Abstract
Background:
Racial differences in cardiovascular disease (CVD) are likely related to differences in clinical and social factors. The relative contributions of these factors to Black-White differences in premature CVD have not been investigated.
Methods:
In Black and White adults aged 18–30 years at baseline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, the associations of clinical, lifestyle, depression, socioeconomic, and neighborhood factors across young adulthood with racial differences in incident premature CVD were evaluated in sex-stratified, multivariable-adjusted Cox proportional hazards models using multiply imputed data assuming missing at random. Percent reduction in the ß estimate (log-hazard ratio [HR]) for race quantified the contribution of each factor group to racial differences in incident CVD.
Results:
Among 2,785 Black and 2,327 White participants followed for median 33.9 years (25th–75th percentile 33.7–34.0), Black (vs. White) adults had higher risk of incident premature CVD (Black women: HR 2.44 [95% CI 1.71–3.49], Black men: HR 1.59 [1.20–2.10] adjusted for age and center). Racial differences were not statistically significant after full adjustment (Black women: HR 0.91 [0.55–1.52], Black men: HR 1.02 [0.70–1.49]). In women, the largest magnitude percent reduction in the ß estimate for race occurred with adjustment for clinical (87%), neighborhood (32%), and socioeconomic (23%) factors. In men, the largest magnitude percent reduction in the ß estimate for race occurred with adjustment for clinical (64%), socioeconomic (50%), and lifestyle (34%) factors.
Conclusions:
In CARDIA, the significantly higher risk for premature CVD in Black vs. White adults was statistically explained by adjustment for antecedent multi-level factors. The largest contributions to racial differences were from clinical and neighborhood factors in women, and clinical and socioeconomic factors in men.
Keywords: Risk factors, Social determinants of health, premature, cardiovascular disease, racial differences
Introduction
The disproportionate burden of cardiovascular disease (CVD) in Black compared with White adults in the United States is well-recognized, is particularly pronounced across young adulthood, and has been especially evident during the Coronavirus Disease-19 (COVID-19) pandemic.1–4 Several interrelated risk factors are known to increase risk for CVD, including clinical,5 lifestyle,6, 7 psychosocial,8 and socioeconomic factors.9 In addition, data have highlighted the importance of the neighborhood environment in risk of CVD, independent of individual-level factors.10 Disparities in CVD between Black and White adults are recognized as arising from complex differences in clinical and social determinants (rather than due to race itself, which is a social construct).11 However, the individual and combined relative contributions of factors to racial differences in incident CVD remains an unanswered question. Quantification of the contribution of individual- and neighborhood-level factors to the difference in CVD may inform tailored multi-level strategies for prevention and reduction of disparities, by identifying the factors that may contribute most to the observed differences. We hypothesized that clinical and social factors contribute to racial differences in incident CVD, so we evaluated the associations and quantified the contributions of clinical, lifestyle, psychosocial, socioeconomic, and neighborhood-level factors across young adulthood with the racial difference in the incidence of premature CVD in the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
Methods
Study Population
Requests to access the dataset used for this analysis may be submitted to the CARDIA Study at https://www.cardia.dopm.uab.edu. CARDIA is a longitudinal observational cohort that began in 1985–1986 with 5,115 women and men age 18–30 years, recruited from four urban metropolitan areas in the United States (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California).12 Recruitment was approximately balanced within centers on sex, race (self-reported as Black or White), age (<25 versus ≥25 years), and education (≤ high school versus > high school). In-person follow-up visits occurred at 2, 5, 7, 10, 15, 20, 25, and 30 (in 2015–2016) years after baseline. CARDIA was approved by institutional review boards at each study center, and participants provided written informed consent at each follow-up examination.
Ascertainment of Cardiovascular Disease
Incident CVD was defined as fatal or nonfatal myocardial infarction, coronary revascularization, hospitalized unstable angina, heart failure, stroke, transient ischemic attack, carotid or peripheral arterial disease requiring intervention, or other fatal heart or atherosclerotic disease. Incident CVD events were recorded during scheduled study examinations or annual phone interviews (with specific inquiry about hospitalizations and outpatient revascularization procedures). Contact for medical history ascertainment is maintained with participants via telephone, mail, or email every 12 months. Over the last 5 years, >90% of the surviving cohort members have been directly contacted and follow up for vital status is virtually complete through related contacts and intermittent National Death Index searches. Event adjudication was by independent review of medical or death records by two physician members of the CARDIA Endpoints Committee following the CARDIA events manual of operations, as previously described.5, 13, 14 For the current analysis, ascertainment of events was complete through August 31, 2018. Given the baseline age range of participants, all incident CVD events were considered premature.
Risk Factor Measurements
Risk factors for CVD were categorized as: individual-level (clinical, lifestyle, depression, socioeconomic), and neighborhood-level factors. Factors were selected from among those that are potentially intervenable, available as study measures, or known to vary by race group.15 Additional details for clinical and lifestyle factor data collection are in the Supplemental Methods.
Clinical Risk Factors
Height and weight (for body mass index [BMI]) and waist circumference were measured at baseline and each follow-up exam. Glucose and lipids (total and high-density lipoprotein [HDL]) cholesterol) were measured after an 8 hour fast. Systolic blood pressure was assessed as the average of the last two of three measurements. Diabetes and hypertension medication use was recorded using a medication inventory questionnaire. Maximum forced vital capacity (FVC) was measured at baseline, and years 2, 5, 10, and 20 as the maximum of five forced spirometry trials.
Lifestyle Factors
Information on lifestyle factors was collected via self-administered questionnaire. Smoking status (current, former, or never) and alcohol consumption data were collected at each study examination. Dietary quality was measured by a diet questionnaire at baseline and years 7 and 20 and quantified according to the American Heart Association’s Life’s Simple 7 criteria for ideal consumption levels of fiber-rich whole grains, fruits and vegetables, fish, sugar-sweetened beverages, and sodium, and was categorized as high, intermediate, or poor quality.16 Physical activity was measured at each examination as the self-reported frequency of participating in moderate- and vigorous-intensity activities over the preceding year, reported as a score in exercise units (a constructed, not physiologic, measure that reflects a composite of amount and intensity for a range of activities).17
Depression Symptoms
Depression symptoms, a major psychosocial factor related to CVD, were measured with the 20-item Center for Epidemiologic Studies-Depression (CES-D) questionnaire at study examination years 5, 10, 15, 20, 25, and 30, with scores ranging from 0 (low-) to 60 (high-depressive symptomatology).18
Socioeconomic Factors
Socioeconomic factors were measured by self-report at baseline and years 5, 7, 10, 15, 20, 25, and 30. Participants’ maximum level of education, current employment status (full-time or not full-time [part-time, in school, home or child caretaker, unemployed, other]), marital status (married or not married), and financial status (in response to a question about household-level difficulty paying for basics, modeled as poor [very hard, hard, or somewhat hard], versus adequate [not very hard], or don’t know) were measured.19 Participants reported their parents’ maximum level of education. Access to care was defined based on self-report of a usual source of medical care (an affirmative response to the question, “Do you have a usual source of care? By that we mean the place you go if you need a checkup or if you are ill.”).
Neighborhood Factors
Neighborhood-level factors were assessed using participants’ addresses at baseline and years 7, 10, 15, 20, and 25, which accounted for changes in address. Residential addresses from CARDIA participants were geocoded and linked to the US census closest to the CARDIA exam year (1980 for the baseline exam, 1990 for years 7 and 10, 2000 for years 15 and 20, and 2010 for year 25).20 Residential racial segregation was quantified as the Getis and Ord local G statistic (Gi*), a measure that estimates the degree to which the racial composition of a neighborhood (specifically, percentage of individuals in a census tract who are Black) and surrounding neighborhoods deviates from the racial composition of the surrounding metropolitan area or county.21–23 The G statistic is operationalized as a z-score, where higher positive values indicate a neighborhood with higher-than-expected percentage of residents who are Black. Neighborhood-level poverty was defined as the census tract-level percentage of population living in poverty, defined by the US Census.
Statistical Analysis
The individual and relative associations of clinical, lifestyle, depression, socioeconomic, and neighborhood factors with racial differences in incident CVD were evaluated with sex-stratified, multivariable-adjusted Cox proportional hazards regression, comparing Black participants to White participants of the same sex. Time at risk was calculated as the time from the baseline examination until the date at which CVD was diagnosed or last contact. The contribution of factors to the Black-White difference in risk for premature incident CVD aggregated in key categories was estimated as the percent reduction in the log-hazard ratio (HR) (“ß,” where eß equals the HR for incident CVD in Black compared with White participants) corresponding to adjustment for a factor group, according to the formula: 100 * ([ß0] – [ßNEW]) / (ß0).24 The percent reduction in the ß estimate in the coefficient representing Black race from ß0 in the referent model to ßNEW in the new model indicates the percent reduction in risk for premature incident CVD on the log scale when comparing Black participants to White participants, after adjustment for the additional factors in the new model.
The base model (model 1 [M1]) was adjusted for age and study center. Three adjustment strategies were employed to understand the individual and combined relative associations of risk factor categories to sex-specific racial differences in incident CVD. First, a sequential adjustment strategy added each risk factor category to the preceding model in the following order: model 2 (M2) was M1 plus adjustment for clinical risk factors; M3 was M2 plus adjustment for lifestyle factors; M4 was M3 plus adjustment for depression symptoms; M5 was M4 plus adjustment for socioeconomic risk factors; and M6 was M5 plus adjustment for neighborhood factors (thus adjusted for all risk factor categories). At each step, the percent reduction in the coefficient estimate for race was evaluated for each sequentially adjusted model, relative to the coefficient estimate for race in M1.
Second, an individual adjustment strategy was applied wherein each risk factor domain individually was added to M1. The percent reduction in the coefficient estimates for race from these 5 models, relative to M1, was estimated. Third, a leave-one-out adjustment strategy was employed. Here, individual exclusion of risk factor domains from the fully adjusted model (M6) was evaluated, i.e., M6 without clinical risk factors, M6 without lifestyle factors, M6 without depression symptoms, etc. The percent reduction in the coefficient estimate for race associated with lack of adjustment for risk factors, relative to the M6 model, was calculated to reflect the remaining difference when a risk factor group was not included in model adjustment. For instance, a model adjusted for clinical, lifestyle, depression, and neighborhood risk factors reflects the estimated excess incident CVD risk in Black versus White participants when not adjusted for socioeconomic risk factors.
Cox proportional hazards models were estimated using risk factors assessed at baseline and updated at each subsequent examination for which updated data were available (i.e., accounting for time-varying covariates). Incorporating time-varying covariates allows for within-person changes in risk factor levels across the follow-up period, relaxing the assumption that the studied factors have a constant effect on the risk of CVD over time. This approach calculates the risk of CVD at each exam year using the updated covariate values, and the overall hazard ratio reflects an average hazard ratio over the follow-up period. In a complementary analysis, regression models were adjusted for covariates measured at baseline only. The proportional hazards assumption was tested by including a model term for the product of race and natural-log of time at risk; no violations of the assumption were observed.
The primary analysis was conducted in the entire CARDIA cohort, minus 1 person who withdrew consent and 2 people who underwent gender change, assuming unavailable data were missing at random. To impute missing risk factor data at baseline and at subsequent visits, we conducted multiple imputation by chained equations with fully conditional specification using the SAS MI package.25 The variables included in the imputation database are listed in the Supplemental Methods. Risk factor data in all domains from all visits were included sequentially in the model specification. Therefore, an individual’s imputed value for a given variable was predicted using data on that variable from past and future examinations as well as all other available risk factor data. We created 10 imputed datasets. Because occurrences that might have occurred after death were not thought to be missing at random, imputed values were set to missing after participants died or were censored. Participants who had a CVD event were not included in models with time-varying covariates after their event, but their covariate data after the CVD event were used for imputation of future covariates to avoid selection bias (selecting on future events). The distribution of covariates between the observed and the imputed datasets at all visits were similar (Table S1). Each regression analysis as described above was performed separately in each of the imputed data sets, and the results were combined using Rubin’s rules.26 A secondary analysis was conducted using the complete-case dataset. SAS version 9.4 was used for analyses.
Results
In the 5,112 included participants, there were 1,480 Black women (mean age at baseline 24.4 [standard deviation 3.9] years), 1,305 White women (mean age 25.4 [3.4] years), 1,157 Black men (mean age 24.2 [3.8] years), and 1,170 White men (mean age 25.4 [3.4] years). Baseline characteristics of participants are detailed in Table 1. Black participants generally had less favorable clinical, lifestyle, socioeconomic, and depression characteristics compared with White participants. The G statistic indicated that Black participants resided in neighborhoods with higher percentage of Black residents and higher percentage of individuals living in poverty. Over a median follow-up of 33.9 (25th–75th percentile, 33.7–34.0) years, there were 222 CVD events in Black participants and 133 CVD events in White participants (Figure 1).
Table 1.
Participant characteristics at baseline (1985–1986) by sex and race
| Women | Men | |||
|---|---|---|---|---|
| Black (N=1480) |
White (N=1305) |
Black (N=1157) |
White (N=1170) |
|
| Age, years, mean (SD) | 24.4 (3.9) | 25.4 (3.4) | 24.2 (3.8) | 25.4 (3.4) |
| Incident CVD cases per 1000 person-years | 2.2 | 1.0 | 3.2 | 2.4 |
| Socioeconomic | ||||
| Education, years, mean (SD) | 13.1 (1.8) | 14.5 (2.3) | 12.9 (1.9) | 14.6 (2.5) |
| Married, N (%) | 292 (21%) | 370 (29%) | 211 (19%) | 264 (23%) |
| Poor financial status, N (%)* | 579 (39%) | 420 (32%) | 474 (41%) | 316 (27%) |
| Employed full-time, N (%) | 678 (46%) | 807 (62%) | 601 (52%) | 792 (68%) |
| Maximum parental education, years, mean (SD) | 13.3 (3.1) | 15.0 (3.1) | 13.5 (3.2) | 15.1 (2.9) |
| Usual source of medical care, N (%) | 1346 (91%) | 1181 (91%) | 944 (82%) | 941 (81%) |
| Neighborhood | ||||
| G statistic of Black segregation, mean (SD) | 4.8 (3.0) | 1.5 (3.2) | 4.9 (3.2) | 1.6 (3.4) |
| Percentage of census tract living in poverty, %, mean (SD) | 24 (13) | 13 (10) | 24 (13) | 13 (10) |
| Depression | ||||
| CES-Depression score, mean (SD) | 13.5 (9.0) | 10.5 (8.1) | 11.6 (7.6) | 9.5 (7.0) |
| Lifestyle | ||||
| Smoking, N (%) | ||||
| Never | 885 (60%) | 686 (53%) | 619 (54%) | 670 (57%) |
| Former | 130 (9%) | 263 (20%) | 109 (9%) | 186 (16%) |
| Current | 465 (31%) | 356 (27%) | 429 (37%) | 314 (27%) |
| Daily alcohol intake, N (%) | ||||
| None | 841 (57%) | 490 (38%) | 380 (32%) | 277 (24%) |
| Moderate | 539 (36%) | 605 (46%) | 620 (54%) | 717 (61%) |
| Heavy | 100 (7%) | 210 (16%) | 157 (14%) | 176 (15%) |
| Physical activity, exercise units, mean (SD) | 278 (226) | 399 (261) | 534 (344) | 509 (302) |
| Diet quality, N (%) | ||||
| High | 115 (8%) | 317 (24%) | 39 (3%) | 146 (13%) |
| Intermediate | 829 (56%) | 777 (60%) | 564 (49%) | 648 (55%) |
| Poor | 536 (36%) | 211 (16%) | 554 (48%) | 376 (32%) |
| Clinical | ||||
| Fasting glucose, mg/dL, mean (SD) | 81 (21) | 81 (13) | 84 (16) | 85 (11) |
| Diabetes medication use, N (%) | 5 (0.3%) | 5 (0.4%) | 2 (0.2%) | 2 (0.2%) |
| Body mass index, kg/m2, mean (SD) | 25.9 (6.6) | 23.1 (4.4) | 24.6 (4.3) | 24.3 (3.6) |
| Waist circumference, cm, mean (SD) | 77 (13) | 72 (9) | 81 (10) | 83 (9) |
| Systolic blood pressure, mmHg, mean (SD) | 108 (10) | 105 (9) | 115 (11) | 114 (10) |
| Antihypertensive medication use, N (%) | 57 (4%) | 14 (1%) | 25 (2%) | 19 (2%) |
| Total cholesterol, mg/dL, mean (SD) | 178 (34) | 176 (31) | 176 (35) | 176 (34) |
| HDL cholesterol, mg/dL, mean (SD) | 55 (13) | 56 (13) | 53 (14) | 47 (11) |
| Forced vital capacity, L, mean (SD) | 3.3 (0.5) | 3.9 (0.5) | 4.6 (0.7) | 5.5 (0.7) |
CES-D: Center for Epidemiologic Studies-Depression, CVD: Cardiovascular disease, HDL: High density lipoprotein, SD: Standard deviation.
Poor financial status determined in response to a question about household-level difficulty paying for basics, modeled as poor [very hard, hard, or somewhat hard], versus adequate [not very hard], or don’t know).
Figure 1. Cumulative incidence of cardiovascular disease in Black and White participants in the CARDIA study, 1985–1986 (baseline exam) to 2018.
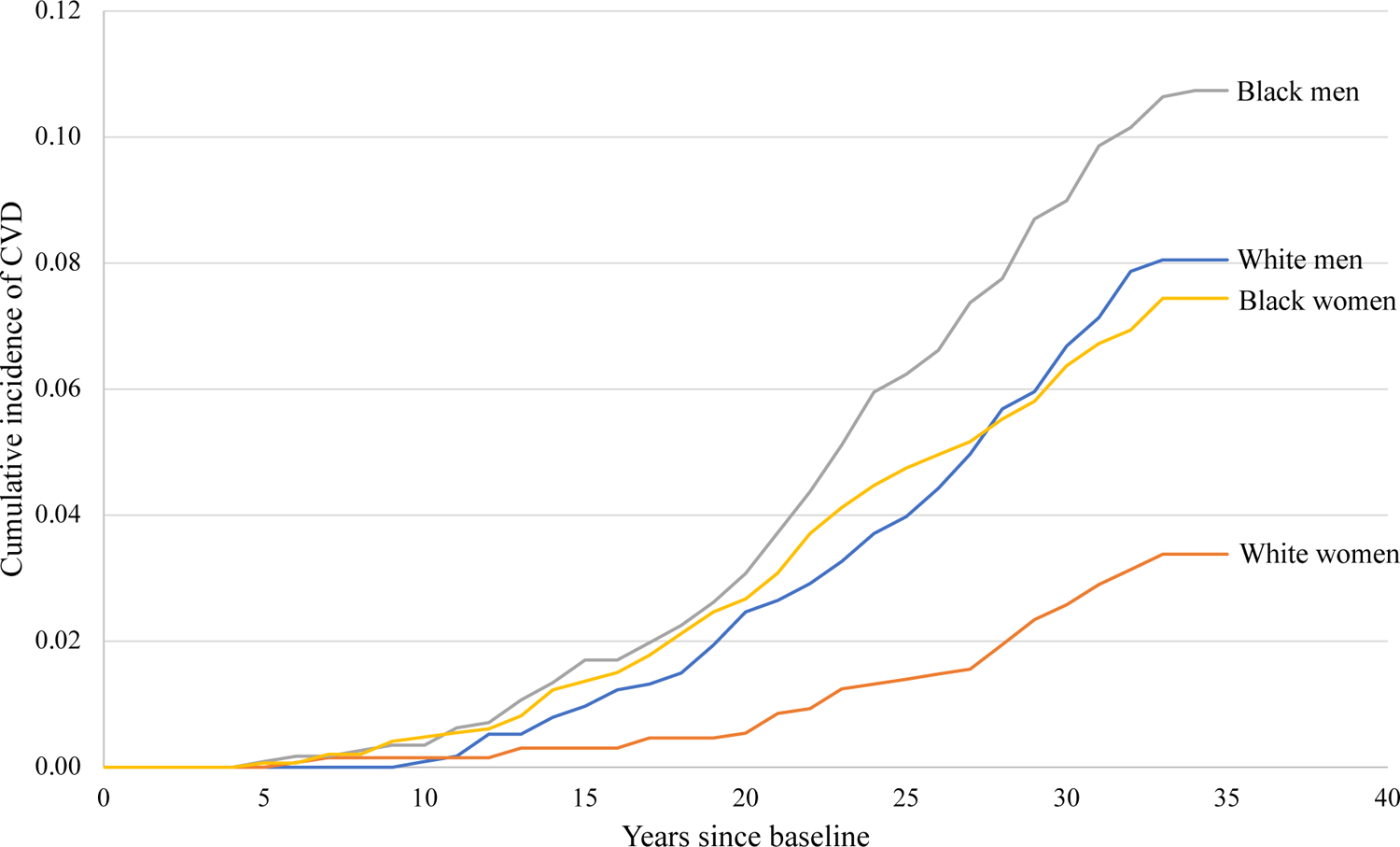
CVD: Cardiovascular disease. Median follow-up was 33.9 years (25th-75th percentile 33.8–34.0) in Black women, 33.8 years (33.7–34.0) in White women, 33.9 years (33.7–34.1) in Black men, and 33.8 years (33.7–34.0) in White men.
The primary analysis of HRs for incident CVD in Black participants compared with White participants, and percent reduction in ß estimates relative to the base model (M1) for time-varying risk factor adjustments, are shown in Figure 2. In M1 (adjusted for age and field center) Black women and men had a higher risk for incident CVD compared with White women and men, respectively (Black women: HR 2.44 [95% CI 1.71 – 3.49]; Black men: HR 1.59 [95% CI 1.20 – 2.10]). In M6 (adjusted for all categories of time-varying risk factors), Black participants no longer had significantly higher risk for incident CVD compared with White participants (women: HR 0.91 [0.55 – 1.52]; men: HR 1.02 [0.70 – 1.49]) after accounting for risk factors in all domains. Adjustment for all categories of risk factors (M6) was associated with an 110% reduction in women and 95% reduction in men in the ß estimate for the risk of incident premature CVD in Black compared with White participants, relative to M1. HRs associated with each individual risk factor in M6 are shown in Table 2.
Figure 2. Hazard ratios for incident cardiovascular disease in Black compared with White participants and percent reduction in ß estimates using time-varying factor data.
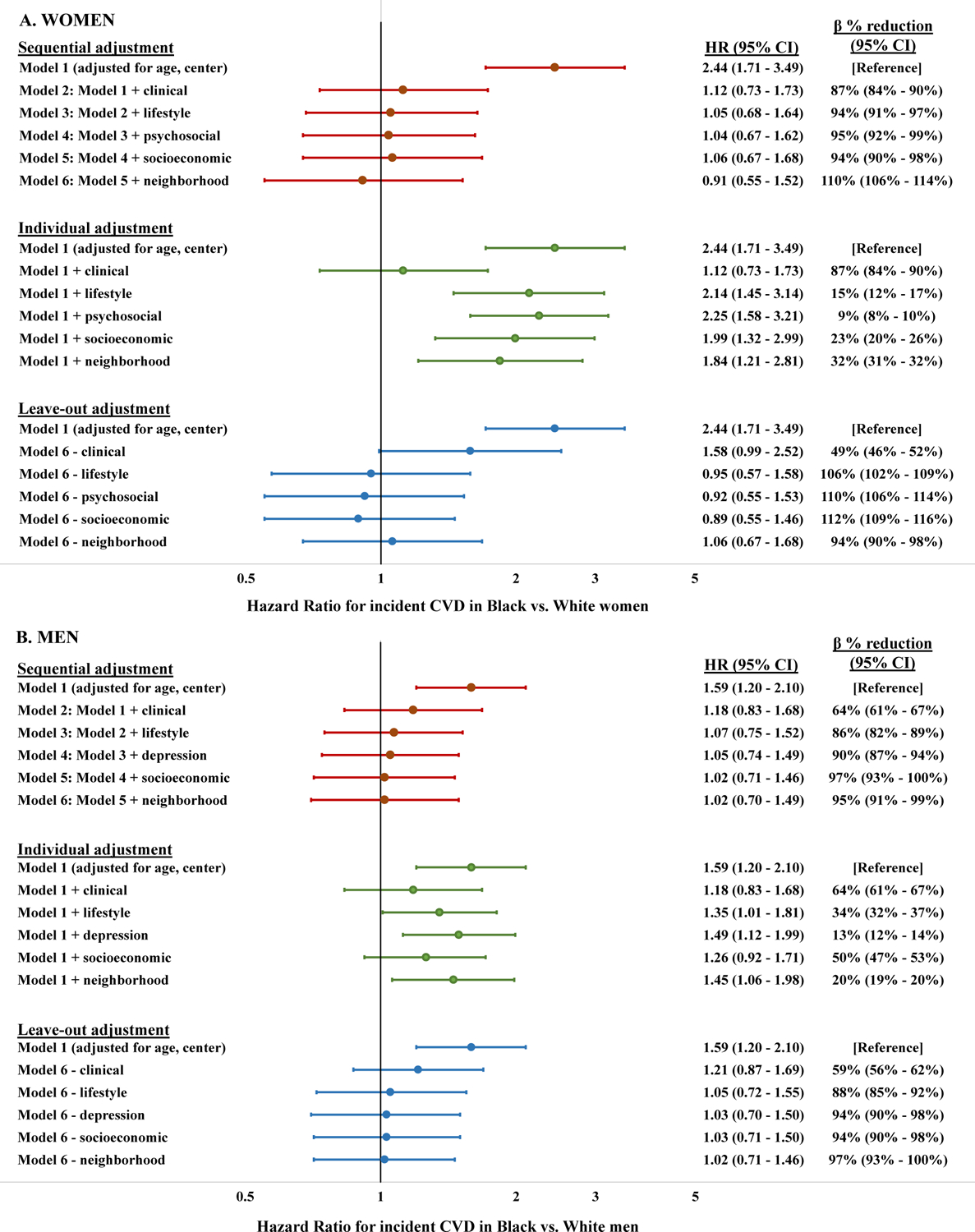
CI: Confidence interval, HR: Hazard ratio. Hazard ratios are adjusted for time-varying factor levels updated across multiple measurements in young adulthood. Clinical factors: Fasting glucose, diabetes medication use, body mass index, waist circumference, systolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, and forced vital capacity. Lifestyle factors: Smoking, alcohol intake, physical activity, diet score. Depression factor: Center for Epidemiologic Studies-Depression score. Socioeconomic factors: Participant and parental educational attainment, marital status, financial status, employment status. Neighborhood factors: G statistic for census tract-level racial segregation and percentage of population living in poverty
Table 2.
Adjusted hazard ratios for incident cardiovascular disease per unit increment of covariates
| Women | Men | |
|---|---|---|
| Black race | 0.91 (0.55 – 1.52) | 1.02 (0.70 – 1.49) |
| Age, per 1 year | 1.02 (0.98 – 1.08) | 1.10 (1.05 – 1.15) |
| Site | ||
| Birmingham, Alabama | [Reference] | [Reference] |
| Chicago, Illinois | 0.91 (0.60 – 1.41) | 0.96 (0.65 – 1.43) |
| Minneapolis, Minnesota | 0.69 (0.41 – 1.15) | 0.72 (0.49 – 1.07) |
| Oakland, California | 0.53 (0.33 – 0.84) | 0.52 (0.34 – 0.82) |
| Socioeconomic | ||
| Participant education, per 1 year | 1.00 (0.93 – 1.08) | 0.96 (0.91 – 1.02) |
| Marital status | ||
| Not married | [Reference] | [Reference] |
| Married | 0.78 (0.52 – 1.17) | 1.03 (0.75 – 1.40) |
| Financial status | ||
| Adequate (Not hard to pay for basics) | [Reference] | [Reference] |
| Poor (Hard to pay for basics) | 0.81 (0.54 – 1.23) | 1.14 (0.79 – 1.64) |
| Employment | ||
| Not employed full-time | [Reference] | [Reference] |
| Employed full-time | 0.61 (0.41 – 0.91) | 0.82 (0.57 – 1.17) |
| Medical care | ||
| Does not have usual source of care | [Reference] | [Reference] |
| Has usual source of care | 0.91 (0.45 – 1.83) | 0.99 (0.62 – 1.60) |
| Maximum parental education, per 1 year | 1.05 (0.99 – 1.12) | 1.03 (0.98 – 1.08) |
| Neighborhood | ||
| G-statistic of Black segregation, per 1 unit | 1.05 (0.97 – 1.13) | 0.97 (0.91 – 1.04) |
| Percentage of census tract living in poverty, per 1% | 1.00 (0.98 – 1.02) | 1.01 (0.99 – 1.02) |
| Depression | ||
| CES-Depression score, per 1 point | 1.01 (0.99 – 1.04) | 1.01 (0.99 – 1.03) |
| Lifestyle | ||
| Smoking | ||
| Never | [Reference] | [Reference] |
| Former | 1.18 (0.77 – 1.80) | 0.75 (0.45 – 1.26) |
| Current | 1.60 (1.04 – 2.46) | 2.13 (1.48 – 3.03) |
| Daily alcohol intake | ||
| None | [Reference] | [Reference] |
| Moderate | 1.04 (0.70 – 1.54) | 0.79 (0.56 – 1.11) |
| Heavy | 0.48 (0.28 – 0.82) | 0.67 (0.42 – 1.07) |
| Physical activity, per 100 exercise units | 1.02 (0.93 – 1.10) | 0.98 (0.93 – 1.05) |
| Diet quality | ||
| High | [Reference] | [Reference] |
| Intermediate | 0.96 (0.54 – 1.71) | 0.83 (0.45, 1.52) |
| Poor | 1.14 (0.57 – 2.31) | 0.92 (0.50, 1.69) |
| Clinical | ||
| Fasting glucose, per 10 mg/dL | 1.07 (1.03 – 1.12) | 1.01 (0.96 – 1.06) |
| Diabetes medication (not using medication) | [Reference] | [Reference] |
| Using medication | 1.59 (0.94 – 2.68) | 1.32 (0.76 – 2.30) |
| Body mass index, per 1 kg/m2 | 0.95 (0.92 – 0.99) | 0.98 (0.92 – 1.04) |
| Waist circumference, per 1 cm | 1.02 (1.00 – 1.04) | 1.02 (0.99 – 1.05) |
| Systolic blood pressure, per 10 mmHg | 1.32 (1.21 – 1.43) | 1.18 (1.06 – 1.31) |
| Antihypertensive medication (not using medication) | [Reference] | [Reference] |
| Using medication | 2.42 (1.54 – 3.79) | 1.78 (1.21 – 2.62) |
| Total cholesterol, per 10 mg/dL | 1.02 (0.97 – 1.07) | 1.08 (1.05 – 1.12) |
| HDL cholesterol, per 10 mg/dL | 0.96 (0.86 – 1.08) | 0.91 (0.81 – 1.04) |
| Forced vital capacity, per 1 L | 0.83 (0.59 – 1.16) | 0.83 (0.67 – 1.03) |
CES-D: Center for Epidemiologic Studies-Depression, CVD: Cardiovascular disease, HDL: High density lipoprotein, SD: Standard deviation. Hazard ratios are adjusted for time-updated covariate measurements.
In the individual risk factor category adjustment strategy in women, adjustment only for clinical risk factors corresponded to the greatest percent reduction (87%) in the ß estimate for the risk of premature incident CVD in Black compared with White participants relative to M1. The next greatest attenuation was associated with adjustment only for neighborhood factors (32% reduction), and only socioeconomic factors (23% reduction). Adjustment for depression symptoms only was associated with the least attenuation in the ß estimate (9%). In men, adjustment only for clinical risk factors corresponded to the greatest percent reduction (64%) in the ß estimate for the risk of premature incident CVD in Black compared with White participants relative to M1, followed by adjustment for only socioeconomic factors (50% reduction) and only lifestyle factors (34% reduction). Adjustment for depression symptoms was associated with the least attenuation in the ß estimate (13%). In the leave-out adjustment strategy in both women and men, omitting adjustment for clinical risk factors (with adjustment for all other domains) was associated with the least attenuation of the premature incident CVD ß estimate for Black compared with White participants (women 49%, men 59%) relative to M1.
Secondary analysis of HRs for incident CVD in Black compared with White participants, and percent reduction in ß estimates for baseline-only risk factor adjustments, is shown in Table S2. After adjusting for baseline levels of all risk factor groups, Black women still had significantly higher risk for incident premature CVD compared with White women (HR 2.29 [1.36 – 3.86] with 7% reduction in ß estimate), but the risk for incident premature CVD in Black men was no longer significantly elevated (HR 1.33 [0.87 – 2.02] with 38% reduction in ß estimate). Percent reduction in the ß estimate for individual risk factor category adjustment was highest with adjustment for only clinical risk factors at baseline in women (36% reduction) and only socioeconomic factors at baseline in men (58% reduction).
Results from additional secondary analysis in the complete-case (non-imputed) dataset (N = 3,320) are provided in the Supplement (baseline characteristics in Table S3; characteristics of included versus excluded participants in Table S4). After adjustment for all factors accounting for time-varying covariates across the study period (Table S5), the risk for incident premature CVD was not statistically significantly higher in Black compared with White women (HR 0.92 [0.48 – 1.75]) or men (HR 1.16 [0.72 – 1.88]). Hazard ratios associated with adjustment for categories of factors using time-varying and baseline-only factors in the complete-case dataset are shown in Tables S6 and S7, respectively.
Discussion
The significantly higher incidence of premature CVD in Black compared with White participants in the CARDIA study was statistically explained by adjustment for multi-level individual- and neighborhood-level factors measured beginning in young adulthood. In women and men, the racial difference in premature CVD was primarily attenuated by differences in clinical risk factors, with smaller magnitudes of attenuation particularly from neighborhood, socioeconomic, and lifestyle factors. This study contextualizes and extends prior findings that implicate the studied risk factors individually as associated with CVD,6–10 by simultaneously evaluating a comprehensive set of interrelated factors that may underlie racial differences in incident premature CVD. In addition, this analysis accounts for changes in factors across young adulthood starting at mean age 25 years with over 30 years of follow-up.
Differences in clinical risk factors even at a young age significantly contribute to disproportionately worse cardiometabolic outcomes in Black compared with White adults in the US.2, 27, 28 For instance, in a prior analysis from CARDIA, clinical risk factors were most strongly associated with the racial difference in diabetes.15 Almost half the racial difference in incident stroke in older adults within the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study was attributable to clinical risk factors, primarily due to differences in blood pressure.29 Our results align with these prior findings, indicating that clinical risk factors across young adulthood statistically explain the greatest magnitude of the racial difference in premature CVD in Black compared with White women and men.
Importantly, this observation does not imply that social determinants of health are less important contributors to racial differences and in fact, upstream social determinants of health (e.g., socioeconomic disadvantage) are likely important contributors to differences in clinical risk factors. Further, the large relative component of the racial difference in premature CVD statistically explained by clinical factors is likely observed, in part, due to the relative proximity of clinical factors to CVD on the causal pathway.30 The findings from the individual adjustment strategy indicate that a substantial portion of the racial difference in incident CVD is still captured by racial differences in socioeconomic and neighborhood-level factors, indicating these upstream factors have an important role in the observed racial differences in CVD outcomes. While individuals with lower socioeconomic position are more likely to develop clinical CVD risk factors, a recent simulation study of the US population found that traditional clinical risk factors explained only 40% of excess CVD events among individuals with low socioeconomic position.31 Future work may seek to identify the extent to which social determinants operate through clinical risk factors to influence differences in incident CVD.
The studied risk factor domains are likely interrelated, the included measures likely incompletely account for the entire effect of a particular domain, and several other factors that were not accounted for may also contribute to differences. For example, structural racism and discrimination are fundamental drivers of health disparities, and may be partly captured by the included risk factor categories (e.g. socioeconomic, psychosocial, neighborhood), but may also operate beyond the contribution of the included factors.32 There were differences between women and men in the amount of the racial differences in incident premature CVD explained by the various factors, which may reflect how social determinants (e.g., health behaviors, psychosocial stressors, socioeconomic position) interact with gender and sex through putative mechanisms of socialization and norms of gender roles, experiences of discrimination and workplace inequality, or traumatic life events.33 Additional contributors to racial differences in premature CVD in women may be include differences in adverse pregnancy outcomes and premature menopause, both of which disproportionately affect Black women and are associated with pre-pregnancy and pre-menopausal clinical cardiovascular risk factors that are accounted for in our analysis.34 Consistent with the identification of Black race as a social (not biological) construct, genetic differences are not implicated in the observed racial differences in incident premature CVD.35
Importantly, the evaluated models assume that the effect of the included factors on incident CVD is similar in Black and White participants. But certain factors, for example neighborhood-level characteristics such as segregation, may not influence health uniformly across racial groups. Efforts to prevent premature CVD that specifically address the risk factor groups identified as important contributors may have the most impact in reducing premature CVD differences and disparities experienced by Black women and men, but local contextualization of potential interventions is a critical step to ensure the role of each factor is appropriately considered. These findings also reveal that risk factors as early as the second decade of life contribute to CVD differences. There was attenuation in the ß estimates associated with adjustment for factors only at baseline, particularly for men, suggesting the potential impact of interventions earlier in life to reduce differences and prevent CVD. Notably, the magnitude of hazard ratios for incident CVD after adjusting for baseline levels of all factors remained relatively large in both women (2.29) and men (1.33).
Our analysis has several limitations. First, these data are observational. Residual confounding from unmeasured factors may influence results, and interventional studies are needed to evaluate the reduction in racial differences and disparities associated with targeting each type of risk factor group. Second, the relatively low number of each subtype of CVD event precludes evaluation of subtypes of incident CVD or fatal versus nonfatal events. Third, adjusting for time-varying clinical factors may introduce bias if Black participants had less access to quality care compared with White participants. Our sensitivity analysis of baseline factors (without time-varying covariate updates) partially addresses this limitation and demonstrated similar relative contribution of each domain of factors to the racial difference observed, and we acknowledge the potential bias introduced by time-dependent confounder feedback when adjusting for time-varying covariates. Fourth, while the covariates included in the models statistically account for the observed racial difference in premature incident CVD, these data may be underpowered to detect a smaller, but still clinically significant, difference in incident CVD. Fifth, the analysis is limited to Black and White adults. The association of risk factors with differences in premature CVD experienced by Hispanic, Asian, and Native American populations are not evaluated, and the contribution of risk factors to differences in different race/ethnic groups may differ. Sixth, we were not able to assess experiences of racial discrimination, as these were not measured until year 7 in CARDIA.
Conclusions
In this cohort study of young adults over >30 years of follow-up, the significantly higher risk for premature CVD in Black vs. White individuals was accounted for by adjustment for multi-level factors. Clinical risk factors across young adulthood were associated with most of the Black-White difference in incident CVD in both women and men. Socioeconomic, neighborhood, and lifestyle factors across young adulthood variably contributed in smaller magnitude to the racial difference in incident CVD. These results may help identify intervention targets beginning in early adulthood to mitigate racial differences in premature CVD.
Supplementary Material
Clinical Perspective.
What is new?
The significantly higher incidence of premature CVD in Black participants compared with White participants in the CARDIA study was significantly and completely attenuated by adjustment for multi-level individual- and neighborhood-level factors measured beginning in young adulthood.
In women, clinical, neighborhood, and socioeconomic factors statistically explained the largest component of the racial difference in incident cardiovascular disease (87%, 32%, and 23%, respectively).
In men, clinical, socioeconomic, and lifestyle factors statistically explained the largest components of the racial difference in incident cardiovascular disease (64%, 50%, and 34%, respectively).
What are the clinical implications?
Interrelated social factors and clinical factors may contribute differently to racial differences in incident cardiovascular disease in women compared with men in the United States.
The factors that statistically explain the largest component of the racial difference in women and men may guide targeted prospective intervention research and public health strategies to reduce racial differences in cardiovascular disease most effectively.
Funding Sources
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). Research reported in this publication was supported, in part, by the National Heart, Lung, and Blood Institute grant number K23HL157766 (NSS) and the National Institutes of Health, grant numbers P30AG059988 and P30DK092939 (SSK). Research reported in this publication was also supported, in part, by the American Heart Association (#19TPA34890060) to SSK. The funding organizations were not involved in design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. This manuscript has been reviewed by CARDIA for scientific content. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding organizations.
Non-standard Abbreviations and Acronyms
- BMI
Body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CES-D
Center for Epidemiologic Studies-Depression
- COVID-19
Coronarvirus Disease-19
- CVD
Cardiovascular disease
- FVC
Forced vital capacity
- HDL
High-density lipoprotein
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
Footnotes
Disclosures
The authors report no disclosures or conflicts of interest.
References
- 1.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr., Willis M, et al. Cardiovascular health in African Americans: A Scientific Statement from the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 2.Shah NS, Molsberry R, Rana JS, Sidney S, Capewell S, O’Flaherty M, Carnethon M, Lloyd-Jones DM and Khan SS. Heterogeneous trends in burden of heart disease mortality by subtypes in the United States, 1999–2018: observational analysis of vital statistics. BMJ. 2020;370:m2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD and Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhala N, Curry G, Martineau AR, Agyemang C and Bhopal R. Sharpening the global focus on ethnicity and race in the time of COVID-19. Lancet. 2020;395:1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perak AM, Ning H, Khan SS, Bundy JD, Allen NB, Lewis CE, Jacobs DR Jr., Van Horn LV and Lloyd-Jones DM. Associations of late adolescent or young adult cardiovascular health with premature cardiovascular disease and mortality. J Am Coll Cardiol. 2020;76:2695–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah RV, Murthy VL, Colangelo LA, Reis J, Venkatesh BA, Sharma R, Abbasi SA, Goff DC Jr., Carr JJ, Rana JS, et al. Association of fitness in young adulthood with survival and cardiovascular risk: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med. 2016;176:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah NS, Leonard D, Finley CE, Rodriguez F, Sarraju A, Barlow CE, DeFina LF, Willis BL, Haskell WL and Maron DJ. Dietary patterns and long-term survival: A retrospective study of healthy primary care patients. Am J Med. 2018;131:48–55. [DOI] [PubMed] [Google Scholar]
- 8.Cohen BE, Edmondson D and Kronish IM. State of the art review: Depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes: Challenges and interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson C, Diez Roux AV, Jacobs DR Jr., Kiefe CI, West D and Williams DR. Neighbourhood characteristics, individual level socioeconomic factors, and depressive symptoms in young adults: the CARDIA study. J Epidemiol Community Health. 2005;59:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amutah C, Greenidge K, Mante A, Munyikwa M, Surya SL, Higginbotham E, Jones DS, Lavizzo-Mourey R, Roberts D, Tsai J, et al. Misrepresenting race - The role of medical schools in propagating physician bias. N Engl J Med. 2021;384:872–878. [DOI] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K and Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 13.CARDIA Study. CARDIA Endpoint Events Manual of Operations. 2020;2022. [Google Scholar]
- 14.Yano Y, Reis JP, Tedla YG, Goff DC Jr., Jacobs DR Jr., Sidney S, Ning H, Liu K, Greenland P and Lloyd-Jones DM. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Cardiol. 2017;2:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ and Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017;318:2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs DR Jr., Hahn LP, Haskell WL, Pirie P and Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 19.Matthews KA, Kiefe CI, Lewis CE, Liu K, Sidney S and Yunis C. Socioeconomic trajectories and incident hypertension in a biracial cohort of young adults. Hypertension. 2002;39:772–6. [DOI] [PubMed] [Google Scholar]
- 20.Kershaw KN, Robinson WR, Gordon-Larsen P, Hicken MT, Goff DC Jr., Carnethon MR, Kiefe CI, Sidney S and Diez Roux AV. Association of changes in enighborhood-level racial residential segregation with changes in blood pressure among Black adults: The CARDIA Study. JAMA Intern Med. 2017;177:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ and Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Circulation. 2015;131:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pool LR, Carnethon MR, Goff DC Jr., Gordon-Larsen P, Robinson WR and Kershaw KN. Longitudinal associations of neighborhood-level racial residential segregation with obesity among Blacks. Epidemiology. 2018;29:207–214. [DOI] [PubMed] [Google Scholar]
- 23.Getis A and Ord JK. The analysis of spatial association by use of distance statistics. Geographical Analysis. 1992;24:189–206. [Google Scholar]
- 24.Lash TL, VanderWeele TJ, Haneuse S and Rothman KJ. Modern Epidemiology. 4th ed: Wolters Kluwer; 2020. [Google Scholar]
- 25.Raghunathan TE, Lepkowski JM, Van Hoewyk J and Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 26.Rubin DB and Schenker N. Multiple imputation in health-care databases: An overview and some applications. Stat Med. 1991;10:585–98. [DOI] [PubMed] [Google Scholar]
- 27.Colantonio LD, Gamboa CM, Richman JS, Levitan EB, Soliman EZ, Howard G and Safford MM. Black-White differences in incident fatal, nonfatal, and total coronary heart disease. Circulation. 2017;136:152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard G, Safford MM, Moy CS, Howard VJ, Kleindorfer DO, Unverzagt FW, Soliman EZ, Flaherty ML, McClure LA, Lackland DT, et al. Racial differences in the incidence of cardiovascular risk factors in older Black and White adults. J Am Geriatr Soc. 2017;65:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42:3369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, et al. Social determinants of risk and outcomes for cardiovascular disease: A Scientific Statement from the American Heart Association. Circulation. 2015;132:873–98. [DOI] [PubMed] [Google Scholar]
- 31.Hamad R, Penko J, Kazi DS, Coxson P, Guzman D, Wei PC, Mason A, Wang EA, Goldman L, Fiscella K, et al. Association of low socioeconomic status with premature coronary heart disease in US adults. JAMA Cardiol. 2020;5:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to Action: Structural racism as a fundamental driver of health disparities: A Presidential Advisory from the American Heart Association. Circulation. 2020;142:e454–e468. [DOI] [PubMed] [Google Scholar]
- 33.O’Neil A, Scovelle AJ, Milner AJ and Kavanagh A. Gender/sex as a social determinant of cardiovascular risk. Circulation. 2018;137:854–864. [DOI] [PubMed] [Google Scholar]
- 34.Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D, American Heart Association Council on Epidemiology and Prevention, et al. Adverse pregnancy outcomes and cardiovascular disease risk: Unique opportunities for cardiovascular disease prevention in women: A Scientific Statement from the American Heart Association. Circulation. 2021;143:e902–e916. [DOI] [PubMed] [Google Scholar]
- 35.Powell-Wiley TM. Disentangling ancestry from social determinants of health in hypertension disparities-An important step forward. JAMA Cardiol. 2021;6:398–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 2015.
- 37.Liu K, Slattery M, Jacobs D Jr., Cutter G, McDonald A, Van Horn L, Hilner JE, Caan B, Bragg C, Dyer A, et al. A study of the reliability and comparative validity of the CARDIA dietary history. Ethn Dis. 1994;4:15–27. [PubMed] [Google Scholar]
- 38.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D Jr., Liu K, Hubert H, Gernhofer N, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–12. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


