL) | 1.0 ± 0.8 | 0.8 ± 0.7 | 1.7 ± 0.7 | .07 | .25 |
| Eosinophils (×103/μL) | 0.0 (0.0‐0.2) | 0.0 (0.0‐0.5) | 0.0 (0.0‐0.2) | .76 | .84 |
| Platelets (×103/μL) | 236 ± 96 | 249 ± 100 | 197 ± 79 | .32 | .51 |
| Albumin (mg/dL) | 3.4 ± 4.7 | 3.5 ± 0.5 | 3.1 ± 0.4 | .94 | .99 |
| ALT (U/L) | 60 (43‐90) | 67 (49‐98) | 38 (24‐77) | .11 | .26 |
| Bilirubin (total) (mg/dL) | 0.1 (0.0‐1.0) | 0.1 (0.0‐1.0) | 0.1 (0.0‐0.2) | .67 | .79 |
| BUN (mg/dL) | 11 (8‐17) | 12 (9‐19) | 9 (7‐10) | .09 | .26 |
| Cholesterol (mg/dL) | 252 (211‐267) | 254 (222‐282) | 224 (197‐251) | .13 | .28 |
| Creatinine (mg/dL) | 0.7 (0.6‐0.9) | 0.7 (0.6‐1.0) | 0.7 (0.4‐0.9) | .68 | .79 |
Note: Data are presented as mean ± SD for normally distributed data and median (IQR) for non‐normally distributed data. Comparisons between data from dogs with aspiration pneumonia and those with bacterial pneumonia were compared with t‐tests, Mann‐Whitney U tests or χ 2. Raw P‐values and those following adjustment for multiple comparisons using the Benjamini‐Hochberg false discovery rate method are presented. P‐values displayed in bold font remained significant at P < .05 after correction for multiple comparisons.
TABLE 3.
Summary of antimicrobials
| Drugs | All dogs (n = 16) | Aspiration pneumonia (n = 12) | Bacterial pneumonia (n = 4) |
|---|---|---|---|
| Amoxicillin, n (%) | 1 (6.25) | 1 (8.3) | 0 |
| Ampicillin sulbactam, n (%) | 16 (100) | 12 (100) | 4 (100) |
| Amoxicillin clavulanate, n (%) | 16 (100) | 12 (100) | 4 (100) |
| Cefazolin, n (%) | 1 (6.25) | 1 (8.3) | 0 |
| Cefpodoxime, n (%) | 1 (6.25) | 0 | 1 (25) |
| Ceftazidime, n (%) | 1 (6.25) | 0 | 1 (25) |
| Clindamycin, n (%) | 1 (6.25) | 0 | 1 (25) |
| Doxycycline, n (%) | 2 (12.5) | 1 (8.3) | 1 (25) |
| Enrofloxacin, n (%) | 15 (93.75) | 12 (100) | 3 (75) |
| Pradofloxacin, n (%) | 1 (6.25) | 0 | 1 (25) |
| Trimethoprim sulfonamide, n (%) | 1 (6.25) | 0 | 1 (25) |
Note: Amoxicillin (Butler Animal Health, Fort Worth, Texas), ampicillin/Sulbactam (Unasyn, Pfizer, New York, New York), amoxicillin clavulanate acid (Clavamox, Zoetis, Kalamazoo, Michigan), cefazolin (Fresnius Kabi, Lake Zurich, Illinois), cefpodoxime (Simplicef, Zoetis, Kalamazoo, Michigan), ceftazidime (Tazicef, Hospira, Lake Forest, Illinois), clindamycin (Aurobindo Pharma USA, East Windsor, New Jersey), doxycycline (Butler Animal Health, Fort Worth, Texas), enrofloxacin (Baytril, Piedmont Animal Health, Greensboro, North Carolina), pradofloxacin (Veraflox, Bayer, Shawnee Mission, Kansas), trimethoprim sulfonamide (Butler Animal Health, Fort Worth, Texas).
3.2. APPs
Median concentrations of CRP and SAA were maximal at admission, whereas median HPT concentration was maximal on day 7. From admission, concentrations of CRP and SAA decreased rapidly over time, whereas those of HPT initially increased before decreasing more gradually (Figure 1A). On average, concentrations of CRP decreased below the upper bound of the reference interval on day 6 approximately, concentrations of SAA decreased to within the reference interval on day 5 approximately, whereas HPT concentrations decreased to below the reference interval on day 28 approximately. Concentrations of CRP and SAA on day 14 and day 28 were not different in dogs that received antimicrobials for ≤14 days compared to >14 days or for dogs that received antimicrobials drugs for ≤28 or >28 days (Figure S1). The times to normalization of CRP (7 [7‐14] days) and SAA concentrations (7 [7‐14] days) were significantly shorter than the duration of AMD administration (17.5 [14.5‐33.5] days; P = .001 and .002, respectively; Figure 2).
FIGURE 2.
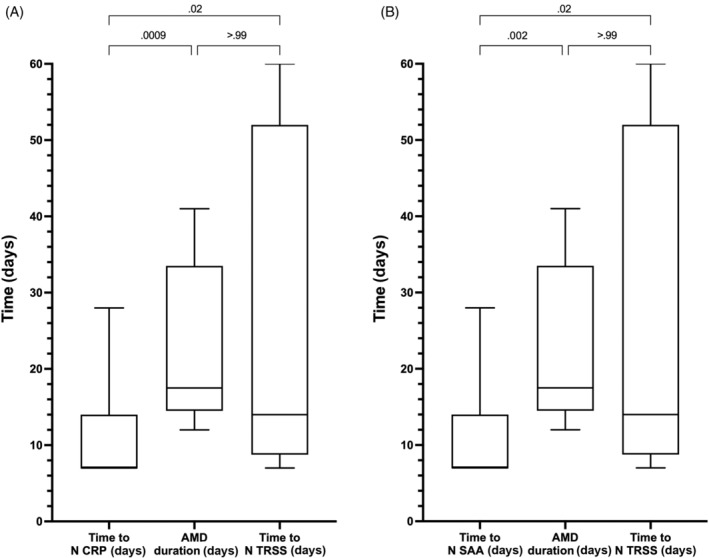
(A) Box/whisker plots of the time (days) to normalization of C‐reactive protein (CRP) concentrations, the duration of antimicrobial drug (AMD) administration and the time to normalization (N) of the thoracic radiograph severity score (TRSS). (B) Box/whisker plots of the time (days) to normalization of serum amyloid A (SA) concentrations, the duration of AMD administration and the time to normalization of the TRSS. Central horizontal lines represent the median, boxes represent the 25% to 75% range, and whiskers represent the minimum and maximum values. Comparisons between these data were performed using the Friedman test with Dunn's post‐hoc correction for multiple comparisons, with corresponding P‐values listed on the figure panels
3.3. Thoracic radiograph interpretation
All dogs had evidence of pneumonia on day 1 (admission). Subsequently, 4 (28.5%) of 14 dogs had radiographic resolution of pneumonia by day 7 and 11 (68.75%) of 16 dogs by day 14. Three (18.75%) dogs had residual evidence of disease on day 28. Of these, 1 dog had megaesophagus secondary to myasthenia gravis, 1 dog had bacterial pneumatoceles and concurrent parvoviral enteritis, and 1 dog had self‐declared poor owner compliance with drug administration (this dog had residual disease on day 60). A TRSS was assigned to all radiographs, with agreement between 2 radiologists present in 48 (61%) of 78 studies, necessitating evaluation of 30 studies by a third radiologist. No agreement among all 3 radiologists occurred in 4 (5.1%) of 78 studies, with the median TRSS used for further analyses. The overall interobserver agreement between radiologists was moderate (κ, .499, P < .0001). The TRSS decreased over time with normalization on average by day 14 (Figure 1B). The sensitivity, specificity, and accuracy of the TRSS (score > 1) for determination of disease (using the original radiograph report as the reference method) are reported in Table 4. The pneumonia score was sensitive for the presence of disease on admission and at days 28 and 60. The specificity of the TRSS was lowest at day 7 and highest at day 28. Accuracy of the TRSS was highest at admission and at day 60 and lowest on day 14 as shown in Table 5.
TABLE 4.
Concentration of acute phase proteins (APP) at the different time points
| APP (reference range) | Day 1 | Day 7 | Day 14 | Day 28 | Day 60 |
|---|---|---|---|---|---|
| CRP (0‐20 mg/L) | 117 (55.3‐143) | 12.8 (8.47‐31.3) | 3.72 (0.1‐13.2) | 1.82 (0.1‐5.25) | 4.04 (0.1‐7.3) |
| SAA (0‐10 mg/L) | 219 (54‐839) | 2.19 (0.1‐19.3) | 0.1 (0.1‐1.52) | 0.1 (0.1‐2.45) | 0.1 (0.1‐2.22) |
| HPT (0‐2 mg/mL) | 2.27 (1.27‐7.93) | 5.33 (2.01‐8.38) | 2.31 (1.81‐3.1) | 2.01 (0.61‐2.55) | 1.35 (0.65‐2.02) |
Note: Values are presented as median (IQR).
Abbreviations: CRP, C‐reactive protein; SAA, serum amyloid A; HPT, haptoglobin.
TABLE 5.
Sensitivity and specificity of the Thoracic Radiographic Severity Scoring (TRSS) system in diagnosing pulmonary disease, using the radiographical report as reference method
| Day 1 | Day 7 | Day 14 | Day 28 | Day 60 | |
|---|---|---|---|---|---|
| Sensitivity (95% CI) | 100% (0.81‐1.00) | 80% (0.49‐0.97) | 60% (0.23‐0.93) | 100% (0.44‐1.00) | 100% (0.81‐1.00) |
| Specificity (95% CI) | 50% (0.89‐0.91) | 63.64% (0.36‐0.85) | 76.92% (0.50‐0.92) | ||
| Accuracy | 100% | 71.4% | 62.5% | 81.3% | 100% |
Note: Accuracy between the radiographical report (normal vs abnormal) and the TRSS (normal [TRSS = 1] vs. abnormal [TRSS > 1]) was determined as a percentage using the radiographical report as reference method.
3.4. APP concentrations versus thoracic radiographs
Dogs with abnormal thoracic radiographs had median CRP concentrations that were higher than the laboratory reference interval (<20 μg/mL), and higher than in dogs with normal radiographs (31.8 μg/mL [9.6‐123.3] vs 2.7 μg/mL [0.1‐9.2]; P < .0001). As shown in Table 6, CRP and SAA normalized earlier than thoracic radiographical abnormalities. Agreement between thoracic radiograph interpretations and abnormal APP concentrations was highest on admission, and lowest on days 14 and 28, before increasing on day 60 (Table 7). Based on ROC curve analysis, CRP was highly discriminant for abnormal thoracic radiographs (AUC, 0.873; 95% CI, 0.798‐0.948; P < .001). The optimal cutoff for discriminating abnormal from normal thoracic radiographs was CRP >9.6 μg/mL with sensitivity of 77.1% and specificity of 81.0% and accuracy of 79.2% (Figure 3A). Median plasma SAA concentration was also higher than the reference interval (<10 μg/mL) and increased in dogs with radiographic evidence of pneumonia compared to dogs with normal thoracic radiographs (22.0 μg/mL [1.5‐241.7] vs 0.1 μg/mL [0.1‐1.9]; P < .0001). Plasma SAA concentration was also highly discriminant for abnormal thoracic radiographs (AUC, 0.819; 95% CI, 0.718‐0.921; P < .0001). The optimal cutoff for discrimination of abnormal thoracic radiographs was SAA >5.9 μg/mL with sensitivity of 68.6% and specificity of 95.1% and accuracy of 82.3% (Figure 3B). The median HPT concentration in dogs with abnormal thoracic radiographs was higher than the reference interval (<2 μg/mL), but HPT concentrations were not different between dogs with normal and abnormal thoracic radiographs (2.0 [1.0‐2.4] vs 2.4 [1.3‐5.6]; P = .51), and HPT concentration was not discriminant for abnormal thoracic radiographs (AUC, 0.631; 95% CI, 0.502‐0.759; P = .05; Figures 3C and 4).
TABLE 6.
Percent of abnormal radiographical report and number of cases with concentration of C‐reactive protein (CRP), serum amyloid A (SAA), and haptoglobin above the reference interval at the different time points
| Day 1 | Day 7 | Day 14 | Day 28 | Day 60 | |
|---|---|---|---|---|---|
| % Radiographic abnormality | 16/16 (100%) | 10/14 (71%) | 5/16 (31%) | 3/16 (19%) | 1/14 (7%) |
| % Abnormal CRP | 14/16 (88%) | 6/14 (43%) | 3/16 (19%) | 1/16 (6%) | 0/14 (0%) |
| % Abnormal SAA | 15/16 (94%) | 7/14 (50%) | 1/16 (6%) | 1/16 (6%) | 3/14 (21%) |
| % Abnormal haptoglobin | 9/16 (56%) | 10/14 (71%) | 10/16 (63%) | 8/16 (50%) | 0/14 (0%) |
| % Any APP abnormal | 15/16 (94%) | 10/14 (71%) | 12/16 (75%) | 8/16 (50%) | 3/14 (21%) |
| % Abnormal CRP or SAA | 15/16 (94%) | 7/14 (50%) | 3/16 (19%) | 1/16 (6%) | 3/14 (21%) |
TABLE 7.
Agreement between thoracic radiograph interpretation (normal vs abnormal) and concentration of acute phase proteins (APP): C‐reactive protein (CRP), serum amyloid A (SAA), and haptoglobin at the different time points
| D1 | Agreement D1 | D7 | Agreement D7 | D14 | Agreement D14 | D28 | Agreement D28 | D60 | Agreement D60 | |
|---|---|---|---|---|---|---|---|---|---|---|
| TXR abnormal | 16/16 (100%) | ‐ | 10/14 (71%) | ‐ | 5/16 (31%) | ‐ | 3/16 (19%) | ‐ | 1/14 (7%) | ‐ |
| CRP abnormal | 14/16 (88%) | 88% | 6/14 (43%) | 43% | 3/16 (19%) | 75% | 1/16 (6%) | 75% | 0/14 (0%) | 93% |
| SAA abnormal | 15/16 (94%) | 94% | 7/14 (50%) | 64% | 1/16 (6%) | 75% | 1/16 (6%) | 75% | 3/14 (21%) | 93% |
| Haptoglobin abnormal | 9/16 (56%) | 56% | 10/14 (71%) | 57% | 10/16 (63%) | 44% | 8/16 (50%) | 44% | 0/14 (0%) | 74% |
| Any APP abnormal | 15/16 (94%) | 94% | 10/14 (71%) | 79% | 12/16 (75%) | 44% | 8/16 (50%) | 44% | 3/14 (21%) | 74% |
| CRP or SAA abnormal | 15/16 (94%) | 94% | 7/14 (50%) | 50% | 3/16 (19%) | 52% | 1/16 (6%) | 52% | 3/14 (21%) | 93% |
Note: Observed agreement was calculated using a 2 × 2 contingency table where radiographical reports were labeled as normal vs abnormal and APP concentration within reference interval (normal) or above reference interval (abnormal) using the formula observed agreement = (A + B/A + B + C + D) × 100, where A = number of dogs with normal radiographs with normal APP, B = number of dogs with abnormal radiographs with abnormal APP, C = number of dogs with normal thoracic radiographs and abnormal APP, and D = number of dogs with abnormal thoracic radiographs and normal APP.
FIGURE 3.
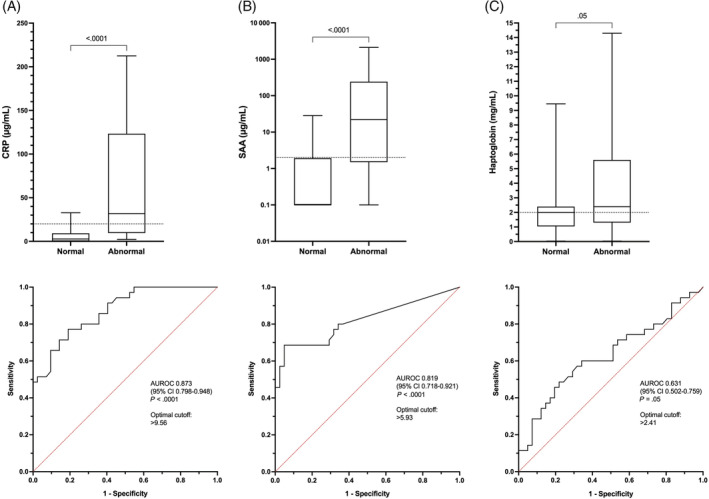
Box/whisker plots and corresponding receiver operating characteristic (ROC) curves for the differentiation of normal vs abnormal thoracic radiographs based on the concentrations of (A) C‐reactive protein (CRP), (B) serum amyloid A (SAA), and (C) haptoglobin. Comparisons between the acute phase protein concentrations in dogs with normal vs abnormal thoracic radiographs were performed using the Mann‐Whitney U test, with corresponding P‐values listed on the figure panels. On each ROC curve, the area under the curve (AUR) is displayed with the corresponding 95% confidence intervals and associated P‐values
FIGURE 4.
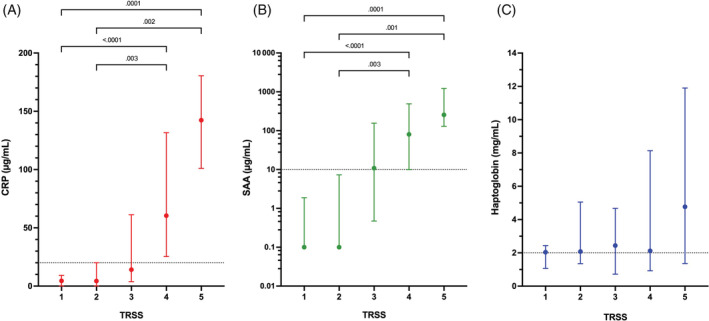
Dotplots comparing concentrations of acute phase proteins (A) C‐reactive protein (CRP), (B) serum amyloid A (SAA), and (C) haptoglobin with the corresponding thoracic radiograph severity score (TRSS) values. For all of the dotplots, the central dot represents the median value, with the error bars representing the interquartile range (25%‐75%). Acute phase protein concentrations for each corresponding TRSS were compared using the Kruskal‐Wallis test with Dunn's post‐hoc correction for multiple comparisons. Corresponding P‐values are displayed on the panels. Note, the P‐values for all of the comparisons for haptoglobin were >.99 and hence none of corresponding P‐values are displayed
3.5. APPs versus TRSS
Concentrations of CRP and SAA were positively correlated with TRSS (CRP r s , 0.643, P < .0001; SAA r s , 0.634, P < .0001) Concentrations of HPT were not significantly correlated with TRSS. The median concentration of CRP was within the reference interval for dogs with TRSS ≤3, such that CRP concentrations above the reference interval were associated with presence of moderate to severe disease (score >3). Concentrations of CRP were higher in dogs with moderate or severe disease (score 4 or 5) compared to those with normal or equivocal radiographs (score 1 or 2; P ≤ .003; Figure 4A). Median SAA concentrations were at or below the reference interval upper bound in dogs with normal, equivocal or mild disease (pneumonia score ≤3). The SAA concentration was significantly increased in dogs with moderate to severe disease (score >3) compared to those with normal or equivocal radiographs (score 1 or 2; P ≤ .003; Figure 4B). Haptoglobin concentrations did not change with TRSS and were only substantially above the reference interval in dogs with severe pneumonia (score 5; Figure 4C). Radiographic resolution of disease (ie, return of TRSS to 1) occurred on average by day 14. Time to normalization of CRP (7 [7‐14] days) and SAA concentrations (7 [7‐14] days) was shorter than the time to normalization of TRSS (14 [8.8‐52] days; P = .02 and .02, respectively).
4. DISCUSSION
We developed a severity scoring system for assessment of thoracic radiographs for dogs with pneumonia and used it to assess changes in severity over time. In parallel, we measured 3 APPs (CRP, SAA, and HPT) serially over a 60‐day period after hospital admission. We aimed to determine if APP concentrations correlated with radiographic disease severity, to determine if APP concentrations normalized contemporaneously with radiographic resolution and to compare the duration of AMD treatment with time to normalization of APP concentrations and radiographic changes. We observed that concentrations of CRP and SAA, but not HPT, were positively correlated with radiographic severity of pneumonia as assessed by TRSS. Graphical analysis indicated that median concentrations for CRP and SAA were within reference intervals by day 7, whereas the median TRSS was not normal (score 1) until day 14. This finding suggests a time lag between resolution of the acute phase response and radiographic resolution of pneumonia. Consistent with this conclusion, the time to normalization of CRP and SAA was significantly shorter than the time to radiographic resolution. Comparison of the duration of AMD administration with time to normalization of APP concentrations suggested that the acute phase response resolves before clinicians discontinue AMDs. No difference was found between time to normalization of TRSS and AMD duration. This finding is consistent with usual clinical practice, where clinicians use thoracic radiographs as a means to determine if the pneumonia has resolved. Our data are consistent with those of a previous study, 20 and suggest that APP measurement may guide the clinician's decision to discontinue AMDs, but our study was not designed to investigate this factor.
Concentrations of CRP and SAA have been widely studied and are increased in dogs with systemic inflammation, critical illness, autoimmune disease, trauma, and neoplasia. 37 , 38 , 39 , 40 , 41 , 42 , 43 In dogs, CRP may be a useful biomarker for the differentiation of dogs with infectious respiratory disease from those with noninfectious disorders, with values >100 μg/mL reported to be 100% specific for bacterial pneumonia. 24 Similarly, a previous study found that CRP >55 μg/mL in dogs with abnormal thoracic radiographs could discriminate aspiration pneumonia from Bordetella bronchiseptica infection. 44 In our study, in a population of dogs treated for pneumonia, CRP >9.5 μg/mL identified dogs with abnormal thoracic radiographs, whereas TRSS was positively correlated with both CRP and SAA concentrations. Our study design enabled us to repeatedly evaluate thoracic radiographs over time and hence provided a wide range of disease severities, within and between dogs. This feature may explain why TRSS was correlated with APP concentrations in our study, whereas concentrations of CRP, SAA, and HPT in dogs with pneumonia did not correlate with illness severity at presentation in a previous study. Direct comparison between our study and previous studies is complicated by differences in the means of determining illness severity (duration of hospitalization and oxygenation compared to APPLEfast and radiographic changes). Serial APP measurements provided insight into the extent and duration of the acute phase response and enabled us to determine the point at which resolution occurred. Three dogs had delayed radiographic resolution secondary to repeated aspiration, severe disease or poor owner compliance. Serial APP measurements indicating a lack of resolution might prompt further investigation in such cases. Serial APP concentrations are valuable for monitoring autoimmune diseases, 45 , 46 and in the postoperative period to permit early recognition of surgical infections. 27 , 47 , 48
Our results suggest that serial measurement of CRP and SAA may enable therapeutic decision‐making in dogs with pneumonia, specifically with regard to interpretation of equivocal radiographs and AMD discontinuation, although further study is required. Measurement of SAA may be superior to CRP in order to maximize sensitivity, specificity, and accuracy of disease detection. Use of APP measurement for patient management limits ionizing radiation exposure, eliminates the need for sedation to facilitate diagnostic imaging, and may provide clinicians with additional confidence that disease is present or that disease has resolved if radiographic interpretation by a board‐certified radiologist is not feasible. In contrast to CRP and SAA, HPT increased and decreased more gradually in our study. Median concentrations were not maximal until day 7, and remained increased longer than did CRP or SAA. These properties may limit the utility of HPT for diagnosis, monitoring or therapeutic decision‐making. In studies of autoimmune disease, HPT did not identify disease resolution, 46 and was not useful for identification of the development of postoperative complications. 47 The cause of persistently increased HPT concentration in infectious diseases is not known, but has been documented previously. 20 , 47
Duration of AMD treatment for pneumonia is predicated on radiographic resolution, with various sources recommending treatment for 1 to 2 weeks after normalization of thoracic radiographs. 3 , 4 , 5 Thoracic radiographs are considered the gold standard for diagnosis of pneumonia in humans, although it can be difficult to distinguish it from non‐bacterial disease processes. 49 In veterinary medicine, no standard definition exists for radiographic diagnosis of pneumonia, and the subjective nature of image interpretation, particularly in equivocal cases, might explain the only moderate agreement among radiologists in our study. In our study, radiologists were blinded to the patient and time point of each radiograph. This design aimed to minimize bias, but may have decreased the ability of the radiologist to make accurate interpretations because the study design prevented comparison with prior images and excluded any clinical data. Given the moderate agreement of the TRSS score, application in clinical practice is not recommended. Its use as a research tool should be further validated. The results of our blinded, randomized radiographic study further supports the use of APP measurement for assessment of dogs with pneumonia because APP concentrations are objective and can be compared to a reference interval. Thus, if radiographic interpretation is challenging, or images cannot be reviewed by a board‐certified radiologist, APP measurement might enhance decision‐making. Based on both the original radiographic reports and the TRSS, our data suggest that a lag exists between clinical resolution of bacterial pneumonia and radiographic resolution, similar to findings in humans. 13 , 14 In humans, delayed radiographic resolution also is associated with disease severity. 14
In children with community‐acquired pneumonia, the value of repeated thoracic radiographs has been questioned. A previous study showed that 30% of evaluations 3 to 7 weeks post‐diagnosis had abnormalities present, with 20% of children having new radiographic changes. However, despite presence of abnormal radiographs, treatment and resolution did not change. 50 In our study, 28.5% of dogs had no radiographic disease on day 7, and 81.5% had radiographic resolution of disease by day 28. This finding is consistent with a previous study of dogs with aspiration pneumonia, in which 50% of dogs had radiographic resolution of disease at day 14 and 84% by 1 month. 6 , 7 Moreover, radiographic resolution of pneumonia was similar regardless of whether dogs received <14 days of AMD or >14 days. In another study, the median duration of treatment in dogs in the <14 days group was 11 days, whereas in the >14 days group it was 21 days. In our study, mean AMD duration was 22 days, significantly longer than the time to normalization of TRSS and APP concentrations. Use of APP measurements to guide AMD treatment in dogs with pneumonia has been reported previously, 20 and our data strongly support this practice. Current ISCAID recommendations for treatment of bacterial pneumonia involve re‐evaluation of treatment after 10 to 14 days. Based on our study, measurement of CRP or SAA or both in place of thoracic radiographs between days 7 and 14 might be superior to radiographs to determine if AMDs can be safely discontinued. Our results do not support the current dogma of treating dogs for 1 to 2 weeks after radiographic resolution. 3 , 4
Strengths of our study include the prolonged follow‐up period that enabled use of dogs as their own controls for both APP measurement and thoracic radiograph interpretation. Similarly, using blinded, randomized radiograph review enhanced the validity of the TRSS and reinforced the notion that radiographic evaluation should be performed in conjunction with clinical history and review to prior imaging results. We measured multiple APPs to maximize discrimination and were able to compare these concentrations serially over time. Our study also had some limitations. Although our repeated assessment provided 78 sets of radiographs to assess, our overall study population was limited (n = 16), which may limit external validity and determination of the optimal APP concentration cutoffs in other patient populations. Similarly, some dogs had concurrent disease processes (eg, gastric dilatation volvulus, parvovirus, skin infections) that may have contributed to the increased APP concentrations observed. In addition, only a small number of dogs had bacterial infections confirmed by positive microbiological growth or a positive PCR of infectious agents. In the absence of lower airway samples for cytology and bacterial culture, clinicians diagnose pneumonia based on historical, clinical and radiographic signs. Given the small number of dogs with bacterial cultures, some dogs may not have been prescribed appropriate AMDs. Use of molecular methods from upper airway samples in dogs with lower respiratory disease may not properly identify the causative agent, and such results should be interpreted cautiously. Furthermore, 12 of 16 dogs had aspiration pneumonia. A recent case series documented successful management and resolution of aspiration pneumopathy without AMDs, even when bacterial cultures were positive in 4 of 14 and CRP concentrations were similar to those reported here. The need for AMDs in dogs with respiratory signs secondary to aspiration may be overestimated. 51 Most dogs in our study had mild to moderate illness severity, with a median APPLEfast score of 14, which corresponds to a predicted mortality of approximately 5%. Our findings with respect to APP concentrations or timing might have been different if we had enrolled a more severely affected patient population. Lastly, the effect of storage time and batch analysis on SAA and HPT on long‐term stability when frozen is unknown may have impacted APP concentrations results.
In summary, we developed a novel severity scoring system for assessment of thoracic radiographs for dogs with pneumonia and were able to demonstrate that normalization of CRP and SAA concentrations occurs significantly earlier than does radiographic resolution and AMD discontinuation. These findings support the use of serial APP measurement to facilitate therapeutic decision‐making regarding AMD treatment duration in dogs with pneumonia. Future studies could evaluate integration of the TRSS with APP measurement into a combined pneumonia score and compare APP‐ and clinician‐guided AMD treatment for dogs with pneumonia.
CONFLICT OF INTEREST DECLARATION
Julie Menard serves as a consultant for the pharmaceutical company Vetoquinol for the launch of 1 of their products. Dr. Menard has also spoken about antimicrobial stewardship and resistance at international conference for which she received payments (European Veterinary Emergency and Critical Care Congres 2020). Dr. Menard is an editor for Frontiers of Veterinary Sciences (Emergency and Critical Care section). No other authors have a conflict of interest.
OFF LABEL ANTIMICROBIAL DECLARATION
Pradofloxacin was used off label in 1 dog. This drug does not have Food and Drug Administration approval in the United States for use in dogs but does have European Medicines Agency approval for use in dogs in Europe.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Cornell University IACUC, protocol #2014‐0053.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1. Left panel: Box/whisker plots of concentration of C reactive protein (CRP)andserum amyloid A (SAA), at day 14 when having received antimicrobial drug (AMD) treatment 〈 14 days or 〉 14 days. Right panel: Box/whisker plots of concentration of C reactive protein (CRP) and serum amyloid A (SAA), at day 28 when having received antimicrobial drug (AMD) treatment 〈 28 days or 〉 28 days. Central horizontal lines represent the median, boxes represent the 25% to 75% range and whiskers represent the minimum and maximum values. Comparisons between these data were performed using the Friedman test with Dunn's post‐hoc multiple comparisons adjustment.
ACKNOWLEDGMENT
This study was funded by the Research Animal Health grant of Cornell University. The authors thank Denise Lalonde‐Paul for her help in scheduling and technical assistance during the study. The authors thank Eiken Chemical Co (Japan) for provision of the SAA reagents and the Acute Phase Protein Laboratory of the University of Miami for support on all the testing and other reagents.
Menard J, Porter I, Lerer A, Robbins S, Johnson PJ, Goggs R. Serial evaluation of thoracic radiographs and acute phase proteins in dogs with pneumonia. J Vet Intern Med. 2022;36(4):1430‐1443. doi: 10.1111/jvim.16448
Funding information Cornell University
REFERENCES
- 1. Robbins SN, Goggs R, Lhermie G, Lalonde‐Paul DF, Menard J. Antimicrobial prescribing practices in small animal emergency and critical care. Front Vet Sci. 2020;7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines working group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med. 2017;31(2):279‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dear JD. Bacterial pneumonia in dogs and cats: an update. Vet Clin North Am – Small Anim Pract. 2020;50(2):447‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanel R, Hansen BD. Pneumonia. In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy. 15th ed. St Louis, MO: Elsevier Saunders; 2014:2137‐2151. [Google Scholar]
- 5. Coté E. Pneumonia. In: Silverstein DC, Hopper K, eds. Small Animal Critical Care Medicine. 2nd ed. St Louis, MO: Elsevier Saunders; 2015:120‐126. [Google Scholar]
- 6. Wayne A, Davis M, Sinnott VB, Bracker K. Outcomes in dogs with uncomplicated, presumptive bacterial pneumonia treated with short or long course antibiotics. Can Vet J. 2017;58(6):610‐613. [PMC free article] [PubMed] [Google Scholar]
- 7. Vientós‐Plotts AI, Masseau I, Reinero CR. Comparison of short‐ versus long‐course antimicrobial therapy of uncomplicated bacterial pneumonia in dogs: a double‐blinded, placebo‐controlled pilot study. Animals. 2021;11(11):3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83(5):353‐359. [PMC free article] [PubMed] [Google Scholar]
- 9. Erdman LK, D'Acremont V, Hayford K, et al. Biomarkers of host response predict primary end‐point radiological pneumonia in Tanzanian children with clinical pneumonia: a prospective cohort study. PLoS One. 2015;10(9):e0137592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang H, Ideh RC, Gitau E, et al. Discovery and validation of biomarkers to guide clinical management of pneumonia in african children. Clin Infect Dis. 2014;58(12):1707‐1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valim C, Ahmad R, Lanaspa M, et al. Responses to bacteria, virus, and malaria distinguish the etiology of pediatric clinical pneumonia. Am J Respir Crit Care Med. 2016;193(4):448‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saghafian‐Hedengren S, Mathew JL, Hagel E, et al. Assessment of cytokine and chemokine signatures as potential biomarkers of childhood community‐acquired pneumonia severity: a nested cohort study in India. Pediatr Infect Dis J. 2017;36(1):102‐108. [DOI] [PubMed] [Google Scholar]
- 13. Bruns AHW, Oosterheert JJ, Prokop M, Lammers JWJ, Hak E, Hoepelman AIM. Patterns of resolution of chest radiograph abnormalities in adults hospitalized with severe community‐acquired pneumonia. Clin Infect Dis. 2007;45(8):983‐991. [DOI] [PubMed] [Google Scholar]
- 14. Bruns AHW, Oosterheert JJ, El Moussaoui R, Opmeer BC, Hoepelman AIM, Prins JM. Pneumonia recovery; discrepancies in perspectives of the radiologist, physician and patient. J Gen Intern Med. 2010;25(3):203‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuetz P, Christ‐Crain M, Thomann R, et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059‐1066. [DOI] [PubMed] [Google Scholar]
- 16. Christ‐Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84‐93. [DOI] [PubMed] [Google Scholar]
- 17. Christ‐Crain M, Jaccard‐Stolz D, Bingisser R, et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363(9409):600‐607. [DOI] [PubMed] [Google Scholar]
- 18. Stolz D, Christ‐Grain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131(1):9‐19. [DOI] [PubMed] [Google Scholar]
- 19. Coelho LM, Salluh JIF, Soares M, et al. Patterns of c‐reactive protein RATIO response in severe community‐acquired pneumonia: a cohort study. Crit Care. 2012;16(2):R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viitanen SJ, Lappalainen AK, Christensen MB, Sankari S, Rajamäki MM. The utility of acute‐phase proteins in the assessment of treatment response in dogs with bacterial pneumonia. J Vet Intern Med. 2017;31(1):124‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kogan DA, Johnson LR, Jandrey KE, Pollard RE. Clinical, clinicopathologic, and radiographic findings in dogs with aspiration pneumonia: 88 cases (2004‐2006). J Am Vet Med Assoc. 2008;233(11):1742‐1747. [DOI] [PubMed] [Google Scholar]
- 22. Radhakrishnan A, Drobatz KJ, Culp WTN, King LG. Community‐acquired infectious pneumonia in puppies: 65 cases (1993‐2002). J Am Vet Med Assoc. 2007;230(10):1493‐1497. [DOI] [PubMed] [Google Scholar]
- 23. Hayes G, Mathews K, Doig G, et al. The acute patient physiologic and laboratory evaluation (APPLE) score: a severity of Il lness stratification system for hospitalized dogs. J Vet Intern Med. 2010;24(5):1034‐1047. [DOI] [PubMed] [Google Scholar]
- 24. Viitanen SJ, Laurila HP, Lilja‐Maula LI, Melamies MA, Rantala M, Rajamäki MM. Serum C‐reactive protein as a diagnostic biomarker in dogs with bacterial respiratory diseases. J Vet Intern Med. 2014;28(1):84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kjelgaard‐Hansen M, Jensen AL, Kristensen AT. Evaluation of a commercially available human C‐reactive protein (CRP) turbidometric immunoassay for determination of canine serum CRP concentration. Vet Clin Pathol. 2003;32(2):81‐87. [DOI] [PubMed] [Google Scholar]
- 26. Christensen M, Jacobsen S, Ichiyanagi T, Kjelgaard‐Hansen M. Evaluation of an automated assay based on monoclonal anti‐human serum amyloid A (SAA) antibodies for measurement of canine, feline, and equine SAA. Vet J. 2012;194(3):332‐337. [DOI] [PubMed] [Google Scholar]
- 27. Löfqvist K, Kjelgaard‐Hansen M, Nielsen MBM. Usefulness of C‐reactive protein and serum amyloid A in early detection of postoperative infectious complications to tibial plateau leveling osteotomy in dogs. Acta Vet Scand. 2018;60(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Escribano D, Cihan H, Martínez‐Subiela S, et al. Changes in serum proteins in dogs with Ehrlichia canis infection. Microbial Pathogenesis. Vol 113. Academic Press; 2017;113:34‐39. doi: 10.1016/j.micpath.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 29. Romiszewski P, Kostro K, Lisiecka U. Effects of subclinical inflammation on C‐reactive protein and haptoglobin levels as well as specific humoral immunity in dogs vaccinated against canine distemper and parvovirus. BMC Vet Res. 2018;14(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brindle E, Fujita M, Shofer J, O'Connor KA. Serum, plasma, and dried blood spot high‐sensitivity C‐reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362(1–2):112‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol. 2012;41(4):441‐453. [DOI] [PubMed] [Google Scholar]
- 32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289‐300. [Google Scholar]
- 33. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37‐46. [Google Scholar]
- 34. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. [PubMed] [Google Scholar]
- 35. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 36. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32‐35. [DOI] [PubMed] [Google Scholar]
- 37. Ohno K, Yokoyama Y, Nakashima K, Setoguchi A, Fujino Y, Tsujimoto H. C‐reactive protein concentration in canine idiopathic polyarthritis. J Vet Med Sci. 2006;68(12):1275‐1279. [DOI] [PubMed] [Google Scholar]
- 38. Griebsch C, Arndt G, Raila J, Schweigert FJ, Kohn B. C‐reactive protein concentration in dogs with primary immune‐mediated hemolytic anemia. Vet Clin Pathol. 2009;38(4):421‐425. [DOI] [PubMed] [Google Scholar]
- 39. Mitchell KD, Kruth SA, Wood RD, Jefferson B. Serum acute phase protein concentrations in dogs with autoimmune hemolytic anemia. J Vet Intern Med. 2009;23(3):585‐591. [DOI] [PubMed] [Google Scholar]
- 40. Gebhardt C, Hirschberger J, Rau S, et al. Use of C‐reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care (San Antonio). 2009;19(5):450‐458. [DOI] [PubMed] [Google Scholar]
- 41. Chan DL, Rozanski EA, Freeman LM. Relationship among plasma amino acids, C‐reactive protein, illness severity, and outcome in critically ill dogs. J Vet Intern Med. 2009;23(3):559‐563. [DOI] [PubMed] [Google Scholar]
- 42. Gommeren K, Desmas I, Garcia A, et al. Inflammatory cytokine and C‐reactive protein concentrations in dogs with systemic inflammatory response syndrome. J Vet Emerg Crit Care. 2018;28(1):9‐19. [DOI] [PubMed] [Google Scholar]
- 43. Hindenberg S, Bauer N, Moritz A. Extremely high canine C‐reactive protein concentrations > 100 mg/l‐prevalence, etiology and prognostic significance. BMC Vet Res. 2020;16(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Canonne AM, Menard M, Maurey C, et al. Comparison of C‐reactive protein concentrations in dogs with Bordetella bronchiseptica infection and aspiration bronchopneumonia. J Vet Intern Med. 2021;35(3):1519‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lowrie M, Penderis J, Eckersall PD, McLaughlin M, Mellor D, Anderson TJ. The role of acute phase proteins in diagnosis and management of steroid‐responsive meningitis arteritis in dogs. Vet J. 2009;182(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 46. Grobman M, Outi H, Rindt H, Reinero C. Serum thymidine kinase 1, canine‐C‐reactive protein, Haptoglobin, and vitamin D concentrations in dogs with immune‐mediated hemolytic anemia, thrombocytopenia, and polyarthropathy. J Vet Intern Med. 2017;31(5):1430‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dabrowski R, Kostro K, Lisiecka U, Szczubiał M, Krakowski L. Usefulness of C‐reactive protein, serum amyloid A component, and haptoglobin determinations in bitches with pyometra for monitoring early post‐ovariohysterectomy complications. Theriogenology. 2009;72(4):471‐476. [DOI] [PubMed] [Google Scholar]
- 48. Kanno N, Hayakawa N, Suzuki S, Harada Y, Yogo T, Hara Y. Changes in canine C‐reactive protein levels following orthopaedic surgery: A prospective study. Acta Vet Scand. 2019;61(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boiselle PM, Tocino I, Hooley RJ, et al. Chest radiograph interpretation of pneumocystis carinii pneumonia, bacterial pneumonia, and pulmonary tuberculosis in HIV‐positive patients: accuracy, distinguishing features, and mimics. J Thorac Imag. 1997;12:47‐53. [DOI] [PubMed] [Google Scholar]
- 50. Virkki R, Juven T, Mertsola J, Ruuskanen O. Radiographic follow‐up of pneumonia in children. Pediatr Pulmonol. 2005;40(3):223‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cook S, Greensmith T, Humm K. Successful management of aspiration pneumopathy without antimicrobial agents: 14 dogs (2014‐2021). J Small Anim Pract. 2021;62(1–6):1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Left panel: Box/whisker plots of concentration of C reactive protein (CRP)andserum amyloid A (SAA), at day 14 when having received antimicrobial drug (AMD) treatment 〈 14 days or 〉 14 days. Right panel: Box/whisker plots of concentration of C reactive protein (CRP) and serum amyloid A (SAA), at day 28 when having received antimicrobial drug (AMD) treatment 〈 28 days or 〉 28 days. Central horizontal lines represent the median, boxes represent the 25% to 75% range and whiskers represent the minimum and maximum values. Comparisons between these data were performed using the Friedman test with Dunn's post‐hoc multiple comparisons adjustment.


