Cardiac magnetic resonance (CMR) imaging has emerged as an invaluable tool for patients with cardiomyopathy and/or arrhythmia. In addition to providing structural and functional information, CMR offers insights into myocardial tissue characteristics and identifies focal changes using post-injection differential time-dependent concentration of gadolinium-based agents in different tissue compositions. Gadolinium is an extracellular contrast agent with a limited volume of distribution in healthy myocardium but increased volume of distribution in conditions with expansion of extracellular space. This, along with delayed washout of contrast from abnormal tissues, leads to an increase in pixel image intensity on CMR, labeled as late-gadolinium enhancement (LGE).
Even in the setting of ischemic cardiomyopathy, thought to be a relatively simple and homogenous condition, a significant proportion of visualized LGE signifies lipomatous metaplasia rather than scar. The heterogeneity in the setting of non-ischemic cardiomyopathy (NICM), an umbrella terminology that refers to multiple conditions, each due to a different myopathic processes resulting in myocardial dysfunction, is far greater. Although various distribution patterns of LGE in patients with NICM are frequently labeled as fibrotic or ‘scarred’, histology-imaging correlations are lacking, and the truth is likely more complicated. For example, myocardial hypertrophy and disarray in hypertrophic cardiomyopathy leads to disorganized myocyte protein apparatus with resultant expansion of interstitium, a non-fibrotic nidus for gadolinium accumulation. LGE may also result from non-collagenous expansion of the interstitium due to infiltration with abnormal protein (amyloidosis), granulomatous deposits (sarcoidosis), or focal inflammation (myocarditis). These subtypes of NICM, however, only represent a minority of cases. In up to half of patients with NICM, LGE can be seen in the mid-myocardium (Figure) and the etiology and histopathologic composition of this LGE have not been established. T1 mapping can measure the burden of global diffuse fibrosis, but current clinical CMR sequences lack the spatial resolution to visualize regional variations in interstitial fibrosis. Given the uncertainty in the underlying pathophysiology of idiopathic NICM, we must consider another hypothesis for the mechanism of LGE, based upon the anatomical orientation and mechanical interaction of myocardial fiber layers.
Figure 1:
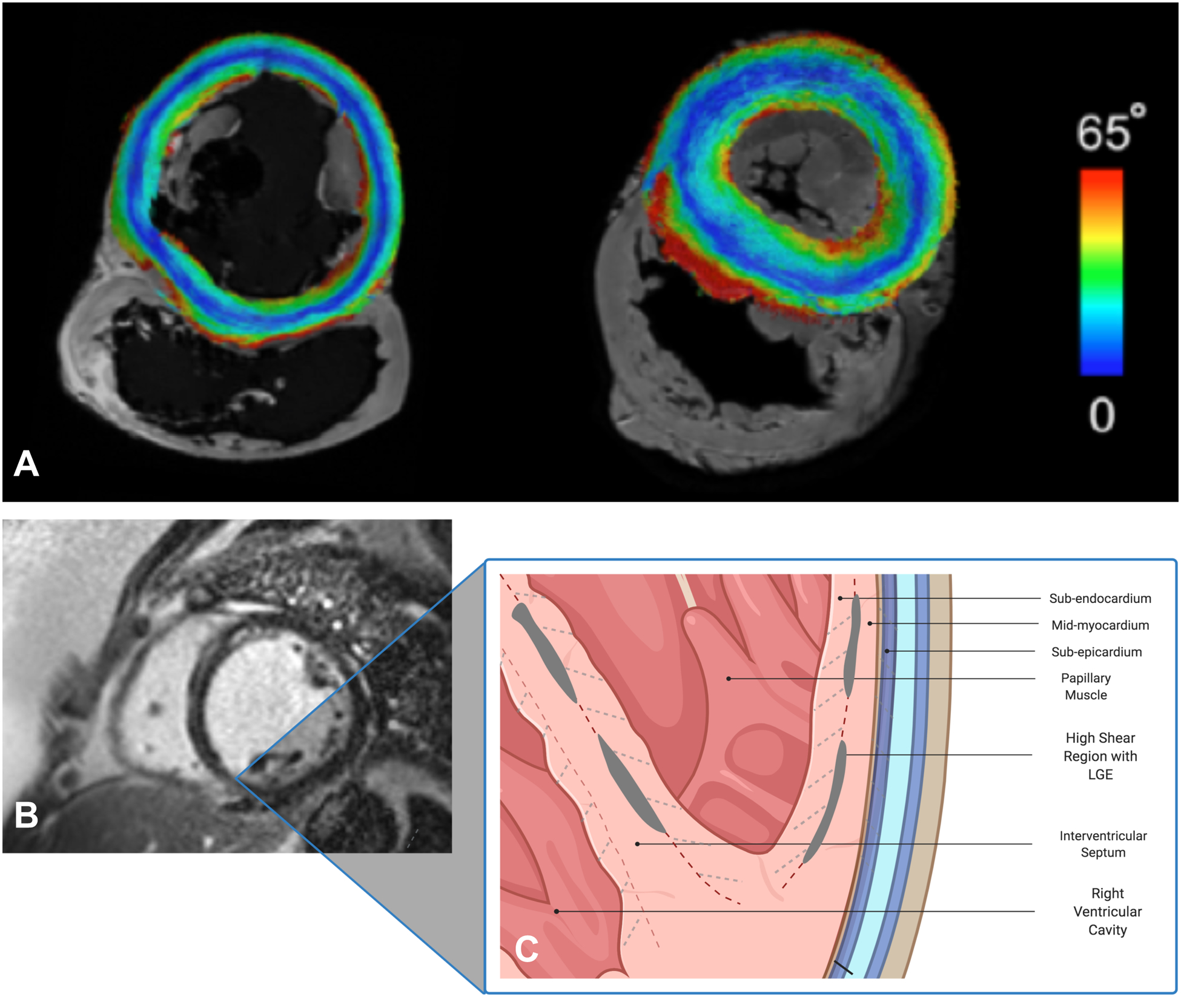
Panel A: Short-axis slice of normal porcine (left) and human (right) hearts on diffusion-tensor CMR with submillimeter resolution showing various myofiber orientations of different myocardial layers from endo- to epicardium (color-coded by the absolute values of the inclination angle 0–65°). Panels B & C: Short-axis CMR image of a patient with dilated NICM showing linear, mid-wall LGE in the septum, inferior and inferolateral walls. In systole, significant shear forces develop along the myocardial sheets cleavage planes with various orientations (dashed lines). The strain created as these fibers separate leads to increased extracellular space and gadolinium accumulation in the mid-myocardium. This process may be exaggerated in patients with non-ischemic cardiomyopathy.). Created with BioRender.com.
The ventricular myocardium consists of three layers of differentially oriented fibers. Quantitatively, sub-endocardial fibers have a positive helix angle (defined as the inclination angle of a myofiber relative to the left ventricular [LV] short axis plane), while sub-epicardial fibers have a negative helix angle.1 Mid-myocardial fibers are circumferential in the short axis plane. During systole, significant shear forces develop along the myocardial sheets cleavage planes, particularly between sub-endocardial and mid-myocardial layers.2 This leads to extension and radial reorientation of myofibers, facilitating myocardial thickening and ejection of blood. The strain created as these fibers separate may lead to increased extracellular space. This process may be exaggerated in patients with NICM who have ongoing collagenous expansion of the extra-cellular space as well as myofiber reorientation due to remodeling in response to LV dilatation. The resultant expansion of the extracellular space may result in gadolinium accumulation, particularly in the mid-myocardium. Another example of this phenomenon is at the right ventricular (RV) septal insertion sites where the intersection of myocardial fibers from both ventricles leads to disorganized myocardial architecture, expansion of the extracellular space and LGE.
Moreover, LGE has been reported in the left atria (LA) of patients without prior history of ablation. Importantly, in such cases, there is no association between LGE and myocardial voltage, a well-established surrogate of myocardial scar.3 Pashakhanloo et al. derived the 3-dimentional myofiber architecture of the human atria using high-resolution diffusion-tensor CMR. In this study, the authors showed spatial heterogeneity of fiber orientation such that two myofiber layers (epicardial and endocardial) with distinct and perpendicular orientations run across the atrium.4 The change in fiber orientation takes place at the mid-myocardium and is not homogeneous across the LA. Heterogeneity in fiber orientation was most prevalent at the LA roof, near the pulmonary veins and at the inferior and anterior walls of the LA. Anatomically, these areas comprise the intersection of major myocardial bundles such as the Bachmann bundle with oblique and circumferential bundles on the anterior LA wall. Interestingly, this mirrors the distribution of LGE in atria of patients with and without atrial fibrillation.5 Additionally, these regions with de-novo LGE, which do not display low voltage, do display increased electrogram fractionation, which lends further support to varying conduction in distinct layers of myocardium with reduced interaction due to expanded inter-layer spacing as identified by LGE.3
Further multimodal correlation studies are needed to explore this hypothesis which has important clinical implications: heterogeneity in fiber orientation and spatial variation of signal dispersion could lead to abrupt electrical conduction disturbances potentially leading to arrhythmias in the absence of myocardial scar.
Clinical Implications.
An important application of CMR is its integration in the prognostication and management of patients with NICM who develop ventricular tachycardia (VT). Electro-anatomical studies in these patients have demonstrated reduced bipolar voltage and abnormal electrical conduction properties within myocardial segments with LGE on CMR. Moreover, critical sites for the maintenance of VT predominantly co-localize with LGE, demonstrating that the latter harbors arrhythmic substrate in NICM. The mechanism underlying this association however, is more complex in NICM compared to ICM. The mechanism of monomorphic VT involves electrical wave front propagation, termed reentry, within slowly conductive or around non-conductive tissue such as scar. In NICM, electrophysiologists are limited in their ability to appropriately map and ablate circuits that are typically smaller and difficult to characterize due to sampling density limitations and presumed critical components deep to mappable surfaces of the myocardium. Therefore, electrophysiologists are often required to adopt an empiric strategy ablating myocardium with abnormal electro-anatomical properties on intra-cardiac mapping or LGE on CMR.
If instead of dense scar, LGE represents expanded extracellular space due to heterogenous fiber orientation and systolic shear between myofiber sheets, then the current therapeutic paradigm of VT and even AF should be questioned. The arrhythmogenicity underlying LGE may not be caused by re-entry around dense scar but rather fueled by abrupt changes in conduction properties between myofiber layers with different orientations and mechanical shear across the cardiac cycle. Delivering empiric ablation energy at these sites may lead to unnecessary necrosis of bystander healthy myocytes on either side of expanded interlayer matrix. Until more is known about the pathophysiology of LGE in idiopathic NICM and AF, we believe that ablation lesions should be targeted based upon electrophysiologic mapping and/or entrainment maneuvers when possible, rather than based only on MRI findings.
Additional studies to delineate the correlation of LGE in the myocardium with myocardial architecture and tissue composition are necessary before ever more destructive ablation tools are directed at the myocardium.
Acknowledgments:
We would like to thank Dr. Farhad Pashakhanloo for his invaluable contribution to this article through his work on imaging myofiber orientation using diffusion-tensor CMR and providing material for the figure.
Disclosures:
SN has disclosed grants from Biosense Webster, ImriCor, ADAS software, and the U.S. National Institutes of Health; SN is a consultant for Circle Software.
Nonstandard Abbreviations and Acronyms
- AF
Atrial Fibrillation
- CMR
Cardiac Magnetic Resonance
- ICM
Ischemic Cardiomyopathy
- LGE
Late-Gadolinium Enhancement
- LV
Left Ventricle
- NICM
Non-Ischemic Cardiomyopathy
- VT
Ventricular Tachycardia
References:
- 1.Mekkaoui C, Huang S, Chen HH, Dai G, Reese TG, Kostis WJ, Thiagalingam A, Maurovich-Horvat P, Ruskin JN, Hoffmann U, et al. Fiber architecture in remodeled myocardium revealed with a quantitative diffusion CMR tractography framework and histological validation. J. Cardiovasc. Magn. Reson 2012;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeGrice IJ, Takayama Y, Covell JW. Transverse Shear Along Myocardial Cleavage Planes Provides a Mechanism for Normal Systolic Wall Thickening. Circ. Res 1995;77:182–193. [DOI] [PubMed] [Google Scholar]
- 3.Kuo L, Zado E, Frankel D, Santangelli P, Arkles J, Han Y, Marchlinski FE, Nazarian S, Desjardins B. Association of Left Atrial High-Resolution Late Gadolinium Enhancement on Cardiac Magnetic Resonance With Electrogram Abnormalities Beyond Voltage in Patients With Atrial Fibrillation. Circ. Arrhythmia Electrophysiol 2020;E007586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pashakhanloo F, Herzka DA, Ashikaga H, Mori S, Gai N, Bluemke DA, Trayanova NA, McVeigh ER. Myofiber architecture of the human atria as revealed by submillimeter diffusion tensor imaging. Circ. Arrhythmia Electrophysiol 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochet H, Mouries A, Nivet H, Sacher F, Derval N, Denis A, Merle M, Relan J, Hocini M, Haïssaguerre M, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J. Cardiovasc. Electrophysiol 2015;26:484–492. [DOI] [PubMed] [Google Scholar]


