Abstract
Reducing negative impacts of stress, for example through mindfulness training, benefits physical and psychological well-being, and is becoming ever more crucial due to large-scale societal uncertainties (e.g., COVID-19). While extensive research has focused on mindfulness-related reductions in self-reported negativity, essentially no research has targeted task-based behavioral outcomes throughout long-term mindfulness trainings. Responses to emotionally ambiguous signals (e.g., surprised expressions), which might be appraised as either positive or negative, provide a nuanced assessment of one’s emotional bias across diverse contexts, offering unique leverage for assessing the effects of mindfulness. Here, we compared the effects of short- and long-term training via Mindfulness-Based Stress Reduction on ratings of faces with a relatively clear (angry, happy) and ambiguous (surprised) valence. Ratings became more positive for ambiguity from the start (Week 1) to end of training (Week 8; p < .001), but there were no short-term effects (from a single class session). This shift towards positivity continued through an additional eight-week follow-up (Week 16; p < .001). Notably, post-training valence bias (Week 8) was uniquely predicted by the non-reactivity facet of mindfulness (p = .01). Together, mindfulness promotes a relatively long-lasting shift toward positivity bias, which is uniquely supported by reduced emotional reactivity.
Keywords: mindfulness, emotional ambiguity, valence bias, non-reactivity, stress
From relatively minor (e.g., bumper-to-bumper traffic) to more significant circumstances (e.g., economic downturns, a global pandemic, racial unrest), our psychological well-being relies on our ability to cope with a diverse and ever-changing set of stressors. Some stress can be beneficial (Jamieson et al., 2018; Yerkes & Dodson, 1908); for instance, pre-race cortisol levels – a physiological marker of stress – are associated with faster swim times in elite athletes (Rano et al., 2019), and stress can be harnessed to increase facilitative, rather than debilitative, competition anxiety (Kerr & Leith, 1993). On the other hand, chronic or uncontrolled stress negatively impacts both physical (Everson-Rose & Lewis, 2005; Low et al., 2009) and psychological well-being (Berghdal & Berghdal, 2002), as well as cognitive function (e.g., working memory deficits; Schoofs et al., 2009). As such, altering our maladaptive stress responses, such as in chronic or uncontrolled stress, via stress reduction techniques can have downstream benefits for both physical (Rosenkranz et al., 2013) and psychological (Britton et al., 2012) well-being.
One well-established method for reducing stress that has garnered increasing attention in recent years is the practice of mindfulness. Mindfulness – a concept rooted in Buddhist tradition – is characterized by both a conscious attention to the present moment (Brown & Ryan, 2003) as well as a non-judgmental and non-reactive attitude towards one’s bodily sensations, thoughts, and emotions (Kabat-Zinn, 1990). Although mindfulness comes in many forms, the most well-studied, standardized mindfulness intervention is an eight-week program known as Mindfulness-Based Stress Reduction (MBSR; Kabat-Zinn, 1990). There is growing evidence that MBSR relieves symptoms of a variety of psychopathologies, including mood and anxiety disorders (e.g., rumination and worry; Hofmann et al., 2010; Querstret et al., 2020) at a rate that is comparable to other evidence-based treatments (e.g., anti-depressants; Goldberg et al., 2018).
Indeed, a large body of literature supports the notion that mindfulness interventions improve symptoms of psychopathology, along with more general psychological well-being, even in non-clinical populations. For instance, a recent meta-analysis revealed that short-term mindfulness interventions (i.e., less than two weeks) reduce self-reported negative affect (Schumer et al., 2018). Another meta-analysis, focused on MBSR specifically, showed similar benefits for indices of self-reported stress, depression and anxiety symptomology, quality of life, and burnout (Khoury et al., 2015). Such reductions in self-reported negative affect and improvements in self-reported well-being may be attributable to greater mindfulness-related flexibility (Carmody et al., 2009; Shapiro et al., 2006; Silberstein et al., 2012) and its associated improvement in emotion regulation abilities (Sayers et al., 2015). Indeed, an integrated mindful emotion regulation framework posits that mindfulness meditation may lead to greater conscious control of one’s cognitions, emotions, and sensations (Chambers et al., 2009; Garland et al., 2011), supporting more flexible and adaptive responses (e.g., positive reappraisals).
Two notable gaps in the mindfulness literature are that many studies do not assess long-term impacts beyond the end of the intervention and that it has relied heavily on self-report questionnaires over task-based behavioral measures (but see Kiken & Shook, 2011, which focused on effects of a brief mindfulness intervention on memory for positive and negative stimuli). In fact, the reliance on self-report questionnaires has been identified as one of the most pernicious sources of biases within the mindfulness literature (Goldberg et al., 2021). Self-report questionnaires are subject to the influences of demand characteristics (e.g., reporting lower stress or higher well-being because it is expected), recall or estimation biases (e.g., reporting more frequent or longer duration mindfulness practice than actually achieved), and differences in the abilities of novices versus more experienced mindfulness practitioners to self-report (e.g., long-term practitioners likely have greater insight into their own experience with mindfulness-related constructs; Davidson & Kaszniak, 2015). In contrast, task-based behavioral measures provide a crucial advance with greater ecological validity than self-report questionnaires, and shed additional light on the impact of MBSR and mindfulness interventions on psychological well-being. Further, task-based behavioral measures include both explicit task performance (e.g., subjective appraisals), which are still moderately subject to demand characteristics, and also implicit measures (e.g., mouse-tracking) that are more resistant to these phenomena.
Mouse-tracking, as an implicit and objective measure, provides a unique window to the mechanisms underlying explicit performance by characterizing response competition – a psychological construct representing parallel activation of competing cognitive representations – within the decision process. For instance, in the context of emotion, competing cognitive representations might result from parallel activation of positive and negative valence representations (e.g., Cacioppo et al., 1997; Norris et al., 2010). The online measurement of response competition via mouse-tracking quantifies the degree of parallel activation in a manner that more traditional measures, like reaction time, cannot. For example, slower reaction time might reflect greater response competition, but it may also reflect a slower accumulation of evidence. As such, mouse-tracking provides a more fine-grained measure for exploring parallel activation of cognitive representations (response competition) and how this parallel activation of multiple representations might change as a function of interventions.
Here, we address these two gaps by exploring the effect of MBSR training on explicit and implicit task-based behavioral measures in a longitudinal study that measures responses to emotional ambiguity. For example, surprised expressions predict both positive (unexpected visit from an old friend) and negative (witnessing a car crash) outcomes, and, in the absence of context, individuals differ in their tendency to appraise these ambiguous signals as positive or negative (Harp et al., 2021; Neta et al., 2009; 2013). This variability in appraisals of emotional ambiguity represent one’s valence bias. Valence bias is a particularly sensitive measure that represents an emotional lens through which one views the world, exerting a powerful influence on decision-making, social interactions, and psychological well-being. For example, the risks and ambiguities inherent in decision-making in financial (e.g., stock market gains and losses), health (e.g., social distancing), and many other domains are likely perceived differently as a function of one’s valence bias. In social contexts, valence bias offers insight into the stereotype-based expectancies that drive social decision-making (Harp et al., 2021; Macrae et al., 1994). For instance, those with a more negative valence bias may maintain negative perceptions of ambiguity, which ultimately leads to confirmatory searches for more negative information in a self-perpetuating manner (Snyder & Swann, 1978; Trope & Thompson, 1997). Last but not least, valence bias has been shown to relate to subclinical levels of internalizing symptomology (see Petro, Tottenham, & Neta, 2021; Neta & Brock, 2021). Thus, valence bias provides a nuanced assessment of one’s propensity to perceive positive or negative emotional valence in situations when both alternatives are equally valid, and across a diverse range of contexts.
Interestingly, accumulating evidence suggests people have a default, initial negativity, and a positive valence bias appears to rely on slower, more controlled processing (Neta & Whalen, 2010; Neta & Tong, 2016; Neta, Berkebile, & Freeman, 2020). In other words, the ability to overcome the initial negativity and appraise emotionally ambiguous signals as positive indicates greater emotional flexibility, and appears to be supported in part by an emotion regulation mechanism. Neuroimaging evidence supports this view as individuals with a more negative valence bias show increased amygdala and decreased prefrontal activity in response to surprised faces, but the inverse pattern was evident in those with a more positive bias (Kim et al., 2003; Petro et al., 2018). In line with these findings, situations that hamper cognitive control, such as elevated stress (Brown et al., 2017) or emotional arousal brought on by unpredictable and adverse events (i.e., a threat of shock paradigm; Neta et al., 2017), lead to a more negative valence bias. As such, assessing valence bias as a function of mindfulness interventions, like MBSR, holds promise for informing the behavioral mechanisms (e.g., greater emotional flexibility) through which mindfulness mitigates negative affect and benefits psychological well-being.
Here, we aim to provide a longitudinal assessment of the effects of a long-term mindfulness intervention, MBSR, on appraisals of emotionally ambiguous social cues (i.e., surprised facial expressions). Specifically, we directly compare the effects of a brief versus longer-term intervention by testing two preregistered hypotheses that MBSR training would promote a more positive valence bias after both (1) acute (pre- to post-class, i.e., brief intervention) and (2) long-term (start to end of course, i.e., longer-term intervention) training. In exploratory analyses, we test for a longer-term shift in valence bias by assessing appraisals of emotional ambiguity eight weeks after the MBSR training is complete. At each time point, we also explore an implicit measure reflecting response competition underlying these appraisals of ambiguity. Further, in order to contextualize any changes over the course of the MBSR training, we also explore which facet of mindfulness, across five established facets (Baer et al., 2006), best predict valence bias after training, and whether a change in these facets is associated with degree of change in valence bias. Such an analysis allows us to explore mechanistic effects of mindfulness on any associated changes in valence bias. Lastly, we assess appraisals of emotionally ambiguous social cues (i.e., surprised facial expressions) in two independent, demographically matched, passage of time control groups. These control groups will be used to address the specificity of any change in appraisals over time as a result of the MBSR intervention.
Method
Participants.
Our preregistration identified a target sample of twenty participants, although funding obtained after this prepregistration permitted the collection of a total of sixty-one participants. These participants were recruited through advertisements distributed by MBSR instructors from courses throughout the United States. Eligibility was based on a lack of prior mindfulness training or active practice (i.e., one hour per week or more), and being at least 18 years of age. All MBSR instructors were formally trained. Two subjects were removed because their MBSR instructor disclosed information regarding predictions for the study, and one additional subject was removed because he was unable to remain awake during data collection for the first session and did not return for the final three sessions. The final sample included 58 participants. All participants provided informed consent in accordance with the Declaration of Helsinki, and the research protocol was approved through the University of Nebraska-Lincoln’s Institutional Review Board (Approval # 20171217871EP).
Additionally, two independent samples from other experiments that included repeated measurements of valence bias (described below, but to be fully reported elsewhere) were identified as passage of time control groups. The first was selected from a large-scale longitudinal study exploring neurocognitive mechanisms of valence bias, and the second was selected from a large-scale longitudinal study exploring the effects of the COVID-19 pandemic on valence bias. The selected subsample reported here was chosen as an age- and gender-matched control group for the best comparison to the MBSR sample (see Table 1). All participants provided informed consent in accordance with the Declaration of Helsinki and all research protocols were approved through the University of Nebraska’s Institutional Review Board for both the Lincoln community sample (Approval # 20141114675EP) and the COVID-19 sample (Approval # 20200520425EP).
Table 1.
Descriptive statistics of demographic variables.
| COVID-19 Sample (n = 58) | ||
|---|---|---|
| M(SD) = 42.86(12.69) Range = 21–69 |
M(SD) = 44.69(16.63) Range = 21–69 |
M(SD) = 39.88(12.09) Range = 20–68 |
| 2 Asian, 4 more than one race, 52 White | 5 Asian, 3 more than one race, 50 White | 3 Asian, 1 Black, 54 White |
| 4 Hispanic/Latino, 53 not Hispanic/Latino, 1 unreported | 4 Hispanic/Latino, 53 not Hispanic/Latino, 1 unreported | 58 not Hispanic/Latino |
| 52 Females, 6 Males | 45 Females, 13 Males | 49 Females, 9 Males |
| Iowa (12), Massachusetts (6), North Carolina (10), Nebraska (5), Nevada (1), Washington (24) | Lincoln, Nebraska | U.S.-based Online Recruitment (Mturk and Social Media) |
Intervention.
The MBSR instructors delivered the standard MBSR training (Kabat-Zinn, 1990) in their own private practice setting. This “gold-standard” course (Van Dam et al., 2018) includes eight weekly classes, each approximately 2.5 hours, and daily homework assignments (e.g., pre-recorded, guided mediations) to encourage mindfulness practice each day throughout the course. In addition, there was a full day (approximately 8 hours) meditation retreat that occurred in the final week of the course. The class size for each MBSR training was not recorded, but we note that the participants in this study represent only a subset of the individuals in any given class (i.e., participation in this study was not required to participate in the MBSR training).
Stimuli.
Face stimuli in the valence bias task (described below) included 72 unique identities (37 female, 35 male) posing one or more of the three expressions (angry, happy, surprised), for a total of 120 faces (60 female, 60 male). These stimuli were selected from the Karolinska Directed Emotional Faces (Lundqvist et al., 1998), Umea (Samuelsson et al., 2012), and NimStim sets (Tottenham et al., 2009). The total stimulus set was divided into five subsets that did not differ in hit rate (F(1, 118) = 0.06, p = .80). Each subset included an equal number of clear (6 angry, 6 happy) and ambiguous (12 surprised) faces that were presented in a pseudorandomized order. Participants were randomly assigned to a counterbalanced order, pairing each subset with one of the five sessions.
In addition, participants completed a color-rating task, in which blue, red, or varying shades of purple circles on a gray background were presented, as a within-subject control for the valence bias task (described below). This task has long been used in mouse-tracking research because mouse trajectories reliably show greater response competition in response to purple stimuli, as opposed to red or blue (e.g., Freeman & Ambady, 2010). Thus, it was included to control for the possibility of participation in the MBSR training resulting in a more generalized shift in processing of ambiguity, uncertainty, or response competition. If the training resulted in general changes to response competition or the processing of conflicting or ambiguous stimuli (rather than a specific effect on the interpretation of a face’s emotional valence, as we hypothesize), then we would expect the training to exert corresponding changes in mouse-trajectory responses to the purple vs. red/blue stimuli.
Participants in the passage of time control conditions saw a subset of the faces described above but did not complete the color-rating task. In addition to the face stimuli, participants in the Lincoln community sample viewed and rated emotional scenes from the International Affective Picture System (IAPS; Lang et al., 2008), as described in prior work (see Neta et al., 2013). Participants in the COVID-19 sample also viewed and rated IAPS scenes, as well as emotional words. A complete list of these additional stimuli is available in Harp et al. (2021). The analyses reported here focus only on ratings of the faces for a direct comparison to the task completed by the MBSR sample.
Procedure.
Participants completed a series of five sessions over a period of sixteen weeks (Table 2). The first session took place before, and the second session immediately after, the first MBSR class. The third and fourth sessions took place before and after the eighth and final MBSR class, and the fifth session took place eight weeks after the final MBSR class (Week 16). Descriptive statistics for the time in hours between sessions is available in Table 3. In each session, participants completed both a valence bias and a color-rating task. Additionally, the Five Facet Mindfulness Questionnaire (FFMQ; Baer et al., 2006) was administered during the first, third, and fifth sessions to assess self-reported mindfulness throughout the training. Participants also provided demographic information during the first session (see Table 2). In addition to these measures, participants completed questionnaires to assess personality, symptoms of mood disorders, emotion regulation tendencies, intolerance of uncertainty, optimism, empathy, and stress. These measures were included for exploratory purposes, beyond the scope of the present findings, and are thus not reported here.
Table 2.
Summary of research sessions and design for the MBSR sample.
| Session # | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | |
|---|---|---|---|---|---|---|
| Tasks | Valence & Color rating | Valence & Color rating | Valence & Color rating | Valence & Color rating | Valence & Color rating | |
| FFMQ | FFMQ | FFMQ | ||||
| Demographics | ||||||
| Class | Before first MBSR class (Pre-Class) | After first MBSR class (Post-Class) | Before last MBSR class (Pre-Class) | After last MBSR class (Post-Class) | Eight weeks after Session 4 (N/A) | |
| Training | Start | Start | End | End | (N/A) | |
| Week | Week 1 | Week 1 | Week 8 | Week 8 | Week 16 | |
| Timeline | Baseline testing | (Undergoing MBSR Training) | Follow-up | |||
Table 3.
Descriptive statistics for time in hours between pre- and post-class sessions at the start and end of training.
| Training | Mean | SD | Minimum1 | Maximum |
|---|---|---|---|---|
| Start (Week 1) | 43.99 hours | 48.69 hours | 2.00 hours | 196.37 hours |
| End (Week 8) | 26.66 hours | 24.36 hours | 3.47 hours | 114.12 hours |
One of the “post-class” data files for two subjects was removed because the pre- and post-class tasks were both completed prior to the MBSR class.
The valence bias and color-rating tasks were completed using a JavaScript-based online implementation of MouseTracker software (Freeman & Ambady, 2010) to collect response and mouse trajectory data. Participants rated each facial expression as either positive or negative and each colored circle as either blue or red in a two-alternative, forced-choice task (Figure 1). Trials were self-paced, with each trial beginning once the participant clicked the “Start” button at the bottom-center of the screen. In the valence bias task, an image of an angry, happy, or surprised face appeared after the start button was pressed and remained on screen until the participant responded. In the color-rating task, an image of a red, blue, or purple circle appeared and remained on screen until the participant responded. Participants responded by clicking a “Positive” or “Negative” response (valence bias task) or “Red” or “Blue” response (color rating task) located at the top corners of the screen with their computer mouse (responses, located left vs. right, were randomized across participants). A reminder to respond quickly was displayed following any trials on which participants did not respond in less than 2000 ms. Participants completed ten practice trials before beginning the valence bias task, after which they completed a total of 72 trials (18 angry, 18 happy, 36 surprise) in the valence bias task and 48 trials (21 red, 21 blue, 6 purple) in the color rating task while their mouse movements were recorded. These recorded trajectories were then used to generate measures of response competition, such as maximum deviation (MD). MD is the maximum perpendicular deviation from a straight-line response trajectory on any given trial, and this measure quantifies response competition as the maximal attraction toward the competing or unselected response (Freeman et al., 2011; Hehman et al., 2015).
Figure 1.
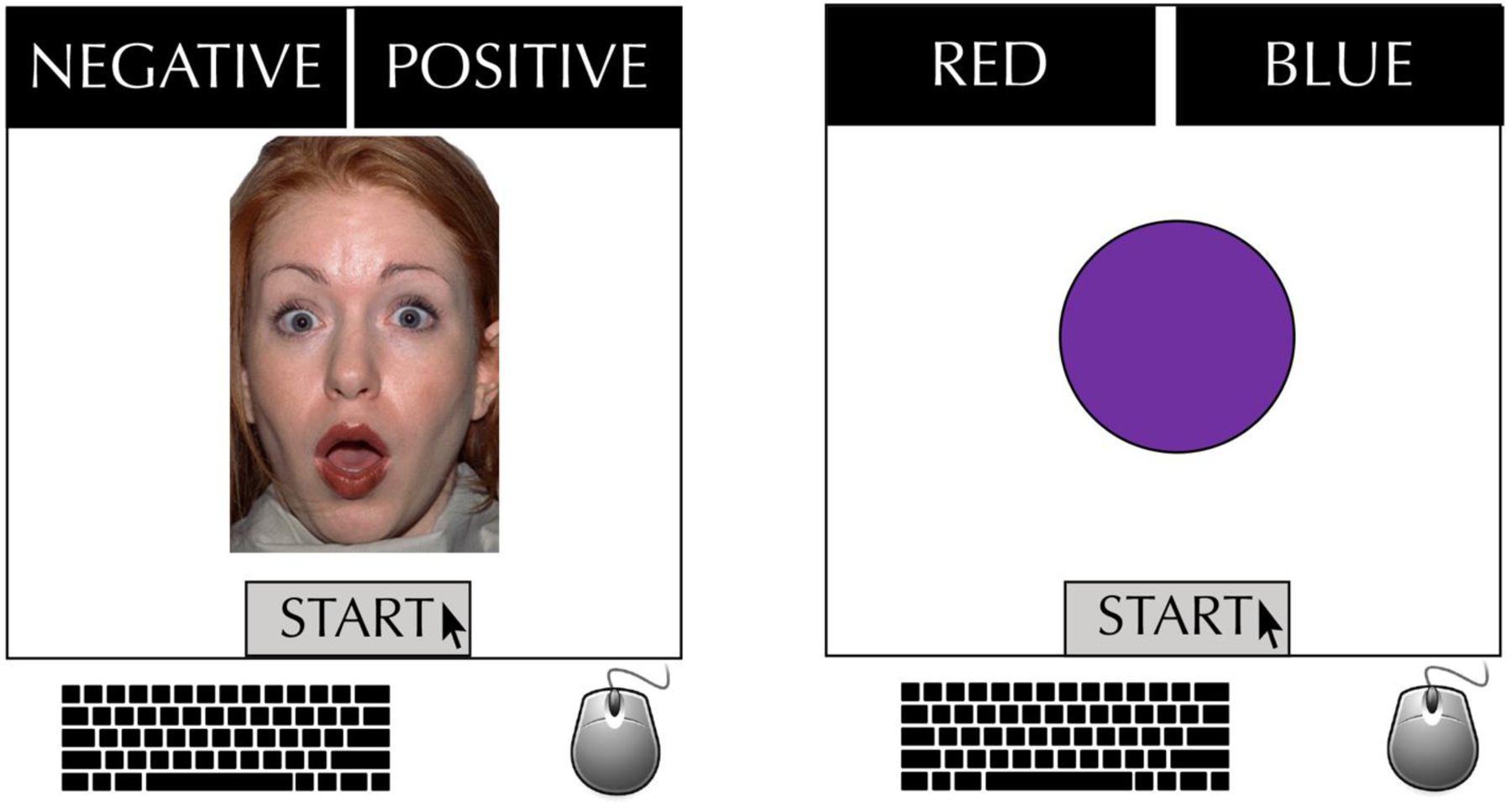
Example trial and set-up for each of the two tasks – valence bias task, with surprise face displayed (01F from NimStim; Tottenham et al., 2009), and color rating task, with purple circle displayed.
All tasks were completed on either the participants’ own computers with internet connection and a mouse or one provided by the research team. Participants using their own computers presumably completed the tasks at home. Those using computers provided by the research team completed the task in an area nearby the training (i.e., on-site). Compensation was awarded intermittently, such that payments occurred after completion of sessions one and two ($10), three and four ($20), and five ($20).
Participants in the passage of time control conditions completed a similar task, with a few differences that are noted below. First, in the Lincoln community sample, two participants completed the task in E-prime (Psychology Software Tools, Pittsburgh, PA, USA) instead of MouseTracker. Additionally, stimuli were displayed for 500 ms rather than indefinitely (there was an intertrial interval of 1500 ms in E-prime, self-paced in MouseTracker). The second session, approximately one year later (M(SD) = 363.26(18.45) days, range = 340–448 days), was completed online via Qualtrics on participants’ own computers. Participants were compensated $5.00 per 30 minutes for the laboratory session and $5.00 total for the online follow-up.
The COVID-19 sample completed the valence bias task in Gorilla Experiment Builder (Anwyl-Irvine et al., 2020) for both sessions. The two sessions were approximately six months apart (M(SD) = 183.77(7.95) days, range = 167–208 days). Face stimuli were displayed for 500 ms and followed by a 1500 ms fixation cross. Failure to make a response within 2000 ms resulted in the task advancing to the next trial, and the trial was removed prior to analysis. Participants used a keyboard button (“A” or “L”) to rate each image. Participants were compensated on a graded scale ($5.00-$10.00 per 30 minutes), depending on the number of previous sessions they had completed.
Analyses.
Data preprocessing, analysis, and plotting were completed using R (R Core Team, 2017) along with the mousetrap (Kieslich et al., 2019), lme4 (Bates et al., 2015), emmeans, (Lenth et al., 2020), lmerTest (Kuznetsova et al., 2017), car (Fox & Weisberg, 2019), and ggplot2 (Wickham, 2016) packages. Our preregistered hypotheses can be found at https://aspredicted.org/i9qy5.pdf and analysis scripts are available at https://osf.io/dx4ke/. Data are available upon request (see Author Note). Across all sessions in the MBSR sample, task data were missing for 79 of the 290 (27%; n = 58 × 5 timepoints) valence bias and color rating timepoints. Similarly, survey data across all sessions were missing for 37 of the 174 (21%; n = 58 × 3 timepoints) survey timepoints. Maximum likelihood estimation was used to account for these missing data. Full information maximum likelihood was used for nested model comparison – that is, when comparing models with different random effects structures – but restricted maximum likelihood estimation was used for all final models and the reported fixed effects. We report 95% CIs and effect sizes when possible, but note that linear mixed effects models do not have an agreed upon method for calculating effect sizes (see Rights & Sterba, 2019, for a discussion). There were no missing behavioral data in either the Lincoln community or the COVID-19 samples.
Valence bias was calculated as percent negative ratings (e.g., if a participant rated eight out of twelve surprise faces as negative, then their valence bias would be 66%). As in previous work, data were screened such that participants failing to rate the clearly valenced faces above 60% accuracy were removed prior to the statistical analyses (n = 0; Neta et al., 2009). Similarly, a color bias (percent red ratings) was also calculated by dividing the number of “red” ratings for each color of circle by the total number of trials for each color (e.g., if a participant rated two out of six purple circles as red, then their color bias is 33%). MD (the extent to which responses deviated from a straight-line trajectory) and initiation time (the time at which the first mouse movement occurred) were also calculated for each trial.
Results
Effects of MBSR on subjective appraisals
Percent Negative Ratings.
To explore percent negative ratings as a function of MBSR training, we tested condition mean values for effects of Expression (angry, happy, surprise), Training (start, end), and Class (pre-, post-) in a linear mixed-effects model. This model revealed a significant effect of Expression (X2(2) = 837.45, p < .001), such that percent negative ratings for angry faces were consistently more negative than surprised faces, which were more negative than happy faces (ps < .001). In addition, there was a significant effect of Training (X2(1) = 4.17, p = .04), which revealed that percent negative ratings became more positive overall from the start to end of training (t = 2.06, p = .04, 95% CI [0.10, 8.12]). Critically, an Expression × Training interaction (X2(2) = 11.76, p = .003) revealed that the more positive ratings at the end of training were driven by a surprise-specific shift towards positivity (t = 3.75, p < .001, 95% CI [4.72, 15.27]), as hypothesized in our pre-registration, while ratings for angry (t = 1.02, 95% CI [−2.56, 7.98], p = .31) and happy faces (t = −0.14, p = .89, 95% CI [−5.65, 4.89]) did not significantly change (Figure 2a).
Figure 2. Behavioral ratings.
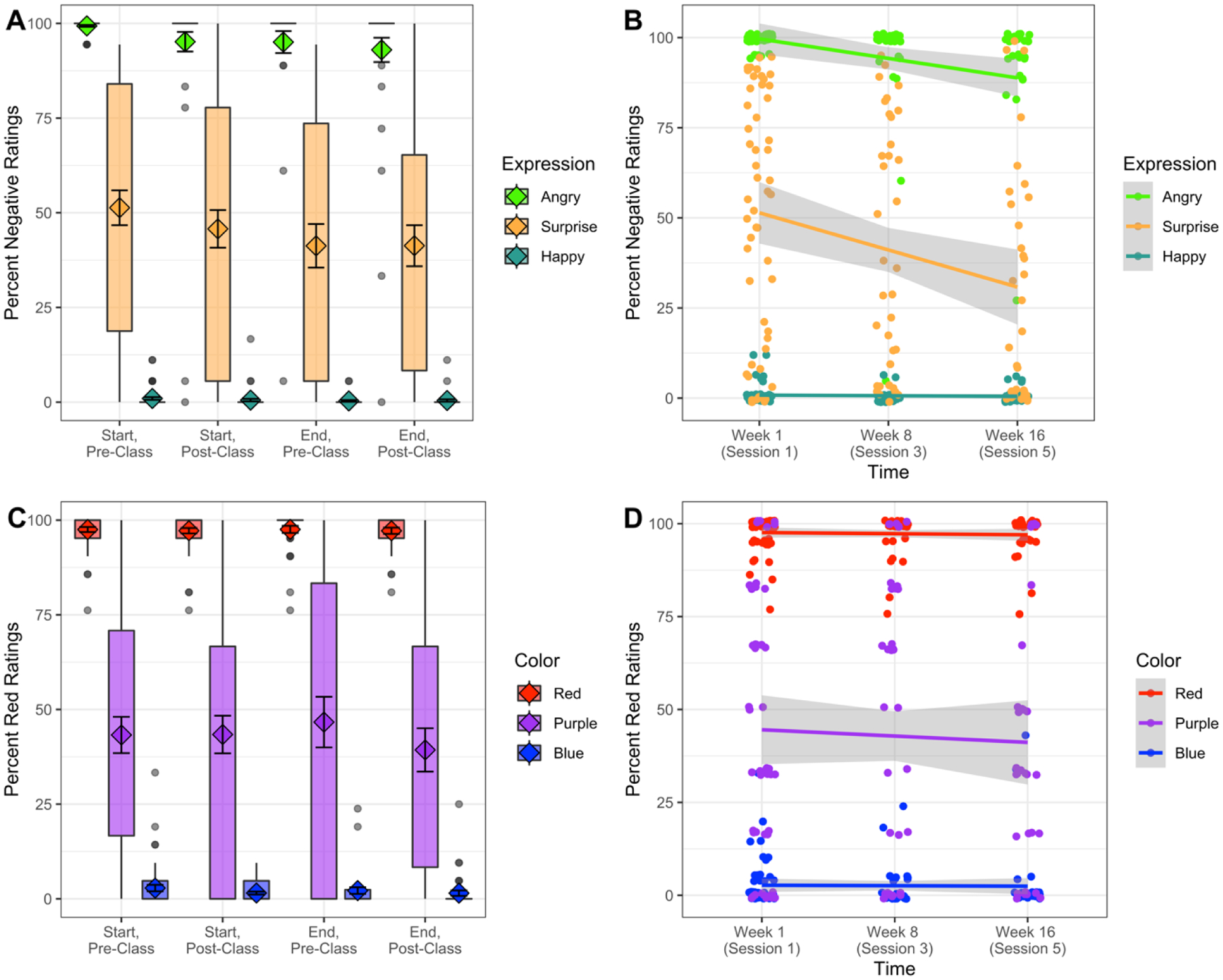
(A, C) Boxplots and (B, D) line graphs of Percent Negative Ratings and Percent Red Ratings at each timepoint. (A) An Expression × Training interaction revealed a surprise-specific shift towards positivity throughout MBSR training (X2(2) = 11.76, p = .003). (B) The rate of change for surprise ratings was greater than for happy (t = 3.96, p < .001) and angry (t = 2.21, p = .03) ratings. (C) There was no evidence of a Color × Training interaction (X2(2) = 0.07, p = .97), (D) nor was the rate of change for percent red ratings of any color statistically different than zero (ts < 1.10, ps > .27). Diamonds and error bars represent the mean and standard error. The edges of the box represent the first and third quartiles of the data, and bars extend to 1.5 multiplied by the interquartile range (IQR). Individual points are data points outside of 1.5 × IQR.
To explore the more long-lasting effects of MBSR on percent negative ratings, we tested for linear effects of Time (Week 1: baseline, Week 8: post-training, Week 16: follow-up) for each Expression (angry, happy, surprise) in a linear mixed-effects model on condition mean values. We removed the post-class timepoints for this analysis (i.e., Session 2 and Session 4) because the pre-class measurements provide a better estimate of baseline bias. This model revealed that the rate of change over time for surprise was significantly different than for happy (t = 3.96, p < .001, 95% CI [5.24, 15.49]) and for angry (t = 2.21, p = .03, 95% CI [0.64, 10.92]), such that ratings of surprise became more positive over time (t = −5.61, p < .001, 95% CI [−14.11, −6.81]), more so than for angry faces (t = −2.50, p = .01, 95% CI [−8.33, −1.03]) and for happy faces, which did not change (t = −0.05, p = .96, 95% CI [−3.74, 3.55]; Figure 2b).
Percent red ratings.
We next explored percent red ratings as a function of MBSR, testing for effects of Color (red, blue, purple), Training (start, end), and Class (pre-, post-) in a linear mixed-effects model. This model revealed an effect of Color (X2(2) = 1,028.55, p < .001), such that red was rated as red more frequently than purple (t = −17.91, p < .001, 95% CI [−60.00, −48.10], which was rated as red more frequently than blue (t = −13.76, p < .001, 95% CI [−47.50, −35.60]). As expected, all other effects and interactions were non-significant (all ps > .4 except for an effect of Class at p = .14; Figure 2c).
To confirm that MBSR was not significantly impacting responses on the color rating task, we next tested for linear effects of Color (red, blue, purple) and Time (Week 1: baseline, Week 8: post-training, Week 16: follow-up). Again, as expected, there were no interactions across the Color conditions (ps > .54) and the slope of time for each condition was not significantly different from zero (ps > .27; Figure 2d).
The role of non-reactivity in promoting positivity.
In a multiple regression, valence bias scores were regressed on each facet of the FFMQ. The regression model included four non-significant predictors and therefore indicated a collective effect at only a trend level (F(5, 37) = 2.12, p = .08, Multiple R2 = 0.22). Non-reactivity was the only significant predictor of valence bias (b = −32.43, p = .01, 95% CI [−56.79, −8.07]); observing (b = 19.45, p = .24, 95% CI [−13.66, 52.65]), acting with awareness (b = 2.02, p = .87, 95% CI [−22.95, 26.99]), non-judgmental (b = 2.15, p = .80, 95% CI [−15.17, 19.46]), and describing (b = −10.69, p = .30, 95% CI [−31.36, 9.97]) facets did not uniquely predict valence bias.
We next explored the changes in valence bias as a function of increased non-reactivity on an individual level. After computing change scores for both valence bias (percent negative ratings for surprise faces; Figure 3a) and non-reactivity as measured with the FFMQ from the start (Week 1; average of pre- and post-class) to the end of training (Week 8; average of pre- and post-class), we correlated the two scores. We found that the degree to which individuals became more non-reactive, or more able to allow feelings to come and go, was significantly associated with a larger shift towards a more positive valence bias at the end of training (r(40) = −.38, p = .01; Figure 3b).
Figure 3. Change in valence bias associated with a change in non-reactivity.
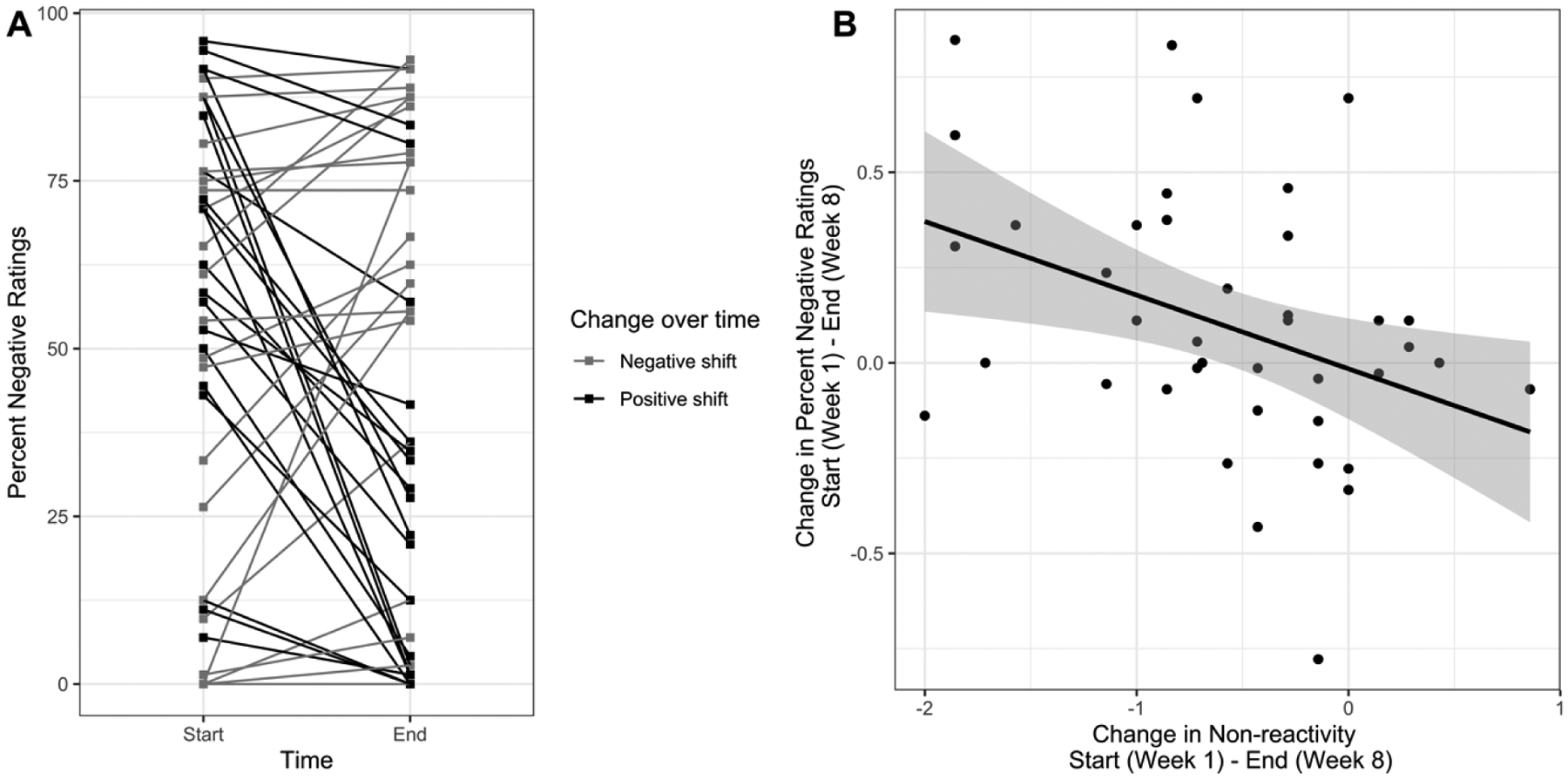
(A) Each line represents the change for an individual participant in percent negative ratings for surprised expressions from the start to end of the MBSR training. Those who became more negative have red lines and those who became more positive have blue lines. (B) Scatterplot of the change in percent negative ratings from the start to end of training with change scores in non-reactivity across the same time (r(40) = −.38, p = .01).
Effects of MBSR on response competition.
Initiation time.
We did not expect initiation times (i.e., when participants initiated a movement) to vary, as participants should initiate movement similarly across conditions. Nonetheless, we explored initiation times as a function of MBSR, testing for effects of Expression (angry, happy, surprise), Training (start, end), and Class (pre-, post-) in a linear mixed-effects model on the trial-level data. There were only effects of Class (X2(1) = 5.53, p = .02) and Class × Time (X2(1) = 6.22, p = .01), such that initiation times tended to be slower overall at post- than pre-class sessions (z = −2.18, p = .03, 95% CI [−57.70, −3.04]), and this effect was stronger at the start (z = −2.60, p = .01, 95% CI [−64.10, −8.95]) than the end of training (z = −1.68, p = .09, 95% CI [−52.50, 4.07]). We also tested for effects of Expression (angry, happy, surprise) and Time (Week 1: baseline; Week 8: post-training; Week 16: follow-up) on initiation times, but initiation time did not differ over time (ps > .36) nor between expressions (ps > .26). Given the pre- to post-class differences, we included initiation time as a covariate in the corresponding analyses of maximum deviation to account for the trajectories initiating at different times, and thus different stages of the decision-making process.
Maximum Deviation (MD).
This analysis examines the effects of MBSR training on participants’ computer mouse trajectories during the valence bias task. We first tested for effects of Expression (angry, happy, surprise), Training (start, end), and Class (pre-, post-) in a linear mixed-effects model fit to the trial-level data. Even when including initiation time as a covariate, there was an effect of Class on MD (X2(1) = 14.08, p < .001), such that computer mouse trajectories tended to be more direct post-class (z = 3.57, p < .001, 95% CI [0.02, 0.07]). Trajectories also tended to be more direct at the end than start of training (X2(1) = 4.52, p = .03; 95% CI [0.00, 0.05]), and an Expression × Training interaction (X2(2) = 19.21, p < .001) revealed that MD for angry expressions marginally increased from the start to the end of training (z = −1.62, p = .10, 95% CI [−0.07, 0.01]), while MD for happy (z = 3.46, p < .001, 95% CI [0.03, 0.10]) and surprise faces (z = 2.53, p = .01, 95% CI [0.01, 0.07]) significantly decreased (Figure 4a–c).
Figure 4.
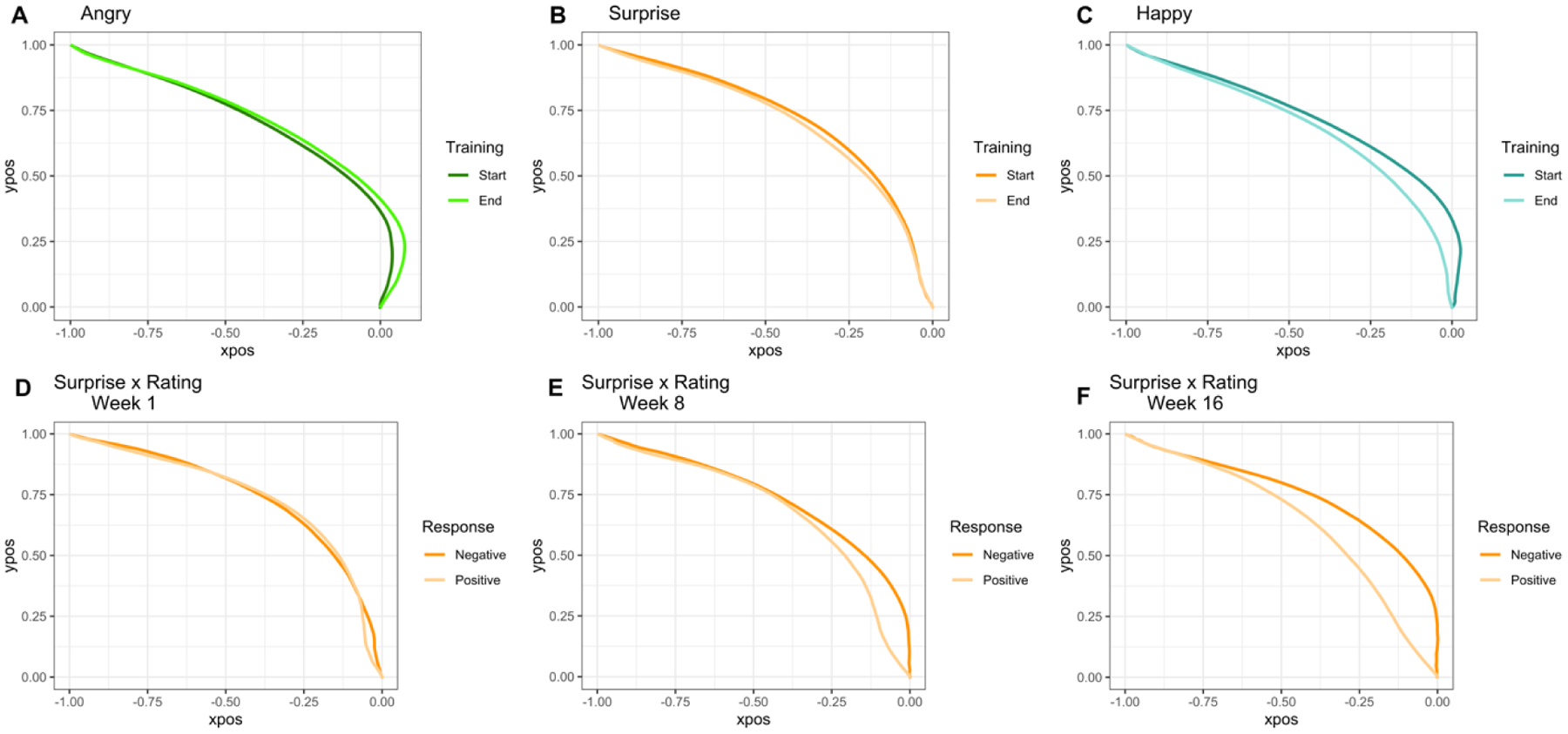
Plots of average trial trajectories for both the start and end of training for each facial expression as well as the trajectories for surprise rated as positive vs. negative at each week. (A-C) An Expression × Training interaction (X2(2) = 19.21, p < .001) revealed that trajectories for happy (p < .001) and surprise faces (p = .01) were more direct at the end than start of the MBSR training, but trajectories for angry trials tended to be less direct (p = .10). (D-F) A Response × Time interaction (p < .001) showed that response competition decreased over time for positive (p < .001) but not negative (p = .50) ratings of surprise.
We also explored more long-lasting effects of MBSR training on these measures of response competition. This model revealed that trajectories were more direct over time (i.e., with increased mindfulness experience) for both surprise (t = −3.69, p < .001, 95% CI [−0.08, −0.02]) and happy trials (t = −4.23, p < .001, 95% CI [−0.10, −0.04]) but not for angry trials (t = 1.31, p = .19, 95% CI [−0.01, 0.05]). To better understand differences in response competition for positive versus negative ratings of surprise faces, we removed the Expression term and instead compared MD by Response (positive, negative) for surprise trials. This analysis revealed an interaction of Response × Time (t = −3.88, p < .001, 95% CI [−0.13, −0.04]), such that response competition decreased over time for positive (t = −5.61, p < .001, 95% CI [−0.13, −0.06]) but not negative (t = 0.67, p = .50, 95% CI [−0.05, 0.03]) ratings of surprise (Figure 4d–f).
Passage of time controls.
Demographic matching.
The demographic variables of age, gender, race, and ethnicity were compared across the three samples (MBSR, Lincoln, COVID-19). A one-way ANOVA on age revealed no differences across the samples (F(2, 171) = 1.81, p = .17). Likewise, there were no significant differences in the gender (X2(2) = 3.15, p = .21), race (X2(6) = 7.27, p = .30), or ethnic (X2(4) = 6.38, p = .17) composition of the samples.
Percent negative ratings.
To explore the possibility that percent negative ratings change over time, outside of an intervention context, we tested for effects of Time (Lincoln sample: Year 1, Year 2; COVID-19 sample: Time 1 (Oct. 9 to Nov. 3, 2020), Time 2 (April 12 to May 10, 2021)) × Expression (angry, happy, surprise) in a linear mixed-effects model on condition mean values in each of the demographically matched samples. In the Lincoln sample, the model revealed an effect of Expression (X2(2) = 894.68, p < .001), such that angry expressions were rated as more negative than surprise expressions (t = 15.29, p < .001, 95% CI [42.63, 55.31) which were rated as more negative than happy expressions (t = −14.62, p < .001, 95% CI [−40.45, −14.62]). As expected, there was neither a main effect of Time (X2(1) = 0.05, p = .82) nor an interaction of Time × Expression (X2(2) = 0.23, p = .89). Likewise, in the COVID-19 sample, there was an effect of Expression (X2(2) = 1,600.72, p < .001), replicating the pattern described for the MBSR and Lincoln samples, but neither an effect of Time (X2(1) = 0.11, p = .74) nor a Time × Expression interaction (X2(2) = 0.88, p = .65).
Last, to further establish that the change in ratings shown in the MBSR sample differed from the Lincoln and COVID-19 samples, an analysis of Sample (MBSR, Lincoln, COVID-19) × Time (Time 1, Time 2) × Expression (angry, happy, surprise) was conducted across all participants, with levels of time as described above for each of the three samples. Crucially, the three-way interaction was significant (X2(4) = 11.97, p = .02), revealing that the MBSR sample rated surprised expressions as significantly more positive at Time 2 than Time 1 (t = 5.03, p < .001, 95% CI [9.37, 21.38]), whereas there was no change in ratings of surprised expressions for the Lincoln (t = 0.44, p = .66, 95% CI [−3.70, 5.82]) or the COVID-19 samples (t = −0.82, p = .41, 95% CI [−6.75, 2.77]).
Discussion
Here, we explored the acute and longer-term effects of MBSR training on both explicit (appraisals) and implicit (mouse-tracking) task-based behavioral measures, leveraging the valence bias task to explore responses to emotional ambiguity. We found that MBSR promoted a long-term shift (start to end of training; through the eight-week follow-up) toward positivity in response to ambiguity, and that this shift was specific to emotional ambiguity rather than a more general shift in response bias, as we did not observe significant changes in appraisals of the clear valence expressions throughout the MBSR training. Although there was some evidence of a potential shift towards positivity for the angry expressions over the longer-term (i.e., at the Week 16 follow-up), the degree of change for surprised expressions was significantly stronger than that of the angry expressions. Further, the response competition underlying the decision-making process reaffirms this shift towards positivity, in that mouse trajectories during positive appraisals of emotionally ambiguous faces were more direct as a function of the MBSR training. This shift in response competition was specific to emotional ambiguity, rather than a more generalized shift shift in processing of ambiguity, uncertainty, or response competition, as there were no effects for the color bias task. Notably, analysis of two separate passage of time (i.e., inactive) control groups supported the notion that the surprise-specific shift towards positivity was likely attributed to the MBSR intervention, rather than some other alternative explanation related to repeated exposure. We discuss these effects as a potential mechanism underlying the benefits of MBSR for psychological well-being and in the context of the initial negativity hypothesis below.
Psychological well-being.
Theoretical (Shapiro et al., 2006) and empirical work (Carmody et al., 2009; Silberstein et al., 2012) suggest that cognitive, emotional, and behavioral flexibility are mechanisms underlying the beneficial effects of MBSR on psychological well-being. However, this work is limited by its reliance on either self-report questionnaires (e.g., Zou et al., 2020), or other methodological concerns described above. Our implementation of a repeated-measures design, which allows for examination of both short- and longer-term effects on behavior, is a notable contribution to current understanding of the effects of MBSR on affective outcomes. Likewise, the present study makes a novel contribution to the literature on emotional flexibility as a function of MBSR training given our use of a task that leverages both explicit, subjective behavioral responses and implicit, objective indices of response competition during the resolution of emotional ambiguity. Indeed, a shift toward a more positive valence bias (and a concomitant decrease in response competition associated with positivity) suggests that MBSR promotes greater flexibility (see more on this below).
The desired effects of MBSR on mood and anxiety psychopathology (Goldberg et al., 2018) may be explained, at least in part, by this shift in valence bias. Indeed, a more positive valence bias has been linked to both physical (Neta et al., 2019) and psychological well-being (Neta et al., 2017; Petro et al., 2021), and promoting positivity in the face of ambiguity likely mitigates negative affect in day-to-day life (Puccetti et al., under review). In other words, a more positive valence bias is likely to impact one’s appraisals of the emotional meaning for many day-to-day events, helping individuals to “see the bright side” of emotional events (e.g., reframing a stressor as a challenge). This new reframing likely, then, contributes to the upward spiral of dispositional mindfulness and positive reappraisal tendencies (Garland et al., 2011).
Interestingly, we found that the extent to which individuals develop a more positive valence bias, indicating greater flexibility in response to emotional ambiguity, was associated with increases in non-reactivity. Further, levels of non-reactivity at the end of the MBSR training uniquely predicted valence bias at that same time point. This facet of mindfulness training is thought to play an important role in reducing depression symptoms (Royuela-Colomer & Calvete, 2016) and mediating MBSR-related improvements in self-reported cognitive flexibility (Zou et al., 2020). Altogether, our results suggest that MBSR-induced non-reactivity is likely the “active ingredient” that promotes the development of a more positive valence bias.
Although speculative, the MBSR-induced change in non-reactivity is likely prompting an increased functional connectivity of brain regions linked to emotion regulation (i.e., increased ventromedial prefrontal cortex activity along with decreased amygdala activity; Kral et al., 2018). Such a change would be expected to promote positivity towards emotional ambiguity, as this pattern of activation in ventromedial prefrontal cortex and amygdala activity are associated with a more positive valence bias (Kim et al., 2003; Petro et al., 2018). Interestingly, these neural effects of non-reactivity may contribute to reduced depressive symptoms (Paul et al., 2013), which are inversely related with a positive valence bias (Petro et al., 2021; Brock & Neta, 2021) and may be suggestive of a mechanism through which mindfulness protects against depressive symptoms (Royuela-Colomer & Calvete, 2016).
This finding has numerous implications for clinical research, suggesting, for instance, that non-reactivity may serve as a potential mechanism for improved psychological well-being and highlighting the need for future research into the relationship between psychological (e.g., emotional) and physiological (e.g., immune) reactivity. Indeed, heightened levels of immune reactivity markers (e.g., C-reactive protein) predict a greater risk for depression (Wium-Andersen et al., 2013), and recent evidence has shown that psychosocial interventions (e.g., cognitive-behavioral therapy, mindfulness-based interventions) reduce these inflammatory immune markers (Black & Slavich, 2016; Shields et al., 2020). Thus, future research should consider both psychological and physiological reactivity in response to emotional ambiguity to further elucidate the brain-body pathways that support psychological well-being.
Initial negativity hypothesis.
Our finding demonstrating that non-reactivity is the “active ingredient” for supporting positivity is also in line with the initial negativity hypothesis. This hypothesis suggests that initial responses to emotional ambiguity tend to be negative and that more positive responses come about through greater deliberation (Neta & Tong, 2016) and recruitment of brain regions involved in emotion regulation (Petro et al., 2018). Given previous research showing that non-reactivity mediates the effects of MBSR on self-reported cognitive flexibility (Zou et al., 2020), it follows that non-reactivity would be associated with a more positive valence bias and an enhanced ability to overcome initial negativity (i.e., more flexible responding). This is also consistent with an integrated mindful emotion regulation framework (Chambers et al., 2009), which suggests that mindfulness meditation allows conscious and flexible engagement with cognitions and emotions in a manner that reduces the initial (here, negative) reactivity to a stimulus. In other words, this flexible awareness and nonreactivity likely facilitates a more controlled and intentional appraisal that putatively overrides the initial negativity and thus contributes to greater positivity.
Additionally, our analysis of computer mouse trajectories, a more objective and implicit window to the decision-making process, highlighted that MBSR training decreased response competition during ratings of emotionally ambiguous (surprise) and clearly positive (happy) facial expressions and marginally increased response competition during ratings of clearly negative (angry) facial expressions. That is, computer mouse trajectories were more direct for the surprised expressions following mindfulness training, suggesting a mindfulness-related override of the initial negativity. This finding is consistent with previous work showing mindfulness-related attenuation of negativity bias (Erisman & Roemer, 2010; Kiken & Shook, 2011). The concomitant decrease in response competition for clearly positive (happy) and a marginal increase for clearly negative (angry) expressions may indicate a shift towards greater attention to positive than negative emotional schema/categories more broadly, or a general reluctance to rate anything as negative (but note that this marginal effect for angry faces was not seen at longer timescales, when including the follow-up 8 weeks after the MBSR course ended). Indeed, this pattern of findings is consistent with the mindfulness-to-meaning theory, which posits that mindfulness promotes greater cognitive flexibility that ultimately promotes reappraisal of negativity and a savoring of positivity (Garland et al., 2011).
Limitations and conclusions.
Although this work represents a novel advancement in understanding the mechanisms through which MBSR shapes emotional bias, it has its limitations. For example, there was neither an active control group nor random assignment to the intervention. However, we included two within-subject controls (i.e., the clear valence emotional expressions and color-rating task) and demonstrated the relative stability of the valence bias measure in two demographically matched passage of time (i.e., inactive) control groups. The results from the within-subject controls suggest that our findings are specific to emotional ambiguity as neither the clear valence expressions (angry, happy) nor the perceptual ambiguity task showed significant changes in behavior as a function of mindfulness training. In other words, the surprise-specific shift in the valence bias task and the lack of training effects in the color-rating task jointly suggest that bias in response to emotional ambiguity, rather than a shift in response bias (given no effect for the clear valence conditions) or a more generalized shift in response competition or conflict (given no effect for the color bias task), was evident following the training.
The inclusion of two inactive control groups, which did not show significant change in valence bias over time, support the notion that the shift throughout the MBSR course and into the follow-up are most likely mindfulness training-related effects and not a result of habituation to the emotionally ambiguous stimuli following repeated task exposure, regression to the mean, or some alternative process. Although both controls shown here involved a longer time scale than that of the MBSR training (i.e., 6–12 months compared to 2–4 months), other research has shown stability in valence bias over a shorter time frame (i.e., approximately one week; Neta et al., 2018). Future work could, and should, address these remaining limitations by randomizing participants to either MBSR or a matched-control training (e.g., Health Enhancement Program; MacCoon et al., 2012), and perhaps consider other behavioral measures of bias to further explore related MBSR training effects.
Additionally, we predicted that even a single MBSR class might contribute to a more positive valence bias, given previous work showing effects of brief mindfulness interventions (Erisman & Roemer, 2010; Kiken & Shook, 2011). However, we observed no such effect, perhaps because the eight-week MBSR training is, at least initially, somewhat effortful and even stressful (Kabat-Zinn, 1990). As such, short-term effort or stress associated with beginning a mindfulness practice may have reduced any short-term positivity gains. Alternatively, the lack of a short-term effect on valence bias may be due to participants failing to complete the post-class task soon enough after class. In other words, participants often needed a reminder the next day to complete the task and the effects of a single mindfulness session may have been transient enough to dissipate by the following day (see Table 3). In fact, the only pre- to post-class effects concerned the response trajectories, and although the data suggest that participants were more decisive after class, the more direct trajectories may, at least in part, reflect either the later trial-wise initiation times after class or practice effects given that participants completed the pre- and post-class sessions relatively quickly.
In conclusion, the present study provides evidence that MBSR training promotes positivity in the face of emotional ambiguity, overriding an initial negativity bias and reducing attraction to negative valence categories/schema throughout the decision-making process. Not only do these findings have important clinical implications, such as understanding the mechanisms of mindfulness-based treatments, they also highlight an accessible behavioral strategy for improving psychological well-being and overcoming negativity during a time of large-scale, societal tension and stressors (e.g., economic downturns, the COVID-19 pandemic, racial unrest). For instance, a more positive valence bias likely facilitates social connection, perhaps through greater empathy (Neta & Brock, 2021) or more secure attachment, and maintaining positive social connections during uncertain times protects against negative physical and mental health outcomes (Nitschke et al., 2020). Thus, trainings like MBSR may not only improve psychological well-being on an individual level but may also contribute to well-being on a broader (societal) scale.
Context
The idea for this set of studies came about from previous work establishing the Initial Negativity Hypothesis, whereby we demonstrated that the initial or default response to emotional ambiguity is negative and the positivity appears to rely on slower, more deliberate processing. Relatedly, other work exploring the bias to appraise emotional ambiguity as positive or negative (i.e., valence bias) has linked greater stress reactivity - cortisol responses - to a more negative valence bias. These findings led us to believe that stress reduction programs which promote slower and more deliberate processing, like MBSR, might promote a more positive valence bias. Here, we tested this hypothesis, showing a sustained, long-term shift towards positivity after mindfulness training. In addition, we found that non-reactivity (one facet of mindfulness) plays a unique role in promoting a more positive bias, and may be a central focus in future work exploring brain-body pathways supporting positivity. Our ongoing research continues to probe the role of non-reactivity in promoting a more positive valence bias in relation to physiological indices of reactivity (e.g., gut-brain axis alterations) and inflammation (e.g., interleukins).
Acknowledgments
This work was supported by the National Institutes of Health (NIMH111640; PI: Neta), Nebraska Tobacco Settlement Biomedical Research Enhancement Funds, and University of Nebraska-Lincoln ENHANCE College of Arts & Sciences funds. We thank Catherine C. Brown for assisting with the collection of contact information for U.S. MBSR instructors, the instructors for sharing recruitment materials, and Hayoung Woo for assistance compiling the data.
Footnotes
Our preregistered hypotheses can be found at https://aspredicted.org/i9qy5.pdf. Analysis scripts are available at https://osf.io/dx4ke/. Data cannot be shared publicly because the participants consented to the report of the data in terms of group means, and not individualized data. The University of Nebraska-Lincoln Institutional Review Board completed review of the original research protocol including data sharing restrictions. Data requests can be sent to David Clausen (dclausen2@unl.edu, 402-472-3366), the current contracts manager in the Office of Sponsored Programs at University of Nebraska-Lincoln. All data sharing requests are first subject to a contract agreement through the Office of Sponsored Programs.
References
- Anwyl-Irvine AL, Massonié J, Flitton A, Kirkham N, & Evershed JK (2020). Gorilla in our midst: An online behavioral experiment builder. Behavior Research Methods, 52, 388–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, & Toney L (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13(1), 27–45. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. doi: 10.18637/jss [DOI] [Google Scholar]
- Berghdal J, & Berghdal M (2002). Perceived stress in adults: Prevalence and association of depression, anxiety and medication in a Swedish population. Stress and Health, 18, 235–241. doi: 10.1002/smi.946 [DOI] [Google Scholar]
- Black DS, & Slavich GM (2016). Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Annals of the New York Academy of Sciences, 1373(1), 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Shahar B, Szepsenwol O, Jacobs WJ (2012). Mindfulness-based cognitive therapy improves emotional reactivity to social stress: Results from a randomized controlled trial. Behavioral Therapy, 43(2), 365–380. doi: 10.1016/j.beth.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CC, Raio CM, & Neta M (2017). Cortisol responses enhance negative valence perception for ambiguous facial expressions. Scientific Reports, 7(1), 15107. doi: 10.1038/s41598-017-14846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, & Ryan R (2003). The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology, 84(4), 822–848. doi: 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, & Berntson GG (1997). Beyond bipolar conceptualizations and measures: The case of attitudes and evaluative space. Personality and Social Psychology Review, 1(1), 3–25. [DOI] [PubMed] [Google Scholar]
- Carmody J, Baer RA, Lykens ELB, & Olendzki N (2009). An empirical study of the mechanisms of mindfulness in a mindfulness-based stress reduction program. Journal of Clinical Psychology, 65(6), 613–626. [DOI] [PubMed] [Google Scholar]
- Chambers R, Gullone E, & Allen NB (2009). Mindful emotion regulation: An integrative review. Clinical Psychology Review, 29, 560–572. doi: S0272735809000865 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, & Kaszniak AW (2015). Conceptual and Methodological Issues in Research on Mindfulness and Meditation. American Psychologist, 70(7), 581–592. doi: 10.1037/a0039512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman SM, & Roemer L (2010). A preliminary investigation of the effects of experimentally-induced mindfulness on emotional responding to film clips. Emotion, 10(1), 72–82. doi: 10.1037/a0017162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose SA, & Lewis TT (2005). Psychosocial factors and cardiovascular diseases. Annual Review of Public Health, 26, 469–500. doi: 10.1146/annurev.publhealth.26.021304.144542 [DOI] [PubMed] [Google Scholar]
- Fox J, & Weisberg S (2019). An R Companion to Applied Regression, Third edition. Sage, Thousand Oaks CA. [Google Scholar]
- Freeman JB, & Ambady N (2010). MouseTracker: Software for studying real-time mental processing using a computer mouse-tracking method. Behavior Research Methods, 42(1), 226–241. doi: 10.3758/BRM.42.1.226 [DOI] [PubMed] [Google Scholar]
- Freeman J, Dale R, & Farmer T (2011). Hand in motion reveals mind in motion. Frontiers in Psychology, 2. doi: 10.3389/fpsyg.2011.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehman E, Stolier RM, & Freeman JB (2015). Advanced mouse-tracking analytic techniques for enhancing psychological science. Group Processes & Intergroup Relations, 18(3), 384–401. doi: 10.1177/1368430214538325 [DOI] [Google Scholar]
- Garland EL, Gaylord SA, & Fredrickson BL (2011). Positive reappraisal mediates the stress-reductive effects of mindfulness: An upward spiral process. Mindfulness, 2, 59–67. doi: 10.1007/s12671-011-0043-8 [DOI] [Google Scholar]
- Goldberg SB, Riordan KM, Sun S, & Davidson RJ (2021). The empirical status of mindfulness-based interventions: A systematic review of 44 meta-analyses of randomized controlled trials. Perspectives on Psychological Science. doi: 10.1177/1745691620968771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, Simpson TL (2018). Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clinical Psychology Review, 59, 52–60. doi: 10.1016/j.cpr.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp NR, & Neta M (2021, November 22). Mindfulness-Based Stress Reduction Triggers a Long-term Shift Toward More Positive Appraisals of Emotional Ambiguity. Retrieved from osf.io/dx4ke [DOI] [PMC free article] [PubMed]
- Harp NR, Brown CC, & Neta M (2021). Spring break or heart break? Extending valence bias to emotional words. Social Psychological and Personality Science, 12(7), 1392–1401. doi: 10.1177/1948550620972296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, & Oh D (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183. doi: 10.1037/a0018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JP, Crum AJ, Goyer P, Marotta M, & Akinola M (2018). Optimizing stress responses with reappraisal and mindset interventions: An integrated model. Anxiety, Stress, & Coping, 31(3), 245–261. doi: 10.1080/10615806.2018.1442615 [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain and illness. New York, NY: Delacorte. [Google Scholar]
- Kerr G, & Leith L (1993). Stress management and athletic performance. The Sport Psychologist, 7(3), 221–231. [Google Scholar]
- Kiken LG, & Shook NJ (2011). Looking up: Mindfulness increases positive judgments and reduces negativity bias. Social Psychological and Personality Science, 2(4), 425–431. doi: 10.1177/1948550610396585 [DOI] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, & Whalen PJ (2003). Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport, 14(18), 2317–2322. doi: 10.1097/00001756-200312190-00006 [DOI] [PubMed] [Google Scholar]
- Khoury B, Sharma M, Rush SE, & Fournier C (2015). Mindfulness-based stress reduction for healthy individuals: A meta-analysis. Journal of Psychosomatic Research, 78(6), 519–528. doi: 10.1016/j.jpsychores.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Kieslich PJ, Henninger F, Wulff DU, Haslbeck JMB, & Schulte-Mecklenbeck M (2019). Mouse-tracking: A practical guide to implementation and analysis. In Schulte-Mecklenbeck M, Kühberger A, & Johnson JG (Eds.), A Handbook of Process Tracing Methods (pp. 111–130). New York, NY: Routledge. [Google Scholar]
- Kral TRA, Schuyler BS, Mumford JA, Rosenkranz MA, Lutz A, & Davidson RJ (2018). Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. NeuroImage, 181, 301–313. doi: 10.1016/j.neuroimage.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest package: Tests in linear mixed effects models.” Journal of Statistical Software, 82(13), 1–26. doi: 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lang P, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual [Technical Report A–8]. University of Florida. [Google Scholar]
- Lenth R (2019). emmeans: Estimated marginal means, aka least-squares means. R package version 1.3.4. https://CRAN.R-project.org/package=emmeansLogie
- Low CA, Salomon K, Matthews KA (2009). Chronic life stress, cardiovascular reactivity, and subclinical cardiovascular disease in adolescents. Psychosomatic Medicine, 71(9), 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, & Öhman A (1998). The Karolinska directed emotional faces—KDEF (CD ROM)., Stockholm: Karolinska Institute, Department of Clinical Neuroscience, Psychology Section. [Google Scholar]
- MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, … Lutz A (2012). The validation of an active control intervention for mindfulness based stress reduction (MBSR). Behaviour Research & Therapy, 50, 3–12. doi: 10.1016Zj.brat.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Milne AB, & Bodenhausen GV (1994). Stereotypes as energy-saving devices: A peek inside the cognitive toolbox. Journal of Personality and Social Psychology, 66(1), 37–47. [Google Scholar]
- Neta M, Berkebile MM, & Freeman JB (2020). The dynamic process of ambiguous emotion perception. Cognition and Emotion, 35(4), 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, & Brock RL (2021). Social connectedness and negative affect uniquely explain individual differences in response to emotional ambiguity. Scientific Reports, 11, 3870. doi: 10.1038/s41598-020-80471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Cantelon J, Haga Z, Mahoney CR, Taylor HA, & Davis CF (2017). The impact of uncertain threat on affective bias: Individual differences in response to emotional ambiguity. Emotion, 17(8), 1137–1143. doi: 10.1037/emo0000349 [DOI] [PubMed] [Google Scholar]
- Neta M, Harp NR, Henley DJ, Beckford SE, & Koehler K (2019). One step at a time: Physical activity is linked to positive interpretations of ambiguity. PLoS ONE, 14(11), e0225106. doi: 10.1371/journal.pone.0225106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Tong TT, & Henley DJ (2018). It’s a matter of time (perspectives): Shifting valence responses to emotional ambiguity. Motivation and Emotion, 42(2), 258–266. [Google Scholar]
- Neta M, Kelley WM, & Whalen PJ (2013). Neural responses to ambiguity involve domain-general and domain-specific emotion processing systems. Journal of Cognitive Neuroscience, 25(4), 547–557. doi: 10.1162/jocn_a_00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Norris CJ, & Whalen PJ (2009). Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion (Washington, D.C.), 9(5), 640–648. doi: 10.1037/a0016819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, & Tong TT (2016). Don’t like what you see? Give it time: Longer reaction times associated with increased positive affect. Emotion, 16(5), 730–739. doi: 10.1037/emo0000181 [DOI] [PubMed] [Google Scholar]
- Neta M, & Whalen PJ (2010). The primacy of negative interpretations when resolving the valence of ambiguous facial expressions. Psychological Science, 21(7), 901–907. doi: 10.1177/0956797610373934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JP, Forbes PAG, Ali N, Cutler J, Apps MA, Lockwood PL, & Lamm C (2020). Resilience during uncertainty? Greater social connectedness during COVID-19 lockdown is associated with reduced distress and fatigue. British Journal of Health Psychology. doi: 10.1111/bjhp.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson G, & Cacioppo JT (2010). The current status of research on the structure of evaluative space. Biological Psychology, 84, 422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul NA, Stanton SJ, Greeson JM, Smoski MJ, & Wang L (2013). Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Social Cognitive and Affective Neuroscience, 8(1), 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauker K, Rule NO, & Ambady N (2010). Ambiguity and social perception. In Social psychology of visual perception (pp. 7–26). Psychology Press. [Google Scholar]
- Petro NM, Tong TT, Henley DJ, & Neta M (2018). Individual differences in valence bias: fMRI evidence of the initial negativity hypothesis. Social Cognitive and Affective Neuroscience, 13(7), 687–698. doi: 10.1093/scan/nsy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro NM, Tottenham N, & Neta M (2021). Exploring valence bias as a metric for frontoamygdalar connectivity and depressive symptoms in childhood. Developmental Psychobiology, 00, 1–16. doi: 10.1002/dev.22084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti NA, Villano W, Stamatis CA, Torrez VF, Neta M, Timpano K, & Heller AS (2020, December 28). Affect instability links task-based negativity bias and variability in depressive symptoms. 10.31234/osf.io/gdszp [DOI]
- Querstret D, Morison L, Dickinson S, Cropley M, & John M (2020). Mindfulness-based stress reduction and mindfulness-based cognitive therapy for psychological health and well-being in nonclinical samples: A systematic review and meta-analysis. International Journal of Stress Management, in press. [Google Scholar]
- Rano J, Fridén C, & Eek F (2019). Effects of acute psychological stress on athletic performance in elite male swimmers. Journal of Sports Medicine and Physical Fitness, 59(6), 1068–1076. doi: 10.23736/S0022-4707.18.08493-1 [DOI] [PubMed] [Google Scholar]
- Rights JD, & Sterba SK (2019). Quantifying explained variance in multilevel models: An integrative framework for defining R-squared measures. Psychological Methods, 24(3), 309–338. 10.1037/met0000184 [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Davidson RJ, MacCoon DG, Sheridan JF, Kalin NH, & Lutz A (2013). A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain, Behavior, and Immunology, 27, 174–184. doi: 10.1016/j.bbi.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royuela-Colomer E, & Calvete E (2016). Mindfulness facets and depression in adolescents: Rumination as a mediator. Mindfulness, 7(5), 1092–1102. doi: 10.1007/s12671-016-0547-3 [DOI] [Google Scholar]
- Samuelsson H, Jarnvik K, Henningsson H, Andersson J, & Carlbring P (2012). The Umeå University database of facial expressions: A validation study. Journal of Medical Internet Research, 14(5), e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers WM, Creswell JD, & Taren A (2015). The emerging neurobiology of mindfulness and emotion processing. In Ostafin BD, Meier BF, & Robinson MD (Eds.). Handbook of Mindfulness and Self-Regulation (pp. 9–22). Springer. [Google Scholar]
- Schoofs D, Wolf OT, & Smeets T (2009). Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behavioral Neuroscience, 123(5), 1066–1075. doi: 10.1037/a0016980 [DOI] [PubMed] [Google Scholar]
- Schumer MC, Lindsay EK, & Creswell JD (2018). Brief mindfulness training for negative affectivity: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology, 86(7), 569–583. doi: 10.1037/ccp0000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, & Freedman B (2006). Mechanisms of mindfulness. Journal of Clinical Psychology, 62, 373–386. [DOI] [PubMed] [Google Scholar]
- Shields GS, Spahr CM, & Slavich GM (2020). Psychosocial interventions and immune system function: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry, 77(10), 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein LR, Tirch D, Leahy RL, & McGinn L (2012). Mindfulness, psychological flexibility and emotional schemas. Journal of Cognitive Therapy, 5, 406–419. [Google Scholar]
- Snyder M, & Swann WB (1978). Hypothesis-testing processes in social interaction. Journal of Personality and Social Psychology, 36(11), 1202–1212. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, & Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. doi: 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope Y, & Thompson EP (1997). Looking for truth in all the wrong places? Asymmetric search of individuating information about stereotyped group members. Journal of Personality and Social Psychology, 73(2), 229–241. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A,… Fox KC (2018). Mind the hype: A critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspectives on Psychological Science, 13, 36–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Wium-Andersen MK, Ørsted DD, & Nielsen SF (2013). Elevated C-Reactive Protein levels, psychological distress, and depression in 73 131 individuals. JAMA Psychiatry, 70(2), 176–184. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, & Dodson JD (1908). The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology, 18, 459–482. [Google Scholar]
- Zou Y, Li P, Hofmann SG, & Liu X (2020). The mediating role of non-reactivity to mindfulness training and cognitive flexibility: A randomized controlled trial. Frontiers in Psychology, 11, 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]


