Abstract
Background: Computed tomography pulmonary angiography (CTPA) as the gold-standard examination in the detection of pulmonary embolism (PE) is contraindicated or unavailable in certain cases. The current study aimed to assess the accuracy of unenhanced CT in the diagnosis of PE.
Methods: This cohort study was conducted between October 2020 and March 2021 in Birjand, Iran, on 195 participants with clinical suspicion of PE examined with multidetector computed tomography (MDCT) scanning and CTPA. The patients were categorized into 2 groups based on the diagnosis PE in CTPA results. Imaging variables in unenhanced CT scans, including hyper/hypodense intraluminal signs, pulmonary trunk enlargements, peripheral wedge-shaped opacities, and pleural effusions, were independently reviewed by 2 radiologists and then compared between the groups.
Results: There were 82 men (42.1%) and 113 women (57.9%) at a mean age ± standard deviation of 56.00±0.24 years. Based on CTPA results, PE was diagnosed in 24.1% of the study population (47/195). However, only 20 cases (42.5%) were detected by MDCT: 17 cases (85.0%) with central PE and 3 cases (15.0%) with peripheral PE. Concerning the intraluminal clot density, 12 patients (60.0%) had hyperdense signs, 3 (15.0%) had hypodense signs, and 5 (25.0%) had mixed hyper/hypodense signs. There was a significant difference between central PE and peripheral PE detected by MDCT. Intraluminal signs had the highest specificity and sensitivity (98.6% and 42.5%, area under the curve =0.734).
Conclusion: Unenhanced MDCT has a remarkable performance in detecting PE, specifically central clots, and can, therefore, be considered an alternative modality when CTPA is not available or indicated.
Key Words: Pulmonary embolism, Multidetector computed tomography, Contrast media, Computed tomography angiography, Sensitivity and specificity
Introduction
Pulmonary embolism (PE) is the third most common cardiovascular pathology after coronary artery disease and stroke, with an incidence rate of 1 per 1000 individuals.1 PE causes 100 000 deaths annually in the United States and is responsible for 5% to 10% of cases of in-hospital mortality.2 Due to its wide range of nonspecific clinical symptoms, PE is always difficult to diagnose accurately, hence the clinical significance of its timely detection and management to substantially decrease the mortality rate from 30% to 2%–10%.3
Since there are no clinical or laboratory findings to confirm the diagnosis of PE, imaging has remained the only diagnostic option to rule out PE in recent decades. Computed tomography pulmonary angiography (CTPA) is the gold-standard examination performed in patients with clinical suspicion of PE. This method visualizes clots in pulmonary arteries as intraluminal filling defects surrounded by enhanced blood following contrast medium (CM) injection. However, this method has such limitations as allergies to iodinated medium, elevated serum creatinine, and the time wasted on renal function tests to be ready, which may worsen the prognosis.
Still, not only can unenhanced CT scanning be a useful alternative modality to detect PE in such patients but also it can reveal other parenchymal and chest wall involvements such as neoplasms, calcifications including chronic emboli and calcified hilar nodes, and the position of interest before CM injection. Since patients with nonspecific cardiopulmonary symptoms commonly undergo unenhanced CT scans, radiologists should take heed of signs suggesting PE, including hyperdense and hypodense intraluminal signs, peripheral wedge-shaped opacities, pulmonary artery trunk enlargements, and pleural effusions.
There are limited reports regarding the validity of non-contrast CT to detect PE, and they are mostly with a retrospective design and a small sample size or only focused on central and hyperattenuating signs.4-7 Thus, the current study aimed to assess accuracy in the diagnosis of PE between unenhanced CT and CTPA as the gold-standard examination.
Methods
The present study was carried out between October 2020 and March 2021. The study protocol was approved by the Institutional Review Board (IRB) of Birjand University of Medical Sciences (Ir.bums.REC.1399.323), and written informed consent was obtained from each patient. A total of 195 consecutive patients aged more than 18 years were enrolled in our study. All patients with clinical suspicion of PE were referred to the radiology department from the other departments of Birjand University of Medical Sciences to undergo CTPA. The exclusion criteria were any contraindications to radiation, including first-trimester pregnancy, elevated serum creatinine, and allergies to CM.
According to our protocol, when a patient is suspected of PE, we perform an unenhanced CT scan prior to CM injection and subsequent CTPA. Accordingly, the unenhanced CT scan and then CTPA were independently reviewed by 2 expert radiologists on a picture archiving and communications system (PACS) workstation. The radiologists were blinded to the patients’ clinical and laboratory history. If there was a disagreement between the 2 radiologists, the patient was excluded. Demographics and information related to imaging variables, including the presence or absence of PE, the location of PE, hyperdense and hypodense intraluminal signs, pulmonary trunk enlargements, peripheral wedge-shaped opacities, and pleural effusions, were collected using a standard questionnaire.
All the examinations were performed by using a Siemens SOMATOM Emotion16-slice multidetector computed tomography (MDCT) scanner (Forchheim, Germany) with 1.5 mm collimations, 4 mm reconstruction intervals, and soft tissue kernels. All the scans ranged from the pulmonary apices to the diaphragm with a craniocaudal direction. The region of interest was placed at the level of the bifurcation of the main pulmonary artery to achieve the maximum enhancement when scanning with the following settings: 120 kV, 185 mAs, 0.6-second rotation time, 1 mm thickness, and soft tissue kernels. All the cases received 100 mL of iodinated CM intravenously with an injection flow rate of 3 mL/s. Regarding the location of the clot, whether it was within the main and lobar pulmonary arteries or the segmental, subsegmental, and more peripheral arteries, PE was categorized into central and peripheral, respectively. As evidenced by CTPA, cases with central PE were classified as the case group and cases with no evidence of PE as the control group.
Categorical data were presented as numbers and percentages. The receiver operating characteristic (ROC) curve analysis was utilized to determine the area under the curve (AUC) and the diagnostic value of unenhanced MDCT in detecting PE as mentioned by DeLong et al by using MedCalc software, version 12.5 (Ostend, Belgium). Additionally, sensitivity, specificity, positive predictive value, and negative predictive value, with confidence intervals of 95%, were analyzed for each of the imaging values for PE diagnosis. IBM SPSS statistical software, version 22 (IBM Corp, New York, USA), was applied to analyze descriptive data. The χ2 test was performed to compare categorical variables between the 2 groups. A P value of less than 0.05 was considered statistically significant.
Results
The study population consisted of 195 patients who had clinical and laboratory findings suggestive of PE. There were 82 men (42.1%) and 113 women (57.9%). The mean age ± standard deviation (SD) of all the cases was 56.00±15.24 years.
Based on CTPA results, 24.1% of the studied patients (47/195) had PE: 16 men (34.0%) and 31 women (66.0%) at an average age of 68.15±63.62 years. In terms of the location of involvement, 28 patients (59.5%) had central PE and 19 (40.5%) peripheral PE.
An unenhanced MDCT scan of PE was positive when our radiologists observed an intraluminal sign (hyperdense, hypodense, or mix) as the primary sign in the present study (Figure 1 & Figure 2). Accordingly, 20 cases (42.5% of the case group and 10.2% of the total population) were detected with PE on MDCT scans, consisting of 17 cases (85.0%) with central PE and 3 cases (15.0%) with peripheral PE. Apropos of the parameter of clot density in unenhanced MDCT results, 12 patients (60.0%) had hyperdense signs, 3 (15.0%) hypodense signs, and 5 (25.0%) mixed hyper/hypodense signs (Table 1). Only 2 false-positive intraluminal signs (1 hyperdense and 1 hypodense) were observed in the MDCT scans of the patients without PE as confirmed by CTPA. There was a significant difference between central PE and peripheral PE as detected by MDCT. Consistently, intraluminal signs were notably distributed more frequently in central pulmonary arteries than in peripheral pulmonary arteries. Our results did not show a remarkable difference in secondary findings from the MDCT scan, including pulmonary trunk enlargements, peripheral wedge-shaped opacities, and pleural effusions, concerning the presence of PE. Pleural effusions, as the most common secondary finding in patients with PE, were found in 31 (68.8%) (Table 2).
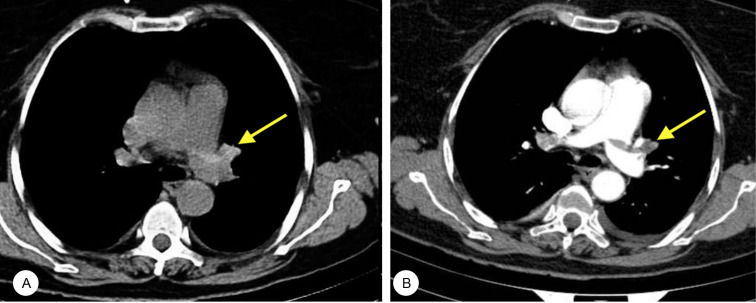
Figure 1.
A) Unenhanced CT shows an intraluminal hyperdense sign in the left pulmonary artery (arrow). B) Contrast-enhanced CTPA shows a filling defect (arrow) in the same artery.
CTPA, Computed tomography pulmonary angiography
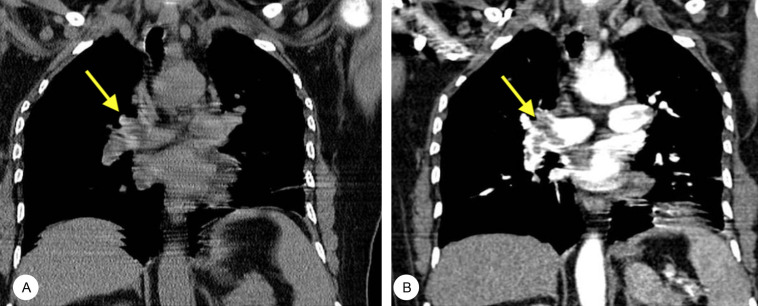
Figure 2.
A) The intraluminal mix-density sign (arrow) is seen in the right pulmonary artery on an unenhanced MDCT scan. B) Post-contrast imaging via CTPA shows the filling defect (arrow) in the right pulmonary artery.
MDCT, Multidetector computed tomography; CTPA, Computed tomography pulmonary angiography
Table 1.
Frequency of intraluminal signs in the studied patients with PE regarding the location of involvement on unenhanced MDCT scans*
| Clot Type | Central | Peripheral | P |
|---|---|---|---|
| Hyperdense | 10 (50.0) | 2 (10.0) | <0.001 |
| Hypodense | 3 (15.0) | 0 | <0.001 |
| Mixed | 4 (20.0) | 1 (5.0) | <0.001 |
| Overall | 17 (85.0) | 3 (15.0) | <0.001 |
PE, Pulmonary embolism; MDCT, Computed tomography pulmonary angiography
Data are presented as n (%).
Table 2.
Frequency of secondary findings in unenhanced MDCT in the studied patients with and without PE*
| Parameter | With PE | Without PE |
|---|---|---|
| Pulmonary artery trunk enlargement | 17 (36.2) | 23 (15.5) |
| Wedge-shaped opacity | 16 (34.0) | 40 (27.0) |
| Pleural effusion | 19 (29.8) | 61 (57.5) |
MDCT, Computed tomography pulmonary angiography; PE, Pulmonary embolism
Data are presented as n (%).
Table 3 shows the validity of imaging parameters in the detection of PE on MDCT scans. The highest specificity and sensitivity were related to intraluminal signs (98.6% and 42.5%, AUC=0.734), followed by pulmonary trunk enlargements (84.4% and 36.1%, AUC=0.603), peripheral wedge-shaped opacities (72.9% and 34.0%, AUC=0.535), and pleural effusions (58.7% and 40.4%, AUC=0.496).
Table 3.
Performance of unenhanced MDCT in the diagnosis of PE
| Parameter | AUC | Sensitivity % | Specificity % | PPV % | NPV % |
|---|---|---|---|---|---|
| Intraluminal symptoms | 0.734 | 42.5 | 98.6 | 90.9 | 84.3 |
| Pulmonary artery trunk enlargement | 0.603 | 36.1 | 84.4 | 42.5 | 80.6 |
| Wedge-shaped opacity | 0.535 | 34.0 | 72.9 | 28.5 | 77.7 |
| Pleural effusion | 0.496 | 40.4 | 58.7 | 23.7 | 75.6 |
MDCT, Computed tomography pulmonary angiography; PE, Pulmonary embolism; AUC, Area under the curve; PPV, Positive predictive value; NPV, Negative predictive value
Discussion
The diagnosis of PE remains challenging to clinicians since PE mainly manifests itself with unspecific cardiopulmonary symptoms, leading to high mortality (30%) if not diagnosed; thereby, early diagnosis can notably reduce the mortality rate (2%–10%).2 CTPA is the gold-standard diagnostic tool to detect PE with a specificity and sensitivity close to 100%.8 In this method, intravenous injection of iodinated CM is used to enhance the blood surrounding the occlusion in the pulmonary arterial circulation appearing as a filling defect in imaging.8 However, this method is associated with several limitations. For instance, its use is limited in patients with allergies to CM and/or renal insufficiency, and renal function tests take a long time to be ready, which may worsen the prognosis.
In such cases, unenhanced MDCT can play a vital role as an alternative diagnostic tool. In addition, non-contrast MDCT provides a picture of pulmonary parenchyma and chest wall that can assist us in identifying problems other than PE such as neoplasms. In many patients with suspected PE, radiologists immediately conduct CTPA precluding the non-contrast CT, while performing a non-contrast CT before PA can help to locate the region of interest. In some cases, patients who have unspecific cardiopulmonary symptoms may undergo CT examinations following a differential diagnosis other than PE, indicating how crucial it is to be aware of imaging signs suggestive of PE. The significance of the latter is demonstrated by a case report recently published. Reinert et al9 described a 61-year-old man admitted with the flu-like syndrome and no clinical response to antibiotic therapy. The patient was suspected of COVID-19, and despite a negative polymerase chain reaction test, he was examined by non-contrast CT. The CT scan revealed an intraluminal hyperdense sign in the left pulmonary artery. Further workup with CTPA confirmed the presence of PE. Nevertheless, there is a paucity of reports on the accuracy of unenhanced CT in the detection of PE, and they are mostly with a retrospective design and a small sample size or only focused on central and hyperattenuating signs.
The present study enrolled 195 patients with clinical suspicion of PE. Based on the results of CTPA, PE was diagnosed in 47 patients (24.1%), of whom 20 (42.5% of the case group) were detectable on unenhanced MDCT scans. These findings are in line with the results of previous works. In a study conducted by Cobelli et al,4 36.4% of the total population had PE based on CTPA results, while only 41.2% of the cases were detected by unenhanced CT.
We observed that 59.5% of PE cases were in central arteries and 40.5% in peripheral arteries. These proportions were changed by the results of unenhanced MDCT as there were 85% central PE and 15% peripheral PE, with a significant difference, indicating the better performance of MDCT in recognizing central PE. The lower accuracy of MDCT in peripheral arteries is a result of cardiopulmonary motion artifacts, partial volume averaging, and low signal-to-noise ratios.6 Consistently, a study carried out by Tatco and Piedad5 revealed that the highest specificity (99.1%) and sensitivity (66.7%) were related to hyperdense lumen signs for central PE, followed by overall PE and peripheral PE. Nonetheless, from a clinical point of view, central clots seem more important because of their greater impact on hemodynamic status and probably death. However, this is a hypothesis, and until the prognosis of patients with peripheral clots is not clear, we must approach patients with central or peripheral clots similarly.
The current study confirmed the intraluminal presence of hyperdense (60%), hypodense (15%), and mix-density (25%) signs in MDCT images. Similarly, intraluminal signs were notably distributed more frequently in central pulmonary arteries than in peripheral pulmonary arteries. An investigation on 51 patients with confirmed PE revealed that 21 cases had luminal signs detected on unenhanced CT images, consisting of 47.6% hyperdense, 23.8% hypodense, and 28.6% mix-density signs.4 The visualization of intraluminal signs depends on the density contrast between the embolus and the circulating blood. The density of emboli is determined by their compositing factors, including concentrated red blood cells and intracellular elements, particularly proteins. In fact, water decrease during acute clot retraction is responsible for the high density of clots. Eventually, density decreases through the progressive degradation of red blood cells and fibrins within chronic emboli.10, 11 Therefore, hyperdense and hypodense signs generally imply the existence of acute and chronic emboli, respectively. However, there are some exceptions. Hematocrit is another factor impacting the relative density of clots seen in MDCT scans. Higher hematocrit comes along with a higher density of the blood flow; thus, acute PE in patients with polycythemia can appear as a hypodense luminal sign compared with the blood flow.12, 13 In this regard, a study showed that the average blood density (42 Hounsfield Units) in hyperdense clots was significantly lower than the average blood density in (54 Hounsfield Units) hypodense clots.4 Another exception is when calcium deposition may lead to the high-density appearance of chronic embolisms. Nevertheless, it is clinically important to distinguish between acute and chronic thromboembolism since the management and therapeutic options vary for each of them.
Our results clarified that intraluminal signs without considering the type of density had the most specificity (98.6%) and sensitivity (42.5%) for the detection of central PE and/or peripheral PE. There were only 2 cases with false-positive results of intraluminal signs in the present work, which could be due to atherosclerotic plaques or anatomic parts near pulmonary arteries, mimicking a hyperattenuating appearance.
Evaluating all clot types, including hyperdense, hypodense, and mix-density clots, in both central and peripheral fields of pulmonary arteries and enrolling an adequate number of patients were the advantages of our work. Regarding the limitations of our study, we did not assess the density and level of the patients’ hematocrit, nor did we use echocardiography to detect right ventricular dilation as an alternative diagnostic modality. We recommend further investigations with a prospective design and the follow-up of tomodensitometry changes and their relationship with the clinical course of the illness.
Conclusion
Unenhanced multidetector computed tomography has an acceptable performance to detect pulmonary embolism, particularly central clots, and should be considered an alternative modality when computed tomography pulmonary angiography is not available or indicated. Furthermore, the intraluminal sign is the main indicator of pulmonary embolism in multidetector computed tomography images, with the highest specificity and sensitivity. We recommend that radiologists and other clinicians be aware of intraluminal signs in unexpected pulmonary embolism cases.
Acknowledgments
We gratefully thank the personnel at the Cardiology Department of Razi Hospital for their support. This study was approved and supported by the Deputyship for Research and Technology at Birjand University of Medical Sciences, Birjand, Iran.
Notes:
This paper should be cited as: Ehsanbakhsh A, Hatami F, Valizadeh N, Khorashadizadeh N, Norouzirad F. Evaluating the Performance of Unenhanced Computed Tomography in the Diagnosis of Pulmonary Embolism. J Teh Univ Heart Ctr 2021;16(4):156-161.
References
- 1.Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. BMJ. 2020;370:m2177. doi: 10.1136/bmj.m2177. [DOI] [PubMed] [Google Scholar]
- 2.Turetz M, Sideris AT, Friedman OA, Triphathi N, Horowitz JM. Epidemiology, pathophysiology, and natural history of pulmonary embolism. Semin Intervent Radiol. 2018;35:92–98. doi: 10.1055/s-0038-1642036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J Jr, Hobbins TE. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–1245. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 4.Cobelli R, Zompatori M, De Luca G, Chiari G, Bresciani P, Marcato C. Clinical usefulness of computed tomography study without contrast injection in the evaluation of acute pulmonary embolism. J Comput Assist Tomogr. 2005;29:6–12. doi: 10.1097/01.rct.0000148274.45419.95. [DOI] [PubMed] [Google Scholar]
- 5.Tatco VR, Piedad HH. The validity of hyperdense lumen sign in non-contrast chest CT scans in the detection of pulmonary thromboembolism. Int J Cardiovasc Imaging. 2011;27:433–440. doi: 10.1007/s10554-010-9673-5. [DOI] [PubMed] [Google Scholar]
- 6.Chien CH, Shih FC, Chen CY, Chen CH, Wu WL, Mak CW. Unenhanced multidetector computed tomography findings in acute central pulmonary embolism. BMC Med Imaging. 2019;19:65. doi: 10.1186/s12880-019-0364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghaye B, Szapiro D, Mastora I, Delannoy V, Duhamel A, Remy J, Remy-Jardin M. Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology. 2001;219:629–636. doi: 10.1148/radiology.219.3.r01jn32629. [DOI] [PubMed] [Google Scholar]
- 8.Goodman LR, Curtin JJ, Mewissen MW, Foley WD, Lipchik RJ, Crain MR, Sagar KB, Collier BD. Detection of pulmonary embolism in patients with unresolved clinical and scintigraphic diagnosis: helical CT versus angiography. AJR Am J Roentgenol. 1995;164:1369–1374. doi: 10.2214/ajr.164.6.7754875. [DOI] [PubMed] [Google Scholar]
- 9.Reinert D, Mönnings P, Schneider R, Lukas C. Hyperdense pulmonary artery sign - detection of pulmonary embolism in patients with suspected COVID-19 using non-contrast chest CT. Radiol Case Rep. 2021;16:1815–1818. doi: 10.1016/j.radcr.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobelli R, Zompatori M, Bresciani P, De Luca G. Visualization of hypoattenuation clots on unenhanced CT of the thorax. AJR Am J Roentgenol. 2004;182:530–531. doi: 10.2214/ajr.182.2.1820530. [DOI] [PubMed] [Google Scholar]
- 11.New PF, Aronow S. Attenuation measurements of whole blood and blood fractions in computed tomography. Radiology. 1976;121(3 Pt. 1):635–640. doi: 10.1148/121.3.635. [DOI] [PubMed] [Google Scholar]
- 12.Foster M, Nolan RL, Lam M. Prediction of anemia on unenhanced computed tomography of the thorax. Can Assoc Radiol J. 2003;54:26–30. [PubMed] [Google Scholar]
- 13.Collins AJ, Gillespie S, Kelly BE. Can computed tomography identify patients with anaemia? Ulster Med J. 2001;70:116–118. [PMC free article] [PubMed] [Google Scholar]