Abstract
Parkinson’s Disease (PD) is a neurodegenerative disorder that manifests as an impairment of motor and non-motor abilities due to a loss of dopamine input to deep brain structures. While there is presently no cure for PD, a variety of pharmacological and surgical therapeutic interventions have been developed to manage PD symptoms. This review explores the past, present and future outlooks of PD treatment, with particular attention paid to deep brain stimulation (DBS), the surgical procedure to deliver DBS, and its limitations. Finally, our group’s efforts with respect to brain mapping for DBS targeting will be discussed.
Index Terms: Parkinson’s disease (PD), Parkinson’s symptoms, Deep brain stimulation (DBS), Essential tremor, Dystonia, Bradykinesia, Treatment resistant depression (TRD), Alzheimer’s, Tourette syndrome (TS), Subthalamic nucleus (STN), Thalamus (VM), globus pallidus interna (GPi)
Parkinson’s Disease (PD)
Overview
Parkinson’s disease is a chronic and degenerative neurological movement disorder with prevalence increasing with age. It is estimated that 1 million people in the U.S and 10 million worldwide are suffering from PD[1, 2].
PD is the result of the loss of function or death of dopaminergic neurons in the substantia nigra in the ventral midbrain. The loss of dopaminergic innervation in deep brain structures results in symptoms such as slow movements (bradykinesia) or lack of movement (akinesia)[3]. Numerous therapies, ranging from drug-based to surgical, have been explored in the hopes of alleviating parkinsonian symptoms. Many of these therapies have shown great promise, in particular, deep brain stimulation (DBS), which involves electrical stimulation of deep brain structures using surgically implanted electrodes. This review will briefly outline clinical presentations of PD, its therapies, the current status of DBS, and anticipated future developments.
Clinical Presentation
PD is traditionally characterized by the motor-based features; the cardinal features of PD are tremor, rigidity, bradykinesia and postural instability which result in involuntary movement. Resting tremors seen in PD are continuous (unlike twitches which are transient), uncontrolled rhythmic movements, predominant in the upper limb and are also observed in the lips, chin and legs, when the muscles are relaxed[4]. Rigidity is defined as hypertonia or increased resistance in the muscles during passive motion of the limbs[4]. The combination of rigidity and tremors can elicit ‘cogwheel rigidity’ which is a jerking movement present with passive motion[4]. Bradykinesia is slowed movement encompassing both fine and gross motor actions and describes a lower amplitude of motion. Decreased eye blinking, altered facial expression, slowed walking, difficulty standing up, and drooling are all considered clinical features of bradykinesia as seen in PD patients [4]. Postural instability manifests in the form of abnormal gait and stooped posture. The combination of these symptoms affects the individual’s daily activities and can result in an inability to feed oneself, difficulty swallowing, writing, getting dressed, among other activities thus lowering their quality of life[4]. While the most notable symptoms of PD are motor-related, non-motor symptoms are also present in some individuals. Some of these symptoms include cognitive issues, behavioral disorders, sensory and autonomic dysfunction, depression, sleep disorders, and dementia, among others[4–6].
The aforementioned symptoms are not limited to PD; thus, appropriate measures must be taken to differentiate PD from other motor and neurological disorders. Certain diseases, including Essential Tremor and Progressive Supranuclear Palsy, and the side effects of medications (i.e. antipsychotics) are often misdiagnosed as PD[4].
Diagnosis
Currently, no diagnostic test exists that can conclusively diagnose PD, which is why the disease is identified at a very late stage, when approximately 80% of dopaminergic neurons have already died[7]. To diagnose PD, clinical and physiologic approaches are required to reach an appropriate conclusion. A clinical diagnosis is based on the presentation of cardinal symptoms while using diagnostic criteria to rule out other potential causes of the present symptoms[4].
Since PD is characterized by the loss of dopaminergic neurons, characterization of this loss can be confirmed on post-mortem pathology. In terms of the pathology associated with PD, the presence of Lewy bodies is considered a morphological marker of the disease. Lewy bodies are fibrous aggregates associated with degeneration of neurons and are found in the dorsal motor nucleus of the vagus nerve and locus coeruleus. An important constituent of Lewy bodies is alpha-synuclein protein prevalent in PD pathology[8].
Imaging can be conducted to confirm or rule out other neurological disorders. Positron Emission Tomography (PET) is has been used for PD diagnosis as well as dopamine transporter imaging and single photon emission computed tomography (DAT-SPECT) to detect dopaminergic neuron degeneration[9]. Additionally, treatment with levodopa (medication for PD) can be tested to see if the individual is responsive, hence confirming the diagnosis[4, 9].
Prognosis
Presently, there is no known cure for PD. While treatment exists, it does not halt progression; treatment alleviates symptoms rather than preventing the inevitable progression of the disease. With aging, the severity of symptoms increases, and new symptoms may emerge. PD is unique to each individual, the possibility of developing dementia as the disease progresses may be present in some patients[10]. The cardinal symptoms may increase in severity while additional non-motor symptoms such as sleep disturbances and mood changes may begin to present, if not already apparent. In terms of life expectancy, those with PD have a shorter life expectancy, however, the causes of death are not due to PD, but as a result of symptoms associated with PD[11].
Therapy
Pharmacological
Medications are prescribed to compensate for the lack of dopamine production to improve motor issues symptoms. The most effective medication prescribed is carbidopa-levodopa which crosses the blood brain barrier and undergoes conversion to dopamine. Other medications include dopamine agonists, monoamine oxidase B inhibitors, catechol O-methyl transferase inhibitors, anticholinergic drugs and Amantadine[5].
As PD progresses the efficacy of pharmacological treatment decreases and the proportion of ‘on’ time to ‘off’ time decreases[12]. Dyskinesias (medication-induced involuntary movements), tremors, and bradykinesias worsen, and additional treatment is required to supplement levodopa-based treatment.
Lesioning
The thalamus is a structure located in the diencephalon of the brain that is important for relaying motor and sensory signals to the cortex. A thalamotomy is a rare procedure, performed under CT or MRI guidance, in which an electrode is surgically introduced to thermally ablate the ventral intermediate nucleus in the thalamus with the desired outcome of eliminating tremors[13]. Thalamotomy is of limited use since it only addresses tremors and no other symptoms[14], and because it is invasive and the effects are irreversible. In most cases, the procedure is performed while the patient is awake. Insertion points are made in the skull with a drill through which probes can enter to destroy brain tissue[7, 15, 16]. It has more recently been supplanted by MR guided focused ultrasound lesioning, which creates a lesion accurately without surgery.[13]
Pallidotomy is another type of brain lesioning technique used in PD treatment, which involves the ablation of parts of the overactive globus pallidus. As with other surgical treatments, pallidotomy is only considered in cases of severe motor impairments and when there is a lack of response to medications. A potential side-effect of levodopa therapy is dyskinesia which can be addressed with pallidotomy. Additionally, pallidotomy can be beneficial in treating bradykinesia, rigidity, and tremors. Pallidotomy is rarely performed because it is a high-risk surgery compared with DBS and can result in irreversible effects related to lesioning of adjacent structures [14, 17]. The subthalamic nucleus is another lesioning target. Subthalamotomy is also seldom performed due to the risks posed[14].
Deep Brain Stimulation (DBS)
Deep Brain Stimulation (DBS) delivers pulses of electrical current to deep brain structures via surgically implanted electrodes and a pulse generator. For PD treatment, the subthalamic nucleus and globus pallidus pars interna are the most common targets. DBS will be described in greater detail in upcoming sections.
Other Therapies
Stem cell therapies for replacing dopaminergic neurons are being explored as a treatment option for PD. Transplanting dopamine producing neurons has led to alleviation of symptoms resulting from the loss of these neurons in patients. Inconsistent results across studies call for further research and development in the field[18].
Recent developments in brain-computer interface technologies and increased appreciation for the potential for plasticity in the adult brain has led to a renewed interest in using neurofeedback to induce and guide adaptive plasticity[19]. It has recently been proposed that the neurophysiological correlates of PD (e.g. beta bursts) may be good targets for neurofeedback-based operant conditioning to modify brain pathology and reduce symptom severity[20, 21, 22].
DBS Benefits and Limitations
Benefits
Pharmacological (Levodopa) symptom control is currently the most common therapy for those afflicted by PD but becomes less effective as the disease progresses. DBS treatment presents a great number of advantages over stand-alone pharmacological treatment[23]. Although DBS is more invasive and riskier than drug-based therapy, it has been proven that a greater improvement in motor symptoms is achieved through DBS than with drug-based therapy alone[24, 25, 26, 27]. Weaver et al. report patients gaining more “on time”, time without dyskinesias, and greater improvement in mobility and activities of daily living than did patients on traditional medical therapy only, which demonstrated DBS improved quality of life compared to other therapeutics[26]. DBS is also advantageous because it is a reversible therapy unlike ablative procedures such as thalamotomy and pallidotomy[28, 29, 30, 31, 32]. DBS imparts minimal damage surrounding nerve cells and tissue as compared to other surgical interventions[33]. Furthermore, DBS targeting and stimulation parameters can be customized to uniquely treat each individual patient for optimal benefit.
Limitations
While DBS presents a great number of advantages, one of its limitations is the risk due to the complexity and invasiveness of the surgery. Some of the risks will be explained further in the coming sections (see Risks). Another limitation of DBS is a marginal impact on non-motor symptoms. Various studies have conflicting results thus more research is required to determine whether DBS can alleviate non-motor PD symptoms[28]. Current DBS practices are also limited in their requirement of regular follow-ups to adjust stimulation parameters. The patient must see a surgeon or another specialist to optimize stimulation parameters according to the symptoms presented as time progresses[34].
DBS may lead to unwanted side effects. Some of these include impulse control limitations, cognitive impairments, dysphonia, dysarthria, depression and apathy, and personality changes[35, 36, 37, 38].
DBS Devices
A DBS system consists of the electrode(s), the implantable pulse generator (IPG), and leads connecting the two. The IPG serves as the pacemaker, initiating, and regulating the pulses and also containing the battery, while the electrode delivers pulses to the site of action[3, 34].
Electrodes
DBS electrodes are composed of a central shaft on which electrode contacts reside; the active sites are manufactured near the distal region of the shaft. Current commercial DBS electrodes have 4 or 8 segmented contacts, with a typical contact length of 1.5 mm and inter-electrode spacing ranging between 0.5 and 1.5 mm depending on the design and manufacturer. The electrode contact is typically a platinum-based metal or alloy such as platinum-iridium. The impedance of current DBS macro electrodes is in the range of 500–1500 Ω when tested at 1 kHz[38, 39, 40].
Implantable Pulse Generators
The second half of a DBS system is the IPG, which is implanted in the chest and connected to the electrodes to modulate the electrical signal. The impulse generator is programmed to dictate the signal amplitude, frequency, and pulse width. The IPG can use either a voltage source or a current source for pulse generation, although each have their advantages and limitations, current sources are gaining popularity in the field[34].
DBS systems require a battery which can be rechargeable or non-rechargeable[40, 41]. The battery life of the DBS system is dependent on electrical characteristics such as the stimulation amplitude and rate as well as the number of contacts, battery capacity and pulse width[38, 42].
IPG programming
In order to achieve the desired therapeutic effect while avoiding side effects, pulse generators must be appropriately configured for localized stimulation. The reach of the electrical field is dictated by both the configuration of the IPG and electrode design. Changes to parameters such as the pulse width and stimulation rate can result in undesired stimulation and side effects[43]. The signal from the pulse generator stimulates the target area based on a predetermined rate (between 2 and 255 Hz), pulse width (20–507 microseconds), and stimulation amplitude (up to 25.5 mA depending on manufacturer)[34]. After the initial setting of these parameters, follow-ups are conducted to reprogram the IPG, replace the battery and evaluate the pharmacological treatment regimen[7].
One of the key parameters for DBS is the frequency of stimulation which can either be high frequency stimulation (HFS, > 100 Hz) or low frequency stimulation (LFS, 60 Hz)[44]. HFS has been used more often and has a longer history of relieving motor symptoms of various neurodegenerative diseases including PD [30, 44]. An alternative to HFS is LFS DBS which is used for treating both motor and non-motor symptoms [45]. Research suggests that LFS might worsen tremors while HFS may resolve tremors - thus further research needs to be conducted to deduce when HFS and LFS should be used[46].
DBS Surgery
Prior to the procedure, at our centre, the patient undergoes a levodopa challenge in which the motor scores are quantified off and on medications. They are also assessed by a neuropsychologist and a psychiatrist. Any outstanding psychiatric issues are optimized prior to consideration of surgery. Patients undergo an MRI for surgical planning within 2 weeks of the surgery (Figs. 1 and 2).
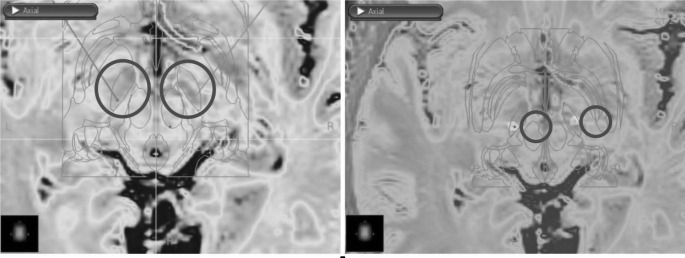
Fig. 1.
Images obtained from the DBS surgery planning station showing coronal (top left), sagittal (top right), and axial (bottom left) pre-operative MRI with an overlayed atlas to identify the STN based on high iron deposits (coloured MRI) and atlas anatomic identification. Bottom right image showing a 3D reconstruction with the planned bilateral electrode insertion trajectory
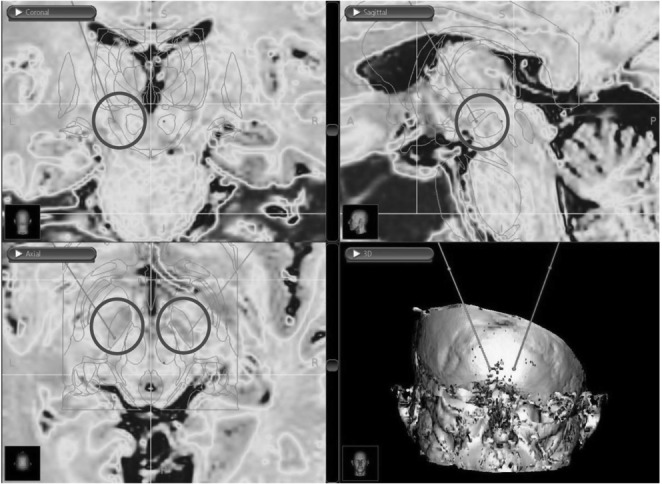
Fig. 2.
Images obtained from the DBS surgery planning station showing pre-operative (left) and post-operative (right) axial MRIs with an overlayed atlas for DBS of the STN
DBS Targets
The standard DBS targets for the treatment of motor disorders include the STN, thalamus (VIM) and globus pallidus interna (GPi)[33]. The target choice depends on the individual’s symptoms[30, 47, 48]. Most commonly, DBS targets the STN or the GPi, both of which are components of the basal-ganglia-thalamo-cortical network. These two nuclei have been described by Almeida et al. to be a part of direct, indirect and hyperdirect pathways in which faulty signaling results in motor and cognitive defects[49]. By electrically stimulating the neural networks involving the STN and GPi, imbalances can be offset to relieve motor symptoms. The STN comprises associative, motor, and limbic regions but only the motor region is targeted [48]. Other research shows additional areas that can be targeted for therapy which include the internal capsule (IC) to thalamic structures such as the somatosensory thalamus, centromedian intralaminar perifascicular complex (CMPf), to the periventricular and periaqueductal grey (PVG/PAG), to the nucleus accumbens (NAc) and anterior cingulate cortex (AC) and the subcallosal cingulate gyrus (SCG) in DBS for unipolar treatment resistant depression (TRD)[50–55].
Globus Pallidus interna (GPi) or Subthalamic (STN) DBS
The two most commonly targeted areas are the GPi and the STN. Studies comparing the two targets have either demonstrated neither target being more advantageous than the other with respect to motor function, or slightly greater improvement with STN stimulation[56]. Non-motor function and side effects were also explored when comparing the two indicating trade-offs associated with stimulating one region over the other[28]. Hence, Follett et al. suggests selecting a target on a case-by-case basis based on nonmotor factors for each individual patient’s specific needs[57].
Some of the nonmotor factors studied were post-DBS medication requirement, depression, visuomotor function, and cognitive function among others. Follet et al’s results demonstrated worsened depression with STN stimulation while the level of depression improved with GPi stimulation[57]. On the other hand, Odekekentetal et al. demonstrated no significant difference between the two on mood[56]. Further studies must be conducted to confirm the advantages of one target over the other[58–61].
Surgical Planning
Accurate placement of the DBS electrode is paramount for optimal treatment efficacy. Electrodes misaligned with anatomic structures can have intolerable side effects[38]. Great care is taken to position the DBS electrode in the target to maximize efficacy and minimize side effects.
Prior to the surgery, the patient will undergo magnetic resonance imaging (MRI) of their head and brain to reveal the individual’s neuroanatomy (Figs. 1 and 2). The neurosurgeon uses their expertise to combine atlas information with indirect targeting and patient anatomy with direct targeting to determine the DBS electrode target and plan the trajectory from the surface of the brain to the target.
In indirect targeting, the surgeon selects the target based on alignment of the patient images with a human brain atlas. A non-linear warping from template space to patient space is calculated, then a standard atlas (in template space) is warped to patient space and atlas structures are overlaid on the patient images (Fig. 1).
In direct targeting, a 1.5 or 3T MRI is used to identify the motor region in the posteriolateral region of the STN directly in the patient images. Using a thalamic parcellation protocol with a T1-weighted brain scan, a T2- weighted coronal scan, and a diffusion-weighted brain scan, the STN is identified as a hypointense structure above the substantia nigra with an approximate volume 125.4 mm3 [62]. Spin echo, susceptibility-weighted and T2-weighted imaging are commonly used in imaging the STN which is biconvex and has an oblique orientation. Some disagreement on the effectiveness of susceptibility-weighted imaging in identifying the STN exists in current literature [62–64].
Using high field diffusion-weighted imaging and pulse sequences, the STN motor region can be better resolved and contrasted, allowing for more precise targeting[62]. These methods of surgical planning can be used to provide patient specific information on the location of the STN. Studies have shown that newly developed 7T MRI technology is able to provide localization that is in agreement with MER localization lending to the possibility of novel imaging technology displacing the need for MER[65].
As with STN imaging sequences, specific sequences have been used for imaging of the GPi. At our centre the Fast Gray Matter Acquisition T1 Inversion Recovery MRI sequence is used to delineate the GPi and GPe. At other centres the use of modified driven equilibrium Fourier transform (MDEFT) MRI sequencing for identification of the GPi has proven effective particularly in distinguishing the structure from the rest of the basal ganglia[65].
The boundaries of the GPi are indicated by the combination of the internal capsule, medial intramedullary lamina, and transition to white matter at the border of the GPi [66]. Ventrally the GPi transitions to white matter, dorsally the GPi is in contact with the internal capsule, the posterior and anterior aspects intersect with the internal capsule and medial intermedullary lamina, the GPi is bound laterally by the medial intermedullary lamina and medially by the internal capsule[66]. Using 3T FLASH T2 imaging is another protocol that has effectively identified the GPi[66, 67].
The planned trajectory must be aligned with the surgical apparatus using frame-based or frameless stereotaxy. In the former, a head frame is placed and immobilized with screws. In the latter, fiducial screws are inserted[46]. After placement of the frame or fiducials, a computed tomography (CT) scan is performed and the CT is co-registered with the MRI, and fiducials are marked in MRI space[68]. The stereotaxy system is then used to determine the position and orientation of the microdrive that will be used to drive the DBS electrode to the target along the planned trajectory.
Intraoperative Mapping
While use of imaging to determine trajectory and location are effective in treatment planning, it is prone to error. The imaging and superposition of the atlas is a theoretical model of what is expected and does not necessarily indicate the exact anatomy at the time of the procedure. Despite dedicated MRI sequences, the target nuclei (e.g. STN, GPi, or VIM) are not perfectly delineated from surrounding tissue, and the subregion of the target nuclei cannot be determined using current imaging techniques. Preoperative imaging cannot account for intraoperative brain shift due to CSF loss and changes in intracranial pressure[69]. This results in a deviation of the DBS target from the planned trajectory. Intraoperative mapping is required to accurately target the region of interest. Brain mapping can be achieved with multiple modalities including imaging and electrophysiology.
Interventional imaging is an emerging technique to assist with electrode targeting refinement[70, 71, 72]. Interventional MRI and CT methods present real-time imaging allowing for target localization. Using a 1.5 T MRI machine the insertion points can be determined as well as confirmation of precise implantation intraoperatively. Using this approach, some have argued that the need for MER is also eliminated[72]. The combination of MRI and CT intraoperatively, termed CT-MRI fusion, allows for use of the modalities pre-, intra- and postoperatively for effective and efficient insertion and implantation of DBS electrodes[71]. Intraoperative ventriculography is another option to confirm the location of the DBS leads (Fig. 3).
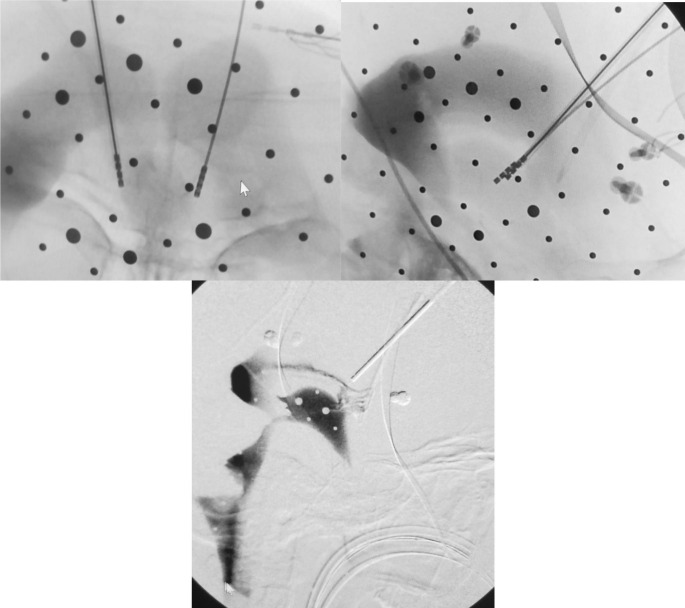
Fig. 3.
Intraoperative anterior thalamic nucleus stimulation surgery ventriculograms showing DBS lead placement. (top left – coronal; top right – sagittal; bottom – sagittal)
Interventional imaging is not indicated for all patients. For many DBS cases, the preferred method for intraoperative targeting refinement is microelectrode recording (MER) mapping of targets based on their neurophysiology. Furthermore, recent studies suggest that optimal DBS electrode locations are not defined precisely by anatomy and neurophysiological definition may be better[73–76]. Precise and accurate localization of neurophysiological targets is also important for adaptive DBS which measures ongoing neurophysiology to adapt stimulation parameters[77, 78].
After stereotactic measures are performed to determine the trajectory, a rigid guide tube is placed into the brain to initiate the desired path. A microdrive is used to advance the electrode precisely, in increments of 100–300 micrometers, through the guide tubes and into the brain during the recording procedure.
MER is a process in which signals of the neurons surrounding the electrode are recorded to determine the precise location of the electrode. Each brain region has a characteristic signal that is elicited both passively and actively through patient behaviour. The DBS team observes the signal visually and aurally to determine which region the electrode is in.
After recording, high-frequency (130–300 Hz) current is passed through low impedance electrodes on the microelectrode shank at increasing intensities. The patient is conscious during this procedure to monitor the impact of the stimulation. The patient may be asked to perform certain cognitive and physical tasks to identify the side effects of microstimulation; side effects indicate which structures are being stimulated which helps to further localize the electrode position in the brain [7, 13].
Current MER and microstimulation mapping techniques and devices require serial examination of each site. Mapping duration is extended when the initial microelectrode pass does not provide enough information to localize the DBS target and additional passes are needed to map more of the subcortical volume. This can be a very time-consuming process that increases the risk of infection, hemorrhages and general discomfort for the patient[79]. New electrode designs that may help to speed this process up are described in part VI. Post-operative imaging can be obtained to confirm placement of the DBS leads (Fig. 4).
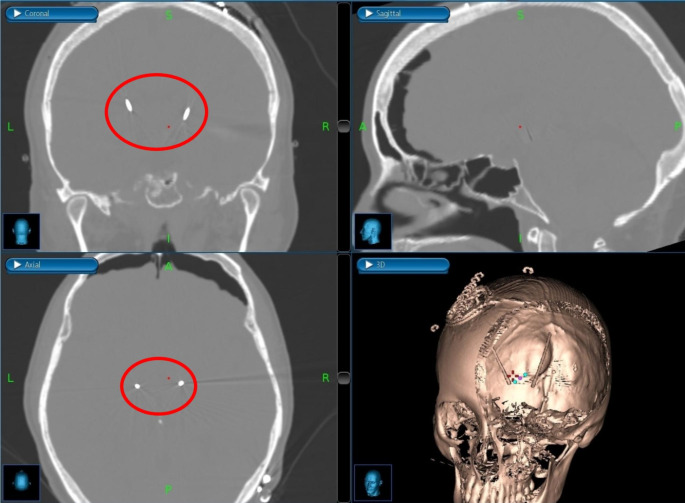
Fig. 4.
Post-operative, bone-window CT head images showing coronal (top left), sagittal (top right), and axial (bottom left) positioning of bilateral, STN DBS leads
DBS Risks
While DBS surgery has a great number of benefits, as with any other complex surgery it has its associated risks. These risks and complications have been categorized into three groups (1) surgical complications (2) hardware-related complications and (3) stimulation-based complications[80]. Some of the surgical complications associated with the surgery are common to most invasive procedures include hemorrhage and infection. Other complications can occur such seizures, cognitive impairment, behavioral and mood changes, and speech problems, which can be attributed to surgical complications or undesired stimulation[80].
Associated hardware complications include skin erosions, lead migration, lead fracture/ failure, IPG malfunction, extension wire damage and failure of other electronic components[81]. Due to the highly complex nature of the surgery, care must be taken in the placement of electrodes, patient selection, IPG programming, postoperative stimulation, and drug-based treatment[82]. Errors in these areas can result in postoperative complications. While there are potential complications associated with DBS, they are less severe and less common than those presented by other surgical therapies for PD. A list of possible complications of DBS surgery and their prevalence is seen in Table I.
Table I.
Post-surgical complications and risk associated
According to one study almost three times as many ‘severe adverse effects’ were reported with DBS compared to treatment with medical therapy[84]. These adverse events include falls, gait disturbance, dyskinesia, motor dysfunction, balance disorder, depression, and dystonia[84]. Additionally, studies have reported increased mood swings, suicide attempts, depression and hypomania[84, 85]. Speech centers in the brain may also be unintentionally affected leading to speech impediments or improvements in any speech disorders present prior to surgery[84], [85]. Of these complications, hemorrhage risk increases with the number of microelectrode passes, infection rate is related to the duration of surgery, and lead misplacement is related to the accuracy and interpretability of the physiological recording. Thus considerable effort contributes to ongoing innovation in microelectrode design to mitigate these risks.
Microelectrodes For Brain Mapping
Current microelectrode design factors
Current microelectrode recording techniques determine microelectrode design factors. The recording electrode must be able to discriminate activity from a small volume of tissue and this requires high impedance (typically 400–2000 kOhm). The stimulation electrode must have relatively low impedance (< 3 kOhm, lower is better) to minimize the voltage required to drive enough current to evoke a response. A common solution to these combined constraints is to use separate recording and stimulation electrodes; the recording electrode is positioned at the microelectrode tip located on an inner core, and the stimulating electrode is positioned 10-mm proximal on an outer sheath. The recording electrode can be retracted within the outer sheath to protect it during insertion through the drive, and so that the recording electrode is not driven deeper than necessary while placing the stimulating electrode.
Another design factor is that the electrode must be rigid to maintain its intended trajectory as it is driven through the tissue. This can be achieved by using a rigid electrode of a large diameter or using a small diameter electrode in a rigid guide tube that terminates a minimum distance above the target. Guide tubes typically terminate 10–15 mm above the target[86]. Recording sample locations span ~ 10 mm above the target to a few mm past the target. Steps through the trajectory can be automated or manually controlled.
Multiple MER leads may be used in an array inserted through guide tubes. Electrodes in the array configuration are typically spaced 2 mm apart and traverse parallel trajectories. More electrodes sample more cortical volume simultaneously to help increase information from each mapping pass to decrease surgical time and improve outcomes, but unnecessary electrodes also increase the risk of tissue damage and potential for hemorrhage[87]. Each surgical team must decide on a compromise between information and risk when deciding how many electrodes to use.
Microelectrodes meeting these specifications have medical licensing approval in all major markets and are available from several different manufacturers.
Future microelectrode designs
There are several different electrode designs used in animal models that enable recording from many more contacts simultaneously with a similar profile to the currently approved electrodes. An example of an electrode with multiple contacts is the Linear Microelectrode Array (LMA) electrode. The LMAs are fabricated using Polyimide Insulated Platinum/Iridium wire that is threaded through Polyimide tubing (another option is a stainless-steel tube) [88]. The tip is 1 mm in length and made of Tungsten, the first contact is placed 0.5 mm from the bottom of the tip while the rest of the contacts are placed subsequently with a spacing of 0.75 micrometer to 1 mm along the length of the electrode[88]. The polyimide tube can be as long as 25 mm allowing 4–24 contacts per shank with an outer diameter of 198 ~ 560 micrometers[88]. There has been much excitement about a new silicon probe known as Neuropixels for recording from a very large number of contact sites in animals[89]. Each probe has 384 recording channels that can access 960 complementary metal–oxide–semiconductor (CMOS), processing-compatible, low-impedance TiN6 sites that tile a single 10-mm long, 70 × 20-µm cross-section shank[89]. These probes enable the placement of 960 sites on a single, 10-mm long, non-tapered shank with 70 × 20-µ m cross-section which is the main advantage over the existing technology[89]. The 12 × 12-µ m sites are arranged in a checkerboard pattern with 4 columns and 25-µ m center-to-center nearest neighbor spacing. Additional features of this probe include low noise, minimal movement related artifacts, and efficient data transmission due to the use of low noise analog amplifiers, multiplexers and digitizers. The use of TiN6 for recording sites addresses stability and fabrication concerns[59, 89]. While the current form factor is not suitable for use in DBS MER, this combination of high-performance electrode technology and scalable chip fabrication methods should be translatable to novel designs appropriate for longer electrodes.
Current Application and Future Outlook
Current Applications
DBS therapy has shown promise in not only alleviating symptoms of PD but also for treatment of other diseases both motor and non-motor.
Studies have shown DBS effectiveness for the following symptoms when targeting particular brain regions:
Essential tremor: DBS targeting the thalamus can resolve hand tremors, however, may increase head and voice tremors[90].
Parkinson’s Disease: DBS targeting the subthalamic nucleus improves slowness, tremors, and rigidity in 70% of patients[90].
Dystonias: DBS targeting the Globus Pallidus has been reported to be very effective[91].
Treatment Resistant Depression
DBS is used as a last resort to treat those with chronic depression who are not responsive to traditional pharmacologic, electroconvulsive, and psychotherapy. High-frequency stimulation of the subgenual cingulate gyrus has demonstrated improvement of depression in treatment resistant depression patients. Testing with a larger sample size needs to be conducted in order to affirm the benefits of DBS for TRD[91].
Alzheimer’s Disease
Research is being conducted in targeted stimulation of the fornix, a part of the hippocampus, and the nucleus basalis of Meynert, in efforts to stimulate memory circuits in the brain studied the impact of DBS for Alzheimer’s and demonstrated improvements in and slowing of cognitive decline in patients receiving continuous stimulation for 12 months[92]. The current state of research is preliminary but shows promise for Alzheimer’s treatment[92, 93].
Tourette Syndrome
Tourette Syndrome is a neurological childhood disease that results in abnormal movements and vocal changes known as tics. Although Bloch et al. discourage the use of DBS, studies have shown improvement in motor and neuropsychiatric symptoms[94]. As with Alzheimer’s disease, DBS treatment for Tourette Syndrome is in its early stages and requires further exploration[95].
Neuropathic Pain
DBS of the periventricular and periaqueductal gray matter, sensory thalamus and internal capsule have proven to alleviate neuropathic pain and provide long-term relief. The mechanism of action is believed to be through the release of opioids in the region of stimulation resulting in pain relief that may not be achieved by pharmacological intervention[96, 97].
Other general non-motor symptoms
Studies have shown that DBS can also impact a variety of non-motor symptoms due to either direct stimulation, or as a byproduct of undesired stimulation[98]. Disorders of Consciousness have been shown to be improved through deep brain stimulation as well as resolution of vegetative state[98]. Some of these symptoms include behavioral, cognitive, and autonomic dysfunction, resulting in sleep disturbances, blood pressure issues, bowel changes, among others[4, 5].
Anticipated technological developments in DBS
There are several different DBS technology advances being developed to improve target localization and to improve electrical current delivery.
Precise DBS electrode localization depends on intraoperative mapping. The utility and efficiency of MER mapping may be improved and simultaneously reduce the burden on the expert through assisted or automated discrimination of DBS targets[99, 100]. Trained machine-learning models can be used to infer target identities from the raw MER data or extracted features[99]. Automatic identification of anatomical identity based on neurophysiology will have synergistic gains with multichannel axial electrode arrays because identification can be performed in real-time at multiple sites in parallel[100–102]. This will enable kinesthetic mapping of many different sites as well as leveraging effective connectivity-based signal features that require many simultaneously acquired signals.
Two avenues of innovation are being pursued to improve electrical current delivery. The first is to modify the geometry of the electrode contacts to direct or shape the electric fields generated by the DBS electrodes. Traditional systems use leads that stimulate a spherical region of the brain and this may stimulate a larger volume than required[101]. This leads to undesired stimulation and leakage currents which can cause unwanted side effects. Directional DBS can direct the current in one of several different directions, reducing the volume of undesired stimulation[101, 102]. Abott’s St. Jude Medical Infinity DBS system and Boston Scientific’s Vercise Directional DBS Systems have the capacity to perform directional. Both systems contain segmented electrodes allowing for targeted stimulation with the option of customized shape, position, length and direction of stimulation allowing for more patient specific stimulation and treatment.
The second innovation is to adjust stimulation parameters based on the ongoing needs of the patient as determined by behavioural or neurophysiological sensors. This is referred to as closed-loop DBS or adaptive DBS[103, 104]. In adaptive DBS, stimulation is turned on when the neurophysiological or behavioural state is determined to require stimulation to reduce symptom presentation and stimulation is turned off when the state suggests that symptoms are no longer of concern. By reducing stimulation delivery when it is not needed, side effects are reduced and the IPG battery can last longer[103, 105, 106].
Adaptive DBS requires additional processing to integrate sensor information and make decisions about stimulation parameters, as well as additional components to communicate with sensors. As of this writing, there are no IPGs capable of supporting adaptive DBS that are approved for human use. There are, however, such devices undergoing investigational testing. The Percept PC from Medtronic, which has been FDA and EU approved, has improved recording capability, the future potential of closed-loop DBS and initial capacity to assess local field potential (LFP) history. The stimulator will be used in conjunction with BrainSense technology which allows for tracking of patient-specific signals and therefore the potential for future customization of treatment. This development has opened the door to more sophisticated closed-loop DBS and presents the potential for therapeutic advancements.
Future outlook
Currently commercially available and clinically used MER electrodes contain some variation of one stimulation site and one recording site. Multiple recording and stimulation sites distributed along the length of the shaft of one shank will permit simultaneous recording and stimulation for brain mapping but no such electrodes are available for human use. The AlphaProbe by Alpha Omega is designed with radially arranged microcontact sites that can record at one depth but do not allow for simultaneous multi-level recording. In order to optimize DBS treatment and mitigate the risks associated with extended MER localization, multiple, longitudinally arranged recording and stimulation sites must be developed to allow for a single pass to delineate the thickness of a target nucleus within the trajectory. This functionality could minimize the need for incremental advancement of the DBS lead thereby decreasing the length of the operation as well as potentially reduce the risk of hemorrhage with multiple passes. The presence of longitudinally arranged contact sites will allow for recording at multiple sites simultaneously which can be processed by machine learning or a DBS expert for feature extraction and determination of the boundaries for the selected nucleus. Our group’s goal is to select appropriate materials for stimulation and recording sites as well as optimize the electrode design to have multiple stimulation-and-recording sites longitudinally arranged on one shank to allow for parallel recording and stimulation of different sites. The aforementioned design will reduce the time spent intraoperatively determining the ideal location for permanent electrode placement. This in turn will decrease the risks of infection as well as lower costs of the procedure.
Conclusions
Parkinson’s Disease is not an easily diagnosed condition due to symptoms that can be attributed to various other neurological and motor diseases. Similarly, treatment of PD also presents various challenges. Medication, while lessening the severity of the symptoms, does not have a long-term impact as the disease continues to progress rendering the medication ineffective. Furthermore, a subset of the affected population may be unresponsive to medication - requiring novel therapy development, in the form of DBS. DBS therapy is gaining popularity for PD treatment as well as other motor-neuron related diseases. Using electrodes and a pulse generator to artificially stimulate neural networks in the brain has shown promise in treating the cardinal motor symptoms of PD. A variety of electrode designs and hardware are being explored to optimize the results obtained from DBS, some of which have been described in this review. Prior to performing DBS each patient must be assessed to determine their anatomy, baseline symptoms, and memory to ensure that the therapy is personalized and to compare pre- and post-surgical conditions, Optimization of the system is being explored in the form of smart DBS, to have a cloud-based database to store data obtained from recording microelectrodes, as well as closed-loop DBS to have automated adjustment of stimulation parameters. Optimization in the MER mapping process is also being conducted. Of particular interest is the investment in longitudinally designed DBS recording electrodes for multi-level feature extraction and nucleus identification. Research on these enhancements is being conducted in the hopes of creating a more efficient and patient friendly system. While DBS is a promising approach, it does have its limitations and further research and development is required to improve current technology and implementation of DBS therapy.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Human and animals rights
This article does contain studies with human participants performed by the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chadwick Boulay, Email: cboulay@uottawa.ca.
Adam Sachs, Email: asachs@toh.ca.
Jeongwon Park, Email: jepark@unr.edu.
References
- 1.“Michael J.Fox foundation for Parkinson’s research,” Michael J.Fox foundation. [Online]. Available: https://www.michaeljfox.org/understanding-parkinsons/i-have-got-what.php.
- 2.Kogan M, McGuire M, Riley J, “Deep Brain Stimulation for Parkinson Disease.,” Neurosurg. Clin. N. Am., vol. 30, no. 2, pp. 137–146, Apr. 2019. [DOI] [PubMed]
- 3.Triarhou LC, “Dopamine and Parkinson’s Disease,” Madame Curie Bioscience Database, 2013. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK6271/.
- 4.Massano J, Bhatia KP. Clinical approach to Parkinson’s disease: features, diagnosis, and principles of management. Cold Spring Harb Perspect Med. 2012;2(6):a008870–0. doi: 10.1101/cshperspect.a008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes MT, “Parkinson’s Disease and Parkinsonism,” Am. J. Med., vol. 132, no. 7, pp. 802–807, Jul. 2019. [DOI] [PubMed]
- 6.Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–45. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 7.Masato A, Plotegher N, Boassa D, Bubacco L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol Neurodegener. 2019;14(1):35. doi: 10.1186/s13024-019-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakabayashi K, Tanji K, Mori F, Takahashi H, “The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of α-synuclein aggregates,” Neuropathology, vol. 27, no. 5, pp. 494–506, Oct. 2007. [DOI] [PubMed]
- 9.Brooks DJ, “Imaging Approaches to Parkinson Disease,” J. Nucl. Med., vol. 51, no. 4, pp. 596–609, Apr. 2010. [DOI] [PubMed]
- 10.Gazewood JD, Richards DR, Clebak K, “Parkinson disease: an update.,” Am. Fam. Physician, vol. 87, no. 4, pp. 267–273, Feb. 2013. [PubMed]
- 11.NEWMAN EJ, “PREVALENCE AND DIAGNOSIS OF PARKINSON’S. DISEASE: A COMMUNITY STUDY,” University of Glasgow.
- 12.Jenner P, “Treatment of the later stages of Parkinson’s disease - pharmacological approaches now and in the future,” Transl. Neurodegener., vol. 4, p. 3, Feb. 2015. [DOI] [PMC free article] [PubMed]
- 13.Schlesinger I, Sinai A, Zaaroor M, “MRI-Guided Focused Ultrasound in Parkinson’s Disease: A Review,” Parkinsons. Dis., vol. 2017, p. 8124624, 2017. [DOI] [PMC free article] [PubMed]
- 14.Lee DJ, Lozano AM. The Future of Surgical Treatments for Parkinson’s Disease. J Parkinsons Dis. 2018;8(s1):S79–83. doi: 10.3233/JPD-181467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Rojas R, et al., “Subthalamotomy for Parkinson’s disease: clinical outcome and topography of lesions.,” J. Neurol. Neurosurg. Psychiatry, vol. 89, no. 6, pp. 572–578, Jun. 2018. [DOI] [PubMed]
- 16.Spindola B, Leite MA, Orsini M, Fonoff E, Landeiro JA, Pessoa BL, “Ablative surgery for Parkinson’s disease: Is there still a role for pallidotomy in the deep brain stimulation era?,” Clin. Neurol. Neurosurg., vol. 158, pp. 33–39, Jul. 2017. [DOI] [PubMed]
- 17.Politis M, Lindvall O. Clinical application of stem cell therapy in Parkinson’s disease. BMC Med. 2012;10(1):1. doi: 10.1186/1741-7015-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly JJ, Wolpaw JR, “Brain-computer interfaces in neurological rehabilitation.,” Lancet. Neurol., vol. 7, no. 11, pp. 1032–1043, Nov. 2008. [DOI] [PubMed]
- 19.Tinkhauser G, Pogosyan A, Tan H, Herz DM, Kuhn AA, Brown P, “Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication.,” Brain, vol. 140, no. 11, pp. 2968–2981, Nov. 2017. [DOI] [PMC free article] [PubMed]
- 20.Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B, “Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys.,” Eur. J. Neurosci., vol. 5, no. 4, pp. 382–389, Apr. 1993. [DOI] [PubMed]
- 21.Khanna P, et al., “Neurofeedback Control in Parkinsonian Patients Using Electrocorticography Signals Accessed Wirelessly With a Chronic, Fully Implanted Device.,” IEEE Trans. Neural Syst. Rehabil. Eng., vol. 25, no. 10, pp. 1715–1724, Oct. 2017. [DOI] [PMC free article] [PubMed]
- 22.Boulay CB, Sachs AJ. In: “Brain-Computer Interfaces for Communication and Rehabilitation Using Intracortical Neuronal Activity from the Prefrontal Cortex and Basal Ganglia in Humans BT - Brain-Computer Interface Research: A State-of-the-Art Summary 4. Guger C, Müller-Putz G, Allison B, editors. Cham: Springer International Publishing; 2015. pp. 19–27. [Google Scholar]
- 23.Deuschl G, et al., “A randomized trial of deep-brain stimulation for Parkinson’s disease.,” N. Engl. J. Med., vol. 355, no. 9, pp. 896–908, Aug. 2006. [DOI] [PubMed]
- 24.Hartwigsen G. The neurophysiology of language: Insights from non-invasive brain stimulation in the healthy human brain. Brain Lang. 2015;148:81–94. doi: 10.1016/j.bandl.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Hogg E, Wertheimer J, Graner S, Tagliati M. Deep Brain Stimulation and Nonmotor Symptoms. ” Int Rev Neurobiol. 2017;134:1045–89. doi: 10.1016/bs.irn.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Weaver FM, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. ” JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams A, et al., “Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial,” Lancet. Neurol., vol. 9, no. 6, pp. 581–591, Jun. 2010. [DOI] [PMC free article] [PubMed]
- 28.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. ” Lancet Neurol. 2009;8(1):67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 29.Vidailhet M, et al., “Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia.,” N. Engl. J. Med., vol. 352, no. 5, pp. 459–467, Feb. 2005. [DOI] [PubMed]
- 30.Wichmann T, Bergman H, DeLong MR, “Basal ganglia, movement disorders and deep brain stimulation: advances made through non-human primate research.,” J. Neural Transm., vol. 125, no. 3, pp. 419–430, Mar. 2018. [DOI] [PMC free article] [PubMed]
- 31.Khan MS, Deng H. Design and Prototyping a Smart Deep Brain Stimulator: An Autonomous Neuro-Sensing and Stimulating Electrode System. IEEE Intell Syst. 2017;32(5):14–27. doi: 10.1109/MIS.2017.3711648. [DOI] [Google Scholar]
- 32.Alomar S, King NKK, Tam J, Bari AA, Hamani C, Lozano AM. Speech and language adverse effects after thalamotomy and deep brain stimulation in patients with movement disorders: A meta-analysis. ” Mov Disord. 2017;32(1):53–63. doi: 10.1002/mds.26924. [DOI] [PubMed] [Google Scholar]
- 33.Fang JY, Tolleson C. The role of deep brain stimulation in Parkinson’s disease: an overview and update on new developments. ” Neuropsychiatr Dis Treat. 2017;13:723–32. doi: 10.2147/NDT.S113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amon A, Alesch F, “Systems for deep brain stimulation: review of technical features.,” J. Neural Transm., vol. 124, no. 9, pp. 1083–1091, Sep. 2017. [DOI] [PMC free article] [PubMed]
- 35.Gimsa J, Habel B, Schreiber U, van Rienen U, Strauss U, Gimsa U, “Choosing electrodes for deep brain stimulation experiments - electrochemical considerations,” J. Neurosci. Methods, vol. 142, pp. 251–265, Apr. 2005. [DOI] [PubMed]
- 36.Morello ANDC, Beber BC, Fagundes VC, Cielo CA, Rieder CRM, “Dysphonia and Dysarthria in People With Parkinson’s Disease After Subthalamic Nucleus Deep Brain Stimulation: Effect of Frequency Modulation.,” J. Voice, Nov. 2018. [DOI] [PubMed]
- 37.Rossi M, Bruno V, Arena J, Cammarota Á, Merello M. Challenges in PD Patient Management After DBS: A Pragmatic Review. Mov Disord Clin Pract. 2018;5(3):246–54. doi: 10.1002/mdc3.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cyron D. Mental Side Effects of Deep Brain Stimulation (DBS) for Movement Disorders: The Futility of Denial. Front Integr Neurosci. 2016;10:17. doi: 10.3389/fnint.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butson CR, Maks CB, McIntyre CC, “Sources and effects of electrode impedance during deep brain stimulation,” Clin. Neurophysiol., vol. 117, no. 2, pp. 447–454, Feb. 2006. [DOI] [PMC free article] [PubMed]
- 40.Medtronic. “Advanced Deep Brain Stimulation Therapy in Canada,” 2010. [Online]. Available: https://www.disabled-world.com/health/neurology/dbs.php.
- 41.Arnulf I, et al., “Effect of low and high frequency thalamic stimulation on sleep in patients with Parkinson’s disease and essential tremor.,” J. Sleep Res., vol. 9, no. 1, pp. 55–62, Mar. 2000. [DOI] [PubMed]
- 42.Wagle Shukla A, Zeilman P, Fernandez H, Bajwa JA, Mehanna R, “DBS Programming: An Evolving Approach for Patients with Parkinson’s Disease,” Park. Dis., vol. 2017, p. 8492619, 2017. [DOI] [PMC free article] [PubMed]
- 43.Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Bötzel K. Deep Brain Stimulation Programming for Movement Disorders: Current Concepts and Evidence-Based Strategies. Front Neurol. 2019;10:410. doi: 10.3389/fneur.2019.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallabhajosula S, et al., “Low-Frequency versus High-Frequency Subthalamic Nucleus Deep Brain Stimulation on Postural Control and Gait in Parkinson’s Disease: A Quantitative Study,” Brain Stimul., vol. 8, Oct. 2014. [DOI] [PubMed]
- 45.Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollak P. Long-term electrical inhibition of deep brain targets in movement disorders. ” Mov Disord. 1998;13(Suppl 3):119–25. doi: 10.1002/mds.870131321. [DOI] [PubMed] [Google Scholar]
- 46.HASSLER R, RIECHERT T, MUNDINGER F, UMBACH W, GANGLBERGER JA. “PHYSIOLOGICAL OBSERVATIONS IN STEREOTAXIC OPERATIONS IN EXTRAPYRAMIDAL MOTOR DISTURBANCES,” Brain, vol. 83, no. 2, pp. 337–350, Jun. 1960. [DOI] [PubMed]
- 47.Okun MS, Vitek JL, “Lesion therapy for Parkinson’s disease and other movement disorders: update and controversies.,” Mov. Disord., vol. 19, no. 4, pp. 375–389, Apr. 2004. [DOI] [PubMed]
- 48.Ramirez-Zamora A, Ostrem JL, “Globus Pallidus Interna or Subthalamic Nucleus Deep Brain Stimulation for Parkinson Disease: A Review.,” JAMA Neurol., vol. 75, no. 3, pp. 367–372, Mar. 2018. [DOI] [PubMed]
- 49.Adams JE, Hosobuchi Y, Fields HL, “Stimulation of internal capsule for relief of chronic pain.,” J. Neurosurg., vol. 41, no. 6, pp. 740–744, Dec. 1974. [DOI] [PubMed]
- 50.McInerney SJ, et al. Neurocognitive Predictors of Response in Treatment Resistant Depression to Subcallosal Cingulate Gyrus Deep Brain Stimulation. Front Hum Neurosci. 2017;11:74. doi: 10.3389/fnhum.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boccard SGJ, et al., “Targeting the affective component of chronic pain: a case series of deep brain stimulation of the anterior cingulate cortex.,” Neurosurgery, vol. 74, no. 6, pp. 627–628, Jun. 2014. [DOI] [PubMed]
- 52.Mallory GW, et al., “The nucleus accumbens as a potential target for central poststroke pain.,” Mayo Clin. Proc., vol. 87, no. 10, pp. 1025–1031, Oct. 2012. [DOI] [PMC free article] [PubMed]
- 53.Duncan GH, Bushnell MC, Marchand S, “Deep brain stimulation: a review of basic research and clinical studies.,” Pain, vol. 45, no. 1, pp. 49–59, Apr. 1991. [DOI] [PubMed]
- 54.Hécaen H, Talairach J, David M, Dell MB, COAGULATIONS LIMITEES DU THALAMUS DANS LES ALGIES DU SYNDROME THALAMIQUE-RESULTATS THERAPEUTIQUES ET PHYSIOLOGIQUES,” Rev Neurol (Paris) 1949;81(11):917–31. [Google Scholar]
- 55.Odekerken VJJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. ” Lancet Neurol. 2013;12(1):37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 56.Follett KA, et al., “Pallidal versus Subthalamic Deep-Brain Stimulation for Parkinson’s Disease,” N. Engl. J. Med., vol. 362, no. 22, pp. 2077–2091, Jun. 2010. [DOI] [PubMed]
- 57.Odekerken VJJ, et al. “GPi vs STN deep brain stimulation for Parkinson disease: Three-year follow-up. ” Neurol. 2016;86(8):755–61. doi: 10.1212/WNL.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 58.Xu F, Ma W, Huang Y, Qiu Z, Sun L. Deep brain stimulation of pallidal versus subthalamic for patients with Parkinson’s disease: a meta-analysis of controlled clinical trials. ” Neuropsychiatr Dis Treat. 2016;12:1435–44. doi: 10.2147/NDT.S105513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obeso JA, Olanow CW, Rodriguez-Oroz MC, Krack P, Kumar R, Lang AE. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. ” N Engl J Med. 2001;345(13):956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 60.Williams NR, Foote KD, Okun MS. “STN vs. GPi Deep Brain Stimulation: Translating the Rematch into Clinical Practice. ” Mov Disord Clin Pract. 2014;1(1):24–35. doi: 10.1002/mdc3.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plantinga BR, et al., “Individualized parcellation of the subthalamic nucleus in patients with Parkinson’s disease with 7T MRI.,” Neuroimage, vol. 168, pp. 403–411, Mar. 2018. [DOI] [PMC free article] [PubMed]
- 62.Chandran AS, Bynevelt M, Lind CRP. Magnetic resonance imaging of the subthalamic nucleus for deep brain stimulation. ” J Neurosurg. 2016;124(1):96–105. doi: 10.3171/2015.1.JNS142066. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Luo X-G, Gao C. “Utility of susceptibility-weighted imaging in Parkinson’s disease and atypical Parkinsonian disorders,” Transl. Neurodegener., vol. 5, p. 17, Oct. 2016. [DOI] [PMC free article] [PubMed]
- 64.Shamir RR, et al., “Microelectrode Recordings Validate the Clinical Visualization of Subthalamic-Nucleus Based on 7T Magnetic Resonance Imaging and Machine Learning for Deep Brain Stimulation Surgery.,” Neurosurgery, vol. 84, no. 3, pp. 749–757, Mar. 2019. [DOI] [PMC free article] [PubMed]
- 65.Nowacki A, et al., “Using MDEFT MRI Sequences to Target the GPi in DBS Surgery,” PLoS One, vol. 10, no. 9, p. e0137868, Sep. 2015. [DOI] [PMC free article] [PubMed]
- 66.Nolte IS, Gerigk L, Al-Zghloul M, Groden C, Kerl HU, “Visualization of the internal globus pallidus: sequence and orientation for deep brain stimulation using a standard installation protocol at 3.0 Tesla.,” Acta Neurochir. (Wien)., vol. 154, no. 3, pp. 481–494, Mar. 2012. [DOI] [PubMed]
- 67.Miyagi Y, Shima F, Sasaki T, “Brain shift: an error factor during implantation of deep brain stimulation electrodes.,” J. Neurosurg., vol. 107, no. 5, pp. 989–997, Nov. 2007. [DOI] [PubMed]
- 68.Tai C-H, et al., “Deep brain stimulation therapy for Parkinson’s disease using frameless stereotaxy: comparison with frame-based surgery.,” Eur. J. Neurol., vol. 17, no. 11, pp. 1377–1385, Nov. 2010. [DOI] [PubMed]
- 69.Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS, “Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy.,” J. Neurosurg., vol. 112, no. 3, pp. 479–490, Mar. 2010. [DOI] [PMC free article] [PubMed]
- 70.Bot M, Bour L, de Bie RM, Contarino MF, Schuurman PR, van den Munckhof P. Can We Rely on Susceptibility-Weighted Imaging for Subthalamic Nucleus Identification in Deep Brain Stimulation Surgery? Neurosurgery. 2016;78(3):353–60. doi: 10.1227/NEU.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 71.Burchiel KJ, McCartney S, Lee A, Raslan AM, “Accuracy of deep brain stimulation electrode placement using intraoperative computed tomography without microelectrode recording.,” J. Neurosurg., vol. 119, no. 2, pp. 301–306, Aug. 2013. [DOI] [PubMed]
- 72.Mirzadeh Z, Chapple K, Lambert M, Dhall R, Ponce FA, “Validation of CT-MRI fusion for intraoperative assessment of stereotactic accuracy in DBS surgery.,” Mov. Disord., vol. 29, no. 14, pp. 1788–1795, Dec. 2014. [DOI] [PubMed]
- 73.Priori A, Foffani G, Rossi L, Marceglia S, “Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations.,” Exp. Neurol., vol. 245, pp. 77–86, Jul. 2013. [DOI] [PubMed]
- 74.Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z, “Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease.,” Brain, vol. 133, no. Pt 7, pp. 2007–2021, Jul. 2010. [DOI] [PubMed]
- 75.van Wijk BCM, et al., “Subthalamic nucleus phase-amplitude coupling correlates with motor impairment in Parkinson’s disease.,” Clin. Neurophysiol., vol. 127, no. 4, pp. 2010–2019, Apr. 2016. [DOI] [PMC free article] [PubMed]
- 76.Horn A, Neumann W-J, Degen K, Schneider G-H, Kuhn AA, “Toward an electrophysiological ‘sweet spot’ for deep brain stimulation in the subthalamic nucleus.,” Hum. Brain Mapp., vol. 38, no. 7, pp. 3377–3390, Jul. 2017. [DOI] [PMC free article] [PubMed]
- 77.Little S, et al., “Adaptive deep brain stimulation in advanced Parkinson disease.,” Ann. Neurol., vol. 74, no. 3, pp. 449–457, Sep. 2013. [DOI] [PMC free article] [PubMed]
- 78.Fenoy AJ, Simpson RKJ, “Risks of common complications in deep brain stimulation surgery: management and avoidance.,” J. Neurosurg., vol. 120, no. 1, pp. 132–139, Jan. 2014. [DOI] [PubMed]
- 79.Bick SKB, et al., “Subthalamic Nucleus Deep Brain Stimulation Alters Prefrontal Correlates of Emotion Induction.,” Neuromodulation, vol. 20, no. 3, pp. 233–237, Apr. 2017. [DOI] [PMC free article] [PubMed]
- 80.Jitkritsadakul O, Bhidayasiri R, Kalia SK, Hodaie M, Lozano AM, Fasano A, “Systematic review of hardware-related complications of Deep Brain Stimulation: Do new indications pose an increased risk?,” Brain Stimul., vol. 10, no. 5, pp. 967–976, Sep. 2017. [DOI] [PubMed]
- 81.Kuhn AA, Volkmann J, “Innovations in deep brain stimulation methodology.,” Mov. Disord., vol. 32, no. 1, pp. 11–19, Jan. 2017. [DOI] [PubMed]
- 82.Clausen J, “Ethical brain stimulation - neuroethics of deep brain stimulation in research and clinical practice.,” Eur. J. Neurosci., vol. 32, no. 7, pp. 1152–1162, Oct. 2010. [DOI] [PubMed]
- 83.Grill WM, “Safety considerations for deep brain stimulation: review and analysis.,” Expert Rev. Med. Devices, vol. 2, no. 4, pp. 409–420, Jul. 2005. [DOI] [PubMed]
- 84.Højlund A, Petersen MV, Sridharan KS, Østergaard K. Worsening of Verbal Fluency After Deep Brain Stimulation in Parkinson’s Disease: A Focused Review. Comput Struct Biotechnol J. 2016;15:68–74. doi: 10.1016/j.csbj.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Picillo M, Vincos GB, Sammartino F, Lozano AM, Fasano A. Exploring risk factors for stuttering development in Parkinson disease after deep brain stimulation. ” Parkinsonism Relat Disord. 2017;38:85–9. doi: 10.1016/j.parkreldis.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 86.Alonso F, Vogel D, Johansson J, Wårdell K, Hemm S. “Electric Field Comparison between Microelectrode Recording and Deep Brain Stimulation Systems-A Simulation Study,” Brain Sci., vol. 8, no. 2, p. 28, Feb. 2018. [DOI] [PMC free article] [PubMed]
- 87.Ben-Haim S, Asaad WF, Gale JT, Eskandar EN, “Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design.,” Neurosurgery, vol. 64, no. 4, pp. 753–754, Apr. 2009. [DOI] [PubMed]
- 88.MicroProbs. “LINEAR MICROELECTRODE ARRAY.” [Online]. Available: https://www.microprobes.com/products/multichannel-arrays/lma.
- 89.Jun JJ, et al., “Fully integrated silicon probes for high-density recording of neural activity,” Nature, vol. 551, no. 7679, pp. 232–236, Nov. 2017. [DOI] [PMC free article] [PubMed]
- 90.Rosa M, et al. Risk of Infection After Local Field Potential Recording from Externalized Deep Brain Stimulation Leads in Parkinson’s Disease. ” World Neurosurg. 2017;97:64–9. doi: 10.1016/j.wneu.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 91.Mayberg HS, et al., “Deep brain stimulation for treatment-resistant depression.,” Neuron, vol. 45, no. 5, pp. 651–660, Mar. 2005. [DOI] [PubMed]
- 92.Laxton AW, Lozano AM, “Deep brain stimulation for the treatment of Alzheimer disease and dementias.,” World Neurosurg., vol. 80, no. 3–4, p. S28.e1-8, 2013. [DOI] [PubMed]
- 93.Laxton AW, et al., “A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease.,” Ann. Neurol., vol. 68, no. 4, pp. 521–534, Oct. 2010. [DOI] [PubMed]
- 94.Porta M, et al., “Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome.,” Neurology, vol. 73, no. 17, pp. 1375–1380, Oct. 2009. [DOI] [PubMed]
- 95.Bloch MH, Leckman JF, “Clinical course of Tourette syndrome,” J. Psychosom. Res., vol. 67, no. 6, pp. 497–501, Dec. 2009. [DOI] [PMC free article] [PubMed]
- 96.Bittar RG, et al., “Deep brain stimulation for pain relief: a meta-analysis.,” J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas., vol. 12, no. 5, pp. 515–519, Jun. 2005. [DOI] [PubMed]
- 97.Sims-Williams H, et al., “Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans.,” Neuroimage, vol. 146, pp. 833–842, Feb. 2017. [DOI] [PMC free article] [PubMed]
- 98.Vanhoecke J, Hariz M, “Deep brain stimulation for disorders of consciousness: Systematic review of cases and ethics.,” Brain Stimul., vol. 10, no. 6, pp. 1013–1023, Nov. 2017. [DOI] [PubMed]
- 99.Wan KR, Maszczyk T, See AAQ, Dauwels J, King NKK, “A review on microelectrode recording selection of features for machine learning in deep brain stimulation surgery for Parkinson’s disease.,” Clin. Neurophysiol., vol. 130, no. 1, pp. 145–154, Jan. 2019. [DOI] [PubMed]
- 100.Rajpurohit V, Danish SF, Hargreaves EL, Wong S. Optimizing computational feature sets for subthalamic nucleus localization in DBS surgery with feature selection. ” Clin Neurophysiol. 2015;126(5):975–82. doi: 10.1016/j.clinph.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 101.Steigerwald F, Muller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. ” Mov Disord. 2016;31(8):1240–3. doi: 10.1002/mds.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Contarino MF, et al., “Directional steering: A novel approach to deep brain stimulation.,” Neurology, vol. 83, no. 13, pp. 1163–1169, Sep. 2014. [DOI] [PubMed]
- 103.Morishita T, Inoue T. Need for multiple biomarkers to adjust parameters of closed-loop deep brain stimulation for Parkinson’s disease. Neural Regen Res. 2017;12(5):747–8. doi: 10.4103/1673-5374.206642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng X-J, Greenwald B, Rabitz H, Shea-Brown E, Kosut R, “Toward closed-loop optimization of deep brain stimulation for Parkinson’s disease: concepts and lessons from a computational model.,” J. Neural Eng., vol. 4, no. 2, pp. L14-21, Jun. 2007. [DOI] [PubMed]
- 105.Rosin B, et al., “Closed-loop deep brain stimulation is superior in ameliorating parkinsonism.,” Neuron, vol. 72, no. 2, pp. 370–384, Oct. 2011. [DOI] [PubMed]
- 106.Parastarfeizabadi M, Kouzani AZ, “Advances in closed-loop deep brain stimulation devices,” J. Neuroeng. Rehabil., vol. 14, no. 1, p. 79, Aug. 2017. [DOI] [PMC free article] [PubMed]