Abstract
Microfluidic methods act as an effective motile sperm separation technique used in infertility treatments. This work presents a standalone microfluidic device to separate motile sperm cells from non-motile sperm cells and debris. The separation mechanism is based on the centrifugal force acting on sperms and the ability of progressive motile sperms to swim upstream. The separation of motile sperm is carried out using a simple T-shaped microchannel which constitutes three reservoirs: one inlet and two outlets. Herein, one of the outlets is kept sealed. The sealed channel leads to a high-velocity gradient and a rheotaxis zone at the T junction resulting in the separation of motile sperms. Separated sperms are isolated in a sealed channel with a low Reynolds number flow so that sperms cannot have a net displacement, which ensures that the sperms do not re-enter the fluid flow. CFD simulation is conducted to study the flow fields inside the channel and experimental investigation is carried to observe the separation behaviour of sperms. The reported device provides 100% sperm separation efficiency and ensures the entrapment of sperm cells for a longer period. A modified colorimetric nitroblue tetrazolium test conducted on separated sperm cells shows that there is only a marginal increase in superoxide (O2−) production, proving normal sperm integrity. This device offers an effective and safe alternative to conventional sperm sorting methods.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13534-022-00229-9.
Keywords: Microfluidics, Sperm sorting, Rheotaxis, Infertility, ROS, Superoxide (O2−)
Introduction
Infertility is not just a health issue but also a social and economic one in today's society, as it has far-reaching implications for a person's entire life. Male infertility concerns arise due to lifestyle, environmental and genetic-related factors. Male infertility is often related to low sperm quality [1]. For successful fertilization, a sperm should have high motility, high sperm count and good morphology. Fertility treatments are provided to overcome these issues using Assisted Reproductive Technologies (ARTs). These technologies include intracytoplasmic sperm injection (ICSI) and in vitro fertilization (IVF) treatments which require sorting of high-quality sperms. Generally, conventional sperm sorting techniques comprise density gradient centrifugation and swim-up assay [2]. Even though they appear promising, their acceptability is limited because of high equipment cost, operator training, time-consuming operations and reduced sperm motility [3, 4]. The selected sperms using these techniques are not filtered through the natural selection mechanism that occurred in the female reproductive tract and are susceptible to DNA fragmentation during centrifugation. DNA fragmentation negatively impacts the fertilization process and nearly leads to a 50% possibility of failure in the fertilization process [5]. These conventional techniques for sperm sorting in ARTs have been used for the last four decades. The success rates of ARTs are observed to be minimal and only 26% of ART treatments result in successful childbirth [6]. Low success rates of ARTs are attributed to the failure of sperm sorting processes to provide a suitable sperm. This demands a drastic need for more efficient sperm sorting techniques. The conventional techniques do not follow the natural selection process, which impacts the success rates of ARTs and in some cases, has a long-standing effect on the produced child. These issues can be addressed by a device that selects the sperms based on the natural selection process and has minimum impact on sperm quality.
Microfluidic methods are emerging as potential and low-cost alternatives for effective sperm selection [7]. Microfluidic techniques use different geometry of microchannels at biologically relevant dimensions (5–300 µm) for sperm sorting based on viscosity gradient, temperature gradient and chemical attractants [6, 8]. These methods are classified based on separation mechanisms as active and passive methods. Active methods use acoustic waves, chemotaxis and thermotaxis for sperm sorting, while in passive methods, sperm sorting takes place using rheotaxis, fluid flow and geometry of channel [8]. Microfluidic sperm sorting techniques provide a more reliable option than conventional sperm sorting methods. Mane et al. [9] presented a study that provides insight into sperm behaviour in different viscous mediums using a microfluidic channel. The issue of DNA fragmentation in sperms is observed in conventional sperm sorting techniques and can be minimized or eliminated using microfluidic methods. Quinn et al. [10] conducted an experimental investigation to study the effect of different sperm selection methods on DNA fragmentation of the sperms. Nearly undetectable levels of DNA fragmentation were observed in motile sperms in their study. Along with DNA fragmentation, the production of ROS (Reactive Oxygen Species) also affects the fertilization process. Lamirande et al. [11] shows that a high concentration of ROS causes a loss in both motility and viability; they also found that ROS is responsible for hyperactivation capacitation or the acrosomal reaction. Atkinson et al. [12] observed that with an increase in oxidative stress, DNA fragmentation also increases, which affects sperm movement and decreases sperm oocyte fusion. Said et al. [13] reported an inverse correlation between the level of O2− and sperm morphology and motility. They also observed that ROS formation is strongly correlated with superoxide O2− production. Yildiz et al. [14] observed improved fertilization rates in patients with recurrent in vitro fertilization. It shows that microfluidic chips decrease DNA fragmentation compared to centrifugation methods.
Several researchers have worked with microfluidic devices to provide effective sperm sorting mechanisms. Cho et al. [15] presented a device to sort sperm based on motility even with small volume samples. This sorting method is based on the ability of motile sperms to swim across the laminar flow streamlines in a channel. Timothy et al. [16] used a K-type channel to separate motile sperms by providing them with an opportunity for inter-streamline crossing in a microfluidic device. Chen et al. [17] used a microfluidic device to separate sperms in a microchannel using differential flow fields. This device also provides a mechanism to quantify sperm quality using resistive pulse detection. Chung et al. [18] described a passively driven microfluidic sperm sorting device. In this device, non-motile and progressive motile sperms are separated using a laminar flow stream, whilst non-motile sperms are washed out in the outlet and progressive sperm swim into the sorting channel. Huang et al. [19] used an integrated microfluidic system to provide efficient motile sperm sorting with laminar flow and a novel design. They observed a maximum of 95.2% viability of the sperms. Rappa et al. [20] experimented using a differential fluid flow chip to study sperm rheotaxis at different physiological flow conditions. It is observed that sperms only show upstream swimming behaviour at specific flow rates and sperms showing rheotaxis behaviour are more motile and faster. Rheotaxis is generated because of a non-uniform flow field in a channel [21, 22]. These non-uniform flow fields can be easily achieved using different microchannel designs in microfluidics and is a potent technique to study rheotaxis [23]. Zaferani et al. [24] introduced a microfluidic device to passively isolate motile sperms using a corral structure in a microchannel. The flow rate provided was such that it introduced a rheotaxis zone at the opening of the corral and encourages sperms to move into the corral. Sarbandi et al. [25] have developed a biomimicry device to separate motile sperms using their upstream movement in micropocket geometries employing rheotaxis. Micropocket geometries mimic the female reproductive tract and were used to isolate the motile sperms swimming against the fluid flow direction.
As evident from the extant literature, there are various devices capable of separating motile sperm from the semen. However, the issue of cost-effectiveness and biological functionality is looked at by only a few studies. In addition, several microfluidic devices involve a complex design, fabrication steps and require a pump to maintain the fluid media at appropriate flow rates. Hence, there exists a considerable scope for improvement. An ideal sperm separation device should be quick, easy, cost-effective, filter all debris, and eliminate toxic substances [26]. The existing sperm separation devices do not fully meet the criteria for an ideal sperm separation device. In view of these shortcomings, we decided to pursue the current investigation and attempt to explore an efficient sperm separation device.
This work presents a microfluidic chip that isolates progressive motile sperms without affecting the biological functionalities. In this device, we sealed one of the T-shaped microchannel reservoirs to create a rheotaxis zone near the T junction and a sperm isolation zone in a sealed branch. The rheotaxis zone near the T junction allows progressive motile sperms to separate from the fluid flow and enter the sealed channel where the flow velocity is negligible. The number of sperms entering the isolation zone and their trajectories are monitored with time and flow velocity. In the isolation zone, sperms are trapped because of low Reynolds number flow allowing for effective entrapment of highly motile sperms. As no non-motile sperms are present in the separated sample, 100% separation can be claimed. Herein, separation efficiency can be defined as the ratio of the number of progressive motile sperms present in a separated sperm sample to the total number of sperms in the separated sperm sample. We also conducted a modified calorimetric nitroblue tetrazolium (NBT) test. The results indicate that separated sperms have good physiology. This separation process does not contribute toward ROS production and ensures normal structural integrity and functional activities of sperms. Compared to previously mentioned sperm sorting devices, the given device has distinct advantages, including requirement of low sample volume (40 µl), no external power requirements, ease of operation, and most importantly minimal ROS. All these features may contribute to higher fertilization rates at reduced costs.
Working principle of device
In this section, we provide a brief discussion on the working principle of the proposed microdevice.
Motile sperm separation in T shaped microchannel
The sperms have to travel through the female reproductive tract to meet the egg for a successful conception. This migration of sperm in the female reproductive system is guided by rheotaxis [23]. The natural selection of motile sperm occurs when they are strong enough to steer their way through the highly viscous cervical fluid. Figure 1a, b show the working principle of sperm separation in the T-shaped microchannel. This device consists of three reservoirs, one inlet and two outlets. One outlet is kept sealed as shown in Fig. 1c, d. A gravity-assisted diffusion flow is induced between the inlet and outlet reservoir by placing a 40 µl liquefied semen drop on the inlet reservoir. As the flow progress, the rheotaxis zone is formed near the T junction due to no flow condition in the sealed channel. This rheotaxis zone provides an opportunity for a motile sperm to swim away from a high-velocity flow (between inlet and outlet reservoir) into a very low-velocity flow in the sealed channel. The sperms entering this rheotaxis zone are more likely to enter the sealed channel. At the junction of the T-shaped microchannel, sperm cells are separated in two ways, as shown in Fig. 1a. The sperm cells travel from the outlet channel to the sealed channel due to upstream swimming of the motile sperms encouraged by the rheotaxis zone [27]. Fluid flow applies drag force (FD) and rotational torque on the sperm body; due to rotational torque, sperms rotate with angular velocity (Ω), and hence sperm cells are reoriented against the flow direction [23, 24, 28]. It is reported that human sperms show rheotaxis behaviour for a flow velocity range of 22 to 102 µm/s [28–30]. At a very high flow rate most of the sperms cannot withstand the drag force from the flow and move downstream. But few sperms attached themselves to the outlet channel wall and travel to the sealed channel. When flow velocity falls in the rheotaxis range, the sperms with strong propulsive force (FP) can overcome the drag force to swim against the flow and steer out of the flow streamlines to enter the sealed channel [25]. Figure 1b shows the upstream movement of sperms having higher propulsive force (FP) than drag force (FD) after their reorientation due to drag force. With the further decrease in flow rate below the rheotaxis range, the flow cannot apply a sufficient amount of drag force for the reorientation of sperms [23, 27, 31]. This leads to sperms travelling in a random direction, reducing the number of sperm cells entering the sealed channel. Further, sperm cells from the inlet channel can also enter the sealed channel by using centrifugal force. Fluid flow in a curved shape path induces centrifugal force perpendicular to flow direction [32]. When flow turns sharply towards the outlet at the T junction, the sperm cells experience a centrifugal force. This force is perpendicular to the flow direction and hence at the T junction, sperms will be pushed into the rheotaxis zone at the mouth of the sealed channel, where they can swim into the low-velocity region. The centrifugal force acting on the sperm body should be higher than the drag force to have a successful separation, as shown in Fig. 1b.
Fig. 1.
Showing sperm locomotion and its dynamics in the T-shaped microchannel with one outlet sealed. a Schematic of separation and isolation of progressive motile sperms. Debris and dead sperm washed into the outlet reservoir while progressive motile sperms from the inlet and outlet channel swim into the sealed channel. b Simplistic view of forces applied on the motile sperm cells during separation, sperm cell in a flow experience drag (FD) and rotational torque. Sperm overcoming the drag with their propulsive force can travel against the flow. Few sperm cells enter into the rheotaxis zone due to centrifugal force (FC). c Three-dimensional representation of T-shaped microfluidic device with inlet, outlet and sealed reservoir. The channel is primed with PBS and then a reservoir is sealed using adhesive tape. A drop of liquified sperm sample placed on the inlet reservoir, inducing a gravity-assisted diffusion flow. d Photograph of the PDMS-based microchannel
Sperm entrapment in a sealed microchannel
The flow velocity in a sealed channel will be negligible. Hence, the viscous forces will dominate the sperm behaviour in the sealed channel and the flow velocity will further decrease as the distance from junction increases and flow at a low Reynolds number (< < 1) will be observed. This can provide an opportunity to isolate the sperm and study its behaviour at a low Reynolds number. Purcell et al. [33] have explained how microorganisms behave in a low Reynolds number. As there is almost no flow into the sealed microchannel, inertia will not play any role in sperm reorientation and therefore sperms randomly enter the sealed channel. Usually, higher flagellar movement will result in higher swimming velocity of the sperm, but at a low Reynolds number, the speed of flagellar movement will not affect the sperm in a Newtonian fluid environment, a swimming organism having time-symmetric motion cannot have net displacement [34–36]. The scallop theorem states that a swimming organism showing time-symmetric body movement cannot move in low Reynold number Newtonian fluid. The scallop theorem is valid for a microorganism having any reciprocal motion for displacement in a Newtonian fluid with no inertial force [37], which is the case with human sperm cells. This phenomenon can play a crucial role in avoiding re-entry of the motile sperms into high-velocity streamlines once they enter the sealed channel.
Materials and method
This section provides details about the microchannel fabrication, experimental setup and procedure employed. All experiments were performed in accordance with the guidelines and regulations approved by the Institute Human Ethical Committee, Birla Institute of Technology and Science-Pilani, K K Birla Goa Campus, India. The guidelines issued by the ethical committee are followed and responsible conduct of research is maintained during this study.
Microchannel fabrication
Figure 1c shows a simple T-shaped microchannel with three branches, reservoirs, and channels with width and height of 200 µm and 50 µm, respectively. Photolithography and soft lithography techniques are used to develop this microchannel. A prebaked negative photoresist (SU-8 2050) was dispensed on a silicon wafer and uniform thickness (50 µm) was achieved by rotating it at 3000 RPM. A mask aligner is placed over it before exposing it to UV light which washes out the unexposed portion and the master mold is prepared. This mold is then used to produce PDMS (Polydimethylsiloxane) microchannels. In this mold, a 10:1 (w/w) mixture of PDMS and a curing agent is poured and kept in a hot air oven at 65 °C for 45 min. When curing is over PDMS channel is removed from the mold and reservoir shapes are punched at the end of each channel. Finally, this PDMS chip is bonded with a glass slide using a 6:1 (w/w) ratio of PDMS and curing agent and heating it at 80 °C for 1 h [38]. Figure 1d shows the PDMS chip bonded on a glass slide.
Experimental setup and procedure
The experimental setup consists of an inverted microscope (CKX53, Olympus) equipped with a digital camera (C11440-36U, ORCA-spark Hamamatsu) to observe and record the sperm behaviour in a T-shaped microchannel. The sperm behaviour and separation inside the PDMS microchannel are monitored and recorded using HCImage Live software. Sperm trajectories in the T-shaped microchannel are tracked using the Manual Tracking plugin of ImageJ software. Using ImageJ software, sperm motion characteristics like VCL and VSL are measured. VCL is the curvilinear velocity calculated by averaging the velocity at each point and VSL (straight-line velocity) is the time-average velocity calculated along the straight line between the initial and final point of the sperm head. The semen is collected from healthy, well-informed and consented human male subjects in a sterile container. After collection of semen, it is kept at 37 °C for 20 min in a hot air oven to liquefy. The semen collection procedure is conducted according to WHO norms [39] and ethical committee guidelines. A T-shaped PDMS channel is then used to create a velocity gradient and rheotaxis zone in the channel. This T-shaped channel is first primed with PBS solution, ensuring no air bubbles present in the microchannel. Once priming is complete, one arm of the T channel is sealed with adhesive tape and the channel is kept at room temperature for 15 min to achieve a steady state. A 40 µl drop of liquefied semen is placed on the inlet reservoir, which causes gravity-assisted diffusion flow from the inlet to the outlet reservoir. This flow driving mechanism is caused by the gravity and diffusion of sperm cells into the PBS-filled microchannel. Here, 40 µl semen drop on the inlet reservoir acts as a head that propagates the flow into the channel and when the flow propagates, the sperm cells in the drop start moving towards the outlet reservoir. This mechanism allowed a laminar flow from inlet to outlet reservoir for a more extended period (> 2 h). This approach eliminates the requirement for any external power source or equipment for the device's operation. This device provides a simple, portable and cost-effective method for sperm sorting.
To study the structural integrity and functional reliability of separated sperms, a modified colorimetric NBT test has been performed. The superoxide (O2−) production in separated sperm is compared with sperm from fresh semen, as superoxide is predominantly responsible for ROS formation [19]. The fresh semen sample and separated sperm were washed using PBS (Phosphate Buffer Saline) by centrifuging it at 800 g for 10 min [40]. The sperm were then resuspended in 100 µl of PBS with the same amount of NBT reagent. The mixture was then incubated at 37 °C for 45 min. This solution was centrifuged twice at 500 g for 10 min with PBS after incubation. Only a cell pellet containing formazan remained when the remaining NBT solution was extracted. To determine the amount of formazan, cells were first solubilized using 60 µl of KOH (Potassium Hydroxide) followed by 60 µl of DMSO (Dimethyl Sulphoxide) solution to dissolve the blue formazan with gentle shaking for 10 min [41]. A 60 µl of KOH and DMSO is used as a negative control. The dissolved NBT solution was then transferred to a microplate and the absorbance was measured using a microplate reader at different wavelengths (530–750 nm).
Result and discussion
In this section we discuss the flow fields generated within the microchannel, details of separation of progressive sperm cells, trajectories of sperm cells, condition of sperms in the sealed outlet, biological validation of the separated sperm cells and a table comparing the present device with few other devices reported in the literature.
Fluid flow fields in sealed T microchannel
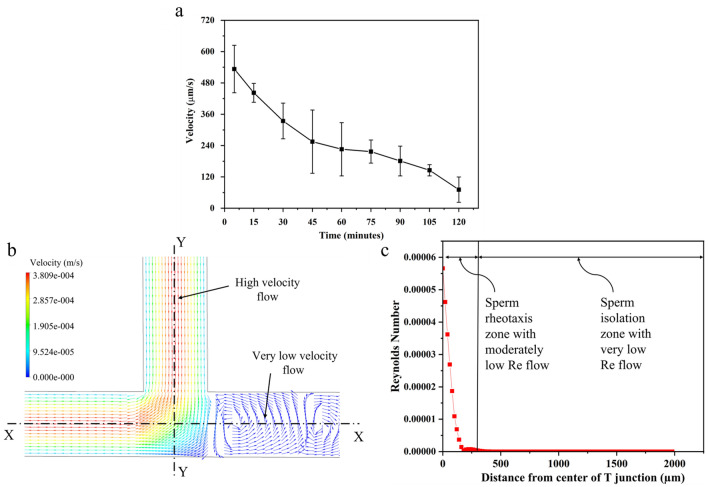
As the flow field of fluids (semen and PBS) has a primitive role in sperm behaviour in the channel, a steady flow computational fluid dynamics (CFD) simulation is conducted using commercial software (ANSYS Fluent). The standard laminar viscous flow model is used and velocity boundary condition is maintained at the inlet while the one outlet remains at atmospheric pressure and the other is sealed. The residual convergence criterion is set to 10–5. The one sealed end T-shaped microchannel geometry is used to study the velocity contours. As in the microchannel, gravity-assisted diffusion flow takes place, the velocity of the flow decreases continuously. To determine the range of fluid flow velocity, the velocity of dead sperm cells is calculated using ImageJ software (manual tracking plugin). As the dead sperm do not have any self-propulsive force, the velocity of the flow medium can be correlated with the dead sperm velocity. It is observed that the velocity of dead sperm cells in the inlet channel decreases from 533.25 µm/s at 5 min to 71 µm/s at 120 min, as shown in Fig. 2a. Hence, the flow velocity is maintained at 255 µm/s (average velocity of dead sperm cells at 45 min) to visualize approximate fluid flow fields in the channel.
Fig. 2.
Flow behaviour in one end sealed T-shaped microchannel. a Dead sperm velocity in PBS primed one end sealed T-shaped microchannel. The decrease in velocity of dead sperm cells indicates the reduction in drag force. b Normalized velocity vectors of flow field obtained from CFD simulation in sealed T-shaped microchannel. A drastic velocity gradient is observed between inlet–outlet flow and sealed channel. c Reynolds number of flow in a sealed channel calculated from the center of T junction. The Reynolds number of flow decreases as we move away from the center of the T junction, this area of decreasing Reynolds acts as a rheotaxis zone
Figure 2b shows the normalized velocity vector plot in the sealed T-shaped microchannel obtained through simulation. The vector plot of flow velocity remains unchanged towards the downstream in the open-end channel. In contrast, a very low velocity is observed in the sealed branch of the microchannel. The low-velocity region near the T junction forms a rheotaxis zone and allows the sperm to swim into the sealed branch of the channel. Figure 2c shows the calculated Reynolds number of the flow in the sealed channel from the center of the T junction.
Separation of progressive motile sperm cells
In this study, gravity-assisted diffusion flow takes place between the inlet and outlet reservoir and velocity decreases continuously with time. The sperm separation is monitored for 2 h. During this period sperm cells traveling from the inlet and outlet channel to the sealed channel for the duration of 5 min are counted at every 30 min.
It can be observed that, as time increases, the flow velocity and drag force on the sperms decreases. Here, initial high velocity inflicts an increased drag force on the sperm bodies; the sperm propulsive force is insufficient to swim against this high drag force [29]. Due to this, sperm swimming from the outlet channel to the sealed channel is minimal in the first 30 min (Fig. 3). Few of the sperms from the outlet channel can attach themselves to the channel wall and successfully swim against the flow. This wall adhesion of sperm cells is observed by several researchers [42–45]. Due to hydrodynamic force, the wall repulses the sperm tail; this tilts the sperm swimming axis and pushes the sperm head towards the wall [46]. The cyclic repulsion and head tilting result in wall adhesion of sperm cells, allowing them to flow against the high-velocity flow and swim out of it more comfortably. It is also seen that number of sperms swimming from the inlet to the sealed channel is nearly constant for the first 60 min. In the first 60 min, high flow velocity generates centrifugal force at the T junction as flow turns towards the outlet reservoir. The centrifugal force pushes the sperm cells into the rheotaxis zone, where progressive motile sperm can swim into the sealed channel. Non-motile sperms and other cells are pushed by the flow in the rheotaxis zone and get sucked back into the main flow as they cannot steer further away from the flow. Hence, almost all the non-motile sperms and other cells move along the flow and towards the outlet reservoir.
Fig. 3.
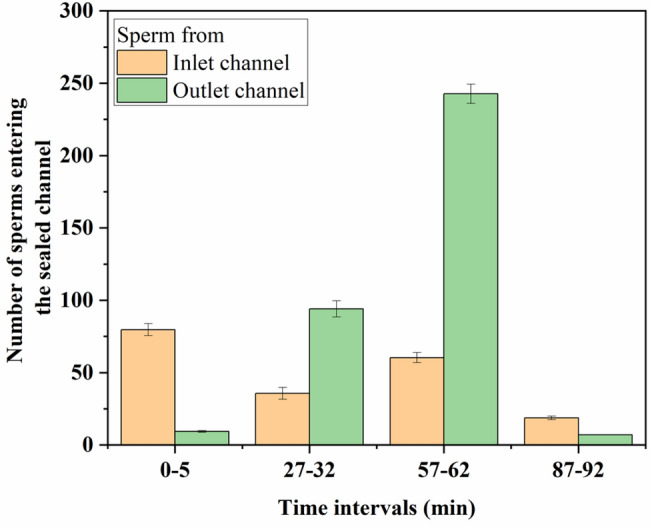
Sperms entering the sealed channel from the inlet and outlet channel. The entry of sperms in a sealed channel was monitored for 5 min at every 30 min interval for 90 min
When the drag force applied by the flow velocity decreases below the sperm propulsion force, a large number of motile sperms in the outlet channel can swim against the flow [31, 33]. The maximum number of sperm cells swim into the sealed channel by swimming against the flow near the wall in this velocity range. During this time, most of the motile sperms are separated from the flow. After 60 min, the flow velocity decreases further; most of the motile sperms are already separated from the flow and velocity is insufficient to generate the centrifugal force to throw sperms into the rheotaxis zone and hence low separation is observed after 60 min. Movie S1 shows the separation of the sperms in a sealed T channel. Even though some of the motile sperm cells cannot enter the sealed channel, all the trapped sperms in the sealed microchannel are motile. Only highly progressive motile sperm can swim against the flow and enter the sealed channel. Hence, the selected sample contains only progressive motile sperms, and 100% separation efficiency can be claimed in this device. Our device can separate around 3500 motile sperms in 90 min from raw semen sample containing 0.84 million sperm cells in total (motile and non- motile). For conventional IVF, approximately 3000–5000 sperms must be cultured with an egg, whereas ICSI uses single sperm for fertilization. Modern IVF techniques, such as the Walking Egg (tWE), require only 2000 sperms for treatment [47].
Swimming trajectories of sperms
The swimming trajectories of sperms help in exploring and understanding the interaction between sperms and flow dynamics. The sperm's response to the surrounding fluid and the forces exerted by the flow can be analyzed by studying the trajectories. The sperms behaviour and separation tendency changes with a change in velocity because of the variation in flow dynamics. Figure 4 shows different paths taken by sperm cells at different time instances, traced using ImageJ software (manual tracking plugin).
Fig. 4.
Experimental images of paths taken by sperm cells at different time instances during the separation process. Sperm trajectories from inlet and outlet channel to sealed channel at a, b 15 min, c, d 45 min and e, f 75 min from initiation of flow. The trajectories of sperm change with the change in flow velocity with time
It is observed that with an increase in time, the flow velocity decreases, and the representative trajectory taken by most sperm cells also changes. The variation in representative trajectories of sperm cells from the inlet channel (Fig. 4a, c, e) shows minimal change with time. When the velocity is minimum at 75 min (Fig. 4e), very few sperms travel from the inlet to the sealed channel. Most of the sperm trajectories from the inlet channel show that sperms separated from the inlet channel move into the sealed channel by attaching themselves to the walls of the sealed channel or swimming at the center axis of the sealed channel. The trajectories of sperm cells from the outlet channel can be seen in Fig. 4b, d, f. At 15 min, the flow velocity is high, which exerts a higher drag on the sperms. Sperms cannot migrate against this high drag force; hence, most sperm cells attach themselves to the wall of the outlet channel or swim close to the wall. In this region, sperms experienced lower drag force than at the centre of the outlet channel, which allowed the sperm to move upstream against the flow. When sperms experience low-velocity streamlines, i.e., after crossing the junction, they detach from the wall and enter the sealed channel by navigating through these low-velocity streamlines. At 45 min, flow velocity and drag force decrease, allowing sperm migration near the centre of the channel (Fig. 4d). At 75 min, due to very low flow velocity, sperms do not experience considerable drag resistance. The sperms can overcome this drag resistance and enter the sealed channel by swimming near both the walls of the outlet channel. These trajectories show the change in the upstream swimming pattern in response to the drag force applied by the flow.
Sperm separation velocity
Figure 5a shows the average velocities of sperms entering the sealed channel at different intervals of flow time. As the flow velocity decreases with time, the flow behaviour and forces applied on sperms also change.
Fig. 5.
a Straight line velocity (VSL) and curvilinear velocity (VCL) of sperms entering the sealed channel with time. No considerable change in VCL of sperm is observed b) Microscopic image of isolated progressive motile sperms in the sealed channel branch of the T-shaped microchannel (250 µm from the T-junction centre as shown in Fig. 2b)
This leads to different swimming velocities of the sperms with time. It can be seen in Fig. 5a that the VSL and VCL of sperms are nearly constant at 15 and 45 min, as the change in flow rate during this time did not affect the sperm velocity. Initially, the flow velocity is adequate to exert a rotational drag on the sperm to reorient it against the flow [29, 31] and hence comparable VSL and VCL are observed. But at 75 min, the low velocity of flow is insufficient to apply a rotational torque on the sperm for reorientation against the flow and the rheotaxis zone also disappears. Lack of specific orientation and rheotaxis response increases the random movement of sperm which reduces sperm VSL. It can also be observed that all the sperm velocities measured in this study are greater than 25 µm/s. It is to be noted that sperm cells with velocities higher than 25 µm/s are considered as progressive motile sperms [48]. Hence, all the separated sperms are progressively motile confirming the claim of 100% separation efficiency.
Sperm cells in sealed channel
The motile sperms move into the sealed channel and get accumulated there. This entrapment of sperms in the sealed channel is a result of the typical behaviour of microorganisms at low Reynold's number. There is no external torque and flow motion in the sealed channel, which leads to higher resistance to the sperm head. The tail experiences greater resistance due to higher viscous force exerted by the surrounding fluid. Even though high tangential force makes fluid penetration hard for the sperm head, the flagellar movement continues. As a result, sperms rotate around the head and realign themselves, moving the tail away from the T junction. The progressive motile sperms trapped in a sealed channel at a low Reynolds number can be seen in Fig. 5b (see Movie S2). It was observed that sperm travel into the sealed channel for some distance, reorient themselves and stop displacement even though flagellar beating is still on. Sperm motion in liquid results from cyclic and symmetric reciprocal flagellar movement. During this movement, sperm flagella regain their original position at a specific time in a cyclic manner; due to this, sperms cannot move in the surrounding fluid, as explained by the scallop theorem [36]. As progressive motile sperms are reoriented at low Reynolds number and cannot have net displacement, no sperm movement is observed except for flagellar beating. A large population accumulated in this channel shows similar behaviour. This entrapment continues for as long as the sealed channel has a negligible velocity that can be maintained for a longer period. Hence, a large number of motile sperms can be isolated.
Assessment of superoxide (O2−) production using the NBT test for biological validation
The working principle of NBT includes the incubation of targeted cells in the presence of tetrazolium salt. The cells allow NBT to enter into the cytoplasm where free radical, i.e., superoxide, converts NBT into blue formazan crystals [49]. The formazan was released using the correct solubilizing agent, and the absorbance of the purple-blue mixture was measured for quantification [50, 51]. The superoxide (O2−) production in the semen sample strongly correlated with the level of ROS in sperms. These levels of superoxide (O2−) affect sperm motility and morphology [13]. In the case of sperms cells, superoxide (O2−) is considered as a primary ROS producer [11]. So, to analyze the level of ROS production, a modified colorimetric test was performed. Figure 6a shows that superoxide (O2−) production for separated sperms and fresh semen sample initially increases with the wavelength and the peak absorbance is observed between 630 and 670 nm. Figure 6b compares the production of superoxide (O2−) in separated sperms and fresh semen at 630 nm. It was observed that superoxide (O2−) formation in separated sperms is only 4% higher compared to the fresh semen sample. The data obtained from the modified NBT test shows that the microfluidic device presented in this study does not contribute towards the significant ROS production. This provides a biological validation to the separation process suggested in this work.
Fig. 6.
Comparison of superoxide (O2−) production in separated sperm cells and sperm cells from fresh semen sample assessed using modified calorimetric NBT test a) O2− production at different wavelengths. b) O2− production at 630 nm for separated sperm, fresh semen sample and control
Comparison with literature
Table 1 shows a comparative study of sperm separation techniques undertaken by various researchers with the present work. Most of the systems reported in literature mention use a syringe pump or hydraulic pressure-driven laminar flow. The separation process is quite expensive when a syringe pump is used, and hydraulic pressure-driven laminar flow demands careful reservoir design and manufacturing to induce the flow.
Table 1.
Comparative study of microfluidic sperm separation techniques
| Researchers | Species | Separation mechanism | Geometry | Flow type | Separation efficiency (%) |
|---|---|---|---|---|---|
| Cho et al. [15] | Human | Inter-streamline swimming of motile sperms | K type | Hydraulic pressure driven laminar flow | 100 |
| Timothy et al. [16] | Human | Inter-streamline swimming of motile sperms | K type | Hydraulic pressure driven laminar flow | 96–98 |
| Sarbandi et al. [25] | Human | Rheotaxis | Straight channel with micropockets | Syringe pump driven laminar flow | 100 |
| Huang et al. [19] | Human | Inter-streamline swimming of motile sperms | Modified K type with multiple outlets | Gravity driven and syringe pump driven laminar flow | 75.06–95.2 |
| Zaferani et al. [24] | Human and Bovine | Rheotaxis | Straight channel with corral system | Syringe pump driven laminar flow | 100 |
| Hwang et al. [46] | Canine | Rheotaxis | Diffuser type | Hydraulic pressure driven laminar flow | – |
| Present work | Human | Rheotaxis | Sealed T type | Gravity assisted diffusion flow | 100 |
Our device used a simple gravity-assisted diffusion flow that does not require a syringe pump or precise reservoirs, significantly lowering the separation cost. A simple T-shaped microchannel is easy to fabricate and reduces the manufacturing complexities. Our device conclusively provides proof of the safety and biological validation of the sperm separation process. This is something that is rarely seen in reported sperm sorting technologies.
Conclusions
This work presented a simple T-shaped microfluidic device that uses gravity-assisted diffusion flow to provide a sperm sorting mechanism using low Reynolds number and rheotaxis phenomenon. The separation of sperms takes place from the inlet and outlet channel to the isolation zone. The separation mechanism involves drag force, centrifugal force and sperm propulsive force. As the centrifugal force decreases with the flow velocity, the number of motile sperms entering the isolation zone through the inlet channel reduces. Also, with the decrease in flow velocity, propulsive force dominates the drag force, resulting in an increased number of sperm traveling from the outlet channel to the isolation zone. The rheotaxis zone vanishes at a very low flow velocity, decreasing sperm migration from the outlet channel to the isolation zone. The flow velocity also influences the path taken by sperms to travel into the isolation zone. A large number of sperm cells move close to the outer wall of the outlet channel at high flow velocity, whereas when the flow velocity is low, sperms can enter into the isolation zone from both walls of the outlet channel. The device provides 100% separation efficiency as only motile sperm cells are observed in the sealed microchannel. The modified colorimetric NBT test shows that the fertilization capabilities of sperm cells remain unharmed during the separation process. This microdevice is simple to construct, cost-effective, easy to operate with minimal training, safe and capable of filtering debris and toxic substances.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by Science and Engineering Research Board (SERB), Department of Science & Technology (DST), Government of India (Start-up Research Grant SRG/2019/000285).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sciarra J. Infertility: an international health problem. Int J Gynaecol Obstet. 1994;46(2):155–163. doi: 10.1016/0020-7292(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 2.Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, Bianchi PG, Bianchi U. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod. 2000;15:1112–1116. doi: 10.1093/humrep/15.5.1112. [DOI] [PubMed] [Google Scholar]
- 3.Antinori M, Licata E, Dani G, Cerusico F, Versaci C, D'Angelo D, Antinori S. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod BioMed Online. 2008;16:835–841. doi: 10.1016/S1472-6483(10)60150-2. [DOI] [PubMed] [Google Scholar]
- 4.Ainsworth C, Nixon B, Aitken RJ. Development of a novel electrophoretic system for the isolation of human spermatozoa. Hum Reprod. 2005;20:2261–2270. doi: 10.1093/humrep/dei024. [DOI] [PubMed] [Google Scholar]
- 5.Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90. doi: 10.4103/1008-682X.182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosrati R, Graham PJ, Zhang B, Riordon J, Lagunov A, Hannam T, Escobedo C, Jarvi K, Sinton D. Microfluidics for sperm analysis and selection. Nat Rev Urol. 2017;14:707–730. doi: 10.1038/nrurol.2017.175. [DOI] [PubMed] [Google Scholar]
- 7.Muratori M, Tarozzi N, Carpentiero F, Danti S, Perrone FM, Cambi M, Casini A, Azzari C, Boni L, Maggi M, Borini A, Baldi E. Sperm selection with density gradient centrifugation and swim up: effect on DNA fragmentation in viable spermatozoa. Sci Rep. 2019;9:7492. doi: 10.1038/s41598-019-43981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh JBY, Marcos B. The study of spermatozoa and sorting in relation to human reproduction. Microfluid Nanofluid. 2015;18:755–774. doi: 10.1007/s10404-014-1520-x. [DOI] [Google Scholar]
- 9.Mane N, Mane S, Shah K, Banarjee A, Tripathi S. Effect of Newtonian and shear thinning medium on human sperm motion within a microchannel. In: 48th national conference on fluid mechanics and fluid power. 2021.
- 10.Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars M, Rosen M. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33(8):1388–1393. doi: 10.1093/humrep/dey239. [DOI] [PubMed] [Google Scholar]
- 11.De Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2:48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- 12.Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 13.Said TM, Agarwal A, Sharma RK, Mascha E, Sikka SC, Thomas AJ. Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil Steril. 2004;82(4):871–877. doi: 10.1016/j.fertnstert.2004.02.132. [DOI] [PubMed] [Google Scholar]
- 14.Yildiz K, Yuksel S. Use of microfluidic sperm extraction chips as an alternative method in patients with recurrent in vitro fertilization failure. J Assist Reprod Genet. 2019;36(7):1423–1429. doi: 10.1007/s10815-019-01480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S. Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem. 2003;75(7):1671–1675. doi: 10.1021/ac020579e. [DOI] [PubMed] [Google Scholar]
- 16.Timothy GS, Brenda C, Laura MK, Shuichi T, Smith DG. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod Biomed Online. 2003;7(1):75–81. doi: 10.1016/S1472-6483(10)61732-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen YA, Huang ZW, Tsai FS, Chen CY, Lin CM, Wo AM. Analysis of sperm concentration and motility in a microfluidic device. Microfluid Nanofluidics. 2011;10(1):59–67. doi: 10.1007/s10404-010-0646-8. [DOI] [Google Scholar]
- 18.Chung Y, Zhu X, Gu W, Smith GD, Takayama S. Microscale integrated sperm sorter. Microfluidic Tech. 2006;321:227–244. doi: 10.1385/1-59259-997-4:227. [DOI] [PubMed] [Google Scholar]
- 19.Huang HY, Fu HT, Tsing HY, Huang HJ, Li CJ, Yao DJ. Motile human sperm sorting by an integrated microfluidic system. J Nanomed Nanotechnol. 2014;5:3. doi: 10.4172/2157-7439.1000199. [DOI] [Google Scholar]
- 20.Rappa KL, Rodriguez HF, Hakkarainen GC, Anchan RM, Mutter GL, Asghar W. Sperm processing for advanced reproductive technologies: where are we today? Biotechnol Adv. 2016;34:578–587. doi: 10.1016/j.biotechadv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Ishimoto K, Gaffney EA. Fluid flow and sperm guidance: a simulation study of hydrodynamic sperm rheotaxis. J R Soc Interface. 2015;12:20150172. doi: 10.1098/rsif.2015.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bretherton FP, Rothschild L. Rheotaxis of spermatozoa. Proc R Soc B Biol Sci. 1961;153:490–502. [Google Scholar]
- 23.Kantsler V, Dunkel J, Blayney M, Goldstein RE. Rheotaxis facilitates upstream navigation of mammalian sperm cells. Elife. 2014;3:e02403. doi: 10.7554/eLife.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaferani M, Cheong SH, Abbaspourrad A. Rheotaxis-based separation of sperm with progressive motility using a microfluidic corral system. PNAS. 2018;115:8272–8277. doi: 10.1073/pnas.1800819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarbandi IR, Lesani A, Zhand MM, Nosrati R. Rheotaxis-based sperm separation using a biomimicry microfluidic device. Sci Rep. 2021;11:18327. doi: 10.1038/s41598-021-97602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003;1:108. doi: 10.1186/1477-7827-1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Liu J, Meriano J, Ru C, Xie S, Luo J, Sun Y. Human sperm rheotaxis: a passive physical process. Sci Rep. 2016;6:23553. doi: 10.1038/srep23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tung C, Ardon F, Roy A, Koch DL, Suarez SS, Wu M. Emergence of upstream swimming via a hydrodynamic transition. Phys Rev Lett. 2015;114:108102. doi: 10.1103/PhysRevLett.114.108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tung C, Hu L, Fiore AG, Ardon F, Hickman DG, Gilbe RO. Microgrooves and fluid flows provide preferential passageways for sperm over pathogen Tritrichomonas foetus. PNAS. 2015;112:5431–5436. doi: 10.1073/pnas.1500541112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miki K, Clapham DE. Article rheotaxis guides Mammalian sperm. Curr Biol. 2013;23:443–452. doi: 10.1016/j.cub.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bukatin A, Kukhtevich I, Stoop N, Dunkel J, Kantsler V. Bimodal rheotactic behavior refects fagellar beat asymmetry in human sperm cells. PNAS. 2015;112:15904–15909. doi: 10.1073/pnas.1515159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darvishi V, Darvishi S, Bavani MB, Navidbakhsh M, Asiaei S. Centrifugal isolation of SARS-CoV-2: numerical simulation for purification of hospitals' air. Biomech Model Mechanobiol. 2021;20:1809–1817. doi: 10.1007/s10237-021-01477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purcell EM. Life at low Reynolds number. Am J Phys. 1977;45:3. doi: 10.1119/1.10903. [DOI] [Google Scholar]
- 34.Netta C, Boyle JH. Swimming at low Reynolds number: a beginners guide to undulatory locomotion. Contemp Phys. 2010;51(2):103–123. doi: 10.1080/00107510903268381. [DOI] [Google Scholar]
- 35.Brennen C, Winet H. Fluid mechanics of propulsion by cilia and flagella. Annu Rev Fluid Mech. 1977;9:339–398. doi: 10.1146/annurev.fl.09.010177.002011. [DOI] [Google Scholar]
- 36.Lauga E. Life around the scallop theorem. Soft Matter. 2011;7(7):3060–3065. doi: 10.1039/C0SM00953A. [DOI] [Google Scholar]
- 37.Liu QY, Tang XY, Chen DD, Xu YQ, Tian F. Hydrodynamic study of sperm swimming near a wall based on the immersed boundary-lattice Boltzmann method. Eng Appl Comput Fluid Mech. 2020;14(1):853–870. [Google Scholar]
- 38.Tripathi S, Prabhakar A, Kumar N, Singh GS, Agrawal A. Blood plasma separation in elevated dimension T-shaped microchannel. Biomed Microdevice. 2013;15(3):415–425. doi: 10.1007/s10544-013-9738-z. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization: WHO laboratory manual for the examination and processing of human semen. 5th ed. 2010.
- 40.Chao HT, Ng HT, Kao SH, Wei YH, Hong CY. Human follicular fluid stimulates the motility of washed human sperm. Arch Androl. 1991;26(2):61–65. doi: 10.3109/01485019108987627. [DOI] [PubMed] [Google Scholar]
- 41.Tunc O, Thompson J, Tremellen K. Development of the NBT assay as a marker of sperm oxidative stress. Int J Androl. 2010;33:13–21. doi: 10.1111/j.1365-2605.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 42.Nosrati R, Driouchi A, Yip CM, Sinton D. Two dimensional slither swimming of sperm within a micrometre of a surface. Nat Commun. 2015;6:8703. doi: 10.1038/ncomms9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith DJ, Gaffney EA, Shum H, Gadelha H, Brown JK. Comment on the article by Elgeti J, Kaupp UB, Gampper G: hydrodynamics of sperm cells near surfaces. Biophys J. 2011;100:2318–2320. doi: 10.1016/j.bpj.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winet H, Bernstein GS, Head J. Observations on the response of human spermatozoa to gravity, boundaries and fluid shear. J Reprod Fertil. 1984;70:511–523. doi: 10.1530/jrf.0.0700511. [DOI] [PubMed] [Google Scholar]
- 45.Elgeti J, Kaupp UB, Gompper G. Hydrodynamics of sperm cells near surfaces. Biophys J. 2010;99(4):1018–1026. doi: 10.1016/j.bpj.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang B, Lee D, Hwang S, Baek J, Kim B. Rheotaxis based high-throughput motile sperm sorting device. Int J Precis Eng Manuf. 2019;20:1037–1045. doi: 10.1007/s12541-019-00144-7. [DOI] [Google Scholar]
- 47.Boshoff GM, Ombelet W, Huyser C. Oocyte insemination with the walking egg simplified IVF culture system—an investigation into reduced sperm numbers, sperm DNA fragmentation and reactive oxygen species formation. Facts Views Vis Obgyn. 2018;10(4):191–197. [PMC free article] [PubMed] [Google Scholar]
- 48.Vasan SS. Semen analysis and sperm function tests: how much to test? Indian J Urol. 2011;27(1):41–48. doi: 10.4103/0970-1591.78424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baehner RL, Boxer LA, Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976;48:309–313. doi: 10.1182/blood.V48.2.309.309. [DOI] [PubMed] [Google Scholar]
- 50.Rook GA, Steele J, Umar S, Dockrell HM. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985;82:161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- 51.Choi HS, Kim JW, Cha YN, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem. 2006;27:31–34. doi: 10.1080/15321810500403722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.