SUMMARY
Transcription factors specify the fate and connectivity of developing neurons. We investigate how a lineage-specific transcription factor, Acj6, controls the precise dendrite targeting of Drosophila olfactory projection neurons (PNs) by regulating the expression of cell-surface proteins. Quantitative cell-surface proteomic profiling of wild-type and acj6 mutant PNs in intact developing brains and a proteome-informed genetic screen identified PN surface proteins that execute Acj6-regulated wiring decisions. These include canonical cell adhesion molecules and proteins previously not associated with wiring, such as Piezo, whose mechanosensitive ion channel activity is dispensable for its function in PN dendrite targeting. Comprehensive genetic analyses revealed that Acj6 employs unique sets of cell-surface proteins in different PN types for dendrite targeting. Combined expression of Acj6 wiring executors rescued acj6 mutant phenotypes with higher efficacy and breadth than expression of individual executors. Thus, Acj6 controls wiring specificity of different neuron types by specifying distinct combinatorial expression of cell-surface executors.
eTOC Blurb
How do transcription factors control neuronal wiring specificity? Xie, Li, et al. combine in situ cell-surface proteomics and genetic analyses to investigate the “transcription factor→cell-surface executor→neuronal wiring” axis and find that lineage-defining transcription factor Acj6 uses unique combinations cell-surface proteins to specify distinct dendrite targeting of different neuron types.
INTRODUCTION
The nervous system is incredibly complex. An adult human brain consists of an average of about 86 billion neurons (Azevedo et al., 2009) forming ~1014 connections. Even in simpler organisms such as the fruit fly, there are around 200,000 neurons in its brain (Raji and Potter, 2021) forming ~107 connections. Precise formation of these neuronal connections ensures proper information processing, which underlies all nervous system functions. Decades of research have identified numerous wiring molecules that specify these neuronal connections.
Most molecules that instruct neuronal wiring discovered thus far fall into two categories—transcription factors and cell-surface proteins (Butler and Tear, 2007; Jan and Jan, 2010; Kolodkin and Tessier-Lavigne, 2011; Sanes and Zipursky, 2020; Santiago and Bashaw, 2014). Transcription factors are central commanders specifying cell fate, morphology, and physiology while cell-surface proteins execute these commands through interaction with cellular environment. In developing neurons, it is presumed that transcription factors control wiring specificity through regulation of cell-surface protein expression. However, causal links between transcription factor and cell-surface wiring proteins have only been established in a few isolated cases (Butler and Tear, 2007; Lee et al., 2015; Peng et al., 2018; Peng et al., 2017; Santiago and Bashaw, 2014; 2017). Moreover, besides one study that linked the function of four cell-surface proteins to the regulation by the transcription factor Even skipped in the development of dorsal motor neurons in Drosophila embryos (Zarin et al., 2014), most reports focus on the regulation of one or two known wiring regulators. Therefore, the number and identity of cell-surface protein(s) a transcription factor regulates to execute its wiring command remain largely unknown (Figure 1A, top). It is also unclear whether a transcription factor regulates the same set or different sets of cell-surface proteins in different neuron types to specify their connectivity (Figure 1A, bottom).
Figure 1. Identification of Acj6-regulated mRNAs and cell-surface proteins.
(A) Central question: how does a transcription factor (TF) control targeting specificity of different neuron types by regulating the expression of cell-surface proteins (CSPs)?
(B) Diagram of cell body locations and glomerular innervation patterns of Drosophila olfactory projection neurons (PNs) derived from either the anterodorsal (adPN, colored in blue) or the lateral (lPN) neuroblast lineage. Acj6 is specifically expressed in adPNs, which target dendrites to a stereotyped subset of glomeruli (colored in blue).
(C and D) Compared to those of wild-type (C), dendrites of acj6−/− adPN neuroblast clones (D) exhibit both loss of innervation (circled in red) and ectopic targeting (circled in white) at a few specific glomeruli. Blue, anti-NCad antibody staining revealing glomerular organization of the antennal lobe. Green, anti-GFP staining of membrane-targeted GFP expressed by PNs.
(E) Schematic of RNA sequencing in developing wild-type (WT) and acj6 mutant adPNs. APF, after puparium formation.
(F) Top five KEGG pathways enriched among genes that are differentially expressed (DE; adjusted p < 0.05) between wild-type and acj6 mutant adPNs.
(G) Schematic of PN surface proteomic profiling in developing wild-type and acj6 mutant brains.
(H) Neutravidin staining of developing PNs expressing HRP after the cell surface biotinylation reaction. Signals are absent intracellularly in PN somata.
Scale bars, 20 μm. D, dorsal; L, lateral.
See also Figure S1.
Here, we systematically addressed these questions using the transcription factor Acj6 (Abnormal chemosensory jump 6) as an example. Acj6 is a member of POU domain transcription factors, which are widely used from C. elegans to mammals to regulate neural development (Certel et al., 2000; Komiyama et al., 2004; Komiyama et al., 2003; Schonemann et al., 1998). In the Drosophila olfactory system, about 50 types of cholinergic excitatory PNs are derived from two distinct neuroblast lineages (Jefferis et al., 2001). Each PN type targets dendrites to a stereotyped antennal lobe glomerulus according to its lineage and birth order (Jefferis et al., 2001; Lin et al., 2012; Marin et al., 2005; Yu et al., 2010). Acj6 is specifically expressed in postmitotic neurons in the anterodorsal PN (adPN) lineage (Figure 1B), whereas another POU domain transcription factor Vvl (Ventral veins lacking, also known as Drifter) is expressed in the lateral PN (lPN) lineage, to instruct lineage-specific dendrite targeting of adPNs and lPNs (Komiyama et al., 2003).
In this study, we profiled the developing PN-surface proteomes of wild-type and acj6-mutant brains by quantitative liquid chromatography-tandem mass spectrometry (LC-MS/MS) and performed a proteome-informed genetic screen to identify cell-surface proteins that execute Acj6’s wiring commands. We discovered that Acj6 instructs PN dendrite targeting by regulating the expression of both canonical wiring molecules with extracellular cell-adhesion domains and unconventional molecules that have been studied mostly in the context of neuronal function, such as the mechanosensitive ion channel Piezo. Overexpressing one or a pair of Acj6 target(s) downregulated on the acj6 mutant PN surface gave rise to specific and combinatorial rescue of acj6 mutant phenotypes, indicating that Acj6 regulates unique combinations of cell-surface proteins in different PN types to specify distinct dendrite targeting. Together, we provide the most comprehensive evidence to date of how a single transcription factor can specify many unique neuronal connections and present functional data demonstrating that neuronal wiring is controlled by a combinatorial code of cell-surface proteins.
RESULTS
Acj6 shapes the PN surface proteomic milieu
Acj6 is required for the proper dendrite targeting of adPNs, as acj6 mutant adPNs exhibit both loss of innervation in glomeruli normally targeted by adPN dendrites and ectopic innervation in glomeruli normally not targeted by these adPN dendrites (Komiyama et al., 2003; Figures 1C and 1D). Acj6 may regulate dendrite targeting through direct transcriptional regulation of cell-surface proteins or through intermediate transcription factors and post-transcriptional mechanisms. To investigate this, we performed RNA sequencing of wild-type and acj6 mutant adPNs at 36–40h after puparium formation (APF), when PN dendrites are actively making wiring decision (Figure 1E; Figures S1A-C; Table S1). We found that genes involved in protein processing in the endoplasmic reticulum and endocytosis were among the top five pathways regulated by Acj6 (Figure 1F), suggesting that post-transcriptional regulation of the PN-surface proteomic landscape by Acj6 may be profound (Figure S1D-F).
Therefore, to capture the entirety of Acj6-regulated cell-surface proteins, we performed PN-surface proteomic profiling in intact wild-type or acj6 mutant fly brains (Figure 1G) at 36–40h APF utilizing our recently developed in situ cell-surface proteomic profiling method (Li et al., 2020). Briefly, membrane-tethered horseradish peroxidases (HRP) expressed on PNs convert the membrane-impermeable biotin-xx-phenol (BxxP) substrate to phenoxyl radicals in the presence of H2O2 (Loh et al., 2016), which promiscuously biotinylate proteins on the PN surface. This approach led to biotinylation of PN-surface proteins with high spatial specificity (Figure 1H).
We applied an 8-plex tandem mass tag (TMT)-based quantitative mass spectrometry strategy to identify those biotinylated proteins (Li et al., 2020; Thompson et al., 2003) (Figure 2A). For each genotype, in addition to two biological replicates, we also included two negative controls, in which either H2O2 or HRP was omitted to account for non-specific bead binders, endogenously biotinylated proteins, and labeling by endogenous peroxidases. Each sample (derived from ~1100 dissected pupal brains) was separately lysed and enriched with streptavidin beads (Figure 2B). After on-bead trypsin digestion and TMT labeling, all samples were pooled for mass spectrometry analysis (Table S2).
Figure 2. Cell-surface proteomes of wild-type and acj6 mutant PNs.
(A) Design of the 8-plex tandem mass tag (TMT)-based quantitative proteomic experiment. Each genotype comprises two biological replicates (blue or pink) and two negative controls omitting either HRP or H2O2 (black). Labels in the TMT row indicate the TMT tag used for each condition.
(B) Streptavidin blot of the post-enrichment bead eluate—3.5% from each sample listed in (A).
(C) Receiver operating characteristic (ROC) analysis showing the separation of true positives from false positives in each biological replicate using the experimental-to-control TMT ratio.
(D) Summary of the cutoff analysis. Proteins with TMT ratios greater than the cutoffs in both biological replicates were retained (‘Intersection’). FDR: false discovery rate.
(E) Venn diagram showing the size of and overlap between wild-type and acj6 mutant PN surface proteomes.
(F) Top five Cellular Component and Biological Process Gene Ontology terms for wild-type (blue) and acj6 mutant (pink) PN surface proteomes.
(G) Volcano plot showing proteins with altered protein levels on acj6 mutant PN surface compared to wild-type PN surface. Proteins downregulated in acj6 mutant [log2(fold change) less than −0.53 and p-value less than 0.05] are colored in blue. Proteins upregulated in acj6 mutant [log2(fold change) greater than 0.53 and p-value less than 0.05] are colored in red.
See also Figures S1 and S2.
Biological replicates in both genotypes showed high correlation (Figure S2A), indicating the robustness of our method. Additionally, loss of Acj6 did not cause global disruption of protein expression (Figure S2B). We ranked proteins by their experimental-to-control TMT ratios in a descending order and found that known plasma membrane proteins were enriched while contaminants were sparse at the top of those ranked lists (bottom left corner, Figure 2C). Therefore, we filtered out contaminants using experimental-to-control TMT ratios (Hung et al., 2014; Li et al., 2020) (Figure 2D; Figure S2C), yielding PN surface proteomes of 459 and 537 proteins for wild-type and acj6 mutant, respectively (Figure 2E). Cellular Component terms classified by Gene Ontology analysis showed that both proteomes consisted of proteins localized at the cell surface, confirming the spatial specificity of our approach (Figure 2F, top). Top Biological Process terms showed enrichment of neural developmental processes in both proteomes (Figure 2F, bottom), matching the developmental stage at which we conducted PN surface profiling.
Many proteins exhibited altered expression on the acj6 mutant PN surface (Figure 2G). These include proteins belonging to classic cell adhesion protein families—Off-track (Otk), Sidestep-V (Side-V), and Dprs in the immunoglobulin (Ig) superfamily, and Reduced ocelli (Rdo) and Tartan (Trn) in the leucine-rich repeat superfamily. We also observed proteins known to participate in neuronal function—the mechanosensitive ion channel Piezo, the dopamine receptors Dop1R1 and Dop2R, a regulator of voltage-gated calcium channel Stolid (Stol), and the voltage-gated calcium channel subunit α1 Cacophony (Cac). Most of these proteins were also identified using a more stringent cutoff criterion (Figures S2D-S2F). Consistent with our RNA-sequencing analysis suggesting possible post-transcriptional regulation of cell-surface proteins by Acj6 (Figure 1F), Acj6 binding site prediction suggested that most genes encoding cell-surface proteins differentially expressed in wild-type and acj6 mutant are unlikely direct targets of Acj6 (Figure S1G).
A proteome-informed genetic screen identified new wiring molecules for PN dendrites
Among the Acj6-regulated PN surface proteins we identified, Trn is the only one known to participate in PN dendrite targeting (Hong et al., 2009). Therefore, we designed a genetic screen to systematically identify Acj6 targets with wiring functions.
We used a split GAL4 intersectional strategy (Luan et al., 2006; Pfeiffer et al., 2010) to reconstitute full-length GAL4 in about half of all adPN types by combining the PN-specific VT033006-GAL4DBD with the adPN lineage-restricted C15-p65AD. This “adPN-GAL4” line was used to knock down (with UAS-RNAi) or overexpress (with UAS-cDNA) candidates whose expressions were down- or up-regulated on the acj6 mutant PN surface, respectively. Due to the limited availability of existing UAS-cDNA lines for overexpression, most candidates we tested were downregulated in acj6 mutant, which means that Acj6 normally promotes their expression.
We scored the innervation degrees of 38 glomeruli in each antennal lobe of each genotype (Figure 3A; Figure S3). To determine whether altering the expression of these Acj6-regulated PN surface proteins caused significant dendrite targeting changes, the innervation extent of each glomerulus in each genotype was compared to control using a Chi-squared test (Figure 3A; Figure S4). In contrast to our previous wiring molecule screening (Ward et al., 2015; Xie et al., 2019), this screening and scoring strategy can monitor dendrite targeting defects in many PN types simultaneously instead of just two to three PN types located in a specific region. This is critical as Acj6 controls dendrite targeting of many PN types and might regulate expression of different wiring molecules in different PN types.
Figure 3. A proteome-informed genetic screen to identify wiring executors of Acj6.
(A) Schematic of the genetic screen and quantification. Dendrite innervation of each glomerulus was scored into three categories: strong, weak, and none. Scorer was blind to genotypes.
(B) Confocal images showing dendrite innervation patterns of adPN−GAL4+ PNs (green) in controls and when acj6 or candidate genes were knocked down by RNAi. Blue, N-cadherin (NCad) staining highlight the glomeruli. Glomeruli with decreased PN dendrite innervation are circled in red while ectopically targeted glomeruli are circled in white. Arrowheads point to the glomeruli exhibiting mistargeting phenotypes.
Scale bars, 20 μm. D, dorsal; L, lateral.
See also Figures S3 and S4.
We identified many new molecules required for PN dendrite targeting (Figure 3B). These include Otk, Neprilysin 3 (Nep3), and Dystroglycan (Dg), which have been previously shown to be required for neurite targeting of other neuron types (Cafferty et al., 2004; Li et al., 2020; Shcherbata et al., 2007; Winberg et al., 2001). In addition, knockdown of Dop1R1, Dop2R, stol, and piezo, which are traditionally thought to mediate neuronal function instead of development, also caused PN wiring defects. Previously uncharacterized genes, such as CG5027, also contributed to the targeting accuracy of PN dendrites.
As with knocking down acj6 itself, knocking down Acj6-regulated cell-surface proteins caused abnormal targeting of adPN dendrites of many glomeruli. Notably, these ectopic targeting or loss of targeting resembled acj6-RNAi in multiple glomeruli including DA4m, DA41, VA71, VA3, and DM4 (Figure 3B). Furthermore, dendrite targeting to an individual glomerulus can be affected by knocking down several different molecules. For example, loss of VA71 innervation was observed when knocking down acj6, otk, or Dop2R, but not piezo, Dop1R1, or ostγ (second row of Figure 3B). These data suggest that many Acj6-regulated cell-surface proteins indeed regulate wiring specificity, and that proper targeting of some PN dendrites requires multiple cell-surface proteins controlled by Acj6. Additionally, knockdown of some candidates also caused unique dendrite mistargeting patterns not observed in acj6-RNAi (Figure S4), suggesting that these molecules also have wiring functions independent of Acj6.
Ig-superfamily protein Otk is a cell-surface wiring executor for Acj6
To establish causal relationships between Acj6 and its targets in regulating wiring specificity (Figure 4A), we tested whether forced expression of specific Acj6-downregulated cell-surface proteins can rescue acj6 mutant phenotypes using mosaic analysis with a repressible cell marker (Lee and Luo, 2001) (MARCM; Figures S5A and S5B) with null mutant alleles and full-length UAS-cDNA transgenes. Besides bypassing Acj6’s transcriptional regulation, the amplification effect of the GAL4/UAS system makes it likely that forced expression of candidate cell-surface proteins would also overcome potential post-transcriptional regulation by Acj6.
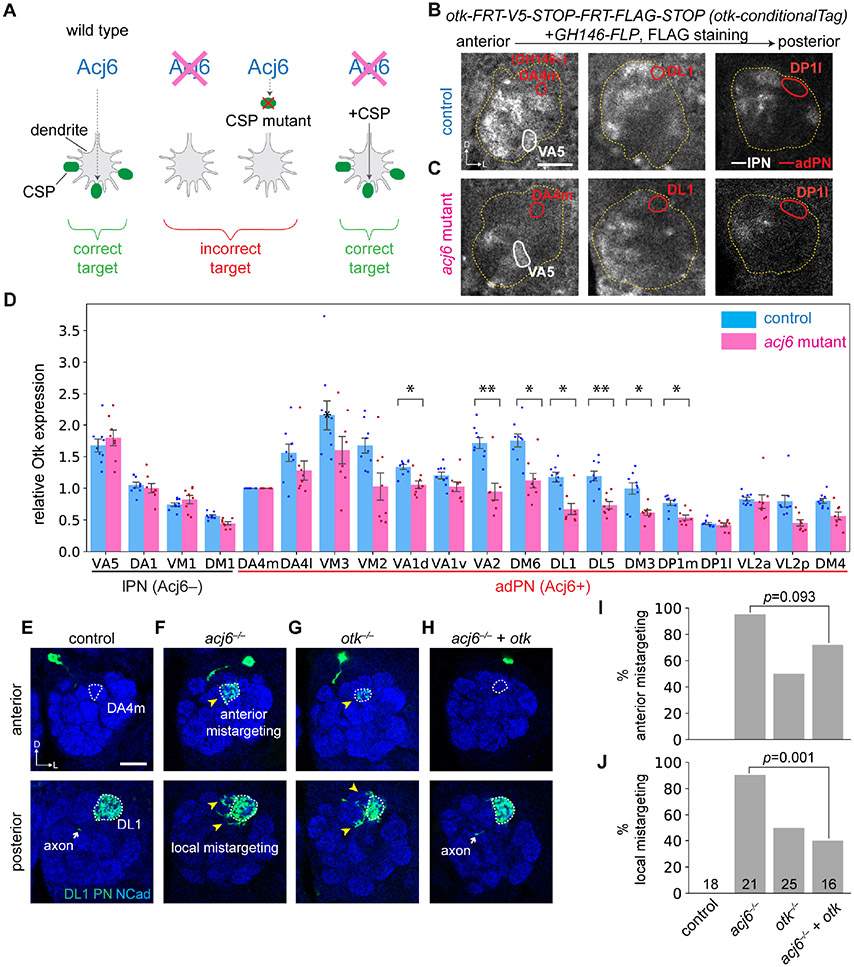
Figure 4. Off-track (Otk) is an Acj6 executor for instructing PN dendrite targeting.
(A) Criteria of Acj6’s cell-surface wiring executors. Loss of either Acj6 or its cell-surface wiring executor would lead to similar dendrite mistargeting. Supplying back the cell-surface wiring executor of Acj6 would rescue acj6 mutant phenotype.
(B and C) Expression of Otk in in a subset of discernible PN types at 42–48 hr APF in wild-type (B) or acj6 mutant (C) brains. Antennal lobe (outlined in yellow) and the DA4m (GH146− adPN), VA5 (lPN), and DL1 (adPN) glomeruli are outlined. GH146-FLP was used to express FLP in the majority of PNs.
(D) Quantification of Otk expression in developing PNs of wild-type (n = 9) and acj6-mutant (n = 8) animals. Fluorescence intensities were normalized to the GH146-FLP negative DA4m glomerulus in each antennal lobe. Mean ± s.e.m. *: p < 0.05; **: p < 0.01 (two-tailed t-test; p-values were adjusted for multiple comparisons using Bonferroni’s method). Note that the fluorescence intensity is always lower in deeper (more posterior) sections due to tissue scattering, so the absolute intensity cannot be compared across different PN types.
(E) Dendrites of wild-type DL1 single-cell clones innervate exclusively the posterior DL1 glomerulus (n = 18).
(F) acj6−/− DL1 single-cell clones mistarget to both nearby glomeruli and a distant anterior glomerulus (n = 21). Yellow arrowhead, mistargeted PN dendrites.
(G) otk−/− DL1 single-cell clones phenocopy acj6−/− (n = 25).
(H) acj6−/− DL1 dendrite mistargeting phenotypes were partially rescued by overexpressing otk (n = 16).
(I and J) Quantification of DL1 single-cell clones that mistargeted at a distance (I) and locally (J). p-values of Chi-squared tests are shown on plots.
Scale bars, 20 μm. D, dorsal; L, lateral. Arrow, PN axons.
See also Figures S5.
We first investigated Otk, a transmembrane protein containing five extracellular Ig-like domains implicated in axon guidance in embryonic motor axons (Winberg et al., 2001) and photoreceptor axons (Cafferty et al., 2004). We generated a conditional tag to examine endogenous Otk expression specifically in PNs (Figures S5C-S5E). We found that Otk was normally expressed in subsets of both Acj6-positive adPNs and Acj6-negative lPNs during development (Figure 4B). In acj6 mutant, there was an apparent overall decrease in Otk signal (Figure 4C). Quantification of fluorescence intensity in individual glomeruli revealed that Otk expression was indeed decreased in many adPNs such as DL1, but not in lPNs such as VA5 (Figure 4D). However, some adPN types such as VA1v and VL2a had similar levels of Otk expression in acj6 mutant and wild-type animals (Figure 4D), and some adPN types such as DP1l had barely detectable Otk expression in wild-type animals (Figure 4B). Thus, Acj6 promotes Otk expression on the surface of a subset of adPNs.
We performed MARCM analysis by deleting otk in either anterodorsal or lateral neuroblast clones (Figure S5B) and found dendrite mistargeting in both (Figures S5F-S5I), consistent with otk expression in both Acj6+ adPNs and Acj6− lPNs. These data validated Otk as a wiring molecule for PN dendrites. To test if Acj6 regulates Otk expression to instruct adPN dendrite targeting, we focused on single-cell clones of DL1 PNs (Figure S5B). In wild-type animals, dendrites of DL1 PNs are confined to a specific glomerulus located in a posterior section of the antennal lobe (Figure 4E, outlined in the bottom panel). Deleting acj6 or otk in DL1 PNs yielded nearly identical mistargeting phenotypes: DL1 PN dendrites mistargeted to both nearby posterior glomeruli, DL5 and DC1, as well as the distant DA4m glomerulus in the anterior antennal lobe (Figures 4F and 4G). Furthermore, both local and long-range mistargeting phenotypes in acj6 mutant were partially rescued by overexpressing otk in DL1 single cell clones (Figures 4H-4J). This incomplete rescue is consistent with the observation that otk−/− clones had lower phenotypic penetrance than acj6−/− clones (Figures 4I and 4J) and suggests that Acj6 controls the expression of additional wiring molecule(s) in DL1 PNs to ensure precise dendrite targeting.
These single-cell clone analyses, together with the observation that Otk expression was decreased in acj6 mutant DL1 PNs (Figures 4B-4D), demonstrated that Acj6 directs precise targeting of DL1 PN dendrites in part by cell-autonomously promoting Otk expression.
Piezo executes Acj6’s wiring command independently of its mechanosensitive ion channel activity
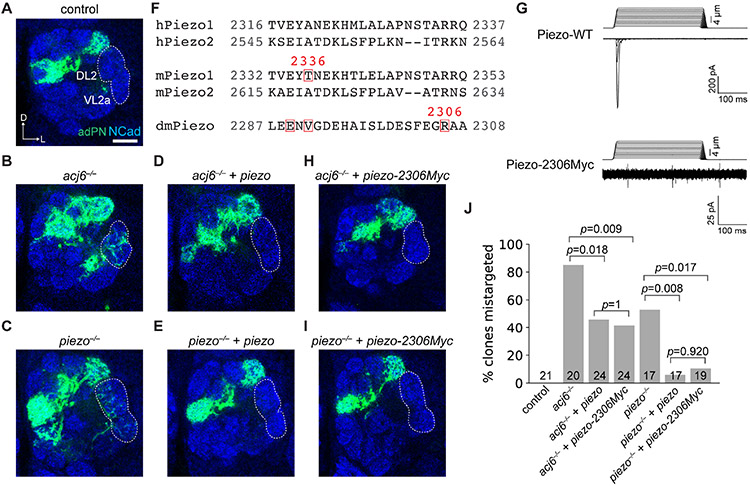
An unexpected observation in our genetic screen was that knocking down the mechanosensitive ion channel, Piezo, disrupted normal PN dendrite targeting (Figure 3B; Figure S4). MARCM analysis using a piezo null mutant confirmed that both acj6−/− and piezo−/− adPNs in neuroblast clones mistargeted their dendrites to the DL2 and VL2a glomeruli (Figures 5A-5C), albeit with a lower penetrance in piezo−/− PNs (Figure 5J). piezo endogenous conditional tag revealed that Piezo was expressed in a sparse set of PNs, and expression of Piezo in some adPNs was downregulated in acj6 mutant animals (Figures S6A and S6B). Furthermore, overexpressing wild-type piezo in adPN neuroblast clones not only rescued piezo mutant phenotypes (Figures 5E and 5J), but also partially rescued acj6 mutant phenotypes (Figures 5D and 5J). Thus, Piezo is an executor for Acj6 in controlling dendrite targeting of a subset of PNs.
Figure 5. Piezo is an Acj6 executor for instructing PN dendrite targeting.
(A–C) Dendrite innervation patterns of wild-type (n = 21) (A), acj6−/− (n = 20) (B), and piezo−/− (n = 17) (C) adPN neuroblast clones. DL2 and VL2a glomeruli are ectopically innervated in acj6 and piezo mutants. Note that this neuroblast clone analysis does not allow us to determine the specific adPN type(s) that produce mistargeted dendrites.
(D and E) acj6−/− (D, n = 24) and piezo−/− (E, n = 17) mutant phenotypes were rescued by overexpressing wild-type piezo.
(F) Sequence alignment of human Piezo1 (hPiezo1), human Piezo2 (hPiezo2), mouse Piezo1 (mPiezo1), mouse Piezo2 (mPiezo2), and fruit fly Piezo (dmPiezo). Red boxes highlight amino acids located N-terminal to Myc insertions.
(G) A Myc tag inserted C-terminal to amino acid position 2306 of fly Piezo abolishes its mechanically activated channel activity. Representative traces of indentation-induced whole-cell currents from piezo-WT or piezo-2306Myc transfected human PIEZO1 knockout HEK293T cells are shown (see Figure S6 for quantification as well as additional data on other Myc insertion mutants).
(H and I) acj6−/− (n = 24) and piezo−/− (n = 19) mutant phenotypes were rescued by overexpressing piezo-2306Myc.
(J) Quantification of adPN dendrites in neuroblast clones that mistargeted to DL2 and VL2 glomeruli, p-values of Chi-squared tests after Yates’ continuity correction are shown.
Scale bars, 20 μm. D, dorsal; L, lateral.
See also Figures S6.
Is the mechanosensitive ion channel activity of Piezo required for instructing PN dendrite targeting? A previous study showed that inserting a Myc tag after amino acid 2336 in mouse Piezo1 abolishes its channel activity (Coste et al., 2015). Therefore, we inserted Myc tags at three amino acid locations in fly Piezo in a region homologous to amino acid 2336 in the mouse Piezo1 (Figure 5F). All three Myc-insertion variants displayed proper cell-surface expression (Figure S6D). However, whole-cell patch clamp recordings showed that all three variants exhibited reduced mechanically activated currents in response to membrane indentation compared with control, and the mechanosensitive ion channel activity of Piezo-2306Myc was completely abolished (Figure 5G; Figures S6E and S6F).
Remarkably, overexpressing piezo-2306Myc in adPN neuroblast clones rescued dendrite targeting deficits of piezo and acj6 mutants to the same extent as overexpressing wild-type piezo (Figures 5D, 5E, and 5H-5J). We further examined the Acj6-negative lPNs and observed that loss of Piezo also caused dendrite mistargeting, which was partially rescued by either wild-type Piezo or Piezo-2306Myc (Figures S6G-S6J). This indistinguishable rescuing capacity suggested that Piezo regulates PN dendrite targeting independently of its mechanosensitive ion channel activity.
Acj6 employs distinct cell-surface executors in different PN types
Data presented so far indicate that Acj6 directs the precise targeting of DL1 PN dendrites in part through Otk (Figure 4) and prevents mistargeting of adPN dendrites to the DL2 and VL2a glomeruli in part through Piezo (Figure 5). To investigate more cell-surface wiring executors of Acj6 in a broader set of PNs, we overexpressed in acj6−/− adPN neuroblast clones six additional cell-surface proteins that were downregulated in acj6 mutant and examined dendrite innervation patterns in a broader set of PNs.
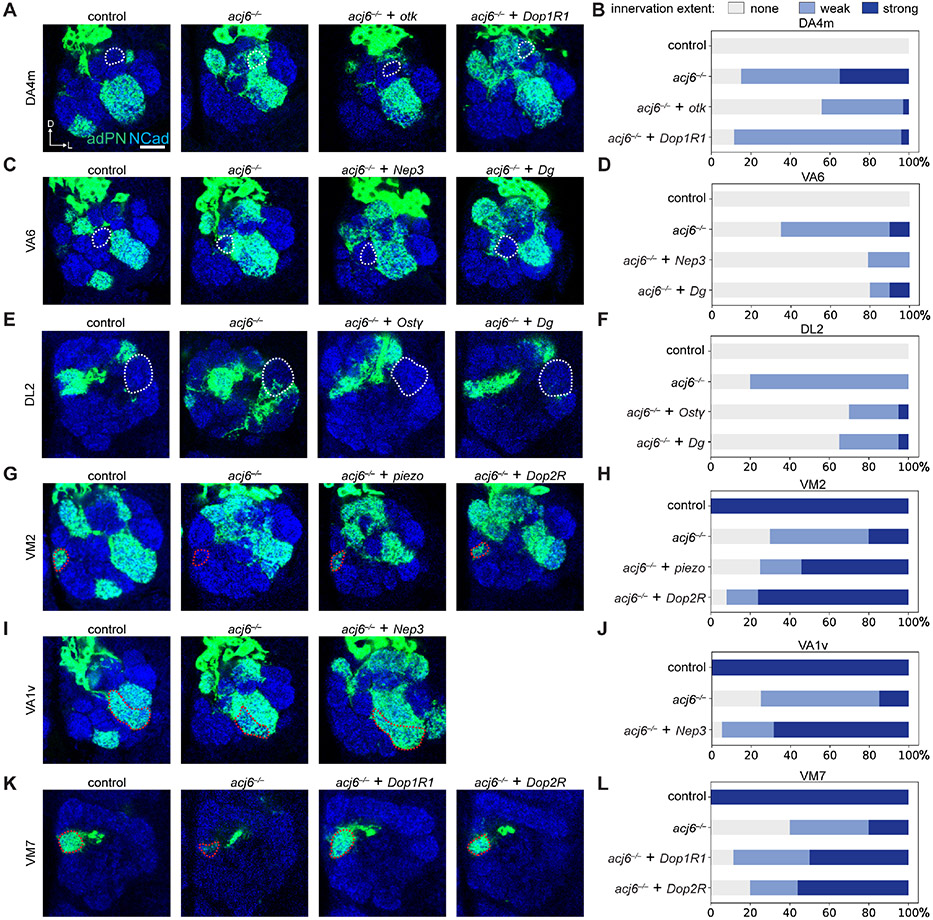
Overexpression of seven out of eight of these cell-surface proteins rescued specific subsets of acj6 mutant phenotypes to different extents (Figure 6; Figure S7A), including ectopic targeting (DA4m, VA6, and DL2; Figures 6A-6F) and loss of innervation (VM2, VA1v, and VM7; Figures 6G-6L). The mistargeting of each PN type in acj6 mutant was rescued by specific cell-surface proteins. For example, loss of VA1v targeting in acj6 adPN neuroblast clones was rescued by Nep3 (Figures 6I and 6J) while loss of VM7 targeting was rescued by the dopamine receptors Dop1R1 and Dop2R (Figures 6K and 6L). From the perspective of individual cell-surface protein, each participated in the targeting of specific glomeruli. For instance, Dg overexpression rescued mistargeting to VA6 and DL2 glomeruli (Figures 6C-6F), whereas Dop1R1 overexpression rescued VM7 targeting and mistargeting to DA4m in acj6 adPN neuroblast clones (Figures 6A, 6B, 6K, and 6L). Thus, Acj6-regulated cell-surface executors exhibited a complex but highly specific correspondence to individual adPN types and their target glomeruli in the developing antennal lobe.
Figure 6. acj6 mutant phenotypes rescued by individual cell-surface executors.
(A and B) Ectopic targeting of adPN dendrites in neuroblast clones (adPN dendrites hereafter) to the DA4m glomerulus in acj6 mutant was partially rescued by overexpressing otk or Dop1R1.
(C and D) Ectopic targeting of adPN dendrites to the VA6 glomerulus in acj6 mutant was partially rescued by overexpressing Nep3 or Dg.
(E and F) Ectopic targeting of adPN dendrites to the DL2 glomerulus in acj6 mutant was partially rescued by overexpressing Ostγ or Dg.
(G and H) Loss of adPN dendrite innervation in the VM2 glomerulus in acj6 mutant was partially rescued by overexpressing piezo or Dop2R.
(I and J) Loss of adPN dendrite innervation in the VA1v glomerulus in acj6 mutant was partially rescued by overexpressing Nep3.
(K and L) Loss of adPN dendrite innervation in the VM7 glomerulus in acj6 mutant was partially rescued by overexpressing Dop1R1 or Dop2R.
Bar graphs show fractions of clones belonging to three categories of innervation extent: strong, weak, and none.
Scale bars, 20 μm. D, dorsal; L, lateral.
See also Figures S7.
To quantitatively assess whether overexpression of Acj6-regulated cell-surface proteins indeed improved wiring precision of acj6−/− adPN dendrites, we performed Chi-squared tests comparing the innervation extent of each glomerulus in each rescue experiment to that in control or acj6−/− (Figure 7A). As shown in each row of Figure 7A, overexpressing a single Acj6-regulated cell-surface protein rescued a subset of acj6−/− phenotypes, consistent with our observation that loss of one cell-surface protein resembled only a subset of acj6−/− phenotypes (Figures 3-5). Notably, phenotypic rescues by different cell-surface proteins appeared to be largely non-overlapping (Figure 7A), indicating that Acj6 uses distinct cell-surface executors in different PN types for dendrite targeting.
Figure 7. Acj6 instructs PN wiring through a cell-surface combinatorial code.
(A and B) Heatmap summarizing changes in the dendrite innervation pattern of adPN neuroblast clones to eleven glomeruli (columns). Dark red (ectopic innervation) and dark blue (loss of innervation) indicate either acj6 mutant phenotype itself or no rescue of acj6 mutant phenotype (p ≥ 0.05 comparing to acj6 mutant innervation extent, by Chi-squared test here and after). White indicates either the wild-type innervation pattern or complete rescue of acj6 mutant phenotype (p ≥ 0.05 comparing to wild-type innervation extent). Light red (partial ectopic innervation) and light blue (partial loss of innervation) indicate that the phenotypic rescue was significant (p < 0.05 comparing to acj6 mutant innervation extent) but still different from wild-type (p < 0.05 comparing to wild-type innervation extent). Letters within the grids indicate panels where detailed quantifications are shown. Dots highlight an example of between-glomeruli additive interaction.
(C–G) Examples of additive (C and D), subtractive (E), and synergistic (F and G) interactions between Acj6-regulated cell-surface proteins.
(H) Summary of the cell-surface wiring executors of Acj6 for instructing correct targeting or preventing ectopic targeting of adPN dendrites to distinct glomeruli.
(I) Wiring specificity of different neuron types is dictated by a cell-surface protein combinatorial code, which is controlled by combinatorial expression of transcription factors—each transcription factor regulates the expression of multiple cell-surface proteins (divergence), and each cell-surface protein is regulated by multiple transcription factors (convergence).
See also Figures S7.
We note that occasionally overexpression of an Acj6-regulated cell-surface protein rescued acj6 mutant phenotypes better than overexpression of Acj6 itself (Figure 7A). Because acj6 undergoes alternative splicing to produce 13 isoforms (Bai and Carlson, 2010; Certel et al., 2000), one possible explanation is that different PN types express different acj6 isoforms to regulate the expression of different cell-surface executors, so overexpressing a specific one (isoform J in our case) may not be able to rescue all acj6 mutant phenotypes. Another possible explanation is the delayed onset of MARCM-based overexpression due to GAL80 perdurance and the earlier action of a transcription factor compared to its downstream cell-surface executors.
Interestingly, overexpressing some cell-surface proteins that were downregulated in acj6 mutant occasionally exacerbated dendrite mistargeting caused by acj6 mutant. For example, the VA1v glomerulus became even less frequently innervated in acj6−/− adPN neuroblast clones overexpressing Dg compared to acj6 clones alone (Figure S7A). Examining the endogenous Dg expression using the conditional tag strategy revealed that Dg was not expressed in VA1v PNs (Figure S7B). Therefore, the worsened phenotype is likely caused by mis-expression of Dg. These results further support that Acj6 regulates different cell-surface proteins in different PN types for their precise wiring.
Acj6 specifies PN dendrite targeting through a combinatorial cell-surface code
The limited rescue of a small subset of acj6 mutant phenotypes by each single cell-surface protein (Figure 7A) prompted us to examine whether combinatorial expression could better rescue wiring defects caused by loss of acj6. We tested seven combinations of two Acj6-regulated cell-surface proteins and found that these combinations indeed led to overall stronger and broader rescue of acj6−/− wiring defects in adPN neuroblast clones (more white and lighter colored squares in Figure 7B than in Figure 7A). A closer examination of these combinations revealed different modes of genetic interactions between Acj6-regulated cell-surface executors, which collectively contributed to the improved rescuing efficacy.
The most frequently observed interaction mode is additive. For each glomerulus, we observed two candidates with partial rescues could “sum up” to produce an almost complete rescue when co-expressed. For instance, overexpression of either Otk or Piezo partially restored innervation to the VM2 glomerulus (Figure 7C and ‘C’ squares in Figure 7A). Co-expression of Otk and Piezo led to wild-type-like innervation in the VM2 glomerulus (Figure 7C and the ‘C’ square in Figure 7B). When Piezo was coupled with Dg, they reduced ectopic targeting to the DL2 glomerulus from −80% in acj6 mutant to less than 20% (Figure 7D and ‘D’ squares in Figures 7A and B). Across different glomeruli, the phenotypic rescue by different cell-surface executors were also additive. For example, Dop2R but not Dg overexpression rescued loss of innervation to the DA3 glomerulus, while Dg but not Dop2R overexpression rescued ectopic targeting to the VA6 glomerulus (Figure 7A, black dots). When Dop2R and Dg were co-expressed, both DA3 and VA6 phenotypes were rescued (Figure 7B, black dots). Lastly, additive interactions can inhibit phenotypic rescue if one candidate counteracts, resulting in a subtractive interaction. As described above, Dg overexpression exacerbated the loss of VA1v innervation phenotype in acj6 mutant (Figure 7E). Co-expressing Dg with Nep3, whose expression rescued loss of innervation to the VA1v glomerulus, diminished the rescue effect of Nep3 (Figure 7E and ‘E’ squares in Figures 7A and 7B).
Besides additive interactions, we also observed synergistic interactions. For example, neither Otk nor Dop1R1 overexpression alone significantly rescued VL2a ectopic targeting in acj6 mutant (Figure 7F and ‘F’ squares in Figure 7A). However, co-expressing both reduced the mistargeting rate from more than 70% in mutant to ~30% (Figure 7F and ‘F’ square in Figure 7B), suggesting that they might both be required for controlling dendrite targeting. In another case, Dop2R overexpression alone could not provide significant rescue of DA4m ectopic targeting but, when co-expressed with Otk, it significantly enhanced rescue by Otk (Figure 7G and ‘G’ squares in Figures 7A and B).
Taken together, combinatorial expressions of Acj6-regulated cell-surface executors deliver stronger and broader rescues of acj6 wiring defects than single expressions. These results reveal that Acj6-regulated executors act combinatorically—both between different PN types and within the same PN types—to instruct dendrite targeting, and different PN types employ distinct combinations of cell-surface proteins for their precise targeting (Figure 7H).
DISCUSSION
Nucleus-residing transcription factors control neuronal connectivity but do not directly regulate wiring at distal neurites. It is therefore a long-standing presumption that transcription factors regulate the expression of cell-surface proteins to execute wiring decisions. Here, we combined in situ cell-surface proteomic profiling and genetic analyses to systematically identify cell-surface executors for the lineage-specific transcription factor Acj6 in the wiring of fly olfactory PNs. We discovered many previously unknown wiring molecules and revealed the operational strategies of Acj6-regulated cell-surface executors in instructing dendrite targeting.
Identifying cell-surface executors of transcription factors
For most transcription factors directing neuronal connectivity, their cell-surface wiring executors remain elusive due to the lack of approaches for systematically identifying them. In cases where the wiring executor(s) of a transcription factor have been identified, often only one or two molecules whose wiring function has been established in that neuron type were investigated (selected examples summarized in Santiago and Bashaw, 2014), precluding the discovery of the repertoire of cell-surface wiring executors controlled by a transcription factor. RNA sequencing provides a way to determine how deleting a transcription factor alters the transcriptome of developing neurons (Jain et al., 2020; Morey et al., 2008; Peng et al., 2018; Peng et al., 2017), but studies comparing transcriptome and proteome of the same cell type often revealed modest to poor correlations (Carlyle et al., 2017; Ghazalpour et al., 2011; Wang et al., 2019), particularly for membrane proteins whose trafficking and turnover is subject to extensive post-translational regulation (Li et al., 2020; MacGurn et al., 2012; Trowbridge et al., 1993). Indeed, comparing wild-type and acj6 mutant adPN transcriptomes suggested that Acj6 could regulate the expression of proteins on PN surface post-transcriptionally (Figures 1E and 1F; Figure S1).
We therefore took advantage of our recently developed cell-surface proteomic profiling (Li et al., 2020) to identify Acj6-regulated wiring executors for PN dendrite targeting by comparing cell-surface proteomes of wild-type and acj6-mutant developing PNs (Figure 2), followed by a proteome-informed genetic screen (Figure 3). Quantitative cell-surface proteomic profiling allowed us to directly examine the protein expression levels at the cell surface, instead of inferring from the mRNA levels (Aebersold and Mann, 2016; Han et al., 2018; Hosp and Mann, 2017; Li et al., 2020; Natividad et al., 2018). Our subsequent functional interrogations identified many Acj6 executors via both loss-of-function and rescue experiments (Figures 4-7), highlighting the effectiveness of our approach for identifying cell-surface executors of a transcription factor.
Since transcription factors often serve as central commanders of cellular functions while cell-surface proteins work as direct executors in cell-cell interactions, the ‘transcription factor → cell-surface executor → physiological function’ framework is of importance for not only neural circuit wiring but also all other biological processes involving cells communicating with their environment. Notably, cell-surface proteins are more accessible than nucleus-residing transcription factors and often exhibit higher specificity for a specific biological process than broadly-acting transcription factors, making cell-surface proteins more favorable targets for drug development. Therefore, systematically identifying cell-surface executors of a transcription factor not only delineates biological mechanisms but also has the potential for discovering more druggable targets.
Piezo instructs neural circuit wiring independently of its mechanosensitive ion channel activity
Since the discovery of Piezo1 and Piezo2 (Coste et al., 2010), numerous functions of the Piezo protein family have been uncovered in a wide range of physiological contexts, including but not limited to somatosensory and interoceptive mechanotransduction and cell volume regulation (reviewed in Murthy et al., 2017; Szczot et al., 2021). Piezo has also been shown to regulate neurodevelopmental processes and axon regeneration—it promotes neurogenesis instead of astrogenesis in human neural stem cells (Pathak et al., 2014), contributes to axon growth and targeting of Xenopus retinal ganglion cells (Koser et al., 2016), and cell autonomously inhibits axon regeneration (Song et al., 2019). Notably, all known functions of Piezo thus far have been attributed to its mechanosensitive ion channel activity.
We found that Piezo instructs PN dendrite targeting, and its mechanically activated ion channel activity is dispensable for this function (Figure 5), suggesting that Piezo can also function independently of being a mechanosensitive ion channel. We note that lack of mechanically activated currents from Piezo-Myc2306 does not distinguish between loss of ion permeation property and loss of mechanosensitivity. Given that Piezo proteins form 900-kDa complexes with a large extracellular surface area, it is possible that currently unknown Piezo ligands and/or receptors mediate the wiring function of Piezo. Systematic identification of Piezo’s molecular partners may reveal how Piezo instructs dendrite targeting and how it functions independently of its mechanosensitive channel activity.
Besides Piezo, our proteome-informed genetic screen discovered several other unconventional wiring molecules for PN dendrite targeting, including the dopamine receptors Dop1R1 and Dop2R, highlighting the functional versatility of these molecules in multiple biological contexts. It will be interesting to explore whether these molecules regulate neuronal wiring via their conventional molecular functions or through currently unknown mechanisms like Piezo.
Acj6 instructs PN wiring through a combinatorial cell-surface code
Because the number of neuronal connections far exceeds the number of wiring molecules, it has been proposed that precise neuronal connections are specified by a combinatorial code—each type of neuron uses a unique combination of wiring molecules, and each molecule is used in multiple neuron types (Hong and Luo, 2014). Here, we observed that many acj6−/− adPN dendrites ectopically targeted to glomeruli normally occupied by other (not labeled) Acj6+ adPNs (Figures 6A-6F), suggesting that Acj6 regulates the expression of distinct cell-surface proteins in different PN types and instructs dendrite targeting through a combinatorial code.
Endogenous protein tagging of three Acj6 executors, Otk, Piezo, and Dg, showed that Acj6 indeed regulates the expression of different cell-surface proteins in different PN types (Figures 4B-4D; Figures S6A and S6B; Figure S7B). Functionally, our systematic examination of Acj6 executors in PN dendrite targeting by RNAi knockdown (Figure 3; Figure S3; Figure S4) and rescue experiments (Figure 6; Figure 7; Figure S7A) revealed that different PN types require different cell-surface proteins for dendrite targeting (columns in Figure 7A), and each molecule often regulates a few distinct PN types (rows in Figure 7A). Moreover, combinatorial expression of Acj6 executors yielded stronger and broader rescues of specific acj6 mutant phenotypes than expressing individual executors (Figure 7B). All these observations support the notion that Acj6 employs unique combinations of cell-surface proteins in different PN types to control dendrite targeting (Figure 7H).
Our findings illustrate a divergent ‘transcription factor → cell-surface executor’ relationship—one transcription factor Acj6 regulates many different cell-surface proteins in distinct neuron types to execute its function (Figure 7I). This could not have been discovered by examining only one or two wiring executors of a transcription factor in a single neuron type. On the other hand, Acj6-regulated cell-surface proteins often also have Acj6-independent functions. For instance, Otk is also expressed by Acj6-negative lPNs (Figures 4B-4D) and is required for lPN dendrite targeting (Figures S5H and S5I). Piezo also participates in lPN dendrite targeting (Figures S6G-S6J). Thus, they must also be regulated by other transcription factors. The fact that Acj6 differentially regulates the same cell-surface proteins in different adPN types also demands the involvement of other transcription factors; otherwise, Acj6 would uniformly regulate each executor in all Acj6+ PN types. Indeed, a previous study has demonstrated that a combination of transcription factors works together to regulation the expression of a set of cell-surface proteins for the accurate axon guidance of dorsal motor neurons (Zarin et al., 2014). Therefore, the ‘transcription factor → cell-surface executor’ relationship is also convergent—multiple transcription factors regulate the expression of one cell-surface protein (Figure 7I). We thus anticipate the existence of two intertwined combinatorial codes—one of the transcription factors, which specifies the other one of cell-surface executors—to determine neuronal wiring specificity.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to the Lead Contact, Liqun Luo (lluo@stanford.edu).
Material availability
All unique reagents generated in this study are available from the Lead Contact.
Data and code availability
Raw sequencing reads of the transcriptomics experiment have been deposited to GEO (GSE200841). Processed RNA-sequencing data is provided in Table S1. Mass spectra from the proteomic experiment have been deposited to MassIVE (MSV000089187). Processed proteomic data is provided in Table S2. Custom analysis code is available at https://github.com/Qijing-Xie/Acj6.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila stocks and genotypes
Flies were maintained on standard cornmeal medium with a 12 hr light–dark cycle at 25°C, except for the RNAi/overexpressing screen in which flies were raised at 29°C. Complete genotypes of flies used in each experiment are described in Table S3. The following lines were used in this study: tubP-GAL80 and UAS-mCD8-GFP (Lee and Luo, 1999), hsFlp (Golic and Lindquist, 1989), FRT19A, FRT40A, and FRT42D (Xu and Rubin, 1993), GH146-Flp (Hong et al., 2009), acj66 (Clyne et al., 1999), otk3 (Winberg et al., 2001), piezoKO (Kim et al., 2012), VT033006-GAL4 (Tirian and Dickson, 2017), GH146-GAL4 (Stocker et al., 1997), C15-p65AD (Xie et al., 2019), UAS-HRP-CD2 (Larsen et al., 2003), UAS-dcr2 (Dietzl et al., 2007), UAS-otk (Winberg et al., 2001), UAS-acj6-J (Bai and Carlson, 2010). UAS-Dg (Robert Ray, Francis Crick Institute), and UAS-Dop2R (Deng et al., 2019). The RNAi lines were generated previously (Dietzl et al., 2007; Ni et al., 2011; Perkins et al., 2015) and acquired from the Bloomington Drosophila Stock Center and the Vienna Drosophila Resource Center. VT033006-GAL4DBD is unpublished reagent generously provided by Yoshi Aso (Janelia Research Campus). UAS-piezo, UAS-piezo-2306Myc, UAS-Dop1R1, UAS-Nep3, UAS-CG5027, UAS-Ostγ, otk-FRT-V5-STOP-FRT-FLAG-STOP, piezo-FRT-V5-STOP-FRT-FLAG-STOP, and Dg-FRT-V5-STOP-FRT-FLAG-STOP were generated in this study.
METHOD DETAILS
Transcriptome profiling of adPNs
Wild-type or acj6 mutant Drosophila brains with mCD8-GFP labeled adPNs using the split GAL4 driver C15-p65AD, VT033006-GAL4DBD were dissected at 36–40 hr after puparium formation in Schneider’s medium (Thermo Fisher). Single-cell suspension was prepared as previously described (Li et al., 2017). One thousand GFP positive cells were sorted for each sample using Fluorescence Activated Cell Sorting (FACS) on a Sony SH800 cell sorter system (Sony Biotechnology). Three biological replicates were collected for each genotype. Full-length poly(A)-tailed RNA was reverse-transcribed and amplified by PCR following the modified SMART-seq2 protocol (Picelli et al., 2014). Sequencing libraries were prepared from amplified cDNA, pooled, and quantified using BioAnalyser (Agilent). Sequencing was performed using the NextSeq 500 Sequencing system (Illumina) with 75 paired-end reads.
Acj6 binding site prediction
Prediction of Acj6 binding site was adapted from previously described method with experimentally validated Acj6 consensus (Bai et al., 2009). For each gene, we scanned through its promoter sequence, encompassing sequence from 500bp upstream to 100bp downstream of its transcription start site (Eukaryotic Promoter Database; Dreos et al., 2017; Dreos et al., 2015). The sum of weight calculated using the positional weight matrix (PWM) was used to score each of the 12bp long DNA sequence on both the sense and antisense strand. Sequences with a score greater than 6 were counted as putative Acj6 binding sites.
Biotinylation of PN-surface proteins
PN surface biotinylation was performed following the previously published method (Li et al., 2020). Briefly, we dissected wild-type or acj6 mutant brains expressing HRP-rCD2 by PNs in pre-chilled Schneider’s medium (Thermo Fisher), removed the optic lobes, and transferred them into 500 μL of the Schneider’s medium in 1.5 mL protein low-binding tubes (Eppendorf) on ice. Brains were washed with fresh Schneider’s medium to remove fat bodies and debris and were incubated in 100 μM of BxxP in Schneider’s medium on ice for one hour. Brains were then labeled with 1 mM (0.003%) H2O2 (Thermo Fisher) for 5 minutes, and immediately quenched by five thorough washes using quenching buffer (10 mM sodium ascorbate [Spectrum Chemicals], 5 mM Trolox [Sigma-Aldrich], and 10 mM sodium azide [Sigma-Aldrich] in phosphate buffered saline [PBS; Thermo Fisher]). After the washes, the quenching solution was removed, and brains were either fixed for immunostaining (see below for details) or were snapped frozen in liquid nitrogen and stored at −80°C for the proteomic sample collection. For the proteomic sample collection, 1100 dissected and biotinylated brains were collected for each experimental group (8800 brains in total).
Collection of biotinylated proteins
For each proteomic sample, there were five tubes each containing ~220 dissected brains. We added 40 μL of high-SDS RIPA (50mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1% Triton X-100, 1x protease inhibitor cocktail [Sigma-Aldrich; catalog # P8849], and 1 mM phenylmethylsulfonyl fluoride [PMSF; Sigma-Aldrich]) to each of those tubes, and grinded the samples on ice using disposable pestles with an electric pellet pestle driver. Tubes containing brain lysates of the same experimental group were spun down, merged, and rinsed with an additional 100 μL of high-SDS RIPA to collect remaining proteins. Samples were then vortexed briefly, sonicated twice for ten seconds each, and incubated at 95°C for five minutes to denature postsynaptic density proteins. 1.2 mL of SDS-free RIPA buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% sodium deoxycholate, 1% Triton X-100, 1x protease inhibitor cocktail and 1 mM PMSF) were added to each sample, and the mixture was rotated for two hours at 4°C. Lysates were then diluted with 200 μL of normal RIPA buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.2% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 1x protease inhibitor cocktail, and 1 mM PMSF), transferred to 3.5 mL ultracentrifuge tubes (Beckman Coulter), and centrifuged at 100,000 g for 30 minutes at 4°C. 1.5 mL of the supernatant was carefully collected for each sample.
400 μL of streptavidin-coated magnetic beads (Pierce; catalog #88817) washed twice using 1 ml RIPA buffer were added to each of the post-ultracentrifugation brain lysates. The lysate and the streptavidin bead mixture were left to rotate at 4°C overnight. On the following day, beads were washed twice with 1 mL RIPA buffer, once with 1 mL of 1 M KCl, once with 1 mL of 0.1 M Na2CO3, once with 1 mL of 2 M urea in 10 mM Tris-HCl (pH 8.0), and again twice with 1 mL RIPA buffer. The beads were resuspended in 1 mL fresh RIPA buffer. 35 μL of the bead suspension was taken out for western blot, and the rest proceeded to on-bead digestion.
Western blot of biotinylated proteins
Biotinylated proteins were eluted from streptavidin beads by the addition of 20 μL elution buffer (2X Laemmli sample buffer, 20 mM DTT, 2 mM biotin) followed by a 10 min incubation at 95°C. Proteins were resolved by 4%–12% Bis-Tris PAGE gels (Thermo Fisher) and transferred to nitrocellulose membrane (Thermo Fisher). After blocking with 3% bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween 20 (TBST; Thermo Fisher) for 1 hour, membrane was incubated with 0.3 mg/mL HRP-conjugated streptavidin for one hour. The Clarity Western ECL blotting substrate (Bio-Rad) and BioSpectrum imaging system (UVP) were used to develop and detect chemiluminescence.
On-bead trypsin digestion of biotinylated proteins
To prepare proteomic samples for mass spectrometry analysis, proteins bound to streptavidin beads were washed twice with 200 μL of 50 mM Tris-HCl buffer (pH 7.5) and twice with 2 M urea/50 mM Tris (pH 7.5) buffer. After washes, the 2 M urea/50 mM Tris (pH 7.5) buffer was removed, and beads were incubated with 80 μL of 2 M urea/50 mM Tris buffer containing 1 mM dithiothreitol (DTT) and 0.4 μg trypsin for 1 hour at 25°C with shaking at 1000 revolutions per minute (rpm). After 1 hour, the supernatant was transferred to a fresh tube. The streptavidin beads were rinsed twice with 60 μL of 2 M urea/50 mM Tris (pH 7.5) buffer and the solution was combined with the on-bead digest supernatant. The eluate was reduced with 4 mM DTT for 30 minutes at 25°C with shaking at 1000 rpm and alkylated with 10 mM iodoacetamide for 45 minutes in the dark at 25°C while shaking at 1000 rpm. An additional 0.5 μg of trypsin was added to the sample and the digestion was completed overnight at 25°C with shaking at 700 rpm. After overnight digestion, the sample was acidified (pH < 3) by adding formic acid (FA) such that the sample contained 1% FA. Samples were desalted on C18 StageTips (3M). Briefly, C18 StageTips were conditioned with 100 μL of 100% MeOH, 100 μL of 50% MeCN/0.1% FA followed by two washes with 100μL of 0.1% FA. Acidified peptides were loaded onto the conditioned StageTips, which were subsequently washed twice with 100 μL of 0.1% FA. Peptides were eluted from StageTips with 50 μL of 50% MeCN/0.1 % FA and dried to completion.
TMT labeling and SCX StageTip fractionation of peptides
8 TMT reagents from a 10-plex reagent kit were used to label desalted peptides (Thermo Fisher) as directed by the manufacturer. Peptides were reconstituted in 100 μL of 50 mM HEPES. Each 0.8 mg vial of TMT reagent was reconstituted in 41 μL of anhydrous acetonitrile and incubated with the corresponding peptide sample for 1 hour at room temperature. Labeling of samples with TMT reagents was completed with the design described in Figure 2A. TMT labeling reactions were quenched with 8 μL of 5% hydroxylamine at room temperature for 15 minutes with shaking, evaporated to dryness in a vacuum concentrator, and desalted on C18 StageTips as described above. For the TMT 8-plex cassette, 50% of the sample was fractionated into 3 fractions by Strong Cation Exchange (SCX) StageTips while the other 50% of each sample was reserved for LC-MS analysis by a single shot, long gradient. One SCX StageTip was prepared per sample using 3 plugs of SCX material (3M) topped with 2 plugs of C18 material. StageTips were sequentially conditioned with 100 μL of MeOH, 100 μL of 80% MeCN/0.5% acetic acid, 100 μL of 0.5% acetic acid, 100 μL of 0.5% acetic acid/500mM NH4AcO/20% MeCN, followed by another 100 μL of 0.5% acetic acid. Dried sample was re-suspended in 250 μL of 0.5% acetic acid, loaded onto the StageTips, and washed twice with 100 μL of 0.5% acetic acid. Sample was trans-eluted from C18 material onto the SCX with 100 μL of 80% MeCN/0.5% acetic acid, and consecutively eluted using 3 buffers with increasing pH—pH 5.15 (50mM NH4AcO/20% MeCN), pH 8.25 (50mM NH4HCO3/20% MeCN), and finally pH 10.3 (0.1% NH4OH, 20% MeCN). Three eluted fractions were re-suspended in 200 μL of 0.5% acetic acid to reduce the MeCN concentration and subsequently desalted on C18 StageTips as described above. Desalted peptides were dried to completion.
Liquid chromatography and mass spectrometry
Desalted, TMT-labeled peptides were resuspended in 9 μL of 3% MeCN, 0.1% FA and analyzed by online nanoflow liquid chromatography tandem mass spectrometry (LC-MS/MS) using a Q Exactive HF-X (Thermo Fisher) coupled on-line to a Proxeon Easy-nLC 1000 (Thermo Fisher). 4 μL of each sample was loaded at 500 nL/min onto a microcapillary column (360 μm outer diameter x 75 μm inner diameter) containing an integrated electrospray emitter tip (10 μm), packed to approximately 24 cm with ReproSil-Pur C18-AQ 1.9 μm beads (Dr. Maisch GmbH) and heated to 50°C. The HPLC solvent A was 3% MeCN, 0.1% FA, and the solvent B was 90% MeCN, 0.1% FA. Peptides were eluted into the mass spectrometer at a flow rate of 200 nL/min. Non-fractionated samples were analyzed using a 260 min LC-MS/MS method with the following gradient profile: (min:%B) 0:2; 1:6; 235:30; 244:60; 245:90; 250:90; 251:50; 260:50 (the last two steps at 500 nL/min flow rate). The SCX fractions were run with 110-minute method, which used the following gradient profile: (min:%B) 0:2; 1:6; 85:30; 94:60; 95:90;100:90; 101:50; 110:50 (the last two steps at 500 nL/min flow rate). The Q Exactive HF-X was operated in the data-dependent mode acquiring HCD MS/MS scans (r = 45,000) after each MS1 scan (r = 60,000) on the top 20 most abundant ions using an MS1 target of 3E6 and an MS2 target of 5E4. The maximum ion time utilized for MS/MS scans was 120 ms (single-shot) and 105 ms (SCX fractions); the HCD normalized collision energy was set to 31; the dynamic exclusion time was set to 20 s, and the peptide match and isotope exclusion functions were enabled. Charge exclusion was enabled for charge states that were unassigned, 1 and > 7.
Mass spectrometry data processing
Collected data were analyzed using the Spectrum Mill software package v6.1 pre-release (Agilent Technologies). Nearby MS scans with the similar precursor m/z were merged if they were within ± 60 s retention time and ± 1.4 m/z tolerance. MS/MS spectra were excluded from searching if they failed the quality filter by not having a sequence tag length 0 or did not have a precursor MH+ in the range of 750 – 4000. All extracted spectra were searched against a UniProt database containing Drosophila melanogaster reference proteome sequences. Search parameters included: ESI QEXACTIVE-HCD-v2 scoring parent and fragment mass tolerance of 20 ppm, 30% minimum matched peak intensity, trypsin allow P enzyme specificity with up to four missed cleavages, and calculate reversed database scores enabled. Fixed modifications were carbamidomethylation at cysteine. TMT labeling was required at lysine, but peptide N termini were allowed to be either labeled or unlabeled. Allowed variable modifications were protein N-terminal acetylation and oxidized methionine. Individual spectra were automatically assigned a confidence score using the Spectrum Mill auto-validation module. Score at the peptide mode was based on target-decoy false discovery rate (FDR) of 1%. Protein polishing auto-validation was then applied using an auto thresholding strategy. Relative abundances of proteins were determined using TMT reporter ion intensity ratios from each MS/MS spectrum and the median ratio was calculated from all MS/MS spectra contributing to a protein subgroup. Proteins identified by 2 or more distinct peptides and ratio counts were considered for the dataset.
Proteomic data cutoff analysis
We used a ratiometric strategy (Hung et al., 2014) to remove contaminants. Briefly, all detected proteins (2332 with 2 or more unique peptides) were annotated as either true-positives (TPs; proteins with plasma membrane annotation), false-positives (FPs; proteins with either cytosol, mitochondrion, or nucleus annotation but without the membrane annotation), or other annotations according to the subcellular localization annotation in the UniProt database. For each experimental group, we calculated the TMT ratios of proteins in this group compared to one of the controls and sorted proteins in a descending order. For each TMT ratio, a true-positive rate (TPR) and a false positive rate (FPR) were calculated by summing up the number of TPs or FPs with a higher ranking and divided them by the total number of TPs or FPs, respectively. The TPRs and FPRs were used to generate the ROC curves. The cutoff for each experimental-to-control group was determined by finding the TMT ratio where [TPR – FPR] is maximized. Proteins with TMT ratio higher than the cutoff were retained for each experimental group. 459 proteins that passed the cutoffs using both 127N/127C and 126/128N ratios were retained for wild-type, and 537 proteins that passed the cutoffs using both 129N/129C and 128C/130N ratios were retained for acj6 mutant in Figure 2. We also tested a more stringent cutoff criterion where we compared each of the experimental group to both controls for each genotype, and only kept proteins that passed the cutoffs in all four possible combinations (Figures S2D-S2F). Gene Ontology analyses were performed on these gene sets using Flymine (Lyne et al., 2007).
Immunocytochemistry
Fly brains were dissected and immunostained according to the previously published protocol (Wu and Luo, 2006a). Briefly, fly brains of the desired genotype and developmental stage were dissected in PBS, transferred to a tube with 1 mL of fixation buffer (4% paraformaldehyde in PBS with 0.006% Triton X-100) on ice, and fixed for 20 minutes by nutating at room temperature. After fixation, brains were washed with PBST (0.3% Triton X-100 in PBS) twice, nutated in PBST for 20 minutes twice, and blocked in 5% normal goat serum in PBST for 1 hour (except for conditional tag flies; see below for details). To visualize the antennal lobe glomeruli and PN dendrites, brains were then incubated in rat anti-Ncad (N-Ex #8; 1:40; Developmental Studies Hybridoma Bank) and chicken anti-GFP (1:1000; Aves Labs) diluted in 5% normal goat serum in PBST for two overnights on a 4°C nutator. After primary antibody incubation, brains were washed four time with PBST (two quick washes and two 20-minute washes) and incubated with secondary antibodies conjugated to Alexa Fluor 488 and 647 (1:250 in 5% normal goat serum; Jackson ImmunoResearch). To visualize biotinylated proteins, brains were incubated with Neutravidin (Thermo Fisher) pre-conjugated to Alexa Fluor 647 (Invitrogen). After the antibody incubation(s), brains were washed four times (two quick washes and two 20-minute washes), mounted with SlowFade antifade reagent (Thermo Fisher), and stored at 4°C before imaging.
For the staining of Otk, Piezo, or Dg conditional tag, the above method produced low signal-to-noise FLAG (or V5) signal, so Alexa 488 Tyramide SuperBoost kit (Thermo Fisher) was used to amplify the signal. Briefly, after dissection, fixation, and washing steps, brains were rinsed with PBS twice and incubated with 3% hydrogen peroxide for 1 hour to quench the activity of endogenous peroxidases. Brains were then washed with PBST three times, blocked for 1 hour in 10% goat serum provided by the kit, and incubated with V5 or FLAG antibody diluted in 10% goat serum for two overnights on a 4°C nutator. After four 20-minute washes using PBST, brains were incubated with the poly-HRP-conjugated secondary antibody provided in the kit for two overnights on a 4°C nutator. Brains were washed four times again with PBST (two quick and two 20-minute washes) and twice with PBS. Afterwards, the brains were incubated with the tyramide solution for 5 minutes at room temperature, and reaction was immediately quenched by three washes using the quenching buffer provided by the kit. Brains were then washed with PBST four times, and NCad staining was performed using standard immunostainng protocol described above.
Image acquisition and processing
Images were obtained with a Zeiss LSM 780 laser-scanning confocal microscope (Carl Zeiss) using a 40x oil objective. Z-stacks were acquired at 1-μm intervals at the resolution of 512x512. Brightness and contrast adjustments as well as image cropping were done using ImageJ.
Genetic screen to identify molecules required for adPN dendrite targeting
The adPN screening line was generated by recombining UAS-dcr2 with UAS-mCD8-GFP on the X chromosome and C15-p65AD with VT033006-GAL4DB on the third chromosome. Virgin female flies from this screening line were crossed to UAS-RNAi or UAS-cDNA males, and the progenies were kept at 25°C for 2 days after egg laying and then transferred to 29°C to enhance expression by the GAL4/UAS system. Brains were dissected, immunostained, and imaged as described above.
To quantify adPN dendrite innervation pattern, individual glomeruli were identified using the NCad staining (based on their stereotyped shapes and positions), and then categorical innervation scores (not innervated, weakly innervated, or strongly innervated) were assigned to each of the identified glomeruli. The genotypes were blinded during scoring. Data was analyzed using python packages Pandas and Scipy. For each glomerulus, we calculated the frequency of each type of innervation and plotted the results as stacked bars in Figure S3. To quantify if knocking down or overexpressing a gene caused significant dendrite innervation changes, Chi-squared tests were performed on the innervation degree frequencies of a glomerulus of a given genotype compared to control. p-values were adjusted for multiple comparisons using Bonferroni correction, and glomeruli whose dendrite innervation were significantly changed (p-value < 0.05) were color blue or red in a heatmap shown in Figure S4.
Generation of endogenous conditional tags
Otk, Piezo, or Dg conditional tag flies were generated based on the previously described method (Li et al., 2020). To make the homology-directed repair (HDR) vectors, a ~2000bp of genomic sequence flanking the stop codon was amplified using Q5 hot-start high-fidelity DNA polymerase (New England Biolabs) and inserted into pCR-Blunt-TOPO vector (Thermo Fisher). The conditional tag cassette (FRT-1xV5-6xStop-loxP-mini-White-loxP-FRT-3xFLAG-6xStop) was amplified from the LRP1 plasmid (Li et al., 2020) and inserted into the TOPO genomic sequence plasmid to replace the stop coding using NEBuilder HiFi DNA assembly master mix (New England Biolabs). CRIPSR guide RNA (gRNA) targeting a locus near the stop codon was designed using the flyCRISPR Target Finder tool and cloned into the pU6-BbsI-chiRNA vector (Addgene #45946) by NEBuilder HiFi DNA assembly master mix.
The HDR and the gRNA vectors were co-injected into vas-Cas9 (Port et al., 2014) fly embryos. G0 flies were crossed to a white− balancer and all white+ progenies were individually balanced. To remove the loxP-flanked miniWhite cassette, each line was crossed to flies with hs-Cre. Fertilized eggs or young larvae were heat shocked twice at 37°C for 1 hour separated a day apart and crossed to a balancer. Their white− progenies were individually balanced and verified by sequencing to obtain gene-FRT-V5-STOP-FRT-FLAG-STOP.
Generation of UAS constructs and transgenic flies
To generate UAS flies, we used Q5 hot-start high-fidelity DNA polymerase (New England Biolabs) to amplify the transcripts of each candidate gene from complementary DNA (cDNA) synthesized using the total RNA of w1118 fly heads (extracted using MiniPrep kit; Zymo Research, R1054). For each candidate gene, we designed more than 2 pairs of PCR primers in the 5’ and 3’ untranslated regions and inserted all resulting PCR products into pCR-Blunt-TOPO vector (Thermo Fisher). The TOPO-transcript vectors of the same gene were sequenced and compared to verify that no error was introduced to the coding sequence during reverse transcription. The verified coding sequences were then amplified and assembled into either the pUAST-attB vector (Nep3) or a modified pUAST-attB vector in which a FLAG tag was added at the 3’ end (Dg, CG5027, DopR, Ostγ, and piezo). To generate Piezo mutants, myc tag was inserted into amino acid position 901, 2289, 2291, and 2306 (Figure 5; Figure S6) in plasmid pUAST-attB-piezo-FLAG using Q5 site-directed mutagenesis kit (New England Biolabs). Each plasmid was sequence confirmed by full-length DNA sequencing.
The pUAST-attB constructs were inserted into either attP40 or attP86Fb (for Piezo constructs) landing sites. G0 flies were crossed to a white− balancer, and all white+ progenies were individually balanced and verified by sequencing.
MARCM-based mosaic analysis
hsFlp-based MARCM analyses were performed following the previously published protocol (Wu and Luo, 2006b). Each fly contains GH146-GAL4, UAS-mCD8-GFP, tubP-GAL80, hsFlp, the desired FRT, a mutant allele distal to the FRT site (or wild-type for control), and, in rescue experiments, one or two UAS-candidate gene (see Table S3 for complete genotypes). To generate adPN neuroblast, lPN neuroblast, or DL1 single-cell MARCM clones, flies were heat shocked at 0–24 hours after larvae hatching for 1 hour at 37°C. More than 150 adult brains were dissected for each genotype to get approximately 20 clones.
Transfection and staining of Drosophila S2 cells
pUAST-attB-piezo-Myc-FLAG constructs contain an intracellular C-terminal FLAG tag and an extracellular Myc tag inserted at different positions. Piezo-901Myc, the equivalent of mPiezo1-897Myc, was used as a control to show wild-type Piezo expression (Coste et al., 2015; Saotome et al., 2018). S2 cells were co-transfected with these Piezo constructs and Actin-GAL4 using FuGENE HD transfection Reagent (Promega). After 48 hours, transfected cells were incubated with rabbit anti-Myc antibody (1:250; Cell Signaling) and mouse anti-FLAG M2 antibody (1:200; Sigma-Aldrich) either before or after 4% PFA fixation and permeabilization with 0.3% Triton in PBS for non-permeabilized and permeabilized condition, respectively. Cells were then washed with PBS, incubated with secondary antibodies, and imaged.
Cell culture and transfection of HEK293T PIEZO1 knockout cells
Human embryonic kidney HEK293T PIEZO1 knockout (HEK-P1KO) cells were grown in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum, and 1% penicillin and streptomycin. Cells were plated on coverslips coated with laminin and transfected with 700 ng of plasmid DNA using lipofectamine 2000 (Thermo Fisher Scientific, Cat No. 11668019) as per manufacturer’s instructions. The cDNA sequence of wild-type and mutant Drosophila Piezos were cloned into pcDNA3.2 vector that has been modified to include an IRES2-mNeonGreen element.
Electrophysiology
Cells were recorded from 48–72 hours following transfection at room temperature. All recordings were done in whole-cell voltage clamp mode using Axopatch 200B amplifier (Axon Instruments). Currents were acquired at membrane holding potential of −80mV, sampled at 20 kHz and filtered at 2 kHz. Leak currents before mechanical stimulations were subtracted off-line from the current traces in Clampex 11. Current traces were analyzed using Clampex 11 and GraphPad Prism.
Extracellular solution was composed of: 133 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 10 mM HEPES, and 10 mM Glucose, 310 mOsm/L, and pH of 7.3 (NaOH). Intracellular solution was composed of: 133mM CsCl, 5mM EGTA, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 4 mM Mg2ATP, and 0.4 mM Na2GTP, 300 mOsm/L, and pH of 7.3 (CsOH). Patch pipettes were made from borosilicate capillaries pulled with Sutter Instruments puller (Model P-97). Pipettes of 1.8–4 MΩ resistance in intracellular solution were used for recordings.
Mechanical stimulation was induced using a blunt glass probe held at ~80° angle, and controlled by a piezoelectric actuator (E625 LVPZT, Physik Instrumente). Cells were indented for 300 ms at 1-micron increments, with a 30 s inter-sweep duration. Seal integrity was continuously monitored by including a 10 ms voltage step from −80 mV to −75 mV, 90 ms prior to indenting the cell.
QUANTIFICATION AND STATISTICAL ANALYSIS
The statistical tests and numbers of independent replicates per experiment are indicated in the figures or figure legends.
RNA-seq data analyses
Reads were aligned to the Drosophila melanogaster genome (r6.10) using STAR (2.4.2) (Dobin et al., 2013). Gene counts were produced using HTseq (0.7.2) with default settings except “-m intersection-strict’ (Anders et al., 2015). To normalize for differences in sequencing depth across individual cells, we rescaled gene counts to transcript counts per million reads (CPM). Genes with less than 1 CPM in more than 3 samples were excluded from further analysis.
Differential gene expression was calculated with R package edgeR (Robinson et al., 2010) and limma (Ritchie et al., 2015). Briefly, data were normalized with the weighted trimmed mean of M-values (TMM) method (Robinson and Oshlack, 2010) and fitted to a linear model with voom and lmFit functions (Ritchie et al., 2015). Finally, empirical Bayes statistics were applied to correct variance of genes with low expression, p-value was adjusted using the Benjamini–Hochberg method. We define differentially expressed genes to be those with adjusted p-value less than 0.05. Enrichment analysis was performed using Enrichr (Kuleshov et al., 2016) and pathway gene sets “KEGG_2019” in the GSEApy python package.
Quantitative comparison of wild-type and acj6 mutant proteomes
For the volcano plots (Figures 2G; Figure S2F) comparing differentially enriched proteins on acj6 mutant PN surface compared to wild-type PN surface, a linear model was fit to account for the variance across replicates for each stage and normalize data by the appropriate negative control samples as previously described (Li et al., 2020).
A protein summary was first generated where each TMT condition was calculated as a log ratio to the median intensity of all the channels, enabling all channels to have the same denominator. Following calculation of the log2 ratio, all samples were normalized by subtracting the median log2 ratio (median centering). For each protein, a linear model was used to calculate the following ratio and the corresponding p-value:
Using log2 transformed TMT ratios, the linear model is as follows:
where MUT (acj6 mutant) and TRT (treatment) are indicator variables representing acj6 mutant (MUT = 1 for mutant, 0 for wildtype) and labeling condition (TRT = 1 for labeled, 0 for negative control) respectively. The above linear model with interaction terms expands to:
Coefficient b3 represents the required (log-transformed) ratio between mutant and wildtype conditions taking into account the appropriate negative controls and replicates. A moderated t-test was used to test the null hypothesis of b3 = 0 and calculate a nominal p-value for each protein. These nominal p-values were then corrected for multiple testing using the Benjamini-Hochberg FDR (BH-FDR) method (Benjamini and Hochberg, 1995). Figure 2G and Figure S2F show the value of b3 along the x-axis and the −log2 (p-value) along the y-axis of the volcano plot.
The linear model along with the associated moderated t-test and BH-FDR correction were implemented using the limma library (Ritchie et al., 2015) in R.
We note that the ratio compression effect of the TMT strategy reported previously (Savitski et al., 2013; Ting et al., 2011) can also compromise the accuracy of our data, and it is not possible for us to estimate the amount of compression without spiked-in standards. However, by using a less complex sample (proximity-labeled rather than whole cell proteomes) and performing offline fractionation prior to MS analysis, we have reduced ratio compression to the best of our ability without sacrificing the number of proteins identified.
Quantification of Otk and Piezo expression in PNs
In ImageJ, individual glomeruli were identified based on the NCad staining, and the average FLAG fluorescence intensities for these glomeruli were measured. The intensities were then normalized to the intensity of the GH146-FLP-negative DA4m glomerulus, and the normalized values were plotted in Figure 4D and Figure S6B. t-test of independence was used to compare expression of Otk or Piezo between wild-type and acj6 mutant glomeruli, and p-values were adjusted using Bonferroni correction.
Quantification of adPN dendrite innervation patterns in MARCM-based mosaic analysis
All images of MARCM clones were given human-unidentifiable names and mixed with all other genotypes before scoring. Individual glomeruli were identified using the NCad staining, and then categorical innervation scores were assigned to identified glomeruli. After scoring, the data were imported into python, and the genotype information was revealed.
To test whether overexpressing a transgene rescued any dendrite mistargeting phenotypes caused by the loss of Acj6, we first performed Chi-squared test comparing each glomerulus of a given genotype with that glomerulus in wild-type: if the p-value was greater than 0.05, we considered it as fully rescued; if the p-value was less than 0.05, we further compared the innervation frequencies of this glomerulus of this genotype with that of acj6 mutant to see if there was significant partial rescue (p-value < 0.05 compared to acj6 mutant). Note that we did not adjust for multiple comparisons here, because the adjustment would render most mistargeting phenotypes in acj6 mutant insignificant compared to control and would also fail to detect cases with obvious phenotypic rescue.
Supplementary Material
Table S1. Processed RNA-seq data rank ordered according to adjusted P-value of the comparisons between acj6 mutant and wild-type samples, related to Figure 1
Table S2. Raw and processed proteome data, related to Figure, related to Figure 2
(A) Wild-type (WT) PN surface proteome by the cutoff shown in Figures 2D and 2E. Proteins are ranked by the TMT ratio 127N:127C in a descending order.
(B) acj6 mutant (acj6−) PN surface proteome by the cutoff shown in Figures 2D and 2E. Proteins are ranked by the TMT ratio 129N:129C in a descending order.
(C) Wild-type (WT) PN surface proteome by the more stringent cutoff shown in Figures S2D and S2E. Proteins are ranked by the TMT ratio 127N:127C in a descending order.
(D) acj6 mutant (acj6−) PN surface proteome by the more stringent cutoff shown in Figures S2D and S2E. Proteins are ranked by the TMT ratio 129N:129C in a descending order.
(E) Protein expression changes comparing WT and acj6 mutant and the corresponding p values. Proteins are ranked by the p value in an ascending order. Note that this list includes all detected proteins prior to the cutoff analysis and thus contains intracellular contaminants.
(F) TMT ratios of all detected proteins prior to the cutoff analysis.
(G to N) Each tab shows one experimental-to-control TMT ratio (see Figure 2A for detail). All detected proteins prior to the cutoff analysis are listed and ranked by the corresponding TMT ratio in a descending order.
Table S3. Genotypes of flies in each experiment, related to Figures 1-7 and STAR Methods
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat anti-NCad | Developmental Studies Hybridoma Bank | RRID: AB 528121 |
| Chicken anti-GFP | Aves Labs | RRID: AB 10000240 |
| Mouse anti-FLAG | Sigma-Aldrich | RRID: AB 259529 |
| Mouse anti-V5 | Sigma-Aldrich | RRID: AB 2556564 |
| Deposited data | ||
| RNA sequencing data | This paper | GEO: GSE200841 |
| Mass spectrometry proteomics data | This paper | MassIVE: MSV000089187 |
| Experimental models: Organisms/strains | ||
| D. melanogaster: tubP-GAL80 | (Lee and Luo, 1999) | RRID: BDSC_9917 |
| D. melanogaster: UAS-mCD8-GFP | (Lee and Luo, 1999) | RRID: DGRC_108068 |
| D. melanogaster: hsFlp | (Golic and Lindquist, 1989) | N/A |
| D. melanogaster: FRT19A | (Xu and Rubin, 1993) | RRID: BDSC_1744 |
| D. melanogaster: FRT40A | (Xu and Rubin, 1993) | N/A |
| D. melanogaster: FRT42D | (Xu and Rubin, 1993) | RRID: BDSC_1802 |
| D. melanogaster: GH146-Flp | (Hong et al., 2009) | N/A |
| D. melanogaster: acj66 | (Clyne et al., 1999) | RRID: BDSC_60686 |
| D. melanogaster: otk3 | (Winberg et al., 2001) | N/A |
| D. melanogaster: piezoKO | (Kim et al., 2012) | RRID: BDSC_58770 |
| D. melanogaster: VT033006-GAL4 | (Tirian and Dickson, 2017) | RRID: VDRC_202281 |
| D. melanogaster: GH146-GAL4 | (Stocker et al., 1997) | RRID: BDSC_30026 |
| D. melanogaster: VT033006-GAL4DBD | Yoshi Aso (unpublished) | N/A |
| D. melanogaster: C15-p65AD | (Xie et al., 2019) | N/A |
| D. melanogaster: UAS-HRP-CD2 | (Larsen et al., 2003) | RRID: BDSC_9906 |
| D. melanogaster: UAS-RNAi lines | (Dietzl et al., 2007; Ni et al., 2011; Perkins et al., 2015) | Stock numbers listed in Figure S3 |
| D. melanogaster: UAS-dcr2 | (Dietzl et al., 2007) | N/A |
| D. melanogaster: UAS-otk | (Winberg et al., 2001) | N/A |
| D. melanogaster: UAS-piezo | This study | N/A |
| D. melanogaster: UAS-piezo-2306Myc | This study | N/A |
| D. melanogaster: UAS-acj6-J | (Bai and Carlson, 2010) | N/A |
| D. melanogaster: UAS-Dg | Robert Ray, Francis Crick Institute | RRID: BDSC_63052 |
| D. melanogaster: UAS-Dop1R1 | This study | N/A |
| D. melanogaster: UAS-Dop2R | (Deng et al., 2019) | RRID: BDSC_86134 |
| D. melanogaster: UAS-Nep3 | This study | N/A |
| D. melanogaster: UAS-CG5027 | This study | N/A |
| D. melanogaster: UAS-Ostγ | This study | N/A |
| D. melanogaster: otk-FRT-V5-STOP-FRT-FLAG-STOP | This study | N/A |
| D. melanogaster: piezo-FRT-V5-STOP-FRT-FLAG-STOP | This study | N/A |
| D. melanogaster: Dg-FRT-V5-STOP-FRT-FLAG-STOP | This study | N/A |
| HEK-PI KO cells | (Lukacs et al., 2015) | N/A |
| Recombinant DNA | ||
| Conditional tag cassette FRT-1xV5-6xStop-loxP-mini-White-loxP-FRT-3xFLAG-6xStop | (Li et al., 2020) | N/A |
| pCR-Blunt-TOPO | Thermo Fisher Scientific | Catalog #: K280020 |
| pU6-BbsI-chiRNA | (Gratz et al., 2014; Gratz et al., 2013) | RRID: Addgene_45946 |
| pUASTattB | (Bischof et al., 2007) | RRID: DGRC_1419 |
| pUAST-piezo-901Myc-FLAG | This study | N/A |
| pUAST-piezo-2289Myc-FLAG | This study | N/A |
| pUAST-piezo-2291Myc-FLAG | This study | N/A |
| pUAST-piezo-2306Myc-FLAG | This study | N/A |
| pcDNA3.2-piezo-IRES2-mNeonGreen | Ardem Patapoutian, Scripps Research Institute | N/A |
| pcDNA3.2-piezo-WT-IRES2-mNeonGreen | This study | N/A |
| pcDNA3.2-piezo-2289Myc-mNeonGreen | This study | N/A |
| pcDNA3.2-piezo-2291Myc-mNeonGreen | This study | N/A |
| pcDNA3.2-piezo-2306Myc-mNeonGreen | This study | N/A |
| Software and algorithms | ||
| Zen | Carl Zeiss | RRID: SCR_013672 |
| ImageJ | National Institutes of Health | RRID: SCR_003070 |
| Illustrator | Adobe | RRID: SCR_010279 |
| Spectrum Mill | Agilent | https://www.agilent.com/Agilent404?s=https://www.agilent.com/en/products/software-informatics/masshunter-suite/masshunter-for-life-science-research/spectrum-mill |
| pClamp | Molecular Devices | RRID: SCR_011323 |
Highlight:
Lineage-defining transcription factor Acj6 shapes neuronal surface proteome
Acj6 regulates diverse cell-surface protein expression for olfactory circuit assembly
Piezo controls neuronal wiring independently of its force-gated ion channel activity
A combinatorial code of cell-surface proteins controls neural circuit wiring
ACKNOWLEDGMENTS
We thank Y. Rao, V. Ruta, Y. Aso, the Bloomington Drosophila Stock Center, and the Vienna Drosophila Resource Center for the fly lines, and A. Patapoutian and Addgene for plasmids. We thank T. Clandinin, J. Kaltschmidt, K. Shen, C. McLaughlin, Z. Li, K. Wong, C. Lyu, Y. Ge, J. Ren, D. Pederick, M. Wagner, and members of the Luo lab for technical support and insightful advice on this study. We thank M. Molacavage for administrative assistance. Q.X. was a Bertarelli Fellow. J.L. is a Jane Coffin Childs postdoctoral fellow. L.L. is an investigator of the Howard Hughes Medical Institute. This work was supported by the National Institutes of Health (R01-DC005982 to L.L. and R01-DK121409 to S.A.C. and A.Y.T.) and the Wu Tsai Neurosciences Institute of Stanford University (Neuro-omics grant to A.Y.T., S.R.Q., and L.L.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- Aebersold R, and Mann M (2016). Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355. 10.1038/nature19949. [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, and Huber W (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, and Herculano-Houzel S (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513, 532–541. 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Bai L, and Carlson JR (2010). Distinct functions of acj6 splice forms in odor receptor gene choice. J Neurosci 30, 5028–5036. 10.1523/JNEUROSCI.6292-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Goldman AL, and Carlson JR (2009). Positive and negative regulation of odor receptor gene choice in Drosophila by acj6. J Neurosci 29, 12940–12947. 10.1523/JNEUROSCI.3525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, and Hochberg Y (1995). Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B 57, 289–300. DOI 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, and Basler K (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104, 3312–3317. 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, and Tear G (2007). Getting axons onto the right path: the role of transcription factors in axon guidance. Development 134, 439–448. 10.1242/dev.02762. [DOI] [PubMed] [Google Scholar]
- Cafferty P, Yu L, and Rao Y (2004). The receptor tyrosine kinase Off-track is required for layer-specific neuronal connectivity in Drosophila. Development 131, 5287–5295. 10.1242/dev.01406. [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Kitchen RR, Kanyo JE, Voss EZ, Pletikos M, Sousa AMM, Lam TT, Gerstein MB, Sestan N, and Nairn AC (2017). A multiregional proteomic survey of the postnatal human brain. Nat Neurosci 20, 1787–1795. 10.1038/s41593-017-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel SJ, Clyne PJ, Carlson JR, and Johnson WA (2000). Regulation of central neuron synaptic targeting by the Drosophila POU protein, Acj6. Development 127, 2395–2405. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, and Carlson JR (1999). The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron 22, 339–347. 10.1016/s0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, and Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, and Patapoutian A (2015). Piezo1 ion channel pore properties are dictated by C-terminal region. Nat Commun 6, 7223. 10.1038/ncomms8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B, Li Q, Liu X, Cao Y, Li B, Qian Y, Xu R, Mao R, Zhou E, Zhang W, et al. (2019). Chemoconnectomics: Mapping Chemical Transmission in Drosophila. Neuron 101, 876–893 e874. 10.1016/j.neuron.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156. 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreos R, Ambrosini G, Groux R, Cavin Perier R, and Bucher P (2017). The eukaryotic promoter database in its 30th year: focus on non-vertebrate organisms. Nucleic Acids Res 45, D51–D55. 10.1093/nar/gkw1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreos R, Ambrosini G, Perier RC, and Bucher P (2015). The Eukaryotic Promoter Database: expansion of EPDnew and new promoter analysis tools. Nucleic Acids Res 43, D92–96. 10.1093/nar/gku1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazalpour A, Bennett B, Petyuk VA, Orozco L, Hagopian R, Mungrue IN, Farber CR, Sinsheimer J, Kang HM, Furlotte N, et al. (2011). Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet 7, e1001393. 10.1371/journal.pgen.1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic KG, and Lindquist S (1989). The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59, 499–509. 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, and O'Connor-Giles KM (2014). Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961–971. 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Wildonger J, Harrison MM, and O'Connor-Giles KM (2013). CRISPR/Cas9-mediated genome engineering and the promise of designer flies on demand. Fly (Austin) 7, 249–255. 10.4161/fly.26566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Li J, and Ting AY (2018). Proximity labeling: spatially resolved proteomic mapping for neurobiology. Curr Opin Neurobiol 50, 17–23. 10.1016/j.conb.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, and Luo L (2014). Genetic control of wiring specificity in the fly olfactory system. Genetics 196, 17–29. 10.1534/genetics.113.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Zhu H, Potter CJ, Barsh G, Kurusu M, Zinn K, and Luo L (2009). Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat Neurosci 12, 1542–1550. 10.1038/nn.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp F, and Mann M (2017). A Primer on Concepts and Applications of Proteomics in Neuroscience. Neuron 96, 558–571. 10.1016/j.neuron.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Hung V, Zou P, Rhee HW, Udeshi ND, Cracan V, Svinkina T, Carr SA, Mootha VK, and Ting AY (2014). Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol Cell 55, 332–341. 10.1016/j.molcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Lin Y, Kurmangaliyev Y, Mirshahidi P, Parrington B, and Zipursky SL (2020). A Global Temporal Genetic Program for Neural Circuit Formation. bioRxiv 09.18.304410. [Google Scholar]
- Jan YN, and Jan LY (2010). Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11, 316–328. 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Marin EC, Stocker RF, and Luo L (2001). Target neuron prespecification in the olfactory map of Drosophila. Nature 414, 204–208. 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, and Patapoutian A (2012). The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212. 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, and Tessier-Lavigne M (2011). Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3, a001727. 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Carlson JR, and Luo L (2004). Olfactory receptor neuron axon targeting: intrinsic transcriptional control and hierarchical interactions. Nat Neurosci 7, 819–825. 10.1038/nn1284. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Johnson WA, Luo L, and Jefferis GS (2003). From lineage to wiring specificity. POU domain transcription factors control precise connections of Drosophila olfactory projection neurons. Cell 112, 157–167. 10.1016/s0092-8674(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa LD, Guck J, et al. (2016). Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci 19, 1592–1598. 10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, et al. (2016). Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44, W90–97. 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CW, Hirst E, Alexandre C, and Vincent JP (2003). Segment boundary formation in Drosophila embryos. Development 130, 5625–5635. 10.1242/dev.00867. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim M, Kim N, Macfarlan T, Pfaff SL, Mastick GS, and Song MR (2015). Slit and Semaphorin signaling governed by Islet transcription factors positions motor neuron somata within the neural tube. Exp Neurol 269, 17–27. 10.1016/j.expneurol.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, and Luo L (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461. 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lee T, and Luo L (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24, 251–254. 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Li H, Horns F, Wu B, Xie Q, Li J, Li T, Luginbuhl DJ, Quake SR, and Luo L (2017). Classifying Drosophila Olfactory Projection Neuron Subtypes by Single-Cell RNA Sequencing. Cell 171, 1206–1220 e1222. 10.1016/j.cell.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Han S, Li H, Udeshi ND, Svinkina T, Mani DR, Xu C, Guajardo R, Xie Q, Li T, et al. (2020). Cell-Surface Proteomic Profiling in the Fly Brain Uncovers Wiring Regulators. Cell 180, 373–386 e315. 10.1016/j.cell.2019.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Kao CF, Yu HH, Huang Y, and Lee T (2012). Lineage analysis of Drosophila lateral antennal lobe neurons reveals notch-dependent binary temporal fate decisions. PLoS Biol 10, e1001425. 10.1371/journal.pbio.1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KH, Stawski PS, Draycott AS, Udeshi ND, Lehrman EK, Wilton DK, Svinkina T, Deerinck TJ, Ellisman MH, Stevens B, et al. (2016). Proteomic Analysis of Unbounded Cellular Compartments: Synaptic Clefts. Cell 166, 1295–1307 e1221. 10.1016/j.cell.2016.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, Peabody NC, Vinson CR, and White BH (2006). Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52, 425–436. 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, Kim HJ, Bandell M, Longo N, Day RW, et al. (2015). Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun 6, 8329. 10.1038/ncomms9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne R, Smith R, Rutherford K, Wakeling M, Varley A, Guillier F, Janssens H, Ji W, McLaren P, North P, et al. (2007). FlyMine: an integrated database for Drosophila and Anopheles genomics. Genome Biol 8, R129. 10.1186/gb-2007-8-7-r129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGurn JA, Hsu PC, and Emr SD (2012). Ubiquitin and membrane protein turnover: from cradle to grave. Annu Rev Biochem 81, 231–259. 10.1146/annurev-biochem-060210-093619. [DOI] [PubMed] [Google Scholar]
- Marin EC, Watts RJ, Tanaka NK, Ito K, and Luo L (2005). Developmentally programmed remodeling of the Drosophila olfactory circuit. Development 132, 725–737. 10.1242/dev.01614. [DOI] [PubMed] [Google Scholar]
- Morey M, Yee SK, Herman T, Nern A, Blanco E, and Zipursky SL (2008). Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature 456, 795–799. 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SE, Dubin AE, and Patapoutian A (2017). Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18, 771–783. 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, McClatchy DB, and Yates JR 3rd (2018). From Synapse to Function: A Perspective on the Role of Neuroproteomics in Elucidating Mechanisms of Drug Addiction. Proteomes 6, E50. 10.3390/proteomes6040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, Yang-Zhou D, Shim HS, Tao R, Handler D, Karpowicz P, et al. (2011). A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods 8, 405–407. 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, and Tombola F (2014). Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A 111, 16148–16153. 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Santiago IJ, Ahn C, Gur B, Tsui CK, Su Z, Xu C, Karakhanyan A, Silies M, and Pecot MY (2018). Drosophila Fezf coordinates laminar-specific connectivity through cell-intrinsic and cell-extrinsic mechanisms. Elife 7, e33962. 10.7554/eLife.33962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YR, Tran NM, Krishnaswamy A, Kostadinov D, Martersteck EM, and Sanes JR (2017). Satb1 Regulates Contactin 5 to Pattern Dendrites of a Mammalian Retinal Ganglion Cell. Neuron 95, 869–883 e866. 10.1016/j.neuron.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, McCall K, Yang-Zhou D, Flockhart I, Binari R, Shim HS, et al. (2015). The Transgenic RNAi Project at Harvard Medical School: Resources and Validation. Genetics 201, 843–852. 10.1534/genetics.115.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, and Rubin GM (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755. 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, and Sandberg R (2014). Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc 9, 171–181. 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- Raji JI, and Potter CJ (2021). The number of neurons in Drosophila and mosquito brains. PLoS One 16, e0250381. 10.1371/journal.pone.0250381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, and Oshlack A (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11, R25. 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, and Zipursky SL (2020). Synaptic Specificity, Recognition Molecules, and Assembly of Neural Circuits. Cell 181, 1434–1435. 10.1016/j.cell.2020.05.046. [DOI] [PubMed] [Google Scholar]
- Santiago C, and Bashaw GJ (2014). Transcription factors and effectors that regulate neuronal morphology. Development 141, 4667–4680. 10.1242/dev.110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, and Bashaw GJ (2017). Islet Coordinately Regulates Motor Axon Guidance and Dendrite Targeting through the Frazzled/DCC Receptor. Cell Rep 18, 1646–1659. 10.1016/j.celrep.2017.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, and Ward AB (2018). Structure of the mechanically activated ion channel Piezo1. Nature 554, 481–486. 10.1038/nature25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitski MM, Mathieson T, Zinn N, Sweetman G, Doce C, Becher I, Pachl F, Kuster B, and Bantscheff M (2013). Measuring and managing ratio compression for accurate iTRAQ/TMT quantification. J Proteome Res 12, 3586–3598. 10.1021/pr400098r. [DOI] [PubMed] [Google Scholar]
- Schonemann MD, Ryan AK, Erkman L, McEvilly RJ, Bermingham J, and Rosenfeld MG (1998). POU domain factors in neural development. Adv Exp Med Biol 449, 39–53. 10.1007/978-1-4615-4871-3_4. [DOI] [PubMed] [Google Scholar]
- Shcherbata HR, Yatsenko AS, Patterson L, Sood VD, Nudel U, Yaffe D, Baker D, and Ruohola-Baker H (2007). Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. EMBO J 26, 481–493. 10.1038/sj.emboj.7601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, Lo TY, Wang F, Li T, Thompson-Peer KL, et al. (2019). The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 102, 373–389 e376. 10.1016/j.neuron.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, and de Belle JS (1997). Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol 32, 443–456. . [DOI] [PubMed] [Google Scholar]
- Szczot M, Nickolls AR, Lam RM, and Chesler AT (2021). The Form and Function of PIEZO2. Annu Rev Biochem 90, 507–534. 10.1146/annurev-biochem-081720-023244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, and Hamon C (2003). Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem 75, 1895–1904. 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- Ting L, Rad R, Gygi SP, and Haas W (2011). MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 8, 937–940. 10.1038/nmeth.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirian L, and Dickson BJ (2017). The VT GAL4, LexA, and split-GAL4 driver line collections for targeted expression in the Drosophila nervous system. bioRxiv, 198648. 10.1101/198648. [DOI] [Google Scholar]
- Trowbridge IS, Collawn JF, and Hopkins CR (1993). Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol 9, 129–161. 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Wang D, Eraslan B, Wieland T, Hallstrom B, Hopf T, Zolg DP, Zecha J, Asplund A, Li LH, Meng C, et al. (2019). A deep proteome and transcriptome abundance atlas of 29 healthy human tissues. Mol Syst Biol 15, e8503. 10.15252/msb.20188503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Hong W, Favaloro V, and Luo L (2015). Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron 85, 1013–1028. 10.1016/j.neuron.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman WW, and Sandelin A (2004). Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet 5, 276–287. 10.1038/nrg1315. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Tamagnone L, Bai J, Comoglio PM, Montell D, and Goodman CS (2001). The transmembrane protein Off-track associates with Plexins and functions downstream of Semaphorin signaling during axon guidance. Neuron 32, 53–62. 10.1016/s0896-6273(01)00446-9. [DOI] [PubMed] [Google Scholar]
- Wu JS, and Luo L (2006a). A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc 1, 2110–2115. 10.1038/nprot.2006.336. [DOI] [PubMed] [Google Scholar]
- Wu JS, and Luo L (2006b). A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat Protoc 1, 2583–2589. 10.1038/nprot.2006.320. [DOI] [PubMed] [Google Scholar]
- Xie Q, Wu B, Li J, Xu C, Li H, Luginbuhl DJ, Wang X, Ward A, and Luo L (2019). Transsynaptic Fish-lips signaling prevents misconnections between nonsynaptic partner olfactory neurons. Proc Natl Acad Sci U S A 116, 16068–16073. 10.1073/pnas.1905832116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, and Rubin GM (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237. [DOI] [PubMed] [Google Scholar]
- Yu HH, Kao CF, He Y, Ding P, Kao JC, and Lee T (2010). A complete developmental sequence of a Drosophila neuronal lineage as revealed by twin-spot MARCM. PLoS Biol 8, e1000461. 10.1371/journal.pbio.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarin AA, Asadzadeh J, Hokamp K, McCartney D, Yang L, Bashaw GJ, and Labrador JP (2014). A transcription factor network coordinates attraction, repulsion, and adhesion combinatorially to control motor axon pathway selection. Neuron 81, 1297–1311. 10.1016/j.neuron.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Processed RNA-seq data rank ordered according to adjusted P-value of the comparisons between acj6 mutant and wild-type samples, related to Figure 1
Table S2. Raw and processed proteome data, related to Figure, related to Figure 2
(A) Wild-type (WT) PN surface proteome by the cutoff shown in Figures 2D and 2E. Proteins are ranked by the TMT ratio 127N:127C in a descending order.
(B) acj6 mutant (acj6−) PN surface proteome by the cutoff shown in Figures 2D and 2E. Proteins are ranked by the TMT ratio 129N:129C in a descending order.
(C) Wild-type (WT) PN surface proteome by the more stringent cutoff shown in Figures S2D and S2E. Proteins are ranked by the TMT ratio 127N:127C in a descending order.
(D) acj6 mutant (acj6−) PN surface proteome by the more stringent cutoff shown in Figures S2D and S2E. Proteins are ranked by the TMT ratio 129N:129C in a descending order.
(E) Protein expression changes comparing WT and acj6 mutant and the corresponding p values. Proteins are ranked by the p value in an ascending order. Note that this list includes all detected proteins prior to the cutoff analysis and thus contains intracellular contaminants.
(F) TMT ratios of all detected proteins prior to the cutoff analysis.
(G to N) Each tab shows one experimental-to-control TMT ratio (see Figure 2A for detail). All detected proteins prior to the cutoff analysis are listed and ranked by the corresponding TMT ratio in a descending order.
Table S3. Genotypes of flies in each experiment, related to Figures 1-7 and STAR Methods
Data Availability Statement
Raw sequencing reads of the transcriptomics experiment have been deposited to GEO (GSE200841). Processed RNA-sequencing data is provided in Table S1. Mass spectra from the proteomic experiment have been deposited to MassIVE (MSV000089187). Processed proteomic data is provided in Table S2. Custom analysis code is available at https://github.com/Qijing-Xie/Acj6.