Abstract
BACKGROUND:
Epidemiologic studies have demonstrated an association between endometriosis and the subsequent development of cardiovascular disease. The direct impact of endometriosis on the progression of atherosclerotic, if any, has not been previously characterized. Endometriosis leads to systemic inflammation that could have consequences for cardiovascular health. Here, we report the effects of endometriosis on the development of atherosclerosis in a murine model.
OBJECTIVE:
To determine the contribution of endometriosis in promoting cardiovascular disease in a murine model of endometriosis.
STUDY DESIGN:
Endometriosis was induced in 18 ApoE-null mice, the standard murine model used to study atherosclerosis. Mice of the same strain were used as controls (N=18) and underwent sham surgery without inducing endometriosis. Formation of endometriotic lesions were confirmed after 25 weeks of induction. Atherosclerotic lesions were subjected to H&E staining followed by measurement of the aortic root luminal area and wall thickness. Total aorta was isolated and Oil Red O (ORO) staining was performed to quantify the lipid deposits/plaque formation, while biochemical assays were carried out in serum to determine the levels of lipids and inflammatory-related cytokines.
RESULTS:
ApoE mice with endometriosis exhibited increased aortic atherosclerosis compared to controls as measured by ORO staining, (7.9% vs 3.1%, respectively; p=0.0004). The endometriosis mice showed a significant 50% decrease in aortic luminal area compared to sham mice (0.85 mm2 vs 1.46 mm2, p=0.03) and a significant increase in aortic root wall thickness (0.22 mm vs 0.15 mm, p=0.04). There were no differences in the lipoprotein profile between mice with endometriosis and sham mice. The serum levels of inflammatory cytokines IL-1α, IL-6, IFN-γ and VEGF were significantly increased in the endometriosis mice.
CONCLUSIONS:
This is the first study using a murine model to determine the effect of endometriosis on atherosclerosis. Inflammation-related cytokines IL1-α, IL-6, IFN-γ and angiogenic factor VEGF released by endometriotic lesions may contribute to the increased cardiovascular risks in women with endometriosis. To reduce the risk of cardiovascular disease, early identification and treatment of endometriosis is essential. Future treatments targeting inflammatory cytokines may help reduce the long-term risk of cardiovascular disease in women with endometriosis.
Keywords: Endometriosis, ApoE mice, atherosclerosis, inflammation, cytokines, plaques, cardiovascular disease
Introduction
Endometriosis is an estrogen dependent inflammatory gynecological disorder characterized by the growth of endometrial tissue outside the uterus that can cause chronic pelvic pain and infertility.1–5 Endometriosis affects 6–10% of reproductive aged women and the lesions are most typically found in the pelvic cavity. 6,7 Although endometriosis originates as a localized syndrome, the process of inflammation proves to be systemic1,8–11, where inflammatory cytokines, such as C-reactive protein (CRP), interleukin-1 (IL-1), interleukin-1 (IL-6), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF), are found to be elevated in both the serum and the peritoneal fluid of women with endometriosis.10,12
More recently, endometriosis has been associated with an increased risk of multiple adverse health conditions including cardiovascular disease, adverse reproductive outcomes, autoimmunity, endocrine disorders, and a variety of cancers.8,13–15 Epidemiological studies have specifically identified increased cardiovascular risk in women with endometriosis, however, the mechanism underlying this has not yet been determined.16–20 The risk may be indirect and linked to loss of estrogen production resulting from oophorectomy or suppressive effects of medical therapies. Alternatively, cardiovascular disease may be the result of direct or indirect effects of endometriosis on vasculature. Atherosclerosis is a complex inflammatory process involving the interface of lipoproteins, monocyte-derived macrophages, and T cells with the vessel wall.21,22 Women with endometriosis show significant elevations in markers of endothelial inflammation and activation.23,24 Recent evidence also suggests higher oxidative stress and an atherogenic lipid profile in women with endometriosis, which may all contribute to the development of cardiovascular disesase.25–27
Hyperlipidemia has long been considered the major risk factor for the development of atherosclerosis. However, in several animal models of atherosclerosis, inflammation was shown to play an essential role in the pathogenesis of this disease, mediating all its stages and driving lipid accumulation in the intima of arteries.28 Multiple inflammatory markers including CRP, serum amyloid A, IL-6 and TNF-α are elevated in cardiovascular diseases and the degree of elevation is associated with worse prognosis.29–31 Endometriosis is also associated with systemic inflammation and an increased number of activated macrophages. Hence, both disease processes share similar pathophysiologic mechanisms.32
Under normal conditions, mice do not develop atherosclerosis and require use of Apolipoprotein E (ApoE)-null mice in research settings.33 ApoE is a ligand that is crucial for the absorption and removal of atherogenic lipoproteins.34 In the absence of ApoE, mice develop hypercholesterolemia and early atherosclerotic lesions.35–37 We report here the development of severe atherosclerosis in a murine model of endometriosis due to increased inflammatory related cytokines.
Materials and Methods
Animals
ApoE-null c57BL/6 female mice of 6–8-week-old age were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) in compliance with an approved Yale Institutional Animal Care and Use Committee (IACUC). All animals received a chow diet ad libitum. All in-vivo experiments were carried out in accordance with Reporting of In Vivo Experiments guidelines.38 After 9 weeks of age, mice were randomly divided into two groups (n=18 per group) and endometriosis was induced and sham surgeries were carried out in the second group as control. These experiments were conducted in two separate replicates consisting of 10 experimental animals and 10 controls in the first set and 8 per group in the second set of experiments.
Induction of endometriosis in ApoE mice
Briefly, both uterine horns were extracted from donor ApoE mice by laparotomy. Uterine horns were separated, and each horn was opened longitudinally and sectioned horizontally resulting in a total of four sections per uterus. Experimental recipient mice (n=18) were anesthetized using inhalation of isoflurane (2.5 L/min) in conjunction with oxygen (1.5 L/min). Two uterine segments were sutured either side of the parietal peritoneum of each experimental mouse using 5–0 polyglactin suture (Vicryl), approximately 1 centimeter apart. The peritoneum and skin of the experimental mouse were closed with 4–0 polyglactin suture. Sham surgeries were performed for the control group using the same surgical procedure without the introduction of donor uterine tissue (n=18). The experimental mice was allowed to develop endometriosis for 25 weeks. Development of the model was confirmed post-transplantation by laparotomy and visualization of endometriotic lesions. After 25 weeks post-transplant and following an overnight fast, the mice were euthanized and the endometriotic lesions removed. Plasma was collected via cardiac puncture and stored at 4°C preceding analysis. Whole aortas were perfused and dissected from the three arches at the aortic arch to the two branches of the iliac arteries, then stored in formalin at 4°C before being subjected to ORO staining.
H & E staining
Lesions collected from mice with endometriosis were fixed in 5% PFA overnight and transferred to 70% ethanol the next day, then paraffin embedded and sectioned into 50-μm thick sections using a vibratome. H&E staining was followed by histologic examination for the confirmation of endometriosis in the ectopic lesions.
Oil red O staining
Oil Red O (ORO) was purchased from VWR Life Science, Solon, OH, USA (Cat. #0684–100G). ORO staining solution (0.25, weight-to-volume ratio) was prepared with 35 mL ORO solution in methanol mixed with 10 mL of 1M sodium hydroxide and filtered with filter paper.39 Whole aortas were washed in 1 mL 78% methanol for 5 minutes on tilted roller paper, incubated in 1 mL of ORO staining solution for 50 minutes on tilted rollers, destained for 5 minutes in 1 mL 78% methanol, and stored in phosphate-buffered saline until ready for mounting and imaging.
Remnant adventitial fat was carefully removed using fine forceps under an Olympus SZX16 microscope. Using micro dissecting spring scissors, the aortas were cut longitudinally and pinned flat with lumen side up onto clear-bottom Sylgard-coated glass dissecting dishes, as described.40 The images were captured using an LEICA DFC295 microscope with a 10450528 0.5x XPF objective lens connected to an Olympus Model U-LH100HG camera at 0.73x with no adjustment to contrast or sharpness. A red threshold was used to analyze the images, determined based on ability to visualize red stain in regions of plaque formation, using ImageJ software.
Histology of aortic root
Aortic root and total aorta was separated and mounted in OCT compound and frozen at −80°C until sections were obtained. Sections (5 μM thickness) were stained with hematoxylin-eosin (H&E) for histological studies to determine the lumen area and wall thickness using imageJ software.
Lipid profile analysis
Serum was collected via cardiac puncture after mice were anesthetized. Blood was allowed to clot for 1 hour at room temperature and supernatant was collected after centrifugation at 5000 rpm for 10 min and stored at −80°C until used. Serum was analyzed for lipoprotein profile at Yale Core Center for Biochemical Assays (TG; Diagnostic Chemicals, Charlottetown, PEI, Canada), and total cholesterol (TC), and high-density lipoproteins (HDL) (TC, HDL, ThermoFisher Scientific Inc., Waltham, MA). HDL was subtracted from TC to yield low-density lipoproteins (LDL).
Cytokine assay
Differential expression of cytokines in serum was determined using Mouse Cytokine/Chemokine 31-Plex Discovery Assay® Array (Cat.# MD31, Eve Technologies Corporation, Calgary, Canada (MD31). Experiments were carried out in duplicates and averaged for cytokines known to be associated with atherosclerosis development (IL1-α, IL-6, IFN-γ, and VEGF) were plotted (Endo vs Sham).
Statistical analysis
GraphPad Prism was used for statistical analyses of the data. Aortic plaque formation was quantified by ORO staining. Two objective luminal areas and aortic wall thickness at equivalent anatomic locations were measured separately for each aorta on ImageJ software, and an average was taken of the resulting data. T test or Mann Whitney test used to determine significant differences between groups for quantitative plaque analyses, serum lipids and serum cytokines.
Results
Lesion formation
Before aortic extraction, we examined the peritoneal cavity of the experimental (endometriosis) and sham (control) ApoE mice for endometriosis lesions at the implantation site or suture site. Mice in the endometriosis group all had noticeable bilateral lesions at 25-weeks after transplantation of uterine tissue as shown in Figure 1A. The lesions were confirmed to be endometriosis using H & E staining that demonstrated growth of glandular and stromal endometrial tissue as shown in Figure 1B. No lesions were found in the corresponding location in control mice that underwent sham surgeries.
Figure 1.
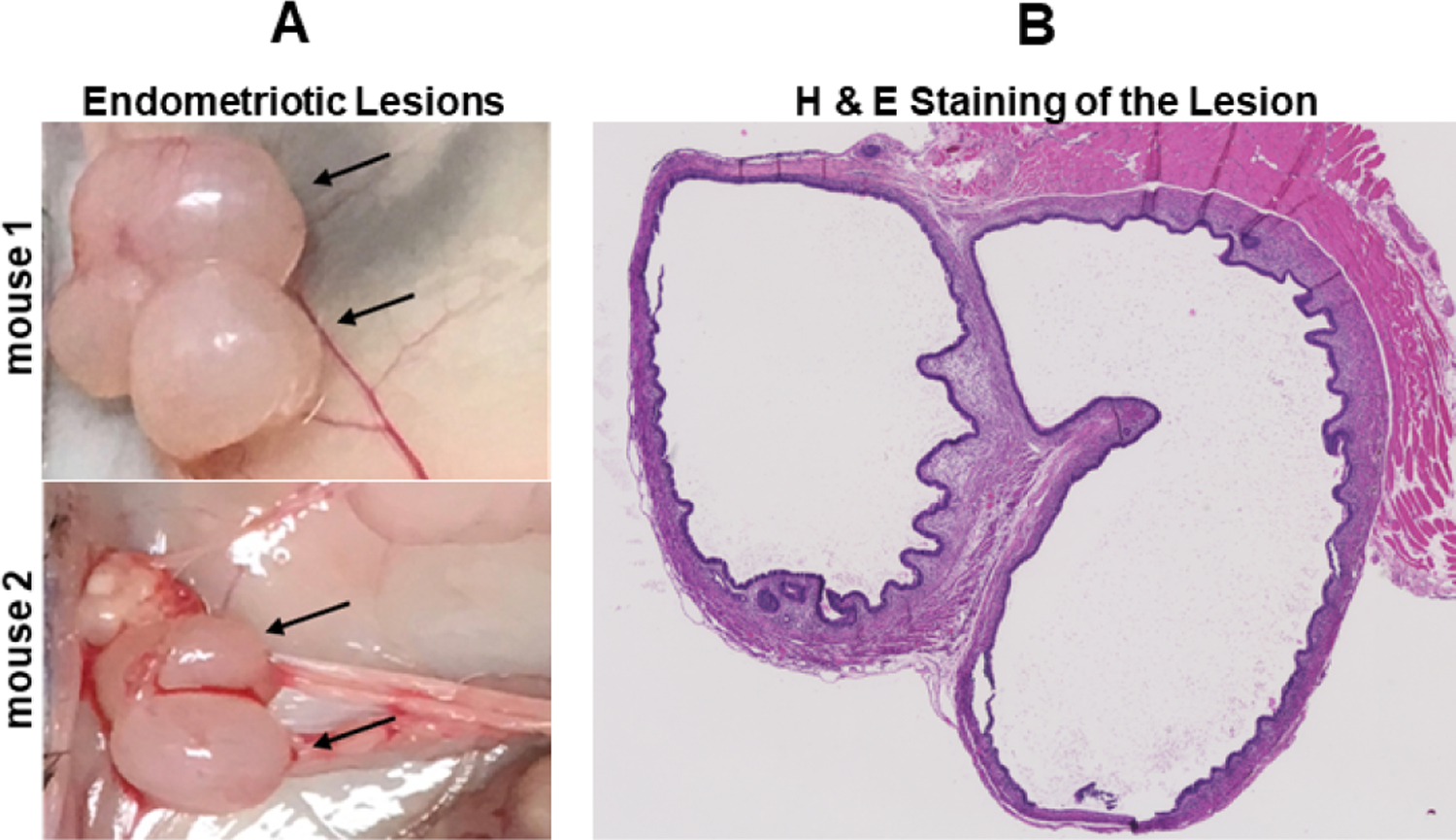
Endometriotic lesion formation. (A) Gross morphology of endometriotic lesions from representative mice. (B) Lesion section stained by H & E showing glandular and stromal tissue, consistent with endometriosis.
Plaque formation in aorta
To examine the effect of endometriosis on atherosclerotic plaque formation, we used ORO staining on the aortas with quantification of coloration threshold as displayed in Figure 2A. The ORO staining showed minimal plaque formation in the sham ApoE null mice. In contrast, a large amount of plaque formation was noted in the endometriosis ApoE null mice. Plaque formation was significantly higher in mice with endometriosis compared to sham (control) mice as shown in Figure 2B, (mean ± SEM: control 3.1 ± 0.9%; endometriosis, 7.9 ± 1.6%, p = 0.0004) as determined by quantification of ORO coloration.
Figure 2.
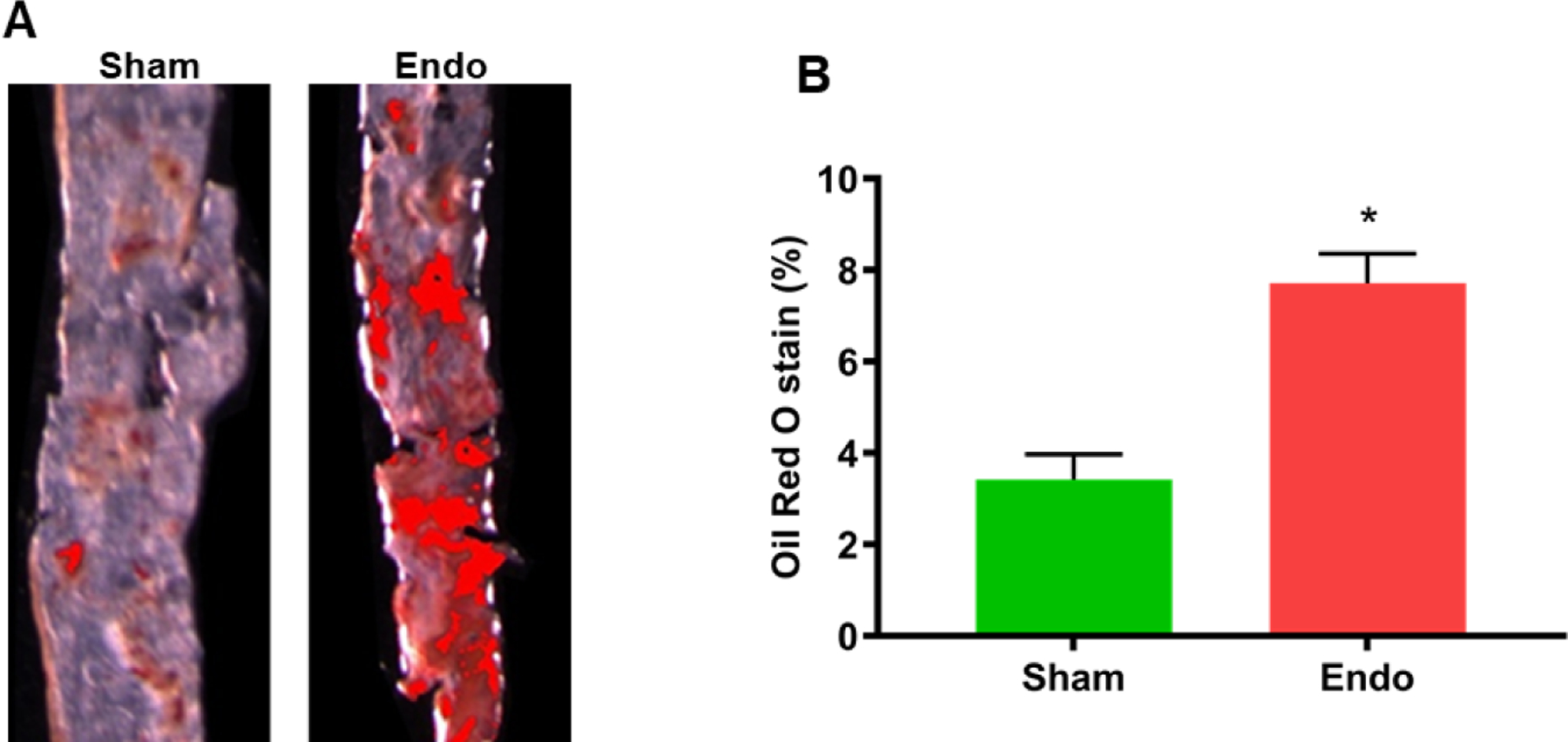
Quantification of plaque formation in the aorta. (A) ORO staining demonstrates plaque formation both in sham and endometriosis ApoE mice. The intensity of the red color is directly proportional to the amount of plaque formation. (B) Percent of ORO staining in whole aorta. There was a significant increase (in atherosclerotic plaque formation in endometriosis group compared to sham group (N=18 per group). Each bar represents the mean ± SEM; * denotes p=0.0004 between the endometriosis and sham groups.
Histology of aortic root
Aortas with the aortic root were extracted from mice and fixed in Optimal Cutting Temperature (OCT) solution Sections were subjected to H&E staining as shown in Figure 3A. The luminal area as well as arterial wall thickness were measured in the H&E stained sections. The luminal area was significantly reduced while artery wall thickness was significantly increased in the endometriosis mice compared to sham controls, as shown in Figure 3B ( lumen area, mean ± SEM: sham 1.46 ± 0.12 mm2; endometriosis, 0.85 ± 0.14 mm2, p=0.03) and Figure 3C (wall thickness, mean ± SEM: sham 0.15 ± 0.01 mm; endometriosis, 0.22 ± 0.01 mm; p=0.04), respectively.
Figure 3.
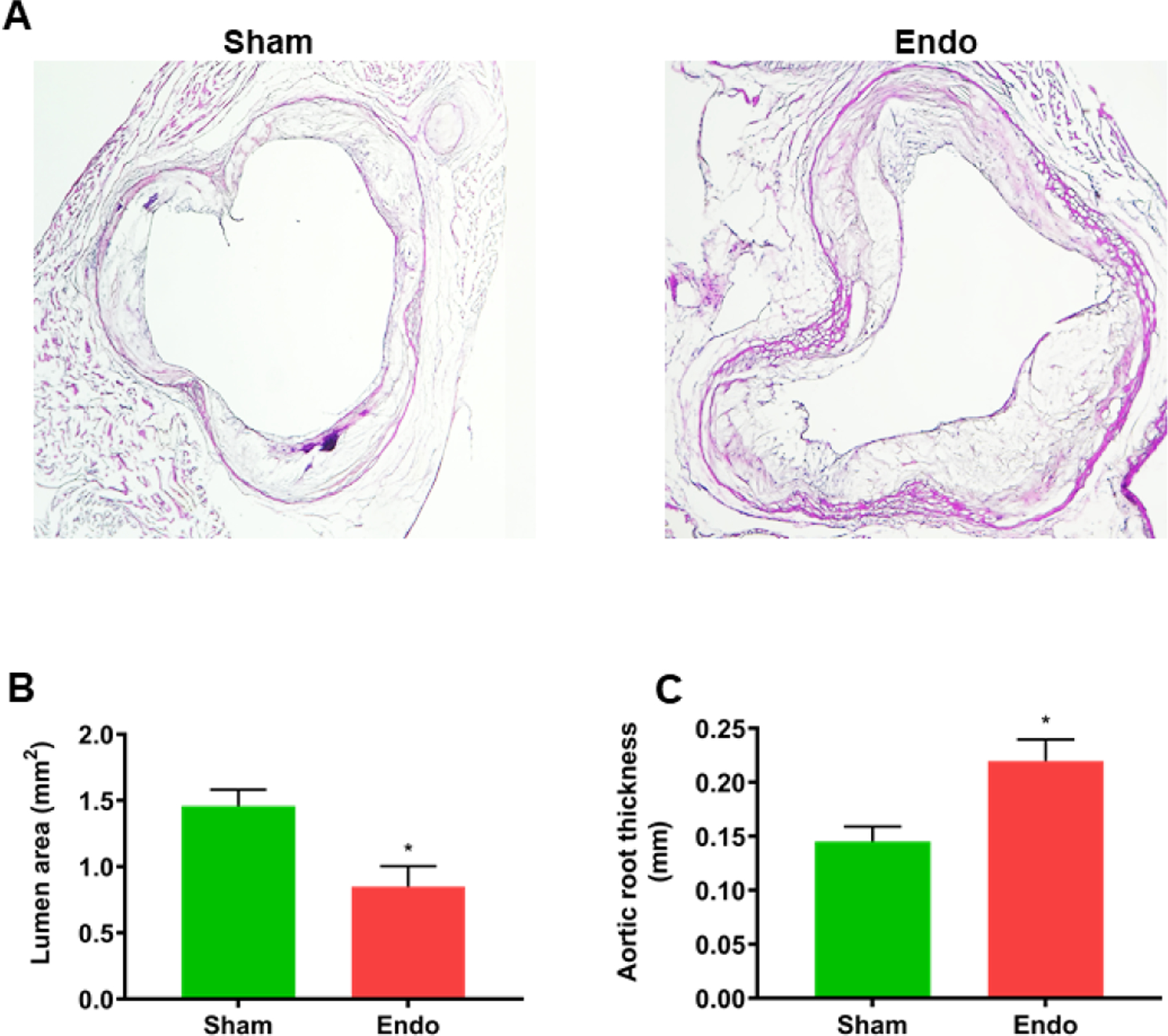
Lumen area and arterial wall thickness in the aortic root. (A) Representative section of the aortic root stained by H & E. (B) Average lumen area measured in H&E stained sections. There was significant reduction in lumen area in endometriosis mice. Each bar represents the mean ± SEM. * denotes p < 0.05 between endometriosis and sham groups (n=18. (B) The average wall thickness of the aortic root measured in H&E stained sections. Wall thickness was significantly increased in endo mice. Each bar represents the mean ± SEM. * denotes p < 0.05 between endometriosis and sham groups (n=18).
Increased cytokine levels in endometriosis ApoE mice
Serum from both endometriosis and sham groups was analyzed for lipoprotein levels including HDL, LDL, TC as well as glucose levels. We observed no significant changes in the levels of these lipoproteins or glucose as shown in Figure 4. We then determined the expression of various inflammation-related cytokines known to be associated with atherosclerosis formation. The levels of cytokines IL1-a, IL-6, IFN-r and VEGEF were each significantly increased (p<0.05) in ApoE mice with endometriosis compared to ApoE mice without endometriosis as shown in Figure 5.
Figure 4.
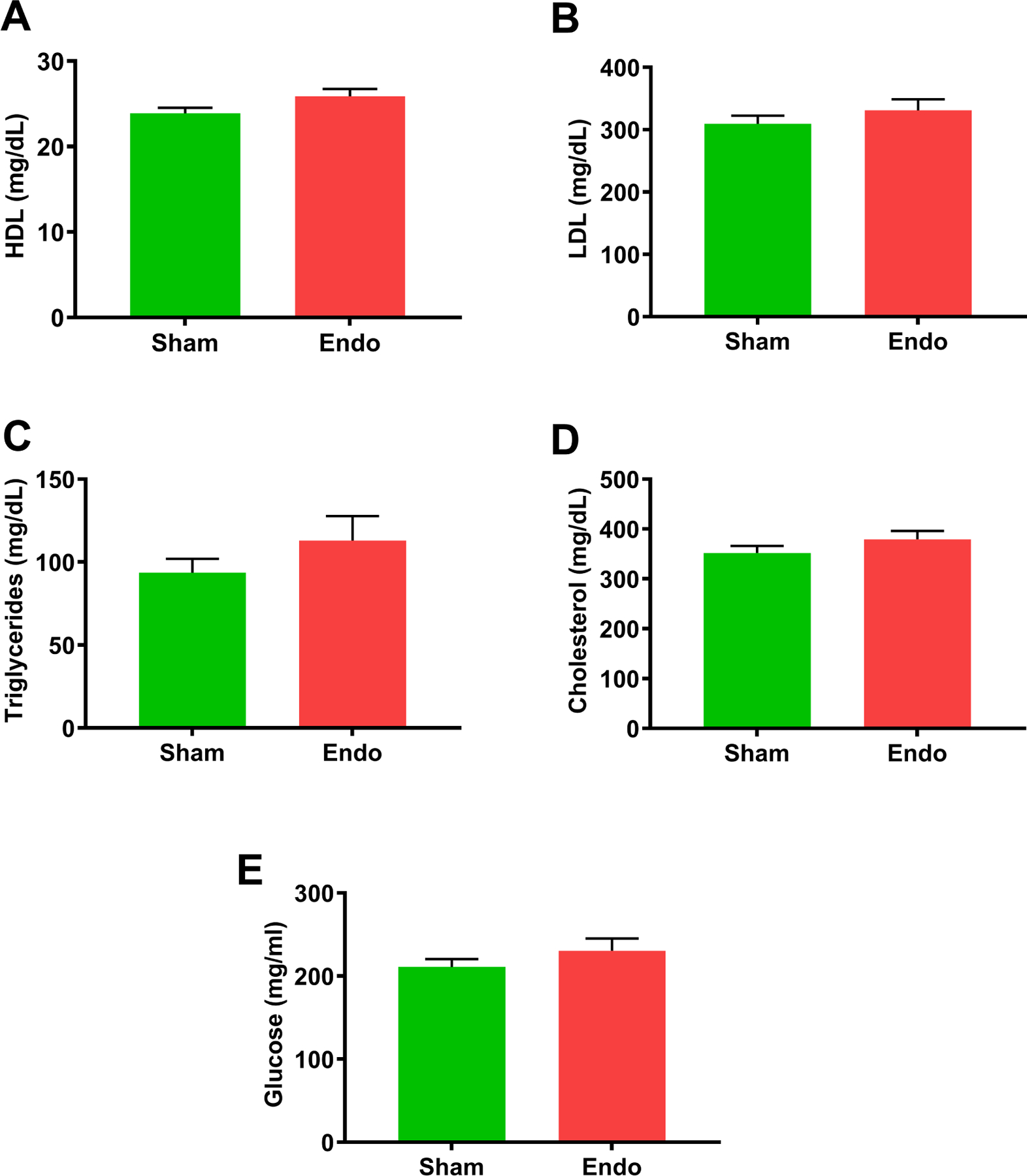
Determination of different lipid levels in serum. (A) HDL (B) LDL (C) Triglycerides (D) Cholesterol (E) Glucose. There were no significance differences in various lipid levels between the sham and endometriosis groups. Data are presented mean ± SEM (n=18).
Figure 5.
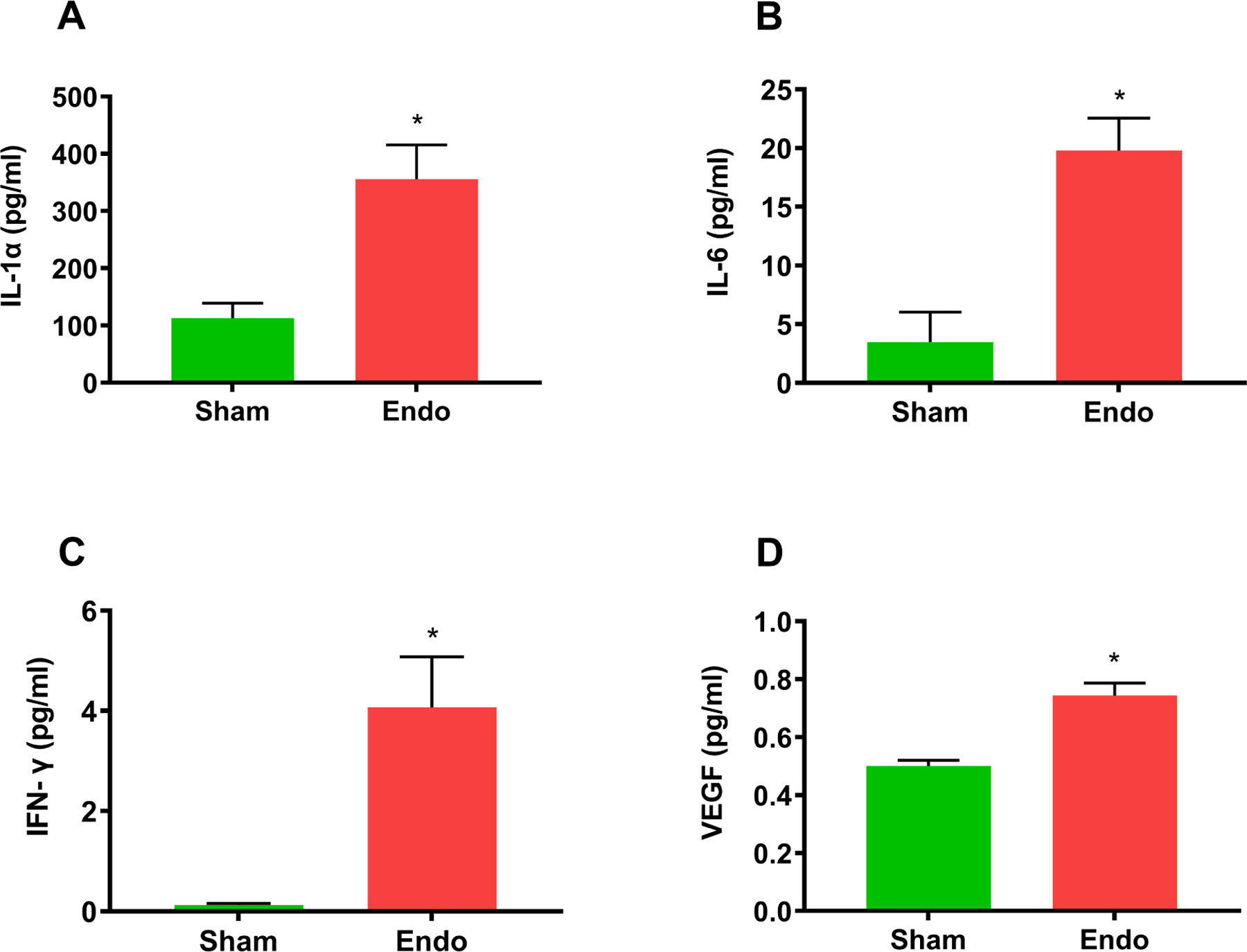
Elevated levels of cytokines. Serum levels of cytokines associated with atherosclerosis. (A) IL1-α, (B) IL-6, (C) IFN-γ, and (D) VEGEF. The levels of all these cytokines were significantly increased in the endometriosis group compared to sham group. Each bar represents the mean ± SEM (n=18). *denotes p < 0.05 between endometriosis and sham groups.
Structured Discussion/ Comment
1. Principal findings
This is the first study examining the relationship between endometriosis and atherosclerosis in a murine model. Mice with endometriosis exhibited significant increases in aortic plaque formation as determined by Oil Red O staining. In addition, a significant decrease in aortic luminal area and a significant increase in aortic wall thickness was seen in endometriosis mice. Biochemical analysis of serum revealed that there were no changes in the lipoprotein profile between mice with endometriosis and sham mice. The serum levels of inflammatory cytokines IL-1α, IL-6, IFN-γ, and in VEGEF were significantly increased in the endometriosis mice.
Results
In this study, we examined the effect of endometriosis on atherosclerosis development in a murine model of endometriosis. We used c57BL6/J female mice with a targeted deletion of ApoE. ApoE null mice are susceptible to arterial plaque formation and constitute the most widely studied animal model of atherosclerosis.41,42 We analyzed the formation of atherosclerotic lesions 25 weeks after the induction of the endometriosis. We confirmed that the formation of endometriotic lesions in peritoneal cavity of ApoE mice was similar to that induced in our previous reports using this endometriosis model in wild type mice.43–45 Control mice showed some plaque formation in agreement with studies previously reporting that ApoE mice develop atherosclerotic plaques.46,47 ORO staining of total aorta isolated from ApoE mice with endometriosis showed significant increases in plaque development in addition to significant structural differences in aortic root anatomy, compared to control ApoE mice. In general, plaque formation increases the thickness of the arterial wall and reduces the luminal volume of the artery, thereby obstructing the flow of blood in atherosclerosis.48
Women with endometriosis are at risk for atherosclerosis and cardiovascular disease.15 Like endometriosis, atherosclerosis is an inflammatory disease with accumulation of lipids in the wall of arteries leading to the development of atherosclerotic lesions.49 These lesions can remain stable for long periods of time, however, they eventually harden, causing arterial rigidity and further narrowing of the lumen. Many risk factors such as hyperlipidemia, hypertension, inflammation, and diabetes can determine the extent and rate of atherosclerosis development.50 Plasma LDL has a major role in the development of atherosclerosis51,52, especially oxidized LDL.53,54 In the present study, we did not observe any significant differences in serum lipoprotein levels including LDL, HDL, TGs, and cholesterol between the two groups of ApoE mice. In the ApoE gene knockout mice the lipid profile is changed compared to normal mice of the same strain35; ApoE KO mice have decreased levels of HDL and increased cholesterol levels, which predispose them to atherosclerosis.55,56 We observed that sham mice developed plaques consistent with anticipated changes in serum lipid levels that results from deletion of ApoE, however not as severely as seen in mice with endometriosis,. Further, we observed no changes in serum glucose levels between the groups, suggesting that diabetes was not a risk factor for atherosclerosis in this murine model of endometriosis.
We hypothesize that the increased number and size of plaques formed in ApoE mice may be due to additional inflammation induced by endometriosis. Endothelial dysfunction triggers the infiltration of monocytes into the artery wall. Monocytes differentiate into macrophages that are filled with excess cholesterol resulting in the formation of foam cells. 57,58 The foam cells in turn trigger inflammation, further damaging the vessel wall. In addition to the contribution from foam cells, endometriotic lesions also contribute to inflammation. We and others have previously demonstrated increased systemic inflammation in women with endometriosis.1,59 Here, our data showed significant increases in the levels of inflammatory cytokines IL1-α, IL-6, IFN-γ, and VEGEF in ApoE mice with endometriosis compared to ApoE mice without endometriosis. These results are consistent with previously reported results in cardiovascular disease where IL1-α, IL-6, IFN-γ, and VEGF levels are significantly increased.60–67 Therefore, the increase in these specific cytokines support a shared inflammatory pathway underlies endometriosis and atherosclerosis. Similarly, increased angiogenic factor VEGF levels promote lesion growth by initiating new vascularization.67,68 Increases in VEGF mediated by endometriosis may further compound the development of atherosclerosis.
Clinical implications
Endometriosis has long been considered a gynecologic disease, however, it has been increasingly shown to be associated with other systemic illnesses, including cardiovascular disease. Women with endometriosis are typically lean and do not have obvious risk factors for cardiovascular disease; it is unknown if the epidemiologic association with cardiovascular disease is directly related to endometriosis or due to other cofounders. Determining that endometriosis causes cardiovascular disease may change efforts to identify and treat the disease, as well as provide a model to test potential preventative measures.
An immense number of confounders prevent direct attribution of cardiovascular disease to endometriosis in human studies. Women with endometriosis often undergo surgeries that impact hormone production and may lead to early menopause. Endometriosis patients also have a lower BMI and altered behavior. They are treated with multiple hormonal medications and anti-inflammatory drugs. These interventions all modify cardiovascular disease, and it is difficult to distinguish the direct effects of endometriosis from our treatment of the disease. An animal model allows for the determination of cause and effect without these confounders.
2. Research Implications
This is the first experimental murine model of endometriosis that demonstrates increased arterial plaque formation caused by endometriosis. While our study has not dissected the complete signaling pathways involved in inflammation that led to plaque formation, these findings support the need for further studies to investigate the underlying molecular mechanisms. We identify atherosclerosis as a possible sequala of endometriosis as well as several novel targets to reduce the risk of cardiovascular disease in this population.
3. Strengths and limitation of the study
We chose an intentionally simple model of endometriosis to clearly demonstrate that endometriosis leads to increased cardiovascular disease. The strength of our study is induction of endometriosis in ApoE null mice, an inbred strain of mice that allowed transplantation of uterine tissue to create endometriosis without immune rejection; this in-vivo murine model allows us to demonstrate severe plaque development due to endometriosis. Further, the model allowed us to study the effect of inflammation induced by endometriosis in promoting the development of cardiovascular disease. There were no changes in the lipid profile while inflammatory-related cytokine levels were significantly increased. We identify endometriosis as leading to atherosclerosis through an inflammatory pathway.
While allowing us to determine a causal relationship between endometriosis and cardiovascular disease, we are also limited by the animal model used to study this disease. The ApoE null mouse model is the standard model used for atherosclerosis research, and accurately mimics human plaque formation, however the model used may not account for all aspects of human endometriosis. Another potential weakness of the study is the variation in size and development of endometriotic lesions. This limits the ability to assess the degree of inflammation induced by each lesion reflected by the levels of inflammatory-related cytokines.
Conclusions
This is the first study demonstrating that ApoE mice with endometriosis exhibit severe plaque formation likely due to endometriosis induced inflammation rather than changes in the lipoprotein profile. Inflammation-related cytokines IL1-α, IL-6, IFN-γ, and angiogenic factor VEGF released by or in response to the lesions may contribute to the increased cardiovascular risks in women with endometriosis. To reduce the risk of cardiovascular disease, early identification and treatment of endometriosis is essential. Future treatments specifically targeting inflammatory cytokines may help reduce the long-term risk of cardiovascular disease in women with endometriosis.
AJOG at a Glance:
A. Why was this study conducted?
Endometriosis has long been considered a gynecologic disease, however, it has recently been demonstrated to be associated with other systemic illnesses, including cardiovascular disease. Women with endometriosis are typically lean and lack obvious risk factors for cardiovascular disease; it is unknown if the epidemiologic association with cardiovascular disease is directly related to endometriosis or due to other cofounders, including drug effects and surgical treatments. Determining a link between endometriosis and cardiovascular disease can identify and treat a previously unidentified at-risk patient population.
B. Key findings
Established endometriotic lesion formation in ApoE−/− mice as evidenced by histology.
Identified more severe plaque development in the aortas of mice with endometriosis compared to controls using Oil Red O (ORO) stain.
Histology studies with H&E staining revealed that thickening of the aortic root wall and decreased luminal area in mice with endometriosis.
Biochemical analysis revealed no change in lipid profile while inflammatory-related cytokines and angiogenic factor levels were significantly increased in endometriosis mice.
C. What does this study add to what is already known?
This is the first study demonstrating increased arterial plaque formation with endometriosis using a murine model. Endometriosis did not affect the lipid profile but did increase production of inflammatory-related cytokines known to promote atherosclerosis. We identify atherosclerosis as a possible sequelae of endometriosis, as well as several novel targets to reduce the risk of cardiovascular disease in women with endometriosis.
Financial support:
Grants from NIH (NIH U54 HD052668 and R01 HD076422 to Dr. Taylor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No potential conflicts of interest.
Condensation
Endometriosis induced inflammation and the development of atherosclerosis in a mouse model, suggesting a causal relationship between endometriosis and cardiovascular disease.
References
- 1.Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet Feb 27 2021;397(10276):839–852. 10.1016/s0140-6736(21)00389-5. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med Jan 15 2009;360(3):268–79. 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Binda MM, Donnez O, Dolmans MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril Oct 2016;106(5):1011–1017. 10.1016/j.fertnstert.2016.07.1075. [DOI] [PubMed] [Google Scholar]
- 4.Macer ML, Taylor HS. Endometriosis and Infertility: A Review of the Pathogenesis and Treatment of Endometriosis-associated Infertility. Obstetrics and Gynecology Clinics of North America December// 2012;39(4):535–549. 10.1016/j.ogc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci Feb 2009;16(2):216–29. 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril Sep 2012;98(3):511–9. 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod Oct 2005;20(10):2698–704. 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 8.Alderman MH 3rd, Yoder N, Taylor HS. The Systemic Effects of Endometriosis. Semin Reprod Med May 2017;35(3):263–270. 10.1055/s-0037-1603582. [DOI] [PubMed] [Google Scholar]
- 9.de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet Aug 28 2010;376(9742):730–8. 10.1016/s0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 10.Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest 2006;62(3):139–47. 10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- 11.Yun BH, Chon SJ, Choi YS, Cho S, Lee BS, Seo SK. Correction: Pathophysiology of Endometriosis: Role of High Mobility Group Box-1 and Toll-Like Receptor 4 Developing Inflammation in Endometrium. PLoS One 2018;13(9):e0203741. 10.1371/journal.pone.0203741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kvaskoff M, Mu F, Terry KL, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update Jul-Aug 2015;21(4):500–16. 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AlAshqar A, Patzkowsky K, Afrin S, Wild R, Taylor HS, Borahay MA. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obstet Gynecol Surv Nov 2019;74(11):661–673. 10.1097/ogx.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes CL, Foster WG, Agarwal SK. The Impact of Endometriosis across the Lifespan of Women: Foreseeable Research and Therapeutic Prospects. Biomed Res Int 2015;2015:158490. 10.1155/2015/158490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Missmer SA, Tu FF, Agarwal SK, et al. Impact of Endometriosis on Life-Course Potential: A Narrative Review. Int J Gen Med 2021;14:9–25. 10.2147/ijgm.S261139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro L, D’Onofrio F, Flore R, Gasbarrini A, Santoliquido A. Endometriosis and atherosclerosis: what we already know and what we have yet to discover. Am J Obstet Gynecol Sep 2015;213(3):326–31. 10.1016/j.ajog.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Li PC, Yang YC, Wang JH, Lin SZ, Ding DC. Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women. J Clin Med Sep 15 2021;10(18). 10.3390/jcm10184173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei CH, Chang R, Wan YH, Hung YM, Wei JC. Endometriosis and New-Onset Coronary Artery Disease in Taiwan: A Nationwide Population-Based Study. Front Med (Lausanne) 2021;8:619664. 10.3389/fmed.2021.619664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taskin O, Rikhraj K, Tan J, Sedlak T, Rowe TC, Bedaiwy MA. Link between Endometriosis, Atherosclerotic Cardiovascular Disease, and the Health of Women Midlife. J Minim Invasive Gynecol Jul-Aug 2019;26(5):781–784. 10.1016/j.jmig.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Tani A, Yamamoto S, Maegawa M, et al. Arterial stiffness is increased in young women with endometriosis. J Obstet Gynaecol 2015;35(7):711–5. 10.3109/01443615.2014.992871. [DOI] [PubMed] [Google Scholar]
- 21.Goetz TG, Mamillapalli R, Sahin C, et al. Addition of Estradiol to Cross-Sex Testosterone Therapy Reduces Atherosclerosis Plaque Formation in Female ApoE−/−Mice. Endocrinology Feb 1 2018;159(2):754–762. 10.1210/en.2017-00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell Feb 23 2001;104(4):503–16. 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 23.Santoro L, D’Onofrio F, Campo S, et al. Regression of endothelial dysfunction in patients with endometriosis after surgical treatment: a 2-year follow-up study. Hum Reprod Jun 2014;29(6):1205–10. 10.1093/humrep/deu074. [DOI] [PubMed] [Google Scholar]
- 24.Santoro L, D’Onofrio F, Campo S, et al. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum Reprod May 2012;27(5):1320–6. 10.1093/humrep/des062. [DOI] [PubMed] [Google Scholar]
- 25.Cirillo M, Coccia ME, Petraglia F, Fatini C. Role of endometriosis in defining cardiovascular risk: a gender medicine approach for women’s health. Hum Fertil (Camb) Apr 30 2021:1–9. 10.1080/14647273.2021.1919764. [DOI] [PubMed] [Google Scholar]
- 26.Scutiero G, Iannone P, Bernardi G, et al. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid Med Cell Longev 2017;2017:7265238. 10.1155/2017/7265238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melo AS, Rosa-e-Silva JC, Rosa-e-Silva AC, Poli-Neto OB, Ferriani RA, Vieira CS. Unfavorable lipid profile in women with endometriosis. Fertil Steril May 1 2010;93(7):2433–6. 10.1016/j.fertnstert.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation Mar 5 2002;105(9):1135–43. 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 29.Berk BC, Weintraub WS, Alexander RW. Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol Jan 15 1990;65(3):168–72. 10.1016/0002-9149(90)90079-g. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med Mar 23 2000;342(12):836–43. 10.1056/nejm200003233421202. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation May 9 2000;101(18):2149–53. 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 32.Hogg C, Horne AW, Greaves E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front Endocrinol (Lausanne) 2020;11:7. 10.3389/fendo.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breslow JL. Mouse models of atherosclerosis. Science May 3 1996;272(5262):685–8. 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 34.Curtiss LK, Boisvert WA. Apolipoprotein E and atherosclerosis. Curr Opin Lipidol Jun 2000;11(3):243–51. 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb Jan 1994;14(1):133–40. 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 36.Osada J, Joven J, Maeda N. The value of apolipoprotein E knockout mice for studying the effects of dietary fat and cholesterol on atherogenesis. Curr Opin Lipidol Feb 2000;11(1):25–9. 10.1097/00041433-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 37.van Dijk KW, Hofker MH, Havekes LM. Dissection of the complex role of apolipoprotein E in lipoprotein metabolism and atherosclerosis using mouse models. Curr Atheroscler Rep Sep 1999;1(2):101–7. 10.1007/s11883-999-0005-y. [DOI] [PubMed] [Google Scholar]
- 38.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol Aug 2010;160(7):1577–9. 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guevara NV, Kim HS, Antonova EI, Chan L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med Mar 1999;5(3):335–9. 10.1038/6585. [DOI] [PubMed] [Google Scholar]
- 40.Andrés-Manzano MJ, Andrés V, Dorado B. Oil Red O and Hematoxylin and Eosin Staining for Quantification of Atherosclerosis Burden in Mouse Aorta and Aortic Root. Methods Mol Biol 2015;1339:85–99. 10.1007/978-1-4939-2929-0_5. [DOI] [PubMed] [Google Scholar]
- 41.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr 1995;15:495–518. 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 42.Coleman R, Hayek T, Keidar S, Aviram M. A mouse model for human atherosclerosis: long-term histopathological study of lesion development in the aortic arch of apolipoprotein E-deficient (E0) mice. Acta Histochem 2006;108(6):415–24. 10.1016/j.acthis.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Mamillapalli R, Dang T, Habata S, Gao XB, Taylor HS. Activation of Hypocretin Neurons in Endometriosis. Reprod Sci Jul 19 2021. 10.1007/s43032-021-00682-4. [DOI] [PubMed] [Google Scholar]
- 44.Pluchino N, Mamillapalli R, Shaikh S, et al. CXCR4 or CXCR7 antagonists treat endometriosis by reducing bone marrow cell trafficking. J Cell Mol Med Feb 2020;24(4):2464–2474. 10.1111/jcmm.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotlyar AM, Mamillapalli R, Flores VA, Taylor HS. Tofacitinib alters STAT3 signaling and leads to endometriosis lesion regression. Mol Hum Reprod Mar 9 2021. 10.1093/molehr/gaab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golforoush P, Yellon DM, Davidson SM. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res Cardiol Nov 30 2020;115(6):73. 10.1007/s00395-020-00829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gargiulo S, Gramanzini M, Mancini M. Molecular Imaging of Vulnerable Atherosclerotic Plaques in Animal Models. Int J Mol Sci Sep 9 2016;17(9). 10.3390/ijms17091511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canton G, Hippe DS, Chen L, et al. Atherosclerotic Burden and Remodeling Patterns of the Popliteal Artery as Detected in the Magnetic Resonance Imaging Osteoarthritis Initiative Data Set. J Am Heart Assoc Jun 2021;10(11):e018408. 10.1161/jaha.120.018408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golforoush P, Yellon DM, Davidson SM. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic research in cardiology 2020;115(6):73–73. 10.1007/s00395-020-00829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet Sep 11-17 2004;364(9438):937–52. 10.1016/s0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 51.Steinberg D, Glass CK, Witztum JL. Evidence mandating earlier and more aggressive treatment of hypercholesterolemia. Circulation Aug 5 2008;118(6):672–7. 10.1161/circulationaha.107.753152. [DOI] [PubMed] [Google Scholar]
- 52.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Dec 15 2012;380(9859):2224–60. 10.1016/s0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res Jan 21 2011;108(2):235–48. 10.1161/circresaha.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navab M, Ananthramaiah GM, Reddy ST, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res Jun 2004;45(6):993–1007. 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Hinder LM, Vincent AM, Hayes JM, McLean LL, Feldman EL. Apolipoprotein E knockout as the basis for mouse models of dyslipidemia-induced neuropathy. Exp Neurol Jan 2013;239:102–10. 10.1016/j.expneurol.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishida T, Choi SY, Kundu RK, et al. Endothelial lipase modulates susceptibility to atherosclerosis in apolipoprotein-E-deficient mice. J Biol Chem Oct 22 2004;279(43):45085–92. 10.1074/jbc.M406360200. [DOI] [PubMed] [Google Scholar]
- 57.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 2009;27:165–97. 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barthwal MK, Anzinger JJ, Xu Q, Bohnacker T, Wymann MP, Kruth HS. Fluid-phase pinocytosis of native low density lipoprotein promotes murine M-CSF differentiated macrophage foam cell formation. PLoS One 2013;8(3):e58054. 10.1371/journal.pone.0058054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, Taylor HS. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab Jan 1 2018;103(1):64–74. 10.1210/jc.2017-01199. [DOI] [PubMed] [Google Scholar]
- 60.Kamari Y, Werman-Venkert R, Shaish A, et al. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis Nov 2007;195(1):31–8. 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 61.Reiss AB, Siegart NM, De Leon J. Interleukin-6 in atherosclerosis: atherogenic or atheroprotective? Clinical Lipidology 2017/January/01 2017;12(1):14–23. 10.1080/17584299.2017.1319787. [DOI] [Google Scholar]
- 62.Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-γ: new insights and therapeutic targets. Trends Cardiovasc Med Jan 2014;24(1):45–51. 10.1016/j.tcm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holm PW, Slart RH, Zeebregts CJ, Hillebrands JL, Tio RA. Atherosclerotic plaque development and instability: a dual role for VEGF. Ann Med 2009;41(4):257–64. 10.1080/07853890802516507. [DOI] [PubMed] [Google Scholar]
- 64.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature May 19 2011;473(7347):317–25. 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 65.McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res Oct 2011;50(4):331–47. 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Qin X, Lu X. Crocin improves endometriosis by inhibiting cell proliferation and the release of inflammatory factors. Biomed Pharmacother Oct 2018;106:1678–1685. 10.1016/j.biopha.2018.07.108. [DOI] [PubMed] [Google Scholar]
- 67.Malutan AM, Drugan T, Costin N, et al. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent Eur J Immunol 2015;40(1):96–102. 10.5114/ceji.2015.50840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rocha AL, Reis FM, Taylor RN. Angiogenesis and endometriosis. Obstet Gynecol Int 2013;2013:859619. 10.1155/2013/859619. [DOI] [PMC free article] [PubMed] [Google Scholar]


