Abstract
Context
Moderate alcohol consumption is associated with decreased risk of cardiovascular disease (CVD) and improvement in cardiovascular risk markers, including lipoproteins and lipoprotein subfractions.
Objective
To systematically review the relationship between moderate alcohol intake, lipoprotein subfractions, and related mechanisms.
Data sources
Following PRISMA, all human and ex vivo studies with an alcohol intake up to 60 g/d were included from 8 databases.
Data extraction
A total of 17 478 studies were screened, and data were extracted from 37 intervention and 77 observational studies.
Results
Alcohol intake was positively associated with all HDL subfractions. A few studies found lower levels of small LDLs, increased average LDL particle size, and nonlinear relationships to apolipoprotein B–containing lipoproteins. Cholesterol efflux capacity and paraoxonase activity were consistently increased. Several studies had unclear or high risk of bias, and heterogeneous laboratory methods restricted comparability between studies.
Conclusions
Up to 60 g/d alcohol can cause changes in lipoprotein subfractions and related mechanisms that could influence cardiovascular health.
Systematic Review Registration
PROSPERO registration no. 98955
Keywords: apoB-containing lipoproteins, cardiovascular disease, cholesterol efflux capacity, high-density lipoprotein cholesterol, paraoxonase
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide.1 In observational studies, regular moderate consumption of alcohol (ethanol) has long been linked to a reduced risk of CVD, especially coronary heart disease (CHD).2 A J- or U-shaped relationship between alcohol intake and incidence of CVD and type 2 diabetes has been reported in several studies.2–4 This association is mainly seen in middle-aged men and postmenopausal women,4,5 with a relative risk for CVD among moderate drinkers vs non-drinkers of approximately 0.80.2,6,7 The lowest risk has been observed at intakes between 2.5 g and 14.9 g of alcohol a day (≤ 1 drink/d) for most CVD outcomes spanning both sexes. However, the risk of CHD might also be reduced at higher intakes.2,5 In some analyses, the lowest risk is observed at intakes of 1–2 drinks per day for men and 0.5–1 drink per day for women, which is sometimes termed “light-to-moderate drinking.”8 This definition is supported by the National Institute of Alcohol Abuse and Alcoholism9 and is in concordance with the maximum recommended intake level in most countries.10
Data from observational studies show that the type of alcoholic beverage appears to be less critical, indicating that ethanol itself has biological activity.11–13 A recent meta-analysis reported similar protective effects of beer and wine consumption but not of spirits.14 This discrepancy could be related to a higher frequency of binge drinking in people who drink spirits rather than beer and wine.14 The effect of alcohol on high-density lipoprotein (HDL) cholesterol, proposed as a group-level alcohol intake biomarker,15 does not seem to differ according to beverage type.16
The underlying, potentially protective mechanisms of alcohol intake have not been fully elucidated.17 Among the effects of moderate alcohol consumption are changes in circulating lipoproteins,17 which have been associated with reduced risk of CHD in both clinical and observational studies.18 An overview of the effect of moderate alcohol consumption on the overall classes of lipoproteins and related apolipoproteins as reported in published studies, is provided in Table 1.17,19–23
Table 1.
Results from meta-analyses of the effect of moderate alcohol intake on the overall classes of lipoproteins and associated apolipoproteins
| Lipoprotein | Huang et al19,a | Brien et al17,b | Rimm et al20,c | Spaggiari et al 21,d | Hannuksela et al 22,23,e |
|---|---|---|---|---|---|
| VLDL-TG | – | – | – | – | ↔ or ↑ |
| TGf | ↔g | ↔ | ↑ | ↔ | ↔ or ↑ |
| HDL-C | ↑ | ↑ | ↑ | ↑ | ↑ |
| LDL-C | ↓ | ↔ | ↔ | ↔ | ↔ or ↓ |
| Lp(a)h | – | ↔ | ↔ | – | ↓ |
| ApoA-I | ↑ | ↑ | ↑ | ↑ | ↑ |
| ApoA-II | – | – | – | – | ↑ |
| ApoBh | – | – | ↔ | – | ↔ or ↓ |
| TC | ↔ | ↔ | – | ↑ | – |
| VLDL-C | – | – | – | – | ↔ |
| IDL-C | – | – | – | – | ↔ |
Meta-analysis of intervention studies: no diagnosed CVD, diabetes, or alcohol dependence; ≤ 30 g/d alcohol (n = 2 with 0.19–0.81 g/kg/d and 0.75 g/kg/d) for ≥ 7 d; TC: n = 17, HDL-C: n = 22, apoA-I: n = 11, LDL-C: n = 17, TG: n = 22.
Meta-analysis of intervention studies: No diagnosed CVD and no heavy drinking; ≤ 90 g/d alcohol for ≥ 7 d; LDL-C and TGs nonsignificantly reduced; TGs increased at > 60 g/d; TC: n = 26, HDL-C: n = 33, apoA-I: n = 16, LDL-C: n = 24, TG: n = 31.
Meta-analysis of intervention studies: no diagnosed CHD, diabetes, or alcohol dependence; <100 g/d alcohol for ≥ 7 d (predicted mean change after 30 g/d used in analysis); HDL-C level increased by 0.103 mmol/L per 30 g of alcohol consumed per day; Lp(a) (n = 4) nonsignificantly decreased; HDL-C: n = 25 (36 data records), apoA-I: 24 data records. ApoB and LDL-C analyses not specified.
Meta-analysis of beer consumption in controlled intervention studies: Healthy, overweight, high cardiovascular risk, hypertension, or healthy; ≤ 41 g/d beer intake; acute studies (n = 5) and ≥ 3 wk; TC: n = 14, HDL-C: n = 18, apoA-I: n = 5, LDL-C: n = 12.
Results from 2 extensive narrative reviews with different study designs. No dose definition available. ApoB might only be reduced at higher intakes.
A review reports a J-shaped relationship to alcohol intake, nadir at intakes of 10–20 g/d (∼1–2 drinks).238
→: unchanged, ↑: increased, ↓: reduced, –: not investigated.
Investigated in few studies only.
Abbreviations: Apo, apolipoprotein; C, cholesterol concentration; CVD, cardiovascular disease; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); VLDL, very-low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
Lipoproteins are a heterogeneous group of lipid-carrying particles in the blood that differ in size, density, composition, metabolism, and biological activity. Within each overall class of lipoprotein, there are several subfractions.24 Findings from some studies suggest the relationship between lipoproteins and CVD risk differs according to the distribution of subclasses.25–27 Depending on the method, subfractions are classified according to various characteristics, including density, charge, apolipoprotein composition, and particle number.28 This heterogeneity makes interpretation of the relationship between CVD and subfractions troublesome.
There is currently no universally accepted definition of lipoprotein subfractions (LPSFs), and comparability between studies is complicated by the use of different methodologies for separating and measuring these structures. These methodologies include analytical ultracentrifugation, gradient gel electrophoresis, nuclear magnetic resonance (NMR), and ion mobility (IM) spectrometry.24 An expert group recently suggested a uniform nomenclature for the HDL subfractions that includes definitions of subfractions measured as particle number and size, but characterization by cholesterol content or apolipoprotein composition is not included.29
Knowledge about the effect of moderate alcohol consumption on the LPSFs could give more insight into the mechanisms involved and provide hypotheses for a potential causal role of alcohol consumption in CVD. Studies of LPSFs are not included in the most recent meta-analysis, which covered the effect of alcohol on 13 biological markers related to CVD risk.17 Current narrative reviews describing the relationship between alcohol intake and lipoproteins with atherosclerosis provide only a few comments on LPSFs.22,23,27,30,31
Nevertheless, several observational studies and short-term intervention trials have investigated the effect of alcohol consumption on LPSFs in both younger and older populations.32–36 A thorough systematic overview of these effects, including the mechanisms by which alcohol potentially alters the LPSFs, has not been published previously, to our knowledge. The primary aim of this systematic review, therefore, was to investigate the influence of moderate alcohol consumption and regular intakes up to 60 g/d on LPSF changes and related mechanisms, and secondarily, whether changes were influenced by study design or health status.
METHODS
Review protocol and registration
This systematic review was conducted in accordance with established Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)37,38 and reported in the PROSPERO database before the systematic search (registration no. 98955).
Eligibility criteria
English-language studies published in peer-reviewed journals were included and assessed for eligibility according to predefined PICOTS criteria (Table 2). Studies investigating oral alcohol intakes ≤ 60 g/d in human adults were included. A broad interval of alcohol intake was chosen to avoid excluding studies in which “moderate” intake was up to 60 g/d. The comparator intervention included no or low alcohol intake. There were no restrictions on diet or medication, but comparable background diets and medication protocols in the intervention and control groups were required. Studies in patients with cancer, genetic lipid disorders, or kidney, pancreatic, and liver diseases, were excluded. Studies in people with alcoholism or heavy drinkers (>60 g/d) were also ineligible.
Table 2.
PICOTS criteria for eligibility of studies
| Criterion | Description |
|---|---|
| Population | Human adults ≥ 18 years of age, including healthy people and those at high risk of cardiovascular disease (eg, people with type 2 diabetes, hyperlipidemia, increased waist circumference, increased fasting blood glucose or reduced glucose tolerance, atherosclerosis, hypertension, metabolic syndrome. Individuals taking lipid-lowering drugs were eligible. |
| Intervention/exposure | Oral consumption of ethanol ≤ 60 g/d. Comparable background diet and medication use in compared groups |
| Comparison | No or low alcohol intake |
| Outcomes | Quantitative changes in lipoprotein subfractions and related physiological mechanisms |
| Timing | Any intervention or exposure period > 3 days, any follow-up period |
| Study design | All types of designs in humans |
Eligible outcomes included all types of LPSFs related to the overall classes of lipoproteins: LDL, HDL, chylomicrons, very-low-density lipoprotein (VLDL), and intermediate-density lipoprotein. The LPSF definitions described in the individual studies were used. All these definitions are listed in Table S6 in the Supporting Information online. In addition, outcomes related to the mechanisms by which alcohol could modulate any LPSF were included. Studies with LPSFs defined according to apolipoprotein content were also eligible, but overall classes of lipoproteins and apolipoproteins were excluded. Quantitative changes in the LPSFs were the primary outcome, and related mechanisms were the secondary outcome. All types of human study designs longer than 3 days and investigating the effect of the exposure or intervention were included.
Despite being part of the initial aim and search strategy (Supplementary Methods in the Supporting Information online), we chose to exclude studies in which overall levels of apolipoproteins were investigated, in addition to animal, cell, and postprandial studies, because of the vast number of additional articles. This decision was made prior to data extraction and before the authors had any knowledge about the study outcomes.
Literature search strategy
A comprehensive systematic literature search was performed in April 2018 of 8 bibliographic databases (Figure 1) according to the predefined eligibility criteria. The search was updated in March 2021, spanning 2018 through March 2021. An email alert was created in the central databases, continuously informing the researchers of recent publications according to the search criteria. In addition, we screened the ClinicalTrials.gov database (www.clinicaltrials.gov) for unpublished literature. The search terms were constructed in blocks and related to exposure (alcohol consumption) and outcomes (LPSFs and mechanisms). Hand searching of reference lists in the included reviews was also performed. The complete search strategy is provided in the Supplementary Methods in the Supporting Information online. The process was verified by an experienced health sciences research librarian and agreed upon by all 4 authors.
Figure 1.
Flowchart of the systematic literature process. *Some articles include outcomes related to both lipoprotein subfractions and mechanisms (n = 13) and are reported twice in this review. Thus, 114 individual papers were included, but 127 data sets were reported. Abbreviations: EMBASE, Excerpta Medica Database; WoF, Web of Science.
Study selection
The study selection was performed in 2 phases after duplicates had been removed in Endnote X8.239 and Covidence.40 Three independent researchers (T.L.W., J.N.E., and K.T.) screened all identified articles for eligibility on the basis of titles and abstracts. Articles that met the inclusion criteria, or articles with uncertain eligibility, were included for full-text screening. In the second phase, screening of articles at the full-text level was undertaken independently by 2 researchers (T.L.W. and K.T.). Any lack of consensus between the authors was settled by a third author (either J.N.E. or L.O.D.) until consensus was reached. Contact by a single email to study authors was made in case of missing or incomplete data.
Data collection and items
Two independent researchers (T.L.W. and K.T.) performed data extraction, and all 4 authors discussed any disagreements. A data extraction form was piloted on the different study designs before data collection. The following data were extracted: first author; publication year; country; sample size and dropouts; age distribution; sex distribution; health status; study design; alcohol intervention or exposure; laboratory measurements; statistical analyses of LPSFs and covariates; and LPSFs outcomes, including direction of the results. Missing data were retrieved via a single email to the authors; we state in the accompanying tables if data proved inaccessible.
Bias risk assessment
The methodological quality of the included articles was assessed independently by 2 researchers (T.L.W. and K.T.). Disagreements were discussed with the remaining authors (J.N.E. and L.O.D.). Assessment was performed at study level and according to the specific study design. For randomized controlled trials (RCTs), The Cochrane Collaboration tool for assessing the risk of bias was used.41 The Risk of Bias Assessment tool for Nonrandomized Studies (RoBANS)42 was used for nonrandomized investigations, and the JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies43 was used for cross-sectional studies. Blinding, by default, was scored as high risk of bias in all intervention studies, because this is not believed to be feasible for alcohol consumption.44,45 Similarly, self-reported alcohol intake was classified as high risk of bias in observational studies because self-reported alcohol intake is regarded an inaccurate methodology, compared with objective biomarkers.46
Data synthesis and analysis
Alcohol intake in grams per day was used and recalculated from grams or ounces per week, when necessary. Amounts reported in grams per kilogram body weight were recalculated on the basis of a standard person weight of 70 kg. Frequency measures were not converted. In observational studies, the alcohol intake was classified into levels and handled as categorical or continuous variables. The lowest alcohol-intake group was often used as the reference group, except in studies using multivariate analysis. It was impossible to delimit the alcohol intake to exactly ≤ 60 g/d, because of the group classifications and the corresponding statistical analyses in the included observational studies. The individual LPSFs were not extracted in absolute values; instead they are described with arrows. Vertical arrows indicate significant increases or decreases (P < 0.05 or significance level used by the study). Horizontal arrows indicate nonsignificant effects. Arrows in brackets indicate trends defined by the review authors (P range, > 0.05 to ≤ 0.1). Outcomes were preferably based on both sexes, but results of each sex separately were included if a combined outcome was not available. Presumed multiple reports from the same study are reported individually but listed after each other in the final table. NMR- and IM lipoprotein outcomes are provided in particle number concentration, abbreviated -P.
RESULTS
Study selection
The number of articles retrieved from the individual databases is shown in Figure 1. A total of 17 478 articles were identified. After removing duplicates, and title and abstract screening was conducted, 579 articles were initially included for full-text screening. The interrater agreement at this stage was moderate (κ = 0.59). Additional articles were retrieved from various sources (Figure 1). Hence, 597 papers were screened at the full-text level, where the interrater agreement was high (κ = 0.82), and a total of 114 articles were included. These were 37 intervention studies and 77 observational studies, totaling 20 510 and 104 773 participants, respectively.
The characteristics of the included studies are outlined in Tables S1–S4 in the Supporting Information online. The vast number of citations in this review reduce readability; therefore, the reader is directed to Table S5 in the Supporting Information online, which lists specific statements supported by multiple articles included in this review, with the appropriate references. Table 3,35,36,47–70Table 4,33,71–123Table 5,13,32,124–131 and Table 616,49,50,52,53,59–62,64,84,104,112,114,132–152 provide an overview of the outcomes from each study based on the distinct types of LPSFs, as illustrated in Figure 2.
Table 3.
Summary of results of alcohol intake on lipoprotein subfractions from all intervention studies
| Lipoprotein subfraction | Increased | No change | Decreased |
|---|---|---|---|
| HDL2-C | Burr et al, 1986 36 ; Clevidence et al, 1995 57 ; Hartung et al, 1990 64 , a ; Kaul et al, 2010 65 ; Masarei et al, 1986 35 , b ; Tabara et al, 2017 66 , c ; Vu et al, 2016 67 , d ; Hartung et al, 199368,e; McConnell et al, 199769 | Moore et al, 1988 70 ; Pikaar et al, 1987 47 , f ; Rumpler et al, 1999 48 , g ; Senault et al, 2000 49 , h ; Hagiage et al, 199250,i; Hartung et al, 198651,j; Nishiwaki et al, 199452; Välimäki et al, 198853,k; Välimäki et al, 199154 | Cox et al, 1993 55 , l |
| HDL2-TM | Haskell et al, 1984 34 , m ; Fraser et al, 198356; Hagiage et al, 199250,i; Välimäki et al, 198853,k; Välimäki et al, 199154 | ||
| HDL2a-TM | Bertière et al, 198658 | ||
| HDL2b-TM | Bertière et al, 198658 | ||
| HDL3-C | Clevidence et al, 1995 57 ; Hartung et al, 1990 64 , a ; Masarei et al, 1986 35 , b ; Senault et al, 2000 49 , h ; Tabara et al, 2017 66 , c ; Nishiwaki et al, 199452; Välimäki et al, 198853,k; Välimäki et al, 199154 | Burr et al, 1986 36 ; Kaul et al, 2010 65 ; Moore et al, 1988 70 ; Pikaar et al, 1987 47 , f ; Rumpler et al, 1999 48 , g ; Vu et al, 2016 67 , d ; Hartung et al, 198651,j; Hartung et al, 199368,e; McConnell et al, 199769 | Cox et al, 1993 55 , l ; Hagiage et al, 199250i |
| HDL3-TM | Bertière et al, 198658; Välimäki et al, 198853,k | Fraser et al, 198356; Hagiage et al, 199250,i; Välimäki et al, 199154 | Haskell et al, 1984 34 , m |
| VHDL | Bertière et al, 198658 | ||
| Pre-β-HDL | Beulens et al, 2004 59 , n | ||
| LpA-I | Beulens et al, 2004 59 , n ; Clevidence et al, 199557; Senault et al, 200049,h; Välimäki et al, 199154 | Gottrand et al, 1999 60 ; Hagiage et al, 199250,i; Serdyuk et al, 200061,o | |
| LpA-I:A-II | Beulens et al, 2004 59 , n ; Clevidence et al, 1995 57 ; Gottrand et al, 1999 60 ; Senault et al, 2000 49 , h ; Välimäki et al, 199154 | Hagiage et al, 199250,i, Serdyuk et al, 200061,o | |
| HDL size | Clevidence et al, 1995 57 | Mukamal et al, 2017 44 , p , De Oliveira E Silva et al, 200062 | |
| VLDL1-C | Kaul et al, 2010 65 | ||
| VLDL2-C | Kaul et al, 2010 65 | ||
| VLDL3-C | Kaul et al, 2010 65 | ||
| LDL size | Sharpe et al, 199563 | Mukamal et al, 2017 44 , p | |
| sdLDL-C | Tabara et al, 2017 66 , c | Vu et al, 2016 67 , d | |
| lbLDL-C | Tabara et al, 2017 66 , c |
Summary of results based on each lipoprotein subfraction investigated. Randomized controlled trials are indicated with bold text. A more detailed description of each study can be found in Table S1 in the Supporting Information online.
Runners, inactive people, and drinkers of 15 g/d and 45 g/d alcohol. Participants were randomized to 1 or 3 beers in each activity category. Here, the results represent within-group changes (ie, the effect of moderate to low vs no alcohol consumption).
Multiple linear regression analysis (adjusted for weight).
A Mendelian randomization study; results represent men. Results for women were similar except no change in sdLDL was found.
A Mendelian randomization study; results represent all drinkers compared with abstainers. From the 2-stage least squares regression analysis (adjusted for age and sex).
Inactive participants. Results for runners (HDL2-C unchanged and HDL3-C increased) are reported in the text, but no P values are provided.
Represent 23 g/d and 46 g/d vs 0 g/d alcohol.
The low-fat diet group. The high-fat diet group: HDL2-C increased and HDL3-C unchanged.
The red wine group: 30 g/d alcohol. LpA-I did not increase in the 30 g/d pure alcohol-with-mineral-water group. A randomized controlled trial, but only within-group results provided in the article.
The nonobese subgroup. No significant changes in the obese participants. Results represent within-group changes. Changes between groups (obese vs control participants) were examined but not extracted.
Inactive participants. Results for runners were similar.
Represent 30 g/d and 60 g/d alcohol compared with abstention, respectively, because results are similar in both periods.
Alcohol restriction compared with habitual, moderate alcohol intake.
From 6 wk of intervention: 0 g/d vs 30 g/d alcohol.
Brackets refer to a trend: P > 0.05 to ≤ 0.1.
Subgroup with the lowest HDL level. The subgroup with high HDL (≥ 50 mg/dL): LpA-I increased.
A 6-month randomized controlled trial: no effect on any LPSF, including particle size (low compliance reported). Results on specific subfractions are not reported in the article but are reported for HDL size and LDL size here as examples.
Abbreviations: C, cholesterol concentration; HDL, high-density lipoprotein; lbLDL, large, buoyant low-density lipoprotein; LDL, low-density lipoprotein; LpA-I, HDL particles with apolipoprotein A1; LpA-I:A-II, HDL particles with apolipoprotein A1 and apolipoprotein A2; sdLDL, small, dense low-density lipoprotein; TM, total mass concentration; VHDL, very-high-density lipoprotein; VLDL, very-low-density lipoprotein.
Table 4.
Summary of results of alcohol intake effects on lipoprotein subfractions from observational studies with electrophoresis, ultracentrifugation, precipitation, immunologic, and enzymatic methods a
| Lipoprotein subfraction | Increased | No change | Decreased |
|---|---|---|---|
| HDL2-C | Fulton-Kehoe et al, 199271,b; Gardner et al, 200082,c; Gaziano et al, 199393; Ito et al, 1995104; Kim et al, 2014115; Lupien et al, 1988120,d; Meilahn et al, 1988121; Miller and Gilson, 1981122,e; Miller et al, 1988123; Moriyama and Takahashi, 201472,f; Mänttäri et al, 199173; Parlesak et al, 201433,g; Patsch et al, 199274; Robinson et al, 198775,h; Steenkamp et al, 199076,i; Rywik et al, 201177,ii; Volcik et al, 200878,j; Walton et al, 199579 | Andrade et al, 199080,k; Bergmann et al, 199781,l; Diehl et al, 198883,m; Haffner et al, 198584,n; Lakshman et al, 199685,o; Marti et al, 198986,p; Meilahn et al, 199187,; Momoseet al, 199488,q; Razay et al, 199289; Rossouw et al, 199290,; Sillanaukee et al, 199391,r; Sillanaukee et al, 200092,s; Vasisht et al, 199294,t; Woo and Lam, 199095 | Skoczyńska et al, 201396 |
| HDL2a-C | Schäfer et al, 200797 | ||
| HDL2b-C | Schäfer et al, 200797 | ||
| HDL2-TM | Williams et al, 198598,u | ||
| HDL2a-TM | Williams et al, 199399 | ||
| HDL2b-TM | Williams et al, 199399 | ||
| HDL3-C | Bergmann et al, 199781,l; Burke et al, 1992100,uu; Diehl et al, 198883,m; Fulton-Kehoe et al, 199271,b; Gardner et al, 200082,c; Gaziano et al, 199393; Haffner et al, 198584,n; Ito et al, 1995104; Kim et al, 2014115; Lupien et al, 1988120,d; Meilahn et al, 1988121; Miller et al, 1988123; Momoseet al, 199488,q; Moriyama and Takahashi, 201472,f; Mänttäri et al, 199173; Patsch et al, 199274; Razay et al, 199289; Robinson et al, 198775,h; Steenkamp et al, 199076,i; Rywik et al, 201177,ii; Schäfer et al, 200797; Sillanaukee et al, 199391,r; Sillanaukee et al, 200092,s; Volcik et al, 200878,j; Walton et al, 199579 | Andrade et al, 199080,k; Marti et al, 198986,p; Meilahn et al, 199187,; Miller and Gilson, 1981122,e; Parlesak et al, 201433,g; Rossouw et al, 199290,; Skoczyńska et al, 201396,Vasisht et al, 199294,t; Woo and Lam, 199095 | |
| HDL3-TM | Williams et al, 198598,u | ||
| HDL3a-TM | Williams et al, 199399 | ||
| HDL3b-TM | Williams et al, 199399 | ||
| HDL3c-TM | Williams et al, 199399 | ||
| HDL-C + apoC-III | Jensen et al, 2012101; Onat et al, 2003105,v; Onat et al, 2009102,w | Koch et al, 2017103 | |
| HDL-C - apoC-III | Jensen et al, 2012101; Koch et al, 2017103 | ||
| Non-HDL + apoC-III | Onat et al, 2003105,v | Onat et al, 2009102,w | |
| LpA-I | Branchi et al, 1997106,$; Kee et al, 1995107,x; Marques-Vidal and Al, 1995108; Marques-Vidal et al, 2001109,y | Luc et al, 2002110; Lyu et al, 2018111; Mansfield, McPherson and Koski, 1999112,z; Perret et al, 2002114,aa; Steinmetz et al, 1990113 | Puchois et al, 1990116 |
| LpA-I:A-II | Branchi et al, 1997106,bb; Marques-Vidal and Al, 1995108; Luc et al, 2002110; Perret et al, 2002114,aa; Puchois et al, 1990116 | Kee et al, 1995107,x; Lyu et al, 2018111; Steinmetz et al, 1990113 | |
| LDL-IVB | Williams and Krauss, 1997117,cc | ||
| LDL-IVA | Williams and Krauss, 1997117,cc | ||
| LDL-IIIB | Williams and Krauss, 1997117,cc | ||
| LDL-IIIA | Williams and Krauss, 1997117,cc | ||
| LDL-II | Williams and Krauss, 1997117,cc | ||
| LDL-I | Williams and Krauss, 1997117,cc | ||
| ISL | Williams and Krauss, 1997 117,cc | ||
| sdLDL-C | Parlesak et al, 201433,g | ||
| lbLDL-C | Parlesak et al, 201433,g | ||
| LDL size | McNamara et al, 1992118,dd | ||
| LpE:B | Marques-Vidal and Al, 1995108, Marques-Vidal et al, 2001109,y | Schiele et al, 2002119,ee | |
| LpE:non-B | Schiele et al, 2002119,ee | ||
| LpC-III:B | Marques-Vidal and Al, 1995108 |
Summary of results based on each lipoprotein subfraction investigated. A more detailed description on each study can be found in Table S2 (in the Supporting Information online).
P < 0.05.
Multiple regression (adjusted for age, body mass index, subscapular-to-triceps ratio, physical activity, smoking, fasting insulin, and use of β-blockers). Results represent female drinkers (intake, ≥ 50 g/d). In male drinkers of ≥ 50 g/d, HDL2-C results not available, HDL3-C increased. Alcohol intake of men and women < 50 g/d compared with nondrinkers: no changes (results on HDL2-C not available for men).
Women. In men, no change in HDL2-C, HDL3-C increased,
Analysis of covariance (adjusted for age, weight, and smoking).
Men. Results for women not available.
Results from the multiple linear regression analysis (adjusted for sex, waist circumference, exercise, and smoking). Results from the analysis of variance were similar.
Per response from study authors, there was no significant association between alcohol and HDL3-C.
Multiple linear regression (adjusted for age, body mass index, waist-to-hip ratio, smoking, and physical activity). Results represent alcohol intake < 13.4 g/d compared with abstention. Drinkers of > 13.4 g/d compared with abstainers: HDL2-C was unchanged, HDL3-C increased.
Multiple linear regression (adjusted for body mass index, triglycerides, smoking, CHO intake), in men. In women: HDL2-C increased, HDL3-C unchanged.
Men and women in the United States. Participants from Poland: in men, both subfractions were increased; in women, there was no change in any subfraction.
White women and men. For the Black participants in all subgroups and for both sexes: HDL3-C increased, HDL2-C was unchanged.
Similar results when those drinking alcohol ≥ 5 to ≤ 20 g/d and > 20 to < 70 g/d were compared with abstainers.
Multiple linear regression (adjusted for age, body mass index, blood pressure, menopausal status, energy intake, cholesterol intake, saturated fatty acid, smoking, and physical activity).
Multiple regression (adjusted for age, body mass index, smoking, physical activity, triglycerides, and social class). Results similar in men and women.
Pearson’s partial correlation and (adjusted for sex, body mass index, smoking, and VLDL-triglycerides), men and women combined.
HDL3-C results not available.
Women. In men, HDL3-C levels were increased, HDL2-C was unchanged.
Men. Results for women were not available.
Alcohol intake of ≥40 g/d compared with abstention. No association found when < 40 g/d was compared with abstention.
Results represent unpaired t tests: >20–40 g/d and > 40 g/d compared with 0–10 g/d. When > 10–20 g/d alcohol intake was compared with the 0–10 g/d, there was no change in HDL3-C levels and HDL2-C levels decreased.
Alcohol intake of approximately 12 g/d (21 g/d for 3–5 d/wk) compared with abstention. Intakes of 44 g/d (62 g/d for 4–6 d/wk) compared with abstention: HDL2-C levels increased, HDL3-C levels were unchanged.
Multiple regression analysis (adjusted for starch and sucrose). Partial Spearman’s correlation analyses (adjusted for smoking, adiposity, and physical) returned similar results.
Results on HDL2-C not available.
Results for men from the correlation analysis. In women, findings were not significant.
Results for men and women.
Alcohol intake > 10 g/d compared with abstainers. Alcohol intake < 10 g/d compared with abstainers: no change in any HDL subfraction.
LpA-I increased in participants from France and participants from Northern Ireland, and by all beverage types (wine, beer, and spirits), except in drinkers of spirits in France. LpE:B levels were unchanged after all beverage types except beer. LpE:B levels increased after drinking beer.
Model 1 (energy, protein, carbohydrates, fat [saturated, monounsaturated, and polyunsaturated], alcohol intake, waist-to-hip ratio, body mass index, and aerobic capacity). LpA-I:A-II result not available.
Alcohol intake ≤ 35 g/d compared with abstainers. Drinkers of > 35 g/d compared with abstainers: no significant change was found in any HDL subfraction.
Multiple linear regression (adjusted for age, body mass index, physical activity, triglycerides, and dietary variables).
LDL-I: ∼ large LDL, LDL-II: ∼ intermediate LDL, LDL-III plus LDL-IV: ∼ small dense LDL.
Brackets refer to a trend: P > 0.05 to ≤ 0.1.
Women. In men, no change in any LPSF.
Pearson’s partial correlation (adjusted for smoking, physical activity, blood pressure, triglycerides, and body mass index).
Linear regression analysis, in women. In men, HDL2-C and HDL3-C levels increased.
Abbreviations: C, cholesterol concentration; HDL, high-density lipoprotein; ISL, intermediate-size lipoprotein; lbLDL, large, buoyant low-density lipoprotein; LDL, low-density lipoprotein; LpA-I, HDL particles with apolipoprotein A1; LpA-I:A-II, HDL particles with apolipoprotein A1 and apolipoprotein A2; LpE:B, apoB-containing lipoproteins with apoE; LpE:non-B, non–apoB-containing lipoproteins with apoE; LpC-III:B, apoB-containing lipoproteins with apoC-III; LPSF, lipoprotein subfraction; sdLDL, small, dense low-density lipoprotein; TM, total mass concentration; VLDL, very-low-density lipoprotein.
Table 5.
Summary of results on lipoprotein subfractions from observational studies with nuclear magnetic resonance and ion mobility methodsa
| Lipoprotein subfraction | Increased | No change | Decreased |
|---|---|---|---|
| Total HDL-P | Mukamal et al, 2007124; Muth et al, 201032,b; Zaid et al, 2018125,c | Millar et al, 2020127 | |
| Very large HDL-P | Du et al, 202013; Würtz et al, 2016126 | Si et al, 2021128 | |
| Large HDL-P | Du et al, 202013,d; Mukamal et al, 2007124; Muth et al, 201032,bSi et al, 2021128; Sonestedt et al, 2012129,e; Würtz et al, 2016126; Zaid et al, 2018125,c | Bogl et al, 2013130; Tighe et al, 2013131 | Millar et al, 2020127 |
| Medium HDL-P | Bogl et al, 2013130; Du et al, 202013,d; Mukamal et al, 2007124; Muth et al, 201032,b; Si et al, 2021128; Tighe et al, 2013131; Würtz et al, 2016126 | Zaid et al, 2018125,c | Millar et al, 2020127 |
| Small HDL-P | Bogl et al, 2013130; Du et al, 202013,d; Si et al, 2021128; Würtz et al, 2016126 | Millar et al, 2020127; Muth et al, 201032,b; Sonestedt et al, 2012129,e; Zaid et al, 2018125,c | Mukamal et al, 2007124; Tighe et al, 2013131 |
| HDL particle size | Du et al, 202013,d; Mukamal et al, 2007124; Muth et al, 201032,b; Würtz et al, 2016126 Zaid et al, 2018125,c | Bogl et al, 2013130; Si et al, 2021128 | Millar et al, 2020127 |
| HDL2-C | Du et al, 202013,d; Si et al, 2021128; Würtz et al, 2016126 | ||
| HDL3-C | Si et al, 2021128; Würtz et al, 2016126 | Du et al, 202013 | |
| Total LDL-P | Millar et al, 2020127; Mukamal et al, 2007124,⤿Δ | ||
| Large LDL-P | Mukamal et al, 2007124 | Bogl et al, 2013130; Millar et al, 2020127; Si et al, 2021128; Sonestedt et al, 2012129,e; Tighe et al, 2013131 | Würtz et al, 2016126 |
| Medium LDL-P | Bogl et al, 2013130 | Du et al, 202013; Si et al, 2021128; Würtz et al, 2016126 | Sonestedt et al, 2012129e |
| Small LDL-P | Bogl et al, 2013130 | Du et al, 202013; Millar et al, 2020127; Si et al, 2021128; Sonestedt et al, 2012129,e; Tighe et al, 2013131; Würtz et al, 2016126 | Mukamal et al, 2007124 |
| Medium small LDL-P | Tighe et al, 2013131 | ||
| Very small LDL-P | Sonestedt et al, 2012129e; Tighe et al, 2013131 | ||
| LDL particle size | Mukamal et al, 2007124; Sonestedt et al, 2012129e | Millar et al, 2020127 | Bogl et al, 2013130; Du et al, 202013; Si et al, 2021128; Würtz et al, 2016126 |
| Large IDL-P | Du et al, 202013 | ||
| Total IDL-P | Si et al, 2021128 | Du et al, 202013; Würtz et al, 2016126 | |
| Total VLDL-P | Mukamal et al, 2007124 | ||
| Extra-large VLDL-P | Si et al, 2021128; Würtz et al, 2016126 | Du et al, 202013 | |
| Very large VLDL-P | Si et al, 2021128; Würtz et al, 2016126 | Du et al, 202013 | |
| Large VLDL-P | Bogl et al, 2013130; Si et al, 2021128; Würtz et al, ., 2016126 | Du et al, 202013; Mukamal et al, 2007124,⤿Δ,Tighe et al, 2013131 | Millar et al, 2020127 |
| Medium VLDL-P | Bogl et al, 2013130; Si et al, 2021128; Würtz et al, 2016126 | Millar et al, 2020127; Tighe et al, 2013131 | Du et al, 202013,d; Mukamal et al, 2007124 |
| Small VLDL-P | Bogl et al, 2013130; Millar et al, 2020127; Si et al, 2021128 | Tighe et al, 2013131; Würtz et al, 2016126 | Du et al, 202013,d, Mukamal et al, 2007124 |
| Very small VLDL-P | Si et al, 2021128; Würtz et al, 2016126 | Du et al, 202013 | |
| VLDL particle size | Bogl et al, 2013130; Mukamal et al, 2007124; Si et al, 2021128; Würtz et al, 2016126 | Du et al, 202013; Millar et al, 2020127 |
Summary of results based on each lipoprotein subfraction investigated. A more detailed description on each study can be found in Table S2 in the Supporting Information online.
P < 0.05.
Multiple linear regression analysis (adjusted for hormone replacement therapy, body mass index, diabetes, smoking, exercise, age). Results similar for men and women. Analysis of variance (ANOVA): Quadratic trend in medium HDL-P and HDL particle size (Δ) and in small HDL-P (⤿). Total HDL-P was not included in the regression analysis, so ANOVA results are reported here.
Women. In men, all HDL subfractions, total HDL-P, and mean HDL-P size increased. Brackets refer to a trend: P > 0.05 to ≤ 0.1.
Significant changes in linear and polynomial regression and after adjustment for age, sex, socio-economic status, region of residence, education, occupation, marital status, smoking, physical activity, cardiorespiratory fitness, diet, depression, and anxiety (model 2).
Women. In men, there was no change in medium LDL and LDL particle size; small HDL levels increased.
Significant quadratic relationships and nonsignificant linear relationships in these outcomes. For some of the other outcomes, significant quadratic relationships are also found, but the linear relationship are reported here due to lower P values.
Abbreviations: C, cholesterol concentration; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; P, particle number concentration; VLDL, very-low-density lipoprotein.
Table 6.
| Mechanism | Increased | No change | Decreased |
|---|---|---|---|
| Cholesterol efflux capacity | Beulens et al, 2004 59 , m ; Králová Lesná et al, 2010132,cPadro et al, 2018133; Senault et al, 200049,d; Sierksma et al, 2004134; van der Gaag et al, 2001135; Hoang et al, 2008136,e; Koekemoer et al, 2017137,f,Saleheen et al, 2015138 | Serdyuk et al, 2000 61 , g ; Perret et al, 2002114 | |
| ABCA1 expression | Hoang et al, 2008136,e | ||
| LCAT: activity | Beulens et al, 2004 59 ; Serdyuk et al, 200061,g | Nishiwaki et al, 1994 52 , h ; Ito et al, 1995104; Riemens et al, 1997139 | |
| LCAT: mass | Goto et al, 2003140 | Albers et al, 1982141,i; Haffner et al, 198584 | |
| LCAT: serum/plasma cholesterol esterification | van der Gaag et al, 2001 135 ; Perret et al, 2002114 | Sierksma et al, 2004 134 | Serdyuk et al, 2000 61 , g |
| CETP: activity | Beulens et al, 2004 59 ; Nishiwaki et al, 199452,h; Serdyuk et al, 200061,g; Alarcon et al, 2004142; Vergeer et al, 2010143; Dullaart et al, 1998144; Ito et al, 1995104; Riemens et al, 1997139 | Kinoshita et al, 1996153 | |
| CET activity: cholesterol transfer to apoB lipoproteins; process of CETP | Serdyuk et al, 2000 61 , g | Sierksma et al, 2004 134 ; Perret et al, 2002114 | Hagiage et al, 1992 50 , j |
| CETP: concentration | Senault et al, 200049,d | ||
| CETP: mass | Goto et al, 2003140; Mansfield, McPherson and Koski, 1999112,k | ||
| PLTP activity | Beulens et al, 2004 59 ; Alarcon et al, 2004142; Riemens et al, 1997139 | ||
| Lp-PLA2 activity | Beulens et al, 2008 145 | Hatoum et al, 2010146 | |
| HL activity | Hartung et al, 1990 64 ; Nishiwaki et al, 199452,h; Välimaki et al, 198853; Alarcon et al, 2004142 | De Oliveira e Silva et al, 200062 | |
| LPL activity | Nishiwaki et al, 1994 52 , h ; Alarcon et al, 2004142; De Oliveira E Silva et al, 200062 | Hartung et al, 1990 64 ; Välimäki et al, 198853 | |
| LPL mass | Nishiwaki et al, 1994a52,h | Goto et al, 2003140 | |
| Specific activity of LPLl | Nishiwaki et al, 1994 52 , h | ||
| ApoA-I TR | De Oliveira e Silva et al, 200062 | ||
| ApoA-I PR | Gottrand et al, 1999 60 | ||
| ApoA-I FCR | De Oliveira e Silva et al, 200062; Gottrand et al, 199960 | ||
| ApoA-II TR | De Oliveira e Silva et al, 200062 | ||
| ApoA-II PR | Gottrand et al, 1999 60 | ||
| ApoA-II FCR | De Oliveira e Silva et al, 200062 | Gottrand et al, 1999 60 | |
| PON activity | Rajdl et al, 2007 147 ; Sierksma et al, 2002148; van der Gaag et al, 199916; Gruppen et al, 2018149; Rao et al, 2003150; Sirivarasai et al, 2011151 | Sarandöl et al, 2003 152 | |
| PON mass | Sierksma et al, 2002 148 ; van der Gaag et al, 199916 |
Summary of results based on each mechanism investigated. A more detailed description on each study can be found in Tables S3 and S4 in the Supporting Information online.
Intervention studies are in bold text.
P < 0.05.
Brackets refer to a trend: P > 0.05 to ≤ 0.1.
The red wine group: 30 g/d alcohol. The 30 g/d pure alcohol-with-mineral-water group: no change in CETP concentration and cholesterol efflux. A randomized controlled trial, but only within-group results provided in the article.
Alcohol intake > 11 g/d alcohol compared with abstention. Results from alcohol intake < 11 g/d vs abstention analysis: cholesterol efflux and ABCA1 expression were unchanged.
Multivariate analysis (adjusted for age, sex, smoking, systolic blood pressure, waist-to-hip ratio, albumin, HDL, LDL, TG, phospholipids, and lipoprotein(a)).
Subgroup with the lowest HDL level. Subgroup with high HDL (≥ 50 mg/dL): LCAT activity increased, no difference in CETP activity.
The group that received alcohol (group 2).
Men and women using hormones. In women not using hormones, LCAT mass decreased (P for trend = 0.07). Multiple linear regression analysis (adjusted for age, body mass index, and smoking).
Nonobese subgroup. Obese subgroup: cholesteryl ester transfer activity unchanged. Results represent within-group changes. Changes between groups (obese vs control participants) were also examined but not extracted.
Multiple regression analysis (adjusted for energy, protein, cholesterol, fat [saturated, monounsaturated, polyunsaturated acids], alcohol intake, waist-to-hip ratio, body mass index, and aerobic capacity). CETP increased in model 2 (adjusted for less covariates).
Specific activity of LPL: amount of substrate that the enzyme converts per mg protein in the enzyme preparation, per unit of time.
ABCA1-dependent cholesterol efflux.
Abbreviations: ABCA1, ATP-binding cassette transporter 1; apo, apolipoprotein; CET, cholesterol ester transfer; CETP, cholesteryl ester transfer protein; CHO, carbohydrates; FCR, fractional catabolic rate; HDL, high-density lipoprotein; HL, hepatic lipase; LCAT, lecithin-cholesterol acyltransferase; LDL, how-density lipoprotein; LPL, hipoprotein lipase; Lp-PLA2, lipoprotein-associated phospholipase A2; PLTP, phospholipase transfer protein; PON, paraoxonase; PR, production rate; TR, transport rate.
Figure 2.
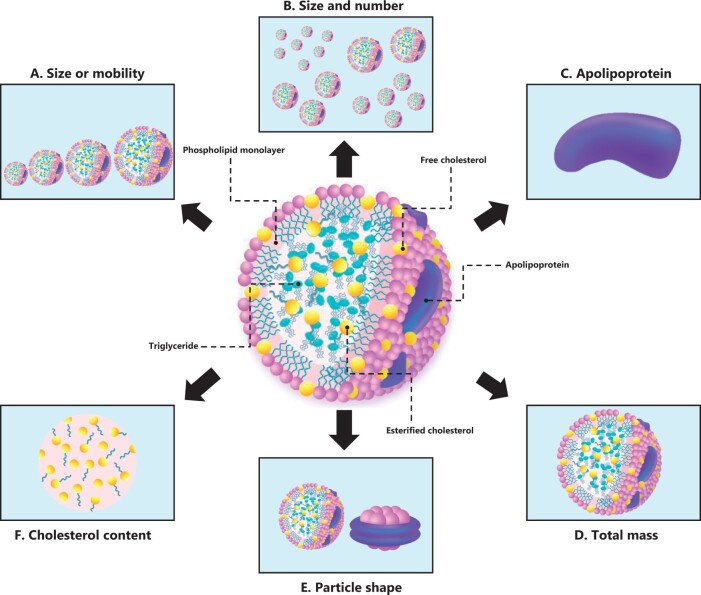
Lipoprotein subfractions included in this review. Lipoprotein subfractions are classified differently depending on the method of assessment. Some of the different types of lipoproteins subfractions included in this review are illustrated here. (A) Subfractions defined according to size or mobility with, for example, gel electrophoresis (eg, low-density lipoprotein [LDL]-I through LDL-IVB). (B) Subfractions defined according to size and particle number per unit volume, measured by nuclear magnetic resonance or ion mobility (eg, small, medium, and large HDL particle number concentration). (C) Subfractions defined by their apolipoprotein content (eg, apoA-I, apoA-II, or apoC-III) with immunoelectrophoresis (eg, LpA-I:A-II, LpC-III:B, or HDL with apoC-III). (D) Subfractions of distinct densities defined by their total mass of each subfraction with, for example, analytical ultracentrifugation or ultracentrifugation followed by enzymatic assays (eg, HDL2-TM, HDL3-TM). (E) Subfractions defined by their shape (spherical or discoidal) with 2-dimensional immunoelectrophoresis (eg, pre-β-HDL). (F) Subfractions of distinct densities defined by their cholesterol cargo; the density measured with ultracentrifugation and the cholesterol content in each subfraction measured with, for example, enzymatic assays (eg, HDL2-C, HDL3-C, VLDL1-C). (Adapted from Camont et al159 and Rizzo et al.28 Illustrations by graphic designer Susanne Riber, www.susanneriber.dk)
Study characteristics and participants
Results from the individual LPSF studies are shown in Tables S1 and S2 in the Supporting Information online. LPSF outcomes were measured in 28 intervention studies, shared among 14 RCTs, 3 nonrandomized trials,52,56,62 9 nonrandomized studies with sequential crossover, and 2 Mendelian randomization studies (MRSs).66,67 We included 64 observational LPSF studies, the majority of which were cross-sectional. Würtz et al126 performed a cross-sectional analysis but also captured metabolic changes at a 6-year follow-up examination. In addition, 2 cohort studies102,118 and 4 case-control studies were included. In all case-control studies and 1 cohort study,102 the alcohol data extracted were from cross-sectional analyses. Last, 1 RCT study is listed among the observational studies, because the only available data on alcohol and LPSFs were cross-sectional and from baseline.131 Multiple reports from the same study were identified; these redundant entries are combined in Table S2 in the Supporting Information online as Kee et al107 and Marques-Vidal et al108; Luc et al154 and Marques-Vidal et al (2001)109; Onat et al (2003)105 and Onat et al (2009)102; and Rossouw et al90 and Steenkamp et al.76
Intervention studies: characteristics.
The intervention studies with LPSF outcomes were published between 1983 and 2017, and study size varied between 5 and 112 participants (Table S1 in the Supporting Information online). The 2 MRSs with 8,400–10,900 participants are listed with the intervention studies. Overall, the age of the participants ranged between 18 and 75 years, and the majority of studies included healthy participants only. Three studies included women only48,51,57 and 17 studies included only men. The shortest duration of the alcohol interventions was 10 days; the longest was 6 months. Alcohol was provided in amounts from 12.6 to 60 g/d, but the majority of studies provided 20–40 g/d and lasted for at least 2 weeks. The type of alcohol consumed varied among studies. Several different laboratory techniques for LPSF analyses were used. Depending on the characterization metric, techniques for separation and quantification included different precipitation techniques, immunogenic assays, radiolabeling, ultracentrifugation techniques, chromatography methods, and more recent technologies such as NMR or IM.
Of the 28 intervention studies with LPSF outcomes, compliance information was found in 10 studies. This information was based on self-reports in 6 studies, on self-reports and blood biomarkers in 3 studies,35,44,55 and on a blood biomarker alone in 1 study.60 Alcohol was served at the study site in 3 studies.56,59,62 Compliance was only explicitly described in 1 of these studies,59 but compliance appears to have been closely monitored in the remaining 2 studies. In general, the studies reported good compliance with the alcohol intervention.
Observational studies: characteristics.
The 64 observational studies investigating LPSF outcomes were published from 1981 to 2021, and number of participants ranged from 25 to 9778 (Table S2 in the Supporting Information online). Participants’ age ranged from 18 to 85 years. Five studies included only women, and 23 studies included only men. The observational studies include 1 IM study129 and 9 NMR studies. The period of self-reported alcohol exposure was highly diverse across studies, with a maximum of 1-year recall. However, exact duration was not consistently reported in all the articles. The results represent alcohol per se, because no specific type of alcohol was investigated in any study. Healthy populations were included in most studies. A range of laboratory methods was used, including enzyme-linked immunosorbent assay, immunoaffinity chromatography, immunoelectrophoresis, IM, or NMR.
Outcomes related to each LPSF
HDL subfractions: intervention studies.
Measured subfractions of HDL included HDL2 and HDL3. The cholesterol content of HDL2, HDL2-C, was measured in 19 intervention studies, and increased HDL2-C levels were found in 9 studies, whereas no change was reported in 9 studies. A decrease was shown in a single study in which the effects of alcohol restriction were compared with moderate intake (Table 3 and Table S1 in the Supporting Information online).55 The reported increases in HDL2-C concentration ranged from 0.039 to 0.155 mmol/L, with a median of 0.06 mmol/L. Studies that showed increasing concentrations of HDL2-C had a median of 45 participants (range, 20–10 893), whereas the median was 25 participants (range, 10–60) in the remaining studies. Increasing HDL2-C levels were shown in studies of both low (range, 13.5–19 g/d)36,69 and higher (range, 45–50 g/d)35,64 amounts of alcohol intake, but no effects on HDL2-C were reported in other studies with alcohol intakes of 45–60 g/d.47,53,54 Increases were observed in studies of shorter duration (2–4 wk) and studies with moderate duration (6–12 wk).35,57,69 The majority of studies reporting increased HDL2-C levels were RCTs or MRSs. Studies of women only57 or men only35,64,68 reported increased concentrations of HDL2-C.
HDL3-C was measured in the same 19 studies as HDL2-C. Increased concentrations were found in 8 studies, and no effect was found in 9 studies. Decreased levels were reported in 2 studies,50,55 1 of which investigated alcohol restriction.55 HDL3-C was increased in the range of 0.049–0.132 mmol/L, with a median increase of 0.078 mmol/L. The median population size of the studies finding positive effects was 45.6 (range, 10–8364), whereas the median population was 36 (range, 12–10 893) in the studies with nonsignificant results. Alcohol doses > 30 g/d were provided in 7 of the studies in which increased HDL3-C concentrations were reported; none of the studies providing amounts < 30 g/d reported any increases. Increased HDL3-C level was reported in studies of 2–4 weeks’ and studies of 6–12 weeks’ duration.35,57 The 9 studies in which researchers reported finding increased HDL3-C concentrations included 1 study of women only57 and 6 studies of men only, and 5 of the studies were RCTs or MRSs.
Total mass concentrations of HDL2 and HDL3 were measured in 6 studies; increased levels of HDL3 were reported in 2 of these (Table 3).53,58 The LpA-I and LpA-I:A-II subfractions were investigated in 7 studies (Table 3). Increased levels of LpA-I and LpA-I:A-II were found in 4 and 5 studies, respectively, whereas no change was found in 350,60,61 and 2 studies,50,61 respectively. The increases ranged from 0.03 to 0.04 g/L (median, 0.035 g/L) for LpA-I, and from 0.03 to 0.248 g/L (median, 0.05 g/L) for LpA-I:A-II. The respective median population size was 34 (range, 10–56) and 12 (range, 5–20) for significant and nonsignificant results, respectively, for increased LpA-I. The corresponding, respective median population sizes for LpA-I:A-II were 24 (range, 5–56) and 17 (range, 14–20). Positive results were primarily found in studies with a randomized, controlled design.
Two intervention studies measured HDL subfractions and HDL size by NMR, with no effects reported for alcohol doses of 15 g/d or ≈ 30 g/d (Table 3).44,62 Low compliance was reported in 1 of these studies.44 A third study showed increased HDL size measured with gradient gel electrophoresis after a dose of 30 g/d.57
Overall, most intervention studies found increased levels of all types of HDL subfractions independent of analytical method, dose, and study duration. These findings were supported by 2 MRSs.66,67 A limited number of studies included participants with disease; therefore, stratification by disease status was not possible. Most of the study populations were healthy, though participants with high CVD risk were included in 2 studes,44,70 and participants with mixed health status were included in 1 study, with 9% having diabetes.67
HDL subfractions: observational studies.
HDL2-C was measured in 36 studies, 21 of which reported that higher alcohol intake was associated with higher HDL2-C levels. No associations were found in 14 studies, and a negative association was found in 1 study (Tables 4 and 5).96 Schäfer et al97 found positive associations of alcohol intake with higher HDL2a-C and HDL2b-C levels. The median population size was 1032 (range, 32–9778) in studies with increasing concentrations of the HDL subfractions with alcohol intake, and 246 (range, 32–1386) in the studies that found no associations. Of the 5 studies conducted with women, a positive association between alcohol intake and HDL2-C concentration was found in 1 study,121 whereas higher HDL2-C levels were found in 7 of the 12 studies conducted with men.
HDL3-C concentration was measured in 37 studies, of which 27 reported positive associations with alcohol intake, and 10 studies found no associations (Tables 4 and 5). The median population size was 932 (range, 30–9778) in studies with positive associations between alcohol intake and HDL3-C, and 290 (range, 32–2044) in studies with no change in HDL3-C. Four of the 5 studies with women only and 8 of the 24 studies with men only reported significant results for HDL3-C relative to alcohol intake.
Two studies investigated the total mass of HDL2a–2b and HDL3–3c, but the findings were inconsistent (Table 4).98,99 In contrast, LpA-I was measured in 10 studies and positive associations between alcohol intake and LpA-I levels were found in 4. No relationships were found in 5 studies, and a negative association was found in 1 study (Table 4).116 The studies that found positive associations between alcohol intake and LpA-I levels had a median size of 395 (range, 100–6729), compared with a median size of 409 (range, 25–8357) in the studies with nonsignificant results. LpA-I:A-II was examined in 8 studies, 5 of which reported positive associations with alcohol intake, and no relationships were reported in 3 studies.107,111,113 The median population size was 344 (range, 46–8357) and 409 (range, 175–536) in the significant and nonsignificant studies, respectively. All except 2 LpA-I and LpA-I:A-II studies111,113 were conducted with men only.
HDL subfractions were defined by the apolipoprotein C-III (apoC-III) content in 4 studies (Table 4 and Table S2 in the Supporting Information online). Koch et al103 found that greater amount of alcohol intake was associated with higher levels of apolipoprotein A-I (apoA-I) in HDL without apoC-III, but not with apoA-I HDL containing apoC-III. Positive associations with HDL cholesterol with and without apoC-III were reported in the study by Jensen et al,101 in which 50% of participants had CHD. Onat et al found positive correlations with both apoC-III in HDL and non-HDL in men but not in women in 1 study,105 and positive associations with apoC-III in HDL in men, and men and women combined, in another study.102
HDL subfractions characterized by size and particle numbers were available from 1 IM study129 and 9 NMR studies involving 233–9778 volunteers (Table 5 and Table S2 in the Supporting Information online). Alcohol intake was associated with increases in all types of HDL subfractions. Nonsignificant associations were found only for a few individual outcomes, and 2 studies reported negative associations between alcohol intake and small HDL-P.124,131 The study by Millar et al127 was the only study with contrasting results; they reported a negative association of alcohol intake with most HDL subfractions. Overall, with increased alcohol intake, the increase in the medium and larger HDL-P occurred more frequently than the increase in the smaller HDL-P, and increased average HDL particle size was reported in 5 studies. Total HDL-P were measured in 3 studies, all of which found increased levels with moderate alcohol intake.32,124,125
Sex-specific analyses were performed in 4 NMR studies. Minor differences between men and women were reported in some of these studies. However, women often had a lower average alcohol intake than did men.32,125,129 Mukamal et al124 found no substantial differences in HDL subfractions between men and women. Du et al13 found no interaction with sex, and Würtz et al126 found similar results for men and women.
In summary, alcohol intake was associated with increased levels of almost all types of HDL subfractions (Tables 4 and 5). The evidence for HDL subfractions characterized by total mass or apoC-III content was sparse (Table 4). Populations of mixed health status were included in 18 studies, and participants with dyslipidemia73 and hypertension85 were included in 2 (Tables S1 and S2 in the Supporting Information online). A health status description was missing in 12 of the included studies. No pattern between disease status and outcomes was found.
LDL subfractions: intervention and observational studies.
LDL subfractions were investigated relative to alcohol intake in 2 intervention studies44,63 and 2 MRSs66,67 (Table 3). No effect on any NMR-measured LPSFs was found in a 6-month RCT with presumed low compliance regarding alcohol intake.44 An increased ratio of LDL-C to apolipoprotein B (apoB) was found in the other study, implying an increase in LDL size with moderate alcohol intake.63 One of the 2 MRSs found decreased levels of large, buoyant LDL cholesterol in Japanese men and women and increases in small, dense LDL cholesterol (sdLDL-C) in men.66 In that study, the aldehyde dehydrogenase 2*1 allele was used as a proxy for alcohol consumption in men but not in women. The other MRS found a trend toward decreased sdLDL-C in both men and women, using 5 different single-nucleotide polymorphisms on different alcohol dehydrogenase genes as exposure markers.67
LDL subfractions were included in 6 observational studies, excluding NMR and IM studies (Table 4). No consistent pattern was observed, but one study found a positive association between alcohol intake and large LDLs155 in men,117 and another study found a decrease in sdLDL-C in association with alcohol intake.33 A trend toward decreased LDL particle size was found in a third study.118 Five studies investigated LPSFs, defined by apolipoprotein E108,109,119 or apoC-III102,105,108 content, but reported inconsistent results. Results from 8 NMR and IM studies were heterogeneous (Table 5); associations in opposite directions and nonsignificant or even nonlinear relationships were reported relative to alcohol intake. Mukamal et al124 found a U-shaped association of alcohol intake with total LDL-P, with a different pattern depending on the particle size. The concentration of large LDL-P was highest in consumers of ≥ 1 drink/wk, and that of small LDL-P was lowest in consumers of 7–13 drinks/wk. The net result was increased average LDL size. The LDL-C measure did not capture the shift in the distribution of LDL subfractions. Generally, the associations were similar in men and women, but stronger in women. Würtz et al126 reported complex relationships to apoB-carrying lipoproteins. Most of these associations had a U-shaped pattern in the first segment of the slopes up to 100 g/wk and lowest lipoprotein levels at ∼ 50 g/wk (7 g/d). The decreasing limb of these curves was generally steeper in women than in men.
VLDL subfractions: intervention and observational studies.
VLDL subfractions were only measured in 1 intervention study, which found no effect of alcohol at a dose of 20 g/for 2 weeks or 35 g/d for 1 week (Table 3).65 Inconsistent results were found in 7 observational NMR studies. Positive and negative associations of alcohol intake with all types of VLDL subfractions and VLDL size were found, but positive associations were reported most frequently (Table 5). Positive associations of alcohol intake with medium and large VLDL-P plus average VLDL particle size was observed in the largest NMR study by Würtz et al126 (N = 9778). The relationship to medium VLDL was nonlinear, with a U-shaped curve at alcohol intakes between 0 and 100 g/wk. In partial agreement with this, Mukamal et al124 reported a quadratic or U-shaped relationship of alcohol intake to large VLDL-P and larger average VLDL particle size, but inverse associations with small, medium, and total VLDL-P. Consumers of 1–13 drinks/wk had the lowest level of large VLDL-P, and the decreased total number of VLDL-P was driven by reduced levels of medium and small VLDL-P.
Mechanisms.
Results from individual studies investigating potential mechanisms for the relationship between alcohol intake and LPSFs are presented in Tables S3 and S4 in the Supporting Information online, and a summary is provided in Table 6. Most intervention and observational studies examined cholesterol ester transfer protein (CETP), hepatic lipase, lecithin-cholesterol acyltransferase (LCAT), phospholipid transfer protein (PLTP), and lipoprotein-associated phospholipase A2 (Lp-PLA2). No consistent relationships with alcohol intake were found for any of these. Results were more robust for cholesterol efflux capacity (CEC) and paraoxonase (PON). CEC was increased with alcohol intake in 9 studies, whereas 2 studies found no change.61,114 A positive association with the cholesterol efflux regulatory protein, ATP-binding cassette transporter A1 (ABCA1) was reported in 1 study.136 PON activity and PON mass were increased in 6 studies, and no change was found in 3.16,148,152 Three studies found increased levels lipoprotein lipase (LPL) activity and mass with alcohol intake,52,62,142 whereas no such changes were reported in 3 other studies.53,64,140
Bias risk assessment of individual studies
Bias risk assessment results for all the included studies are shown in Figures S1–S6 in the Supporting Information online. Several of the RCTs with LPSF outcomes were older and inadequately reported, and none of the studies were of high quality in terms of bias risk (Figure S1 in the Supporting Information online). For example, randomization procedures were not described adequately in any of the included RCTs, resulting in unclear risk of selection bias. Likewise, several studies had unclear or high risk of reporting bias caused by a lack of consistency between planned and reported outcomes. Because of the nature of alcohol interventions, the risk of performance bias is high in all RCTs. Last, all RCTs had unclear or high risk of other bias due to shortcomings such as unidentified carryover effects in cross-over studies57,60; baseline imbalances55; potential selection bias due to insufficient description of participant recruitment36,60,64; or potential confounding from changes in body weight, physical activity, smoking status, or dietary intake. The background diet was only fully controlled in 4 studies and partially in 1.59 In the remaining studies, participants were asked not to change their dietary intakes.
The different nonrandomized investigations (Figure S2 in the Supporting Information online) were a mix of (1) non-RCTs with a crossover or parallel design, (2) nonrandomized studies with sequential crossover, (3) case-control studies, and (4) cohort studies.102,118 Most of these studies had unclear or high risk of selection bias due to inadequate description or selection of participants, compromising external validity, or insufficient consideration or handling of potential confounders, compromising internal validity. A priori, all the studies were assessed with high risk of performance bias due to inadequate possibilities for blinding for the drinking of alcohol. In contrast, the risk of detection bias was consistently considered low due to the objective nature of lipoprotein outcomes. Only a few studies had unclear risk of attrition bias or unclear or high risk of reporting bias. Overall, only 2 of the randomized studies were of high quality regarding bias risk.101,115
Overall, many observational studies were assessed to have lower risk of bias, including the most recently published NMR studies. However, some of these studies were older and did not follow current reporting standards (Figure S3 in the Supporting Information online). The descriptions of eligibility criteria and study populations were generally insufficient. Alcohol exposure was measured via self-report and scored with high risk of bias on the validity and reliability of the exposure variable. In contrast, all studies except 169 identified potential confounders and adjusted for them in their analyses. In a few included studies, researchers performed inappropriate statistical analyses or did not describe them. The LPSFs were generally measured reliably (ie, with laboratory methods found reliable when the study was conducted), but some included studies did not specify the laboratory method used.81,100,120
No obvious systematic patterns relating risk of bias with study outcomes in any study designs were observed. Only a few studies had low risk of bias, and these appeared more frequently among the more recent observational studies.
DISCUSSION
To our knowledge, this systematic review is the first to examine LPSFs after moderate alcohol intake. Most intervention studies provided alcohol in an amount of 20–40 g/d for at least 2 weeks, even though the limit for eligibility was set to ≤ 60 g/d. The reviewed articles explored several different LPSFs, mainly characterized according to total size and mobility; size and number; apolipoprotein content; total mass; shape; or cholesterol content and size (Figure 2). Alcohol in amounts up to 60 g/d was related to increased levels of almost all HDL subfractions measured, independent of the analytical approach. Results on LDL subfractions were sparse, especially from intervention studies. However, a few observational studies and MRSs found reduced levels of sdLDL-C, increased average LDL size, and U-shaped or other nonlinear relationships for alcohol intake and apoB-containing lipoproteins. Mechanistic studies show a pretty clear pattern of increased CEC and PON activities across all study designs. Because these was an insufficient number of relevant studies, it was impossible to fulfill the aim of analyzing data according to disease status.
Comparison with other studies
High-density lipoproteins.
The overall classes of lipoproteins were not included in the search strategy because they have been covered in 3 previous meta-analyses of intervention studies summarizing the effect of moderate drinking on several biological markers of CVD risk (Table 2). These meta-analyses mainly included healthy individuals, and the dose of alcohol provided was up to 100 g in some of the included studies.17,20 They all reported increased levels of HDL-C and apoA-I (Table 2), in concordance with findings in several nonsystematic reviews.22,23,27,30 On the basis of these results, we hypothesized that the increase in HDL could be explained by a more robust increase in some types of HDL subfractions than in others. Most studies in the present review do not support this hypothesis, because an increase with moderate alcohol intake is commonly reported for all the HDL subfractions. That said, increased levels of HDL3-C were reported more frequently than for the larger HDL2-C, especially in observational studies (Tables 3–5).
On the other hand, increases in medium and larger HDL-P compared with the smaller HDL-P occurred more often in the NMR and IM studies (Table 5). Würtz et al126 found more pronounced associations of alcohol intake and the medium and small HDLs in the largest NMR study, underlining that there are no consistent differences between the effects of moderate alcohol intake on the different HDL subfractions. Similarly, no clear distinction was found for LpA-I compared with LpA-I:A-II, and the reported effect sizes of HDL2-C and HDL3-C do not differ substantially. These findings are further corroborated by Hannuksela et al22 in their narrative review, whereas Brinton et al27 reported more significant increases in HDL3 compared with HDL2. In neither of these reviews was the evidence gathered systematically, and the definition of moderate alcohol intake was not clear in these reports, which undermines the weight of their conclusions.
Low-density lipoproteins.
The LDL subfractions have not been included in previous meta-analyses of moderate drinking, and results on total LDL-C are inconsistent.17,19,20 No effect on LDL-C was found in 2 of these meta-analyses,17,20 whereas Huang et al19 showed decreased levels in a meta-analysis of studies with alcohol doses up to only 30 g/d. The inconclusive findings on total LDL could be due to opposing changes in small and large LDL subfractions or to nonlinear dose-response relationships masking physiologically relevant findings. The evidence is sparse in the present review, but some studies indicate decreased levels of smaller LDLs,33,67,124 increased levels of lbLDL,117,124 and an increase in overall LDL particle size (Tables 3–5). 63,124,129 However, this pattern needs further substantiation. In addition, standardization of subfraction classification is required, because a direct comparison of different types of smaller LDLs, such as sdLDL-C33,67 and small LDL-P124 is troublesome. Results on LDL subfractions were not included in 2 narrative reviews, but the authors reported decreased or unchanged total mass of LDL or total LDL-C with moderate drinking.22,27 No clear definition of types of LDL particles, alcohol dose, or duration of alcohol intake was made in these reviews, which complicates direct comparison. It has been suggested that changes in LDL particles seem to vary more with population, sex, and drinking pattern than changes in HDL particles.27 Despite the original aim, too few studies investigating LDL subspecies were included to allow for stratified analyses on population characteristics.
Very-low-density lipoproteins.
Results on VLDL subfractions come primarily from NMR studies and are sparse and inconsistent (Table 5). VLDL was not included in the meta-analyses of alcohol intervention studies.17,19,20 Under normal circumstances, VLDL particles carry most of the plasma triglycerides (TGs) in their core,156 and larger VLDL particles generally contain more TG.157 Overall TG levels were unchanged after moderate alcohol intake in 2 meta-analyses of intervention studies.17,19 In contrast, increased TG levels were shown with alcohol intake in an older meta-analysis (Table 2).20 Brien et al17 reported increased TG levels only when alcohol intake exceeded 60 g/d. Likewise, in 1 included observational study, the authors found decreased levels of all VLDL subfractions and overall TG levels in drinkers of 7–13 drinks/wk compared with abstainers.124 Results from 2 of the largest observational NMR studies found positive or U-shaped associations of alcohol with medium or large VLDLs.124,126 Taken together, TG levels could be unaltered or decreased after moderate alcohol consumption and increased at higher intakes. A decreased TG level would be in line with the well-known inverse relationship between TG levels and HDL-C.158 However, if the HDL-C level increases in a linear manner with alcohol consumption,20 and the relationship to TG is U-shaped, there is no longer concordance with the inverse-relationship hypothesis. Effects on VLDL subfractions need further examination. Even though U-shaped patterns with alcohol intake were observed, results were inconsistent.
Clinical implications
HDL and CVD.
HDL subfractions not classified solely by their dynamic cholesterol content and mechanistic or functional outcomes of HDL have been suggested as potential biomarkers for assessing cardiovascular risk.159,160 Traditionally, HDL-C has been used in cardiovascular risk equations.161 The inverse association between HDL-C and CHD is well known162 and has been ascribed to the significant role of HDL in reverse cholesterol transport.159 In recent years, the causal role of HDL-C in the development of atherosclerosis has been questioned.163 Several clinical drug trials have shown no or negative effects on CVD outcomes despite increased HDL-C levels,164,165 and an MRS did not prove a causal relationship between HDL-C and myocardial infarction.166 In addition, the association of HDL-C with all-cause mortality and CVD has been shown to be J-shaped, with a minimum in risk at 54–58 mg/dL and 68–71 mg/dL, respectively,167 and the relationship between alcohol and HDL-C is linear.20 High levels of dysfunctional HDL-C may even increase the risk of CVD.168 When investigating the potential beneficial effects of moderate drinking on cardiovascular health, it would seem, therefore, too simplistic to include HDL-C exclusively.160 Several antiatherogenic roles of HDL that are not captured by measuring the cholesterol content have also been described. These include anti-inflammatory, vasodilatory, and antioxidative functions, and they may be subfraction specific.159 Examining the effect of alcohol on HDL subfractions, therefore, is relevant.
In this review, it was not confirmed that increases in the small rather than the large HDLs could explain a potential cardiovascular benefit of moderate drinking. Some studies found that HDL3-C is more frequently increased than HDL2-C, but increases in the medium and large HDL-P were reported more often in other studies. In addition, increased HDL size was reported by several studies (Tables 3 and 5) Authors of a previous nonsystematic literature review concluded that HDL2-C and HDL3-C did not significantly improve risk prediction over HDL-C but also pointed to the inadequacy of cholesterol measurements to sufficiently identify HDL heterogeneity and CVD risk.25 Several larger, secondary analyses examining the relationship between the different types of HDL subfractions and CVD risk have been conducted.169–174 With 1 exception,172 their results indicate stronger inverse associations of CVD with smaller HDL subfractions, such as HDL3-C and small HDL-P, compared with larger HDL subfractions and overall HDL-C. That said, only a few of these analyses were adjusted for apoB.170 Findings relating to the role of LpA-I and LpA-I:A-II in CVD risk are less convincing,29,110,175 and these subfractions were not included in a proposed new nomenclature of HDL subfractions.29 Authors of a narrative review suggested a more beneficial role of smaller HDLs and refer to their importance in CEC and their antioxidant, anti-inflammatory, and antithrombotic actions.159 However, the clinical relevance of specific HDL subfractions still needs to be determined.159
LDL and CVD.
LDL-C has been firmly established as a causal factor for atherosclerosis and CVD, with convincing evidence from genetic studies, prospective epidemiological studies, MRSs, and RCTs of LDL-lowering therapies.176 The alcohol-induced change in LDL subfractions is much less clear than for the HDL subfractions. Still, some evidence for reduced levels of smaller LDL, increased levels of larger LDL, and increases in overall LDL particle size was seen. Whether LDL subfractions are better predictors of risk than LDL-C is debatable.24 Small LDLs enter the arterial wall more easily177 and bind more avidly to arterial wall macrophages and proteoglycans and glycosaminoglycans.178 They have a lower affinity for the LDL receptor, causing longer residence time.179 Overall, the majority of studies have associated smaller LDL with higher CVD risk,24,180 compared with larger LDL.181,182 A recent narrative review found independent associations to CVD clinical outcomes for small LDL subfractions and LDL-P,180 despite minor inconsistencies between studies. Smaller LDLs do often coexist with higher TG levels and lower levels of HDL-C (the atherogenic lipid triad), however, so disentangling an independent effect of small LDLs is difficult.24
Quantification of LDL subfractions may strengthen our understanding of the association between moderate alcohol intake and CVD, but improved risk predictions compared with standard lipid measurements have yet to be made.180 Several studies reported elevated levels of sdLDL in disorders such as diabetes and metabolic syndrome,183 which are metabolic states with a high prevalence of the atherogenic lipid triad.184 Several alcohol studies have shown inverse associations between moderate alcohol intake and metabolic syndrome,185 glycemic markers,186 and diabetes.3,187 These results strengthen the hypothesis that moderate alcohol intake could have beneficial health effects partially due to changes in lipoproteins such as smaller LDLs. That said, a cause-and-effect relationship is still missing.
In this review, interesting post hoc results on overall apoB levels were also observed. All lipoprotein particles carrying apoB, and the cholesterol content within them, play a central role in atherosclerosis.177,188 ApoB has been proposed as a better marker of atherogenic risk than HDL-C and LDL-C,189 and apoB measurements are now included in clinical guidelines.18,190 LDL-P can be used as a surrogate for apoB under normal circumstances.189 One of the included NMR studies found a negative association of alcohol with apoB,13 and another study showed a U-shaped relationship, with a nadir at intakes between 50 and 100 /wk.126 Total LDL-P was inversely related to alcohol consumption in a quadratic pattern in another study, with the lowest level at 1–6 drinks/wk.124 Conversely, older intervention studies have found unchanged apoB levels,20 but results on apoB were not included in the 2 most recent meta-analysis of intervention studies (Table 2).17,19 These results might have clinical implications for moderate alcohol intake and should be investigated further.
VLDL and CVD.
Large and total VLDL-P have been associated with insulin resistance,191,192 and increased large VLDL-P levels have been positively associated with markers of CVD severity in smaller cohorts.193,194 A higher concentration of large VLDL-P is also associated with a decrease in small LDL-P levels and an overall increase in the number of LDL particles, changes that are important for CVD risk.192 Equally, all VLDL-P measured by NMR and a higher average VLDL particle size were positively associated with a higher risk of incidence of CVD in the Women’s Health Study.172 Positive associations to all types of VLDL subfractions and increased VLDL size were seen here (Table 5), implying a potential downside of moderate drinking. At the same time, nonlinear results were found in some observational NMR studies. A positive association to large VLDL-P and a U-shaped relationship to medium VLDL-P was found in 1 study,126 and Mukamal et al124 found a quadratic or U-shaped relationship to large VLDL-P. As for the TGs described previously in the present review, these results could indicate either decreased or unchanged VLDL subfractions after moderate alcohol intake with increased levels at higher intakes, though this is speculative. Mukamal et al124 also found negative associations to small, medium, and total VLDL-P, which could be beneficial (Table 5), but the changes in VLDL subfractions after moderate drinking, and the clinical relevance, still have to be clarified.
Apolipoprotein C-III.
ApoC-III resides on lipoproteins like HDL, LDL, and VLDL,195 and stimulates atherogenesis directly via mechanisms such as recruitment of monocytes and activation of endothelial cells.196 In this review, results on HDL with and without apoC-III were limited and inconsistent, but alcohol intake was positively associated with HDL lacking apoC-III in 2 studies (Table 4). 101,103 On the other hand, 3 studies found higher levels of HDL containing apoC-III.101,102,105 These studies included diverse population types and different laboratory procedures for HDL subclassification, making direct comparison troublesome. One of the studies classified the HDL subfractions by cholesterol content101 (Table S2 in the Supporting Information online). Another study that was excluded from this review because the analysis included people with alcoholism also reported increasing levels of non-apoB lipoproteins (HDLs) containing apoC-III, termed LpC-III, with higher levels of alcohol intake (P ≤ 0.001).197
The clinical impact of apoC-III in HDL on CVD risk is not fully clarified. However, analyses from the Nurses’ Health Study and the Health Professionals Follow-up Study showed that HDL-C lacking apoC-III was inversely associated with CHD risk, and HDL-C containing apoC-III was directly associated with CHD risk. The results remained significant after adjustment for TGs and apoB.101 These results were confirmed in another analysis of 4 different cohorts.198 When residing on apoB-containing lipoproteins, apoC-III modulates TG metabolism through delayed TG lipolysis and inhibited catabolism of TG-rich lipoproteins.199 In the present review, 3 observational studies investigated apoB-containing lipoproteins containing apoC-III and found positive associations105 or nonsignificant results102,108 with alcohol intake (non-HDL plus apoC-III and LpC-III:B) (Table 4). A study not included here found more or less similar levels of apoC-III in apoB-containing lipoproteins (LpC-III:B) even though the levels were significant in the overall analysis of variance. In summary, the evidence is limited, and the influence of moderate alcohol intake on apoC-III levels should be addressed in future studies.
Biological mechanisms
The metabolism of lipids and lipoproteins is complex, and only a limited number of regulating factors have been examined after moderate alcohol intake.200 The primary proteins and enzymes involved (ie, LCAT, CETP, PLTP, LPL, and hepatic lipase)22 were systematically included in this review (Table 6). No evident increase in LCAT was observed after moderate alcohol intake. LCAT is responsible for the maturation of the HDLs. After activation by apoA-I, and in cooperation with the ABCA1 transporter, LCAT converts free cholesterol from blood or tissues into cholesteryl esters, making them ready for storage in the core of lipoproteins.201 The small, discoidal pre-β-HDL is the primary substrate for LCAT, an enzymes that catalyzes the conversion and maturation of small HDLs into larger, spherical HDLs. On this basis, it has been postulated that LCAT is a critical enzyme in reverse cholesterol transport, where cholesterol is removed from the periphery and transported back to the liver.201
Another regulatory protein in this pathway is CETP. CETP transfers cholesteryl esters from HDL to apoB-containing lipoproteins in exchange for TGs.202 Inhibition of CETP can increase HDL-C levels165 and would be a plausible mechanism for how moderate drinking could increase HDL levels. However, this is not supported by findings of the present review (Table 6). PLTP is also unchanged after moderate drinking in studies from the present review. PLTP is another member of the lipid transfer protein family and mediates the exchange of phospholipids and other lipids between lipoproteins.203 PLTP mediates the transfer of phospholipids from apoB-containing lipoproteins to HDL, and PLTP deficiency reduces HDL-C levels. In addition, PLTP might also have a role in macrophage cell cholesterol efflux, although results from animal studies have been conflicting.203
Likewise, the literature on moderate alcohol intake and LPL activity or mass is conflicting (Table 6). LPL hydrolyses TG in chylomicrons and VLDL and plays an essential role in TG metabolism.200 In the present review, we found evidence for increasing or unchanged LPL activity, which is concordant with decreased or unchanged TG levels, respectively.17,124 A previous narrative review reported increased LPL in heavy drinkers and unchanged or increased levels in moderate drinkers.22 At excessive alcohol intakes, TG synthesis may exceed LPL activity, whereas this might be more or less outbalanced in moderate drinkers.204 The same review found unchanged or reduced hepatic lipase activity after moderate alcohol intake.22 Hepatic lipase is a lipolytic enzyme that catalyzes the hydrolysis of TGs and phospholipids in IDL, LDL, and HDL, leading to smaller particles.200 Hepatic lipase also stimulates hepatic uptake of cholesteryl esters from larger HDLs, thereby converting them to smaller HDL particles that can potentially take up more cholesterol from other cells.205 This review demonstrates increased levels of smaller HDLs after alcohol intake, which could theoretically be explained by increased hepatic lipase activity, but the current evidence does not confirm this (Table 6).
Because the initial step in HDL synthesis requires its main structural component, apoA-I, increased flux of apoA-I could explain the increased HDL levels. An increased apoA-I transport rate was only found in 1 of the studies reviewed here (Table 6).62 More convincing results have been noted for CEC, the initial step in reverse cholesterol transport, and a measure of HDL function.206 Moderate alcohol intake consistently induced increased CEC, which was shown for doses of 15–40 g/d in the intervention studies (Table 6). The increase in CEC could explain the concomitant increase in HDL-C and HDL-C subfractions after moderate drinking. However, the metabolic fate of cholesterol after removal from the arterial wall is complex.200 The role of CEC has been reviewed in detail elsewhere.159 Briefly, small and larger HDLs are probably capable of promoting CEC, depending on the efflux regulatory protein: ABCA1, ATP-binding cassette transporter G1, or scavenger receptor class B type 1.159 For example, smaller HDLs, such as apoA-I and pre-β-HDL, are inducers of CEC via interaction with ABCA1, and both of these are increased after moderate alcohol intake.17,19,20,59
Adiponectin, which is also increased with moderate alcohol intake,17 upregulates ABCA1 expression and thus facilitates apoA-I–mediated CEC.207 In addition, the phospholipid content of HDL has been associated with the capacity of HDL to promote CEC.206 Compositional changes in HDL are beyond the scope of this review. However, it is worth noting that some of the included studies found increased phospholipid content of the HDL molecule after moderate alcohol intake.33,97,134,208 Thus, specific particle characteristics of the HDL subfraction, stimulated by moderate drinking, could potentially be inducers of cholesterol efflux.
The methods for CEC estimation have several limitations because ex vivo experiments may not entirely resemble a living organism.206 However, in theory, CEC may be a more relevant marker than HDL-C for removal of cholesterol from macrophages, and it has been suggested to be the key pathway by which HDL reduces the cholesterol content of the arterial wall and thus prevents atherosclerosis.159 CEC has also been inversely associated with CVD risk in several studies, independent of HDL-C and apoA-I.138,206 However, measurement of CEC says little about the fate of the cholesterol molecules following efflux from macrophages or other cells. Nonetheless, the potential cardioprotective effects of moderate drinking might involve stimulation of the initial step in reverse cholesterol transport. This function may be more relevant than the overall abundance of HDL-C.
Antiatherogenic effects of HDLs may not only be attributable to the reverse cholesterol pathway, because antioxidant effects have also been described.159 In this context, the esterase enzyme PON may be important. PON is attached to HDL and protects LDL and HDL from oxidative damage.209 PON activity or mass was consistently increased in the reviewed studies and could be among the mechanisms underlying the reduced CVD risk in moderate drinkers. Its clinical relevance is still questionable, but a negative association with CVD risk has been shown.210 Furthermore, PON has been suggested to be a stimulator of CEC.211 Another enzyme attached to both LDL and HDL is Lp-PLA2. Lp-PLA2 has been suggested to be an inflammatory marker that contributes to atherogenesis by increasing inflammatory processes in the arterial intima,212 and Lp-PLA2 has independently been associated with CHD and ischemic stroke.213 Unlike PON, Lp-PLA2 is unchanged, according to the results reviewed here, but the evidence is limited.
Limitations of the study
This review presents an LPSF perspective of the relationship between moderate drinking and CVD, and other biological markers and mechanisms besides lipoproteins may be equally important.17 In addition, our results apply primarily to healthy men and women aged 18–85 years, whereas the assumed beneficial effect of moderate drinking generally concerns middle-aged individuals.5
Furthermore, the upper limit of ≤ 60 g/d of alcohol is high compared with current recommendations for alcohol intake.10 This limit was set to avoid excluding important studies using this threshold as moderate alcohol intake and because some studies used > 1 alcohol dose or increasing doses. Higher doses than 60 g/d have also been included in other meta-analyses investigating moderate alcohol intake.17,20 The cutoff was also set as a compromise between the available evidence and the nadir of the U-shaped risk estimate; 1 of the most recent meta-analyses showed higher all-cause mortality risk compared with abstaining only when the alcohol dose exceeded 60 g/d.2 On the other hand, it is acknowledged that such drinking limits may have changed over the years. The broad definition could have compromised our comparisons between the intervention studies, but most studies provided 20–40 g/d alcohol (Tables S1 and S4 in the Supporting Information online).
In the PICOTS criteria, the comparator intervention is defined as “no” or “low” alcohol intake. Three RCTs with alcohol intakes in the comparators groups > 0 g/d were included because these studies did not use the control group in their analyses but analyzed the results according to a before-and-after design.35,55,64 In addition, the reference groups in few observational studies were defined as individuals drinking a range of alcohol amounts, including 0 g/d, but with upper limits of 5 g/d,97 10 g/d,92,96 22 g/d,113 or 28 g/d.85 Removing these studies from our analyses did not change our overall conclusions. Of note, many observational studies used regression analyses without reference groups and thus did not include any comparator group. In addition, several observational studies exceeded 60 g/d because of the use of regression analyses without dose restriction.
This review does not include potential differential effects of specific beverage types, and data from the majority of the observational studies did not allow for such an analysis. Ethanol per se has been suggested to be the element primarily responsible for the potential benefits of moderate intakes.11,12 However, a recent, large observational analysis of spirits drinkers suggested no causal relationship between moderate drinking and CVD.214 Besides the type of beverage, drinking pattern is also crucial because of the toxic effects of higher doses of alcohol.215 Regular drinking vs binge drinking was not examined in this review and was not part of the aim.
The included studies also defined the LPSFs differently, complicating comparability (Table S6 in the Supporting Information online). For example, LpA-I is found in both the HDL2 and HDL3 density range, and LpA-I:A-II is mainly found in the HDL3 density range.216 The investigated LPSFs were categorized (Figure 2), but definitions still differ between analytical methods.99,124 Even definitions of LPSFs in studies using the same methodology varied.13,124 This variation also involved HDL definitions, and some studies48,58,70 used a broader density range than the more commonly used range of 1.063–1.210 g/mL.159
The analytical methods for LPSF measurements have limitations, too. Ultracentrifugation has been the gold standard, although outcomes are operator dependent and the procedures time consuming.217,218 In addition, ultracentrifugation may cause loss of apolipoproteins and redistribution of subspecies due to buffer components and shear force.218,219 Compared with precipitation, losses of apoA-I are assumed to be higher by ultracentrifugation.220 Other laboratory methods focus on particle number concentrations based on size and density, such as NMR and IM. The lipoprotein particle number concentration has been suggested as a better measure of risk than the cholesterol cargo,221 and clinical studies have indicated that HDL-P may provide more information of CVD status than HDL-C.222 NMR lipoprotein analyses are less labor-intensive, high-throughput measurements.223 Ultracentrifugation is often used for calibration of the NMR spectral model, so the method basically predicts what would be measured by ultracentrifugation, improving comparability between these methods.223 Overall, standardization of the different NMR methods, including using a standard, unique reference material, is needed.224
Discrepancies between NMR and IM measurements have also been found. NMR studies in healthy cohorts have predicted an average HDL particle concentration of approximately 32–34 μmol/L,225,226 whereas IM studies have reported average levels of approximately 5–6 μmol/L.227,228 Such differences are critical for the validity and comparability of the particle number measurements as a clinical metric. Although these differences indicate that comparison of absolute quantities is compromised in this review, a comparability study showed that the positive association between sdLDLs and coronary artery stenosis was consistently found by 4 independent methods (ie, NMR, IM, gradient gel electrophoresis, and vertical auto profile), although the correlations among the methods varied significantly.181 Taken together, differences between laboratory methods and their limitations may explain some of the inconsistency in results between studies and underline the need for standardization.
Another limitation is the high frequency of unclear or high risk of bias in eligible studies, particularly in the intervention studies and trials. More specifically, older studies had short descriptions of the applied methods and results, complicating their interpretation. Compliance was not often measured, and suitable biomarkers of alcohol intake still need to be implemented. Compliance was not even discussed in several intervention studies, and only a few studies reported control of diet, physical activity, body weight, or smoking habits. Furthermore, not all the crossover studies examined carryover effects. Last, it is impossible to judge to what extent results were influenced by study size and power, because none of the studies reported any power calculations for LPSFs.
Although the observational studies generally had lower bias risk, they were prone to intentional and unintentional recall bias due to self-reported alcohol intake. Also, the definition of a standard drink differed among studies. Therefore, data on alcohol intake were extracted in grams per day or week. Furthermore, nonlinear relationships between alcohol intake and LPSFs were only directly explored in 3 observational studies.13,32,124 It has also been suggested that LPSF analyses should be adjusted for other LPSFs and overall classes of lipoproteins due to collinearity,24 which was generally not practiced.
The included MRSs also had limitations. Even though MRSs are assumed to be less prone to confounding and reverse causation than conventional epidemiologic studies, such limitations may exist.229 For example, the instrumental variables used in the 2 MRSs66,67 included here, alcohol dehydrogenase and aldehyde dehydrogenase, could potentially directly influence the outcome, because the enzymes are involved in the metabolism of alcohol. In Japanese men that the aldehyde dehydrogenase 2 variant is related to reduced alcohol intake and lower body weight, a potential consequence of lower calorie intake from alcohol.230 No bias-risk assessment tool specific to MRSs is available; thus, the results of these studies may be biased.
Future perspectives
High-quality RCTs that target LPSFs, apoB, and apoB-containing lipoproteins after moderate alcohol intake are needed. Studies should include individuals at elevated risk of CVD, because lipoprotein structure and function may change in disease states.168 Studies investigating mechanisms within these types of populations are few. From the reviewed studies, a daily alcohol intake of 20 g/d might be sufficient to cause changes in LPSFs, though few studies showed changes in specific subfractions at lower intake levels (Table S1 in the Supporting Information online). In recent years, observational studies questioning any safe level of drinking have been published.231,232 Intervention studies, therefore, should aim for low amounts of alcohol, preferably no more than 1 drink/d (10–15 g/d). Standardization of laboratory methods to ease comparison within and between methodologies is needed,233 and consensus nomenclatures for all LPSFs are crucial.
HDL and its subspecies may still be of interest in cardiovascular research, even if HDL-C is not casually related to atherosclerotic CVD.234 Focus has shifted from the HDL-C hypothesis to an HDL function hypothesis. Among the HDL functions of interest are mechanisms related to reverse cholesterol transport.160 Studies tracking cholesterol from macrophages to hepatic uptake and onward to fecal excretion have been conducted in animals but need confirmation in humans.235 Furthermore, “omics” analyses of the HDL lipidome and proteome may support future CVD diagnostics and provide more information on the composition of a broader range of subpopulations.168,236
LPSFs may eventually be included as cardiovascular biomarkers in risk prediction models relevant for moderate drinkers. A recently developed diabetes risk index comprises several NMR-measured LPSFs and has been associated with insulin resistance and increased risk of developing type 2 diabetes.237 Such risk markers will likely expand to other diseases soon.
Conclusions
Alcohol in doses from 12–60 g/d is related to higher levels of all types of HDL subfractions, independent of study design. Effects on total HDL-C, therefore, seem unrelated to any specific subfraction. The influence of moderate drinking on LDL and VLDL subfractions is still speculative; however, some observational studies found nonlinear associations of alcohol intake with potential beneficial associations in the moderate drinking range. A few studies of different designs found reduced levels of small LDLs, higher levels of large LDLs, and increased LDL particle size. Moderate alcohol intake consistently increases CEC and PON activity, both of which have been associated with reduced CVD risk and with HDL’s antiatherosclerotic functions. More research is needed to study effects in women and in people with diabetes and other cardiometabolic conditions. At present, evidence is lacking on the influence of moderate drinking on functional metrics of HDL, apoB-containing lipoproteins, and subfractions classified by their content of essential apolipoproteins, such as apoC-III.
Supplementary Material
Acknowledgments
We thank especially Anne Berit Bagger and Anne Cathrine Trumpy, librarians at Copenhagen University Library, for support during the systematic search. We are very grateful for their help in handling bibliographic databases and retrieving articles for full-text screening.
Author contributions. T.L.W. wrote the protocol, supervised by J.N.E. and L.O.D. T.L.W. conducted the literature search, and T.L.W., K.T., and J.N.E. screened the literature and agreed on exclusion and inclusion of studies. T.L.W. and K.T. performed the data extraction. T.L.W. wrote the manuscript; all authors revised and provided intellectual feedback regarding the manuscript. All authors approved the final manuscript.
Funding. This work was supported by a PhD scholarship sponsored in part by a Carlsberg Foundation Semper Ardens grant on biomarkers to L.O.D. [grant no. CF15-0574], and in part by National Institute of Alcohol Abuse and Alcoholism to L.O.D. [grant no. U10AA025286]. Salaries for T.L.W., J.N.E., and L.O.D. were partly supported by grants from the Carlsberg Foundation and National Institute of Alcohol Abuse and Alcoholism.
Declaration of interest. The funders had no role in the planning or conduct of the systematic search or interpretation of the results. The authors have no other conflicts of interest to report.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 Lipoprotein subfractions in intervention studies
Table S2 Lipoprotein subfractions in observational studies
Table S3 Mechanisms in intervention studies
Table S4 Mechanisms in observational studies
Table S5 Statements from the manuscript including citations
Table S6 Definitions of lipoprotein subfractions as provided in the individual intervention and observational studies
Figure S1 Bias risk assessment from randomized controlled trials on lipoprotein subfractions
Figure S2 Bias risk assessment from nonrandomized investigations on lipoprotein subfractions
Figure S3 Bias risk assessment from cross-sectional studies on lipoprotein subfractions
Figure S4 Bias risk assessment from randomized controlled trials on mechanisms
Figure S5 Bias risk assessment from nonrandomized investigations on mechanisms
Figure S6 Bias risk assessment from cross-sectional studies on mechanisms
Text S1 Systematic literature search strategy
Text S2 PRISMA checklist moderate alcohol consumption and lipoprotein subfractions
References
- 1. Celermajer DS, Chow CK, Marijon E, et al. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol. 2012;60:1207–1216. [DOI] [PubMed] [Google Scholar]
- 2. Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:D671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Yu F, Zhou Y, et al. Association between alcohol consumption and the risk of incident type 2 diabetes : A systematic review and dose-response meta-analysis 1. Am J Clin Nutr. 2016;103:818–829. [DOI] [PubMed] [Google Scholar]
- 4. O’Keefe JH, Bybee KA, Lavie CJ.. Alcohol and cardiovascular health. The razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–1014. [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto C, Miedema MD, Ofman P, et al. An expanding knowledge of the mechanisms and effects of alcohol consumption on cardiovascular disease. J Cardiopulm Rehabil Prev. 2014;34:159–171. [DOI] [PubMed] [Google Scholar]
- 6. Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev. 1993;15:328–351. [DOI] [PubMed] [Google Scholar]
- 7. Corrao G, Rubbiati L, Bagnardi V, et al. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95:1505–1523. [DOI] [PubMed] [Google Scholar]
- 8. De Lange DW, Van De Wiel A.. Drink to prevent: review on the cardioprotective mechanisms of alcohol and red wine polyphenols. Semin Vasc Med. 2004;4:173–186. [DOI] [PubMed] [Google Scholar]
- 9.National Institute on Alcohol Abuse and Alcoholism (NIAAA). Drinking levels defined. Available at: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed March 18, 2021.
- 10.International Alliance for Responsible Drinking (IARD). Drinking guidelines: general population. Available at: https://iard.org/science-resources/detail/Drinking-Guidelines-General-Population. 2019. Accessed December 12, 2020.
- 11. Cleophas TJ. Wine, beer and spirits and the risk of myocardial infarction: a systematic review. Biomed Pharmacother. 1999;53:417–423. [DOI] [PubMed] [Google Scholar]
- 12. Rimm EB, Klatsky A, Grobbee D, et al. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits? BMJ. 1996;312:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du D, Bruno R, Blizzard L, et al. The metabolomic signatures of alcohol consumption in young adults. Eur J Prev Cardiol. 2020;27:840–849. [DOI] [PubMed] [Google Scholar]
- 14. Costanzo S, Di Castelnuovo A, Donati MB, et al. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: a meta-analysis. Eur J Epidemiol. 2011;26:833–850. [DOI] [PubMed] [Google Scholar]
- 15. Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. [DOI] [PubMed] [Google Scholar]
- 16. van der Gaag MS, van Tol A, Scheek LM, et al. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomised intervention study in middle-aged men. Atherosclerosis. 1999;147:405–410. [DOI] [PubMed] [Google Scholar]
- 17. Brien SE, Ronksley PE, Turner BJ, et al. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:3210–3227. [Google Scholar]
- 19. Huang Y, Li Y, Zheng S, et al. Moderate alcohol consumption and atherosclerosis: meta-analysis of effects on lipids and inflammation. Wien Klin Wochenschr. 2017;129:835–843. [DOI] [PubMed] [Google Scholar]
- 20. Rimm EB, Williams P, Fosher K, et al. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spaggiari G, Cignarelli A, Sansone A, et al. To beer or not to beer: a meta-analysis of the effects of beer consumption on cardiovascular health. PLoS One. 2020;15:e0233619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hannuksela ML, Rämet ME, Nissinen AET, et al. Effects of ethanol on lipids and atherosclerosis. Pathophysiology. 2004;10:93–103. [DOI] [PubMed] [Google Scholar]
- 23. Hannuksela ML, Liisanantti MK, Savolainen MJ.. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit Rev Clin Lab Sci. 2002;39:225–283. [DOI] [PubMed] [Google Scholar]
- 24. Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21:305–311. [DOI] [PubMed] [Google Scholar]
- 25. Superko HR, Pendyala L, Williams PT, et al. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol. 2012;6:496–523. [DOI] [PubMed] [Google Scholar]
- 26. Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brinton EA. Effects of ethanol intake on lipoproteins. Curr Atheroscler Rep. 2012;14:108–114. [DOI] [PubMed] [Google Scholar]
- 28. Rizzo M, Otvos J, Nikolic D, et al. Subfractions and subpopulations of HDL: an update. Curr Med Chem. 2014;21:2881–2891. [DOI] [PubMed] [Google Scholar]
- 29. Rosenson RS, Brewer HB, Chapman MJ, et al. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410. [DOI] [PubMed] [Google Scholar]
- 30. Brinton EA. Effects of ethanol intake on lipoproteins and atherosclerosis. Curr Opin Lipidol. 2010;21:346–351. [DOI] [PubMed] [Google Scholar]
- 31. Li JM, Mukamal KJ.. An update on alcohol and atherosclerosis. Curr Opin Lipidol. 2004;15:673–680. [DOI] [PubMed] [Google Scholar]
- 32. Muth ND, Laughlin GA, von Mühlen D, et al. High-density lipoprotein subclasses are a potential intermediary between alcohol intake and reduced risk of cardiovascular disease: the Rancho Bernardo Study. Br J Nutr. 2010;104:1034–1042. [DOI] [PubMed] [Google Scholar]
- 33. Parlesak A, Eckoldt J, Winkler K, et al. Intercorrelations of lipoprotein subfractions and their covariation with lifestyle factors in healthy men. J Clin Biochem Nutr. 2014;54:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haskell WL, Camargo C, Williams PT, et al. The effect of cessation and resumption of moderate alcohol intake on serum high-density-lipoprotein subfractions. N Engl J Med. 1984;310:805–810. [DOI] [PubMed] [Google Scholar]
- 35. Masarei JRL, Puddey IB, Rouse IL, et al. Effects of alcohol consumption on serum lipoprotein-lipid and apolipoprotein concentrations. Results from an intervention study in health subjects. Atherosclerosis. 1986;60:79–87. [DOI] [PubMed] [Google Scholar]
- 36. Burr ML, Fehily AM, Butland BK, et al. Alcohol and high-density-lipoprotein cholesterol: a randomized controlled trial. Br J Nutr. 1986;56:81–86. [DOI] [PubMed] [Google Scholar]
- 37. Shamseer L, Moher D, Clarke M, et al. ; the PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647–25. [DOI] [PubMed] [Google Scholar]
- 38. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 39.Endnote X8.2 (Bld 11343, desktop version). Clarivate analytics. Philadelphia, P. Available at: www.endnote.com. Accessed December 12, 2019.
- 40.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at: www.covidence.org. Accessed December 12, 2019.
- 41. Higgins JPT, Altman DG, Sterne AAC, eds. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (Updated March 2011). The Cochrane Collaboration; 2011. Available at: www.handbook.cochrane.org. [Google Scholar]
- 42. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. [DOI] [PubMed] [Google Scholar]
- 43. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. Adelaide, South Australia: The Joanna Briggs Institute; 2017. https://reviewersmanual.joannabriggs.org/. [Google Scholar]
- 44. Mukamal KJ, Na B, Mu L, et al. Lessons and challenges from a 6-month randomized pilot study of daily ethanol consumption. Curr Dev Nutr. 2017;1:e000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Conrad M, McNamara P, King A.. Alternative substance paradigm: effectiveness of beverage blinding and effects on acute alcohol responses. Exp Clin Psychopharmacol. 2012;20:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iglesias K, Lannoy S, Sporkert F, et al. Performance of self-reported measures of alcohol use and of harmful drinking patterns against ethyl glucuronide hair testing among young Swiss men. PLoS One. 2020;15:e0244336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pikaar NA, Wedel M, van der Beek EJ, et al. Effects of moderate alcohol consumption on platelet aggregation, fibrinolysis, and blood lipids. Metabolism. 1987;36:538–543. [DOI] [PubMed] [Google Scholar]
- 48. Rumpler WV, Clevidence BA, Muesing RA, et al. Changes in women’s plasma lipid and lipoprotein concentrations due to moderate consumption of alcohol are affected by dietary fat level. J Nutr. 1999;129:1713–1717. [DOI] [PubMed] [Google Scholar]
- 49. Senault C, Betoulle D, Luc G, et al. Beneficial effects of a moderate consumption of red wine on cellular cholesterol efflux in young men. Nutr Metab Cardiovasc Dis. 2000;10:63–69. http://www.ncbi.nlm.nih.gov/pubmed/10919170. [PubMed] [Google Scholar]
- 50. Hagiage M, Marti C, Rigaud D, et al. Effect of a moderate alcohol intake on the lipoproteins of normotriglyceridemic obese subjects compared with normoponderal controls. Metabolism. 1992;41:856–861. [DOI] [PubMed] [Google Scholar]
- 51. Hartung GH, Reeves RS, Foreyt JP, et al. Effect of alcohol intake and exercise on plasma high-density lipoprotein cholesterol subfractions and apolipoprotein A-I in women. Am J Cardiol. 1986;58:148–151. [DOI] [PubMed] [Google Scholar]
- 52. Nishiwaki M, Ishikawa T, Ito T, et al. Effects of alcohol on lipoprotein lipase, hepatic lipase, cholesteryl ester transfer protein, and lecithin: cholesterol acyltransferase in high-density lipoprotein cholesterol elevation. Atherosclerosis. 1994;111:99–109. [DOI] [PubMed] [Google Scholar]
- 53. Välimäki M, Taskinen M-RR, YLIKAHRI R, et al. Comparison of the effects of two different doses of alcohol on serum lipoproteins, HDL-subfractions and apolipoproteins A-I and A-II: a controlled study. Eur J Clin Invest. 1988;18:472–480. [DOI] [PubMed] [Google Scholar]
- 54. Välimäki M, Laitinen K, Ylikahri R, et al. The effect of moderate alcohol intake on serum apolipoprotein A-I-containing lipoproteins and lipoprotein (a). Metabolism. 1991;40:1168–1172. [DOI] [PubMed] [Google Scholar]
- 55. Cox KL, Puddey IB, Morton AR, et al. The combined effects of aerobic exercise and alcohol restriction on blood pressure and serum lipids: a two-way factorial study in sedentary men. J Hypertens. 1993;11:191–201. [DOI] [PubMed] [Google Scholar]
- 56. Fraser GE, Anderson JT, Foster N, et al. The effect of alcohol on serum high density lipoprotein (HDL). A controlled experiment. Atherosclerosis. 1983;46:275–286. [DOI] [PubMed] [Google Scholar]
- 57. Clevidence BA, Reichman ME, Judd JT, et al. Effects of alcohol consumption on lipoproteins of premenopausal women. Arterioscler Thromb Vasc Biol. 1995;15:179–184. [DOI] [PubMed] [Google Scholar]
- 58. Bertière MC, Betoulle D, Apfelbaum M, et al. Time-course, magnitude and nature of the changes induced in HDL by moderate alcohol intake in young non-drinking males. Atherosclerosis. 1986;61:7–14. [DOI] [PubMed] [Google Scholar]
- 59. Beulens JWJ, Sierksma A, van Tol A, et al. Moderate alcohol consumption increases cholesterol efflux mediated by ABCA1. J Lipid Res. 2004;45:1716–1723. [DOI] [PubMed] [Google Scholar]
- 60. Gottrand F, Beghin L, Duhal N, et al. Moderate red wine consumption in healthy volunteers reduced plasma clearance of apolipoprotein AII. Eur J Clin Invest. 1999;29:387–394. [DOI] [PubMed] [Google Scholar]
- 61. Serdyuk AP, Metelskaya VA, Ozerova IN, et al. Effects of alcohol on the major steps of reverse cholesterol transport. Biochemistry (Mosc). 2000;65:1310–1315. [PubMed] [Google Scholar]
- 62.De Oliveira e Silva ER, Foster D, Harper MM, et al. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–2352. [DOI] [PubMed] [Google Scholar]
- 63. Sharpe PC, McGrath LT, McClean E, et al. Effect of red wine consumption on lipoprotein (a) and other risk factors for atherosclerosis. QJM. 1995;88:101–108. [PubMed] [Google Scholar]
- 64. Hartung GH, Foreyt JP, Reeves RS, et al. Effect of alcohol dose on plasma lipoprotein subfractions and lipolytic enzyme activity in active and inactive men. Metabolism. 1990;39:81–86. [DOI] [PubMed] [Google Scholar]
- 65. Kaul S, Belcik T, Kalvaitis S, et al. Effect of modest alcohol consumption over 1-2 weeks on the coronary microcirculation of normal subjects. Eur J Echocardiogr. 2010;11:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tabara Y, Arai H, Hirao Y, et al. The causal effects of alcohol on lipoprotein subfraction and triglyceride levels using a Mendelian randomization analysis: the Nagahama study. Atherosclerosis. 2017;257:22–28. [DOI] [PubMed] [Google Scholar]
- 67. Vu KN, Ballantyne CM, Hoogeveen RC, et al. Causal role of alcohol consumption in an improved lipid profile: the Atherosclerosis Risk in Communities (ARIC) study. PLoS One. 2016;11:e0148765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hartung GHH, Lawrence SJ, Reeves RS, et al. Effect of alcohol and exercise on postprandial lipemia and triglyceride clearance in men. Atherosclerosis. 1993;100:33–40. [DOI] [PubMed] [Google Scholar]
- 69. McConnell MV, Vavouranakis I, Wu LL, et al. Effects of a single, daily alcoholic beverage on lipid and hemostatic markers of cardiovascular risk. Am J Cardiol. 1997;80:1226–1228. [DOI] [PubMed] [Google Scholar]
- 70. Moore RD, Smith CR, Kwiterovich PO, et al. Effect of low-dose alcohol use versus abstention on apolipoproteins A-I and B. Am J Med. 1988;84:884–890. [DOI] [PubMed] [Google Scholar]
- 71. Fulton-Kehoe DL, Eckel RH, Shetterly SM, et al. Determinants of total high density lipoprotein cholesterol and high density lipoprotein subfraction levels among Hispanic and non-Hispanic white persons with normal glucose tolerance: the San Luis Valley diabetes study. J Clin Epidemiol. 1992;45:1191–1200. [DOI] [PubMed] [Google Scholar]
- 72. Moriyama K, Takahashi E.. Relationships of high-density lipoprotein 2 and 3 cholesterols with lifestyle habits in Japanese adults. Ningen Dock Int.2014;1:54–62. [Google Scholar]
- 73. Mänttäri M, Koskinen P, Manninen V, et al. Lifestyle determinants of HDL2- and HDL3-cholesterol levels in a hypercholesterolemic male population. Atherosclerosis. 1991;87:1–8. [DOI] [PubMed] [Google Scholar]
- 74. Patsch W, Sharrett AR, Sorlie PD, et al. The relation of high density lipoprotein cholesterol and its subfractions to apolipoprotein A-l and fasting triglycerides: the role of environmental factors. Am J Epidemiol. 1992;136:546–557. [DOI] [PubMed] [Google Scholar]
- 75. Robinson D, Ferns GA, Bevan EA, et al. High density lipoprotein subfractions and coronary risk factors in normal men. Arteriosclerosis. 1987;7:341–346. [DOI] [PubMed] [Google Scholar]
- 76. Steenkamp HJ, Jooste PL, Benadé AJ, et al. Relationship between high density lipoprotein subfractions and coronary risk factors in a rural white population. Arteriosclerosis. 1990;10:1026–1031. [DOI] [PubMed] [Google Scholar]
- 77. Broda Gyna, Davis CE, Pajak A, et al. Poland and United States collaborative study on cardiovascular epidemiology. Arterioscler Thromb Vasc Biol. 1996;16:339–349. [DOI] [PubMed] [Google Scholar]
- 78. Volcik KA, Ballantyne CM, Fuchs FD, et al. Relationship of alcohol consumption and type of alcoholic beverage consumed with plasma lipid levels: differences between Whites and African Americans of the ARIC Study. Ann Epidemiol. 2008;18:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Walton C, Lees B, Crook D, et al. Body fat distribution, rather than overall adiposity, influences serum lipids and lipoproteins in healthy men independently of age. Am J Med. 1995;99:459–464. [DOI] [PubMed] [Google Scholar]
- 80. Andrade RJ, Escolar J, Valdivielso P, et al. Apolipoprotein distribution in plasma HDL subfractions in alcohol consumers. Drug Alcohol Depend. 1990;26:161–168. [DOI] [PubMed] [Google Scholar]
- 81. Bergmann S, Siegert G, Wahrburg U, et al. Influence of menopause and lifestyle factors on high density lipoproteins in middle-aged women. Menopause. 1997;4:52–61. [Google Scholar]
- 82. Gardner CD, Tribble DL, Young DR, et al. Population frequency distributions of HDL, HDL(2), and HDL(3) cholesterol and apolipoproteins A-I and B in healthy men and women and associations with age, gender, hormonal status, and sex hormone use: the Stanford Five City Project. Prev Med (Baltim). 2000;31:335–345. [DOI] [PubMed] [Google Scholar]
- 83. Diehl AK, Fuller JH, Mattock MB, et al. The relationship of high density lipoprotein subfractions to alcohol consumption, other lifestyle factors, and coronary heart disease. Atherosclerosis. 1988;69:145–153. [DOI] [PubMed] [Google Scholar]
- 84. Haffner SM, Applebaum-Bowden D, Wahl PW, et al. Epidemiological correlates of high density lipoprotein subfractions, apolipoproteins A-I, A-II, and D, and lecithin cholesterol acyltransferase. Effects of smoking, alcohol, and adiposity. Arteriosclerosis. 1985;5:169–177. [DOI] [PubMed] [Google Scholar]
- 85. Lakshman MR, Reda D, Materson BJ, et al. Comparison of plasma lipid and lipoprotein profiles in hypertensive black versus white men. Am J Cardiol. 1996;78:1236–1241. [DOI] [PubMed] [Google Scholar]
- 86. Marti B, Suter E, Riesen WF, et al. Anthropometric and lifestyle correlates of serum lipoprotein and apolipoprotein levels among normal non-smoking men and women. Atherosclerosis. 1989;75:111–122. [DOI] [PubMed] [Google Scholar]
- 87. Meilahn EN, Kuller LH, Matthews KA, Wing RR, et al. Potential for increasing high-density lipoprotein cholesterol, subfractions HDL2-C and HDL3-C, and apoprotein Al among middle-age women. Prev Med (Baltim). 1991;20:462–473. [DOI] [PubMed] [Google Scholar]
- 88. Momose Y, Une H, Esaki H.. Relation between lifestyle factors and high-density lipoprotein cholesterol subfraction levels among healthy adults living in a rural district. J Jpn Assoc Rural Med. 1994;43:1–7. [Google Scholar]
- 89. Razay G, Heaton KW, Bolton CH, et al. Alcohol consumption and its relation to cardiovascular risk factors in British women. BMJ. 1992;304:80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rossouw JE, Lai-Tung MT, Jooste PL, et al. Alcohol intake in relation to lipids, lipoproteins and blood pressure. S Afr Med J. 1992;82:246–250. [PubMed] [Google Scholar]
- 91. Sillanaukee P, KOIVULA T, Jokela H, et al. Relationship of alcohol consumption to changes in HDL‐subfractions. Eur J Clin Invest. 1993;23:486–491. [DOI] [PubMed] [Google Scholar]
- 92. Sillanaukee P, Koivula T, Jokela H, et al. Alcohol consumption and its relation to lipid-based cardiovascular risk factors among middle-aged women: the role of HDL3 cholesterol. Atherosclerosis. 2000;152:503–510. [DOI] [PubMed] [Google Scholar]
- 93. Gaziano JM, Buring JE, Breslow JL, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–1834. [DOI] [PubMed] [Google Scholar]
- 94. Vasisht S, Pant MC, Srivastava LM.. Effect of alcohol on serum lipids & lipoproteins in male drinkers. Indian J Med Res. 1992;96:333–337. [PubMed] [Google Scholar]
- 95. Woo J, Lam CW.. Serum lipid profile in an elderly Chinese population. Arteriosclerosis. 1990;10:1097–1101. [DOI] [PubMed] [Google Scholar]
- 96. Skoczyńska A, Wojakowska A, Turczyn B, et al. Lipid pattern in middle-aged inhabitants of the Lower Silesian region of Poland. The PURE Poland sub-study. Ann Agric Environ Med. 2013;20:317–324. [PubMed] [Google Scholar]
- 97. Schäfer C, Bode C, Bode JC, et al. Beyond HDL-cholesterol increase: phospholipid enrichment and shift from HDL 3 to HDL 2 in alcohol consumers. J Lipid Res. 2007;48:1550–1558. [DOI] [PubMed] [Google Scholar]
- 98. Williams PT, Dreon D, Krauss RM, et al. Associations of diet and alcohol intake with high-density lipoprotein subclasses. Metabolism. 1985;34:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Williams PT, Vranizan KM, Austin MA, et al. Associations of age, adiposity, alcohol intake, menstrual status, and estrogen therapy with high-density lipoprotein subclasses. Arterioscler Thromb. 1993;13:1654–1661. [DOI] [PubMed] [Google Scholar]
- 100. Burke V, Puddey IB, Beilin LJ, et al. Changes in markers of alcohol intake in man below “safe” drinking levels. Alcohol. 1992;27:677–683. [PubMed] [Google Scholar]
- 101. Jensen MK, Rimm EB, Furtado JD, et al. Apolipoprotein C‐III as a potential modulator of the association between HDL‐cholesterol and incident coronary heart disease. J Am Heart Assoc. 2012;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Onat A, Hergenç G, Ayhan E, et al. Serum apolipoprotein C-III in high-density lipoprotein: a key diabetogenic risk factor in Turks. Diabet Med. 2009;26:981–988. [DOI] [PubMed] [Google Scholar]
- 103. Koch M, Furtado JD, Jiang GZ, et al. Associations of anthropometry and lifestyle factors with HDL subspecies according to apolipoprotein C-III. J Lipid Res. 2017;58:1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ito T, Nishiwaki M, Ishikawa T, et al. CETP and LCAT activities are unrelated to smoking and moderate alcohol consumption in healthy normolipidemic men. Jpn Circ J. 1995;59:541–546. [DOI] [PubMed] [Google Scholar]
- 105. Onat A, Hergenç G, Sansoy V, et al. Apolipoprotein C-III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 2003;168:81–89. [DOI] [PubMed] [Google Scholar]
- 106. Branchi A, Rovellini A, Tomella C, et al. Association of alcohol consumption with HDL subpopulations defined by apolipoprotein A-I and apolipoprotein A-II content. Eur J Clin Nutr. 1997;51:362–365. [DOI] [PubMed] [Google Scholar]
- 107. Kee F, Weston J, McCrum EE, et al. The effect of diet on lipid, apoprotein and lipoparticle variation in the ECTIM study in Belfast. Rev Epidemiol Sante Publique. 1995;43:18–25. [PubMed] [Google Scholar]
- 108. Marques-Vidal P, Cambou JP, Nicaud V, et al. Cardiovascular risk factors and alcohol consumption in France and Northern Ireland. Atherosclerosis. 1995;115:225–232. [DOI] [PubMed] [Google Scholar]
- 109. Marques-Vidal P, Montaye M, Haas B, et al. Relationships between alcoholic beverages and cardiovascular risk factor levels in middle-aged men, the PRIME study. Atherosclerosis. 2001;157:431–440. [DOI] [PubMed] [Google Scholar]
- 110. Luc G, Bard J-M, Ferrières J, et al. Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease: the PRIME study. Arterioscler Thromb Vasc Biol. 2002;22:1155–1161. [DOI] [PubMed] [Google Scholar]
- 111. Lyu L-C, Schaefer EJ, Ordovas JM, et al. Association of sex, adiposity, and diet with HDL subclasses in middle-aged Chinese. Am J Clin Nutr. 2001;74:64–71. [DOI] [PubMed] [Google Scholar]
- 112. Mansfield E, McPherson R, Koski KG.. Diet and waist-to-hip ratio: important predictors of lipoprotein levels in sedentary and active young men with no evidence of cardiovascular disease. J Am Diet Assoc. 1999;99:1373–1379. [DOI] [PubMed] [Google Scholar]
- 113. Steinmetz J, Choukaife A, Visvikis S, et al. Biological factors affecting concentrations of serum LpAI lipoprotein particles in serum, and determination of reference limits. Clin Chem. 1990;36:677–680. [PubMed] [Google Scholar]
- 114. Perret B, Ruidavets J-B, Vieu C, et al. Alcohol consumption is associated with enrichment of high-density lipoprotein particles in polyunsaturated lipids and increased cholesterol esterification rate. Alcoholism Clin Exp Res. 2002;26:1134–1140. [DOI] [PubMed] [Google Scholar]
- 115. Kim D, Burt AA, Ranchalis JE, et al. Effects of dietary components on high-density lipoprotein measures in a cohort of 1,566 participants. Nutr Metab (Lond). 2014;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Puchois P, Ghalim N, Zylberberg G, et al. Effect of alcohol intake on human apolipoprotein A-I-containing lipoprotein subfractions. Arch Intern Med. 1990;150:1638–1641. [PubMed] [Google Scholar]
- 117. Williams PT, Krauss RM.. Associations of age, adiposity, menopause, and alcohol intake with low-density lipoprotein subclasses. Arterioscler Thromb Vasc Biol. 1997;17:1082–1090. [DOI] [PubMed] [Google Scholar]
- 118. McNamara JR, Jenner JL, Li Z, et al. Change in LDL particle size is associated with change in plasma triglyceride concentration. Arterioscler Thromb. 1992;12:1284–1290. [DOI] [PubMed] [Google Scholar]
- 119. Schiele F, Vincent-Viry M, Starck M, et al. Apolipoprotein E in apolipoprotein B (apo B)- and non-apo B-containing lipoproteins in 3523 participants in the Stanislas cohort: biological variation and genotype-specific reference limits. Clin Chem. 2002;48:291–300. [PubMed] [Google Scholar]
- 120. Lupien PJ, Moorjani S, Jobin J, et al. Smoking, alcohol consumption, lipid and lipoprotein levels. Can J Cardiol. 1988;4:102–107. [PubMed] [Google Scholar]
- 121. Meilahn EN, Kuller LH, Stein EA, et al. Characteristics associated with apoprotein and lipoprotein lipid levels in middle-aged women. Arteriosclerosis. 1988;8:515–520. [DOI] [PubMed] [Google Scholar]
- 122. Miller GJ, Gilson RJC.. Similarity in males and females of HDL2 and HDL3 cholesterol concentration in a Caribbean rural community. Atherosclerosis. 1981;40:75–80. [DOI] [PubMed] [Google Scholar]
- 123. Miller NE, Bolton CH, Hayes TM, et al. Associations of alcohol consumption with plasma high density lipoprotein cholesterol and its major subfractions: the Caerphilly and Speedwell Collaborative Heart Disease Studies. J Epidemiol Community Health. 1988;42:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mukamal KJ, Mackey RH, Kuller LH, et al. Alcohol consumption and lipoprotein subclasses in older adults. J Clin Endocrinol Metab. 2007;92:2559–2566. [DOI] [PubMed] [Google Scholar]
- 125. Zaid M, Miura K, Okayama A, et al. ; INTERLIPID and INTERMAP Research Groups. Associations of high-density lipoprotein particle and high-density lipoprotein cholesterol with alcohol intake, smoking, and body mass index ― the INTERLIPID study. Circ J. 2018;82:2557–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Würtz P, Cook S, Wang Q, et al. Metabolic profiling of alcohol consumption in 9778 young adults. Int J Epidemiol. 2016;45:1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Millar SR, Harrington JM, Perry IJ, et al. Protective lifestyle behaviours and lipoprotein particle subclass profiles in a middle-to older-aged population. Atherosclerosis. 2020;314:18–26. [DOI] [PubMed] [Google Scholar]
- 128. Si J, Li J, Yu C, et al. Improved lipidomic profile mediates the effects of adherence to healthy lifestyles on coronary heart disease. Elife. 2021;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sonestedt E, Wirfält E, Wallström P, et al. High disaccharide intake associates with atherogenic lipoprotein profile. Br J Nutr. 2012;107:1062–1069. [DOI] [PubMed] [Google Scholar]
- 130. Bogl LH, Pietiläinen KH, Rissanen A, et al. Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutr Metab Cardiovasc Dis. 2013;23:1071–1078. [DOI] [PubMed] [Google Scholar]
- 131. Tighe P, Duthie G, Brittenden J, et al. Effects of wheat and oat-based whole grain foods on serum lipoprotein size and distribution in overweight middle aged people: a randomised controlled trial. PLoS One. 2013;8:e70436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Králová Lesná I, Suchánek P, Stávek P, et al. May alcohol-induced increase of HDL be considered as atheroprotective? Physiol Res. 2010;59:407–413. [DOI] [PubMed] [Google Scholar]
- 133. Padro T, Muñoz-García N, Vilahur G, et al. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients. 2018;10:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sierksma A, Vermunt SHF, Lankhuizen IM, et al. Effect of moderate alcohol consumption on parameters of reverse cholesterol transport in postmenopausal women. Alcohol Clin Exp Res. 2004;28:662–666. [DOI] [PubMed] [Google Scholar]
- 135. van der Gaag MS, van Tol A, Vermunt SH, et al. Alcohol consumption stimulates early steps in reverse cholesterol transport. J Lipid Res. 2001;42:2077–2083. [PubMed] [Google Scholar]
- 136. Hoang A, Tefft C, Duffy SJ, et al. ABCA1 expression in humans is associated with physical activity and alcohol consumption. Atherosclerosis. 2008;197:197–203. [DOI] [PubMed] [Google Scholar]
- 137. Koekemoer AL, Codd V, Masca NGD, et al. Large-scale analysis of determinants, stability, and heritability of high-density lipoprotein cholesterol efflux capacity. Arterioscler Thromb Vasc Biol. 2017;37:1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Riemens SC, van Tol A, Hoogenberg K, et al. Higher high density lipoprotein cholesterol associated with moderate alcohol consumption is not related to altered plasma lecithin: cholesterol acyltransferase and lipid transfer protein activity levels. Clin Chim Acta. 1997;258:105–115. [DOI] [PubMed] [Google Scholar]
- 140. Goto A, Sasai K, Suzuki S, et al. Plasma concentrations of LPL and LCAT are in putative association with females and alcohol use which are independent negative risk factors for coronary atherosclerosis among Japanese. Clin Chim Acta. 2003;329:69–76. [DOI] [PubMed] [Google Scholar]
- 141. Albers JJ, Bergelin RO, Adolphson JL, et al. Population-based reference values for lecithin-cholesterol acyltransferase (LCAT). Atherosclerosis. 1982;43:369–379. [DOI] [PubMed] [Google Scholar]
- 142. Alarcon SBK, Oliveira HCF, Harada LM, et al. Moderate hyperalphalipoproteinaemia in a Brazilian population is related to lipoprotein lipase activity, apolipoprotein A-I concentration, age and body mass index. Clin Sci. 2004;106:11–17. [DOI] [PubMed] [Google Scholar]
- 143. Vergeer M, Boekholdt SM, Sandhu MS, et al. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation. 2010;122:470–477. [DOI] [PubMed] [Google Scholar]
- 144. Dullaart RPF, Beusekamp BJ, Riemens SC, et al. High-density lipoprotein cholesterol is related to the TaqIB cholesteryl ester transfer protein gene polymorphism and smoking, but not to moderate alcohol consumption in insulin-dependent diabetic men. Scand J Clin Lab Invest. 1998;58:251–258. [DOI] [PubMed] [Google Scholar]
- 145. Beulens JWJ, van den Berg R, Kok FJ, et al. Moderate alcohol consumption and lipoprotein-associated phospholipase A2 activity. Nutr Metab Cardiovasc Dis. 2008;18:539–544. [DOI] [PubMed] [Google Scholar]
- 146. Hatoum IJ, Nelson JJ, Cook NR, et al. Dietary, lifestyle, and clinical predictors of lipoprotein-associated phospholipase A2 activity in individuals without coronary artery disease. Am J Clin Nutr. 2010;91:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rajdl D, Racek J, Trefil L, et al. Effect of white wine consumption on oxidative stress markers and homocysteine levels. Physiol Res. 2007;56:203–212. [DOI] [PubMed] [Google Scholar]
- 148. Sierksma A, Gaag MS, Tol A, et al. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcoholism Clin Exp Res. 2002;26:1430–1435. [DOI] [PubMed] [Google Scholar]
- 149. Gruppen EG, Bakker SJL, James RW, et al. Serum paraoxonase-1 activity is associated with light to moderate alcohol consumption: the PREVEND cohort study. Am J Clin Nutr. 2018;108:1283–1290. [DOI] [PubMed] [Google Scholar]
- 150. Rao MN, Marmillot P, Gong M, et al. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism. 2003;52:1287–1294. [DOI] [PubMed] [Google Scholar]
- 151. Sirivarasai J, Kaojarern S, Sura T, et al. Biochemical, environmental, and genetic factors associated with paraoxonase (PON1) activity. Biochem Genet. 2011;49:364–368. [DOI] [PubMed] [Google Scholar]
- 152. Sarandöl E, Serdar Z, Dirican M, et al. Ö. Effects of red wine consumption on serum paraoxonase/arylesterase activities and on lipoprotein oxidizability in healthy-men. J Nutr Biochem. 2003;14:507–512. [DOI] [PubMed] [Google Scholar]
- 153. Kinoshita M, Teramoto T, Shimazu N, et al. CETP is a determinant of serum LDL-cholesterol but not HDL-cholesterol in healthy Japanese. Atherosclerosis. 1996;120:75–82. [DOI] [PubMed] [Google Scholar]
- 154. Luc G, Bard J-M, Evans A, et al. The relationship between apolipoprotein AI-containing lipoprotein fractions and environmental factors: the Prospective Epidemiological Study of Myocardial Infarction (PRIME study). Atherosclerosis. 2000;152:399–405. [DOI] [PubMed] [Google Scholar]
- 155. Ivanova EA, Myasoedova VA, Melnichenko AA, et al. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev. 2017;2017;2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sniderman AD, Couture P, Martin SS, et al. Hypertriglyceridemia and cardiovascular risk: a cautionary note about metabolic confounding. J Lipid Res. 2018;59:1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Adiels M, Packard C, Caslake MJ, et al. A new combined multicompartmental model for apolipoprotein B-100 and triglyceride metabolism in VLDL subfractions. J Lipid Res. 2005;46:58–67. [DOI] [PubMed] [Google Scholar]
- 158. Chapman MJ, Ginsberg HN, Amarenco P, et al. ; for the European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Camont L, Chapman MJ, Kontush A.. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. [DOI] [PubMed] [Google Scholar]
- 160. Rader DJ. New therapeutic approaches to the treatment of dyslipidemia. Cell Metab. 2016;23:405–412. [DOI] [PubMed] [Google Scholar]
- 161. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 162. Gordon DJ, Rifkind BM.. High-density lipoprotein–the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. [DOI] [PubMed] [Google Scholar]
- 163. Rader DJ, Tall AR.. The not-so-simple HDL story: is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–1346. [DOI] [PubMed] [Google Scholar]
- 164. Investigators A-H, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 165. Armitage J, Holmes MV, Preiss D.. Cholesteryl ester transfer protein inhibition for preventing cardiovascular events: JACC review topic of the week. J Am Coll Cardiol. 2019;73:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhong GC, Huang SQ, Peng Y, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: a pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiolog. 2020;27:1187–1203. [DOI] [PubMed] [Google Scholar]
- 168. Kajani S, Curley S, McGillicuddy FC.. Unravelling HDL—looking beyond the cholesterol surface to the quality within. Int J Mol Sci. 2018;19:1971–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Martin SS, Khokhar AA, May HT, et al. ; Lipoprotein Investigators Collaborative (LIC). HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the lipoprotein investigators collaborative. Eur Heart J. 2015;36:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Joshi PH, Toth PP, Lirette ST, et al. ; Lipoprotein Investigators Collaborative (LIC) Study Group. Association of high-density lipoprotein subclasses and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. Eur J Prev Cardiol. 2016;23:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Albers JJ, Slee A, Fleg JL, et al. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis. 2016;251:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mora S, Otvos JD, Rifai N, et al. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. [DOI] [PubMed] [Google Scholar]
- 174. McGarrah RW, Craig DM, Haynes C, et al. High-density lipoprotein subclass measurements improve mortality risk prediction, discrimination and reclassification in a cardiac catheterization cohort. Atherosclerosis. 2016;246:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Asztalos BF, Demissie S, Cupples LA, et al. LpA-I, LpA-I: A -II HDL and CHD-risk: the Framingham Offspring Study and the Veterans Affairs HDL Intervention Trial. Atherosclerosis. 2006;188:59–67. [DOI] [PubMed] [Google Scholar]
- 176. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Borén J, Williams KJ.. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol. 2016;27:473–483. [DOI] [PubMed] [Google Scholar]
- 178. Camejo G, Hurt-Camejo E, Wiklund O, et al. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139:205–222. [DOI] [PubMed] [Google Scholar]
- 179. Lund-Katz S, Laplaud PM, Phillips MC, et al. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry. 1998;37:12867–12874. [DOI] [PubMed] [Google Scholar]
- 180. Kjellmo C, Hovland A, Lappegård K.. CVD risk stratification in the PCSK9 era: is there a role for LDL subfractions? Diseases. 2018;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Williams PT, Zhao X-Q, Marcovina SM, et al. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis. 2014;233:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Campos H, Moye LA, Glasser SP, et al. Low-density lipoprotein size, pravastatin treatment, and coronary events. JAMA. 2001;286:1468–1474. [DOI] [PubMed] [Google Scholar]
- 183. Rizzo M, Berneis K.. Small, dense low-density-lipoproteins and the metabolic syndrome. Diabetes Metab Res Rev. 2007;23:14–20. [DOI] [PubMed] [Google Scholar]
- 184. Toth PP, Barter PJ, Rosenson RS, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. [DOI] [PubMed] [Google Scholar]
- 185. Tresserra-Rimbau A, Medina-Remón A, Lamuela-Raventós RM, et al. ; on behalf of the PREDIMED Study Investigators. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br J Nutr. 2015;113:S121–S130. [DOI] [PubMed] [Google Scholar]
- 186. Schrieks IC, Heil ALJ, Hendriks HFJ, et al. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care. 2015;38:723–732. [DOI] [PubMed] [Google Scholar]
- 187. Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32:2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019;4:1287– 1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Mach F, Baigent C, Catapano AL, et al. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;2019:1–78. [DOI] [PubMed] [Google Scholar]
- 191. Jiang ZG, De Boer IH, Mackey RH, et al. Associations of insulin resistance, inflammation and liver synthetic function with very low-density lipoprotein: the Cardiovascular Health Study. Metabolism. 2016;65:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. [DOI] [PubMed] [Google Scholar]
- 193. Colhoun HM, Otvos JD, Rubens MB, et al. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diabetes. 2002;51:1949–1956. [DOI] [PubMed] [Google Scholar]
- 194. Freedman DS, Otvos JD, Jeyarajah EJ, et al. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1046–1053. [DOI] [PubMed] [Google Scholar]
- 195. Taskinen MR, Packard CJ, Borén J.. Emerging evidence that ApoC-III inhibitors provide novel options to reduce the residual CVD. Curr Atheroscler Rep. 2019;21: doi:10.1007/s11883-019-0791-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kawakami A, Aikawa M, Alcaide P, et al. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114:681–687. [DOI] [PubMed] [Google Scholar]
- 197. Lecomte E, Herbeth B, Paille F, et al. Changes in serum apolipoprotein and lipoprotein profile induced by chronic alcohol consumption and withdrawal: determinant effect on heart disease? Clin Chem. 1996;42:1666–1675. [PubMed] [Google Scholar]
- 198. Jensen MK, Aroner SA, Mukamal KJ, et al. High-density lipoprotein subspecies defined by presence of apolipoprotein C-III and incident coronary heart disease in four cohorts. Circulation. 2018;137:1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Khetarpal SA, Qamar A, Millar JS, et al. Targeting ApoC-III to reduce coronary disease risk. Curr Atheroscler Rep. 2016;18: doi:10.1007/s11883-016-0609-y [DOI] [PubMed] [Google Scholar]
- 200. Feingold KR, Grunfeld C, et al. Introduction to lipids and lipoproteins. In: Feingold KR, Anawalt B, Boyce A., eds. Endotext. South Dartmouth, MA: MDText.com, Inc; 2000. Available at: https://www.ncbi.nlm.nih.gov/books/NBK305896/. [Google Scholar]
- 201. Ossoli A, Simonelli S, Vitali C, et al. Role of LCAT in atherosclerosis. J Atheroscler Thromb. 2016;23:119–127. [DOI] [PubMed] [Google Scholar]
- 202. Tall AR, Rader DJ.. Trials and tribulations of CETP inhibitors. Circ Res. 2018;122:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jiang XC. Phospholipid transfer protein: its impact on lipoprotein homeostasis and atherosclerosis. J Lipid Res. 2018;59:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Schneider J, Liesenfeld A, Mordasini R, et al. Lipoprotein fractions, lipoprotein lipase and hepatic triglyceride lipase during short-term and long-term uptake of ethanol in healthy subjects. Atherosclerosis. 1985;57:281–291. [DOI] [PubMed] [Google Scholar]
- 205. Thuren T. Hepatic lipase and HDL metabolism. Curr Opin Lipidol. 2000;11:277–283. [DOI] [PubMed] [Google Scholar]
- 206. Anastasius M, Kockx M, Jessup W, et al. Cholesterol efflux capacity: an introduction for clinicians. Am Heart J. 2016;180:54–63. [DOI] [PubMed] [Google Scholar]
- 207. Tsubakio-Yamamoto K, Matsuura F, Koseki M, et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2008;375:390–394. [DOI] [PubMed] [Google Scholar]
- 208. Würtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk. Circulation. 2015;131:774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Aviram M. HDL–associated paraoxonase 1 (PON1) and dietary antioxidants attenuate lipoprotein oxidation, macrophage foam cells formation and atherosclerosis development. Pathophysiol Haemost Thromb. 2006;35:146–151. [DOI] [PubMed] [Google Scholar]
- 210. Shunmoogam N, Naidoo P, Chilton R, et al. Paraoxonase (PON)-1: a brief overview on genetics, structure, polymorphisms and clinical relevance. Vasc Health Risk Manag. 2018;14:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. White CR, Anantharamaiah GM.. Cholesterol reduction and macrophage function. Curr Opin Lipidol. 2017;28:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Ali M, Madjid M.. Lipoprotein-associated phospholipase A2: a cardiovascular risk predictor and a potential therapeutic target. Future Cardiol. 2009;5:159–173. [DOI] [PubMed] [Google Scholar]
- 213. Oei H-HS, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111:570–575. [DOI] [PubMed] [Google Scholar]
- 214. Millwood IY, Walters RG, Mei XW, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet. 2019;393:1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Holahan CJ, Schutte KK, Brennan PL, et al. Episodic heavy drinking and 20-year total mortality among late-life moderate drinkers. Alcohol Clin Exp Res. 2014;38:1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Kontush A, Chapman MJ.. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. [DOI] [PubMed] [Google Scholar]
- 217. Monsonis-Centelles S, Hoefsloot HCJ, Engelsen SB, et al. Repeatability and reproducibility of lipoprotein particle profile measurements in plasma samples by ultracentrifugation. Clin Chem Lab Med. 2019;58:103–115. doi: 10.1515/cclm-2019-0729. [DOI] [PubMed] [Google Scholar]
- 218. Munroe WH, Phillips ML, Schumaker VN.. Excessive centrifugal fields damage high density lipoprotein. J Lipid Res. 2015;56:1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kunitake ST, Kane JP.. Factors affecting the integrity of high density lipoproteins in the ultracentrifuge. J Lipid Res. 1982;23:936–940. [PubMed] [Google Scholar]
- 220. Hafiane A, Genest J.. High density lipoproteins: measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015;3:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Davidson WS, HDL-C vs H-P.. How changing one letter could make a difference in understanding the role of high-density lipoprotein in disease. Clin Chem. 2014;60:e1–e3. [DOI] [PubMed] [Google Scholar]
- 222. MacKey RH, Greenland P, Goff DC, et al. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Aru V, Lam C, Khakimov B, et al. Quantification of lipoprotein profiles by nuclear magnetic resonance spectroscopy and multivariate data analysis. TrAC - Trends Anal Chem. 2017;94:210–219. [Google Scholar]
- 224. Langlois MR, Chapman MJ, Cobbaert C, et al. ; for the European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of LDL cholesterol. A Consensus Statement from EAS and EFLM. Clin Chem. 2018;64:1006–1033. [DOI] [PubMed] [Google Scholar]
- 225. El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. [DOI] [PubMed] [Google Scholar]
- 226. Mora S, Glynn RJ, Ridker PM.. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Krauss RM, Wojnooski K, Orr J, et al. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J Lipid Res. 2012;53:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Musunuru K, Orho-Melander M, Caulfield MP, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29:1975–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Smith GD, Ebrahim S.. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 230. Nakamura Y, Amamoto K, Tamaki S, et al. Genetic variation in aldehyde dehydrogenase 2 and the effect of alcohol consumption on cholesterol levels. Atherosclerosis. 2002;164:171–177. [DOI] [PubMed] [Google Scholar]
- 231. Wood AM, Kaptoge S, Butterworth AS, et al. ; Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Griswold MG, Fullman N, Hawley C, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Feingold KR, Grunfeld C, et al. Utility of advanced lipoprotein testing in clinical practice. In: Feingold KR, Anawalt B, Boyce A, eds. Endotext. South Dartmouth, MA: MDText.com, Inc; 2000. [Google Scholar]
- 234. Rader DJ, Hovingh GK.. HDL and cardiovascular disease. Lancet. 2014;384:618–625. [DOI] [PubMed] [Google Scholar]
- 235. Rader DJ, Alexander ET, Weibel GL, et al. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. von Zychlinski A, Kleffmann T.. Dissecting the proteome of lipoproteins: new biomarkers for cardiovascular diseases? Transl Proteomics. 2015;7:30–39. [Google Scholar]
- 237. Flores-Guerrero JL, Gruppen EG, Connelly MA, et al. A newly developed diabetes risk index, based on lipoprotein subfractions and branched chain amino acids, is associated with incident type 2 diabetes mellitus in the PREVEND cohort. J Clin Med. 2020;9:2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kovář J, Zemánková K.. Moderate alcohol consumption and triglyceridemia. Physiol Res. 2015;64(suppl 3):S371–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.