Abstract
Background and Objectives:
We previously developed an Africa specific donor health questionnaire (ASDHQ) based on local risk factors and designed a scoring scheme. This study assessed the performance of a new DHQ by comparing the HIV status in accepted versus deferred donors by the ASDHQ and comparing the rate of risk deferrals with historical data.
Materials and Methods:
Data were collected during a cross-sectional study conducted over 15 months at three referral hospital based blood services in Cameroon. The ASDHQ was administered to blood donors aged from 18 to 65 years in the same screening conditions as the routine questionnaire. The main outcomes of the study were ASDHQ sensitivity and specificity with regard to HIV laboratory testing, and donor deferral rates for each of the routine screening algorithms and for the ASDHQ.
Results:
Overall, 71/11,120 (0.6%) were confirmed as HIV-positive. The mean ASDHQ score was 95.80±4.4 in HIV negative donors and 94.80±4.4 in HIV-positive donors (p=0.05). The optimal cut-off provided by the ROC curve for the best performance of the ASDHQ was 95.04. Using this optimal cut-off, the ASDHQ sensitivity and specificity were 57% and 53% respectively (AUC=0.58 [0.51, 0.64], p=0.028). Using the ASDHQ, the HIV prevalence was 0.7% in deferred donors and 0.6% in accepted donors.
Conclusion:
The ASDHQ might be efficient only in specific conditions that maximize truthful donor’s responses, requiring each blood service to create an environment of trust and transparency to increase donors’ compliance and improve the accuracy of the questionnaire.
Keywords: HIV, Africa, Donor health questionnaire, blood safety
Introduction
Despite several years of international support, African blood services have some of the poorest blood safety indicators in the world as reported by several multicenter surveys (1–3). Recently some multicenter studies have shown that the risk of HIV transmission by transfusion ranges between 1 in 456 and 1 in 90,200, which is much higher than in high-income countries(4,5). To reduce blood-borne HIV transmission, four main strategies need to be considered: more effective identification of blood donors at high risk of HIV infection, better laboratory screening for HIV, pathogen reduction of blood products and reduced blood utilization. Blood donor risk screening comes first in the overall process of collecting safe blood products. The identification and exclusion of donors with high-risk behaviors leads to a significant yet poorly quantified reduction in the risk of infections for the blood recipients and ensures donor safety as well. However, medical selection is inappropriately conducted in several African blood services and the donor health questionnaire (DHQ) used for it is frequently inefficient(6,7).
To design an African specific donor health questionnaire based on local risk factors, we conducted a case-control study in Cameroon in 2017 and gathered risk factor data using audio computer-assisted self-interviews (ACASI). We identified 16 HIV local risk factors and developed an Africa specific donor health questionnaire (ASDHQ) and designed a scoring scheme to distinguish between HIV-positive and HIV-negative cases using receiver operating characteristics curves. Donors who scored over 82.2 on a 100 point HIV risk score were more likely to be HIV negative than those who scored less (8). However, to validate the new scoring system it needed to be implemented in a real-world setting.
This study assessed the performance of a new DHQ by determining its sensitivity and specificity, comparing the HIV status in accepted versus deferred donors by the ASDHQ and comparing the rate of risk deferrals with historical data. We also determined the operational acceptability of the new ASDHQ by collecting qualitative data from the donors. We hypothesized that the ASDHQ questionnaire would reduce the rate of risk deferrals compared to historical data, increase HIV prevalence in deferred donors compared to HIV prevalence in accepted donors.
Methods:
Study design and settings
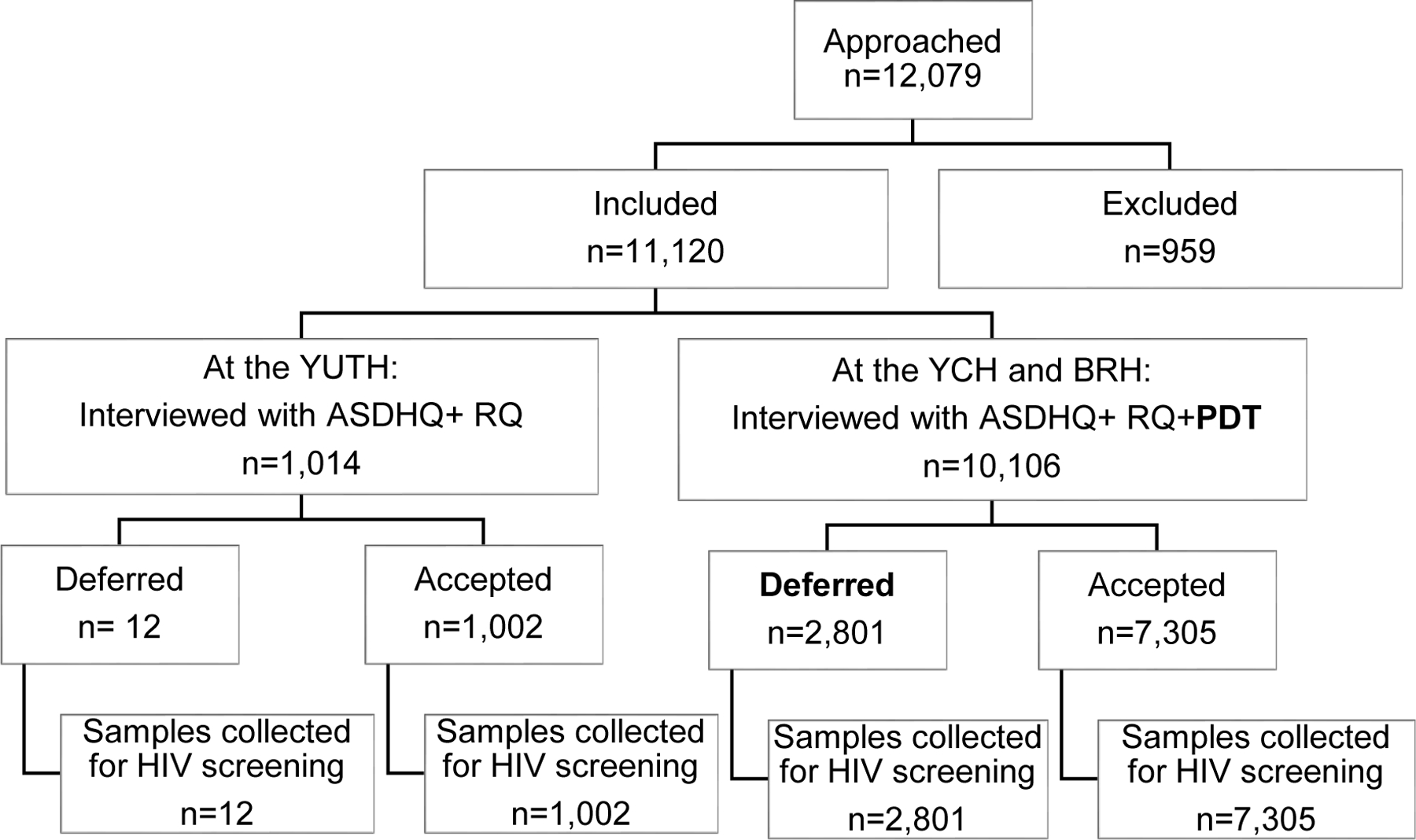
Data used in this study were collected during a cross-sectional study conducted over 15 months at three referral hospital based blood services in Cameroon: the Yaounde University Teaching Hospital (YUTH), the Blood bank of the Yaounde Central Hospital (YCH) and the Bafoussam Regional Hospital blood service (BRH). The sampling was consecutive. The 3 centers are all located in urban areas. They collected between 2,000 to 10,000 blood units per year. They have less than 30% of VNRBD. NAT is not performed on blood samples in Cameroon.
Study population and data collection procedures
Following eligibility assessment and informed consent, prospective blood donors were included in the study. Blood donors aged from 18 to 65 years were recruited at the clinics during their routine medical screening that included a routine donor questionnaire (RQ) at the YUTH, and a RQ plus pre-donation testing (PDT) at the YCH and BRH. The ASDHQ was then administered in the same screening conditions as the RQ. The donor deferral decision was based only on the routine screening criteria. Investigators collected 5 ml of whole blood specimens from all the accepted and deferred blood donors for further HIV testing in the laboratory. Investigators also completed a paper-based laboratory tracking form in which they documented PDT and ELISA results performed on-site by the facilities themselves in addition to other specimens collected.
Measurements and laboratory analysis
The RQ used by the facilities was an empirical questionnaire based on 24 questions with yes/no response possibilities. The ASDHQ is a comprehensive questionnaire of 16 questions designed previously by the authors to be administered within 15 minutes. Both questionnaires are available upon request to the authors. The ASDHQ was designed to discriminate HIV positive donors (Score <82.2) from HIV negative donors (Score=82.2 or more) (8). Both the RQ and ASDHQ are available upon request. Each plasma specimen was first analyzed by the facilities themselves using their routine algorithm for HIV diagnosis. It included two rapid determination tests (RDT) in a serial algorithm as described in Fig. 1. Each plasma specimen was tested before donation at the YCH and BRH and after donation at the YUTH with a rapid test (RDT 1 – Alere Determine HIV-1/2, Matsudo, Japan); each specimen was also tested after donation in all three centers with either ELISA Ab (Human Diagnostics Worldwide, Wiesbaden, Germany) or ELISA Ag/Ab (Murex HIV Ag/Ab, Diasorin SpA, Saluggia, Italy). If at least one test was reactive the donor was considered as at- risk to HIV and deferred. If both tests were non-reactive, the donors was considered HIV negative and the blood unit safe for transfusion with regard to HIV. Samples reported discordant (at least one test reactive) or positive (two different assays reactive) were retested by the research team with the Oraquick HIV-1/2 (Orasure Technologies, Inc., Bethlehem, PA, USA), Geenius Bio-Rad HIV 1&2 (Bio-Rad, Marnes-la-Coquette, France) and/or the RNA detection for confirmation according to an appropriate algorithm (Figure 2). The confirmatory algorithm was developed following results from a study conducted in the same setting a year before and consistent with the WHO confirmatory approach (9). Historical data on donor deferral rate and HIV prevalence were collected from blood center registers and published papers(10–12). We also interviewed 30 blood donors to measure the mean time spent to fill the questionnaire and their perception and their acceptability of the questionnaire using a set of 5 open-ended questions.
Figure 1:
Selection and testing procedures in the 3 participating facilities
YUTH: Yaounde University Teaching Hospital
YCH: Yaounde Central Hospital
BRH: Bafoussam Regional Hospital
ASDHQ: African specific donor heath questionnaire;
RQ: Routine Questionnaire;
PDT : Predonation testing
Figure 2:

Testing outcomes in the 3 participating facilities
YUTH: Yaounde University Teaching Hospital
YCH: Yaounde Central Hospital
BRH: Bafoussam Regional Hospital
ASDHQ: African specific donor heath questionnaire;
RQ: Routine Questionnaire;
RDT1: First rapid determination testing (pre or post donation)
RDT2: Second rapid determination testing (post donation)
Variables, outcomes and analysis
The main outcomes of the study were the ASDHQ sensitivity and the specificity with regard to HIV laboratory testing, donor deferral rates for each of the routine screening algorithms and for the ASDHQ at the recommended cut-off of 82.2 (8), the area under curve (AUC) and the optimal performance cut-off. The internal consistency (reliability) of the questionnaire was tested by calculating Cronbach’s alpha. The best threshold of distinguishing between HIV positive and HIV negative donors was selected by Receiver Operating Characteristics (ROC) curves based on calculated sensitivity and specificity. The cut-off score was adjusted to get the highest sensitivity. We measured and compared deferred donor rate, the HIV prevalence in accepted donors and in deferred donors for the different donor screening approaches (RQ, the ASDHQ the RQ+PDT and PDT alone).
The sociodemographic data of the population were described using descriptive statistics: median and interquartile ranges (IQR) for continuous variables as well as counts and proportions for categorical variables. The outcomes described above were determined as proportions measured using EPI info 7.2.6 and Microsoft Excel. Sample size calculations were performed using the following baseline parameters: alpha = 0.05, 1-beta = 0.80, an annual study population of 15,000 donors, a baseline deferral rate of 13% and a baseline HIV prevalence of 2% in accepted donors. The proportion of blood donor deferrals and HIV prevalence in different time periods and in the deferred versus accepted blood donors was compared using Chi² or Fisher’s exact tests as appropriate. P values less than 0.05 were considered statistically significant.
Ethical considerations
The study protocol was approved by the Cameroonian National Ethical Committee and the University of California San Francisco Committee on Human Research. All the collaborative centers provide an agreement for data and material sharing.
Results
Study population
A total of 11,120 blood donors were included in the study with 1,014 (9.1%), 8,718 (78.4%), 1,388 (12.5%) from the YUTH, the YCH and BRH respectively. In total 9,374 (84.5%) were male and 9,560 (86.0%) were family replacement blood donors. The mean age of the study population was 29.94±8.24 (18–64).
Main findings
Overall, 209/11,120 (1.9%) blood donors were reactive to RDT1 and 71/11,120 (0.6%) were confirmed HIV positive. The confirmed HIV prevalence was 0.7 % (7/1,014), 0.7% (60/8,718) and 0.3% (4/1,388) in YUTH, YCH and BRH respectively. The prevalence of HIV was 0.7% (64/9,374) in males versus 0.4% (7/1,746) in females (p=0.11). Of the 16 HIV risk variables included in the ASDHQ, only five were associated with HIV status: sex with a man who had sex with another man, use of illegal drugs, history of treatment on the street such as pedicure/manicure, dental care and body piercings (all p<0.05) (Table 1).
Table 1:
HIV statutes according to responses to risk factors questions in the study population
| HIV Statut | Frequently | Occasionally | Rarely | Never |
|---|---|---|---|---|
| C2 : Sexual intercourse without condom during the past 12 months (P=0.26) | ||||
| Negative | 1947(99.1) | 1514(99.4) | 1740(99.1) | 4464(99.4) |
| Positive | 17(0.9) | 9(0.6) | 15(0.9) | 26(0.6) |
| Total (%) | 1964(20.2) | 1523(15.6) | 1755(18.0) | 4490(46.2) |
| C3 : Anal sex during the past 12 months (P=0.72) | ||||
| Negative | 28(96.6) | 108(100.0) | 82(98.8) | 9447(99.3) |
| Positive | 1(3.4) | 0(0.0) | 1(1.2) | 65(0.7) |
| Total (%) | 29 (0.3) | 108(1.1) | 83(0.9) | 9512(97.7) |
| C4 : Sex with people you are not officially married to during the past 12 months (P=0.36) | ||||
| Negative | 936(98.9) | 1157(99.3) | 1387(99.4) | 6185(99.4) |
| Positive | 10(1.1) | 8(0.7) | 9(0.6) | 40(0.6) |
| Total (%) | 946(9.7) | 1165(12.0) | 1396(14.3) | 6225(64.0) |
| C6 : Sex with sex workers during the past 12 months (P=0.29) | ||||
| Negative | 38(100.0) | 77(100.0) | 178(99.4) | 9372(99.3) |
| Positive | 0(0.0) | 0(0.0) | 1(0.6) | 66(0.7) |
| Total (%) | 38(0.4) | 77(0.8) | 179(1.8) | 9438(97.0) |
| C7 : Sex with drug users during the past 12 months (P=0.49) | ||||
| Negative | 64(100.0) | 28(96.6) | 56(98.2) | 9517(99.3) |
| Positive | 0(0.0) | 1(3.4) | 1(1.8) | 65(0.7) |
| Total (%) | 23(0.4) | 25(0.4) | 36(0.6) | 6264(98.6) |
| C10 : Sex with a man who had sex with another man (P=0.000) | ||||
| Negative | 10(100.0) | 59(100.0) | 38(100.0) | 9558(99.3) |
| Positive | 0(0.0) | 0(0.0) | 0(0.0) | 67(0.7) |
| Total (%) | 7(0.1) | 17(0.3) | 33(0.5) | 6293(99.1) |
| C12 : Sex with somebody who spent at least a night in jail (P=0.40) | ||||
| Negative | 13(100.0) | 23(100.0) | 81(97.6) | 9548(99.3) |
| Positive | 0(0.0) | 0(0.0) | 2(2.4) | 65(0.7) |
| Total (%) | 8(0.1) | 18(0.3) | 34(0.5) | 6291(99.1) |
| C15 : Treated for sexually transmitted infection (P=0.15) | ||||
| Negative | 62(98.4) | 285(98.3) | 734(99.2) | 8584(99.4) |
| Positive | 1(1.6) | 5(1.7) | 6(0.8) | 55(0.6) |
| Total (%) | 38(0.6) | 190(3.0) | 537(8.4) | 5584(88.0) |
| C17 : Use non injected illegal drugs (P=0.000) | ||||
| Negative | 26(100.0) | 64(100.0) | 97(100.0) | 9478(99.3) |
| Positive | 0(0.0) | 0(0.0) | 0(0.0) | 24(0.7) |
| Total (%) | 21(0.3) | 47(0.7) | 55(0.9) | 6225(98.1) |
| C18 : Use injected illegal drugs (P=0.000) | ||||
| Negative | 12(100.0) | 18(100.0) | 27(100.0) | 9608(99.3) |
| Positive | 0(0.0) | 0(0.0) | 0(0.0) | 67(0.7) |
| Total (%) | 13(0.2) | 13(0.2) | 16(0.3) | 6308(99.3) |
| C21 : Undergo treatment on the street such as pedicure/manicure, tooth care (p=0.028) | ||||
| Negative | 171(99.4) | 429(98.4) | 792(98.8) | 8273(99.4) |
| Positive | 1(0.6) | 7(1.6) | 10(1.2) | 49(0.6) |
| Total (%) | 117(1.8) | 319(5.1) | 535(8.4) | 5380(84.7) |
| C22 : Undergo acupuncture (p=0.57) | ||||
| Negative | 10(100.0) | 25(100.0) | 49(100.0) | 9581(99.3) |
| Positive | 0(0.0) | 0(0.0) | 0(0.0) | 67(0.7) |
| Total (%) | 10(0.2) | 19(0.3) | 33(0.5) | 6286(99.0) |
| C23 : Tattoo yourself (p=0.23) | ||||
| Negative | 11(100.0) | 42(97.7) | 155(98.7) | 9457(99.3) |
| Positive | 0(0.0) | 1(2.3) | 2(1.3) | 64(0.7) |
| Total (%) | 9(0.1) | 30(0.5) | 112(1.8) | 6199(97.6) |
| C24 : Piercings on your body (p=0.000) | ||||
| Negative | 13(100.0) | 39(100.0) | 159(100.0) | 9454(99.3) |
| Positive | 0(0.0) | 0(0.0) | 0(0.0) | 67(0.7) |
| Total (%) | 12(0.2) | 30(0.5) | 112(1.8) | 6197(97.5) |
| C25: Scarifications on your body (0.65) | ||||
| Negative | 13(100.0) | 43(97.7) | 268(99.3) | 9341(99.3) |
| Positive | 0(0.0) | 1(2.3) | 2(0.7) | 64(0.7) |
| Total (%) | 15(0.3) | 45(0.7) | 238(3.7) | 6053(95.3) |
| C30: If you are a woman, are you excised? (p=0.45) | ||||
| No | Yes | |||
| Negative | 64(98.5) | 1472(99.6) | ||
| Positive | 1(1.5) | 6(0.4) | ||
| Total (%) | 6(0.6) | 17(1.8) | ||
The ASDHQ score ranged from 30 to 100 with an average of 95.79±4.40. The mean ASDHQ score was 95.80±4.4 in HIV negative donors and 94.80±4.4 in HIV positive donors (p=0.05) (Table 2). Using the planned cut-off of 82.2, the sensitivity of the ASDHQ was 0%. The optimal cut-off provided by the ROC curve for the best performance of the ASDHQ was 95.04. Using this optimal cut-off, the ASDHQ sensitivity and specificity were 57% and 53% respectively (AUC=0.58 [0.51, 0.64], p=0.028) (Figure 3). A total of 2,801 (25.2%) blood donors were deferred in the three sites. The deferral rate for the routine questionnaire alone was 23.9 (2,611/10,918) versus 2.0% (202/10,106) for the RDT alone, 43.1 (4,806/11,120) for the ASDHQ at the optimal cut-off of 95.04. Using the ASDHQ, the HIV prevalence was 0.7% in deferred donors and 0.6% in accepted donors (Table 3).
Table 2:
Mean score (over 100) of the ASDHQ by Center and per HIV status
| HIV Status | CHUY | HCY | HRB | Total | P-values |
|---|---|---|---|---|---|
| HIV Negative | 95.70±4.6 | 95.93±4.4 | 95.02±3.8 | 95.80±4.4 | |
| HIV Positive | 95.72±2.9 | 94.84±4.6 | 92.50±2.88 | 94.80±4.4 | |
| P-value | 0.99 | 0.055 | 0.18 | 0.05 | |
| Total | 95.70±4.6 | 95.93±4.4 | 95.00±3.8 | 95.79±4.4 | P=0.000 |
Figure 3:
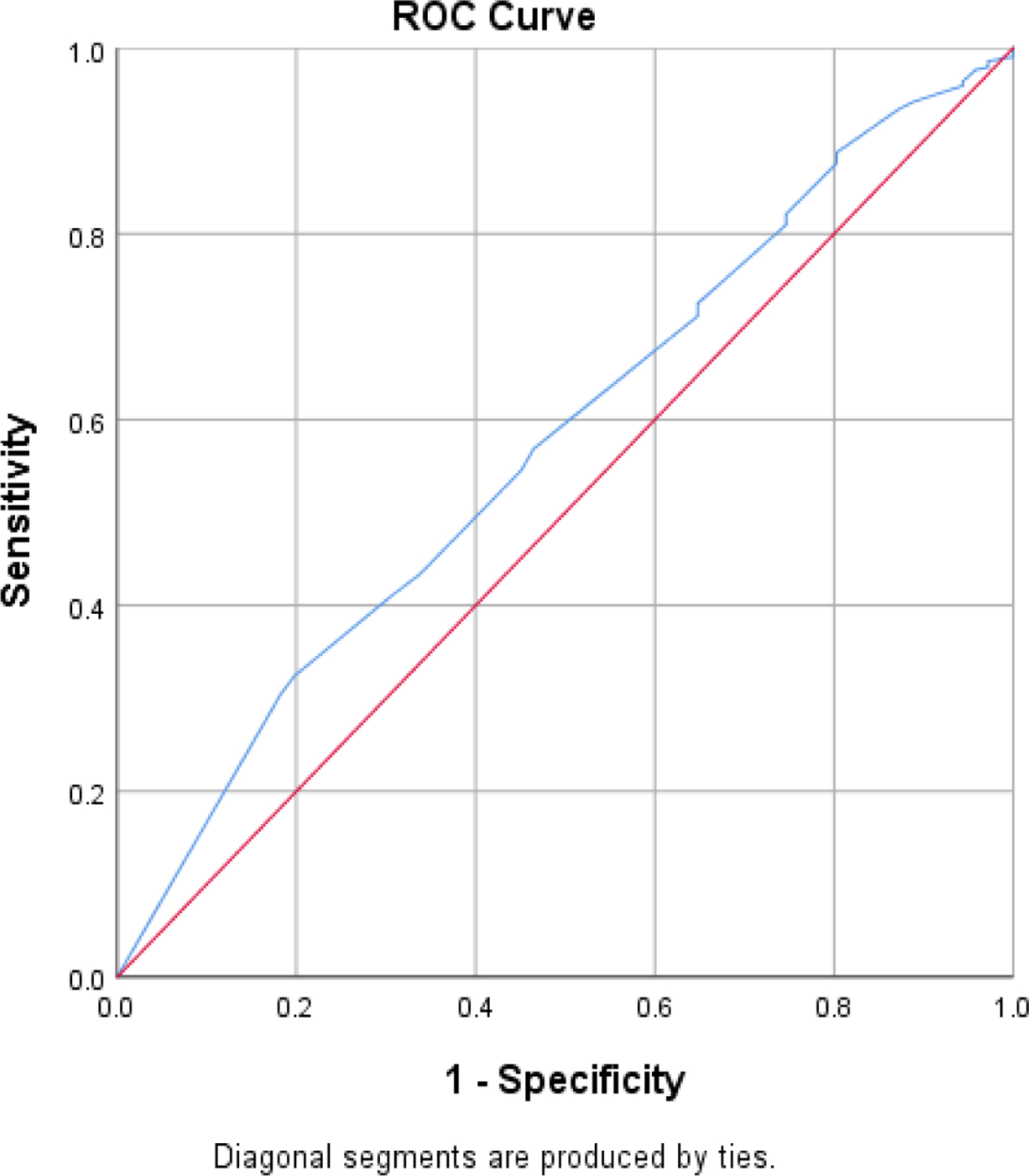
The ASDHQ area under the curve using the ROC
Table 3:
Deferral rate and HIV status is accepted and deferred blood donors
| Donor screening approach | Deferral rate n(%) |
HIV in Accepted n(%) | HIV in Deferred n(%) | Sens/Spec (%) | AUC |
|---|---|---|---|---|---|
| Routine questionnaire alone (n=10,918)π | 2,611 (23.9) | 7 (0.6) | 29 (0.2) | 40.8/76.6 | |
| RDT alone (pre-test) (n=10,106)µ | 202 (2.0) | 0 (0) | 64 (0.6) | 100/98.1 | |
| Routine questionnaire + RDT (pre-test) (n=10,106)µ | 2,801(25.2) | 0 (0) | 64 (0.6) | 100/72.0 | |
| ASDHQ cut-off 82.2* (n=11,120) |
46 (0.4) | 71 (0.6) | 0 (0) | 0/100 | |
| ASDHQ cut-off 95.04 (n=11,120) |
4,806 (43.1) | 34 (0.7) | 37 (0.6) | 57/53 | 0.58 [0.51, 0.64] p=0.028 |
Tagny & al, 2012,
All sites,
HCY+HRB
The sensitivity and specificity of the RQ were respectively 40.8% and 76.6%. The sensitivity of the RDT1 was 100% (71/71) and the specificity was 98.90% (10,911/11,049). The sensitivity of the RDT1 was 100% in pre-donation testing in YCH and BRH and 100% in post donation testing in YUTH. A total of 138/9,904 donors were false positive with the pre-donation testing and were excluded for donation. The positive predictive value and the negative predictive values of the RDT1 were 34% (71/209) and 100% (10,911/10,911) respectively.
The mean time required to fill the questionnaire was 14 minutes (5–30minutes). Concerning the acceptability of the methods, 11/30 blood donors declared they had difficulty understanding all the questions, 28/30 declared the questionnaire is useful for the safety of the recipient and 17/30 needed additional explanations during the screening process.
Discussion
The study revealed that the ASDHQ performed no better than the RQ, and both performed poorly in discriminating HIV negative blood donors and positive blood donors. The study also revealed that the ASDHQ has a high deferral rate in a context of low HIV prevalence. Finally, rapid testing performed either before or after donation had high sensitivity and specificity.
The sensitivity and specificity of any DHQ is important because they describe the ability of the questionnaire to identify HIV-positive donors while allowing HIV negative donors to donate blood. The low sensitivity of DHQ increases the risk of collecting blood from at-risk donors while low specificity would result in the exclusion of too many safe donors and adversely impact the blood supply. Our literature review revealed few assessments of DHQ in sub-Saharan Africa but the few that we did find reported that DHQs are inefficient as they were developed without assessment of local HIV risk factors (6,7). This study also confirmed the poor performance of the RQ used in Cameroon. The poor performance of the RQ was the evidence that supports the development of the ASDHQ from locally based risk factors using a rigorous case-control study based on recommended HIV diagnosis algorithm in a quality-assured laboratory (8).
Despite the good performance of the ASDHQ in the pilot study and an adjustment to its cutoff, the ASDHQ also performed poorly and worse than in the previous study. The ASDHQ scores were higher than expected with no significant difference in score and HIV prevalence between the HIV negative group and the HIV positive group. Several risk factors that were significantly associated with the HIV positive status in the first study were no longer associated with HIV status in the present study. This may be due to the differences in the screening environment in the two studies, the lower HIV prevalence in this validation phase and to the difference in the study design. Indeed, the pilot study was a case control study with a large number of HIV cases and was administered using a computer-assisted program which was more confidential and conducive to donor attention and thus to more accurate responses.
Audio-computed assisted systems for donor health screening have been shown previously to produce more truthful responses compared to standard written questionnaire and interview(13–16) and people may be more likely to respond truthfully about risk behaviors when questioning is anonymous(17). Research on sexual behavior, smoking, alcohol, and drug use, has found that use of a computer-assisted questionnaire increased reporting of “stigmatized” behaviors compared to face-to-face interviews(18,19). Previous research has also suggested that the limitations of screening questionnaires in identifying ineligible donors may in part be due to the donors’ failure to carefully read the instructions or understand the information (20), particularly donors with low educational status. In our study, a significant number of donor declare that they needed more explanations. The donor screening using ASDHQ may then be more efficient if it is conducted in an environment that maximizes the quality of the responses. Despite an ASDHQ sensitivity of 0.77 and specificity of 0.73 reported by Fonkou & al (unpublished data), the ASDHQ could not be reliable in routine environment in Cameroon because the authors conducted the assessment on a small sample size using RDT as the only test for HIV diagnosis.
Surprisingly, the HIV prevalence in Cameroonian blood donors and blood donations was lower compared to the previous studies confirming the steady decrease since 20 years(11,21). This is consistent with the national and international publications on the trends of HIV in Cameroon (22–24). The effect of coordinated National and International programs against HIV/AIDS on general population and blood donors in particular may be the main explanation. The decrease in HIV prevalence may also be explained by the increase in the proportion of VNRBD but also by the progressive implementation of good practices during donor deferral in African blood services, better training programmes and subsequent appointment of trained staff in the facilities under programmes (1,25,26). Considering the mean HIV residual risk of 1/2,028 reported in Cameroon recently(5), 5 blood donors of the study population may have been falsely negative but this will not change significantly the prevalence.
The predonation screening (RDT1) had a 100% sensitivity. This raises the hypothesis that testing HIV before donation might be an option in Cameroon. This hypothesis needs to be confirmed by a study appropriately designed to assess laboratory screening methods. Indeed, several studies had previously confirmed the performance of predonation screening especially when it is conducted in a quality manner (27,28), but other studies demonstrated the limitations of RDT in African blood services(29,30). About 140/10,000 donors were false positive and unnecessary excluded for donation, consistent with previous reports that up to 10% of RDT reactivity are false positive in Cameroon(5,21,31).
In summary, despite good cost-effectiveness of predonation testing is reported in high TTI prevalence settings in Africa(32), the cost effectiveness in low HIV prevalence settings is still to be assessed. Moreover, stigmatization after HIV donor notification immediately after the donor testing may limit acceptability of the PDT by benevolent donors and impact on regular blood donation.
We recognize some limitations in this study. The low HIV prevalence may have limited an accurate assessment of the ASDHQ. But the HIV prevalence was still higher than in other countries suggesting that this assessment outcome is likely to be the same in many settings with lower HIV prevalence. The study was not designed to assess RDT but its outcomes appear to support the need to confirm the cost benefit of the predonation testing. For a better assessment of the questionnaire, we could have been included used implementation science design instead of just observing the use of the questionnaire in the confirmatory algorithm but the probability of finding a NAT+ sample among HIV antibody negative samples was likely very low. This step was important to measure its relevance prior to its full implementation. Finally, donor perception regarding the truthfulness of their responses on the ASDHQ was not assessed although we hypothesized that unadmitted risk factors were the likely explanation for its inability to discriminate HIV positives and negatives.
This study has public health and governmental policy implications regarding the blood donor screening strategies to adopt in similar environments. We brought to light that the donor health questionnaires and the screening processes in Cameroon or in similar environments perform poorly. The ASDHQ might be efficient only in specific conditions that maximize truthful donor’s responses, requiring each blood service to create an environment of trust and transparency to increase donors’ compliance and improve accuracy of the questionnaire. Given deficiencies of the donor history questionnaire, both improved donor recruitment with transition to voluntary donor pool, as well as strengthening of laboratory testing of blood donations for transfusion transmissible pathogens, is needed. The study argues for more attention to RDTs than the DHQs in family replacement blood donations and progressive decrease of HIV prevalence in blood donors. Finally, the donation settings. The study also suggests that blood services may wish to assess pre-donation testing strategies for HIV and other TTIs, taking into account cost-effectiveness and donor acceptability.
Highlights.
The study found that the Africa-Specific Donor Health Questionnaire (ASDHQ) performed poorly in discriminating HIV-negative blood donors from HIV-positive blood donors.
The study also showed that the ASDHQ was associated with a high deferral rate in the context of low HIV prevalence.
The ASDHQ might be efficient only in specific conditions that maximize truthful donor responses, requiring each blood service to create an environment of trust and transparency to increase donor compliance and improve the accuracy of the questionnaire.
Acknowledgments:
We thank the Bafoussam Regional Hospital, the Yaoundé Central Hospital Blood service and the University Teaching Hospital for their authorization. C.T.T., G.N., D.M. and E.M. designed the study; C.T.T., G.I., C.A., M.N., C.N., F.N., A.F.S., C.G., G.E. conducted the study; C.T.T., GN, and EM performed statistical analysis; C.T.T. and G.N.T. wrote the manuscript; and G.I., G.N., D.M. and E.M. revised the manuscript.
Funding:
This research was supported by a grant from the National Institutes of Health, UCSF-Gladstone Center for AIDS Research, P30AI027763.
This research was partially supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW010345 to Dr. Murphy. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors have disclosed no conflicts of interest.
References
- 1.World Health Organization. Current status on blood safety and availability in the WHO african region: Report of 2013 survey WHO Regional office for Africa, Brazzaville, Gongo; 2016. [Google Scholar]
- 2.World Health Organization. Action framework to advance universal access to safe, effective and quality-assured blood products 2020–2023 © World Health Organization; 2020; 2020. [Google Scholar]
- 3.World Health Organization. Global Safety Report on Safety and Availability WHO, Geneva, Switzerland; 2017. [Google Scholar]
- 4.Lefrère J-J, Dahourouh H, Dokekias AE, Kouao MD, Diarra A, Diop S, et al. Estimate of the residual risk of transfusion-transmitted human immunodeficiency virus infection in sub-Saharan Africa: a multinational collaborative study. Transfusion (Paris) 2011. Mar;51(3):486–92. [DOI] [PubMed] [Google Scholar]
- 5.Guekeng E, Nsagha D, Zofou D, Njunda A, Nanfack A, Fokam J, et al. Residual risk of HIV transmission through blood transfusion in five blood banks in Cameroon. Journal of Medical Research 2020;158–65. [Google Scholar]
- 6.Tagny CT, Kouao MD, Touré H, Gargouri J, Fazul AS, Ouattara S, et al. Transfusion safety in francophone African countries: an analysis of strategies for the medical selection of blood donors. Transfusion (Paris) 2012. Jan;52(1):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouegnigan Rerambiah L, Biyoghe AS, Bengone C, Djoba Siawaya JF. Evaluation of blood donors questionnaire in a developing country: The case of Gabon. Transfus Clin Biol J Soc Francaise Transfus Sang 2014. Jun;21(3):116–9. [DOI] [PubMed] [Google Scholar]
- 8.Tagny CT, Nguefack-Tsague G, Fopa D, Ashu C, Tante E, Ngo Balogog P, et al. Risk factors for human immunodeficiency virus among blood donors in Cameroon: evidence for the design of an Africa-specific donor history questionnaire. Transfusion (Paris) 2017;57(8):1912–21. [DOI] [PubMed] [Google Scholar]
- 9.Tagny CT, Bissim M, Djeumen R, et al. The use of the GeeniusTM HIV-1/2 Rapid confirmatory test for the enrolment of patients and blood donors in the WHO Universal Test and Treat Strategy in Cameroon, Africa. Vox Sang 2020; [DOI] [PubMed] [Google Scholar]
- 10.Tagny CT, Kouao MD, Touré H, Gargouri J, Fazul AS, Ouattara S, et al. Transfusion safety in francophone African countries: an analysis of strategies for the medical selection of blood donors. Transfusion (Paris) 2012. Jan;52(1):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagny CT, Ndoumba A, Laperche S, Murphy E, Mbanya D. Reducing risks of Transfusion-transmitted infections in a resource-limited hospital-based blood bank: the case of the Yaoundé University Teaching Hospital, Cameroon. ISBT Sci Ser 2016. Aug;11(2):82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tagny CT, Diarra A, Yahaya R, Hakizimana M, Nguessan A, Mbensa G, et al. Characteristics of blood donors and donated blood in sub-Saharan Francophone Africa. Transfusion (Paris) 2009. Aug;49(8):1592–9. [DOI] [PubMed] [Google Scholar]
- 13.Sellors JW, Hayward R, Swanson G, Ali A, Haynes RB, Bourque R, et al. Comparison of deferral rates using a computerized versus written blood donor questionnaire: a randomized, cross-over study [ISRCTN84429599]. BMC Public Health 2002. Aug 21;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman M, Ram SS, Yi Q-L, Mazerall J, O’brien SF. The donor health assessment questionnaire: potential for format change and computer-assisted self-interviews to improve donor attention. Transfusion (Paris) 2007. Sep;47(9):1595–600. [DOI] [PubMed] [Google Scholar]
- 15.Goldman M, Ram SS, Yi Q-L, O’Brien SF. The Canadian donor health assessment questionnaire: can it be improved? Transfusion (Paris) 2006. Dec;46(12):2169–75. [DOI] [PubMed] [Google Scholar]
- 16.Locke SE, Kowaloff HB, Hoff RG, Safran C, Popovsky MA, Cotton DJ, et al. Computer interview for screening blood donors for risk of HIV transmission. MD Comput Comput Med Pract 1994. Feb;11(1):26–32. [PubMed] [Google Scholar]
- 17.Williams AE, Thomson RA, Schreiber GB, Watanabe K, Bethel J, Lo A, et al. Estimates of infectious disease risk factors in US blood donors. Retrovirus Epidemiology Donor Study. JAMA 1997. Mar 26;277(12):967–72. [PubMed] [Google Scholar]
- 18.Aquilino WS, Wright DL, Supple AJ. Response effects due to bystander presence in CASI and paper-and-pencil surveys of drug use and alcohol use. Subst Use Misuse 2000. Jun;35(6–8):845–67. [DOI] [PubMed] [Google Scholar]
- 19.Des Jarlais DC, Paone D, Milliken J, Turner CF, Miller H, Gribble J, et al. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: a quasi-randomised trial. Lancet Lond Engl 1999. May 15;353(9165):1657–61. [DOI] [PubMed] [Google Scholar]
- 20.Doll LS, Petersen LR, White CR, Ward JW. Human immunodeficiency virus type 1-infected blood donors: behavioral characteristics and reasons for donation. The HIV Blood Donor Study Group. Transfusion (Paris) 1991. Oct;31(8):704–9. [DOI] [PubMed] [Google Scholar]
- 21.Tagny CT, Mbanya D, Leballais L, Murphy E, Lefrère J-J, Laperche S. Reduction of the risk of transfusion-transmitted human immunodeficiency virus (HIV) infection by using an HIV antigen/antibody combination assay in blood donation screening in Cameroon. Transfusion (Paris) 2011. Jan;51(1):184–90. [DOI] [PubMed] [Google Scholar]
- 22.UNAIDS. UNAIDS data 2020 [Internet] Joint United Nations Programme on HIV/AIDS (UNAIDS); 2020. [cited 2021 Jul 7]. Available from: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf [Google Scholar]
- 23.UNAIDS. Country factsheets, Cameroon [Internet] [cited 2021 Nov 11]. Available from: https://www.unaids.org/fr/regionscountries/countries/cameroon
- 24.CNLS – DLM. Profil des Estimations et projections en matière de VIH/sida au Cameroun Com Natl Lutte Contre SIDA Ministère Santé Publique. 2012; [Google Scholar]
- 25.Tagny CT, Laperche S, Murphy EL, Francophone Africa Network for Transfusion Medicine Research. Updated characteristics of blood services, donors and blood products in 11 French-speaking African countries. Vox Sang 2018. Aug 19; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz LM, Donnelly JJ, Gresens CJ, Holmberg JA, MacPherson J, Zacharias PJK, et al. Report of a workshop on ensuring sustainable access to safe blood in developing countries: International Blood Safety Forum, March 24, 2017. Transfusion (Paris) 2018. May;58(5):1299–306. [DOI] [PubMed] [Google Scholar]
- 27.Owusu-Ofori S, Temple J, Sarkodie F, Anokwa M, Candotti D, Allain J-P. Predonation screening of blood donors with rapid tests: implementation and efficacy of a novel approach to blood safety in resource-poor settings. Transfusion (Paris) 2005. Feb;45(2):133–40. [DOI] [PubMed] [Google Scholar]
- 28.Lieshout-Krikke RW, Zaaijer HL, van de Laar TJW. Predonation screening of candidate donors and prevention of window period donations. Transfusion (Paris) 2015. Feb;55(2):373–8. [DOI] [PubMed] [Google Scholar]
- 29.Laperche S, Francophone African Group for Research in Blood Transfusion. Multinational assessment of blood-borne virus testing and transfusion safety on the African continent. Transfusion (Paris) 2013. Apr;53(4):816–26. [DOI] [PubMed] [Google Scholar]
- 30.Pruett CR, Vermeulen M, Zacharias P, Ingram C, Tayou Tagny C, Bloch EM. The use of rapid diagnostic tests for transfusion infectious screening in Africa: a literature review. Transfus Med Rev 2015. Jan;29(1):35–44. [DOI] [PubMed] [Google Scholar]
- 31.Candotti D High rate of HCV and HIV false-positive results in serological screening can impair blood supply in Sub- Saharan Africa. Transfusion 2019;In press. [DOI] [PubMed] [Google Scholar]
- 32.Dosunmu AO, Akinbami AA, Ismail AK, Olaiya MA, Uche EI, Aile IK. The cost-effectiveness of predonation screening for transfusion transmissible infections using rapid test kits in a hospital-based blood transfusion centre. Niger Postgrad Med J 2017. Sep;24(3):162–7. [DOI] [PubMed] [Google Scholar]


