Abstract
Inflammatory agents, microbial products or stromal factors pre-activate or prime neutrophils to respond to activating stimuli in a rapid and aggressive manner. Primed neutrophils exhibit enhanced chemotaxis, phagocytosis and respiratory burst when stimulated by secondary activating stimuli. We previously reported that Triggering Receptor Expressed on Myeloid cells-1 (TREM-1) mediates neutrophil effector functions such as increased superoxide generation, transepithelial migration and chemotaxis. However, it is unclear whether TREM-1 is required for the process of priming itself or for primed responses to subsequent stimulation. To investigate this, we utilized in vitro and in vivo differentiated neutrophils that were primed with TNF-α and then stimulated with the particulate agonist, opsonized zymosan (OpZ). Bone marrow progenitors isolated from WT and Trem-1−/− mice were transduced with estrogen regulated Homeobox8 (ER-Hoxb8) fusion transcription factor and differentiated in vitro into neutrophils following estrogen depletion. The resulting neutrophils expressed high levels of TREM-1 and resembled mature in vivo differentiated neutrophils. The effects of priming on phagocytosis and oxidative burst were determined. Phagocytosis did not require TREM-1 and was not altered by priming. In contrast, priming significantly enhanced OpZ-induced oxygen consumption and superoxide production in WT but not Trem-1−/− neutrophils indicating that TREM-1 is required for primed oxidative burst. TREM-1-dependent effects were not mediated during the process of priming itself as priming enhanced degranulation, ICAM-1 shedding and IL-1ß release to the same extent in WT and Trem-1−/− neutrophils. Thus, TREM-1 plays a critical role in primed phagocytic respiratory burst and mediates its effects following priming.
Keywords: ER-Hoxb8 neutrophils, EPR spectroscopy, TNF-α, Receptors
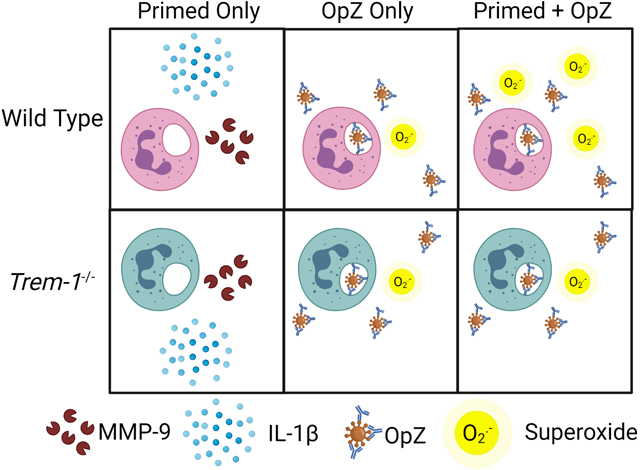
Graphical Abstract
Introduction
Neutrophils are one of the first immune cells to be recruited to sites of injury and are critical to host defense against invading pathogens. The vascular endothelium, when activated by microbial products or endogenous danger signals, modifies adhesion molecule expression to capture circulating neutrophils and facilitate their migration through endothelial barriers to the affected tissue [1]. Neutrophil transit from the circulation to inflamed sites exposes them to various inflammatory agents and chemotactic factors that pre-activates or “primes” them to respond rapidly and aggressively to secondary activating stimuli. As a result, the extravasated neutrophil differs both phenotypically and functionally from the quiescent circulating neutrophil and the naïve bone marrow derived neutrophil (BMDN). Following stimulation, primed neutrophils exhibit enhanced chemotaxis, phagocytosis, oxidative burst, degranulation, neutrophil extracellular trap (NET) extrusion, and delayed apoptosis [1–3]. This behavioral plasticity enables the host to tightly regulate neutrophil effector functions in a spatiotemporal manner targeting only the site of inflammation while protecting the remaining tissue from unwarranted damage.
Neutrophil priming, which occurs in sepsis, acute respiratory distress syndrome, and following traumatic injury, dramatically impacts neutrophil responses in vivo [4–6]. In vitro studies of neutrophil priming are typically performed with primary neutrophils harvested from the circulation or bone marrow. While such studies have yielded valuable insights, they are potentially limited by inadvertent neutrophil priming during isolation, the inability to genetically manipulate these cells, a limited supply of these neutrophils and their short life span. In vitro differentiated ER-Hoxb8 neutrophils circumvent many of these drawbacks as they can be generated in large numbers from genetic murine models with relative ease and in a reliable and reproducible manner [7]. Bone marrow progenitors are conditionally immortalized by retroviral transduction with estrogen receptor Homeobox8 (ER-Hoxb8) fusion transcription factor that enforces an estrogen-dependent differentiation block [7]. Following estrogen withdrawal these progenitors differentiate into mature neutrophils in the presence of stem cell factor (SCF). These in vitro differentiated neutrophils (ER-Hoxb8 neutrophils) have been used successfully to investigate neutrophil functions such as oxidative burst, degranulation, chemotaxis, fungal killing, and calcium signaling [8–12]. They can be derived from the bone marrow of transgenic and knockout murine models and are amenable to CRISPER/Cas9 editing as well as viral transduction [9, 10, 13]. Moreover, they have been used in adoptive transfer experiments to recapitulate the functional properties of mature primary neutrophils [13].
Neutrophil priming increases expression of receptors that recognize exogenous and endogenous danger signals and augments neutrophil responses [2]. Crosstalk between these receptors coordinates neutrophil responses to these signals. Triggering receptor expressed on myeloid cells-1 (TREM-1) is a member of the IgG superfamily expressed on neutrophils [14, 15]. TREM-1 synergizes with pattern recognition receptors (PRRs) such as Toll-like receptors (TLR) and Nod-like receptors (NLR) to augment cytokine production, reactive oxygen species (ROS) and NET formation [16–21]. TREM-1 facilitates transepithelial neutrophil migration in the infected lung and augments neutrophil responses in vivo [22]. Moreover, TREM-1 regulates NADPH oxidase (NOX) -mediated superoxide production in exudate neutrophils [23]. However, the role of TREM-1 in neutrophil priming is unstudied. We hypothesized that priming augments agonist-induced superoxide production in a TREM-1-dependent manner.
We addressed our hypothesis using ER-Hoxb8 neutrophils. We generated matching ER-Hoxb8 neutrophil models from WT bone marrow progenitors as well as from bone marrow progenitors isolated from our previously described Trem-1−/− murine model [7, 22]. Our data demonstrate that ER-HoxB8 neutrophils demonstrate characteristic features of mature neutrophils and express high levels of surface TREM-1. Expression of cell surface receptors such as TNFRI/II involved in TNF-α priming was comparable in ER-Hoxb8 neutrophils and mature circulating and exudate neutrophils but significantly lower in BMDN. Phagocytosis was not dependent on TREM-1 expression. ER-Hoxb8 and circulating neutrophils demonstrated rapid and robust OpZ-induced oxygen consumption and superoxide production. Unprimed WT and TREM-1 deficient neutrophils stimulated with OpZ generated similar amounts of superoxide. Priming with TNF-α, however, significantly augmented these responses in a TREM-1 dependent manner in both ER-Hoxb8 and circulating neutrophils. Because primed but unstimulated WT and TREM-1 deficient neutrophils exhibited similar levels of degranulation, ICAM-1 shedding and IL-1ß release, our findings suggest that TREM-1 is not required for the process of priming but, instead, mediates its effects following priming. Thus, our observations reveal an important role of TREM-1 in primed neutrophil oxidative burst and support the use of ER-Hoxb8 neutrophils as a valid model to investigate mature neutrophil functions.
Methods
Mice
TREM-1-deficient mice were generated as previously described [23]. All animals used in this study were bred in a barrier facility at The University of Iowa. All protocols involving mice were approved by the University of Iowa Institutional Animal Care and Use Committee and were carried out in accordance with institutional guidelines and regulations. Heterozygous littermates were intercrossed to generate closely related mice that are either WT or homozygous for the TREM-1/3 deletion.
Isolation of bone marrow-derived neutrophils (BMDN).
BMDN were isolated from bone marrows of 7–9-week-old male and female WT and Trem-1−/− mice by negative selection using EasySep Mouse Neutrophil Enrichment kit (Stem Cell Technologies Inc). After isolation, neutrophils were kept on ice in Ca2+/Mg 2+-free HBSS (HBSS−/−) until use and used within 4 hours of isolation. Purity of neutrophils was assessed by determining enrichment of Ly6G+ stained cells.
Isolation of peritoneal exudate neutrophils.
Peritoneal exudate neutrophils were isolated as described previously [23]. Briefly, exudate neutrophils were harvested 16–18 hours after intraperitoneal injection of WT and Trem-1−/− mice with 1 ml/mouse of 4% thioglycollate (Difco). After hemolyzing, washing, and filtering, exudate cells were counted and used immediately for immunostaining.
Isolation of circulating neutrophils.
WT and Trem-1−/− mice were anesthetized with ketamine/xylazine and blood was collected by submandibular or cardiac puncture into tubes containing 10U/mL heparin. Blood was hemolyzed with 1x RBC Lysis Buffer (eBioscience) and diluted to 50 mL with Ca2+/Mg 2+-free PBS (PBS−/−), centrifuged at 300 g for 10 min at 4°C to collect cell pellets. Cells were counted and used immediately for neutrophil isolation by negative selection using EasySep Mouse Neutrophil Enrichment kit (Stem Cell Technologies Inc).
ER-HoxB8 immortalized progenitor cells.
Bone marrow isolated from the femurs of 11–12-week-old female WT and Trem-1−/− mice were sent to David B. Sykes (Massachusetts General Hospital, Boston) for isolation of bone marrow progenitors and retroviral transduction with ER-Hoxb8 retrovirus as previously described [7]. Bone marrow progenitors were conditionally immortalized by the ER-Hoxb8 fusion transcription factor and maintained in the presence of SCF and ß-estradiol as previously described [7]. In the presence of SCF (supplied as 4% conditioned media from an SCF producing CHO cell line), cells were committed to the differentiation to neutrophils following the withdrawal of ß-estradiol and inactivation of the ER-Hoxb8 protein. CHO cells were a kind gift from D.B. Sykes. The culture media included RPMI (GIBCO) supplemented with 10% FCS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO), 0.5μM estrogen (Sigma) and 4% SCF-containing CHO conditioned medium. Media was replaced every other day. For differentiation into mature neutrophils, 4 million progenitor cells were washed twice in estrogen-free media and resuspended in 100 mL of estrogen-free media for five days. Cells were collected by gently transferring to conical bottom tubes, centrifuging for 7 min at 250 g and washing twice with PBS-/−. Cells were counted using a hemocytometer and used for experiments outlined below.
Opsonization of Zymosan.
Zymosan (Sigma) was opsonized with WT C57Bl/6 mouse serum as previously described [23]. All stimulations were performed in phenol red-free HBSS containing Ca2+/Mg 2+ and 5.56mM glucose (HBSS+/+).
Priming.
Neutrophils were primed for 30 min at 37 °C with 0.33–2 μg/mL of murine TNF-α (R&D Systems) in HBSS+/+. Thereafter, cells were stimulated with OpZ at the indicated MOI. Unprimed neutrophils received buffer alone.
Cell surface receptor expression
Cells were immunolabeled with TREM-1-APC (R&D Systems), CD11b-A488 (CR3) (Biolegend) and Ly6G-BV421 (BioLegend) (panel1) or CD11b-A488 (CR3) (Biolegend), CD120a-APC (TNFRI) (BioLegend), CD120b-PE (TNFRII) (BioLegend), CD16/32-APC-Cy7 (FcγRII/III) (BioLegend) and ICAM-1-PE-Cy7 (Biolegend) (panel 2). Neutrophils (0.1 million) were resuspended in 100 μL HBSS+/+ containing 5.56mM glucose in 96-well round-bottomed plates and primed for 30 min at 37 °C with 2 μg/mL TNF-α. Unprimed control cells received buffer alone. After washing, resting or TNF-α primed neutrophils were washed with PBS−/− and stained for 10 min with the cell viability marker, Zombie Aqua (BioLegend). After Zombie Aqua staining, cells were blocked for 10 min with PBS−/− containing 2% FCS and 2% serum of the respective antibody host species. Cells were subsequently stained for 15 min in the continued presence of blocking buffer. After immunolabeling, all cells were washed twice, resuspended in MACS buffer (PBS−/− + 2% FBS + 1 mM EDTA) and analyzed on Cytek Aurora spectral flow cytometer. Data were analyzed with FlowJo v10 software. All incubations were performed at room temperature, protected from light.
Phagocytosis
ER-Hoxb8 neutrophils and circulating neutrophils (0.1 million) were resuspended in 100 μL HBSS+/+ containing 5.56mM glucose in 96-well round-bottomed plates and primed for 30 min at 37°C with 2 μg/mL TNF-α. Unprimed control cells received buffer alone. After washing, cells were labeled with the cell viability marker, Zombie NIR (Biolegend) for 10 min in the dark, washed and placed on ice. OpZ-A488 (ThermoFisher Scientific) was added to cells (MOI=5) and the plate was centrifuged for 5 min at 4 °C at 300 g to synchronize phagocytosis. The plate was then transferred to 37°C and cells were incubated with OpZ-A488 (MOI=5) for 30 min. Cells were fixed with 2% PFA to stop phagocytosis and then washed with MACS buffer. After blocking with 2% rat serum, cells were immunolabeled with anti-Ly6G-PE and anti-CD11b-APC (BioLegend) for 15 min in the dark. Cells were washed and analyzed using Cytek Aurora spectral flow cytometer. Data were analyzed with FlowJo v10 software.
Oxygen Consumption.
The rate of cellular oxygen uptake was monitored using an ESA BioStat multi-electrode system (Clark Electrode) (ESA Products, Dionex Corp., Chelmsford, MA, USA) in conjunction with a YSI oxygen probe (5331) and glass reaction chamber vials in a YSI bath assembly (5301) (Yellow Springs Instruments, Yellow Springs, OH, USA), all at 37 °C. Oxygen consumption rate (OCR) was measured in both primed and unprimed ER-Hoxb8 neutrophils, (250μL containing 8 × 106) resuspended in PBS+/+/+ (with CaCl2/MgCl2/D-glucose/sodium pyruvate) (GIBCO). Cells were primed with either 50μL of 2μg/mL, 50μL of 6μg/mL or 50μL of 12μg/mL TNF- α for a final TNF- α concentration of 0.33μg/mL, 1μg/mL, or 2μg/mL, respectively. Control unprimed cells received 50μL PBS+/+/+. Cells were incubated at 37 °C for 30 min and then transferred to the glass reaction chamber containing 2.7 mL PBS+/+/+. Oxygen consumption was continuously monitored in the following sequence: (1) baseline for about 10 min and (2) after addition of 120μL OpZ (1 million particles/μL) (MOI 15) for another 10 min. Raw amperometric data were imported into Excel and GraphPad Prism to calculate slopes representing OCR expressed in units of attomoles O2 cell−1 s−1 [24]. Data from 5 independent experiments were pooled and the average baseline OCR was calculated. OpZ-induced OCR was expressed as fold induction over average baseline OCR.
Mitochondrial Respiration.
Cellular oxygen utilization extracellular acidification rates were determined using a Seahorse Bioscience XF96 extracellular flux analyzer (Agilent-Seahorse, North Billerica, MA, USA). Briefly, ER-HoxB8 neutrophils were suspended at a density of 5 × 106 cells/mL in RPMI 1640 with 1mM HEPES, pH 7.4 (Agilent-Seahorse) containing 10 mM glucose (Sigma), 2mM L-glutamine (Life Technologies) and 1mM sodium pyruvate (Life Technologies), pH 7.40. Fifty μL (250,000 cells) were then placed into XF96 V3 PS microculture plates (Agilent-Seahorse) treated with Cell-Tak™ (BD Biosciences, Bedford MA) cell and tissue adhesive. The cells were then adhered to the bottom of the plates after a brief spin in a centrifuge following the manufacturer’s recommendations. Twenty-five μL of 3μg/mL TNF-α was then added to a final concentration of 1μg/mL and cells were incubated for 30 min at 37 °C. Control cells received 25μL buffer alone. Additional medium was then added to each well to a final volume of 175μL. Cells were stimulated with 25μL of 150 × 106 particles/mL OpZ (MOI 15). Experiments were performed following standard Seahorse/Agilent Biosciences protocols. The final concentrations of mitochondrial inhibitors used were 10μM each of Rotenone (Sigma) and Antimycin A (Sigma). Oxygen consumption rate (OCR) was determined using standard approaches for this technology and imported into Excel to calculate OCR. OCR was expressed in units of attomoles O2 cell−1 s−1 [24]. Data from 3 experiments were pooled and plotted in GraphPad Prism.
EPR spectroscopy.
EPR spectra, using spin trapping with DMPO (5,5-dimethyl-1-pyrroline-N-oxide from Dojindo Molecular Technologies, Inc., Rockville, MS) were acquired from resting and stimulated cells before and after priming [25, 26]. ER-Hoxb8 neutrophils, 75μL of 20 × 106/mL (1.5 million) suspended in PBS were added to a tube containing either 75μL of 4μg/mL TNF-α or 75 μLPBS+/+/+ (with CaCl2/MgCl2/D-glucose sodium pyruvate) (GIBCO) and incubated for 30 min at 37°C. Circulating neutrophils,66 μL of 10 × 106/mL (660,000) suspended in PBS were added to a tube containing either 66μL of 4μg/mL TNF-α or 66μL PBS+/+/+ and incubated for 30 min at 37°C. Then DETAPAC final concentration 1mM, DMPO final concentration 50mM, and OpZ (MOI of 15) were added, and the mixture brought up to 300μL with additional PBS+/+/+. At specified intervals, the contents of the tube were mixed gently and about 40μL (approximately 200,000 ER-Hoxb8 neutrophils or 88,000 circulating neutrophils) were drawn into a 100 mm x 0.8 mm ID capillary tube. One end of the capillary tube was sealed with Critoseal tube sealant and the remaining mixture was then placed back in the incubator. The sealed capillary tube containing the sample was rinsed with water, dried, placed in a quartz (250 × 3 mm inner diameter) EPR tube (Wilmad Lab-Glass, Vineland NJ) and centered in an ER 4119HS resonator of a Bruker EMX EPR spectrometer (Bruker BioSpin; Billerica, MA). Spectra were obtained at room temperature (24–27 °C). Typical EPR parameters were as follows: 3478 G center field; 80 G sweep width; 9.78 GHz microwave frequency; 20mW power; receiver gain 1 × 105; modulation frequency of 100 kHz; modulation amplitude of 1.0 G; conversion time of 20.48ms; and time constant of 81.928ms with spectra collected in the additive mode with 4 sequential (Y-Resolution) 10 X-scans; each scan comprised of a 1024-point spectrum. EPR signal height was quantified by measuring peak heights (max-min) of DMPO-OH and DMPO-OOH peaks using tools available in the Bruker EMX software. The 1st downfield peak was identified as DMPO-OH and the 2nd downfield peak was identified as DMPO-OOH as shown in Figure 5A. DMPO-OH and DMPO-OOH signal heights were converted to respective concentrations using a xanthine oxidase system and 3-carboxyl-proxyl as a standard as previously described [27] except that tools available in the Bruker EMX software were used for double integration of spectral and subsequent peak area determination. Non-linear regression analysis was used to compare rates of DMPO-OH formation between unprimed and primed neutrophils. Specificity of the signal was verified by preincubating neutrophils with SOD (100U/mL).
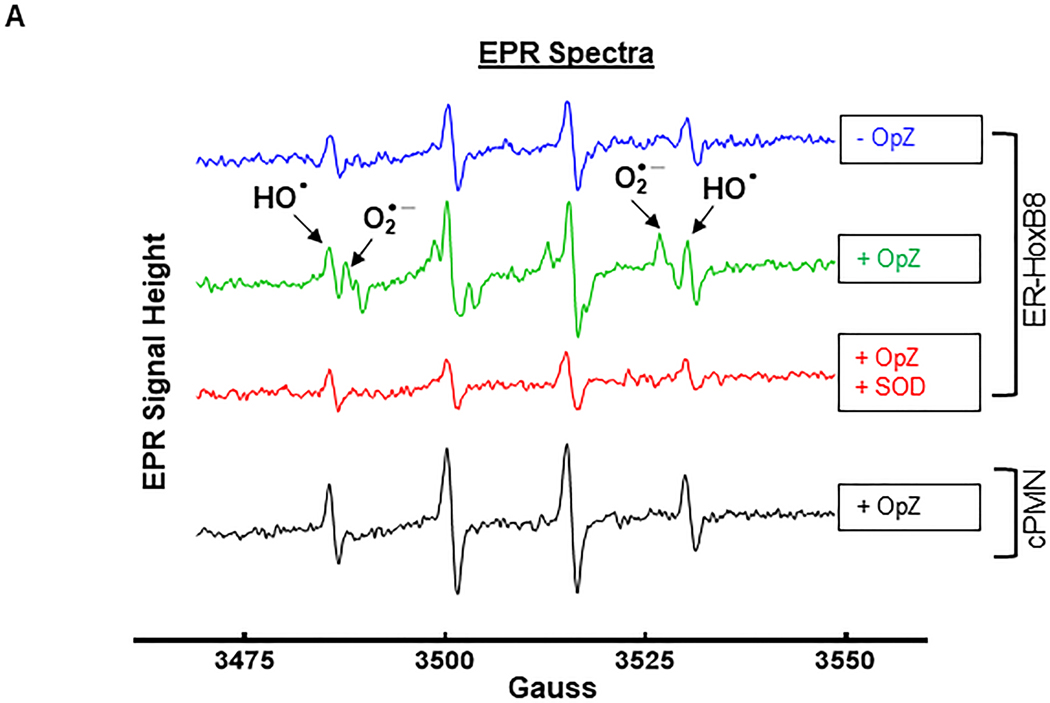
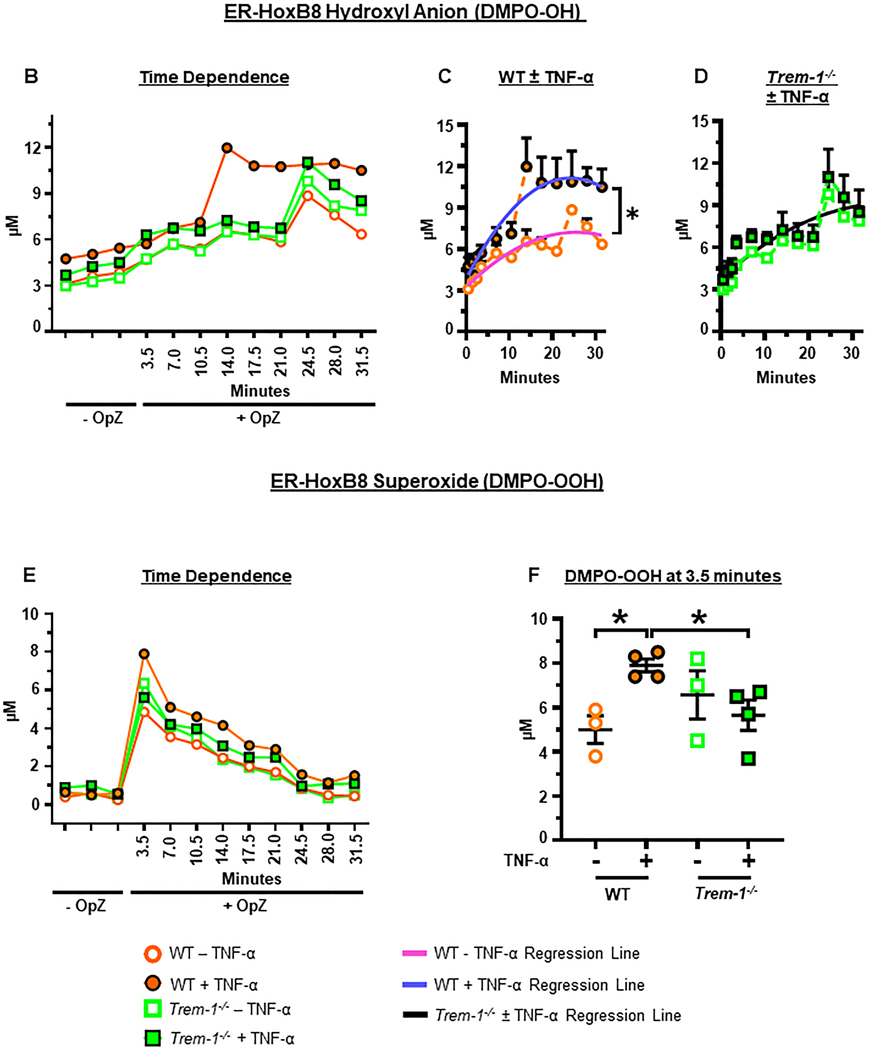
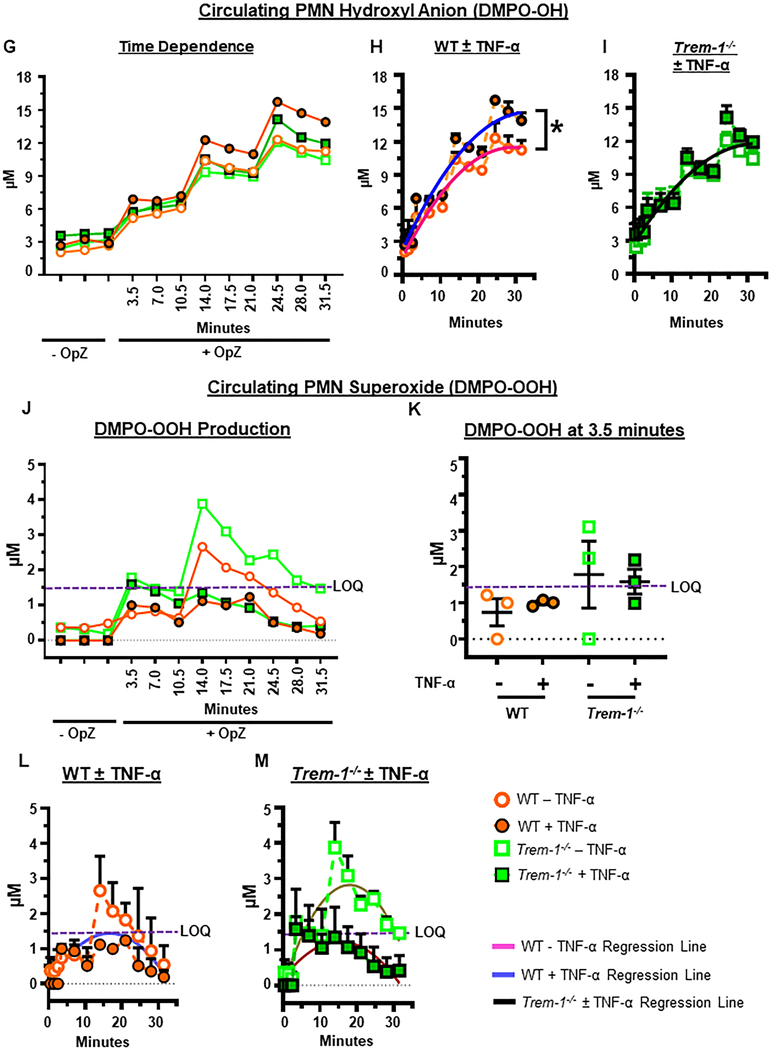
Figure 5. Priming enhances OpZ-induced superoxide anion and hydroxyl radical production in a TREM-1-dependent manner.
ER-Hoxb8 and circulating neutrophils (cPMN) were primed for 30 min with TNF-α (2 μg/mL) and stimulated with OpZ (MOI = 15). A. EPR spectra of resting and OpZ-stimulated neutrophils in the presence or absence of SOD (100 U/mL). B. Time-dependent formation of DMPO-OH in OpZ stimulated WT and Trem-1−/− ER-Hoxb8 neutrophils following TNF-α priming. C&D. Data shown in B were subjected to nonlinear (quadratic) regression analyses to calculate rates of DMPO-OH formation in primed and unprimed WT (C) and Trem-1−/− (D) neutrophils (solid lines). E. Time-dependent formation of DMPO-OOH in OpZ stimulated WT and Trem-1−/− ER-Hoxb8 neutrophils following TNF-α priming. F. Superoxide production after 3.5min of OpZ stimulation in primed and unprimed neutrophils. G. Time-dependent formation of DMPO-OH in OpZ stimulated WT and Trem-1−/− cPMN following TNF-α priming. H&I. Data shown in G were analyzed by nonlinear (quadratic) regression analyses to calculate rates of DMPO-OH formation in primed and unprimed WT (H) and Trem-1−/− (I) cPMN (solid lines). J. Time-dependent formation of DMPO-OOH in OpZ stimulated WT and Trem-1−/− cPMN following TNF-α priming. K. Superoxide production after 3.5min of OpZ stimulation in primed and unprimed neutrophils. L&M. Data shown in J were analyzed by nonlinear (quadratic) regression analyses to calculate rates of DMPO-OOH formation in primed and unprimed WT (L) and Trem-1−/− (M) cPMN (solid lines). Statistical analyses=In B, E, and G statistical differences between WT and Trem-1−/− at each time point were assessed by multiple unpaired t-tests. False discovery rate (Benjamin, Krieger and Yekutieli method) with Q = 0.1% was used to correct for multiple comparisons. In C,D,H,I,L&M the null hypothesis that one curve fits all data sets was tested. It was rejected in C,H,L&M where significance of difference between slopes was tested using Extra sum-of-squares F test. In D and I a single regression line fits both data sets and indicates that DMPO-OH was not altered by TNF-a priming (black solid line). Differences in F&K were calculated by one-way ANOVA. Mean ± SEM, n=3–4, *p<0.05.
Release of Superoxide.
Release of superoxide was measured by reduction of ferricytochrome c as previously described but with the following modifications [28]. Cells (0.1 million/well) were incubated in a 96-well plate for 30 min at 37 °C with 1 or 2 μg/mL TNF-α or buffer alone in the presence of ferricytochrome c (Sigma) (0.1 mM final). The plate was placed in a plate reader that was maintained at 37 °C. After priming, the plate containing primed neutrophils was transferred to ice and OpZ (MOI = 15) was added. The plate was centrifuged for 5 min at 300 g at 4 °C to synchronize phagocytosis and absorbance at 550 nm was monitored at regular intervals at 37°C. Specificity of the superoxide signal was verified by including SOD (150U/well) in a separate set of wells. SOD-inhibitable reduction of ferricytochrome c was calculated as the difference in OD of samples with and without SOD. Time-dependent increase in SOD-inhibitable signal was subjected to regression analysis and the slope was calculated to determine the rate of superoxide release. Data were analyzed using GraphPad Prism.
IL-1ß and MMP-9 Release.
WT and TREM-1 deficient neutrophils were primed for 30 min with 2μg/mL of TNF-α and collected by centrifugation for 10 min at 300 g at 4°C. Control cells received buffer alone. The supernatant or conditioned medium containing IL-1ß and degranulation products such as MMP-9 was concentrated using 10kDa molecular weight cut off Amicon Ultra 4 centrifugal filters (Millipore Sigma) to collect >10kDa molecular weight components. Concentrated media volumes corresponding to equivalent numbers of neutrophils were heated for 10 min with Laemmli Sample buffer containing DTT, resolved by SDS/PAGE and transferred to PVDF or nitrocellulose membranes. After blocking with 5% non-fat dry milk in TRIS buffered saline containing 0.1% Tween-20 (TTBS), membranes were immunoblotted with anti-MMP-9 (R&D Systems #AF909) or anti-IL-1ß (R&D Systems #AF401) antibodies. Membranes washed and incubated with the anti-goat-HRP (R&D Systems #HAF017). After washing, HRP signal was detected with SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific). The signal was digitally captured using FugiFilm Luminescent Image Analyzer LAS4000 (Fuji Medical Systems). Densitometric quantitation of bands was performed with Gelanalyzer Software (GelAnalyzer 19.1 (www.gelanalyzer.com) by Istvan Lazar Jr., PhD and Istvan Lazar Sr., PhD, CSc).
Statistical Analyses.
All statistical analyses were performed using GraphPad Prism 9. Paired Student’s ttest was used to compare unprimed and primed neutrophil responses of a given genotype. One-way ANOVA with correction for multiple comparisons was used to compare genotypes or ER-Hoxb8 with other neutrophils. The specific tests used for each experiment are indicated in the corresponding figure legends.
Results
ER-Hoxb8 myeloid progenitor cells differentiate into neutrophils in the absence of estrogen.
Morphological and functional characteristics of in vitro differentiated ER-Hoxb8 neutrophils were compared to BMDN, circulating, and tissue infiltrating neutrophils. Each of these populations represent different states of pre-activation and maturation. Hence, these cells differ widely in their responses to activating stimuli [29]. SCF-dependent ER-Hoxb8 myeloid progenitors were differentiated as previously described [7]. During five days of estrogen withdrawal, differentiation into neutrophils was assessed by monitoring the acquisition of morphological features and cell surface proteins characteristic of neutrophils. On day five cells exhibited characteristic multilobular nuclei (Figure 1A). In both WT and TREM-1 deficient ER-Hoxb8 neutrophils estrogen withdrawal induced CD11b expression in a time-dependent manner (Figure 1B), Ly6G expression was detected in both genotypes only after five days of estrogen withdrawal (Figure 1C). As expected, TREM-1 expression was observed after 5 days of estrogen withdrawal in WT but not TREM-1-deficient ER-Hoxb8 neutrophils (Figure 1D). These results are consistent with the generation of a pure population of mature neutrophils.
Figure 1. ER-Hoxb8 myeloid progenitor cells differentiate into neutrophils in the absence of estrogen.
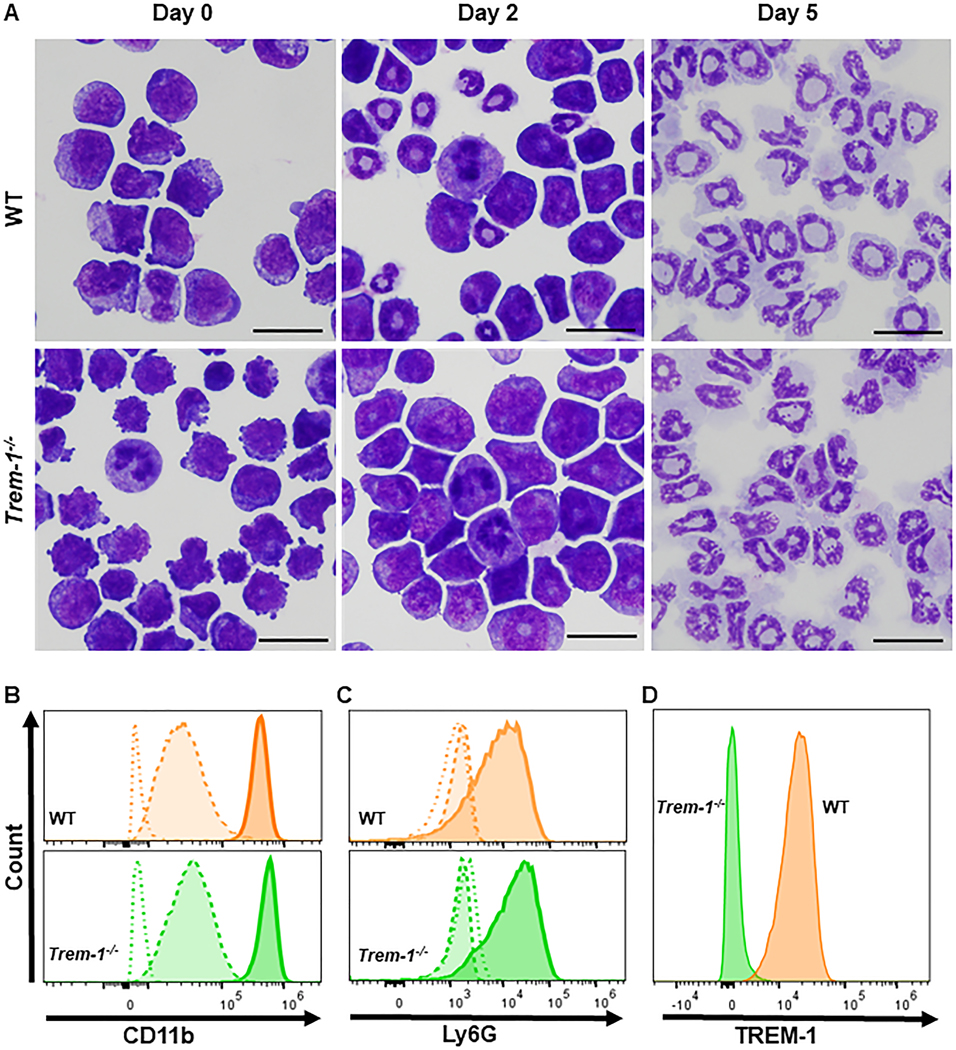
A. Differentiation of ER-Hoxb8 myeloid progenitors to ER-HoxB8 neutrophils as demonstrated by Hema 3 stained cytospins (bar=10μm). B. Cell surface CD11b. C. Ly6G expression on days 0 (open/dotted line), 2 (light/dashed line), and 5 (dark/solid line) of estrogen withdrawal. D. Surface TREM-1 expression in WT and Trem-1−/− ER-Hoxb8 neutrophils.
Higher TREM-1, TNFRI and TNFRII expression in ER-Hoxb8 and mature neutrophils compared to BMDN.
Priming is characterized by increased surface expression of receptors, NOX subunits and enhanced phagocytosis [1, 2, 30]. We next assessed the competence of ER-Hoxb8 neutrophils in exhibiting characteristic features of primed neutrophils. We chose to prime ER-Hoxb8 neutrophils with TNF-α as its role in neutrophil priming is well established [1, 3]. We measured cell surface expression of TREM-1, TNFRI, and TNFRII in unprimed neutrophils to assess whether they were capable of being primed with TNF-α. In addition, we evaluated the effects of priming on phagocytosis and surface expression of TREM-1, FcγRII/III, and CR3.
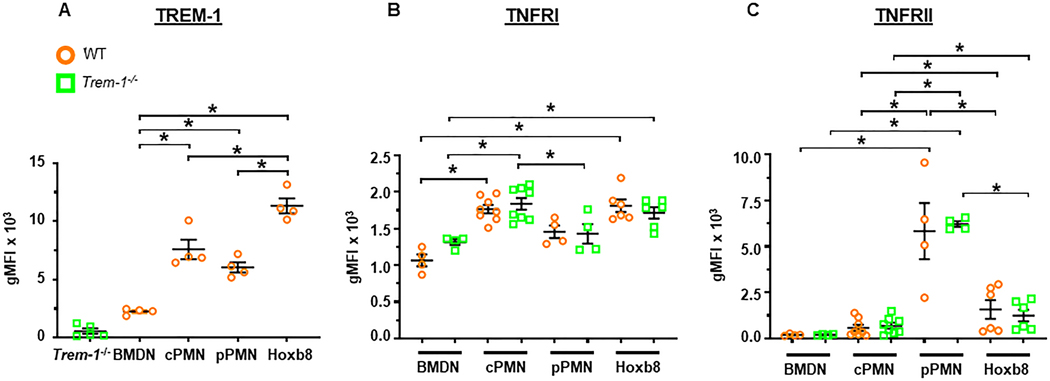
We first determined whether ER-Hoxb8 neutrophils express surface TREM-1 at levels comparable to in vivo differentiated neutrophils. Interestingly, cell surface TREM-1 expression was found to be the highest in ER-Hoxb8 neutrophils and the least in BMDN (Figure 2A). TREM-1 levels were intermediate in circulating and peritoneal neutrophils. As expected, TREM-1 was undetectable in TREM-1 deficient neutrophils.
Figure 2. Higher TREM-1, TNFRI and TNFRII expression in ER-Hoxb8 and mature neutrophils compared to BMDN.
Surface receptor expression in BMDN, circulating neutrophils (cPMN), ER-Hoxb8 neutrophils (Hoxb8) and peritoneal exudate neutrophils (pPMN). A. TREM-1. B. TNFRI. C. TNFRII. Mean±SEM, n = 3–8, *p<0.05. Statistical analyses=one-way ANOVA with Sidak’s multiple comparisons test.
Consistent with the above results, cell surface TNFRI/II expression was the least in BMDN (Figure 2B,C). TNFRI was similar in WT ER-Hoxb8, WT circulating and WT peritoneal exudate neutrophils (Figure 2B). TNFRI was higher in TREM-1 deficient circulating neutrophils compared to TREM-1 deficient peritoneal exudate neutrophils. In contrast, surface TNFRII expression was highest in peritoneal neutrophils, high in ER-Hoxb8 neutrophils and intermediate in circulating neutrophils (Figure 2C). WT and TREM-1 deficient neutrophils displayed no differences in TNFRI or TNFRII expression.
These observations demonstrate that ER-Hoxb8 neutrophils resemble mature neutrophils and are a suitable model for investigating the effects of TREM-1 in TNF-α primed neutrophils.
TREM-1 is not required for phagocytosis.
We next determined the functional consequences of TREM-1 deficiency in ER-Hoxb8 neutrophils and circulating neutrophils following priming. We first examined the effects of priming on TREM-1, FcγRII/III and CR3 surface expression. Priming with TNF-α decreased surface TREM-1 expression in both WT ER-Hoxb8 and circulating neutrophils in contrast to endotoxin-mediated TREM-1 upregulation (Figure 3A) [17].
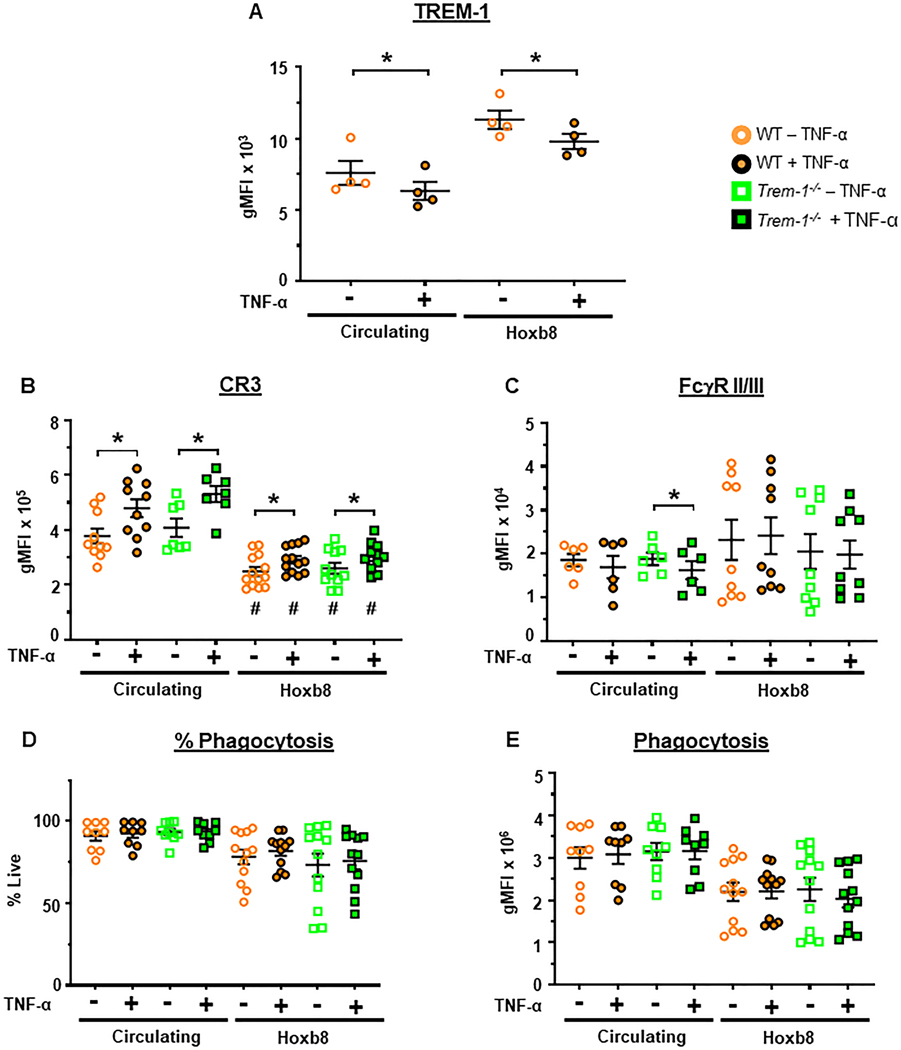
Figure 3. TREM-1 is not required for phagocytosis.
Phagocytosis and cell surface receptor expression were determined in circulating and ER-Hoxb8 neutrophils (Hoxb8) before and after priming for 30 min with TNF-α (2μg/mL). A. TREM-1. B. CR3/CD11b. C. FcgRII/III (CD16/32). D. Percent of cells that phagocytosed OpZ-AF-488 (MOI = 5). E. Phagocytosis of OpZ-AF-488, gMFI. Mean±SEM, n = 4–13. Statistical analyses=paired ttest for differences between primed and unprimed neutrophils of a given genotype and one-way ANOVA followed by Sidak’s multiple comparisons test for differences between circulating and ER-Hoxb8 neutrophils. *p<0.05, #p<0.05 vs corresponding circulating neutrophil genotype.
CR3 and FcγRII/III receptors are involved in phagocytosis of opsonized particles [31]. CR3, a granular component, is trafficked from intracellular granules to cell surfaces following priming [32, 33]. FcγR function is enhanced following priming and may be a consequence of increased surface expression [1, 34]. We, therefore, examined the effects of priming on cell surface expression of these receptors in WT and TREM-1 deficient neutrophils. CR3 increased with priming in both circulating and ER-Hoxb8 neutrophils (Figure 3B). Higher levels of surface CR3 expression was observed in circulating neutrophils compared to ER-Hoxb8 neutrophils. FcγRII/III expression was equivalent in circulating and ER-Hoxb8 neutrophils (Figure 3C). It was not significantly altered by priming except in TREM-1 deficient circulating neutrophils. In these neutrophils, priming modestly decreased FcγRII/III expression. TREM-1 deficiency did not alter either CR3 or FcγRII/III expression in either circulating or ER-Hoxb8 neutrophils.
Based on these observations, we questioned whether TNF-α priming increases phagocytosis in these neutrophil populations. Despite increased CR3 expression phagocytosis was unaltered by priming in both circulating neutrophils and ER-Hoxb8 cells as measured by percentage of cells phagocytosing and particle uptake (Figure 3DE). Greater than 75% of circulating neutrophils phagocytosed OpZ (Figure 3D). In contrast, phagocytosis tended to be lower and more variable in ER-Hoxb8 neutrophils (Figure 3D,E). Thus TREM-1 expression did not alter phagocytosis following TNF-α priming consistent with lack of TREM-1-dependent changes in CR3 and FcγRII/III expression.
The above results demonstrate that ER-Hoxb8 neutrophils display high levels of surface TREM-1 and are more comparable to circulating and exudate neutrophils than to the immature BMDN in terms of TNFRI and TNFRII expression. However, compared to circulating neutrophils, ER-Hoxb8 neutrophils express fewer cell surface CR3 which may be associated with their trend towards lower phagocytosis of particulate stimuli. No TREM-1 dependent effects were observed in either receptor expression or phagocytosis. Taken together, these results demonstrate the maturity of ER-Hoxb8 neutrophils and that they are a competent model to investigate the role of TREM-1 in TNF-α primed responses such as neutrophil oxidative burst.
OpZ-induced oxygen consumption is enhanced by priming in a TREM-1-dependent manner and is independent of mitochondrial activity.
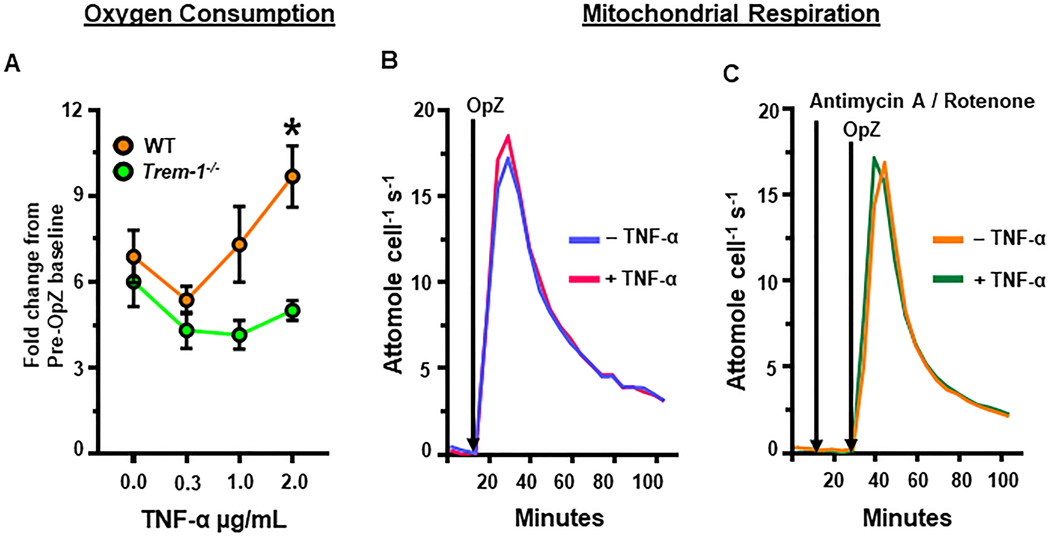
The most well described consequence of priming is enhanced neutrophil oxidative burst following activation by a secondary stimulus. This is characterized by increased NOX activity, superoxide generation, and oxygen consumption [3, 30, 35]. We previously demonstrated that TREM-1 is important for OpZ-induced oxygen consumption and NOX activation in peritoneal exudate neutrophils [23]. Exudate neutrophils are extravasated, primed and activated and, therefore, functionally different from circulating neutrophils or BMDN [29, 36–38]. As TREM-1 is known to amplify inflammatory signaling in infection, a condition in which neutrophils are primed and activated, we hypothesized that TREM-1 may promote neutrophil oxidative burst following priming and stimulation with a particulate agonist. ER-Hoxb8 neutrophils were primed with TNF-α and then stimulated with OpZ. Oxygen consumption was measured over a twenty-minute period using a Clarke electrode. Data are expressed as fold change relative to unstimulated neutrophils. As expected, OpZ stimulation increased oxygen consumption by about 6-fold in unprimed WT and TREM-1 deficient neutrophils (Figure 4A 0μg/mL TNF-α). No difference in oxygen consumption was noted between WT and TREM-1 deficient neutrophils in the absence of priming. TNF-α priming augmented OpZ-induced oxygen consumption in WT neutrophils in a dose-dependent manner. TREM-1 deficient neutrophils did not exhibit such a primed response. At 2μg/mL TNF-α, OpZ-induced oxygen consumption was significantly greater in WT compared to TREM-1 deficient neutrophils.
Figure 4. OpZ-induced oxygen consumption is enhanced by priming in a TREM-1-dependent manner and is independent of mitochondrial activity.
A. OpZ-induced oxygen consumption in WT and Trem-1−/− ER-Hoxb8 neutrophils primed for 30 min with 0.3, 1 or 2 μg/mL TNF-α was measured using the Clarke electrode. Statistical analyses= one-way ANOVA with Brown-Forsythe and Welch correction for unequal variances and Dunnett’s T3 correction for multiple comparisons. B. Oxygen consumption by WT ER-Hoxb8 neutrophils in the absence of mitochondrial inhibitors using the Seahorse Bioscience extracellular flux analyzer. C. Oxygen consumption by WT ER-Hoxb8 neutrophils in the presence of Antimycin A and Rotenone. Mean±SEM, n = 3–9, *p<0.05 vs Trem-1−/−+2μg/mL TNF-α and vs WT+0.3μg/mL TNF-α.
The contribution of the mitochondrial electron transport chain to oxygen consumption in stimulated ER-Hoxb8 neutrophils is not known. We investigated the role of mitochondria in OpZ-induced oxygen consumption in this model using the Seahorse Bioscience extracellular flux analyzer. Primed and unprimed WT ER-Hoxb8 neutrophils were pre-incubated with rotenone and antimycin A to inhibit Complex I and Complex III activity, respectively. They were then stimulated with OpZ. As observed with peritoneal exudate neutrophils, inhibition of mitochondrial activity did not attenuate OpZ-induced oxygen consumption in ER-HoxB8 cells (Figure 4B,C) [23].
Taken together, these results demonstrate that TREM-1 is important for enhanced OpZ-induced oxygen consumption in primed neutrophils. As this is not due to increased mitochondrial respiration, our results strongly suggest that TREM-1 promotes NOX-mediated superoxide generation.
Priming enhances OpZ-induced superoxide production in a TREM-1-dependent manner.
NOX is the main source of superoxide in neutrophils [23, 39]. It transfers electrons from NADPH across cell membranes to oxygen to form superoxide. Priming agents enhance the rate and amount of superoxide generated by NOX following exposure to secondary activating stimuli [3]. Based on the above results we hypothesized that priming enhances OpZ-induced superoxide generation in a TREM-1-dependent manner. To address this hypothesis, we employed EPR spectroscopy using DMPO as a spin trap. This is a specific method that has been successfully used for the detection of superoxide anion and its derivative, the hydroxyl radical [23, 40, 41]. This technique circumvents problems associated with the non-specificity of fluorescent and chemiluminescent redox sensitive probes that are widely used as tools to evaluate ROS generation.
After priming, cells were stimulated with OpZ (MOI = 15) in the presence of the spin trap DMPO: the spin adducts of the hydroxyl radical (HO•) (DMPO-OH; aN = aβH = 14.9 G) and superoxide anion (O2•-) (DMPO-OOH; aN = 14.2 G, aβH = 11.3 G, aβH = 1.2 G) were observed using EPR spectroscopy. Representative spectra from WT ER-Hoxb8 and circulating neutrophils under resting (-OpZ) and stimulated (+OpZ) conditions are shown (Figure 5A). Upon stimulation with OpZ the temporal evolution of the DMPO spin adducts was monitored between 3.5- and 31.5-min. Resting cells demonstrated a small DMPO-OH adduct signal, likely derived from baseline O2•- production and other oxidants. As expected, OpZ stimulated cells demonstrated increases in HO• and O2•- DMPO adducts, as seen by increased intensity of each species in the EPR spectra. Addition of SOD to cells stimulated with OpZ reduced the DMPO-OOH adduct peaks confirming the specificity of the signal as O2•−.
OpZ induced very rapid increases in both DMPO-OH and DMPO-OOH adducts in ER-Hoxb8 neutrophils (Figure 5B,E). DMPO-OH adduct signal (HO•) increased with time in both primed and unprimed neutrophils (Figure 5B). However, priming enhanced the rate of DMPO-OH adduct produced in WT neutrophils such that peak production was observed 10 min earlier in these cells compared to unprimed WT neutrophils or TREM-1 deficient neutrophils (Figure 5B,C). In contrast, priming did not alter the rate of DMPO-OH formation in TREM-1 deficient neutrophils (Figure 5B,D).
DMPO-OOH (O2•-) generation peaked at 3.5 min in all neutrophils and declined to near basal levels by 31.5 min (Figure 5E). Significantly more DMPO-OOH adduct was detected after priming in WT but not TREM-1-deficient neutrophils (Figure 5E,F). As we were unable to acquire readings before 3.5 min, it is possible that O2•- production peaked before 3.5 min and that our measurements are an underestimate of the true increases caused by priming. No difference in DMPO-OH or DMPO-OOH formation was observed between the two genotypes in unprimed cells.
Superoxide anion generation declined rapidly after 3.5 min whereas the production of DMPO-OH plateaued after 14 min in primed WT neutrophils and after 24.5 min in other cells. While this may reflect the rapid decay of DMPO-OOH (t1/2 ~ 60 s), compared to decay of DMPO-OH (t1/2 ~ 4000 s), it may also represent conversion of some DMPO-OOH to DMPO-OH by glutathione peroxidase or other such enzymes [26]. We did not measure other ROS species in this study. As priming augments degranulation, it is possible that superoxide was shunted via hydrogen peroxide to MPO-mediated hypochlorous acid formation [1, 42]. Taken together, our results indicate that ER-Hoxb8 neutrophils are capable of rapid superoxide generation and that TREM-1 is required for priming to enhance OpZ-induced superoxide generation.
We next evaluated the role of TREM-1 in superoxide generation by mature in vivo isolated circulating neutrophils. Similar to ER-Hoxb8 neutrophils, circulating neutrophils demonstrate robust generation of DMPO-OH that increased linearly with time up to 24.5 min following OpZ stimulation (Figure 5G). No difference in DMPO-OH was detected between unprimed WT and unprimed TREM-1 deficient neutrophils. In contrast to ER-Hoxb8 neutrophils, earlier peak production of DMPO-OH was not observed in WT circulating neutrophils. However, as observed with ER-Hoxb8 neutrophils, the rate of DMPO-OH formation increased significantly after priming only in WT but not TREM-1 deficient neutrophils (Figure 5G-I). Therefore, these results indicate that priming increases OpZ-induced hydroxyl radical production in a TREM-1 dependent manner. In contrast to ER-Hoxb8 neutrophils, however, the superoxide anion spin adduct, DMPO-OOH, was barely above detection limits (LOQ) in unprimed circulating neutrophils and undetectable in primed circulating neutrophils (Figure 5J-M). The reasons for this are not entirely clear. Hydroxyl radical is derived from superoxide and hydrogen peroxide and primed WT circulating neutrophils generated higher amounts of hydroxyl radical than primed TREM-1 deficient circulating neutrophils (p=0.059). Moreover, compared to ER-Hoxb8 neutrophils, circulating neutrophils produced more DMPO-OH (10μM vs 14μM at 31.5 min). Based on this our results suggest that in circulating neutrophils priming promotes OpZ-induced superoxide and/or conversion of superoxide to hydroxyl radical in a TREM-1-dependent manner. Our inability to detect superoxide production directly is probably a consequence of rapid scavenging of superoxide by other enzymes such as SOD and MPO to form hydrogen peroxide and hypochlorous acid. This shunt may occur at a faster rate in circulating neutrophils compared to ER-Hoxb8 neutrophils.
Taken together, the above results support the role of TREM-1 in mediating OpZ-induced superoxide in primed neutrophils. Moreover, they demonstrate that the oxidative responses of ER-Hoxb8 neutrophils are comparable to mature circulating neutrophils with slight differences in kinetics.
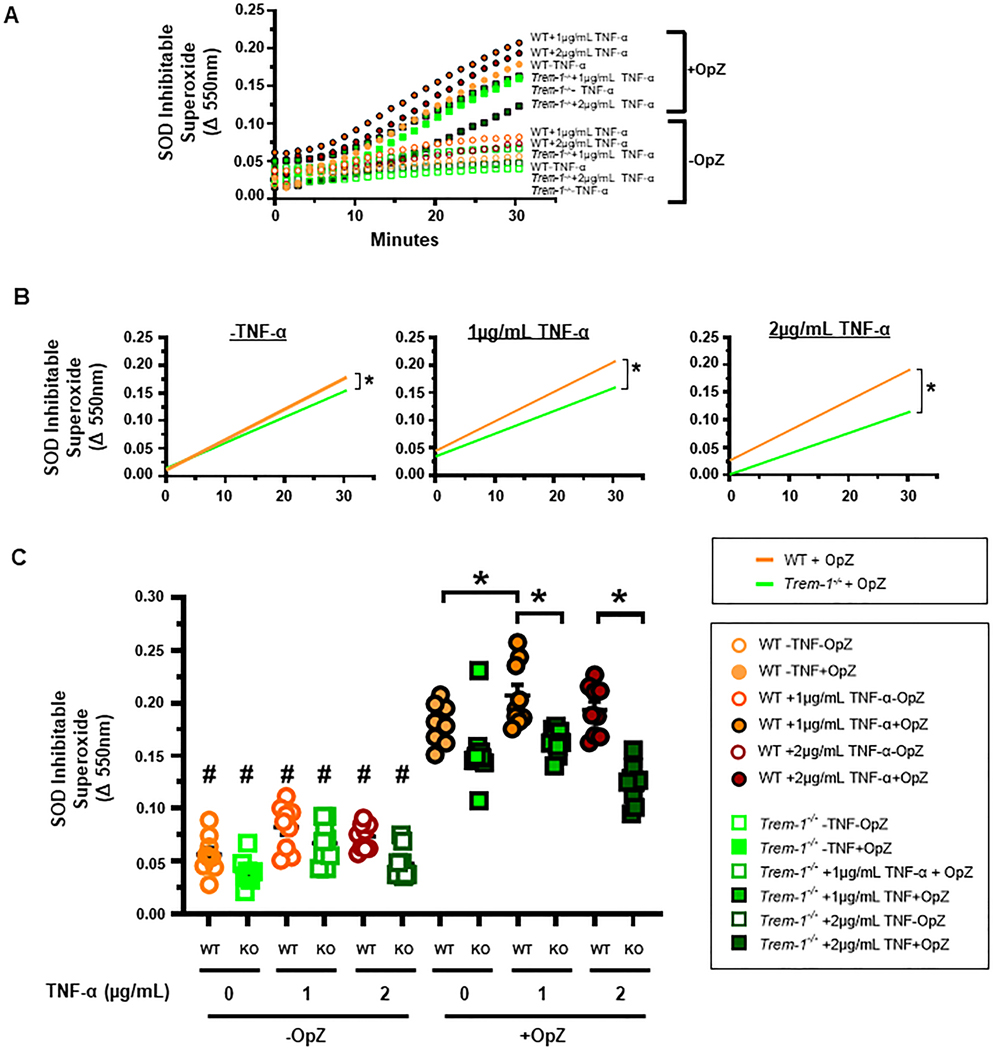
As DMPO can cross the lipid bilayer, the above results represent superoxide generated in both intracellular and extracellular compartments. Extracellular ROS are important in neutrophil migration [43, 44]. Release of superoxide to the extracellular space from OpZ-stimulated neutrophils has been previously reported [28]. As priming increased total superoxide generation in a TREM-1-dependent manner, we next investigated the role of TREM-1 in superoxide release to the extracellular space from WT and TREM-1 deficient ER-Hoxb8 neutrophils using SOD-inhibitable reduction of ferricytochrome c. Stimulation with OpZ enhanced superoxide release from both primed and unprimed neutrophils in a linear manner up to 30 min (Figure 6A). Priming with 1 or 2 μg/mL TNF-α increased the rate of superoxide released into the extracellular space in WT compared to TREM-1 deficient neutrophils (Figure 6B). As a result, after 30 min of OpZ stimulation significantly more superoxide was released from WT neutrophils compared to TREM-1 deficient neutrophils (Figure 6C). In addition, priming with 1 μg/mL TNF-α enhanced OpZ-induced superoxide release in WT but not TREM-1 deficient neutrophils (Figure 6C). In unprimed neutrophils, the rate of OpZ-induced superoxide release was modestly higher in WT compared to TREM-1 deficient neutrophils (Figure B). However, this did not result in significant differences between the two genotypes in the amount of superoxide released after 30 min of OpZ stimulation (Figure 6C). Thus, these results demonstrate that superoxide release after priming and OpZ stimulation is TREM-1 dependent. In aggregate, the above results obtained with EPR spectroscopy and cytochrome c reduction demonstrate that TREM-1 is required for priming to augment superoxide generation in response to particulate agonists.
Figure 6. Priming enhances OpZ-induced superoxide release in a TREM-1-dependent manner.
ER-Hoxb8 neutrophils were primed for 30 min with TNF-α (1 or 2 μg/mL) and stimulated with OpZ at MOI=15. A. Time-dependent release of extracellular SOD-inhibitable superoxide. B. Rate of SOD-inhibitable superoxide release. Simple linear regression with post hoc tests were performed to determine significant differences between slopes and/or intercepts. C. Release of SOD-inhibitable superoxide after 30 min of OpZ stimulation. Differences between various groups were determined by one-way ANOVA with Sidak’s multiple comparisons test. Mean±SEM, n = 9, *p<0.05, #p<0.05 vs corresponding +OpZ group.
TREM-1 is not required for neutrophil priming.
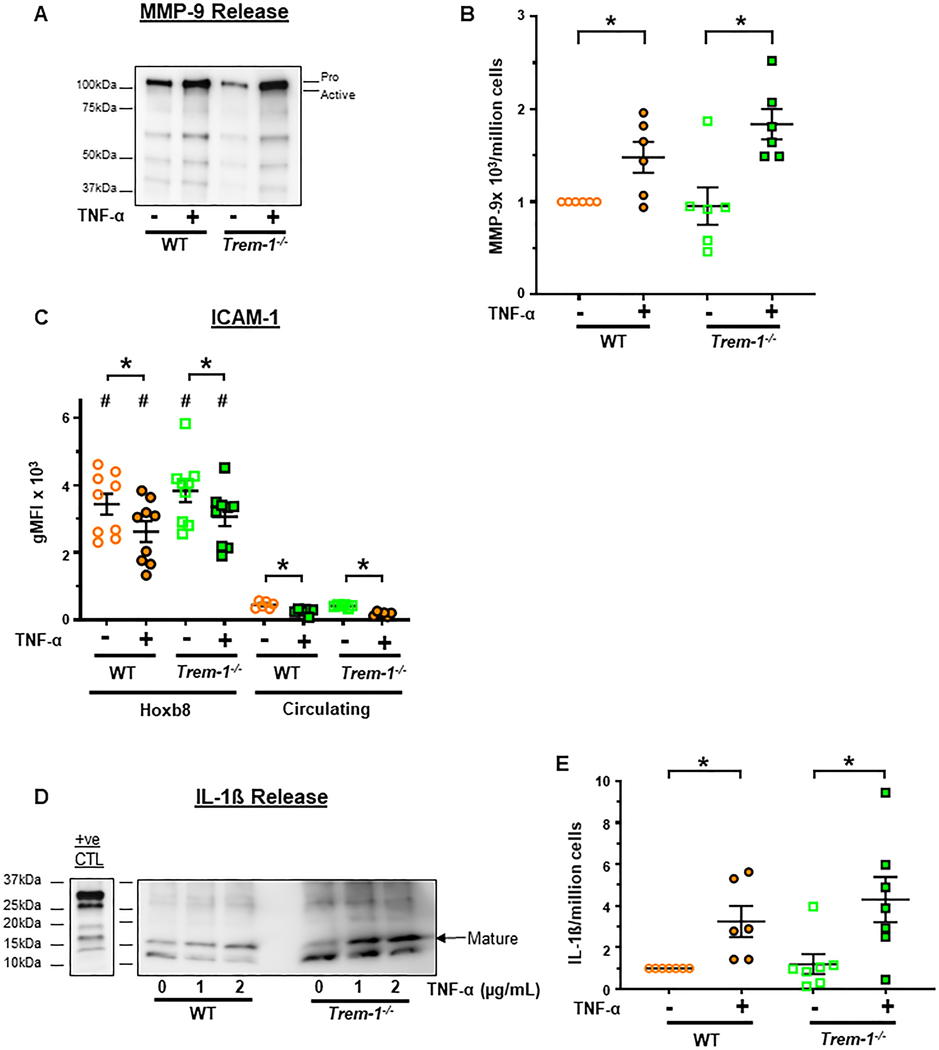
We next questioned whether TREM-1 dependent effects on superoxide generation are mediated during the process of neutrophil priming itself. In our studies, neutrophils were primed with TNF-α for only 30 min. Therefore, we focused on non-transcriptional markers of priming such as degranulation, surface receptor expression and cytokine release [45–48]. Matrix metalloproteinase-9 (MMP-9) is present in secondary and tertiary granules and is released during the process of degranulation following TNF-α priming [46, 49]. In addition, MMP-9 is reported to cleave surface receptors such as ICAM-1 [50, 51]. We, therefore, questioned whether surface ICAM-1 is shed after priming. Furthermore, as pro-IL-1ß is reported to be cleaved to its mature biologically active form by serine proteases or MMP-9 contained in neutrophil granules, we measured release of mature IL-1ß from primed neutrophils [52–56]. Our results demonstrate that TNF-α priming significantly increased MMP-9 release from primed ER-Hoxb8 neutrophils (Figure 7A,B). As expected, surface ICAM-1 expression was decreased after priming in both ER-Hoxb8 and circulating neutrophils although ER-Hoxb8 neutrophils expressed significantly more ICAM-1 (Figure 7C). Lastly, priming increased the secretion of mature IL-1ß consistent with cleavage of pro-IL-1ß to the mature isoform (Figure 7D,E). No significant differences in any of the three markers of priming were noted between WT and TREM-1 deficient neutrophils. In addition, CR3, a component of neutrophil secondary and tertiary granules and secretory vesicles, is known to be trafficked to the cell surface as a result of granule exocytosis after TNF-α priming [32, 33]. Consistent with this, we demonstrate that priming increased CR3 expression (Figure 3B) and, as with MMP-9, ICAM-1 expression and IL-1ß secretion, we did not observe any significant differences between WT and TREM-1 deficient neutrophils. These results suggest that TREM-1 is not required for neutrophil priming at least as assessed by degranulation, surface receptor expression and IL-1ß release.
Figure 7. TREM-1 is not required for degranulation and IL-1ß release following neutrophil priming.
ER-Hoxb8 and circulating neutrophils were primed for 30 min with 2μg/mL TNF-α. A. Representative immunoblot of MMP-9 release into the media of primed and unprimed ER-Hoxb8 neutrophils. B. Densitometric quantitation of pro- and active MMP-9 in the media from 6 independent experiments normalized to WT-TNF-α. C. Surface ICAM-1 expression. D. Representative immunoblot of IL-1ß released into media of primed and unprimed ER-Hoxb8 neutrophils. IL-1ß in BMDN primed for 3 hours with 0.5μg/mL LPS and stimulated for 45 min with 3mM ATP served as a positive control. E. Densitometric quantitation of mature IL-1ß release from 7 independent experiments normalized to WT-TNF-α. Mean±SEM. *p<0.05. Statistical analyses= paired ttest for differences between primed and unprimed responses of a given genotype and one-way ANOVA followed by Sidak’s multiple comparisons test for differences between other groups.
Discussion
Our findings reveal an important role for TREM-1 in primed neutrophil oxidative burst. Using circulating neutrophils and the in vitro differentiated ER-Hoxb8 neutrophils that exhibit multiple effector functions characteristic of primary neutrophils we report that TREM-1 is required for primed superoxide generation following particulate stimulation [8–13, 57, 58]. These effects appear to be mediated after priming as TREM-1 is not required for the process of priming itself. Furthermore, using primary neutrophils isolated from three separate sites, each representing different stages of neutrophil activation and maturation, we demonstrate that ER-Hoxb8 neutrophils resemble mature neutrophils such as extravasated and circulating neutrophils more than BMDN and express high levels of TREM-1.
Neutrophil priming can occur within minutes to enable rapid responses to environmental cues [2, 59]. This process does not involve de novo protein synthesis but instead promotes granular mobilization and expression of pre-formed receptors on cell surfaces [32, 33]. Neutrophils can also be primed to induce transcriptional changes that trigger longer lasting and exaggerated responses to activating stimuli. Such responses are typically observed after longer exposures to priming agents [48]. Priming conditions employed in this study were of short duration during which consistent changes in IL-1β protein or mRNA abundance were not detected (data not shown). Instead, we observed more rapid responses such as increased granule mobilization and degranulation as evidenced by enhanced surface CR3 expression and MMP-9 release. MMP-9, reported to cleave ICAM-1, may have accounted for decreased ICAM-1 expression after priming [50, 51]. In addition, TREM-1 has an MMP-9 cleavage site and incubation of neutrophils with activated MMP-9 increases shedding of membrane TREM-1 [60]. Thus, increased MMP-9 release after priming may also have accounted for our observation of decreased surface TREM-1 expression in primed neutrophils. In conditions where neutrophils are recruited in large numbers to sites of inflammation, secretion of mature IL-1ß is mediated by proteases released as a result of neutrophil degranulation [61]. In our study, TNF-α priming of ER-Hoxb8 neutrophils resulted in significant increase in the secretion of mature IL-1ß that may have been due to cleavage of pro-IL-1β to mature IL-1β by serine proteases or MMP-9 released from granules during priming [53–56, 61]. Importantly, no differences in these markers were evident between WT and TREM-1 deficient neutrophils suggesting that TREM-1 is not required for neutrophil priming. Priming enables migration of neutrophils across endothelia to sites of infection or injury in a process that is mediated, in part, by granule exocytosis and release of metalloproteinases [1]. The lack of TREM-1 dependent changes in MMP-9 release from primed neutrophils suggests that TREM-1 is not required for transendothelial migration. This is consistent with our previous observations demonstrating that TREM-1 is important in transepithelial but not transendothelial migration [22].
An important target of neutrophil priming is NOX. Several priming agents, including TNF-α, prime NOX for rapid and aggressive action following exposure to a secondary stimulus [3, 30]. One mechanism by which this occurs is by increased granule exocytosis during priming and exposure of membrane NOX sub-units, gp91phox and p22phox (cytochrome b558) on plasma membranes [62, 63]. This would increase potential docking sites for cytosolic NOX subunits, p47phox, p40phox, p60phox and Rac, and permit assembly of the multimeric NOX enzyme. However, as TREM-1 was not required for granule mobilization as evidenced by lack of effect on surface CR3 expression, this step may not be regulated by TREM-1. Priming also triggers membrane translocation of cytosolic subunits by p42/44MAPK- and p38MAPK-mediated phosphorylation of p47phox on S345 [64]. TNF-α and TREM-1 trigger signaling cascades that converge on multiple kinases, including AKT, p42/44MAPK and p38MAPK [64–66]. Therefore, TREM-1 could potentiate the effects of TNF-α priming by enhancing p42/44MAPK and/or p38MAPK activation and thereby increase phosphorylation of p47phox. Lack of commercially available antibodies specific to murine p47phox phosphorylated on S345 hindered our ability to directly assess the effects of priming on NOX. It is possible that murine p47phox may be regulated at other sites during priming and a thorough examination of p47phox phosphorylation sites that may be regulated by TREM-1 is warranted. Our results suggest that TREM-1 mediates its effects during agonist stimulation following priming rather than during the process of priming itself. In exudate neutrophils that are primed in vivo, AKT is activated in a TREM-1 dependent manner following OpZ stimulation [23]. TREM-1 may, therefore, promote NOX activation in TNF-α primed neutrophils via AKT-mediated p47phox phosphorylation and translocation to membranes following particulate stimulation. Alternatively, other steps in the assembly of NOX such as activation of Rac or other kinases such as PKC that could be targets of TREM-1 modulation cannot be ruled out.
Multiple studies show ER-Hoxb8 neutrophils generate ROS in response to soluble and particulate agonists [8, 10–13, 58]. In response to integrin activation, however, ER-Hoxb8 neutrophils, plated on fibronectin-coated surfaces, released less extracellular hydrogen peroxide than BMDN suggesting that ER-Hoxb8 neutrophils are not as competent as in vivo neutrophils in generating superoxide [12]. This effect may be agonist-specific and may reflect lower rate of conversion of superoxide to hydrogen peroxide rather than NOX activity itself. Consistent with our results, McDonald et al. report that the particulate agonist, OpZ, induced ROS within minutes of exposure in ER-Hoxb8 neutrophils [13]. This study utilized a probe that detects hydroxyl radical, peroxynitrite and hypochlorous acid. As all three derivatives arise from superoxide, their results suggest rapid generation of superoxide in these cells and are consistent with our observations. To specifically measure superoxide anion and hydroxyl radical production, we utilized the highly specific method of EPR spin trapping [26, 40, 67]. We find that ER-Hoxb8 neutrophils require TREM-1 to augment superoxide production following priming. Superoxide generation in these neutrophils occurred within minutes of OpZ stimulation and due to this rapid rate, we could not capture signals before 3.5 min of stimulation. It is therefore highly likely that we missed detecting earlier peak production of spin adducts. As a result, we may be underestimating true augmentation in the rate and amount of OpZ-induced superoxide following priming. We demonstrate comparable effects of priming on oxidant production in circulating neutrophils. In these neutrophils, TREM-1 enhanced the rate of hydroxyl radical generation following priming. While we were unable to directly capture the superoxide spin adduct, DMPO-OOH, in circulating neutrophils, we found that these neutrophils could convert superoxide to hydroxyl radical which was generated in slightly higher amounts than ER-Hoxb8 neutrophils. It is possible that circulating neutrophils convert superoxide to its derivatives at a faster rate than the rate at which they generate superoxide, thus lowering the overall steady state levels to below EPR detectable limits. This would account for us not being able to capture DMPO-OOH adducts after 3.5 min of stimulation in these cells. Thus, as with ER-Hoxb8 neutrophils, we may be underestimating true rates of superoxide production in primed circulating neutrophils stimulated with OpZ. EPR spectroscopy of DMPO adducts reflects total superoxide generated in response to priming and stimulation. We report that TREM-1 was also important for extracellular superoxide. As extracellular superoxide is important in neutrophil migration, this observation may explain our earlier finding that TREM-1-dependent superoxide was required for neutrophil chemotaxis [23, 43, 44].
Heterogenous neutrophil populations with unique phenotypic and functional properties have been observed in various compartments such as the bone marrow, blood and tissue [68]. Plasticity at the transcriptional, post-transcriptional and functional levels enables neutrophils to be primed and respond in an agonist- and tissue-specific manner [1]. We observed marked differences in cell surface receptor expression amongst the three in vivo populations of neutrophils studied and we discovered that ER-Hoxb8 neutrophils more closely resemble mature circulating and tissue exudate neutrophils. An important finding is that surface TREM-1 expression is high in ER-Hoxb8 neutrophils but low in BMDN. This suggests that the bone marrow may not provide the appropriate environmental signals for optimal TREM-1 expression and BMDN may not, therefore, be an appropriate model to investigate TREM-1 dependent functions. Our findings, instead, support the use of ER-Hoxb8 neutrophils as a model of mature neutrophils to investigate TREM-1 dependent regulation of neutrophil effector functions. Our novel observation that TREM-1 is critical for augmented OpZ-induced superoxide generation in primed neutrophils offers insight into mechanisms by which this innate immune receptor regulates neutrophil function. Further investigation of its potential targets, mechanisms of action and regulation of its expression are warranted. As ER-Hoxb8 neutrophils can be genetically manipulated unlike in vivo differentiated mature neutrophils, regulation of NOX subunit phosphorylation, translocation and expression by TREM-1 can be investigated in these cells using genetic approaches.
Acknowledgments
Research reported in this publication was supported by NIH grant 5R01HL121105 (J.K.T.), NCI award P01CA217797 (G.R.B.), NCI award K08CA201640 (D.B.S.) and an Ash Scholar Award (D.B.S.). The data presented were obtained at the Flow Cytometry Facility, and the ESR Facility, which are core research facilities at The University of Iowa funded through user fees and the financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center (P30CA086862), and Iowa City Veteran’s Administration Medical Center.
Abbreviations
- BMDN
bone marrow derived neutrophils
- CR 3
complement receptor 3
- DETAPAC
diethylenetriaminepentaacetic acid
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- EPR
electron paramagnetic resonance
- ER-Hoxb8
estrogen receptor-Homeobox8 fusion transcription factor
- FcγR
Fc gamma receptor
- ICAM-1
Intercellular Adhesion Molecule-1
- IL-1ß
Interleukin-1ß
- MMP-9
matrix metalloproteinase-9
- MOI
multiplicity of infection
- NOX
NADPH oxidase
- O2•-
superoxide anion
- OCR
oxygen consumption rate
- HO•
hydroxyl radical
- OpZ
opsonized zymosan
- ROS
reactive oxygen species
- SCF
stem cell factor
- SOD
superoxide dismutase
- TLR4
Toll-like receptor 4
- TNFRI
TNF-α receptor I
- TNFRII
TNF-α receptor II
- TREM-1
Triggering Receptor Expressed on Myeloid Cells-1
Footnotes
Authorship
J.K-T. formulated hypotheses, interpreted data, wrote, and edited manuscript
D. B. S. generated ER-Hoxb8 progenitors, interpreted data, edited manuscript
G.R.B Designed experiments, interpreted data, wrote, and edited manuscript.
B.A.W. Designed experiments, interpreted data, and edited manuscript.
K. K. maintained mouse colony and ER-Hoxb8 myeloid progenitors and neutrophils in culture, prepared BMDN, circulating and peritoneal exudate neutrophils, conducted experiments, interpreted data, prepared figures, edited manuscript
J. B. designed and conducted experiments, interpreted data, prepared figures, edited manuscript
S. B. designed and conducted experiments, interpreted data, prepared figures, edited manuscript
S. M. formulated hypotheses, maintained ER-Hoxb8 myeloid progenitors and neutrophils in culture, designed and conducted experiments, interpreted data, wrote, and edited manuscript
Conflict of Interest Disclosure
D.B.S. is a co-founder and holds equity in Clear Creek Bio, is a consultant and holds equity in SAFI Biosolutions, and is a consultant for Keros Therapeutics. Other authors declare no conflicts of interest.
References
- 1.Miralda I, Uriarte SM, McLeish KR Multiple Phenotypic Changes Define Neutrophil Priming. Front Cell Infect Microbiol. 2017; 7:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condliffe AM, Kitchen E, Chilvers ER Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clinical science (London, England : 1979) 1998; 94:461–471. [DOI] [PubMed] [Google Scholar]
- 3.El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunological reviews. 2016; 273:180–193. [DOI] [PubMed] [Google Scholar]
- 4.Bass DA, Olbrantz P, Szejda P, Seeds MC, McCall CE Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. Journal of immunology (Baltimore, Md. : 1950). 1986; 136:860–866. [PubMed] [Google Scholar]
- 5.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. Am Rev Respir Dis. 1992; 146:990–996. [DOI] [PubMed] [Google Scholar]
- 6.Ogura H, Tanaka H, Koh T, Hashiguchi N, Kuwagata Y, Hosotsubo H, Shimazu T, Sugimoto H. Priming, second-hit priming, and apoptosis in leukocytes from trauma patients. J Trauma. 1999; 46:774–781; discussion 781–773. [DOI] [PubMed] [Google Scholar]
- 7.Wang GG, Calvo KR, Pasillas MP, Sykes DB, Hacker H, Kamps MP Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat Methods. 2006; 3:287–293. [DOI] [PubMed] [Google Scholar]
- 8.Chu JY, McCormick B, Mazelyte G, Michael M, Vermeren S. HoxB8 neutrophils replicate Fcgamma receptor and integrin-induced neutrophil signaling and functions. J Leukoc Biol. 2019; 105:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopke A, Scherer A, Kreuzburg S, Abers MS, Zerbe CS, Dinauer MC, Mansour MK, Irimia D. Neutrophil swarming delays the growth of clusters of pathogenic fungi. Nat Commun. 2020; 11:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negoro PE, Xu S, Dagher Z, Hopke A, Reedy JL, Feldman MB, Khan NS, Viens AL, Alexander NJ, Atallah NJ, Scherer AK, Dutko RA, Jeffery J, Kernien JF, Fites JS, Nett JE, Klein BS, Vyas JM, Irimia D, Sykes DB, Mansour MK Spleen Tyrosine Kinase Is a Critical Regulator of Neutrophil Responses to Candida Species. mBio. 2020; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orosz A, Walzog B, Mocsai A. In Vivo Functions of Mouse Neutrophils Derived from HoxB8-Transduced Conditionally Immortalized Myeloid Progenitors. Journal of immunology (Baltimore, Md. : 1950). 2021; 206:432–445. [DOI] [PubMed] [Google Scholar]
- 12.Saul S, Castelbou C, Fickentscher C, Demaurex N. Signaling and functional competency of neutrophils derived from bone-marrow cells expressing the ER-HOXB8 oncoprotein. J Leukoc Biol. 2019; 106:1101–1115. [DOI] [PubMed] [Google Scholar]
- 13.McDonald JU, Cortini A, Rosas M, Fossati-Jimack L, Ling GS, Lewis KJ, Dewitt S, Liddiard K, Brown GD, Jones SA, Hallett MB, Botto M, Taylor PR In vivo functional analysis and genetic modification of in vitro-derived mouse neutrophils. FASEB J. 2011; 25:1972–1982. [DOI] [PubMed] [Google Scholar]
- 14.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nature immunology. 2006; 7:1266–1273. [DOI] [PubMed] [Google Scholar]
- 15.Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacology & therapeutics. 2017; 177:81–95. [DOI] [PubMed] [Google Scholar]
- 16.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. Journal of immunology (Baltimore, Md. : 1950). 2000; 164:4991–4995. [DOI] [PubMed] [Google Scholar]
- 17.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001; 410:1103–1107. [DOI] [PubMed] [Google Scholar]
- 18.Boufenzer A, Carrasco K, Jolly L, Brustolin B, Di-Pillo E, Derive M, Gibot S. Potentiation of NETs release is novel characteristic of TREM-1 activation and the pharmacological inhibition of TREM-1 could prevent from the deleterious consequences of NETs release in sepsis. Cellular & Molecular Immunology. 2021; 18:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortin CF, Lesur O, Fulop T Jr. Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int Immunol. 2007; 19:41–50. [DOI] [PubMed] [Google Scholar]
- 20.Klesney-Tait J. and Colonna M. Uncovering the TREM-1-TLR connection. American journal of physiology. Lung cellular and molecular physiology. 2007; 293:L1374–1376. [DOI] [PubMed] [Google Scholar]
- 21.Ornatowska M, Azim AC, Wang X, Christman JW, Xiao L, Joo M, Sadikot RT Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. American journal of physiology. Lung cellular and molecular physiology. 2007; 293:L1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klesney-Tait J, Keck K, Li X, Gilfillan S, Otero K, Baruah S, Meyerholz DK, Varga SM, Knudson CJ, Moninger TO, Moreland J, Zabner J, Colonna M. Transepithelial migration of neutrophils into the lung requires TREM-1. The Journal of clinical investigation. 2013; 123:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baruah S, Murthy S, Keck K, Galvan I, Prichard A, Allen LH, Farrelly M, Klesney-Tait J. TREM-1 regulates neutrophil chemotaxis by promoting NOX-dependent superoxide production. J Leukoc Biol. 2019; 105:1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner BA, Venkataraman S, Buettner GR The rate of oxygen utilization by cells. Free Radic Biol Med. 2011; 51:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britigan BE, Rosen GM, Thompson BY, Chai Y, Cohen MS Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986; 261:17026–17032. [PubMed] [Google Scholar]
- 26.Buettner GR and Mason RP Spin-trapping methods for detecting superoxide and hydroxyl free radicals in vitro and in vivo. Methods Enzymol. 1990; 186:127–133. [DOI] [PubMed] [Google Scholar]
- 27.Buettner GR On the reaction of superoxide with DMPO/.OOH. Free Radic Res Commun. 1990; 10:11–15. [DOI] [PubMed] [Google Scholar]
- 28.DeLeo FR, Allen LA, Apicella M, Nauseef WM NADPH oxidase activation and assembly during phagocytosis. Journal of immunology (Baltimore, Md. : 1950). 1999; 163:6732–6740. [PubMed] [Google Scholar]
- 29.Follin P, Briheim G, Dahlgren C. Mechanisms in neutrophil priming: characterization of the oxidative response induced by formylmethionyl-leucyl-phenylalanine in human exudated cells. Scandinavian journal of immunology. 1991; 34:317–322. [DOI] [PubMed] [Google Scholar]
- 30.DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. The Journal of clinical investigation. 1998; 101:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman SA and Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunological reviews. 2014; 262:193–215. [DOI] [PubMed] [Google Scholar]
- 32.Forsberg M, Lofgren R, Zheng L, Stendahl O. Tumour necrosis factor-alpha potentiates CR3-induced respiratory burst by activating p38 MAP kinase in human neutrophils. Immunology. 2001; 103:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tandon R, Sha’afi RI, Thrall RS Neutrophil beta2-integrin upregulation is blocked by a p38 MAP kinase inhibitor. Biochem Biophys Res Commun. 2000; 270:858–862. [DOI] [PubMed] [Google Scholar]
- 34.Belostocki K, Park MS, Redecha PB, Masuda E, Salmon JE, Pricop L. FcgammaRIIa is a target for modulation by TNFalpha in human neutrophils. Clin Immunol. 2005; 117:78–86. [DOI] [PubMed] [Google Scholar]
- 35.Guthrie LA, McPhail LC, Henson PM, Johnston RB Jr. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984; 160:1656–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itou T, Collins LV, Thorén FB, Dahlgren C, Karlsson A. Changes in activation states of murine polymorphonuclear leukocytes (PMN) during inflammation: a comparison of bone marrow and peritoneal exudate PMN. Clinical and vaccine immunology : CVI. 2006; 13:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oved JH, Paris AJ, Gollomp K, Dai N, Rubey K, Wang P, Spruce LA, Seeholzer SH, Poncz M, Worthen GS Neutrophils promote clearance of nuclear debris following acid-induced lung injury. Blood. 2021; 137:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yost CC Neutrophils clear sterile inflammatory debris. Blood. 2021; 137:293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nauseef WM Nox enzymes in immune cells. Semin Immunopathol. 2008; 30:195–208. [DOI] [PubMed] [Google Scholar]
- 40.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012; 52:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauseef WM Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochimica et biophysica acta. 2014; 1840:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogt KL, Summers C, Chilvers ER, Condliffe AM Priming and de-priming of neutrophil responses in vitro and in vivo. European journal of clinical investigation. 2018; 48 Suppl 2:e12967. [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016; 16:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niethammer P, Grabher C, Look AT, Mitchison TJ A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009; 459:996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishimoto TK, Jutila MA, Berg EL, Butcher EC Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989; 245:1238–1241. [DOI] [PubMed] [Google Scholar]
- 46.Potera RM, Jensen MJ, Hilkin BM, South GK, Hook JS, Gross EA, Moreland JG Neutrophil azurophilic granule exocytosis is primed by TNF-alpha and partially regulated by NADPH oxidase. Innate Immun. 2016; 22:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walcheck B, Kahn J, Fisher JM, Wang BB, Fisk RS, Payan DG, Feehan C, Betageri R, Darlak K, Spatola AF, Kishimoto TK Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature. 1996; 380:720–723. [DOI] [PubMed] [Google Scholar]
- 48.Yao Y, Matsushima H, Ohtola JA, Geng S, Lu R, Takashima A. Neutrophil priming occurs in a sequential manner and can be visualized in living animals by monitoring IL-1beta promoter activation. Journal of immunology (Baltimore, Md. : 1950). 2015; 194:1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakrabarti S, Zee JM, Patel KD Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J Leukoc Biol. 2006; 79:214–222. [DOI] [PubMed] [Google Scholar]
- 50.Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002; 21:5213–5223. [DOI] [PubMed] [Google Scholar]
- 51.Tong S, Neboori HJ, Tran ED, Schmid-Schonbein GW Constitutive expression and enzymatic cleavage of ICAM-1 in the spontaneously hypertensive rat. J Vasc Res. 2011; 48:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black RA, Kronheim SR, Cantrell M, Deeley MC, March CJ, Prickett KS, Wignall J, Conlon PJ, Cosman D, Hopp TP, et al. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988; 263:9437–9442. [PubMed] [Google Scholar]
- 53.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proceedings of the National Academy of Sciences of the United States of America. 1999; 96:6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O’Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007; 130:918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, van der Meer JW, Dinarello CA, van den Berg WB Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009; 60:3651–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. Journal of immunology (Baltimore, Md. : 1950). 1998; 161:3340–3346. [PubMed] [Google Scholar]
- 57.Amini P, Stojkov D, Felser A, Jackson CB, Courage C, Schaller A, Gelman L, Soriano ME, Nuoffer JM, Scorrano L, Benarafa C, Yousefi S, Simon HU Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat Commun. 2018; 9:2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faller S, Hausler F, Goeft A, von Itter MA, Gyllenram V, Hoetzel A, Spassov SG Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung injury. Sci Rep. 2018; 8:14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright HL, Moots RJ, Bucknall RC, Edwards SW Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford). 2010; 49:1618–1631. [DOI] [PubMed] [Google Scholar]
- 60.Weiss G, Lai C, Fife ME, Grabiec AM, Tildy B, Snelgrove RJ, Xin G, Lloyd CM, Hussell T. Reversal of TREM-1 ectodomain shedding and improved bacterial clearance by intranasal metalloproteinase inhibitors. Mucosal Immunol. 2017; 10:1021–1030. [DOI] [PubMed] [Google Scholar]
- 61.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010; 6:e1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLeish KR, Merchant ML, Creed TM, Tandon S, Barati MT, Uriarte SM, Ward RA Frontline Science: Tumor necrosis factor-alpha stimulation and priming of human neutrophil granule exocytosis. J Leukoc Biol. 2017; 102:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uriarte SM, Rane MJ, Luerman GC, Barati MT, Ward RA, Nauseef WM, McLeish KR Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. Journal of immunology (Baltimore, Md. : 1950). 2011; 187:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. The Journal of clinical investigation. 2006; 116:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aggarwal BB Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003; 3:745–756. [DOI] [PubMed] [Google Scholar]
- 66.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009; 41:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buettner GR and Oberley LW Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978; 83:69–74. [DOI] [PubMed] [Google Scholar]
- 68.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019; 19:255–265. [DOI] [PubMed] [Google Scholar]