Abstract
Background:
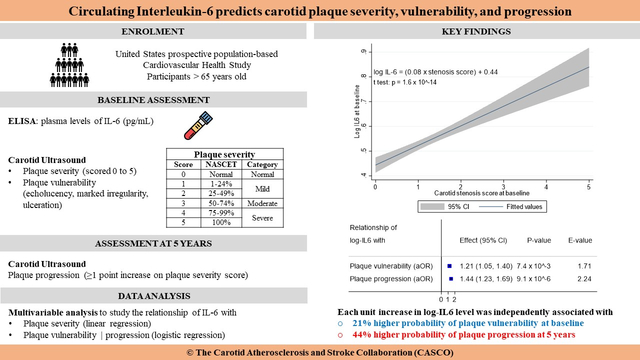
Interleukin-6 (IL-6) has important roles in atherosclerosis pathophysiology. To determine if anti-IL-6 therapy warrants evaluation as an adjuvant stroke prevention strategy in patients with carotid atherosclerosis, we tested whether circulating IL-6 levels predict carotid plaque severity, vulnerability, and progression in the prospective population-based Cardiovascular Health Study.
Methods:
Duplex carotid ultrasound was performed at baseline and 5 years. Baseline plaque severity was scored 0 to 5 based on North American Symptomatic Carotid Endarterectomy Trial grade of stenosis. Plaque vulnerability at baseline was the presence of markedly irregular, ulcerated or echolucent plaques. Plaque progression at 5 years was a ≥1 point increase in stenosis severity. The relationship of baseline plasma IL-6 levels with plaque characteristics was modeled using multivariable linear (severity) or logistic (vulnerability and progression) regression. Risk factors of atherosclerosis were included as independent variables. Stepwise backward elimination was used with p>0.05 for variable removal. To assess model stability, we computed the E-value or minimum strength of association (odds ratio scale) that unmeasured confounders must have with log IL-6 and the outcome to suppress the association. We performed internal validation with 100 bootstrap samples.
Results:
There were 4334 participants with complete data (58.9% women, mean age: 72.7 ± 5.1 years), including 1267 (29.2%) with vulnerable plaque and 1474 (34.0%) with plaque progression. Log IL-6 predicted plaque severity (β = 0.09, p=1.3 × 10−3), vulnerability (OR = 1.21, 95% CI: 1.05–1.40, p=7.4 × 10−3, E-value = 1.71) and progression (OR = 1.44, 95% CI: 1.23–1.69, p=9.1 × 10−6, E-value 2.24). In participants with >50% predicted probability of progression, mean log IL-6 was 0.54 corresponding to 2.0 pg/mL. Dichotomizing IL-6 levels did not affect the performance of prediction models.
Conclusions:
Circulating IL-6 predicts carotid plaque severity, vulnerability, and progression. The 2.0 pg/mL cut-off could facilitate the selection of individuals that would benefit from anti-IL-6 drugs for stroke prevention.
Keywords: interleukin-6, carotid stenosis, atherosclerosis, stroke, inflammation, Risk Factors, Primary Prevention, Epidemiology, Ultrasound, Atherosclerosis
Graphical Abstract
INTRODUCTION
Abnormal lipid profile and chronic systemic inflammation are key features of atherosclerosis, a major contributor to the burden of cardiovascular disease.1 Historically, interventions to slow the progression of atherosclerosis have focused on controlling vascular risk factors and reducing circulating levels of cholesterol.2 Recent clinical and experimental studies have demonstrated that anti-inflammatory drugs targeting the nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3 (NLRP3) inflammasome can decrease cardiovascular events independent of lipid lowering and blood pressure control.3 Additional analyses have revealed that this atheroprotective effect is mediated by the reduction in circulating levels of interleukin-6 (IL-6).4,5 Furthermore, genetic studies substantiate the causal relationship between IL-6 signaling and atherosclerotic cardiovascular disease.6–10 These observations position human monoclonal antibodies targeting IL-6 as promising adjuvant agents to prevent ischemic stroke in patients with carotid atherosclerosis.11–13
The potential of anti-inflammatory drugs to prevent carotid atherosclerosis-related stroke warrants further evaluation. To date, trials assessing anti-inflammatory drugs to reduce cardiovascular risk have used composite endpoints and not specifically stroke caused by carotid atherosclerosis. In a subgroup analysis of the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) trial,3 canakinumab did not show benefit for stroke prevention potentially because of non-atherothrombotic causes of stroke. Second, there is no population-based study demonstrating the specific association of IL-6 levels with carotid atherosclerosis-related ischemic stroke. Third, a recent population-based study with a modest sample size demonstrated that high levels of IL-6 are associated with carotid plaque progression but did not demonstrate the relationship of IL-6 with plaque severity and vulnerability.14
To know if therapies targeting IL-6 are worth evaluating as an adjuvant primary or secondary stroke prevention strategy in patients with carotid atherosclerosis, it is important to demonstrate the relationship between levels of IL-6 and high-risk plaque features associated with stroke risk.15 Our primary objective was to investigate if circulating levels of IL-6 are independently associated with plaque severity, vulnerability, and progression at 5 years in the Cardiovascular Health Study (CHS). As a secondary objective, we aimed to identify a candidate threshold for the use of circulating IL-6 as a biomarker for clinical decision-making in the management of carotid atherosclerosis.
METHODS
Data availability
All requests to access the CHS dataset should be addressed directly to the National Heart Lung, and Blood Institute via the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC, https://biolincc.nhlbi.nih.gov/home/). For reproducibility purposes, the file containing all the commands used for data curation and analysis are available from the corresponding author upon reasonable request. The list of variables extracted to conduct this study is provided in Supplementary Table S1.
Study design and participants
The Cardiovascular Health Study (CHS) is a prospective population-based cohort study aiming to identify risk factors of cardiovascular disease in people aged 65 years or older, recruited and followed up between 1989 and 1999.16 Eligible participants were non-institutionalized, able to give informed consent, not requiring a proxy respondent at baseline, and expected to remain in their area of residence for at least 3 years following enrolment. Individuals who were wheelchair-dependent, institutionalized or receiving anticancer treatment at baseline were excluded. For the analyses reported in this article, we excluded participants with a missing value of baseline circulating IL-6 levels or incomplete carotid ultrasound data (baseline and follow up at 5 years).
Clinical and laboratory assessment
All participants underwent clinical and laboratory evaluations at baseline to identify the presence and severity of cardiovascular risk factors as well as subclinical and clinical cardiovascular disease. The diagnosis of prevalent cardiovascular diseases at baseline and during follow up was centrally adjudicated.16 The definitions of hypertension, diabetes, dyslipidemia, and hyperuricemia used in this study are provided in the Supplemental Methods. Collection of blood samples at baseline was performed via venipuncture after a 12-hour fast. Further details on sample processing are available in the Supplemental Methods and previous publications.16–18 Plasma IL-6 levels were measured by enzyme-linked immunosorbent assay (ELISA, High Sensitivity Quantikine kit, R&D Systems, Minneapolis, MN, USA). The plasma samples used for IL-6 ELISA were prepared using ethylenediaminetetraacetic acid (EDTA) and were run in duplicates. The detectable limit was 0.10 pg/mL, the intra-assay coefficient of variability was 6.3% and the inter-assay coefficient of variability was 7%.19,20
Carotid ultrasound assessment
Duplex ultrasonography of both carotid arteries was performed at baseline and at 5 years with a Toshiba SSA-270A ultrasound device (Toshiba American Medical Systems, Tustin, CA, USA) equipped with 5.0 MHz transducer. Details of the assessment and the quality assurance process are available in the Supplemental Methods. Analytic measurements included the grade of stenosis based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria, a plaque irregularity score, and a description of plaque echogenicity focusing on the carotid bulb and proximal internal carotid arteries.16 Definitions of plaque severity, irregularity, echogenicity, vulnerability, and progression are provided in the Supplemental Methods.21–23
Statistical analysis
Natural logarithmic transformation was applied to continuous variables with a non-Gaussian distribution, and the resulting log-transformed variables were used for subsequent analyses unless stated otherwise. The relationship of IL-6 with cardiovascular risk factors was assessed by comparing the mean log IL-6 between groups defined by the presence of each risk factor using the Student t test.
The relationship of IL-6 with plaque characteristics was modeled using univariable and multivariable linear regression for maximum plaque severity at baseline and multivariable logistic regression for plaque vulnerability at baseline and plaque progression at 5 years. Selection of independent variables to include in the multivariable models was based on available evidence of association with atherosclerosis24,25. Considering our previous work suggesting that the presence of high-risk features is not dependent on plaque severity, we did not include plaque severity in the model to predict baseline plaque vulnerability.15 However, we considered the baseline ipsilateral plaque severity score and the baseline ipsilateral plaque vulnerability in the logistic regression model to predict plaque progression at 5 years and explored the interaction with log IL6.
In all multivariable regression analyses, a stepwise backward elimination process was applied with p>0.05 for removal of variables based on tests for the significance of regression coefficients. In this process, variables are removed in decreasing order of p-values. The significance of individual regression coefficients was determined by a t-test (linear regression) or a Wald test (logistic regression). All quantitative variables were included in regression models as continuous to optimize the statistical power. To further assess the stability of all logistic regression models, we computed the E-value defined as the minimum strength of association on the odds ratio scale that an unmeasured confounder must have with both log IL-6 and the dependent variable (baseline vulnerability or progression at 5 years) to fully suppress the observed association, conditional on the measured covariates.26 Moreover, we computed optimism-adjusted odds ratios using the heuristic shrinkage method 27 and performed bootstrapping-based internal validation.28 The reported optimism-adjusted area under the receiver operating characteristic curve (AUC), calibration in-the-large index (CITL), and calibration slope were computed after applying a bootstrap shrinkage factor derived from an internal validation process with 100 bootstrap samples.
In a secondary analysis, we attempted to define a candidate clinical threshold for plasma IL-6 levels. The approach is described in the Supplemental Methods.29,30
All statistical tests were two-sided and unpaired, with a significance threshold of p ≤ 0.05. Data analyses were performed using Stata software, version 17 (StataCorp LLC, College Station, TX, USA). Data were analyzed from September 9, 2021, to October 28, 2021.
Ethical considerations and reporting
The CHS was approved by the Institutional Review Board of the University of Washington and each of the participating field centers. All participants provided written informed consent.16
This study is part of the Carotid Atherosclerosis and Stroke Collaboration (CASCO) research initiative. The CASCO research protocol was approved by the University of Alberta Human Research Ethics Board (Pro00106520).
This report is compliant with the Transparent Reporting of a multivariable prediction model forIndividual Prognosis Or Diagnosis (TRIPOD) and the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statements.31,32
RESULTS
Characteristics of the participants
Of the 5888 participants of the CHS, 4334 (58.9% women) had complete data for baseline plasma IL-6 levels and carotid ultrasound assessment. The mean age was 72.7 ± 5.1 years (Supplementary Table S2). A detailed description of the study population is available in the appendix (Supplemental Results, Supplementary Figures S1–S5, and Supplementary Tables S2–S4). Plasma IL-6 levels were higher in participants with cardiovascular risk factors (Figure 1 and Supplementary Figure S6).
Figure 1: Relationship of IL-6 with cardiovascular risk factors.
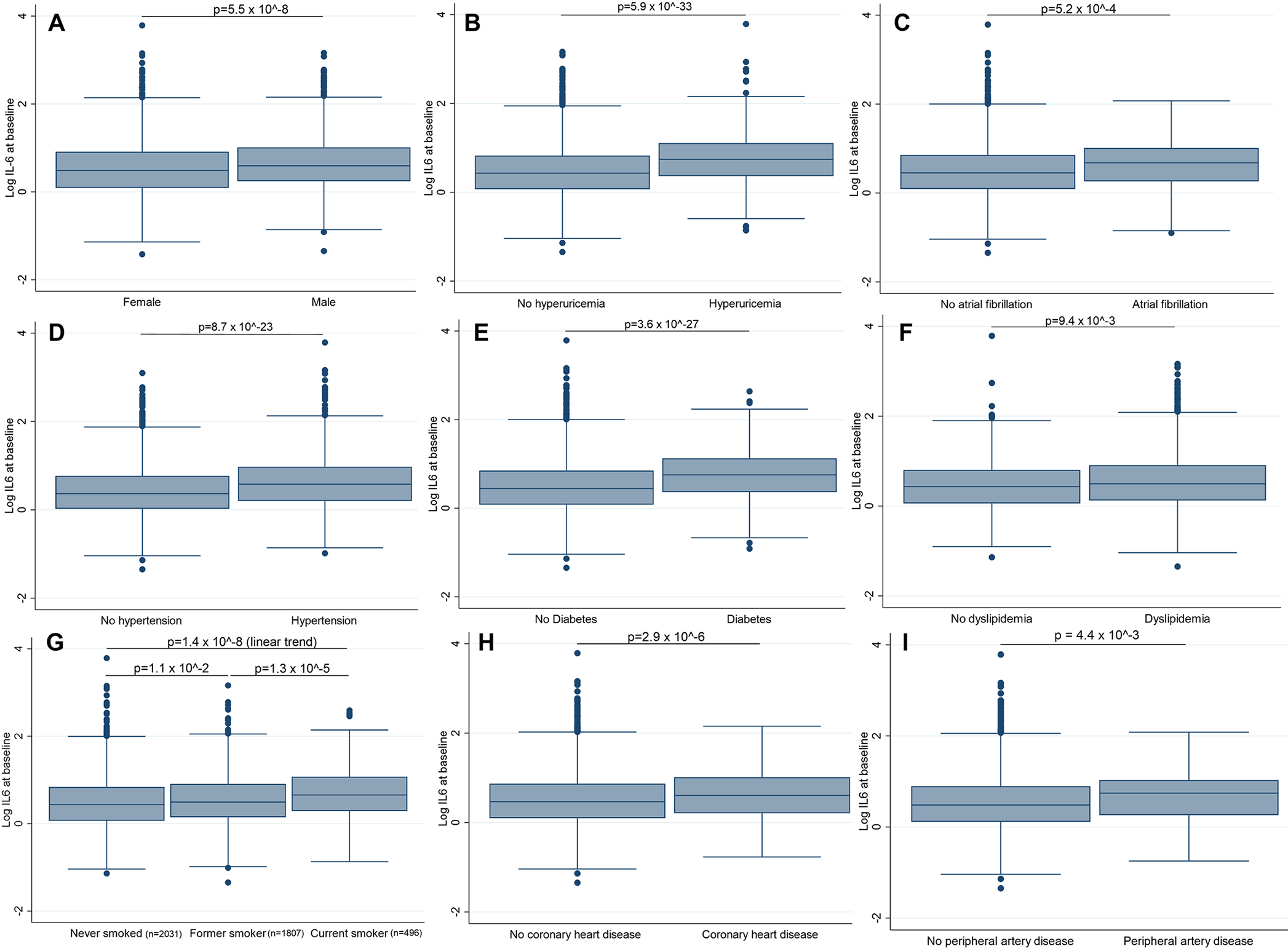
Panels illustrate the comparison of mean log IL-6 across categories of sex (A), hyperuricemia (B), atrial fibrillation (C), hypertension (D), diabetes mellitus (E), dyslipidemia (F), smoking status (G), coronary artery disease (H), and peripheral artery disease (I). The prevalence of each cardiovascular risk factor is available in Supplementary Table S1. Counts are provided when they cannot be derived from the table. All p-values are derived from unadjusted two-sample Student t tests. Violin plots are available in Supplementary Figure S6.
Relationship of plasma IL-6 with plaque severity at baseline
In the univariable linear regression model, log-IL-6 increased by 0.08 for each point increment on the plaque severity score (Figures 2A and 2B, Supplementary Figure S7).
Figure 2: Relationship of IL-6 with the grade of carotid stenosis.
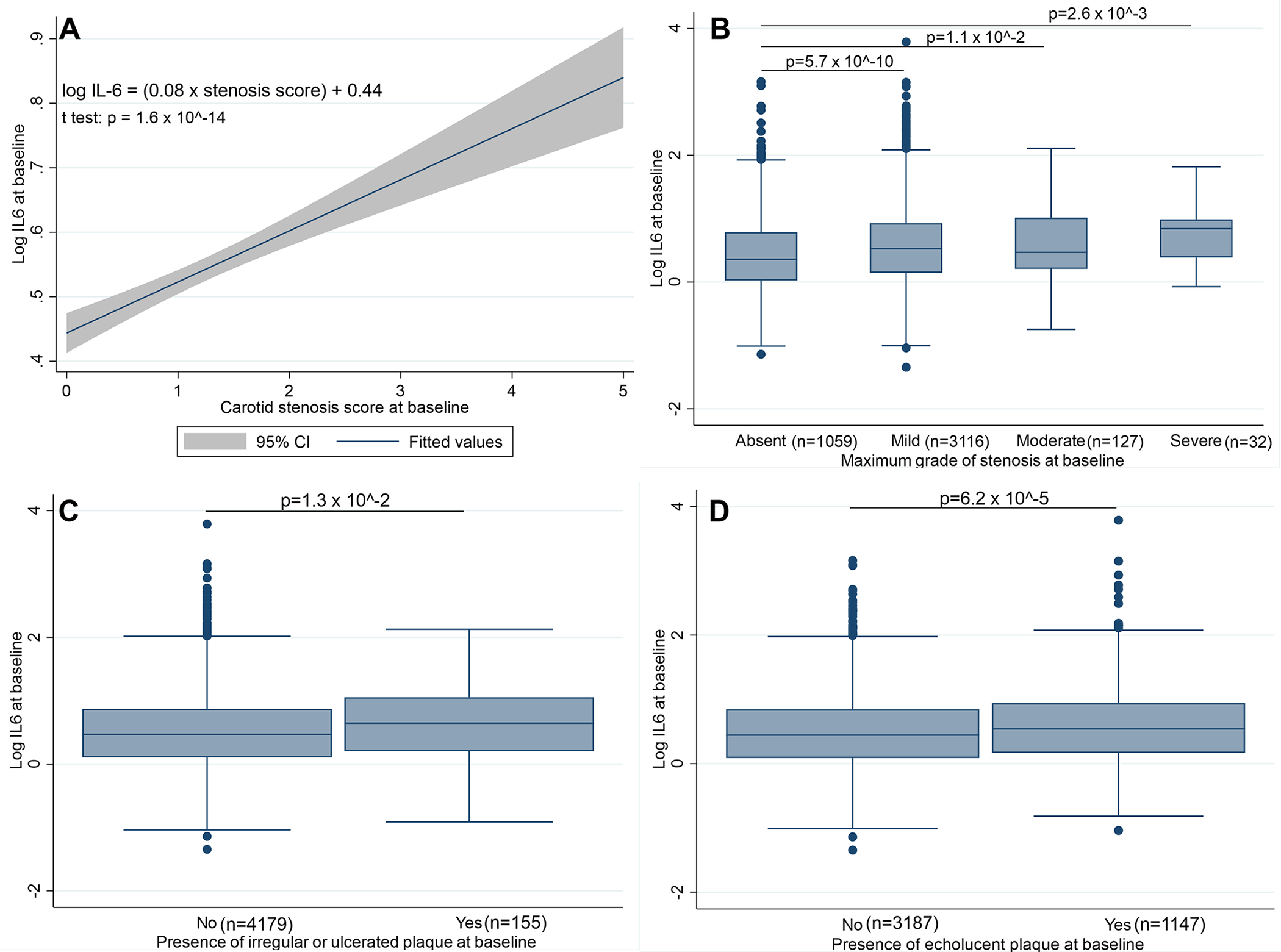
A: Univariable linear regression of log IL-6 over the baseline carotid stenosis score (β = 0.08, p-value of the t test for significance of the coefficient p=1.6 × 10−14).
B: Distribution of log IL-6 across categories of stenosis severity. All p-values are derived from unadjusted two-sample Student t tests.
C: Comparison of mean log IL-6 in patients with versus without markedly irregular or ulcerated carotid plaques. The p-value is derived from an unadjusted two-sample Student t test.
D: Comparison of mean log IL-6 in patients with versus without echolucent carotid plaques. The p-value is derived from an unadjusted two-sample Student t test.
Panels B-D are also presented as violin plots in Supplementary Figure S7.
In the multivariable linear regression analysis, log IL-6 was independently associated with plaque severity (β = 0.09, p=0.001, Table 1). Peripheral artery disease, smoking, dyslipidemia, prior stroke or TIA, and hypertension were the most important predictors of plaque severity with standardized beta coefficients of 0.33, 0.33, 0.24, 0.22, and 0.17 (Table 1). The distribution of the standardized residuals of the model is provided in Supplementary Figure S8. The results of the linear regression after excluding patients with history of cardiovascular disease (n = 979) or after using LDL-cholesterol and statin therapy as independent variables are provided in Supplementary Tables S5 and S6).
Table 1:
Multivariable linear regression model for the association of IL-6 with carotid plaque severity at baseline
| Independent variables | β1* | 95% CI | p-value | β2† |
|---|---|---|---|---|
| Log IL-6 | 0.09 | 0.03 – 0.14 | 1.3 × 10−3 | 0.08 |
| Age | 0.02 | 0.02 – 0.03 | 9.8 × 10−13 | 0.02 |
| Male | 0.11 | 0.05 – 0.18 | 5.5 × 10−4 | 0.14 |
| Black (African American) | −0.21 | (−0.29) – (−0.13) | 5.4 × 10−7 | −0.20 |
| Hypertension | 0.16 | 0.10 – 0.22 | 1.5 × 10−6 | 0.17 |
| Diabetes mellitus | 0.11 | 0.02 – 0.20 | 2.0 × 10−2 | 0.07 |
| Dyslipidemia | 0.24 | 0.13 – 0.35 | 2.3 × 10−5 | 0.24 |
| Current smoker | 0.32 | 0.22 – 0.42 | 2.1 × 10−10 | 0.33 |
| Hyperuricemia | 0.10 | 0.01 – 0.18 | 3.2 × 10−2 | 0.06 |
| Coronary heart disease | 0.16 | 0.07 – 0.25 | 7.0 × 10−4 | 0.13 |
| Peripheral artery disease | 0.31 | 0.10 – 0.51 | 2.9 × 10−3 | 0.33 |
| Prior stroke or TIA | 0.20 | 0.05 – 0.35 | 7.3 × 10−3 | 0.22 |
| Intercept | −0.94 | (−1.41) – (−0.48) | 7.9 × 10−5 | −0.81 |
IL-6: interleukin-6; NA: not applicable; TIA: transient ischemic attack.
Non-standardized coefficients (linked to change in stenosis severity score per 1 unit increase)
Standardized coefficients (linked to change in stenosis severity score per 1 standard deviation increase)
Treatment with antiplatelet drugs (p=0.85), atrial fibrillation, cystatin-based glomerular filtration rate, alcohol consumption, body mass index, log C-Reactive Protein, treatment with anti-inflammatory drugs (p=0.06) were consecutively removed from the model automatically due to coefficients with p-value >0.05.
Fisher F test for significance of the model: F = 24.1, df = 12, p = 6 × 10−52. Maximum Cook distance = 0.03. Maximum variance inflation factor = 1.15.
The p values for significance of the regression coefficients were determined by a t-test.
Relationship of plasma IL-6 with plaque vulnerability at baseline
Plasma IL-6 levels were higher in participants with a vulnerable carotid plaque (mean log IL-6 of 0.59 versus 0.50, p=4.4 × 10−6). They were also higher in patients with markedly irregular or ulcerated carotid plaques (mean log IL-6 of 0.65 versus 0.52, p=1.3 × 10−2, Figure 2C) and in patients with an echolucent carotid plaque (mean log IL-6 of 0.59 versus 0.50, p=6.2 × 10−5, Figure 2D).
In the multivariable logistic regression (Table 2), log IL-6 was independently associated with the presence of a vulnerable carotid plaque at baseline (OR = 1.21, 95% CI: 1.05–1.40, p=7.4 × 10−3). There was a 12% increase in the probability of having a vulnerable carotid plaque per standard deviation increase in log IL-6 (Table 2 and Figure 3), thus making log IL6 one of the most important contributors to plaque vulnerability. The logistic regression model displayed good calibration (Figure 4A). The association of log IL-6 with plaque vulnerability remained significant after adjustment for optimism (OR = 1.18, 95% CI: 1.05–1.33, Table 2).
Table 2:
Multivariable logistic regression model for the association of IL-6 with carotid plaque vulnerability at baseline
| Independent variables | OR1 (95% CI) * | p-value | OR2† | OR3 (95% CI) ‡ |
|---|---|---|---|---|
| Log IL-6 | 1.21 (1.05 – 1.40) | 7.4 × 10−3 | 1.12 | 1.18 (1.05 – 1.33) |
| Male | 1.22 (1.02 – 1.44) | 3.1 × 10−2 | 1.10 | 1.18 (1.01 – 1.37) |
| Dyslipidemia | 1.55 (1.11 – 2.17) | 1.0 × 10−2 | 1.13 | 1.45 (1.09 – 1.92) |
| Hyperuricemia | 1.36 (1.09 – 1.70) | 5.9 × 10−3 | 1.12 | 1.30 (1.07 – 1.56) |
| Intercept (baseline odds) | 0.26 (0.19 – 0.37) | 3.5 × 10−15 | NA | 0.49 (0.36 – 0.70) |
IL-6: interleukin-6; NA: not applicable.
Non-standardized odds ratio (linked to change in odds per 1 unit increase)
Standardized odds ratio (linked to change in odds per 1 standard deviation increase)
Optimism-adjusted odds ratio using the heuristic shrinkage method.
Coronary heart disease (p=0.94), diabetes mellitus, race, history of stroke or transient ischemic attack, log C-reactive protein, cystatin-based glomerular filtration rate, atrial fibrillation, alcohol consumption, age, treatment with anti-inflammatory drugs, hypertension, peripheral artery disease, treatment with antiplatelet drugs, body mass index, smoking status (p=0.08) were consecutively removed from the model automatically due to coefficients with p-value >0.05.
Likelihood ratio chi-squared test for significance of the model: χ2 = 37.3, df = 4, p = 1.6 ×10−7. Area under the Receiver Operating Characteristic curve = 0.57. Count R2 = 67%. Proportion of patients correctly classified = 66%. Maximum Cook distance = 0.03. Maximum variance inflation factor = 1.09.
The p values for significance of the odds ratios were determined by a Wald test.
Figure 3: Linear relationship of IL-6 with the probability of carotid plaque vulnerability and progression.
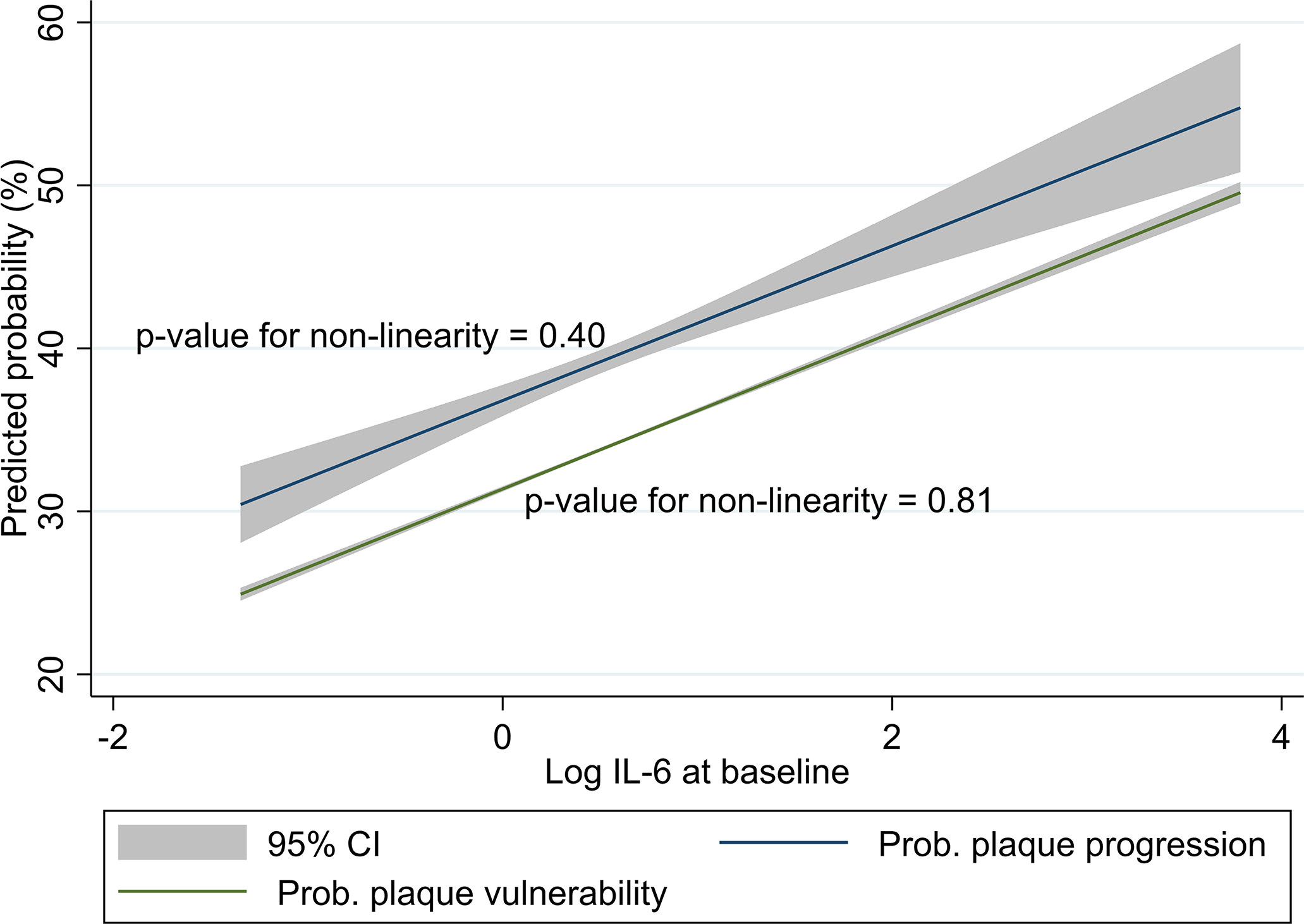
The curves are derived from the optimism-adjusted multivariable logistic regression models reported in Tables 2 and 3.
Figure 4: Calibration plots for the logistic regression models to predict plaque vulnerability and plaque progression.
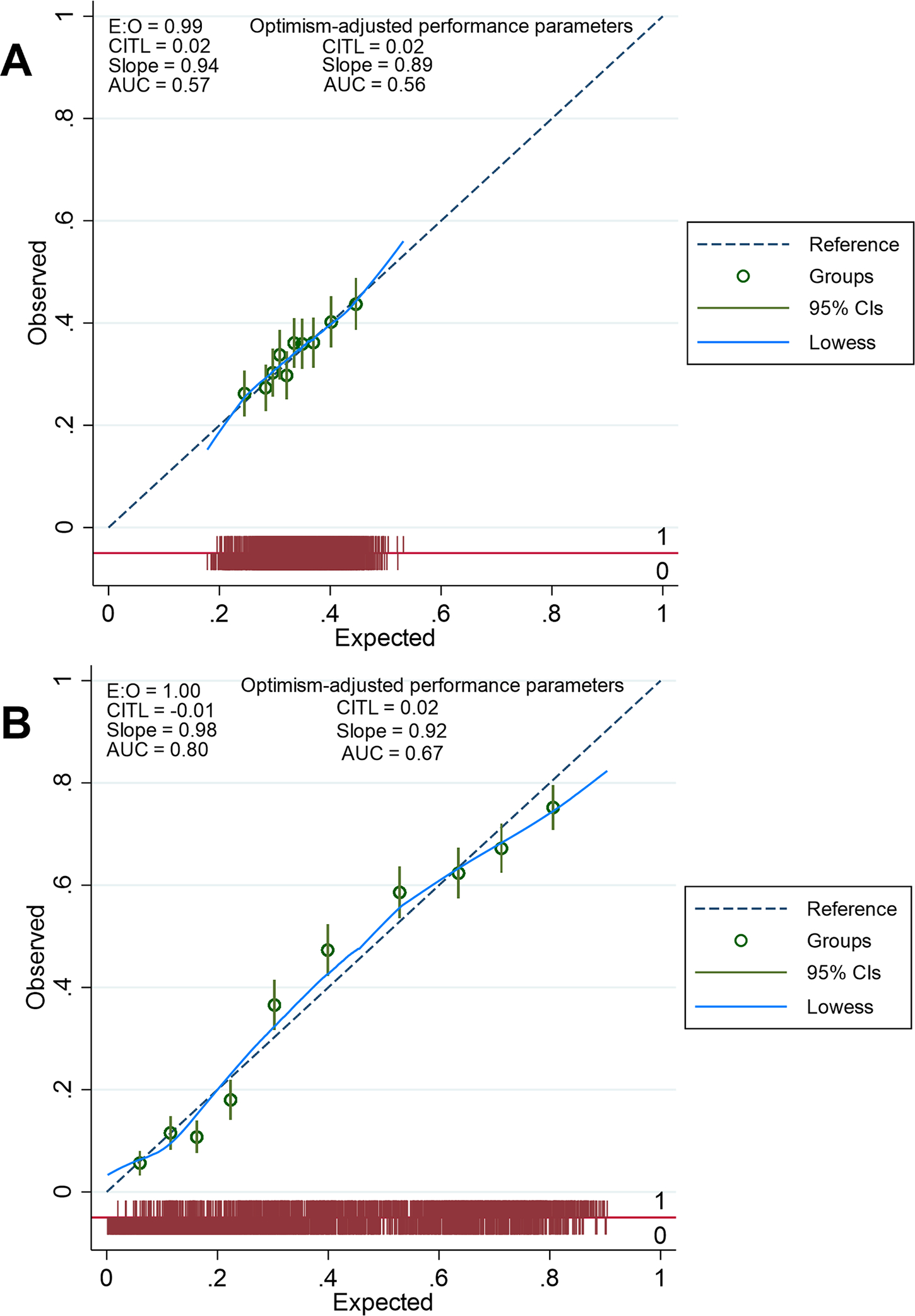
E:O = ratio of expected and observed probabilities
CITL = Calibration-in-the-large indicates whether predictions are systematically too low (CITL>0) or systematically too high (CITL<0).
AUC = Area under the receiver operating characteristic curve
The 45° reference line represents the line of perfect agreement between the model and the data (equality of observed and predicted probabilities). The groups are created using deciles of risk as cut points (10 risks groups). The Locally Weighted Scatterplot Smoothing (lowess) is the smoothed calibration line across individuals displayed on the bar graph at the bottom of each plot.
Optimism-adjusted performance parameters are obtained after applying a bootstrap shrinkage factor derived from an internal validation process with 100 bootstrap samples.
A: Calibration plot for the logistic regression model to predict plaque vulnerability
B: Calibration plot for the logistic regression model to predict plaque progression
In the sensitivity analysis, the E-value was 1.71 suggesting that, to explain away the observed OR of 1.22, an unmeasured confounder would need to be associated with both log IL-6 and plaque vulnerability with an OR of at least 1.71 each, above and beyond the measured confounders (Supplementary Figure S9–A). The results of the logistic regression after excluding patients with history of cardiovascular disease or after using LDL-cholesterol and statin therapy as independent variables are provided in Supplementary Tables S7 and S8).
Relationship of plasma IL-6 with plaque progression at 5 years
In the multivariable logistic regression (Table 3), log IL-6 was independently associated with plaque progression at 5 years (OR = 1.44, 95% CI: 1.23–1.69, p=9.1 × 10−6). There was no interaction between log IL-6 and ipsilateral baseline plaque severity score (OR for the interaction term = 0.92, p=0.39) or ipsilateral baseline plaque vulnerability (OR for the interaction term = 1.01, p=0.95). There was a 24% increase in the probability of carotid plaque progression per standard deviation increase in log IL-6 (Table 3 and Figure 3), thus making log IL-6 the second most important contributor to plaque progression after dyslipidemia. The probability of carotid plaque progression decreased by 73% per standard deviation increase in baseline ipsilateral plaque severity score (Table 3). Progression was less often reported for carotid plaques with high-risk features at baseline (OR = 0.77, 95% CI: 0.61 – 0.97, Table 3). The logistic regression model displayed good calibration (Figure 4B). The association of log IL-6 with plaque progression remained significant after adjustment for optimism (OR = 1.34, 95% CI: 1.18–1.53, Table 3).
Table 3:
Multivariable logistic regression model for the association of IL-6 with carotid plaque progression at 5 years
| Independent variables | OR1 (95% CI) * | p-value | OR2† | OR3 (95% CI) ‡ |
|---|---|---|---|---|
| Log IL-6 | 1.44 (1.23 – 1.69) | 9.1 × 10−6 | 1.24 | 1.34 (1.18 – 1.53) |
| Current smoker | 1.64 (1.20 – 2.25) | 1.6 × 10−3 | 1.16 | 1.50 (1.16 – 1.93) |
| Dyslipidemia | 2.32 (1.64 – 3.30) | 2.3 × 10−6 | 1.26 | 1.98 (1.50 – 2.63) |
| Diabetes mellitus | 1.46 (1.09 – 1.95) | 1.1 × 10−2 | 1.13 | 1.36 (1.07 – 1.72) |
| Hypertension | 1.37 (1.12 – 1.66) | 1.5 × 10−3 | 1.17 | 1.29 (1.10 – 1.51) |
| Coronary heart disease | 1.37 (1.03 – 1.83) | 3.0 × 10−2 | 1.12 | 1.29 (1.03 – 1.63) |
| Male | 1.28 (1.05 – 1.56) | 1.3 × 10−2 | 1.13 | 1.22 (1.04 – 1.43) |
| Age (years) | 1.03 (1.01 – 1.05) | 6.4 × 10−3 | 1.14 | 1.02 (1.01 – 1.04) |
| Vulnerability at baseline (ipsilateral) | 0.77 (0.61 – 0.97) | 2.0 × 10−2 | 0.89 | 0.81 (0.67 – 0.97) |
| Stenosis score at baseline (ipsilateral) | 0.24 (0.21 – 0.28) | 1.7 × 10−92 | 0.27 | 0.32 (0.28 – 0.35) |
| Intercept (baseline odds) | 0.08 (0.02 – 0.37) | 9.8 × 10−4 | NA | 0.52 (0.13–2.41) |
IL-6: interleukin-6; NA: not applicable.
Non-standardized odds ratio (linked to change in odds per 1 unit increase)
Standardized odds ratio (linked to change in odds per 1 standard deviation increase)
Optimism-adjusted odds ratio using the heuristic shrinkage method.
The first interaction term (Log IL-6 # ipsilateral vulnerability at baseline, p = 0.99), atrial fibrillation, treatment with anti-inflammatory drugs, race, history of stroke or transient ischemic attack, alcohol consumption, the second interaction term (Log IL-6 # ipsilateral baseline stenosis score), body mass index, log C-reactive protein, treatment with antiplatelet drugs, peripheral artery disease, hyperuricemia, and cystatin-based glomerular filtration rate (p=0.06) were consecutively removed from the model automatically due to coefficients with p-value >0.05.
Likelihood ratio chi-squared test for significance of the model: χ2 = 712.2, df = 10, p = 1.5 × 10−146. Area under the Receiver Operating Characteristic curve = 0.80. Count R2 = 73%. Proportion of patients correctly classified = 73.4%. Maximum Cook distance = 0.07. Maximum variance inflation factor = 1.15.
The p values for significance of the odds ratios were determined by a Wald test.
In the sensitivity analysis, the E-value was 2.24 suggesting that, to explain away the observed OR of 1.44, an unmeasured confounder would need to be associated with both log IL-6 and plaque progression with an OR of at least 2.24 each, above and beyond the measured confounders (supplementary Figure S9–B). The results of the logistic regression after excluding patients with history of cardiovascular disease or after using LDL-cholesterol and statin therapy as independent variables are provided in Supplementary Tables S9 and S10.
Selection of a clinical threshold for plasma IL-6 levels
In participants with a >50% predicted probability of plaque progression based on the optimism-adjusted multivariable logistic regression model (Table 3), the median (IQR) level of plasma IL-6 was 1. 63 (1.12 – 2.46) pg/mL and the mean (95% CI) of log IL-6 was 0.54 (0.52 – 0.56), corresponding to a plasma IL-6 level of 1.72 pg/mL. Therefore, the threshold of 2.0 pg/mL was used to define high plasma IL-6 levels. There were 1584 (36.6%) participants with high plasma IL-6 at baseline. High IL-6 levels at baseline where significantly associated with all cardiovascular risk factors (Supplementary Table S11). At baseline, there was a higher prevalence of severe stenosis (1.3% versus 0.4%, p=6 × 10−4) and vulnerable plaques (32.0% versus 27.6%, p=2.3 × 10−3) in participants with high IL-6 levels (Supplementary Table S11). The rate of plaque progression at 5 years was similar in patients with high or low IL-6 levels at baseline (34.6% versus 33.7%, p=0.54). The prevalence of treatment with anti-inflammatory drugs was similar in participants with high versus low IL-6 levels at baseline (Supplementary Table S11).
In the multivariable logistic regression analysis, high IL-6 levels at baseline were independently associated with the presence of vulnerable carotid plaque (OR = 1.21, 95% CI: 1.02–1.45, p=0.03, E-value = 1.71, Supplementary Table S12) and with carotid plaque progression at 5 years (OR = 1.25, 95% CI: 1.01–1.57, p=0.04, E-value = 1.81, Supplementary Table S13). Dichotomization did not significantly affect the coefficients, performance, calibration, discrimination, and stability of the logistic regression models (Supplementary Tables S12 and S13). The diagnostic accuracy of the multivariable logistic regression model for identification of patients with plaque progression at 5 years was better than that of IL-6 used as a standalone biomarker with the 2 pg/mL threshold (Supplementary Table S14).
DISCUSSION
This study shows that circulating IL-6 is an independent predictor of carotid plaque severity, vulnerability, and progression. The standardized odds ratios for log IL-6 in the multivariable logistic regression models show that inflammation is a major contributor to the risk of plaque vulnerability and progression. This observation has three important implications. First, achieving an optimal level of plasma cholesterol and controlling existing cardiovascular risk factors might not be sufficient to suppress the risk of stroke associated with carotid atherosclerosis in the absence of a treatment specifically targeting inflammation. This requires further investigation in trials where information on the control status of various cardiovascular risk factors, and not just the prescription of drugs, is properly recorded and factored into the interpretation of trial results. Second, carotid plaque imaging biomarkers could represent valid surrogate endpoints in trials of anti-IL-6 drugs for stroke prevention. Third, it is important to accelerate the integration of IL-6 assays in routine clinical practice by defining and validating a cut-off to improve risk stratification in patients with carotid atherosclerosis and identify patients who could benefit from anti-IL6 drugs in addition to current best medical therapy.
In this regard, we propose a non-arbitrary risk-informed threshold allowing the dichotomization of IL-6 levels and their use in prediction models or scores without log transformation and without loss of predictive performance. If validated, this threshold could be used to assess residual inflammatory risk as is the case with CRP. IL-6 is targeted by several specific compounds already approved or under development,13 and has stronger genetic, experimental, clinical, and epidemiological evidence for a causal relationship to atherosclerotic cardiovascular disease than CRP.12 Moreover, preliminary evidence suggest that specific anti-IL-6 drugs like Ziltivekimab have a better safety profile than other anti-inflammatory drugs with respect to myelosuppression (increased risk of infections and bleeding), dyslipidemia, and toxicity to the liver and the kidney.11 The renal toxicity of colchicine limits its prescription to patients with chronic kidney disease who derive a greater absolute cardiovascular benefit from anti-inflammatory drugs than those with normal renal function.11,33 Anti-IL-6 drugs would be of particular interest in patients with carotid plaques at perceived higher risk of stroke who are not eligible for surgical revascularization. This includes patients with multiple comorbidities and patients with mild or moderate carotid stenosis, especially if high-risk features are present.15,25
IL-6 is a soluble proinflammatory cytokine secreted by activated monocytes, macrophages, endothelial cells, adipocytes, fibroblasts, T helper 2 cells, typically after stimulation by interleukin-1 or tumor necrosis factor.12,34,35 In the classical signaling pathway, IL-6 binds to membrane-bound IL-6 receptors on hepatocytes and stimulates the production of acute phase reactants such as fibrinogen, plasminogen activator inhibitor that inhibits fibrinolysis, and CRP. In the alternate signaling pathway, the alpha subunit of the transmembrane IL-6 receptor is cleaved by a disintegrin and metalloproteinase with thrombospondin motifs 17 (ADAMTS17) and forms a complex with IL-6. This complex can bind the gp130 protein on other cell types such as endothelial and smooth muscle cells to promote atherogenesis.12,34 This dual pro-atherogenic and pro-thrombotic effects of IL-6 likely explain why higher plasma concentrations are found in patients with severe carotid stenosis, carotid occlusion and in patients with vulnerable or progressive carotid atherosclerotic lesions. Surprisingly, in our study, the univariable analysis did not find significantly higher levels of IL-6 in patients diagnosed with plaque progression at 5 years. This is explained by the fact that plaques causing a higher grade of stenosis are less likely to progress.36 Indeed, we report a 73% decrease in the probability of plaque progression per standard deviation increase in the ipsilateral baseline stenosis severity score. After adjusting for the ipsilateral baseline grade of stenosis, the significant association between IL-6 and carotid plaque progression became apparent. The inverse relationship between plaque vulnerability and plaque progression is another intriguing finding that warrants further investigations. It suggests that vulnerable plaques are less likely to progress because of a higher risk of rupture that causes cerebrovascular events and potentially justifies surgery. However, this hypothesis could not be tested in the absence of information on carotid procedures during follow-up.
The association of IL-6 with all cardiovascular risk factors reported in this study suggests that cardiovascular risk factors exert their pro-atherothrombotic effect at least in part by promoting IL-6-mediated arterial inflammation and remodeling. For instance, previous studies have shown that lowering blood pressure decreases circulating IL-6 levels37 and that IL-6 secretion is induced by hyperglycemia, hyperuricemia, and smoking.38–40 Our findings also suggest that the relationship of IL-6 with stroke risk reported in previous observational studies41,42 might be driven by the formation, progression, and destabilization of carotid atherosclerotic plaques. This hypothesis warrants further investigation in population-based cohorts. Furthermore, the conflicting results regarding stroke prevention using various anti-inflammatory drugs in recent trials3,43,44 support the need for more granular subgroup analyses by stroke subtypes in future trials. It is possible that drugs targeting the NLRP3-interleukin 1-interleukin 6-CRP signaling pathway are more efficient for the prevention of large artery atherosclerosis-related stroke than other stroke subtypes.
Altogether, this study provides new pieces of evidence to suggest a causal relationship between circulating IL-6 levels and carotid plaque severity, vulnerability, and progression according to Bradford-Hill criteria.45 It shows that the association between circulating IL-6 levels and carotid plaque vulnerability and progression is strong (criterion 1) given the 12% and 24% increase in the odds per standard deviation increase in log IL-6. The association is also specific (criterion 3) and displays a linear dose-response gradient (criterion 5). Indeed, the association is independent of all known risk factors of atherosclerosis even after restricting the analysis to participants without history of cardiovascular disease and the association is unlikely to be offset by an unobserved confounder. When considering the computed E-values, the existence of an unmeasured confounder that would have a stronger association with plaque vulnerability and plaque progression than dyslipidemia seems unlikely. Additionally, the multivariable logistic regression model using high plasma IL-6 levels to predict plaque progression correctly classifies >70% of the participants. The other causality criteria could be inferred from previous publications, notably consistency (criterion 2),46–49 temporality (criterion 4),14,46 plausibility and coherence (criteria 6 and 7).12 Furthermore, experimental studies in humans and animals have shown that inhibition of the IL-6 signaling pathway decreases the progression of systemic atherosclerotic disease and prevents cardiovascular events (criterion 8) 4,5,50. Therefore, by analogy (criterion 9), one could hypothesize that the same would be true when looking specifically at carotid plaques in humans. Further experimental evidence could come from the Colchicine for Prevention of Vascular Inflammation in Non-cardio Embolic Stroke trial (CONVINCE, NCT02898610) and the Research Study to Look at How Ziltivekimab Works Compared to Placebo in People with Cardiovascular Disease, Chronic Kidney Disease, and Inflammation (ZEUS, NCT05021835).
Limitations
Our study has some limitations. First, despite the large sample size, the study population was restricted to elderly patients, which means that our results may not be generalizable to the entire population. However, patients aged >65 years old are likely the most relevant subgroup for the study of predictors of carotid atherosclerosis across the spectrum of stenosis severity given the relatively low prevalence of moderate and severe stenosis in younger adults.51 Second, we could not incorporate information on whether participants underwent carotid revascularization procedures between baseline and 5-year follow-up ultrasound examinations. This might have led to underestimation of the number of participants with plaque progression and reduced statistical power for the multivariable logistic regression analysis. Nevertheless, we believe the number of participants undergoing surgical revascularization would only represent a small percentage of the study population comprised mostly of healthy and asymptomatic individuals. Furthermore, the procedures performed to adjust for optimism, especially bootstrapping, suggest that our results would not be affected by fluctuations in the number of patients diagnosed with plaque progression at 5 years. The absence of information on carotid procedures during follow-up also precluded a reliable investigation of the relationship between circulating IL-6 levels and plaque regression. Third, it was not possible to incorporate data on the occurrence of cerebrovascular events during follow-up in this study to also investigate the relationship of circulating IL-6 with stroke risk and test the hypothesis that the risk is mediated through carotid atherosclerosis. Nevertheless, the association between circulating IL-6 levels and the risk of stroke is already well-established from previous population-based studies.41,42 Fourth, our analyses were not adjusted for the concurrent presence of conditions that could affect IL-6 levels either at baseline or during follow-up. However, patients with cancer were excluded from the CHS and all multivariable models were adjusted for CRP levels and treatment with anti-inflammatory drugs that are surrogate indicators of conditions such as infection, cancer, or auto-immune diseases that modulate IL-6 secretion. Moreover, we computed E-values to demonstrate that unmeasured confounders are likely not able to offset the reported associations. Fifth, our model for plaque progression at 5-years is based on a single measurement of IL-6 levels at baseline which may not reflect fluctuations of IL-6 levels during follow-up. Nevertheless, available evidence suggest that IL-6 levels are largely genetically determined, and the population variance of IL-6 might be explained mostly by inter-personal rather than intra-personal fluctuations.7,52 Last, the proportion of patients treated with statins in the CHS was low. Given that statins also have anti-inflammatory properties, further studies are needed to determine their effect on the relationship between circulating IL-6 levels and carotid plaque features.
Conclusion
This study provides new evidence for a causal relationship between circulating levels of IL-6 and three key features of high-risk carotid atherosclerosis: severity of stenosis, baseline plaque vulnerability, and long-term plaque progression. It also defines a threshold of 2.0 pg/mL to identify individuals with a higher probability of plaque vulnerability and progression. This threshold could be used to select patients who might derive greater stroke prevention benefits from anti-IL-6 drugs in future studies.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is known?
Interleukin-6 (IL-6) has important roles in atherosclerosis pathophysiology as demonstrated by clinical, experimental, and genetic studies but its relationship with carotid plaque severity and vulnerability has never been established.
Results of the Canakinumab Antiinflammatory Thrombosis Outcome Study suggest that the atheroprotective effect of anti-inflammatory drugs is mediated by the reduction of IL-6 levels.
Several trials have demonstrated the safety and efficacy of anti-cytokine drugs for cardiovascular prevention but their relevance for the specific prevention of carotid atherosclerosis-related stroke remains unclear and there is no validated cut-off to facilitate the use of IL-6 as a biomarker for clinical decision making.
What new information does this article contribute?
There is an independent linear relationship of circulating interleukin-6 with carotid plaque severity, vulnerability, and progression.
Inflammation is a major contributor to the risk of plaque progression independent of dyslipidemia and other cardiovascular risk factors.
The non-arbitrary risk-informed cut-off (2.0 pg/mL) allows the dichotomization of circulating IL-6 levels and their use in clinical prediction models without log transformation and without loss of predictive performance.
While IL-6 is an established independent predictor of stroke, current stroke prevention strategies focus on controlling cardiovascular risk factors and reducing circulating levels of cholesterol. The relevance of anti-cytokine therapies for the prevention of carotid atherosclerosis-related stroke remains unclear. To determine if anti-IL-6 drugs could be utilized in stroke prevention in patients with carotid atherosclerosis, the relationship between IL-6 levels and high-risk plaque features associated with stroke risk were examined. This is the first large population-based study that concurrently demonstrates the independent linear relationship of baseline IL-6 levels with carotid plaque severity, vulnerability, and progression and identifies a specific cut-off value.
Acknowledgments
The authors are grateful to the University of Alberta Research Services Office (Canada) for reviewing and approving the data transfer agreements.
Sources of funding
There was no targeted funding for this study.
The Cardiovascular Health Study (CHS) was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC45133, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85085, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). Further contract details are available at https://chs-nhlbi.org/pubs/PubAcknowGuidelines. A full list of principal CHS investigators and institutions can be found at https://chs-nhlbi.org/pi.
ABBREVIATIONS AND ACRONYMS
- ADAMTS 17
disintegrin and metalloproteinase with thrombospondin motifs 17
- AUC
Area under the receiver operating characteristic curve
- CANTOS
Canakinumab Antiinflammatory Thrombosis Outcome Study
- CHS
Cardiovascular Health Study
- CITL
calibration-in-the-large index
- CRP
C-reactive protein
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- gp130
glycoprotein 130
- HDL-C
high-density lipoprotein cholesterol
- IL-6
interleukin-6
- LDL-C
low-density lipoprotein cholesterol
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- NLRP3
nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3
- TIA
transient ischemic attack
Footnotes
Disclosures
Joseph Kamtchum-Tatuene was funded by the Faculty of Graduate Studies and Research Doctoral Recruitment Scholarship, the Bank of Montreal Graduate Scholarship, the Alberta Graduate Excellence Scholarship, the Faculty of Medicine and Dentistry Motyl Graduate Studentship in Cardiac Sciences, the Alberta Innovates Graduate Scholarship, the Department of Medicine Ballermann Translational Research Fellowship, the Izaak Walton Killam Memorial Scholarship, and the Andrew Stewart Memorial Graduate Prize. All awards, prizes, and scholarships managed by the University of Alberta.
Luca Saba reports no conflicts of interest.
Mirjam R. Heldner reports grants from the Swiss Heart Foundation and the Bangerter Foundation, and Advisory Board participation for Amgen.
Michiel H. F. Poorthuis reports no conflicts of interest.
Gert J. de Borst reports no conflicts of interest.
Tatjana Rundek is funded by grants from the National Institutes of Health (R01 NS040807, R01 NS029993, R01 MD012467, RF1AG074306, U24 NS107267, P30 AG066506, HHSN268200625234C, U19 AG056169), the National Center for Advancing Translational Sciences (UL1 TR002736, KL2 TR002737), and the Florida Department of Health.
Stavros K. Kakkos reports no conflicts of interest.
Seemant Chaturvedi serves on the Executive Committee of the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2, NCT02089217).
Raffi Topakian has received fees for serving as Advisory Board Member (Alexion, Roche, Sanofi-Aventis) and conference support from the industry (Daiichi-Sankyo, Pfizer, Roche), all within the last three years.
Joseph F. Polak reports no conflicts of interest.
Glen C. Jickling receives research grant support from Canadian Institutes of Health Research (CIHR), Heart and Stroke Foundation, Alberta University Hospital Foundation, Canada Foundation for Innovation (CFI), and National Institutes of Health (NIH).
SUPPLEMENTAL MATERIALS
REFERENCES
- 1.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgozoglu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, MacFadyen JG, Thuren T, Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1beta inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J. 2020;41:2153–2163. [DOI] [PubMed] [Google Scholar]
- 6.Georgakis MK, Malik R, Gill D, Franceschini N, Sudlow CLM, Dichgans M, Invent Consortium CIWG. Interleukin-6 Signaling Effects on Ischemic Stroke and Other Cardiovascular Outcomes: A Mendelian Randomization Study. Circ Genom Precis Med. 2020;13:e002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgakis MK, Malik R, Li X, Gill D, Levin MG, Vy HMT, Judy R, Ritchie M, Verma SS, Regeneron Genetics C, et al. Genetically Downregulated Interleukin-6 Signaling Is Associated With a Favorable Cardiometabolic Profile: A Phenome-Wide Association Study. Circulation. 2021;143:1177–1180. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, Guo Y, Chung C, Peasey A, Pfister R, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai T, Zhang Y, Ho YL, Link N, Sun J, Huang J, Cai TA, Damrauer S, Ahuja Y, Honerlaw J, et al. Association of Interleukin 6 Receptor Variant With Cardiovascular Disease Effects of Interleukin 6 Receptor Blocking Therapy: A Phenome-Wide Association Study. JAMA Cardiol. 2018;3:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin MG, Klarin D, Georgakis MK, Lynch J, Liao KP, Voight BF, O’Donnell CJ, Chang KM, Assimes TL, Tsao PS, et al. A Missense Variant in the IL-6 Receptor and Protection From Peripheral Artery Disease. Circ Res. 2021;129:968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, Lo L, Kling D, Pergola P, Raj D, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397:2060–2069. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Rane M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ Res. 2021;128:1728–1746. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltoft A, Arntzen KA, Wilsgaard T, Mathiesen EB, Johnsen SH. Interleukin-6 is an independent predictor of progressive atherosclerosis in the carotid artery: The Tromso Study. Atherosclerosis. 2018;271:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Kamtchum-Tatuene J, Noubiap JJ, Wilman AH, Saqqur M, Shuaib A, Jickling GC. Prevalence of High-Risk Plaques and Risk of Stroke in Patients With Asymptomatic Carotid Stenosis: A Meta-analysis. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. [DOI] [PubMed] [Google Scholar]
- 20.Jenny NS, Tracy RP, Ogg MS, Luong le A, Kuller LH, Arnold AM, Sharrett AR, Humphries SE. In the elderly, interleukin-6 plasma levels and the −174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–2071. [DOI] [PubMed] [Google Scholar]
- 21.Polak JF, O’Leary DH, Kronmal RA, Wolfson SK, Bond MG, Tracy RP, Gardin JM, Kittner SJ, Price TR, Savage PJ. Sonographic evaluation of carotid artery atherosclerosis in the elderly: relationship of disease severity to stroke and transient ischemic attack. Radiology. 1993;188:363–370. [DOI] [PubMed] [Google Scholar]
- 22.Polak JF, Shemanski L, O’Leary DH, Lefkowitz D, Price TR, Savage PJ, Brant WE, Reid C. Hypoechoic plaque at US of the carotid artery: an independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology. 1998;208:649–654. [DOI] [PubMed] [Google Scholar]
- 23.Saba L, Saam T, Jager HR, Yuan C, Hatsukami TS, Saloner D, Wasserman BA, Bonati LH, Wintermark M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559–572. [DOI] [PubMed] [Google Scholar]
- 24.Poorthuis MHF, Sherliker P, Morris DR, Massa MS, Clarke R, Staplin N, Lewington S, de Borst GJ, Bulbulia R, Halliday A. Development and Internal Validation of a Risk Score to Detect Asymptomatic Carotid Stenosis. Eur J Vasc Endovasc Surg. 2021;61:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamtchum-Tatuene J, Nomani AZ, Falcione S, Munsterman D, Sykes G, Joy T, Spronk E, Vargas MI, Jickling GC. Non-stenotic Carotid Plaques in Embolic Stroke of Unknown Source. Front Neurol. 2021;12:719329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA. 2019;321:602–603. [DOI] [PubMed] [Google Scholar]
- 27.Van Houwelingen JC, Le Cessie S. Predictive value of statistical models. Stat Med. 1990;9:1303–1325. [DOI] [PubMed] [Google Scholar]
- 28.Steyerberg EW, Harrell FE Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 29.Cleves MA. From the help desk: Comparing areas under receiver operating characteristic curves from two or more probit or logit models. Stata J. 2002;2:301–313. [Google Scholar]
- 30.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 31.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH. Inhibition of Interleukin-1beta by Canakinumab and Cardiovascular Outcomes in Patients With Chronic Kidney Disease. J Am Coll Cardiol. 2018;71:2405–2414. [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Rocha VZ. All roads lead to IL-6: A central hub of cardiometabolic signaling. Int J Cardiol. 2018;259:213–215. [DOI] [PubMed] [Google Scholar]
- 35.Loppnow H, Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990;85:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakkos SK, Nicolaides AN, Charalambous I, Thomas D, Giannopoulos A, Naylor AR, Geroulakos G, Abbott AL, Asymptomatic Carotid S, Risk of Stroke Study G. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg. 2014;59:956–967 e951. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez-Oliva G, Fernandez-Real JM, Zamora A, Vilaseca M, Badimon L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Hum Hypertens. 2005;19:457–462. [DOI] [PubMed] [Google Scholar]
- 38.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. [DOI] [PubMed] [Google Scholar]
- 39.Lyngdoh T, Marques-Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, Vollenweider P. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6:e19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunyer J, Forastiere F, Pekkanen J, Plana E, Kolz M, Pistelli R, Jacquemin B, Bruske-Hohlfeld I, Pitsavos C, Bellander T, et al. Interaction between smoking and the interleukin-6 gene affects systemic levels of inflammatory biomarkers. Nicotine Tob Res. 2009;11:1347–1353. [DOI] [PubMed] [Google Scholar]
- 41.Jenny NS, Callas PW, Judd SE, McClure LA, Kissela B, Zakai NA, Cushman M. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology. 2019;92:e2375–e2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadopoulos A, Palaiopanos K, Bjorkbacka H, Peters A, de Lemos JA, Seshadri S, Dichgans M, Georgakis MK. Circulating Interleukin-6 Levels and Incident Ischemic Stroke: A Systematic Review and Meta-analysis of Prospective Studies. Neurology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838–1847. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okazaki S, Sakaguchi M, Miwa K, Furukado S, Yamagami H, Yagita Y, Mochizuki H, Kitagawa K. Association of interleukin-6 with the progression of carotid atherosclerosis: a 9-year follow-up study. Stroke. 2014;45:2924–2929. [DOI] [PubMed] [Google Scholar]
- 47.Puz P, Lasek-Bal A. Repeated measurements of serum concentrations of TNF-alpha, interleukin-6 and interleukin-10 in the evaluation of internal carotid artery stenosis progression. Atherosclerosis. 2017;263:97–103. [DOI] [PubMed] [Google Scholar]
- 48.Puz P, Lasek-Bal A, Ziaja D, Kazibutowska Z, Ziaja K. Inflammatory markers in patients with internal carotid artery stenosis. Arch Med Sci. 2013;9:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thakore AH, Guo CY, Larson MG, Corey D, Wang TJ, Vasan RS, D’Agostino RB Sr., Lipinska I, Keaney JF Jr., Benjamin EJ, et al. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study). Am J Cardiol. 2007;99:1598–1602. [DOI] [PubMed] [Google Scholar]
- 50.Akita K, Isoda K, Sato-Okabayashi Y, Kadoguchi T, Kitamura K, Ohtomo F, Shimada K, Daida H. An Interleukin-6 Receptor Antibody Suppresses Atherosclerosis in Atherogenic Mice. Front Cardiovasc Med. 2017;4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O’Leary DH, Rosvall M, Sitzer M, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41:1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worns MA, Victor A, Galle PR, Hohler T. Genetic and environmental contributions to plasma C-reactive protein and interleukin-6 levels--a study in twins. Genes Immun. 2006;7:600–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All requests to access the CHS dataset should be addressed directly to the National Heart Lung, and Blood Institute via the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC, https://biolincc.nhlbi.nih.gov/home/). For reproducibility purposes, the file containing all the commands used for data curation and analysis are available from the corresponding author upon reasonable request. The list of variables extracted to conduct this study is provided in Supplementary Table S1.


