Abstract
Sinorhizobium meliloti is usually cultured in rich media containing yeast extract. It has been suggested that some components of yeast extract are also required for growth in minimal medium. We tested 27 strains of this bacterium and found that none were able to grow in minimal medium when methods to limit carryover of yeast extract were used during inoculation. By fractionation of yeast extract, two required growth factors were identified. Biotin was found to be absolutely required for growth, whereas previously the need for this vitamin was considered to be strain specific. All strains also required supplementation with cobalt or methionine, consistent with the requirement for a vitamin B12-dependent homocysteine methyltransferase for methionine biosynthesis.
Bacteria of the genera Rhizobium and Sinorhizobium fix nitrogen in a highly evolved symbiotic relationship within nodules on the roots of legumes. One of the best studied is Sinorhizobium meliloti, which fixes nitrogen in symbiosis with alfalfa (Medicago sativa). Rhizobia are readily isolated from nodules and have been cultured on a variety of media, usually rich media containing high concentrations of yeast extract such as yeast mannitol broth (20) or tryptone yeast extract medium (4).
Growth of rhizobia in synthetic media was the subject of numerous studies prior to 1970 (1, 3, 10, 14, 20). Early workers in the field recognized that yeast extract, or other complex plant or animal extracts, stimulated the growth of rhizobia (22), but the identity of the requirements or their necessity for growth of different strains has never been resolved. The stimulatory effect was initially postulated to be due to the presence of a heat-stable active substance termed coenzyme R. It is notable that in 1940, West and Wilson (23) described experiments in which they demonstrated that biotin actually exhibited properties comparable to those of coenzyme R. These and other workers concluded that biotin was only stimulatory for most strains and was absolutely required by only a few (1, 24). In contrast, Jordan (14) later concluded that none of the common vitamins, in particular biotin, could initiate growth of a variety of rhizobia and demonstrated that most rhizobia, including S. meliloti, could be grown in a defined medium without vitamins but containing certain amino acids, namely histidine, cysteine, and methionine. Since these early studies there has been little clarification of the nutrient requirements of the fast-growing rhizobia, including S. meliloti, and supplementation of defined media varies between laboratories. For example, the commonly used S. meliloti strain 1021 has not been noted to require any added growth factors, yet it has been cultured in defined media with various vitamin and mineral supplements (5, 7–9, 15, 16, 21).
S. meliloti strains require factors present in yeast extract for growth.
In our experience, cultures of S. meliloti 1021 and JJ1c10 grown in M9 (18) liquid medium supplemented only with a carbon source do not grow at reproducible growth rates, and their growth rates decrease with serial subculturing. These observations suggested that growth was dependent on unidentified growth factors carried over in the inoculum. Attempts to demonstrate the need for an unidentified factor by either depleting the medium or washing the inoculum were unsuccessful. However, we found that when a small number of freshly grown cells from M9 plates was used as inoculum, M9 liquid cultures remained clear (A620 < 0.05), while portions of the culture supplemented with 50 μg of yeast extract/ml showed turbid growth (A620 > 1.0). In this communication we describe experiments to identify two components of yeast extract required as medium supplements for growth of S. meliloti cells and demonstrate that the requirement for these growth factors is characteristic of the species.
Twenty-seven S. meliloti strains from a variety of sources have been used in this study (Table 1), although 1021 and JJ1c10 were used for most testing and screening. For these studies 20 mM succinate was used as the carbon source in M9 medium. Rich medium was tryptone yeast extract medium (4). Strain JJ1c10 cultures were additionally supplemented with 0.5 μg of pantothenic acid/ml, which was found to be a requirement for this strain. Yeast extract (Difco, Detroit, Mich.) was made up as an autoclaved 1% stock and added at 50 μg/ml. Cultures were grown at 30°C with shaking, and growth was measured spectrophotometrically at A620 after 24 to 60 h. As shown in Table 1, all strains were found to require yeast extract supplementation for growth in M9 medium when a minimal amount of cells from M9 plates was used as inoculum.
TABLE 1.
S. meliloti strains: supplements required for growth in minimal medium
| Strain | Source | Growth in M9 medium (A620)a with:
|
||
|---|---|---|---|---|
| None | Yeast extract | Met + Cys + YE biotin | ||
| JJ1c10 | V. N. Iyer, Carleton University, Ottawa, Canada | 0.03 | 1.45 | 1.42 |
| 1021 | T. M. Finan, McMaster University, Hamilton, Canada | 0.03 | 1.11 | 1.45 |
| NRG185 | W. A. Rice, Beaverlodge Research Station | 0.02 | 0.93 | 0.96 |
| 102F34 | J. C. Burton, Nitragin Co., Milwaukee, Wis. | 0.02 | 0.97 | 0.75 |
| Balsac | L. M. Bordeleau, Sainte-Foy Research Station | 0.00 | 0.71 | 0.32 |
| Rm41 | A. Kondorosi, Szeged, Hungary | 0.03 | 1.00 | 0.85 |
| Elora2 | J. C. Sirois, CBRI, Ottawa, Canada | 0.01 | 0.58 | 0.98 |
| Kirby2 | J. C. Sirois, CBRI, Ottawa, Canada | 0.00 | 0.58 | 0.67 |
| R304 | M. R. Purdom, Marandellas, Zimbabwe | 0.01 | 0.39 | 1.17 |
| R423 | M. R. Purdom, Marandellas, Zimbabwe | 0.00 | 0.42 | 0.93 |
| 2012 | M. Dye, Rothamsted Experimental Station | 0.00 | 0.44 | 0.68 |
| DMG117 | C. T. Corke, University of Guelph, Guelph, Canada | 0.02 | 0.60 | 1.29 |
| DMG118 | C. T. Corke, University of Guelph, Guelph, Canada | 0.01 | 0.64 | 1.27 |
| MB5 | S. M. Lesley, CBRI, Ottawa, Canada | 0.02 | 0.67 | 0.33 |
| Cavan2 | J. C. Sirois, CBRI, Ottawa, Canada | 0.02 | 0.64 | 0.49 |
| Harrow2 | J. C. Sirois, CBRI, Ottawa, Canada | 0.03 | 0.78 | 0.56 |
| Kirby5 | J. C. Sirois, CBRI, Ottawa, Canada | 0.07 | 0.83 | 0.81 |
| R762 | Our field isolate | 0.06 | 0.90 | 0.94 |
| U45 | J. Brockwell, CSIRO, Canberra, Australia | 0.03 | 0.55 | 0.97 |
| MB1 | S. M. Lesley, CBRI, Ottawa, Canada | 0.01 | 0.71 | 0.79 |
| MB6 | S. M. Lesley, CBRI, Ottawa, Canada | 0.02 | 0.56 | 0.67 |
| MBA25 | S. M. Lesley, CBRI, Ottawa, Canada | 0.02 | 0.54 | 0.93 |
| BT5 | S. M. Lesley, CBRI, Ottawa, Canada | 0.06 | 0.21 | 0.79 |
| YG2 | S. M. Lesley, CBRI, Ottawa, Canada | 0.02 | 0.72 | 0.86 |
| 1642 | E. Bromfield, PRC, Ottawa, Canada | 0.02 | 0.73 | 1.17 |
| R763 | Our field isolate | 0.02 | 0.86 | 0.74 |
| I-6-5 | S. M. Lesley, CBRI, Ottawa, Canada | 0.01 | 0.57 | 0.57 |
Each strain was tested by inoculating a minimal medium culture with cells from minimal M9 agar. The culture was subdivided, additionally supplemented as indicated, and shaken at 30°C for 2 to 3 days before growth was monitored. Values shown are the averages of three measurements (standard deviation < 0.01). None, no additional supplements; Yeast extract, 50 μg of yeast extract per ml; Met + Cys + YE biotin, 5 μg of each of methionine and cysteine per ml and 5 ng of biotin purified from yeast extract per ml.
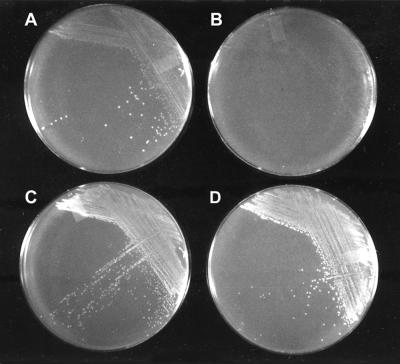
In contrast to their growth in liquid M9 medium, JJ1c10 and 1021 grew well on M9 agar plates supplemented only with a carbon source and could be serially propagated on these plates. This suggested that agar may contribute factors required for their growth. This was demonstrated by using plates prepared using 1.2% agarose (FMC BioProducts, Rockland, Maine) instead of 1.7% agar (Difco) and supplemented with 50 μg of yeast extract/ml or left unsupplemented. After 3 days, the S. meliloti strains did not form colonies on M9 agarose plates unless they were supplemented (Fig. 1). Extended incubation of the plates without yeast extract resulted in the appearance of microcolonies after 5 to 7 days, probably due to trace amounts of required growth factors still present in the agarose.
FIG. 1.
Supplements required for growth of S. meliloti on agar and agarose media. Strain JJ1c10 was streaked on plates containing M9 medium with succinate and pantothenic acid and incubated for 48 h. Plates included agar (A), agarose (B), agarose plus yeast extract (C), and agarose plus methionine and biotin (D).
We attempted, without success, to identify the required growth factor(s) by testing for growth of JJ1c10 and 1021 in media supplemented with common vitamins and minerals individually and in mixtures. Some compounds were found to decrease or inhibit the growth of control cultures such that tests of mixtures often produced ambiguous results with low and variable levels of growth (A260 < 0.2). For these reasons it was unclear if the factor was present in the test mixtures but antagonized by other compounds, if multiple factors were required, or if the factor was an unusual compound not tested. Because of these ambiguities, we instead attempted to identify the factor(s) directly by fractionation of yeast extract.
Yeast extract contains two factors necessary for growth of S. meliloti cells.
Chemical fractionation of yeast extract showed that the growth factors were quantitatively extractable in glacial acetic acid, ethanol, or methanol. The methanol-soluble fraction was then separated by reverse-phase (RP) chromatography using a 0 to 100% methanol gradient on a Whatman C18 ODS-3 Prep column. A single peak (A205) which permitted growth of strain 1021 or JJ1c10 was further purified using a 100 to 0% methanol gradient on a Zorbax NH2 column, followed by use of a Whatman RP ODS-3 column in 20% methanol. Active fractions were dried and dissolved in water and then separated on a Pharmacia Superose-12 column in water. The active factor, corresponding to a single peak, was approximately 10,000-fold purified by these fractionations.
During the purification, the test cultures for screening fractions were additionally supplemented with 0.2% Casamino Acids (Difco) to compensate for nonspecific growth stimulation due to variations in richness between different fractions of the yeast extract. Casamino Acid supplementation alone did not permit growth of the S. meliloti test strains. Unexpectedly, it was found that when Casamino Acids were not included, increasing amounts of the partially purified factor from successive intermediate purification steps were required to achieve growth comparable to that obtained with yeast extract. Our most purified preparation of factor from Superose columns was completely unable to support growth of S. meliloti cells in minimal media without Casamino Acids. These results are consistent with the interpretation that yeast extract contains two S. meliloti growth factors, one of which we had directly purified and a second which is present in, or replaceable by, Casamino Acids. We subsequently verified the presence of a second growth factor in yeast extract by testing the RP high-performance liquid chromatography (HPLC) fractions for the ability to support growth without Casamino Acids but in the presence of the purified growth factor.
Biotin is essential for growth of S. meliloti cells.
The purified growth factor was identified as biotin by testing individual vitamins for their ability to replace the factor in media supplemented with Casamino Acids. We also directly demonstrated that the purified factor from yeast extract was identical to biotin by nuclear magnetic resonance (NMR) spectroscopy using a Bruker AM500 NMR spectrometer. Biotin was routinely added at 100 ng/ml, although tests with different concentrations of biotin using JJ1c10 and 1021 showed that only 1 ng/ml was required for cultures to grow to stationary phase (A620 = 1.0 to 2.0).
We tested the complete genomic sequence of S. meliloti (http://sequence.toulouse.inra.fr/rhime/Complete/doc/Complete.html) for open reading frames encoding homologs of the enzymes catalyzing the conversion of pimeloyl-coenzyme A plus alanine to biotin (6). The four enzymes of this pathway, encoded by bioF, bioA, bioD, and bioB, are common to most bacteria. We used the sequences of these proteins from Escherichia coli K-12, Bacillus subtilis, and Mesorhizobium loti for BLASTp searches at an expect value of 0.0001 (2). No BioD or BioB homologs were found. For the BioF and BioA proteins the best hits (<35% identity) were to proteins of the same enzyme class but otherwise unrelated to biotin synthesis. Similar results were obtained using sequences for the E. coli BioC and BioH proteins, which are required for unknown steps prior to pimeloyl-coenzyme A synthesis. These results suggest that S. meliloti lacks the entire known biotin biosynthetic pathway.
Streit et al. (19) reported the isolation of biotin auxotrophs of S. meliloti 1021 and mapped the mutations. These workers isolated their mutants using agar plates, although our results indicate that agar plates contain sufficient biotin for growth. It seems likely that their mutants require a higher concentration of biotin for growth rather than being true auxotrophs. The presence of biotin in commercial agar preparations has also been reported by Graham (10).
Identification of methionine or cobalt as a growth requirement for S. meliloti cells.
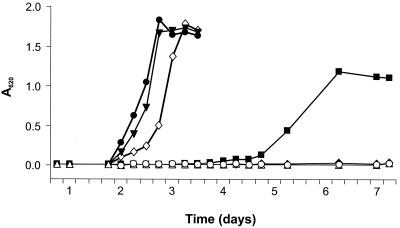
To identify the factor present in Casamino Acids, individual amino acids (5 μg/ml) were tested for their ability to support growth in minimal media in the presence of biotin. Methionine was identified as the required growth component, although cysteine also was able to support poorer growth (A620 < 0.2). However, commercial methionine (minimum 98% or SigmaUltra >99%; Sigma Chemical Co.) was found to support growth of the S. meliloti strains without the addition of biotin, suggesting that biotin was present as a contaminant. To demonstrate a dependence on both factors, methionine was further purified by HPLC on a 50-cm C18 RP column (Partisil 10 ODS-3 M/9-50cm; Whatman) using a 0 to 50% methanol gradient. As shown in Fig. 2, the HPLC-purified methionine permitted no growth without biotin, even during prolonged incubation. Methionine and biotin were found to be sufficient for growth and able to replace yeast extract. An equivalent result was obtained using plates prepared with agarose (Fig. 1).
FIG. 2.
Supplements required for growth of S. meliloti in liquid media. Strain 1021 was inoculated into M9 medium containing succinate and incubated with shaking for 8 days. Growth (A620) was measured in triplicate (standard deviation, <0.01). Cultures were either left unsupplemented (♦) or were further supplemented with biotin (■), HPLC-purified methionine (○), cobalt (▵), biotin and methionine (⋄), biotin and cobalt (▾), or biotin, methionine, and cobalt (●).
In most bacteria, synthesis of methionine from homocysteine is catalyzed by the enzyme homocysteine methyltransferase. The most efficient version of this enzyme requires vitamin B12 as a cofactor (13, 17). In bacteria the requirement for cobalt is primarily due to its presence in vitamin B12 and the important role of this vitamin in methionine biosynthesis. It was found that cobalt (cobalt chloride, 5 ng/ml) could be substituted for methionine to permit growth of S. meliloti strains in minimal medium (Fig. 2). In contrast, we found that trace impurities provide sufficient cobalt for growth of E. coli in M9 medium, indicating that a higher concentration of cobalt is necessary for S. meliloti cell growth. A requirement for cobalt for methionine synthesis in S. meliloti has been described by Inukai et al. (11). We also tested supplementation using 10 ng of vitamin B12/ml, methylcobalamin and coenzyme B12 (Sigma), and 5-μg/ml concentrations of the methionine precursors cystathionine and homocysteine (Sigma), but significant growth was not observed compared to that with cobalt or methionine.
Vitamin B12 is also required for the activity of a B12-dependent ribonucleotide reductase found in rhizobia, Lactobacillus leichmannii, and several other bacteria, but not in E. coli (12). In the presence of methionine alone (i.e., without cobalt), the ribonucleotide reductase was found to be synthesized at higher levels in S. meliloti cells. This was reported to affect cell morphology but not growth (12). For this reason it is probably preferable to always supplement the cells with cobalt.
Methionine can also be synthesized in many bacteria by a second homocysteine methyltransferase induced in the absence of cobalt, which does not require vitamin B12, but this enzyme is extremely inefficient (13, 17). We tested for slow growth of S. meliloti cells in minimal medium containing biotin but neither cobalt nor methionine during extended incubations (Fig. 2). After 5 days, a slower growth (doubling time > 10 h) ensued, and the cultures did not grow to the same density as supplemented cultures. Although it cannot be excluded that this growth was due to residual cobalt present in these cultures, these results could also be due to a B12-independent homocysteine methyltransferase, such that methionine or cobalt might not be strictly required for growth. However, for practical culturing purposes this supplementation is necessary.
The requirement for biotin and either cobalt or methionine is characteristic of most S. meliloti strains.
As shown in Table 1, the 27 independent S. meliloti isolates were able to grow in minimal medium supplemented with methionine, cysteine, and purified biotin instead of yeast extract. Figure 2 shows the growth of S. meliloti 1021 cells with combinations of pure biotin, methionine, and cobalt as supplements. Similar tests have been done using purified components using strains JJ1c10, Rm41, Kirby 5, Balsac, 102F34, NRG185, and R762, demonstrating that all have an absolute requirement for biotin. Although all strains also required either cobalt or methionine, there was some apparent strain preference for one compound over the other, as indicated by relative lag times before growth. As these strains were able to grow only with the purified components, the requirement for these growth factors appears to be a general characteristic of S. meliloti.
Acknowledgments
We thank John Nikiforuk for NMR analysis of yeast extract fractions. We thank Yiu-Kwok Chan, Eden Bromfield, and Les Barran for critical reading of the manuscript.
REFERENCES
- 1.Allen E K, Allen O N. Biochemical and symbiotic properties of the rhizobia. Bacteriol Rev. 1950;14:273–330. doi: 10.1128/br.14.4.273-330.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Bergersen F J. The growth of Rhizobium in synthetic media. Aust J Biol Sci. 1961;14:349–360. [Google Scholar]
- 4.Beringer J E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 5.David M, Domergue O, Pognonec P, Kahn D. Transcription patterns of Rhizobium meliloti symbiotic plasmid pSym: identification of nifA-independent fix genes. J Bacteriol. 1987;169:2239–2244. doi: 10.1128/jb.169.5.2239-2244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg M. Biosynthesis of biotin and lipoic acid. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium, cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 544–550. [Google Scholar]
- 7.Engelke T H, Jagadish M N, Puhler A. Biochemical and genetical analysis of Rhizobium meliloti mutants defective in C4-dicarboxylate transport. J Gen Microbiol. 1987;133:3019–3029. [Google Scholar]
- 8.Finan T M, Kunkel B, de Vos G F, Signer E R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glazebrook J, Meiri G, Walker G C. Genetic mapping of symbiotic loci on the Rhizobium meliloti chromosome. Mol Plant-Microbe Interact. 1992;5:223–227. doi: 10.1094/mpmi-5-223. [DOI] [PubMed] [Google Scholar]
- 10.Graham P H. Vitamin requirements of root nodule bacteria. J Gen Microbiol. 1963;30:245–248. [Google Scholar]
- 11.Inukai S, Sato K, Shimizu S. The relationship of cobalt requirement to vitamin B12-dependent methionine synthesis in Rhizobium meliloti. Agric Biol Chem. 1977;41:2229–2234. [Google Scholar]
- 12.Inukai S, Sato K, Shimizu S. Purification and properties of vitamin B12-dependent ribonucleotide reductase from Rhizobium meliloti. Agric Biol Chem. 1979;43:637–646. [Google Scholar]
- 13.Jeter R, Escalante-Semerena J C, Roof D, Olivera B, Roth J. Synthesis and use of vitamin B12. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium, cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 551–556. [Google Scholar]
- 14.Jordan D C. Studies on the legume root nodule bacteria. III. Growth factor requirements for effective, ineffective and parasitic strains. Can J Bot. 1952;30:693–700. [Google Scholar]
- 15.Kiss G B, Vincze E, Kalman Z, Forrai T, Kondorosi A. Genetic and biochemical analysis of mutants affected in nitrate reduction in Rhizobium meliloti. J Gen Microbiol. 1979;113:105–118. [Google Scholar]
- 16.Meade H M, Long S R, Ruvkun G B, Brown S E, Ausubel F M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowbury R J. Methionine biosynthesis and its regulation. In: Herrmann K M, Somerville R L, editors. Amino acids, biosynthesis and genetic regulation. Reading, Mass: Addison-Wesley Publishing Company; 1983. pp. 191–211. [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Streit W R, Joseph C M, Phillips D A. Biotin and other water-soluble vitamins are key growth factors for alfalfa root colonization by Rhizobium meliloti 1021. Mol Plant-Microbe Interact. 1996;9:330–338. doi: 10.1094/mpmi-9-0330. [DOI] [PubMed] [Google Scholar]
- 20.Vincent J M. A manual for the practical study of the root-nodule bacteria. Oxford, United Kingdom: Blackwell Scientific Publications; 1970. [Google Scholar]
- 21.Watson R J. Analysis of the C4-dicarboxylate transport genes of Rhizobium meliloti: nucleotide sequence and deduced products of dctA, dctB, and dctD. Mol Plant-Microbe Interact. 1990;3:174–181. doi: 10.1094/mpmi-3-174. [DOI] [PubMed] [Google Scholar]
- 22.West P M, Wilson P W. Effect of biotin concentrates on growth of Rhizobium and related species. J Bacteriol. 1939;38:110–111. [Google Scholar]
- 23.West P M, Wilson P W. Biotin as a growth stimulant for the root nodule bacteria. Enzymologia. 1940;8:152–162. [Google Scholar]
- 24.Wilson J B, Wilson P W. Biotin as a growth factor for rhizobia. J Bacteriol. 1942;43:329–341. doi: 10.1128/jb.43.3.329-341.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]