Abstract
Background:
Guidelines recommend screening for primary aldosteronism in patients diagnosed with hypertension and obstructive sleep apnea. Recent studies have shown that adherence to these recommendations is extremely low. It has been suggested that cost is a barrier to implementation. No analysis has been done to rigorously evaluate the cost-effectiveness of widespread implementation of these guidelines.
Methods:
We constructed a decision-analytic model to evaluate screening of the hypertensive obstructive sleep apnea population for primary aldosteronism as per guideline recommendations in comparison with current rates of screening. Probabilities, utility values, and costs were identified in the literature. Threshold and sensitivity analyses assessed robustness of the model. Costs were represented in 2020 US dollars and health outcomes in quality-adjusted life-years. The model assumed a societal perspective with a lifetime time horizon.
Results:
Screening per guideline recommendations had an expected cost of $47,016 and 35.27 quality-adjusted life-years. Continuing at current rates of screening had an expected cost of $48,350 and 34.86 quality-adjusted life-years. Screening was dominant, as it was both less costly and more effective. These results were robust to sensitivity analysis of disease prevalence, test sensitivity, patient age, and expected outcome of medical or surgical treatment of primary aldosteronism. The screening strategy remained cost-effective even if screening were conservatively presumed to identify only 3% of new primary aldosteronism cases.
Conclusions:
For patients with hypertension and obstructive sleep apnea, rigorous screening for primary aldosteronism is cost-saving due to cardiovascular risk averted. Cost should not be a barrier to improving primary aldosteronism screening adherence.
Background
Primary aldosteronism (PA) is a frequent etiology of secondary hypertension. Although previously thought to be a rare diagnosis with a relatively benign impact, it is now recognized to represent 5% to 10% of all hypertension cases and 12% to 22% of resistant hypertension.1-5 Further, PA bears a risk profile that is substantially higher than that of essential hypertension.2,6-8 A proportion of these patients—those with unilateral etiologies of hyperaldosteronism such as aldosteronoma or unilateral hyperplasia—may achieve cure with surgery, and those who are eligible for medical management benefit meaningfully from early targeted treatment.9
Underdiagnosis of PA is a major public health issue.10,11 As hypertension affects 32% to 46% of people in the United States, 5% to 10% of all hypertensive patients represents 10 to 16 million people in this country alone who could benefit from an accurate, timely diagnosis of PA.12 Global prevalence of hypertension is estimated at 1 billion people with proportional potential benefit.13
Delayed intervention risks avoidable target organ injury. Hyperaldosteronism provokes vascular and cardiac fibrosis, and the resulting increased complication rates are independent of blood pressure levels.14 Among blood-pressure-matched hypertensive patients, those with PA have significantly higher rates of poor cardiovascular outcomes, including coronary artery disease, stroke, atrial fibrillation, and heart failure.6,7,15 Targeted treatment with adrenalectomy or mineralocorticoid receptor antagonists has been shown to equilibrate cardiovascular complications between these groups.16,17 Younger patients appear to benefit most from treatment, supporting the concept of a direct relationship between duration of disease and morbidity.10,18
Obstructive sleep apnea (OSA) is increasingly recognized to coexist with PA, with data suggesting a causal link owing to aldosterone induced peripharyngeal fluid retention that increases airway resistance.19,20 This has led both the Endocrine Society and the American Heart Association to advocate for PA screening among hypertensive patients with an OSA diagnosis in their most recent guidelines.2,12 Adherence to these guidelines has been shown to be extremely poor. Ruhle et al reported a screening rate of only 3.0% for PA in hypertensive patients with OSA.21 Among patients with hypertension and any indication for PA screening, Sivarajah et al reported a screening rate of just 1.3%. Among those with hypertension and OSA, this figure was 1.7%.22 European rates of screening are also poor. Among 500 primary care providers in Italy and Germany, Mulatero et al documented awareness of PA screening eligibility at 8% and 13%, respectively.23 Cost is often suggested as a barrier to implementation of the guideline recommendations, and a cost-effectiveness analysis has been called for.10
We hypothesized that, given the expense of arguably avoidable downstream cardiac outcomes, screening for PA in the OSA population might be more financially sound than assumed. No previous analysis has considered this question, so we developed a decision-analytic model to evaluate screening of the hypertensive OSA population for PA.
Methods
Model structure
We developed a Markov model using TreeAge Pro (TreeAge Software, Inc, Williamstown, MA) that allowed us to compare a robust screening program adhering to Endocrine Society consensus guidelines with contemporary documented rates of screening in the hypertensive OSA population. Screening was defined as an aldosterone-renin ratio plus confirmatory testing in the form of a saline infusion test. Because not all patients actually require confirmatory testing, this overestimates the cost of the screening strategy. We chose this conservative estimate because it would add pressure to the model contrary to our hypothesis.
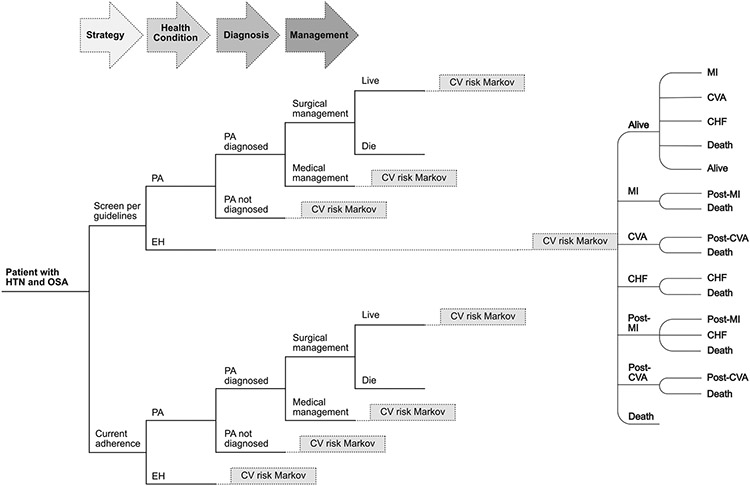
The model structure (Fig 1) was developed to reflect management of hypertension in the United States. The initial branch point is screening per Endocrine Society consensus guidelines (per guideline, PG) versus current published rates of adherence to the guidelines (current adherence, CA). Thereafter, the model unfolds to reflect likelihood of PA diagnosis, subtype classification, and results of medical and surgical management, and then flows to a cardiovascular morbidity and mortality module as developed by Smith et al.24 Transition probabilities, costs, and health state utilities are based on literature review, and the model cycles annually. The model assumes a societal perspective with a lifetime time horizon. A 3% discounting is applied to costs and QALYs as is standard practice to acknowledge that future costs as well as effects tend to be less valued than current ones. The base case is a 40-year-old adult with hypertension and OSA diagnoses. PA is diagnosed in adults 20 to 60 years old, with a mean age of diagnosis reported in the late fourth decade of life. Patients with PA typically have a history of hypertension for several years before formal diagnosis; therefore, we chose 40 years old as a reasonable estimation of the relevant at-risk population for the base case scenario.25
Fig 1.
Model schematic: Simplified model. CHF, congestive heart failure; CV, cardiovascular; CVA, cerebrovascular accident; EH, essential hypertension; HTN, hypertension; OSA, obstructive sleep apnea; PA, primary aldosteronism; MI, myocardial infarction.
Important assumptions underlie this model. First, all patients are surgical candidates, and, second, all those who are diagnosed with surgically treatable disease undergo surgery. Third, all patients identified as having non–surgically treatable disease receive medical treatment. Finally, we assume constant annual risk of progression to cardiovascular outcomes, though it is likely that these risks actually increase over time given the progressive deleterious effects of hypertension and aldosterone exposure. We did not find data in the literature to characterize this increase accurately. Therefore, we are probably overestimating life expectancy in both arms of the model. Arguably in this case, we could have used a non-lifetime time horizon to ameliorate the effects of this assumption, but given the long-term and significant mortality effects of essential hypertension and PA, we decided that it was more accurate to use the lifetime time horizon and acknowledge this tension.
This project was exempted from institutional review board approval because it involved no human subjects nor protected health information.
Transition probabilities
Best estimates for transition probabilities were derived from the literature (Table I). Cardiovascular risk transition probabilities represented Framingham Study data used in previously published cost-effectiveness work.24 Mortality and complication rates, stratified by age, pertaining to laparoscopic adrenalectomy were sourced from previously published cost-effectiveness studies and were originally derived from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample.33
Table I.
Model inputs including base case values and ranges used in sensitivity analysis
| Transition probabilities | Base | Range | Source |
|---|---|---|---|
| Probability PA diagnosis without screening | 0.03 | 0.5–1.5x base | 21 |
| Sensitivity of ARR test | 0.8 | 0.50–1 | 26 |
| Specificity of SIT | 0.75 | 0.75–1 | 27 |
| Probability of surgical management | 0.43 | 0.33–0.60 | 27 |
| Probability of PA in OSA population | 0.215 | 0.089–0.34 | 20,28 |
| Probability of CHF in EH | 0.0009 | 0.5–1.5x base | 6 |
| Probability of CHF in PA | 0.003 | 0.5–1.5x base | 6 |
| Probability CVA in EH | 0.0041 | 0.5–1.5x base | 6 |
| Probability of CVA in PA | 0.0109 | 0.5–1.5x base | 6 |
| Probability MI in EH | 0.0033 | 0.5–1.5x base | 6 |
| Probability of MI in PA | 0.0061 | 0.5–1.5x base | 6 |
| Transition CHF to death (annual) | 0.43 | 0.344–0.516 | 24,29 |
| Transition CVA to death (annual) | 0.069 | 0.055–0.083 | 24,30 |
| Transition MI to death | 0.071328306 | 0.057–0.086 | 24,31 |
| Transition post-CVA to death (annual) | 0.236 | 0.189–0.283 | 24,30 |
| Transition post-MI to CHF (annual) | 0.021759765 | 0.017–0.026 | 24,31 |
| Transition post-MI to death (annual) | 0.028583536 | 0.023–0.034 | 24,31 |
| Hazard ratio for surgical treatment of PA | 1.25 | 1.0–3.0 | 7,18,32 |
| Hazard ratio for medical treatment of PA | 1.91 | 1.0–3.0 | 9 |
| Complication risk, laparoscopic adrenalectomy | 0.8–1.2x base | 33 | |
| Age ≤40 | 0.062 | ||
| Age 41–60 | 0.079 | ||
| Age 61–70 | 0.085 | ||
| Age >70 | 0.219 | ||
| Mortality risk, laparoscopic adrenalectomy | 0.8–1.2x base | 33 | |
| Age ≤40 | 0.003 | ||
| Age 41–60 | 0.001 | ||
| Age 61–70 | 0.005 | ||
| Age >70 | 0.029 | ||
| Costs (in 2020 USD) | Base | Range | Source |
| Cost associated with death (1 ×) | 16,579 | 13,264–16,579 | 24,34 |
| Annual cost of medical HTN treatment | 317 | 0.5–1.5x base | 35 |
| Cost of yearly office visit | 46 | 0.5–1.5x base | 36 |
| Cost of CVA, initial year | 36,665 | 29,332–43,997 | 24,37 |
| Cost of MI, initial year | 70,414 | 56,330–84,497 | 24,34 |
| Annual cost CHF management | 15,033 | 12,026–18,039 | 24,38 |
| Annual cost post-CVA | 35,929 | 28,744–43,115 | 24,39 |
| Annual cost post-MI | 4,532 | 3,626–5,439 | 24,40 |
| Cost of screening for PA (ARR + SIT) | 188 | 0.5–1.5x base | 36 |
| Cost of CT scan | 283 | 0.5–1.5x base | 36 |
| Cost of AVS | 2,963 | 0.5–1.5x base | 27,28 |
| Cost of laparoscopic adrenalectomy, with complication | 0.8–1.2x base | 33 | |
| Age ≤40 | 16,935 | ||
| Age 41–60 | 19,609 | ||
| Age 61–70 | 17,896 | ||
| Age >70 | 20,448 | ||
| Cost of laparoscopic adrenalectomy, uncomplicated | 0.8–1.2x base | 33 | |
| Age ≤40 | 10,280 | ||
| Age 41–60 | 11,404 | ||
| Age 61–70 | 11,513 | ||
| Age >70 | 12,457 | ||
| Health Utilities (in QALYs) | Base | Range | Source |
| Utility of 1 year of healthy life | 1 | n/a | |
| Annual utility CHF | 0.71 | 0.568–0.852 | 24,41 |
| Utility of CVA, initial year | 0.64 | 0.512–0.768 | 24,42 |
| Utility of MI, initial year | 0.7 | 0.56–0.84 | 24,43 |
| Post-CVA annual utility | 0.66 | 0.528–0.792 | 24,42 |
| Post-MI annual utility | 0.88 | 0.704–0.95 | 24,44 |
| Utility of death | 0 | n/a |
All costs are in 2020 United States dollars. All health utilities are represented in quality-adjusted life-years (QALYs).
ARR, aldosterone-renin ratio; AVS, adrenal venous sampling; CHF, congestive heart failure; CVA, cerebrovascular accident; EH, essential hypertension; HTN, hypertension; MI, myocardial infarction; OSA, obstructive sleep apnea; PA, primary aldosteronism; QALY, quality-adjusted life-year; SIT, saline infusion test; USD, US dollars.
Overall, PA confers a higher risk of cardiovascular events than essential hypertension regardless of treatment. In this model, cardiovascular risks associated with surgically treated and medically treated PA are represented as hazard ratios multiplied by the risks associated with essential hypertension. A published hazard ratio was available for medically treated PA in the base case (1.91), but due to a lack of clear consensus in the literature and variation in success related to treatment regimens (eg, Hundemer et al reported that PA patients treated with mineralocorticoid antagonists had a cardiovascular risk profile that was 3 times that of essential hypertension patients if the plasma renin activity remained suppressed but became equivalent to essential hypertension patients when the plasma renin activity was no longer suppressed), a wide interval was used in sensitivity analysis.6,9 Surgically treatable disease is in some cases curative, but published rates of success vary widely (27%–94%) and depend on the definitions of cure used (complete clinical success, complete biochemical success, normotensive status with or without medications).7,18,32 Delay to diagnosis, among other factors, may impact the efficacy of treatment and may be an underlying source of this variability. Again, we selected a representative base case and used a wide interval in sensitivity analysis to determine how more extreme estimates would impact model predictions.
Published estimates of the prevalence of PA in OSA patients vary substantially. In a study of 325 consecutive newly diagnosed patients with hypertension, Di Murro et al reported an OSA diagnosis in 53 patients, and a concurrent PA diagnosis in 18 patients, for a prevalence of 34%. In contrast, Buffalo et al reported an 8.9% prevalence of PA among 203 hypertensive patients with OSA. Both studies suffer from small sample size. We chose to take a simple mean of these prevalence estimates as our base case and used the values reported in these studies for the range of our sensitivity analysis.
Costs
Cost inputs, including screening, testing, and age-stratified costs related to adrenalectomy, were identified from the literature and from the United States Medicare reimbursement schedule.26,36 All costs were adjusted to 2020 United States dollars using the Consumer Price Index for urban consumers.
Health state utilities
Effectiveness was represented in quality-adjusted life-years (QALYs), a standard measure used in health economic studies. QALYs are equivalent to the product of a health utility value pertaining to a health state and the length of time spent in that state. By convention, 1 year of fully healthy life is equivalent to 1 QALY, and death is equivalent to 0 QALYs. Health state utilities were derived from the literature.
Analysis
Base case and sensitivity analyses were performed. As is standard practice for cost-effectiveness analysis, our aim was to compare strategies and determine whether either was dominant (less costly and more effective) or, if not, to characterize the tradeoff of cost for effectiveness by identifying an incremental cost-effectiveness ratio (ICER). Univariate or first-order sensitivity analyses—in which a variable is altered independently along its range while all others are held at their base case estimates—were conducted on all input variables, and those with notable effect on the model were further interrogated with threshold analysis. Ranges for sensitivity analysis were identified in the literature. When ranges were unavailable in the literature, a range of 0.5 to 1.5 times the base case estimate was used. Monte Carlo probabilistic sensitivity analysis, in which multiple variables are varied along their ranges simultaneously, was performed to examine parameter uncertainty. Willingness-to-pay, the societally determined value at which an intervention is deemed cost-effective, was set at $150,000 per QALY in accordance with guidance for healthcare cost-effectiveness in the United States published by the American College of Cardiology and the American Heart Association.45
Results
Base case
In the base case, PG was the dominant (less costly and more effective) strategy, with a cost of $47,016 and utility of 35.27 QALYs. CA cost $48,350 and produced a utility of 34.86 QALYs (Table II). Therefore, a robust screening program for identifying PA in hypertensive patients with OSA was the optimal strategy.
Table II.
Base case outcomes
| Strategy | Cost (2020 USD) |
Incremental cost (2020 USD) |
Effectiveness (QALYs) |
Incremental effectiveness (QALYs) |
Incremental cost-effectiveness ($/QALY) |
|---|---|---|---|---|---|
| Per Guideline (PG) | 47,016 | n/a | 35.27 | 0.41 | 0 |
| Current Adherence (CA) | 48,350 | 1,334 | 34.86 | n/a | Dominated |
The Per Guideline strategy was dominant—more effective and less costly—in the base case.
QALY, quality-adjusted life-year; USD, US dollars.
Sensitivity analysis
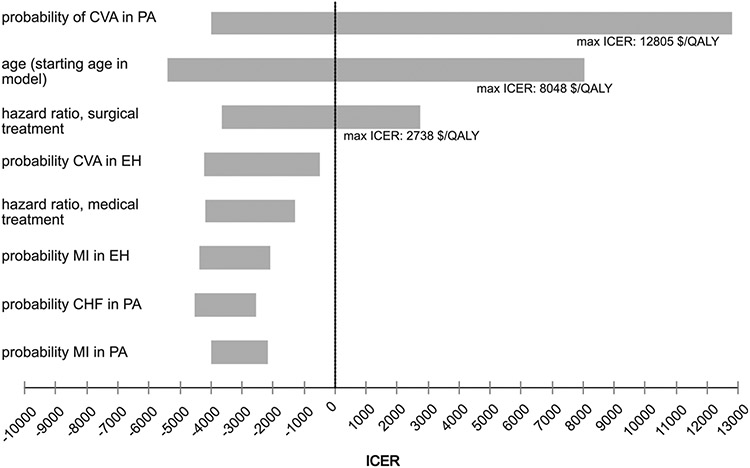
In univariate (first-order) sensitivity analysis, in which the model is run for each individual variable along the entirety of its anticipated range, 3 variables produced a positive ICER at some point along their ranges: the probability of stroke in patients with PA, patient age (starting age for the model), and the hazard ratio related to surgical treatment. ICERs were no higher than $13,000/QALY for any of these variables. These results are presented in a tornado diagram, which is a pictorial representation of individual univariate analyses presented together so that their impact on the model can be compared (Fig 2). Specifically, as the risk of stroke in patients with PA decreases, PG ceases to be dominant but remains cost-effective at a point estimate of 0.007. The highest ICER produced by the variable’s expected value range is $12,805/QALY. Patient age refers to the starting age as patients enter the model. As this age increases, PG ceases to be dominant and becomes cost-effective at a point estimate of 76.7 years. The highest ICER produced along this variable’s range is $8,048/QALY. As surgical treatment becomes less effective at ameliorating the effects of PA, screening ceases to be dominant at a point estimate of 2.59 and is instead cost-effective with a maximum ICER of $2,738/QALY. No thresholds were identified beyond which the CA strategy was optimal.
Fig 2.
Tornado diagram.The tornado diagram is a composite representation of multiple univariate sensitivity analyses. Here the 8 inputs with the greatest effect are shown. Only 3 variables produced an ICER in univariate analysis. These are labeled with the maximum ICER produced along each variable’s range. CHF, congestive heart failure; CVA, cerebrovascular accident; EH, essential hypertension; ICER, incremental cost-effectiveness ratio; MI, myocardial infarction; PA, primary aldosteronism.
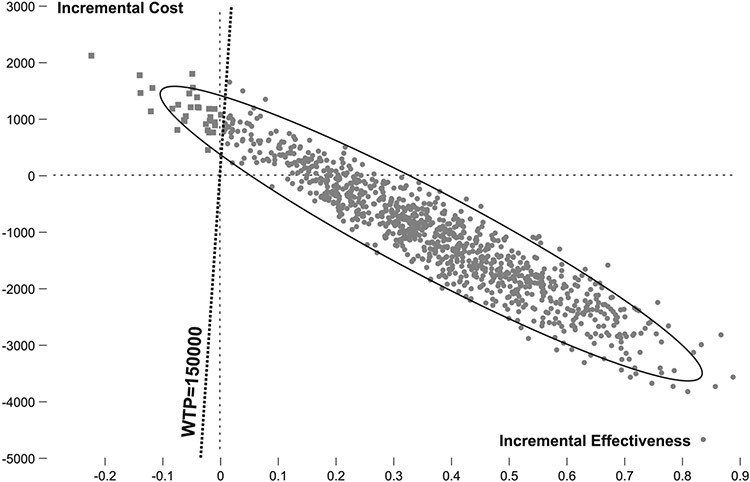
Monte Carlo probabilistic sensitivity analysis (second-order sensitivity analysis) was performed to assess parameter uncertainty in the model (Fig 3). In this technique, multiple inputs are varied along their anticipated ranges simultaneously. Project Evaluation and Review Technique distributions were used. In 84% of 5,000 iterations, PG was found to be dominant, and in an additional 13%, PG was cost-effective at a willingness-to-pay threshold of $150,000. Therefore, in 97% of 5,000 iterations, PG was the optimal strategy.
Fig 3.
Probabilistic sensitivity analysis. Results of the PSA are shown on an incremental cost-effectiveness plane. In 97% of 1,000 iterations, the Per Guideline (PG) strategy was either dominant (represented in the right lower quadrant) or cost-effective (represented in the right upper quadrant, to the right of the willingness-to-pay line). Circles represent iterations in which PG is superior, and squares represent iterations in which the Current Adherence strategy is superior.
An extended threshold analysis was conducted to determine how effective PG would have to be in the base case to remain cost-effective. This was done by exploring the behavior of a variable that represented the probability that PA would be diagnosed with the proposed screening regimen, which was calculated from the sensitivity of the aldosterone-renin ratio and specificity of the saline infusion test. Using a range of 0 to 100% revealed a threshold of 3%. If PG screening identified only 3% of new cases of PA in the OSA population, it would remain cost-effective. Given that the low end of the expected range for this variable is calculated to be 38%, this should be highly feasible.
Discussion
PA is a major public health issue with available effective intervention options that may reduce morbidity and mortality. Increased cardiac risk associated with untreated PA is well--established,3,6,9 and surgically correctable PA is a curable disease.32 Enriched prevalence of PA in the OSA population makes this group a potential target for focused surveillance and intervention.2,20
Adherence to guidelines that encourage increased screening for PA in the OSA population is poor, and cost has been suggested as a barrier.21,22 Here we demonstrate that a robust screening program for PA in hypertensive patients with OSA is cost-saving, and even at the outer reaches of sensitivity analysis, it remains definitively cost-effective.
The base case of our model suggests that a robust screening program for PA in the OSA population is more effective and less costly than current practice, owing to the substantial monetary and health utility costs of resulting cardiac morbidity and mortality. In first-order sensitivity analysis, there are some scenarios in which PG is not dominant but remains cost-effective with ICERs that are comfortably within generally accepted standards of cost-effectiveness. Second-order sensitivity analysis, accounting for parameter uncertainty, still identifies robust screening as the optimal pathway.
These results are consistent with existing literature describing cost-effectiveness with regard to PA. Lubitz et al conducted a cost-effectiveness analysis examining several strategies for screening for PA and subtype diagnosis in the resistant hypertensive population in the United States. This group reported that screening with an aldosterone to renin ratio and CT scan followed by AVS was cost-effective in this population at $82,000/QALY.27 Sato et al performed a cost-effectiveness analysis evaluating screening for PA in Japan in patients with stage I–III hypertension, reporting a cost of 4,923,385 JPY/life-year (approximately $45,000/life-year), which was cost-effective.46 Velasco et al showed a cost-saving benefit of screening patients with resistant hypertension, excluding those patients whose diagnosis of resistant hypertension may have been in error due to medication nonadherence.47 Studies have shown that for those forms of PA that are surgically correctable, surgery delivers lifetime costs that are lower than medical treatment.48,49
Our analysis is limited by the inherent nature of any modeling study—certain assumptions must be made that may oversimplify complex clinical scenarios and decision-making processes. Furthermore, conclusions drawn from the model are only as valuable as its inputs are accurate. Specifically, there were several variables for which clear, discrete values were unavailable. This was due to differences in practice with regard to clinical evaluation and medical treatment of PA, variable definitions of successful medical and surgical treatment, and variably reported rates of successful treatment with surgical and medical therapy.10,50 This required us to choose best estimates for the base case and to rely on very wide confidence intervals in sensitivity analysis to compensate for uncertainty. The consequences of delayed diagnosis are also not represented in this model but are clearly important clinically. Duration of hypertension has been associated with less treatable disease, arguing once again in favor of the PG strategy.2
It is unclear why more screening in the hypertensive OSA population is not taking place, as indicated by recent studies.21,22 The cost-prohibitive nature of widespread screening has been suggested as one reason. Our study investigated this question and demonstrated that cost should not be a factor. Indeed, screening may be cost-saving over a long-time horizon. Current guidelines are sound, reasonable, and have the potential to save lives. If cost is not the issue, then one can proceed to identifying and addressing other barriers with thoughtful interventions. Awareness among referring providers may be another factor that deserves investigation and action.
For patients with hypertension and OSA, screening for PA is cost-saving due to cardiovascular risk averted. In efforts to address this major public health issue, cost should not be a barrier to improving PA screening adherence in the OSA population.
Biographies
Dr. Michael Yeh (Los Angeles): I recently learned about this association between hyperaldosteronism and obstructive sleep apnea. What is the direction of causality, and what is the biological mechanism?
Dr. Kathryn Chomsky-Higgins Menut: There's evidence that it's bi-directional, but this idea of aldosterone causing accumulation of pharyngeal edema as a causative element towards the development of obstructive sleep apnea, that is the best description that we have found and is the leading theory at this point.
Dr. Michael Yeh (Los Angeles): I know that the more we screen for primary aldosteronism, the more we find, and yet the fraction of surgically remediable cases goes down the more we screen, because we find more hyperplasia. How did that figure into your cost analysis?
Dr. Kathryn Chomsky-Higgins Menut: I think the really important point is that even by finding more hyperplasia, that is also treatable with targeted treatments. If we identify primary aldosteronism that is only treatable medically, where there is not a unilateral cause, then we can treat that with mineralocorticoid receptor antagonists. There are data from matched cohorts of people with essential hypertension and people with primary aldosteronism. For blood pressure matched cohorts, the people with primary aldosteronism have worse outcomes. But, importantly, with targeted treatment, particularly in younger patients, both with surgery and with medical treatment, they can be brought back down to the risk level of essential hypertension. I think regardless of whether we can treat them surgically, it's just as important to identify patients who can be treated medically.
Dr. Amanda Laird (New Brunswick): Is there an age at which screening would no longer be cost-effective? Or is cost-effectiveness more related to comorbid conditions?
Dr. Kathryn Chomsky-Higgins Menut: Well, in our study, we used the base case age of 40 and also did a sensitivity analysis that found that at about age 76, doing a comprehensive screening program is not dominant but is still cost-effective. Now, that's assuming a patient who is a surgical candidate. In this particular analysis, we did not account for other comorbid conditions.
Dr. Michael Yeh (Los Angeles): Because yours is the first of several cost-effectiveness papers, can you articulate for the audience the difference between dominant and cost-effective?
Dr. Kathryn Chomsky-Higgins Menut: Dominant is less costly and more effective. In these analyses, you might be expecting that one treatment is going to cost a little bit more, but you're going to get enough effectiveness to make it worthwhile, essentially. But even better is if a treatment is both more effective and it costs less. That's what we want in the base case.
Dr. Mahes Sivarajah (New York): You’ve shown that cost isn't the issue. What do you think are the barriers? What would you propose to improve our screening rates?
Dr. Kathryn Chomsky-Higgins Menut: First of all, thank you for your paper that I read in making my analysis. Second, I think this is ripe for a quality improvement initiative.
There are no excuses. We have identified that for screening, you want something easy to do that's relatively low cost and low risk for which you have an actual targeted treatment. That is true here in primary aldosteronism. That is the reason why it's in these guidelines.
Clearly, we need more outreach to the primary care community, and I think this is a real opportunity to build a program to do that, perhaps with AAES. So awareness is the primary need. I hope that with this cost-effectiveness analysis we have shown that cost is not an excuse to avoid more screening.
Footnotes
Submitted as a Societal Paper associated with the American Association of Endocrine Surgeons (AAES) meeting to be held April 25–27, 2021, virtual.
Conflict of interest/Disclosure
Dr Menut disclosed that she previously received consulting fees from Prescient Surgical. Dr Pearlstein reported no financial interests nor potential conflicts of interest. Dr Conroy reported no financial interests nor potential conflicts of interest. Dr Roman reported no financial interests nor potential conflicts of interest. Dr Shen reported no financial interests nor potential conflicts of interest. Dr Gosnell reported no financial interests nor potential conflicts of interest. Dr Sosa is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by GlaxoSmithKline, Novo Nordisk, Astra Zeneca, and Eli Lilly. Institutional research funding is received from Exelixis and Eli Lilly. Dr Duh reported no financial interests nor potential conflicts of interest. Dr Suh is a consultant for Medtronic and Prescient Surgical.
References
- 1.Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 3.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. [DOI] [PubMed] [Google Scholar]
- 4.Mulatero P, Stowasser M, Loh K-C, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. [DOI] [PubMed] [Google Scholar]
- 5.Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies: a review of the current literature. Horm Metab Res. 2012;44:157–162. [DOI] [PubMed] [Google Scholar]
- 6.Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. [DOI] [PubMed] [Google Scholar]
- 7.Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad J-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. [DOI] [PubMed] [Google Scholar]
- 8.Stowasser M, Sharman J, Leano R, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab. 2005;90:5070–5076. [DOI] [PubMed] [Google Scholar]
- 9.Hundemer GL. Primary aldosteronism: cardiovascular outcomes pre- and post-treatment. Curr Cardiol Rep. 2019;21:93. [DOI] [PubMed] [Google Scholar]
- 10.Libianto R, Fuller PJ, Young MJ, Yang J. Primary aldosteronism is a public health issue: challenges and opportunities. J Hum Hypertens. 2020;34:478–486. [DOI] [PubMed] [Google Scholar]
- 11.Funder JW. Primary aldosteronism as a public health issue. Lancet Diabetes Endocrinol. 2016;4:972–973. [DOI] [PubMed] [Google Scholar]
- 12.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–115. [DOI] [PubMed] [Google Scholar]
- 13.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zennaro M-C, Boulkroun S, Fernandes-Rosa FL. Pathogenesis and treatment of primary aldosteronism. Nat Rev Endocrinol. 2020;16:578–589. [DOI] [PubMed] [Google Scholar]
- 15.Mulatero P, Monticone S, Bertello C, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833. [DOI] [PubMed] [Google Scholar]
- 16.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. [DOI] [PubMed] [Google Scholar]
- 17.Rossi GP, Maiolino G, Flego A, et al. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71:585–591. [DOI] [PubMed] [Google Scholar]
- 18.Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pimenta E, Calhoun DA, Oparil S. Sleep apnea, aldosterone, and resistant hypertension. Prog Cardiovasc Dis. 2009;51:371–380. [DOI] [PubMed] [Google Scholar]
- 20.Di Murro A, Petramala L, Cotesta D, et al. Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2010;11:165–172. [DOI] [PubMed] [Google Scholar]
- 21.Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery. 2019;165:221–227. [DOI] [PubMed] [Google Scholar]
- 22.Sivarajah M, Beninato T, Fahey TJ. Adherence to consensus guidelines for screening of primary aldosteronism in an urban healthcare system. Surgery. 2020;167:211–215. [DOI] [PubMed] [Google Scholar]
- 23.Mulatero P, Monticone S, Burrello J, Veglio F, Williams TA, Funder J. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34:2253–2257. [DOI] [PubMed] [Google Scholar]
- 24.Smith L, Atherly A, Campbell J, Flattery N, Coronel S, Krantz M. Cost-effectiveness of a statewide public health intervention to reduce cardiovascular disease risk. BMC Public Health. 2019;19:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young WF. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126–148. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51:386–394. [DOI] [PubMed] [Google Scholar]
- 27.Lubitz CC, Economopoulos KP, Sy S, et al. Cost-effectiveness of screening for primary aldosteronism and subtype diagnosis in the resistant hypertensive patients. Circulation. 2015;8:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buffolo F, Monticone S, Tetti M, Mulatero P. Primary aldosteronism in the primary care setting. Curr Opin Endocrinol Diabetes Obes. 2018;25:155–159. [DOI] [PubMed] [Google Scholar]
- 29.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. [DOI] [PubMed] [Google Scholar]
- 30.Saposnik G, Hill MD, O’Donnell M, et al. Variables associated with 7-day, 30-day, and 1-year fatality after ischemic stroke. Stroke. 2008;39:2318–2324. [DOI] [PubMed] [Google Scholar]
- 31.Velagaleti RS, Pencina MJ, Murabito JM, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorselaars WMCM, Nell S, Postma EL, et al. Clinical outcomes after unilateral adrenalectomy for primary aldosteronism. JAMA Surg. 2019;154, e185842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TS, Cheung K, Roman SA, Sosa JA. A cost-effectiveness analysis of adrenalectomy for nonfunctional adrenal incidentalomas: is there a size threshold for resection? Surgery. 2012;152:1125–1132. [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29:693–704. [DOI] [PubMed] [Google Scholar]
- 35.Dehmer SP, Maciosek MV, LaFrance AB, Flottemesch TJ. Health benefits and cost-effectiveness of asymptomatic screening for hypertension and high cholesterol and aspirin counseling for primary prevention. Ann Fam Med. 2017;15:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services. Medicare physician fee schedule. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed January 24, 2021. [Google Scholar]
- 37.Luengo-Fernandez R, Gray AM, Rothwell PM. Costs of stroke using patient-level data: a critical review of the literature. Stroke. 2009;40:e18–23. [DOI] [PubMed] [Google Scholar]
- 38.Greiner MA, Hammill BG, Fonarow GC, et al. Predicting costs among Medicare beneficiaries with heart failure. Am J Cardiol. 2012;109:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engel-Nitz NM, Sander SD, Harley C, Rey GG, Shah H. Costs and outcomes of noncardioembolic ischemic stroke in a managed care population. Vasc Health Risk Manag. 2010;6:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsey SD, Clarke LD, Roberts CS, Sullivan SD, Johnson SJ, Liu LZ. An economic evaluation of atorvastatin for primary prevention of cardiovascular events in type 2 diabetes. Pharmacoeconomics. 2008;26:329–339. [DOI] [PubMed] [Google Scholar]
- 41.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. [DOI] [PubMed] [Google Scholar]
- 42.Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81:1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsevat J, Goldman L, Soukup JR, et al. Stability of time-tradeoff utilities in survivors of myocardial infarction. Med Decis Making. 1993;13:161–165. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–2322. [DOI] [PubMed] [Google Scholar]
- 46.Sato M, Morimoto R, Seiji K, et al. Cost-effectiveness analysis of the diagnosis and treatment of primary aldosteronism in Japan. Horm Metab Res. 2015;47:826–832. [DOI] [PubMed] [Google Scholar]
- 47.Velasco A, Chung O, Raza F, et al. Cost-effectiveness of therapeutic drug monitoring in diagnosing primary aldosteronism in patients with resistant hypertension. J Clin Hypertens (Greenwich). 2015;17:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sywak M, Pasieka JL. Long-term follow-up and cost benefit of adrenalectomy in patients with primary hyperaldosteronism. Br J Surg. 2002;89:1587–1593. [DOI] [PubMed] [Google Scholar]
- 49.Reimel B, Zanocco K, Russo MJ, et al. The management of aldosterone-producing adrenal adenomas: does adrenalectomy increase costs? Surgery. 2010;148:1178–1185:discussion 1185. [DOI] [PubMed] [Google Scholar]
- 50.Wachtel H, Kelz RR. Association of outcome definitions with success following adrenalectomy for primary aldosteronism. JAMA Surg. 2019;154:e185843. [DOI] [PubMed] [Google Scholar]