Abstract
Streptococcus iniae was recovered from diseased rainbow trout (Oncorhynchus mykiss, Walbaum) previously vaccinated against streptococcosis. PCR and serological methods indicate the presence of a new serotype in the diseased fish.
The fish pathogen Streptococcus iniae (11) is endemic in various parts of the world, including Israel (6) and North America (10). S. iniae infection of rainbow trout (Oncorhynchus mykiss) produces a disease which substantially affects the brain, with only minor pathological changes in other organs (4). Recently, S. iniae has been isolated from diseased humans suffering from cellulitis, meningitis, and bacteremias, indicating a threat to public health (16).
A specific S. iniae vaccine became available in 1995. From 1995 to 1997, all Israeli trout farms in the Upper Galilee, (which share water reservoirs) routinely vaccinated their entire stocks (roughly 3 million fish/year), reducing S. iniae-related mortalities from 50% annually to less than 5% (5). However, massive new outbreaks of the disease were recorded in 1997. Unlike the previous pathological manifestations, diseased fish exhibited multisystem organ involvement and diffuse internal hemorrhages. Brains samples were collected from diseased fish and streaked on Columbia agar base (Difco) supplemented with 5% (vol/vol) defibrinated sheep blood. Beta-hemolytic gram-positive cocci were detected following an incubation of 24 to 48 h at 24°C. Conventional identification schemes (API 20 STREP; BioMerieux SA, Marcy l'Etoie, France) suggested that all isolates (of 100 collected over 24 months) were S. iniae. The new isolates, unlike the previous isolates, were shown to be arginine dehydrolase (ADH) negative. Definitive identification was accomplished by PCR, using S. iniae 16S rDNA-specific primers (Zlotkin et al. [17]), which revealed the 300-bp S. iniae-specific PCR product in all isolates.
Six early ADH-positive isolates collected in 1989 (Dan 1), 1991 (Dan 3 and Dan 4), 1992 (Dan 15 and Dan 16), and 1995 (Dan 35) [collected prior to the vaccination program] and seven recent ADH-negative-isolates obtained from vaccinated fish in 1997 (KFP 173), 1998 (KFP 186), 1999 (KFP 198, KFP 199, and KFP 206), and 2000 (KFP 400 and KFP 404) were randomly taken for further characterization.
Primers U-1 and U-1500 (Escherichia coli 16S rDNA sequence [GenBank accession no. J01859], positions 7 to 27 and 1516 to 1540, respectively) were used for 16S rDNA amplification and sequencing of isolates Dan 1, Dan 4, Dan 35, KFP 173, KFP 186, and KFP 198 and S. iniae ATCC 29178 (isolated in 1976 from a diseased Amazonian dolphin (11)). The rDNA sequence analyses confirmed that the six isolates were S. iniae. All had an identical sequence (GenBank accession no. AF335573) and differed from that of S. iniae ATCC 29178 (GenBank accession no. AF335572) in six bases (99.6% homology). EcoRI and HindIII digests of DNAs extracted from early and recent isolates resulted in identical restriction fragment length polymorphism ribotype patterns (data not shown), indicating that all isolates cluster in the Israeli S. iniae rank (6). The use of additional endonucleases (PvuII or KpnI) did not provide strain-to-strain differentiation (data not shown).
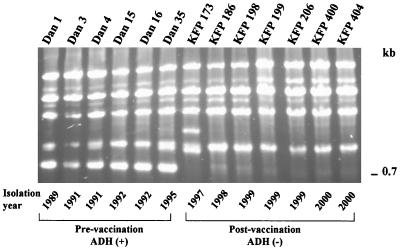
The random amplified polymorphic DNA (RAPD) technique (Fig. 1) was used to distinguish early from recent isolates. Primer p14 (5′GATCAAGTCC), previously proven useful for discrimination among group A streptococcol strains, was used (Neeman et al. [8]). All early (1991 to 1995) isolates produced a band with an estimated length of 750 bp. No such band was found in the PCR product of the recent isolates (Fig. 1).
FIG. 1.
RAPD patterns of representative isolates collected between 1991 and 1999.
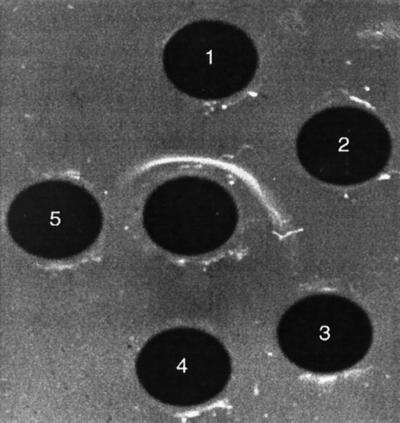
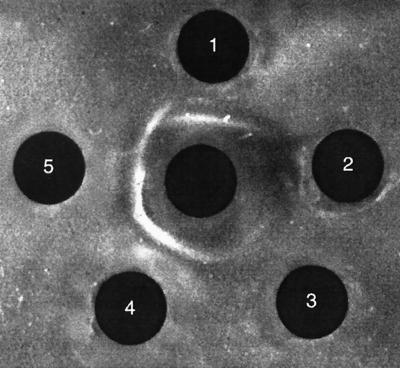
Hyperimmune sera for serological differentiation were obtained by three monthly immunizations of rainbow trout with formalin-fixed S. iniae Dan 1 or S. iniae KFP 173 bacterin (5). Specific group antigens were extracted by exposure of the bacteria to 0.2 N HCl for 10 min at 100°C (7), and immunodiffusion tests were performed by the agar-gel method of Ouchterlony (9). As shown in Fig. 2 and 3, sera against S. iniae Dan 1 reacted with all early isolates; sera against S. iniae KFP 173 reacted with all recent isolates. While sera against Dan 1 failed to recognize recent strains, sera against KFP 173 recognized early strains in a manner that indicates partial identity (Fig. 3).
FIG. 2.
Ouchterlony agar-gel immunodiffusion. Carbohydrate antigen extracts of Dan 1 and Dan 4 early isolates (wells 1 and 2, respectively), and KFP 173 and KFP 186 recent isolates (wells 3 and 4, respectively), and the fish pathogen Lactococcus garvieae ATCC 43921T (well 5, negative control) were reacted with anti-Dan 1 serum (central well).
FIG. 3.
Ouchterlony agar-gel immunodiffusion. Carbohydrate antigen extracts of KFP 173, KFP 186, and KFP 196 recent isolates (wells 5, 4, and 3, respectively); Dan 1 early isolate (well 1); and the fish pathogen Lactococcus garvieae ATCC 43921T (well 2, negative control) were reacted against anti-KFP 173 serum (central well).
Partial immunological cross-recognition between early and recent isolates was also demonstrated in agglutination tests, in which 50-μl samples of formalin-killed S. iniae Dan 1 and KFP 173, diluted to an optical density of 0.8 at 540 nm, were reacted with serial dilutions of specific sera. Sera raised against S. iniae Dan 1 precipitated the early isolates at dilutions of up to 1:2,560 but precipitated none of the recent ones. However, sera raised against S. iniae KFP 173 recognized all of the recent strains (titers of 1:2,560), as well as all of the early strains, which were precipitated at low serum dilutions (1:20). Recent S. iniae isolates were therefore designated serotype II strains, while early S. iniae strains were designated serotype I strains.
Contrary to eradication schemes, vaccination of large stocks cannot eliminate the pathogen. We have previously shown that, while vaccination drastically reduced mortality, the pathogen still remained in some of the fish or in the environment (5). As a consequence of the selective pressure induced by the vaccination, a second serotype appears to have taken over. The RAPD analysis (Fig. 1) supports this assumption, demonstrating that a shift from a stable environment containing S. iniae serotype I isolates to an environment containing serotype II isolates has occurred.
Serological tests indicating a shift in the capsular composition provide further support to the assumption that selection favoring the emergence of a vaccine-resistant serotype has occurred (Fig. 2 and 3). Capsular polysaccharides have long been recognized as a major virulence factor of many streptococci. The onset of disease caused by group B streptococci is strictly dependent on the capsular type. The vast majority of neonatal group B streptococcal meningitis cases and the majority of cases of late-onset disease are related to type III strains (3, 14). But the classic example is that of Streptococcus pneumoniae, in which differences between serotypes also result in adverse terms of activation of the alternative pathway of complement (12), deposition and degradation of complement components on the capsule (2), resistance to phagocytosis, and ability to induce antibodies (15). In the disease caused by S. pneumoniae, it has been shown that protection is related to anticapsular antibodies present in serum (1, 13).
To summarize, the long-term massive vaccination of rainbow trout against serotype I resulted in an outbreak of serotype II, which is able to evade the protective response elicited by vaccination with type I strains.
Acknowledgments
We thank Shira Yaron for her assistance.
This work was supported by a joint American-Israeli grant (BARD IS-2727-96R).
REFERENCES.
- 1.Alonso De Velasco E, Dekker B A, Verheul A F, Feldman R G, Verhoef J, Snippe H. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J Infect Dis. 1995;172:562–565. doi: 10.1093/infdis/172.2.562. [DOI] [PubMed] [Google Scholar]
- 2.Angel C S, Ruzek M, Hostetter M K. Degradation of C3 by Streptococcus pneumoniae. J Infect Dis. 1994;170:600–608. doi: 10.1093/infdis/170.3.600. [DOI] [PubMed] [Google Scholar]
- 3.Baker C J, Edwards M S. Group B streptococci infections. In: Remington J S, Klein J O, editors. Infectious disease of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 980–1054. [Google Scholar]
- 4.Eldar A, Ghittino C. Lactococcus garvieae and Streptococcus iniae infections in rainbow trout Oncorhynchus mykiss: similar, but different diseases. Dis Aquat Org. 1999;36:227–231. doi: 10.3354/dao036227. [DOI] [PubMed] [Google Scholar]
- 5.Eldar A, Horovitcz A, Bercovier H. Development and efficacy of a vaccine against Streptococcus iniae infection in farmed rainbow trout. Vet Immunol Immunopathol. 1997;56:175–183. doi: 10.1016/s0165-2427(96)05738-8. [DOI] [PubMed] [Google Scholar]
- 6.Eldar A, Lawhon S, Frelier P F, Assenta L, Simpson B R, Varner P W, Bercovier H. Restriction fragment length polymorphisms of 16S rDNA and of whole rRNA genes (ribotyping) of Streptococcus iniae strains from the United States and Israel. FEMS Microbiol Lett. 1997;151:155–162. doi: 10.1111/j.1574-6968.1997.tb12564.x. [DOI] [PubMed] [Google Scholar]
- 7.Lancefield R C. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med. 1933;57:406–409. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neeman R, Keller N, Barzilai A, Korenman Z, Sela S. Prevalence of internalisation-associated gene, prtF1, among persisting group-A streptococcus strains isolated from asymptomatic carriers. Lancet. 1998;352:1974–1977. doi: 10.1016/S0140-6736(97)12452-7. [DOI] [PubMed] [Google Scholar]
- 9.Ouchterlony O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–9. [PubMed] [Google Scholar]
- 10.Perera R P, Johnson S K, Collins M D, Lewis D H. Streptococcus iniae associated with mortality of Tilapia nilotica × T. aurea hybrids. J Aquat Anim Health. 1995;6:335–340. [Google Scholar]
- 11.Pier G B, Madin S H. Streptococcus iniae sp. nov., a beta-hemolytic streptococcus isolated from an Amazon freshwater dolphin, Inia geoffrensis. Int J Syst Bacteriol. 1976;26:545–553. [Google Scholar]
- 12.Silvennoinen-Kassinen S, Koskela M. Optimal conditions for the opsonophagocytosis test with Streptococcus pneumoniae serotypes 3, 6A, 7F and 19F and human granulocytes. Acta Pathol Microbiol Immunol Scand Sect C. 1986;94:105–111. doi: 10.1111/j.1699-0463.1986.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 13.Smit P, Oberholzer D, Hayden-Smith S, Koornhof H J, Hilleman M R. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977;238:2613–2616. [PubMed] [Google Scholar]
- 14.Takahashi S, Adderson E E, Nagano Y, Nagano N, Briesacher M R, Bohnsack J F. Identification of a highly encapsulated, genetically related group of invasive type III group B streptococci. J Infect Dis. 1998;177:1116–1119. doi: 10.1086/517408. [DOI] [PubMed] [Google Scholar]
- 15.van Dam J E, Fleer A, Snippe H. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek. 1990;58:1–47. doi: 10.1007/BF02388078. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein M R, Litt M, Kertesz D A, Wyper P, Rose D, Coulter M, McGeer A, Facklam R, Ostach C, Willey B M, Borczyk A, Low D E. Invasive infections due to a fish pathogen, Streptococcus iniae. S. iniae Study Group. N Engl J Med. 1997;337:589–594. doi: 10.1056/NEJM199708283370902. [DOI] [PubMed] [Google Scholar]
- 17.Zlotkin A, Hershko H, Eldar A. Possible transmission of Streptococcus iniae from wild fish to cultured marine fish. Appl Environ Microbiol. 1998;64:4065–4067. doi: 10.1128/aem.64.10.4065-4067.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]