Abstract
Background:
Sarcopenia is associated with complications and inferior oncologic outcomes in solid tumors. Axial computed tomography (CT) scans can be used to evaluate sarcopenia, however manual quantification is laborious. We sought to validate an automated method of quantifying muscle cross-sectional area (CSA) in patients with pancreatic adenocarcinoma (PDAC).
Methods:
Mid-L3 CT images from patients with PDAC were analyzed: CSAs of skeletal muscle (SM) were measured using manual segmentation and the software AutoMATiCA, and then compared with linear regression.
Results:
Five-hundred-twenty-five unique scans were analyzed. There was robust correlation between manual and automated segmentation for L3 CSA (R2 0.94, P<0.001). Bland-Altman analysis demonstrated a consistent overestimation of muscle CSA by AutoMATiCA with a mean difference of 5.7%. A correction factor of 1.06 was validated using a unique test dataset of 36 patients with non-PDAC peripancreatic malignancies.
Conclusions:
Automated muscle CSA measurement with AutoMATiCA is highly efficient and yields results highly correlated with manual measurement. These findings support the potential use of high-throughput sarcopenia analysis with abdominal CT scans for both clinical and research purposes.
Keywords: Sarcopenia, Pancreatic Ductal Adenocarcinoma, Body Composition
Introduction
Sarcopenia, defined as low muscle mass, is a known predictor of poor oncologic outcomes in several types of cancer including head & neck squamous cell carcinoma, hepatocellular carcinoma, and pancreatic adenocarcinoma (PDAC) and is associated with increased postoperative complications.(1–4) A 2020 meta-analysis of patients undergoing gastrointestinal surgery found an odds ratio of 3.01 for post-operative complications for patients with sarcopenia.(5) Sarcopenia can be measured in multiple ways, including computed tomography (CT) imaging, dual energy X-ray absorptiometry, or bioelectrical impedance analysis.(6) In patients with cancer, CT imaging is of particular utility and convenience as they likely undergo regular imaging as part of their treatment course and muscle compartment size can be calculated from this cross-sectional imaging. Several studies have demonstrated that axial CT imaging can be reliably used for evaluation of sarcopenia at several vertebral levels, most commonly L3.(7) However, quantification through this method is laborious and logistically challenging, as each L3 image must be manually segmented to separate out muscle bellies from the surrounding adipose tissue and osseous structures.
In recent years there have been several attempts to use machine learning to expedite this analysis process. Paris et al. recently published a new automated method to perform this body composition analysis.(8) The Automated Muscle and Adipose Tissue Composition Analysis (AutoMATiCA) is a Python-based software that uses a neural network to automatically segment an L3-level axial CT image into the following discrete tissue types: skeletal muscle, intermuscular adipose tissue, visceral adipose tissue, and subcutaneous adipose tissue, calculating both cross sectional area and density via Hounsfield units. Scans can be systematically analyzed in under a minute, greatly increasing throughput, and inter-rater reliability through reliance on a single algorithm. AutoMATiCA has been shown to correlate well with manually-assessed body composition quantifications; however, validation was initially performed in a predominantly noncancer population with 300 of 893 being designated as “critically ill” and 253 being organ donors. Of the 230 patients with a cancer diagnosis, 181 had clear cell renal cell carcinoma; only 49, or 5.5 % of the total population, had PDAC.
Sarcopenia, found at diagnosis or developed throughout the course of treatment, is extremely common in PDAC.(9) Muscle loss in this disease is multifactorial, and the underlying mechanisms remain somewhat poorly understood; however, it is known that these patients have worse medical and surgical outcomes even in the setting of high overall body weight.(10) For example, Kurita et al showed that sarcopenic patients undergoing FOLFIRINOX treatment had a significantly decreased overall survival (11.3 vs 17.0 months) and a 50% decrease in time to treatment failure, and Joglekar et al showed that sarcopenia was an independent predictor of poorer outcomes including grade III complications, for patients undergoing pancreatectomy for PDAC.(2, 11) Because the manual quantification of sarcopenia is laborious, it is not of good clinical utility and limits the pace of research in this area. Automated analysis offers an opportunity to much more efficiently study the relationship between PDAC and sarcopenia; we sought to independently validate this process specifically for use in this high mortality disease.
Material & Methods
Study Population
Patients with PDAC treated at our institution between 2007 and 2018 who were consented to the Oregon Pancreas Tissue Registry were identified. Additional clinicopathologic data, including staging, tumor characteristics, height, weight, and body mass index (BMI) were obtained via our institutional National Surgical Quality Improvement Project (NSQIP) database and institutional cancer registry, which is accredited by the American College of Surgeon’s Commission on Cancer (CoC).
Body Composition Analysis
Computed tomography (CT) scans with IV contrast containing axial images including the mid-L3 level were identified for selected patients. The CT at diagnosis was captured in all patients, along with all longitudinal CTs when available. DICOM files of the mid-L3 level were abstracted as previously described by Mourtzakis et al. and quality checked.(6) Images were analyzed manually using sliceOmatic software (Tomovision, Canada) for the following variables: skeletal muscle cross sectional area (CSA), intermuscular adipose tissue (IMAT) CSA, visceral adipose tissue (VAT) CSA, subcutaneous adipose tissue (SAT) CSA, and skeletal muscle Hounsfield units (HU). Skeletal muscle CSA was computed by combining the CSAs of psoas, paraspinal and abdominal wall musculature. The same DICOM files were similarly fed into the AutoMATiCA software and identical datapoints tabulated by an individual blinded to the results of the manual quantification. Sarcopenia was defined according to Prado et al., setting a skeletal muscle index threshold of 38.5 cm2/m2 as the cutoff for women and 52.4 cm2/m2 as the cutoff for men.(12)
Statistical Analysis
Descriptive statistics were tabulated separately for male and female patients, utilizing Chi squared testing and Student’s t-test for group comparisons of categorical and continuous variables, respectively. Linear regression was performed, comparing scan-specific values from manual and AutoMATiCA quantification for the five analyzed outcomes, with Pearson’s correlation coefficients and residuals calculated for each. Regression lines were forced to intersect the origin.
Validation of Correction Factor
Due to differences in the method of AutoMATiCA calculation of muscle CSA (the primary variable of interest) compared to the manual method, a correction factor was necessary. This correction factor was validated with an additional subset of patients with anatomically approximate tumors (distal cholangiocarcinoma and ampullary carcinoma) treated during the same time period at our institution. Using the correction factor calculated from patients with PDAC, the slope of the line of best fit was compared prior to and following correction for the validation data set.
Results
Clinicopathologic Characteristics
A total of 346 consented patients with PDAC were identified, 148 female and 198 male, with a median age at diagnosis of 64 and median BMI of 25.8 (Table 1). Metastases were identified at diagnosis in 107 patients. Of the 238 nonmetastatic patients, 75 were designated AJCC 8th edition stage I, 130 stage II, and 34 stage III. Based on manual quantification, 187 (54%) of patients were sarcopenic at time of diagnosis.
Table 1:
Clinicopathologic Characteristics of Patients with Pancreatic Adenocarcinoma Analyzed for Body Composition
| Variable | Total (n=346); N (%) |
|---|---|
| Age at Diagnosis, years; median [IQR] | 64 [57–72] |
| Race | |
| White Non-Hispanic | 320 (92.5) |
| Other | 26 (7.5) |
| Height, meters; median [IQR] | 1.70 [1.63–1.80] |
| Weight at Diagnosis, kg; median [IQR] | 76.3 [65.3–88.5] |
| Body Mass Index, kg/m 2 ; median [IQR] | 25.8 [22.8–29.3] |
| Tumor Grade | |
| Grade I | 8 (2.3) |
| Grade II | 87 (25.1) |
| Grade III | 80 (23.1) |
| Unknown | 171 (49.4) |
| Lymphovascular Invasion | |
| Absent | 49 (14.2) |
| Present | 86 (24.9) |
| Unknown/Indeterminate | 211 (61.0) |
| Tumor Size, cm; median [IQR] | 3.5 [2.7–4.4] |
| AJCC T Stage | |
| T1 | 24 (6.9) |
| T2 | 79 (22.8) |
| T3 | 166 (48.0) |
| T4 | 55 (15.9) |
| Tx | 22 (6.4) |
| Nodal Status | |
| Node Negative | 56 (16.2) |
| Node Positive | 140 (40.5) |
| Not Evaluated | 150 (43.4) |
| AJCC 8th Edition Stage Group | |
| I | 75 (21.7) |
| II | 130 (37.6) |
| III | 34 (9.8) |
| IV | 107 (30.9) |
| L3 Skeletal Muscle Index, cm 2 /m 2 ; median [IQR] | 43.9 [39.0–49.6] |
| Sarcopenic at Diagnosis | 187 (54.0) |
Abbreviations: IQR=Interquartile Range;
AJCC=American Joint Commission on Cancer
Performance of AutoMATiCA and Manual Segmentation
In order to evaluate the performance of AutoMATiCA relative to manual segmentation on the 525 analyzed CT scans, we performed linear regression for L3 muscle CSA as measured by both techniques. AutoMATiCA displayed an excellent correlation with manual segmentation (R2=0.94, P<0.001, Figure 1A). AutoMATiCA also performed excellently for subcutaneous, visceral, intramuscular adipose tissues, and muscle Hounsfield units (Figure 1B–E, R2=0.96, 0.96, 0.91, 0.91, respectively, P<0.001).
Figure 1:
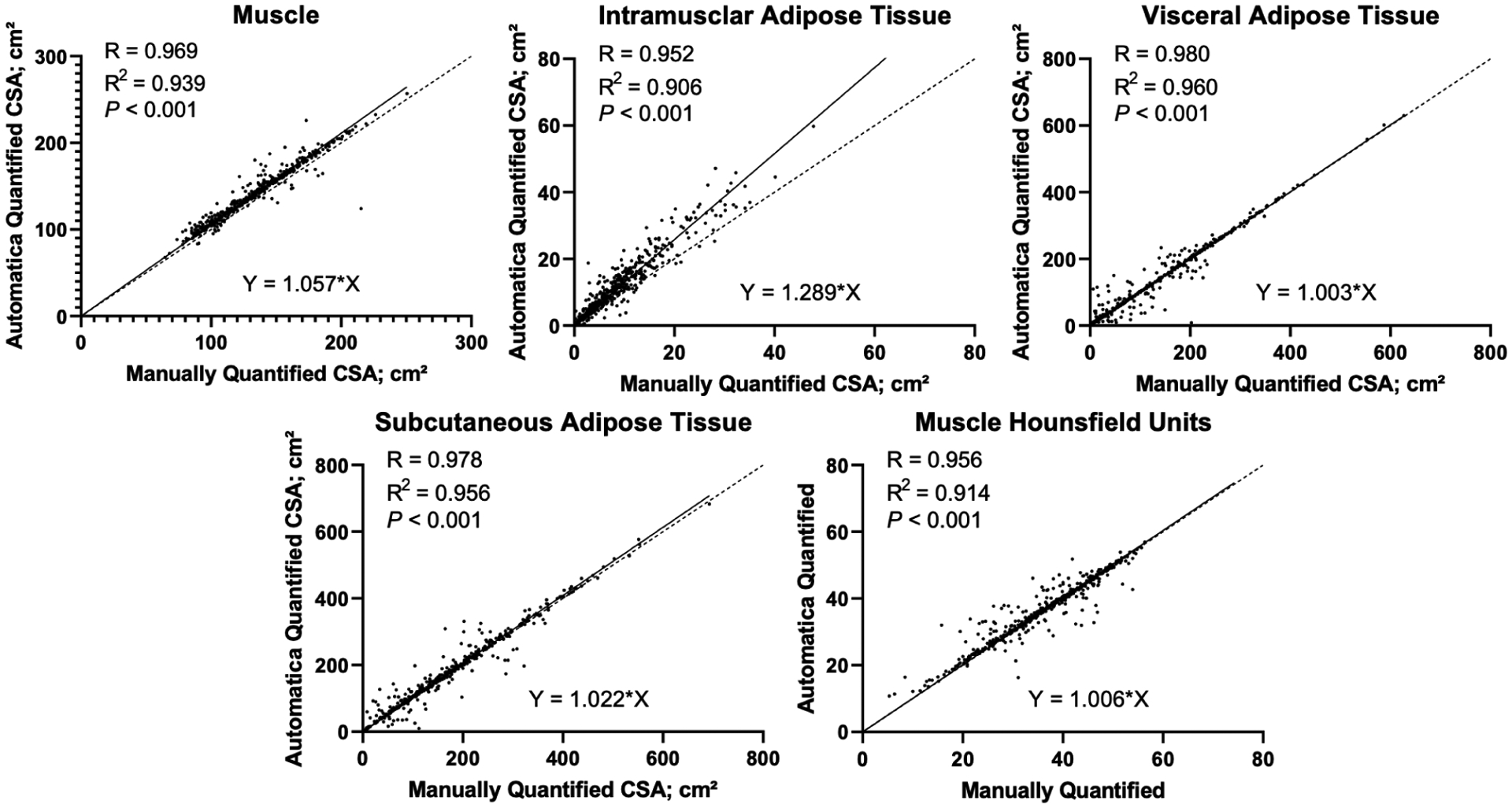
Linear correlation between manual and automatic segmentation of 525 scans from patients with PDAC for each tissue type: skeletal muscle (A) intramuscular adipose tissue (B), visceral adipose tissue (C), and subcutaneous adipose tissue (D) cross sectional areas, as well as muscle Hounsfield units (E).
Derivation of Correction Factor for AutoMATiCA Segmentation
Bland-Altman analysis demonstrated a consistent overestimation of muscle CSA by AutoMATiCA with a mean difference of 7.89 ± 7.94 cm2 or 5.7% ± 5.7% of manual mean. When comparing axial CT images segmented manually with AutoMATiCA, it was clear that AutoMATiCA consistently overestimated muscle CSA by incorrectly measuring non-muscular tissue around the linea alba, spine, and between the paraspinal muscles (Figure 2). As such, a correction factor of 1.06 was pre-specified for validation in a test dataset.
Figure 2:
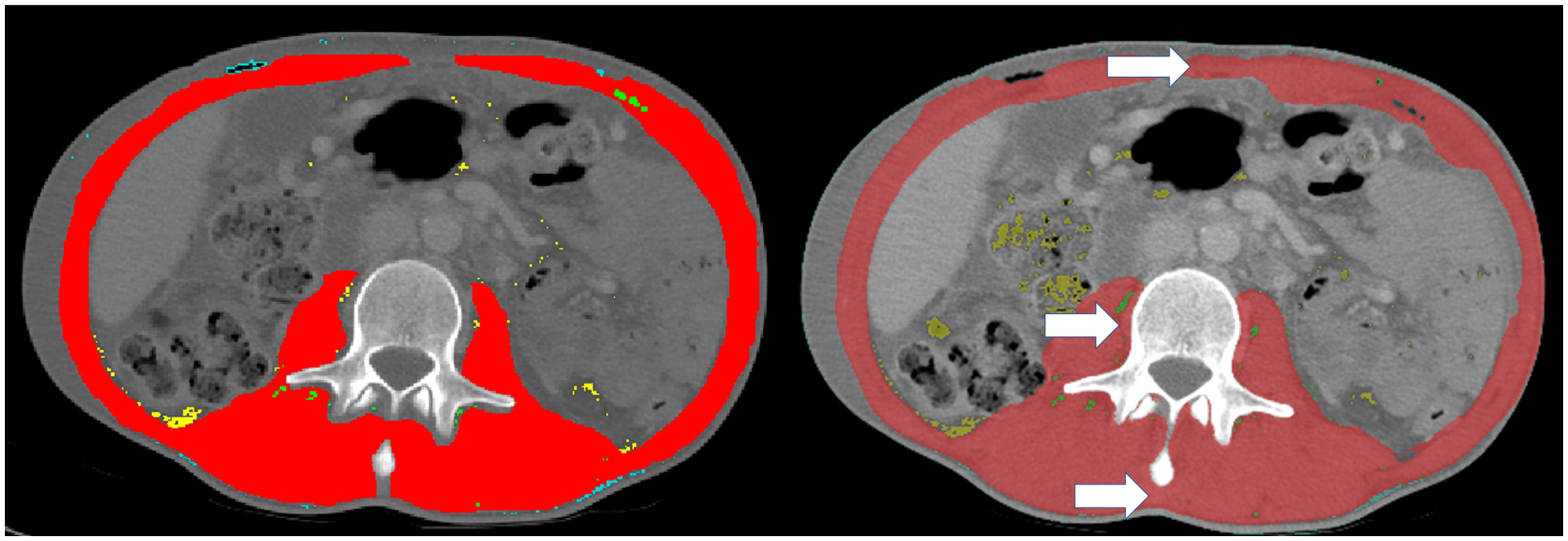
Representative manual segmentation (A) and automatic segmentation (B) of muscle cross sectional area (red). White arrows depict areas of routinely overestimated muscle tissue at linea alba (top), spinous process (bottom), and tissue beside vertebral body (left). Uncommonly overestimated areas include enhanced intraperitoneal fat (yellow arrow).
Validation of Correction Factor
Using scans from 36 consented patients with distal cholangiocarcinoma or ampullary carcinoma, we evaluated the performance of AutoMATiCA for measuring muscle CSA both before and after correction utilizing the pre-specified correction factor of 1.06. The overestimation of muscle CSA was again noted, and application of the correction factor changed the slope of the regression line to 1 (Figure 3).
Figure 3:
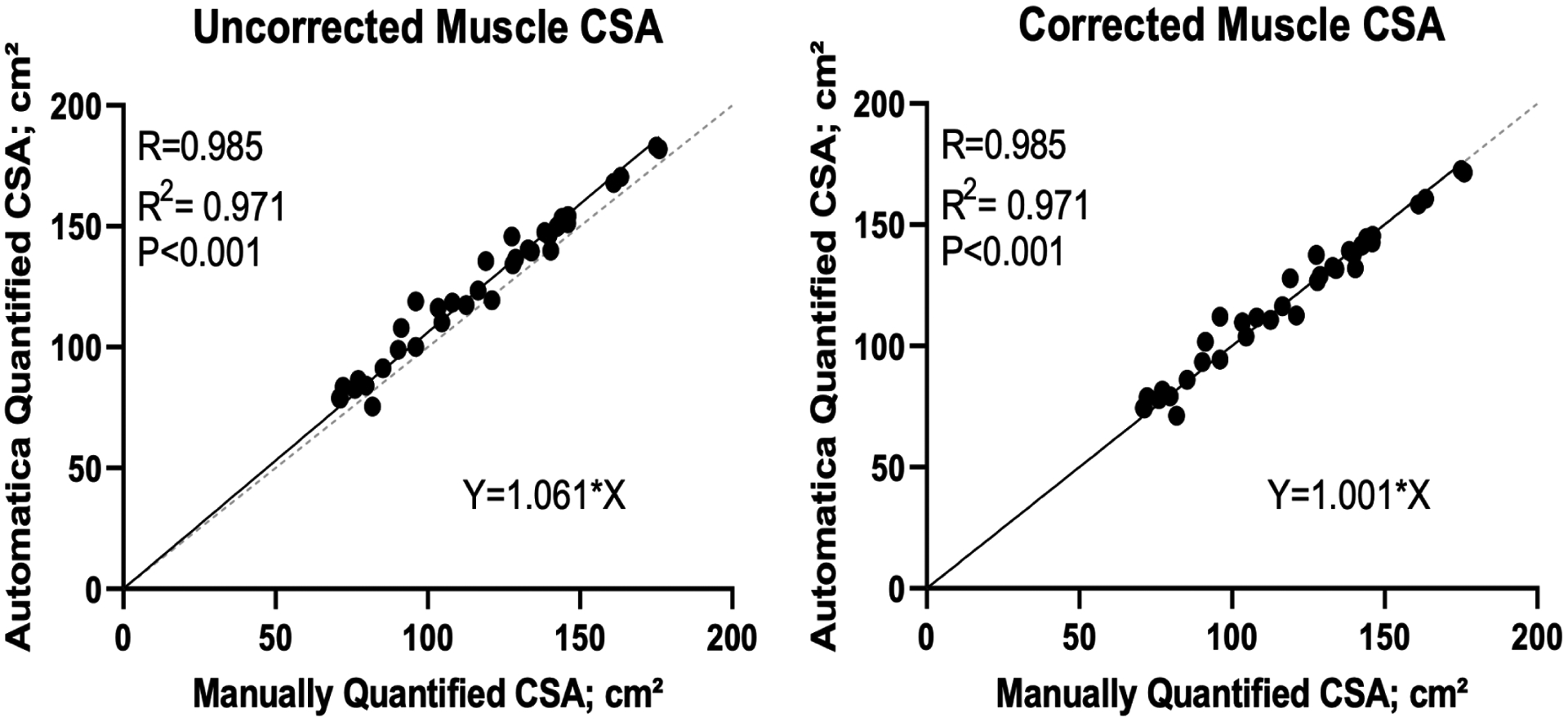
Linear correlation between manual and automatic segmentation of skeletal muscle cross sectional area in 36 scans from patients with distal cholangiocarcinoma or ampullary carcinoma before (A) and after (B) correction.
In order to understand how the correction factor affected accuracy for AutoMATiCA-derived results relative to manually-derived results, we evaluated pre-specified thresholds for sarcopenia within our dataset. L3 skeletal muscle indices (SMIs), which are used to contextualize muscle mass by patient size, were derived by dividing L3 skeletal muscle CSAs by patient height in meters squared. These values were then normalized to yield Z scores, and two thresholds for sarcopenia were chosen: one standard deviation above and below the mean for men and women. For a threshold set one standard deviation above the mean, L3 SMI cutoff thresholds were 46.80 for women and 52.92 for men, compared with 33.14 for women and 36.03 for men for the threshold one standard deviation below the mean. Per manual quantification, 421 and 47 scans met criteria for sarcopenia when the SMI threshold was set one standard deviation above and below the mean, respectively, compared to 361 and 17 scans for uncorrected AutoMATiCA results and 413 and 40 scans for corrected AutoMATiCA results, respectively. Compared with manual results, for the threshold set one standard deviation above mean L3 SMI uncorrected AutoMATiCA results had 63 false negatives (FN), 3 false positives (FP), 101 true negatives (TN), and 358 true positives (TP), yielding an accuracy of 87.4%; corrected AutoMATiCA results had 16 FN, 8 FP, 96 TN, and 405 TP, yielding an accuracy of 95.4%. For the L3 SMI threshold set one standard deviation below the mean, uncorrected AutoMATiCA results had 31 FN, 1 FP, 477 TN, and 16 TP, yielding an accuracy of 93.9%; corrected AutoMATiCA results had 11 FN, 4 FP, 474 TN, and 36 TP, yielding an accuracy of 97.1%.
Discussion
We found that automated analysis with the AutoMATiCA program correlates extremely well with manual segmentation, a relationship that holds true across all tissue types except for intramuscular adipose tissue. For skeletal muscle specifically, the key measure of sarcopenia, the software does not appropriately recognize specific areas such as the linea alba, posterior to the spinous process and immediately adjacent to the vertebral column as non-muscular tissue, leading to a larger calculated CSA proportional to total actual muscle CSA. If left uncorrected, this results in non-generalizability between studies using manual and AutoMATiCA segmentation. To address this, we validated a correction factor utilizing a second population of patients with hepatobiliary cancers, suggesting this overestimation is a pervasive phenomenon that can be systematically rectified.
While it is evident that sarcopenia is an important clinical entity in PDAC, significant questions remain about the underlying pathophysiology and causative relationship. For example, a systematic review in 2015 failed to find that sarcopenia or cachexia were independently correlated with decreased overall survival in PDAC.(9) Another 2016 study found that in a population of patients with unresectable pancreatic cancer, sarcopenia alone did not predict decreased overall survival; however, myosteatosis, or fat infiltration into skeletal muscle, did.(13) More recently, a retrospective study in 2021 demonstrated that decreased skeletal muscle volume and radiologic density were both poor prognostic factors for overall survival, and were associated with Grade 3 or higher chemotherapy toxicity.(14) What is clear, is that patients with PDAC are at increased risk for nutritional deficiencies due to the physiologic roles of the pancreas, and continued loss of skeletal muscle over the course of treatment is a poor prognostic factor. (15, 16) More thoroughly elucidating these relationships will require body composition analysis, which unfortunately is very laborious when performed manually and therefore AutoMATiCA has the ability to advance the pace of investigation through increased efficiency.
Automated segmentation will also facilitate the possibility of clinical implementation, as links between sarcopenia and outcomes are better understood and the clinical utility of monitoring muscle mass becomes more apparent. Shin et al showed in 2021 that patients with locally advanced pancreatic cancer who were able to maintain skeletal muscle during FOLFIRINOX therapy had a greater chance of resection and increased overall survival. (17) In the future, AutoMATiCA may be used to efficiently monitor body composition in a way that could guide treatment decisions including surgical candidate selection as well as for possible pre- and rehabilitation studies. Early interventions in both nutrition and exercise have showed promise for patients with PDAC for both quality of life measures and overall survival, but work remains to continue to understand how to optimize these interventions. (18, 19) Our hope is that this research and eventual clinical implementation could be guided by the automated body composition analysis validated here.
This study is limited by its retrospective design, and lack of correlation with physiologic or muscle-based measures of sarcopenia. Given the close correlation between these two methodologies, it is unlikely that a clinically significant difference exists, however this may be a subject for further study. Notably, the degree of discordance between AutoMATiCA and manual segmentation is dependent on patient characteristics and the study-specific SMI threshold set for sarcopenia; higher SMI thresholds are more likely to yield false negative designations of sarcopenia due to proportional overestimation of muscle CSA by AutoMATiCA. Accuracy can be significantly improved by the inclusion of a correction factor to account for this phenomenon.
Conclusion
In conclusion, this work independently validates AutoMATiCA for tissue compartment segmentation analysis in patients with pancreatic cancer and supports its use for more efficient research in this field in the future, with a validated correction factor to allow for comparison to prior manual segmentation data.
Highlights:
AutoMATiCA and manual measurements of body composition are strongly correlated
Overestimation of muscle area by AutoMATiCA can be reliably adjusted for
AutoMATiCA is a reliable tool for the study of sarcopenia in pancreatic cancer
Funding:
This research was supported by a grant from the National Cancer Institute (CA245188), awarded to Dr. Grossberg. Dr. Sutton received salary support from the Brenden-Colson Center for Pancreatic Care at Oregon Health & Science University. Funders had no part in the preparation of the manuscript nor conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Amini N, Spolverato G, Gupta R, et al. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg. 2015. Sep;19(9):1593–602. Epub 2015/05/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joglekar S, Asghar A, Mott SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015. May;111(6):771–5. Epub 2015/01/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakanishi R, Oki E, Sasaki S, et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today. 2018. Feb;48(2):151–7. Epub 2017/07/13. eng. [DOI] [PubMed] [Google Scholar]
- 4.Stone L, Olson B, Mowery A, et al. Association Between Sarcopenia and Mortality in Patients Undergoing Surgical Excision of Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg. 2019. Jul 1;145(7):647–54. Epub 2019/06/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pipek LZ, Baptista CG, Nascimento RFV, et al. The impact of properly diagnosed sarcopenia on postoperative outcomes after gastrointestinal surgery: A systematic review and meta-analysis. PLoS One. 2020;15(8):e0237740. Epub 2020/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008. Oct;33(5):997–1006. Epub 2008/10/17. eng. [DOI] [PubMed] [Google Scholar]
- 7.Bundred J, Kamarajah SK, Roberts KJ. Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford). 2019. Dec;21(12):1603–12. Epub 2019/07/04. eng. [DOI] [PubMed] [Google Scholar]
- 8.Paris MT, Tandon P, Heyland DK, et al. Automated body composition analysis of clinically acquired computed tomography scans using neural networks. Clin Nutr. 2020. Oct;39(10):3049–55. Epub 2020/02/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozola Zalite I, Zykus R, Francisco Gonzalez M, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology. 2015. Jan–Feb;15(1):19–24. Epub 2014/12/20. eng. [DOI] [PubMed] [Google Scholar]
- 10.Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009. Nov 15;15(22):6973–9. Epub 2009/11/06. eng. [DOI] [PubMed] [Google Scholar]
- 11.Kurita Y, Kobayashi N, Tokuhisa M, et al. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology. 2019. Jan;19(1):127–35. Epub 2018/11/27. eng. [DOI] [PubMed] [Google Scholar]
- 12.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008. Jul;9(7):629–35. Epub 2008/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 13.Rollins KE, Tewari N, Ackner A, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016. Oct;35(5):1103–9. Epub 2015/09/29. eng. [DOI] [PubMed] [Google Scholar]
- 14.Kim IH, Choi MH, Lee IS, et al. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: a retrospective observational study. BMC Cancer. 2021. Jan 18;21(1):77. Epub 2021/01/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basile D, Parnofiello A, Vitale MG, et al. The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2019. Apr;10(2):368–77. Epub 2019/02/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi Y, Oh DY, Kim TY, et al. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS One. 2015;10(10):e0139749. Epub 2015/10/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin DW, Kim MA, Lee JC, et al. Maintenance of skeletal muscle mass during FOLFIRINOX is a favorable prognostic factor in pancreatic cancer patients. BMC Res Notes. 2021. Jul 15;14(1):272. Epub 2021/07/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trestini I, Carbognin L, Sperduti I, et al. Prognostic impact of early nutritional support in patients affected by locally advanced and metastatic pancreatic ductal adenocarcinoma undergoing chemotherapy. Eur J Clin Nutr. 2018. May;72(5):772–9. Epub 2018/03/28. eng. [DOI] [PubMed] [Google Scholar]
- 19.Luo H, Galvão DA, Newton RU, et al. Exercise Medicine in the Management of Pancreatic Cancer: A Systematic Review. Pancreas. 2021. Mar 1;50(3):280–92. Epub 2021/04/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]


