Introduction
Approximately one third of U.S. adults experience intimate partner aggression (IPA) during their lifetimes (Breiding et al., 2008; Peterson et al., 2018), which can result in high rates of morbidity, mortality, and poor health and treatment outcomes, particularly when co-occurring with alcohol use disorder (AUD; Afifi et al., 2009; Bonomi et al., 2006; Centers for Disease Control and Prevention, 2003; Ellsberg et al., 2008; Lipsky et al., 2011; Lipsky et al., 2010). AUD is a well-established risk factor for IPA (Leonard and Quigley, 2017). Rates of IPA perpetration are substantially higher among individuals with AUD compared to those without AUD (Chermack et al., 2010; Duke et al., 2018; Smith et al., 2012). The link between heavy drinking, which is common among people with AUD, and IPA has been supported in separate literatures conducted primarily among healthy participants without AUD. Both laboratory studies and studies conducted in naturalistic settings provide evidence of the causal link between alcohol intoxication and subsequent IPA perpetration (Crane et al., 2016; Derrick and Testa, 2017; Eckhardt et al., 2021; Testa and Derrick, 2013). Evidence also suggests a dose-response effect of alcohol consumption on IPA (Duke et al., 2011), with IPA being most frequent and severe during heavy drinking episodes (Graham et al., 2011; Murphy et al., 2005; Testa et al., 2003). At the event level, intoxicated IPA is most commonly bidirectional between partners and characterized by a high degree of symmetry and reactive aggression between partners (Leisring and Grigorian, 2016; Straus, 2008). Thus, it is important to examine co-occurring AUD and IPA (AUD/IPA) through a dyadic lens.
Despite its clear negative sequelae, only two treatments have been tested with a priori goals to address alcohol and IPA outcomes concurrently. Neither study required AUD diagnosis and neither treatment is for couples. First, Stuart and colleagues (2013) found that among hazardous drinking men, a single 90-minute session of Motivational Interviewing outperformed a court-mandated batterer intervention program on alcohol and psychological IPA, but not physical IPA outcomes. Significant effects did not remain at 12-month follow-up. Second, Murphy and colleagues (2018) found that among hazardous drinking men, four sessions of Motivational Enhancement did not outperform a time-matched control condition on any outcomes. Other studies have tested different non-pharmacologic treatments but these studies have either not had minimal alcohol-related inclusion criteria (Romero-Martínez et al., 2016), have specifically excluded participants with AUD diagnosis (Taft et al., 2013), or did not require IPA for inclusion. For example, Behavioral Couples Therapy for Alcoholism and Drug Abuse (BCT) has optional IPA content and has shown a promising ability to reduce IPA among couples with AUD. However, IPA has been examined only in secondary analyses of these trials, where approximately two-thirds of the participants reported IPA (O’Farrell et al., 2003; Schumm et al., 2009).
Progress to develop effective IPA-specific interventions generally has been limited by lengthy in-person sessions, high dropout rates, treatment resistance, poor working alliance, and low readiness to change (Daly and Pelowski, 2000; Daniels and Murphy, 1997; Levesque et al., 2008; Taft et al., 2003; Taft and Murphy, 2007; Taft et al., 2001). Pharmacologic interventions might improve reach, accessibility, feasibility, and effectiveness in this field, but to our knowledge, fluoxetine is the only medication that has been tested as a strategy for reducing IPA among individuals with AUD (N=60; George et al., 2011). Twelve weeks of fluoxetine augmentation of a cognitive behavioral treatment program led to greater reductions in partner report of AUD patients’ irritability and IPA perpetration compared to placebo. Given the lack of efficacious non-pharmacologic interventions, and scarcity of medications investigated, novel treatment options that target both AUD and IPA simultaneously are needed to exert more robust effects on AUD/IPA.
Oxytocin: Modulator of Social Behavior and Aggression
Oxytocin is one promising treatment for AUD/IPA. Oxytocin has gained much attention in the psychiatric literature due to findings suggesting that it can reduce stress reactivity, enhance prosocial behavior and positive affect, and restore sensitivity to natural rewards such as interpersonal relationships (MacDonald and MacDonald, 2010; McGregor and Bowen, 2012; Sippel et al., 2017). Two competing hypotheses explain these outcomes. The proposed study was designed in accordance with the prosocial hypothesis, which was the prevailing model at the time of the study’s design. This model posits that oxytocin selectively enhances affiliative and positive prosocial behavior (MacDonald and MacDonald, 2010; Mitchell et al., 2015) via neurobiological stress reduction and anxiolytic pathways. These two constructs are known underpinnings of both alcohol craving within AUD and IPA, suggesting that oxytocin is a strong candidate to ameliorate IPA among couples with AUD. More recently, the social salience hypothesis (Shamay-Tsoory and Abu-Akel, 2016), was advanced as a more comprehensive and integrative extension of the prosocial hypothesis. This model posits that oxytocin is more likely to amplify one’s preexisting adaptive and maladaptive social tendencies equally rather than prosocial tendencies specifically. Under this model, oxytocin’s effects are likely to be informed by individual and social contextual differences such as in/out group membership or familiarity, stable personality characteristics, and psychopathology.
Despite a vast literature examining the effects of oxytocin on various social cognitive processes and behaviors including empathy, trust, cooperation, generosity, altruism, and emotion regulation (Bartz et al., 2011; Evans et al., 2014; Koch et al., 2019; Kosfeld et al., 2005; Zak et al., 2007), few studies have investigated its effects on human aggression. Extant studies have focused on healthy community samples (see de Jong and Neumann, 2017, for review), and none have directly examined oxytocin’s effects on IPA behaviors specifically, which prevents generalizability to the large population of individuals with AUD/IPA. Consistent with the prosocial hypothesis, some studies demonstrate that oxytocin may reduce general aggression (Berends et al., 2019; Romney et al., 2019; Zhu et al., 2019). However, one laboratory study (DeWall et al., 2014) found evidence of a modest increase in IPA “inclinations” (i.e., self-reported probability of engaging in IPA) following intranasal oxytocin administration among healthy undergraduate men. Consistent with the social salience hypothesis, this effect was only observed among men with higher trait aggression. Similar findings from other studies suggest that oxytocin might increase general aggression, but only under certain conditions such as provocation (Ne’eman et al., 2016; Pfundmair et al., 2018).
Oxytocin: AUD Pathophysiology and Treatment
A separate literature suggests that oxytocin plays an important role in the pathophysiology of AUD and could be a promising pharmacotherapy for this condition (Lee et al., 2016; Lee and Weerts, 2016; Peris et al., 2020). Oxytocin is specifically hypothesized to disrupt the negative reinforcement (i.e., drinking to cope; Koob, 2013) that contributes to the development and maintenance of AUD (King et al., 2020; McGregor and Bowen, 2012; Pedersen, 2017; Tunstall et al., 2019). Promising preclinical research indicates that oxytocin attenuates alcohol self-administration and stress-induced alcohol relapse (Becker et al., 2017; Bowen et al., 2011; King et al., 2017a; King et al., 2017b; MacFadyen et al., 2016; Stevenson et al., 2017) as well as stress-induced reinstatement of alcohol seeking (King and Becker, 2019). Similarly, three preliminary clinical trials found that oxytocin attenuated alcohol withdrawal symptoms and craving, respectively, although the effect only emerged among more anxiously attached participants in one of these studies (Melby et al., 2019; Mitchell et al., 2016; Pedersen et al., 2013). Conversely, the three other preliminary trials conducted to date found no effects of oxytocin on alcohol outcomes in treatment-seeking or non-treatment seeking samples (Melby et al., 2021; Stauffer et al., 2019; Vena et al., 2018). All clinical studies to date have utilized small sample sizes, few are pre-registered trials with a priori power analyses, and few are balanced by sex. Notably, no studies to date have examined the impact of oxytocin on alcohol outcomes in a sample of couples with AUD.
The Current Study
Taken together, evidence suggests that while the current state of the translational oxytocin science is complex, investigation of its potential to mitigate alcohol craving and IPA concurrently in a clinical sample of romantic dyads is warranted. The current trial was designed to fill this gap in the literature. We examined the effects of a single dose of intranasal oxytocin (40 international units [IU]) on cue-induced alcohol craving and both subjective aggression and laboratory task-based IPA among couples with current AUD and physical IPA. Because previous literature attributes oxytocin’s behavioral effects, in part, to its ability to attenuate stress-related hypothalamic-pituitary-adrenal axis (HPA) dysregulation (Cardoso et al., 2014), we also examined the effects of oxytocin on salivary cortisol response. Consistent with the prosocial hypothesis, we expected that participants in the oxytocin condition, as compared to the placebo condition, would report less cue-induced subjective alcohol craving, subjective aggression, laboratory task-based IPA, and cortisol reactivity in a laboratory paradigm.
Materials and Methods
Participants
One hundred couples (N = 200 individuals) were enrolled using 1:1 randomization. Both partners within a couple were assigned to the same condition (oxytocin or placebo). Inclusion criteria indicated that one or both partners in each couple met DSM-5 (American Psychiatric Association, 2013) criteria for current AUD as assessed by the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). In order to enroll a high-risk sample of couples and to ensure integrity of the laboratory aggression task, participants were also required to endorse physical IPA (i.e., hitting, slapping) that occurred at any point during their current relationship, as assessed at the telephone screening using the physical IPA subscale items from the Revised Conflict Tactics Scale (CTS-2; Straus et al., 1996). Participants who reported severe and unilateral IPA in the current relationship were excluded from participating for safety; however, only one couple was excluded for this reason. Participants with conditions known to correlate with aggression and IPA (e.g., posttraumatic stress disorder or intermittent explosive disorder) were not excluded. Participants were also required to be stabilized on psychotropic medications for at least four weeks before enrolling. Additional exclusion criteria included pregnancy or breastfeeding, seizure disorder, current suicidal or homicidal ideation warranting a higher level of care, or alcohol withdrawal as indicated by a score ≥8 on the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar; Sullivan et al., 1989). Because the study measured salivary cortisol, participants were excluded if they had a health condition likely to influence HPA functioning (e.g., hematological, endocrine, renal, or pulmonary disease; synthetic glucocorticoid or exogenous steroid therapy; psychotic, bipolar, or eating disorders) or if both partners in a dyad had a body mass index ≥ 39. See Figure 1 for the CONSORT diagram.
Figure 1.
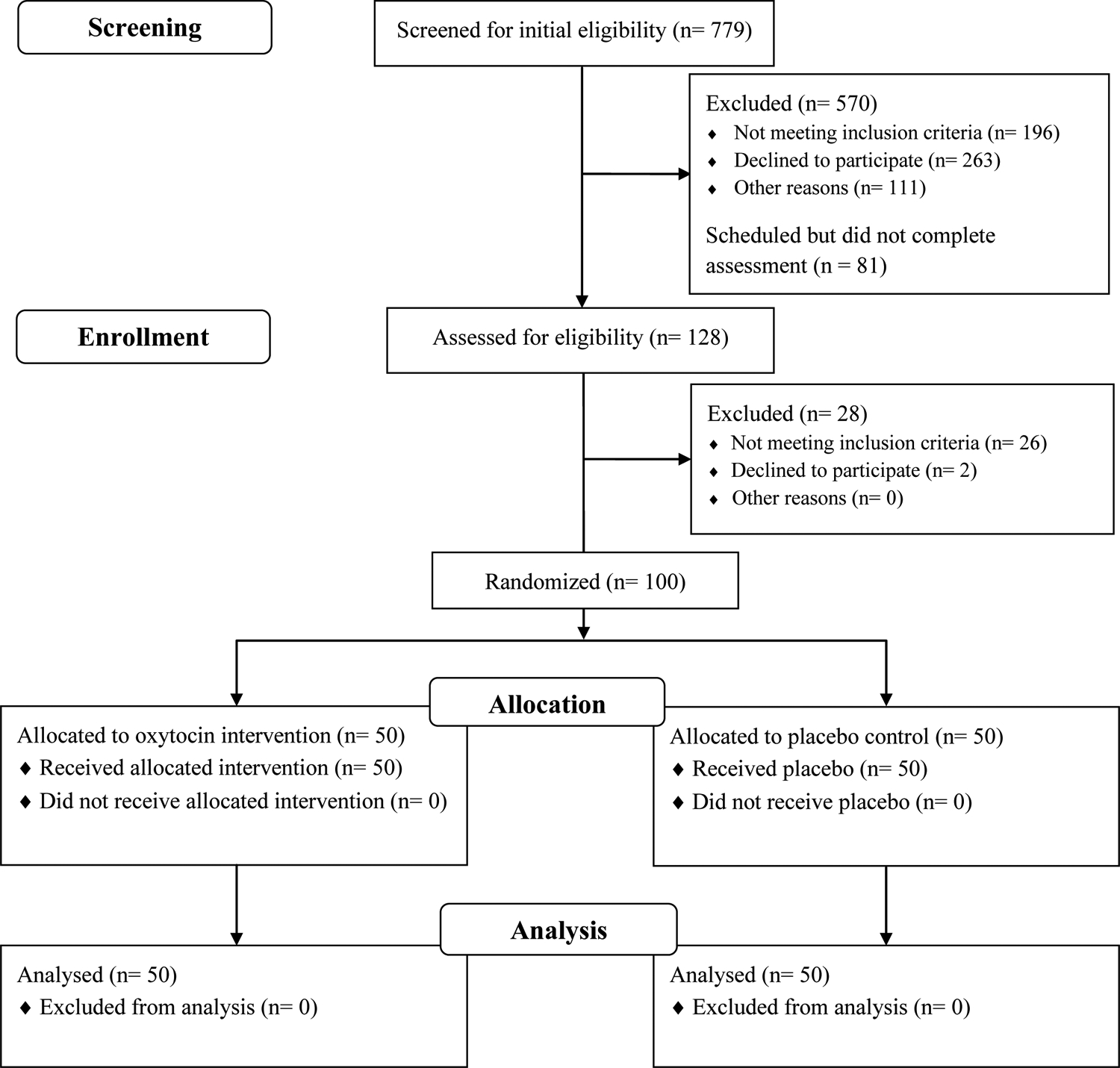
CONSORT flow diagram. n = number of couples
Measures
Clinical characteristics.
The Timeline Follow Back (TLFB; Sobell and Sobell, 1992) characterized participants’ alcohol consumption in the past 60 days and the Alcohol Use Disorder Identification Test (AUDIT; Saunders et al., 1993) assessed alcohol problem severity. The AUDIT is a 10-item self-report scale assessing alcohol problem severity during the past 12 months. Total scores range from 0 – 40, with higher scores suggesting greater alcohol-related problems. Cronbach’s alpha =.89. The Dyadic Adjustment Scale (DAS; Spanier, 1976) is a 32-item self-report questionnaire that assesses various features of relationship adjustment. Higher scores reflect greater relationship adjustment, with scores ≤ 97 indicating clinically significant levels of relationship distress. Cronbach’s alpha =.94. The Revised Conflict Tactics Scale (CTS-2; Straus et al., 1996) is a 78-item self-report scale used to assess psychological and physical IPA perpetration and victimization during the past 6 months. Cronbach’s alpha for the psychological IPA subscale was .75; the physical IPA subscale was .86.
Subjective alcohol craving and aggression.
A modified version of the Within Session Rating Scale (Childress et al., 1986) assessed subjective alcohol craving and aggression. This 100mm Visual Analogue Scale (VAS) was anchored from 0 (not at all) to 10 (extremely). Participants completed the VAS at eight time points (described below). The question assessing craving asked “How strong is your current craving for alcohol?” and the question assessing aggression asked “How aggressive are you currently feeling?”.
Laboratory task-based IPA.
We employed a modified version of the Taylor Aggression Paradigm (TAP; Giancola and Zeichner, 1995; Taylor, 1967) that has demonstrated safety and validity in numerous prior studies (Giancola and Parrott, 2008; Parrott et al., 2015). The modified TAP involved participants engaging in a fictitious reaction time competition with their partner, as opposed to a confederate, as originally designed. Participants were told that a winning trial required them to deliver a shock to their partner that ranged from 1 to 10 intensity for a duration of their choosing. A losing trial resulted in them receiving a shock from their partner. In reality, all participants received an identical sequence of “winning” or “losing” trials (and corresponding shocks) generated by the TAP software (Eckhardt et al., 2015). Shocks were administered through two electrodes attached to the index and middle fingers of the nondominant hand and were calibrated to control for individual differences in pain threshold that could influence shock selection. IPA was operationalized as the average intensity and duration of shocks administered in response to “losing” trials.
Salivary cortisol.
Cortisol was measured using the Salimetrics Passive Drool enzyme immunoassay kit. This kit has a 0.91 correlation with serum samples and a sensitivity of <0.007 ug/dL. Saliva samples were stored on ice and immediately placed in storage at −20°C until assay. Saliva was collected at the same time points as subjective craving and aggression.
Procedures
Enrollment occurred between July 2017 to April 2021. This study was approved by the local academic and Veterans Administration institutional review boards and conducted in accordance with the Helsinki Declaration as revised 1989. Recruitment was conducted via internet and flyer advertisements and local clinic referrals. Participants underwent an initial phone screening, private written informed consent, and a baseline eligibility assessment. Following their arrival to the laboratory, participants were escorted to separate rooms and remained in separate rooms for the duration of the laboratory visit until debriefing. To maximize participant privacy and safety as well as rigor of the laboratory paradigms, participants also surrendered their cell phones to research staff in order to ensure that they were not communicating during the laboratory visit. Baseline assessments included a urine drug screen and pregnancy test (for participants of childbearing potential), breathalyzer to confirm blood alcohol concentration = 0.00, and interview and self-report measures. Participants had the option to complete the laboratory procedures on the same day as the baseline or to schedule a separate visit.
Participants were instructed to abstain from caffeine, nicotine, alcohol, or other substances of abuse the day of the laboratory procedures. Participants who used nicotine products were offered a nicotine patch for their visit. Participants completed baseline measures of subjective craving and aggression and provided a saliva sample at minute 0 (Time 1) and minute 5 (Time 2). At minute 10, participants received a 40 IU intranasal dose of oxytocin or placebo (i.e., saline). At minute 40 (Time 3), another subjective assessment and saliva sample were obtained. At minute 45, the alcohol cue paradigm began followed by the Taylor Aggression Paradigm (TAP; described below) at minute 65. Participants completed assessments immediately after the alcohol cue (Time 4) and TAP (Time 5), and 15- (Time 6), 30-(Time 7), and 60-minutes (Time 8) following the TAP. See Table 2 for timeline.
Table 2.
Laboratory Procedures Timeline
| Duration | Procedure | |
|---|---|---|
| Study Baseline Portion | ||
| 2–2.5 hours | Informed consent and eligibility assessment battery | |
| Study Laboratory Portion | ||
| Time 1 | Minute 0 | Baseline measurement #1 (VAS, cortisol) |
| Time 2 | Minute 5 | Baseline measurement #2 (VAS, cortisol) |
| Minute 10 | Oxytocin or placebo administered | |
| Time 3 | Minute 40 | Post-medication assessment (VAS, cortisol) |
| Minute 45 | Alcohol cue | |
| Time 4 | Minute 50 | Post-alcohol cue assessment (VAS, cortisol) |
| Minute 65 | TAP | |
| Time 5 | Minute 70 | Immediate post-task assessment (VAS, cortisol) |
| Time 6 | Minute 85 | 15-minute post-task assessment (VAS, cortisol) |
| Time 7 | Minute 100 | 30-minute post-task assessment (VAS, cortisol) |
| Time 8 | Minute 130 | 60-minute post-task assessment (VAS, cortisol) |
| Minute 135 | Participant debriefed, compensated, and discharged | |
Note. VAS = Visual Analog Scale; TAP = Taylor Aggression Paradigm.
COVID-19 modifications.
All participants enrolled after March 17, 2020 (n = 16 couples) completed informed consent and eligibility assessments via telehealth. Participants were shipped supplies (i.e., urine drug screen cups, pregnancy tests, and saliva alcohol strips) prior to the baseline appointment. Participants verbally confirmed that they had adequate privacy to complete study procedures and were queried if any privacy concerns arose (e.g., staff heard someone opening a door or talking in the background). If eligible, participants presented in-person to complete laboratory procedures, but did not provide saliva samples to minimize risk for COVID-19 transmission.
Alcohol cue task.
For the alcohol cue task (Monti et al., 1987), participants were instructed via an audio recording to spend 3 minutes holding and smelling a control beverage (i.e., water). The audio recording prompted participants to complete the corresponding VAS immediately following this 3-minute period. A 3-minute rest period followed next and after the rest period, participants’ preferred alcoholic beverage was presented in an appropriate glass/container. Participants were instructed to spend 3 minutes holding and smelling the beverage. Instructions for the water and alcohol cues were identical.
Modified TAP.
In this study, the TAP consisted of 34 trials (17 wins and 17 losses) for a duration of 12–14 minutes. Participants received shocks during each “losing” trial. Shocks were 1 second in duration and sequenced in two blocks that escalated in intensity from 55–65% (low provocation) to 90–100% (high provocation) of the participant’s pain threshold. Afterwards, while still separated from their partner, participants were asked a standardized series of scripted questions as a manipulation check. Participants were also debriefed on the deception including being informed that their partner did not administer the shocks, that their responses were “normal” and consistent with those of others in the study, and it was explained that deception occurred because some people might artificially alter their responses if they are aware that the purpose of the TAP was to assess IPA. Participants were also assessed for their understanding of the deception. No participants required exclusion from the study due to a failed manipulation check.
Data Analytic Plan
General linear mixed models (GLMMs) were used to examine study outcomes using the PROC Mixed procedure in SAS v9.4 (Cary, NC). All models contained random dyad effects to account for clustering within dyads and random participant effects with an autoregressive type 1 covariance structure to account for within-subject clustering over time. For TAP intensity and duration, outcomes were modeled as a function of treatment condition (oxytocin or placebo), while adjusting for sex, baseline diagnosis of current AUD (dichotomized present/not present), and trial number. TAP trial number was treated as a categorical variable due to the lack of linear trends over time. For subjective craving, subjective aggression, and cortisol outcomes, models expressed outcomes (at Time 3 to Time 8) as a function of group, time, and group x time, while adjusting for sex, baseline AUD, and the baseline value of the outcome (defined as the average of the Time 1 and Time 2 values). For the models examining subjective craving and aggression, time was treated as a categorical variable due to the lack of linear trends over time. As a check that the cue did, in fact, increase craving, within-group contrasts were also constructed to compare craving before and after the cue. For the model examining cortisol, time was treated as a continuous variable, since a linear trend was deemed appropriate.
The study was designed to have 80% power to detect moderate within-group changes in TAP shock intensity: around 0.4 units [±10%−20%], assuming alpha=0.05 and 2-sided hypothesis testing. The design also provided 80% power to detect moderate between-group differences (0.4 units) but with a larger alpha level (0.25). This is consistent with the exploratory nature of the study due to the lack of prior studies examining the effects of oxytocin on alcohol craving or aggression using the TAP.
Results
Sample Characteristics
Complete demographic and clinical characteristics are presented in Table 1. One hundred-six (53%) participants identified as female, and 94 (47%) participants identified as male. The sample included n = 92 (92%) different-sex couples and n = 8 (8.0%) same-sex couples. No participants identified as transgender, gender non-binary, or having another gender identity. Participants had a mean age of 35.4 years (SD = 11.3). Most participants identified as white (n = 138, 74.3%); 19.1% (n = 44) identified as Black; and 7.0% (n=14) identified as Hispanic or Latino. Twenty-nine (14.5%) participants were military veterans. Most participants (n = 158, 79%) met diagnostic criteria for current AUD. Both partners had AUD in 58/100 couples in this sample. Out of the past 60 days assessed by the TLFB, participants reported an average of 47.24% drinking days (SD = 33.95) and 25.09% (SD = 31.72) heavy drinking days (≥ 4 drinks for women, ≥ 5 for men). Nearly all participants reported at least one instance of psychological IPA perpetration (n = 192, 96.0%; M = 26.2, SD = 25.9) and victimization (n = 194, 97.0%, M = 27.3, SD = 27.9), respectively, in the past six months. Most participants reported at least one instance of physical IPA perpetration (n = 107, 53.5%, M = 6.9, SD = 19.4) and victimization (n = 109, 54.5%, M = 8.6, SD = 21.9), respectively, in the past six months. No between-group differences in demographic or clinical characteristics were statistically significant (i.e., p > 0.05).
Table 1.
Demographic and Clinical Characteristics by Treatment Condition (N = 200)
| Characteristics | Overall | Group | |||
|---|---|---|---|---|---|
| N=200 | Placebo | Oxytocin | |||
| n=100 | n=100 | ||||
| Demographics | |||||
| Age (years) | 35.4 ± 11.3 | 34.6 ± 11.0 | 36.2 ± 11.5 | ||
| Sex, n (%) | |||||
| Male | 94 (47.0) | 47 (47.0) | 47 (47.0) | ||
| Female | 106 (53.0) | 53 (53.0) | 53 (53.0) | ||
| Employment, n (%) | |||||
| Unemployed | 35 (17.5) | 13 (13.0) | 22 (22.0) | ||
| Part-time | 43 (21.5) | 28 (28.0) | 15 (15.0) | ||
| Full-time | 101 (50.5) | 50 (50.0) | 51 (51.0) | ||
| Student | 11 (5.5) | 5 (5.0) | 6 (6.0) | ||
| Retired | 2 (1.0) | — | 2 (2.0) | ||
| Disabled | 5 (2.5) | 2 (2.0) | 3 (3.0) | ||
| Missing | 3 (1.5) | 2 (2.0) | 1 (1.0) | ||
| Household Income: median (interquartile range) | $50,000 ($30,000 – $80,000) | $50,000 ($30,000 – $70,000) | $50,000 ($30,000 – $80,000) | ||
| Race, n (%) | |||||
| White | 138 (74.3) | 74 (74.0) | 64 (64.0) | ||
| Black/African American | 44 (19.1) | 19 (19.0) | 25 (25.0) | ||
| More than One Race | 11 (4.0) | 4 (4.0) | 7 (7.0) | ||
| Asian | 3 (1.5) | 1 (1.0) | 2 (2.0) | ||
| Native American/Alaskan Native | 2 (1.0) | 1 (1.0) | 1 (1.0) | ||
| Missing | 2 (1.0) | 1 (1.0) | 1 (1.0) | ||
| Ethnicity, n (%) | |||||
| Non-Hispanic/Latino/a/x | 184 (93.5) | 93 (93.0) | 91 (91.0) | ||
| Hispanic/Latino/a/x | 14 (7.0) | 5 (5.0) | 9 (9.0) | ||
| Missing | 2 (1.0) | 2 (2.0) | — | ||
| Relationship Status, n (%) | |||||
| Cohabitating | 107 (53.5) | 53 (53.0) | 54 (54.0) | ||
| Married | 71 (32.2) | 32 (32.0) | 39 (39.0) | ||
| Dating | 22 (15.0) | 15 (15.0) | 7 (7.0) | ||
| Relationship Length (months) | 89.2 ± 93.5 | 81.3 ± 88.4 | 97.0 ± 98.1 | ||
| Clinical Characteristics | |||||
| Alcohol Use Disorder, n (%) | 158 (79.0) | 76 (76.0) | 82 (82.0) | ||
| Mild | 50 (25.0) | 20 (20.0) | 30 (30.0) | ||
| Moderate | 33 (19.1) | 19 (19.0) | 14 (14.0) | ||
| Severe | 74 (37.2) | 37 (37.0) | 37 (37.0) | ||
| No AUD | 43 (21.5) | 24 (24.0) | 19 (19.0) | ||
| Alcohol Problem Severity | 11.0 ± 7.6 | 10.9 ± 7.9 | 11.1 ± 7.4 | ||
| Dyadic Adjustment | 105.6 ± 21.1 | 105.3 ± 21.0 | 105.9 ± 21.2 | ||
| Psychological IPV Perpetration | 29.5 ± 28.1 | 32.4 ± 29.9 | 26.6 ± 25.9 | ||
| Psychological IPV Victimization | 30.8 ± 30.1 | 34.4 ± 31.9 | 27.3 ± 27.9 | ||
| Minor Physical IPV Perpetration | 5.3 ± 14.5 | 5.0 ± 15.7 | 5.7 ± 13.3 | ||
| Minor Physical IPV Victimization | 6.5 ± 15.9 | 5.7 ± 15.6 | 7.4 ± 16.2 | ||
| Severe Physical IPV Perpetration | 1.6 ± 15.9 | 2.0 ± 6.9 | 1.3 ± 4.5 | ||
| Severe Physical IPV Victimization | 2.1 ± 7.8 | 1.8 ± 6.4 | 2.4 ± 9.0 | ||
| Sexual Coercion Perpetration | 4.9 ± 12.6 | 4.3 ± 12.8 | 5.5 ± 12.4 | ||
| Sexual Coercion Victimization | 4.7 ± 11.7 | 4.5 ± 12.8 | 4.9 ± 10.6 | ||
| Injury Perpetration | 1.0 ± 4.2 | 0.5 ± 1.3 | 1.5 ± 5.7 | ||
| Injury Victimization | 1.4 ± 5.3 | 0.6 ± 1.4 | 2.3 ± 7.3 | ||
| Own Negotiation | 68.1 ± 37.0 | 72.0 ± 38.1 | 64.1 ± 35.5 | ||
| Partner Negotiation | 63.5 ± 35.7 | 65.0 ± 36.8 | 61.9 ± 34.7 | ||
| Laboratory Responses | |||||
| Frequency of ‘10’ shocks* | 2.3 ± 2.8 | 2.0 ± 2.0 | 2.7 ± 3.4 | ||
| Mean Shock Intensity | 4.6 ± 2.1 | 4.3 ± 2.0 | 4.8 ± 2.2 | ||
| Mean Shock Duration | 410.0 ± 115.0 | 414.4 ± 117.0 | 405.5 ± 113.2 | ||
| Mean Alcohol Craving | 2.6 ± 2.0 | 2.4 ± 1.9 | 2.7 ± 2.1 | ||
Note. All values are expressed as n (%) and mean ± standard deviation, unless otherwise stated. No between-group differences were statistically significant (i.e., p>0.05 for all comparisons). IPV = Intimate partner violence.
The number of times (out of n=16 TAP trials) in which the subject shocked their partner at the highest level (i.e., a ‘10’).
Preliminary Analyses
As a check that the alcohol cue elicited increases in craving, pre- and post-cue craving scores were compared within each group. Mean craving scores increased from 2.25 to 3.46 points (mean increase ± SE: 1.21 ± 0.12, p < 0.0001) between time point 3 (before the alcohol cue) and 4 (after the alcohol cue) in the placebo group and from 2.35 to 3.25 points (mean increase ± SE: 0.90 ± 0.12, p < 0.0001) in the oxytocin group, confirming that the cues had their intended effect. Similarly, mean subjective aggression scores increased from 1.17 to 1.58 points (mean increase ± SE: 0.41 ± 0.09, p < 0.0001) between time point 4 and 5 in the placebo group and from 1.36 to 1.68 points (mean increase ± SE: 0.31 ± 0.10, p = 0.001) in the oxytocin group.
Analyses of Primary Outcomes
Results indicate that there were no statistically significant differences between the oxytocin and placebo groups on any of the outcomes examined. The longitudinal trends over time were similar between groups, and the groups did not significantly differ from one another at any time point (p > 0.05; Figure 2). In both treatment groups, mean craving scores peaked immediately after the alcohol cue (Time 4) and gradually attenuated (Figure 2a). Similar patterns were noted for mean aggression scores, which peaked at Time 5 (post TAP) in both groups before attenuation (Figure 2b). TAP intensity levels were generally lower during the first half of the TAP trials when provocation was low. Means ranged from 2.5 to 4.5, compared with the latter half of the TAP trials, where means ranged from 4.6 to 7.1 (Figure 2c). Mean TAP durations were highest in both groups during TAP trial #1 (placebo mean [95% CI] = 618 ms [562 to 673]; oxytocin mean [95% CI] = 653 [584 to 721]; Figure 2d). Mean TAP durations on the subsequent trials were lower in both groups, with means ranging from 360ms to 440ms and from 362ms to 411ms in the placebo and oxytocin groups, respectively (p > 0.05 for between-group comparisons at all time points; Figure 2d). Mean cortisol levels were similar between groups at baseline (placebo mean [95% CI] = 0.17 μL/dL [0.14 to 0.20]; oxytocin mean = 0.17μL/dL [0.12 to 0.21]; p > 0.05), and the means declined at similar rates in both treatment groups (Figure 2e).
Figure 2:
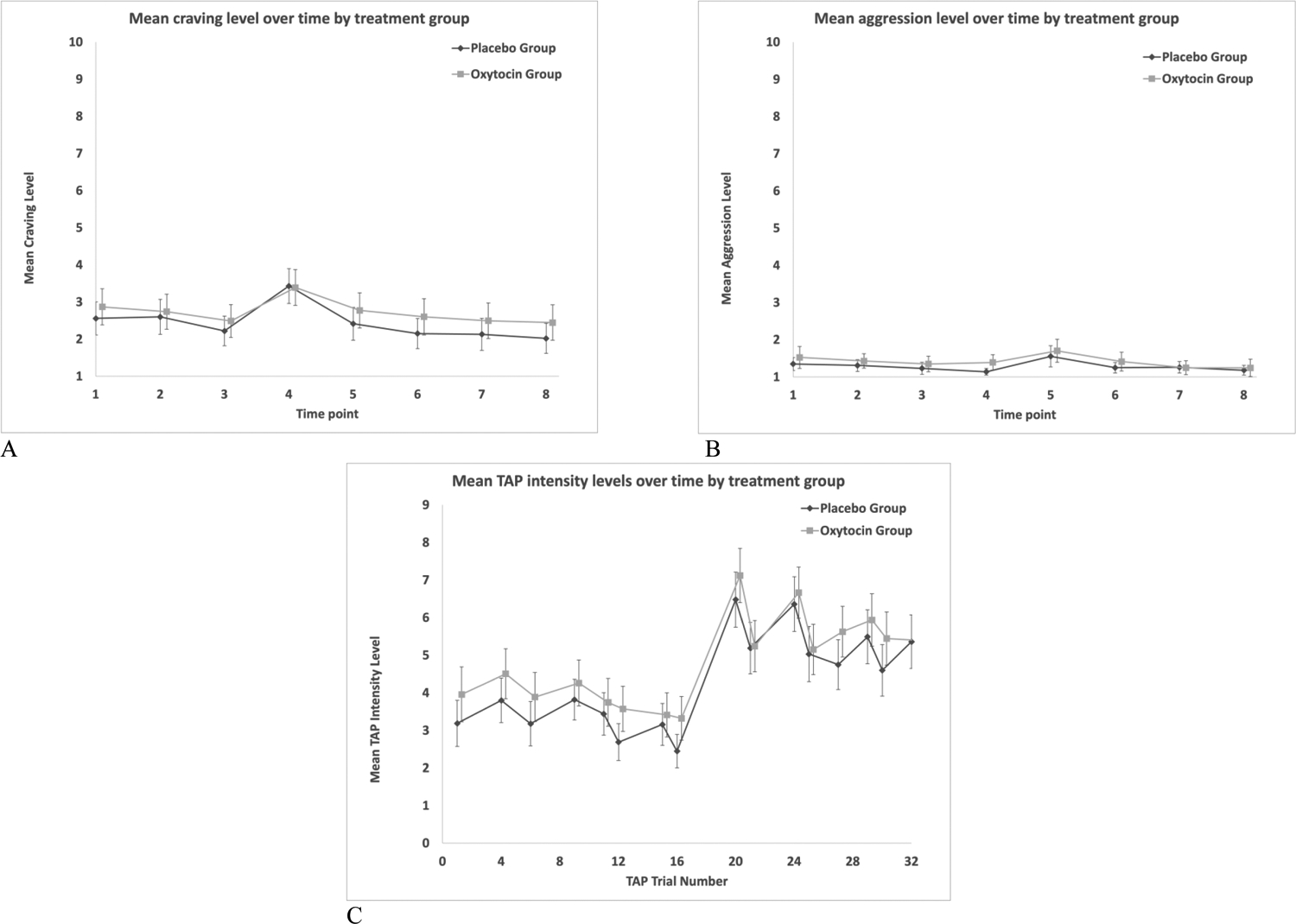

A) Trends over time in the two treatment groups for craving; B) Trends over time in the two treatment groups for aggression; C) Trends over time in the two treatment groups for TAP intensity; D) Trends over time in the two treatment groups for TAP duration; E) Trends over time in the two treatment groups for salivary cortisol. The mean cortisol levels depicted in panel E are based on a subset of participants in each group (i.e., n=52 to n=58 for placebo; n=57 to n=60 for oxytocin), due to COVID-19 restrictions that precluded in-person contact for part of the study time period. In each panel, the error bars reflect 95% confidence intervals.
Oxytocin Safety and Adverse Events
No serious adverse events were reported during the trial. In total, seven adverse events occurred. Six were unrelated to study procedures, and one (a nosebleed following medication self-administration) was “probably” related to study procedures.
Discussion
This was the first randomized, placebo-controlled trial evaluating the effects of intranasal oxytocin on subjective alcohol craving and aggression, laboratory-based IPA, and salivary cortisol responding among couples with AUD and IPA. Contrary to hypotheses, participants in the oxytocin condition did not evidence lower subjective alcohol craving or aggression, laboratory-based IPA, or salivary cortisol as compared to participants in the placebo group. However, consistent with extant literature, safety of the medication was well supported.
The findings contribute to the literature by demonstrating for the first time, in a rigorously conducted randomized placebo-controlled trial in a clinical sample, that oxytocin is not likely to increase aggression. This is a critical finding given the emergence of the social salience model of oxytocin and preliminary research in non-clinical samples suggesting that oxytocin could have iatrogenic, and specifically aggressogenic, effects depending on individual and contextual factors such as trait aggressive tendencies and provocation, respectively (DeWall et al., 2014; Ne’eman et al., 2016; Pfundmair et al., 2018). The significance of this outcome is particularly notable given that we studied a high-risk sample of couples with AUD/IPA, whom the social salience hypothesis may have predicted would display increased aggressive under the influence of oxytocin, either due to dispositional or contextual factors. Our findings may not align with limited previous research that found aggressogenic effects of oxytocin because IPA, as measured here, is not synonymous with self-reported trait aggression or IPA inclinations considering that urges to enact aggression can be inhibited.
The lack of iatrogenic effects is also a valuable finding considering that this project overcame several persistent methodological limitations in the existing oxytocin literature. This project was designed and implemented specifically to test the present hypotheses rather than to examine oxytocin effects on craving or aggression as a secondary or exploratory analysis and as such, was rigorously controlled and included a targeted sampling and assessment plan. Because past studies of oxytocin’s effects on both craving and aggression have focused on healthy individuals, examining effects in a high risk, clinically relevant sample of couples was necessary to more fully understand and clarify inconsistent findings from a complex literature. This study suggests that in a population in urgent need of novel intervention strategies, intranasal oxytocin was safe, but a single 40 IU dose was neither iatrogenic nor effective in mitigating the primary outcomes in a laboratory setting. One important contribution of this project is that it was conducted among romantic couples using a validated dyadic adaptation of the TAP, and thus incorporated critical social contextual components that were overlooked by previous studies of oxytocin and aggression. Although the current study was designed according to the prosocial hypothesis, this methodological advancement provides important opportunities to examine potential modifiers of these outcomes in alignment with the social salience hypothesis. Although no laboratory task exactly mimics naturalistic behaviors, the TAP approach used here represents an improvement on most other aggression paradigms employed in the oxytocin literature to date. Another methodological strength of the current study, lending confidence to the results, is the validity of the behavioral manipulations employed. Subjective alcohol craving increased substantially following the alcohol cue, suggesting that it was effective in priming craving. Similarly, no participants were excluded for deception penetration, and as expected, an increase in TAP shock intensity was observed mid-procedure, when the software increases the intensity of shocks administered to participants. These patterns mitigate concerns that the null findings could be attributed to ineffective laboratory procedures. Future studies can improve on the current design by priming IPA through a laboratory couple conflict task to more closely approximate IPA events in naturalistic settings.
The methodological advancements demonstrated in this study are particularly important because the extant clinical oxytocin literature has received increased scrutiny in recent years (Leng and Ludwig, 2016). The proliferation of contradictory outcomes in human trials in addition to a growing list of dispositional and contextual factors that moderate oxytocin response have led to important questions about oxytocin pharmacokinetics, bioavailability, dose-response relationships, and intranasal delivery methods that will require deliberate, systematic methodologies to answer. Deeper scientific issues have also been raised including publication bias and rigor and reproducibility standards (Quintana et al., 2021; Ryabinin and Fulenwider, 2021; Walum et al., 2016). These challenges must be addressed to mitigate existing translational complexities and limitations for oxytocin and other neuropeptides. The present study achieves these standards and provides important clarity to a rapidly expanding literature.
Limitations and Future Directions
Despite important methodological strengths, some limitations of the current study warrant consideration. First, this study included an a priori power analysis, but our ability to fully estimate anticipated power was limited by the absence of prior studies examining the effects of oxytocin on aggression using the TAP. The inclusion of a power analysis reduces some persistent power concerns in the oxytocin-aggression and IPA literatures (Hyatt et al., in press), but ultimately, this study was powered to detect only large differences between the oxytocin and placebo groups. It was also not designed to detect whether certain factors moderated drug response. True group differences may be subtler than originally hypothesized. This could partly explain why our findings do not support the prosocial hypothesis as we expected (i.e., oxytocin did not reduce IPA as observed in some studies of general aggression (Berends et al., 2019; Romney et al., 2019; Zhu et al., 2019). As previously noted, these studies focused on healthy community samples and did not examine IPA specifically. Oxytocin’s effects may be too subtle to override practiced patterns of behavior in highly salient relationships across an entire sample and standardized protocol. As seen in studies of individuals (versus couples), more highly powered studies will be poised to examine individual- and dyadic factors that may moderate oxytocin response in order to eventually delineate which couples may be more likely to experience the therapeutic (and/or iatrogenic) effects of oxytocin.
Second, while at least one partner within each dyad had to meet diagnostic criteria for AUD, not all participants were required to have AUD. Although the majority did have AUD, there was substantial variability in severity (mild, moderate, or severe) which lends increased confidence to the strength of the sampling plan to lead to generalizable findings. It is possible that limiting to a more severe AUD or alcohol treatment-seeking sample would have yielded different alcohol craving outcomes. Because discordant within-dyad alcohol consumption is a known correlate of IPA and relationship functioning (Leonard et al., 2014; Quigley et al., 2013), it is also possible that couples in which both partners had AUD might differ from AUD-discordant couples on outcomes. This question warrants exploration in secondary post-hoc analysis.
Another consideration is that couples were required to have had physical IPA in their current relationship, however, the TAP does not measure important metrics of dyadic behavior such as psychological IPA, which is known to have significant negative consequences on mental and physical health (Basile et al., 2004; Carbone-López et al., 2006). Future studies are needed to evaluate whether oxytocin might influence psychological IPA specifically. Additionally, these couples evidenced better relationship adjustment than expected in light of the sampling criteria and the recency of psychological and physical IPA endorsed on the CTS-2. Findings might have been different if couples were required to have clinically significant relationship distress (i.e., DAS scores < 97; Spanier, 1976). Because negative emotional states such as anger are strongly associated in a causal manner with aggressive acts (Norlander and Eckhardt, 2005), future studies examining the effects of oxytocin on IPA can improve on the current approach by assessing a wider variety of emotion and cognitions. Although the TAP includes provocation, the social salience hypothesis suggests that more thorough dyadic provocation such as a problem solving task, or individual differences such as a history of general aggression or high trait aggression, might influence these null findings. Unfortunately, this study lacks data regarding participants’ trait-level aggression propensity, and it lacks a healthy control group on which to make comparisons about a wide range of individual differences. There also is no minimal clinically significant difference available in the TAP literature, or regarding subjective aggression in response to alcohol cues among participants with AUD. These scientific advances would be helpful to the oxytocin and alcohol-related IPA literature. Future studies should also more thoroughly assess sexual identity, as IPA is both highly prevalent among members of the LGBTQ+ community and subject to numerous additional intervention barriers (Calton et al., 2016; Whitfield et al., 2021).
An important line of recent preclinical research suggests that intranasally-delivered oxytocin has the ability to cross the blood-brain barrier (Lee et al., 2020; Oppong-Damoah et al., 2019; Zaman et al., 2018), challenging longstanding translational questions regarding oxytocin pharmacokinetics and effectiveness of intranasal delivery (Guastella et al., 2013; Lee and Jayant, 2019). Evidence of these effects in humans is promising, but many opportunities remain to delineate ideal administration routes (Guastella et al., 2013; Quintana et al., 2018). It is possible that a single dose is insufficient to overcome salient social or substance cues, such as partner conflict and alcohol craving in the context of AUD; thus, future studies using a more intensive repeated dosing design are warranted (Horta et al., 2020). It may also prove effective to pair oxytocin with non-pharmacologic talk therapies that can optimally leverage its effects (Flanagan and Mitchell, 2019) and to consider a precision medicine framework to identify individuals most likely to respond to oxytocin (Andari et al., 2017).
Conclusions
A single dose of intranasal oxytocin (40 IU) was safe and well tolerated in this sample but did not result in reduced craving or aggression. Importantly, findings from this rigorously designed and implemented trial contradict some emerging literature suggesting that oxytocin might increase aggression propensity. Further study is warranted to determine if more intensive dosing or administration approaches are more likely to yield clinically meaningful outcomes.
Supplementary Material
Role of the Funding Source.
This manuscript is the result of work supported, in part, by the National Institute on Alcohol Abuse and Alcoholism (K23AA023845, K23AA027307, and T32AA007474), and the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment.
Footnotes
Declarations of interest: None
References
- Afifi TO, MacMillan H, Cox BJ, Asmundson GJG, Stein MB, Sareen J, 2009. Mental Health Correlates of Intimate Partner Violence in Marital Relationships in a Nationally Representative Sample of Males and Females. Journal of Interpersonal Violence 24(8), 1398–1417 10.1177/0886260508322192 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (5th ed.). Author, Arlington, VA. [Google Scholar]
- Andari E, Hurlemann R, Young LJ, 2017. A precision medicine approach to oxytocin trials. Behavioral Pharmacology of Neuropeptides: Oxytocin, 559–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN, 2011. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences 15(7), 301–309 10.1016/j.tics.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Basile KC, Arias I, Desai S, Thompson MP, 2004. The differential association of intimate partner physical, sexual, psychological, and stalking violence and posttraumatic stress symptoms in a nationally representative sample of women. Journal of Traumatic Stress 17(5), 413–421 [DOI] [PubMed] [Google Scholar]
- Becker HC, King CE, Griffin WC, 2017. Oxytocin reduces alcohol self-administration and stress-induced alcohol relapse behavior in mice. Alcohol 60, 218 10.1016/j.alcohol.2017.02.250 [DOI] [Google Scholar]
- Berends YR, Tulen J, Wierdsma AI, van Pelt J, Feldman R, Zagoory-Sharon O, de Rijke YB, Kushner SA, van Marle H, 2019. Intranasal administration of oxytocin decreases task-related aggressive responses in healthy young males. Psychoneuroendocrinology 106, 147–154 10.1016/j.psyneuen.2019.03.027 [DOI] [PubMed] [Google Scholar]
- Bonomi AE, Thompson RS, Anderson M, Reid RJ, Carrell D, Dimer JA, Rivara FP, 2006. Intimate Partner Violence and Women’s Physical, Mental, and Social Functioning. American Journal of Preventative Medicine 30(6), 458–466 10.1016/j.amepre.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS, 2011. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PloS one 6(11), e27237–e27237 10.1371/journal.pone.0027237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiding MJ, Black MC, Ryan GW, 2008. Prevalence and Risk Factors of Intimate Partner Violence in Eighteen U.S. States/Territories, 2005. American Journal of Preventative Medicine 34(2), 112–118 10.1016/j.amepre.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Calton JM, Cattaneo LB, Gebhard KT, 2016. Barriers to help seeking for lesbian, gay, bisexual, transgender, and queer survivors of intimate partner violence. Trauma, Violence, & Abuse 17(5), 585–600 [DOI] [PubMed] [Google Scholar]
- Carbone-López K, Kruttschnitt C, Macmillan R, 2006. Patterns of intimate partner violence and their associations with physical health, psychological distress, and substance use. Public Health Reports 121(4), 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, Kingdon D, Ellenbogen MA, 2014. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: moderation by method and mental health. Psychoneuroendocrinology 49, 161–170 10.1016/j.psyneuen.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2003. Costs of intimate partner violence against women in the United States. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Chermack ST, Grogan-Kaylor A, Perron BE, Murray RL, De Chavez P, Walton MA, 2010. Violence among men and women in substance use disorder treatment: A multi-level event-based analysis. Drug and Alcohol Dependence 112(3), 194–200 10.1016/j.drugalcdep.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP, 1986. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. British Journal of Addiction 81(5), 655–660 10.1111/j.1360-0443.1986.tb00385.x [DOI] [PubMed] [Google Scholar]
- Crane CA, Godleski SA, Przybyla SM, Schlauch RC, Testa M, 2016. The proximal effects of acute alcohol consumption on male-to-female aggression: A meta-analytic review of the experimental literature. Trauma, Violence, & Abuse 17(5), 520–531 10.1177/1524838015584374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JE, Pelowski S, 2000. Predictors of dropout among men who batter: A review of studies with implications for research and practice. Violence and Victims 15(2), 137–160 [PubMed] [Google Scholar]
- Daniels JW, Murphy CM, 1997. Stages and processes of change in batterers’ treatment. Cognitive and Behavioral Practice 4(1), 123–145 [Google Scholar]
- de Jong TR, Neumann ID, 2017. Oxytocin and aggression. Behavioral pharmacology of neuropeptides: oxytocin, 175–192 10.1007/7854_2017_13 [DOI] [Google Scholar]
- Derrick JL, Testa M, 2017. Temporal effects of perpetrating or receiving intimate partner aggression on alcohol consumption: A daily diary study of community couples. Journal of Studies on Alcohol and Drugs 78(2), 213–221 10.15288/jsad.2017.78.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, Gillath O, Pressman SD, Black LL, Bartz JA, Moskovitz J, Stetler DA, 2014. When the Love Hormone Leads to Violence Oxytocin Increases Intimate Partner Violence Inclinations Among High Trait Aggressive People. Social Psychological and Personality Science 5(6), 691–697 10.1177/1948550613516876 [DOI] [Google Scholar]
- Duke AA, Giancola PR, Morris DH, Holt JC, Gunn RL, 2011. Alcohol dose and aggression: Another reason why drinking more is a bad idea. Journal of Studies on Alcohol and Drugs 72(1), 34–43 10.15288/jsad.2011.72.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AA, Smith K, Oberleitner L, Westphal A, McKee SA, 2018. Alcohol, drugs, and violence: A meta-meta-analysis. Psychology of Violence 8(2), 238 10.1037/vio0000106 [DOI] [Google Scholar]
- Eckhardt CI, Parrott DJ, Sprunger JG, 2015. Mechanisms of Alcohol-Facilitated Intimate Partner Violence. Violence against women 21(8), 939–957 10.1177/1077801215589376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt CI, Parrott DJ, Swartout KM, Leone RM, Purvis DM, Massa AA, Sprunger JG, 2021. Cognitive and Affective Mediators of Alcohol-Facilitated Intimate-Partner Aggression. Clinical Psychological Science 9(3), 385–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsberg M, Jansen HA, Heise L, Watts CH, Garcia-Moreno C, 2008. Intimate partner violence and women’s physical and mental health in the WHO multi-country study on women’s health and domestic violence: an observational study. The Lancet 371(9619), 1165–1172 10.1016/s0140-6736(08)60522-x [DOI] [PubMed] [Google Scholar]
- Evans SL, Dal Monte O, Noble P, Averbeck BB, 2014. Intranasal oxytocin effects on social cognition: A critique. Brain Research 1580, 69–77 10.1016/j.brainres.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Mitchell JM, 2019. Augmenting treatment for posttraumatic stress disorder and co-occurring conditions with oxytocin. Current Treatment Options in Psychiatry 6(2), 132–142 10.1007/s40501-019-00171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Phillips MJ, Lifshitz M, Lionetti TA, Spero DE, Ghassemzedeh N, Doty L, Umhau JC, Rawlings RR, 2011. Fluoxetine treatment of alcoholic perpetrators of domestic violence: a 12-week, double-blind, randomized, placebo-controlled intervention study. The Journal of Clinical Psychiatry 72(1), 60–65 10.4088/JCP.09m05256gry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Parrott DJ, 2008. Further evidence for the validity of the Taylor Aggression Paradigm. Aggressive Behavior 34(2), 214–229 10.1002/ab.20235 [DOI] [PubMed] [Google Scholar]
- Giancola PR, Zeichner A, 1995. Construct validity of a competitive reaction-time aggression paradigm. Aggressive Behavior 21(3), 199–204 [DOI] [Google Scholar]
- Graham K, Bernards S, Wilsnack SC, Gmel G, 2011. Alcohol may not cause partner violence but it seems to make it worse: A cross national comparison of the relationship between alcohol and severity of partner violence. Journal of Interpersonal Violence 26(8), 1503–1523 10.1177/0886260510370596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB, 2013. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38(5), 612–625 10.1016/j.psyneuen.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Horta M, Kaylor K, Feifel D, Ebner NC, 2020. Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neuroscience & Biobehavioral Reviews 108, 1–23 10.1016/j.neubiorev.2019.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt CS, Crowe ML, West SJ, Vize CE, Carter NT, Chester DS, Miller JD, in press. An empirically based power primer for laboratory aggression research. Aggressive Behavior 10.1002/ab.21996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Becker HC, 2019. Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice. Psychopharmacology 236(9), 2613–2622 10.1007/s00213-019-05233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Gano A, Becker HC, 2020. The role of oxytocin in alcohol and drug abuse. Brain Research 1736, 146761 10.1016/j.brainres.2020.146761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC, 2017a. Oxytocin Reduces Ethanol Self-Administration in Mice. Alcoholism: Clinical and Experimental Research 41(5), 955–964 10.1111/acer.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, McGinty JF, Becker HC, 2017b. Effects of oxytocin on stress-induced reinstatement of alcohol-seeking in mice with and without a history of stress. Alcohol 60, 231–232 10.1016/J.ALCOHOL.2017.02.305 [DOI] [Google Scholar]
- Koch S, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2019. Effects of intranasal oxytocin on distraction as emotion regulation strategy in patients with post-traumatic stress disorder. European Neuropsychopharmacology 29(2), 266–277 [DOI] [PubMed] [Google Scholar]
- Koob GF, 2013. Addiction is a reward deficit and stress surfeit disorder. Frontiers in Psychiatry 4, 1–18 10.3389/fpsyt.2013.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E, 2005. Oxytocin increases trust in humans. Nature 435(7042), 673–676 [DOI] [PubMed] [Google Scholar]
- Lee MR, Jayant RD, 2019. Penetration of the blood-brain barrier by peripheral neuropeptides: new approaches to enhancing transport and endogenous expression. Cell and Tissue Research 375(1), 287–293 10.1007/s00441-018-2959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, Leggio L, 2016. Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs 30, 109–123 10.1007/s40263-016-0313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Shnitko TA, Blue SW, Kaucher AV, Winchell AJ, Erikson DW, Grant KA, Leggio L, 2020. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nature Communications 11(1), 1–10 10.1038/s41467-020-15942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Weerts EM, 2016. Oxytocin for the treatment of drug and alcohol use disorders. Behavioural Pharmacology 27(8), 640–648 10.1097/FBP.0000000000000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisring PA, Grigorian HL, 2016. Self-defense, retaliation, and gender: Clarifying motivations for physical partner violence. Journal of Family Violence 31(8), 949–953 [Google Scholar]
- Leng G, Ludwig M, 2016. Intranasal oxytocin: Myths and delusions. Biological Psychiatry 79(3), 243–250 10.1016/j.biopsych.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Leonard KE, Quigley BM, 2017. Thirty years of research show alcohol to be a cause of intimate partner violence: future research needs to identify who to treat and how to treat them. Drug and Alcohol Review 36(1), 7–9 10.1111/dar.12434 [DOI] [PubMed] [Google Scholar]
- Leonard KE, Smith PH, Homish GG, 2014. Concordant and discordant alcohol, tobacco, and marijuana use as predictors of marital dissolution. Psychology of Addictive Behaviors 28(3), 780 10.1037/a0034053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque DA, Velicer WF, Castle PH, Greene RN, 2008. Resistance among domestic violence offenders: Measurement development and initial validation. Violence Against Women 14(2), 158–184 [DOI] [PubMed] [Google Scholar]
- Lipsky S, Caetano R, Roy-Byrne P, 2011. Triple jeopardy: impact of partner violence perpetration, mental health and substance use on perceived unmet need for mental health care among men. Social Psychiatry and Psychiatric Epidemiology 46(9), 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky S, Krupski A, Roy-Byrne P, Lucenko B, Mancuso D, Huber A, 2010. Effect of co-occurring disorders and intimate partner violence on substance abuse treatment outcomes. Journal of Substance Abuse Treatment 38(3), 231–244 10.1016/j.jsat.2009.12.005 [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM, 2010. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry 18(1), 1–21 10.3109/10673220903523615 [DOI] [PubMed] [Google Scholar]
- MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J, 2016. Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacology Biochemistry and Behavior 140, 27–32 10.1016/j.pbb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT, 2012. Breaking the loop: Oxytocin as a potential treatment for drug addiction. Hormones and Behavior 61(3), 331–339 10.1016/j.yhbeh.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Melby K, Gråwe RW, Aamo TO, Salvesen Ø, Spigset O, 2019. Effect of intranasal oxytocin on alcohol withdrawal syndrome: A randomized placebo-controlled double-blind clinical trial. Drug and Alcohol Dependence 197, 95–101 10.1016/j.drugalcdep.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Melby K, Gråwe RW, Aamo TO, Skovlund E, Spigset O, 2021. Efficacy of Self-Administered Intranasal Oxytocin on Alcohol Use and Craving After Detoxification in Patients With Alcohol Dependence. A Double-Blind Placebo-Controlled Trial. Alcohol and Alcoholism 56(5), 565–572 10.1093/alcalc/agaa133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Weinstein D, Arcuni P, Laxamana J, Woolley J, 2015. The effects of intranasal oxytocin on social cognition, implicit preferences and craving in alcoholics. Drug and Alcohol Dependence 146, e42 10.1016/j.drugalcdep.2014.09.485 [DOI] [Google Scholar]
- Mitchell JM, Arcuni PA, Weinstein D, Woolley JD, 2016. Intranasal oxytocin selectively modulates social perception, craving, and approach behavior in subjects with alcohol use disorder. Journal of Addiction Medicine 10(3), 182–189 10.1097/ADM.0000000000000213 [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR, 1987. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol 96, 122–126 10.1037//0021-843x.96.2.122 [DOI] [PubMed] [Google Scholar]
- Murphy CM, Ting LA, Jordan LC, Musser PH, Winters JJ, Poole GM, Pitts SC, 2018. A randomized clinical trial of motivational enhancement therapy for alcohol problems in partner violent men. Journal of Substance Abuse Treatment 89, 11–19 10.1016/j.jsat.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CM, Winters J, O’Farrell TJ, Fals-Stewart W, Murphy MM, 2005. Alcohol consumption and intimate partner violence by alcoholic men: comparing violent and nonviolent conflicts. Psychology of Addictive Behaviors 19(1), 35. [DOI] [PubMed] [Google Scholar]
- Ne’eman R, Perach-Barzilay N, Fischer-Shofty M, Atias A, Shamay-Tsoory SG, 2016. Intranasal administration of oxytocin increases human aggressive behavior. Hormones and Behavior 80, 125–131 10.1016/j.yhbeh.2016.01.015 [DOI] [PubMed] [Google Scholar]
- Norlander B, Eckhardt C, 2005. Anger, hostility, and male perpetrators of intimate partner violence: A meta-analytic review. Clin Psychol Rev 25(2), 119–152 10.1016/j.cpr.2004.10.001 [DOI] [PubMed] [Google Scholar]
- O’Farrell TJ, Fals-Stewart W, Murphy M, Murphy CM, 2003. Partner violence before and after individually based alcoholism treatment for male alcoholic patients. Journal of Consulting and Clinical Psychology 71(1), 92 10.1037//0022-006x.71.1.92 [DOI] [PubMed] [Google Scholar]
- Oppong-Damoah A, Zaman RU, D’Souza MJ, Murnane KS, 2019. Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Hormones and Behavior 108, 20–29 10.1016/j.yhbeh.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott DJ, Miller CA, Hudepohl AD, 2015. Immediate and short-term reactions to participation in laboratory aggression research. Psychology of Violence 5, 209–216 10.1037/a0035922 [DOI] [Google Scholar]
- Pedersen CA, 2017. Oxytocin, Tolerance, and the Dark Side of Addiction, International Review of Neurobiology. pp. 239–274. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov‐Polevoi A, Casey RL, Fender T, Garbutt JC, 2013. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism: Clinical and Experimental Research 37(3), 484–489 10.1111/j.1530-0277.2012.01958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Steck MR, Krause EG, 2020. Oxytocin treatment for alcoholism: Potential neurocircuitry targets. Neuropharmacology 171, 108091 10.1016/j.neuropharm.2020.108091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C, Kearns MC, McIntosh WL, Estefan LF, Nicolaidis C, McCollister KE, Gordon A, Florence C, 2018. Lifetime economic burden of intimate partner violence among US adults. American Journal of Preventive Medicine 55(4), 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfundmair M, Reinelt A, DeWall CN, Feldmann L, 2018. Oxytocin strengthens the link between provocation and aggression among low anxiety people. Psychoneuroendocrinology 93, 124–132 10.1016/j.psyneuen.2018.04.025 [DOI] [PubMed] [Google Scholar]
- Quigley BM, Crane CA, Testa M, 2013. Dyadic alcohol use as a moderator of the relationship between partner conflict and subsequent day relationship functioning: a daily diary analysis. Alcoholism: Clinical and Experimental Research 37, 24A–24A 10.1111/acer.12162 [DOI] [Google Scholar]
- Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, Becker B, 2021. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Molecular Psychiatry 26(1), 80–91 10.1038/s41380-020-00864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Smerud KT, Andreassen OA, Djupesland PG, 2018. Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Therapeutic Delivery 9(7), 515–525 10.4155/tde-2018-0002 [DOI] [PubMed] [Google Scholar]
- Romero-Martínez Á, Lila M, Martínez M, Pedrón-Rico V, Moya-Albiol L, 2016. Improvements in empathy and cognitive flexibility after court-mandated intervention program in intimate partner violence perpetrators: The role of alcohol abuse. International Journal of Environmental Research and Public Health 13(4), 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romney C, Hahn-Holbrook J, Norman GJ, Moore A, Holt-Lunstad J, 2019. Where is the love? A double-blind, randomized study of the effects of intranasal oxytocin on stress regulation and aggression. International Journal of Psychophysiology 136, 15–21 10.1016/j.ijpsycho.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Fulenwider HD, 2021. Alcohol and Oxytocin: scrutinizing the relationship. Neuroscience & Biobehavioral Reviews 10.1016/j.neubiorev.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J,B, Aasland O,G, Babor T,F, De La, F J,R, Grant M, 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 88(6), 791–804 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Schumm JA, O’Farrell TJ, Murphy CM, Fals-Stewart W, 2009. Partner violence before and after couples-based alcoholism treatment for female alcoholic patients. Journal of Consulting and Clinical Psychology 77(6), 1136 10.1037/a0017389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A, 2016. The social salience hypothesis of oxytocin. Biological Psychiatry 79(3), 194–202 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59, 22–33 [PubMed] [Google Scholar]
- Sippel LM, Allington CE, Pietrzak RH, Harpaz-Rotem I, Mayes LC, Olff M, 2017. Oxytocin and stress-related disorders: neurobiological mechanisms and treatment opportunities. Chronic Stress 1, 1–15 10.1177/2470547016687996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Homish GG, Leonard KE, Cornelius JR, 2012. Intimate partner violence and specific substance use disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychology of Addictive Behaviors 26(2), 236–245 10.1037/a0024855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back, Measuring alcohol consumption. Springer, pp. 41–72. [Google Scholar]
- Spanier GB, 1976. Measuring Dyadic Adjustment: New Scales for Assessing the Quality of Marriage and Similar Dyads. Journal of Marriage and Family 38(1), 15–28 10.2307/350547 [DOI] [Google Scholar]
- Stauffer CS, Meinzer NK, Morrison T, Wen J, Radanovich L, Leung D, Niles A, O’Donovan A, Batki SL, Woolley JD, 2019. Effects of oxytocin administration on cue-induced craving in co-occurring alcohol use disorder and PTSD: a within-participant randomized clinical trial. Alcoholism: Clinical and Experimental Research 10.1111/acer.14217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM, Bohidar AE, Ndem JR, Vogel IR, O’Kane CM, 2017. Oxytocin reduces alcohol consumption in prairie voles. Physiology & Behavior 179, 411–421 10.1016/j.physbeh.2017.07.021 [DOI] [PubMed] [Google Scholar]
- Straus MA, 2008. Dominance and symmetry in partner violence by male and female university students in 32 nations. Children and Youth Services Review 30(3), 252–275 10.1016/j.childyouth.2007.10.004 [DOI] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy SUE, Sugarman DB, 1996. The Revised Conflict Tactics Scales (CTS2): Development and Preliminary Psychometric Data. Journal of Family Issues 17(3), 283–316 10.1177/019251396017003001 [DOI] [Google Scholar]
- Stuart GL, Shorey RC, Moore TM, Ramsey SE, Kahler CW, O’Farrell TJ, Strong DR, Temple JR, Monti PM, 2013. Randomized clinical trial examining the incremental efficacy of a 90-minute motivational alcohol intervention as an adjunct to standard batterer intervention for men. Addiction 108(8), 1376–1384 10.1111/add.12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, 1989. Assessment of Alcohol Withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction 84(11), 1353–1357 10.1111/j.1360-0443.1989.tb00737.x [DOI] [PubMed] [Google Scholar]
- Taft C, Murphy CM, King DW, Musser PH, DeDeyn JM, 2003. Process and treatment adherence factors in group cognitive behavioral therapy for partner violent men. Journal of Consulting and Clinical Psychology 71(4), 812–820 [DOI] [PubMed] [Google Scholar]
- Taft CT, Macdonald A, Monson CM, Walling SM, Resick PA, Murphy CM, 2013. “Strength at home” group intervention for military populations engaging in intimate partner violence: Pilot findings. Journal of Family Violence 28(3), 225–231 10.1007/s10896-013-9496-y [DOI] [Google Scholar]
- Taft CT, Murphy CM, 2007. The working alliance in intervention for partner violence perpetrators: Recent research and theory. Journal of Family Violence 22(1), 11–18 [Google Scholar]
- Taft CT, Murphy CM, Elliott JD, Morrel TM, 2001. Attendance-enhancing procedures in group counseling for domestic abusers. Journal of Counseling Psychology 48(1), 51 [Google Scholar]
- Taylor SP, 1967. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. Journal of Personality 35(2), 297–310 10.1111/j.1467-6494.1967.tb01430.x [DOI] [PubMed] [Google Scholar]
- Testa M, Derrick JL, 2013. A Daily Process Examination of the Temporal Association Between Alcohol Use and Verbal and Physical Aggression in Community Couples. Psychology of Addictive Behaviors 28(1), 127–138 10.1037/a0032988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa M, Quigley BM, Leonard KE, 2003. Does alcohol make a difference? Within-participants comparison of incidents of partner violence. Journal of Interpersonal Violence 18(7), 735–743 10.1177/0886260503253232 [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Kirson D, Zallar LJ, McConnell SA, Vendruscolo J, Ho CP, Oleata CS, Khom S, Manning M, Lee MR, 2019. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS biology 17(4), e2006421 10.1371/journal.pbio.2006421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena A, King A, Lee R, de Wit H, 2018. Intranasal oxytocin does not modulate responses to alcohol in social drinkers. Alcoholism: Clinical and Experimental Research 42(9), 1725–1734 10.1111/acer.13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ, 2016. Statistical and Methodological Considerations for the Interpretation of Intranasal Oxytocin Studies. Biol Psychiatry 79(3), 251–257 10.1016/j.biopsych.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield DL, Coulter R, Langenderfer-Magruder L, Jacobson D, 2021. Experiences of intimate partner violence among lesbian, gay, bisexual, and transgender college students: The intersection of gender, race, and sexual orientation. Journal of Interpersonal Violence 36(11–12), 6040–6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S, 2007. Oxytocin increases generosity in humans. PloS one 2(11), e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman RU, Mulla NS, Gomes KB, D’Souza C, Murnane KS, D’Souza MJ, 2018. Nanoparticle formulations that allow for sustained delivery and brain targeting of the neuropeptide oxytocin. International Journal of Pharmaceutics 548(1), 698–706 10.1016/j.ijpharm.2018.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Liu C, Li T, Xu Z, Fung B, Feng C, Wu H, Luo Y, Wang L, 2019. Intranasal oxytocin reduces reactive aggression in men but not in women: A computational approach. Psychoneuroendocrinology 108, 172–181 10.1016/j.psyneuen.2019.06.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


