Abstract
Stress-induced condensation of mRNA and protein into massive cytosolic clusters is conserved across eukaryotes. Known as stress granules when visible by imaging, these structures remarkably have no broadly accepted biological function, mechanism of formation or dispersal, or even molecular composition. As part of a larger surge of interest in biomolecular condensation, studies of stress granules and related RNA/protein condensates have increasingly probed the biochemical underpinnings of condensation. Here, we review open questions and recent advances, including the stages from initial condensate formation to accumulation in mature stress granules; mechanisms by which stress-induced condensates form and dissolve; and surprising twists in understanding the RNA components of stress granules and their role in condensation. We outline grand challenges in understanding stress-induced RNA condensation, centering on the unique and substantial barriers in the molecular study of cellular structures, such as stress granules, for which no biological function has been firmly established.
Graphical Abstract
eTOC blurb
Stress granules (SGs), canonical membraneless organelles, have seen a surge of activity in identifying their protein/RNA components and biophysical underpinnings. Glauninger et al. review our understanding of SGs and their biomolecular condensate precursors, emphasizing conceptual and methodological challenges arising from the lack of established biological functions for these conserved structures.
From humans and other vertebrates to single-celled yeasts, from plants to protozoa, the onset of primordial stresses such as heat shock, oxidizing agents, hypoxia, and starvation is rapidly followed by the intracellular condensation and accumulation of myriad proteins and mRNAs in cytosolic clusters (Cherkasov et al., 2013; Decker and Parker, 2012; Farny et al., 2009; Jain et al., 2016; Kedersha et al., 2000, 1999; Kramer et al., 2008; Nover et al., 1989; Wallace et al., 2015). These enigmatic structures, called stress granules when they grow large enough to resolve by microscopy, have become standard examples of so-called membraneless organelles alongside nucleoli, processing (P) bodies, paraspeckles, and others (Alberti and Carra, 2018; Boeynaems et al., 2018; Brangwynne, 2013; Gomes and Shorter, 2019; Guo and Shorter, 2015; Lyon et al., 2020; Mitrea and Kriwacki, 2016). Stress granules and their condensed molecular precursors have become a nexus of extraordinary recent activity because of the involvement of protein and RNA liquid-liquid phase separation (LLPS) in their formation (Guillén-Boixet et al., 2020; Molliex et al., 2015; Riback et al., 2017; Sanders et al., 2020; Van Treeck et al., 2018; Wheeler et al., 2016; Yang et al., 2020) and hints that dysregulation of condensation and stress granule formation contribute to disease (Bosco et al., 2010; Patel et al., 2015).
Yet despite sustained and vigorous inquiry, a remarkable array of foundational questions remain unanswered. What do stress granules do, if anything? What are the functional consequences of condensation, and what functions do specific mechanisms of condensation, such as LLPS, carry out? (Throughout this review we explicitly intend “condensate” to be a catch-all term for membraneless clusters without any further stipulation as to their structure, process of formation, or adaptive significance (Box 1), largely following standard usage (Banani et al., 2017; Lyon et al., 2020).) What biological roles are played by molecular-level condensation events versus subsequent merging of these condensates into larger, microscopically visible structures? How do condensation and accumulation occur, and are these processes mediated mainly by intrinsic molecular forces or extrinsic cellular machinery such as cytoskeleton-associated motors? To what extent are stress-triggered condensation and stress granule accumulation processes and participants conserved over evolutionary time?
Box 1: What is a condensate?
Biomolecular condensates are membraneless clusters of biomolecules such as proteins and nucleic acids. Classic examples are nucleoli, stress granules, P bodies, and germline P granules, among many others.
“Biomolecular condensate” serves as an umbrella term for these structures which is agnostic as to their specific size, function, mechanism of formation, material state, or method of experimental study. The term arose, in part, due to the growing realization that more specific terms referring to mechanism (e.g., liquid-liquid phase separation [LLPS]), material state (e.g. droplet, hydrogel), or function (compartment, membraneless organelle) often implied more than is presently known.
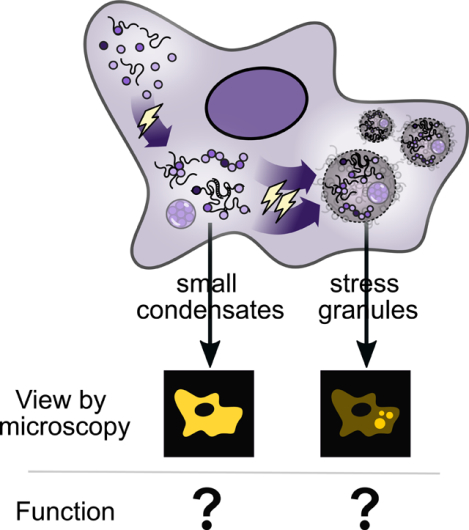
Importantly, many biomolecular condensates have been near-exclusively studied by specific methods. Stress granules, for example, are operationally defined by formation of foci resolvable by fluorescence microscopy that contain specific marker proteins and poly(A)+ RNA. Failure to detect microscopic foci is routinely taken to indicate the absence of stress granules, even though submicroscopic assemblies may be present. Rather than overturn this well-established operational definition, here we use the umbrella term condensates to refer to assemblies whether or not they are visible by microscopy. We use “accumulation” as a general term for processes in which smaller condensates are brought together to form larger structures.
Among the deepest challenges in studying stress granules is that, in the absence of molecular functions and cellular phenotypes, the phenomenon itself is operationally rather than biologically defined: a stress granule consists of anything which forms microscopically visible foci which colocalize with established stress granule markers (cf. Box 1). Although these structures have been hypothesized to play a variety of cellular roles, their function remains unclear (Buchan et al., 2011; Ivanov et al., 2019; Kedersha and Anderson, 2002, 2009; Kedersha et al., 2000). That stress granules are termed “membraneless organelles,” where the latter word explicitly means a cellular structure which performs distinct functions, has served to create the unfortunate impression that this fundamental question has been answered.
This question of function applies not only to stress granules, but also to the broader study of cytoplasmic ribonucleoprotein (RNP) foci including P-bodies, RNA transport granules, and P granules. In some cases, such as RNA transport granules in neurons, the question of function has been more directly addressed (Kiebler and Bassell, 2006; Pushpalatha and Besse, 2019). However, in many cases function is still presented as a model. P-bodies were long presumed to be sites of RNA degradation (Aizer et al., 2014; Franks and Lykke-Andersen, 2007; Sheth and Parker, 2003), but this model has been challenged (Eulalio et al. 2007; Hubstenberger et al. 2017). Additionally, work on G3BP1 aggregates in axons shows that condensates composed of canonical stress granule proteins may play a role under non stress conditions, introducing basal stress granule-like condensates (Sahoo et al., 2018, 2020). The questions and challenges regarding stress granules raised here apply to other biomolecular condensates, purported membraneless organelles, and contexts beyond cell stress.
As efforts to develop a parts list for stress granules (Buchan et al., 2011; Cherkasov et al., 2015; Jain et al., 2016; Wallace et al., 2015) have proceeded alongside attempts to recapitulate in vitro certain molecular events such as stress-reactive condensation and RNA recruitment (Begovich and Wilhelm, 2020; Iserman et al., 2020; Riback et al., 2017; Van Treeck et al., 2018), evidence has emerged for multiple quasi-independent contributing pathways, multiple molecular stages, and multiple levels of organization in stress granules and their precursors. This will serve as our jumping-off point. Given the multiple levels of molecular organization known to contribute to stress-induced RNA condensation, how do these levels interrelate, and at what level are adaptive features best understood?
Throughout this review, we intend a larger question to lurk in the reader’s mind. How can the characterization, interrogation, isolation, and reconstitution of stress-induced protein/RNA condensates and stress granules be effectively guided and evaluated in the absence of established functions, biological activities, or cellular phenotypes?
Multiple stages of stress-induced RNA condensation and stress granule formation
What is the relationship between protein/mRNA biomolecular condensation and stress granule formation? Although these processes are sometimes considered synonymous, and although how initial condensates accumulate in microscopically visible foci remains largely unknown, the existence of multiple stages in stress granule formation has long been understood (Figure 1). Existing models commonly reflect hierarchical organization in stress granules, with some stable components (“core”) surrounded by more dynamic components (“shell”) (Jain et al., 2016; Wheeler et al., 2016), or nanoscopic “seeds” interacting and merging to form stress granules (Padrón et al., 2019; Panas et al., 2016).
Figure 1: Stress-triggered protein/mRNA condensation and stress granule formation occur in stages, depend on stress intensity and identity, and involve multiple types of molecular interactions.
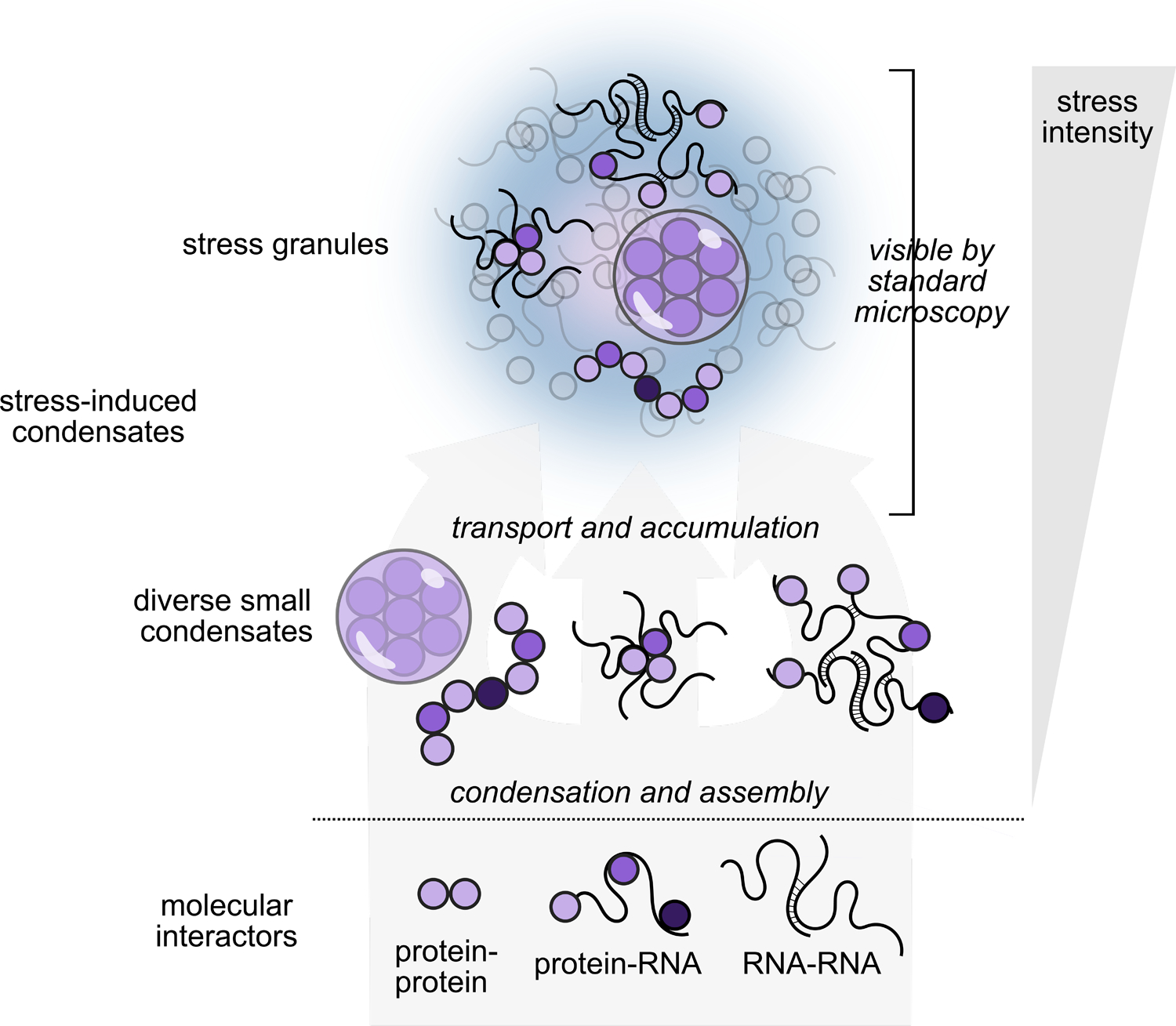
Severe stress causes the accumulation of diverse small condensates into stress granules observable as cytosolic foci by standard microscopy.
Evidence for these multiple stages comes from several independent sources. First, individual core markers for stress granules such as poly(A)-binding protein, G3BP, and Ded1 can be purified recombinantly and will autonomously condense in response to stress-associated physiological cues (e.g. heat shock, presence of long ribosome-free mRNA) in vitro (Guillén-Boixet et al., 2020; Iserman et al., 2020; Kroschwald et al., 2018; Riback et al., 2017; Yang et al., 2020). These in vitro results suggest that condensation in vivo may not depend on interactions between a large set of stress granule components, at least at initial stages.
Second, although formation of canonical microscopically visible stress granules can be blocked by translation elongation inhibitors (Kedersha et al., 2000; Nadezhdina et al., 2010; Namkoong et al., 2018; Wallace et al., 2015), the stress-triggered condensation, as measured by biochemical fractionation, of stress granule components such as poly(A)-binding protein proceeds virtually unaffected by such inhibition, indicating that accumulation of condensates into stress granules is a separate step (Wallace et al., 2015). This suggests that formation of canonical stress granules involves cell-biological transport processes which bring multiple components together in the cytosol (Panas et al., 2016). In support of this model, depolymerization of microtubules disrupts stress granule accumulation (Ivanov et al., 2003a, 2003b), and stress granules tether to the endoplasmic reticulum and lysosomes using specific factors for intracellular transport (Liao et al., 2019). Similarly, in contrast to in vitro ATP-independent condensation processes, ATP-driven mechanisms are required for stress granule formation in cells (Jain et al., 2016). Transport and accumulation of small condensates and other components is a separate process from the initial condensation events which also accompany stress.
Finally, the appearance of canonical stress granulesstress granules generally depends on stress intensity and duration, and in important cases, low levels of stress cause condensation of protein constituents but not their stress granule accumulation. For example, heat shock in budding yeast leads to biochemically detectable condensation of certain proteins after 8 minutes at 37 °C or 42 °C, and accumulation of certain proteins in cytosolic foci, but formation of classic stress granules marked by poly(A)-binding protein requires pushing temperatures to 44–46 °C at this timescale (Cherkasov et al., 2013; Wallace et al., 2015). Limitations of imaging techniques may contribute to this discrepancy to some degree (see our discussion of grand challenges below), and exciting developments of improved microscopy-based methods—such as lattice light-sheet microscopy or fluorescence cross-correlation spectroscopy—may help minimize these concerns in the future (Guillén-Boixet et al., 2020; Peng et al., 2020). But the differential accumulation of protein factors at different levels of stress intensity (Grousl et al., 2013) rules out simplistic notions that, for example, stress granules are merely small at first and grow larger with intensifying stress. More evidence for an ordered assembly of stress granules comes from time-resolved proximity labeling experiments, which identified the interactome of the stress granule component eIF4A1 during heat shock of HEK293 cells (Padrón et al., 2019). This study found that certain canonical stress granule components interacted with eIF4A1 before others. Thus, assembly proceeds in separable stages, ending with accumulation in large foci under severe stress.
The existence of assembly stages naturally raises the question: at what stages might specific functions be carried out? A deeper question haunting the field is: what do stress granules actually do?
Elusive functions of stress granules and stress-triggered RNA condensation
No commonly accepted function for stress granules yet exists. Many functions have been proposed, implicating stress granules in a range of roles, including sequestration of mRNAs and proteins; protection of mRNAs and proteins from degradation; promotion of enzymatic activities by increasing local concentration; minimization of cellular energy expenditure; and acting in translational quality control, signaling, and cargo delivery (Aronov et al., 2015; Buchan and Parker, 2009; Escalante and Gasch, 2021; Ivanov et al., 2019; Kedersha and Anderson, 2002; Kedersha et al., 2013; Mahboubi and Stochaj, 2017; Moon et al., 2020). Stress granules have also been implicated in suppressing cell death by sequestering pro-apoptotic factors such as receptor of activated C kinase 1 (RACK1) (Arimoto et al., 2008; Tsai and Wei, 2010). Similarly, a recent study found that stress granule formation suppressed pyroptosis, a form of cell death associated with inflammation, by sequestering the protein DEAD-box helicase 3 X-linked (DDX3X) (Samir et al., 2019). However, the large variety of functions proposed for stress granules, combined with some conflicting findings, have made it difficult to form an overarching model of stress granule function (Mateju and Chao, 2021).
For instance, an oft-speculated function for RNA condensation is transiently protecting transcripts from degradation during stress (Hubstenberger et al., 2017; Moon et al., 2019; Sorenson and Bailey-Serres, 2014), yet other work finds no effect on mRNA half-life following stress granuleinhibition (Bley et al., 2015). Another model holds that RNA condensation contributes to selective translation of non-condensed transcripts. Stress-induced transcripts are often translated in the midst of global translational shutoff. Some transcripts that are highly translated during stress, such as HSP70 and HSP90, do not associate with stress granules, suggesting a connection between translation and escaping condensation (Kedersha and Anderson, 2002; Stöhr et al., 2006; Zid and O’Shea, 2014). Certain translation initiation factors also condense, raising the possibility that a combination of protein and RNA sequestration can help promote selective translation during stress (Iserman et al., 2020; Wallace et al., 2015). However, stress granules are not required for global translational shutoff, so this selective translation would occur on top of a more dominant effect (Escalante and Gasch, 2021). Additionally, translation has been observed inside stress granules, complicating this model (Mateju et al., 2020).
A potential resolution to these conflicting results may be that particular functions are carried out at specific stages of organization. For example, stabilization of RNA by sequestration can conceivably occur at the pre-microscopic condensate level whereas other proposed functions may require collection of components into a larger and more molecularly diverse body (Figure 2). Hypothetically, a study in which perturbations block stress granule accumulation but not initial condensation, with no effect on RNA stabilization, would reach different conclusions than a study in which perturbations block both processes. An expanded understanding of assembly stages, a deepened grasp of the molecular drivers of these stages, and a widened array of perturbations capable of targeting specific stages and molecular determinants will be needed to sort out these questions.
Figure 2: Formation of canonical stress granules (visible by standard microscopy, composed of a large number of components) may not be required for many attributed functions.
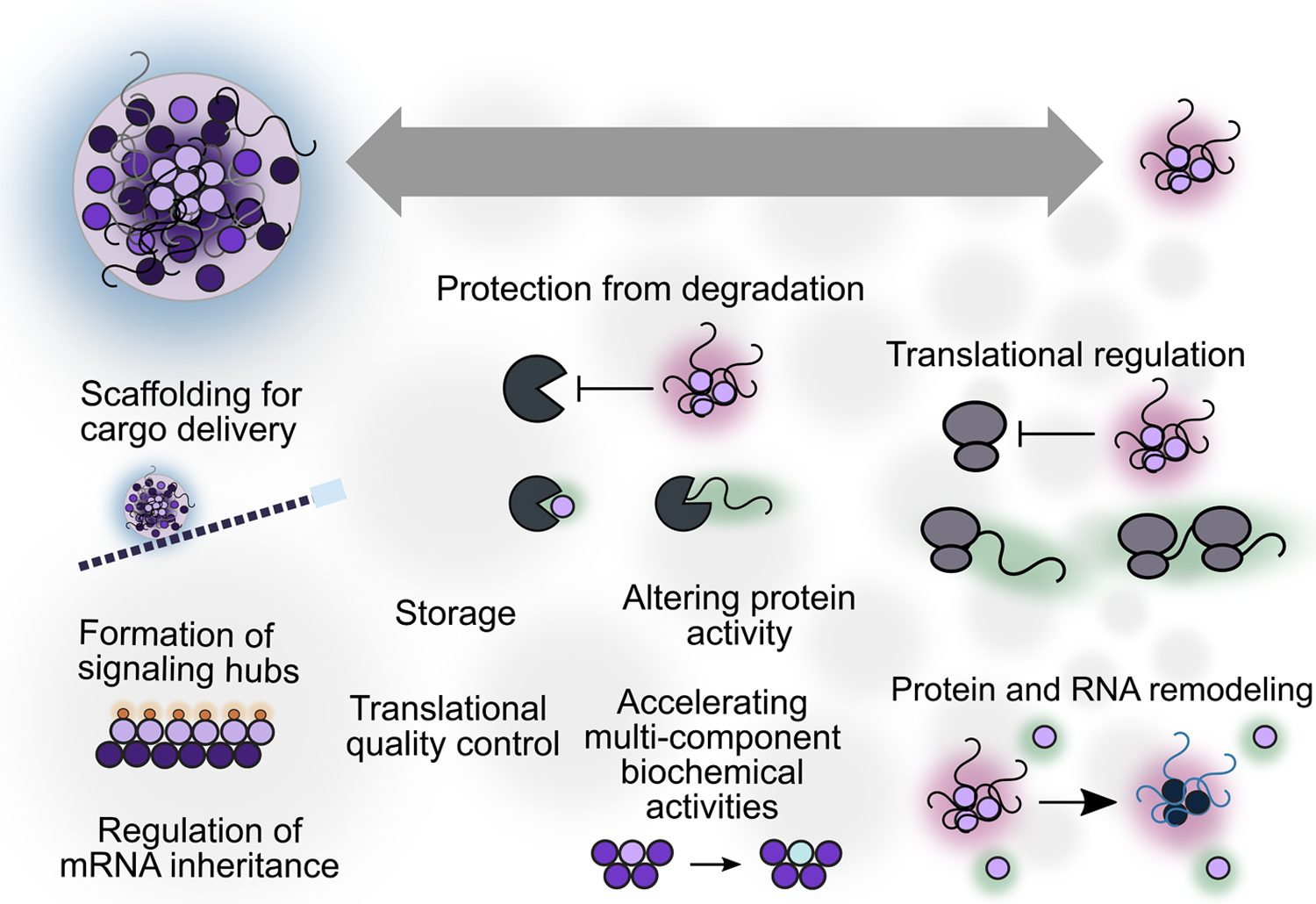
Many roles could in principle be accomplished by small RNA/protein condensates consisting of a sharply restricted subset of components assembled into submicroscopic condensates. The diagram provides speculative positioning of functions on the size spectrum because strong hypotheses regarding which functions require large foci are lacking.
Less discussed in the field are the issues inherent in studying biological phenomena whose functional contributions, if any, are unclear. Purification and reconstitution strategies, deprived of an activity-based standard for measuring success, must instead rely on morphological or compositional metrics whose relationship with biological function remains to be established (Begovich and Wilhelm, 2020; Freibaum et al., 2021). The lack of functional insight is compounded by the remarkable lack of standard cellular phenotypes in the study of stress granules. Because not all of a given protein or RNA localizes to stress granules, determining a function must come from specifically perturbing condensation behavior without influencing activity, localization, or expression level. Even at the condensate level, phenotypes have been difficult to establish, although an allelic series of mutations which suppress poly(A)-binding protein’s heat-triggered condensation in vitro and in vivo also suppress growth during heat stress (Riback et al., 2017). The rarity of such phenotypes, particularly for stress granules, has led to a lingering question of whether stress granules may often simply be byproducts of other cellular changes (Mateju and Chao, 2021).
Informing functions of stress-triggered condensation through the lens of disease
Some promising directions in uncovering stress granule function have come through study of disease contexts. Stress granules are induced by viral infection, where their formation has been proposed to help restrict viral replication (Eiermann et al., 2020). In fact, many viruses have developed strategies for preventing stress granule formation by, for instance, sequestering or cleaving key stress granule components (Katoh et al., 2013; White et al., 2007). What function do stress granules serve that viruses are so intent on disrupting? One possibility is that stress granules could sequester viral RNA, similar to their proposed function in storing cellular mRNAs (Burgess and Mohr, 2018; Law et al., 2019). However, as discussed above, it is difficult to conclude whether recruitment of viral RNA to stress granules is required for proposed functions without mutations that specifically perturb stress granule formation while preserving separate molecular functions of stress granule components. One such perturbation comes from recent work showing that chikungunya virus promotes stress granule disassembly through the ADP-ribosylhydrolyase activity of nonstructual protein 3 (nsP3) (Abraham et al., 2018; Akhrymuk et al., 2018; Jayabalan et al., 2021). Removing this activity from nsP3 preserves stress granules during infection, providing a manipulatable system for future studies of stress granule function without deletion of any host machinery.
The stressful environments inhabited by tumors—such as nutrient deprivation, hypoxia, increased reactive oxygen species, and perturbed protein folding resulting from the dysregulation of metabolism and growth in malignancy—makes cancer biology a useful model for studying the functions of stress-induced condensation (Ackerman and Simon, 2014; Anderson et al., 2015; Clarke et al., 2014; Gorrini et al., 2013). Moreover, certain chemotherapy drugs trigger cancer cells to form stress granules, which are generally thought to be pro-survival, leading to condensation modulation as a potential target for therapeutics (Fournier et al., 2010; Gao et al., 2019; Kaehler et al., 2014). In contrast, another chemotherapy agent, sodium selenite, triggers non-canonical stress granules lacking certain components whose stress granule localization has been linked to cell survival. These non-canonical stress granules have thus been suggested to be less functional in the stress response (Fujimura et al., 2012). Additional work aimed at understanding the precise differences in stress-induced condensation between the considered pro-survival canonical and the non-canonical stress granules, at both the stress granule and pre-microscopic condensate level, will help inform the functions of condensation in response to stress and perhaps even inform the importance of its organization at the size/spatial levels.
Further underscoring the potential role of condensation in the pathogenesis of cancer, recent work studying myeloid malignancies has shown that specific driver mutations upregulate stress granule formation, which is linked to increased stress adaptation and cancer development (Biancon et al., 2022). Additionally, work with disease mutations related to neurodegenerative diseases suggests a relationship between maladaptive protein aggregates and adaptive condensates like stress granules, suggesting that maladaptive aggregates may occur when stress granules are not properly disassembled (Gal et al., 2016; Gwon et al., 2021; Mackenzie et al., 2017). Even so, our understanding of these maladaptive protein aggregates will be limited without a deeper understanding of the function of adaptive condensates. Without understanding the functions of stress-induced condensation, we can only speculate on the pathophysiology of persistent stress granules.
While many studies of stress granules focus on proteins which, when fluorescently tagged, are easily visible microscopically, RNA sits at the center of stress granule formation and function. We thus begin with a consideration of how our understanding of RNA’s role has changed as new methods have come into use.
The role of RNA: old observations and emerging results
The accumulation of poly(A)-RNA is among the defining features of stress granules. Moreover, the role of mRNA in stress granule formation has long been known. Among the most crucial experiments is the demonstration that translational inhibition affects stress granule formation in a mechanistically specific way: elongation inhibitors such as cycloheximide and emetine, which freeze ribosomes on mRNA, block stress granule formation, whereas puromycin, which prematurely terminates translation and frees mRNA of ribosomes, promotes stress granule formation (Bounedjah et al., 2014; Kedersha et al., 2000; Namkoong et al., 2018; Wallace et al., 2015). Inhibition of transcription also inhibits stress granule formation (Bounedjah et al., 2014; Khong et al., 2017a), further underscoring the role of RNA, at least at the accumulation stage.
But which RNAs? How does RNA contribute to condensation and stress granule formation? To what extent does RNA drive condensation or accumulation, and to what extent is it passively dragged along?
Early important results showed that prominent stress-induced mRNAs are selectively excluded from stress granules in both plant and mammalian cells (Kedersha and Anderson, 2002; Nover et al., 1989; Stöhr et al., 2006; Zid and O’Shea, 2014). Because stress granules are, by most metrics, accumulation sites for translationally repressed mRNAs, and because it is both biologically appealing and empirically established in some systems that stress-induced transcripts are well-translated (Preiss et al., 2003; Zid and O’Shea, 2014), these early results placed stress granules at the center of translational regulation during stress.
But these foundational results have not survived into the recent era dominated by high-throughput studies, where transcriptome-scale effects can be observed. Modern studies do not find substantial depletion of stress-induced mRNAs from stress granules; instead, recent studies employing diverse approaches have converged on transcript length as the key correlate of mRNA recruitment to stress granules. Messenger RNA length is the dominant correlate of their enrichment in the transcriptome associated with purified stress granule cores and stress-associated RNA granules (Khong et al., 2017b; Matheny et al., 2019, 2021; Namkoong et al., 2018); in in vitro systems, increasing RNA length promotes RNA/protein phase separation organized by the stress-granule hub G3BP1 (Guillén-Boixet et al., 2020; Yang et al., 2020); and single-molecule studies show that mRNA length correlates with the dwell-time of mRNAs on stress granules and other condensed structures (Moon et al., 2019).
An increased concentration of ribosome-free mRNA following stress-induced translational shutdown is considered the key trigger for stress granule formation (Hofmann et al., 2020), and inhibition of translation initiation triggers condensation, such as in stress, eIF2α phosphorylation, or inhibition of the initiation factor eIF4A (Buchan et al., 2008; Iserman et al., 2020; Kedersha et al., 1999; Mazroui et al., 2006; Riback et al., 2017). (Fig. 3). This model is supported by several lines of evidence: 1) global translation initiation downregulation and subsequent polysome collapse is associated with RNA condensation during stress (Cherkasov et al., 2013), 2) prevention of polysome collapse during stress blocks stress granule formation (Kedersha et al., 2000), 3) transfection of translationally arrested cells with free mRNA triggers stress granule formation (Bounedjah et al., 2014), 4) inhibiting eIF4A, an essential translation initiation factor, promotes stress granule formation (Dang et al., 2006; Low et al., 2005; Mazroui et al., 2006; Tauber et al., 2020a). Alongside these data, early and still-current alternative models in which RNA length plays a minimal role exist. For example, stalled preinitiation complexes (PICs) which accumulate during stress may in part form the core of stress granules (Kedersha et al., 2002) (Fig. 3).
Figure 3: The mechanisms of stress-triggered condensation and stress granule formation remain an area of active inquiry.
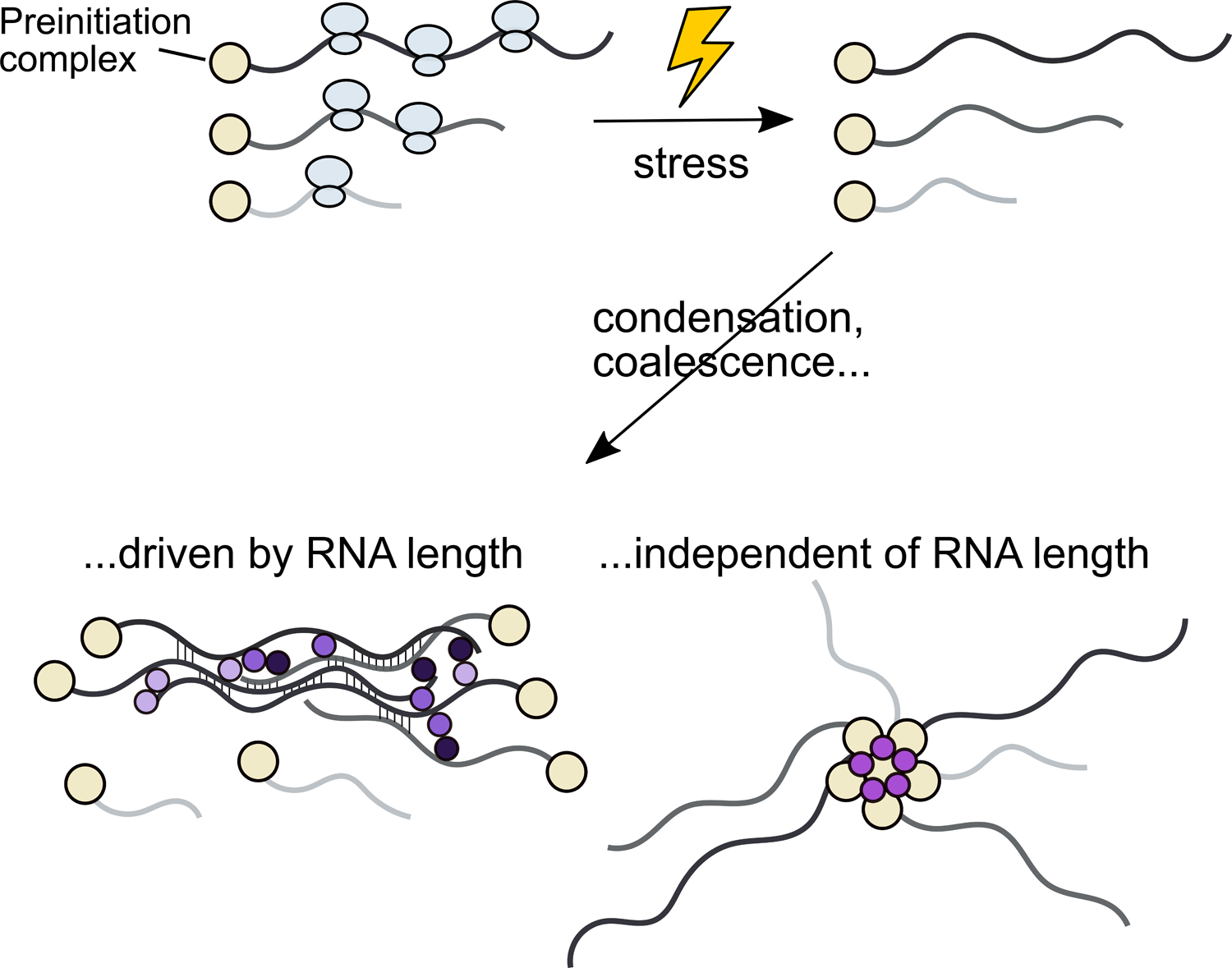
Treatments which inhibit translation initiation (often by phosphorylation of eIF2ɑ), producing ribosome-free mRNA, cause stress granule formation in a wide range of systems and circumstances. Substantial recent work implicates long RNAs in condensation and formation of stress granules, a result which is biophysically plausible yet functionally puzzling.
Beyond ribosome-free RNA, a role of RNA length makes intuitive biophysical sense, because the number of opportunities for either RNA-RNA or protein-RNA interactions—i.e., valence—naturally scales with length, all else being equal (Jain and Vale, 2017). Evidence for a role from RNA-RNA interactions is circumstantial, resting on partial recapitulation of some stress granule transcriptome features in vitro using only purified RNA (Van Treeck et al., 2018), the dependence of in vitro phase separation on long, unfolded RNAs (Guillén-Boixet et al., 2020; Yang et al., 2020) and RNA helicases (Tauber et al., 2020a). Further discussion of the available evidence supporting the roles of RNA-RNA or protein-RNA interactions can be found in several informative reviews (Campos-Melo et al., 2021; Hofmann et al., 2020; Ripin and Parker, 2021; Van Treeck and Parker, 2018).
Even though a dominant role for RNA length is sensible biophysically, it is puzzling biologically. The overwhelming consensus holds that stress granules are accumulation sites for mRNA whose translation is suppressed during stress. Yet the length-driven model (and existing results supporting it) suggests that induction of long transcripts during stress would be futile for protein production, because long transcripts would be immediately recruited into translationally silent stress granules. However, while evidence that long transcripts are translationally silenced during stress after their stress granule recruitment is lacking, it has been hypothesized that shorter transcripts may be associated with rapid responses, which could help resolve the paradox (Lopes et al., 2021).
However, an important caveat is that mRNA length is also a natural confounding variable in experiments and analyses. Sedimentation by centrifugation is employed in most transcriptome-scale studies aimed at isolating stress granule-associated mRNAs, mirroring the use of sedimentation in proteome-scale studies of stress granule-associated proteins (Cherkasov et al., 2015; Jain et al., 2016; Wallace et al., 2015). But unlike proteins, long RNAs, due to their size—an mRNA weighs roughly an order of magnitude more than the protein it encodes—will tend to sediment whether or not they are in a condensate. Consequently, comparing stress and non-stress conditions is crucial to determining the extra sedimentation due to stress. However, as others have pointed out (Namkoong et al., 2018), the original study (Khong et al., 2017b) reporting yeast and mammalian stress granule transcriptomes, and reporting the profound effect of length, did not include non-stress controls. Long RNAs may stick nonspecifically to affinity reagents in pulldowns due to their valence or increased structure (Sanchez de Groot et al., 2019). Although subsequent controlled work in mammalian cells has confirmed the accumulation of longer RNAs in granules following ER or oxidative stress (Matheny et al., 2019; Namkoong et al., 2018), the effects are more modest, and no non-stress control is yet available in yeast. Reduced translational efficiency (TE) has also been reported to be a major contributor to stress granule RNA accumulation. However, the two measures of TE used—codon optimality and ribosome density—have long been known to be inversely correlated with transcript length (Arava et al., 2005; Duret and Mouchiroud, 1999; Weinberg et al., 2016), raising the question of whether TE is a causal contributor to mRNA recruitment or a spurious correlation. Sedimentation-independent methods to examine recruitment of mRNAs, such as mRNA fluorescence in situ hybridization (FISH) in intact cells, have covered only a handful of targets (Khong et al., 2017b; Matheny et al., 2019), reported only a modest stress granule recruitment effect from length, and concluded that “length, per se, is not the major driving force in stress granule enrichment” (Matheny et al., 2021). Large-scale, well-controlled, systematic studies of the effect of length will be useful in resolving lingering uncertainty.
Given the sharp change in the apparent biology of RNA recruitment to stress granules from early to present-day studies, the limited set of transcriptome-scale studies available at this writing, and the challenging nature of isolating molecular components of functionally ill-defined structures, the RNA components of stress-induced condensates and stress granules will continue to be an area of intense investigation.
Mechanisms of dissolution
How do stress-induced RNA condensates dissolve after stress, as cells return to basal operations? Dissolution appears to be a regulated, controlled process that relies on specific proteins (Hofmann et al., 2020; Marmor-Kollet et al., 2020). Proteins categorized as molecular chaperones and autophagic proteins have been implicated in stress granule dissolution, as have proteins associated with post-translational modifications (PTMs) such as sumoylation, ubiquitination, and phosphorylation (Buchan et al., 2013; Cherkasov et al., 2013; Gwon et al., 2021; Keiten-Schmitz et al., 2020; Marmor-Kollet et al., 2020; Maxwell et al., 2021; Shattuck et al., 2019; Yoo et al., 2021). Work in yeast has revealed that heat-induced (42 °C) protein aggregates are entirely reversible, which is incompatible with autophagy and suggests that different fates occur in different stresses (Wallace et al., 2015). Recent work shows that molecular chaperones can dissolve stress-triggered protein condensates orders of magnitude more efficiently than misfolded reporter proteins in vitro, suggesting that molecular chaperones may have evolved to interact with stress-induced condensates (Yoo et al., 2021). Additionally, recent work in mammalian cells has shown that stress granules can be eliminated through either an autophagy-independent disassembly process or autophagy-dependent degradation, depending on the severity and acuteness of the initial stress (Gwon et al., 2021; Maxwell et al., 2021). This work suggests that the disassembly of stress granules is related to the initial stress, suggesting that different methods of assembly may require different methods of disassembly.
The kinetics of stress granule dissolution may be tied to a functional role, such as translational control. If stress-induced condensates are sites of storage, the contents must be disassembled in a timely manner. It has been proposed that stress granules dissolve in discrete steps, where an initial shell is pulled away followed by a core, with particular proteins being recruited at distinct stages (Wheeler et al., 2016). Proteins necessary for cell recovery from stress, such as translation initiation factors, may need to be dispersed earlier than other stress granule core proteins that are dissolved more slowly. In fact, proper disassembly of stress granules was shown to be required for recovering cellular activities, such as translation, after stress (Maxwell et al., 2021). The dissolution of stress-induced condensates may be related to maladaptive insoluble protein aggregates that are often associated with diseases, motivating a further understanding of the mechanism and function of dissolution (Hofmann et al., 2020).
However, as the function of stress granules remains unclear, the lack of functional assays demands careful experimental perturbations and cautious conclusions. For example, condensates that are no longer visible by microscopy may still occupy a conformation distinct from a monomeric form. New findings about the material state and assembly process of stress-induced condensates will illuminate the dissolution process, addressing questions such as whether the multiple steps of dissolution are equivalent to the stages of assembly or if a change in material state may lead to a different dissolution process. On this front, the role of liquid-liquid phase separation in stress granule formation may have crucial consequences for how these structures dissolve.
Examining the role of liquid-liquid phase separation in stress-induced condensation
Liquid-liquid phase separation (LLPS) is a thermodynamically driven mechanism by which a solution of a compound demixes into a dilute and a dense phase above a certain critical concentration (Hyman et al., 2014). A host of stress granule-associated proteins have been shown to undergo phase separation in vivo and in vitro (Guillén-Boixet et al., 2020; Iserman et al., 2020; Kroschwald et al., 2018; Molliex et al., 2015; Riback et al., 2017; Sanders et al., 2020; Yang et al., 2020), and it is widely held that stress granule assembly is driven by LLPS (reviewed in Hofmann et al., 2020). Recent work has converged on G3BP as a central node in LLPS-driven stress granule formation (Guillén-Boixet et al., 2020; Sanders et al., 2020; Yang et al., 2020), yet G3BP is dispensable for stress granule formation in response to certain stressors, such as heat and osmotic shock (Kedersha et al., 2016; Matheny et al., 2021). Thus, G3BP-focused models of stress granule formation may overly simplify the complex process of stress-induced condensation.
Using LLPS as an assembly mechanism provides key advantages beneficial for responding to stress. The ultra-cooperativity of LLPS enables proteins to precisely sense and respond to small changes in their environments (Yoo et al., 2019). For instance, in yeast Ded1 autonomously condenses in response to temperature stress. Ded1 from a cold-adapted yeast condenses at lower temperatures than that of S. cerevisiae, while Ded1 from a thermophilic yeast condenses at higher temperatures (Iserman et al., 2020). This correlates with the fact that each yeast species has evolved to trigger its heat shock response relative to its environmental niche. Other key advantages of LLPS include that it enables passive (energy-independent) cellular reorganization and that it is reversible. Following the removal of the stress stimulus, LLPS would no longer be energetically favored, and the system would spontaneously return to basal conditions.
Biomolecular condensation can result in the concentration of protein and RNA molecules into phases with a variety of material states. How could a condensate’s material state—how liquid-like or solid-like it is—affect its function? More solid-like condensates have been linked to disease, as pathogenic mutations of certain condensing proteins such as FUS increase aging and a loss of liquid-like properties over time (Patel et al., 2015). This thinking extends to RNA condensates as well, as it has been proposed that RNA helicases prevent RNA-RNA entanglement to maintain a liquid-like condensed state (Tauber et al., 2020a, 2020b). Further, the viscoelasticity of the nucleolus has been linked with enabling the vectorial release of properly folded ribosomes (Riback et al., 2022). Yet, the material state of stress-induced condensates does not appear to be widely conserved across eukaryotes, which like other evolutionarily variable features would usually be taken as evidence that the material state is not central to function. For instance, yeast stress granules are more solid-like than those of metazoa (Kroschwald et al., 2015), although there are methodological caveats (Wheeler et al., 2016). Reconstituted heat-induced condensates of the yeast stress granule protein Pab1 are solids (Riback et al., 2017) which are not spontaneously reversible, even though these condensates are readily dispersed by endogenous molecular chaperones (Yoo et al., 2021). Even within an organism, pH-induced condensates of the yeast stress granule protein Pub1 are more liquid-like than those induced by heat shock—and only the heat-induced condensates depend on chaperones (Kroschwald et al., 2018)—yet both conditions are thought to be physiologically relevant.
The apparent lack of conservation of the material state can be rationalized when we consider that a condensate’s material state appears irrelevant for many of the functions ascribed to stress granules. For example, if the role of stress-induced condensation is to temporarily store housekeeping mRNA to enable the preferential translation of stress-response messages, how liquid-like the storage compartment is may be of minor importance. Additionally, if the function is to sequester certain proteins to perturb a given signaling pathway in the cytoplasm, the key feature is to deplete the protein from the dilute phase, and the liquidity of the dense phase is less relevant. On the other hand, if the material state is particularly relevant for the potential pathogenicity of condensates, then the evolutionary pressures on material state in different organisms may differ substantially even if stress granules have a conserved cellular function.
Hazards in defining stress granule composition
Defining the composition of stress granules is complicated by a number of factors, even setting aside the existential problem of what constitutes a biologically important structure in the absence of well-established functions and phenotypes. Nevertheless, the obvious consistency and evolutionary conservation of the accumulation of some proteins and RNAs into large foci has led to a sustained effort to identify lists of molecular components involved in the lifecycle of stress granules. Individual mRNAs and proteins can be localized to microscopically visible foci of stress granule markers (Cherkasov et al., 2015; Khong et al., 2017b; Mateju et al., 2020; Moon et al., 2019, 2020; Wallace et al., 2015; Wilbertz et al., 2019). On a larger scale, the stress granule interactome has been defined using a variety of techniques, many of which rely on using individual stress granule components, such as poly(A)-binding protein, G3BP1, TIA1, and eIF4A, as bait proteins and then assessing the mRNAs and proteins which interact with that bait. The interactors have been identified through immunoprecipitations, purification of particles containing a bait fused to a fluorescent protein, and by biotin proximity labeling (Hubstenberger et al., 2017; Khong et al., 2017b; Namkoong et al., 2018; Padrón et al., 2019; Somasekharan et al., 2020). Additionally, proximity labeling methods have found similar interactomes between stress granule proteins prior to stress and during stress (Markmiller et al., 2018; Youn et al., 2018). This may indicate that stress granules are mainly stabilized by enhancements of basal interactions, or that the interactions which distinguish stress granules are labile or refractive to these methods.
The different levels of organization in stress-triggered condensation and stress granule formation, along with diverse methods whose relative accuracy can be difficult to establish given the ill-defined nature of the target, combine to create a challenging experimental landscape (Fig. 4). Unlike a membrane-bound mitochondrion or a relatively compositionally stable ribosome, stress-induced condensates and stress granules lack features which might simplify their description.
Figure 4:
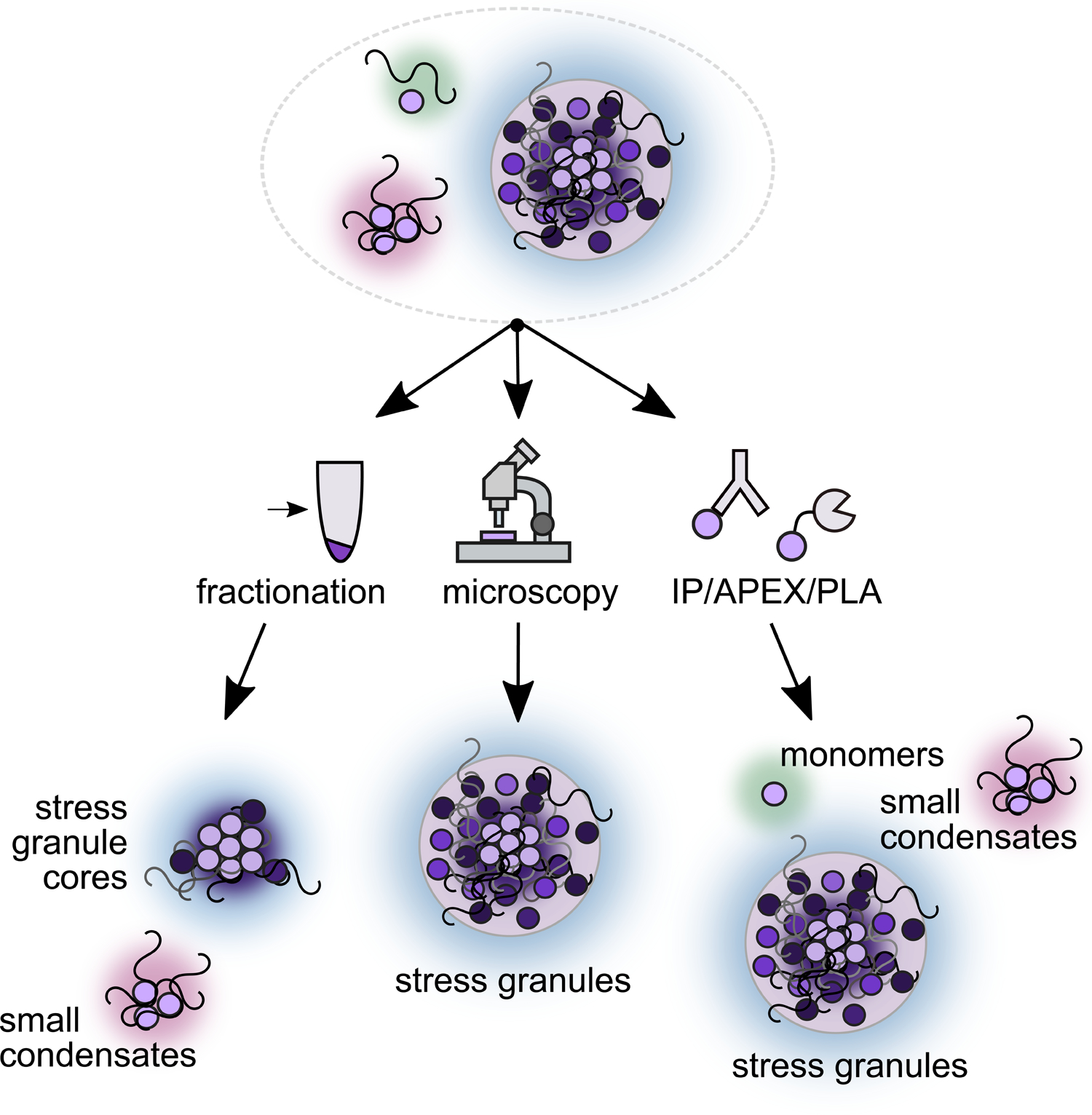
Different methods used to probe stress-induced condensation capture and report on different stages of stress-induced condensation and stress granule formation, providing complementary information.
A hallmark of biomolecular condensation is that many of the components of the condensate individually associate through weak, dynamic interactions (Alberti and Hyman, 2021). No biologically clear cutoff for interaction strength exists, making it unclear how to decide if a given component is part of the structure or not. For instance, many transcripts have been observed to associate only briefly with stress granule proteins (Wilbertz et al., 2019). How long must an mRNA reside at a stress granule to be considered a component? Additionally, consistent but weak associations may be lost during the isolation steps necessary for sequencing, mass spectrometry, or other biochemical methods. Perhaps certain molecular components form a scaffold to which client proteins are recruited (Campos-Melo et al., 2021; Shiina, 2019; Zhang et al., 2019). Differences in interaction strength may reveal biologically important differences; for example, major molecular chaperones associate with stress granules by colocalization (Cherkasov et al., 2013), but do not co-fractionate with stress-triggered condensates (Wallace et al., 2015). Should such chaperones be considered a component of stress granules, merely associates, or something else? Here again, functional assays would sharpen these distinctions in crucial ways.
Because stress granules are operationally defined as microscopic foci marked by specific proteins, the definition of the structure is unfortunately entwined with technical limitations and with compositional preconceptions. Failure to observe foci microscopically, for example at low levels of stress, are consistent with two distinct biological possibilities: the absence of condensates entirely, or the formation of structures below the diffraction limit which still retain key properties of larger condensates (Guzikowski et al., 2019). Likewise, failure to observe colocalization with a specific marker molecule may reflect legitimate biological variation either in the marker itself or in the structure being marked.
Finally, the composition of stress granules is not static, but depends on the nature of the stress and also changes over time (Aulas et al., 2017; Buchan et al., 2011; Padrón et al., 2019; Reineke and Neilson, 2019; Zhang et al., 2019). Cells have evolved a variety of strategies to deal with changing environments. In the face of brief stresses, it may be advantageous to store transcripts until the stress has passed, allowing for a faster restoration of growth, whereas prolonged stress may necessitate more drastic reprogramming of cellular processes (Arribere et al., 2011). Consequently, deciding whether a molecular species is or is not a part of the stress granule transcriptome/proteome, reducing the problem to a yes or no, may obscure more biology than it illuminates.
Grand challenges in studying stress-induced protein/mRNA condensation
As is now apparent, stress granules and their molecular precursors represent an exemplary system in which field-level challenges find crisp expression. Here we identify grand challenges in the study of these structures (Figure 5).
Figure 5:
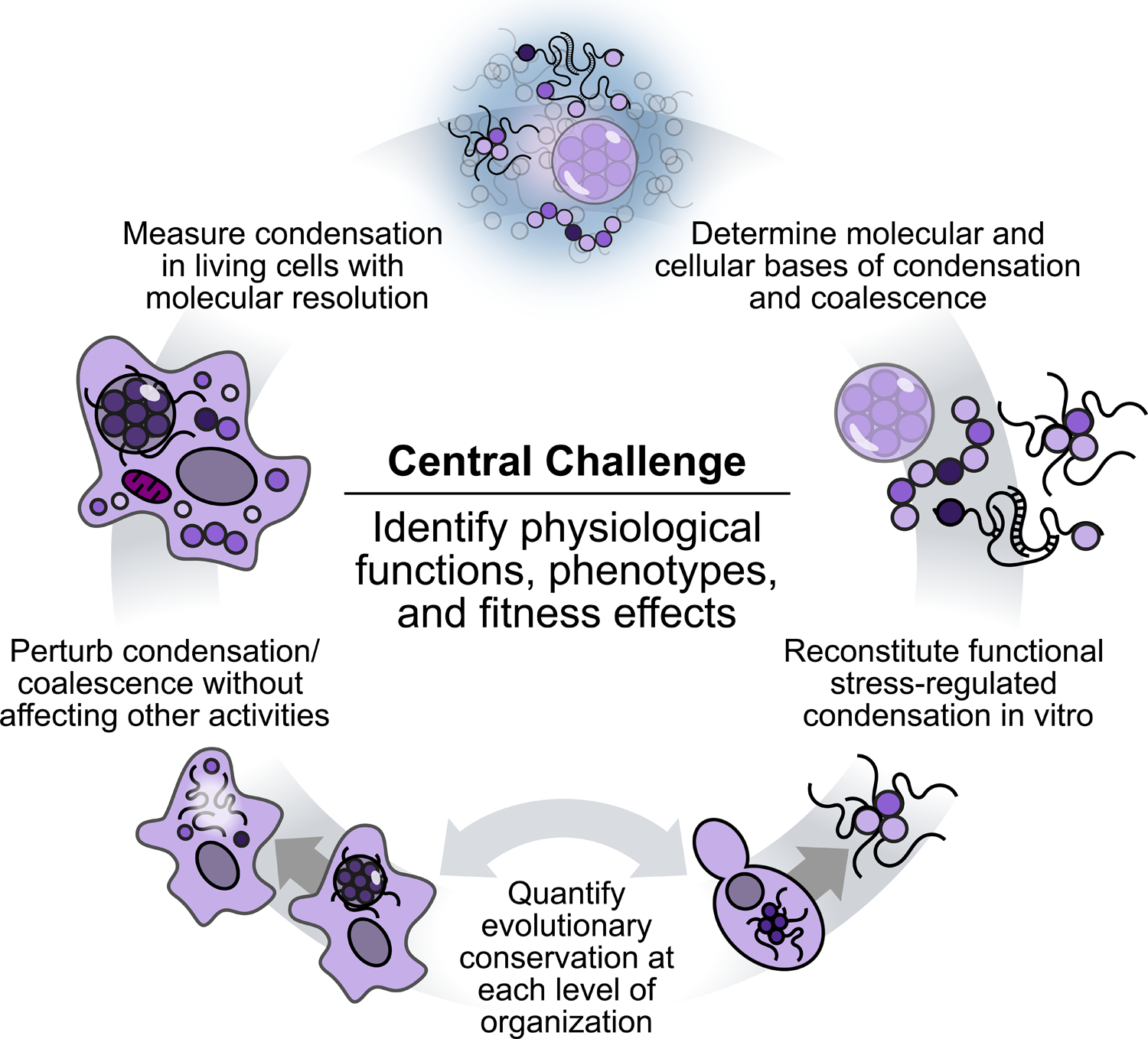
Grand challenges in the study of stress granules and stress-induced condensation.
The first central challenge is to identify the functions of stress-induced condensates and stress granules, and determine how these functions are executed. Of particular importance is the identification of fitness-related cellular phenotypes. The near-total reliance on molecular or imaging phenotypes, in the absence of function- and fitness-related phenotypes (growth, survival, differentiation, activity), has become tolerated in ways that may hinder progress. For example, given that canonical stress granules only become microscopically visible during severe stress in some important cases (Grousl et al., 2009; Wallace et al., 2015), the reliance on microscopic methods may blind us to wide swaths of functional phenomena. In addition, the identification of a cellular phenotype would make it possible to design genetic screens that search for factors that are not just involved in focus formation but are integral to stress granule function.
Similarly, the use of inducers which robustly and reliably produce stress granules but are of uncertain physiological relevance, such as the broadly popular sodium arsenite, may have hidden disadvantages. If cells have not evolved to respond to a trigger, the cellular response is likely to lack organizational and molecular features which characterize responses to more physiological triggers such as heat, hypoxia, and osmotic shock. Even for these stresses, intensities which exceed physiological levels are in routine experimental use. Moreover, to validate a potent inducer such as sodium arsenite phenotypically against physiological inducers remains challenging until a phenotype or function of physiological stress granules is itself firmly established. Surmounting this central functional challenge will require sustained searches, a focus on physiology to match the extraordinary attention given to biophysics, and perhaps new thinking to identify a set of standardized phenotypes for functional studies.
Surrounding this central challenge lurk many other intertwined grand challenges (Fig. 5). Some are well-established: determining the molecular bases of condensation and accumulation, and measuring molecular-scale condensation in living cells. Success on the latter would allow us, for the first time, to observe all the stages of stress-triggered condensation in vivo, even under mild stress conditions where large canonical stress granules do not form (Fig. 1).
In attempting to discern the molecular determinants of condensation and stress granule formation, less discussed is the crucial difficulty—another grand challenge—of perturbing these phenomena cleanly, that is, without disrupting other activities. By analogy, study of an enzyme might involve, in order of decreasing disruption, a gene knockout, a temperature-sensitive mutation, a catalytic mutation, or development of a specific and reversible inhibitor. Despite considerable strides in this direction for stress granules (including screens for gene knockouts which disrupt stress granules (Yang et al., 2014)), at this moment the search for clean perturbations remains almost entirely open.
In the absence of defined functions, another clear grand challenge looms: biochemical reconstitution of stress granule activities and functions. Reconstitution demonstrates the sufficiency of specific molecules and conditions to recapitulate cellular behavior. At present, all efforts have necessarily focused on reconstitution of traits without any unambiguous link to cellular fitness or adaptive function. Our situation in the stress granule field is remarkably different from historical efforts to purify specific biochemical fractions or molecules which could recapitulate an observed cellular activity.
Finally, the evolutionary conservation of stress granules provides powerful motivation for their study. But how conserved are they? To what degree are the following conserved: specific components and stages; molecular determinants such as domains; biophysical forces; formation and dispersal pathways; regulators; and ultimate functions? Answering these questions would meet our final grand challenge (Fig. 5). Serious efforts to use evolutionary approaches, and to move beyond a handful of model organisms, has the potential to dramatically accelerate progress in our understanding of these enigmatic structures and processes. To the extent that stress granules are not merely reliable side-effects of some other biological process, consistent contributions to cellular and organismal fitness will be the decisive factors in their preservation across the tree of life.
These grand challenges underscore that the field of stress granule biology is at a pivotal point. As we approach the 40-year mark since stress granules were first observed in tomato plants (Nover et al., 1983), we are due to move toward a deeper understanding of stress granules. Armed with clearly defined challenges, we can tackle the fundamental unknowns that still remain. Massive parallel surges in our understanding of composition and assembly mechanisms, both cell-biologically and biophysically, appear poised to drive a positive feedback loop of research integrating studies of assembly at multiple biological scales, mechanistic studies of the impact of condensation on mRNA lifecycles, and finally the fitness advantages that stress-induced condensation imparts.
Acknowledgements
We thank members of the Drummond and Sosnick labs for critical comments on the manuscript. Research reported in this publication was supported by the National Institutes of Health through the National Institute of General Medical Sciences (awards GM126547, GM127406, GM144278) and the National Institute of Environmental Health Sciences (award F30ES032665). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Abraham R, Hauer D, McPherson RL, Utt A, Kirby IT, Cohen MS, Merits A, Leung AKL, and Griffin DE (2018). ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc. Natl. Acad. Sci. U. S. A 115, E10457–E10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman D, and Simon MC (2014). Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol. 24, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizer A, Kalo A, Kafri P, Shraga A, Ben-Yishay R, Jacob A, Kinor N, and Shav-Tal Y (2014). Quantifying mRNA targeting to P bodies in living human cells reveals a dual role in mRNA decay and storage. Journal of Cell Science. [DOI] [PubMed] [Google Scholar]
- Akhrymuk I, Frolov I, and Frolova EI (2018). Sindbis Virus Infection Causes Cell Death by nsP2-Induced Transcriptional Shutoff or by nsP3-Dependent Translational Shutoff. J. Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, and Carra S (2018). Quality Control of Membraneless Organelles. J. Mol. Biol 430, 4711–4729. [DOI] [PubMed] [Google Scholar]
- Alberti S, and Hyman AA (2021). Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol 22, 196–213. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N, and Ivanov P (2015). Stress granules, P-bodies and cancer. Biochim. Biophys. Acta 1849, 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arava Y, Boas FE, Brown PO, and Herschlag D (2005). Dissecting eukaryotic translation and its control by ribosome density mapping. Nucleic Acids Res. 33, 2421–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, and Takekawa M (2008). Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol 10, 1324–1332. [DOI] [PubMed] [Google Scholar]
- Aronov S, Dover-Biterman S, Suss-Toby E, Shmoish M, Duek L, and Choder M (2015). Pheromone-encoding mRNA is transported to the yeast mating projection by specific RNP granules. J. Cell Biol 209, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere JA, Doudna JA, and Gilbert WV (2011). Reconsidering Movement of Eukaryotic mRNAs between Polysomes and P Bodies. Mol. Cell 44, 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulas A, Fay MM, Lyons SM, Achorn CA, Kedersha N, Anderson P, and Ivanov P (2017). Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci 130, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, and Rosen MK (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begovich K, and Wilhelm JE (2020). An In Vitro Assembly System Identifies Roles for RNA Nucleation and ATP in Yeast Stress Granule Formation. Mol. Cell [DOI] [PubMed] [Google Scholar]
- Biancon G, Joshi P, Zimmer JT, Hunck T, Gao Y, Lessard MD, Courchaine E, Barentine AES, Machyna M, Botti V, et al. (2022). Precision analysis of mutant U2AF1 activity reveals deployment of stress granules in myeloid malignancies. Mol. Cell 82, 1107–1122.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bley N, Lederer M, Pfalz B, Reinke C, Fuchs T, Glaß M, Möller B, and Hüttelmaier S (2015). Stress granules are dispensable for mRNA stabilization during cellular stress. Nucleic Acids Res. 43, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, et al. (2018). Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 28, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ Jr, Sapp P, McKenna-Yasek D, Brown RH Jr, and Hayward LJ (2010). Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet 19, 4160–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounedjah O, Desforges B, Wu T-D, Pioche-Durieu C, Marco S, Hamon L, Curmi PA, Guerquin-Kern J-L, Piétrement O, and Pastré D (2014). Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res. 42, 8678–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP (2013). Phase transitions and size scaling of membrane-less organelles. J. Cell Biol 203, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, and Parker R (2009). Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36, 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Muhlrad D, and Parker R (2008). P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol 183, 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Yoon J-H, and Parker R (2011). Stress-specific composition, assembly and kinetics of stress granules in Saccharomyces cerevisiae. J. Cell Sci 124, 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Kolaitis R-M, Taylor JP, and Parker R (2013). Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153, 1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HM, and Mohr I (2018). Defining the Role of Stress Granules in Innate Immune Suppression by the Herpes Simplex Virus 1 Endoribonuclease VHS. J. Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Melo D, Hawley ZCE, Droppelmann CA, and Strong MJ (2021). The Integral Role of RNA in Stress Granule Formation and Function. Front Cell Dev Biol 9, 621779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasov V, Hofmann S, Druffel-Augustin S, Mogk A, Tyedmers J, Stoecklin G, and Bukau B (2013). Coordination of translational control and protein homeostasis during severe heat stress. Curr. Biol 23, 2452–2462. [DOI] [PubMed] [Google Scholar]
- Cherkasov V, Grousl T, Theer P, Vainshtein Y, Gläßer C, Mongis C, Kramer G, Stoecklin G, Knop M, Mogk A, et al. (2015). Systemic control of protein synthesis through sequestration of translation and ribosome biogenesis factors during severe heat stress. FEBS Lett. [DOI] [PubMed] [Google Scholar]
- Clarke HJ, Chambers JE, Liniker E, and Marciniak SJ (2014). Endoplasmic reticulum stress in malignancy. Cancer Cell 25, 563–573. [DOI] [PubMed] [Google Scholar]
- Dang Y, Kedersha N, Low W-K, Romo D, Gorospe M, Kaufman R, Anderson P, and Liu JO (2006). Eukaryotic initiation factor 2alpha-independent pathway of stress granule induction by the natural product pateamine A. J. Biol. Chem 281, 32870–32878. [DOI] [PubMed] [Google Scholar]
- Decker CJ, and Parker R (2012). P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol 4, a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret L, and Mouchiroud D (1999). Expression pattern and, surprisingly, gene length shape codon usage in Caenorhabditis, Drosophila, and Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 96, 4482–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiermann N, Haneke K, Sun Z, Stoecklin G, and Ruggieri A (2020). Dance with the Devil: Stress Granules and Signaling in Antiviral Responses. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante LE, and Gasch AP (2021). The role of stress-activated RNA-protein granules in surviving adversity. RNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farny NG, Kedersha NL, and Silver PA (2009). Metazoan stress granule assembly is mediated by P-eIF2alpha-dependent and -independent mechanisms. RNA 15, 1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M-J, Gareau C, and Mazroui R (2010). The chemotherapeutic agent bortezomib induces the formation of stress granules. Cancer Cell Int. 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks TM, and Lykke-Andersen J (2007). TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU-rich elements. Genes Dev. 21, 719–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Messing J, Yang P, Kim HJ, and Taylor JP (2021). High-fidelity reconstitution of stress granules and nucleoli in mammalian cellular lysate. J. Cell Biol 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K, Sasaki AT, and Anderson P (2012). Selenite targets eIF4E-binding protein-1 to inhibit translation initiation and induce the assembly of non-canonical stress granules. Nucleic Acids Res. 40, 8099–8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal J, Kuang L, Barnett KR, Zhu BZ, Shissler SC, Korotkov KV, Hayward LJ, Kasarskis EJ, and Zhu H (2016). ALS mutant SOD1 interacts with G3BP1 and affects stress granule dynamics. Acta Neuropathol. 132, 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Jiang L, Gong Y, Chen X, Ying M, Zhu H, He Q, Yang B, and Cao J (2019). Stress granule: A promising target for cancer treatment. Br. J. Pharmacol 176, 4421–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E, and Shorter J (2019). The molecular language of membraneless organelles. J. Biol. Chem 294, 7115–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrini C, Harris IS, and Mak TW (2013). Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov 12, 931–947. [DOI] [PubMed] [Google Scholar]
- Grousl T, Ivanov P, Frydlova I, Vasicova P, Janda F, Vojtova J, Malinska K, Malcova I, Novakova L, Janoskova D, et al. (2009). Robust heat shock induces eIF2alphaphosphorylation-independent assembly of stress granules containing eIF3 and 40S ribosomal subunits in budding yeast, Saccharomyces cerevisiae. J. Cell Sci 122, 2078–2088. [DOI] [PubMed] [Google Scholar]
- Grousl T, Ivanov P, Malcova I, Pompach P, Frydlova I, Slaba R, Senohrabkova L, Novakova L, and Hasek J (2013). Heat shock-induced accumulation of translation elongation and termination factors precedes assembly of stress granules in S. cerevisiae. PLoS One 8, e57083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén-Boixet J, Kopach A, Holehouse AS, Wittmann S, Jahnel M, Schlüßler R, Kim K, Trussina IREA, Wang J, Mateju D, et al. (2020). RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 181, 346–361.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, and Shorter J (2015). It’s Raining Liquids: RNA Tunes Viscoelasticity and Dynamics of Membraneless Organelles. Mol. Cell 60, 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzikowski AR, Chen YS, and Zid BM (2019). Stress-induced mRNP granules: Form and function of processing bodies and stress granules. Wiley Interdiscip. Rev. RNA 10, e1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwon Y, Maxwell BA, Kolaitis R-M, Zhang P, Kim HJ, and Taylor JP (2021). Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science 372, eabf6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S, Kedersha N, Anderson P, and Ivanov P (2020). Molecular mechanisms of stress granule assembly and disassembly. Biochim. Biophys. Acta Mol. Cell Res 1868, 118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A, Courel M, Bénard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot J-B, Munier A, Fradet M, et al. (2017). P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol. Cell 68, 144–157.e5. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, and Jülicher F (2014). Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol 30, 39–58. [DOI] [PubMed] [Google Scholar]
- Iserman C, Altamirano CD, Jegers C, Friedrich U, Zarin T, Fritsch AW, Mittasch M, Domingues A, Hersemann L, Jahnel M, et al. (2020). Condensation of Ded1p Promotes a Translational Switch from Housekeeping to Stress Protein Production. Cell 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Kedersha N, and Anderson P (2019). Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb. Perspect. Biol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov PA, Chudinova EM, and Nadezhdina ES (2003a). Disruption of microtubules inhibits cytoplasmic ribonucleoprotein stress granule formation. Exp. Cell Res 290, 227–233. [DOI] [PubMed] [Google Scholar]
- Ivanov PA, Chudinova EM, and Nadezhdina ES (2003b). RNP stress-granule formation is inhibited by microtubule disruption. Cell Biol. Int 27, 207–208. [DOI] [PubMed] [Google Scholar]
- Jain A, and Vale RD (2017). RNA phase transitions in repeat expansion disorders. Nature 546, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, and Parker R (2016). ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 164, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayabalan AK, Adivarahan S, Koppula A, Abraham R, Batish M, Zenklusen D, Griffin DE, and Leung AKL (2021). Stress granule formation, disassembly, and composition are regulated by alphavirus ADP-ribosylhydrolase activity. Proc. Natl. Acad. Sci. U. S. A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehler C, Isensee J, Hucho T, Lehrach H, and Krobitsch S (2014). 5-Fluorouracil affects assembly of stress granules based on RNA incorporation. Nucleic Acids Res. 42, 6436–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Okamoto T, Fukuhara T, Kambara H, Morita E, Mori Y, Kamitani W, and Matsuura Y (2013). Japanese encephalitis virus core protein inhibits stress granule formation through an interaction with Caprin-1 and facilitates viral propagation. J. Virol 87, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, and Anderson P (2002). Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans 30, 963–969. [DOI] [PubMed] [Google Scholar]
- Kedersha N, and Anderson P (2009). Regulation of translation by stress granules and processing bodies. Prog. Mol. Biol. Transl. Sci 90, 155–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, and Anderson P (2000). Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol 151, 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, and Anderson P (2002). Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13, 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Ivanov P, and Anderson P (2013). Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci 38, 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, Thomas M, Lieberman J, McInerney GM, Ivanov P, et al. (2016). G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J. Cell Biol 212, 845–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, and Anderson P (1999). RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol 147, 1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiten-Schmitz J, Wagner K, Piller T, Kaulich M, Alberti S, and Müller S (2020). The Nuclear SUMO-Targeted Ubiquitin Quality Control Network Regulates the Dynamics of Cytoplasmic Stress Granules. Mol. Cell 79, 54–67.e7. [DOI] [PubMed] [Google Scholar]
- Khong A, Kerr CH, Yeung CHL, Keatings K, Nayak A, Allan DW, and Jan E (2017a). Disruption of Stress Granule Formation by the Multifunctional Cricket Paralysis Virus 1A Protein. J. Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, and Parker R (2017b). The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol. Cell 68, 808–820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler MA, and Bassell GJ (2006). Neuronal RNA granules: movers and makers. Neuron 51, 685–690. [DOI] [PubMed] [Google Scholar]
- Kramer S, Queiroz R, Ellis L, Webb H, Hoheisel JD, Clayton C, and Carrington M (2008). Heat shock causes a decrease in polysomes and the appearance of stress granules in trypanosomes independently of eIF2(alpha) phosphorylation at Thr169. J. Cell Sci 121, 3002–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwald S, Maharana S, Mateju D, Malinovska L, Nüske E, Poser I, Richter D, and Alberti S (2015). Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife 4, e06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwald S, Munder MC, Maharana S, Franzmann TM, Richter D, Ruer M, Hyman AA, and Alberti S (2018). Different Material States of Pub1 Condensates Define Distinct Modes of Stress Adaptation and Recovery. Cell Rep. 23, 3327–3339. [DOI] [PubMed] [Google Scholar]
- Law LMJ, Razooky BS, Li MMH, You S, Jurado A, Rice CM, and MacDonald MR (2019). ZAP’s stress granule localization is correlated with its antiviral activity and induced by virus replication. PLoS Pathog. 15, e1007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y-C, Fernandopulle MS, Wang G, Choi H, Hao L, Drerup CM, Patel R, Qamar S, Nixon-Abell J, Shen Y, et al. (2019). RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Cell 179, 147–164.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes I, Altab G, Raina P, and de Magalhães JP (2021). Gene Size Matters: An Analysis of Gene Length in the Human Genome. Front. Genet 12, 559998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low W-K, Dang Y, Schneider-Poetsch T, Shi Z, Choi NS, Merrick WC, Romo D, and Liu JO (2005). Inhibition of eukaryotic translation initiation by the marine natural product pateamine A. Mol. Cell 20, 709–722. [DOI] [PubMed] [Google Scholar]
- Lyon AS, Peeples WB, and Rosen MK (2020). A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, Annu K, Baker M, Perkerson RB, Kurti A, et al. (2017). TIA1 Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Promote Phase Separation and Alter Stress Granule Dynamics. Neuron 95, 808–816.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboubi H, and Stochaj U (2017). Cytoplasmic stress granules: Dynamic modulators of cell signaling and disease. Biochim. Biophys. Acta Mol. Basis Dis 1863, 884–895. [DOI] [PubMed] [Google Scholar]
- Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, Luo E-C, Krach F, Yang D, Sen A, et al. (2018). Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell 172, 590–604.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor-Kollet H, Siany A, Kedersha N, Knafo N, Rivkin N, Danino YM, Moens TG, Olender T, Sheban D, Cohen N, et al. (2020). Spatiotemporal Proteomic Analysis of Stress Granule Disassembly Using APEX Reveals Regulation by SUMOylation and Links to ALS Pathogenesis. Mol. Cell 80, 876–891.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateju D, and Chao JA (2021). Stress granules: Regulators or by-products? FEBS J. [DOI] [PubMed] [Google Scholar]
- Mateju D, Eichenberger B, Voigt F, Eglinger J, Roth G, and Chao JA (2020). Single-Molecule Imaging Reveals Translation of mRNAs Localized to Stress Granules. Cell 183, 1801–1812.e13. [DOI] [PubMed] [Google Scholar]
- Matheny T, Rao BS, and Parker R (2019). Transcriptome-Wide Comparison of Stress Granules and P-Bodies Reveals that Translation Plays a Major Role in RNA Partitioning. Mol. Cell. Biol 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny T, Van Treeck B, Huynh TN, and Parker R (2021). RNA partitioning into stress granules is based on the summation of multiple interactions. RNA 27, 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell BA, Gwon Y, Mishra A, Peng J, Nakamura H, Zhang K, Kim HJ, and Taylor JP (2021). Ubiquitination is essential for recovery of cellular activities after heat shock. Science 372, eabc3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui R, Sukarieh R, Bordeleau M-E, Kaufman RJ, Northcote P, Tanaka J, Gallouzi I, and Pelletier J (2006). Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation. Mol. Biol. Cell 17, 4212–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrea DM, and Kriwacki RW (2016). Phase separation in biology; functional organization of a higher order. Cell Commun. Signal 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, and Taylor JP (2015). Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SL, Morisaki T, Khong A, Lyon K, Parker R, and Stasevich TJ (2019). Multicolour single-molecule tracking of mRNA interactions with RNP granules. Nat. Cell Biol 21, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SL, Morisaki T, Stasevich TJ, and Parker R (2020). Coupling of translation quality control and mRNA targeting to stress granules. J. Cell Biol 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadezhdina ES, Lomakin AJ, Shpilman AA, Chudinova EM, and Ivanov PA (2010). Microtubules govern stress granule mobility and dynamics. Biochim. Biophys. Acta 1803, 361–371. [DOI] [PubMed] [Google Scholar]
- Namkoong S, Ho A, Woo YM, Kwak H, and Lee JH (2018). Systematic Characterization of Stress-Induced RNA Granulation. Mol. Cell 70, 175–187.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Scharf KD, and Neumann D (1983). Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell. Biol 3, 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Scharf KD, and Neumann D (1989). Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol. Cell. Biol 9, 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrón A, Iwasaki S, and Ingolia NT (2019). Proximity RNA Labeling by APEX-Seq Reveals the Organization of Translation Initiation Complexes and Repressive RNA Granules. Mol. Cell 75, 875–887.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas MD, Ivanov P, and Anderson P (2016). Mechanistic insights into mammalian stress granule dynamics. J. Cell Biol 215, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. (2015). A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Peng S, Li W, Yao Y, Xing W, Li P, and Chen C (2020). Phase separation at the nanoscale quantified by dcFCCS. Proc. Natl. Acad. Sci. U. S. A 117, 27124–27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss T, Baron-Benhamou J, Ansorge W, and Hentze MW (2003). Homodirectional changes in transcriptome composition and mRNA translation induced by rapamycin and heat shock. Nat. Struct. Biol 10, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Pushpalatha KV, and Besse F (2019). Local Translation in Axons: When Membraneless RNP Granules Meet Membrane-Bound Organelles. Front Mol Biosci 6, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke LC, and Neilson JR (2019). Differences between acute and chronic stress granules, and how these differences may impact function in human disease. Biochem. Pharmacol 162, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback JA, Katanski CD, Kear-Scott JL, Pilipenko EV, Rojek AE, Sosnick TR, and Drummond DA (2017). Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell 168, 1028–1040.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback JA, Eeftens JM, Lee DSW, Quinodoz SA, Beckers L, Becker LA, and Brangwynne CP (2022). Viscoelastic RNA entanglement and advective flow underlie nucleolar form and function. [DOI] [PMC free article] [PubMed]
- Ripin N, and Parker R (2021). Are stress granules the RNA analogs of misfolded protein aggregates? RNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo PK, Lee SJ, Jaiswal PB, Alber S, Kar AN, Miller-Randolph S, Taylor EE, Smith T, Singh B, Ho TS-Y, et al. (2018). Axonal G3BP1 stress granule protein limits axonal mRNA translation and nerve regeneration. Nat. Commun 9, 3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo PK, Kar AN, Samra N, Terenzio M, Patel P, Lee SJ, Miller S, Thames E, Jones B, Kawaguchi R, et al. (2020). A Ca2+-Dependent Switch Activates Axonal Casein Kinase 2α Translation and Drives G3BP1 Granule Disassembly for Axon Regeneration. Curr. Biol 30, 4882–4895.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samir P, Kesavardhana S, Patmore DM, Gingras S, Malireddi RKS, Karki R, Guy CS, Briard B, Place DE, Bhattacharya A, et al. (2019). DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez de Groot N, Armaos A, Graña-Montes R, Alriquet M, Calloni G, Vabulas RM, and Tartaglia GG (2019). RNA structure drives interaction with proteins. Nat. Commun 10, 3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, Riback JA, Bracha D, Eeftens JM, Iwanicki A, Wang A, et al. (2020). Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 181, 306–324.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck JE, Paul KR, Cascarina SM, and Ross ED (2019). The prion-like protein kinase Sky1 is required for efficient stress granule disassembly. Nat. Commun 10, 3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U, and Parker R (2003). Decapping and Decay of Messenger RNA Occur in Cytoplasmic Processing Bodies. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N (2019). Liquid- and solid-like RNA granules form through specific scaffold proteins and combine into biphasic granules. J. Biol. Chem 294, 3532–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasekharan SP, Zhang F, Saxena N, Huang JN, Kuo I-C, Low C, Bell R, Adomat H, Stoynov N, Foster L, et al. (2020). G3BP1-linked mRNA partitioning supports selective protein synthesis in response to oxidative stress. Nucleic Acids Res. 48, 6855–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson R, and Bailey-Serres J (2014). Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A 111, 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr N, Lederer M, Reinke C, Meyer S, Hatzfeld M, Singer RH, and Hüttelmaier S (2006). ZBP1 regulates mRNA stability during cellular stress. J. Cell Biol 175, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber D, Tauber G, Khong A, Van Treeck B, Pelletier J, and Parker R (2020a). Modulation of RNA Condensation by the DEAD-Box Protein eIF4A. Cell 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber D, Tauber G, and Parker R (2020b). Mechanisms and Regulation of RNA Condensation in RNP Granule Formation. Trends Biochem. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai N-P, and Wei L-N (2010). RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cell. Signal 22, 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck B, and Parker R (2018). Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies. Cell 174, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck B, Protter DSW, Matheny T, Khong A, Link CD, and Parker R (2018). RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl. Acad. Sci. U. S. A 201800038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace EWJ, Kear-Scott JL, Pilipenko EV, Schwartz MH, Laskowski PR, Rojek AE, Katanski CD, Riback JA, Dion MF, Franks AM, et al. (2015). Reversible, Specific, Active Aggregates of Endogenous Proteins Assemble upon Heat Stress. Cell 162, 1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg DE, Shah P, Eichhorn SW, Hussmann JA, Plotkin JB, and Bartel DP (2016). Improved Ribosome-Footprint and mRNA Measurements Provide Insights into Dynamics and Regulation of Yeast Translation. Cell Rep. 14, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JR, Matheny T, Jain S, Abrisch R, and Parker R (2016). Distinct stages in stress granule assembly and disassembly. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Cardenas AM, Marissen WE, and Lloyd RE (2007). Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2, 295–305. [DOI] [PubMed] [Google Scholar]
- Wilbertz JH, Voigt F, Horvathova I, Roth G, Zhan Y, and Chao JA (2019). Single-Molecule Imaging of mRNA Localization and Regulation during the Integrated Stress Response. Mol. Cell 73, 946–958.e7. [DOI] [PubMed] [Google Scholar]
- Yang P, Mathieu C, Kolaitis R-M, Zhang P, Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q, et al. (2020). G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 181, 325–345.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Shen Y, Garre E, Hao X, Krumlinde D, Cvijović M, Arens C, Nyström T, Liu B, and Sunnerhagen P (2014). Stress Granule-Defective Mutants Deregulate Stress Responsive Transcripts. PLoS Genet. 10, e1004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Triandafillou C, and Drummond DA (2019). Cellular sensing by phase separation: Using the process, not just the products. J. Biol. Chem 294, 7151–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Bard JAM, Pilipenko E, and Allan Drummond D (2021). Chaperones directly and efficiently disperse stress-triggered biomolecular condensates. [DOI] [PMC free article] [PubMed]
- Youn J-Y, Dunham WH, Hong SJ, Knight JDR, Bashkurov M, Chen GI, Bagci H, Rathod B, MacLeod G, Eng SWM, et al. (2018). High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies. Mol. Cell 69, 517–532.e11. [DOI] [PubMed] [Google Scholar]
- Zhang P, Fan B, Yang P, Temirov J, Messing J, Kim HJ, and Taylor JP (2019). Chronic optogenetic induction of stress granules is cytotoxic and reveals the evolution of ALS-FTD pathology. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, and O’Shea EK (2014). Promoter sequences direct cytoplasmic localization and translation of mRNAs during starvation in yeast. Nature 514, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


