Abstract
Background:
Black adults have a higher incidence of peripheral artery disease (PAD) and limb amputations than White adults in the United States. Given that peripheral endovascular intervention (PVI) is now the primary revascularization strategy for PAD, it is important to understand whether racial differences exist in PVI incidence and outcomes.
Methods:
Data from fee-for-service (FFS) Medicare beneficiaries ≥66 years of age from 2016 to 2018 were evaluated to determine age- and sex-standardized population-level incidences of femoropopliteal PVI among Black and White adults over the three-year study period. Patients’ first inpatient or outpatient PVIs were identified via claims codes. Age- and sex-standardized risks of the composite outcome of death and major amputation within one year of PVI were examined by race.
Results:
Black adults underwent 928 PVIs per 100,000 Black beneficiaries compared with 530 PVIs per 100,000 White beneficiaries (risk ratio [RR] = 1.75, 95% CI 1.73–1.77, p<0.01). Black adults who underwent PVI were younger (mean age 74.5 vs. 76.4 years, p<0.01), more likely to be female (52.8% vs.42.7%, p<0.01), and had a higher burden of diabetes (70.6% vs 56.0%, p<0.01), chronic kidney disease (67.5% vs 56.6%, p<0.01), and heart failure (47.4% vs 41.7%, p<0.01) than White adults. When analyzed by indication for revascularization, Black adults were more likely to undergo PVI for CLTI than White adults (13,023/21,352 [61.0%] vs. 59,956/120,049 [49.9%], p<0.01). There was a strong association between Black race and the composite outcome at one year (OR = 1.21, 95% CI 1.16–1.25). This association persisted after adjustment for socioeconomic status (OR = 1.08, 95% CI 1.03–1.13) but was eliminated after adjustment for comorbidities (OR = 0.96, 95% CI 0.92–1.01).
Conclusions:
Among FFS Medicare beneficiaries, Black adults had substantially higher population-level PVI incidence and were significantly more likely to experience adverse events following PVI than White adults. The association between Black race and adverse outcomes appears to be driven by a higher burden of comorbidities. This analysis emphasizes the critical need for early identification and aggressive management of PAD risk factors and comorbidities to reduce Black-White disparities in the development and progression of PAD and the risk of adverse events following PVI.
Keywords: peripheral endovascular revascularization, peripheral artery disease, disparities
Introduction
Peripheral artery disease (PAD) is a common and debilitating condition.1 Atherosclerosis of the lower extremities may result in pain, ulceration, and tissue loss, as well as functional limitations. Amputation, an end-stage complication of PAD, has major physical, economic, and emotional repercussions.2 PAD is also an indicator of systemic disease and carries a poor prognosis with an estimated all-cause mortality of 33.1% over ten years.3 Patients with PAD, even those who are asymptomatic, have a high risk of cardiovascular and cerebrovascular events.4 Despite the prevalence and significant health consequences of PAD, there is a low national awareness of the condition resulting in widespread underdiagnosis and undertreatment.5
Black adults in the United States bear a disproportionate burden of PAD. Starting in the fifth decade of life, Black adults have rates of PAD that are at least double those of White adults and this disparity increases with age.6 In addition to having a higher burden of PAD, Black adults with PAD are significantly more likely to undergo major and minor lower extremity amputation than White adults with PAD.7 Black adults are routinely undertreated for PAD and PAD risk factors.8,9
Revascularization is central to the management of patients with advanced PAD – those with medication-refractory claudication and chronic limb-threatening ischemia (CLTI). Revascularization strategies for PAD have undergone a significant evolution over the last decade with peripheral endovascular intervention (PVI) now the primary modality of revascularization for patients with PAD as opposed to open surgical approaches.10 This has resulted in notable changes to the populations treated with PVI including more revascularization of patients with milder disease (i.e. claudication as opposed to CLTI) and more revascularization performed in the outpatient setting.11,12
Given the changing landscape of interventional therapies for PAD and the significant racial disparities among those with PAD, it is critical to better understand racial differences in patients treated with PVI and in outcomes associated with PVI. This analysis leverages Medicare claims data to answer the following two questions. First, how does the incidence of femoropopliteal PVI differ between Black and White Medicare beneficiaries on a population level? Second, how does the incidence of adverse events such as major amputation and death following PVI compare between these groups?
Methods
The data that support this analysis are available from the corresponding author upon request. This study was evaluated by the Institutional Review Board at Beth Israel Deaconess Medical Center, which waived the need for approval as human subject research was not involved.
Study Population
To evaluate population-level incidence of femoropopliteal PVI per 100,000 fee-for-service (FFS) Medicare beneficiaries from 2016 to 2018, all individual FFS Medicare beneficiaries were included in the study cohort. Within this population, all patients who underwent femoropopliteal PVI were identified. Patients who underwent outpatient PVI were identified in 100% samples of the Carrier FFS files and the institutional outpatient files using CPT insurance claims codes (Figure S1). For those who underwent inpatient revascularization, PVI was identified using ICD-10-PCS insurance claims codes in the CMS Medicare Provider Analysis and Review (MedPAR) files. Patients were excluded if they had less than one year of Medicare FFS enrollment prior to their index procedure (which allows for identification of comorbidities), were not consistently enrolled in Medicare FFS, or were of non-White or non-Black race.
Next, the cohort described above that underwent PVI, with the additional exclusion of patients who did not maintain enrollment in Medicare FFS for one year after PVI, was used to examine outcomes (Figure S1). For patients who underwent multiple procedures during the study period, only the first procedure was included in the analysis. If bilateral PVIs were performed during the index procedure, this was considered a single PVI in the analysis. Prescriptions for cardiovascular medications were evaluated in patients with Medicare Part D coverage.
Baseline Characteristics
Patient race was obtained from the Medicare enrollment database (EDB) in the Master Beneficiary Summary File (MBSF). The EDB has high validity for identifying Black and White races.13 Race is reported at the time of enrollment in Medicare. Baseline demographics (e.g. age and sex) were determined as of the index procedure date. Clinical comorbidities were ascertained using the Chronic Conditions Data Warehouse (CCW).14 The CCW has data on 27 comorbidities identified using a lookback period of one to three years and using claims from multiple clinical care settings (i.e. inpatient and outpatient care settings). In addition to these comorbidities, ICD-9-CM and ICD-10-CM claims codes were applied over a one-year lookback period to identify current or prior tobacco use and prior lower extremity amputation (Table S3). Similarly, indications for PVI were evaluated using claims codes over the one-year period preceding the index PVI and were categorized as claudication, CLTI, or other indications (defined as absence of claudication or CLTI claims codes). Hospital characteristics were retrieved from the 2016 American Heart Association Annual Survey File which includes hospital teaching status, region, and bed capacity.
In order to further account for measures of socioeconomic status, we distinguished between patients in rural versus urban residences by matching their zip codes with the 2010 rural-urban commuting area (RUCA) codes from U.S. census tract data.15 We determined whether patients were from distressed communities through linkage with the distressed community index (DCI). The DCI assesses economic well-being at the zip code level using seven socioeconomic indicators.16 Higher DCI scores are indicative of greater levels of socioeconomic distress. We also identified low-income Medicare beneficiaries based on dual enrollment in Medicare and Medicaid for at least one month in a given year.17
Cardiovascular medication utilization at the time of PVI was evaluated among patients enrolled in Medicare Part D during the study period. Medication use was categorized by angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs), angiotensin receptor neprilysin inhibitors (ARNIs), anticoagulants, P2Y12 inhibitors, beta-blockers, lipid-lowering agents, diabetes medications, diuretics, aldosterone receptor antagonists (MRAs), and phosphodiesterase 3 (PDE3) inhibitors.
Outcomes
The primary outcome for the first aim of this analysis was population-level incidence of PVI among included Medicare beneficiaries. The primary outcome for the second aim of this analysis was the incidence of adverse events among those who underwent PVI defined as a composite of death or major lower extremity amputation within one year of PVI. If a patient underwent multiple PVIs, the composite outcome was determined relative to the first PVI. Major lower extremity amputations were ascertained using CPT and ICD-10-PCS claims codes (Table S2). Death was ascertained using vital status data from the CMS Vital Status File.
Statistical Analyses
Baseline patient and hospital characteristics were presented as continuous variables with mean ± standard deviation and were compared using Student’s t-test. Categorical variables were presented as frequencies and percentages and were compared with Pearson’s chi-square tests.
To compare PVI use over the three-year study period, the incidence of PVI use per 100,000 beneficiaries was calculated for each race group. To avoid the influence of outliers, each patient could only contribute one (the first) procedure to the analysis. The direct standardization method was then applied to standardize incidence of PVI use by age and sex using the age and sex distribution of the Medicare FFS population in the year 2016 as a reference (Table S4). Age- and sex-standardized risks for each race group were reported and relative risks with 95% confidence intervals were estimated to compare risks between Black and White patients. Subgroup analyses were performed by indication for PVI. Findings were also stratified by United States census region to evaluate for regional consistency of findings.
To evaluate the risks of the composite endpoint of death and major amputation within one year across race groups, similar methods of age- and sex-adjustment as described above were applied to the population of patients who underwent PVI and had one year of follow-up. In order to understand the impact of regional, socioeconomic, and comorbidity characteristics on one-year outcomes by Black versus White race, stepwise variable selection was used. In each model, variable selection was used with a significance level of 0.1 for entering predictors into the model and 0.1 for removing predictors from the model. Race (Black versus White) was forced into each model. Odds ratios with 95% confidence intervals were reported for each model. The order of variables included age and sex, DCI (highly distressed defined as top quartile of DCI scores), dual enrollment in Medicare and Medicaid, residence in rural-urban location, hospital and procedure variables, and comorbidities. The full list of these variables can be found in Table S5.
All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, North Carolina), using a two-sided p-value less than 0.05 to define significance.
Results
PVI Incidence by Race
Of 40,393,685 FFS Medicare patients included, 215,320 patients underwent PVI during the study period and remained after exclusions, which resulted in 0.86% of Black Medicare beneficiaries having undergone PVI compared with 0.51% of White Medicare beneficiaries. After standardizing by age and sex, 928 (95% CI 918–939) per 100,000 Black Medicare beneficiaries underwent PVI compared with 530 (95% CI 527–532) per 100,000 White Medicare beneficiaries over the three-year study period (Figure 1). The age- and sex-standardized relative risk of undergoing PVI as a Black adult versus a White adult was 1.75 (95% CI 1.73–1.77, p<0.01). This pattern was consistent across all census regions of the United States (Figure 1).
Figure 1. Age- and sex-adjusted population-level incidence of PVI in Black and White Medicare beneficiaries from 2016–2018.
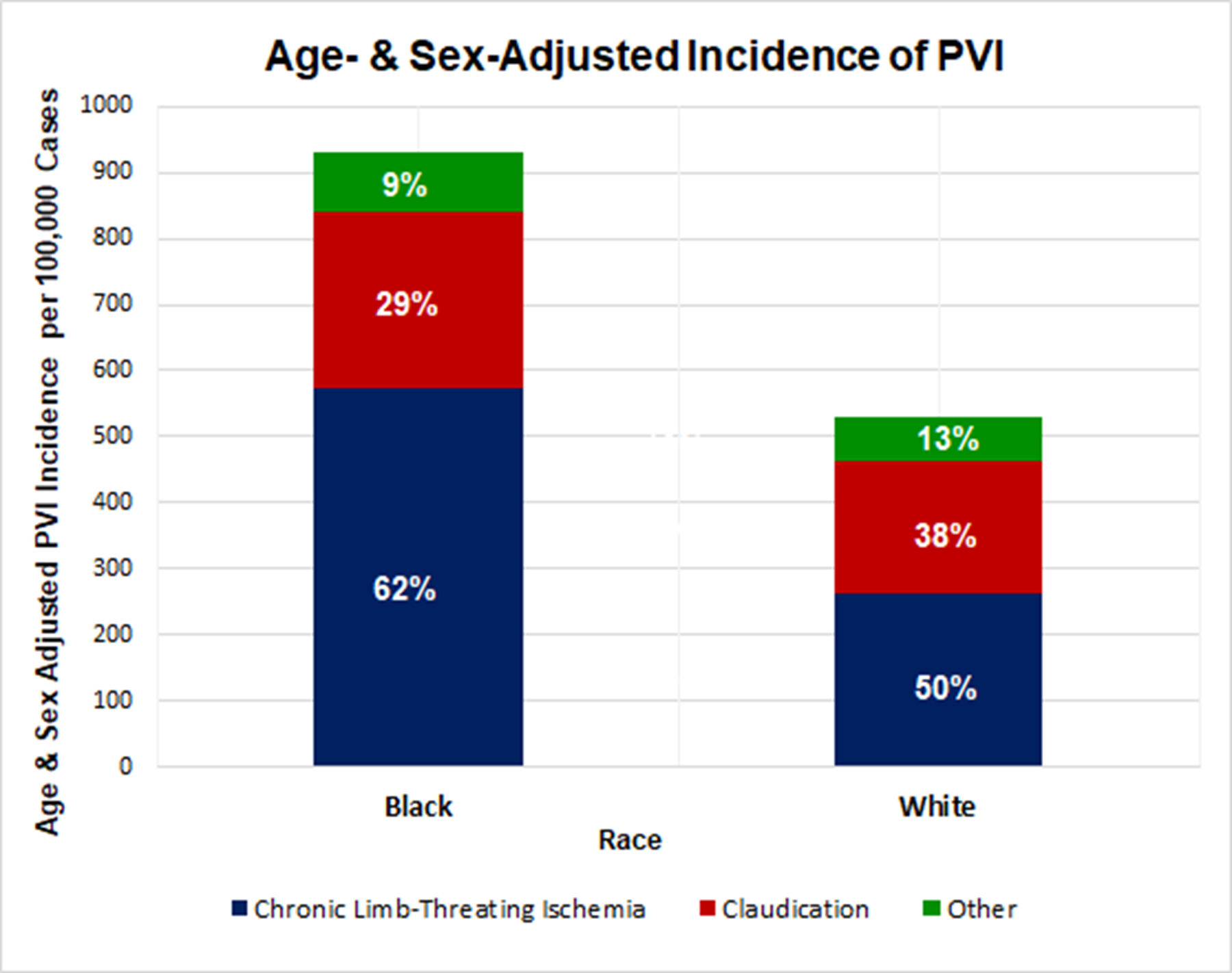
Black adults have a higher incidence of PVI than White adults from 2016 to 2018. Black beneficiaries underwent 928 PVIs per 100,000 Black beneficiaries compared with 530 PVIs per 100,000 White beneficiaries over the three-year study period (risk ratio [RR] = 1.75 for Black versus White, 95% CI 1.73–1.77, p<0.01).
When stratified by indication for PVI, a greater proportion of Black adults were treated with CLTI compared with White adults (61.0% vs. 49.9%, p<0.01), suggesting that Black adults presented for PVI with a greater severity of disease (Figure 2). A lower proportion of Black adults were referred for PVI due to claudication compared with White adults (29.6% versus 37.6%, p<0.01).
Figure 2. Relative risk of undergoing PVI in Black and White Medicare beneficiaries from 2016–2018 divided by census regions of the U.S.
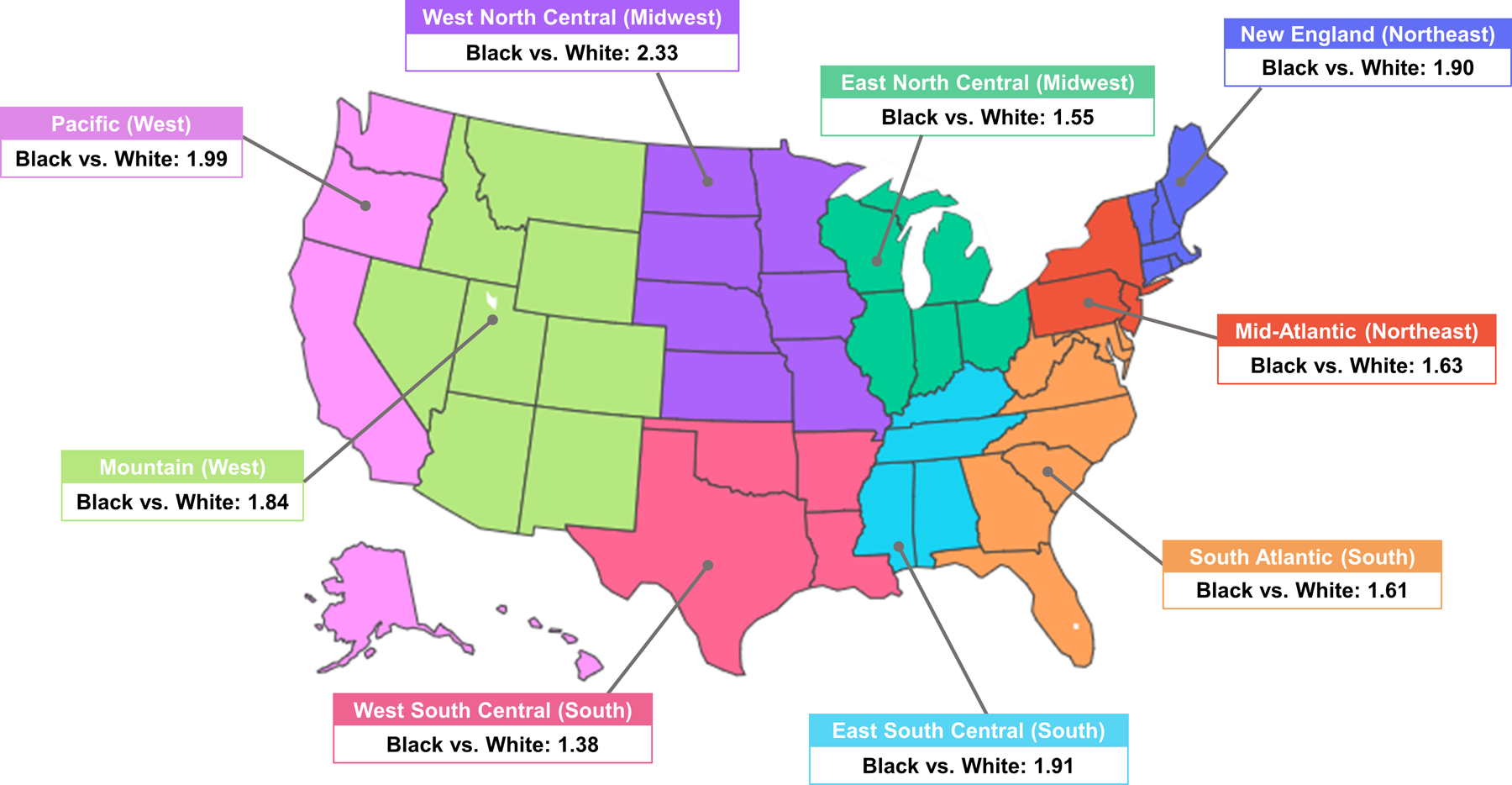
Black adults are more likely to undergo PVI in all regions of the United States. There is a statistically significant difference in PVI incidence between Black and White adults in every census region (Table 1).
Outcomes Following PVI by Race
After further exclusion of patients who underwent PVI but did not have at least one year of follow-up data, 141,401 patients remained for evaluating outcomes (Figure S1). Of these patients, 21,352 (15.1%) were Black and 120,049 (84.9%) were White (Table 1). Black adults were younger (average age of 74.5 vs. 76.4 years, p<0.01) and more likely to be female (52.8% vs. 42.7%, p<0.01) than were White adults. Black adults also had a higher prevalence of diabetes (70.6% vs. 56.0%, p<0.01), chronic kidney disease (67.5% vs. 56.6%, p<0.01), heart failure (47.4% vs. 41.7%, p<0.01), and stroke (14.2% vs. 10.2%, p<0.01) compared with White adults. Black adults were more likely to be dually enrolled in Medicare and Medicaid (42.3% vs. 16.4%, p<0.01) and were more likely to be from distressed communities (49.6% vs. 20.3%, p<0.01) than were White adults (Table 1). White adults were more likely to live in rural locations than were Black adults (4.5% vs. 1.6%, p<0.01). Of the 141,401 patients, 104,699 (74.0%) had medication data available at the time of PVI.
Table 1.
Baseline characteristics of patients who underwent femoropopliteal endovascular revascularization stratified by race.
| All Race Groups (N=141,401) |
Black (N=21,352) |
White (N=120,049) |
P-Value | |
|---|---|---|---|---|
| Age, mean (SD) | 76.1±7.9 | 74.5±7.9 | 76.4±7.8 | <.001 |
| Female, % | 44.2 | 52.8 | 42.7 | <.001 |
| Dual Enrollment, % | 20.3 | 42.3 | 16.4 | <.001 |
| DCI ≥ 75th Percentile, % | 24.7 | 49.6 | 20.3 | <.001 |
| Rural, % | 4.0 | 1.6 | 4.5 | <.001 |
| PAD Symptoms | ||||
| CLTI, % | 51.6 | 61.0 | 49.9 | <.001 |
| Claudication, % | 36.4 | 29.6 | 37.6 | <.001 |
| Other, % | 12.0 | 9.4 | 12.5 | <.001 |
| Comorbidities | ||||
| Chronic Kidney Disease, % | 58.2 | 67.5 | 56.6 | <.001 |
| Diabetes, % | 58.2 | 70.6 | 56.0 | <.001 |
| Hyperlipidemia, % | 81.1 | 78.5 | 81.5 | <.001 |
| Hypertension, % | 92.6 | 94.5 | 92.2 | <.001 |
| Tobacco, % | 47.3 | 45.8 | 47.6 | <.001 |
| Acute Myocardial Infarction, % | 5.1 | 4.8 | 5.1 | 0.037 |
| Atrial Fibrillation, % | 20.4 | 12.4 | 21.8 | <.001 |
| Heart Failure, % | 42.6 | 47.4 | 41.7 | <.001 |
| COPD/Bronchiectasis, % | 34.1 | 27.9 | 35.2 | <.001 |
| Ischemic Heart Disease, % | 74.6 | 70.8 | 75.3 | <.001 |
| Prior Lower Extremity Amputation, % | 7.2 | 11.2 | 6.5 | <.001 |
| Stroke/Transient Ischemic Attack, % | 10.8 | 14.2 | 10.2 | <.001 |
| Hospital Characteristics | ||||
| Teaching Hospital, % | 79.3 | 84.8 | 78.4 | <0.001 |
| Region | ||||
| Northeast Central, % | 13.4 | 11.5 | 13.7 | <.001 |
| Southeast Central, % | 6.2 | 8.7 | 5.8 | <.001 |
| Northwest Central, % | 5.7 | 2.1 | 6.3 | <.001 |
| Southwest Central, % | 10.0 | 11.0 | 9.8 | <.001 |
| Mid Atlantic, % | 8.8 | 7.3 | 9.1 | <.001 |
| Mountain, % | 3.5 | 0.8 | 3.9 | <.001 |
| Northeast, % | 3.2 | 1.1 | 3.5 | <.001 |
| Other, % | 0.1 | 0.1 | 0.1 | <.001 |
| Pacific, % | 33.8 | 36.1 | 33.4 | <.001 |
| South Atlantic, % | 15.3 | 21.4 | 14.2 | <.001 |
| Bed Size | ||||
| 6 to 24, % | 0.1 | 0.1 | 0.1 | <.001 |
| 25 to 49, % | 0.7 | 0.5 | 0.7 | <.001 |
| 50 to 99, % | 3.4 | 1.8 | 3.6 | <.001 |
| 100 to 199, % | 40.0 | 43.0 | 39.5 | <.001 |
| 200 to 299, % | 14.7 | 10.4 | 15.5 | <.001 |
| 300 to 399, % | 13.3 | 12.7 | 13.4 | <.001 |
| 400 to 499, % | 8.1 | 8.3 | 8.0 | <.001 |
| ≥ 500, % | 19.8 | 23.1 | 19.2 | <.001 |
Abbreviations: CLI=Chronic Limb-Threatening Ischemia; COPD=Chronic Obstructive Pulmonary Disease; DCI=Distressed Community Index; PAD=Peripheral Artery Disease; SD=Standard Deviation
Despite more cardiovascular comorbidities and greater severity of PAD, a lower percentage of Black adults was prescribed key cardiovascular medications. This included lower use of statins (55.3% vs. 60.0%), PCSK9 inhibitors (0.1% vs. 0.2%), and ACE-inhibitors (26.2% vs 30.4%) (Table 2). Furthermore, Black adults were prescribed antiplatelet medications at lower rates (44.5% vs. 48.1%).
Table 2.
Rates of prescriptions of key cardiovascular medications by race.
| All Race Groups (N=104,699) |
Black (N=16,998) |
White (N=87,701) |
P-Value | |
|---|---|---|---|---|
| ACE Inhibitor/ARB, % | 44.83 | 40.85 | 45.61 | <.001 |
| ACE Inhibitor, % | 29.74 | 26.20 | 30.42 | <.001 |
| ARB, % | 15.66 | 15.30 | 15.73 | 0.158 |
| ARNI, % | 0.30 | 0.22 | 0.32 | 0.038 |
| Anticoagulant, % | 15.77 | 11.15 | 16.66 | <.001 |
| VKA, % | 8.54 | 5.95 | 9.04 | <.001 |
| DOAC, % | 6.94 | 4.92 | 7.33 | <.001 |
| Other, % | 1.54 | 0.96 | 1.65 | <.001 |
| Antiplatelet, % | 47.52 | 44.51 | 48.10 | <.001 |
| Beta-blocker, % | 52.94 | 50.02 | 53.50 | <.001 |
| Cholesterol | ||||
| Bile Acid Binding, % | 0.71 | 0.49 | 0.75 | <.001 |
| Fibrates, % | 4.22 | 1.41 | 4.76 | <.001 |
| Statins, % | 59.22 | 55.32 | 59.97 | <.001 |
| PCSK9 Inhibitors, % | 0.19 | 0.11 | 0.21 | 0.005 |
| Other, % | 3.41 | 2.62 | 3.56 | <.001 |
| Diabetes | ||||
| Biguanides, % | 15.70 | 13.38 | 16.14 | <.001 |
| DPP-4, % | 5.53 | 5.99 | 5.43 | 0.003 |
| GLP-1 Agonists, % | 1.09 | 0.82 | 1.14 | <.001 |
| Insulin, % | 18.51 | 22.17 | 17.80 | <.001 |
| Meglitinides, % | 0.61 | 0.62 | 0.60 | 0.823 |
| SGLT2 Inhibitor, % | 0.97 | 0.71 | 1.02 | <.001 |
| Sulfonylureas, % | 11.92 | 10.97 | 12.11 | <.001 |
| Thiazolidinediones, % | 1.58 | 1.52 | 1.60 | 0.447 |
| Other, % | 0.16 | 0.18 | 0.16 | 0.593 |
| Diuretics | ||||
| Carbonic, % | 0.02 | 0.04 | 0.02 | 0.047 |
| Loop Diuretics, % | 24.86 | 22.11 | 25.40 | <.001 |
| Thiazides, % | 16.60 | 18.81 | 16.17 | <.001 |
| Potassium Sparing, % | 6.25 | 5.61 | 6.37 | <.001 |
| MRA (%) | 0.14 | 0.08 | 0.15 | 0.016 |
| Nitrates (%) | 8.16 | 9.67 | 7.86 | <.001 |
| PDE3 inhibitor (%) | 6.68 | 6.55 | 6.71 | 0.438 |
Abbreviations: ACE=angiotensin-converting enzyme; ARB=angiotensin receptor blockers; ARNI=angiotensin receptor-neprilysin inhibitor; DOAC=direct oral anticoagulants; DPP-4=dipeptidyl peptidase-4; GLP-1=glucagon-like peptide-1; MRA=mineralocorticoid receptor antagonists; PCSK9=proprotein convertase subtilisin/kexin type 9; PDE3=phosphodiesterase-3; SGLT2=sodium-glucose co-transporter-2; VKA=vitamin K antagonists
After adjustment for age and sex, Black adults who underwent PVI were more likely to experience the composite outcome of major amputation or death compared with White adults (25.0% [95% CI 24.45–25.61] vs 18.6% [95% CI 18.39–18.85]) (Table 3). This translated into Black adults having a 34% greater likelihood of experiencing major amputation or death within one year after PVI (95% CI 1.31–1.38, p<0.01). Furthermore, after stratification for indication for PVI, Black adults with CLTI who underwent PVI were more likely to experience the composite outcome of major amputation or death compared with White adults with CLTI (33.7% [95% CI 32.89–34.53] vs. 27.6% [95% CI 27.23–28.01]), which equated to a 22% increased risk of experiencing the composite outcome for Black versus White adults with CLTI (95% CI 1.19–1.26, p<0.01). Similar findings of increased risks among Black adults were observed for PVI indications of claudication and other (Table 3).
Table 3.
Incidence of the composite outcome of death and major amputation stratified by race and indication for peripheral endovascular intervention (PVI).
| Race | Age and Sex-adjusted Outcome Incidence | Chronic Limb-Threatening Ischemia | Claudication | Other |
|---|---|---|---|---|
| Black, % | 25.03 (24.45, 25.61) |
33.71 (32.89, 34.53) |
8.56 (7.86, 9.27) |
21.30 (19.49, 23.10) |
| White, % | 18.62 (18.39, 18.85) |
27.62 (27.23, 28.01) |
7.08 (6.82, 7.33) |
17.94 (17.29,18.59) |
Variables Associated with Increased Risk of Major Amputation or Death among Black Adults
Black race was significantly associated with worse one-year outcomes following PVI after adjustment for age and sex (Table 4). This association persisted following sequential adjustment for individual and regional socioeconomic measures (dual Medicare-Medicaid enrollment, distressed community status, and rural residence) as well as hospital and procedural factors. However, the association between Black race and the composite outcome was no longer apparent after adjustment for comorbidities (OR = 0.96, 95% CI 0.92–1.01).
Table 4.
Sequential regression models examining associations between key variables and the composite outcome of death and amputation.
| Candidate Variables | Odds Ratio (95% Confidence Interval) |
|---|---|
| Black vs White | |
| Age, Sex | 1.21 (1.16, 1.25) |
| Age, Sex Distressed Community Index (DCI) |
1.18 (1.13, 1.23) |
| Age, Sex DCI Dual Medicare/Medicaid Enrollment |
1.08 (1.03, 1.13) |
| Age, Sex DCI Dual Medicare/Medicaid Enrollment Rural/Urban |
1.07 (1.03, 1.12) |
| Age, Sex DCI Dual Medicare/Medicaid Enrollment Rural/Urban Hospital/Procedural Variables |
1.05 (1.01, 1.10) |
| Age, Sex DCI Dual Medicare/Medicaid Enrollment Rural/Urban Hospital/Procedural Variables Comorbidities |
0.96 (0.92, 1.01) |
All candidate variables were included in all models with the exception of the final model. In the final model, the following variables were not selected: dual Medicare/Medicaid enrollment, rural-urban location, breast cancer, endometrial cancer, ischemic heart disease. See Table S5 for specific hospital/procedure variables and comorbidities included in the model.
Discussion
The use of PVI has increased dramatically over time, and it has become a first-line modality of revascularization for PAD. Despite the importance of PVI in PAD care, there are limited data regarding Black-White disparities in PVI use and in clinical outcomes associated with PVI. This nationwide analysis of the Medicare population, identified marked differences in population-level PVI incidence between Black and White adults. Black adults underwent significantly more PVIs than White adults. Among patients undergoing PVI, Black adults were treated for more advanced stages of PAD than White adults. In addition, Black adults were significantly more likely to experience major amputation or death following PVI than White adults. The association between Black adults and adverse outcomes was no longer significant after adjusting for comorbid illness demonstrating that Black-White differences in comorbidities explain a significant portion of this relationship.
The finding that Black adults are more likely to undergo PVI at the population level than White adults indicates that Black adults have a higher burden of advanced PAD than White adults. This finding likely reflects a higher prevalence of PAD in the Black population, which has been previously documented.6
While all patients who undergo PVI have an advanced form of PAD, a higher proportion of Black adults underwent PVI for the indication of CLTI than White adults even within the cohort of patients who underwent PVI. CLTI is the most severe form of PAD and carries the highest risk of both death and amputation.18 Within one year of receiving a diagnosis of CLTI, only 50% of patients are alive and have not undergone amputation.18
After undergoing PVI for PAD, Black adults were significantly more likely to experience adverse outcomes than White adults. This was true when Black and White adults who underwent PVI for the same indication (CLTI, claudication, or other) were compared. These results parallel those of a prior study using California discharge records from 2005–2009 which found that Black adults had significantly higher amputation rates than White adults following endovascular procedures for PAD.19
There are several systemic and structural issues that may contribute broadly to the identified disparities. Black adults have lower average wealth, lower average income, and higher rates of unemployment, and are more likely to live in poverty than White adults.20,21 Black adults have lower access to education, which, along with low household income, has been associated with the development of PAD.22 Black adults are less likely to have health insurance23,24 and have more limited health care access compared with White adults.25 Structural racism perpetuates these health disparities.21
One important way in which these systemic and structural factors lead to worse outcomes in Black patients is through comorbidities. Black-White disparities in the prevalence of PAD risk factors including hypertension,26 diabetes, and end-stage renal disease are well-documented.27
In this analysis, the extent to which variables including comorbidities, measures of individual and regional wealth, geographic variation, and hospital and procedure-related factors were able to explain the association between race and adverse outcomes was evaluated. Black race was no longer associated with an increase in adverse outcomes once comorbidities were included in the variable selection model. This suggests a critical need for targeted, upstream intervention to reduce the disproportionate development of PAD in the Black population, reduce the disproportionate development of advanced disease within the PAD population, and improve outcomes for patients with advanced PAD who require PVI. Prior studies have demonstrated the meaningful impact of management of comorbid conditions on PAD outcomes. In particular, poor diabetes control has been associated with greater risks of major and more proximal amputations.28,29 This analysis found that Black adults had a higher burden of risk factors, such as diabetes and chronic kidney disease, known to be associated with both the development of PAD and with worse outcomes in those with PAD. In addition, Black adults were less likely than White adults to be prescribed key medical therapies for the treatment of PAD and for risk factors for PAD. Notable examples that are known to improve outcomes for patients with PAD include statins,30 PCSK9 inhibitors,31 and ACE inhibitors.32 This race-based difference in medical therapies for PAD was particularly striking as this analysis evaluated medication utilization at the time of PVI when PAD is advanced and requires intervention. This analysis also found that Black adults were more likely to have low individual wealth and reside in distressed communities.
The complex interplay of social determinants of health, structural racism, and risk factors results in the downstream effects of a higher burden of PAD in the Black population, and, eventually, more advanced PAD and a higher incidence of procedures on a population-level than in White adults. The American Heart Association recently published a policy statement calling for a 20% reduction in amputations by the year 2030.33 Achieving this goal will require particular attention to racial disparities in all aspects of PAD care.
Limitations
There are notable strengths and limitations of this study. Strengths include that the Medicare database likely captures a large proportion of patients with PAD nationwide as PAD typically manifests in older age when most U.S. adults are insured by Medicare. In addition, this analysis captures care in multiple clinical settings and uses validated algorithms to ascertain important procedural and comorbidity data.
Important limitations include that this analysis examined the use of PVI among the total Medicare population as opposed to exclusively those with PAD. This was necessary because Medicare insurance claims codes are unlikely to accurately define a cohort of patients with PAD due to the frequent diagnosis and management of PAD in the outpatient setting and the possibility of diagnosis prior to enrollment in Medicare. As such, this analysis is unable to assess the frequency of PVI in to patients with PAD. In addition, this analysis included only FFS Medicare beneficiaries and, therefore, the results may not be generalizable to other populations. The Medicare database does not provide granular anatomic or imaging details to better understand the severity of disease or all procedural details. Furthermore, this analysis only included patients who underwent femoropopliteal PVI, as this is the most frequent site of endovascular therapy. It is possible that these findings may not be generalizable to revascularizations of other arterial segments, and future studies may be needed to examine the reproducibility of these findings in other arterial segments. Also, although important sociodemographic and clinical characteristics were included in the regression analysis, this may still be subject to some degree of unmeasured confounding. Lastly, the constraints of the Medicare database do not allow for robust analysis of the impact of ethnicity on the racial differences seen.
Conclusions
In this nationwide analysis of the FFS Medicare population, there was a substantial difference in the population-level PVI incidence between Black and White adults – Black adults had a significantly higher incidence of PVI than White adults. In addition, Black adults were significantly more likely to experience death or major amputation within one year following PVI than White adults. The relationship between Black race and adverse events following PVI appears to be driven, in part, by the greater burden of comorbidities in Black adults compared with White adults. Therefore, this analysis emphasizes the critical need for increased clinical and health system efforts targeting the Black population to reduce the downstream effects of a higher prevalence of PAD, more advanced PAD, and a greater likelihood of major amputation or death following PVI in the Black population relative to the White population.
Supplementary Material
Clinical Perspective.
What is new?
This is a large, nationwide analysis documenting a higher incidence of peripheral vascular interventions in Black adults compared with White adults.
Black adults have a significantly higher risk of major amputation or death following PVI than White adults.
The increase in risk of adverse outcomes in Black adults compared with White adults following PVI appears to be explained by a higher burden of medical comorbidities in Black adults.
Black adults undergoing PVI are less likely to be treated with guideline-directed medical therapy for PAD than White adults.
What are the clinical implications?
Early diagnosis and aggressive treatment of risk factors and medical comorbidities are crucial for eliminating the striking racial disparities in PAD.
Special attention must be paid to ensure that all patients with PAD, and in particular Black adults, are treated with guideline-directed medical therapies.
Sources of Funding
Dr. Secemsky is funded by the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH/NHLBI K23HL150290). The Smith Center has received unrestricted research funding from Boston Scientific.
Abbreviations:
- CMS
Centers for Medicare & Medicaid Services
- CLTI
Chronic limb threatening ischemia
- FFS
Fee-for-service
- PVI
Peripheral Vascular Intervention
Footnotes
Conflicts of Interest/Disclosures
Dr. Krawisz receives grant support from the John S. LaDue Memorial Fellowship. Dr. Yeh reports grant support from AstraZeneca, Abbott Vascular, BD Bard, Boston Scientific, Cook Medical, Medtronic, Microport, and Philips; consulting fees from Abbott Vascular, AstraZeneca, Boston Scientific, Medtronic, Shockwave, and Zoll. Dr. Jaff is a part-time employee of Boston Scientific; he is an advisor to Gilde; he is an equity investor in Efemoral, Embolitech, R3 Vascular, Vactronix, Venarum. Dr. Giri reports research funds from Boston Scientific, Inari Medical, Abiomed, and BioSense Webster; he is on the advisory Boards for Boston Scientific, Inari Medical, and Astra Zeneca. Dr. Julien reports stock in Johnson & Johnson and Shockwave medical. Dr. Secemsky reports grant support from NIH/NHLBI K23HL150290, the Food & Drug Administration, Harvard Medical School’s Shore Faculty Development Award, AstraZeneca, BD, Boston Scientific, Cook, CSI, Laminate Medical, Medtronic and Philips; he reports consulting fees from Abbott, Bayer, BD, Boston Scientific, Cook, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and VentureMed. All other authors have nothing to disclose.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Atherton R, Robertson N. Psychological adjustment to lower limb amputation amongst prosthesis users. Disabil Rehabil 2006;28:1201–1209. doi: 10.1080/09638280600551674 [DOI] [PubMed] [Google Scholar]
- 3.Sartipy F, Sigvant B, Lundin F, Wahlberg E. Ten Year Mortality in Different Peripheral Arterial Disease Stages: A Population Based Observational Study on Outcome. Eur J Vasc Endovasc Surg 2018;55:529–536. doi: 10.1016/j.ejvs.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH., Aboyans V. Epidemiology of Peripheral Artery Disease. Circ Res 2015; 116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849 [DOI] [PubMed] [Google Scholar]
- 5.Berger JS, Ladapo JA. Underuse of Prevention and Lifestyle Counseling in Patients With Peripheral Artery Disease. J Am Coll Cardiol 2017; 69:2293–2300. doi: 10.1016/j.jacc.2017.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-Specific Prevalence of Peripheral Arterial Disease in the United States. Am J Prev Med 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 7.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED. Temporal Trends and Geographic Variation of Lower Extremity Amputation in Patients with Peripheral Artery Disease: Results from U.S. Medicare 2000–2008. J Am Coll Cardiol 2012;60:2230–2236. doi: 10.1016/j.jacc.2012.08.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipworth L, Fazio S, Kabagambe EK, Munro HM, Nwazue VC, Tarone RE, McLaughlin JK, Blot WJ, Sampson UKA. A prospective study of statin use and mortality among 67,385 blacks and whites in the Southeastern United States. Clin Epidemiol 2013;6:15–25. doi: 10.2147/CLEP.S53492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035 [DOI] [PubMed] [Google Scholar]
- 10.Rowe VL, Weaver FA, Lane JS, Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998–2006. J Vasc Surg 2010;51:21S–26S. doi: 10.1016/j.jvs.2009.09.066 [DOI] [PubMed] [Google Scholar]
- 11.Jones WS, Mi X, Qualls LG, Vemulapalli S, Peterson ED, Patel MR, Curtis LH. Trends in Settings for Peripheral Vascular Intervention and the Effect of Changes in the Outpatient Prospective Payment System. J Am Coll Cardiol 2015;65:920–927. doi: 10.1016/j.jacc.2014.12.048 [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee D, Hashemi H, Contos B. The disproportionate growth of office-based atherectomy. J Vasc Surg 2017;65:495–500. doi: 10.1016/j.jvs.2016.08.112 [DOI] [PubMed] [Google Scholar]
- 13.Jarrín OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of Race and Ethnicity Codes in Medicare Administrative Data Compared With Gold-standard Self-reported Race Collected During Routine Home Health Care Visits. Med Care 2020;58:e1–e8. doi: 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chronic Conditions Data Warehouse. Center for Medicare and Medicaid Services https://www2.ccwdata.org/web/guest/condition-categories. Accessed August 1, 2020.
- 15.2010 Rural-Urban Commuting Area (RUCA) Codes. United States Department of Agriculture Economic Research Service https://www.ers.usda.gov/data-products/ruralurban-commuting-area-codes/documentation/. Accessed March 10, 2021.
- 16.Economic Innovation Group: Distressed Community Index https://eig.org/dci. Accessed August 20, 2020.
- 17.Wadhera RK, Wang Y, Figueroa JF, Dominici F, Yeh RW, Joynt Maddox KE. Mortality and Hospitalizations for Dually Enrolled and Nondually Enrolled Medicare Beneficiaries Aged 65 Years or Older, 2004 to 2017. JAMA 2020;323:961–969. doi: 10.1001/jama.2020.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073 [DOI] [PubMed] [Google Scholar]
- 19.Loja MN, Brunson A, Li CS, Carson JG, White RH, Romano PS, Hedayati N. Racial disparities in outcomes of endovascular procedures for peripheral arterial disease: an evaluation of California hospitals, 2005–2009. Ann Vasc Surg 2015;29:950–959. doi: 10.1016/j.avsg.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firebaugh G, Acciai F. For blacks in America, the gap in neighborhood poverty has declined faster than segregation. Proc Natl Acad Sci 2016;113:13372–13377. doi: 10.1073/pnas.1607220113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to Action: Structural Racism as a Fundamental Driver of Health Disparities: A Presidential Advisory From the American Heart Association. Circulation 2020;142 e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 22.Pande RL, Creager MA. Socioeconomic Inequality and Peripheral Artery Disease Prevalence in US Adults. Circ Cardiovasc Qual Outcomes 2014;7:532–539. doi: 10.1161/CIRCOUTCOMES.113.000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berchick ER, Barnett JC, Upton RD. Health Insurance Coverage in the United States: 2018. United States Census Bureau 2019;44:P60–267. [Google Scholar]
- 24.Sohn H. Racial and Ethnic Disparities in Health Insurance Coverage: Dynamics of Gaining and Losing Coverage Over the Life-Course. Popul Res Policy Rev 2017;36:181–201. doi: 10.1007/s11113-016-9416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copeland VC. African Americans: Disparities in Health Care Access and Utilization. Health Soc Work 2005;30:265–270. doi: 10.1093/hsw/30.3.265 [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal R, Chiu N, Wadhera RK, Moran AE, Raber I, Shen C, Yeh RW, Kazi DS. Racial/Ethnic Disparities in Hypertension Prevalence, Awareness, Treatment, and Control in the United States, 2013 to 2018. Hypertension 2021;78:1719–1726. doi: 10.1161/HYPERTENSIONAHA.121.17570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham G Disparities in Cardiovascular Disease Risk in the United States. Curr Cardiol Rev 2015;11:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pemayun TGD, Naibaho RM, Novitasari D, Amin N, Minuljo TT. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital-based case–control study. Diabet Foot Ankle 2015;6:29629. doi: 10.3402/dfa.v6.29629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman MP, Clark CJ, Craven TE, Davis RP, Williams TK, Velazquez-Ramirez G, Hurie JB, Edwards MS. Effect of Intensive Glycemic Control on Risk of Lower Extremity Amputation. J Am Coll Surg 2018;227:596–604. doi: 10.1016/j.jamcollsurg.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 30.Shipra A, Anjali K, Zachary OB, DeMartino RR, Brewster LP, Goodney PP, Wilson PWF. Association of Statin Dose With Amputation and Survival in Patients With Peripheral Artery Disease. Circulation 2018;137:1435–1446. doi: 10.1161/CIRCULATIONAHA.117.032361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376:1713–1722. doi/full/ 10.1056/nejmoa1615664 [DOI] [PubMed] [Google Scholar]
- 32.Khan SZ, O’Brien-Irr MS, Rivero M, Blochle R, Cherr GS, Dryjski ML, Dosluoglu HH, Lukan J, Rowe VL, Harris LM. Improved survival with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic limb-threatening ischemia. J Vasc Surg 2020;72:2130–2138. doi: 10.1016/j.jvs.2020.02.041 [DOI] [PubMed] [Google Scholar]
- 33.Creager MA, Matsushita K, Arya S, Beckman JA, Duval S, Goodney PP, Gutierrez AT, Kaufman JA, Joynt Maddox KE, Pollak AW, et al. Reducing Nontraumatic Lower-Extremity Amputations by 20% by 2030: Time to Get to Our Feet: A Policy Statement From the American Heart Association. Circulation 2021;143:e875–e891. doi: 10.1161/CIR.0000000000000967 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


