Abstract
BACKGROUND:
Disparities in adjuvant treatment between Black and White women with endometrial cancer exist and contribute to worse outcomes among Black women. However, factors leading to disparate treatment receipt are understudied.
OBJECTIVE:
We examined whether patient refusal of adjuvant treatment (chemotherapy or radiation) differed between Black and White women and whether treatment refusal mediated racial disparities in survival among women with endometrial cancer.
METHODS:
We used the National Cancer Database, a hospital-based cancer registry, to identify non-Hispanic Black and non-Hispanic White women diagnosed with endometrial cancer from 2004 to 2016 who either received or refused recommended radiation or chemotherapy. We used logistic regression to estimate multivariable-adjusted odds ratios and 95% confidence intervals for associations between race and treatment refusal. We also examined predictors of treatment refusal in race-specific models. Accelerated failure time models were used to estimate absolute differences in overall survival by race. We used causal mediation analysis to estimate the proportion of racial differences in overall survival attributable to racial differences in adjuvant treatment refusal. We considered the overall study population as well as strata defined by histology, and adjusted for sociodemographic, tumor, and facility characteristics.
RESULTS:
Our analysis included 75,447 endometrial cancer patients recommended to receive radiation and 60,187 endometrial cancer patients recommended to receive chemotherapy, among which 6.4% and 11.4% refused treatment, respectively. Among Black women recommended for radiation or chemotherapy, 6.4% and 9.6% refused, respectively. Among White women recommended for radiation or chemotherapy, 6.4% and 11.8% refused, respectively. After adjusting for sociodemographic variables, facility characteristics, and tumor characteristics, Black women were in fact more likely to refuse chemotherapy than White women (adjusted odds ratio=1.26, 95% confidence interval=1.15, 1.37), but no difference in radiation refusal was observed (adjusted odds ratio=1.00, 95% confidence interval=0.91, 1.11). Some predictors of radiation refusal varied by race, namely income, education, histology, stage, and chemotherapy receipt (p-interactions<0.05), while predictors of chemotherapy refusal were generally similar between Black and White women. Among women recommended for radiation, Black women survived an average of 4.3 years shorter than White women, none of which appeared to operate through differences in radiation refusal. Among women recommended for chemotherapy, Black women survived an average of 3.2 years less than White women, of which 1.9 months (4.9%) could potentially be attributed to differences in chemotherapy refusal.
CONCLUSIONS:
We observed differences in chemotherapy refusal by race, and those differences may be responsible for up to about two months of the overall 3.2-year survival disparity between White and Black women; radiation refusal did not explain any of the 4.3-year disparity among women recommended for radiation. Treatment refusal accounts for at most a small fraction of the total racial disparity in endometrial cancer survival. While a better understanding of the reasons for patient treatment refusal and subsequent intervention may help improve outcomes for some women, other causes of disparate outcomes, particularly those reflecting the social determinants of health, must be investigated.
Keywords: chemotherapy, disparities, hospital-based cancer registry, race, radiation treatment, uterus neoplasm
Condensation:
Black women with endometrial cancer are more likely than White women to refuse adjuvant chemotherapy; however, this explains only a small portion of the total Black-White survival disparity.
INTRODUCTION
Uterine cancer, primarily endometrial cancer (EC), is the most common gynecologic malignancy diagnosed in the U.S. and is characterized by one of the worst racial disparities of all solid tumors: between 2014 and 2018, annual mortality was 8.9 per 100,000 non-Hispanic Black women compared to 4.5 per 100,000 non-Hispanic White women, constituting a 98% higher likelihood of death among Black women (1). Several factors contribute to Black-White disparities in mortality: Black women are more likely to be diagnosed with poor prognosis tumors (2), have a higher burden of comorbidities (3), and are less likely to receive treatment. Although the literature related to treatment disparities among women with EC is somewhat inconsistent, likely due to different analytic methods and heterogeneous study populations, in general, Black women receive EC treatment at lower rates than White women. For example, hysterectomy and bilateral salpingo-oophorectomy, the mainstay of EC treatment, is less common among Black as compared with White EC patients in some studies (4–6), but not others (7). Similarly, the typical trend of adjuvant treatment use – which includes radiation, chemotherapy, or both – is that Black women less often receive adjuvant treatment (8, 9), although some conflicting studies exist (7, 10, 11). Most recently, studies that evaluate receipt of the full course of guideline-concordant treatment demonstrate significantly lower receipt among Black women (12–14). Missing from our understanding are the reasons underlying unequal EC treatment, yet this information is a prerequisite for reducing survival disparities arising from unequal treatment.
One component that may contribute to racial differences in treatment receipt is refusal of recommended treatment. Studies of other cancer types have demonstrated that Black cancer patients are more likely to refuse recommended treatment, which could partially explain the lower prevalence of treatment receipt (15–19). In a National Cancer Database (NCDB) study, Parsons and colleagues reported no significant difference in radiation refusal comparing White and Black women with EC, but that radiation refusal was associated with worse overall survival (20). We sought to expand this analysis by also examining Black-White differences in refusal of adjuvant chemotherapy and assessing whether refusal of radiation or chemotherapy contributes to racial disparities in overall survival (OS) using a causal mediation analysis. In addition, we examined predictors (sociodemographic, facility characteristics, and tumor factors) of refusal of adjuvant radiation or chemotherapy in race-specific models to identify potential leverage points for future interventions aimed at decreasing treatment refusal.
MATERIALS AND METHODS
Data Source
Data were obtained from the NCDB, a hospital-based cancer registry containing data from over 1500 facilities accredited by the American College of Surgeons’ Commission on Cancer (CoC) (21). Approximately 70% of all malignant cancers diagnosed in the United States are included in this dataset. While the NCDB collects a large proportion of incident cancer diagnoses in the United States, selection bias may exist as only hospitals approved by the CoC contribute data to the NCDB. Available data elements, including sociodemographic characteristics, tumor characteristics, attributes of the treatment facilities, treatment, and survival outcomes, are abstracted from patient medical records by Certified Tumor Registrars (22). For cases with missing data elements, registrars may contact the treating physicians to obtain the necessary data to complete the record. Data submitted to the NCDB undergo rigorous data quality checks in line with standards set by the American College of Surgeons CoC. Case records that do not meet requirements are identified and returned to the hospital (22). All data are de-identified, and the study was considered exempt by the Ohio State University Institutional Review Board (IRB).
Study population
We conducted a retrospective cohort study of women diagnosed with EC [International Classification of Disease for Oncology, Third Edition (ICD-O-3) primary site codes: C54.0-C54.3, C54.8-C54.9, C55.9] between 2004 and 2016 using data from the NCDB (21, 22). We identified 423,657 women ≥ 18 years of age at diagnosis who self-reported non-Hispanic White (hereafter, White) or non-Hispanic Black (hereafter, Black) race. We excluded women from this analysis for the following reasons: no surgical procedure (n=34,116); subtotal hysterectomy (n=4,346); surgery not otherwise specified (n=866); missing stage (n=68,380); or histology types not classifiable as endometrioid/adenocarcinoma (ICD-O-3 morphology codes 8140, 8380–8383, 8210, 8211, 8260–8263, 8560, 8570], serous (ICD-O-3 8441, 8460, 8461), carcinosarcoma (ICD- O-3 8950, 8951, 8980, 8981), mixed epithelial (ICD- O-3 8323, 8255), or clear cell (ICD- O-3 8310) (n=9,303) or ungraded endometrioid (n=32,713). We further excluded 5,821 women with missing information on facility location, 21,384 women with missing zip code-level income, and 78 women who did not have information on follow-up time (or zero months), resulting in a sample size of 246,650 (Figure 1). Further exclusions for the analyses of radiation refusal and chemotherapy refusal are described below.
Figure 1.
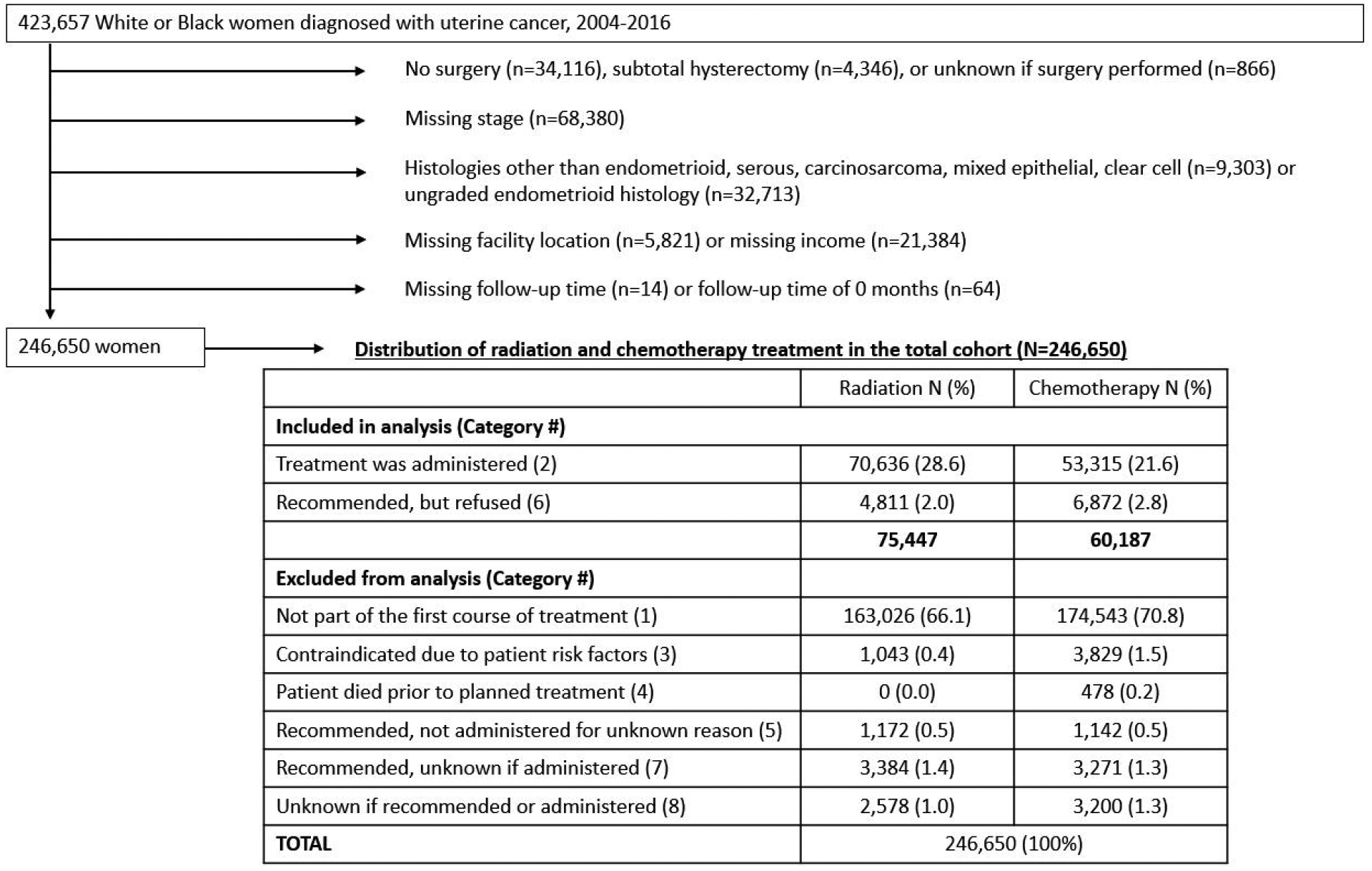
Study flow chart demonstrating cohort selection and distribution of treatment status in the overall study population
Treatment refusal
Radiation and chemotherapy treatment were categorized as follows: (1) none, not recommended as part of the planned first course of therapy, (2) treatment received as part of planned first course of therapy, (3) treatment was not recommended due to contraindications, (4) treatment was not administered because the patient died prior to planned recommended therapy, (5) treatment was not administered, but was recommended, unknown why not administered, (6) treatment was recommended but refused by patient, (7) treatment was recommended but unknown whether administered, (8) it is unknown whether treatment was recommended or administered. Figure 1 shows the distribution of these treatment categories in the sample of 246,650 women.
As our goal was to understand how treatment refusal impacts survival, the radiation and chemotherapy analyses were limited to those women who were recommended to have each specific treatment and either received (category 2) or refused (category 6) the recommended treatment. Our definition of treatment refusal did not include patients who did not receive recommended treatment because of contraindications nor cases where treatment was recommended but not received for an unknown reason. In analyses of radiation refusal, we included 75,447 women who were recommended to receive radiation, while in analysis of chemotherapy refusal, we included 60,187 women who were recommended to receive chemotherapy. In Supplemental Table 1, we compared demographic characteristics between women who were included in the analyses (recommended to receive treatment and either received or refused with non-missing data on exclusion criteria) to women who were recommended to receive treatment and either received or refused but were excluded due to missing data on exclusion criteria (e.g., missing surgery, missing stage, etc.). In both analyses (refusal of radiation and refusal of chemotherapy) excluded women were more likely to be Black, younger at diagnosis, have comorbidities, be uninsured and less likely to have private insurance.
Covariates
Information on the following covariates was included: age at diagnosis (<50, 50–69, ≥70), Charlson-Deyo comorbidity score (0, 1, or ≥2), type of health insurance (none, private, Medicaid, Medicare), median income in zip code of residence in two categories (<$48 000 or ≥$48 000), percentage of residents by zip code who did not graduate from high school (≥21%, 13%–20.9%, 7%–12.9%, <7%), facility location (Northeast, Midwest, Mountain, Pacific, South), facility type (community cancer, comprehensive community cancer, academic/research, integrated network cancer), 2009 American Joint Commission on Cancer pathologic stage (I, II, III, IV), and grade (1, 2, 3, applicable for endometrioid EC only). We combined grade and histology to create a histologic subtype variable with the following categories: low-grade endometrioid, high-grade endometrioid, serous, clear cell, and carcinosarcoma. Median household income for each patient’s area of residence is estimated by matching the zip code of the patient recorded at the time of diagnosis against files derived from the 2012 American Community Survey data, spanning years 2008–2012 and adjusted for 2012 inflation. Household income is categorized as quartiles based on equally proportioned income ranges among all US zip codes; we grouped the lower two and upper two quartiles. Overall survival (OS) was calculated as the time from the date of diagnosis to the date of death; among women alive at the end of follow-up, the date of last contact was used as the censoring time.
Statistical analysis
We used multivariable-adjusted logistic regression to estimate adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for associations between refusal of adjuvant treatment and epidemiological, tumor, and facility characteristics in the overall study population and among Black and White women separately. We also examined the association between race and refusal of adjuvant treatment stratified by histology, based on the hypothesis that treatment refusal among women with certain histological diagnoses would be more harmful for survival. All variables shown in Table 1 were significantly related to treatment refusal in univariable models (p<0.05, data not tabled) and were therefore included in multivariable models. We included the same adjustment factors in the race-specific and histology-stratified analyses in order to facilitate comparisons. To test whether associations differed by race, we included a multiplicative interaction term between each assessed predictor and race in the multivariable models.
Table 1.
Multivariable-adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for associations between epidemiological, facility, and tumor characteristics and refusal of adjuvant radiation or chemotherapy
| Radiation (N=75,447) | Chemotherapy (N=60,187) | |||||||
|---|---|---|---|---|---|---|---|---|
| Received n=70,636 | Refused n=4,811 | Received n=53,315 | Refused n=6,872 | |||||
| n (%) | aOR (95% CI)1 | p | n (%) | aOR (95% CI)2 | p | |||
| Race | 0.98 | <0.0001 | ||||||
| White | 62,200 (88.1%) | 4,231 (87.9%) | 1.00 | 44,187 (82.9%) | 5,907 (86.0%) | 1.00 | ||
| Black | 8,436 (11.9%) | 580 (12.1%) | 1.00 (0.91, 1.11) | 9,128 (17.1%) | 965 (14.0%) | 1.26 (1.15, 1.37) | ||
| Age at diagnosis | <0.0001 | <0.0001 | ||||||
| <50 | 4,063 (5.8%) | 132 (2.7%) | 1.00 | 3,428 (6.4%) | 337 (4.9%) | 1.00 | ||
| 50–69 | 44,183 (62.6%) | 2,311 (48.0%) | 1.47 (1.22, 1.76) | 34,385 (64.5%) | 3,358 (48.9%) | 1.56 (1.37, 1.78) | ||
| ≥70 | 22,390 (31.7%) | 2,368 (49.2%) | 2.33 (1.92, 2.82) | 15,502 (29.1%) | 3,177 (46.2%) | 3.59 (3.11, 4.14) | ||
| Charlson Comorbidity Score | 0.001 | <0.0001 | ||||||
| 0 | 53,679 (76.0%) | 3,442 (71.5%) | 1.00 | 40,582 (76.1%) | 4,892 (71.2%) | 1.00 | ||
| 1 | 13,541 (19.2%) | 1,038 (21.6%) | 1.10 (1.03, 1.19) | 10,155 (19.1%) | 1,537 (22.4%) | 1.16 (1.08, 1.24) | ||
| ≥2 | 3,416 (4.8%) | 331 (6.9%) | 1.26 (1.12, 1.42) | 2,578 (4.8%) | 443 (6.5%) | 1.22 (1.09, 1.37) | ||
| Insurance | <0.0001 | <0.0001 | ||||||
| None | 1,856 (2.6%) | 160 (3.3%) | 1.00 | 1,640 (3.1%) | 212 (3.1%) | 1.00 | ||
| Private | 31,587 (44.7%) | 1,514 (31.5%) | 0.56 (0.47, 0.67) | 23,920 (44.9%) | 2,319 (33.8%) | 0.63 (0.54, 0.75) | ||
| Medicaid | 2,911 (4.1%) | 207 (4.3%) | 0.79 (0.64, 0.99) | 2,576 (4.8%) | 297 (4.3%) | 0.84 (0.69, 1.03) | ||
| Medicare | 32,571 (46.1%) | 2,857 (59.4%) | 0.67 (0.56, 0.80) | 23,868 (44.8%) | 3,919 (57.0%) | 0.84 (0.71, 1.00) | ||
| Income | 0.002 | 0.03 | ||||||
| <$48,000 | 27,004 (38.2%) | 1,996 (41.5%) | 1.00 | 21,179 (39.7%) | 2,847 (41.4%) | 1.00 | ||
| ≥$48,000 | 43,632 (61.8%) | 2,815 (58.5%) | 0.89 (0.83, 0.96) | 32,136 (60.3%) | 4,025 (58.6%) | 0.92 (0.86, 0.99) | ||
| Education (% without high school diploma) | 0.63 | 0.17 | ||||||
| ≥21% | 9,663 (13.7%) | 660 (13.7%) | 1.00 | 7,987 (15.0%) | 985 (14.3%) | 1.00 | ||
| 13% - 20.9% | 17,843 (25.3%) | 1,272 (26.4%) | 1.06 (0.96, 1.18) | 13,639 (25.6%) | 1,777 (25.9%) | 1.01 (0.92, 1.11) | ||
| 7% - 12.9% | 24,859 (35.2%) | 1,691 (35.2%) | 1.07 (0.96, 1.19) | 18,084 (33.9%) | 2,468 (35.9%) | 1.04 (0.94, 1.15) | ||
| < 7% | 18,271 (25.9%) | 1,188 (24.7%) | 1.08 (0.95, 1.21) | 13,605 (25.5%) | 1,642 (23.9%) | 0.95 (0.85, 1.07) | ||
| Facility Type | <0.0001 | <0.0001 | ||||||
| Community Cancer Program | 3,433 (4.9%) | 180 (3.7%) | 1.00 | 2,246 (4.2%) | 346 (5.0%) | 1.00 | ||
| Academic/Research Program | 29,606 (41.9%) | 2,036 (42.3%) | 1.44 (1.22, 1.69) | 24,500 (46.0%) | 2,661 (38.7%) | 0.74 (0.64, 0.84) | ||
| Comprehensive Community Cancer Program | 27,054 (38.3%) | 1,817 (37.8%) | 1.28 (1.09, 1.51) | 18,770 (35.2%) | 2,788 (40.6%) | 0.97 (0.85, 1.11) | ||
| Integrated Network Cancer Program | 10,543 (14.9%) | 778 (16.2%) | 1.45 (1.23, 1.73) | 7,799 (14.6%) | 1,077 (15.7%) | 0.93 (0.80, 1.07) | ||
| Facility Location | <0.0001 | <0.0001 | ||||||
| Northeast | 20,155 (28.5%) | 1,160 (24.1%) | 1.00 | 13,535 (25.4%) | 1,647 (24.0%) | 1.00 | ||
| South | 21,156 (30.0%) | 1,334 (27.7%) | 1.05 (0.96, 1.15) | 18,178 (34.1%) | 2,068 (30.1%) | 0.72 (0.66, 0.78) | ||
| Midwest | 20,497 (29.0%) | 1,573 (32.7%) | 1.31 (1.20, 1.42) | 14,583 (27.4%) | 2,129 (31.0%) | 1.02 (0.95, 1.11) | ||
| Mountain | 2,454 (3.5%) | 165 (3.4%) | 1.17 (0.98, 1.39) | 1,843 (3.5%) | 272 (4.0%) | 1.10 (0.95, 1.29) | ||
| Pacific | 6,374 (9.0%) | 579 (12.0%) | 1.64 (1.47, 1.82) | 5,176 (9.7%) | 756 (11.0%) | 1.08 (0.97, 1.19) | ||
| Histology | <0.0001 | <0.0001 | ||||||
| Low-grade endometrioid | 34,101 (48.3%) | 2,299 (47.8%) | 1.00 | 12,381 (23.2%) | 3,245 (47.2%) | 1.00 | ||
| High-grade endometrioid | 14,906 (21.1%) | 843 (17.5%) | 0.90 (0.83, 0.98) | 9,798 (18.4%) | 1,110 (16.2%) | 0.43 (0.40, 0.47) | ||
| Serous | 7,676 (10.9%) | 526 (10.9%) | 1.99 (1.79, 2.22) | 13,601 (25.5%) | 872 (12.7%) | 0.16 (0.14, 0.17) | ||
| Carcinosarcoma | 5,491 (7.8%) | 468 (9.7%) | 1.84 (1.64, 2.05) | 8,102 (15.2%) | 781 (11.4%) | 0.24 (0.22, 0.26) | ||
| Mixed epithelial | 6,715 (9.5%) | 530 (11.0%) | 1.73 (1.56, 1.92) | 7,266 (13.6%) | 636 (9.3%) | 0.25 (0.23, 0.28) | ||
| Clear cell | 1,747 (2.5%) | 145 (3.0%) | 1.67 (1.39, 2.00) | 2,167 (4.1%) | 228 (3.3%) | 0.27 (0.23, 0.31) | ||
| Stage | <0.0001 | <0.0001 | ||||||
| I | 39,220 (55.5%) | 2,938 (61.1%) | 1.00 | 16,189 (30.4%) | 4,253 (61.9%) | 1.00 | ||
| II | 10,532 (14.9%) | 524 (10.9%) | 0.68 (0.62, 0.75) | 3,686 (6.9%) | 613 (8.9%) | 0.79 (0.71, 0.87) | ||
| III | 18,474 (26.2%) | 1,173 (24.4%) | 1.64 (1.52, 1.78) | 23,628 (44.3%) | 1,593 (23.2%) | 0.22 (0.21, 0.24) | ||
| IV | 2,410 (3.4%) | 176 (3.7%) | 2.05 (1.73, 2.43) | 9,812 (18.4%) | 413 (6.0%) | 0.12 (0.11, 0.14) | ||
| Radiation | --- | <0.0001 | ||||||
| No | --- | --- | --- | 24,030 (45.1%) | 4,931 (71.8%) | 1.00 | ||
| Yes | --- | --- | --- | 27,561 (51.7%) | 1,828 (26.6%) | 0.26 (0.25, 0.28) | ||
| Chemotherapy | <0.0001 | --- | ||||||
| No | 41,913 (59.3%) | 4,081 (84.8%) | 1.00 | --- | --- | --- | ||
| Yes | 27,561 (39.0%) | 688 (14.3%) | 0.16 (0.15, 0.18) | --- | --- | --- | ||
Multivariable aOR adjusted for: race (White, Black), age (≤ 50, 50–69, ≥70), Charlson comorbidity score (0, 1, ≥2), insurance (None, Private, Medicaid, Medicare, Other Government, unknown), income (<$48,000 ≥$48,000), education (≥21%, 13%–20.9%, 7%–12.9%, <7%), facility type (Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, Integrated Network Cancer Program), facility location (Northeast, South, Midwest, Mountain, Pacific), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, mixed epithelial, clear cell), stage (I, II, III, IV), chemotherapy (no, yes, unknown)
Multivariable aOR adjusted for: race (White, Black), age (≤ 50, 50–69, ≥70), Charlson comorbidity score (0, 1, ≥2), insurance (None, Private, Medicaid, Medicare, Other Government, unknown), income (<$48,000 ≥$48,000), education (≥21%, 13%–20.9%, 7%–12.9%, <7%), facility type (Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, Integrated Network Cancer Program), facility location (Northeast, South, Midwest, Mountain, Pacific), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, mixed epithelial, clear cell), stage (I, II, III, IV), radiation (no, yes, unknown)
We used Kaplan-Meier curves and log-rank tests to evaluate the overall association between race and OS as well as the joint effect of race and refusal of adjuvant radiation or chemotherapy on OS. To quantify how much of the difference in survival by race might operate through different patterns of treatment refusal, we performed a causal mediation analysis (23). We applied the simulation-based structural equation modeling approach implemented in the R mediation package (24–26). Mediation analysis explicitly examines how a third intermediate variable, the mediator (i.e., treatment refusal), is related to the observed exposure-outcome (race-overall survival) relationship. Because the hazard ratio in a Cox proportional hazards model does not necessarily have a causal interpretation when the outcome is not rare (27, 28), we fit accelerated failure time (AFT) models assuming a Weibull error distribution, and our outcome of interest is mean survival time. Mediation analysis seeks to partition the total effect (here, the mean difference in survival time between Black and White women) into the average causal mediation effect (ACME) and the average direct effect (ADE). The ACME, which captures how much of the effect of race operates through the mediator (treatment refusal), is of central interest; elsewhere the terminology natural indirect effect or pure/total indirect effect is used (29, 30). The ACME is the difference in mean survival time that we expect if the distribution of race in our population remained the same, but the value of the mediator changed from what we expect it to be for each woman if her race was changed from White to Black. We model each woman’s likelihood of treatment refusal via logistic regression as a function of race and other covariates and use this model to estimate how a woman’s probability of treatment refusal changes if all variables were fixed except race, which is changed from White to Black. Thus, the ACME captures how much of the difference in mean survival time between Black and White women is attributable to racial differences in patterns of treatment refusal, adjusting for potential confounders. The ADE is the remaining effect of race on survival -- i.e., how much of the mean difference between Black and White women’s survival operates through pathways other than treatment refusal. Elsewhere the terminology natural direct effect or pure/total direct effect is used (29, 30).
Separately for chemotherapy and radiation, we estimated mediation effects overall and within categories defined by tumor histology. The mediator and outcome models were adjusted for the confounders listed in Table 1. All analyses were performed in SAS version 9.4 or R version 4.0.2. All p-values were two-sided.
RESULTS
Among those in our analysis recommended to receive radiation (n=75,447) or chemotherapy (n=60,187), 6.4% and 11.4% refused treatment, respectively. Table 1 shows the distribution of epidemiological, facility, and tumor characteristics by refusal of radiation or chemotherapy in the overall study population, along with multivariable-adjusted ORs and 95% CIs for associations with treatment refusal. Among Black and White women recommended for radiation, 6.4% refused in each race category. In the multivariable model, race was not associated with radiation refusal (aOR=1.00, 95% CI=0.91, 1.11). Among Black and White women recommended for chemotherapy, 9.6% and 11.8% refused, respectively. After adjustment for important potential confounders, Black women were in fact more likely than White women to refuse chemotherapy (aOR=1.26, 95% CI=1.15, 1.37). Older age at diagnosis was associated with higher odds of refusing radiation (≥70 vs <50, aOR= 2.33, 95% CI, 1.92, 2.82) and chemotherapy (≥70 vs <50, aOR= 3.59, 95% CI, 3.11, 4.14), while a higher number of comorbidities increased the odds of radiation and chemotherapy refusal by approximately 25%. Compared to treatment at a community cancer program, treatment at other facility types was associated with higher odds of radiation refusal (aOR range: 1.28–1.45), while treatment at an academic/research-designated facility was associated with lower odds of chemotherapy refusal (aOR=0.74, 95% CI=0.64, 0.84). Radiation refusal was higher in the Midwest (aOR=1.31, 95% CI=1.20, 1.42) and the Pacific region (aOR=1.64, 95% CI=1.47, 1.82) compared with the Northeast, while chemotherapy refusal was lower among those treated in the South (aOR=0.72, 95% CI=0.66, 0.78). Women diagnosed with high-grade endometrioid EC (aOR=0.90, 95% CI=0.83, 0.98) were less likely than women with low-grade endometrioid EC to refuse radiation, while women diagnosed with non-endometrioid subtypes were more likely to refuse radiation (aOR range: 1.67–1.99). Chemotherapy refusal was significantly lower among women diagnosed with high-grade endometrioid or non-endometrioid subtypes compared to women diagnosed with low-grade endometrioid (aOR range: 0.16–0.43). Finally, women with stage III or IV diagnoses were more likely to refuse radiation compared with stage I cases, while stage was inversely related with odds of chemotherapy refusal. Receipt of chemotherapy was inversely associated with radiation refusal (aOR=0.16, 95% CI=0.15, 0.18) and receipt of radiation was inversely associated with chemotherapy refusal (aOR=0.26, 95% CI=0.25, 0.28). Associations between race and adjuvant treatment refusal stratified by histology are shown in Supplemental Table 2. Among women diagnosed with serous tumors, Black women were more likely than White women to refuse radiation (OR=1.36, 95% CI=1.07, 1.72) and chemotherapy (OR=1.51, 95% CI=1.27, 1.81).
Table 2 shows predictors of radiation refusal stratified by race. Some divergent associations were noted between Black and White women, namely for income, education, histology, stage, and chemotherapy receipt (p-interactions<0.05). Zip code-level income was not associated with radiation refusal among Black women (aOR=0.98, 95% CI=0.77, 1.24), yet among White women, higher area-level income was significantly associated with lower odds of refusing radiation (aOR=0.88, 95% CI=0.81, 0.95). Significant racial differences between histology and radiation refusal were observed (p-interaction=0.01). Among both Black and White women, higher odds of radiation refusal were noted for women with non-endometrioid tumors compared to women diagnosed with low-grade endometrioid disease; however, the magnitude was higher among Black women. Conversely, for stage, we observed that Black and White women diagnosed with stages III or IV tumors were more likely than those with stage I tumors to refuse radiation, yet the magnitude was greater among White women. Finally, chemotherapy receipt was associated with lower odds of radiation refusal among Black (aOR=0.10, 95% CI=0.08, 0.13) and White women (aOR=0.17, 95% CI=0.16, 0.19).
Table 2.
Multivariable-adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for associations between epidemiological, facility, and tumor characteristics and refusal of adjuvant radiation by race/ethnicity
| Black (n=9,016) | White (n=66,431) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Received n=8,436 | Refused n=580 | Received n=62,200 | Refused n=4,231 | |||||
| n (%) | aOR (95% CI) | p | n (%) | aOR (95% CI) | p | p-int | |||
| Age at diagnosis | 0.001 | <0.0001 | 0.83 | ||||||
| <50 | 442 (5.2%) | 15 (2.6%) | 1.00 | 3,621 (5.8%) | 117 (2.8%) | 1.00 | |||
| 50–69 | 5,656 (67.1%) | 319 (55.0%) | 1.43 (0.83, 2.47) | 38,527 (61.9%) | 1,992 (47.1%) | 1.47 (1.21, 1.78) | |||
| ≥70 | 2,338 (27.7%) | 246 (42.4%) | 2.04 (1.16, 3.60) | 20,052 (32.2%) | 2,122 (50.2%) | 2.38 (1.94, 2.92) | |||
| Charlson Comorbidity Score | 0.78 | 0.0001 | 0.71 | ||||||
| 0 | 5,760 (68.3%) | 368 (63.5%) | 1.00 | 47,919 (77.0%) | 3,074 (72.7%) | 1.00 | |||
| 1 | 2,091 (24.8%) | 160 (27.6%) | 1.06 (0.86, 1.29) | 11,450 (18.4%) | 878 (20.8%) | 1.11 (1.02, 1.20) | |||
| ≥2 | 585 (6.9%) | 52 (9.0%) | 1.10 (0.80, 1.51) | 2,831 (4.6%) | 279 (6.6%) | 1.28 (1.13, 1.47) | |||
| Insurance | 0.01 | <0.0001 | 0.40 | ||||||
| None | 409 (4.9%) | 31 (5.3%) | 1.00 | 1,447 (2.3%) | 129 (3.1%) | 1.00 | |||
| Private | 3,202 (38.0%) | 163 (28.1%) | 0.65 (0.43, 0.99) | 28,385 (45.6%) | 1,351 (31.9%) | 0.54 (0.44, 0.65) | |||
| Medicaid | 722 (8.6%) | 48 (8.3%) | 0.83 (0.51, 1.35) | 2,189 (3.5%) | 159 (3.8%) | 0.79 (0.61, 1.01) | |||
| Medicare | 3,847 (45.6%) | 331 (57.1%) | 0.79 (0.52, 1.20) | 28,724 (46.2%) | 2,526 (59.7%) | 0.64 (0.53, 0.78) | |||
| Income | 0.83 | 0.001 | 0.01 | ||||||
| <$48,000 | 5,359 (63.5%) | 362 (62.4%) | 1.00 | 21,645 (34.8%) | 1,634 (38.6%) | 1.00 | |||
| ≥$48,000 | 3,077 (36.5%) | 218 (37.6%) | 0.98 (0.77, 1.24) | 40,555 (65.2%) | 2,597 (61.4%) | 0.88 (0.81, 0.95) | |||
| Education (% without high school diploma) | 0.33 | 0.94 | 0.05 | ||||||
| ≥21% | 2,837 (33.6%) | 177 (30.5%) | 1.00 | 6,826 (11.0%) | 483 (11.4%) | 1.00 | |||
| 13% - 20.9% | 3,134 (37.2%) | 219 (37.8%) | 1.09 (0.87, 1.36) | 14,709 (23.7%) | 1,053 (24.9%) | 1.04 (0.92, 1.16) | |||
| 7% - 12.9% | 1,779 (21.1%) | 128 (22.1%) | 1.22 (0.91, 1.63) | 23,080 (37.1%) | 1,563 (36.9%) | 1.03 (0.91, 1.16) | |||
| < 7% | 686 (8.1%) | 56 (9.7%) | 1.42 (0.96, 2.10) | 17,585 (28.3%) | 1,132 (26.8%) | 1.04 (0.91, 1.18) | |||
| Facility Type | 0.003 | 0.0001 | 0.11 | ||||||
| Community Cancer Program | 290 (3.4%) | 9 (1.6%) | 1.00 | 3,143 (5.1%) | 171 (4.0%) | 1.00 | |||
| Comprehensive Community Cancer Program | 2,404 (28.5%) | 142 (24.5%) | 2.10 (1.04, 4.23) | 24,650 (39.6%) | 1,675 (39.6%) | 1.25 (1.06, 1.47) | |||
| Academic/Research Program | 4,491 (53.2%) | 332 (57.2%) | 2.81 (1.41, 5.58) | 25,115 (40.4%) | 1,704 (40.3%) | 1.37 (1.16, 1.62) | |||
| Integrated Network Cancer Program | 1,251 (14.8%) | 97 (16.7%) | 2.59 (1.27, 5.27) | 9,292 (14.9%) | 681 (16.1%) | 1.40 (1.17, 1.67) | |||
| Facility Location | 0.17 | <0.0001 | 0.77 | ||||||
| Northeast | 2,044 (24.2%) | 129 (22.2%) | 1.00 | 18,111 (29.1%) | 1,031 (24.4%) | 1.00 | |||
| South | 4,369 (51.8%) | 290 (50.0%) | 1.00 (0.79, 1.27) | 16,787 (27.0%) | 1,044 (24.7%) | 1.05 (0.96, 1.16) | |||
| Midwest | 1,611 (19.1%) | 126 (21.7%) | 1.18 (0.90, 1.56) | 18,886 (30.4%) | 1,447 (34.2%) | 1.31 (1.21, 1.43) | |||
| Mountain | 42 (0.5%) | 2 (0.3%) | 0.73 (0.16, 3.27) | 2,412 (3.9%) | 163 (3.9%) | 1.18 (0.99, 1.40) | |||
| Pacific | 370 (4.4%) | 33 (5.7%) | 1.54 (1.00, 2.35) | 6,004 (9.7%) | 546 (12.9%) | 1.64 (1.46, 1.83) | |||
| Histology | <0.0001 | <0.0001 | 0.01 | ||||||
| Low-grade endometrioid | 2,199 (26.1%) | 124 (21.4%) | 1.00 | 31,902 (51.3%) | 2,175 (51.4%) | 1.00 | |||
| High-grade endometrioid | 1,797 (21.3%) | 92 (15.9%) | 1.05 (0.79, 1.40) | 13,109 (21.1%) | 751 (17.8%) | 0.90 (0.82, 0.98) | |||
| Serous | 1,831 (21.7%) | 148 (25.5%) | 3.53 (2.69, 4.64) | 5,845 (9.4%) | 378 (8.9%) | 1.78 (1.57, 2.01) | |||
| Carcinosarcoma | 1,463 (17.3%) | 115 (19.8%) | 2.25 (1.70, 2.97) | 4,028 (6.5%) | 353 (8.3%) | 1.85 (1.63, 2.10) | |||
| Mixed epithelial | 844 (10.0%) | 72 (12.4%) | 2.93 (2.13, 4.03) | 5,871 (9.4%) | 458 (10.8%) | 1.64 (1.47, 1.83) | |||
| Clear cell | 302 (3.6%) | 29 (5.0%) | 2.41 (1.55, 3.76) | 1,445 (2.3%) | 116 (2.7%) | 1.59 (1.30, 1.94) | |||
| Stage | <0.0001 | <0.0001 | 0.05 | ||||||
| I | 4,262 (50.5%) | 327 (56.4%) | 1.00 | 34,958 (56.2%) | 2,611 (61.7%) | 1.00 | |||
| II | 1,336 (15.8%) | 73 (12.6%) | 0.73 (0.55, 0.95) | 9,196 (14.8%) | 451 (10.7%) | 0.68 (0.61, 0.75) | |||
| III | 2,471 (29.3%) | 150 (25.9%) | 1.37 (1.10, 1.71) | 16,003 (25.7%) | 1,023 (24.2%) | 1.67 (1.54, 1.82) | |||
| IV | 367 (4.4%) | 30 (5.2%) | 1.81 (1.18, 2.76) | 2,043 (3.3%) | 146 (3.5%) | 2.07 (1.72, 2.49) | |||
| Chemotherapy | <0.0001 | <0.0001 | 0.001 | ||||||
| No | 4,031 (47.8%) | 487 (84.0%) | 1.00 | 37,882 (60.9%) | 3,594 (84.9%) | 1.00 | |||
| Yes | 4,243 (50.3%) | 90 (15.5%) | 0.10 (0.08, 0.13) | 23,318 (37.5%) | 598 (14.1%) | 0.17 (0.16, 0.19) | |||
Multivariable aOR adjusted for: age (≤ 50, 50–69, ≥70), Charlson comorbidity score (0, 1, ≥2), insurance (None, Private, Medicaid, Medicare, Other Government, unknown), income (<$48,000 ≥$48,000), education (≥21%, 13%–20.9%, 7%–12.9%, <7%), facility type (Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, Integrated Network Cancer Program), facility location (Northeast, South, Midwest, Mountain, Pacific), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, mixed epithelial, clear cell), stage (I, II, III, IV), chemotherapy (no, yes, unknown)
Table 3 shows predictors of chemotherapy refusal stratified by race. Most predictors for chemotherapy refusal were similar between Black and White women; however, histology and stage showed significant interactions (p-interaction<0.05). Among both Black and White women, lower odds of chemotherapy refusal were noted for women with non-endometrioid tumors compared to women diagnosed with low-grade endometrioid disease. We also noted that stage was inversely associated with chemotherapy refusal among Black and White women. Although other significant interactions were not detected, we did observe that all forms of insurance were associated with lower odds of chemotherapy refusal among White women (aOR range: 0.61–0.81) while insurance status was unrelated to chemotherapy refusal among Black women. In addition, zip code-level income was not associated with chemotherapy refusal among Black women (aOR=0.99, 95% CI=0.81, 1.20), yet among White women, living in areas of higher income was significantly associated with lower odds of refusing chemotherapy (aOR=0.91, 95% CI=0.85, 0.99).
Table 3.
Multivariable-adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for associations between epidemiological, facility, and tumor characteristics and refusal of adjuvant chemotherapy by race/ethnicity
| Black (n=10,093) | White (n=50,094) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Received n=9,128 | Refused n=965 | Received n=44,187 | Refused n=5,907 | |||||
| n (%) | aOR (95% CI) | P | n (%) | aOR (95% CI) | p | p-int | |||
| Age at diagnosis | <0.0001 | <0.0001 | 0.32 | ||||||
| <50 | 408 (4.5%) | 31 (3.2%) | 1.00 | 3,020 (6.8%) | 306 (5.2%) | 1.00 | |||
| 50–69 | 6,365 (69.7%) | 524 (54.3%) | 1.93 (1.28, 2.91) | 28,020 (63.4%) | 2,834 (48.0%) | 1.53 (1.33, 1.75) | |||
| ≥70 | 2,355 (25.8%) | 410 (42.5%) | 4.27 (2.77, 6.58) | 13,147 (29.8%) | 2,767 (46.8%) | 3.53 (3.03, 4.12) | |||
| Charlson Comorbidity Score | 0.01 | <0.0001 | 0.19 | ||||||
| 0 | 6,313 (69.2%) | 600 (62.2%) | 1.00 | 34,269 (77.6%) | 4,292 (72.7%) | 1.00 | |||
| 1 | 2,199 (24.1%) | 287 (29.7%) | 1.28 (1.09, 1.50) | 7,956 (18.0%) | 1,250 (21.2%) | 1.13 (1.05, 1.22) | |||
| ≥2 | 616 (6.8%) | 78 (8.1%) | 1.14 (0.88, 1.49) | 1,962 (4.4%) | 365 (6.2%) | 1.25 (1.09, 1.42) | |||
| Insurance | 0.01 | <0.0001 | 0.75 | ||||||
| None | 473 (5.2%) | 43 (4.5%) | 1.00 | 1,167 (2.6%) | 169 (2.9%) | 1.00 | |||
| Private | 3,456 (37.9%) | 271 (28.1%) | 0.77 (0.54, 1.09) | 20,474 (46.3%) | 2,048 (34.7%) | 0.61 (0.50, 0.73) | |||
| Medicaid | 831 (9.1%) | 88 (9.1%) | 1.05 (0.70, 1.58) | 1,745 (4.0%) | 209 (3.5%) | 0.79 (0.62, 1.00) | |||
| Medicare | 4,110 (45.0%) | 547 (56.7%) | 0.99 (0.70, 1.41) | 19,758 (44.7%) | 3,372 (57.1%) | 0.81 (0.67, 0.98) | |||
| Income | 0.89 | 0.02 | 0.18 | ||||||
| <$48,000 | 5,709 (62.5%) | 616 (63.8%) | 1.00 | 15,470 (35.0%) | 2,231 (37.8%) | 1.00 | |||
| ≥$48,000 | 3,419 (37.5%) | 349 (36.2%) | 0.99 (0.81, 1.20) | 28,717 (65.0%) | 3,676 (62.2%) | 0.91 (0.85, 0.99) | |||
| Education (% without high school diploma) | 0.59 | 0.10 | 0.11 | ||||||
| ≥21% | 3,046 (33.4%) | 321 (33.3%) | 1.00 | 4,941 (11.2%) | 664 (11.2%) | 1.00 | |||
| 13% - 20.9% | 3,341 (36.6%) | 353 (36.6%) | 1.01 (0.85, 1.21) | 10,298 (23.3%) | 1,424 (24.1%) | 1.00 (0.89, 1.11) | |||
| 7% - 12.9% | 1,938 (21.2%) | 197 (20.4%) | 1.02 (0.80, 1.28) | 16,146 (36.5%) | 2,271 (38.5%) | 1.02 (0.92, 1.15) | |||
| < 7% | 803 (8.8%) | 94 (9.7%) | 1.22 (0.89, 1.66) | 12,802 (29.0%) | 1,548 (26.2%) | 0.93 (0.82, 1.05) | |||
| Facility Type | 0.05 | <0.0001 | 0.13 | ||||||
| Community Cancer Program | 266 (2.9%) | 34 (3.5%) | 1.00 | 1,980 (4.5%) | 312 (5.3%) | 1.00 | |||
| Comprehensive Community Cancer Program | 2,432 (26.6%) | 255 (26.4%) | 0.82 (0.54, 1.23) | 16,338 (37.0%) | 2,533 (42.9%) | 1.00 (0.86, 1.15) | |||
| Academic/Research Program | 5,120 (56.1%) | 518 (53.7%) | 0.75 (0.50, 1.12) | 19,380 (43.9%) | 2,143 (36.3%) | 0.74 (0.64, 0.85) | |||
| Integrated Network Cancer Program | 1,310 (14.4%) | 158 (16.4%) | 0.98 (0.64, 1.50) | 6,489 (14.7%) | 919 (15.6%) | 0.92 (0.78, 1.07) | |||
| Facility Location | 0.001 | <0.0001 | 0.30 | ||||||
| Northeast | 2,136 (23.4%) | 208 (21.6%) | 1.00 | 11,399 (25.8%) | 1,439 (24.4%) | 1.00 | |||
| South | 4,856 (53.2%) | 482 (50.0%) | 0.80 (0.66, 0.96) | 13,322 (30.2%) | 1,586 (26.9%) | 0.70 (0.64, 0.77) | |||
| Midwest | 1,633 (17.9%) | 211 (21.9%) | 1.09 (0.87, 1.36) | 12,950 (29.3%) | 1,918 (32.5%) | 1.02 (0.94, 1.11) | |||
| Mountain | 60 (0.7%) | 14 (1.5%) | 2.08 (1.07, 4.05) | 1,783 (4.0%) | 258 (4.4%) | 1.10 (0.91, 1.25) | |||
| Pacific | 443 (4.9%) | 50 (5.2%) | 1.00 (0.70, 1.41) | 4,733 (10.7%) | 706 (12.0%) | 1.09 (0.97, 1.21) | |||
| Histology | <0.0001 | <0.0001 | 0.05 | ||||||
| Low-grade endometrioid | 771 (8.5%) | 202 (20.9%) | 1.00 | 11,610 (26.3%) | 3,043 (51.5%) | 1.00 | |||
| High-grade endometrioid | 1,352 (14.8%) | 150 (15.5%) | 0.40 (0.31, 0.51) | 8,446 (19.1%) | 960 (16.3%) | 0.44 (0.41, 0.48) | |||
| Serous | 3,298 (36.1%) | 247 (25.6%) | 0.20 (0.16, 0.26) | 10,303 (23.3%) | 625 (10.6%) | 0.15 (0.13, 0.16) | |||
| Carcinosarcoma | 2,200 (24.1%) | 219 (22.7%) | 0.28 (0.22, 0.35) | 5,902 (13.4%) | 562 (9.5%) | 0.23 (0.21, 0.26) | |||
| Mixed epithelial | 1,109 (12.2%) | 99 (10.3%) | 0.27 (0.20, 0.35) | 6,157 (13.9%) | 537 (9.1%) | 0.25 (0.22, 0.28) | |||
| Clear cell | 398 (4.4%) | 48 (5.0%) | 0.34 (0.24, 0.48) | 1,769 (4.0%) | 180 (3.1%) | 0.26 (0.22, 0.30) | |||
| Stage | <0.0001 | <0.0001 | <0.0001 | ||||||
| I | 2,846 (31.2%) | 509 (52.8%) | 1.00 | 13,343 (30.2%) | 3,744 (63.4%) | 1.00 | |||
| II | 728 (8.0%) | 108 (11.2%) | 0.93 (0.73, 1.18) | 2,958 (6.7%) | 505 (8.6%) | 0.77 (0.69, 0.86) | |||
| III | 3,630 (39.8%) | 259 (26.8%) | 0.35 (0.30, 0.41) | 19,998 (45.3%) | 1,334 (22.6%) | 0.20 (0.19, 0.22) | |||
| IV | 1,924 (21.1%) | 89 (9.2%) | 0.17 (0.13, 0.22) | 7,888 (17.9%) | 324 (5.5%) | 0.11 (0.10, 0.13) | |||
| Radiation | <0.0001 | <0.0001 | 0.41 | ||||||
| No | 4,549 (49.8%) | 691 (71.6%) | 1.00 | 19,481 (44.1%) | 4,240 (71.8%) | 1.00 | |||
| Yes | 4,243 (46.5%) | 260 (26.9%) | 0.30 (0.25, 0.35) | 23,318 (52.8%) | 1,568 (26.5%) | 0.26 (0.24, 0.28) | |||
Multivariable aOR adjusted for: age (≤ 50, 50–69, ≥70), Charlson comorbidity score (0, 1, ≥2), insurance (None, Private, Medicaid, Medicare, Other Government, unknown), income (<$48,000 ≥$48,000), education (≥21%, 13%–20.9%, 7%–12.9%, <7%), facility type (Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, Integrated Network Cancer Program), facility location (Northeast, South, Midwest, Mountain, Pacific), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, mixed epithelial, clear cell), stage (I, II, III, IV), radiation (no, yes, unknown)
Among women recommended to receive radiation or chemotherapy, Black women had significantly worse OS than White women (Figures 2A and 2B). The joint effect of race and radiation refusal is shown in Figure 3. In this unadjusted comparison, Black women who refused radiation had significantly worse OS compared with all other groups (log-rank p<0.0001). Median OS for Black and White patients who received radiation was 108.3 months (95% CI =103.0, 115.4) and 161.7 months (95% CI=158.8, 165.6), respectively while for Black and White patients who refused radiation, median OS was 66.9 months (95% CI 55.7, 77.2) and 108.1 months (95% CI= 99.3, 115.8), respectively. Similar observations were noted for chemotherapy, where Black women who refused chemotherapy had significantly worse OS than all other groups (log-rank p<0.0001, Figure 4). Median OS for Black and White patients who received chemotherapy was 51.0 months (95% CI=48.6, 53.5) and 107.7 months (95% CI=104.6, 111.2), respectively while for Black and White patients who refused chemotherapy, median OS was 41.3 months (95% CI 36.5, 47.1) and 73.9 months (95% CI=69.5, 78.4), respectively.
Figure 2.
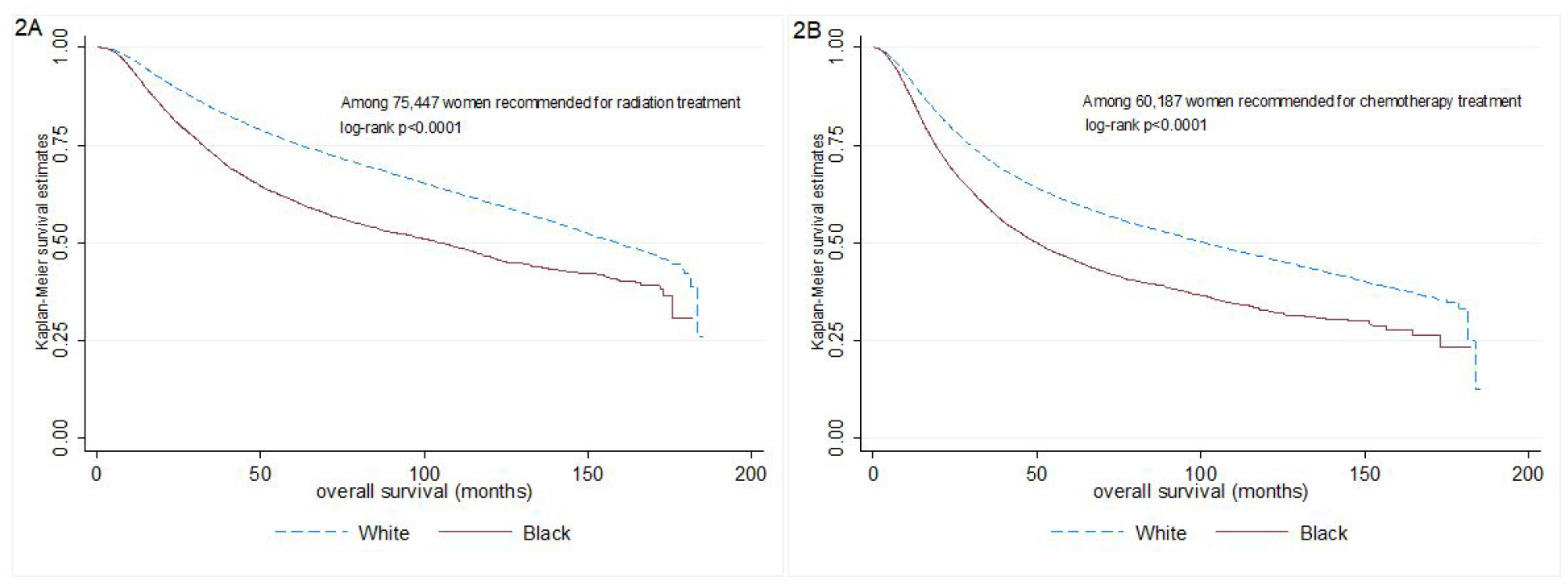
Kaplan-Meier overall survival curves according to race among a.) women recommended to receive radiation (n=75,447) and b.) women recommended to receive chemotherapy (n=60,187)
Figure 3.
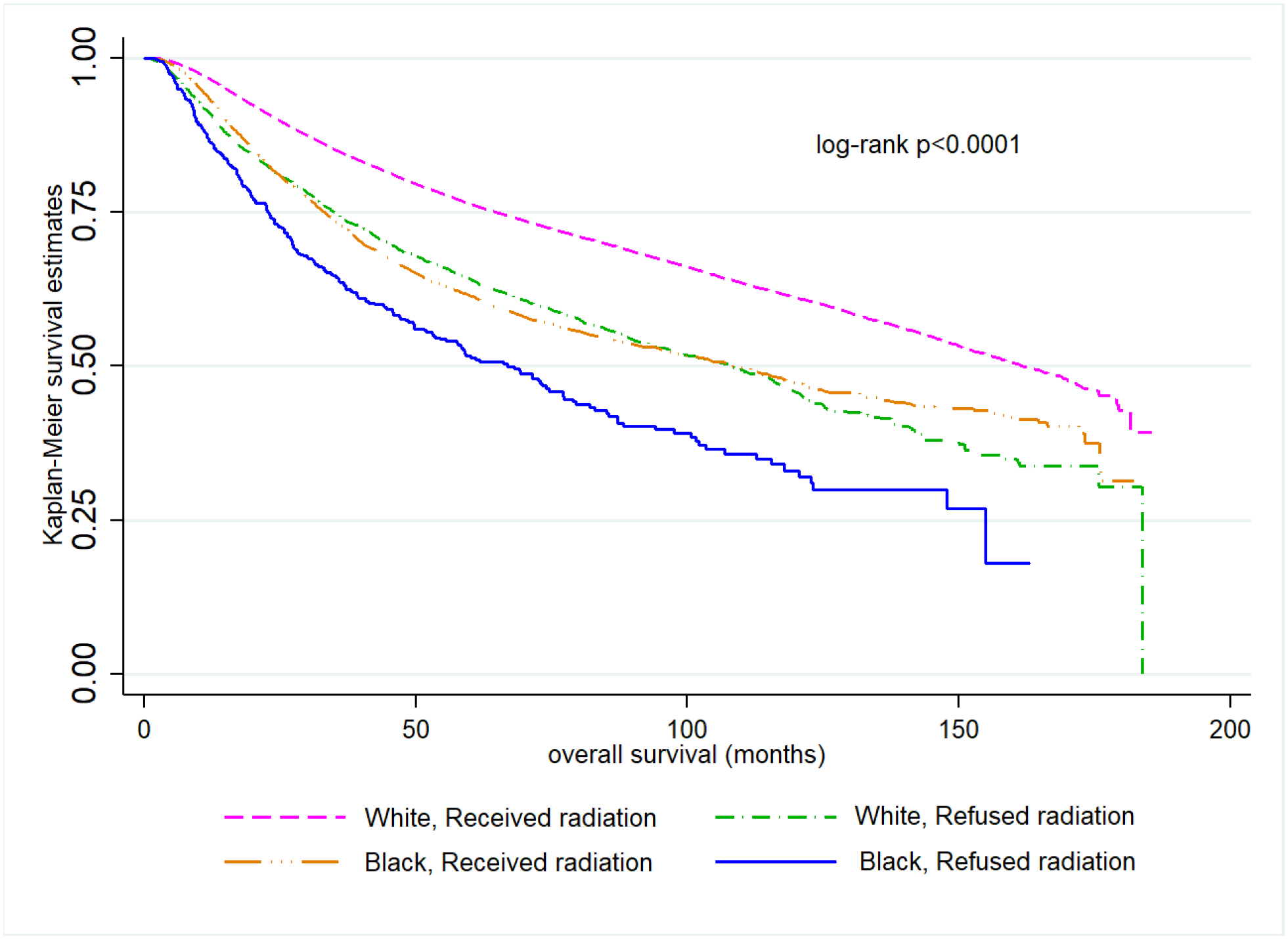
Kaplan–Meier overall survival curves comparing Black and White women who either received or refused radiation (n=75,447)
Figure 4.
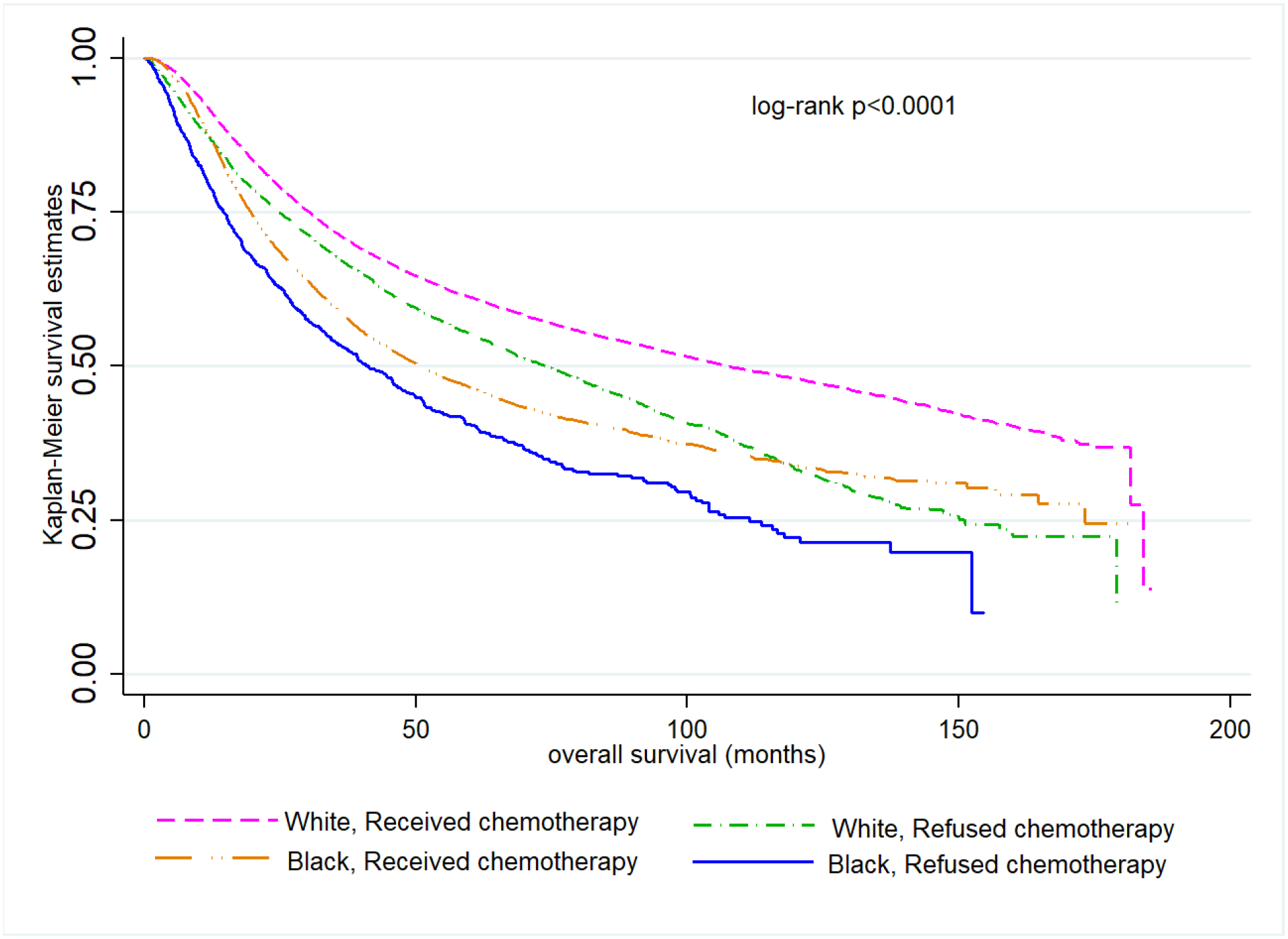
Kaplan–Meier overall survival curves comparing Black and White women who either received or refused chemotherapy (n=60,187)
Mediation of the race-OS relationship by refusal of radiation or chemotherapy is shown in Table 4. In the overall study population recommended for radiation, mean OS for Black women was 51.3 months shorter than for White women. Racial differences in treatment refusal did not explain any of this disparity (average causal mediation effect 0.01 months; 95% CI= −0.35, 0.32; percentage mediated=0.0%), which is unsurprising because we did not observe racial differences overall in refusal of radiation. Results were similar in the histology-stratified models, except among serous tumors where there was a marginally significant association: among women with serous EC, Black women had 15.86 months (95% CI= −26.12, −5.33) shorter mean OS with 0.3 months (95% CI= −0.75, 0.00; proportion mediated=1.8%) potentially attributable to radiation refusal differences.
Table 4.
Accelerated failure time models for associations between race and overall survival with mediation by adjuvant treatment refusal in the overall study population and stratified by histologic subtype
| Treatment | Histology | Overall N | % Black | % White | Mean Overall Survival Difference (95% CI) Black vs. White (Months) | Average Causal Mediation Effect (95% CI) of Treatment refusal (Months) | Percentage Mediated | Mediation p-value |
|---|---|---|---|---|---|---|---|---|
| Women recommended for radiation | All Patients | 75,447 | 12.0% | 88.0% | −51.27 (−58.86, −43.63) | −0.01 (−0.35, 0.32) | 0.00% | 0.96 |
| Low-grade endometrioid | 36,400 | 6.4% | 93.6% | −38.76 (−55.01, −21.42) | 0.38 (−0.14, 0.98) | −0.90% | 0.14 | |
| High-grade endometrioid | 15,749 | 12.0% | 88.0% | −51.10 (−64.18, −37.78) | 0.14 (−0.40, 0.69) | −0.30% | 0.58 | |
| Serous | 8,202 | 24.1% | 75.9% | −15.86 (−26.12, −5.33) | −0.30 (−0.75, −0.00) | 1.80% | 0.05 | |
| Carcinosarcoma | 5,959 | 26.5% | 73.5% | −31.91 (−40.89, −22.80) | 0.26 (−0.33, 0.86) | −0.80% | 0.38 | |
| Mixed Epithelial | 7,245 | 12.6% | 87.4% | −88.61 (−108.56, −69.49) | −0.30 (−1.30, 0.54) | 0.30% | 0.51 | |
| Clear Cell | 1,892 | 17.5% | 82.5% | −11.06 (−46.84, 27.97) | −0.84 (−3.45, 0.81) | 1.60% | 0.35 | |
| Women recommended for chemotherapy | All Patients | 60,187 | 16.8% | 83.2% | −38.56 (−43.05, −34.00) | −1.90 (−2.76, −1.09) | 4.90% | <0.001 |
| Low-grade endometrioid | 15,626 | 6.2% | 93.8% | −38.33 (−61.79, −13.86) | −1.96 (−4.49, 0.31) | 4.90% | 0.09 | |
| High-grade endometrioid | 10,908 | 13.8% | 86.2% | −44.50 (−54.67, −34.30) | −0.48 (−2.23, 1.12) | 1.10% | 0.58 | |
| Serous | 14,473 | 24.5% | 75.5% | −19.08 (−24.63, −13.39) | −1.31 (−2.04, −0.63) | 6.80% | <0.001 | |
| Carcinosarcoma | 8,883 | 27.2% | 72.8% | −22.69 (−28.20, −17.14) | −0.58 (−1.40, 0.18) | 2.50% | 0.14 | |
| Mixed Epithelial | 7,902 | 15.3% | 84.7% | −71.37 (−84.60, −58.00) | −0.47 (−1.75, 0.66) | 0.60% | 0.44 | |
| Clear Cell | 2,395 | 18.6% | 81.4% | −32.96 (−53.02, −12.81) | −2.07 (−5.07, 0.24) | 5.90% | 0.08 |
Accelerated failure time models and treatment refusal (logistic regression) models are adjusted for: age (≤ 50, 50–69, ≥70), Charlson comorbidity score (0, 1, ≥2), insurance (None, Private, Medicaid, Medicare/Other Government,unknown ), income (<$48,000 ≥$48,000), education (≥21%, 13%–20.9%, 7%–12.9%, <7%), facility type (Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, Integrated Network Cancer Program), facility location (Northeast, South, Midwest, Mountain, Pacific), histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, mixed epithelial, clear cell), stage (I, II, III, IV), and whether also treated with chemotherapy (yes, no, or unknown) in the radiation analysis or whether also treated with radiation (yes, no, or unknown) in the chemotherapy analysis.
In the overall study population recommended for chemotherapy, mean OS for Black women was 38.56 (95% CI= −43.05, −34.00) months shorter than for White women. Of this disparity, 1.9 months (95% CI= −2.76, −1.09; percentage mediated=4.9%) of OS may be attributable to racial differences in chemotherapy refusal patterns (p<0.001). In the histology-stratified models, significant mediation was observed among serous tumors: among women with serous EC recommended for chemotherapy, Black women had 19.08 months (95% CI= −24.63, −13.39) shorter mean OS, with 1.31 months (95% CI= −2.04, −0.63; percentage mediated=6.8%) potentially attributable to chemotherapy refusal differences (p<0.001).
COMMENT
Principal findings
In this large hospital-based cancer registry study of women with EC, we examined racial differences in refusal of adjuvant treatment and the extent to which treatment refusal mediated OS differences between Black and White women. No difference in radiation refusal was detected; however, Black women were significantly more likely than White women to refuse chemotherapy in multivariable-adjusted models. Our causal mediation analysis demonstrated that refusal of radiation and chemotherapy explained at most small proportions of the race-OS disparity. As hypothesized, the proportion of the race-OS relationship mediated by treatment refusal varied by histologic subtype, with radiation and chemotherapy refusal mediating a significantly higher portion of the race-OS relationship among women diagnosed with serous EC tumors. Our race-stratified logistic regression analyses revealed similar predictors of chemotherapy refusal but distinct patterns of radiation refusal between Black and White women. To our knowledge, this is the first study to examine the role of treatment refusal as a mediator of racial disparities in survival among women with EC, and our results suggest that chemotherapy refusal is a significant, albeit small contributor to disparate survival.
Results in the context of what is known and clinical implications
In this analysis, we examined the role of clinician-documented reports of radiation or chemotherapy refusal in mediating racial disparities in EC survival. The body of literature focused on racial disparities in refusal of cancer treatment suggests that Black patients are more likely to refuse recommended adjuvant therapy (15, 19). For example, using data from the Surveillance, Epidemiology, End Results (SEER) Program, Aizer et al. (19) reported that non-white race was associated with refusal of recommended radiation among individuals diagnosed with eight common cancer types (including uterine). Similarly, Hamadi and colleagues (15) evaluated radiation refusal for 12 different types of non-metastatic cancer (including uterine) and found that Black patients had a 35% higher odds of radiation refusal compared to White patients. Further, radiation refusal was associated with a 40% higher risk of all-cause death.
Our results related to radiation refusal are similar to a prior study conducted in the NCDB. Among 80,000 women diagnosed with EC between 2004 and 2015, Parsons et al. also observed no Black-White differences in radiation refusal in the overall study population. Moreover, factors reflecting socioeconomic barriers, including non-private insurance and living in zip codes with a higher proportion of low income individuals, were associated with radiation refusal, as were advanced stage disease and aggressive histology - findings similar to the present analysis (20). In a departure from Parsons et al., we characterized predictors of radiation refusal according to race and noted that area-level income and insurance status were less relevant predictors of radiation refusal for Black as compared with White women. This observation is in line with the theory of diminished returns, which posits that material resources (insurance, income, education) that should minimize negative health consequences (in this case, treatment refusal) are absent for Black Americans but present for White Americans (31). Specifically, for similar levels of these material resources, countervailing effects of other negative factors, such as systemic racism, lower quality of care, etc., make these resources less protective for Black women. Further, we identified that aggressive tumor histology was associated with higher odds of radiation refusal among both Black and White women, yet the magnitude was greater among Black women. We suspect that when clinicians recommend both radiation and chemotherapy for women with non-endometrioid subtypes, the strongest recommendation would likely be for chemotherapy due to the increased risk of recurrence and distant metastases for high-risk histologies. If patients tolerate chemotherapy poorly, they may be less willing to undergo additional consolidation radiation, even if recommended. However, as prior randomized controlled trials have not shown a survival benefit of multimodal adjuvant treatment for EC, it is not surprising that the observed racial disparity is not mediated by refusal of recommended radiation (32–34).
Unlike the prior study, we also examined facility characteristics as predictors of radiation refusal, and we observed that treatment at facilities designated as comprehensive community cancer programs, academic/research programs, or integrated network cancer programs compared with treatment at community cancer programs were associated with higher odds of radiation refusal among Black and White women. This is a potentially counterintuitive finding as we typically observe superior treatment delivery and outcomes in academic centers and comprehensive cancer centers (35, 36). It is possible that in academic/research settings, comprehensive cancer centers, or integrated network cancer programs, providers do a more thorough job of describing the potential downsides of recommended therapies, leading to greater refusal. Additional research to explain this result is warranted. Moreover, among White women, treatment at facilities located in the Midwest or Pacific as compared to the Northeast was associated with higher odds of radiation refusal. These patterns may speak to structural barriers associated with receipt of care at these facility types, regional differences in provider recommendations, or large travel distance to facilities. The specific mechanisms underlying these associations should be explored in future studies.
Our observation that Black women with EC had 26% higher odds of refusing chemotherapy compared to White women is a novel finding. The evaluation of factors associated with chemotherapy refusal among Black and White women identified many similarities: older age at diagnosis was associated with higher odds of refusal while advanced stage, aggressive histologic subtypes, and undergoing radiation treatment were associated with higher odds of receiving recommended chemotherapy among both groups of women. Chemotherapy is a mainstay for women diagnosed with advanced stage disease or non-endometrioid histology. Neither zip code level income nor insurance status were associated with chemotherapy refusal among Black women, yet these factors were protective among White women, again implicating unequal gain of similar resources (31).
In EC, unequal treatment is a known contributor to racial disparities in outcomes. One narrative used to explain racial differences in receipt of cancer-related treatment is that medical mistrust among Black individuals prompts greater treatment refusal, which in turn has negative impacts on survival. While we did indeed observe higher odds of chemotherapy refusal among Black women, our causal mediation analyses revealed that in the overall study population, chemotherapy refusal mediated only 4.9% of survival disparities between Black and White women. This proportion varied somewhat by histologic subtype: among women with serous tumors, an aggressive subtype more commonly diagnosed among Black women, 6.8% of survival disparities between Black and White women may be attributable to chemotherapy refusal. Moreover, while radiation refusal did not differ between Black and White women with EC overall, this was a marginally significant mediator of survival disparities in the subgroup of women with serous tumors, potentially explaining 1.8% of the difference. Given the poor survival associated with serous EC and the consistently higher likelihood of development among Black women, an urgent need exists to eliminate barriers to receipt of high-quality cancer care as the stakes of not receiving adequate treatment may be even higher among Black women as compared with other groups. However, although reducing treatment refusal will undoubtedly improve outcomes, our data do not support the narrative that treatment refusal among Black women is a large contributor to the disparity in mortality.
Research implications
Literature exploring reasons underlying refusal of cancer treatment generally have identified factors such as concern about side effects, financial concerns, transportation issues, and negative treatment experiences of friends and family - many of these constitute modifiable barriers, presenting an opportunity to improve outcomes and reduce treatment barriers. Qualitative work in this area is clearly needed. Indeed, no qualitative studies examine the treatment decision-making process among women with EC.
Strengths and limitations
Several limitations of this study should be noted. First, this analysis was based on the documentation of clinicians, which may be affected by reporting bias. We also lacked information on the specific reason for refusal of adjuvant treatment. Treatment refusal is likely a catch-all for various scenarios, which have distinct etiologies and potential solutions. At one end of the spectrum, women may refuse to engage in recommended treatment due to feeling anxious and overwhelmed, which may cause them to disengage entirely from the treatment process. In the middle of the spectrum are those women who want to engage in the recommended treatments but lack the resources to attend frequent treatment visits, and thus may be erroneously labelled as refusing treatment. At the far end of the spectrum are those women who have fully engaged in the treatment decision-making process and have decided to forego treatment based on preferences (e.g., prioritization of perceived quality of life vs. quantity). Qualitative reports from the patient and provider perspective will be key in understanding the complex etiologies of the EC treatment decision-making process. Other limitations include our inability to report disease-specific survival, lack of information on additional potential confounders, and lack of individual-level data for education and income. Strengths of our study include the large sample size of women with EC, particularly Black women, investigation of how patient characteristics relate to treatment refusal differently for White and Black women, and a statistically rigorous mediation approach that estimates mediation in the counterfactual framework when the outcome is not rare.
Conclusions
Black women with EC are more likely than White women to refuse adjuvant chemotherapy. This treatment refusal may partially contribute to dramatic survival disparities observed between these two groups. Efforts to further elucidate the reasons for refusal could lead to improved provider understanding of patient decision making and may allow physicians and the health care system to improve the effective uptake of crucial recommendations.
Supplementary Material
AJOG at a Glance.
A. Why was this study conducted?
Black women with endometrial cancer have worse survival than White women. We assessed the hypotheses that Black women are more likely than White women to refuse adjuvant treatment and that treatment refusal mediates a portion of the survival disparity among women with endometrial cancer.
B. What are the key findings?
Black women with endometrial cancer were more likely than White women to refuse recommended chemotherapy but not radiation. Of the substantial disparities in overall survival between Black and White women, only a small proportion were attributable to differences in chemotherapy refusal.
C. What does this study add to what is already known?
Although Black women with endometrial cancer are more likely than White women to refuse adjuvant chemotherapy, this explains only a small fraction of racial disparities in endometrial cancer survival.
Financial Support:
This work was supported by the National Cancer Institute (K01CA21845701A1 to ASF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no relevant conflicts of interest.
REFERENCES
- 1.Islami F, Ward EM, Sung H, Cronin KA, Tangka FKL, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J Natl Cancer Inst 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol Biomarkers Prev 2015;24(9):1407–15. [DOI] [PubMed] [Google Scholar]
- 3.Ruterbusch JJ, Ali-Fehmi R, Olson SH, Sealy-Jefferson S, Rybicki BA, Hensley-Alford S, et al. The influence of comorbid conditions on racial disparities in endometrial cancer survival. Am J Obstet Gynecol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol 2003;21(22):4200–6. [DOI] [PubMed] [Google Scholar]
- 5.Sud S, Holmes J, Eblan M, Chen R, Jones E. Clinical characteristics associated with racial disparities in endometrial cancer outcomes: A surveillance, epidemiology and end results analysis. Gynecol Oncol 2018;148(2):349–356. [DOI] [PubMed] [Google Scholar]
- 6.Rauh-Hain JA, Buskwofie A, Clemmer J, Boruta DM, Schorge JO, del Carmen MG. Racial disparities in treatment of high-grade endometrial cancer in the Medicare population. Obstet Gynecol 2015;125(4):843–51. [DOI] [PubMed] [Google Scholar]
- 7.Elshaikh MA, Munkarah AR, Robbins JR, Laser BS, Bhatt N, Cogan C, et al. The impact of race on outcomes of patients with early stage uterine endometrioid carcinoma. Gynecol Oncol 2013;128(2):171–4. [DOI] [PubMed] [Google Scholar]
- 8.Liu JR, Conaway M, Rodriguez GC, Soper JT, Clarke-Pearson DL, Berchuck A. Relationship between race and interval to treatment in endometrial cancer. Obstet Gynecol 1995;86(4 Pt 1):486–90. [DOI] [PubMed] [Google Scholar]
- 9.Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control 2009;16(1):53–6. [DOI] [PubMed] [Google Scholar]
- 10.Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecol Oncol 2011;122(1):63–8. [DOI] [PubMed] [Google Scholar]
- 11.Felix AS, Cohn DE, Brasky TM, Zaino R, Park K, Mutch DG, et al. Receipt of adjuvant endometrial cancer treatment according to race: an NRG Oncology/Gynecologic Oncology Group 210 Study. Am J Obstet Gynecol 2018;219(5):459.e1–459.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaspers M, Llamocca E, Quick A, Dholakia J, Salani R, Felix AS. Black and Hispanic women are less likely than white women to receive guideline-concordant endometrial cancer treatment. Am J Obstet Gynecol 2020;223(3):398.e1–398.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang AB, Huang Y, Hur C, Tergas AI, Khoury-Collado F, Melamed A, et al. Impact of quality of care on racial disparities in survival for endometrial cancer. Am J Obstet Gynecol 2020;223(3):396.e1–396.e13. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez VE, LeBrón AMW, Chang J, Bristow RE. Guideline-adherent treatment, sociodemographic disparities, and cause-specific survival for endometrial carcinomas. Cancer 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamidi M, Moody JS, Kozak KR. Refusal of radiation therapy and its associated impact on survival. Am J Clin Oncol 2010;33(6):629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapp J, Tuminello S, Alpert N, Flores RM, Taioli E. Disparities in surgery for early-stage cancer: the impact of refusal. Cancer Causes Control 2019;30(12):1389–1397. [DOI] [PubMed] [Google Scholar]
- 17.Tohme S, Kaltenmeier C, Bou-Samra P, Varley PR, Tsung A. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann Surg Oncol 2018;25(12):3427–3435. [DOI] [PubMed] [Google Scholar]
- 18.Gopal N, Kozikowski A, Barginear MF, Fishbein J, Pekmezaris R, Wolf-Klein G. Reasons for Chemotherapy Refusal or Acceptance in Older Adults With Cancer. South Med J 2017;110(1):47–53. [DOI] [PubMed] [Google Scholar]
- 19.Aizer AA, Chen MH, Parekh A, Choueiri TK, Hoffman KE, Kim SP, et al. Refusal of curative radiation therapy and surgery among patients with cancer. Int J Radiat Oncol Biol Phys 2014;89(4):75664. [DOI] [PubMed] [Google Scholar]
- 20.Parsons MW, Francis S, Maurer KA, Grant J, Gaffney DK. Refusal of Radiation Results in Inferior Survival in Endometrial Cancer. Am J Clin Oncol 2020;43(6):399–410. [DOI] [PubMed] [Google Scholar]
- 21.Merkow RP, Rademaker AW, Bilimoria KY. Practical Guide to Surgical Data Sets: National Cancer Database (NCDB). JAMA Surg 2018;153(9):850–851. [DOI] [PubMed] [Google Scholar]
- 22.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15(3):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods 2010;15(4):309–34. [DOI] [PubMed] [Google Scholar]
- 25.Imai K, Keele L, Yamamoto T. Identification, Inference and Sensitivity Analysis for Causal Mediation Effects. Statistical Science 2010;25(1):51–71, 21. [Google Scholar]
- 26.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. 2014 2014;59(5):38. [Google Scholar]
- 27.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology 2011;22(4):582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelfand LA, MacKinnon DP, DeRubeis RJ, Baraldi AN. Mediation Analysis with Survival Outcomes: Accelerated Failure Time vs. Proportional Hazards Models. Front Psychol 2016;7:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins J, Green P, Hjort N, Richardson S. Highly structured stochastic systems. 2003. [Google Scholar]
- 30.Pearl J Direct and indirect effects. arXiv preprint arXiv:1301.2300 2013. [Google Scholar]
- 31.Assari S Unequal Gain of Equal Resources across Racial Groups. Int J Health Policy Manag 2018;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N Engl J Med 2019;380(24):2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19(3):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J Clin Oncol 2019:Jco1801575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu QD, Zhou M, Peddi P, Medeiros KL, Zibari GB, Shokouh-Amiri H, et al. Influence of facility type on survival outcomes after pancreatectomy for pancreatic adenocarcinoma. HPB (Oxford) 2017;19(12):1046–1057. [DOI] [PubMed] [Google Scholar]
- 36.Carey RM, Fathy R, Shah RR, Rajasekaran K, Cannady SB, Newman JG, et al. Association of Type of Treatment Facility With Overall Survival After a Diagnosis of Head and Neck Cancer. JAMA Netw Open 2020;3(1):e1919697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


