Abstract
Tobacco products present a deadly combination of nicotine addiction and carcinogen exposures resulting in millions of cancer deaths per year in the world. A plethora of smokeless tobacco products lead to unacceptable exposures to multiple carcinogens including the tobacco-specific nitrosamine N'-nitrosonornicotine, a likely cause of the commonly occurring oral cavity cancers observed particularly in South-East Asian countries. Cigarettes continue to deliver a large number of carcinogens including tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons, and volatile organic compounds. The multiple carcinogens in cigarette smoke are responsible for the complex mutations observed in critical cancer genes. The exposure of smokeless tobacco users and smokers to carcinogens and toxicants can now be monitored by urinary and DNA adduct biomarkers that may be able to identify those individuals at highest risk for cancer so that effective cancer prevention interventions can be initiated. Regulation of levels of carcinogens, toxicants, and nicotine in tobacco products and evidence-based tobacco control efforts are now recognized as established pathways to preventing tobacco related cancer.
Table of contents summary
This review discusses carcinogens in smokeless tobacco products and cigarette smoke and biomarkers that may be able to identify those individuals at highest risk for tobacco-related cancers. The review also discusses regulation of levels of carcinogens and nicotine in these products as current approaches to cancer prevention
Introduction
This review, updated from the previous one1 will address chemical mechanisms and prevention of frequently fatal human cancers induced by two worldwide addictions - smokeless tobacco use and cigarette smoking. Although there is general awareness that tobacco products cause cancer, there are nevertheless more than one billion users of these products in the world. We will discuss chemical mechanisms by which smokeless tobacco and cigarette smoking cause cancer, focusing on carcinogenic constituents, their DNA adducts and mutational consequences, biomarkers of these processes, and regulatory approaches to prevention including reduction of tobacco-specific nitrosamines in smokeless tobacco and nicotine in cigarettes. These mechanisms proceed in a well- established sequence from repeated carcinogen exposure due to nicotine addiction to metabolically activated carcinogens to formation of DNA adducts and consequent critical mutations in growth control genes resulting in cancer (Figure 1).1,2 We also describe how we can use biomarkers not only to understand the carcinogenic process but also examine their association with cancer risk. And finally, we discuss selected means to prevent and reduce the harm caused by these addictive and deadly products.
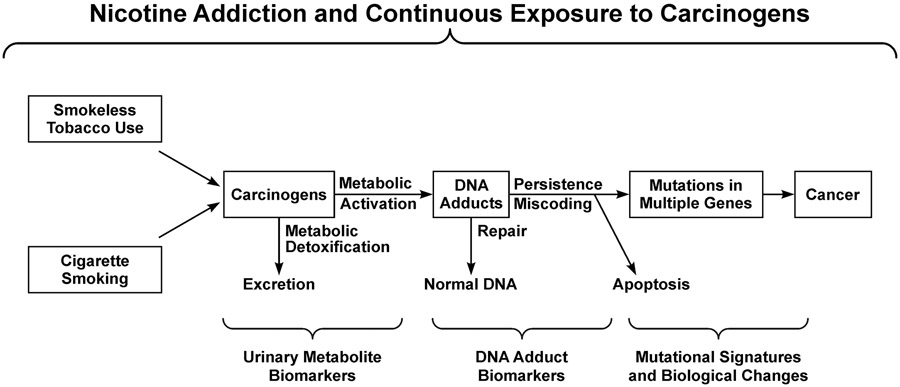
Figure 1 ∣. Overall established scheme relating smokeless tobacco use and cigarette smoking, as driven by nicotine addiction, to cancer.
Long term use of tobacco products results in continuous exposure to carcinogens, formation of DNA adducts, and multiple mutations in critical cancer control genes.1,2 Biomarkers and mutational signatures can elucidate each step.
Massive scope of the problem
Smokeless tobacco
The worldwide prevalence and distribution of smokeless tobacco use is perhaps underappreciated. There are more than 350 million smokeless tobacco users in the world; 67% men, living in 121 countries.3 Nearly 95% live in developing countries; 82.7% in the World Health Organization (WHO) South-East Asia Region, where smokeless tobacco is sometimes the predominant form of tobacco use, exceeding cigarette smoking.3 More than 90% of smokeless tobacco users live in 11 countries: India (237.4 million users), Bangladesh (30.9 million), Myanmar (12.6 million), Pakistan (10.1 million), USA (9.6 million), China (4.1 million), Indonesia (3.2 million), Nepal (2.7 million), Madagascar (2.6 million), Germany and Uzbekistan (2.4 million each).3 Norway, Sweden, Yemen, and parts of Africa are also relatively high prevalence areas.4 In India, the prevalence of current smokeless tobacco use among adults was 25.9% overall, 32.9% among men and 18.4% among women.3
The International Agency for Research on Cancer (IARC) concluded that smokeless tobacco causes cancers of the oral cavity, esophagus, and pancreas in humans;5 the U.S. National Cancer Institute and the American Cancer Society concur with this evaluation.6,7 The evidence supporting these conclusions, from studies in laboratory animals and epidemiologic studies, has been reviewed.5 A systematic review and meta-analysis of epidemiologic studies from South-East Asia demonstrated a combined odds ratio of 4.7 for oral cancer in users of these products.8 A recent review further summarizes this convincing body of research.9 Consistent with the high prevalence of smokeless tobacco use, the incidence of oral cancer in South-East Asia is one of the highest in the world, and it has been estimated that approximately 50% of oral cancer in India is due to smokeless tobacco use, amounting to about 35,000 cancers per year.10 Worldwide, attributable disability adjusted life years (DALYs) for upper aerodigestive tract cancer from smokeless tobacco use amounted to an estimated 1.5 million11 - 2 million,12 mostly from the South-East Asia region.
Extraordinarily diverse types of smokeless tobacco products with varying carcinogenic properties are consumed in the world. Monographs published by the U.S. Centers for Disease Control and Prevention (CDC) and the IARC describe some of the many types of these products.4,13 Broadly, they can be classified as pre-made or custom made. Pre-made products can be further subdivided into two types. The first are made in a manufacturing environment and commercially distributed in sealed packaging, commonly found in the U.S. and other western countries as well as India and other parts of Asia. The second are those handmade in cottage industries and non-traditional environments and having inconsistent types of packaging; these are more common in, for example, the South-East Asia region. Custom made products are produced individually for immediate consumption according to a customer’s preference. Smokeless tobacco products have been divided into 4 categories.4 Category 1 products contain mainly tobacco. Category 2 products have tobacco and alkaline modifiers, which raise the pH facilitating absorption of nicotine. Category 3 contain tobacco, alkaline modifiers, and areca nut, as in the well-known betel quids consumed in South-East Asia. Category 4 contains tobacco and additional substances such as stimulants, flavoring agents, or spices. Examples of products in each category are presented in the CDC monograph.4
Cigarette smoking
Despite the harmful effects of cigarette smoking being nearly universally known, there were 933 million smokers in the world in 2015.14 Over 80% of the world’s smokers live in low and middle income countries.14 It is estimated that about two thirds of lung cancer mortality worldwide is due to smoking.15 There were about 2.2 million new cases of lung cancer and 1.8 million deaths from lung cancer in 2020, accounting for 11.4% diagnosed and 18.0% deaths, respectively, of all cancer sites.15 Lung cancer was the leading cause of cancer-related death worldwide in men and the second leading cause (after breast cancer) in women. It was the leading cause of cancer-related death in men in 93 countries and in women in 25 countries. Convincingly, international variation in lung cancer rates and trends mainly reflect tobacco usage.15 Cigarette smoking is also a cause of cancers of the oral cavity, pharynx, larynx, esophagus, nasal cavity, pancreas, bladder, stomach, liver, kidney, ureter, cervix, colorectum, and ovary (mucinous), as well as of myeloid leukemia.2,5 The devastating effects of cigarette smoking result from the combination of rapid delivery of nicotine to the brain, leading to its highly reinforcing effects and high potential to addict16 along with multiple carcinogens in the smoke that accompany each dose of nicotine.
Collectively, then, these highly addictive tobacco products continue to cause a worldwide epidemic of morbidity and death from cancer. We also note that all smokeless and combustible tobacco products including those not discussed here (various types of smokeless tobacco, cigars, cigarillos, etc.) are established causes of cancer, although the risk may vary depending on product type (combustible or noncombustible) and within categories of tobacco products, particularly with smokeless tobacco. Whether smokeless or combustible, the problem is the tobacco.
While not the focus of this article, bidi smoking and waterpipe use are additional popular international tobacco use practices. Bidis are small hand-rolled tobacco products commonly smoked in South-East Asia where it is likely that there are greater than 50 million users.17 There are millions of waterpipe users in the world. In waterpipe smoking, the tobacco is heated and the smoke is drawn through water which may contain flavoring additives.18 Each of these habits entails exposure to tobacco smoke toxicants and carcinogens.
Carcinogens, DNA adducts and mutations
Tobacco-specific nitrosamines
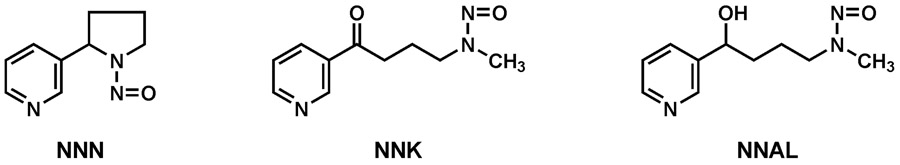
All commercial tobacco products including smokeless tobacco, cigarette smoke, and cigar smoke contain tobacco-specific nitrosamines, which form during the curing and processing of tobacco.19-22 Seven tobacco-specific nitrosamines have been identified in unburned, cured tobacco, but 3 of these – N'-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its major metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) - have received by far the most attention23,24 (Figure 2). The major source of tobacco-specific nitrosamines is cured and processed tobacco, where they are formed by the reaction of tobacco alkaloids with nitrite; there is also evidence for their endogenous formation, particularly by nitrosation of nornicotine.25,26 NNN and NNK are the most carcinogenic of the tobacco-specific nitrosamines; their mean levels in U.S. brands of cigarette tobacco were 1901 ng/g and 523 ng/g tobacco, respectively, while the levels in cigarette mainstream smoke (the smoke taken in by the smoker as estimated by machine smoking) were 189 ng and 122 ng per cigarette, respectively.20 Relative levels of NNN and NNK in cigarette smoke are dependent on the type of tobacco used.27 NNAL, also highly carcinogenic, is present in the urine of all people who use tobacco products, and is also commonly detected in the urine of non-smokers exposed to secondhand tobacco smoke.28 NNN and NNK, which always occur together, are considered carcinogenic to humans by IARC.13 NNN, NNK, and NNAL are typically strong nitrosamine carcinogens, inducing tumors in laboratory animals through a genotoxic mechanism after metabolic activation in specific tissues. Tumor induction is generally independent of the route of administration and occurs in a dose-responsive manner including at low doses relevant to the levels of exposure of people who use tobacco products.13 Some particularly relevant carcinogenicity data for these compounds is presented below.
Figure 2 ∣. Structures of NNN, NNK, and NNAL.
NNN and NNK are carcinogenic tobacco-specific nitrosamines found in all tobacco products. NNAL is the major metabolite of NNK and, like NNK, a potent pulmonary carcinogen. NNAL is found in the urine of all tobacco users.
Tobacco-specific nitrosamines and other carcinogens in smokeless tobacco users
The concentrations of NNN and other tobacco-specific nitrosamines in some smokeless tobacco products widely consumed in South-East Asia are remarkably high.9,29,30 Thus, in khaini products consumed in India, Stanfill et al reported NNN concentrations of 37.7-48.1 μg/g wet weight of tobacco, and Stepanov et al reported levels of 16.8-29.4 μg/g.21,31 Nasrin et al found NNN concentrations as high as 59 μg/g in zarda products, and 25 μg/g in gul, a powdered tobacco snuff product, both consumed in Bangladesh.32 These high levels of NNN (and other tobacco-specific nitrosamines) are in contrast to average amounts of NNN found in 56 Northern European snus products, 0.6 μg/g,33 while amounts in brands sold in the U.S. are typically 1-4 μg/g.34,35 Levels of other tobacco-specific nitrosamines were similarly lower in Northern European and U.S. brands than those in the products from South-East Asia. Thus, concentrations of NNN and other tobacco-specific nitrosamines in smokeless tobacco continue to be a problem in U.S. brands, although generally considerably less than those found in South-East Asia. Because of the ability of NNN to induce tumors of the oral mucosa (described further below), this suggests an association with the high incidence of oral cavity cancer in South-East Asia.
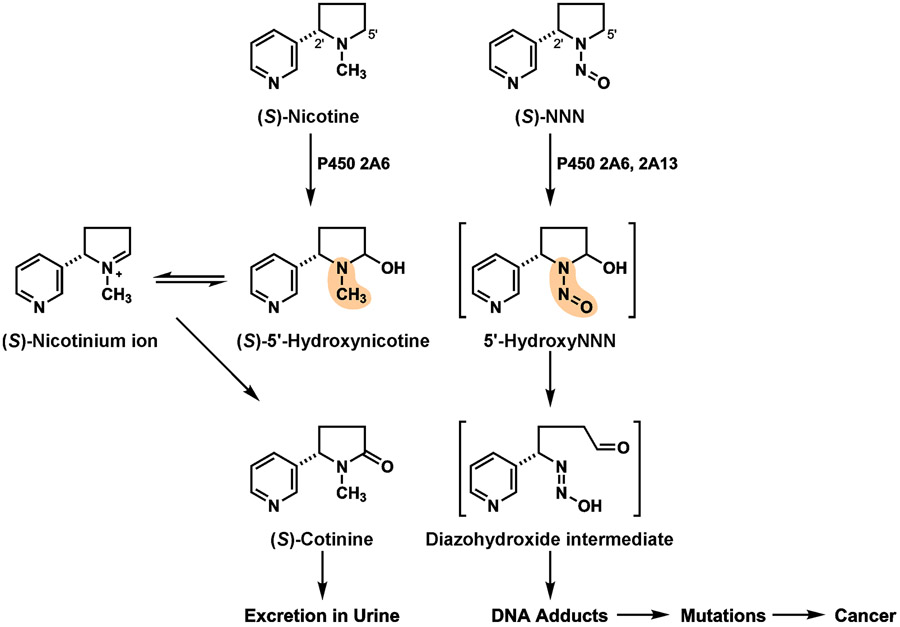
Considerable evidence indicates that NNN in particular is likely to play a critical role as a cause of cancer in smokeless tobacco products, which all have something in common: they contain both nicotine and NNN. Nicotine is the addictive constituent of all tobacco products including smokeless tobacco.36,37 NNN, the main component of smokeless tobacco that induces cancer of the oral cavity and esophagus, is closely related structurally to nicotine.38,39 The only structural difference is that nicotine has an N-methyl group while NNN has an N-nitroso- group (Figure 3). This seemingly minor difference causes a major difference in biological activity. Nicotine binds to nicotinic acetylcholine receptors causing addiction; NNN binds far less readily to these receptors and its concentration in tobacco is more than 1,000 times less than that of nicotine.40 Nicotine is metabolized by cytochrome P450 2A6 (CYP2A6) at its 5'-position to α-hydroxynicotine, which is in equilibrium with the nicotinium ion (Figure 3); these are further oxidized by aldehyde oxidase and CYP2A6 to cotinine, the major metabolite of nicotine.41,42 The nicotinium ion is not reactive with DNA.43 NNN is similarly metabolized at its 5'-position by CYP2A6 and CYP2A13, resulting in the formation of 5'-hydroxyNNN.44 This highly reactive intermediate undergoes ring opening to a diazohydroxide, which readily reacts with DNA, forming adducts that can cause miscoding when DNA is replicated thereby leading to permanent mutations and eventually cancer.45 The DNA adducts formed from diazohydroxide intermediates produced in the metabolism of NNN have been extensively characterized in rats treated with NNN; these adducts result from both 2'-hydroxylation and 5'-hydroxylation of NNN.45,46
Figure 3 ∣. Metabolism of (S)-nicotine and (S)-NNN by 5'-hydroxylation.
Metabolism of (S)-nicotine and (S)-NNN by this pathway are similar, yet lead to drastically different results. The red atoms in the structures are the different functional groups that explain the results. For NNN, after 5'-hydroxylation by CYP2A6 or CYP2A13, the properties of the N-N=O group in 5'-hydroxyNNN cause immediate transformation to the DNA damaging diazohydroxide intermediate illustrated, leading to DNA adducts and cancer. In contrast, the highlighted N-CH3 group of nicotine does not have this effect, but rather is further oxidized to the innocuous compound (S)-cotinine, which is stable and is excreted in urine. (Brackets indicate unstable intermediates).
Nicotine and NNN have chiral carbons at their 2'-position, as indicated in Figure 3. Nicotine in tobacco is >99% (S)-nicotine while (S)-NNN is the predominant enantiomer of NNN, comprising 57-83% of NNN in smokeless tobacco products.47,48 A carcinogenicity study of the NNN enantiomers in F-344 rats demonstrated that (S)-NNN, administered in the drinking water at a dose of 14 ppm for 17 months, is a powerful oral cavity and esophageal carcinogen, causing a total of 89 benign and malignant oral cavity tumors, including tumors of the tongue, oral mucosa, soft palate, epiglottis, and pharynx, in a group of 20 rats; and 122 esophageal tumors in these same rats, while (R)-NNN was less active but enhanced the carcinogenicity of (S)-NNN.39 These results clearly demonstrate the powerful oral carcinogenicity of (S)-NNN in rats, while there is no conclusive evidence that (S)-nicotine is carcinogenic.49 The total dose of (S)-NNN used in this study was less than that calculated for 30 years of human use of a smokeless tobacco product containing 3 μg NNN per gram tobacco.39 (S)-NNN preferentially formed pyridyloxobutyl DNA adducts in the oral mucosa and esophagus of F-344 rats treated identically to those in the carcinogenicity study.46 No other known smokeless tobacco constituent induces tumors in the oral mucosa.5,13
Taken together with the high levels of NNN in multiple types of smokeless tobacco products consumed in South-East Asia as noted above, the collective data strongly suggest that NNN is a cause of oral and esophageal cancer in smokeless tobacco users. But NNN is not the only carcinogen in smokeless tobacco products. As summarized previously and in recent publications, smokeless tobacco products also contain other carcinogens including multiple nitrosamines, certain polycyclic aromatic hydrocarbons (PAHs), formaldehyde, acrolein, and metals such as Cd, but none of these carcinogens induce oral tumors in laboratory animals and are consistently found in these products at levels as high as those of NNN.5,30,50,51 Thus, we conclude that the path to prevention is clear: find ways to prevent the uptake and promote cessation of use of these products in addition to drastically reducing or eliminating NNN from smokeless tobacco products. Approaches to decreasing NNN in smokeless tobacco include plant breeding, agronomics, and tobacco processing and storage.35
Tobacco-specific nitrosamines in cigarette smokers
While the carcinogenic properties of cigarette smoke to the lung and oral cavity (and other tissues) of smokers are undoubtedly collectively due to exposure to multiple carcinogens, co-carcinogens, inflammatory agents and oxidants, there is little doubt that NNK and NNN are very important in this process based on their levels in smoke and carcinogenic activities. All of the properties noted above for NNN are also relevant to cigarette smokers and to cancers of the oral cavity and esophagus in smokers. NNK and NNAL, potent lung carcinogens, are particularly relevant to cigarette smoking and lung cancer. As reviewed previously, the lung is the major target organ of NNK in the rat, hamster, mouse, ferret, and mink.52 NNK readily induces adenocarcinoma of the lung, the major cancer type seen in smokers, independent of the route of administration and at low doses in these laboratory animals. Thus, lung tumors are induced in F-344 rats when NNK is given in the drinking water, by subcutaneous injection, gavage, oral swabbing, or even by intravesicular administration. Lung tumors are always induced preferentially over tumors in other tissues; this is due to efficient metabolic activation of NNK in the lung by cytochrome P450 enzymes, resulting in the formation of methyl- and pyridyloxobutyl-DNA adducts in the lung and consequent mutation of oncogenes. Other effects of NNK such as activation of the α7 nicotinic acetylcholine receptor/extracellular-signal-regulated kinase/contactin 1 pathway and regulation of gene expression through DNA methyltransferase 1-mediated epigenetic modifications have also been described.53,54,55 Extensive dose-response data are available for induction of lung tumors by NNK in rats; the lowest total dose in these studies, only 1.8 mg/kg, administered by subcutaneous injection, induced a significant number of lung tumors; this dose was not dissimilar from the lifetime dose of NNK in a smoker.52,56 One carcinogenicity study carried out more recently deserves mention.57 In this study, NNK and enantiomers of NNAL were administered in the drinking water to F-344 rats at a dose of 5 ppm for 20 months resulting in a total dose of 130 mg/kg body weight. All three compounds as well as racemic NNAL (10 ppm) induced a high incidence of lung tumors, both adenoma and carcinoma; all treated animals had lung lesions while none were observed in the controls. These results again illustrated the powerful pulmonary carcinogenicity of NNK and NNAL, further supporting their role as causes of lung cancer in users of tobacco products.
Multiple studies have examined the mutational properties of DNA adducts induced by NNK and NNAL; these include not only pyridyloxobutyl and pyridylhydroxybutyl adducts, but also methyl DNA adducts, aldehyde DNA adducts, and adducts that generate apurinic sites. As previously noted, the adducts that contribute to the genotoxic effects of NNK and NNAL depend on the context, such as the relative amounts of each DNA alkylating pathway in a given cell, the activity of repair enzymes, and the gene targeted for mutation.54,58 Thus, the relationship between tobacco-specific nitrosamine DNA adducts and cancer in people who use tobacco products is complex and requires further study.
Continuing interest in tobacco-specific nitrosamines
It has been more than 40 years since we first identified tobacco-specific nitrosamines as products of the reaction of nicotine with nitrite and as constituents of tobacco.59-61 There has been sustained research interest in the presence of nitrosamines in tobacco products and the carcinogenic properties of these compounds.62-64 Even the tobacco industry, after their usual initial attempts to discredit this research, joined academic and government scientists to investigate methods to monitor and decrease occurrence of nitrosamines in both tobacco and smoke from cigarettes, and determine their mechanisms of action. Three recently published studies are briefly summarized here. Investigators from the U.S. Food and Drug Administration (FDA) conducted the first comprehensive inhalation study of NNK, comparing exposure to NNK and its metabolism to NNAL in rats treated with NNK by 3 different routes: intraperitoneal injection, oral gavage, or nose only inhalation for 1h using single doses ranging from 5 x 10−5 to 50 mg/kg. NNK was rapidly absorbed and metabolized extensively to NNAL after NNK administration by all routes, but NNK metabolism to NNAL appeared to be more efficient via inhalation than by the other routes. However, NNK significantly increased DNA damage in multiple tissues via all three routes.63 A second study quantified nicotine and tobacco-specific N-nitrosamine levels in 64 snus products made by 10 manufacturers in the United States and Northern Europe. Levels of NNN and NNK were higher in snus sold in the United States (1360 ng/g, standard error = 207) than in Northern Europe (836 ng/g, standard error = 132) likely reflecting the more stringent manufacturing processes used in Northern Europe.33 Another study tested the effects of co-administration of formaldehyde, acetaldehyde, or CO2 by nose only inhalation on lung tumor development in A/J mice treated with NNK by intraperitoneal injection. The mice treated with aldehydes had more adenomas with dysplasia or progression than those given only NNK, while CO2 treatment increased the number of NNK-induced lung adenomas.64 These results demonstrate that gas phase constituents of tobacco smoke can enhance the lung carcinogenicity of NNK. While the mechanisms of enhancement are not clear and require further study, the presence of considerable amounts of these compounds in tobacco smoke (CO2 is 12.5% of smoke) suggest that the effects of cigarette smoke inhalation may be far greater than estimated by consideration of only individual constituent concentrations.
Carcinogens formed by combustion
While carcinogenic tobacco-specific nitrosamines are present in both smokeless tobacco and cigarette smoke, combustion products are generally far more prevalent in cigarette smoke because they are often volatile and are generated by burning tobacco at temperatures as high as 910 - 920 °C. Important among these are the PAHs. Multiple well-established PAH carcinogens are found in tobacco smoke, including benzo[a]pyrene (BaP) (Table 1), benz[a]anthracene, 5-methylchrysene, dibenz[a,h]anthracene, dibenz[a,l]pyrene, indeno[1,2,3-cd]pyrene, benzofluoranthenes and others.65,66 BaP is considered carcinogenic to humans by IARC.67 Additionally, more than 500 other PAHs have been identified or partially identified in cigarette smoke.68 Other combustion products found in cigarette smoke include aldehydes such as formaldehyde, acetaldehyde, and acrolein; heterocycles such as furan; volatile nitrosamines such as dimethylnitrosamine; aromatic amines such as 4-aminobiphenyl and 2-naphthylamine; heterocyclic aromatic amines such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP); and volatile hydrocarbons such as 1,3-butadiene and benzene.69 Thus, most carcinogens in cigarette smoke are combustion products. PAHs are perhaps the most extensively studied of all tobacco smoke carcinogens and considerable data, reviewed previously, indicate that they are among the most important causes of cancer in cigarette smokers.1,5,16,67
Table 1 ∣.
Structures of some compounds mentioned in the text.
| Compound | Structure | Function |
|---|---|---|
| Benzo[a]pyrene (BaP) |
|
Highly carcinogenic polycyclic aromatic hydrocarbon found in all combustion products including tobacco smoke |
| 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB) |
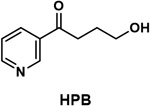
|
Tobacco-specific compound which can be released from DNA or hemoglobin of people who use tobacco products and has been used as a monitor of tobacco-specific DNA damage |
| BaP diol epoxide-N2-deoxyguanosine (BPDE-N2-dG) |
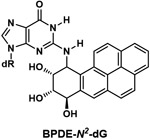
|
Major DNA adduct formed by metabolic activation of BaP via its 7,8-diol-9,10-epoxide |
| Guanine N7 adduct of 3,4-epoxy-1-butene (EB-GII) |
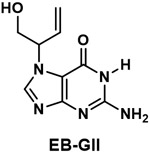
|
DNA adduct formed from the carcinogen 1,3-butadiene |
| Major DNA adduct of acrolein (ɣ-OHPdG) |
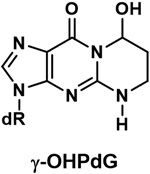
|
DNA adduct formed from acrolein |
| (Z)-7-[1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]cyclopentyl]hept-5-enoic acid (8-iso-PGF2α) |
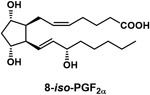
|
Biomarker of oxidative damage found in the urine and blood of all humans |
| Phenanthrene tetraol (PheT) |
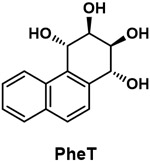
|
Metabolite of the polycyclic aromatic hydrocarbon phenanthrene (Phe) formed by the diol epoxide metabolism pathway and excreted in the urine. It has been used as a biomarker of metabolic activation of polycyclic aromatic hydrocarbons. |
DNA adducts from cigarette smoking
DNA adduct formation is the critical step in mutagenesis induced by tobacco (Figure 1). There is no doubt that DNA adducts resulting from exposure to the complex mixture of genotoxic compounds in tobacco products are central in the carcinogenic process.70-72 When DNA repair systems in the cell are inefficient or error prone in their response to DNA adducts, the adducts can persist, causing mutations when adducted bases are misread, and the wrong base is inserted by DNA polymerases. The result is a permanent mutation, which may occur in critical genes involved in growth control, ultimately leading to cancer. Thus, DNA adducts form the requisite link between exposure to carcinogens or their metabolically formed electrophiles and mutations in critical genes, although the relationship between mutations and specific DNA adducts is complex.73 Multiple studies have reported DNA adduct levels in cells and tissues of smokers compared to non-smokers, and most of these studies demonstrate significantly higher levels of DNA adducts in smokers, but some of the methods used such as immunoassays and 32P-postlabelling are only semi-quantitative or have not identified, or misidentified, the relevant carcinogens. Difficulties in obtaining adequate amounts of relevant tissue DNA and questions of timing of adduct formation and persistence are additional challenges in most DNA adduct studies, which continue to be relatively small. Comprehensive reviews of these studies are available.74-77 We focus here on selected recent studies that have used validated mass spectrometric methods for DNA adduct analysis; this topic has also been reviewed recently.55
Tobacco-specific DNA adducts.
Metabolism by α-hydroxylation at the 2'-position of NNN or the methyl group of NNK produces a reactive intermediate that pyridyloxobutylates DNA. When this DNA is treated with acid, 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB) (Table 1) is released and can be quantified by mass spectrometry. In this study, a highly sensitive high resolution mass spectrometric method for analysis of HPB released from DNA was applied it to oral cell samples obtained from 65 current daily cigarette smokers, 30 with head and neck squamous cell carcinoma (HNSCC)and 35 cancer free controls.78 Significantly higher levels of HPB were released from the DNA of smokers with HNSCC compared to those who were cancer free. These intriguing results require confirmation in larger studies and could lead to a test for early detection and/or prevention of these disfiguring and often fatal cancers.
BaP-DNA adducts.
The major DNA adduct of BaP is BaP diol epoxide-N2-deoxyguanosine (BPDE-N2-dG) (Table 1), but reliable detection and quantitation of this intact adduct in human tissues was lacking until recently. We developed a liquid chromatography-nanoelectrospray-tandem high resolution mass spectrometry method for its analysis in human lung tissue.79 The detection limit was 1 adduct per 1011 nucleotides (corresponding to about 1 amol of the adduct). Application of this method to human lung tissue DNA from 29 individuals resulted in an average level of 3.1 adducts per 1011 nucleotides in smokers, 3 times higher than in non-smokers. While this study identified the presence of the intensively studied BPDE-N2-dG as the intact adduct in pulmonary DNA of smokers, further studies with larger sample sizes are required. In addition, as mentioned above, BaP is only one of multiple carcinogenic PAHs in cigarette smoke, so quantitation of total PAH-DNA adducts in DNA from smokers is an important goal.
1,3-Butadiene-DNA adducts.
1,3-Butadiene is a simple volatile hydrocarbon, which is considered carcinogenic to humans by IARC, and is one of the most abundant carcinogens in cigarette smoke. A metabolite of 1,3-butadiene, 3.4-epoxy-1-butene (EB), reacts with DNA to give adducts, among which is a guanine N7 adduct named EB-GII (Table 1). This adduct is removed from DNA either by spontaneous depurination or repair and excreted in urine. It has been quantified in cigarette smokers as a biomarker of 1,3-butadiene exposure and metabolism; its levels decrease upon smoking cessation.80
Acrolein-DNA adducts.
Acrolein is an abundant and highly toxic compound in cigarette smoke; it is considered probably carcinogenic to humans by IARC. Multiple studies show that acrolein reacts with DNA to form exocyclic 1,N2-deoxyguanosine adducts, detected in various human tissues.81,82 Oral cells are a target for DNA adduct formation in cigarette smokers, since the oral cavity is the first site of exposure.77 In our recent study, average levels of the exocyclic adduct (8R/S)-3-(2'-deoxyribos-1,-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)-one (ɣ-OHPdG) (Table 1) were 27 times higher in oral cells of smokers than non-smokers.83 However, no difference was seen in the levels of these adducts in lung tissue or leukocytes from smokers vs. non-smokers,84,85 and further studies are required to investigate these differences.
Mutational signatures and mutated genes
The majority of studies on mutational signatures potentially traceable to specific DNA adducts and carcinogen exposures have been performed using samples from smokers. In one study, somatic mutations were measured and compared in over 5000 tumor samples from cigarette smokers and non-smokers.86 Five mutational signatures were increased in smokers compared to non-smokers. Important among these was the mutational signature assigned to BaP (and likely other PAHs) exposure, called SBS4 in the Catalogue Of Somatic Mutations In Cancer (COSMIC) database (https://cancer.sanger.ac.uk/cosmic), which was observed particularly in lung and larynx cancers. These results are consistent with the massive amount of data implicating PAHs as critically important carcinogenic agents in tobacco smoke, as noted above. Overall, however, the generation of mutations from cigarette smoking as measured in this study was complex and not fully understood, likely reflecting the complexity of cigarette smoke and the contribution and interactions of multiple genotoxic and carcinogenic agents, and it is still not clear which other constituents contribute to the observed changes.86 A further study sequenced whole genomes derived from normal bronchial epithelial cells in smokers and non-smokers.87 Smoking added thousands of mutations per cell including driver mutations and the signature assigned to BaP exposure. Significant within-person variation in mutational burden was observed, also reflecting the complexity of carcinogen exposure from tobacco smoke.87 One recent study examined exome sequences and copy number profiles in 660 lung adenocarcinoma and 484 lung squamous cell carcinoma (SCC) tumor and normal pairs; 38 genes were significantly mutated in adenocarcinoma and 20 in SCC.88 Multiple mutated genes were discovered, with distinct mutational patterns in adenocarcinoma and SCC. Among the multiple significantly mutated genes in adenocarcinoma were KRAS, KEAP1, EGFR, STK11, SMARCA4, RBM10, TP53, NF1 and RB1 while in SCC they were TP53, RB1, CDKN2A, NFE2L2, PTEN, and MLL2. These results are completely consistent with the many carcinogens in tobacco smoke, most of which form multiple different types of DNA adducts.
Fewer studies have characterized changes in samples originating from smokeless tobacco users. While a comprehensive genomic characterization of HNSCCs has been published, it is not specifically focused on smokeless tobacco users.89 The India Project Team of the International Cancer Genome Consortium reported on the mutational landscape of gingivo-buccal oral SCC,90 prevalent in regions such as India where tobacco chewing is common. Significantly and frequently altered genes specific to this cancer type included USP9X, MLL4, ARID3, UNC13C and TRPM3 while others were shared with HNSCCs, including TP53, FAT1, CASP8, HRAS, and NOTCH1. A high proportion of G-T transversions was observed. Additional smokeless tobacco-associated genetic alterations were identified in cell lines established from gingivo-buccal oral SCC; these included PCLO, FAT3 and SYNE2 as well as oncogenic PIK3CA mutations.90 A further study of human papillomavirus (HPV) negative early stage tongue cancer patients who were habitual chewers of betel nuts, areca nuts, lime or tobacco revealed common G-T transversions and mutations in TP53, NOTCH1, CDKN2A, HRAS, USP6, PIK3CA, CASP8, FAT1, APC, and JAK1 among others.91 Some of the same mutational spectra as well as others were identified by exome sequencing of oral SCC in users of shammah, an Arabian smokeless tobacco preparation.92 The relationship of these changes to DNA adduct formation by 5'-hydroxylation of NNN, the major metabolism pathway observed in human enzyme systems, is unclear.45 DNA adducts formed by this pathway have been characterized (Figure 3), but their mutational consequences are unknown.45,93,94
Urinary metabolite biomarkers
Biomarkers of exposure can potentially identify those cigarette smokers at highest risk for cancer. These individuals can be targeted for intensive smoking cessation interventions and lung cancer screening. The quantitation of urinary metabolites of cigarette smoke constituents as biomarkers of exposure, and in some cases, biomarkers of cancer risk, has matured in recent years mainly due to advances in high throughput liquid chromatography-mass spectrometry techniques, allowing relatively large studies with thousands of urine samples to be performed. These are significant advances permitting tobacco exposure science to proceed from the earlier approaches, which depended mainly on machine measurement of smoke constituents. We will present some recent studies as examples of the application of several urinary biomarkers: total nicotine equivalents (TNE), NNAL, phenanthrene metabolites, and (Z)-7-[1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]cyclopentyl]hept-5-enoic acid (8-iso-PGF2α), which is a biomarker of oxidative damage (Table 1).
TNE
Urinary levels of nicotine and several of its metabolites comprise the TNE biomarker. Various combinations have been used but one recent study is illustrative.95 The combination of the molar quantities of nicotine, cotinine, 3'-hydroxycotinine and their glucuronide conjugates plus nicotine-N-oxide, cotinine-N-oxide, nornicotine and norcotinine in urine accounts for more than 90% of the nicotine dose and is therefore not substantially affected by metabolic differences between individuals. Comparison of this panel to the total urinary molar levels of nicotine, cotinine, and 3'-hydroxycotinine and their glucuronides demonstrated a strong correlation; thus omission of the other technically more demanding minor urinary metabolite assays did not affect the overall results. TNE have been quantified in smokers from 5 ethnic groups with differing risks for lung cancer in the Multiethnic Cohort Study: African Americans, Native Hawaiians, White Americans, Latino Americans, and Japanese Americans.96 The highest levels of TNE were found in African Americans, with intermediate levels in White Americans, and the lowest in Japanese Americans, consistent with their lung cancer risk, but the amounts in Native Hawaiians and Latino Americans did not clearly relate to lung cancer risk. Further studies demonstrated that low activity forms of CYP2A6, the principal nicotine metabolizing enzyme, accounted for the lower levels of TNE in Japanese Americans because more unchanged nicotine remained in the body, thus alleviating the need for more intense smoking.97 The reduced risk of lung cancer in smokers with lower CYP2A6 activity was explained by lower consumption of cigarettes, less intense smoking and reduced CYP2A6-catalyzed activation of NNK98 while greater CYP2A6 activity causes smokers to smoke more and therefore have a higher risk for lung cancer.99
NNAL plus its glucuronides (total NNAL)
NNAL, a major and highly carcinogenic metabolite of NNK, is found in free form and as its glucuronide in the urine of all smokeless tobacco users and cigarette smokers. Total NNAL levels in the Population Assessment of Tobacco and Health (PATH) study, a U.S.-representative longitudinal cohort study of approximately 46,000 adults and youth, age 12 and older, were highest in the urine of approximately 11,000 every day users of smokeless tobacco (993 ng/g creatinine), followed by cigarette smokers (285 ng/g creatinine), and e-cigarette users (6.3 ng/g creatinine); these amounts compared to 1.0 ng/g creatinine in non-users, consistent with the tobacco-specificity of NNK and its relatively high levels in smokeless tobacco.100 (Creatinine is a waste product from normal metabolism that is commonly used as a denominator in biomarker studies.) Similar results were obtained in the U.S. National Health and Nutrition Examination Survey (NHANES) study, based on a different nationally representative sample of the U.S. population. The urinary level of total NNAL in smokeless tobacco users averaged 583 ng/g creatinine while in exclusive cigarette smokers it was 218 ng/g creatinine.101 Levels of total NNAL in urine often correlate with amounts of TNE.100 As in the analysis of TNE in the Multiethnic Cohort Study, urinary levels of total NNAL in 2252 smokers were consistent with lung cancer risk in African Americans (highest), Whites (intermediate), and Japanese Americans (lowest), but not in Native Hawaiians and Latinos.102
PAHs
Urinary phenanthrene metabolites, 1-hydroxypyrene, and related PAHs have been monitored in the NHANES study as biomarkers of PAH exposure. Consistently, levels of these metabolites are significantly higher in smokers than in non-smokers, and cigarette smoking remains one of the major sources of human exposure to PAHs.103 Urinary phenanthrene tetraol (PheT, Table 1) is an excellent biomarker of PAH exposure and of metabolic activation of PAHs by the well-established diol epoxide pathway that results in DNA adduct formation by multiple PAH carcinogens including BaP.104 The metabolism of phenanthrene to PheT mimics that of BaP to its ultimate carcinogen, but phenanthrene metabolites are more readily measured in urine because their concentrations are about 1000 times higher than those of BaP metabolites.105,106 We took advantage of this strategy to determine whether the activation of the aryl hydrocarbon receptor (AHR) and resulting induction of cytochrome P450 enzymes by cigarette smoking, which has been known for more than 50 years,107 resulted in the increased metabolic activation or detoxification of this representative PAH, as measured by the amounts of deuterated PheT and phenanthrene phenols in the urine of 170 smokers and 57 non-smokers given defined doses of deuterated phenanthrene, thus avoiding possible complications due to exposure to phenanthrene in the environment.108 This clearly demonstrated that cigarette smoking increases the metabolic activation of phenanthrene via the diol epoxide pathway, consistent with the conclusion that cigarette smoking enhances the metabolic activation and carcinogenicity of PAHs via activation of the AHR and induction of the cytochrome P450s CYP1A1, CYP1A2, and CYP1B1.
Urinary biomarkers and cancer risk
Collaborative studies with Professor Jian-Min Yuan, who leads the Shanghai Cohort Study and the Singapore Chinese Health Study, both of which are prospective molecular epidemiology studies, have resulted in important findings relating urinary biomarkers and CYP2A6 genetic polymorphisms to lung cancer risk in cigarette smokers.98,109,110 The Shanghai Cohort Study collected a single urine sample and followed more than 18,000 men since 1986-1989 and the Singapore Chinese Health Study obtained a single urine sample from more than 63,000 Chinese men and women followed since 1993-1998. These single void urine samples were stored frozen until biomarker analysis in the laboratory, beginning in 2007. The relationships of the urinary biomarkers to lung cancer risk were determined in subjects, all cigarette smokers. The results were adjusted for smoking intensity and duration. In the Shanghai Cohort Study, the smoking-adjusted odds ratio of lung cancer for the highest vs. lowest quartile of TNE was 4.71, while for total NNAL it was 3.15, both significant. Similar results were obtained in the Singapore Chinese Health Study. In both studies, lower nicotine metabolism [as determined by CYP2A6 phenotype (3'-hydroxycotinine: total cotinine ratio) and genotype] was also significantly associated with a reduced risk of lung cancer.98,109 Further studies demonstrated significant relationships of urinary PheT to lung cancer risk and urinary NNN to esophageal cancer risk;110 however urinary mercapturic acid metabolites of volatile organic compounds such as acrolein, benzene and 1,3-butadiene, were not related to lung cancer risk in smokers, after correction for urinary cotinine and years of smoking.111 Thus, selected urinary biomarkers and importantly, CYP2A6 genotype, as shown in the Transdisciplinary Research in Cancer of the Lung (TRICL) consortium,99 have the potential to identify smokers at high risk for cancer, a critical element in cancer prevention.
While these biomarkers relate directly to carcinogen exposure, 8-iso-PGF2α is an accepted urinary biomarker of oxidative damage, likely involved in co-carcinogenesis and tumor promotion by cigarette smoke. 8-iso-PGF2α was also significantly associated with lung cancer risk among smokers and former smokers, but not non-smokers, in the Shanghai Cohort Study.112 Overall, these relationships of urinary biomarkers of carcinogen exposure and oxidative damage recapitulate in smokers the known effects of cigarette smoke constituents observed in experimental studies with laboratory animals.39,52,113,114
In summary, these biomarker studies in cigarette smokers are consistent with the hypothesis that NNK and PAHs are causative agents for lung cancer, that NNN is a causative agent for esophageal cancer, and that oxidative damage as represented by 8-iso-PGF2α is important in human lung carcinogenesis. The studies also demonstrate that TNE and CYP2A6 polymorphisms are potentially important biomarkers of cancer risk. We envision that a combination of biomarkers could be incorporated into a predictive algorithm for cancer risk to improve lung cancer screening and hopefully prevent fatal lung cancers.
Regulatory approaches
We recognize that there are multiple critically important approaches to prevention of cancer due to tobacco use, including public health strategies (e.g., taxation), recognition and treatment of preneoplastic lesions of the oral cavity, low dose CT lung cancer screening, and others.115-117 Each of these could be the basis of a separate and extensive review. In this review, we focus on examples of the approaches that we have explored in our interdisciplinary research, mainly involving regulatory strategies related to reducing harmful constituents in tobacco products (Articles 9 and 10 of the WHO Framework Convention on Tobacco Control).118
Regulating smokeless tobacco
One approach to reducing cancer risk is to establish standards for the levels of harmful constituents in smokeless tobacco.119 This approach has been implemented by Swedish Match, a tobacco company that manufactures snus, and is referred to as the GOTHIATEK standard.120 This company regulates their smokeless tobacco products to meet certain standards such as 0.95 μg/g NNN + NNK, which is a likely reason that there is a lower risk of oral and esophageal cancer from smokeless tobacco use in Sweden compared South-East Asia.121,122 But few smokeless tobacco manufacturers have established limits on harmful constituents in their products. In 2009, the WHO Study Group on Tobacco Product Regulations recommended, under Article 9 of the WHO Framework Convention on Tobacco Control, that industry manufactured smokeless tobacco products should not exceed 2 μg/g of dry tobacco weight for NNN plus NNK and 5 ng/g for BaP, and that levels of arsenic, cadmium and lead in tobacco should be monitored by regulatory authorities.123 In 2017, the U. S. FDA issued an Advanced Notice of Proposed Rulemaking (ANPRM) proposing a limit of NNN of 1.0 μg/g of dry tobacco weight.124 The FDA deemed that there was insufficient evidence linking NNK to cancer in smokeless tobacco users and thereby focused the product standards on NNN (although reduction in NNN is likely to also lead to a reduction in NNK). Additionally, because NNN levels could increase over time in smokeless tobacco products, the rule would also require an expiration date on each batch of smokeless tobacco products and the manufacturers would have to show that the NNN levels were sustained below the allowable limit through the expiration date. Furthermore, instructions for storage would also be provided on the labelling if storage conditions, which are known to influence NNN levels,124 impacted NNN levels in a given product. It is important to recognize that implementation of product standards does not indicate that the product is safe and tobacco companies should not be allowed to directly or indirectly promote or market their products in this manner, or declare that the product has been approved by the government.123
The NNN product standard is expected to lead to a reduced incidence of oral cancer,124 but unfortunately there have been few countries that have imposed any standards. Furthermore, a product standard may be very difficult to implement in some countries such as those in South-East Asia, where the rate of smokeless tobacco use is high and there are a plethora of different smokeless tobacco products, some which are handmade by consumers or street vendors. Potentially, educating consumers and vendors about the varying amounts of carcinogens in smokeless tobacco and factors that might contribute to the formation of carcinogens such as tobacco type, processing, and storage might be a first step, as reviewed by the WHO.125,126
Other means to reduce the prevalence of smokeless tobacco use include restricting sales to minors, requiring prominent health warning labels, restricting or banning advertising, promotion or sponsorship of smokeless tobacco products, taxation and pricing policies, providing public education and promoting or provision of evidence based smokeless tobacco cessation treatments (for extensive reviews, see references 125 and 4).
Regulating cigarettes
Reducing nicotine in cigarettes to minimally addictive levels would likely lead to a reduction in prevalence of smoking by significantly dampening the trajectory toward cigarette dependence among naïve tobacco users and facilitating the cessation of cigarette use among smokers127,128 A substantial body of scientific literature demonstrates that if nicotine in cigarettes is reduced by about 95%, then reduction in number of cigarettes smoked, cigarette dependence and exposure to carcinogens and toxicants129-131 are observed along with an increase in quit attempts131 or smoking cessation.129 Reductions in smoking behaviors and dependence have been observed among young adults,132 smokers of low socioeconomic status133 or who have been diagnosed with psychiatric disorders133,134 or have a history of substance use135,136 and among non-daily smokers137,138 (see the WHO Advisory Note139 and Technical Report140 for comprehensive reviews of the literature). It has been estimated that in the United States alone, adoption of this approach would reduce the prevalence of smoking to 1.4% and avert cigarette-caused death by 8.5 million by the year 2100.141 This growing body of evidence led the U.S. FDA in March of 2018 to release an ANPRM on reducing nicotine in cigarettes (and potentially other combusted products) to minimally addictive or non-addictive levels.142 This ANPRM aligns with policy as described in the article entitled “A Nicotine-Focused Framework for Public Health.”128 written by a former FDA Commissioner and the Director for the FDA Center for Tobacco Products, which states that, “The public health benefits of implementing a nicotine reduction policy for combustible cigarettes could be enormous” because “nicotine is … responsible for getting smokers addicted to cigarette smoking … and keeping them addicted long-term.” It also concludes that “the availability of potentially less harmful tobacco products could reduce risk while delivering satisfying levels of nicotine for adults who still need or want it.” While the availability of these ‘harm reduction’ products (e.g., electronic cigarettes) has been controversial because of their uptake among youth, they may have a significant role in reducing cigarette smoking among smokers and warrant further consideration for their role in improving public health.143 In 2021, the New Zealand government announced requested public consultation on dramatically reducing nicotine in cigarettes in an effort to achieve Smokefree Aotearoa New Zealand by 2025.144 It is important to note that not all countries, particularly low- and middle-income countries, have adequate resources and infrastructure to implement a nicotine product standard for cigarettes and other combusted tobacco products.139,140,145 Other means for reducing tobacco harm have included gradually reducing toxicants and carcinogens in cigarettes,146 removing cigarette filter ventilation,147 banning flavors in cigarettes including menthol,148-150 but the greatest public health benefit is likely to occur through both taking away the addictiveness of cigarettes and implementing other evidence-based tobacco control efforts, such as those provided in MPOWER (https://www.who.int/initiatives/mpower). MPOWER is a resource developed by the WHO Tobacco Free Initiative as part of the WHO Framework Convention on Tobacco Control. MPOWER stands for: Monitor tobacco use and prevention policies, Protect people from tobacco smoke; Offer help to quit tobacco use; Warn about the dangers of tobacco; Enforce bans on tobacco advertising, promotion and sponsorship; Raise taxes on tobacco. A comprehensive approach to tobacco control should prevent the loss of millions of lives.
Conclusions
The morbidity and mortality caused by tobacco products continues to be unacceptably high. As summarized here, we now understand the chemical mechanisms involved in sufficient detail to design preventive approaches including reduction of exposure to key carcinogens and toxicants including nicotine through product modification and regulation, and identification of susceptible individuals who can be targeted for cessation and early detection of cancer. A great deal of progress has been made, particularly with respect to the prevalence of cigarette smoking, but we need to persist in order to counteract nicotine addiction and ensure that substantially less harmful products fully replace the current dangerous ones.
Acknowledgements
The authors' research is supported by National Cancer Institute grants P01 CA-138338 (SSH), R01 CA-081301 (SSH) and P01 CA-217806 (DKH) and by National Institute on Drug Abuse grant U54 DA-031659 (DKH). Studies reported here were accomplished by an outstanding team of researchers. We thank all team members for their contributions.
Glossary
- DNA adducts
Compounds formed by the reaction of DNA bases with certain electrophilic intermediates generated during metabolism, or with inherently reactive substances
- odds ratio
A statistic that explains the association between two events
- areca nut
The seed of the Areca palm which grows in Southeast Asia and is a common constituent of a quid known as paan which is chewed by millions of people in the region
- Canadian Intense conditions
A machine cigarette smoking regime that prescribes a puff volume of 55 mL, duration of 2 sec, two puffs per min frequency, a bell-shaped puff profile, and the additional requirement that ventilation holes in the cigarettes are blocked
- Agronomics
The branch of economics dealing with the distribution, management, and productivity of land
- apurinic sites
Sites in DNA lacking the usual guanine or adenine bases. The sites can be formed when certain relatively unstable DNA adducts such as 7-methyldeoxyguanosine lose their purine base (in this case 7-methylguanine) due to spontaneous decomposition or hydrolysis
- Advanced Notice of Proposed Rulemaking
A document that an agency such as the U.S. FDA may choose to issue before it is ready to issue a Notice of Proposed Rulemaking (NPRM)
- cigarette filter ventilation
The practice of incorporating holes near the cigarette filter to allow air to mix with the smoke stream leading to overall lower constituent concentrations. It is a defective design because the holes may be blocked during smoking but not during machine measurement of smoke constituents.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1. Hecht SS Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nat. Rev. Cancer 3, 733–744 (2003). This was the forerunner to the present article; although there are similarities, the field has advanced considerably
- 2. U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress. A Report of the Surgeon General. (U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014). Summarizes the health effects of smoking based on 50 years of research.
- 3.Sinha D, Agarwal N & Gupta P Prevalence of smokeless tobacco use and number of users in 121 countries. Br. J. Med. Med. Res 9, 1–20, doi: 10.9734/BJMMR/2015/16285 (2015). [DOI] [Google Scholar]
- 4. National Cancer Institute and Centers for Disease Control and Prevention. Smokeless Tobacco and Public Health: A Global Perspective. NIH Publication No. 14-7983. (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Institutes of Health, National Cancer Institute, 2014). Summarizes information on worldwide smokeless tobacco products and their health effects.
- 5.International Agency for Research on Cancer. Personal Habits and Indoor Combustions. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100E. (IARC, 2012). [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Smokeless Tobacco and Cancer <https://www.cancer.gov/about-cancer/causes-prevention/risk/tobacco/smokeless-fact-sheet> (2010).
- 7.American Cancer Society. Health Risks of Smokeless Tobacco, <https://www.cancer.org/healthy/stay-away-from-tobacco/health-risks-of-tobacco/smokeless-tobacco.html> (2020). [PubMed]
- 8.Khan Z, Tonnies J & Muller S Smokeless tobacco and oral cancer in South Asia: A systematic review with meta-analysis. J. Cancer Epidemiol 2014, 394696, doi: 10.1155/2014/394696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warnakulasuriya S & Straif K Carcinogenicity of smokeless tobacco: Evidence from studies in humans & experimental animals. Indian J. Med. Res 148, 681–686, doi: 10.4103/ijmr.IJMR_149_18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warnakulasuriya S Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 45, 309–316, doi: 10.1016/j.oraloncology.2008.06.002 (2009). [DOI] [PubMed] [Google Scholar]
- 11. Sinha DN et al. Global burden of all-cause and cause-specific mortality due to smokeless tobacco use: Systematic review and meta-analysis. Tob. Control 27, 35–42, doi: 10.1136/tobaccocontrol-2016-053302 (2018). Systematic review and meta analysis of studies investigating the association between smokeless tobacco use and all cause mortality
- 12.Siddiqi K et al. Global burden of disease due to smokeless tobacco consumption in adults: An updated analysis of data from 127 countries. BMC Med. 18, 222, doi: 10.1186/s12916-020-01677-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer. Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 89, pp 41–583 (IARC, 2007). [PMC free article] [PubMed] [Google Scholar]
- 14. GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389, 1885–1906, doi: 10.1016/S0140-6736(17)30819-X (2017). Provides critical data on smoking and disease burden worldwide
- 15.Sung H et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin, doi: 10.3322/caac.21660 (2021). [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General., (Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010). [PubMed] [Google Scholar]
- 17.Duong M et al. Effects of bidi smoking on all-cause mortality and cardiorespiratory outcomes in men from south Asia: an observational community-based substudy of the Prospective Urban Rural Epidemiology Study (PURE). Lancet Glob Health 5, e168–e176, doi: 10.1016/S2214-109X(17)30004-9 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Bhatnagar A et al. Water Pipe (Hookah) Smoking and Cardiovascular Disease Risk: A Scientific Statement From the American Heart Association. Circulation 139, e917–e936, doi: 10.1161/CIR.0000000000000671 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawler TS, Stanfill SB, Zhang L, Ashley DL & Watson CH Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food Chem. Toxicol 57, 380–386 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards SH et al. Tobacco-specific nitrosamines in the tobacco and mainstream smoke of U.S. commercial cigarettes. Chem. Res. Toxicol 30, 540–551, doi: 10.1021/acs.chemrestox.6b00268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanfill SB et al. Chemical characterization of smokeless tobacco products from South Asia: Nicotine, unprotonated nicotine, tobacco-specific N'-Nitrosamines, and flavor compounds. Food Chem. Toxicol 118, 626–634, doi: 10.1016/j.fct.2018.05.004 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Edwards SH et al. Tobacco-specific nitrosamines in the tobacco and mainstream smoke of commercial little cigars. Chem. Res. Toxicol 34, 1034–1045, doi: 10.1021/acs.chemrestox.0c00367 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Hecht SS & Hoffmann D Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 9, 875–884 (1988). [DOI] [PubMed] [Google Scholar]
- 24.Hecht SS, Stepanov I & Carmella SG Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res 49, 106–114, doi: 10.1021/acs.accounts.5b00472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stepanov I et al. Evidence for endogenous formation of N'-nitrosonornicotine in some long term nicotine patch users. Nicotine Tob. Res 11, 99–105 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knezevich A, Muzic J, Hatsukami DK, Hecht SS & Stepanov I Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N'-nitrosonornicotine in humans. Nicotine Tob. Res 15, 591–595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding YS et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol. Biomarkers Prev 17, 3366–3371, doi: 10.1158/1055-9965.EPI-08-0320 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res 22, 1086–1097, doi: 10.1093/ntr/ntz132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta AK, Tulsyan S, Bharadwaj M & Mehrotra R Grass roots approach to control levels of carcinogenic nitrosamines, NNN and NNK in smokeless tobacco products. Food Chem. Toxicol 124, 359–366, doi: 10.1016/j.fct.2018.12.011 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Kumar A et al. Regulation of toxic contents of smokeless tobacco products. Indian J. Med. Res 148, 14–24, doi: 10.4103/ijmr.IJMR_2025_17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepanov I et al. High levels of tobacco-specific N-nitrosamines and nicotine in Chaini Khaini, a product marketed as snus. Tob. Control 24, e271–274, doi: 10.1136/tobaccocontrol-2014-051744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasrin S, Chen G, Watson CJW & Lazarus P Comparison of tobacco-specific nitrosamine levels in smokeless tobacco products: High levels in products from Bangladesh. PLoS One 15, e0233111, doi: 10.1371/journal.pone.0233111 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawler TS et al. Chemical analysis of snus products from the United States and northern Europe. PLoS One 15, e0227837, doi: 10.1371/journal.pone.0227837 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatsukami DK et al. Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prev. Res 8, 20–26, doi: 10.1158/1940-6207.CAPR-14-0250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldham MJ et al. Variability of TSNA in U.S. tobacco and moist smokeless tobacco products. Toxicol. Rep 7, 752–758, doi: 10.1016/j.toxrep.2020.05.008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Surgeon General. The Health Consequences of Smoking: Nicotine Addiction. DHHS publication (CDC) 88-8406. (U.S. Dept. of Health and Human Services, U.S. Government Printing Office, 1988). [Google Scholar]
- 37.Hatsukami D, Stead LF & Gupta PC Tobacco addiction. Lancet 371, 2027–2038 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann D, Raineri R, Hecht SS, Maronpot R & Wynder EL Effects of N'-nitrosonornicotine and N'-nitrosoanabasine in rats. J. Natl. Cancer Inst 55, 977–981 (1975). [DOI] [PubMed] [Google Scholar]
- 39. Balbo S et al. (S)-N'-Nitrosonornicotine, a constituent of smokeless tobacco, is a powerful oral cavity carcinogen in rats. Carcinogenesis 34, 2178–2183 (2013). Demonstration of the oral carcinogenesis of (S)-N'-nitrosonornicotine in rats
- 40.Schuller HM Nitrosamines as nicotinic receptor ligands. Life Sci. 80, 2274–2280 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hukkanen J, Jacob P III & Benowitz NL Metabolism and disposition kinetics of nicotine. Pharmacol. Rev 57, 79–115 (2005). [DOI] [PubMed] [Google Scholar]
- 42. Murphy SE Biochemistry of nicotine metabolism and its relevance to lung cancer. J. Biol. Chem 296, 100722, doi: 10.1016/j.jbc.2021.100722 (2021). Currrent review of nicotine metabolism
- 43.Castagnoli N, Rimoldi J, Bloomquist J & Castagnoli K Potential metabolic bioactivation pathways involving cyclic tertiary amines and azaarenes. Chem. Res. Toxicol 10, 924–940 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Wong HL, Murphy SE & Hecht SS Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N'-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem. Res. Toxicol 18, 61–69 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Zarth AT, Upadhyaya P, Yang J & Hecht SS DNA adduct formation from metabolic 5'-hydroxylation of the tobacco-specific carcinogen N'-nitrosonornicotine in human enzyme systems and in rats. Chem. Res. Toxicol 29, 380–389, doi: 10.1021/acs.chemrestox.5b00520 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L et al. Quantitation of pyridyloxobutyl-DNA adducts in tissues of rats treated chronically with (R)- or (S)- N'-nitrosonornicotine (NNN) in a carcinogenicity study. Chem. Res. Toxicol 26, 1526–1535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carmella SG, McIntee EJ, Chen M & Hecht SS Enantiomeric composition of N'-nitrosonornicotine and N'-nitrosoanatabine in tobacco. Carcinogenesis 21, 839–843 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Stepanov I, Yershova K, Carmella S, Upadhyaya P & Hecht SS Levels of (S)-N'-nitrosonornicotine in U.S. tobacco products. Nicotine Tob. Res 15, 1305–1310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haussmann HJ & Fariss MW Comprehensive review of epidemiological and animal studies on the potential carcinogenic effects of nicotine per se. Crit. Rev. Toxicol 46, 701–734, doi: 10.1080/10408444.2016.1182116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song MA et al. Chemical and toxicological characteristics of conventional and low-TSNA moist snuff tobacco products. Toxicol. Lett 245, 68–77, doi: 10.1016/j.toxlet.2016.01.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arain SS et al. Scalp hair and blood cadmium levels in association with chewing gutkha, mainpuri, and snuff, among patients with oral cancer in Pakistan. J. Oral Pathol. Med 44, 707–713, doi: 10.1111/jop.12283 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Hecht SS Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol 11, 559–603 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Ge GZ, Xu TR & Chen C Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim Biophys Sin (Shanghai) 47, 477–487, doi: 10.1093/abbs/gmv041 (2015). [DOI] [PubMed] [Google Scholar]
- 54. Peterson LA Context matters: Contribution of specific DNA adducts to the genotoxic properties of the tobacco-specific nitrosamine NNK. Chem. Res. Toxicol 30, 420–433, doi: 10.1021/acs.chemrestox.6b00386 (2017). Overview of the consequences of DNA damage by NNK
- 55.Ma B, Stepanov I & Hecht SS Recent studies on DNA adducts resulting from human exposure to tobacco smoke. Toxics 7, 16, doi: 10.3390/toxics7010016, doi: 10.3390/toxics7010016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belinsky SA, Foley JF, White CM, Anderson MW & Maronpot RR Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 50, 3772–3780 (1990). [PubMed] [Google Scholar]
- 57.Balbo S et al. Carcinogenicity and DNA adduct formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F-344 rats. Carcinogenesis 35, 2798–2806, doi: 10.1093/carcin/bgu204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du H, Leng J, Wang P, Li L & Wang Y Impact of tobacco-specific nitrosamine-derived DNA adducts on the efficiency and fidelity of DNA replication in human cells. J. Biol. Chem 293, 11100–11108, doi: 10.1074/jbc.RA118.003477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoffmann D, Hecht SS, Ornaf RM & Wynder EL N'-Nitrosonornicotine in tobacco. Science 186, 265–267 (1974). First identification of N'-nitrosonornicotine in tobacco
- 60. Hecht SS et al. Reaction of nicotine and sodium nitrite: Formation of nitrosamines and fragmentation of the pyrrolidine ring. J. Org. Chem 43, 72–76 (1978). First demonstration of the formation of NNK from nicotine
- 61.Hecht SS et al. Tobacco-specific nitrosamines: formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J. Natl. Cancer Inst 60, 819–824 (1978). [DOI] [PubMed] [Google Scholar]
- 62.Bhutani P, Murray MT, Sommer CW, Wilson KA & Wetmore SD Structural rationalization for the nonmutagenic and mutagenic bypass of the tobacco-derived O4-4-(3-pyridyl)-4-oxobut-1-yl-thymine lesion by human polymerase eta: A multiscale computational study. Chem. Res. Toxicol 34, 1619–1629, doi: 10.1021/acs.chemrestox.1c00063 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Hu SC et al. Toxicokinetic and genotoxicity study of NNK in male Sprague-Dawley rats following nose-only inhalation exposure, intraperitoneal injection, and oral gavage. Toxicol. Sci, doi: 10.1093/toxsci/kfab049 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Peterson LA et al. Coexposure to inhaled aldehydes or carbon dioxide enhances the carcinogenic properties of the tobacco-specific nitrosamine 4-methylnitrosamino-1-(3-pyridyl)-1-butanone in the A/J mouse lung. Chem. Res. Toxicol 34, 723–732, doi: 10.1021/acs.chemrestox.0c00350 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snook ME, Severson RF, Arrendale RF, Higman HC & Chortyk OT Multi-alkyated polynuclear aromatic hydrocarbons of tobacco smoke: separation and identification. Beiträge Tabakforsch 9, 222–247 (1978). [Google Scholar]
- 66.Rodgman A & Perfetti T The Chemical Components of Tobacco and Tobacco Smoke. 1483–1784 (CRC Press, 2009). [Google Scholar]
- 67.International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 92, pp 35–818 (IARC, 2010). [PMC free article] [PubMed] [Google Scholar]
- 68.Snook ME, Severson RF, Arrendale RF, Higman HC & Chortyk OT The identification of high molecular weight polynuclear aromatic hydrocarbons in a biologically active fraction of cigarette smoke condensate. Beiträge zur Tabakforschung 9, 79–101 (1977). [Google Scholar]
- 69.International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 83, pp 33–1413 (IARC, 2004). [PMC free article] [PubMed] [Google Scholar]
- 70.Basu AK DNA damage, mutagenesis and cancer. Int. J. Mol. Sci 19, 970, doi: 10.3390/ijms19040970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delaney JC & Essigmann JM Biological properties of single chemical-DNA adducts: A twenty year perspective. Chem. Res. Toxicol 21, 232–252 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Geacintov NE & Broyde S Repair-resistant DNA lesions. Chem. Res. Toxicol 30, 1517–1548, doi: 10.1021/acs.chemrestox.7b00128 (2017). Current review of repair resistant DNA lesions
- 73.Leemans CR, Snijders PJF & Brakenhoff RH The molecular landscape of head and neck cancer. Nat. Rev. Cancer 18, 269–282, doi: 10.1038/nrc.2018.11 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Phillips DH Smoking-related DNA and protein adducts in human tissues. Carcinogenesis 23, 1979–2004 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Phillips DH & Venitt S DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int. J. Cancer 131, 2733–2753 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Boysen G & Hecht SS Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutation Res. 543, 17–30 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Hecht SS Oral cell DNA adducts as potential biomarkers for lung cancer susceptibility in cigarette smokers. Chem. Res. Toxicol 30, 367–375, doi: 10.1021/acs.chemrestox.6b00372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khariwala SS et al. High level of tobacco carcinogen-derived DNA damage in oral cells is an independent predictor of oral/head and neck cancer risk in smokers. Cancer Prev. Res 10, 507–513, doi: 10.1158/1940-6207.CAPR-17-0140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villalta PW, Hochalter JB & Hecht SS Ultrasensitive high-resolution mass spectrometric analysis of a DNA adduct of the carcinogen benzo[a]pyrene in human lung. Anal. Chem 89, 12735–12742, doi: 10.1021/acs.analchem.7b02856 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jokipii Krueger CC et al. Urinary N7-(1-hydroxy-3-buten-2-yl) guanine adducts in humans: temporal stability and association with smoking. Mutagenesis 35, 19–26, doi: 10.1093/mutage/gez030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung FL, Young R & Hecht SS Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 44, 990–995 (1984). [PubMed] [Google Scholar]
- 82.Minko IG et al. Chemistry and biology of DNA containing 1,N2-deoxyguanosine adducts of the α,ß-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem. Res. Toxicol 22, 759–778 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Paiano V et al. Quantitative liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry analysis of acrolein-DNA adducts and etheno-DNA adducts in oral cells from cigarette smokers and nonsmokers. Chem. Res. Toxicol 33, 2197–2207, doi: 10.1021/acs.chemrestox.0c00223 (2020). Demonstrates high levels of DNA adducts in oral cell DNA of smokers
- 84.Yang J, Balbo S, Villalta PW & Hecht SS Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung DNA from smokers and nonsmokers. Chem. Res. Toxicol 32, 318–325, doi: 10.1021/acs.chemrestox.8b00326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang S, Balbo S, Wang M & Hecht SS Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem. Res. Toxicol 24, 119–124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Alexandrov LB et al. Mutational signatures associated with tobacco smoking in human cancer. Science 354, 618–622, doi: 10.1126/science.aag0299 (2016). Mutational signatures associated with smoking in human cancer
- 87.Yoshida K et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272, doi: 10.1038/s41586-020-1961-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Campbell JD et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet 48, 607–616, doi: 10.1038/ng.3564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517, 576–582, doi: 10.1038/nature14129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.India Project Team of the International Cancer Genome Consortium. Mutational landscape of gingivo-buccal oral squamous cell carcinoma reveals new recurrently-mutated genes and molecular subgroups. Nat Commun 4, 2873, doi: 10.1038/ncomms3873 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Upadhyay P et al. Genomic characterization of tobacco/nut chewing HPV-negative early stage tongue tumors identify MMP10 asa candidate to predict metastases. Oral Oncol. 73, 56–64, doi: 10.1016/j.oraloncology.2017.08.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Hebshi NN et al. Exome sequencing of oral squamous cell carcinoma in users of Arabian snuff reveals novel candidates for driver genes. Int. J. Cancer 139, 363–372, doi: 10.1002/ijc.30068 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y & Hecht SS Identification of an N'-nitrosonornicotine-specific deoxyadenosine adduct in rat liver and lung DNA. Chem. Res. Toxicol 34, 992–1003, doi: 10.1021/acs.chemrestox.1c00013 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Carlson ES, Zarth AT, Upadhyaya P & Hecht SS Investigation of 2'-deoxyadenosine-derived adducts specifically formed in rat liver and lung DNA by n'-nitrosonornicotine metabolism. Chem. Res. Toxicol 34, 1004–1015, doi: 10.1021/acs.chemrestox.1c00012 (2021). [DOI] [PubMed] [Google Scholar]
- 95.Benowitz NL, St Helen G, Nardone N, Cox LS & Jacob P Urine metabolites for estimating daily intake of nicotine from cigarette smoking. Nicotine Tob. Res 22, 288–292, doi: 10.1093/ntr/ntz034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murphy SE et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis 35, 2526–2533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Murphy SE et al. Tobacco biomarkers and genetic/epigenetic analysis to investigate ethnic/racial differences in lung cancer risk among smokers. NPJ Precis. Oncol 2, 17, doi: 10.1038/s41698-018-0057-y (2018). Review of tobacco biomarkers and genetic/epigenetic analysis of ethnic differences in lung cancer in smokers
- 98.Yuan JM et al. CYP2A6 genetic polymorphisms and biomarkers of tobacco smoke constituents in relation to risk of lung cancer in the Singapore Chinese Health Study. Carcinogenesis 38, 411–418, doi: 10.1093/carcin/bgx012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park S-L et al. Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risk of lung cancer and effect on their smoking behavior. Carcinogenesis 37, 269–279, doi: 10.1093/carcin/bgw012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xia B et al. Tobacco-specific nitrosamines (NNAL, NNN, NAT, and NAB) exposures in the US Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013-2014). Nicotine Tob. Res, doi: 10.1093/ntr/ntaa110 (2020). Current data on tobacco-specific nitrosamine biomarkers in U.S. tobacco users
- 101.Rostron BL, Chang CM, van Bemmel DM, Xia Y & Blount BC Nicotine and toxicant exposure among U.S. smokeless tobacco users: Results from 1999 to 2012 National Health and Nutrition Examination Survey data. Cancer Epidemiol. Biomarkers Prev 24, 1829–1837, doi: 10.1158/1055-9965.EPI-15-0376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park SL et al. Variation in levels of the lung carcinogen NNAL and its glucuronides in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. Cancer Epidemiol. Biomarkers Prev 24, 561–569, doi: 10.1158/1055-9965.EPI-14-1054 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jain RB Contributions of dietary, demographic, disease, lifestyle and other factors in explaining variabilities in concentrations of selected monohydroxylated polycyclic aromatic hydrocarbons in urine: Data for US children, adolescents, and adults. Environ. Pollut 266, 115178, doi: 10.1016/j.envpol.2020.115178 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Conney AH Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G.H.A. Clowes Memorial Lecture. Cancer Res. 42, 4875–4917 (1982). [PubMed] [Google Scholar]
- 105.Zhong Y, Carmella SG, Hochalter JB, Balbo S & Hecht SS Analysis of r-, t-8,9, c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine: A biomarker for directly assessing carcinogenic polycyclic aromatic hydrocarbon exposure plus metabolic activation. Chem. Res. Toxicol 24, 73–80, doi: 10.1021/tx100287n (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hochalter JB, Zhong Y, Han S, Carmella SG & Hecht SS Quantitation of a minor enantiomer of phenanthrene tetraol in human urine: Correlations with levels of overall phenanthrene tetraol, benzo[a]pyrene tetraol, and 1-hydroxypyrene. Chem. Res. Toxicol 24, 262–268 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welch RM, Harrison YE, Conney AH, Poppers PJ & Finster M Cigarette smoking: stimulatory effect on metabolism of 3,4-benzpyrene by enzymes in human placenta. Science 160, 541–542 (1968). [DOI] [PubMed] [Google Scholar]
- 108.Luo K et al. Cigarette smoking enhances the metabolic activation of the polycyclic aromatic hydrocarbon phenanthrene in humans. Carcinogenesis, doi: 10.1093/carcin/bgaa137 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan JM et al. Genetic determinants of cytochrome P450 2A6 and biomarkers of tobacco smoke exposure in relation to risk of lung cancer development in the Shanghai Cohort Study. Int. J. Cancer 138, 2161–2171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan JM, Butler LM, Stepanov I & Hecht SS Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 74, 401–411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuan JM et al. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis 33, 804–809 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yuan JM et al. Relationship of the oxidative damage biomarker 8-epi-prostaglandin F2α to risk of lung cancer development in the Shanghai Cohort Study. Carcinogenesis 39, 948–954, doi: 10.1093/carcin/bgy060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hecht SS Tobacco smoke carcinogens and lung cancer, in Chemical Carcinogenesis (ed Penning TM) pp 53–74 (Springer, 2011). [Google Scholar]
- 114.Hecht SS Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst 91, 1194–1210 (1999). [DOI] [PubMed] [Google Scholar]
- 115.Abati S, Bramati C, Bondi S, Lissoni A & Trimarchi M Oral cancer and precancer: A narrative review on the relevance of early diagnosis. Int. J. Environ. Res. Public Health 17, doi: 10.3390/ijerph17249160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoffman RM, Atallah RP, Struble RD & Badgett RG Lung cancer screening with low-dose CT: A meta-analysis. J. Gen. Intern. Med 35, 3015–3025, doi: 10.1007/s11606-020-05951-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Selph S et al. Primary care-relevant interventions for tobacco and nicotine use prevention and cessation in children and adolescents: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 323, 1599–1608, doi: 10.1001/jama.2020.3332 (2020). [DOI] [PubMed] [Google Scholar]
- 118.WHO Framework Convention on Tobacco Control. WHO Framework Convention on Tobacco Control: Guidelines for Implementation of Article 5.3 of the WHO Framework Convention on Tobacco Control, <https://www.who.int/fctc/guidelines/article_5_3.pdf> (2008).
- 119. Berman ML & Hatsukami DK Reducing tobacco-related harm: FDA's proposed product standard for smokeless tobacco. Tob. Control 27, 352–354, doi: 10.1136/tobaccocontrol-2016-053612 (2018). U.S. FDA's regulatory approaches to smokeless tobacco harm reduction
- 120.Swedish Match AB. GOTHIATEK limits for undesired components, <https://www.swedishmatch.com/Snus-and-health/GOTHIATEK/GOTHIATEK-standard/> (2016).
- 121.Stepanov I & Hatsukami D Call to establish constituent standards for smokeless tobacco products. Tob. Reg. Sci 2, 9–30, doi: 10.18001/TRS.2.1.2 (2016). [DOI] [Google Scholar]
- 122.Wyss AB et al. Smokeless tobacco use and the risk of head and neck cancer: Pooled analysis of US studies in the INHANCE consortium. Am. J. Epidemiol 184, 703–716, doi: 10.1093/aje/kww075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.World Health Organization. WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Third report of a WHO study group. WHO Technical Report Series, (World Health Organization, 2009). [PubMed] [Google Scholar]
- 124.U.S. Food and Drug Administration. Tobacco product standard for N-nitrosonornicotine level in finished smokeless tobacco products. Fed. Regist 82, 8004–8053 (2017). [Google Scholar]
- 125.World Health Organization. Smokeless Tobacco Products: Research Needs and Regulatory Recommendations. , in Report on the Scientific Basis of Tobacco Product Regulation: Fifth Report of the WHO Study Group. WHO Technical Report Series Ch. 2,pp 17–30 (World Health Organization, 2015). [Google Scholar]
- 126.World Health Organization. WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Seventh Report of a WHO Study Group. WHO Technical Report Series, vol 1015, (World Health Organization, 2019). [PubMed] [Google Scholar]
- 127.Benowitz NL & Henningfield JE Establishing a nicotine threshold for addiction. N. Engl. J. Med 331, 123–125 (1994). [DOI] [PubMed] [Google Scholar]
- 128.Gottlieb S & Zeller M A Nicotine-focused framework for public health. N. Engl. J. Med 377, 1111–1114, doi: 10.1056/NEJMp1707409 (2017). [DOI] [PubMed] [Google Scholar]
- 129. Hatsukami DK et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA 320, 880–891, doi: 10.1001/jama.2018.11473 (2018). Randomized clinical trial demonstrating the effects of immediate reduction in nicotiine content of cigarettes on biomarkers of smoke exposure.
- 130.Hammond D & O'Connor RJ Reduced nicotine cigarettes: smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol. Biomarkers Prev 23, 2032–2040, doi: 10.1158/1055-9965.EPI-13-0957 (2014). [DOI] [PubMed] [Google Scholar]
- 131.Donny EC et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med 373, 1340–1349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cassidy RN et al. Age moderates smokers' subjective response to very-low nicotine content cigarettes: Evidence from a randomized controlled trial. Nicotine Tob. Res 21, 962–969, doi: 10.1093/ntr/nty079 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Higgins ST et al. Changes in cigarette consumption with reduced nicotine content cigarettes among smokers with psychiatric conditions or socioeconomic disadvantage: 3 randomized clinical trials. JAMA Netw. Open 3, e2019311, doi: 10.1001/jamanetworkopen.2020.19311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tidey JW et al. Effects of 6-week use of very low nicotine content cigarettes in smokers with serious mental illness. Nicotine Tob. Res 21, S38–S45, doi: 10.1093/ntr/ntz133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pacek LR et al. Evaluation of a reduced nicotine product standard: Moderating effects of and impact on cannabis use. Drug Alcohol Depend. 167, 228–232, doi: 10.1016/j.drugalcdep.2016.08.620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dermody SS et al. The impact of smoking very low nicotine content cigarettes on alcohol use. Alcohol Clin. Exp. Res 40, 606–615, doi: 10.1111/acer.12980 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shiffman S, Kurland BF, Scholl SM & Mao JM Nondaily smokers' changes in cigarette consumption with very low-nicotine-content cigarettes: A randomized double-blind clinical trial. JAMA Psychiatry 75, 995–1002, doi: 10.1001/jamapsychiatry.2018.1831 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shiffman S, Scholl SM & Mao JM Very-low-nicotine-content cigarettes and dependence among non-daily smokers. Drug Alcohol Depend. 197, 1–7, doi: 10.1016/j.drugalcdep.2018.12.021 (2019). [DOI] [PubMed] [Google Scholar]
- 139. World Health Organization. WHO Study Group on Tobacco Product Regulation: Global Nicotine Reduction Strategy. WHO Technical Report Series, (World Health Organization, 2015). WHO's approach to global nicotine reduction in tobacco products
- 140.Wayne GF, Donny E & Ribisl KM A Global Nicotine Reduction Strategy: State of the Science, in WHO Study Group on Tobacco Product Regulation: Report on the Scientific Basis of Tobacco Product Regulation: Seventh Report of a Who Study Group WHO Technical Report Series Ch. 4,pp 75–110 (World Health Organization, 2019). [Google Scholar]
- 141.Apelberg BJ et al. Potential public health effects of reducing nicotine levels in cigarettes in the United States. N. Engl. J. Med 378, 1725–1733, doi: 10.1056/NEJMsr1714617 (2018). [DOI] [PubMed] [Google Scholar]
- 142.U.S. Food and Drug Administration. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Fed. Regist 83, 11818–11843 (2018). [Google Scholar]
- 143.Glynn TJ, Hays JT & Kemper K E-Cigarettes, harm reduction, and tobacco control: A path forward? Mayo Clin. Proc, doi: 10.1016/j.mayocp.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 144.Ministry of Health. Proposals for a Smokefree Aotearoa 2025 Action Plan: Discussion Document. 5 (Ministry of Health, 2021). [Google Scholar]
- 145.Hatsukami DK, Xu D & Ferris Wayne G Regulatory approaches and implementation of minimally addictive combusted products. Nicotine Tob. Res, doi: 10.1093/ntr/ntab138 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hecht SS & Hatsukami DK A regulatory strategy for reducing exposure to toxicants in cigarette smoke, in WHO study group on tobacco product regulation: Report on the scientific basis of tobacco product regulation: Seventh report of a WHO study group WHO Technical Report Series Ch. 5,pp 111–124 (World Health Organization, 2019). [Google Scholar]
- 147.Song MA et al. Cigarette filter ventilation and its relationship to increasing rates of lung adenocarcinoma. J. Natl. Cancer Inst 109, doi: 10.1093/jnci/djx075 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tobacco Products Scientific Advisory Committee. Menthol cigarettes and public health: Review of the scientific evidence and recommendations. (Center for Tobacco Products; Food and Drug Adminstration, 2011). [Google Scholar]
- 149.Food and Drug Administration. Preliminary scientific evaluation of the possible public health effects of menthol versus nonmenthol cigarettes. (2013). [Google Scholar]
- 150.World Health Organization. Advisory note: Banning menthol in tobacco products: WHO study group on Tobacco Product Regulation (TobReg). WHO Technical Report Series, (World Health Organization, 2016). [Google Scholar]