Abstract
Background:
Transient expression of proteins in mammalian cells is a key technique for many functional and structural studies of human and higher eukaryotic genes as well as for the production of recombinant protein therapeutics. Maximizing the expression efficiency to achieve a higher expression yield is desirable and may be even critical when, for instance, an expressed protein must be characterized at the single-cell level.
New Methods:
Our goal was to develop a simple method by which protein expression yield in human embryonic kidney (HEK)-293 cells could be enhanced with a brief treatment of dimethyl sulfoxide (DMSO) solution.
Results:
By expressing green fluorescent protein (GFP) as a reporter protein using the calcium phosphate transfection method and imaging a large population of cells, we found that a 5-min exposure of 10 % DMSO to HEK-293 cells, 4 h after transfection of the protein of interest, leads to ~1.6-fold increase in the expression yield without causing any appreciable cytotoxicity. By expressing an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and separately a kainate receptor in HEK-293 cells and measuring glutamate-induced whole-cell current response, we also found that such a brief DMSO treatment did not affect channel activity.
Conclusion:
This method is simple, efficient and inexpensive to use for enhancing transient transfection yield in HEK-293 cells.
Keywords: Protein expression, DMSO, HEK-293, GFP
1. Introduction
Dimethyl sulfoxide (DMSO) is a dipolar, aprotic molecule and it exists as a clear, colorless liquid at ambient temperature. Besides its utilities in chemical reactions, DMSO is routinely used as a solvent. One such a use is to dissolve small hydrophobic drug molecules (Szmant, 1975), due to its amphipathic property. Yet, DMSO is extensively used as a cryopreservative for cells and tissues because of its capability of membrane penetration, which leads to intracellular water displacement (Ashwood-Smith, 1964; Cheng et al., 2015; Gibb et al., 2015; Lovelock and Bishop, 1959; Meryman et al., 1977; Santos et al., 2003). DMSO can readily enter cells through both osmosis and its ability to modulate cell membrane permeability (Edashige, 2017). Among all other cryoprotectants, DMSO is thought to be unique in its ability of dehydrating phospholipid head groups in the membrane, while raising the membrane phase transition temperature (Sydykov et al., 2018). The ability of DMSO to modulate membrane permeability has been explored to increase expression of recombinant proteins in mammalian cells. For example, expression of human monoclonal antibody in Chinese hamster ovary (CHO) cells was increased when 0.2 % DMSO was added to the medium at the height of the cell density (Ling et al., 2003). A 4-minute (min) exposure of DMSO at 30 % v/v concentration to chicken embryo fibroblast cells led to an increase in transient transfection efficiency (Kawai and Nishizawa, 1984). In all these cases, a higher uptake of DNA plasmids through a higher permeability of cell membranes, due to DMSO treatment, is thought to contribute to the enhanced yield of protein expression (Kawai and Nishizawa, 1984).
In this study, we investigated whether the exposure of DMSO to human embryonic kidney (HEK)-293 cells would result in an increase in transfection efficiency. If so, we wanted to further establish the optimal concentration of DMSO and the optimal length of time of DMSO exposure to achieve the enhancement of transfection yield without causing cytotoxicity. A higher concentration and/or a longer exposure of DMSO to living cells can be damaging or cytotoxic (Awan et al., 2020), since DMSO reduces membrane rigidity and, at high concentrations, it can even induce pore formation in membrane (Wang et al., 2007). HEK-293 cells are a commonly used cell line for expression of recombinant proteins (Dalton and Barton, 2014). As compared with other mammalian cells, such as CHO cells, HEK-293 cells exhibit high transfection efficiency and faithful translation (Backliwal et al., 2008; Bollin et al., 2011; Meissner et al., 2001; Thomas and Smart, 2005; Wurm, 2004). In particular, HEK-293 cells have post-translational modification machineries required for proper folding, assembly, and post-translational modifications of recombinant proteins, especially membrane proteins. Protein expression in HEK-293 cells is quick, which usually peaks ~48-hours (h). The small cell size with minimal processes is also advantageous for electrophysiological studies of channel proteins (Lemtiri--Chlieh and Ali, 2013). The fact that HEK-293 cells have some neuronal characteristics further benefits studies of receptors and proteins found in the central nervous system (Hu et al., 2018; Stepanenko and Dmitrenko, 2015). To date, heterologous expression of membrane proteins, such as receptors and ion channels, for biological applications and characterizations, particularly for single-cell electrophysiology, remains a challenge. A high expression of proteins can be critical for studies of a membrane protein at the single-cell level. Therefore, improving the expression efficiency of a recombinant protein in HEK-293 cells is still a worthy effort.
The following experiment was designed to investigate whether the exposure of DMSO to HEK-293 cells would lead to an increase in transfection efficiency and expression yield. We transiently transfected GFP (Chalfie et al., 1994; Tsien, 1998) as a visual marker or reporter protein so that we could determine the transient transfection efficiency by measuring the percentage of GFP-expressing cells or green cells. We also transiently transfected an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor and separately a kainate receptor subunit in HEK-293 cells, and measured their receptor activities using whole-cell recording. The purpose was to examine if DMSO exposure to HEK-293 cells affected the function of an expressed protein. AMPA and kainate receptors are two subtypes of glutamate ionotropic receptor proteins, and they are indispensable for brain development and activity, such as memory and learning (Dingledine et al., 1999). Under both a bright-field and fluorescence detection (Lin et al., 2015), we also measured the number of cells in the population as a function of time so that we could monitor any potential damage to the cells due to the exposure of DMSO. Finally, we chose a calcium phosphate protocol for transient transfection of HEK-293 cells (Chen and Okayama, 1987). While a number of lipid-based transfection reagents are commercially available, the calcium phosphate procedure remains a simple, efficient and inexpensive method for transfecting eukaryotic cells in culture (Graham and van der Eb, 1973). It has been widely used with many adherent and non-adherent cell lines (Chen and Okayama, 1987; Jordan et al., 1996).
2. Materials and methods
2.1. Plasmid DNAs and cell culture
All the cDNA plasmids that encode GFP, large T antigen (TAg), GhiA2Qflip (i.e., unedited at the Q/R site and flip isoform) and GluK2 were propagated in DH5α E. coli host. They were purified with Qiagen kit. HEK-293 T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (Atlanta Biologicals) and penicillin-streptomycin (50 units/mL and 50 μg/mL, respectively, final concentrations). HEK-293 cells were generally cultured at 7 % CO2 and 37 °C. In some experiments, we moved the culture one day after transfection to a 33 °C incubator based on a protocol we reported earlier (Lin et al., 2015).
2.2. Transient transfection and DMSO treatment
HEK-293 cells were grown in 35-mm dishes and some were in 48-well plates. A standard transient transfection protocol was followed, namely, the HEK-293 cells were plated one day prior to transfection. On the next day or on the day of transfection, HEK-293 cells were ~70 % confluent. Calcium phosphate co-precipitation method (Chen and Okayama, 1987) was used. TAg was co-transfected in all cultures, since co-transfection of TAg enhances the yield of the expressed protein (Huang et al., 2005). For imaging study, GFP was transfected as the reporter gene. For the electrophysiological study of GluA2Qflip AMPA and GluK2 kainate receptors, GFP was co-transfected as a cell marker in that only GFP-expressing cells or green cells were used for whole-cell recording. DMSO solutions were prepared in phosphate-buffered saline (PBS) under sterile conditions.
At the 4th hour post-transfection, the culture was washed with PBS twice and then exposed to a DMSO solution at various concentrations or PBS alone as controlat room temperature. After the DMSO solution was removed, the cells were washed twice with PBS before the normal medium was returned to the dish. The untreated control was performed in the same way. All experiments were performed in triplicate, and triplicate data sets were used for data analysis.
2.3. Cell imaging and quantification
Cells were imaged on a Carl Zeiss Axiovert S200 microscope under both bright field and green fluorescence channel. Images were captured using a Sony NEX 3 digital camera with an adapter which has 1.74X magnification (Model Y1S-EA, McCan Imaging Inc.). In a single 35-mm dish, a pair of the bright-field and fluorescence images were taken at the same spot, and three sets of images were taken at three randomly selected spots or locations. These images were captured under a 20X magnification, giving rise to a total magnification of 20 × 1.74 = 34.8X. By a method we previously published (Lin et al., 2015), all images were analyzed using Image J (http://rsb.info.nih.gov/ij/). Each image contained 2288 × 1520 pixels and each pixel was scored with a value in the range of 0–255 on an 8-bit digital scale. The maximum background intensity, where no green color was shown, was 29 on this scale. A typical green cell contains approximately 3000 pixels and the fluorescence intensity was greater than 29 but smaller than 255. Each green cell was assigned to one of the three fluorescence intensity ranges, and categorized accordingly: an intensity between 29 and 100 was classified as light green, 100–200 was medium green, and over 200 was bright green. The number of green cells and the number of total cells were counted in each view. It was assumed that the overall green intensity indicated overall protein expression. Thus, based on these numbers, the percentage of green cells or expression yield was calculated. (Lin et al., 2015) (an example is shown in Fig. 1C).
Fig. 1.
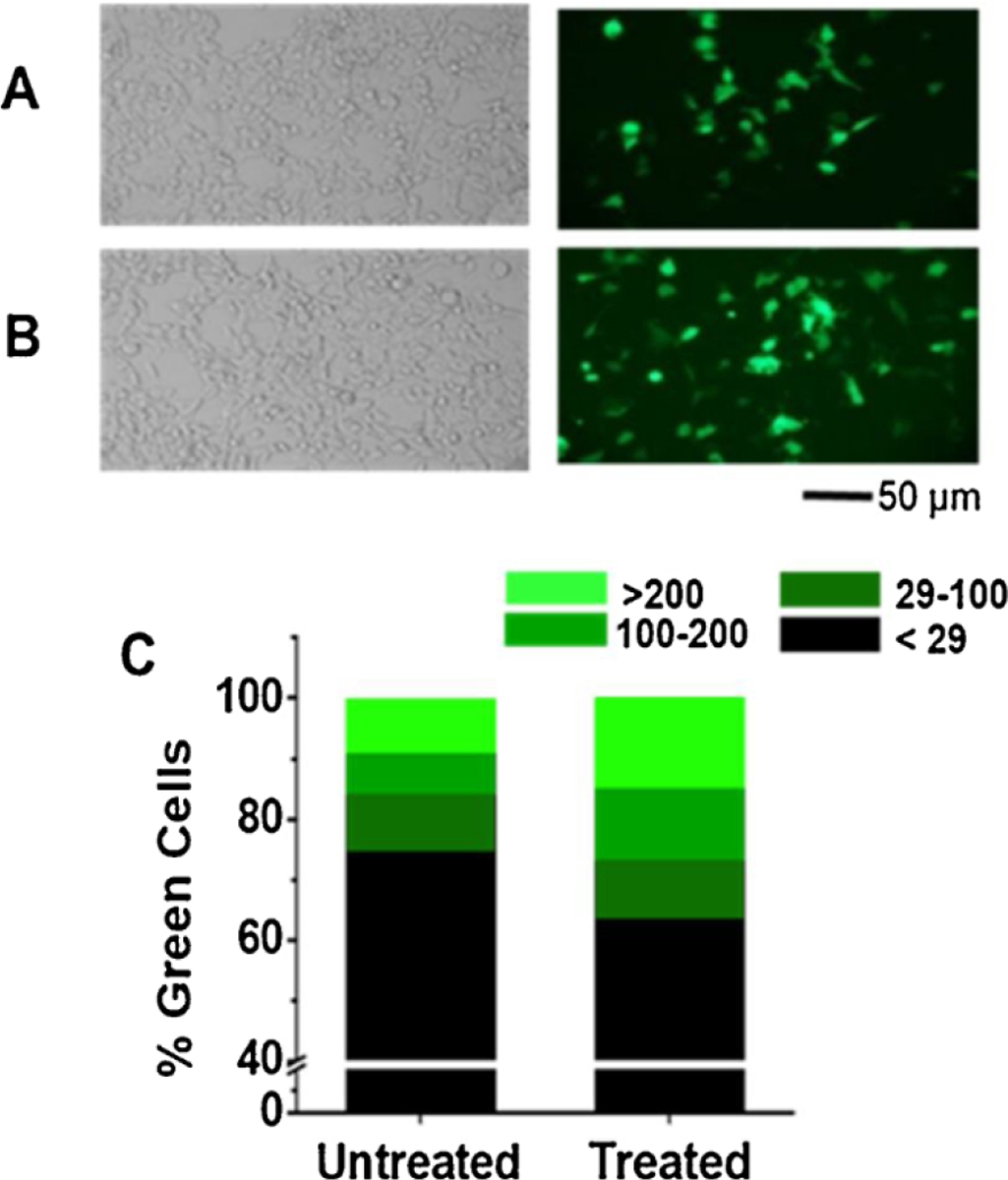
Effect of DMSO on transient transfection of GFP in HEK-293T cells. The cells were incubated at 37 °C for 4 h after transfection before they were treated with 10 % DMSO in PBS for 5 min at room temperature. Dishes that were only exposed to PBS without DMSO, which we termed untreated dishes, were used as control. (A) Untreated- and (B) DMSO-treated cells under bright-field (left panels) and fluorescence view (right panels). These images were taken 48 h after transfection. Each view contains approximately 170 cells. (C) A typical HEK-293 cell in 34.8X magnification contained 3000 ± 800 pixels. The green color intensity was characterized on a scale of 0 to 255 in a JPEG file, recorded in an 8 bit byte. On this scale, a cell with an average brightness between 29 and 100 was labeled low green intensity, colored as dark green bar. A cell with an average brightness between 100 and 200 was considered medium intensity, colored as green. Bright green was used to label a cell with an average brightness between 200 and 255. On the same scale, a cell without any appreciable fluorescent green color registered an intensity of <29. Thus, black color was assigned to these cells or non-green cells. The percentage of each green group was calculated based on the total cell number, which was determined from the bright-field view of the same viewing area. The white line in each column in (C) indicates a break in the y axis from 10 % to 30 %.
2.4. Cell viability assay
AlamarBlue HS reagent (Invitrogen, ThermoFisher A50100) was used for cytotoxicity and cell viability assay. Cells were seeded into 48-well plate (Corning, Costar flat bottom). The alamarBlue reagent was added to a 10 % concentration in complete DMEM. A blank was prepared in a clean well with complete DMEM and 10 % alamarBlue reagent. Monochromator-based fluorescence intensity was measured using a Biotek Synergy H1 Hybrid Reader at the bottom optics position, with the gain set to scale to high wells, and with excitation wavelength of 560 nm and emission wavelength of 590 nm. The fluorescence value of the blank well was subtracted from all fluorescence readings. Fluorescence of the control (PBS-treated cells) was normalized to 100 % and viability values of the DMSO treated cells were calculated as a percentage using the control. All viability experiments were done in triplicate.
2.5. Whole-cell current recording
Whole-cell recording was used to assess whether a 5-min, 10 % DMSO exposure would affect the channel activity of both GluA2Qflip and GluK2, each of which formed a functional, homomeric channel when individually expressed in HEK-293 cells (Li et al., 2003a, b). Specifically, HEK-293 cells were first transfected with cDNA plasmid encoding a receptor, and 4 h later, the cells were exposed to 10 % DMSO for 5 min. The transfected cells were maintained at 37 °C for 48 h before they were used for whole-cell recording. The amplitude of the whole-cell current response to glutamate and the desensitization rate constant were measured from receptor-expressing cells treated with DMSO, whereas untreated cells were used as control. The procedure for the whole-cell recording was described previously (Li et al., 2003b; Pei et al., 2007). In brief, recording electrodes were prepared from glass capillaries (World Precision Instruments). Electrode solution contained 110 mM CsF, 30 mM CsCl, 4 mM NaCl, 0.5 mM CaCl2, 5 mM EGTA, and 10 mM HEPES (pH 7.4 adjusted by CsOH). When filled with electrode solution, the resistance was approximately 3 ΜΩ. External buffer contained 150 mM NaCl, 3 mM KCl, 1 mM CaCl2,1 mM MgCl2, 10 mM HEPES (pH 7.4 adjusted with HCl). Recordings were made at 22 °C with a cell clamped at −60 mV using an Axopatch-200B amplifier, with a cutoff frequency of 2 kHz by a built-in 4-pole, low pass Bessel filter. Whole-cell current traces were digitized at a 5 kHz sampling frequency using a Digidata 1322A by Axon Instruments. Patch clamp data was acquired using pClamp 9 software.
3. Results
3.1. Effect of DMSO
To investigate the effect of DMSO on transient expression of recombinant proteins in HEK-293 cells, we started with transfecting GFP. As compared to the control (Fig. 1A, right panel), exposing DMSO to the transfected culture 4 hafter transfection appeared to increase the number of green cells (Fig. 1B, right panel). As shown, more green cells or precisely more bright-green cells were found from DMSO-treated dishes as compared to untreated dishes (Fig. 1C and its legend). It should be noted that cells were exposed to DMSO 4 h after they were transfected with the GFP plasmid. Ordinarily, we would layer the transfection solution on top of the cells and return the culture to a 37 °C incubator. After 4 h, the transfection solution would be replaced with normal or complete growth medium. We did try mixing DMSO with the transfection solution, and exposed the mixture to the cells. However, applying the mixture of the transfection solution and DMSO to the cells resulted in much fewer living cells afterwards, possibly because incubating DMSO for 4 h would be toxic to cells. In addition, we found mixing DMSO with the transfection solution led to some precipitation in the mixture. Therefore, we decided to wait for 4 h before the cells were subject to DMSO treatment. In other words, 4 h after the transfection, we would take a transfected dish out of the incubator for medium replacement; it was at that time when the dish was first washed with PBS, and then treated with DMSO. After the DMSO treatment, the dish was washed again with PBS and replenished with normal growth medium before it was returned to the incubator. In so doing, we would also be able to vary the DMSO exposure time, as described below.
3.2. Optimum DMSO concentration and exposure time
Next, we attempted to establish the optimum concentration of DMSO to treat the transfected HEK-293 cells and the optimum length of exposure time. These experiments were important because DMSO exposure could damage cells at a higher concentration or for a longer exposure time. It is known that even at a lower concentration, a longer exposure of DMSO to cells could be cytotoxic (Galvao et al., 2014; Hanslick et al., 2009). To determine the optimum DMSO concentration, we tested 4 different concentrations of DMSO in PBS, ranging from 5 to 20 % (% of v/v; Fig. 2) for 5 min, along with the control or untreated dish (i.e., a dish that was only treated with PBS buffer). As seen (Fig. 2A), 10 % DMSO seemed to generate a higher percentage of green cells. In contrast, the 5 % DMSO solution did not lead to a significant increase in green cells, as compared to the untreated control. On the other hand, exposing DMSO solution of higher than 10 % resulted in considerably fewer green cells. Furthermore, at 10 % DMSO concentration, we observed the highest percentage of the bright green cells.
Fig. 2.
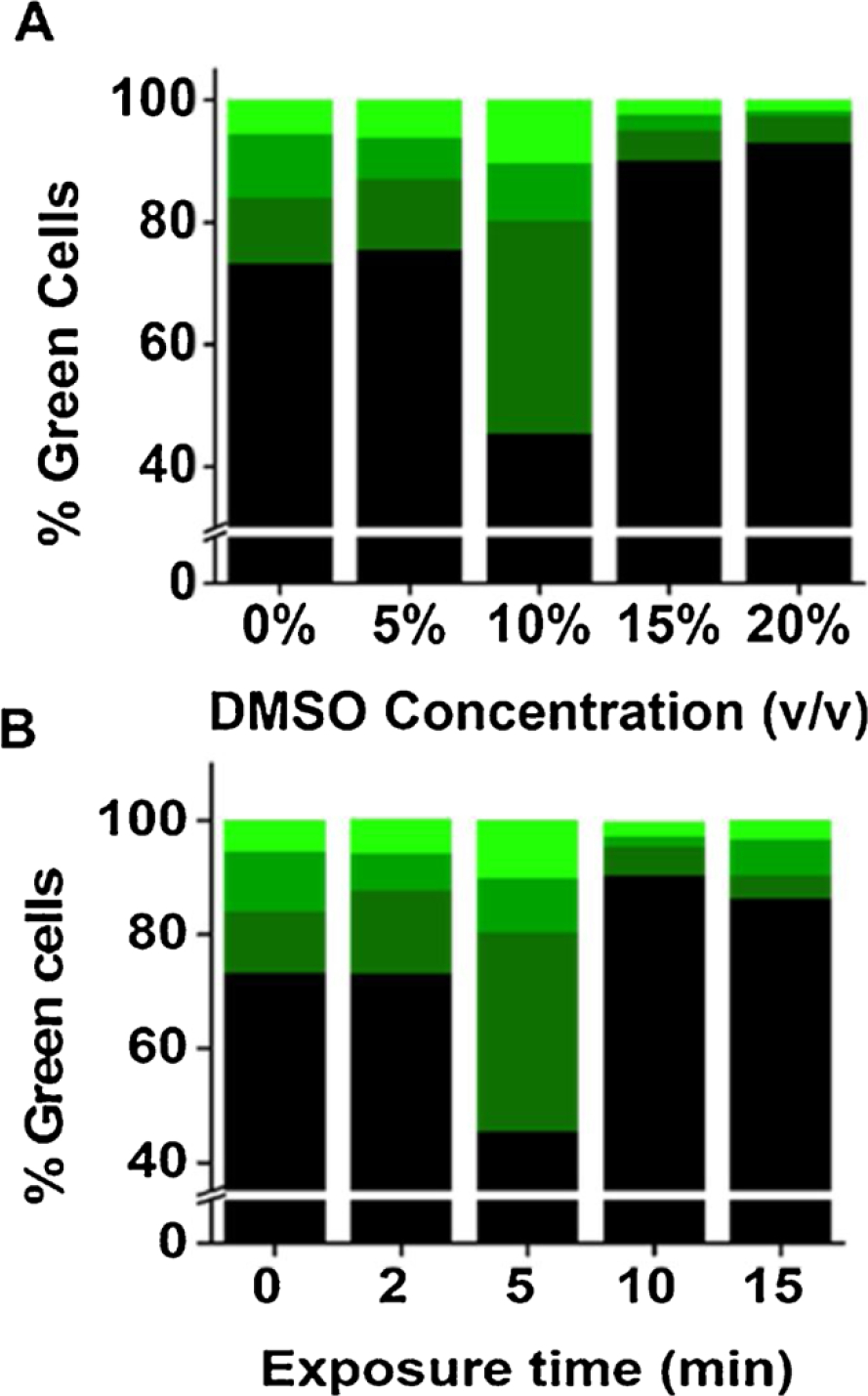
Determining optimum concentration of DMSO and exposure time. (A) Different concentrations of DMSO (i.e., 5 %−20 %) were used to treat HEK-293 cells transfected with GFP. Exposure time was kept 5 min in all experiments. The dishes were then washed with PBS, replenished with the complete medium, and maintained at 37 °C. Images were taken 48 h after transfection. For determining the percentage of green cells and intensity, three images from three randomly chosen areas were taken under both blight-field and fluorescence view for each dish. (B) Similarly, HEK-293 cells transfected with GFP were exposed to 10 % DMSO but with different exposure time, i.e., from 2 to 15 min. Images were taken 48 h after transfection and used for determining the percentage of green cells.
By fixing the DMSO concentration at 10 %, we then varied exposure time. Specifically, the cells were incubated with 10 % DMSO solution from 2 to 15 min at room temperature. We found that the optimum incubation time or exposure time was ~5 min (Fig. 2B). The shorter incubation time (i.e., 2 min) had very little effect on the number of green cells as compared with the control. In contrast, exposing DMSO longer than 5 min resulted in fewer green cells. These results (Figs. 1 and 2) allowed us to conclude that exposing transfected cells with 10 % DMSO for 5 min was considered optimal.
3.3. Time course of GFP expression post-DMSO exposure
We then monitored the GFP expression over time for dishes treated with DMSO. Specifically, we measured the percentage of green cells over a 6-day period from DMSO-treated and untreated dishes. Our data showed that the expression of GFP over this time period was consistently higher in the DMSO-treated cells (Fig. 3A), as compared with untreated ones (Fig. 3B). Furthermore, both DMSO-treated and untreated cells had the highest level of GFP expression on day 2 (Fig. 3C). Based on the percentage of the green cells on day 2, we found that on average, there were ~1.6-fold more green cells in a dish treated with a 5-min, 10 % DMSO, 4 h post-transfection.
Fig. 3.
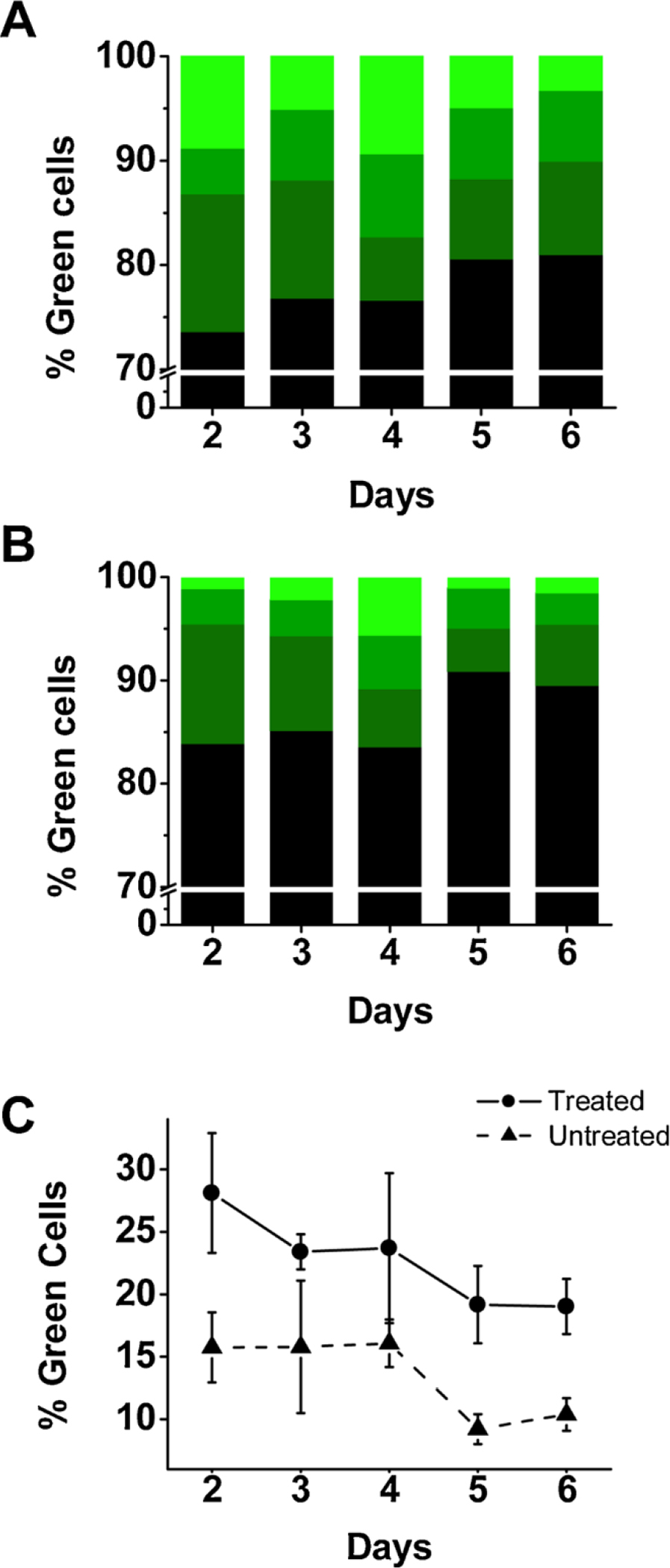
Time course of GFP expression post-DMSO exposure. The percentage of GFP-expressing green cells in dishes treated with 10 % DMSO for 5 min (A), 4 h after transfection, and untreated dishes (B). The percentage of green cells was determined over 6 days by imaging a dish under blight-field and fluorescence view. Plotted is the average percentage of green cells calculated from three images per dish and three dishes in total. The white line indicates a break in the y axis from 10 % to 70 %. Unlike in either (A) or (B) where three green color intensities are displayed, (C) shows the total percentage of green cells over the 6-day period for treated cells (solid circle) and untreated cells (solid triangle).
Starting from day 3, the overall fluorescence intensity of the two cultures gradually decreased (Fig. 3C). It should be noted that we also used lower temperature, precisely 33 °C, for cell maintenance after allowing the transfected culture to recover at 37 °C overnight. This hypothermic condition, which we reported previously (Lin et al., 2015), allowed us to preserve more cells, as compared with 37 °C. The fact that untreated cells also showed a similar rate of decline in the overall percentage of green cells (Fig. 3C, lower line) after day 2, which was consistent with the observation we reported earlier for a regular HEK-293 culture (or without DMSO treatment) (Lin et al., 2015), further suggested that a brief exposure (i.e., 5 min) of DMSO to the transfected HEK-293 cells did not affect cell health, at least not appreciably. Taken together, the decline in the percentage of green cells from day 2 was most likely due to intrinsic protein degradation and/or cell division. In fact, a previous study reported that the half-life of the attenuation of GFP in mammalian cells is about 26 h (Corish and Tyler-Smith, 1999). It should be noted that it is a general practice that in transient transfection of a recombinant protein in mammalian cells such as HEK-293 cells, the cells are typically harvested or used 24–72 h after transfection, given the expression of protein generally peaks during this period of time. What we observed (Fig. 3) fell within this time frame.
3.4. Assay of cell viability for DMSO-treated culture
We further asked whether a 5-min exposure of DMSO to HEK-293 cells would cause cytotoxicity and then affect cell viability. For this experiment, we used alamarBlue reagent. This reagent detects the level of oxidation during respiration, thus providing a quantitative measure of cell viability and cytotoxicity (O’Brien et al., 2000). In addition, when the reagent is mixed with cells, the cells remain viable and healthy, unaffected by the presence of the reagent. As such, the cell viability can be monitored over a period of time. In our experiment, we mixed alamarBlue agent with the transfected cells grown in 48-well plate right after the DMSO treatment, and measured the fluorescence on a microplate reader. As seen (Fig. 4A), a 5-min DMSO treatment did not cause any change of fluorescence as compared with untreated control. A 10-min exposure of the same DMSO concentration (10 %) to cells led to a slight reduction of fluorescence intensity as compared to the control, although the reduction was statistically insignificant (p = 0.92). Because we observed that DMSO treated cells were prone to be washed away during medium replacement (from DMSO solution to complete growth medium), we re-seeded the cells in each well with the identical cell count (100,000/well) before we measured the fluorescence intensity. Furthermore, the 5-min DMSO treatment caused no adverse effect on the health and growth of the transfected HEK-293 cells, evidenced by the fluorescence intensity on days 2 and 3 (Fig. 4C), as compared to the control (Fig. 4B). In fact, on day 3 or at the end of this experiment, we dislodged the cells and re-counted the number of cells for each set of the experiments. We found the control and the 5-min DMSO treated cells contained an average of 1.2 × 107 ± 1.9 × 105 and 1.2 × 107 ± 4.7 × 105 cells per well, respectively (the difference is statistically insignificant; p = 0.88 for 5 min vs. control). In contrast, the 10-min DMSO treated cells grew as well; but in the end, there were on average fewer cells (i.e., 1.2 × 107 ± 8.5 × 105 cells per well), although the difference was not significant as compared to the control (p = 0.61) (Fig. 4D). This result suggested that even with a 10-min exposure, 10 % DMSO treatment did not significantly affect the cell count and the growth. Taken together, we determined it was safe to use a 5-min exposure time for DMSO treatment, because a 5-min exposure of 10 % DMSO solution to the transfected HEK-293 cells showed no detectable cytotoxicity, and did not affect either cell viability or growth.
Fig. 4.
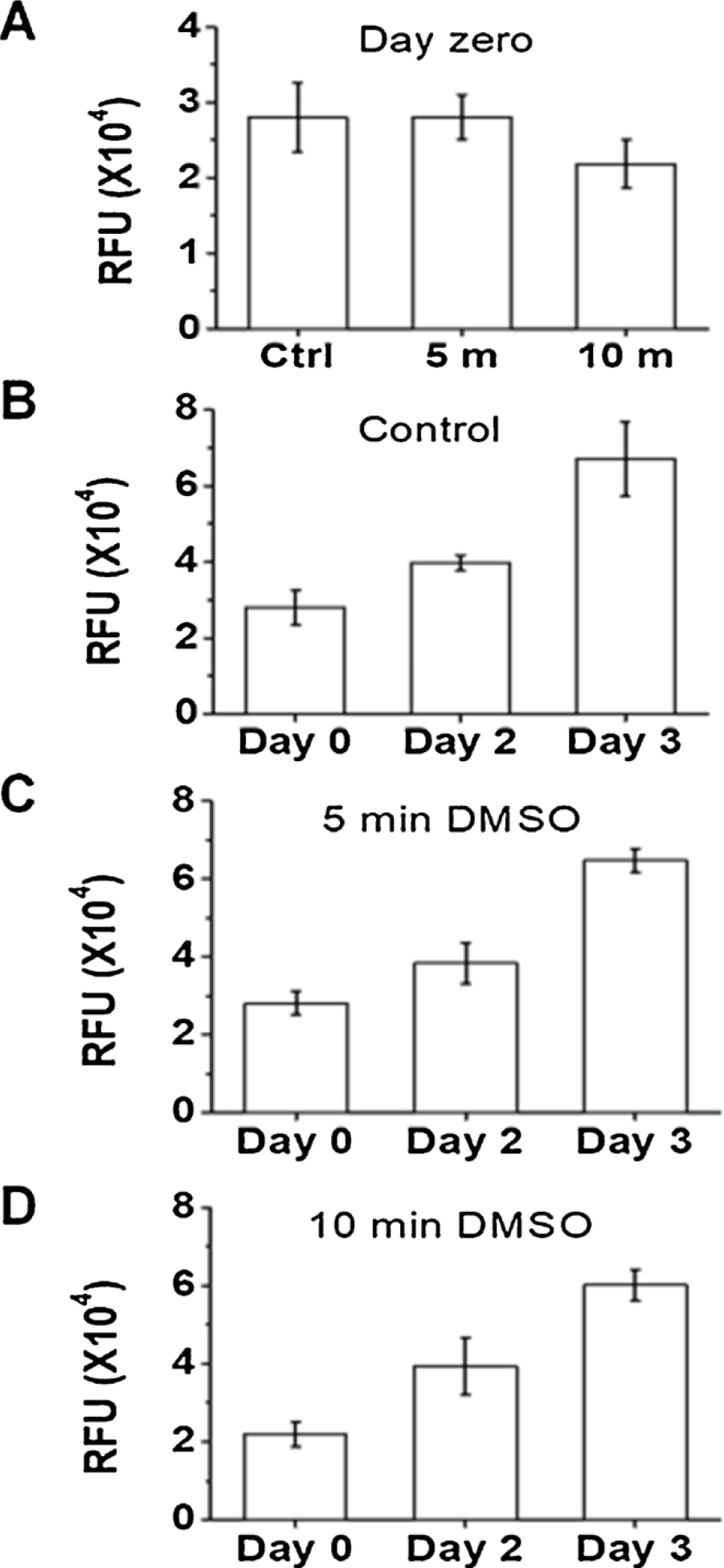
Assay of cell viability and growth for DMSO-treated HEK-293 cells using alamarBlue. After 10 % DMSO exposure, cells were recounted and reseeded in 48-well plate with 100,000 cells/well, and all assays were done in triplicate. Immediately following reseeding, alamarBlue was added to the wells at a 10 % v/v concentration. The cells were incubated with alamarBlue for 2.5 h before fluorescence readout at 560 nm exciation and 590 nm emission on a microplate reader; this fluorescence readout was set as day zero. (A) displays the fluorescence intensity on day zero for control (Ctrl), 5-min and 10-min exposure of 10 % DMSO to HEK-293 cells transfected with GFP. The fluorescence readout is plotted in relative fluorescence unit (RFU). In (B), the fluorescence intensity of the control well was measured and plotted for days 0, 2 and 3. (C) and (D) are fluorescence intensities from wells treated with 10 % DMSO for 5 min and 10 min, respectively. Error bars represent the standard deviation from the mean. Other experimental detail is provided in Materials and Methods.
3.5. Whole-cell recording assay of ion channel activity with DMSO-treated HEK-293 cells
HEK-293 cells are routinely used to express membrane proteins and receptors. To ensure that the brief exposure of 10 % DMSO would not affect the function of a recombinant protein, we expressed and then tested the activity of GluA2Qflip, an AMPA receptor subunit, and separately GluK2, a kainate receptor subunit, in HEK-293 cells. It is known that transient expression of each of these receptor subunits in HEK-293 cells leads to the formation of a functional, homomeric channel, and the channel activity can be characterized from an electrophysiological study using whole-cell recording (note that the GluA2 channel we expressed was the unedited isoform or Q (glutamine) isoform in the Q/R (arginine) editing site) (Li et al., 2003a, b). As a control, we also expressed and tested the same receptors from HEK-293 cells that were not exposed to DMSO.
As shown (Fig. 5A), the whole-cell current rose in response to glutamate, the activating ligand, indicating the opening of the channels. On longer time scale, the current fell towards the baseline due to channel desensitization. The rate constant (kdes) of channel desensitization is one of the fundamental properties of an ion channel (Katz and Thesleff, 1957). Based on the recording of a set of DMSO-treated cells and also untreated cells, we found that kdes for GluA2Qflip measured from the DMSO-treated cells was 125 ± 13 s−1, whereas kdes was 133 ± 17 s−1 for the same receptor but from untreated cells. Likewise, kdes for GluK2 kainate receptors measured from the DMSO-treated cells was 125 ± 15 s−1, whereas kdes was 119 ± 14 s−1 for the same receptor from untreated cells. Furthermore, the magnitude of kdes we observed here for either GluA2Q or GluK2 channel is consistent with the value we reported earlier (Han et al., 2017; Li et al., 2003a, b; Wen et al., 2017). These results therefore demonstrated that the channel desensitization rate for either an AMPA receptor or a kainate receptor was unaffected when HEK-293 cells that expressed a receptor were exposed to 10 % DMSO for 5 min. In other words, the brief treatment of transfected HEK-293 cells with DMSO did not affect the property of these receptors.
Fig. 5.
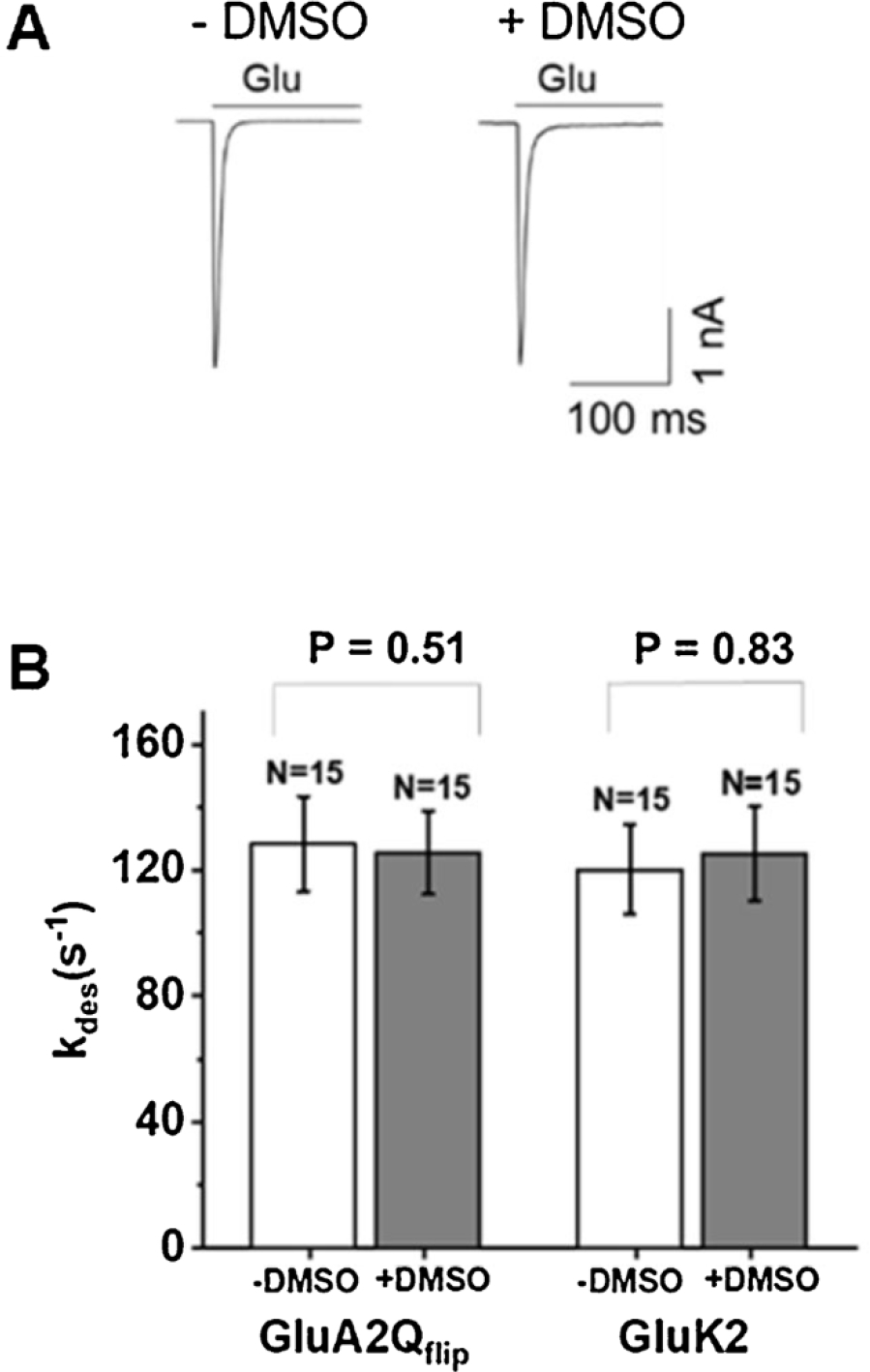
Whole-cell recording assay of AMPA and kainate receptor activities with DMSO-treated and untreated HEK-293 cells. Shown in (A) is a pair of representative whole-cell current response to glutamate mediated through GluK2 kainate receptors expressed in HEK-293 cells. Left trace was from a cell without DMSO treatment (or −DMSO), whereas the trace on the right was from a cell treated with DMSO (or + DMSO). The two traces are scaled to the same amplitude for visual inspection of the channel desensitization rate. The upper bar shows the time scale of a 0.5-mM glutamate application. In (B), the two columns on the left show the channel desensitization rate constant (kdes) at 3 mM glutamate measured from 15 cells (or N = 15) expressing GluA2Qflip AMPA receptors from untreated and treated dishes. Similarly, the two columns on the right represent kdes, measured at 0.5 mM glutamate concentration, for GluK2 kainate receptors expressed in HEK-293 cells. An average kdes is shown with an error bar representing the standard deviation from the mean. Also shown on the top for each receptor type is the p value obtained from a paired two-tailed Student’s t test.
A brief treatment of DMSO had no adverse effect on cells for whole-cell recording assay. Specifically, no difference in patching, seal formation, and seal duration was observed between DMSO-treated and untreated cells. This may not be surprising given that a 5-min DMSO exposure produced no adverse effect in either cell viability or growth (Fig. 4). Furthermore, cells were cultured for another 48 h before they were used for whole-cell recording. It should be noted that 48 h after transfection is the typical time point for the beginning of using transfected cells in electrophysiology. In addition, by using the current amplitude as a measure of the number of the receptors per transfected cell, we found that receptor-expressing, DMSO-treated cells exhibited a ~1.4-fold higher whole-cell current amplitude than untreated cells (Supplementary Fig. 1). This result suggested that DMSO-treated cells expressed roughly 1.4-fold more receptors per cell. This was because whole-cell current amplitude depends on the unitary channel conductance and the number of channels expressed in the cell as well as ligand concentration. Since the unitary conductance level and the ligand concentration we used (i.e., 3 mM) are the same, the whole-cell amplitude is proportional to the number of the receptor channels/cell. We note that the 1.4-fold increase estimated from the receptor-mediated whole-cell current amplitude was similar with the ratio (i.e., ~1.6-fold) determined front fluorescence imaging using GFP as a reporter protein (Fig. 2).
4. Discussion
The productivity of transiently transfected mammalian cultures for expression of recombinant proteins depends on a number of factors. These include the type of the host cell, rate of gene transcription and translation, the stability of gene expression, posttranslational modification in the cell, and the transfection efficiency (Blasey et al., 1995; Chen and Okayama, 1987; Curiel, 1994; Griffiths and Racher, 1994). Once a cell source or cell line is chosen, manipulating transfection efficiency is perhaps one of the most effective ways to improve the yield of the gene product. Among various attributes that have made HEK-293 cells an attractive choice of transient gene expression system is the fact that these cells can be readily manipulated (Meissner et al., 2001; Thomas and Smart, 2005). Based on this idea, we investigated, and report here, the effect of briefly exposing DMSO to transfected cells on the productivity of the transient expression yield. As we have shown in this study, exposing HEK-293 cells with 10 % DMSO for 5 min, 4 h post-transfection of a recombinant protein of interest, enhances the expression yield by ~1.6-fold without any negative effect on either cell viability or growth.
One important finding from our study is that the exposure of DMSO to cells should occur 4 h after the cells have already been exposed to a transfection reagent; in our case, we used calcium phosphate DNA co-precipitation. Under this condition, a 4 h incubation of the transfection mixture with cells is thought to be sufficient to allow absorption of the foreign DNA on the cell surface (Kawai and Nishizawa, 1984). This is the time at which we would normally replace the transfection solution with complete medium before returning the transfected culture to an incubator. A 5-min treatment of the culture with DMSO is therefore just an additional step prior to the complete medium exchange. Care should be taken, however, during medium replacement after the DMSO treatment, since DMSO-treated cells are prone to be washed away during medium exchange. This would be especially important if one wishes to harvest the entire culture as compared with just using a much smaller number of cells for electrophysiology. On this note, we also attempted initially the use of the same protocol, i.e., 5-min 10 % DMSO treatment, with suspension-adapted HEK-293S cells (Reeves et al., 2002). We found similar enhancement in HEK-293S cells but the culture dish must be first coated with collagen; otherwise a significant amount of the cells would be lost during the removal of DMSO solution and washing of the dish before the regular growth medium was returned. Obviously, separation of DMSO solution front the dish by pipetting the solution out is amenable to static culture. Overall, the method we have described is simple to implement, considering that calcium phosphate DNA co-precipitation protocol is a simple, inexpensive way of introducing foreign genes into mammalian cells. In fact, calcium phosphate transfection has been one of the most popular methods of introducing a gene ever since Graham and Van der Eb reported this method for transfecting adenovirus DNAs and simian virus 40 into mammalian cells in 1973 (Graham and van der Eb, 1973).
A 5-min treatment of the transfected HEK-293 cells with 10 % DMSO, which leads to an increase of transfection efficiency by ~1.6-fold, is most likely attributed to the ability of DMSO molecules to increase membrane permeability, by osmosis and DMSO-induced dehydration of phospholipid head groups on the membrane (Edashige, 2017; Sydykov et al., 2018). Consequently, more cDNA plasmids would be able to enter the cell or precisely taken up through endocytosis and finally enter the nucleus (Craig, 1998; Kawai and Nishizawa, 1984). However, a higher DMSO concentration or a longer exposure of DMSO is cytotoxic (Awan et al., 2020). Even a lower concentration of DMSO but a prolonged exposure or to the extreme, a chronic incubation of DMSO with the culture medium, can be damaging to cells (Kloverpris et al., 2010). In this study, we showed that although prolonging the exposure time from 5 to 10 min of the same DMSO concentration (i.e., 10 %) does not lead to a lower cell count, at least not significantly (Fig. 4A and C), it does lead to a lower percentage of protein expression, as seen in the GFP experiment (Fig. 2B). In this case, the reduced production of protein (Fig. 2B), but not the cell number (Fig. 4A), could be attributed to a negative impact of DMSO treatment on even transcription (Moskot et al., 2019). It is possible that overexposure of DMSO could inhibit ribosomal function of the cells (Reisner and Bucholtz, 1977). As long as the exposure time is kept brief or precisely 5 min, such a procedure produces a higher transient transfection yield without any adverse effect on either cell viability, cell growth (Fig. 4A, B and C) or the functional property of an protein expressed in HEK-293 cells (Fig. 5).
Supplementary Material
Acknowledgements
We acknowledged the initial data collection by Deon Teh.
Funding sources
This work was supported in part by grants from National Institutes of Health (1R21NS106392 and R56AG06601501) and also from Department of Defense (W81XWH2010385).
Abbreviations:
- AMP A
α-amino-3-hydiOxy-5-methyl-4-isoxazolepiopionic acid
- CHO cells
Chinese hamster ovary cells
- DMSO
dimethyl sulfoxide
- GFP
green fluorescent protein
- HEK-293 cells
human embiyonic kidney-293 cells
- TAg
large T-antigen
- PBS
phosphate-buffered saline
Footnotes
CRediT authorship contribution statement
Janet Lynch: Conceptualization, Methodology, Investigation, Writing - original draft. JiWoo Chung: Investigation. Zhen Huang: Conceptualization, Methodology, Investigation, Writing - review & editing. Vincen Pierce: Investigation. Noah S. Saunders: Investigation. Li Niu: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing - original draft.
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jneumeth.2020.109058.
References
- Ashwood-Smith MJ, 1964. Low temperature preservation of mouse lymphocytes with dimethyl sulfoxide. Blood 23, 494–501. [PubMed] [Google Scholar]
- Awan M, Buriak I, Fleck R, Fuller B, Goltsev A, Kerby J, Lowdell M, Mericka P, Petrenko A, Petrenko Y, Rogulska O, Stolzing A, Stacey GN, 2020. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 15, 1463–1491. [DOI] [PubMed] [Google Scholar]
- Backliwal G, Hildinger M, Chenuet S, Wulhfard S, De Jesus M, Wurm FM, 2008. Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/1 by transient transfection under serum-free conditions. Nucleic Acids Res. 36, e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasey HD, Aubry JP, Mazzei GJ, Bernard AR, 1995. Large scale transient expression with COS cells. Cytotechnology 18, 183–192. [DOI] [PubMed] [Google Scholar]
- Bollin F, Dechavanne V, Chevalet L, 2011. Design of Experiment in CHO and HEK transient transfection condition optimization. Protein Expr. Purif. 78, 61–68. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC, 1994. Green fluorescent protein as a marker for gene expression. Science 263, 802–805. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H, 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Song J, Pas J, Meijer LH, Han S, 2015. DMSO induces dehydration near lipid membrane surfaces. Biophys. J. 109, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corish P, Tyler-Smith C, 1999. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 12, 1035–1040. [DOI] [PubMed] [Google Scholar]
- Craig A, 1998. Transfecting cultured neurons. Cult. Nerve Cell. 2, 79–111. [Google Scholar]
- Curiel DT, 1994. High-efficiency gene transfer mediated by adenovirus-polylysine-DNA complexes. Ann. N.Y. Acad. Sci. 716, 36–56 discussion −8. [DOI] [PubMed] [Google Scholar]
- Dalton AC, Barton WA, 2014. Over-expression of secreted proteins from mammalian cell lines. Protein Sci. 23, 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF, 1999. The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61. [PubMed] [Google Scholar]
- Edashige K, 2017. Permeability of the plasma membrane to water and cryoprotectants in mammalian oocytes and embryos: its relevance to vitrification. Reprod. Med. Biol. 16, 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF, 2014. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 28, 1317–1330. [DOI] [PubMed] [Google Scholar]
- Gibb SL, Matthay M, Nizzi F, Marlowe M, Jones B, Pati S, 2015. Exposure of mesenchymal stem cells to dmso post-thaw affects cell viability and signaling. Cytotherapy 17. S46–S. [Google Scholar]
- Graham FL, van der Eb AJ, 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52, 456–467. [DOI] [PubMed] [Google Scholar]
- Griffiths JB, Racher AJ, 1994. Cultural and physiological factors affecting expression of recombinant proteins. Cytotechnology 15, 3–9. [DOI] [PubMed] [Google Scholar]
- Han Y, Lin CY, Niu L, 2017. Functional roles of the edited isoform of GluA2 in GluA2-Containing AMPA receptor channels. Biochemistry 56, 1620–1631. [DOI] [PubMed] [Google Scholar]
- Hanslick JL, Lau K, Noguchi KK, Olney JW, Zorumski CF, Mennerick S, Farber NB, 2009. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol. Dis. 34, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Han J, Li H, Zhang X, Liu LL, Chen F, Zeng B, 2018. Human embryonic kidney 293 cells: a vehicle for biopharmaceutical manufacturing, structural biology, and electrophysiology. Cell. Tissues Organs 205, 1–8. [DOI] [PubMed] [Google Scholar]
- Huang Z, Li G, Pei W, Sosa LA, Niu L, 2005. Enhancing protein expression in single HEK 293 cells. J. Neurosci. Methods 142, 159–166. [DOI] [PubMed] [Google Scholar]
- Jordan M, Schallhom A, Wurm FM, 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Thesleff S, 1957. A study of the desensitization produced by acetylcholine at the motor end-plate. J. Physiol. 138, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S, Nishizawa M, 1984. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol. Cell. Biol. 4, 1172–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloverpris H, Fomsgaard A, Handley A, Ackland J, Sullivan M, Goulder P, 2010. Dimethyl sulfoxide (DMSO) exposure to human peripheral blood mononuclear cells (PBMCs) abolish T cell responses only in high concentrations and following coincubation for more than two hours. J. Immunol. Methods 356, 70–78. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Ali R, 2013. Characterization of heterologously expressed transporter genes by patch- and voltage-clamp methods: application to cyclic nucleotide-dependent responses. Methods Mol. Biol. 1016, 67–93. [DOI] [PubMed] [Google Scholar]
- Li G, Oswald RE, Niu L, 2003a. Channel-opening kinetics of GluR6 kainate receptor. Biochemistry 42, 12367–12375. [DOI] [PubMed] [Google Scholar]
- Li G, Pei W, Niu L, 2003b. Channel-opening kinetics of GluR2Q(flip) AMPA receptor: a laser-pulse photolysis study. Biochemistry 42, 12358–12366. [DOI] [PubMed] [Google Scholar]
- Lin CY, Huang Z, Wen W, Wu A, Wang C, Niu L, 2015. Enhancing protein expression in HEK-293 cells by lowering culture temperature. PLoS One 10, e0123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling WL, Deng L, Lepore J, Cutler C, Cannon-Carlson S, Wang Y, Voloch M, 2003. Improvement of monoclonal antibody production in hybridoma cells by dimethyl sulfoxide. Biotechnol. Prog. 19, 158–162. [DOI] [PubMed] [Google Scholar]
- Lovelock JE, Bishop MW, 1959. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 183, 1394–1395. [DOI] [PubMed] [Google Scholar]
- Meissner P, Pick H, Kulangara A, Chatellard P, Friedrich K, Wurm FM, 2001. Transient gene expression: recombinant protein production with suspension-adapted HEK293-EBNA cells. Biotechnol. Bioeng. 75, 197–203. [DOI] [PubMed] [Google Scholar]
- Meryman HT, Williams RJ, Douglas MS, 1977. Freezing injury from “solution effects” and its prevention by natural or artificial cryoprotection. Cryobiology 14, 287–302. [DOI] [PubMed] [Google Scholar]
- Moskot M, Jakobkiewicz-Banecka J, Kloska A, Piotrowska E, Narajczyk M, Gabig-Ciminska M, 2019. The role of dimethyl sulfoxide (DMSO) in gene expression modulation and glycosaminoglycan metabolism in lysosomal storage disorders on an example of mucopolysaccharidosis. Int. J. Mol. Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Wilson I, Orton T, Pognan F, 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426. [DOI] [PubMed] [Google Scholar]
- Pei W, Huang Z, Niu L, 2007. GluR3 flip and flop: differences in channel opening kinetics. Biochemistry 46, 2027–2036. [DOI] [PubMed] [Google Scholar]
- Reeves PJ, Callewaert N, Contreras R, Khorana HG, 2002. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. U.S.A. 99, 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner AH, Bucholtz C, 1977. The in vivo effect of dimethyl sulphoxide (DMSO) on protein synthesis and the polyribosome profile in Paramecium. J. Cell. Physiol. 90, 169–177. [DOI] [PubMed] [Google Scholar]
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C, 2003. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 65, 1035–1041. [DOI] [PubMed] [Google Scholar]
- Stepanenko AA, Dmitrenko VV, 2015. HEK293 in cell biology and cancer research: phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 569, 182–190. [DOI] [PubMed] [Google Scholar]
- Sydykov B, Oldenhof H, de Oliveira Barros L, Sieme H, Wolkers WF, 2018. Membrane permeabilization of phosphatidylcholine liposomes induced by cryopreservation and vitrification solutions. Biochim. Biophys. Acta Biomembr. 1860, 467–474. [DOI] [PubMed] [Google Scholar]
- Szmant HH, 1975. Physical properties of dimethyl sulfoxide and its function in biological systems. Ann. N.Y. Acad. Sci. 243, 20–23. [DOI] [PubMed] [Google Scholar]
- Thomas P, Smart TG, 2005. HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 51, 187–200. [DOI] [PubMed] [Google Scholar]
- Tsien RY, 1998. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544. [DOI] [PubMed] [Google Scholar]
- Wang X, Hua TC, Sun DW, Liu B, Yang G, Cao Y, 2007. Cryopreservation of tissue-engineered dermal replacement in Me2SO: toxicity study and effects of concentration and cooling rates on cell viability. Cryobiology 55, 60–65. [DOI] [PubMed] [Google Scholar]
- Wen W, Lin CY, Niu L, 2017. R/G editing in GluA2Rflop modulates the functional difference between GluAl flip and flop variants in GluAl/2R heteromeric channels. Sci. Rep. 7, 13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm FM, 2004. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 22, 1393–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


