Abstract
Background
Cranial nerve-related diseases such as brain tumors, Alzheimer’s disease, and epilepsy are serious diseases that continue to threaten human. Brain-related diseases are increasing worldwide, including in the United States and Korea, and these increases are closely related to the exposure to harmful substances and excessive stress caused by rapid industrialization and environmental pollution. Drug delivery to the brain is very important for the effective prevention and treatment of brain-related diseases. However, due to the presence of the blood–brain barrier and the extensive first-pass metabolism effect, the general routes of administration such as oral and intravenous routes have limitations in drug delivery to the brain. Therefore, as an alternative, the nasal-brain drug delivery route is attracting attention as a route for effective drug delivery to the brain.
Areas covered
This review includes physiological factors, advantages, limitations, current application status, especially in clinical applications, and the necessary factors for consideration in formulation development related to nasal-brain drug delivery.
Expert opinion
The nasal-brain drug delivery route has the advantage of enhancing drug delivery to the brain locally, mainly through the olfactory route rather than the systemic circulation. The nasal-brain lymphatic system has recently attracted attention, and it has been implied that the delivery of anticancer drugs to the brain nervous system is possible effectively. However, there are limitations such as low drug permeability, as well as nasal mucosa and the mucociliary system, as obstacles in nasal-brain drug delivery. Therefore, to overcome the limitations of nasal-brain drug delivery, the use of nanocarriers and mucoadhesive agents is being attempted. However, very few drugs have been officially approved for clinical application via the nasal-brain drug delivery route. This is probably because the understanding of and related studies on nasal-brain drug delivery are limited. In this review, we tried to explore the major considerations and target factors in drug delivery through the nasal-brain route based on physiological knowledge and formulation research information. This will help to provide a mechanistic understanding of drug delivery through the nasal-brain route and bring us one step closer to developing effective formulations and drugs in consideration of the key factors for nasal-brain drug delivery.
Keywords: Nasal-brain, Drug delivery, Brain disease, Olfactory route, Lymphatic system, Nanocarrier
Introduction
Cranial nerve-related diseases have continuously threatened human life. Neurological disorders in the brain range from migraine headaches to brain tumors, Alzheimer’s, dementia, Parkinson’s, and epilepsy. According to a recent report (Feigin et al. 2021), Alzheimer’s, dementia, and migraine were among the three most burdensome neurological diseases in the United States (US) from 1990 to 2017. Approximately 16% of the US population (about 20 million people) have brain impairment, with Alzheimer’s and epilepsy the most common in adults, excluding stroke (Pal 2018), followed by Parkinson’s disease and brain tumors (Pal 2018). Approximately 100 million people worldwide have neurological disorders, accounting for 20% of the total global disease burden (Aleya and Uddin 2020). The most common neurological disorders worldwide are Alzheimer’s, dementia, Parkinson’s, seizures (related to epilepsy), and brain tumors (Aleya and Uddin 2020).
According to one study (Prince et al. 2016), about 47 million people worldwide have dementia, of which 37 million have been diagnosed with Alzheimer’s. And approximately 5.5 million people in the US have Alzheimer’s and dementia, which was reported as the sixth highest cause of death in elderly people (Agrawal et al. 2018). Unfortunately, Alzheimer’s and dementia-related deaths among the elderly in the US are expected to continue to increase by 2050, reaching 131 million (Agrawal et al. 2018). According to the Global Burden of Disease report (James et al. 2018), there were 1.2 million cases of Parkinson’s disease in 2017. And in 2016, 6.1 million people with Parkinson’s were reported worldwide, and the age-standardized prevalence increased by 21.7% between 1990 and 2016 (Feigin et al. 2019). It is predicted that the burden due to Parkinson’s will increase significantly in the coming decades (Wanneveich et al. 2018). According to a report by the World Health Organization (WHO) (2022), approximately 50 million people worldwide have epilepsy, and approximately 5 million people are diagnosed with epilepsy each year. And it has been reported that the risk of premature death in people with epilepsy is up to three times higher than that in the general population (WHO 2022). An estimated 700,000 people in the US currently live with brain tumors (NBTS 2022). And roughly 88,970 people are expected to be diagnosed with a brain tumor in 2022 (NBTS 2022). A recent study (Kratzer et al. 2022) reported that the US government has invested huge amounts of money (a total of $137.8 billion) each year since the National Cancer Act of 1971 began, and as a result, lung cancer mortality in 2019 was 44% lower than its 1993 peak. However, the incidence of death from brain cancer in 2019 was higher than in 1971 (Kratzer et al. 2022).
Cranial nerve-related diseases have common characteristics that they have a fatal impact on the quality of life of patients and are difficult to cure. Moreover, the social and economic burdens of treatment and management are relatively high compared to other diseases. The reason for the increase in the incidence of cranial nerve-related diseases is probably related to environmental pollution caused by rapid industrialization and the widespread exposure to harmful substances. Recent studies reported that exposure to harmful substances due to environmental pollution was associated with the occurrence of cranial nerve diseases. Exposure to air pollution, which causes chronic oxidative stress in the brain and nervous system, was reported as an important cause of Alzheimer’s disease (Aleya and Uddin 2020). Exposure to heavy metals and pesticides from soil contamination can lead to degenerative brain disease and cancer by increasing cranial nervous system protein folding and aggregation (Aleya and Uddin 2020). Other factors associated with the increased incidence of cranial nerve diseases may be aging and stress. The increase in the incidence of age-related cranial nerve diseases may be partially related to the life expectancy of humans, which has increased under the tremendous advances in medicine. In addition, continuous exposure to various external stresses causes chronic oxidative damage and inflammation in the brain nervous system, which may lead to brain diseases.
The development of pharmacologically effective drugs to treat the increasing number of cranial nerve-related diseases has continued. One such example is the development of drugs that inhibit acetylcholine esterase to relieve Alzheimer’s symptoms. Attempts have also been made to deliver developed therapeutic drugs to the brain. This is because local therapeutic drug delivery to the brain is considered a key factor in reducing side effects in other tissues and effectively controlling the pathogenesis of cranial nervous system diseases. However, drug delivery to the brain has been limited by several major factors. There is a physical brain barrier called the blood–brain barrier (BBB), which blocks harmful substances from entering the brain. Therefore, when a drug is administered by oral and intravenous (IV) routes, which are common administration routes, there is great difficulty in passing the drug into the brain through the BBB. Not only is it difficult for drugs to penetrate the BBB, which has a tight junction between cells, but also the amount of drugs that can reach the brain is limited due to extensive metabolism and excretion during systemic circulation. Therefore, in most cases, the drug concentration in the brain is not sufficient to generate an appropriate therapeutic effect. Cerebrospinal-fluid (CSF) injections have been the most widely tried method clinically to deliver drugs to the brain by bypassing the BBB (Pardridge 2020). However, the injection of a drug into the CSF limits drug penetration into the brain parenchyma because the CSF is rapidly expelled from the brain into the blood (Pardridge 2020). Thus, CSF injections of drugs were reported to be equivalent in effectiveness to slow IV infusions (Pardridge 2020).
Recently, the nasal-brain administration route has attracted attention as an effective alternative for drug delivery to the brain. This is because as studies on the physiological and neurological systems of the nasal-brain are reported, the pathways for drug delivery from the nasal cavity to the brain are being explored more clearly than in the past. Recently, the nasal-brain lymphatic system has been proposed as a novel target for neurological disorders (Sun et al. 2018). This, too, may have been made possible by the clear identification of the physiological system of the nasal-brain. The nasal-brain drug delivery route has the advantage of being able to deliver drugs to the brain locally over a relatively short physical distance. It will be a useful route of administration because the drug can be delivered to the brain using the olfactory nerve system in a non-invasive way, and it can also be applied to relieve some of the symptoms related to the loss of smell. However, practical studies and information on nasal-brain drug delivery are still scarce, probably because an understanding of the mechanism and action is relatively lacking compared to that of the common drug administration routes such as oral and IV routes, and some limitations are difficult to overcome such as the difficulty of selective drug delivery to specific brain regions, the complexity of the cranial nervous system, formulation stability in the administration route, physically limited intranasal space, and the need for a detailed evaluation of potential toxicity and side effects. It may also be because studies on effective formulations such as intranasal solutions, mucoadhesives, nanoformulations, and prodrugs applicable to the nasal cavity are limited.
Therefore, through this review, we wanted to provide information to facilitate an understanding of nasal-brain drug delivery pathway and emphasize the importance of this pathway because information on the nasal-brain drug delivery pathway has not yet been universalized and there is a need to close the knowledge gap. We also wanted to explore the key factors that should be considered in future nasal-brain drug delivery studies because much remains to be accomplished in formulation development for nasal-brain drug delivery. The information provided through this review is expected to help in accelerating the activation and practical application of formulation research in nasal-brain drug delivery and effectively enable the treatment of intractable brain diseases.
Pathways of drug movement from the nasal cavity to the brain
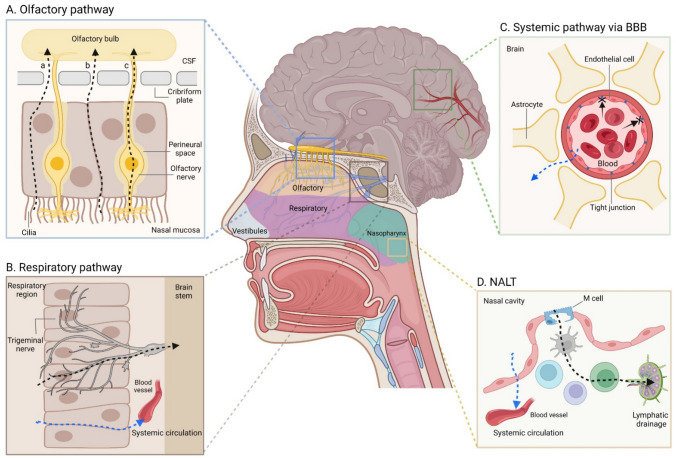
In this review, the physiological pathways and structures related to drug delivery from the nasal cavity to the brain are presented by dividing them into cellular and lymphoid types. Figure 1 [created using BioRender software (Toronto, ON, Canada)] shows a schematic diagram of the physiological systems involved in nasal-brain drug delivery. The direct routes of drug delivery from the nasal cavity (black dotted arrows in Fig. 1A, B, and D) to the brain and indirect routes (blue dotted arrows in Fig. 1B–D) through the systemic circulation were described.
Fig. 1.
Schematic diagram of the physiological systems involved in drug delivery from the nasal cavity to the brain. The olfactory pathway (A), the respiratory pathway (B), the systemic pathway through the BBB (C), and NALT (D) are presented as possible pathways for nasal-brain drug delivery. BBB and NALT are blood–brain-barrier and nasopharynx-associated lymphoid tissue, respectively. In A, a–c represent transcellular (through olfactory epithelial cells), paracellular (through olfactory epithelial cells), and olfactory nerve pathways, respectively. The black dotted arrows in A, B, and D indicate direct drug delivery routes from the nasal cavity to the brain. The blue dotted arrows in B–D indicate indirect drug delivery pathways from the nasal cavity to the brain
Physiological and anatomical systems
The structure of the nasal cavity can be divided into nasal vestibules, and the respiratory, olfactory, and nasopharyngeal regions (Gänger and Schindowski 2018) (Fig. 1). Positionally, the nasal vestibules refer to the anterior part of the nasal cavity and mainly play a role related to the removal of drugs and foreign substances (Gänger and Schindowski 2018). The relevant parts for general drug delivery are the respiratory region and the olfactory region located in the middle and upper parts of the nasal cavity (Gänger and Schindowski 2018). The respiratory region is involved in the cognition of several senses and respiration, and the olfactory region mainly plays a role in olfactory perception (Gänger and Schindowski 2018). Anatomically, the cribriform plate of the ethmoid bone lies between the nasal cavity and the brain, where the olfactory bulbs and olfactory sensory neurons are located (Sun et al. 2018). And based on the cribriform plate, CSF flows in the direction of the brain. The nasal mucosa is located in the direction of the nasal cavity (Sun et al. 2018). Since olfactory neurons located in the olfactory region are directly involved in olfactory transmission from the nasal cavity to the brain (Fig. 1A), the route through the olfactory nerve may be utilized as a pathway for drug delivery to the brain (Bahadur et al. 2020). Drug delivery through the olfactory region enables direct drug transmission to the brain without going through the systemic circulation (Bahadur et al. 2020) because physiologically, the olfactory neurons are connected from the nasal mucosa to the brain. Drug delivery through the olfactory region is largely possible via the olfactory nerve as an intraneuronal pathway or the olfactory epithelial cells present around the nerve pathway (Bahadur et al. 2020). In addition, transcellular methods including endocytosis and simple diffusion and paracellular methods through the junctions between cells provide another mechanism of drug transport through olfactory epithelial cells (Bahadur et al. 2020) (Fig. 1A-a–c). Previous studies (Agrawal et al. 2018; Gänger and Schindowski 2018) reported that drug delivery by the intraneuronal route was relatively slower than delivery through olfactory epithelial cells. That is, drug delivery through the olfactory nerve was possible within 1.5–6 h, and drug delivery through olfactory epithelial cells was possible within a few minutes (Gänger and Schindowski 2018). Of course, the speed and extent of drug delivery through the olfactory nerve and olfactory epithelial cells may differ depending upon the physicochemical properties of the drug. However, in general, the route through the olfactory epithelial cells has more advantages in terms of speed.
The respiratory region occupies the largest portion of the nasal cavity, where capillaries are abundantly distributed (Gänger and Schindowski 2018). Therefore, a drug delivered to the respiratory region is absorbed into the blood and enters the systemic circulation, which becomes a possible indirect route for drug delivery to the brain through the BBB (Gänger and Schindowski 2018). And in the respiratory region, the trigeminal nerve is mainly responsible for pain and temperature sensation. The trigeminal nerve is divided into three branches and each branch is connected to the brain stem and olfactory bulb (Chatterjee et al. 2019; Gänger and Schindowski 2018). Therefore, drug delivery to the trigeminal nerve can be a direct route of drug delivery from the nasal cavity to the brain (Fig. 1B). Although most of the trigeminal nerve is present in the respiratory region of the nasal cavity, it has been reported that some trigeminal nerves are also present in the olfactory region (Gänger and Schindowski 2018). Thus, all drugs reaching the trigeminal nerves present in the respiratory and olfactory regions will be delivered directly to the brain.
In addition, drugs that do not stay in the nasal cavity and pass through the airway or esophagus would be indirectly delivered to the brain through the systemic circulation. However, for drugs to be delivered to the brain through the systemic circulation, they would have to cross the BBB (Fig. 1C) and the amount of drugs indirectly delivered is probably less than that delivered directly from the nasal cavity to the brain due to the tightness of the BBB and extensive drug metabolism and/or elimination in the body.
Nasal-brain lymphatic system
Recent reports (Albayram et al. 2022; Pardeshi and Belgamwar 2013; Sun et al. 2018) have confirmed the presence of a lymphatic system between the nasal cavity and the brain. Olfactory nerves exist in the cribriform plate mentioned above, and perineural spaces exist around the olfactory nerves, which allow CSF to flow into the nasal mucosa (Sun et al. 2018). Therefore, the perineural spaces around the olfactory nerves are called the olfactory/nasal lymphatic route and are the main drainage routes for CSF and interstitial-fluid (ISF) generated from the brain parenchyma (Fig. 1A). The olfactory/nasal lymphatic route ultimately leads to cervical lymph nodes (Sun et al. 2018). That is, the olfactory/nasal lymphatic route physiologically allows CSF and ISF to flow from the subarachnoid space through the perineural spaces and nasal lymphatics to the cervical lymph nodes. According to past reports (Sun et al. 2018; Thiebaud et al. 2011), transporters such as P-glycoproteins (P-gp) and organic anion transporters (OAT) as well as various xenobiotic-metabolizing enzymes exist in the olfactory perineural space as a defense mechanism from foreign substances. The main role of the olfactory/nasal lymphatic route is to maintain the water balance in the brain and export antigens to the cervical lymph nodes via the CSF (Sun et al. 2018). The olfactory/nasal lymphatic route was also utilized as a pathway for immune cells to migrate from the brain parenchyma to cervical lymph nodes (Hsu et al. 2021). According to past reports (Pardeshi and Belgamwar 2013; Sun et al. 2018), the olfactory/nasal lymphatic route is also used as a route through which brain waste is excreted nasally and externally. A previous study reported that the excretion of waste through the olfactory/nasal lymphatic route decreased with aging, and thereby increased brain and nervous system diseases (Sun et al. 2018). The function and activation degree of the nasal-brain lymphatic system is affected by factors such as aging, genetics, the sleep–wake cycle, and body posture, leading to the onset of brain diseases such as neurovascular, neurodegenerative, neuroinflammatory disorders, and brain tumors.
The lymphatic system, which exists between the nasal cavity and the brain, would be useful as a route for drug delivery. In particular, the nasal route would be very useful for delivering genes and peptide drugs to the CSF and brain parenchyma in the treatment of intractable brain and nervous system diseases because generally administered (through oral or IV) small molecules, as well as high molecular weight drugs, have very limited transport from the blood vessels to the brain parenchyma due to the BBB (Sun et al. 2018). Using the olfactory/nasal lymphatic route, intranasally administered drugs can be delivered relatively easily to the CSF and then effectively distributed to brain tissue and related nervous systems through the dynamics (including CSF-ISF exchange) of central nervous system (CNS) fluids. The olfactory/nasal lymphatic route may be able to overcome the limitations of the BBB and maintain effective drug concentrations in the brain while avoiding or reducing the peripheral side effects of brain-targeted drugs.
Other systems
At the back of the nasal cavity is the nasopharyngeal region, where the lymphatic system called nasopharynx-associated lymphoid tissue (NALT) is located (Gänger and Schindowski 2018) (Fig. 1D). NALT is a type of mucosa-associated lymphoid tissue (MALT) and ultimately leads to cervical lymph nodes (Gänger and Schindowski 2018). Therefore, direct drug delivery to the brain can occur through brain lymphatics or indirect drug delivery can occur through the systemic circulation. According to past reports (Chatterjee et al. 2019; Gänger and Schindowski 2018; Selvaraj et al. 2018), NALT is mainly known as an immune organ that blocks the invasion of foreign pathogens in the nasal cavity and upper respiratory tract. Therefore, drug transition to NALT may be a pathway that can effectively exert drug effects related to immune functions in the body. M-cells were reported to exist in NALT (Chatterjee et al. 2019; Selvaraj et al. 2018) (Fig. 1D). Considering that nanoparticle absorption is possible through M-cells (Jeong et al. 2021a), NALT could be effective as a lymphatic delivery route for nanoformulations. The nasal cavity is a system connected to the gastrointestinal and respiratory systems (Gänger and Schindowski, 2018). Therefore, nasally administered drugs are able to move to the lungs or gastrointestinal tract, and in this case, indirect drug delivery to the brain through the systemic circulation is possible.
Advantages of the nasal-brain drug delivery system
The advantages of nasal-brain drug delivery compared to other existing drug administration routes were largely considered in pharmacological and clinical applications.
Pharmacological benefits
The nasal-brain drug delivery route is a relatively physically short and simple delivery route compared to drug delivery to the brain through the systemic circulation and is an effective route that can overcome the obstacles of the BBB. That is, drug delivery to the brain through the systemic circulation must pass through the BBB, which has many defense mechanisms such as tight junctions and P-gp. However, the nasal-brain route bypasses the BBB and enables direct drug delivery to the brain through the olfactory nerve. In addition, the nasal-brain drug delivery path is a route that can significantly improve bioavailability in the brain by avoiding extensive metabolism and the loss of drugs through the gastrointestinal tract or systemic circulation. This suggests that the nasal-brain administration route can effectively maintain therapeutic drug concentrations in the brain while minimizing clinically required drug doses. As a result, targeted drug delivery to the brain will be possible with reduced drug side effects in the periphery. In addition, rapid drug delivery to the brain and the expression of drug effects will be possible. A previous report (Ugwoke et al. 2001) suggested intranasal drug administration as a viable, non-invasive strategy for delivering drugs to the brain. Moreover, nasal drug administration has often been suggested as the most feasible alternative to parenteral injections (Ugwoke et al. 2001), probably because the high permeability of the nasal epithelium allows it to be applied to high molecular weight substances (about 1000 Da or more), and the fast drug absorption rate was similar to the profile of IV injections (Ugwoke et al. 2001).
Due to the higher permeability in the nasal mucosa than in the BBB, the nasal-brain drug delivery route is attracting attention as an effective route for delivering peptides and protein drugs, which are very difficult to deliver to the brain through the systemic circulation. In addition, the nasal cavity has been proposed as a route of delivery of various biological substances, such as oligonucleotides, viral vectors, and stem cells, to the cranial nervous system (Lochhead and Thorne 2012). For example, calcitonin, a polypeptide hormone of 3500 Da, is being applied as a nasal spray for the treatment of postmenopausal osteoporosis (Zhang and Zhang 2010). Insulin reaching 5800 Da was widely distributed to the brain within 1 h of intranasal administration to mice, and the highest detection was confirmed in the olfactory bulb and trigeminal nerve (Francis et al. 2008). Galanin-like peptide, a neuropeptide of 6500 Da, was confirmed to be distributed to the cranial nervous system including the olfactory bulb, anterior brain, hippocampus, hypothalamus, cerebellum, brain stem, and CSF after nasal administration to mice, and the level was approximately 20 times higher than when administered intravenously (Nonaka et al. 2008). These results indicate that the nasal-brain pathway could deliver substances from the nasal cavity to the brain with the high permeability of high molecular weight substances, as well as those with low molecular weights.
In addition, drugs with various physicochemical properties such as hydrophilicity, hydrophobicity, and ionicity can be effectively delivered from the nasal cavity to the brain through proper formulation. Past reports confirmed that cationic peptides, basic fibroblast growth factor (Feng et al. 2012), as well as cationic lipid nanoemulsions (Kim et al. 2000) and nanoparticles (Hanafy et al. 2015), were delivered from the nasal cavity to the brain. Hydrophilic drugs can be administered intranasally even in simple solution form through solubilization, and hydrophobic drugs can be delivered to the brain relatively easily through nanoformulations and prodrug formulations.
Nasal-brain drug delivery would be very useful when toxicity due to excessive exposure to drug metabolites is possible. According to one past study (Qian et al. 2014), intranasal drug administration increased parent drug transport to the brain and decreased exposure to metabolites in both plasma and brain compared to oral administration. The decrease in the concentration of metabolites in the brain and plasma following nasal drug administration was probably because first-pass metabolism was largely avoided compared to other routes of administration such as oral and IV routes.
From a pharmaco-mechanical perspective, nasal-brain drug delivery could offer a great advantage in the treatment of some complex diseases. In the case of brain tumors, the brain tumor directly affects the olfactory nerve, which is often accompanied by the loss of smell (Ship and Chávez 2002). The nasal-brain administration route will deliver drugs to olfactory cells, so it will be possible to treat not only brain tumors but also olfactory damage. Thus, a therapeutic effect for complex diseases such as brain tumors may be possible from intranasal administration by combining anti-inflammatory drugs or neuro-stimulating drugs to improve the sense of smell with anticancer drugs. As key evidence, previous studies (Francis et al. 2008; Nonaka et al. 2008) have already reported that intranasal drug delivery led to drug delivery to the olfactory bulb, one of the olfactory nervous systems.
Through improved formulation, the controlled release of drugs from the nasal cavity to the brain will be possible. For example, it has been reported that polymers such as pluronic F-127 had a controlled release mechanism through the dissolution and diffusion of drugs depending upon their concentration (Moore et al. 2000). Increasing concentrations of pluronic F-127 in the formulation tended to decrease the drug release rate (Moore et al. 2000). For drugs whose therapeutic benefit is to maintain a certain drug concentration in the cranial nervous system for a long time, controlled release applications in the nasal mucosa will have advantages such as faster on/off and higher delivery rates than other administration routes.
Advantages in clinical applications and medication compliance
The nasal-brain route is a non-invasive drug delivery path that does not cause pain or burden the patient, and the self-administration method is relatively simple, so it does not impose a workload on the medical staff. Therefore, the nasal-brain route of administration can be easily applied without much difficulty in clinical practice. A formulation improvement could allow for the sustained release of drugs from the nasal mucosa to the brain, which would enable a reduction in the number of doses. In addition, the high bioavailability of drug delivery from the nasal cavity to the brain could reduce the amount of drug administered per dose. This will provide effective treatment results with improved patient adherence to taking the medication and will enable cost savings. The nasal-brain route provides an alternative to allowing drugs to be absorbed into the body faster than the oral route, which can be especially useful for patients with gastrointestinal problems such as irritable bowel syndrome and Crohn’s disease or those with difficulty swallowing tablets. Rapid drug delivery through the nasal-brain route will also be useful in emergencies such as seizures and acute pain, which require rapid drug action.
Applicable formulations
The formulations applicable to the nasal-brain drug delivery route include intranasal solutions, mucosal adhesion types, nanoformulations, and prodrugs. Their primary purpose is to bypass the BBB and deliver drugs directly to the brain via the nasal/olfactory route.
Intranasal solution
The route of administration has been used advantageously to deliver a drug to brain nervous system targets. Clinical attempts have been made by injecting a drug-dissolved solution into the nasal cavity because the efficiency of drug delivery to the brain is usually higher through this route than by oral or IV administration. Intranasal solutions are mainly delivered through a nasal spray, and although it is possible through drops, there is a limitation in that the clearance from the nasal cavity is faster than spray (Hardy et al. 1985). When 99mTc-labelled human serum albumin solution was applied to the nasal cavity in the form of a spray or drops, it was confirmed that the degree of deposition in the nasal cavity of spray was higher than that of the drop, and the clearness was slower (Hardy et al. 1985). The same bioavailability as that of rectal administration was confirmed in the nasal spray application of diazepam, and in the therapeutic effect (seizure) and adverse events, nasal dosing was superior to existing oral and rectal administration routes (Hogan et al. 2020). This suggests that diazepam, which requires delivery to the cranial nervous system for sedation and seizure stabilization, could act effectively via the nasal-brain drug delivery route. An intranasal solution formulation in which a surfactant (dodecyl-β-d-maltopyranoside), which reversibly loosens cell–cell junctions (Lipton et al. 2018), is added together with the drug (diazepam) has been attempted so that the drug injected into the nasal cavity can be delivered to the brain through the nasal mucosa (Hogan et al. 2020). A previous report (Munjal et al. 2017) showed that dodecyl-β-d-maltopyranoside induced a rapid and reversible decrease in transepithelial/transendothelial electrical resistance values by altering tight junctions to promote uptake, enhancing the permeation of the pericellular marker 3H-mannitol. A study on the nasal application of the same drug with a difference in the formulation was conducted. A comparison of the formulations showed that the bioavailability of diazepam intranasal solution and the intranasal suspension was approximately 97% and 67%, respectively (Agarwal et al. 2013). These results suggest that drug solubilization is a very important factor in terms of formulation for effective drug delivery from the nasal cavity to the brain. An intranasal sodium citrate solution was applied to patients to target the olfactory cleft to improve the sense of smell in viral hyposmia (Whitcroft et al. 2016). An improvement was confirmed in the patient group compared to the placebo group (Whitcroft et al. 2016). Considering that the olfactory nerve is directly connected to the brain by nasal mucosa, the results indirectly suggested that intranasal drug administration would be effective in the treatment of various intractable cranial nervous system diseases. The solution form in which ovalbumin protein was simply dissolved in aqueous phosphate-buffered saline was administered nasally and delivered to the brain (Migliore et al. 2010). Following the intranasal administration of aqueous ovalbumin, the highest intracerebral ovalbumin concentration was achieved within 1 h (Migliore et al. 2010). Although not to a high degree compared to the test liposomal formulation, the intranasal administration of aqueous ovalbumin clearly confirmed intracerebral protein transport. This was a very interesting result, where protein macromolecules were delivered into the brain relatively easily through the characteristics of the intranasal administration route without a special formulation technique because it represents the promising delivery of antibodies or other protein drugs to the brain. In addition, in the case of the water-soluble drug ketamine, effective drug efficacy was confirmed compared to the placebo group even when simply diluted with saline and administered nasally (Diaz 1997). That is, intranasal administration of ketamine solution pleasantly and rapidly separated children from parents, cooperatively accommodated monitoring and mask inhalation induction. The administration did not prolong post-anesthesia recovery or delay discharge from the hospital (Diaz 1997). Ketamine is a drug commonly used clinically for the relief of seizure symptoms, and the findings suggest that the intranasal administration of ketamine solution enabled delivery to the cranial nervous system.
Aside from drug delivery to the brain, clinical trials of zolmitriptan intranasal solution through nasal spray showed faster blood absorption than oral formulations, with drug detection in the plasma within 5 min after administration, suggesting nasal spray as an effective alternative for rapid drug efficacy in clinical practice (Yates et al. 2002). It has been suggested that naloxone administered for the treatment of acute opioid poisoning could also be applied as an intranasal solution using nasal spray, avoiding the first-pass effect of more than 90% and achieving rapid drug effects at the same time (Wermeling 2013). Furthermore, rapid and effective drug efficacy was confirmed from the nasal spray application of an aqueous-buffered solution of fentanyl to relieve pain in cancer patients (Mercadante et al. 2014). That is, an intranasal spray of fentanyl solution provided significant analgesia within 10 min without causing the associated side effects (Mercadante et al. 2014).
Mucoadhesive formulations
In order to effectively deliver drugs from the nasal cavity to the brain, research on mucosal-adhesive formulations that stay in the nasal mucosa for drug delivery have been conducted since the past. This is because drugs that enter the nasal cavity are removed largely by mucociliary clearance from the vestibular region, and the physical approach of nasal mucosal adhesion has become an effective alternative to overcome this barrier (Vyas et al. 2006). Nasal mucosal adhesion formulations have the advantage of continuous drug delivery to the brain by attaching to the nasal mucosa for a relatively long time (Ugwoke et al. 2001). Allowing the drug to stay in the respiratory and/or olfactory regions of the nasal cavity would be effective in maximizing drug delivery to the brain. This is because the drugs attached to the respiratory and/or olfactory regions could be delivered to the brain through several mechanisms such as the olfactory nerve, trigeminal nerve, and lymphatic and vascular paths. Systems suitable for nasal mucoadhesive formulations include viscous formulations, mucoadhesive polymers, hydrogels, and in situ gelations, which will decrease the mucociliary clearance and increase the retention of drugs in the nasal cavity (Ugwoke et al. 2001).
For example, tacrine, an Alzheimer’s therapeutic drug, was formulated using the thermosensitive polymer pluronic F-127 and administered intranasally (Qian et al. 2014). This was an attempt to deliver drugs from the nasal cavity to the brain using an in situ gelation system that undergoes sol–gel transition according to temperature. The nasal administration of the in situ gelation formulation having a sol–gel transition temperature of 28.5 °C increased intranasal drug retention compared to the solution form, and the drug delivery efficiency to the brain was also higher than that of oral administration (Qian et al. 2014). In another study, the intranasal administration of mucoadhesive microemulsion loaded with tacrine showed that the brain bioavailability was more than twice that of intranasal tacrine solution (Jogani et al. 2008). The results suggest that nasal mucosal adhesion or retention of the formulation is a very important factor in drug delivery to the brain via the intranasal route.
Chitosan was applied as a strategy to improve the mucosal adhesion of the formulation. In a past study (Illum et al. 2002), a chitosan-morphine nasal formulation showed 5–6 times higher bioavailability than simple morphine solution and improved drug absorption through the nasal mucosa by increasing intranasal drug retention. The results of another previous report (Cho et al. 2011) suggested that chitosan significantly contributed to mucosal adhesion and increased shear viscosity, and played a major role in the approximately 18 times higher bioavailability than a simple solution. Another adhesive molecule, hyaluronic acid, has also been applied in formulations to improve nasal-brain drug delivery by increasing intranasal mucosal adhesion (Horvát et al. 2009). The formulation was delivered to major brain parts of the olfactory bulb, frontal and parietal cortexes, hippocampus, cerebellum, midbrain, and pons, and hyaluronan was proposed as a mucoadhesive non-toxic biomolecule capable of increasing the brain penetration of hydrophilic drugs (Horvát et al. 2009). In addition, it has been suggested that small lectin molecules with a high specific binding ability to l-fucose receptors, which are highly expressed in olfactory mucosa, can be coated on the surface of a formulation to increase adhesion in the nasal mucosa and improve drug delivery to the brain through the olfactory nerve (Bies et al. 2004). The application of wheat germ agglutinin as a mucoadhesive agent to deliver neuroprotective peptides from the nasal cavity to the brain significantly increased intracerebral drug delivery compared to the formulation without wheat germ agglutinin (Gao et al. 2007). Interestingly, the formulation conjugated with wheat germ agglutinin showed a higher binding affinity to olfactory mucosa than to the respiratory region (Gao et al. 2007), implying that the intranasal administration of wheat germ agglutinin-applied formulations as mucoadhesives would be useful for targeted delivery to the brain.
Additionally, hydrogel systems using carriers such as poloxamer-188 could be applied for the mucosal adhesion and controlled release of nasally administered drugs. A previous study (Anderson et al. 2001) suggested that hydrogels had great advantages in nasal administration due to their high hydration and adequate physical strength and adhesion. Hydrogel also had the ability to load significant amounts of both hydrophilic and hydrophobic drugs, as well as control the release of drugs through matrix swelling (Anderson et al. 2001).
Nanoformulations
Examples of the application of nanoformulations in nasal drug delivery to the brain are presented in Table 1. The applied nanoformulations ranged from nanoparticles (Elnaggar et al. 2015; Eskandari et al. 2011; Hanafy et al. 2016; Meng et al. 2018; Salem et al. 2019; Seju et al. 2011; Shah et al. 2016; Zhang et al. 2014), to nanoemulsions (Abdou et al. 2017; Bahadur and Pathak 2012; Boche and Pokharkar 2017; Espinoza et al. 2019; Haider et al. 2018; Iqbal et al. 2019; Jiang et al. 2019; Kumar et al. 2008, 2016; Mahajan et al. 2014; Nasr 2016; Pandey et al. 2015; Pathak et al. 2014; Sood et al. 2014; Yadav et al. 2015), liposomes (Li et al. 2012; Yang et al. 2013), nanosuspensions (Md et al. 2014), niosomes (Khallaf et al. 2020; Mathure et al. 2018; Rinaldi et al. 2019), and nanostructured lipid carriers (Eskandari et al. 2011). The substances encapsulated in the nanoformulations were mainly therapeutic drugs for Alzheimer’s, dementia, Parkinson’s, epilepsy, and various psychiatric disorders, which pharmacologically and mechanistically targeted the brain nervous system. Attempts have been made to encapsulate drugs in various nanocarriers to deliver drugs from the nasal cavity to the brain with the aim of reaching a therapeutic concentration sufficiently (at brain). This is because it has been confirmed through numerous studies that nanocarriers can significantly improve the physiologically stable aspect of drug delivery. The nanocarriers surrounding the drug prevent the drug from being decomposed by various enzymes and external stressors, increasing the degree of effective drug reaching the target and improving the therapeutic effect (Jeong et al. 2021a). In addition, due to the appropriate degree of hydrophobicity of the nanocarriers and the surfactant applied during the formulation process, nanocarriers can move easily through the cell membrane into the target tissue. Past studies on the intranasal administration of nanoformulations (Table 1) confirmed that drug delivery to the brain was improved in nanoformulations compared to existing solution formulations. Nanoformulations also have great advantages in drug delivery in terms of structural engineering flexibility. When nanocarriers are used in drug delivery, selective targeting of the desired tissues is possible through surface functionalization such as attaching specific ligands to the carrier surface. This aspect can be utilized when targeting and adsorbing in areas such as olfactory and respiratory regions related to direct delivery to the brain via the nasal administration of formulations or delivering drugs to specific brain regions. For example, it has been suggested that the application of a specific lectin as a ligand to the surface of a nanoparticulated formulation for nasal-brain drug delivery could increase formulation adsorption and permeation into the olfactory region of the nasal mucosa (Gabor et al. 2004), and the application of specific lectin ligands may improve drug transport by promoting endocytosis in brain epithelium (Gabor et al. 2004). Lectins are proteins of non-immune origin that specifically bind to the carbohydrate moieties of glycoproteins and glycolipids (Pastor et al. 1992), so they are very good biocompatible ligands for nasal-brain drug delivery. In nasal-brain drug delivery, odorranalectin has been reported as an applicable lectin molecule, and when odorranalectin was conjugated with a drug, drug delivery to the brain was increased three-fold or more, thereby improving the therapeutic effect on cognitive function (Wu et al. 2012). This effect was probably because odorranalectin specifically binds to the l-fucose receptor, which is highly expressed in nasal olfactory mucosa (Li et al. 2008).
Table 1.
Applications of nanoformulations in drug delivery to the brain via the nasal cavity
| Drug | Indication | Formulation (main components/manufacturing method) | Physical size | Study information | Effects | References |
|---|---|---|---|---|---|---|
| bFGF | Alzheimer’s disease | NP (PEG-PLGA, Lectin/Solvent evaporation method) | 118.7 nm | In vivo biodistribution, PK and PD studies in SD-rats |
Brain drug delivery ↑ Cognitive, memory, and learning ability ↑ 1.79–5.17-fold ↑ in the AUC of the brain Cholinergic function ↑ Brain delivery ↑ compared to IV injection of bFGF and IN administration of bFGF solution |
Zhang et al. (2014) |
| Buspirone hydrochloride | Anxiety disorders | NS (Span 60, Cholesterol/Thin film evaporation method) | 181.9 ± 0.36 nm | Ex vivo permeation studies using sheep nasal mucosa |
Permeation of buspirone hydrochloride through sheep nasal mucosa was high in 8 h at 83.49% w/w Application of NS proved the potential of IN delivery of buspirone hydrochloride |
Mathure et al. (2018) |
| Curcumin | Alzheimer’s disease | NE (Capmul MCM, Captex 500, Cremophor EL, Tween 80, Chitosan/Spontaneous emulsification method) | 37.8 ± 3.1 nm | Ex vivo diffusion studies | MA NE showed ↑ flux and permeation | Sood et al. (2014) |
| Cyclosporine A | Neuroprotective | NE (Flax-seed oil, Tween 80, Lipoid E80/Ultrasonication method) | 158.47 ± 3.02 nm | In vivo brain uptake study in SD-rats | The brain/blood targeting ratios for IN NE were 13.6 and 449 times higher than for IN solution and IV NE, respectively | Yadav et al. (2015) |
| Donepezil | Alzheimer’s disease | NE(Capryol 90, Labrasol, Transcutol, Pluronic F-127/Spontaneous emulsification method) | 127.13 ± 4.14 nm | Ex vivo permeation study using porcine nasal mucosa | Drug permeation ↑ for IN NE | Espinoza et al. (2019) |
| Donepezil | Alzheimer’s disease | NSP (Chitosan, Tripolyphosphate/Ionic-crosslinking method) | 150–200 nm | In vivo PK studies in SD-rats |
Drug concentration (147.5 ng/mL) ↑ in the brain rapidly (2 h) NSP had a higher Cmax and AUC in the brain and plasma than IN free drug |
Md et al. (2014) |
| GH | Alzheimer’s disease | NP(Chitosan, Tween 80, Tripolyphosphate/Ionic gelation method) | 48.3–68.3 nm | In vivo pharmacological and toxicological studies in Wistar-rats |
Nasal GH/chitosan complex NPs exhibited a significant ↓ in AChE protein levels in rat brains compared to oral and nasal GH solutions Drug retention time ↑ in the nasal cavity Memory and brain function ↑ No toxic effect on brain cells |
Hanafy et al. (2016) |
| GH | Alzheimer’s disease | LS (Soya phosphatidylcholine, Cholesterol, PG/Thin film homogenization method) | 112 ± 8 nm | In vivo PK and pharmacological studies in SD-rats |
Efficiency of AChE inhibition of GH was greatly ↑ by IN administration compared to oral administration, especially GH-loaded flexible LSs Cmax and AUC0→10 for IN administration of GH-loaded flexible LSs were 3.52 and 3.36 times higher than those of orally administered GH Tmax was greatly ↓ from 1.5 h for oral administration to 0.75 h for IN administration of GH-loaded flexible LSs |
Li et al. (2012) |
| HupA | Alzheimer’s disease | NP (PLGA, Chitosan, Lactoferrin/Solvent evaporation method) | 153.2 ± 13.7 nm | In vivo biodistribution studies in Kunming mice | Drug distribution ↑ in the brain | Meng et al. (2018) |
| HupA | Alzheimer’s disease | NE (IPM, Capryol 90, Cremophor EL, Labrasol, Lactoferrin/Spontaneous emulsification method) | 15.24 ± 0.67 nm | In vivo PK and biodistribution studies in rats |
IN administration of LF-HupA-NE to rats significantly enhanced drug delivery to the brain compared to HupA-NE AUC in the brain for LF-HupA-NE was significantly higher (p < 0.05) compared to HupA-NE and HupA solution |
Jiang et al. (2019) |
| Letrozole | Epilepsy | NE (Triacetin, Tween 80, PEG-400/Spontaneous emulsification method) | 95.59 ± 2.34 nm |
Ex vivo diffusion study using goat nasal mucosa In vivo PD and biodistribution studies in Swiss-albino mice |
Drug permeation for NE was significantly higher than that of drug solution Brain drug concentration of IN NE was significantly (about 20-fold) higher than that of IP solution IN NE enhanced the onset time of seizures and reduced the incidence of status epilepticus IN NE showed better protection of the hippocampus from neurotoxicity compared to solution |
Iqbal et al. (2019) |
| Nimodipine | Dementia | NE (Capmul MCM, Labrasol, Transcutol P, Carbopol 934 P/Spontaneous emulsification method) | 250 ± 76.7 nm |
Ex vivo drug permeation studies In vivo PK and biodistribution studies in rats |
Drug permeation (sevenfold) ↑ of NE compared to drug suspension Brain and plasma concentrations ↑ of nimodipine from in situ gelling MA microemulsion compared to NE and nimodipine suspension |
Pathak et al. (2014) |
| Olanzapine | Schizophrenia | NS (Chitosan, Cholesterol, Span 80/Thin film hydration method) | 201.3–1446 nm | In vivo biodistribution studies in rats | Nasal NS showed a threefold ↑ in drug concentration in the brain compared to IN solution | Khallaf et al. (2020) |
| Olanzapine | Schizophrenia | NP (PLGA, Poloxamer 407/Nanoprecipitation method) | 91.2 ± 5.2 nm |
Ex vivo permeation studies using sheep nasal mucosa In vivo PK study using albino rats |
PK studies showed 6.35 and 10.86-times higher uptake of IN delivered by NPs than olanzapine solution delivered through IV and IN routes, respectively Olanzapine concentrations ↑ in the brain |
Seju et al. (2011) |
| Paroxetine | Depression | NE (Capmul MCM, Solutol HS, PG/Spontaneous emulsification method) | 58.47 ± 3.02 nm |
Ex vivo drug permeation studies Behavioral, biochemical, and histopathological studies in Wistar-rats |
Permeation studies revealed a 2.57-fold ↑ in permeation (in paroxetine-loaded NE) compared to paroxetine suspension Treatment of depressed rats with paroxetine NE (IN administered) significantly improved behavioral activities compared to orally administered paroxetine suspension Damage and degeneration ↓ of the vesicular nuclei of brain tissues |
Pandey et al. (2015) |
| Pentamidine | Parkinson’s disease | NS (Chitosan, DCP, Cholesterol/Thin layer evaporation method) | 300.7 ± 17.2 nm | In vivo pharmacological studies in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced Parkinson’s disease mice | Glial-related neuroinflammation ↓ in IN NS | Rinaldi et al. (2019) |
| Piperine | Alzheimer’s disease | NP (Chitosan, Tripolyphosphate, Poloxamer 188/Ionic gelation method) | 248.50 nm | In vivo pharmacological studies in AD-induced Wistar-rats | Piperine-NPs significantly improved cognitive function as efficiently as standard drug | Elnaggar et al. (2015) |
| Quetiapine | Antipsychotic | NE (Capmul MCM, Tween 80, Transcutol P, PG/Ultrasonication method) | 144 ± 0.5 nm | In vivo drug distribution study in Wistar-rats |
IN administration of quetiapine-loaded NE had shorter Tmax than IV administration Drug transport efficiency ↑ and direct nose-to-brain drug transport were achieved by NE |
Boche and Pokharkar (2017) |
| Quetiapine | Antipsychotic | NP (Chitosan, Tripolyphosphate/Ionic gelation method) | 131.08 ± 7.45 nm |
Ex vivo permeation studies using goat nasal mucosa In vivo PK study using SD-rats |
Diffusion of quetiapine ↑ with NP system compared to pure drug solution Brain/blood ratio ↑ and twofold higher nasal bioavailability in the brain with quetiapine-NPs compared to drug solution |
Shah et al. (2016) |
| Resveratrol | Parkinson’s disease | NE (Labrafac Lipophile, Cremophor RH40/Spontaneous emulsification method) | 176.3 ± 3.5 nm | In vivo biodistribution study in Wistar-rats | IN NE increased the AUC in the brain by sevenfold compared to IN solution | Nasr (2016) |
| Resveratrol | Alzheimer’s Disease | Gold NP (Gold-III chloride, Trisodium citrate/Simple reduction method) | 10.30 ± 2.4 nm |
Ex vivo permeation studies In vivo pharmacological studies in rats |
Behavioral acquisition and spatial memory function ↑ in amnestic rats Accumulation of gold NPs was observed in the rat brain |
Salem et al. (2019) |
| Risperidone | Alzheimer’s disease | NE (Capmul MCM, Tween 80, Transcutol, PG/Spontaneous emulsification method) | 16.7 ± 1.21 nm | In vivo biodistribution, PK, and PD studies in Swiss-albino rats |
Drug concentration (0.11%/g) and AUC (0.48 h·%/g) ↑ in the brain Tmax (1 h) ↓ in the brain Locomotor activity ↓ in the risperidone NE IN administration group compared to the risperidone NE IV administration group Major radioactivity accumulation was seen in the brain following IN administration of MA risperidone NE compared to IV administration of risperidone NE |
Kumar et al. (2008) |
| Rivastigmine | Alzheimer’s disease | NE (Capmul MCM, Tween 80, Transcutol-P/Spontaneous emulsification method) | 35.75 ± 0.21 nm |
Ex vivo diffusion study using goat nasal mucosa In vivo PK and biodistribution studies in Wistar-rats |
Drug permeation of NE was greater than that of solution IN NE exhibited significantly higher drug concentrations in the brain than IN solution and IV NE |
Haider et al. (2018) |
| Rivastigmine | Alzheimer’s disease | LS (Cell-penetrating peptides, DSPE-PEG/Thin film evaporation method) | 178.9 ± 11.7 nm |
In vitro drug permeation studies In vivo PK and PD studies in SD-rats |
Cell-penetrating peptide modified LSs enhanced permeability across the BBB in murine brain microvascular endothelial cell model in vitro IN application of rivastigmine formulations significantly increased the distribution of rivastigmine into the plasma and CNS regions compared to IV administration of rivastigmine solution IN administration showed lagging but intense inhibition of AChE and BChE activities compared to IV administration |
Yang et al. (2013) |
| Saquinavir mesylate | Infection | NE (Capmul MCM, Tween 80, PEG 400/Spontaneous emulsification method) | 176.3 ± 4.21 nm |
Ex vivo drug permeation studies in vivo biodistribution studies |
Optimized NE showed ↑ in drug permeation rate compared to plain drug suspension Drug concentrations ↑ in the brain after IN administration of NE compared to IV drug suspension |
(Mahajan et al. (2014) |
| Selegiline | Parkinson’s disease | NE (Grape seed oil, Sefsol 218, Tween 80, Lauroglycol 90/High-pressure homogenization method) | 61.43 ± 4.10 nm |
Ex vivo diffusion study using porcine nasal mucosa Behavioral activity of Parkinson’s disease in Wistar-rats |
NE improved drug permeation by 3.7-fold compared to drug solution IN NE showed ↑ in behavioral activities compared to IN and IV drug solution |
Kumar et al. (2016) |
| Valproic acid | Seizure | NLC (Cetyl palmitate, Poloxamer 188, OD, Soy lecithin S100/Solvent diffusion and evaporation method) | 154 ± 16 nm | In vivo PK and PD studies in Wistar-rats |
Brain/plasma concentration ratio was much higher (about five times) in IN NLCs than IP NLCs A similar protective effect was observed in rats treated with IN and IP NLCs |
Eskandari et al. (2011) |
| Ziprasidone HCl | Antipsychotic | NE (Capmul MCM, Labrasol, Transcutol, Chitosan/Spontaneous emulsification method) | 145.24 ± 4.75 nm |
Ex vivo diffusion study In vivo PD study in Wistar-rats |
Drug diffusion ↑ than the solution PD study revealed the superiority of NE in the locomotor activity and paw tests |
Bahadur and Pathak (2012) |
| Zolmitriptan | Migraine | NE (Capryol PGMC, Kolliphore RH40, Transcutol-P, Chitosan/Ultrasonication method) | 54.63 ± 3.24 nm |
Ex vivo drug permeation study using sheep nasal mucosa In vivo PK and biodistribution studies in SD-rats |
NE showed permeability coefficients ↑ than the solution IN NE showed AUC ↑ and Tmax ↓ in the brain compared to IN and IV solution |
Abdou et al. (2017) |
IN intranasal; IV intravenous; IP intraperitoneal; PLGA polylactic-co-glycolic acid; PEG poly-ethylene glycol; PG propylene glycol; IPM isopropyl myristate; DCP dicetyl phosphate; DSPE 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; PGMC propylene-glycol monocaprylate; OD octyldodecanol; PK Pharmacokinetic; PD Pharmacodynamic; MA mucoadhesive; NP nanoparticle; NS niosome; NSP nanosuspension; SD Sprague–Dawley; AUC area under the curve; Tmax time to reach maximum concentration; Cmax maximum concentration; LF lactoferrin; NE nanoemulsion; LS liposome; NLC nanostructured lipid carrier; bFGF basic fibroblast growth factor; HupA Huperzine A; GH galantamine hydrobromide; AChE acetylcholinesterase; BChE butyrylcholinesterase; CNS cranial nervous system
One of the major components of nanocarriers frequently used in nasal-brain drug delivery is chitosan. In previous reports (Abd Elgadir et al. 2015; Baldrick 2010), chitosan was selected as the most suitable candidate for the application of a nanocarrier system in nasal-brain drug delivery because chitosan has several advantages such as biodegradability, low toxicity, and high stability for application in drug delivery systems (Baldrick 2010). In addition, chitosan has strong mucosal adhesion, so when administered intranasally, many drugs can be delivered to the brain with relatively low physical clearance (Alexander et al. 2014). In this regard, by measuring the amount of mucin adsorbed on the surface of chitosan-complexed nanoparticles, the ability of chitosan-based nanocarriers to adhere to the mucosal was confirmed (Hanafy et al. 2015). According to that report, strong mucosal adhesion persisted up to 60 min after the administration of chitosan-complexed nanoparticles. And microscopic observation confirmed that a significant amount of chitosan-complexed nanoparticles was delivered to various parts of the brain, including the olfactory bulb, approximately 1 h after nasal administration (Hanafy et al. 2015). Consequently, past studies (Abd Elgadir et al. 2015; Hanafy et al. 2015) have suggested that the application of chitosan as a component of nanocarriers in nasal-brain drug delivery was highly effective.
Prodrugs
As a strategy for delivering drugs from the nasal cavity to the brain, attempts have been made to administer drugs in prodrug form into the nasal cavity. The formulation as a prodrug has been carried out to increase solubility in aqueous media, mainly where the parent drug has limited solubility because drug solubilization is a major factor that affects the delivery of a large amount of drug by a single intranasal dose without relatively large fluctuations between dosings (Di Mauro 2008).
According to one past report (Al-Ghananeem et al. 2002), 17β-estradiol was formulated as a prodrug to improve drug delivery to the cranial nervous system through intranasal administration. When 17β-estradiol was administered orally, first-pass metabolism in the gastrointestinal tract and liver reached approximately 95% (Al-Ghananeem et al. 2002; Bawarshi-Nassar et al. 1989), and although prodrug administration was attempted, oral bioavailability was not significantly improved (Al-Ghananeem et al. 2002; Hussain et al. 1988). Alternatively, transdermal administration has been performed, but systemic and frequent skin irritation side effects were reported (Al-Ghananeem et al. 2002). Therefore, an attempt was made to administer 17β-estradiol intranasally in solubilized prodrug ester form by attaching hydrophilic functional groups to 17β-estradiol, which is highly lipophilic (Al-Ghananeem et al. 2002). The results confirmed rapid in vivo absorption and high bioavailability, and high drug delivery to the brain (Al-Ghananeem et al. 2002). However, systemic side effects are associated with 17β-estradiol, a kind of hormone, so 17β-estradiol was delivered locally to the cranial nervous system via the nasal-brain administration route. When 17β-estradiol in the prodrug ester form was administered intravenously and nasally, the concentration of 17β-estradiol in CSF was approximately 4–9 times higher when administered intranasally than by the IV route (Al-Ghananeem et al. 2002). In another study, curcumin, which has antioxidant and anticancer properties, was formulated as an ester-form prodrug and applied nasally for delivery to the brain (Di Mauro 2008). Due to the extensive in vivo metabolism (especially in the liver and kidneys) of curcumin, which is highly lipophilic, the amount that can reach the brain through the systemic circulation is very low, at less than 0.1% of the dose (Di Mauro 2008). Therefore, attempts have been made to effectively deliver curcumin using the nasal-brain route (Di Mauro 2008). Both the nasally administered 17β-estradiol and curcumin prodrugs are likely to deliver the parent drug to the brain by similar mechanisms. That is, when the drug in prodrug form reaches the olfactory region, the prodrug is metabolized to the parent lipophilic drug and can easily diffuse into the brain tissue. The metabolism from prodrug to parent drug occurs mainly through chemical or enzymatic hydrolysis reactions with esterase present in the nasal mucosa and brain (Di Mauro 2008).
Limitations of nasal-brain drug delivery
The limitations of nasal-brain drug delivery were largely related to the evaluation of the effectiveness of selective delivery to the brain and the consideration of safety aspects.
Delivery efficiency
Intranasal drug administration is generally very limited in terms of dosage compared to other routes of administration. One previous study (Emirzeoglu et al. 2012) reported that the nasal volumes in men and women aged 18–40 years were 7.01 ± 0.18 cm3 and 5.95 ± 0.10 cm3, respectively. These were very small volumes compared to the skin, gastrointestinal tract, and blood vessels, suggesting that the amount of drug that could be administered to the nasal cavity is limited compared to oral, IV, transdermal, and subcutaneous administration. This may be supported by the low administrable volumes in the nasal cavity of approximately 25–200 μL (Pandey et al. 2020). When an excessive amount of drug is administered to the nasal passages, the nasal cavity is easily saturated (Pandey et al. 2020), which may increase drug transport into the systemic circulation and cause side effects.
In intranasal drug administration, swallowing or drainage may occur, not the intended injection into the nasal mucosa, depending upon the administration methods such as the administration angle and respiration level. This is an area of concern that can lead to large inter-individual and/or inter-trial variability in drug delivery to the brain. According to a past report (Foo et al. 2007), in the case of nasal administration using nasal spray, the device plume angle and administration angle were major factors affecting nasal deposition efficiency. In terms of effectiveness, the intranasal injection of a solution or administration as a spray will definitely have the advantage of delivering the drug to the brain, avoiding the first-pass effect, and providing a quick effect. However, there are still many problems to be solved in terms of accurate usage and dosage settings because nasally administered solutions and sprays flow downward, and/or the drug cannot stay in the desired nasal cavity area.
As for formulation limitations, the stability aspect of formulations applied for drug delivery from the nasal cavity to the brain may be a potential problem. That is, the stability of the drug loaded in the formulation becomes a problem due to physical and chemical stresses from the outside, so the effective drug concentration delivered to the brain can be very low. Another aspect is that hydrophilic drugs will have limited absorption from the nasal mucosa into the cranial nervous system through paracellular or transcellular mechanisms. Therefore, for drugs with high hydrophilicity, methods such as the incorporation of permeation enhancers and/or formulations using nanocarriers will be indispensable.
As a limitation in terms of the route, there are difficulties in applying intranasal as a route for drug delivery to the brain in some special cases because if there is a disease in the nasal cavity, direct administration to the site is difficult. In addition, there is a possibility that the administered drug will disappear rapidly due to physical nasal-mucosal clearance including mucosal ciliary effects and rapid airflow in the nasal environment. Therefore, additives such as nasal mucoadhesives should be considered essential in the drug formulation process.
As mentioned earlier, there are four major routes (olfactory, respiratory, systemic via BBB, and NALT pathways) for drug delivery from the nasal cavity to the brain (Fig. 1), and depending upon the route, drugs can be delivered to the brain either directly or indirectly. However, since the intranasal sites related to the four routes are different from each other, drug delivery to the brain through the nasal cavity has a limitation in that it is essentially dependent upon the site where the drug or formulation is seated after nasal administration. Therefore, a formulation method such as using mucoadhesive may be an effective alternative to overcoming the drug attachment site-dependent drug delivery problem.
The cranial nervous system is a very complex system, and the pathogenesis and key causes of most cranial nervous-related diseases have not yet been clearly identified (Feigin et al. 2021). Therefore, it is still necessary to study the key pathogenesis factors and related mechanisms of various cranial nervous system diseases. And through this, the development of targeting technology for a specific area within the brain nervous system will be required. It will be necessary to not only increase the drug delivery from the nasal cavity to the brain but also to minimize the side effects by allowing the drug transferred into the brain to act only on a specific target within the brain nervous system.
Safety issues
Formulations and drugs can exert potentially biotoxic effects on the nasal cavity and brain because frequent and continuous intranasal drug administration may cause irritating and damage not only to the nasal mucosa but also to the olfactory nervous system distributed there. In particular, there is a concern that the continuous use of a permeation enhancer that promotes the permeation of the formulation by causing reversible expansion between cells in the nasal mucosa may increase the influx of external pathogens and cause cell transformation. In addition, in the case of a mucoadhesive applied to the nasal mucosa for physical maintenance of the formulation, components can cause toxicity to the mucociliary system by long-term retention. Although no life-threatening side effects were reported in the preclinical and clinical studies of nasal-brain drug delivery introduced above (see section “Applicable formulations”), most of these were the results of single or short-term exposure to the formulations. For example, a hydrogel composed of pluronic F-127 and poloxamer 188 administered as a nasal mucosal adhesive was maintained without mucociliary toxicity for 14 days after application (Chen et al. 2013). And in relation to the nasal-brain drug delivery application of galanthamine-loaded liposomes, an in vitro toxicity test on the rat pheochromocytoma cell line incubated with the formulation for 12 h did not show any significant cytotoxicity (Li et al. 2012). However, since most of the drugs to be delivered to the brain require long-term application for many years, sub-chronic or chronic toxicity and long-term safety evaluation of the formulations are essential.
Potentially fatal systemic side effects can result from direct drug delivery from the nasal cavity to the brain. Excessive and rapid drug delivery to the brain may disturb the immune system of the cranial nervous system and cause drug toxicity. Therefore, not only formulation studies, but also pharmacometrics studies related to clinical doses and dosage settings for each formulation need to be conducted together to minimize the side effects and maximize the therapeutic effects. However, such studies are relatively difficult compared to other routes such as oral and IV administration due to the lack of mechanistic clarity of the nasal-brain drug delivery route and the existence of uncertainties that the degree of drug delivery to the brain may vary greatly by various variables (such as formulation characteristics, administration methods, and physiological variability between individuals). Thus, many issues need to be overcome and resolved before the nasal-brain drug delivery route can be applied clinically for specific drugs. Sufficient verification and confirmation of the safety and effectiveness of nasal-brain drug delivery agents are required.
Clinical application cases and research status
Formulation development and several clinical studies have been reported in relation to nasal drug administration. The nasal route has been chosen for effective systemic drug delivery, as well as local therapeutic purposes. Clinical trials to deliver various therapeutic drugs targeting the cranial nervous system to the brain by focusing on the nasal-brain pathway are underway.
Table 2 shows examples of drugs that have been marketed and used for drug application in the nasal cavity to date. Onzetra® Xsail® (Al-Salama and Scott 2016), and Spravato® (Jalloh 2020), which were relatively recently approved by the Food and Drug Administration (FDA) and marketed, are nasal drugs applicable to migraine and depressive disorders, respectively, and are commonly used formulations to relieve the symptoms of neurological disorders. Trudhesa® was also recently approved for marketing by the FDA as a nasal spray formulation for acute relief of migraine (FDA 2021b). Therefore, considering that the pharmacological action point of these drugs is the cranial nervous system, the three formulations of Trudhesa®, Onzetra® Xsail®, and Spravato® will be closely related to drug delivery from the nasal cavity to the brain. In addition, some drugs have been approved for intranasal administration for topical treatment and adjuvant therapy not targeting the brain nervous system. Patanase® (FDA 2009), Optivar® (Wolff et al. 2007), and Nasonex® (Berkowitz et al. 1999) are formulations prescribed to treat inflammatory and allergic symptoms in the nasal passages. Narcan® has been approved by the FDA for the acute treatment of symptoms of opioid poisoning (FDA 2015). The marketing of Narcan® suggests that rapid drug delivery to the CNS is possible via the nasal passages. In addition, with respect to viral vaccines, FluMist® has been approved for marketing by the FDA for the purpose of preventing influenza infection (FDA 2021a), and HeberNasvac® has been marketed from Cuba for the prevention and/or treatment of hepatitis B (Pentón-Arias and Aguilar-Rubido 2021). Both FluMist® and HeberNasvac® are nasal spray formulations, and their mechanism of action will be highly relevant to activating the immune system via the nasal passages. And Natesto® (Rogol et al. 2016) is a formulation prescribed to systemically compensate for testosterone in patients with hormone deficiencies and related conditions. These uses suggest that the nasal delivery route would be efficient not only for the topical treatment of nasal-related diseases, but also for hormone supply through the systemic circulation. In addition, Miacalcin® (FDA 2017a), Noctiva® (FDA 2017b), Baqsimi® (FDA 2019), and Synarel® (FDA 2012) have been approved by the FDA as hormone adjuvant therapy through intranasal administration of peptide or hormonal proteinaceous substances. Suprefact®, formulated as a nasal solution of buserelin, a synthetic peptide analogue of a luteinizing hormone-releasing hormone (LHRH) agonist, is marketed in Canada for the treatment of sex hormone-related endometriosis and prostate cancer (Rohrer et al. 2018). Consequently, the marketing of Miacalcin®, Noctiva®, Baqsimi®, Synarel®, and Suprefact® for nasal applications suggested an effective delivery of peptides and hormonal proteins through the nasal passages.
Table 2.
Examples of nasal preparations marketed for drug delivery through the nasal cavity
| Drug | Route | Disease | Information | Approval | References |
|---|---|---|---|---|---|
| Azelastine | IN | Allergy |
Commercial name, Optivar® (nasal spray formulation) Optivar® is an antihistamine that is only available by prescription Relieves symptoms such as stuffy or runny nose, itching, sneezing, allergic rhinitis, hay fever, vasomotor rhinitis, and other upper respiratory allergies |
FDA approved Optivar® as an antihistamine medication in 2000 | Wolff et al. (2007) |
| Buserelin | IN | Endometriosis & hormone-dependent advanced carcinoma of the prostate gland |
Commercial name, Suprefact® (nasal solution formulation) Suprefact® is a medication which is used primarily in the treatment of prostate cancer and endometriosis (through the reduction of sex hormones) |
Suprefact® was approved for sale in Canada as hormone supplementary medication | Rohrer et al. (2018) |
| Calcitonin | IN | Osteoporosis |
Commercial name, Miacalcin® (nasal spray formulation) Miacalcin® is indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause |
FDA approved Miacalcin® as hormone supplementary medication in 2003 | FDA (2017a) |
| Desmopressin | IN | Nocturnal polyuria |
Commercial name, Noctiva® (nasal spray formulation) Noctiva® is indicated for the management of nocturnal polyuria in adults who awaken at least two times per night to void |
FDA approved Noctiva® as hormone supplementary medication in 2017 | FDA (2017b) |
| Dihydroergotamine mesylate | IN | Migraine |
Commercial name, Trudhesa® (nasal spray formulation) Trudhesa® is indicated for the acute treatment of migraine with or without aura in adults |
FDA approved Trudhesa® as a migraine medication in 2021 | FDA (2021b) |
| Esketamine | IN | Depressive disorder |
Commercial name, Spravato® (nasal spray formulation) Spravato® is a medication prescribed for adults with treatment-resistant depression and adults with major depressive disorder with suicidal thoughts or behaviors |
FDA approved Spravato® as antidepressant medication in 2019 | Jalloh (2020) |
| Hepatitis B virus vaccine | IV | Chronic hepatitis B virus infection |
Commercial name, HeberNasvac® (nasal spray formulation) HeberNasvac® is a therapeutic recombinant vaccine for immunotherapy against chronic hepatitis B virus infection and prevention of its possible consequences |
HeberNasvac® was approved for sale in Cuba as a medication for preventing hepatitis B virus infection | Pentón-Arias and Aguilar-Rubido (2021) |
| Influenza virus vaccine | IN | Influenza (for prevention) |
Commercial name, FluMist® (nasal spray formulation) FluMist® Quadrivalent is a vaccine indicated for active immunization for the prevention of influenza disease caused by influenza A subtype viruses and type B viruses contained in the vaccine |
FDA approved FluMist® for the prevention of influenza illness in 2003 | FDA (2021a) |
| Glucagon | IN | Severe hypoglycemia |
Commercial name, Baqsimi® (nasal powder formulation) Baqsimi® is indicated for the treatment of severe hypoglycemia in patients with diabetes ages 4 years and above |
FDA approved Baqsimi® as hormone supplementary medication in 2019 | FDA (2019) |
| Mometasone | IN | Allergy & rhinitis |
Commercial name, Nasonex® (nasal spray formulation) Relieves symptoms such as sneezing, runny, stuffy, and itchy nose It is also used to treat nasal polyps (swelling of the lining of the nose) |
FDA approved Nasonex® as an antiallergy medication in 1997 | Berkowitz et al. (1999) |
| Nafarelin | IN | Central precocious puberty |
Commercial name, Synarel® (nasal solution formulation) Synarel® is indicated for treatment of central precocious puberty (gonadotropin-dependent precocious puberty) in children of both sexes Synarel® is also used to treat symptoms of endometriosis such as pelvic pain, menstrual cramps, and painful intercourse |
FDA approved Synarel® as hormone supplementary medication in 1998 | FDA (2012) |
| Naloxone | IN | Opioid overdose |
Commercial name, Narcan® (nasal spray formulation) Narcan® is indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression |
FDA approved Narcan® as an antidote to opiate addiction in 2015 | FDA (2015) |
| Olopatadine | IN | Allergy & rhinitis |
Commercial name, Patanase® (nasal spray formulation) Patanase® is indicated for the relief of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 6 years of age and older |
FDA approved Patanase® as an antihistamine medication in 2008 | FDA (2009) |
| Sumatriptan | IN | Migraine |
Commercial name, Onzetra® Xsail® (nasal powder formulation) Onzetra® Xsail® is a prescription medication approved for the acute treatment of migraine, with or without aura in adults |
FDA approved Onzetra® Xsail® as a migraine medication in 2016 | Al-Salama and Scott (2016) |
| Testosterone | IN | Hormone disorder |
Commercial name, Natesto® (nasal gel formulation) Natesto® is a prescription medicine that contains testosterone and is used to treat adult males who have low or no testosterone due to certain medical conditions |
FDA approved Natesto® as hormone supplementary medication in 2014 | Rogol et al. (2016) |
IN intranasal; FDA Food and Drug Administration
The nasal delivery of a limited number of drugs has been evaluated in several trials. Table 3 presents examples of the clinical applications of drug delivery through the nasal cavity. Most of the trials performed have been on drugs used to relieve the symptoms of mental disorders. Intranasal delivery of insulin to the brain not only alleviated diabetic neurological diseases but was clinically confirmed to be effective in relieving symptoms in patients with Alzheimer’s and cognitive impairment (Benedict et al. 2011; Craft et al. 2012; Schmidt et al. 2009). Clinical trials of hyposmia patients showed that the nasal administration of insulin was effective in improving the sense of smell (Rezaeian 2018). And improved brain function was reported from the intranasal administration of vasopressin (Brunnlieb et al. 2013; Pietrowsky et al. 1996a; Rilling et al. 2012). The intranasal administration of psychotropic drugs such as midazolam (Hosseini Jahromi et al. 2012; Javadzadeh et al. 2012; Klein et al. 2011; Tsze et al. 2017), remimazolam (Pesic et al. 2020), diazepam (Gizurarson et al. 1999), ketamine (Frey et al. 2019; Hosseini Jahromi et al. 2012; Shimonovich et al. 2016), esketamine (Daly et al. 2018), fentanyl (Frey et al. 2019), and dexmedetomidine (Zhang et al. 2013) showed significant and rapid sedative effects in most clinical studies. These findings suggest that the clinical effect was improved by increasing drug delivery from the nasal cavity to the brain nervous system, which is the point of action of the drugs. The nasal administration of glutathione to Parkinson’s patients showed satisfactory tolerability and safety, and there was no significant deterioration in behavioral evaluation (Mischley et al. 2015). Therefore, these results set the clinical basis for the long-term intranasal application of glutathione in a larger number of patients in the future. The nasal administration of cholecystokinin-8 to healthy adults confirmed the delivery of peptides to the brain (Pietrowsky et al. 1996b). And the nasal administration of the antidote naloxone to opiate-addicted patients demonstrated effective clinical effects such as a higher level of consciousness than when administered intravenously (Sabzghabaee et al. 2014). Overall, a significant number of clinical studies have concluded that intranasal drug administration was effective for symptom relief and as an alternative to conventional routes such as oral, IV, and subcutaneous routes. In particular, intranasal drug administration was reported to be more positive in the treatment of diseases related to the cranial nervous system. These results imply that drug delivery to the brain via the nasal cavity is clearly possible and very useful clinically.
Table 3.
Examples of clinical applications of drug delivery through the nasal cavity
| Drug | Route | Disease | Study information | Effects | References |
|---|---|---|---|---|---|
| AVP | IN | – | In a double-blind, crossover study, healthy men (n = 15) received 20 IU of AVP IN three times, 1.5 IU of AVP IV, or saline solution | The results provide functional evidence that in the human brain, the effects of peptides like AVP may be facilitated after IN administration compared to IV administration | Pietrowsky et al. (1996a) |
| AVP | IN | – | Twenty IU of AVP was nasally administered in a randomized, placebo-controlled, double-blind manner to 36 healthy men using a between-subjects design |
Activation in the right superior temporal sulcus was increased by AVP treatment No differences were found at the behavioral level between AVP treatment and placebo |
Brunnlieb et al. (2013) |
| AVP & OT | IN | – | Ninety-one healthy men 18–22 years old received IN OT (n = 27), IN AVP (n = 27) or IN placebo (n = 36) in a double-blind, placebo-controlled study |
OT also enhanced left amygdala activation in response to reciprocated cooperation AVP strongly increased cooperation in response to a cooperative gesture by the partner compared to both placebo and OT Both OT and AVP increased amygdala functional connectivity with the anterior insula relative to placebo, which may increase the amygdala’s ability to elicit visceral somatic markers that guide decision making |
Rilling et al. (2012) |
| CCK | IN | – |
IN (10 μg) and IV (0.25 and 2.5 μg) administered CCK on auditory event-related potential in 20 healthy subjects The study was designed as a placebo-controlled, double-blind within-subject cross-over comparison |
Adrenocorticotropic hormone concentrations reached significance selectively following the IN mode of CCK administration This study provides functional evidence for the direct access of peptides to the human brain after IN administration |
Pietrowsky et al. (1996b) |
| COVID-19 vaccine | IN | – |
Adenovirus type 5 vector based COVID-19 vaccine (Ad5-nCoV) was administered to 130 healthy adults in a randomized, open-label, phase 1 trial IM single dose, IM two doses, IN high dose, IN low dose, IM + IN (n = 26 in each group) |
A single dose of aerosolized IN vaccine, equivalent to a fifth of the IM dose could induce strong cellular response An aerosolized IN booster vaccination at 28 days after first IM dose induced strong IgG and neutralizing antibody responses |
Wu et al. (2021) |
| Dexmedetomidine | IN | Vascular malformation | Placebo-controlled randomized clinical trial of 60 patients 18–60 years old who received dexmedetomidine 1 μg/kg IN |
After nasal administration, adequate sedation was achieved within 30–45 min IN dexmedetomidine was a sedative under local anesthesia and was suggested as a non-invasive and well-tolerated alternative to IV administration |
Zhang et al. (2013) |
| Diazepam | IN | – | Nine healthy subjects participated in an open crossover study on IN versus IV administration of diazepam (2 mg) | IN administered diazepam may be an effective alternative to IV administration for the relief of seizures | Gizurarson et al. (1999) |
| Esketamine | IN | Depressive disorder | Randomized, double-blind clinical trial of 67 adults 20–64 years old with treatment-resistant depression |
Significant improvement in depressive symptoms was observed after 1 week of IN esketamine, 28–84 mg administered twice weekly, with a significant ascending dose–response relationship Improvement appeared to be sustained with reduced dosing frequency for up to 9 weeks Results of this clinical trial of IN esketamine for treatment-resistant depression support study in larger trials |
Daly et al. (2018) |
| Glucagon | IN | Hypoglycemia |
Randomized, open-label, two-period, crossover trial of type-1 diabetic 66 patients 18–64 years old Participants were randomly assigned to receive either IN glucagon (3 mg) or IM glucagon (1 mg) |
IN glucagon was as efficacious and well tolerated as IM glucagon for the treatment of insulin-induced hypoglycemia in adults IN glucagon has been suggested to be as useful as IM glucagon as a rescue treatment for severe hypoglycemia |
Suico et al. (2020) |
| Glutathione | IN | Parkinson’s disease | Thirty Parkinson’s disease patients were randomized to either placebo (saline), 300 mg/day, or 600 mg/day IN glutathione in three divided daily doses |
After 3 months, all groups met tolerability criteria These data supported the safety and tolerability of IN glutathione in the patient population |
Mischley et al. (2015) |
| Insulin | IN | Alzheimer’s disease |
One hundred and four adults with amnestic mild cognitive impairment (n = 64) or mild-to-moderate Alzheimer’s disease (n = 40) Participants received placebo (n = 30), 20 IU of insulin (n = 36), or 40 IU of insulin (n = 38) for 4 months, administered with a nasal drug delivery device |
The pilot trial results demonstrated that the administration of IN insulin stabilized or improved cognition and function Safety profiles and compliance were excellent for this short-term intervention Taken together, these results provide an impetus for future clinical trials of IN insulin therapy and for further mechanistic studies of insulin’s role in the pathogenesis of Alzheimer’s disease |
Craft et al. (2012) |
| Insulin | IN | Alzheimer’s disease | Randomized clinical trial of 289 adults with mild cognitive impairment or Alzheimer’s disease dementia |
No cognitive or functional benefits were observed with IN insulin treatment compared to placebo over a 12-month period in the primary analyses The results suggest that further investigation of IN delivery devices is needed, as well as consideration of the therapeutic benefit of IN insulin in the treatment of persons with mild cognitive impairment and Alzheimer’s disease dementia |
Craft et al. (2020) |
| Insulin | IN | – | In a double-blind, placebo-controlled, balanced within-subject comparison, 19 healthy normal-weight men (18–26 years old) were IN-administered 160 IU human insulin after an overnight fast |
IN insulin increased postprandial energy expenditure compared to placebo Enhancing brain insulin signaling by means of IN insulin administration enhanced acute thermoregulatory and glucoregulatory responses to food intake, suggesting that central nervous insulin contributes to the control of whole-body energy homeostasis in humans |
Benedict et al. (2011) |
| Insulin | IN | Hyposmia |
Thirty-eight patients 18–70 years old with hyposmia Patients were randomly divided into two parallel groups IN insulin gel foam (40 IU) and saline-soaked gel foam were delivered into the olfactory cleft for the test and placebo groups, respectively |
IN insulin (40 IU) administration may trigger improvement in the olfactory sense and also appears to be free of significant adverse events | Rezaeian (2018) |
| Insulin | IN | Gene deletion | Six children with 22q13 deletion syndrome received IN insulin over a period of 1 year | Long-term administration of IN insulin may benefit motor development, cognitive functions, and spontaneous activity in children with 22q13 deletion syndrome | Schmidt et al. (2009) |
| Ketamine | IN | Pain | Ninety patients (18–70 years) experiencing moderate-severe acute traumatic pain participated in a single-center, randomized, prospective, parallel clinical trial where 1.0 mg/kg ketamine was administered IN | IN ketamine showed effective efficacy and safety | Shimonovich et al. (2016) |
| Ketamine & fentanyl | IN | Pain | Randomized clinical trial of 90 children 8–17 years old with pain due to traumatic limb injury |
Both ketamine and fentanyl administered IN provided effective analgesic effects Nasal administration of ketamine and fentanyl was suggested as an appropriate alternative to opioids for pain associated with acute limb injury |
Frey et al. (2019) |
| Ketamine & midazolam | IN | Anxiety |
One hundred and twenty children 2–8 years old, double-blinded study 0.2 mg/kg midazolam, 0.5 and 3 mg/kg ketamine administered IN |
IN administrations of midazolam and ketamine were effective in reducing preoperative pediatric anxiety | Hosseini Jahromi et al. (2012) |
| Midazolam | IN | Pain & trauma |
Randomized, single-blinded, 3-arm, superiority clinical trial of 99 children 1–7 years old Children undergoing laceration repair requiring 0.5 mg/kg IN midazolam (5 mg/mL) were block-randomized to receive midazolam using 1 of 3 volumes of administration: 0.2, 0.5, or 1 mL |
Sedative effects were observed within approximately 5 min after administration | Tsze et al. (2017) |
| Midazolam | IN | Seizure |
Sixty patients (0.17–15 years) participated IN midazolam was administered at 0.2 mg/kg |
The time needed to control seizures using IN midazolam (3.16 ± 1.24 min) was statistically shorter than IV diazepam (6.42 ± 2.59 min) | Javadzadeh et al. (2012) |
| Midazolam | IN | Pain | One hundred and sixty-nine children 0.5–7 years old needing non-parenteral sedation for laceration repair | IN midazolam was an effective and useful alternative to oral midazolam for sedation for laceration repair | Klein et al. (2011) |
| Mupirocin | IN | Infection |
Three hundred ninety-five patients who underwent abdominal digestive surgery were assigned randomly into two groups: a treated group (193) and controls (202) Patients in the treated group were given 30 mg mupirocin calcium hydrate ointment topically to each nostril three times a day on each of the 3 days before surgery. The untreated group received no mupirocin treatment |
IN mupirocin treatment had no significant impact on surgical-site infection after digestive surgery | Suzuki et al. (2003) |
| Naloxone | IN | Opiate addiction |
One hundred opioid overdose patients were assigned by random allocation into two study groups (n = 50) Both groups received 0.4 mg naloxone: one group IN and the other IV |
Patients who were administered IN naloxone demonstrated significantly higher levels of consciousness than those in the IV group IN naloxone was as effective as IV naloxone in reversing both respiratory depression and depressive effects on the central nervous system caused by opioid overdose |
Sabzghabaee et al. (2014) |
| OT | IN | Social anxiety disorder | Twenty-five social anxiety disorder patients in a randomized, double-blind, placebo-controlled trial. The test group was administered 24 IU of OT |
Participants administered OT showed improved positive evaluations of appearance and speech performance as exposure treatment sessions progressed The administration of OT improved mental representations of self, following exposure therapy |
Guastella et al. (2009) |
| OT | IN | Autism |
One hundred and six ASD patients 18–48 years old participated in a randomized, parallel-group, multicenter, placebo-controlled, double-blind trial Participants were randomly assigned to 6-week IN OT (48 IU/day, n = 53) or placebo (n = 53) groups |
Plasma OT was only elevated from baseline to endpoint in the OT group compared to the placebo group No significant difference in the prevalence of adverse events was observed between the groups The findings suggested the possibility of OT to treat ASD repetitive behavior |
Yamasue et al. (2020) |
| OT | IN | Autism |
Twenty-five male ASD patients 15–33 years old participated in a double-blind, cross-over, placebo-controlled study A single dose of OT or placebo was applied IN |
OT increased bilateral amygdala responsiveness during the physical pain task for both painful and neutral stimuli However, there were no effects of OT treatment |
Mayer et al. (2021) |
| Remimazolam | IN | – |
Ten healthy male volunteers 18–45 years old participated in a randomized, double-blind, 9-period cross-over design study Single IN doses of 10, 20, and 40 mg remimazolam as powder or solution with IN placebo |
IN administration of remimazolam was safe and caused sedative effects However, IN remimazolam caused severe pain and discomfort |
Pesic et al. (2020) |
| RSV vaccine | IN | – | Forty-eight healthy volunteers 18–49 years old participated in a randomized, double-blind, placebo-controlled trial |
Induced systemic plasmablast responses and significant, durable increases in RSV-specific serum antibodies in healthy subjects Therefore, it was proposed as a non-replicating IN RSV subunit vaccine that induces a sustained antibody response in human volunteers |
Ascough et al. (2019) |
IN intranasal; IV intravenous; IM intramuscular; IgG Immunoglobulin G; AVP arginine-vasopressin; OT oxytocin; ASD autism spectrum disorder; CCK cholecystokinin; RSV respiratory syncytial virus
In addition, in order to obtain an effective systemic response, rather than the main purpose of the action in the brain, clinical trials for intranasal administration of virus vaccines and hormones have been conducted. A clinical trial in which a respiratory syncytial virus (RSV) vaccine was administered intranasally confirmed that the immune response in the body was increased compared to placebo (Ascough et al. 2019). And intranasal administration of adenovirus type 5 vector based COVID-19 vaccine (Ad5-nCoV) has been confirmed to have an effect equivalent to or superior to that of intramuscular injection (Wu et al. 2021). In addition, glucagon, an important hormone for raising blood glucose levels, has been confirmed to be effective in diabetic patients suffering from acute hypoglycemia (Suico et al. 2020). These suggested that, as mentioned above, the delivery of peptide hormone drugs and vaccines into the body through the nasal cavity is sufficiently effective and feasible.
However, not all cases of intranasal drug administration showed satisfactory clinical effects. That is, in some cases, no significant improvement was confirmed in terms of the targeted clinical effect according to intranasal drug administration. As an example, although intranasal administration of oxytocin (OT) has been shown to increase the delivery of OT to the cranial nervous system (Guastella et al. 2009; Mayer et al. 2021; Yamasue et al. 2020), no significant clinical effects on alleviating psychiatric disorders has been identified in some cases (Mayer et al. 2021). And although it was not a study targeting the cranial nervous system, intranasal administration of mupirocin to patients undergoing abdominal digestive surgery did not sufficiently prevent infections in the surgical site (Suzuki et al. 2003). In the case of OT and mupirocin, the clinical usefulness of intranasal administration may become clearer as clinical trials are applied to more subjects in the future and route-related mechanism studies are performed.
Key targets and major consideration factors for nasal-brain drug delivery
The major factors to be considered for drug delivery from the nasal cavity to the brain can be largely divided into formulation aspects and other safety and clinical application aspects.
Formulation aspects
For nasal-brain drug delivery, the physicochemical properties of candidate drugs should be considered first. This means that the water solubility of the drug and the log P derived from the octanol/water partition coefficient are very important formulation considerations (Wermeling 2013) because the application and usage of surfactants for the solubilization of drugs depends upon the degree of water solubility of the drug. log P is a value indicating the degree of lipophilicity and represents the probability that a compound will diffuse across biological membranes. Therefore, the higher the log P, the greater the probability that the drug will penetrate the nasal mucosa and olfactory cell layers and enter the brain parenchyma. Drugs that have been successful in nasal-brain drug delivery tended to be water-soluble and have sufficient lipophilic properties to readily cross biological membranes (Wermeling 2013).
The adaptation of the nasal-brain delivery route for poorly soluble drugs may require additional solubilization strategies, including the use of organic cosolvents, excipients such as cyclodextrins or other agents from water-soluble inclusion complexes, or emulsion preparations. And for drugs whose penetration of the nasal mucosa is restricted, membrane penetration can be improved using surfactants or polymers. The use of permeation enhancers may be necessary to further enhance drug penetration through the cell membrane. In this regard, a previous study (Nonaka et al. 2008) co-administering α-cyclodextrin as an absorption enhancer confirmed that the effect of galanin-like peptide delivery from the nasal passages to the brain was increased by 2–3 times.
As confirmed in previous reports (Cho et al. 2011; Horvát et al. 2009; Illum et al. 2002; Jogani et al. 2008; Qian et al. 2014), the proportion of the formulation deposited on the nasal mucosa should be high. In past studies (Cho et al. 2011; Horvát et al. 2009; Illum et al. 2002; Jogani et al. 2008; Qian et al. 2014), when a mucoadhesive agent was applied to the formulation compared to the case where it was not, mucoadhesive application showed significantly higher bioavailability and efficiency of drug delivery to the brain. In addition, when in situ gelation, a type of mucoadhesive strategy, was applied to intranasal formulations, a higher drug delivery effect of Huperzine A, a neuroprotective agent, to the brain was seen compared to oral and IV formulations (Zhao et al. 2007). Viscosity enhancing agents, such as methylcellulose, can promote drug retention in the nasal cavity by slowing the ciliary movement of mucus. As a result, the application of mucoadhesive and viscous agents to formulations may minimize the extent to which the drug passes from the nasal cavity to the pharynx and is removed. One study found that positively charged chitosan groups could interact with negatively charged cell membranes (Borchard et al. 1996). Therefore, it will be possible to increase drug delivery to the brain by imparting an appropriate positive charge to the formulation to improve nasal mucosal adhesion.
As presented as a limitation of nasal-brain drug delivery (see section “Pharmacological benefits”), the amount of drug that can be administered nasally is limited. One past study (Wermeling 2013) reported that the nasal cavity could hold approximately 100–150 μL of nasal formulations without causing an immediate outflow in the front of the nose or down the nasopharynx. Therefore, formulation studies focusing on targeting and drug stability are needed so that a large amount of drug can be delivered from the nasal cavity to the brain even with a small amount of formulation applied. In addition, in the case of nanoformulations, a specific loading strategy may be applied to maximize the amount of drug loaded into the carrier. For example, in the case of low molecular weight heparin with a negative charge on the surface, polyethylenimines (PEI) with a positive charge were applied to increase drug loading via ionic interaction, and at the same time, the drug absorption into the nasal cavity was increased by neutralizing the electrical properties (Yang et al. 2006).
To provide a consistent drug effect between subjects and trials, it is necessary to consider the variability due to the nasal administration of the formulation, especially in terms of the device used. Thus, the development of a device that can place a drug in an appropriate place, such as the olfactory or respiratory region in the nasal cavity, while minimizing variability between applications, will be an important part of effective nasal-brain drug delivery. The examples of applicable devices are needle-less syringes, high-pressure sprays, breath actuators, and electronic atomizers (Agrawal et al. 2018).
Finally, since drugs and formulations must be stable in the device during processing such as sterilization and storage, antioxidants such as ascorbic acid and α-tocopherol (Astete et al. 2011), which are stabilizers, need to be additionally considered. It is also necessary to evaluate the stability of the formulation in relation to the degradation of the drug under various physicochemical conditions. This will include not only the instability of the formulation due to external temperature changes and light, but also the degree of drug degradation by various hydrolytic enzymes present in the nasal mucosa (Thiebaud et al. 2011). And in relation to the long-term storage stability of the formulation, a non-zero zeta potential of more than ± 20–30 mV should be considered (Jang et al. 2019, 2020; Jeong et al. 2018). A previous report (Wermeling 2013) suggested that it may be useful to design formulations to be isotonic or slightly hypertonic to optimize intranasal absorption and drug tolerability to minimize the heterogeneity of the nasal mucosa in terms of osmotic pressure and prevent the burst release and loss of the drug due to the destruction of the dosage form.
Although there have been no reports on optimal particle and/or molecular sizes for the nasal-brain drug delivery route, the physical size of the formulation is likely to be one of the important factors for nasal-brain drug delivery because although the nasal mucosa has relatively high permeation flexibility compared to other biological membranes, there may be a cut-off size that limits permeation due to physiological factors such as the diameter of the olfactory nerve neurons. Past studies (Huang and Donovan 1996; Rejman et al. 2004) reported a difference in the degree of passage through the cell membrane depending upon the size of the particles. Particles with a diameter of 50–100 nm were internalized into cells faster than 20-nm nanoparticles, although the mechanism was unclear (Rejman et al. 2004). Also, there was a difference in the main mechanism of passage through the cell membrane according to particle size. For nanoparticles of 200–1000 nm, caveolae-mediated endocytosis was confirmed to be the main permeation mechanism. One study (Huang and Donovan 1996) confirmed that 10-nm carboxylated polystyrene particles and 200-nm amine-modified polystyrene particles passed through the rabbit nasal respiratory epithelium mainly paracellularly and transcellularly, respectively. Therefore, past reports (Huang and Donovan 1996; Rejman et al. 2004) implied that a particle size of 100 nm or less was sufficient to transport drugs through the nasal mucosa to the brain. In general, it is very difficult for high molecular weight peptides and proteins to penetrate cell membranes, and it is known that the brain, which is an important organ in the body, is thoroughly equipped with defense mechanisms to prevent foreign substances from entering arbitrarily (Agrawal et al. 2018). These features have been a major limitation in the delivery of high molecular weight pharmaceuticals to the cranial nervous system. However, previous studies (Francis et al. 2008; Nonaka et al. 2008; Zhang and Zhang 2010) experimentally confirmed that high molecular weight peptides and/or proteins equivalent to several thousand Daltons were delivered to the brain through the nasal mucosa. This means that drug delivery from the nasal cavity to the brain was relatively unconstrained by formulation or drug size compared to other routes of administration, suggesting a significant advantage of the nasal-brain drug delivery route. To deliver the drug to the brain when the dosage form is administered orally or intravenously, it must have a small particle size of approximately 50–100 nm or less or 600 Da or less, which can pass through the BBB without much difficulty (Banks 2009). Some particles with sizes of approximately 200 nm and/or molecules larger than 600 Da may also be able to cross the BBB through mechanisms of clathrin-mediated endocytosis or transmembrane diffusion, but to a very limited extent (Ceña and Játiva 2018). Therefore, for formulations delivered to the brain through BBB penetration, the physical size becomes a very important factor in delivery efficiency. However, since the nasal-brain delivery route has relatively great flexibility in terms of particle and/or molecular size, the electrical properties of the surface, such as the interaction between the positive charge on the formulation surface and the negative charge on the mucosal cells, may be more important than the size factor in delivery efficiency (Borchard et al. 1996).
There are many immune cells in the brain lymphatic system, as well as in the nasal mucosa (Pandey et al. 2020). Therefore, it has been suggested that the nasal-brain route is effective for vaccine delivery (Pandey et al. 2020). According to one report (Jackson and Herbst-Kralovetz 2012), the intranasal administration of murabutide, a synthetic immunomodulator, improved the humoral and mucosal immune responses to virus-like particles. The presence of the immune system suggested that nanoformulations administered through the nasal-brain route could be extensively phagocytosed and cleared by immune cells. Consequently, the degree of clearance of nanoformulations by the immune system should be considered in nasal-brain drug delivery. For example, in nanoparticles and nanoemulsions of similar sizes (Jang et al. 2019, 2020), the in vivo clearance of nanoparticles was larger than that of nanoemulsions, and opsonization was proposed as one of the causes (Jeong et al. 2021b). In this regard, past reports (Owens III and Peppas, 2006; Wani et al. 2020) have suggested that the surface strength of nanoparticles was relatively easier to opsonize in the body than materials with soft surfaces such as nanoemulsions, and as a result, nanoparticles could be extensively phagocytosed by the immune system. Therefore, formulation selection taking into account opsonization related to immune recognition and immune system evasion strategies such as polyethylene glycol (PEG)ylation need to be implemented in nasal-brain drug delivery. The PEGylation of formulations would be an effective strategy to improve drug stability and significantly reduce immunogenicity (Veronese and Mero 2008).
Other factors including clinical applications and safety
As mentioned earlier (see section “Nasal-brain lymphatic system”), the olfactory/nasal lymphatic route mainly involves the draining of brain CSF into cervical lymph nodes (Pardeshi and Belgamwar 2013; Sun et al. 2018). Therefore, drug delivery from the nasal cavity to the brain using the olfactory/nasal lymphatic route should be in the opposite direction of lymphatic drainage. Consequently, for effective drug delivery to the brain parenchyma, research on formulations and related mechanisms with the driving force to overcome lymphatic drainage needs to be prioritized.
It is necessary to consider the physiological conditions of the nasal cavity in the nasal application of formulations because the absorption of formulations from the nasal mucosa can vary greatly depending upon the physiological state of the nasal passages. Thus, in some cases, such as inflammation or swelling of the nasal mucosa, the absorption of the formulations will be greatly decreased or increased. As the physical spacing of the nasal mucosal cells is widened, the absorption of formulations may be increased abruptly or the absorption may be hindered by secretions related to inflammation.
Regarding patient compliance in clinical practice, the route of administration of the formulation is nasal, so the use of excipients with any unpleasant odor or causing aggressive discomfort in the nasal cavity will be extremely limited. In terms of safety, formulations using non-immunogenic substances with excellent biocompatibility are required. Examples include chitosan, lectin, and lecithin, which are natural products and are non-immunogenic in the body. Synthetic polymers such as PEG, polylactic acid (PLA), and polylactic-co-glycolic acid (PLGA), have been actively applied in the nanoformulations of various drugs because they are biodegradable without significant accumulation in the body, and have almost no reported fatal toxicity to date (Jeong et al. 2018).
Future prospects
There are still many hurdles to overcome for successful nasal-brain drug delivery. Therefore, it is expected that studies to improve the limitations and elucidate the mechanisms of the pathways involved will continue. Efforts are needed to clarify the mechanisms of drug delivery from the nasal cavity to the brain so that formulations can reach key points in nasal-to-brain drug delivery. And it is necessary to design a formulation with physical strength so that formulations that have reached key points in the nasal cavity are not easily removed by physicochemical factors and maintain proper bonding strength. Adhesiveness may be applicable as a controlled release formulation for cases where it is necessary to maintain a sustained drug concentration in terms of neurological effects. To date, nasal-brain drug delivery studies have mainly focused on drug transport to the brain, and lacked information on mechanistic approaches that allow drugs (transmitted to the cranial nervous system) to act on specific elements within brain tissue. Therefore, targeted research that allows drugs to act only on specific factors to reduce side effects and increase therapeutic effects is expected to be actively applied to the nasal-brain delivery route. The results of research on target factors that are the key to the onset and treatment of various cranial nervous diseases can be used to reduce side effects in areas unrelated to lesions by targeting only specific elements within the cranial nervous system.
Delivering high molecular weight pharmaceuticals such as peptides and proteins to the brain, especially via the BBB, has been a huge challenge. However, according to past reports (Francis et al. 2008; Nonaka et al. 2008; Zhang and Zhang 2010), high molecular weight substances were transported to the brain via the nasal-brain route, and the levels were significantly higher than that of other routes of administration. Therefore, the nasal-brain route is expected to be studied more actively as a delivery route for high molecular weight drugs. Many recent studies have applied various nanocarriers, targeting ligands, and mucoadhesive agents to formulations to increase the degree of drug transport to the brain through the nasal mucosa (Agrawal et al. 2018; Bahadur et al. 2020; Pandey et al. 2020). In addition, to overcome the physicochemical factors that limit drug delivery, electrical neutralization or solubilization using appropriate additives and stealth strategies to avoid extensive clearance by the immune system are being attempted. It is expected that the design of novel strategies for nasal-brain drug delivery will continue in the future with the development of formulation technology.
According to previous reports (Agrawal et al. 2018; Bahadur et al. 2020; Chatterjee et al. 2019; Pandey et al. 2020), a significant number of nasal-brain drug delivery studies are still at the preclinical level, especially in rodents. However, the cases extended to the actual clinical stage are very limited compared to the number of preclinical studies, and only had a few drug types. This is probably because most of the studies on formulations for nasal-brain drug delivery remain at the laboratory level, and there are limitations in batch scale-up and difficulties in uniform production and validation. Therefore, in future nasal-brain drug delivery studies, efforts will continue to expand and apply clinically to more and different drugs based on the hopeful results from preclinical studies.
Conclusion
The treatment of neurological diseases such as brain tumors, Alzheimer’s, Parkinson’s, and seizures has long been a major challenge. This may be deeply related to the fact that drug delivery to the brain has been very limited, in addition to the lack of a clear cure point for these diseases due to the complexity of the cranial nervous system. As an effective alternative for delivery, the nasal-brain drug delivery route has been proposed, and the results of studies on drug delivery to the brain via the nasal cavity have been continuously reported. However, there are still questions about the nasal-brain pathway, and the systematization of related information is insufficient. Therefore, based on the importance of the nasal-brain drug delivery pathway and the need for further research, this review focused on the topical drug delivery to the brain via the nasal cavity and explored related content. The nasal-brain route has several attractive points: it is a non-invasive route and avoids the first-pass effect and the BBB, which are the biggest obstacles to reaching therapeutic drug concentrations in the brain. In particular, the ability to deliver high molecular weight drugs to the brain is very surprising and interesting. The nasal-brain route of administration is expected to be actively used in the treatment of intractable neurological diseases through effective drug delivery to the brain. The physiological information and pathway characteristics including the advantages and limitations presented in this review will be useful for understanding the nasal-brain pathway as a drug delivery route. In addition, the proposed factors to be considered in the nasal-brain drug delivery will be useful in the development of related formulations and clinical applications in the future.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (No. 2022R1A2C1010245).
Declarations
Conflict of interest
All authors (S.H. Jeong, J.H. Jang, and Y.B. Lee) declare that they have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Seung-Hyun Jeong and Ji-Hun Jang have contributed equally to this article.
References
- Abd Elgadir M, Uddin MS, Ferdosh S, Adam A, Chowdhury AJK, Sarker MZI. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal. 2015;23:619–629. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou EM, Kandil SM, Miniawy HMFE. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int J Pharm. 2017;529:667–677. doi: 10.1016/j.ijpharm.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105:362–367. doi: 10.1016/j.eplepsyres.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, Alexander A. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Albayram MS, Smith G, Tufan F, Tuna IS, Bostancıklıoğlu M, Zile M, Albayram O. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022;13:1–14. doi: 10.1038/s41467-021-27887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A, Khan J, Saraf S, Saraf S. Formulation and evaluation of chitosan-based long-acting injectable hydrogel for PEGylated melphalan conjugate. J Pharm Pharmacol. 2014;66:1240–1250. doi: 10.1111/jphp.12262. [DOI] [PubMed] [Google Scholar]
- Aleya L, Uddin MS. Environmental pollutants and the risk of neurological disorders. Environ Sci Pollut Res. 2020;27:44657–44658. doi: 10.1007/s11356-020-11272-3. [DOI] [PubMed] [Google Scholar]
- Al-Ghananeem AM, Traboulsi AA, Dittert LW, Hussain AA. Targeted brain delivery of 17β-estradiol via nasally administered water soluble prodrugs. AAPS PharmSciTech. 2002;3:40–47. doi: 10.1208/pt030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salama ZT, Scott LJ. Sumatriptan nasal powder: a review in acute treatment of migraine. Drugs. 2016;76:1477–1484. doi: 10.1007/s40265-016-0641-9. [DOI] [PubMed] [Google Scholar]
- Anderson BC, Pandit NK, Mallapragada SK. Understanding drug release from poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) gels. J Control Release. 2001;70:157–167. doi: 10.1016/S0168-3659(00)00341-2. [DOI] [PubMed] [Google Scholar]
- Ascough S, Vlachantoni I, Kalyan M, Haijema B-J, Wallin-Weber S, Dijkstra-Tiekstra M, Ahmed MS, van Roosmalen M, Grimaldi R, Zhang Q. Local and systemic immunity against Respiratory Syncytial virus induced by a novel intranasal vaccine. A randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. 2019;200:481–492. doi: 10.1164/rccm.201810-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astete CE, Dolliver D, Whaley M, Khachatryan L, Sabliov CM. Antioxidant poly(lactic-co-glycolic) acid nanoparticles made with α-tocopherol–ascorbic acid surfactant. ACS Nano. 2011;5:9313–9325. doi: 10.1021/nn102845t. [DOI] [PubMed] [Google Scholar]
- Bahadur S, Pathak K. Buffered nanoemulsion for nose to brain delivery of ziprasidone hydrochloride: preformulation and pharmacodynamic evaluation. Curr Drug Deliv. 2012;9:596–607. doi: 10.2174/156720112803529792. [DOI] [PubMed] [Google Scholar]
- Bahadur S, Pardhi DM, Rautio J, Rosenholm JM, Pathak K. Intranasal nanoemulsions for direct nose-to-brain delivery of actives for CNS disorders. Pharmaceutics. 2020;12:1230–1256. doi: 10.3390/pharmaceutics12121230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010;56:290–299. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9:1–5. doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawarshi-Nassar RN, Hussain AA, Crooks PA. Nasal absorption and metabolism of progesterone and 17 beta-estradiol in the rat. Drug Metab Dispos. 1989;17:248–254. [PubMed] [Google Scholar]
- Benedict C, Brede S, Schiöth HB, Lehnert H, Schultes B, Born J, Hallschmid M. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes. 2011;60:114–118. doi: 10.2337/db10-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz R, Bernstein D, LaForce C, Pedinoff A, Rooklin A, Damaraju C, Mesarina-Wicki B, Nolop K. Onset of action of mometasone furoate nasal spray (NASONEX®) in seasonal allergic rhinitis. Allergy. 1999;54:64–69. doi: 10.1034/j.1398-9995.1999.00713.x. [DOI] [PubMed] [Google Scholar]
- Bies C, Lehr C-M, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev. 2004;56:425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Boche M, Pokharkar V. Quetiapine nanoemulsion for intranasal drug delivery: evaluation of brain-targeting efficiency. AAPS PharmSciTech. 2017;18:686–696. doi: 10.1208/s12249-016-0552-9. [DOI] [PubMed] [Google Scholar]
- Borchard G, Lueβen HL, de Boer AG, Verhoef JC, Lehr C-M, Junginger HE. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Control Release. 1996;39:131–138. doi: 10.1016/0168-3659(95)00146-8. [DOI] [Google Scholar]
- Brunnlieb C, Münte TF, Krämer U, Tempelmann C, Heldmann M. Vasopressin modulates neural responses during human reactive aggression. Soc Neurosci. 2013;8:148–164. doi: 10.1080/17470919.2013.763654. [DOI] [PubMed] [Google Scholar]
- Ceña V, Játiva P. Nanoparticle crossing of blood–brain barrier: a road to new therapeutic approaches to central nervous system diseases. Nanomedicine. 2018;13:1513–1516. doi: 10.2217/nnm-2018-0139. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Gorain B, Mohananaidu K, Sengupta P, Mandal UK, Choudhury H. Targeted drug delivery to the brain via intranasal nanoemulsion: available proof of concept and existing challenges. Int J Pharm. 2019;565:258–268. doi: 10.1016/j.ijpharm.2019.05.032. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhi F, Jia X, Zhang X, Ambardekar R, Meng Z, Paradkar AR, Hu Y, Yang Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol. 2013;65:807–816. doi: 10.1111/jphp.12043. [DOI] [PubMed] [Google Scholar]
- Cho H-J, Balakrishnan P, Park E-K, Song K-W, Hong S-S, Jang T-Y, Kim K-S, Chung S-J, Shim C-K, Kim D-D. Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J Pharm Sci. 2011;100:681–691. doi: 10.1002/jps.22314. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Raman R, Chow TW, Rafii MS, Sun C-K, Rissman RA, Donohue MC, Brewer JB, Jenkins C, Harless K. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77:1099–1109. doi: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2018;75:139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mauro TM (2008) Intranasally administering curcumin prodrugs to the brain to treat Alzheimer’s disease, Google Patents.
- Diaz J. Intranasal ketamine preinduction of paediatric outpatients. Paediatr Anaesth. 1997;7:273–278. doi: 10.1046/j.1460-9592.1997.d01-93.x. [DOI] [PubMed] [Google Scholar]
- Elnaggar YS, Etman SM, Abdelmonsif DA, Abdallah OY. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci. 2015;104:3544–3556. doi: 10.1002/jps.24557. [DOI] [PubMed] [Google Scholar]
- Emirzeoglu M, Sahin B, Celebi M, Uzun A, Bilgic S, Tontus H. Estimation of nasal cavity and conchae volumes by stereological method. Folia Morphol. 2012;71:105–108. [PubMed] [Google Scholar]
- Eskandari S, Varshosaz J, Minaiyan M, Tabbakhian M. Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: in vivo pharmacodynamic studies using rat electroshock model. Int J Nanomed. 2011;6:363–371. doi: 10.2147/IJN.S15881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza LC, Silva-Abreu M, Clares B, Rodríguez-Lagunas MJ, Halbaut L, Cañas M-A, Calpena AC. Formulation strategies to improve nose-to-brain delivery of donepezil. Pharmaceutics. 2019;11:64–79. doi: 10.3390/pharmaceutics11020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2009) Information for Patanase nasal spray. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021861s002lbl.pdf. Accessed 17 June 2022
- FDA (2012) Information for Synarel nasal solution. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019886s030lbl.pdf. Accessed 17 June 2022
- FDA (2015) Information for Narcan nasal spray. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208411lbl.pdf. Accessed 17 June 2022
- FDA (2017a) Information for Miacalcin nasal spray. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017a/020313s036lbl.pdf. Accessed 17 June 2022
- FDA (2017b) Information for Noctiva nasal spray. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017b/201656lbl.pdf. Accessed 17 June 2022
- FDA (2019) Information for Baqsimi nasal powder. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210134s000lbl.pdf. Accessed 17 June 2022
- FDA (2021a) Information for FluMist quadrivalent. https://www.fda.gov/vaccines-blood-biologics/vaccines/flumist-quadrivalent. Accessed 17 June 2022
- FDA (2021b) Information for Trudhesa nasal spray. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021b/213436s000lbl.pdf. Accessed 17 June 2022
- Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Vos T, Alahdab F, Amit AML, Bärnighausen TW, Beghi E, Beheshti M, Chavan PP, Criqui MH, Desai R. Burden of neurological disorders across the US from 1990–2017: a global burden of disease study. JAMA Neurol. 2021;78:165–176. doi: 10.1001/jamaneurol.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Zhang C, Shao X, Liu Q, Qian Y, Feng L, Chen J, Zha Y, Zhang Q, Jiang X. Enhancement of nose-to-brain delivery of basic fibroblast growth factor for improving rat memory impairments induced by co-injection of β-amyloid and ibotenic acid into the bilateral hippocampus. Int J Pharm. 2012;423:226–234. doi: 10.1016/j.ijpharm.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Foo MY, Cheng Y-S, Su W-C, Donovan MD. The influence of spray properties on intranasal deposition. J Aerosol Med. 2007;20:495–508. doi: 10.1089/jam.2007.0638. [DOI] [PubMed] [Google Scholar]
- Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, Tuor UI, Glazner G, Hanson LR, Frey WH. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain. 2008;131:3311. doi: 10.1093/brain/awn288. [DOI] [PubMed] [Google Scholar]
- Frey TM, Florin TA, Caruso M, Zhang N, Zhang Y, Mittiga MR. Effect of intranasal ketamine vs fentanyl on pain reduction for extremity injuries in children: the PRIME randomized clinical trial. JAMA Pediatr. 2019;173:140–146. doi: 10.1001/jamapediatrics.2018.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor F, Bogner E, Weissenboeck A, Wirth M. The lectin–cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv Drug Deliv Rev. 2004;56:459–480. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Gänger S, Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018;10:116–143. doi: 10.3390/pharmaceutics10030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zhang W, Rong Z, Chen H, Jiang X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release. 2007;121:156–167. doi: 10.1016/j.jconrel.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Gizurarson S, Gudbrandsson FK, Jonsson H, Bechgaard E. Intranasal administration of diazepam aiming at the treatment of acute seizures: clinical trials in healthy volunteers. Biol Pharm Bull. 1999;22:425–427. doi: 10.1248/bpb.22.425. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Haider MF, Khan S, Gaba B, Alam T, Baboota S, Ali J, Ali A. Optimization of rivastigmine nanoemulsion for enhanced brain delivery: in-vivo and toxicity evaluation. J Mol Liq. 2018;255:384–396. doi: 10.1016/j.molliq.2018.01.123. [DOI] [Google Scholar]
- Hanafy AS, Farid RM, ElGamal SS. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: preparation and detection in rat brain. Drug Dev Ind Pharm. 2015;41:2055–2068. doi: 10.3109/03639045.2015.1062897. [DOI] [PubMed] [Google Scholar]
- Hanafy AS, Farid RM, Helmy MW, ElGamal SS. Pharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016;23:3111–3122. doi: 10.3109/10717544.2016.1153748. [DOI] [PubMed] [Google Scholar]
- Hardy J, Lee S, Wilson C. Intranasal drug delivery by spray and drops. J Pharm Pharmacol. 1985;37:294–297. doi: 10.1111/j.2042-7158.1985.tb05069.x. [DOI] [PubMed] [Google Scholar]
- Hogan RE, Gidal BE, Koplowitz B, Koplowitz LP, Lowenthal RE, Carrazana E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61:455–464. doi: 10.1111/epi.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvát S, Fehér A, Wolburg H, Sipos P, Veszelka S, Tóth A, Kis L, Kurunczi A, Balogh G, Kürti L. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur J Pharm Biopharm. 2009;72:252–259. doi: 10.1016/j.ejpb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Hosseini Jahromi S, Hosseini Valami S, Adeli N, Yazdi Z. Comparison of the effects of intranasal midazolam versus different doses of intranasal ketamine on reducing preoperative pediatric anxiety: a prospective randomized clinical trial. J Anesth. 2012;26:878–882. doi: 10.1007/s00540-012-1422-6. [DOI] [PubMed] [Google Scholar]
- Hsu M, Sandor M, Fabry Z. Current concepts on communication between the central nervous system and peripheral immunity via lymphatics: what roles do lymphatics play in brain and spinal cord disease pathogenesis? Biol Futura. 2021;72:45–60. doi: 10.1007/s42977-021-00066-4. [DOI] [PubMed] [Google Scholar]
- Huang Y, Donovan M. Microsphere transport pathways in the rabbit nasal mucosa. Int J Pharm Adv. 1996;1:298–309. [Google Scholar]
- Hussain MA, Aungst BJ, Shefter E. Prodrugs for improved oral β-estradiol bioavailability. Pharm Res. 1988;5:44–47. doi: 10.1023/A:1015863412137. [DOI] [PubMed] [Google Scholar]
- Illum L, Watts P, Fisher A, Hinchcliffe M, Norbury H, Jabbal-Gill I, Nankervis R, Davis S. Intranasal delivery of morphine. J Pharmacol Exp Ther. 2002;301:391–400. doi: 10.1124/jpet.301.1.391. [DOI] [PubMed] [Google Scholar]
- Iqbal R, Ahmed S, Jain GK, Vohora D. Design and development of letrozole nanoemulsion: a comparative evaluation of brain targeted nanoemulsion with free letrozole against status epilepticus and neurodegeneration in mice. Int J Pharm. 2019;565:20–32. doi: 10.1016/j.ijpharm.2019.04.076. [DOI] [PubMed] [Google Scholar]
- Jackson EM, Herbst-Kralovetz MM. Intranasal vaccination with murabutide enhances humoral and mucosal immune responses to a virus-like particle vaccine. PLoS ONE. 2012;7:e41529. doi: 10.1371/journal.pone.0041529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalloh M. Esketamine (Spravato) for treatment-resistant depression. Am Fam Physician. 2020;101:339–340. [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-H, Jeong S-H, Lee Y-B. Preparation and in vitro/in vivo characterization of polymeric nanoparticles containing methotrexate to improve lymphatic delivery. Int J Mol Sci. 2019;20:3312–3335. doi: 10.3390/ijms20133312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-H, Jeong S-H, Lee Y-B. Enhanced lymphatic delivery of methotrexate using W/O/W nanoemulsion: in vitro characterization and pharmacokinetic study. Pharmaceutics. 2020;12:978–995. doi: 10.3390/pharmaceutics12100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadzadeh M, Sheibani K, Hashemieh M, Saneifard H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr. 2012;22:1–8. [PMC free article] [PubMed] [Google Scholar]
- Jeong S-H, Jang J-H, Cho H-Y, Lee Y-B. Soft-and hard-lipid nanoparticles: a novel approach to lymphatic drug delivery. Arch Pharm Res. 2018;41:797–814. doi: 10.1007/s12272-018-1060-0. [DOI] [PubMed] [Google Scholar]
- Jeong S-H, Jang J-H, Lee Y-B. Oral delivery of topotecan in polymeric nanoparticles: lymphatic distribution and pharmacokinetics. J Control Release. 2021;335:86–102. doi: 10.1016/j.jconrel.2021.05.017. [DOI] [PubMed] [Google Scholar]
- Jeong S-H, Jang J-H, Lee Y-B. Pharmacokinetic comparison between methotrexate-loaded nanoparticles and nanoemulsions as hard- and soft-type nanoformulations: a population pharmacokinetic modeling approach. Pharmaceutics. 2021;13:1050–1075. doi: 10.3390/pharmaceutics13071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu C, Zhai W, Zhuang N, Han T, Ding Z. The optimization design of lactoferrin loaded hupa nanoemulsion for targeted drug transport via intranasal route. Int J Nanomed. 2019;14:9217–9234. doi: 10.2147/IJN.S214657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogani VV, Shah PJ, Mishra P, Mishra AK, Misra AR. Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis Assoc Disord. 2008;22:116–124. doi: 10.1097/WAD.0b013e318157205b. [DOI] [PubMed] [Google Scholar]
- Khallaf RA, Aboud HM, Sayed OM. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J Liposome Res. 2020;30:163–173. doi: 10.1080/08982104.2019.1610435. [DOI] [PubMed] [Google Scholar]
- Kim TW, Chung H, Kwon IC, Sung HC, Jeong SY. In vivo gene transfer to the mouse nasal cavity mucosa using a stable cationic lipid emulsion. Mol Cells. 2000;10:142–147. doi: 10.1007/s10059-000-0142-1. [DOI] [PubMed] [Google Scholar]
- Klein EJ, Brown JC, Kobayashi A, Osincup D, Seidel K. A randomized clinical trial comparing oral, aerosolized intranasal, and aerosolized buccal midazolam. Ann Emerg Med. 2011;58:323–329. doi: 10.1016/j.annemergmed.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer TB, Siegel RL, Miller KD, Sung H, Islami F, Jemal A. Progress against cancer mortality 50 years after passage of the National Cancer Act. JAMA Oncol. 2022;8:156–159. doi: 10.1001/jamaoncol.2021.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm. 2008;358:285–291. doi: 10.1016/j.ijpharm.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Kumar S, Ali J, Baboota S. Design Expert® supported optimization and predictive analysis of selegiline nanoemulsion via the olfactory region with enhanced behavioural performance in Parkinson’s disease. Nanotechnology. 2016;27:435101. doi: 10.1088/0957-4484/27/43/435101. [DOI] [PubMed] [Google Scholar]
- Li J, Wu H, Hong J, Xu X, Yang H, Wu B, Wang Y, Zhu J, Lai R, Jiang X. Odorranalectin is a small peptide lectin with potential for drug delivery and targeting. PLoS ONE. 2008;3:e2381. doi: 10.1371/journal.pone.0002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol. 2012;34:272–279. doi: 10.1016/j.etap.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Munjal S, Brand-Schieber E, Rapoport AM. DFN-02 (sumatriptan 10 mg with a permeation enhancer) nasal spray vs placebo in the acute treatment of migraine: a double-blind, placebo-controlled study. Headache. 2018;58:676–687. doi: 10.1111/head.13309. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21:148–154. doi: 10.3109/10717544.2013.838014. [DOI] [PubMed] [Google Scholar]
- Mathure D, Madan JR, Gujar KN, Tupsamundre A, Ranpise HA, Dua K. Formulation and evaluation of niosomal in situ nasal gel of a serotonin receptor agonist, buspirone hydrochloride for the brain delivery via intranasal route. Pharm Nanotechnol. 2018;6:69–78. doi: 10.2174/2211738506666180130105919. [DOI] [PubMed] [Google Scholar]
- Mayer AV, Wermter A-K, Stroth S, Alter P, Haberhausen M, Stehr T, Paulus FM, Krach S, Kamp-Becker I. Randomized clinical trial shows no substantial modulation of empathy-related neural activation by intranasal oxytocin in autism. Sci Rep. 2021;11:1–13. doi: 10.1038/s41598-021-94407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md S, Ali M, Ali R, Bhatnagar A, Baboota S, Ali J. Donepezil nanosuspension intended for nose to brain targeting: in vitro and in vivo safety evaluation. Int J Biol Macromol. 2014;67:418–425. doi: 10.1016/j.ijbiomac.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Meng Q, Wang A, Hua H, Jiang Y, Wang Y, Mu H, Wu Z, Sun K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int J Nanomed. 2018;13:705–718. doi: 10.2147/IJN.S151474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadante S, Prestia G, Adile C, Casuccio A. Intranasal fentanyl versus fentanyl pectin nasal spray for the management of breakthrough cancer pain in doses proportional to basal opioid regimen. J Pain. 2014;15:602–607. doi: 10.1016/j.jpain.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J Pharm Sci. 2010;99:1745–1761. doi: 10.1002/jps.21939. [DOI] [PubMed] [Google Scholar]
- Mischley LK, Leverenz JB, Lau RC, Polissar NL, Neradilek MB, Samii A, Standish LJ. A randomized, double-blind phase I/II a study of intranasal glutathione in Parkinson’s disease. Mov Disord. 2015;30:1696–1701. doi: 10.1002/mds.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Croy S, Mallapragada S, Pandit N. Experimental investigation and mathematical modeling of Pluronic® F127 gel dissolution: drug release in stirred systems. J Control Release. 2000;67:191–202. doi: 10.1016/S0168-3659(00)00215-7. [DOI] [PubMed] [Google Scholar]
- Munjal S, Brand-Schieber E, Allenby K, Spierings EL, Cady RK, Rapoport AM. A multicenter, open-label, long-term safety and tolerability study of DFN-02, an intranasal spray of sumatriptan 10 mg plus permeation enhancer DDM, for the acute treatment of episodic migraine. J Headache Pain. 2017;18:1–8. doi: 10.1186/s10194-017-0740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016;23:1444–1452. doi: 10.3109/10717544.2015.1092619. [DOI] [PubMed] [Google Scholar]
- NBTS (2022) Quick brain tumor facts. https://brain.tumor.org/brain-tumor-information/brain-tumor-facts/
- Nonaka N, Farr SA, Kageyama H, Shioda S, Banks WA. Delivery of galanin-like peptide to the brain: targeting with intranasal delivery and cyclodextrins. J Pharmacol Exp Ther. 2008;325:513–519. doi: 10.1124/jpet.107.132381. [DOI] [PubMed] [Google Scholar]
- Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Pal S. Incidence and prevalence of major neurologic disorders. New York: Jobson Publishing LLC; 2018. pp. 24–24. [Google Scholar]
- Pandey YR, Kumar S, Gupta BK, Ali J, Baboota S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: formulation, behavioural and biochemical estimation. Nanotechnology. 2015;27:025102. doi: 10.1088/0957-4484/27/2/025102. [DOI] [PubMed] [Google Scholar]
- Pandey V, Gadeval A, Asati S, Jain P, Jain N, Roy RK, Tekade M, Soni V, Tekade RK. Drug delivery systems. Amsterdam: Elsevier; 2020. Formulation strategies for nose-to-brain delivery of therapeutic molecules; pp. 291–332. [Google Scholar]
- Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. 2013;10:957–972. doi: 10.1517/17425247.2013.790887. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood–brain barrier and delivery of protein and gene therapeutics to brain. Front Aging Neurosci. 2020;11:373–399. doi: 10.3389/fnagi.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor L, Frutos M, Grana L, Ramos D, Gallego-Huidobro J, Calvo A. Histochemical study of glycoconjugates in the nasal mucosa of the rat and guinea pig. Histochem J. 1992;24:727–736. doi: 10.1007/BF01460825. [DOI] [PubMed] [Google Scholar]
- Pathak R, Prasad Dash R, Misra M, Nivsarkar M. Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm Sin B. 2014;4:151–160. doi: 10.1016/j.apsb.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentón-Arias E, Aguilar-Rubido JC. Cuban prophylactic and therapeutic vaccines for controlling hepatitis B. MED Rev. 2021;23:21–29. doi: 10.37757/MR2021.V23.N1.6. [DOI] [PubMed] [Google Scholar]
- Pesic M, Schippers F, Saunders R, Webster L, Donsbach M, Stoehr T. Pharmacokinetics and pharmacodynamics of intranasal remimazolam—a randomized controlled clinical trial. Eur J Clin Pharmacol. 2020;76:1505–1516. doi: 10.1007/s00228-020-02984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrowsky R, Strüben C, Mölle M, Fehm HL, Born J. Brain potential changes after intranasal vs. intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol Psychiatry. 1996;39:332–340. doi: 10.1016/0006-3223(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Pietrowsky R, Thiemann A, Kern W, Fehm HL, Born J. A nose-brain pathway for psychotropic peptides: evidence from a brain evoked potential study with cholecystokinin. Psychoneuroendocrinology. 1996;21:559–572. doi: 10.1016/S0306-4530(96)00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. Improving healthcare for people living with dementia: coverage, quality and costs now and in the future. World Alzheimer Rep. 2016;2016:140. [Google Scholar]
- Qian S, Wong YC, Zuo Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int J Pharm. 2014;468:272–282. doi: 10.1016/j.ijpharm.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/bj20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeian A. Effect of intranasal insulin on olfactory recovery in patients with hyposmia: a randomized clinical trial. Otolaryngol Head Neck Surg. 2018;158:1134–1139. doi: 10.1177/0194599818764624. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi F, Seguella L, Gigli S, Hanieh P, Del Favero E, Cantù L, Pesce M, Sarnelli G, Marianecci C, Esposito G. inPentasomes: an innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice. J Control Release. 2019;294:17–26. doi: 10.1016/j.jconrel.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Rogol A, Tkachenko N, Bryson N. Natesto™, a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016;4:46–54. doi: 10.1111/andr.12137. [DOI] [PubMed] [Google Scholar]
- Rohrer J, Lupo N, Bernkop-Schnürch A. Advanced formulations for intranasal delivery of biologics. Int J Pharm. 2018;553:8–20. doi: 10.1016/j.ijpharm.2018.10.029. [DOI] [PubMed] [Google Scholar]
- Sabzghabaee AM, Eizadi-Mood N, Yaraghi A, Zandifar S. Naloxone therapy in opioid overdose patients: intranasal or intravenous? A randomized clinical trial. Arch Med Sci. 2014;10:309–314. doi: 10.5114/aoms.2014.42584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem HF, Kharshoum RM, Abou-Taleb HA, Naguib DM. Brain targeting of resveratrol through intranasal lipid vesicles labelled with gold nanoparticles: in vivo evaluation and bioaccumulation investigation using computed tomography and histopathological examination. J Drug Target. 2019;27:1127–1134. doi: 10.1080/1061186X.2019.1608553. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Kern W, Giese R, Hallschmid M, Enders A. Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome: an exploratory clinical trial. J Med Genet. 2009;46:217–222. doi: 10.1136/jmg.2008.062141. [DOI] [PubMed] [Google Scholar]
- Seju U, Kumar A, Sawant K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: in vitro and in vivo studies. Acta Biomater. 2011;7:4169–4176. doi: 10.1016/j.actbio.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Selvaraj K, Gowthamarajan K, Karri VVSR. Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif Cells Nanomed Biotechnol. 2018;46:2088–2095. doi: 10.1080/21691401.2017.1420073. [DOI] [PubMed] [Google Scholar]
- Shah B, Khunt D, Misra M, Padh H. Application of Box–Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route*. Int J Biol Macromol. 2016;89:206–218. doi: 10.1016/j.ijbiomac.2016.04.076. [DOI] [PubMed] [Google Scholar]
- Shimonovich S, Gigi R, Shapira A, Sarig-Meth T, Nadav D, Rozenek M, West D, Halpern P. Intranasal ketamine for acute traumatic pain in the Emergency Department: a prospective, randomized clinical trial of efficacy and safety. BMC Emerg Med. 2016;16:1–9. doi: 10.1186/s12873-016-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ship JA, Chávez EM. Special senses: disorders of taste and smell. Oral Med. 2002;52:277. [Google Scholar]
- Sood S, Jain K, Gowthamarajan K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf B. 2014;113:330–337. doi: 10.1016/j.colsurfb.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Suico JG, Hövelmann U, Zhang S, Shen T, Bergman B, Sherr J, Zijlstra E, Frier BM, Plum-Mörschel L. Glucagon administration by nasal and intramuscular routes in adults with type 1 diabetes during insulin-induced hypoglycaemia: a randomised, open-label, crossover study. Diabetes Ther. 2020;11:1591–1603. doi: 10.1007/s13300-020-00845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B-L, Wang L-h, Yang T, Sun J-y, Mao L-l, Yang M-f, Yuan H, Colvin RA, Yang X-y. Lymphatic drainage system of the brain: a novel target for intervention of neurological diseases. Prog Neurobiol. 2018;163:118–143. doi: 10.1016/j.pneurobio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kamigaki T, Fujino Y, Tominaga M, Ku Y, Kuroda Y. Randomized clinical trial of preoperative intranasal mupirocin to reduce surgical-site infection after digestive surgery. Br J Surg. 2003;90:1072–1075. doi: 10.1002/bjs.4269. [DOI] [PubMed] [Google Scholar]
- Thiebaud N, Menetrier F, Belloir C, Minn A-L, Neiers F, Artur Y, Le Bon A-M, Heydel J-M. Expression and differential localization of xenobiotic transporters in the rat olfactory neuro-epithelium. Neurosci Lett. 2011;505:180–185. doi: 10.1016/j.neulet.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Tsze DS, Ieni M, Fenster DB, Babineau J, Kriger J, Levin B, Dayan PS. Optimal volume of administration of intranasal midazolam in children: a randomized clinical trial. Ann Emerg Med. 2017;69:600–609. doi: 10.1016/j.annemergmed.2016.08.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol. 2001;53:3–22. doi: 10.1211/0022357011775145. [DOI] [PubMed] [Google Scholar]
- Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315–329. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- Vyas TK, Babbar A, Sharma R, Singh S, Misra A. Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting. J Pharm Sci. 2006;95:570–580. doi: 10.1002/jps.20480. [DOI] [PubMed] [Google Scholar]
- Wani TU, Raza SN, Khan NA. Nanoparticle opsonization: forces involved and protection by long chain polymers. Polym Bull. 2020;77:3865–3889. doi: 10.1007/s00289-019-02924-7. [DOI] [Google Scholar]
- Wanneveich M, Moisan F, Jacqmin-Gadda H, Elbaz A, Joly P. Projections of prevalence, lifetime risk, and life expectancy of Parkinson’s disease (2010–2030) in France. Mov Disord. 2018;33:1449–1455. doi: 10.1002/mds.27447. [DOI] [PubMed] [Google Scholar]
- Wermeling DP. A response to the opioid overdose epidemic: naloxone nasal spray. Drug Deliv Transl Res. 2013;3:63–74. doi: 10.1007/s13346-012-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcroft K, Merkonidis C, Cuevas M, Haehner A, Philpott C, Hummel T. Intranasal sodium citrate solution improves olfaction in post-viral hyposmia. Rhinology. 2016;54:368–374. doi: 10.4193/Rhino16.054. [DOI] [PubMed] [Google Scholar]
- WHO (2022) Epilepsy. https://www.who.int/news-room/fact-sheets/detail/epilepsy
- Wolff SC, Brubaker K, Navratil T, Fulcher E, Lankford J, Boyer J. Anticholinergic effects of antihistamine drugs used in the clinic. J Allergy Clin Immunol. 2007;119:S153. doi: 10.1016/j.jaci.2006.11.535. [DOI] [Google Scholar]
- Wu H, Li J, Zhang Q, Yan X, Guo L, Gao X, Qiu M, Jiang X, Lai R, Chen H. A novel small odorranalectin-bearing cubosomes: preparation, brain delivery and pharmacodynamic study on amyloid-β25–35-treated rats following intranasal administration. Eur J Pharm Biopharm. 2012;80:368–378. doi: 10.1016/j.ejpb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, Zhu T, Zhang J, Luo L, Fan P. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21:1654–1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Gattacceca F, Panicucci R, Amiji MM. Comparative biodistribution and pharmacokinetic analysis of cyclosporine-A in the brain upon intranasal or intravenous administration in an oil-in-water nanoemulsion formulation. Mol Pharm. 2015;12:1523–1533. doi: 10.1021/mp5008376. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Okada T, Munesue T, Kuroda M, Fujioka T, Uno Y, Matsumoto K, Kuwabara H, Mori D, Okamoto Y. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol Psychiatry. 2020;25:1849–1858. doi: 10.1038/s41380-018-0097-2. [DOI] [PubMed] [Google Scholar]
- Yang T, Hussain A, Bai S, Khalil IA, Harashima H, Ahsan F. Positively charged polyethylenimines enhance nasal absorption of the negatively charged drug, low molecular weight heparin. J Control Release. 2006;115:289–297. doi: 10.1016/j.jconrel.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z-Z, Zhang Y-Q, Wang Z-Z, Wu K, Lou J-N, Qi X-R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int J Pharm. 2013;452:344–354. doi: 10.1016/j.ijpharm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Yates R, Nairn K, Dixon R, Seaber E. Preliminary studies of the pharmacokinetics and tolerability of zolmitriptan nasal spray in healthy volunteers. J Clin Pharmacol. 2002;42:1237–1243. doi: 10.1177/009127002762491325. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Zhang F. Intranasal delivery of calcitonin gene-related peptide reduces cerebral vasospasm in rats. Front Biosci. 2010;2:1502–1513. doi: 10.2741/e209. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bai X, Zhang Q, Wang X, Lu L. The safety and efficacy of intranasal dexmedetomidine during electrochemotherapy for facial vascular malformation: a double-blind, randomized clinical trial. J Oral Maxillofac Surg. 2013;71:1835–1842. doi: 10.1016/j.joms.2013.06.202. [DOI] [PubMed] [Google Scholar]
- Zhang C, Chen J, Feng C, Shao X, Liu Q, Zhang Q, Pang Z, Jiang X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer's disease. Int J Pharm. 2014;461:192–202. doi: 10.1016/j.ijpharm.2013.11.049. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yue P, Tao T, Qh CHEN. Drug brain distribution following intranasal administration of Huperzine a in situ gel in rats 3. Acta Pharmacol Sin. 2007;28:273–278. doi: 10.1111/j.1745-7254.2007.00486.x. [DOI] [PubMed] [Google Scholar]