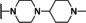Abstract
The transient receptor potential cation channel 5 (TRPC5) plays an important role in numerous cellular processes. Due to this, it has gained considerable attention over the past few years as a potential therapeutic target. Recently, TRPC5 has been shown to be involved in the regulation of podocyte survival, indicating a potential treatment option for chronic kidney disease. In addition, a recent study has shown TRPC5 to be expressed in human sensory neurons and suggests that TRPC5 inhibition could be an effective treatment for spontaneous and tactile pain. To understand these processes more fully, potent and selective tool compounds are needed. Herein we report further exploration of the 2‐aminobenzimidazole scaffold as a potent TRPC5 inhibitor, culminating in the discovery of 16 f as a potent and selective TRPC5 inhibitor.
Keywords: transient receptor potential cation channel 5, TRPC5, benzimidazole, chronic kidney disease, pain
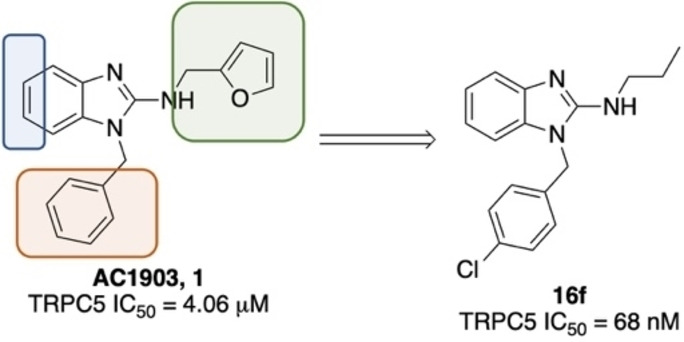
Inhibitors with potential! Medicinal chemistry optimization of the original hit compound, AC1903, 1, led to the formation of a next‐generation transient receptor potential cation channel 5 (TRPC5) inhibitor, 16 f. We profiled this compound in in vitro and in vivo pharmacokinetic assays as well as additional selectivity assays.
Introduction
The transient receptor potential (TRP) channel family act as molecular sensors and are engaged in various roles. This larger TRP class is further subdivided into six subgroups: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRP (polycystin) and TRPML (mucolipin).[ 1 , 2 ] The TRPC channels are Ca2+ permeable nonselective cation channels and are a collection of seven different proteins. These channels are subdivided into four subclasses: TRPC1, TRPC4/5, TRPC3/6/7, and TRPC2 based on their sequence similarity.[ 1 , 2 ] The TRPC4/5 and TRPC3/6/7 subclasses share ∼80 % amino acid homology. Individual channels can function as either homo‐ or heterotetramers consisting of TRPC5 family channels and other TRP channels. This family of proteins is also referred to as receptor‐operated channels due to their activation by PLC after stimulation with receptor tyrosine kinases or GPCRs. Recently, the TRPC3, TRPC4, TRPC5, and TRPC6 structures have been solved using Cryo‐EM which will provide valuable information for the structural basis for understanding the mechanism of these channels.[ 3 , 4 , 5 ]
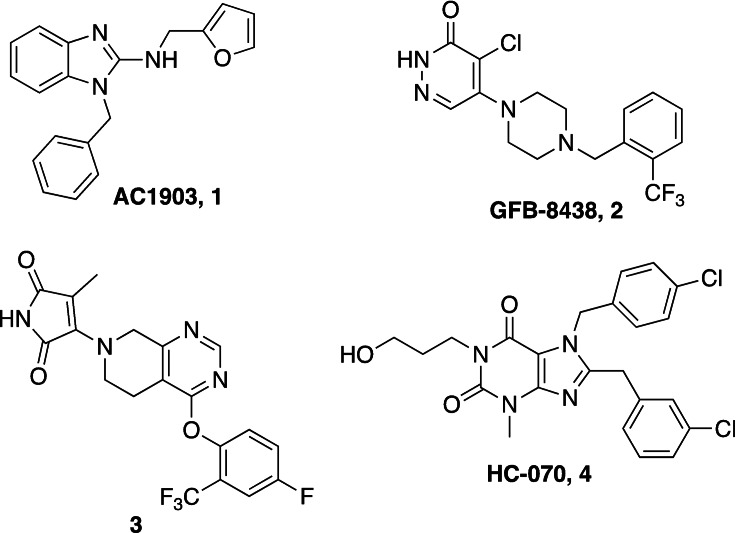
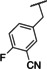
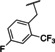
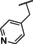
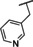
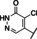
Recently, our laboratory and others have developed new TRPC5 tool compounds and have shown the role that TRPC5 plays in chronic kidney disease (CKD), specifically focal segmental glomerulosclerosis (FSGS).[ 6 , 7 ] AC1903, 1, was the first reported selective TRPC5 antagonist and this was shown to block TRPC5 channel activity in glomeruli of proteinuric rats (Figure 1). [8] Administration of 1 was able to suppress proteinuria, prevent podocyte loss in an animal model of FSGS, and provide benefit in a rat model of hypertensive proteinuric kidney disease. [8] This was the first small molecule TRPC5 antagonist to provide in vivo benefit in an animal model. [9] Next, the discovery and characterization of GFB‐8438, 2, from Goldfinch Bio, was reported and was shown to reduce both total protein and albumin concentrations in urine, a key biomarker for CKD. [10] Goldfinch has since advanced another compound, GFB‐887, into Phase 2 clinical trials. More recently, 3 was discovered and it also showed benefit against protamine sulfate (PS)‐induced podocyte injury in vitro. [11] In addition to CKD, TRPC5 antagonists have shown activity in animal models of depression and pain. Namely, 1 was active in vivo against inflammatory mechanical and spontaneous pain in mice. [12] And, HC‐070, 4, was active in animal models of anxiety and depression. [13]
Figure 1.
Previously reported TRPC5 inhibitors.
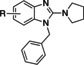
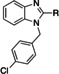
The furan bearing benzimidazole compound AC1903, 1, played a significant role in establishing that TRPC5 inhibition prevents and reverses podocyte injury in FSGS. [8] However, much improvement needs to be made toward its potency and pharmacokinetic liabilities owing to the presence of the furan ring. In this study to identify new inhibitors, we focused our attention on additional analogs around the benzimidazole core. These compounds included a 2‐position N‐linked alkyl or a saturated N‐heterocycle containing benzimidazole. We started our exploration on an N‐1‐benzyl containing benzimidazole and tried various 2‐position substitutions.
Results and Discussion
Chemical synthesis of benzimidazole analogs
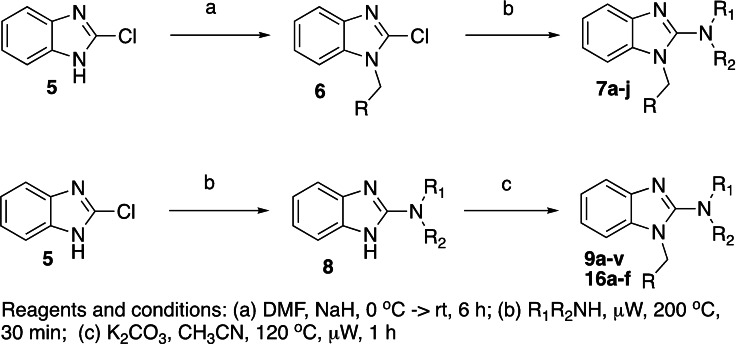
The synthesis of the target compounds was achieved following a procedure that we previously published. [14] The commercially available 2‐chlorobenzimidazole, 5, was alkylated with an appropriately substituted benzyl bromide (DMF, NaH, 0 °C) to yield 6 (Scheme 1). Next, the amino compounds were reacted with the substituted 2‐chlorobenzimidazole under microwave conditions (μW, 200 °C, 30 min) to obtain the final targets 7 a‐j. Similarly, the reaction sequence could be reversed to explore the southern portion of the molecule.
Scheme 1.
Synthesis of 2‐N‐alkylbenzimidazoles.
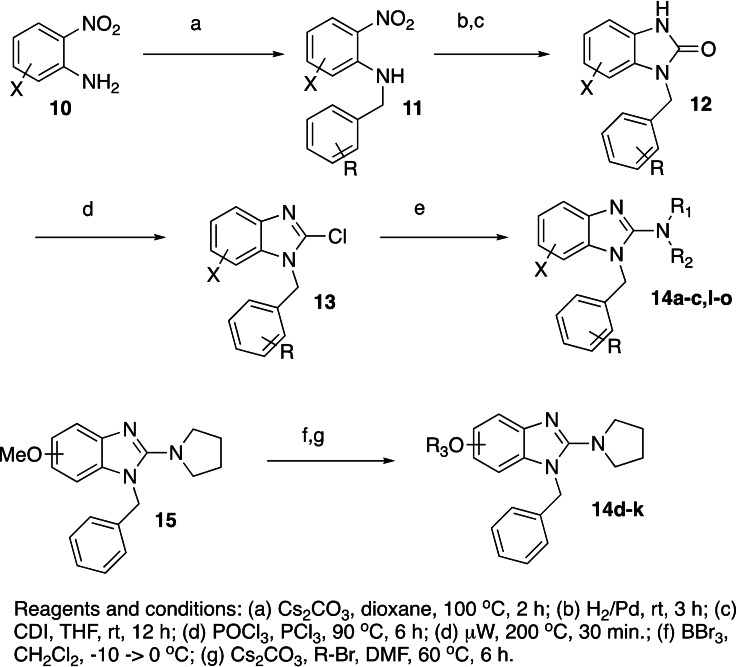
The 5‐ and 6‐substituted analogs were synthesized as outlined in Scheme 2. To this end, the 2‐nitroaniline, 10, was alkylated with BnBr to yield 11. Next, the nitro group was reduced (H2/Pd) and then cyclized with 1,1’‐carbonyldimidazole (THF, rt) to yield the benzimidazole‐2‐one, 12, which can be converted to the 2‐chloro derivative, 13 (POCl3, PCl3, 90 °C). [15] The final targets, 14 a‐c,i–o, were completed following the procedure outlined in Scheme 1. The O‐linked benzimidazole analogs, 14 d‐k were accessed after demethylation of 15 (BBr3) followed by alkylation (Cs2CO3, R−Br, DMF, 60 °C). The synthetic procedures outlined above allow for modular synthesis of the final targets and easy access to multiple points of diversification.
Scheme 2.
Synthesis of 5‐ and 6‐substituted benzimidazoles.
Biological activity of benzimidazole derivatives
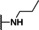
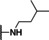
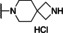
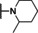
Previously, our work centered around replacing the furan with other heteroaryl moieties [14] ; however, this work looks to find a replacement of the furan with alkyl or cyclic alkyl groups (Table 1). The previously identified, 1, was used as the standard control and the % inhibition at 3 μM was normalized to 100 %. We triaged compounds by assessing % inhibition of the TRPC5 channel using the Syncropatch instrument to have a higher throughput capacity for SAR, which is then followed up with IC50 determinations of active compounds. Replacing the methylfuran moiety with six‐membered compounds 4‐methylpiperidine, 7 a, and piperidine, 7 b, were only moderately active (∼50 % inhibition) compared to 1. Reducing the ring size to the 5‐membered pyrrolidine, 7 c, resulted in a compound equipotent to 1 with an IC50=4.30 μM. Since pyrrolidine can also be susceptible to oxidative metabolism, we tried to incorporate a 2‐methyl substitution, which provided compounds that were equipotent with 1 (100 % inhibition at 3 μM) with the (S)‐7 d or (R)‐7 e stereoisomers showed a slight preference for 7 e. Any attempt to elongate the right hand side with the help of even bulkier heterocycles such as N‐4‐(1‐methylpiperidin‐4‐yl)piperazine, 7 h, and diazaspiro[3.5]nonane, 7 i, were not productive. Adding a two‐carbon spacer to 7 c resulted in a compound, 7 j, with moderate activity. However, the addition of straight chain alkyl groups proved to be a productive change as N‐propyl, 7 f, and N‐isopentyl, 7 g, were potent with an IC50 of 0.72 μM and 1.3 μM, respectively.
Table 1.
SAR of the right‐hand portion.
|
| |||
|---|---|---|---|
|
Cmpd |
R |
% Inhibition @ 3 μM[a] |
TRPC5 IC50 [μM][a,b] |
|
1 AC1903 |
|
100 |
4.06±0.91 |
|
7 a |
|
49.1 |
ND |
|
7 b |
|
64.9 |
ND |
|
7 c |
|
114.6 |
4.30±1.63 |
|
7 d |
|
33.0 |
ND |
|
7 e |
|
101.8 |
ND |
|
7 f |
|
119.9 |
0.72±0.21 |
|
7 g |
|
94.3 |
1.30±0.31 |
|
7 h |
|
−14.7 |
ND |
|
7 i |
|
3.0 |
ND |
|
7 j |
|
91.6 |
ND |
[a] The % inhibition values were recorded in cells (n >10) using the Syncropatch (Nanion). Experiments were recorded (minimum) on one session over two different assay plates. AC1903 and DMSO were added as control compounds on every assay plate. [b] ND: Not determined.
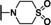
Moving forward, we explored the southern benzylic substitution on the active pyrrolidine moiety, 7 c. The pyrrolidine 7 c was chosen as this provided an easier synthetic handle to explore the southern portion. Active compounds would then be followed up with an additional SAR around the 2‐position. We started with evaluating the southern portion using electron‐withdrawing and electron‐donating groups (Table 2). The fluoro and chloro compounds (9 a‐e) provided potent compounds and no discernible difference between the halogens (i. e., F and Cl with same substitution). The 4‐substitution appears to be more potent (9 b, 1.49 μM vs. 9 c, 0.59 μM and 9 d, 0.72 μM vs. 9 e, 0.44 μM) and each were better than the 2‐substituted analog. A variety of substitutions on the benzyl system provided more potent compounds than 1 with 4‐methyl (9 k, 0.90 μM), 4‐chloro‐2‐fluoro (9 n, 0.84 μM), and 3‐cyano‐4‐fluoro (9 o, 0.54 μM) being of interest. Interestingly, switching the chloro and fluoro to the 2‐chloro‐4‐fluoro derivative, 9 m, was not active. Addition of a heteroaryl in the form of pyridine (9 q‐s) was not active, although addition of a 2‐chloro (9 t, 3.11 μM) did regain some potency which may indicate the basicity of the pyridine nitrogen is important. Any attempt to mask the benzylic methylene with a carbonyl or sulfone, 9 u and 9 v, yielded inactive compounds.
Table 2.
SAR of the southern portion.
|
| |||
|---|---|---|---|
|
Cmpd |
R |
% Inhibition@3 μM[a] |
TRPC5 IC50 [μM][a,b] |
|
1 AC1903 |
|
100 |
4.06±0.91 |
|
9 a |
|
194.8 |
2.69 |
|
9 b |
|
226.3 |
1.49±0.31 |
|
9 c |
|
254.4 |
0.59±0.14 |
|
9 d |
|
216.8 |
0.72±0.21 |
|
9 e |
|
277.0 |
0.44±0.14 |
|
9 f |
|
213.3 |
1.15±0.25 |
|
9 g |
|
124.8 |
1.78±0.43 |
|
9 h |
|
26.0 |
ND |
|
9 i |
|
17.1 |
ND |
|
9 j |
|
74.2 |
ND |
|
9 k |
|
136.7 |
0.902±0.340 |
|
9 l |
|
164.0 |
1.90 |
|
9 m |
|
86.7 |
ND |
|
9 n |
|
242.1 |
0.84±0.23 |
|
9 o |
|
117.7 |
0.54±0.23 |
|
9 p |
|
7.4 |
ND |
|
9 q |
|
−5.2 |
ND |
|
9 r |
|
41.2 |
ND |
|
9 s |
|
97.4 |
ND |
|
9 t |
|
126.8 |
3.11±0.94 |
|
9 u |
|
18.8 |
ND |
|
9 v |
|
−43.0 |
ND |
[a] The % inhibition values were recorded in cells (n >10) using the Syncropatch (Nanion). Experiments were recorded (minimum) on one session over two different assay plates. AC1903 and DMSO were added as control compounds on every assay plate. [b] ND: Not determined.
Next, we tried to diversify the benzimidazole core itself by incorporating various substituents on the C‐5 and C‐6 positions of the phenyl ring in the benzimidazole compound. The 5,6‐dimethylbenzimidazole (14 b, 101 % inhibition, IC50=12.3 μM) had moderate activity (Table 3). Unfortunately, this portion of the molecule did not allow for modification as nearly all the compounds evaluated were not active. Free hydroxy provided an excellent handle to make ethers of varied length; however, elongation beyond a simple methoxy resulted in a reduction of activity. Next, we tried to incorporate the chloro pyridazinone moiety from the Goldfinch Bio compounds to see if it would be tolerated. We attached it to C‐5 via a C−C bond as in 14 n but the activity was not as active as 1 or the reported Goldfinch compounds. [10]
Table 3.
Substitution around the benzimidazole core.
|
| |||
|---|---|---|---|
|
Cmpd |
R |
% Inhibition@3 μM[a] |
TRPC5 IC50 [μM][a,b] |
|
1 AC1903 |
|
100 |
4.06±0.91 |
|
14 a |
5,6‐diCl |
19.1 |
ND |
|
14 b |
5,6‐diMe |
101 |
12.3 |
|
14 c |
6‐F |
76.4 |
ND |
|
14 d |
6‐OMe |
120,2 |
ND |
|
14 e |
6‐OH |
119.8 |
ND |
|
|
|
|
|
|
14 f |
6‐ |
111.1 |
ND |
|
|
|
|
|
|
14 g |
6‐ |
78.0 |
ND |
|
14 h |
5‐OMe |
125.1 |
1.15±0.33 |
|
14 i |
5‐OH |
119.1 |
ND |
|
|
|
|
|
|
14 j |
5‐ |
56.7 |
ND |
|
|
|
|
|
|
14 k |
5‐ |
68.1 |
ND |
|
14 l |
5‐Br |
63.8 |
ND |
|
14 m |
5‐Cl |
108.3 |
ND |
|
|
|
|
|
|
14 n |
5‐ |
115.2 |
7.55±2.52 |
[a] The % inhibition values were recorded in cells (n >10) using the Syncropatch (Nanion). Experiments were recorded (minimum) on one session over two different assay plates. AC1903 and DMSO were added as control compounds on every assay plate. [b] ND: Not determined.
Finally, we wanted to merge the active N‐1 substitution 4‐chlorobenzyl with some active and diverse C‐2 substitution (Table 4). The 2‐ or 4‐methylpiperidine, 16 a and 16 b, were not active, nor was the thiomorpholine‐1,1‐dioxide, 16 c. Reinvestigating the 2‐methyl pyrrolidine, the (R) stereoisomer, 16 e, (IC50=2.89 μM) was more active compared to (S)‐enantiomer, 13 d. The most significant improvement was observed with the N‐propyl containing moiety, 16 f, which turned out to be the most active compound in the series with an IC50=0.068 μM. 16 f is the first sub 100 nanomolar compound in the benzimidazole core containing series of molecules.
Table 4.
Further SAR around the benzimidazole core.
|
| |||
|---|---|---|---|
|
Cmpd |
R |
% Inhibition@3 μM[a] |
TRPC5 IC50 [μM][a,b] |
|
1 AC1903 |
|
100 |
4.06±0.91 |
|
16 a |
|
11.9 |
ND |
|
16 b |
|
46.1 |
ND |
|
16 c |
|
15.5 |
ND |
|
16 d |
|
100.4 |
ND |
|
16 e |
|
119.1 |
2.89±0.70 |
|
16 f |
|
126.5 |
0.068±0.106 |
[a] The % inhibition values were recorded in cells (n >10) using the Syncropatch (Nanion). Experiments were recorded (minimum) on one session over two different assay plates. AC1903 and DMSO were added as control compounds on every assay plate. [b] ND: Not determined.
Next, we further evaluated selected compounds based on liver microsome stability and plasma protein binding (Table 5).[ 16 , 17 , 18 ] Most of the compounds tested were not stable in either human or mouse liver microsomes. Even addition of the 4‐chlorine to block the para‐oxidation of the phenyl ring system did not provide any beneficial effects. This could be due to oxidation of the pyrrolidine moiety. Addition of the 3‐cyano‐4‐fluoro groups in 9 o did improve the stability in both human and mouse, although only modestly. All the compounds tested displayed good free fraction (>2%) in both human and mice, except for 9 e and 9 n. In addition, we profiled select compounds (9 o and 16 f) for selectivity against the closely related TRPC4 channel and we tested them against the Psychoactive Drug Screening Panel at the University of North Carolina, Chapel Hill. [19] This panel consists of 45 receptors and transporters that are of importance in the CNS. 9 o was active against PBR (Ki=399 nM) with no other receptor Ki<7.0 μM. 16 f was active against H1 (Ki=6.2 nM), 5‐HT2B (Ki=249 nM), α2B (Ki=677 nM) and α2C (Ki=434 nM) showing these compounds are predominantly selective for TRPC5, except for the noted exceptions above (Supporting Information Table 1).
Table 5.
In vitro PK parameters of selective compounds.
|
Cmpd |
hCLINT |
hCLHEP |
mCLINT |
mCLHEP |
hPPB |
mPPB |
|---|---|---|---|---|---|---|
|
|
mL/min/kg |
|
|
|
%f u |
|
|
7c |
164.2 |
17.9 |
529.7 |
77.0 |
4.4 |
3.6 |
|
7f |
103.4 |
16.8 |
796.3 |
80.9 |
4.1 |
10.8 |
|
9e |
277.6 |
18.7 |
4257 |
88.2 |
1.3 |
1.3 |
|
9n |
239.2 |
18.5 |
3195 |
87.6 |
1.0 |
2.17 |
|
9o |
38.1 |
13.2 |
370.0 |
72.5 |
6.8 |
5.3 |
|
16f |
127.3 |
17.4 |
866.8 |
81.6 |
1.4 |
3.6 |
Lastly, we evaluated a few key compounds in an in vivo cassette experiment utilizing a 4‐in‐1 experiment design (Table 6).[ 20 , 21 ] In order to conserve resources as well as animals, we chose to do the in vivo PK experiments in rats so that we could serially sample as well as utilize the cassette design. Based on the balance of the potency and in vitro PK properties, we chose 9 o and 16 f for the cassette. As can be seen in Table 6, both compounds displayed high clearance with short half‐lives (CL >55 mL/min/kg; t1/2 <1 hr). The compounds also had good Vss (1.8–2.3 L/kg) and good exposure. As TRPC5 is of interest for a multitude of CNS disorders, we also profiled the compounds to determine the extent of brain penetration. Both compounds had good brain penetration (Kp >0.5) with 16 f being highly brain penetrant (Kp=2.1).
Table 6.
In vivo cassette for selected compounds.
|
|
9o |
16f |
|---|---|---|
|
Rat cassette (0.25 mpk) a,b,c |
|
|
|
CL (mL/min/kg) |
61.3 |
57.8 |
|
T1/2 (h) |
0.64 |
0.721 |
|
C0 (ng/mL) |
221 |
140 |
|
MRTInf<? >obs |
0.642 |
0.632 |
|
Vss (L/kg) |
1.8 |
2.3 |
|
AUC (h×ng/mL0 |
262 |
291 |
|
Plasma |
69.7 |
77.5 |
|
Brain |
41.8 |
163 |
|
Kp (B : P) |
0.6 |
2.10 |
Conclusion
In conclusion, we described the further synthesis and biological evaluation of a series of 2‐aminobenzimidazole compounds as TRPC5 inhibitors. This work builds upon our previous disclosure of 1 and the new SAR revealed that pyrrolidine or N‐alkyl modifications or replacements of the furan moiety were tolerated. Further evaluation using the Syncropatch for IC50 determination revealed that 16 f was the best compound that was identified. Further selectivity and PK studies indicate 16 f is a selective and brain penetrant compound that can be used to further interrogate TPRC5 in brain disorders.
Experimental Section
General Methods
All 1H & 13C NMR spectra were recorded on Bruker AV‐400 (500 MHz) instrument. Chemical shifts are reported in ppm relative to residual solvent peaks as an internal standard set to δH 3.31 and δC 49.00 (CD3OD), 2.50 and 39.52 ((CD3)2SO), and 7.26 and 77.16 (CDCl3). Data are reported as follows: chemical shift, multiplicity (br=broad, s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet), coupling constant (Hz), and integration. Low resolution mass spectra were obtained on an Agilent 1260 LCMS with electrospray ionization, with a gradient of 5–95 % MeCN in 0.1 % formic acid water over 4 min. Analytical thin layer chromatography was performed on LuxPlate silica gel 60 F254 plates. Visualization was accomplished with UV light, and/or the use of ninhydrin, anisaldehyde and ceric ammonium molybdate solutions followed by charring on a hot plate. Chromatography on silica gel was performed using Silica Gel 60Å (230–400 mesh) from Sorbent Technologies. Solvents for extraction, washing and chromatography were HPLC grade. All reagents were purchased from Aldrich Chemical Co. (or similar) and were used without purification. All reagents and solvents were commercial grade and purified prior to use when necessary.
Final compounds were purified on a Gilson preparative reversed‐phase HPLC system comprised of a 322 aqueous pump with solvent‐selection valve, 334 organic pump, GX‐271 liquid hander, two column switching valves, and a 159 UV detector. UV wavelength for fraction collection was user‐defined, with absorbance at 254 nm always monitored. Column: Phenomenex Axia‐packed Luna C18, 50 x 21.2 mm, 5 μm. For Acidic Method: Mobile phase: CH3CN in H2O (0.1 % formic acid). Gradient conditions: 2.0 min equilibration, followed by user‐defined gradient (starting organic percentage, ending organic percentage, duration, typically 15 mins), hold at 95 % CH3CN in H2O (0.1 % TFA) for 2 min, 20 mL/min, 23 °C. Normal phase chromatography purifications were performed with a Biotage IsoleraTM One instrument. Microwave synthesis was performed using an Anton Paar Monowave 400 instrument.
General Procedure A
1‐Benzyl‐2‐chloro‐1H‐benzo[d]imidazole (6). In a round bottom flask fitted with magnetic stirrer, 2‐chloro‐1H‐benzimidazole, 2, (0.50 g, 3.2 mmol) was dissolved in DMSO (3 mL), and then NaH (60 %; 0.19 g, 4.9 mmol) was added at 0 °C and stirred for 1 h. Benzyl bromide (0.67 g, 3.9 mmol) was added to the suspension and the reaction was stirred at rt for 12 h. Ice cold water (15 mL) was added to the mixture the product precipitates, collected via filtration. Filtrate was washed with water and dried under vacuum to give desired product as white solid. Yield=0.75 g, 88 % (white solid). LCMS: RT=2.69 min., >98 % @ 215 and 254 nm, m/z=243.0 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J=7.4 Hz, 1H), 7.35–7.24 (m, 5H), 7.16 (d, J=6.8 Hz, 2H), 5.38 (s, 2H). 13C NMR (125 MHz, CDCl3) δ 141.33, 134.83, 128.99, 128.22, 126.76, 123.53, 123.05, 119.18, 109.98, 47.94.
1‐Benzyl‐N‐(furan‐2‐ylmethyl)‐1H‐benzo[d]imidazol‐2‐amine (1). A 10 mL microwave vial with stir bar was charged with 1‐benzyl‐2‐chloro‐1H‐benzo[d]imidazole (50.0 mg, 0.200 mmol) and furfuryl amine (0.20 g, 2.0 mmol). The mixture was subjected to microwave irradiation at 200 °C for 30 min. The mixture was diluted with ethyl acetate (50 mL) and washed with water (25 mL) and brine (25 mL). The organic layer was dried over sodium sulphate, evaporated, and purified by reverse phase chromatography (0‐100 % water/ACN). Fractions collected were evaporated to yield desired product. Yield=30 mg, 48 % (white solid). LCMS: RT=2.07 min., >98 % @ 215 and 254 nm, m/z=304.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.58 (d, J=7.8 Hz, 1H), 7.34 (m, 4H), 7.17 (t, J=9.2 Hz, 3H), 7.13–7.05 (m, 2H), 6.31 (s, 1H), 6.23 (s, 1H), 5.13 (s, 2H), 4.71 (d, J=5.4 Hz, 2H), 4.34 (s, 1H). 13C NMR (125 MHz, CDCl3) δ 153.76, 151.70, 142.14, 135.29, 134.88, 129.19, 128.14, 126.43, 121.56, 120.03, 116.85, 110.40, 107.46, 107.41, 45.72, 40.51.
1‐Benzyl‐2‐(4‐methylpiperidin‐1‐yl)‐1H‐benzo[d]imidazole (7 a). Yield=191 mg, 95 % (off‐white solid). LCMS: RT=2.15 min., >98 % @ 215 and 254 nm, m/z=306.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J=7.9 Hz, 1H), 7.23 (dtd, J=19.6, 6.6, 6.0, 3.3 Hz, 3H), 7.11 (t, J=6.8 Hz, 3H), 7.00 (td, J=7.6, 2.1 Hz, 1H), 6.93 (d, J=8.0 Hz, 1H), 5.17–5.01 (m, 2H), 3.46–3.37 (m, 2H), 2.93 (t, J=12.1 Hz, 2H), 1.64 (d, J=13.1 Hz, 2H), 1.56 (q, J=11.5, 10.0 Hz, 2H), 1.29 (ddd, J=24.6, 12.5, 8.8 Hz, 3H), 0.93 (dd, J=6.6, 1.9 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 158.97, 141.74, 136.51, 135.46, 128.89, 127.52, 126.12, 121.77, 121.19, 117.90, 109.31, 51.27, 47.70, 34.02, 30.65, 21.87.
1‐Benzyl‐2‐(piperidin‐1‐yl)‐1H‐benzo[d]imidazole (7 b). Yield=139.7 mg, 86 % (off‐white solid). LCMS: RT=2.05 min., >98 % @ 215 and 254 nm, m/z=292.0 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J=7.9 Hz, 1H), 7.31 (dt, J=13.7, 6.8 Hz, 3H), 7.19 (dt, J=6.7, 3.1 Hz, 3H), 7.07 (t, J=7.6 Hz, 1H), 7.00 (d, J=7.9 Hz, 1H), 5.20 (s, 2H), 3.26–3.18 (m, 4H), 1.69 (p, J=5.8 Hz, 4H), 1.62 (q, J=7.1, 5.9 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 159.10, 141.72, 136.54, 135.46, 128.93, 127.57, 126.17, 121.83, 121.24, 117.97, 109.37, 51.96, 47.75, 25.77, 24.22.
1‐Benzyl‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (7 c). Yield=15.3 mg, 8 % (light tan solid). LCMS: RT=2.01 min., >98 % @ 215 and 254 nm, m/z=278.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J=7.9 Hz, 1H), 7.43 (dd, J=8.1, 6.3 Hz, 2H), 7.38 (dd, J=8.5, 5.7 Hz, 1H), 7.30–7.21 (m, 3H), 7.16–7.09 (m, 1H), 7.09–7.03 (m, 1H), 5.38 (s, 2H), 3.78–3.53 (m, 4H), 2.12–1.88 (m, 4H). 13 C NMR (100 MHz, CDCl3) δ 157.51, 142.68, 137.35, 136.55, 129.22, 127.79, 126.29, 122.11, 120.31, 116.95, 108.60, 50.84, 48.11, 25.96.
(S)‐1‐Benzyl‐2‐(2‐methylpyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (7 d). Yield=23.2 mg, 40 % (off‐white solid). LCMS: RT=2.303 min., >98 % @ 254 and 285 nm, m/z=292.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J=7.9 Hz, 1H), 7.52–7.30 (m, 5H), 7.25 (d, J=1.5 Hz, 1H), 7.12 (dtd, J=15.5, 7.9, 1.2 Hz, 2H), 5.39 (d, J=17.1 Hz, 1H), 5.34 (d, J=17 Hz, 1H), 4.39 (dp, J=8.1, 6.2 Hz, 1H), 3.68 (td, J=8.9, 7.1 Hz, 1H), 3.36 (td, J=8.7, 3.8 Hz, 1H), 2.31–2.18 (m, 1H), 2.08–1.86 (m, 2H), 1.66 (ddt, J=12.2, 9.5, 7.7 Hz, 1H), 1.33 (d, J=6.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 157.48, 142.61, 137.17, 136.13, 129.08, 127.71, 126.25, 122.02, 120.54, 117.27, 108.85, 56.93, 52.62, 47.96, 33.85, 25.32, 20.44.
(R)‐1‐Benzyl‐2‐(2‐methylpyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (7 e). Yield=15.3 mg, 18 % (yellow oil). LCMS: RT=2.334 min., >98 % @ 254 and 285 nm, m/z=292.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ δ 7.54 (d, J=7.9 Hz, 1H), 7.29–7.18 (m, 3H), 7.14–7.06 (m, 3H), 6.96 (dtd, J=15.1, 7.9, 1.3 Hz, 2H), 5.48–4.94 (m, 2H), 4.24 (dp, J=8.2, 6.2 Hz, 1H), 3.53 (td, J=8.9, 7.1 Hz, 1H), 3.21 (td, J=8.7, 3.8 Hz, 1H), 2.10 (dtd, J=11.0, 6.8, 3.9 Hz, 1H), 1.90–1.70 (m, 2H), 1.51 (ddt, J=12.3, 9.5, 7.7 Hz, 1H), 1.17 (d, J=6.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 157.52, 142.62, 137.22, 136.18, 129.26, 127.77, 127.77, 126.50, 122.09, 120.61, 117.33, 108.90, 57.01, 52.70, 48.03, 33.91, 25.39, 20.50.
1‐Benzyl‐N‐propyl‐1H‐benzo[d]imidazol‐2‐amine (7 f). Yield=14 mg, 58 % (white solid). LCMS: RT=2.04 min., >98 % @ 215 and 254 nm, m/z=266.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.55 (d, J=7.8 Hz, 1H), 7.38–7.31 (m, 3H), 7.19 (d, J=6.8 Hz, 2H), 7.18–7.14 (m, 1H), 7.11–7.04 (m, 2H), 5.13 (s, 2H), 4.18 (s, 1H), 3.47 (dd, J=12.9, 6.9 Hz, 2H), 1.66–1.57 (m, 2H), 0.88 (t, J=7.4 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 154.17, 141.74, 135.35, 134.62, 129.15, 128.13, 126.47, 121.47, 119.81, 116.31, 107.20, 45.71, 45.07, 22.87, 11.11.
1‐Benzyl‐N‐isopentyl‐1H‐benzo[d]imidazol‐2‐amine (7 g). Yield=12 mg, 11 % (off‐white solid). LCMS: RT=3.07 min., >98 % @ 215 and 254 nm, m/z=294.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.55 (d, J=7.8 Hz, 1H), 7.34 (dt, J=15.2, 5.2 Hz, 3H), 7.18–7.13 (m, 3H), 7.08–7.05 (m, 2H), 5.08 (s, 2H), 4.17 (s, 1H), 3.51 (dd, J=12.8, 7.0 Hz, 2H), 1.58–1.51 (m, 1H), 1.47 (dd, J=14.4, 7.0 Hz, 2H), 0.88 (d, J=6.5 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 154.41, 142.21, 135.53, 134.80, 129.15, 128.09, 126.51, 121.39, 119.70, 116.42, 107.22, 45.66, 41.74, 38.55, 25.76, 22.50.
1‐Benzyl‐2‐(4‐(1‐methylpiperidin‐4‐yl)piperazin‐1‐yl)‐1H‐benzo[d]imidazole (7 h). Yield=15 mg, 19 % (yellow solid). LCMS: RT=1.73 min., >98 % @ 215 and 254 nm, m/z=390.2 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.60 (d, J=7.9 Hz, 1H), 7.32 (t, J=7.2 Hz, 2H), 7.29–7.25 (m, 1H), 7.18 (t, J=7.6 Hz, 1H), 7.14 (d, J=7.3 Hz, 2H), 7.08 (t, J=7.6 Hz, 1H), 7.00 (d, J=7.9 Hz, 1H), 5.20 (s, 2H), 3.35 (d, J=9.5 Hz, 2H), 3.28–3.24 (m, 4H), 2.65 (s, 6H), 2.63 (s, 3H), 2.54–2.45 (m, 1H), 1.96 (d, J=3.9 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 168.13, 157.65, 141.04, 135.99, 135.27, 129.03, 127.73, 125.96, 122.15, 121.70, 117.84, 109.45, 50.50, 48.59, 47.65, 43.27, 25.33. (10 % CD3OD was added for increasing solubility).
7‐(1‐Benzyl‐1H‐benzo[d]imidazol‐2‐yl)‐2,7‐diazaspiro[3.5]nonane (7 i). Yield=13 mg, 20 % (off‐white solid). LCMS: RT=1.72 min., >98 % @ 215 and 254 nm, m/z=333.1 [M+H]+. 1H NMR (500 MHz, DMSO‐d6 ) δ 7.60 (d, J=7.8 Hz, 1H), 7.40 (t, J=7.5 Hz, 3H), 7.36–7.32 (m, 2H), 7.29 (t, J=6.0 Hz, 3H), 5.47 (s, 2H), 3.73 (d, J=5.3 Hz, 4H), 3.49–3.47 (m, 4H), 1.97‐1.92 (m, 4H). 13C NMR (125 MHz, DMSO‐d6 ) δ 172.26, 135.26, 132.65, 129.47, 128.44, 126.71, 124.93, 124.34, 111.87, 55.40, 54.29, 49.26, 46.70, 36.08, 33.54.
1‐Benzyl‐2‐(2‐(pyrrolidin‐1‐yl)ethyl)‐1H‐benzo[d]imidazole (7 j). Yield=21.0 mg, 30 % (yellow solid). LCMS: RT=2.12 min., >98 % @ 215 and 254 nm, m/z=306.18 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J=7.5 Hz, 1H), 7.33–7.27 (m,4H), 7.23 (dd, J=10.9, 5.3 Hz, 2H), 7.07 (d, J=7.0 Hz, 2H), 5.40 (s, 2H), 3.14–3.08 (m, 2H), 3.07–3.04 (m, 2H), 2.65–2.57 (m, 4H), 1.81 (dd, J=6.5, 3.1 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 142.31, 135.75, 135.49, 129.06, 128.02, 126.51, 122.95, 122.39, 119.26, 109.81, 53.94, 52.87, 46.99, 23.39.
1‐(2‐Fluorobenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 a). Yield=79 mg, 50 % (off‐white solid). LCMS: RT=2.051 min., >98 % @ 215 and 254 nm, m/z=296.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J=7.9 Hz, 1H), 7.33–7.26 (m, 1H), 7.15 (dt, J=21.8, 8.2 Hz, 2H), 7.04 (t, J=7.5 Hz, 2H), 6.98 (d, J=7.9 Hz, 1H), 6.91 (t, J=7.7 Hz, 1H), 5.34 (s, 2H), 3.59 (d, J=6.5 Hz, 4H), 2.01–1.90 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 160.97, 158.52, 135.59, 129.26, 127.39, 124.75, 123.94, 122.31, 120.52, 116.48, 108.15, 50.47, 42.08, 25.66.
1‐(3‐Fluorobenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 b). Yield=92 mg, 58 % (off‐white solid). LCMS: RT=1.922 min., >98 % @ 215 and 254 nm, m/z=296.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J=7.9 Hz, 1H), 7.30 (td, J=8.0, 5.8 Hz, 1H), 7.17 (td, J=7.7, 1.2 Hz, 1H), 7.06–7.01 (m, 1H), 7.01–6.91 (m, 3H), 6.87 (dt, J=9.7, 2.2 Hz, 1H), 5.27 (s, 2H), 3.61–3.53 (m, 4H), 1.99–1.90 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 164.49, 162.03, 139.44, 135.64, 130.61, 122.28, 121.36, 120.50, 116.56, 114.78, 113.04, 112.82, 108.20, 50.65, 47.44, 25.65.
1‐(4‐Fluorobenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 c). Yield=76 mg, 48 % (off‐white solid). LCMS: RT=2.036 min., >98 % @ 215 and 254 nm, m/z=296.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J=7.8 Hz, 1H), 7.19–7.08 (m, 3H), 7.06–6.98 (m, 3H), 6.95 (d, J=7.8 Hz, 1H), 5.25 (s, 2H), 3.60–3.50 (m, 4H), 1.97–1.88 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 163.39, 160.95, 156.56, 141.52, 135.72, 132.48, 127.38, 122.14, 120.35, 116.59, 116.03, 115.81, 108.23, 50.63, 47.23, 25.64.
1‐(3‐Chlorobenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 d). Yield=73 mg, 60 % (off‐white solid). LCMS: RT=2.11 min., >98 % @ 215 and 254 nm, m/z=312.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J=7.9 Hz, 1H), 7.25 (d, J=5.6 Hz, 2H), 7.20–7.12 (m, 2H), 7.06–6.98 (m, 2H), 6.94 (d, J=7.8 Hz, 1H), 5.24 (s, 2H), 3.58–3.49 (m, 4H), 1.99–1.86 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 156.83, 141.91, 139.08, 135.86, 135.01, 130.29, 127.88, 125.96, 123.93, 122.14, 120.33, 116.70, 108.15, 50.63, 47.37, 25.65.
1‐(4‐Chlorobenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 e). Yield=72 mg, 43 % (off‐white solid). LCMS: RT=2.084 min., >98 % @ 215 and 254 nm, m/z=312.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J=7.9 Hz, 1H), 7.32–7.28 (m, 2H), 7.16 (td, J=7.6, 1.2 Hz, 1H), 7.08 (d, J=8.2 Hz, 2H), 7.02 (td, J=7.6, 1.1 Hz, 1H), 6.93 (d, J=7.9 Hz, 1H), 5.24 (s, 2H), 3.59–3.49 (m, 4H), 1.97–1.89 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 156.63, 141.64, 135.74, 135.37, 133.46, 129.16, 127.16, 122.17, 120.35, 116.64, 108.17, 50.63, 47.28, 25.64.
1‐(4‐Methoxybenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 f). Yield=85 mg, 52 % (off white solid). LCMS: RT=2.057 min., >98 % @ 215 and 254 nm, m/z=308.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J=7.8 Hz, 1H), 7.19–7.12 (m, 1H), 7.02 (dt, J=23.9, 8.2 Hz, 4H), 6.88–6.82 (m, 2H), 5.24 (s, 2H), 3.78 (s, 3H), 3.62–3.57 (m, 4H), 1.96–1.90 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 159.07, 128.60, 126.93, 122.14, 120.45, 116.28, 114.38, 108.44, 55.31, 50.65, 47.35, 25.66.
1‐(3‐Fluoro‐4‐methoxybenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 g). Yield=59 mg, 34 % (off‐white solid). LCMS: RT=1.928 min., >98 % @ 215 and 254 nm, m/z=326.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J=7.9 Hz, 1H), 7.15 (td, J=7.6, 1.3 Hz, 1H), 7.02 (td, J=7.6, 1.1 Hz, 1H), 6.97–6.79 (m, 4H), 5.19 (s, 2H), 3.86 (s, 3H), 3.59–3.51 (m, 4H), 1.97–1.89 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 156.83, 153.84, 151.38, 146.97, 141.96, 135.88, 129.81, 122.01, 121.50, 120.21, 116.66, 113.78, 108.19, 56.32, 50.58, 46.97, 25.64.
1‐(3‐Chloro‐4‐(trifluoromethoxy)benzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 h). Yield=40 mg, 38 % (off‐white solid). LCMS: RT= 2.2 min., >98 % @ 215 and 254 nm,m/z= 396.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J=7.6 Hz, 1H), 7.34 (d, J=1.9 Hz, 1H), 7.31 (d, J=7.5 Hz, 1H), 7.22 (t, J=7.6 Hz, 1H), 7.12–7.05 (m, 2H), 6.97 (d, J=7.9 Hz, 1H), 5.29 (s, 2H), 3.65‐3.57 (m, 4H), 1.99 (t, J=6.6 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 162.34, 144.70, 128.19, 125.13, 123.12, 116.61, 108.11, 50.79, 46.89, 25.67.
2‐(Pyrrolidin‐1‐yl)‐1‐(2‐(trifluoromethoxy)benzyl)‐1H‐benzo[d]imidazole (9 i). Yield=35 mg, 37 % (off‐white solid). LCMS: RT= 2.31 min., >98 % @ 215 and 254 nm,m/z= 362.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.61 (d, J=7.9 Hz, 1H), 7.40–7.35 (m, 2H), 7.25–7.18 (m, 2H), 7.06 (t, J=7.5 Hz, 1H), 6.96 (dd, J=11.7, 7.9 Hz, 2H), 5.37 (s, 2H), 3.58 (t, J=6.3 Hz, 4H), 1.98–1.94 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 176.99, 146.19, 129.52, 129.02, 127.53, 127.33, 122.36, 120.80, 120.50, 116.57, 108.08, 50.40, 43.10, 25.65.
2‐(Pyrrolidin‐1‐yl)‐1‐(2‐(trifluoromethyl)benzyl)‐1H‐benzo[d]imidazole (9 j). Yield=39.4 mg, 43 % (white powder). LCMS: RT=2.265 min., >98 % @ 215 and 254 nm, m/z=346.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.74 (dd, J=7.0, 2.0 Hz, 1H), 7.56 (d, J=7.9 Hz, 1H), 7.46–7.35 (m, 2H), 7.16 (td, J=7.6, 1.2 Hz, 1H), 7.01 (td, J=7.6, 1.1 Hz, 2H), 6.89 (d, J=7.8 Hz, 1H), 5.46 (s, 2H), 3.56–3.47 (m, 4H), 1.96–1.85 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 156.88, 142.09, 135.87, 135.44, 132.65, 127.60, 126.82, 126.37, 122.22, 120.28, 116.72, 108.00, 50.31, 44.80, 25.62.
1‐(4‐Methylbenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 k). Yield=35 mg, 23 % (off‐white solid). LCMS: RT=2.133 min., >98 % @ 215 and 254 nm, m/z=292.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J=7.8 Hz, 1H), 7.15 (dd, J=12.6, 7.9 Hz, 3H), 7.06–6.94 (m, 4H), 5.25 (s, 2H), 3.64–3.54 (m, 4H), 2.33 (s, 3H), 1.97–1.88 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 156.41, 137.30, 135.81, 133.71, 129.63, 125.65, 122.04, 120.33, 116.35, 108.40, 50.62, 47.65, 25.65, 21.07.
1‐(3‐Methylbenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 l). Yield=72 mg, 45 % (off white solid). LCMS: RT=2.08 min., >98 % @ 215 and 254 nm, m/z=292.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J=7.8 Hz, 1H), 7.20 (t, J=7.6 Hz, 1H), 7.14 (td, J=7.9, 7.5, 1.4 Hz, 1H), 7.08 (d, J=7.6 Hz, 1H), 7.03–6.92 (m, 4H), 5.23 (s, 2H), 3.60–3.49 (m, 4H), 2.30 (s, 3H), 1.96–1.86 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 157.15, 142.26, 138.66, 137.01, 136.28, 128.80, 128.26, 126.35, 122.84, 121.78, 120.01, 116.55, 108.33, 50.54, 47.80, 25.66, 21.48.
1‐(2‐Chloro‐4‐fluorobenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 m). Yield=74 mg, 42 % (off‐white solid). LCMS: RT=2.161 min., >98 % @ 215 and 254 nm, m/z=330.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J=7.9 Hz, 1H), 7.24–7.14 (m, 2H), 7.07–7.00 (m, 1H), 6.93–6.80 (m, 3H), 5.27 (s, 2H), 3.56–3.49 (m, 4H), 1.98–1.89 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 163.13, 160.64, 156.55, 135.55, 132.43, 130.30, 128.18, 122.38, 120.47, 117.33, 116.67, 114.79, 107.98, 50.37, 45.53, 25.65.
1‐(4‐Chloro‐2‐fluorobenzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 n). Yield=81 mg, 46 % (off‐white solid). LCMS: RT=2.166 min., >98 % @ 215 and 254 nm, m/z=330.0 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J=7.9 Hz, 1H), 7.20–7.13 (m, 2H), 7.06–7.01 (m, 2H), 6.95 (d, J=7.8 Hz, 1H), 6.84 (t, J=8.2 Hz, 1H), 5.27 (s, 2H), 3.60–3.51 (m, 4H), 1.99–1.89 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 160.70, 158.23, 156.66, 141.70, 135.60, 134.28, 128.27, 125.15, 122.33, 120.46, 116.75, 116.20, 107.96, 50.47, 41.66, 25.65.
2‐Fluoro‐5‐((2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazol‐1‐yl)methyl)benzonitrile (9 o). Yield=47 mg, 55 %) LCMS: RT= 2.06 min., >98 % @ 215 and 254 nm,m/z= 321.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.50 (d, J=7.9 Hz, 1H), 7.44 (dd, J=5.6, 2.0 Hz, 1H), 7.38–7.33 (m, 1H), 7.21–7.15 (m, 2H), 7.05 (dd, J=11.2, 4.1 Hz, 1H), 6.90 (d, J=7.9 Hz, 1H), 5.27 (s, 2H), 3.51 (q, J=6.3 Hz, 4H), 1.95 (dd, J=8.2, 5.2 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 163.55, 161.48, 134.92, 133.93, 132.55, 132.48, 130.64, 122.77, 120.89, 117.38, 117.22, 116.30, 113.41, 107.99, 102.16, 102.04, 50.56, 46.61, 25.58.
1‐(4‐Fluoro‐2‐(trifluoromethyl)benzyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 p). Yield=20.5 mg, 21 % (white powder). LCMS: RT=2.317 min., >98 % @ 215 and 254 nm, m/z=364.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J=7.9 Hz, 1H), 7.47 (dd, J=8.6, 2.7 Hz, 1H), 7.18 (t, J=7.6 Hz, 1H), 7.12 (td, J=8.2, 2.7 Hz, 1H), 7.03 (t, J=7.6 Hz, 1H), 6.96 (dd, J=8.8, 5.2 Hz, 1H), 6.88 (d, J=7.8 Hz, 1H), 5.42 (s, 2H), 3.52 (td, J=6.6, 5.4, 3.0 Hz, 4H), 1.96–1.89 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 162.72, 160.24, 156.54, 141.69, 135.53, 131.08, 129.02, 122.48, 120.52, 119.31, 116.76, 114.51, 107.92, 50.39, 44.34, 25.63.
1‐(Pyridin‐4‐ylmethyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 q). Yield=16.0 mg, 14 % (white solid). LCMS: RT=0.94 min., >98 % @ 215 and 254 nm, m/z=279.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 8.63 (d, J=4.4 Hz, 2H), 7.74–7.69 (m, 1H), 7.26 (t, J=7.7 Hz, 1H), 7.12 (d, J=6.2 Hz, 1H), 7.10 (d, J=5.6 Hz, 2H), 6.97 (d, J=7.9 Hz, 1H), 5.36 (s, 2H), 3.69–3.61 (m,4H), 2.01–1.96 (m, J=16.3 Hz, 4H).
1‐(Pyridin‐3‐ylmethyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 r). Yield=22.0 mg, 20 % (white solid). LCMS: RT=0.94 min., >98 % @ 215 and 254 nm, m/z=279.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 8.58 (d, J=5.8 Hz, 2H), 7.59 (d, J=7.9 Hz, 1H), 7.41 (d, J=7.9 Hz, 1H), 7.27–7.24 (m, 1H), 7.20 (t, J=7.6 Hz, 1H), 7.07 (t, J=7.6 Hz, 1H), 7.00 (d, J=7.9 Hz, 1H), 5.34 (s, 2H), 3.59 (t, J=6.5 Hz, 4H), 1.99–1.95 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 149.51, 147.58, 133.44, 123.96, 123.13, 121.37, 116.10, 108.32, 50.95, 45.74, 25.67.
1‐(Pyridin‐2‐ylmethyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 s). Yield=20.0 mg, 18 % (white solid). LCMS: RT=1.2 min., >98 % @ 215 and 254 nm, m/z=279.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 8.64–8.61 (m, 1H), 7.65–7.59 (m, 2H), 7.24 (dd, J=7.1, 5.2 Hz, 1H), 7.21–7.17 (m, 1H), 7.08–7.04 (m, 1H), 7.01 (d, J=7.7 Hz, 1H), 6.95 (d, J=7.9 Hz, 1H), 5.42 (s, 2H), 3.62 (t, J=6.6 Hz, 4H), 1.97–1.93 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 156.65, 149.70, 137.27, 122.64, 122.31, 120.45, 120.29, 116.38, 108.11, 50.52, 49.76, 25.65.
1‐((6‐Chloropyridin‐3‐yl)methyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 t). Yield=19.0 mg, 23 % (yellow solid). LCMS: RT=1.2 min., >98 % @ 215 and 254 nm, m/z=279.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 8.34 (s, 1H), 7.58 (d, J=7.9 Hz, 1H), 7.36 (dd, J=8.3, 2.4 Hz, 1H), 7.30–7.27 (m, 1H), 7.19 (t, J=7.6 Hz, 1H), 7.07 (t, J=7.6 Hz, 1H), 6.97 (d, J=7.9 Hz, 1H), 5.30 (s, 2H), 3.58 (d, J=6.4 Hz, 4H), 1.97 (dd, J=8.1, 5.1 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 156.27, 151.07, 147.51, 136.52, 135.22, 131.42, 124.67, 122.56, 120.72, 116.77, 108.00, 50.79, 45.01, 25.64.
Phenyl(2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazol‐1‐yl)methanone (9 u). Yield=4 mg, 3 % (off‐white solid). LCMS/13 C 1H NMR (500 MHz, CDCl3) δ 7.90 (d, J=7.7 Hz, 2H), 7.68 (t, J=7.5 Hz, 1H), 7.52 (t, J=7.6 Hz, 3H), 7.11 (t, J=7.7 Hz, 1H), 6.81 (t, J=7.7 Hz, 1H), 6.52 (d, J=8.1 Hz, 1H), 3.49 (s, 4H), 1.91 (dt, J=11.9, 5.8 Hz, 5H).
1‐(Phenylsulfonyl)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (9 v). Yield=96 mg, 55 % (white powder). LCMS: RT=2.779 min., >98 % @ 215 and 254 nm, m/z=329.1 [M]+. 1H NMR (500 MHz, CDCl3) δ 7.89 (d, J=8.0 Hz, 1H), 7.60 (dd, J=8.4, 1.4 Hz, 2H), 7.49 (t, J=7.5 Hz, 1H), 7.33 (d, J=7.8 Hz, 2H), 7.24 (dd, J=7.8, 1.3 Hz, 1H), 7.19 (td, J=7.6, 1.2 Hz, 1H), 7.10 (td, J=7.7, 1.4 Hz, 1H), 3.75 (td, J=6.7, 5.4, 3.0 Hz, 4H), 2.02–1.97 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 157.50, 136.40, 134.10, 132.91, 128.80, 126.91, 125.96, 121.86, 116.87, 116.25, 52.77, 25.70.
1‐Benzyl‐5,6‐dichloro‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 a). Yield=27.0 mg, 48 % (white solid). LCMS: RT=2.24 min., >98 % @ 215 and 254 nm, m/z=346.0[M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.61 (s, 1H), 7.40–7.35 (m, 1H), 7.34–7.31 (m, 1H), 7.11 (d, J=7.2 Hz, 2H), 7.01 (s, 1H), 5.26 (s, 2H), 3.62–3.56 (m, 4H), 1.95 (d, J=6.2 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 156.79, 135.80, 135.14, 129.21, 127.97, 125.78, 125.45, 123.52, 116.98, 109.59, 50.36, 48.11, 25.67.
1‐Benzyl‐5,6‐dimethyl‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 b). Yield=14.0 mg, 42 % (white solid). LCMS: RT=2.17 min., >98 % @ 215 and 254 nm, m/z=306.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.67 (s, 1H), 7.42–7.34 (m, 3H), 7.10 (d, J=7.1 Hz, 2H), 6.82 (s, 1H), 5.43 (s, 2H), 3.87 (t, J=6.5 Hz, 4H), 2.30 (s, 3H), 2.26 (s, 3H), 2.01 (t, J=6.5 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 148.55, 134.79, 133.81, 132.65, 130.13, 129.57, 128.51, 127.95, 125.13, 114.17, 109.71, 51.20, 48.09, 25.68, 20.33, 19.84.
1‐Benzyl‐6‐fluoro‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 c) Yield=25.0 mg, 44 % (white solid). LCMS: RT=2.12 min., >98 % @ 215 and 254 nm, m/z=296.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.44 (dd, J=8.6, 4.7 Hz, 0.5H), 7.35 (dd, J=13.8, 7.6 Hz, 2H), 7.33–7.30 (m, 1H), 7.23 (dd, J=9.7, 2.0 Hz, 0.5H), 7.16 (d, J=7.3 Hz, 2H), 6.91–6.82 (m, 1H), 6.75–6.66 (m, 1H), 5.27 (d, J=10.7 Hz, 2H), 3.61–3.49 (m, 4H), 1.94 (t, J=6.5 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 158.5, 143.2, 138.5, 136.58, 128.98, 127.63, 125.71, 109.9, 108.08, 106.87, 103.15, 95.9, 50.5, 47.97, 25.64.
1‐Benzyl‐6‐methoxy‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 d). Yield=26.0 mg, 47 % (white solid). LCMS: RT=2.1 min., >98 % @ 215 and 254 nm, m/z=308.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.45 (d, J=8.6 Hz, 1H), 7.35 (t, J=7.3 Hz, 2H), 7.31 (d, J=7.1 Hz, 1H), 7.19 (d, J=7.3 Hz, 2H), 6.78 (dd, J=8.6, 2.4 Hz, 1H), 6.54 (d, J=2.3 Hz, 1H), 5.25 (s, 2H), 3.77 (s, 3H), 3.50 (d, J=6.7 Hz, 4H), 1.95–1.89 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 157.07, 154.81, 136.86, 136.73, 136.51, 128.90, 127.46, 125.82, 116.92, 108.62, 94.50, 55.98, 50.66, 47.82, 25.56.
1‐Benzyl‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazol‐6‐ol (14 e). To a solution of 14 d (100 mg, 0.3 mmol) in CH2Cl2 (4 mL) at −10 °C was added boron tribromide (BBr3, 72 μL, 0.81 mmol) the reaction was stirred at 0 °C for 3 hours. The crude product was evaporated, and the product purified by normal phase flash chromatography. Yield=60.0 mg, 63 % (off‐white solid). LCMS: RT=1.93 min., >98 % @ 215 and 254 nm, m/z=294.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.22–7.17 (m, 3H), 7.14 (d, J=8.5 Hz, 1H), 6.96 (d, J=7.1 Hz, 2H), 6.60 (d, J=8.5 Hz, 1H), 6.44 (s, 1H), 5.05 (s, 2H), 3.56–3.49 (m, 4H), 1.87–1.82 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 168.49, 153.32, 135.68, 134.51, 129.12, 127.92, 125.59, 114.41, 111.41, 96.61, 50.46, 47.72, 25.58.
1‐Benzyl‐6‐isobutoxy‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 f). A solution of 14 d (75 mg, 0.26 mmol), isobutyl bromide (33 μL, 0.31 mmol) and cesium carbonate (169 mg, 0.520 mmol) in DMF (1.5 mL) was stirred at 60 °C for 6 hours. After the completion of the reaction the crude product was evaporated, and the product purified by normal phase flash chromatography. Yield=30.0 mg, 33 % (off‐ white solid). LCMS: RT=2.15 min., >98 % @ 215 and 254 nm, m/z=350.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.36 (d, J=8.6 Hz, 1H), 7.26 (t, J=7.3 Hz, 2H), 7.22 (d, J=6.9 Hz, 1H), 7.09 (d, J=7.4 Hz, 2H), 6.69 (dd, J=8.6, 2.2 Hz, 1H), 6.46 (d, J=2.2 Hz, 1H), 5.16 (s, 2H), 3.57 (t, J=5.6 Hz, 2H), 3.42 (t, J=6.5 Hz, 4H), 1.95 (dt, J=13.3, 6.6 Hz, 1H), 1.85–1.80 (m, 4H), 0.91 (d, J=6.7 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 154.61, 136.89, 129.01, 128.93, 127.48, 125.78, 116.80, 109.53, 95.26, 75.41, 50.68, 47.79, 28.41, 25.57, 19.33.
1‐Benzyl‐6‐(2‐methoxyethoxy)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 g). Yield=22.0 mg, 37 % (off‐white solid). LCMS: RT=2.15 min., >98 % @ 215 and 254 nm, m/z=352.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.35 (d, J=8.6 Hz, 1H), 7.25 (t, J=7.3 Hz, 2H), 7.22–7.19 (m, 1H), 7.07 (d, J=7.3 Hz, 2H), 6.71 (dd, J=8.6, 2.4 Hz, 1H), 6.50 (d, J=2.3 Hz, 1H), 5.14 (s, 2H), 3.99–3.96 (m, 2H), 3.63–3.60 (m, 2H), 3.43 (t, J=6.7 Hz, 4H), 3.33 (s, 3H), 1.85–1.81 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 153.96, 136.74, 136.57, 128.92, 127.50, 125.77, 116.69, 109.33, 95.83, 71.20, 68.15, 59.18, 50.65, 47.83, 25.57.
1‐Benzyl‐5‐methoxy‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 h). Yield=26.0 mg, 47 % (white solid). LCMS: RT=2.06 min., >98 % @ 215 and 254 nm, m/z=308.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.25–7.22 (m, 2H), 7.19 (dd, J=8.3, 6.2 Hz, 1H), 7.07 (d, J=7.3 Hz, 2H), 7.04 (d, J=2.3 Hz, 1H), 6.74 (d, J=8.6 Hz, 1H), 6.54 (dd, J=8.6, 2.4 Hz, 1H), 5.16 (s, 2H), 3.74 (s, 3H), 3.46 (t, J=6.7 Hz, 4H), 1.84–1.80 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 157.50, 156.02, 142.92, 137.05, 130.51, 128.91, 128.76, 127.65, 127.49, 127.33, 125.76, 108.48, 108.04, 100.92, 55.88, 50.49, 47.91, 25.63.
1‐Benzyl‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazol‐5‐ol (14 i). Yield=175.0 mg, 91 % (white solid). LCMS: RT=1.98 min., >98 % @ 215 and 254 nm, m/z=294.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 9.04 (s, 1H), 8.72 (s, 1H), 7.30–7.24 (m, 3H), 7.07–7.01 (m, 3H), 6.63 (d, J=9.3 Hz, 2H), 5.20 (s, 2H), 3.69–3.61 (m, 4H), 1.94–1.90 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 168.26, 155.05, 150.52, 135.64, 133.22, 129.16, 128.86, 128.12, 127.99, 125.76, 125.42, 111.01, 109.63, 99.80, 50.34, 47.78, 25.58.
1‐Benzyl‐5‐isobutoxy‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 j). Yield=21.0 mg, 35 % (yellow oil). LCMS: RT=2.45 min., >98 % @ 215 and 254 nm, m/z=350.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.24 (t, J=7.4 Hz, 2H), 7.20 (d, J=7.3 Hz, 1H), 7.08–7.05 (m, 2H), 7.03 (s, 1H), 6.74 (d, J=8.5 Hz, 1H), 6.55 (dd, J=8.5, 2.3 Hz, 1H), 5.16 (s, 2H), 3.67 (d, J=6.6 Hz, 2H), 3.46 (t, J=6.7 Hz, 4H), 2.05–1.97 (m, 1H), 1.85–1.82 (m, 4H), 0.94 (d, J=6.7 Hz, 6H). Rotamers observed 13C NMR (125 MHz, CDCl3) δ 155.64, 137.09, 130.39, 128.89, 128.84, 128.66, 128.04, 127.46, 125.88, 125.77, 108.99, 108.43, 101.89, 75.38, 50.50, 47.89, 28.24, 25.63, 25.59, 19.37, 19.34.
1‐Benzyl‐5‐(2‐methoxyethoxy)‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 k). Yield=23.0 mg, 39 % (clear oil). LCMS: RT=2.14 min., >98 % @ 215 and 254 nm, m/z=352.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.26 (t, J=7.3 Hz, 2H), 7.22–7.20 (m, 1H), 7.08 (d, J=4.1 Hz, 1H), 7.06 (d, J=2.0 Hz, 2H), 6.76–6.73 (m, 1H), 6.61 (dd, J=8.6, 2.3 Hz, 1H), 5.17 (s, 2H), 4.08–4.05 (m, 2H), 3.70–3.66 (m, 2H), 3.49–3.47 (m, 4H), 3.38 (s, 3H), 1.84 (d, J=2.0 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 155.21, 136.92, 128.94, 127.54, 125.74, 109.34, 108.56, 101.67, 71.20, 68.04, 59.18, 50.52, 47.93, 25.63.
1‐Benzyl‐5‐bromo‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 l). Yield=130.0 mg, 78 % (yellow solid). LCMS: RT=2.5 min., >98 % @ 215 and 254 nm, m/z=356.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.55 (d, J=1.7 Hz, 1H), 7.25 (t, J=7.2 Hz, 2H), 7.22 (d, J=7.1 Hz, 1H), 7.04 (d, J=7.3 Hz, 2H), 7.01 (dd, J=8.3, 1.8 Hz, 1H), 6.72 (d, J=8.3 Hz, 1H), 5.18 (s, 2H), 3.48 (t, J=6.7 Hz, 4H), 1.86–1.83 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 157.60, 144.00, 136.56, 135.29, 129.01, 127.67, 125.65, 122.51, 119.35, 114.61, 109.28, 50.36, 47.91, 25.67.
1‐Benzyl‐5‐chloro‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (14 m). Yield=180.0 mg, 80.0 % (white solid). LCMS: RT=2.2 min., >98 % @ 215 and 254 nm, m/z=312.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.52 (d, J=1.9 Hz, 1H), 7.37–7.33 (m, 2H), 7.31 (t, J=5.0 Hz, 1H), 7.13 (d, J=7.1 Hz, 2H), 6.97 (dd, J=8.4, 1.9 Hz, 1H), 6.85 (d, J=8.4 Hz, 1H), 5.28 (s, 2H), 3.59 (t, J=6.7 Hz, 4H), 1.96–1.92 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 157.25, 136.44, 129.03, 127.71, 127.36, 125.61, 120.03, 116.24, 108.83, 50.41, 47.94, 25.66.
5‐(1‐Benzyl‐2‐(pyrrolidin‐1‐yl)‐1H‐benzo[d]imidazol‐5‐yl)‐4‐chloropyridazin‐3(2H)‐one (14 n). Yield=7.0 mg, 14 % (white solid). LCMS: RT=2.54 min., >98 % @ 215 and 254 nm, m/z=406.1 [M+H]+. 1H NMR (500 MHz, DMSO‐d6 ) δ 13.48 (s, 1H), 7.98 (s, 1H), 7.53 (d, J=1.4 Hz, 1H), 7.35 (t, J=7.1 Hz, 2H), 7.29 (t, J=6.4 Hz, 2H), 7.19–7.12 (m, 3H), 5.48 (s, 2H), 3.55–3.54 (m, 4H), 1.87 (t, J=6.6 Hz, 4H). 13C NMR (125 MHz, DMSO‐d6 ) δ 158.34, 157.42, 142.89, 138.84, 138.02, 137.69, 131.47, 129.27, 127.80, 126.37, 126.02, 120.58, 116.50, 109.09, 50.13, 47.35, 25.60.
1‐(4‐Chlorobenzyl)‐2‐(2‐methylpiperidin‐1‐yl)‐1H‐benzo[d]imidazole (16 a). Yield =16 mg, 26 % (clear oil). LCMS: RT=2.358 min., >98 % @215 and 254 nm, m/z=340.1 [M +H]+. 1H NMR (500 MHz, CDCl3) δ 7.68 (dd, J=25.0, 7.9 Hz, 1H), 7.32–7.28 (m, 2H), 7.25–7.20 (m, 1H), 7.17–7.09 (m, 3H), 7.05 (d, J=7.9 Hz, 1H), 5.33–5.16 (m, 2H), 3.51–3.43 (m, 1H), 3.08 (tdd, J=12.1, 10.1, 4.1 Hz, 2H), 1.89–1.75 (m, 3H), 1.70–1.60 (m, 2H), 1.58–1.38 (m, 2H), 1.02 (dd, J=12.6, 6.4 Hz, 3H).13C NMR (125 MHz, CDCl3) δ 158.12, 141.63, 135.18, 134.06, 133.39, 129.00, 127.85, 121.95, 121.72, 118.64, 109.57, 54.75, 51.00, 46.37, 33.10, 25.96, 22.33, 18.30.
1‐(4‐Chlorobenzyl)‐2‐(4‐methylpiperidin‐1‐yl)‐1H‐benzo[d]imidazole (16 b). Yield=48.5 mg, 79 % (white solid). LCMS: RT=2.358 min., >98 % @215 and 254 nm, m/z=340.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J=7.9 Hz, 1H), 7.33–7.27 (m, 2H), 7.23–7.17 (m, 1H), 7.15–7.06 (m, 3H), 6.97 (d, J=7.9 Hz, 1H), 5.17 (s, 2H), 3.45 (d, J=12.5 Hz, 2H), 2.99 (td, J=12.3, 2.2 Hz, 2H), 1.72 (dd, J=12.7, 1.9 Hz, 2H), 1.65–1.54 (m, 1H), 1.36 (ddd, J=24.4, 12.4, 3.8 Hz, 2H), 1.00 (d, J=6.5 Hz, 3H).13C NMR (125 MHz, CDCl3) δ 158.92, 141.77, 135.24, 135.05, 133.42, 129.13, 127.53, 121.98, 121.35, 118.10, 109.14, 51.34, 47.15, 34.03, 30.67, 21.87.
4‐(1‐(4‐Chlorobenzyl)‐1H‐benzo[d]imidazol‐2‐yl)thiomorpholine‐1,1‐dioxide (16 c). Yield=28.2 mg, 42 % (yellow oil). LCMS: RT=2.392 min., >98 % @215 and 254 nm, m/z=376.0 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J=7.9 Hz, 1H), 7.35 (d, J=8.4 Hz, 2H), 7.30–7.25 (m, 1H), 7.22–7.17 (m, 1H), 7.09 (d, J=8.3 Hz, 3H), 5.24 (s, 2H), 3.83–3.76 (m, 4H), 3.32–3.24 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 154.97, 140.82, 134.89, 134.17, 134.03, 129.50, 127.23, 122.81, 122.63, 118.65, 109.56, 50.45, 49.30, 47.05.
(S)‐1‐(4‐Chlorobenzyl)‐2‐(2‐methylpyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (16 d). Yield=38.1 mg, 65 % (white solid). LCMS: RT=2.227 min., >98 % @215 and 254 nm, m/z=326.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.60 (d, J=7.9 Hz, 1H), 7.29 (dd, J=7.5, 5.0 Hz, 2H), 7.21–7.16 (m, 1H), 7.10 (d, J=8.4 Hz, 2H), 7.08–7.03 (m, 1H), 6.98 (d, J=7.8 Hz, 1H), 5.22 (dd, J=56.1, 17.3 Hz, 2H), 4.34–4.25 (m, 1H), 3.61–3.53 (m, 1H), 3.25 (td, J=8.7, 3.8 Hz, 1H), 2.18 (ddd, J=18.8, 6.8, 3.9 Hz, 1H), 1.97–1.81 (m, 2H), 1.63–1.54 (m, 1H), 1.24 (d, J=6.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 157.12, 142.35, 135.62, 135.40, 133.29, 129.00, 127.38, 121.91, 120.38, 117.14, 108.38, 56.71, 52.42, 47.11, 33.56, 25.05, 20.14.
(R)‐1‐(4‐Chlorobenzyl)‐2‐(2‐methylpyrrolidin‐1‐yl)‐1H‐benzo[d]imidazole (16 e). Yield=27.5 mg, 47 % (tan solid). LCMS: RT=2.213 min., >98 % @215 and 254 nm, m/z=326.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.60 (d, J=7.9 Hz, 1H), 7.30 (dd, J=8.1, 5.6 Hz, 3H), 7.21–7.16 (m, 1H), 7.10 (d, J=8.4 Hz, 2H), 7.08–7.03 (m, 1H), 6.99 (d, J=7.8 Hz, 1H), 5.22 (dd, J=55.7, 17.3 Hz, 2H), 4.33–4.25 (m, 1H), 3.61–3.54 (m, 1H), 3.25 (td, J=8.7, 3.8 Hz, 1H), 2.17 (tt, J=14.5, 5.8 Hz, 1H), 2.00–1.81 (m, 2H), 1.64–1.53 (m, 1H), 1.24 (d, J=6.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 157.11, 142.33, 135.61, 135.39, 133.30, 130.35, 129.00, 128.28, 127.37, 121.91, 120.38, 117.14, 108.38, 56.71, 52.42, 47.11, 33.56, 25.05, 20.13.
1‐(4‐Chlorobenzyl)‐N‐propyl‐1H‐benzo[d]imidazol‐2‐amine (16 f). Yield=25.0 mg, 57 % (white solid). LCMS: RT=2.13 min., >98 % @ 215 and 254 nm, m/z=300.1 [M+H]+. 1H NMR (500 MHz, CDCl3) δ 7.53 (d, J=7.9 Hz, 1H), 7.31 (d, J=8.4 Hz, 2H), 7.17–7.13 (m, 1H), 7.09 (d, J=8.4 Hz, 2H), 7.07–7.01 (m, 2H), 5.08 (s, 2H), 4.33 (s, 1H), 3.47 (dd, J=12.5, 6.9 Hz, 2H), 1.67–1.59 (m, 2H), 0.90 (t, J=7.4 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 154.08, 141.89, 134.45, 133.99, 133.94, 129.31, 127.80, 121.67, 119.93, 116.42, 107.20, 45.20, 45.08, 22.94, 11.21.
Pharmacology
Cells
TRPC5 was stably expressed by cloning mouse TRPC5 into the pCDNA5/FRT/TO plasmid then co‐transfecting with the pOG44 plasmid into Flp‐In T‐REx −293 cells (Invitrogen) followed by 200 ug/mL HygromycinB selection. Cells were maintained in Advanced DMEM/F12 (Invitrogen #10565‐042) supplemented with 10 % FBS along with 15 μg/mL Blasticidin S (Life #A11139‐03) and 200 μg/mL Hygromycin B (Life #10687‐010). For Syncropatch experiments 0.5‐1 μg/mL Doxycycline (1 mg/mL stock doxycycline hyclate, Sigma #D9891‐10G) was added 3 days prior to Syncropatch recordings. On the day of Syncropatch recordings, the cells were lifted using 4 minute incubations at 37 °C with accutase (Sigma #A6964‐100 ML).
Syncropatch pharmacology
The potency of the compounds was measured by inhibition of riluzole‐induced TRPC5 currents as measured on the automated patch clamp platform Syncropatch 384PE (Nanion). Extracellular solution was composed of: 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 2 mM MgCl2, 2 mM CaCl2 and 10 mM glucose. Intracellular solution was composed of: 20 mM CsCl, 90 mM CsSO4, 5 mM EGTA, 2 mM CaCl2, 10 mM HEPES, 10 mM glucose, 4 mM Na2‐ATP and 0.5 mM Na‐GTP. A ramp protocol of 200 ms from −100 mV to +100 mV was pulsed at 5–10s intervals to measure TRPC5 currents. Baseline currents were recorded followed by addition of riluzole to a final concentration of 30 μM to activate currents. Only cells that developed the characteristic doubly‐ rectifying current of TRPC5 homomers were taken for subsequent analysis. The compound being tested was then added while maintaining 30 μM of riluzole in solution. Cells were discarded if there was noticeable runup or rundown in the current generated from the last 3 voltage ramps before compound addition. Percent inhibition was calculated as the average of the 3 ramps after compound addition (once the current had stabilized) divided by the average of the 3 ramps before compound addition. IC50’s were calculated by using the three parameters nonlinear regression (curve fit) function of Prism 7 (GraphPad).
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This work was generously supported by a grant from the US National Institutes of Health (NIDDK: R01DK103658) to C.R.H. The authors would like to thank Q2 Solutions (Indianapolis, IN USA) and Pharmaron, Inc. (Louisville, KY USA) for the in vitro and in vivo DMPK experiments.
S. Sharma, J. L. Pablo, K. T. Tolentino, W. Gallegos, J. Hinman, M. Beninato, M. Asche, A. Greka, C. R. Hopkins, ChemMedChem 2022, 17, e202200151.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1. Clapham D. E., Nature 2003, 426, 517–524. [DOI] [PubMed] [Google Scholar]
- 2. Montell C., Birnbaumer L., Flockerzi V., Cell 2002, 108, 595–598. [DOI] [PubMed] [Google Scholar]
- 3. Tang Q., Guo W., Zheng L., Wu J.-X., Liu M., Zhou X., Zhang X., Chen L., Cell Res. 2018, 28, 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duan J., Li J., Zeng B., Chen G.-L., Peng X., Zhang Y., Wang J., Clapham D. E., Li Z., Zhang J., Nat. Commun. 2018, 9, 3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duan J., Li J., Chen G.-L., Ge Y., Liu J., Xie K., Peng X., Zhou W., Zhong J., Zhang Y., Xu J., Xue C., Liang B., Zhu L., Liu W., Zhang C., Tian X.-L., Wang J., Clapham D. E., Zeng B., Li Z., Zhang J., Sci. Adv. 2019, 5, eaaw7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma S., Hopkins C. R., J. Med. Chem. 2019, 62, 7589–7602. [DOI] [PubMed] [Google Scholar]
- 7. Schaldecker T., Kim S., Tarabanis C., Tian D., Hakroush S., Castonguay P., Ahn W., Wallentin H., Heid H., Hopkins C. R., Lindsley C. W., Riccio A., Buvall L., Weins A., Greka A., J. Clin. Invest. 2013, 123, 5298–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou Y., Castonguay P., Sidhom E.-H., Clark A. R., Dvela-Levitt M., Kim S., Sieber J., Wieder N., Jung J. Y., Andreeva S., Reichardt J., Dubois F., Hoffman S. C., Basgen J. M., Montesinos M. S., Weins A., Johnson A. C., Lander E. S., Garrett M. R., Hopkins C. R., Greka A., Science 2017, 358, 1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung J.-J., Shaw A. S., Science 2017, 358, 1256. [DOI] [PubMed] [Google Scholar]
- 10. Yu M., Ledeboer M. W., Daniels M., Malojcic G., Tibbitts T. T., Coeffet-Le Gal M., Pan-Zhou X.-R., Westerling-Bui A., Beconi M., Reilly J. F., Mundel P., Harmange J.-C., ACS Med. Chem. Lett. 2019, 10, 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Z., Chen L., Tian H., Liu M., Jiang S., Shen J., Wang K., Cao Z., Bioorg. Med. Chem. Lett. 2022, 61, 128612. [DOI] [PubMed] [Google Scholar]
- 12. Sadler K. E., Moehring F., Shiers S. I., Laskowski L. J., Mikesell A. R., Plautz Z. R., Brezinski A. N., Mecca C. M., Dussor G., Price T. J., McCorvy J. D., Stucky C. L., Sci. Transl. Med. 2021, 13, eabd7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Just S., Chenard B. L., Ceci A., Strassmaier T., Chong J. A., Blair N. T., Gallaschun R. J., del Camino D., Cantin S., D'Amours M., Eickmeier C., Fanger C. M., Hecker C., Hessler D. P., Hengerer B., Kroker K. S., Malekiani S., Mihalek R., McLaughlin J., Rast G., Witek J., Sauer A., Pryce C. R., Moran M. M., PLoS One 2018, 13, e0191225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma S. H., Pablo J. L., Montesinos M. S., Greka A., Hopkins C. R., Bioorg. Med. Chem. Lett. 2018, 29, 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kilchmann F., Marcaida M. J., Kotak S., Schick T., Boss S. D., Awale M., Gönczy P., Reymond J.-L., J. Med. Chem. 2016, 59, 7188–7211. [DOI] [PubMed] [Google Scholar]
- 16. Obach R. S., Drug Metab. Dispos. 1999, 27, 1350–1359. [PubMed] [Google Scholar]
- 17. Obach R. S., Baxter J. G., Liston T. E., Silber B. M., Jones B. C., MacIntyre F., Rance D. J., Wastall P., J. Pharmacol. Exp. Ther. 1997, 283, 46–58. [PubMed] [Google Scholar]
- 18. Chang G., Steyn S. J., Umland J. P., Scott D. O., ACS Med. Chem. Lett. 2010, 1, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Besnard J., Ruda G. F., Setola V., Abecassis K., Rodriguez R. M., Huang X. P., Norval S., Sassano M. F., Shin A. I., Webster L. A., Simeons F. R., Sojanovsky L., Prat A., Seidah N. G., Constam D. B., Bickerton G. R., Read K. D., Wetsel W. C., Gilbert I. H., Roth B. L., Hopkins A. L., Nature 2012, 492, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bridges T. M., Morrison R. D., Byers F. W., Luo S., Daniels J. S., Pharmacol. Res. Perspect. 2014, 2, e00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith N. F., Raynaud F. I., Workman P., Mol. Cancer Ther. 2007, 6, 428–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.