Abstract
Background
Schizophrenia is a disabling psychotic disorder characterised by positive symptoms of delusions, hallucinations, disorganised speech and behaviour; and negative symptoms such as affective flattening and lack of motivation. Cognitive behavioural therapy (CBT) is a psychological intervention that aims to change the way in which a person interprets and evaluates their experiences, helping them to identify and link feelings and patterns of thinking that underpin distress. CBT models targeting symptoms of psychosis (CBTp) have been developed for many mental health conditions including schizophrenia. CBTp has been suggested as a useful add‐on therapy to medication for people with schizophrenia. While CBT for people with schizophrenia was mainly developed as an individual treatment, it is expensive and a group approach may be more cost‐effective. Group CBTp can be defined as a group intervention targeting psychotic symptoms, based on the cognitive behavioural model. In group CBTp, people work collaboratively on coping with distressing hallucinations, analysing evidence for their delusions, and developing problem‐solving and social skills. However, the evidence for effectiveness is far from conclusive.
Objectives
To investigate efficacy and acceptability of group CBT applied to psychosis compared with standard care or other psychosocial interventions, for people with schizophrenia or schizoaffective disorder.
Search methods
On 10 February 2021, we searched the Cochrane Schizophrenia Group's Study‐Based Register of Trials, which is based on CENTRAL, MEDLINE, Embase, four other databases and two trials registries. We handsearched the reference lists of relevant papers and previous systematic reviews and contacted experts in the field for supplemental data.
Selection criteria
We selected randomised controlled trials allocating adults with schizophrenia to receive either group CBT for schizophrenia, compared with standard care, or any other psychosocial intervention (group or individual).
Data collection and analysis
We complied with Cochrane recommended standard of conduct for data screening and collection. Where possible, we calculated risk ratio (RR) and 95% confidence interval (CI) for binary data and mean difference (MD) and 95% CI for continuous data. We used a random‐effects model for analyses. We assessed risk of bias for included studies and created a summary of findings table using GRADE.
Main results
The review includes 24 studies (1900 participants). All studies compared group CBTp with treatments that a person with schizophrenia would normally receive in a standard mental health service (standard care) or any other psychosocial intervention (group or individual). None of the studies compared group CBTp with individual CBTp. Overall risk of bias within the trials was moderate to low.
We found no studies reporting data for our primary outcome of clinically important change. With regard to numbers of participants leaving the study early, group CBTp has little or no effect compared to standard care or other psychosocial interventions (RR 1.22, 95% CI 0.94 to 1.59; studies = 13, participants = 1267; I2 = 9%; low‐certainty evidence). Group CBTp may have some advantage over standard care or other psychosocial interventions for overall mental state at the end of treatment for endpoint scores on the Positive and Negative Syndrome Scale (PANSS) total (MD –3.73, 95% CI –4.63 to –2.83; studies = 12, participants = 1036; I2 = 5%; low‐certainty evidence). Group CBTp seems to have little or no effect on PANSS positive symptoms (MD –0.45, 95% CI –1.30 to 0.40; studies =8, participants = 539; I2 = 0%) and on PANSS negative symptoms scores at the end of treatment (MD –0.73, 95% CI –1.68 to 0.21; studies = 9, participants = 768; I2 = 65%). Group CBTp seems to have an advantage over standard care or other psychosocial interventions on global functioning measured by Global Assessment of Functioning (GAF; MD –3.61, 95% CI –6.37 to –0.84; studies = 5, participants = 254; I2 = 0%; moderate‐certainty evidence), Personal and Social Performance Scale (PSP; MD 3.30, 95% CI 2.00 to 4.60; studies = 1, participants = 100), and Social Disability Screening Schedule (SDSS; MD –1.27, 95% CI –2.46 to –0.08; studies = 1, participants = 116). Service use data were equivocal with no real differences between treatment groups for number of participants hospitalised (RR 0.78, 95% CI 0.38 to 1.60; studies = 3, participants = 235; I2 = 34%). There was no clear difference between group CBTp and standard care or other psychosocial interventions endpoint scores on depression and quality of life outcomes, except for quality of life measured by World Health Organization Quality of Life Assessment Instrument (WHOQOL‐BREF) Psychological domain subscale (MD –4.64, 95% CI –9.04 to –0.24; studies = 2, participants = 132; I2 = 77%). The studies did not report relapse or adverse effects.
Authors' conclusions
Group CBTp appears to be no better or worse than standard care or other psychosocial interventions for people with schizophrenia in terms of leaving the study early, service use and general quality of life. Group CBTp seems to be more effective than standard care or other psychosocial interventions on overall mental state and global functioning scores. These results may not be widely applicable as each study had a low sample size. Therefore, no firm conclusions concerning the efficacy of group CBTp for people with schizophrenia can currently be made. More high‐quality research, reporting useable and relevant data is needed.
Keywords: Adult, Humans, Cognitive Behavioral Therapy, Cognitive Behavioral Therapy/methods, Hallucinations, Hallucinations/etiology, Hallucinations/therapy, Psychotic Disorders, Psychotic Disorders/therapy, Quality of Life, Schizophrenia, Schizophrenia/drug therapy
Plain language summary
Group cognitive behavioural therapy for schizophrenia
Review question
Is group cognitive behavioural therapy (talking therapy) that targets psychoses (CBTp) more effective than standard care (the care a person would normally receive) or other forms of talking therapies (for example, counselling, supportive therapy; either group or individual) for people with schizophrenia?
What is schizophrenia?
Schizophrenia is a disabling serious mental illness. It is characterised by delusions (bizarre beliefs), hallucinations (seeing or hearing things that are not there), negative symptoms (social withdrawal, apathy) and disorganised behaviour. Medications known as antipsychotics are the main treatment but are not always very effective at helping with the negative symptoms of schizophrenia. CBTp has been suggested as a useful add‐on treatment to medication for people with mental illness, but evidence for its effectiveness for people with schizophrenia is uncertain. CBTp is usually an individual therapy but can also be delivered to a group of people, which may be more cost‐effective. It is important to find out if group CBTp for people with schizophrenia is effective and acceptable.
What did we do?
The Information Specialist of Cochrane Schizophrenia ran an electronic search of their specialised register up to 10 February 2021 for clinical trials that randomised group CBTp versus standard care or other psychosocial therapies (either group or individual) in people with schizophrenia.
What did we find?
We found 24 studies involving 1900 participants that met the review requirements and provided usable data. All studies compared group CBTp with standard care. Our evidence suggests there is no substantial difference between group CBTp and standard care or other psychosocial interventions in terms of numbers of participants leaving the study early. Group CBTp may be better than standard care or other psychosocial interventions for overall mental state for total symptoms of schizophrenia. Group CBTp seems to show no difference on positive and negative symptoms of schizophrenia compared to standard care or other psychosocial interventions. Group CBTp seems to be better than standard care or other psychosocial interventions on global functioning. There were no real differences between treatment groups for number of participants hospitalised for group CBTp compared to standard care or other psychosocial interventions.
Conclusions
Group CBTp seems to be better than standard care or other psychosocial interventions on total symptoms of schizophrenia and global functioning.
What are the limitations of the evidence?
Studies with larger sample sizes and low risk of bias should be conducted. Longer‐term outcomes need to be addressed to establish whether any effect is transient or long‐lasting. Trials should improve their quality of data reporting.
Summary of findings
Summary of findings 1. Group cognitive behavioural therapy for psychosis compared to standard care for schizophrenia.
| Group cognitive behavioural therapy compared to standard care and other psychosocial interventions for schizophrenia | ||||||
| Patient or population: people with schizophrenia Setting: inpatient and outpatient Intervention: group cognitive behavioural therapy Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with group cognitive behavioural therapy for psychosis | |||||
| Clinically important change, shown as improved – as defined by individual studies | No data available | — | — | — | — | |
| Relapse | No data available | — | — | — | — | |
| Leaving the study early: for any reason – end of treatment | Study population | RR 1.22 (0.94 to 1.59) | 1267 (13 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 155 per 1000 | 195 per 1000 (144 to 259) | |||||
| Mental state: overall: average endpoint score – end of treatment (PANSS total) | The mean mental state: overall: average endpoint score – end of treatment (PANSS total) was 0 | MD 3.73 lower (4.63 lower to 2.83 lower) | — | 1036 (12 RCTs) | ⊕⊕⊝⊝ Lowa,c | — |
| Days in hospital | No data available | — | — | — | — | |
| Quality of life: clinically important change – as defined by individual studies. | No data available | — | — | — | — | |
| Functioning: overall: global assessment of functioning – average endpoint score – end of treatment (GAF) | The mean functioning: overall: global assessment of functioning – average endpoint score – end of treatment (GAF) was 0 | MD 3.61 lower (6.37 lower to 0.84 lower) | — | 254 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GAF: Global Assessment of Functioning; MD: mean difference; PANSS: Positive and Negative Syndrome Scale; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to high risk of performance bias for all studies and unclear selection bias for most studies. bDowngraded one level due to imprecision: confidence interval ranged from no difference to appreciable benefit with standard care. cDowngraded publication bias domain one level as the funnel plot on PANSS total outcome (Figure 1) was asymmetrical, increasing the risk for publication bias.
Background
Description of the condition
Schizophrenia is a disabling psychotic disorder with a point prevalence of between 2.8 and 4.5 per 1000 population (Charlson 2018; Tandon 2008), and an annual incidence of 15 per 100,000 (Jauhar 2022; Tandon 2008). It is characterised by delusions, hallucinations, negative symptoms and disorganised behaviour (APA 2013). Although prognosis can be debilitating for some people with a reduction in life expectancy (McCutcheon 2020), it is possible to achieve recovery (van Os 2009). Medication remains the mainstay of treatment (McCutcheon 2020; van Os 2009). However, not all patients respond well to medication and a significant number remain symptomatic (McCutcheon 2020; Mueser 2004). Cognitive behavioural therapy (CBT) has been suggested as a useful add‐on to medication, particularly for those who show active symptoms (Bighelli 2018; Laws 2018; Turkington 2006). However, the evidence is far from conclusive (Jones 2004; Laws 2018). The National Institute for Health and Care Excellence (NICE) in the UK has nevertheless recommended that CBT should be offered to all people with schizophrenia (NICE 2014).
Description of the intervention
CBT is a short‐term, problem‐focused, psychological intervention (Williams 2002), based on the cognitive model. This model was created by Aaron Beck, and was originally developed for depression (Beck 1979). It is based on the principle that psychological symptoms are linked to maladaptive patterns of thinking which, in turn, influence behaviours and emotions (Beck 1979). CBT is a structured, problem‐oriented approach (Kingdon 1998; Stuart 1998), and is based on collaborative empiricism, which is a goal‐driven process that is discussed and agreed by the clinician and the patient (Beck 1979). Several studies have shown that CBT is one of the most effective forms of treatment for mental health problems (Fordham 2021; Otto 2004). CBT has been suggested as a highly effective method of treatment for a variety of mental health disorders (Butler 2006; Fordham 2021).
The CBT model targeting symptoms of psychosis (CBTp) was developed in the late 1980s and early 1990s (Kingdon 1998). Individual studies have shown that cognitive‐based therapies can be used to manage schizophrenia (Gledhill 1998; Wykes 1999), although meta‐analyses show that the evidence is far from conclusive (Jones 2004; Jones 2018). Jones 2018 suggests that CBT for schizophrenia did not reduce relapse and readmission to hospital compared with standard care. However, it did reduce risk of staying in hospital (Jones 2018). It may be less effective than other forms of interventions such as mindfulness‐based psychoeducation (Mc Glanaghy 2021). Moreover, it appears that CBT for schizophrenia may be more useful in the short‐term, but less so in the medium‐ to long‐term (Jones 2004; Laws 2018). One recent meta‐analysis has shown that the effectiveness CBTp has improved for delusion over time (Sitko 2020).
While CBT for schizophrenia was mainly developed as an individual treatment (Lawrence 2006; Lecomte 2016), there has been some understanding of how a group approach may be more cost‐effective (Lawrence 2006). Group delivery of CBTp is an adaptation to the current method of individual CBTp, to allow groups of people to receive this treatment as a cohort. This model of delivery has the advantage of allowing a greater number of individuals to be treated by a single therapist, which has the potential of reducing costs while maintaining efficacy and allowing greater access to treatment (Lawrence 2006; Lecomte 2016). Other studies suggest that group CBTp has a positive impact on people with schizophrenia (Wykes 1999). In contrast, Barraclough 2006 concluded that group CBTp is effective for reducing hallucinations and delusions, but not as effective for reducing the negative thoughts and feelings the person with schizophrenia may have.
How the intervention might work
CBT for psychosis could work by giving people with schizophrenia skills and tools to help them cope with their psychotic symptoms (Morrison 2010; Spencer 2020). Moreover, it can help patients identify key cognitions, behaviours, emotions and images that are fundamental to their symptoms (Morrison 2010; Spencer 2020). By identifying maladaptive thoughts, emotions and behaviours, the person with schizophrenia is, potentially, in a better position to change them. It is also possible that working on a CBTp model can help instil hope in recovery, reduce distress and improve quality of life (Morrison 2010). The converse could also be true. Greater understanding of maladaptive patterns of thinking could equally instil feelings of helplessness, loss, grief and distress with subsequent repercussions on quality of life (Hesdon 2004).
More specifically, CBT delivered by group for people with schizophrenia could have the additional effect of being applied in a group setting, thus indirectly improving social competence in a population who traditionally have been socially isolated and excluded (Lawrence 2006; Lecomte 2016). Also, shared experience of tackling symptoms can be very powerful in a group setting. There can also be an opportunity of modelling positive behaviours and coping strategies, and also enhance motivation by observational learning (Lecomte 2016).
Why it is important to do this review
Group CBT for psychosis could be both effective and cost‐effective and a possible alternative to individual CBT. The UK National Health Service (NHS) has suggested that people with schizophrenia should receive individual therapy sessions (Bechdolf 2004). Due to the lack of resource this is unlikely to happen – and, perhaps less so as more evidence emerges as to the little or no benefits – and even drawbacks – of CBT for people with schizophrenia when compared with other psychological therapies (Jones 2010). However, the group experience may have benefits that individual therapy simply cannot provide and therefore, a review of best evidence is indicated. Group CBTp can be an important part of a stepped care model for the treatment of schizophrenia spectrum disorder (Kopelovich 2019).
This review is part of a series of Cochrane Reviews aiming to provide a comprehensive overview of the evidence for the effectiveness of CBT for people with schizophrenia. The protocol of this review was published in 2012 (Guaiana 2012).
Objectives
To investigate efficacy and acceptability of group CBT applied to psychosis compared with standard care or other psychosocial interventions, for people with schizophrenia or schizoaffective disorder.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials (RCT). If a trial was described as 'double blind' but implied randomisation, we included the trial in a sensitivity analysis (see Sensitivity analysis). We excluded quasi‐randomised studies, such as those allocating by alternate days of the week. Where people were given additional treatments within group CBT, we included the data if the adjunct treatment was evenly distributed between groups and it was only the group CBT that was randomised.
Types of participants
Adults aged 16 years and over, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder by any means of diagnosis. We did not exclude trials that included participants who had a concurrent secondary diagnosis of another psychiatric disorder but we excluded trials that included participants who had a concomitant medical illness. We only included studies where at least 80% of participants had a diagnosis of schizophrenia or schizoaffective disorder.
We were interested in ensuring that information was relevant to the current care of people with schizophrenia so proposed to clearly highlight the current clinical state (acute, early postacute, partial remission, remission) and stage (prodromal, first episode, early illness, persistent), and whether the studies primarily focused on people with particular problems (e.g. negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Group cognitive behavioural therapy for schizophrenia (CBTp)
Defined as a structured psychotherapeutic intervention, carried out in any group setting, and based on the CBT model applied to psychosis (Beck 1979). The key components are:
a structured approach to each session;
homework assignments on monitoring thoughts and target symptoms;
examination of evidence for and against a distressing thought;
encouragement in developing alternatives in thinking about a belief;
exploration of the relationship between thoughts, emotions and behaviours;
teaching techniques to cope with target symptoms, such as delusions, hallucinations and negative symptoms (Kingdon 2005).
Despite the above criteria, it is often difficult to define CBTp precisely. CBTp interventions that do not meet the key component criteria listed above were considered 'less defined CBTp', while CBTp interventions that met the same criteria were defined as CBTp. We aimed to perform a sensitivity analysis excluding studies where less‐defined criteria were applied to investigate whether the definition resulted in a difference in outcomes.
CBT for schizophrenia could be applied as a stand‐alone intervention or in combination with medication.
2. Standard care
We included interventions described as 'treatment as usual' and wait‐list control interventions as standard care. This is the care participants would normally receive in a standard mental health service and could consist of antipsychotic medication treatment alone or a combination of medication and additional psychosocial interventions of any type (other than group CBTp).
3. Other interventions
As a comparator we also considered other psychosocial interventions of any type.
Types of outcome measures
We aimed to divide all outcomes into short‐term (less than six months), medium‐term (seven to 12 months) and long‐term (over one year).
Primary outcomes
1. Global state
1.1 Clinically important change, shown as improved – as defined by individual studies.
Secondary outcomes
1. Global state
1.1 Any change – as defined by individual studies. 1.2 Relapse. 1.3 Average endpoint or change score on global state scale.
2. Leaving the study early
2.1 For any reason. 2.2 For specific reason.
3. Mental state
3.1 Overall
3.1.1 Clinically important change overall mental state – as defined by individual studies. 3.1.2 Any change in overall mental state – as defined by individual studies. 3.1.3 Average endpoint or change score on mental state scale.
3.2 Specific
3.2.1 Clinically important change in specific symptoms (e.g. positive or negative) – as defined by individual studies. 3.2.2 Any change in specific symptoms (e.g. positive or negative). 3.2.3 Depression. 3.2.4 Average endpoint or change scores on positive or negative symptom scales.
4. Compliance
4.1 Taking drug treatment. 4.2 Adherence to non‐drug treatments.
5. Adverse effects/events
5.1 At least one adverse effect/event. 5.2 Incidence of specific adverse effects. 5.3 Average endpoint or change scores on adverse effect scales. 5.4 Dependency. 5.5 Death: suicide or natural causes.
6. Service utilisation outcomes
6.1 Hospital admission. 6.2 Days in hospital.
7. Quality of life
7.1 Clinically important change – as defined by individual studies. 7.2 Any change – as defined by individual studies. 7.3 General impression of carer/rater or family member. 7.4 Average endpoint/change score on quality of life scales.
8. Functioning – social and living skills
8.1 Clinically important change in functioning – as defined by individual studies. 8.2 Any change in functioning – as defined by individual studies. 8.3 Average endpoint/change score, on social skills/functioning scales. 8.4 Occupational status.
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group's Study‐Based Register of Trials
On 8 January 2018, 28 January 2019, 7 May 2020 and 10 February 2021, the information specialist searched the register using the following search strategy:
*Cogniti* in Intervention Field of STUDY
In such a study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Roberts 2021; Shokraneh 2017; Shokraneh 2021). This allows rapid and accurate searches that reduce waste in the next steps of systematic reviewing (Shokraneh 2019).
Following the methods from Cochrane (Lefebvre 2019), this register is compiled by systematic searches of major resources (CENTRAL, MEDLINE, Embase, CINAHL, ClinicalTrials.Gov, ISRCTN, PsycINFO, PubMed, World Health Organization (WHO) ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, handsearches, grey literature and conference proceedings (Shokraneh 2020; see Schizophrenia Group's website at schizophrenia.cochrane.org/register-trials). There is no language, date, document type or publication status limitations for inclusion of records into the register.
Searching other resources
1. Personal communication
We contacted experts in the field by email or mail to find potential suitable studies for the review, or to find out whether there are studies in progress.
See Table 2 for details of how authors of the studies awaiting classification were contacted.
1. Contact of authors of studies awaiting classification.
| Study name | Contacted | Replied | Provided data | Notes |
| Brenner 1987 | No | No | No | Could not find author's contact details |
| Chung 2001 | Yes | No | No | — |
| Classen 1993 | No | No | No | — |
| Klingberg 2001 | Yes | No | No | — |
| Kraemer 1987 | No | No | No | Could not find author's contact details |
| McLeod 2007 | Yes | No | No | — |
| Shafiei 2011 | No | No | No | — |
2. Reference checking
Finally, we checked the reference lists of the included studies, previous systematic reviews written in English, Italian, French, German, Spanish, Chinese for published reports and citations of unpublished research. We checked the references of all included studies via Science Citation Index for articles which have cited the included study.
Data collection and analysis
Selection of studies
Five review authors (MA, FT, IE, CZ, WL) independently inspected citations from the searches and identified relevant abstracts. Where disputes arose, we acquired the full report for more detailed scrutiny. We then obtained full reports of the abstracts meeting the review criteria and the same five review authors inspected them. Where it was not possible to resolve disagreement by discussion, we attempted to contact the study authors for clarification.
Data extraction and management
1. Extraction
Teams of two review authors (from MA, FT, GG, IE, CZ, WL) independently extracted data from all included studies. We discussed any disagreements, documented decisions and, if necessary, contacted study authors for clarification. With any remaining problems, another member of the review group (AP) helped clarify issues and we documented the final decisions. Whenever possible, we extracted data presented only in graphs and figures but we only included the data if two review authors independently had the same result. We attempted to contact authors through an open‐ended request to obtain missing information or for clarification whenever necessary. If studies were multicentre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We used Covidence to select and extract data from the studies.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and
the measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should have either been a self‐report or completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, but we noted if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. However, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2022).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the problem of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion:
standard deviations (SDs) and means were reported in the paper or obtainable from the authors;
when a scale started from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution) (Altman 1996);
if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases, skew was present if 2 SD > (S – Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and endpoint and these rules can be applied. When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to determine whether data are skewed or not. We entered skewed data from studies of fewer than 200 participants into additional tables rather than into an analysis. Skewed data pose less of a problem when using means if the sample size is large, and therefore, we entered such data into the analyses.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that could be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we attempted to convert outcome measures to dichotomous data. This could be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS; Overall 1962) or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data such that the area to the left of the line of no effect indicated a favourable outcome for group CBT. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'not improved'), we reported data where the left of the line indicated an unfavourable outcome. We noted this in the relevant graphs.
Assessment of risk of bias in included studies
Teams of two review authors (from MA, FT, GG, IE, CZ, WL) independently assessed risk of bias using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2022). This set of criteria is based on evidence of associations between overestimates of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, we made the final rating by consensus, with the involvement of another member of the review group (AP). Where there were inadequate details of randomisation and other characteristics of trials provided, we contacted the study authors to request further information. We reported non‐concurrence in quality assessment, but if disputes arose as to which category a trial was to be allocated, we resolved this by discussion.
We noted the level of risk of bias in both the text of the review and in the risk of bias summary and risk of bias graph (Figure 2; Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive than odds ratios (Boissel 1999), and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes, we estimated MD between groups with 95% CIs. We preferred not to calculate effect size measures (SMD). However, if studies used scales of very considerable similarity, we presumed there was a small difference in measurement, and we calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. First, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992), whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where primary studies did not account for clustering, we presented data in a table, with a * symbol to indicate the presence of a probable unit of analysis error. We sought to contact first authors of studies to obtain intraclass correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering was incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC (design effect = 1 + (m – 1) × ICC) (Donner 2002). If the ICC was not reported, we assumed it to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added and combined them in the two‐by‐two table. If data were continuous, we combined data following the formula in Section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). Where the additional treatment arms were not relevant, we did not reproduce the data but listed them in the Characteristics of included studies table.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we did not reproduce these data or use them within analyses, except for the outcome leaving the study early. However, if more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented the data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, we used the rate of those who stayed in the study – in that particular arm of the trial – for those who did not. We aimed to undertake a sensitivity analysis to test how prone the primary outcomes were to change when comparing 'completer' data only with the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50% and completer‐only data are reported, we reproduced these.
3.2 Standard deviations
If SDs were not reported, we first tried to obtain the missing values from the study authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and CIs were available for group means, and either a P value or a t value were available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022): when only the SE was reported, we calculated SDs using the formula SD = SE × square root (n). Sections 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions present detailed formulae for estimating SDs from P values, t or F values, CIs, ranges or other statistics (Higgins 2022). If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies could introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. Nevertheless, we examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that some studies would use the method of last observation carried forward (LOCF) within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data had been used in the trial, if less than 50% of the data have been assumed, we reproduced these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations that we had not predicted. When such situations or participant groups arose, we discussed them fully in the text.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods that we had not predicted. When such methodological outliers arose, we discussed them fully in the text.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 statistic alongside the Chi2 P value. The I2 statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 statistic depends on magnitude and direction of effects and strength of evidence for heterogeneity (e.g. P value from Chi2 test, or a CI for I2 statistic). We interpreted an I2 statistic estimate of 50% or greater accompanied by a statistically significant Chi2 statistic as evidence of substantial levels of heterogeneity (Higgins 2022). When there were substantial levels of heterogeneity in the primary outcome, we explored reasons for it (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we aimed to seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. However, there is a disadvantage to the random‐effects model. It puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect these studies can either inflate or deflate the effect size. We used the random‐effects model for all analyses. However, readers are able to choose to inspect the data using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses – primary outcomes
We note that subgroup analyses are often exploratory in nature and should be interpreted with caution. First, because it often involves multiple analyses, this can lead to false positive results. Second, these analyses lack power and would more likely result in false‐positive results. Keeping in mind the above reservations, we aimed to perform the following subgroup analyses solely for the primary outcome.
Number of sessions offered (six sessions or fewer, seven to 12 sessions, more than 12 sessions).
People at their first episode of schizophrenia versus those with previous episodes of schizophrenia.
Efficacy in the short‐term (six months or less) versus efficacy in the long‐term (over six months).
1.2 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of group CBT for people with schizophrenia in general. In addition, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, we reported this. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and remove outlying studies to see if homogeneity was restored. Where unanticipated clinical or methodological heterogeneity were obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
We planned the sensitivity analyses for the primary outcome only.
1. Implication of randomisation
We aim to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes, we aimed to include these studies and if there was no substantive difference when the implied randomised studies are added to those with better descriptions of randomisation, then we used all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported results and discuss them but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with completer data only. We aimed to undertake a sensitivity analysis to test how prone results were to change when comparing 'completer' data only with the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included the data from these trials in the analysis.
4. Imputed values
We undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials.
If there were substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately
5. Fixed‐effect and random‐effects models
We synthesised all data using a random‐effects model; however, we planned to use a fixed‐effect model to evaluate whether the greater weights assigned to larger trials with greater event rates altered the significance of the results compared with the more evenly distributed weights in the random‐effects model (see Differences between protocol and review).
6. Dropout rates
We excluded studies with dropout rates of more than 50%.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret findings (Schünemann 2021) and use the GRADE profiler (GRADE Profiler) to import data from Review Manager 5 (Review Manager 2014) to prepare the summary of findings table (Table 1).
The summary of findings table provides outcome‐specific information concerning the overall certainty of the evidence from each included study in the comparison, the magnitude of effect of the interventions examined and the sum of available data on all outcomes we considered important to patient‐care and decision‐making.
In the protocol, we planned to use the following outcome measures for the summary of findings table.
Global state – clinically significant improvement in global state – as defined by each of the studies.
Leaving the study early.
-
Mental state.
Clinically significant response in mental state – as defined by each of the studies.
Relapse.
-
Service utilisation outcomes.
Hospital admission.
Days in hospital.
Quality of life.
Significant change in quality of life – as defined by each of the studies.
However, we selected the following main outcomes for inclusion in the summary of findings table (see Differences between protocol and review for reasons):
Clinically important change, shown as improved – as defined by individual studies.
Relapse.
Leaving the study early: for any reason.
Mental state: overall: average endpoint score – end of treatment (PANSS total).
Days in hospital.
Quality of life: clinically important change – as defined by individual studies.
Functioning: overall: global assessment of functioning – average endpoint score – end of treatment (Global Assessment of Functioning (GAF)).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
Initially, we identified 1324 references (764 studies) through database searching. After removing duplicates, we screened titles and abstracts of 741 records and excluded 579 records that were obviously irrelevant. We retrieved full‐text copies of the remaining 162 records (106 studies). We excluded 114 records (73 studies) overall. We excluded the studies for a variety of reasons (see Figure 4 for more information). In particular, we excluded 69 studies because the intervention was not group CBTp. We included 37 references referring to 24 studies in the qualitative and quantitative synthesis. We classified seven studies as awaiting classification since we could not obtain useful data for the analysis (see Table 2 for more information). Two studies are ongoing. The literature search was last updated in February 2021. See Figure 4 for PRISMA flow diagram.
4.
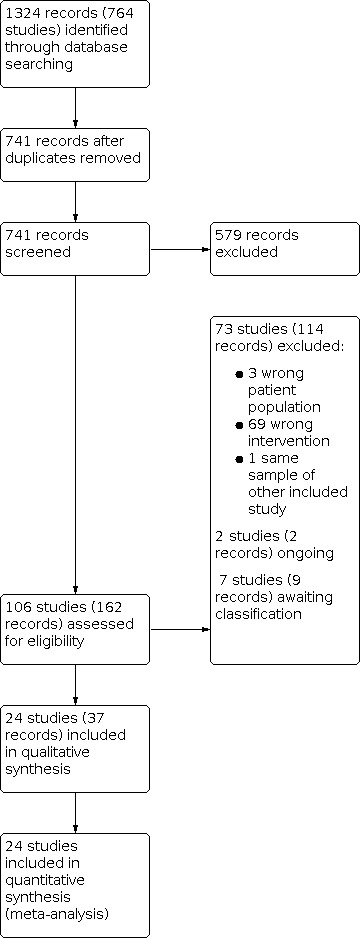
Study flow diagram.
Included studies
We included 24 studies in this systematic review. All the studies were published trials except for Jones 2014 (a PhD thesis dissertation).
1. Design and duration
Most RCTs had two arms except for Granholm 2020 and Mortan Sevi 2020, which included three arms. However, we just included two arms for those studies, as the third arm was not relevant to the outcomes.
2. Participants
All studies included participants with a diagnosis of schizophrenia or schizoaffective disorder, or both.
All studies randomised participants aged at least 16 years or older, but the age ranges of participants varied: two studies recruited older participants (over 42 years) (Granholm 2007; Granholm 2013); the remaining studies recruited people between the ages of 16 and 65 years.
3. Size
Overall, the studies included 1900 participants in active treatment arms. Of these, 949 were randomised to various forms of group CBTp. Of the remaining 951 participants, 495 were randomised to standard care, 228 to medication alone, 147 to supportive therapy, 48 to a psychoeducational group, 17 to mobile device control and 16 to wait‐list control. The mean sample size for both arms was about 40 participants (range six to 73).
4. Setting
Five studies recruited participants from multiple centres (Barrowclough 2006; Chadwick 2016; Granholm 2013; Penn 2009; Wykes 2005). The remaining studies recruited participants from one centre only. All studies were conducted in a single nation: the USA (Daniels 1998; Granholm 2007; Granholm 2013; Granholm 2014; Granholm 2020; Penn 2009), China (Deng 2014; Guan 2016; Li 2013a; Li 2013b; Qi 2012; Qu 2016; Shen 2007; Shi 2015; Song 2012; Song 2014; Tao 2015; Zhou 2015), the UK (Barrowclough 2006; Chadwick 2016; Wykes 2005), Turkey (Mortan Sevi 2020), and Germany (Bechdolf 2004).
Three studies enrolled both inpatients and outpatients (Granholm 2013; Song 2014; Wykes 2005). Eleven studies recruited inpatients only (Barrowclough 2006; Bechdolf 2004; Deng 2014; Guan 2016; Li 2013a; Li 2013b; Qi 2012; Qu 2016; Shi 2015; Song 2012; Zhou 2015). Nine studies recruited outpatients only (Chadwick 2016; Daniels 1998; Granholm 2007; Granholm 2020; Jones 2014; Mortan Sevi 2020; Penn 2009; Shen 2007; Tao 2015).
5. Interventions and comparators
For this review, we defined group CBTp as a group intervention targeting psychotic symptoms, based on the cognitive model. In group CBTp, people work collaboratively on coping with distressing hallucinations, analysing evidence for their delusions, problem‐solving skills and social skills. As for concomitant medication, three studies did not report whether participants received any medication (Daniels 1998; Granholm 2020; Song 2012), while in the remaining 15 studies, all or most of the participants received concomitant medication.
The comparator interventions (control psychological therapies and medications) described in the studies can be considered the treatment that a person with schizophrenia would usually receive in a standard mental health service. For this reason, we grouped the comparator interventions together, and we considered them as "standard care" for analyses.
One study compared group CBTp to a control psychoeducational group (Bechdolf 2004). Twelve studies compared group CBTp to standard care (Barrowclough 2006; Chadwick 2016; Deng 2014; Granholm 2007; Li 2013a; Mortan Sevi 2020; Qu 2016; Song 2012; Song 2014; Tao 2015; Wykes 2005; Zhou 2015). Two studies compared group CBTp with a wait‐list control (Daniels 1998; Jones 2014). Three studies compared group CBTp to a form of supportive therapy (Granholm 2013; Granholm 2014; Penn 2009). Five studies compared group CBTp to medication alone (Guan 2016; Li 2013b; Qi 2012; Shen 2007; Shi 2015).
Two studies were three‐arm trials. Mortan Sevi 2020 compared group CBTp with standard care and a modified CBT called COPE‐CBT, while Granholm 2020 compared group CBTp with a mobile‐assisted group CBTp and an electronic device control. However, we excluded the third arms (COPE‐CBT for Mortan Sevi 2020 and electronic device control for Granholm 2020) from the analysis as we deemed them as not part of a CBTp treatment.
6. Outcomes
6.1 General
All studies provided efficacy data (either as dichotomous or as continuous outcome) that could be entered into a meta‐analysis.
The studies used a variety of scales to assess clinical response, functioning and quality of life.
Thirteen studies also reported the number of participants leaving the study early, due to any reason, while the remaining studies did not report this outcome (Barrowclough 2006; Bechdolf 2004; Chadwick 2016; Deng 2014; Granholm 2007; Granholm 2013; Granholm 2014; Li 2013a; Mortan Sevi 2020; Penn 2009; Shi 2015; Tao 2015; Wykes 2005).
6.2 Outcome scales providing useable data
6.2.1 Mental state
Positive and Negative Syndrome Scale (PANSS) (Kay 1987)
The PANSS was developed from the BPRS and the Psychopathology Rating Scale. It is used to evaluate the positive, negative and general symptoms in schizophrenia. The scale has 30 items, and each item is rated on a 7‐point scoring system varying from 'absent' (1) to 'extreme' (7). Higher scores indicate more pronounced symptomatology.
Brief Psychiatric Rating Scale (BPRS) (Overall 1962)
The BPRS is a scale used to measure the severity of psychiatric symptoms, including psychotic symptoms. The scale usually has 18 items (depending on the version the number of items varies from 16 to 24), and each item is rated on a 7‐point scoring system varying from 'not present' (1) to 'extremely severe' (7). Higher scores indicate more pronounced symptomatology.
Psychotic Symptom Rating Scale (PSYRATS) (Haddock 1999)
The PSYRATS is a medical scale used to measure symptom severity in schizophrenia. It consists of two scales rating auditory hallucinations and delusions. It has 11 items related to auditory hallucinations (frequency, duration, location, loudness, beliefs regarding origin of voices, amount of negative content of voices, degree of negative content of voices, amount of distress, intensity of distress, disruption to life caused by voices and controllability of voices) and six items related to delusions (amount of preoccupation with delusions, duration of preoccupation with delusions, conviction, amount of distress, intensity of distress and disruption to life caused by beliefs). Each symptom is rated on a scale from 0 to 4. Minimum score for PSYRATS is 0 and maximum score is 68. Higher scores indicate more pronounced symptomatology.
Symptom Checklist‐90‐Revised (SCL‐90‐R) (Derogatis 1992)
This scale is used to evaluate a broad range of psychological problems and symptoms of psychopathology. It consists of 90 items and yielding nine scores along primary symptom dimensions and three scores among global distress indices. The primary symptom dimensions are: somatisation, obsessive‐compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, psychoticism and a category of "additional items". The three indices are global wellness index, hardiness and symptom free. Higher scores indicate more pronounced symptomatology.
Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen 1991)
The SAPS is used to measure the positive symptoms of schizophrenia. It consists of four subscales that evaluate four different aspects of positive symptoms: hallucinations, delusions, bizarre behaviour and positive formal thought disorder. Higher scores indicate more pronounced symptomatology.
Scale for the Assessment of Negative Symptoms (SANS) (Andreasen 1991)
The SANS is used to measure the negative symptoms of schizophrenia. It consists of five subscales that evaluate five different aspects of negative symptoms: alogia, affective blunting, avolition‐apathy, anhedonia‐asociality and attentional impairment. Higher scores indicate more pronounced symptomatology.
Auditory Hallucinations Rating Scale (AHRS) (Hoffman 2003)
The AHRS is an English‐language structured tool developed to measure the effect of repetitive transcranial magnetic stimulation in hallucinating people with schizophrenia. It has seven items. It aims at assessing hallucination frequency, number of distinct speaking voices, perceived loudness, vividness, attentional salience (the degree to which hallucinations captured the attention of the patient), length of hallucinations (single words, phrases, sentences, or extended discourse) and degree of distress. Higher scores indicate more pronounced symptomatology.
Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983)
The HADS is used to detect states of depression and anxiety in the setting of a hospital medical outpatient clinic. The anxiety and depressive subscales are also measures of severity of the emotional disorder. Higher scores indicate more pronounced symptomatology.
Hamilton Rating Scale for Depression (HAMD) (Hamilton 1967)
The HAMD is 17‐item scale used to assess the severity of depression. Each item is rated on 3‐ or 5‐point scale. Higher score indicates severe depression. Higher scores indicate more pronounced symptomatology.
Hamilton Anxiety Rating Scale (HARS) (Hamilton 1959)
The HARs is 14‐item scale used to assess the severity of anxiety. Each item was rated on 5‐point scale. Higher score indicates severe anxiety. Higher scores indicate more pronounced symptomatology.
Beck Depression Inventory‐II (BDI‐II) (Beck 1996)
The BDI‐II scale is a 21‐item, self‐report questionnaire which measures the severity of depressive symptoms. Higher scores indicate more pronounced symptomatology.
Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979)
The MADRS is a 10‐item, self‐report questionnaire that measures the severity of depressive symptoms. It is designed to be sensitive to change resulting from antidepressant therapy. Higher scores indicate more pronounced symptomatology.
Beck Anxiety Inventory (BAI) (Beck 1990)
The BAI is a 21‐item self‐report questionnaire that measures the intensity of depressive symptoms. Higher scores indicate more pronounced symptomatology.
Social Avoidance and Distress Scale (SAD) (Watson 1969)
The SAD is a 280‐item true/false scale that measures aspects of social anxiety including distress, discomfort, fear and avoidance. Higher scores indicate more pronounced symptomatology.
6.2.2. Quality of life
Modular System for Quality of Life (MSQoL) (Pukrop 2000)
The MSQoL is an integrative questionnaire that consists of one "G‐factor" (life in general) and six specific dimensions (physical health, vitality, psychosocial relationships, material resources, affect, leisure time). This basic structure represents a core module measured by 47 items. Higher scores indicate better quality of life.
Quality of Life Scale (QLS) (Heinrichs 1984)
The QLS is a 21‐item scale based on a semi‐structured interview designed to assess deficit symptoms in interpersonal relations, instrumental role, intrapsychic foundations, common object and activities. Higher scores indicate better quality of life.
WHOQOL‐BREF (WHOQOL Group 1996)
The WHOQOL‐BREF is an abbreviated version of the World Health Organization Quality of Life Assessment Instrument (WHOQOL‐100; WHOQOL Group 1994). It is a 26‐item quality of life assessment consisting of four domains: Physical, Psychological, Social relationships and Environment. Higher scores indicate better quality of life.
General Quality of Life Inventory‐74 (GQOLI‐74) (Lu 2009)
The GQOLI‐74 was developed based on the WHOQOL‐100 and was modified for use in a Chinese population. The inventory comprises 74 items that can be grouped into 20 facets and covers the following four domains: Physical well‐being (sleep and energy, pain and physical discomfort, eating function, sexual function, daily living capability); Psychological well‐being (psychological distress, negative feelings, positive feelings, cognitive function, body image); Social well‐being (social support, interpersonal relationships, work and study capacity, recreational and leisure activities, marriage and family); and Material well‐being (housing situation, community services, living environment, financial situation). Patients’ responses are converted to scores on a 0 to 100 scale for each domain and each facet. Higher scores indicate better quality of life.
6.2.3 Social functioning
Global Assessment of Functioning (GAF) (APA 2000)
GAF rates the social, occupational and psychological functioning on a hypothetical continuum of mental health illness. The lowest scores are 1 to 10 corresponding to 'Persistent danger of severely hurting self or others OR persistent inability to maintain minimal personal hygiene OR serious suicidal act with clear expectation of death' and the highest scores are 91 to 100 corresponding to 'No symptoms, life's problems never seem to get out of hand, is sought out by others because of his or her many positive qualities'. Higher scores indicate better social functioning.
Social Behaviour Schedule (SBS) (Wykes 1986)
The SBS is a 30‐item scale which measures 21 different social behaviours and nine activities. Higher scores indicate worse social functioning.
Social Functioning Scale (SFS) (Birchwood 1990)
The SFS measures social role and behavioural functioning across seven basic areas of community functioning: social engagement, interpersonal behaviour, prosocial activities, recreation, independence and employment. Higher scores indicate better social functioning.
Independent Living Skills Survey (ILSS) (Wallace 2000)
The ILSS is a comprehensive, objective, performance‐focused measure of the basic functional living skills of individuals with severe and persistent mental illness to obtain a view of their community adjustment. Higher scores indicate better social functioning.
Personal and Social Performance Scale (PSP) (Morosini 2000)
This scale assesses functioning across four dimensions (socially useful activities, personal and social relationships, self‐care, disturbing and aggressive behaviours) rather than one global score. It is a revision of is a revision of the Social and Occupational Functioning Assessment Scale (SOFAS; APA 1994). Higher scores indicate better social functioning.
Inpatient Psychiatric Rehabilitation Outcome Scale (IPROS) (Li 1994)
The IPROS aims at objectively evaluating inpatient rehabilitation programmes. It has five subscales: performance in occupational therapy, daily activities, socialisation, personal hygiene and level of interest in external events. Evaluators (physicians or nurses) observe patients for one week before coding items on a 5‐point scale. Higher scores indicate worse social functioning.
Social Disability Screening Schedule (SDSS) (Liu 2005)
The SDSS scale is composed of 10 items, which is used to assess social function. Each item is scored from 0 = healthy or very minor defects to 2 = severe defect. Higher scores indicate worse social functioning.
Excluded studies
Overall, we excluded 73 studies from the systematic review (see Figure 4 and Characteristics of excluded studies table for more information).
Awaiting classification
We identified five studies awaiting classification (Brenner 1987; Chung 2001; Klingberg 2001; Kraemer 1987; McLeod 2007; see Studies awaiting classification table for details), as it was not possible to obtain data based on the title, abstract, full‐text or a combination of these. We tried to contact the authors of most of those studies to request data, but were unsuccessful. See Table 2 for details on the way we contacted authors.
Ongoing studies
The search of the Cochrane Schizophrenia Trial registry found two ongoing studies (IRCT 20180817040818N; NCT04144075; see Characteristics of ongoing studies table for more information).
Risk of bias in included studies
See Characteristics of included studies table, Figure 2, and Figure 3.
The overall methodological quality of the studies was medium to low; every study was judged as having high or unclear risk of bias in at least one domain (see Figure 2 and Figure 3 for summary graphs).
Allocation
We judged five studies that reported details of the randomisation procedure at low risk of bias (Bechdolf 2004; Guan 2016; Penn 2009; Song 2014; Zhou 2015). The remaining studies did not report details about the randomisation procedure, and so we judged them as having unclear risk of bias.
Barrowclough 2006, Granholm 2014, Penn 2009, and Wykes 2005 described measures to prevent participants or trial personnel from knowing the forthcoming allocation, and we judged them at low risk of bias. The remaining studies report no details on allocation concealment, and we judged them at unclear risk of bias.
Blinding
Given the nature of the intervention, blinding of participants and personnel was not attainable. Therefore, we considered all studies at high risk of bias for the blinding of participants and personnel risk of bias.
Concerning detection bias, 10 studies specified that raters were blind to treatment allocation, and were at low risk of bias (Barrowclough 2006; Bechdolf 2004; Chadwick 2016; Daniels 1998; Granholm 2007; Granholm 2013; Granholm 2014; Granholm 2020; Penn 2009; Wykes 2005). Thirteen studies provided no information on blinding of raters, and were at unclear risk of bias (Deng 2014; Guan 2016; Li 2013a; Li 2013b; Mortan Sevi 2020; Qi 2012; Qu 2016; Shen 2007; Shi 2015; Song 2012; Song 2014; Tao 2015; Zhou 2015). One study clearly specified that it was not possible for the experimenter to be blind to the treatment allocation (Jones 2014). This could have created bias in assessment. Therefore, we judged it at high risk of bias.
Incomplete outcome data
Overall rate of participants leaving the study early was with group CBTp and standard care. All studies except for one (Daniels 1998) did not seem to have differences in attrition between the groups, and were at low risk of bias. Daniels 1998 showed contradictory information on the number of participants, mentioning 40 in the "Subjects" section and 20 on the "Treatment" section. We judged this study at high risk of bias.
Selective reporting
None of the studies had published protocols. Three studies did not report all the data, and were at high risk of bias (Daniels 1998; Granholm 2007; Granholm 2013). Daniels 1998 mentioned using PANSS as a screening tool for positive symptoms, but the author did not report the scores. Granholm 2007 reported some outcomes in unusable diagrams. Granholm 2013 did not report data on PANSS negative outcome result. The remaining studies showed no evidence of selective outcome reporting. We judged them at low risk of bias.
Other potential sources of bias
We found no evidence of other potential source of bias for all the included studies.
Effects of interventions
See: Table 1
Primary outcome
1. Global state
1.1 Clinically important change, shown as improved
No studies reported global state: clinically important change – as defined by individual studies.
Secondary outcomes
1. Global state
1.1 Any change
No studies reported global state: any change – as defined by individual studies.
1.2 Relapse – end of treatment
No studies reported global change: relapse.
1.3 Average endpoint or change score on global state scale
No studies reported global change: average endpoint or change score on global state scale.
2. Leaving the study early
2.1 Leaving the study early: for any reason – end of treatment
There was no clear difference between CBTp and standard care and other psychosocial interventions for numbers of participants leaving the study early (RR 1.22, 95% CI 0.94 to 1.59; studies = 13, participants = 1267; I2 = 9%; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 1: Leaving the study early: for any reason – end of treatment
The funnel plot based on this outcome was symmetrical, making the risk of publication bias low (Figure 5).
5.

Leaving the study early: for any reason – end of treatment.
2.2 Leaving the study early: for specific reason – end of treatment
No studies reported leaving the study early: for specific reason.
3. Mental state
3.1 Mental state: overall
3.1.1 Mental state: overall: clinically important change overall mental state – as defined by individual studies – end of treatment
No studies reported mental state: overall: clinically important change overall mental state – as defined by individual studies – end of treatment.
3.1.2 Mental state: overall: any change in overall mental state – as defined by individual studies
No studies reported mental state: overall: any change in overall mental state – as defined by individual studies.
3.1.3a Mental state: overall: average endpoint score – end of treatment (PANSS total, high = poor)
Results from PANSS total final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –3.73, 95% CI –4.63 to –2.83; studies = 12, participants = 1036; I2 = 5%; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 2: Mental state: overall: average endpoint score – end of treatment (PANSS total, high = poor)
The funnel plot based on this outcome was asymmetrical, increasing the risk for publication bias (Figure 1).
1.

Mental state: overall: average endpoint score – end of treatment (PANSS total, high = poor).
3.1.3b Mental state: overall: average endpoint score – end of treatment (BPRS total, high = poor)
Results from BPRS total final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –4.26, 95% CI –7.50 to –1.02; studies = 3, participants = 180; I2 = 65%; Analysis 1.3). Heterogeneity was high.
1.3. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 3: Mental state: overall: average endpoint score – end of treatment (BPRS total, high = poor)
3.1.3c Mental state: overall: average endpoint score – end of treatment (PSYRATS total, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on PSYRATS total scores at the end of treatment (MD –0.45, 95% CI –3.23 to 2.33; studies = 2, participants = 166; I2 = 0%; Analysis 1.4).
1.4. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 4: Mental state: overall: average endpoint score – end of treatment (PSYRATS total, high = poor)
3.1.3d Mental state: overall: average endpoint score – end of treatment (SCL‐90‐R, high = poor)
Results from SCL‐90‐R total final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –33.26, 95% CI –51.26 to –15.26; studies = 1, participants = 82; Analysis 1.5).
1.5. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 5: Mental state: overall: average endpoint score – end of treatment (SCL‐90‐R, high = poor)
3.2 Mental state: specific
3.2.1 Clinically important change in specific symptoms (e.g. positive or negative) – as defined by individual studies
No studies reported clinically important change in specific symptoms (e.g. positive or negative) – as defined by individual studies.
3.2.2 Any change in specific symptoms (e.g. positive or negative)
No studies reported any change in specific symptoms (e.g. positive or negative).
3.2.3a Mental state: specific: depression – average endpoint score – end of treatment (BDI‐II, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on BDI‐II scores at the end of treatment (MD –0.46, 95% CI –3.29 to 2.37; studies = 5, participants = 229; I2 = 0%; Analysis 1.14).
1.14. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 14: Mental state: specific: depression – average endpoint score – end of treatment (BDI‐II, high = poor)
3.2.3b Mental state: specific: depression – average endpoint score – end of treatment (HAMD, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on HAMD scores at the end of treatment (MD 0.80, 95% CI –2.26 to 3.86; studies = 1, participants = 65; Analysis 1.15).
1.15. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 15: Mental state: specific: depression – average endpoint score – end of treatment (HAMD, high = poor)
3.2.3c Mental state: specific: depression – average endpoint score – end of treatment (MADRS, high = poor)
Results from MADRS total final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –2.18, 95% CI –3.80 to –0.56; studies = 1, participants = 56; Analysis 1.16).
1.16. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 16: Mental state: specific: depression – average endpoint score – end of treatment (MADRS, high = poor)
3.2.3d Mental state: specific: depression – average endpoint score – end of treatment (HADS depression, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on HADS depression subscores at the end of treatment (MD –1.75, 95% CI –3.59 to 0.09; studies = 1, participants = 93; Analysis 1.17).
1.17. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 17: Mental state: specific: depression – average endpoint score – end of treatment (HADS depression, high = poor)
3.2.4a Mental state: specific: positive symptoms – average endpoint score – end of treatment (PANSS positive, high = poor)
Six studies included PANSS positive symptoms average endpoint scores (Bechdolf 2004; Li 2013b; Qi 2012; Shen 2007; Shi 2015; Tao 2015). However, they showed skewness and, therefore, were removed from the analysis (see Table 3 for more information). The analysis with the remaining studies showed that there was no clear difference between group CBTp and standard care and other psychosocial interventions (MD –0.45, 95% CI –1.30 to 0.40; studies = 8, participants = 539; I2 = 0%; Analysis 1.6).
2. Mental state: specific: positive symptoms – average endpoint score – end of treatment (PANSS positive, high = poor): studies with skewed data.
| Study ID | Intervention | Mean | SD | n |
| Bechdolf 2004 | CBTp group | 11.3 | 4.2 | 31 |
| Standard care | 11.4 | 4.5 | 40 | |
| Li 2013b | CBTp group | 10.1 | 3.9 | 60 |
| Standard care | 11.2 | 3.8 | 60 | |
| Qi 2012 | CBTp group | 11.62 | 5.27 | 30 |
| Standard care | 11.94 | 5.46 | 40 | |
| Shen 2007 | CBTp group | 7.36 | 0.621 | 28 |
| Standard care | 8.75 | 2.351 | 28 | |
| Shi 2015 | CBTp group | 7.96 | 3.12 | 56 |
| Standard care | 9.69 | 3.37 | 58 | |
| Tao 2015 | CBTp group | 8.44 | 1.95 | 59 |
| Standard care | 9.31 | 2.9 | 57 |
CBTp: cognitive behavioural therapy model targeting symptoms of psychosis; n: number of participants; PANSS: Positive and Negative Syndrome Scale; SD: standard deviation.
1.6. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 6: Mental state: specific: positive symptoms – average endpoint score – end of treatment (PANSS positive, high = poor)
3.2.4b Mental state: specific: positive symptoms – average endpoint score – end of treatment (SAPS, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on SAPS positive symptoms scores at the end of treatment (MD –0.15, 95% CI –3.24 to 2.95; studies = 2, participants = 39; I2 = 0%; Analysis 1.7).
1.7. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 7: Mental state: specific: positive symptoms – average endpoint score – end of treatment (SAPS, high = poor)
3.2.4c Mental state: specific: delusions – average endpoint total score – end of treatment (PSYRATS delusions, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on PSYRATS delusions scores at the end of treatment (MD –3.39, 95% CI –8.50 to 1.71; studies = 2, participants = 71; I2 = 50%; Analysis 1.8). Heterogeneity was high.
1.8. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 8: Mental state: specific: average endpoint total score – delusions – end of treatment (PSYRATS delusions, high = poor)
3.2.4d Mental state: specific: voices – average endpoint total score – end of treatment (PSYRATS voices, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on PSYRATS voices scores at the end of treatment (MD –5.94, 95% CI –18.04 to 6.16; studies = 2, participants = 71; I2 = 75%; Analysis 1.9). Heterogeneity was high.
1.9. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 9: Mental state: specific: average endpoint total score – voices – end of treatment (PSYRATS voices, high = poor)
3.2.4e Mental state: specific: voices – average endpoint total score – end of treatment (AHRS, high = poor)
Results from AHRS total final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –5.30, 95% CI –7.49 to –3.11; studies = 1, participants = 103; Analysis 1.10).
1.10. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 10: Mental state: specific: average endpoint total score – voices – end of treatment (AHRS, high = poor)
3.2.4f Mental state: specific: negative symptoms – average endpoint score – end of treatment (PANSS negative, high = poor)
Two studies included PANSS negative symptoms average endpoint scores (Shen 2007; Wykes 2005). However, they showed skewness and, therefore, were removed from the analysis (see Table 4 for more information). The analysis with the remaining studies showed that there was no clear difference between group CBTp and standard care and other psychosocial interventions (MD –0.73, 95% CI –1.68 to 0.21; studies = 9, participants = 768; I2 = 65%; Analysis 1.11). Heterogeneity was high.
3. Mental state: specific: negative symptoms – average endpoint score – end of treatment (PANSS negative, high = poor): studies with skewed data.
| Study ID | Intervention | Mean | SD | n |
| Shen 2007 | CBTp group | 9.96 | 3.01 | 28 |
| Standard care | 9.71 | 3.90 | 28 | |
| Wykes 2005 | CBTp group | 17.0 | 16.6 | 41 |
| Standard care | 16.6 | 7.3 | 39 |
CBTp: cognitive behavioural therapy model targeting symptoms of psychosis; n: number of participants; PANSS: Positive and Negative Syndrome Scale; SD: standard deviation.
1.11. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 11: Mental state: specific: negative symptoms – average endpoint score – end of treatment (PANSS negative, high = poor)
3.2.4g Mental state: specific: negative symptoms – average endpoint score – end of treatment (SANS, high = poor)
Results from SANS total final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –5.34, 95% CI –6.05 to –4.64; studies = 4, participants = 180; I2 = 0%; Analysis 1.12).
1.12. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 12: Mental state: specific: negative symptoms – average endpoint score – end of treatment (SANS, high = poor)
3.2.4h Mental state: specific: depression/anxiety – average endpoint score – end of treatment (HADS total, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on HADS scores at the end of treatment (MD –0.53, 95% CI –3.58 to 2.52; studies = 1, participants = 90; Analysis 1.13).
1.13. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 13: Mental state: specific: depression/anxiety – average endpoint score – end of treatment (HADS total, high = poor)
3.2.4i Mental state: specific: anxiety – average endpoint score – end of treatment (BAI, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on BAI scores at the end of treatment (MD –0.49, 95% CI –14.53 to 13.54; studies = 2, participants = 77; I2 = 66%; Analysis 1.18). Heterogeneity was high.
1.18. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 18: Mental state: specific: anxiety – average endpoint score – end of treatment (BAI, high = poor)
3.2.4j Mental state: specific: anxiety – average endpoint score – end of treatment (HADS anxiety, high = poor)
There was no clear difference between group CBTp and standard care on HADS anxiety subscores at the end of treatment (MD –0.71, 95% CI –2.33 to 0.91; studies = 1, participants = 93; Analysis 1.19).
1.19. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 19: Mental state: specific: anxiety – average endpoint score – end of treatment (HADS anxiety, high = poor)
3.2.4k Mental state: specific: anxiety – average endpoint score – end of treatment (HARS, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on HARS anxiety subscores at the end of treatment (MD –6.30, 95% CI –13.81 to 1.21; studies = 1, participants = 20; Analysis 1.20).
1.20. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 20: Mental state: specific: anxiety – average endpoint score – end of treatment (HARS, high = poor)
3.2.4l Mental state: specific: social anxiety – average endpoint score – end of treatment (SAD, high = poor)
Results from SAD total final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –7.33, 95% CI –9.73 to –4.93; studies = 1, participants = 82; Analysis 1.21).
1.21. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 21: Mental state: specific: social anxiety – average endpoint score – end of treatment (SAD, high=poor)
4. Compliance
4.1 Taking drug treatment
No studies reported taking drug treatment.
4.2 Adherence to non‐drug treatments
No studies reported adherence to non‐drug treatments.
5. Adverse effects/events
5.1 At least one adverse effect/event
No studies reported at least one adverse effect/event.
5.2 Incidence of specific adverse effects
No studies reported incidence of specific adverse effects.
5.3 Average endpoint or change scores on adverse effect scales
No studies reported average endpoint or change scores on adverse effect scales.
5.4 Adverse effects/events: dependency
No studies reported dependency.
5.5 Adverse effects/events: death: suicide or natural causes
No studies reported death: suicide or natural causes.
6. Service utilisation outcomes
6.1 Number of participants hospitalised during the study (hospital admissions)
There was no clear difference between CBTp and standard care and other psychosocial interventions for numbers of participants hospitalised during the study (RR 0.78, 95% CI 0.38 to 1.60; studies = 3, participants = 235; I2 = 34%; Analysis 1.22).
1.22. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 22: Service use: number of people hospitalised during the study
6.2 Average number days spent in hospital during the study
The data for average number days spent in hospital during the study were skewed and were not analysed as there was only one study (see Table 5 for more information).
4. Service use: average number days spent in hospital during the study: studies with skewed data.
| Study ID | Interventions | Mean | SD | n |
| Barrowclough 2006 | CBTp group | 11.9 | 36.7 | 55 |
| Standard care | 5.1 | 15.1 | 55 |
CBTp: cognitive behavioural therapy model targeting symptoms of psychosis; n: number of participants; PANSS: Positive and Negative Syndrome Scale; SD: standard deviation.
7. Quality of life
7.1 Clinically important change – end of treatment
No studies reported quality of life: clinically important change – as defined by individual studies – end of treatment.
7.2 Any change – end of treatment
No studies reported quality of life: any change – as defined by individual studies – end of treatment.
7.3 General impression of carer/rater or family member – end of treatment
No study reported quality of life: general impression of carer/rater or family member – end of treatment.
7.4a Average endpoint score – end of treatment (MSQoL, high = good)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on MSQoL scores at the end of treatment (MD 1.80, 95% CI –10.17 to 13.77; studies = 1, participants = 63; Analysis 1.23).
1.23. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 23: Quality of life: average endpoint score – end of treatment (MSQoL, high = good)
7.4b Average endpoint score – end of treatment (QLS, high = good)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on QLS scores at the end of treatment (MD –6.58, 95% CI –15.84 to 2.69; studies = 2, participants = 40; I2 = 0%; Analysis 1.24).
1.24. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 24: Quality of life: average endpoint score – end of treatment (QLS, high = good)
7.4c Average endpoint score – end of treatment (WHOQOL‐BREF – Psychological domain subscale, high = good)
Results from WHOQOL‐BREF Psychological domain subscore slightly favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –4.64, 95% CI –9.04 to –0.24; studies = 2, participants = 132; I2 = 77%; Analysis 1.25). Heterogeneity was high.
1.25. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 25: Quality of life: average endpoint score – end of treatment (WHOQOL‐BREF – Psychological domain subscale, high = good)
7.4d Average endpoint score – end of treatment (GQOLI‐74 – Psychological domain subscale, high = good)
Results from GQOLI‐74 final score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –4.83, 95% CI –8.20 to –1.46; studies = 1, participants = 60; Analysis 1.26).
1.26. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 26: Quality of life: average endpoint score – end of treatment (GQOLI‐74, high = good)
8. Functioning
8.1 Clinically important change in functioning – end of treatment
No studies reported functioning: clinically important change in functioning – as defined by individual studies – end of treatment.
8.2 Any change in functioning
No studies reported functioning: any change in functioning – as defined by individual studies.
8.3a Functioning: overall: global assessment of functioning – average endpoint score – end of treatment (GAF, high = good)
Results from GAF score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –3.61, 95% CI –6.37 to –0.84; studies = 5, participants = 254; I2 = 0%; moderate‐certainty evidence; Analysis 1.27).
1.27. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 27: Functioning: overall: global assessment of functioning – average endpoint score – end of treatment (GAF, high = good)
8.3b Functioning: specific: living skills – average endpoint score – end of treatment (ILSS, high = good)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on ILSS scores at the end of treatment (MD 0.01, 95% CI –0.03 to 0.02; studies = 4, participants = 231; I2 = 0%; Analysis 1.28).
1.28. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 28: Functioning: specific: living skills – average endpoint score – end of treatment (ILSS, high = good)
8.3c Functioning: specific: social – average endpoint score – end of treatment (SBS, high = poor)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on SBS scores at the end of treatment (MD –1.30, 95% CI –4.59 to 1.99; studies = 1, participants = 79; Analysis 1.29).
1.29. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 29: Functioning: specific: social – average endpoint score – end of treatment (SBS, high = poor)
8.3d Functioning: specific: social – average endpoint score – end of treatment (SFS, high = good)
There was no clear difference between group CBTp and standard care and other psychosocial interventions on SFS scores at the end of treatment (MD –1.11, 95% CI –9.03 to 6.81; studies = 2, participants = 140; I2 = 0%; Analysis 1.30).
1.30. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 30: Functioning: specific: social – average endpoint score – end of treatment (SFS, high = good)
8.3e Functioning: specific: social – average endpoint score – end of treatment (PSP, high = good)
Results from PSP score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD 3.30, 95% CI 2.00 to 4.60; studies = 1, participants = 100; Analysis 1.31).
1.31. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 31: Functioning: specific: social – average endpoint score – end of treatment (PSP, high = good)
8.3f Functioning: specific: social – average endpoint score – end of treatment (IPROS, high = poor)
Results from IPROS score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –8.90, 95% CI –12.62 to –5.18; studies = 1, participants = 80; Analysis 1.32).
1.32. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 32: Functioning: specific: social – average endpoint score – end of treatment (IPROS, high = poor)
8.3g Functioning: specific: social – average endpoint score – end of treatment (SDSS, high = poor)
Results from SDSS score favoured group CBTp over standard care and other psychosocial interventions at the end of treatment (MD –1.27, 95% CI –2.46 to –0.08; studies = 1, participants = 116; Analysis 1.33).
1.33. Analysis.

Comparison 1: Group cognitive behavioural therapy (CBT) versus standard care, Outcome 33: Functioning: specific: social – average endpoint score – end of treatment (SDSS, high = poor)
8.4 Occupational status
No studies reported occupational status.
Subgroup analyses
The planned subgroup analyses were not performed since there were no data on the primary outcome (see Subgroup analysis and investigation of heterogeneity).
Sensitivity analyses
The planned sensitivity analyses were not performed since there were no data on the primary outcome (see Sensitivity analysis).
Discussion
Summary of main results
1. General
This review included 24 studies with 1900 participants that compared group CBTp with standard care or other psychosocial interventions in people with schizophrenia or related disorders. The included studies were published from 1998 to 2020 and varied widely in duration (five weeks to one year). The total of 24 included studies with 1900 participants is a small base upon which to judge the effectiveness of group CBTp. Each study did not have a very large sample size. Trials with small sample sizes may lack sufficient power to detect a small to moderate effect, and thus results from such trials are often inconclusive, even when a real effect does exist. We decided to focus on end of treatment outcomes.
2. Treatment effects
2.1 Global state: clinically important change, shown as improved – as defined by individual studies
No studies indicated reported clinically important change as defined by the authors, so we could draw no conclusion on our primary outcome.
2.2 Leaving the study early: for any reason – end of treatment
Thirteen studies included data on leaving the study early. There seems to be no difference in dropout rates between group CBTp and standard care or other psychosocial interventions. However, given the low number and quality of trials, it is difficult to draw firm conclusions. The confidence in the estimate is low.
2.3 Mental state: overall – average endpoint score – end of treatment (PANSS total)
Twelve studies included data for PANSS total scores at the end of treatment. There seems to be some advantage of group CBTp overall over standard care or other psychosocial interventions on total PANSS. However, given the small number of participants in each study, no firm conclusion on the efficacy of group CBTp can be drawn. In addition, funnel plot analysis showed some evidence of publication bias. CBTp seems to be a promising treatment overall, but we need more robust data and further investigation on the probability of publication bias. The confidence in the estimate is low.
2.4 Mental state: overall: average endpoint score – end of treatment (BPRS total)
Three studies included data for BPRS scores at the end of treatment. There seems to be some advantage of group CBTp overall over standard care or other psychosocial interventions on total BPRS scores. However, data may not be generalisable as the number of studies and participants (180 in total) was small. In addition, results showed a high level of heterogeneity. This may be because the populations included, the lengths of the interventions and the control groups were different.
2.5 Mental state: specific: positive symptoms – average endpoint score – end of treatment (PANSS positive)
Fourteen studies included data for PANSS positive symptoms scores at the end of treatment. However, six studies showed skewness and were excluded from the analysis. After removing the studies with skewed data, CBTp group showed no clear advantage over standard care or other psychosocial interventions.
2.6 Mental state: specific: negative symptoms – average endpoint score – end of treatment (PANSS negative)
Eleven studies included data for PANSS negative symptoms scores at the end of treatment. However, two studies showed skewness and were excluded from the analysis. After removing the studies with skewed data, group CBTp showed no advantage over standard care or other psychosocial interventions for negative symptoms. Moreover, heterogeneity was high, possibly because of the different subpopulations of people with schizophrenia included, and the fact that response of negative symptoms to group CBTp may be less consistent and heavily dependent on context and population.
2.7 Mental state: specific: negative symptoms – average endpoint score – end of treatment (SANS)
Four studies included data for SANS. Interestingly, when negative symptoms were measured with SANS, instead of the PANSS, group CBTp showed some advantage over standard care or other psychosocial interventions. However, the sample size was small (180 participants in total), and it is very difficult to draw clear conclusions based on this analysis. One study in particular seemed to have participants with much higher endpoint SANS score than the other three studies included in this outcome (Song 2012).
2.8 Mental state: depression
Several scales were used to measure depression in the studies included in this review. The most common rating scale used to measure depression was the BDI‐II with five studies. Group CBTp offered no clear advantage of standard care or other psychosocial interventions on depression measured by BDI‐II. Depression was also measured with HAMD, MADRS and HADS Depression but each scale had only study using it, so no meaningful conclusions could be drawn by the analyses of these rating scales outcomes.
2.9 Overall: global assessment of functioning – average endpoint score – end of treatment (GAF)
Five studies included data for GAF. Group CBTp offers some advantage over standard care or other psychosocial interventions in global functioning. However, the sample size was quite small (254 participants in total), so it may be not be possible to draw firm conclusions on the efficacy of group CBTp. The confidence in the estimate was moderate.
2.10 Other outcomes
The other outcomes with data included in this review (PSYRATS total, delusions and voices endpoint scores, SAPS endpoint score, SCL‐90‐R endpoint score, AHRS endpoint score, all anxiety endpoint scores, all service utilisation and quality of life outcomes, including number of participants hospitalised, and SBS, SFS, PSP, IPROS, SDSS endpoint scores) had one to three studies each, with a small sample size or high heterogeneity (or both), which was not sufficient to draw clear conclusions on the effect of group CBTp over standard care or other psychosocial interventions. More research is needed, particularly on quality of life outcomes, as studies used several scales that measure different aspects. The outcome 'days in hospital' was skewed and so was not analysable. This may have limited the applicability of our data.
2.11 Secondary outcomes with no available data
We had no data on:
-
global state:
any change – as defined by individual studies;
relapse – end of treatment;
average endpoint or change score on global state scale;
-
leaving the study early:
for specific reasons – end of treatment;
-
mental state:
clinically important change overall mental state – as defined by each of the studies – end of treatment;
any change in overall mental state – as defined by individual studies;
clinically important change in specific symptoms (e.g. positive or negative) – as defined by each of the studies;
any change in specific symptoms (e.g. positive or negative) – as defined by each of the studies;
-
compliance:
taking drug treatment;
adherence to non‐drug treatments;
-
adverse effects/events:
at least one adverse effect/event;
incidence of specific adverse effects;
average endpoint or change scores on adverse effect scales;
dependency;
death: suicide or natural causes;
-
quality of life:
clinically important change – as defined by individual studies;
any change – as defined by individual studies;
general impression of carer/rater or family member;
-
functioning:
clinically important change in functioning – as defined by individual studies;
any change in functioning – as defined by individual studies.
Overall completeness and applicability of evidence
This review examined group CBTp compared to standard care or other psychosocial interventions in people with schizophrenia. We considered standard care to be the care participants would normally receive in a standard mental health service, including medication. Several psychosocial interventions were also compared to group CBTp such as psychoeducational groups and supportive therapy. It is uncertain whether data can be generalised. The sample size of each included study was small, and our analysis could be underpowered at least for some outcomes. This is the main limitation of this review. The main finding is that there is no clear difference between group CBTp and standard care in terms of rates of participants leaving the study early. Total symptoms scores as well as positive symptoms outcomes seem to be associated with a better outcome for group CBTp.
In addition, one relevant issue is that we grouped a mix of CBTp interventions. Even if they are all based on the CBT model, they may have some differences. We decided not to include meta‐cognitive training in this review (Moritz 2007). This training is a well‐established and valid CBT‐based model, but we judged it deserves to be investigated separately. Moreover, at least three systematic reviews that specifically investigated meta‐cognitive training have been published (Eichner 2016; Liu 2018; van Oosterhout 2016). We will conduct a Cochrane Review specifically focused on meta‐cognitive training and including more recent studies.
As for the CBTp interventions, Barrowclough 2006 and Wykes 2005 applied cognitive therapy principles and are more in line with a classic CBTp model. They are also more structured. The CBTp model described in Granholm 2007 and Granholm 2013 is more focused on social skills. The studies conducted in China seemed to use a more standard CBTp model (Deng 2014; Guan 2016; Li 2013a; Li 2013b; Qi 2012; Qu 2016; Shen 2007; Shi 2015; Song 2012; Song 2014; Tao 2015; Zhou 2015). Since there is no unified model of group CBTp, some inconsistency in the findings may be explained by the different model of CBTp employed.
In addition, the overall participants group may be heterogeneous, since some studies were carried out among inpatients, some recruited a mix of inpatients and outpatients and some only recruited outpatients. Level of severity of schizophrenia may have also been different across the studies included.
Another aspect worth mentioning, which can limit the applicability of the evidence, is the fact that duration of treatment was variable, ranging from a few weeks to 12 months. It is recommended that CBT is given for at least 16 sessions (NICE 2014). Schizophrenia is a chronic illness; many of the patients included in this study are not first‐episode psychosis and may require a longer time to recover. This may be one of the possible reasons why there could have been differences in the outcomes. Also, we decided to focus on end of treatment outcomes. It may be possible that CBTp, if administered for a long period of time (i.e. six months or longer), may be effective. These different timepoints were analysed together and this could have added heterogeneity.
We consider that the aspects mentioned above may limit the applicability of evidence and more studies are needed to further explore the efficacy of group CBTp.
Quality of the evidence
We chose seven outcomes for assessing the quality of evidence and to construct the summary of findings tables.
The chosen outcomes are:
clinically important change, shown as improved – as defined by individual studies;
relapse;
leaving the study early: for any reason;
mental state: overall: average endpoint score – end of treatment (PANSS total);
days in hospital;
quality of life: clinically important change – as defined by individual studies;
functioning: overall: global assessment of functioning – average endpoint score – end of treatment (GAF).
See Table 1.
The certainty of the evidence was low to moderate based on GRADE (Schünemann 2021). We downgraded the evidence by one level for all outcomes due to high risk of performance bias for all studies and unclear selection bias for most studies. There was no heterogeneity in the rate of participants leaving the study early, PANSS total and GAF, so we did not downgrade further. The interventions tested, and the population included in the studies, are representative of the interventions and populations in clinical settings. Therefore, we decided not to downgrade the evidence for all outcomes included for indirectness. We found some imprecision on leaving the study early and therefore we downgraded certainty by one level. Exploration of the funnel plot for the 'PANSS total symptoms' outcome showed some evidence of asymmetry, suggesting some degree of publication bias (see Figure 1), and, therefore, we downgraded the certainty of the evidence by one level. We found no data on: clinically important change, shown as improved (as defined by individual studies), relapse, days in hospital and quality of life: clinically important change – as defined by individual studies outcomes.
Potential biases in the review process
We documented and justified changes to our methods from the published protocol in the Differences between protocol and review section (Guaiana 2012).
We decided to pool all the interventions comparing group CBTp into a single 'standard care or other psychosocial interventions' category. We considered this was necessary, otherwise the analysis would have been too fragmented, and would have included too few studies for each outcome if we had to consider each control comparison separately. However, the control interventions were different, ranging from medication only to psychoeducation groups. This may have added a source of bias and heterogeneity in our review.
The search was mainly based on the Cochrane Schizophrenia Group's register of trials. This is largely made up of searches of published literature. It is possible that there are unpublished studies. There seems to be some evidence of publication bias on one outcome (PANSS total, Figure 1) but not on other outcomes.
All the included studies had a limited sample size, which could have made our review underpowered.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first meta‐analysis specifically focused on group CBTp. A previous Cochrane meta‐analysis, published in 2012 (Jones 2012) and updated in 2018 (Jones 2018) focused on CBTp in general, and included some studies on group CBTp. They concluded that, based on the data available, relative to standard care alone, adding CBT to standard care appears to have no effect on long‐term risk of relapse. The authors also stated that the quality of evidence available was poor, and it was not possible to make firm conclusions until more high‐quality data were available. Another systematic review focused on low‐intensity CBTp for psychosis (Hazell 2016), defined as CBTp lasting fewer than 16 sessions. The authors found that low‐intensity CBTp can be a promising treatment as it showed a decrease in symptoms of psychosis. This is somewhat in line with the findings of our review, as there were some differences between treatments. One systematic review on all group treatments in schizophrenia (including group CBTp) was published in 2020 (Burlingame 2020). It included some of the studies that were included in this review but not the studies in Chinese language, included in our review. Burlingame 2020 concluded that group treatment as a whole works in schizophrenia, although they did not find a difference between group CBTp and standard care in their analysis. This is in line with our findings. Burlingame 2020 found that the most effective group treatments for schizophrenia were cognitive remediation and social skills training.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
There was no clear difference between cognitive behavioural therapy targeting symptoms of psychosis (CBTp) and standard care and other psychosocial interventions for numbers of participants leaving the study early (dropouts).
Based on the limited data available, group CBTp seem not to differ from standard care and other psychosocial interventions in effectiveness regarding outcomes on delusions, hallucinations, other positive symptoms or negative symptoms, and social functioning.
There is some very limited evidence that group CBT may have some efficacy in positive symptoms, but this is based on a limited number of studies.
2. For clinicians
The data from this meta‐analysis do not allow us to draw firm conclusions on whether group CBTp can be useful in the treatment for schizophrenia. However, we will try to discuss some tentative implications for practice stemming from this review.
Another aspect we would like to discuss is the relationship between group and individual CBTp. Does group CBTp need to be supplemented with individual CBTp? We consider that in order for group CBTp to work, patients need to create an identity within the group and learn from interaction with other group members. However, this may be difficult due to the nature of schizophrenia, an illness where social cognition may be impaired (Kurtz 2016). Some form of preparation to group work with individual CBTp may be necessary. Otherwise, it may be difficult to differentiate group CBTp from the aspecific factors associated with control groups. The main author of one of the included studies mentions that individual CBTp has advantages that may not be reachable in a group (Wykes 2005). One of these advantages is flexibility to respond to a wide range of symptoms (Wykes 2005). Moreover, another relevant aspect of individual therapy is that it allows a patient to more easily overcome suspiciousness that is linked to theory of mind deficits; it can also be better suited to create a solid therapeutic alliance, which should necessarily come before the technical CBTp intervention. Therapeutic alliance cannot be based on simply explaining a model to a group of people (Pinto 2008). Individual therapy can prepare patients for the group. To our understanding, no study has included individual therapy sessions before the group intervention. This may be a possible reason why there were minimal differences in our review between group CBTp and control groups (Pinto 2008).
Group homogeneity can also be a very important factor. In one of the studies we included, Wykes and co‐authors say: "Group CBT treatments may … only be effective if there is some homogeneity of experience to share within the group" (Wykes 2005). Schizophrenia can present in several ways. For example, there are people with schizophrenia who are distressed by unpleasant auditory hallucinations, while others may be struggling with delusional thinking. It may not be useful to combine different subpopulations in a therapeutic group, as they may not relate to each other's experience thus decreasing the efficacy of the group CBTp. In this case, it may be helpful to screen patients before group CBTp and try to create groups that are as homogeneous as possible in terms of symptomatology or social functioning (or both). Maybe one of the reasons for small effect size is that the patients have been included in a group without individual screening of symptoms and functions.
Finally, the length of treatment should also be carefully considered. Only some studies included in this review had a length of treatment of 24 weeks or more. Other studies included in our review treated patients for less than eight weeks. For example, treatments for other severe and chronic mental disorders such as dialectical behaviour therapy for borderline personality disorder can last for 13 to 14 months (Dimeff 2007). Other psychosocial interventions for schizophrenia, such as family interventions, last for more than 24 weeks (Burlingame 2020). Given the chronic nature of schizophrenia (van Os 2009), we consider that it is reasonable to consider the possibility of a group CBTp length of treatment of one year or more.
3. For managers and policy makers
In terms of planning for resources, group CBTp may not be used yet as a standard care for people with schizophrenia. Since the studies were characterised by a high risk of bias and a low‐ to moderate‐certainty evidence, it may be possible that the studies were underpowered to detect a difference.
Implications for research.
1. General
We consider that consistently following the CONSORT guidance for reporting of clinical trials would help increase the quality of data available in this review (Moher 2001).
2. Specific
2.1 Reviews
It will be advisable that quality of studies is improved by increasing the sample size and better reporting of results.
2.2 Trials
Future studies should aim to employ a blind rater for outcome assessment. Since blinding of participants and personnel is not possible in group CBTp studies, at least the raters should be masked.
Studies should consider clinically important change, shown as improved (as defined by individual studies) as a primary outcome.
Studies should also explicitly try to include a more homogeneous population within people with schizophrenia (e.g. including people with mainly auditory hallucinations or mainly paranoid delusions). This may allow better understanding on the effect of CBTp on the various symptoms. Social functioning should consistently be included as an outcome measure so that it will be possible to fully assess the effect of CBTp.
Given the current limitations in the literature, we propose a design for a new randomised trial (see Table 6). What is also very important in the proposed future trials is that all outcomes are fully reported.
5. Design of a future study.
| Methods | Allocation: randomised (clearly described) Blinding: single‐blind (outcome assessors blinded to treatment) Duration: up to 52 weeks Design: parallel Setting: anywhere |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder n = 100 Age: any Gender: males and females Inclusion criteria: standardised diagnostic criteria for schizophrenia or schizoaffective disorder Exclusion criteria: specific contraindication to evaluated treatments |
| Interventions | 1. Group CBTp 2. Standard care |
| Outcomes | Primary outcomes 1. Global state 1.1 Clinically important change (as defined by individual studies) Secondary outcomes 1. Leaving the study early 1.1 For any reason 1.2 For specific reason 2. Mental state 2.1 Clinically important change overall mental state – as defined by individual studies 2.2 Relapse 2.3 Average endpoint or change score, or both, on mental state scales 2.4 Average endpoint or change scores, or both, on positive or negative symptom scales, or both 2.5 Specific clinically important change in positive or negative symptoms, or both, – as defined by individual studies 3. Adverse effects/events 3.1 Incidence of clinically important depression/anxiety and psychotic symptoms 3.2 Average endpoint and change scores on adverse effect scales 3.3 Death: suicide or natural causes 4. Service utilisation outcomes 4.1 Hospital admission 4.2 Days in hospital 5. Quality of life 5.1 Clinically important change – as defined by each of the studies 5.2 General Impression of carer/rater or family member 6. Social functioning 6.1 Average endpoint or change score, or both, on social skills/functioning scales 6.2 Occupational status |
CBTp: cognitive behavioural therapy model targeting symptoms of psychosis.
History
Protocol first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 26 January 2018 | Amended | All three awaiting studies have been excluded. |
| 8 January 2018 | Amended | Search was updated and 3 studies (4 references) were added to Studies awaiting classification section of the review. |
| 21 June 2016 | Amended | Search was updated and 23 studies (39 references) were added to Studies awaiting classification section of the review. |
| 26 February 2014 | Amended | Search was updated and 21 new references were added to 'classification pending references' section of the review. |
Acknowledgements
We would like to acknowledge the contribution of Victoria Suntharalingam, Anna Clara Morelli and Debbie Chiodo to the development of the protocol.
The Cochrane Schizophrenia Editorial Base is situated across the University of Melbourne, Australia, Technical University of Munich, Germany and University of Nottingham, UK and produces and maintains standard text for use in the methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required. We would also like to acknowledge the Cochrane Schizophrenia Group (in particular Alessandro Rodolico and Marianna Purgato) for their assistance in the final steps of this review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Irene Bighelli, Technical University of Munich.
Managing Editor (provided editorial guidance to authors, edited the article): Hui Wu, Technical University of Munich.
CRG Advisor (provided editorial guidance to authors and editors): Marianna Purgato, University of Verona.
Contact Editors (provided editorial guidance to authors, edited the article): Alessandro Rodolico, University of Catania.
Copy Editor (copy‐editing and production): Anne Lawson, Copy Edit Support, Cochrane.
Information Specialist (search strategy and search results): Farhad Shokraneh, Systematic Review Consultants.
Peer‐reviewers* (provided comments and recommended an editorial decision): Masahiro Banno, Seichiryo Hospital; Genevieve Gariepy, McGill University (clinical/content review).
The previous CSzG editorial team also supported this work, including selecting peer reviewers, collating comments, etc.
*The peer‐reviewers were members of Cochrane Schizophrenia, and provided peer‐review comments on this article, but were not otherwise involved in the editorial process or decision making for this article.
Data and analyses
Comparison 1. Group cognitive behavioural therapy (CBT) versus standard care.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrowclough 2006.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single Duration: 6 months Design: parallel Setting: inpatients Country: UK |
|
| Participants | Diagnosis: persistent positive symptoms of schizophrenia or schizoaffective disorder according to DSM‐IV n = 113 Gender: 82 men, 31 women Age: mean 38.83 years; range 18–55 years History: duration of illness mean 13.67 years. Patients were taking concomitant psychotropic medication Exclusion criteria: substance misuse and learning disability not identified as the primary problem |
|
| Interventions | 1. Group CBTp: group CBTp + standard care, n = 57 Group CBTp was covering the following themes in 18 sessions: Session 1, introduction to the CBT approach to psychosis Session 2, what is CBT? Session 3, identification of patient problems (delusional beliefs and voices were the main focus) Session 4, formulating problems in terms of thoughts, feelings and behaviours Session 5, negative thinking patterns and thought monitoring Sessions 6, 7 and 8, thought challenging Sessions 9, 10 and 11, behavioural strategies: experiments and action plans Sessions 12 and 13, stress, arousal and medication Sessions 14 and 15, staying‐well plans Session 16, emergency staying‐well plans Sessions 17 and 18, follow‐up and revision 18 sessions in total, 2 hours each in duration 2. Standard care: standard care alone, n = 56 Defined as: "based on the care programme approach to case management, and includes maintenance antipsychotic medication, out‐patient and community follow‐up, and access to community‐based rehabilitative activities such as day centres and drop‐in centres". |
|
| Outcomes | Leaving study early Number of participants hospitalised during study Overall mental state (PANSS total score) Positive symptoms (PANSS positive subscore) Negative symptoms (PANSS negative subscore) Anxiety and depression: (HADS total score) Social functioning (SFS score) Global state (GAF score) Average number of days spent in hospital Not used: Hopelessness (BHS score) Self‐esteem (RSE score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised design". |
| Allocation concealment (selection bias) | Low risk | Quote: "They were then allocated to the two conditions using a programme operated by an individual independent of the research team, following the minimisation method of stratification for chronicity". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were conducted by independent assessors who were masked to allocation of participants"; "maintenance of the mask was performed, efforts were made to maintain masking, including locating research and therapy staff in separate offices, providing separate locations for assessment and therapy notes, and reminding participants not to disclose their group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of differences in attrition between group CBTp and standard care. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of any other source of bias. |
Bechdolf 2004.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: open label Duration: 8 weeks Design: parallel Setting: inpatients Country: Germany |
|
| Participants | Diagnosis: schizophrenia or related disorder according to ICD‐10 criteria (diagnoses: F20, F23, F25) n = 88 Gender: 40 men, 48 women Age: range 18–64 years; mean: group CBTp: 32.2 years; control: 31.4 years History: average time since diagnosis: group CBTp: 56.7 months; control: 50 months. They had, on average, been hospitalised 2–3 times and had a mean time since diagnosis > 4 years. Participant were taking concomitant psychotropic medications Exclusion criteria: primary diagnosis of drug or alcohol dependence, organic brain disease, learning disability or hearing impairment |
|
| Interventions | 1. Group CBTp: group CBTp + standard care, n = 40 Group CBT treatment based on the approach by Tarrier and coworkers who used coping strategy enhancement, problem solving and relapse prevention in people with psychosis (Tarrier 1993). 16 sessions (group CBTp), 60–90 minutes per session 2. Group psychoeducational programme, n = 48 8 sessions in 8 weeks. Sessions followed a semi‐structured format and lasted 60–90 minutes, occasionally interrupted by a 5‐ to 10‐minute break. It covered: symptoms of psychosis, models of psychosis, effects and adverse effects of medication, maintenance medication, early symptoms of relapse, relapse prevention. Approach was primarily didactic and included the following strategies: formulation, guided discovery and motivational interviewing. |
|
| Outcomes | Leaving study early Number of participants hospitalised during study Positive symptoms (PANSS positive subscore) Negative symptoms (PANSS negative subscore) QoL (general MSQoL score) Not used: QoL: other MSQoL (physical health, vitality, psychosocial QoL, material QoL, spare time QoL, affective QoL scores) Compliance with medication |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted by computer‐generated random numbers for blocks of eight participants. The results were placed in sealed envelopes and only opened at the time of treatment allocation". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "we made attempts to blind assessments by carrying out most of the assessments by independent raters (C.K. and S.S.), who were not involved in treatment", "Although attempts were made to blind ratings, raters could have found out about the treatment conditions of patients during the assessments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Authors provided sufficient data and procedure is evident. |
| Other bias | Low risk | No evidence of any other source of bias. |
Chadwick 2016.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single Duration: 4 months Design: parallel Setting: 2 mental health services Country: UK |
|
| Participants | Diagnosis: diagnosis of schizophrenia or schizoaffective disorder. n = 108 Gender: 54 men, 54 women Age: median: 42 years; range 18–65 years History: median onset age of hearing voices 21 years (range 5–55 years). Patient were taking concomitant psychotropic medications Exclusion criteria: known organic illness; primary diagnosis of substance misuse |
|
| Interventions | 1. Group PBCT + standard care, n = 54 All sessions began with mindfulness practice and discussion. Mindfulness practice in PBCT is brief (10 minutes), with frequent guidance that includes reference to psychotic experience, and combines focused attention on body and breath with open awareness. Sessions 1–3 socratically drew out participants' voice hearing experiences (onset, impact, meaning, distress and coping) and framed them using the ABC cognitive model. Sessions 4–6 explored personal control, socratically weakening voice omnipotence and enhancing autonomy. Sessions 7–12 added focus on identifying and decentring from negative schemata, and building positive schematic beliefs (including using experiential 2 chair work) alongside recognition that the self is complex and changing. Participants were encouraged to practice mindfulness daily at home, using a supplied 10‐minute recording, and each week 1 further homework was set relating to work on voices or self (e.g. session 6: keeping a record of times when I chose what to do in spite of the voices). 12 sessions, 1.5 hours per session 2. Standard care, n = 54 2‐ to 3‐monthly outpatient appointments with their psychiatrist, antipsychotic medication and contact with care team members every 2 weeks, all of which were documented in accordance with the study protocol |
|
| Outcomes | Leaving study early Overall psychotic symptoms (PSYRATS total scores) Anxiety (HADS anxiety subscore) Depression (HADS depression subscore) Not used: General distress (CORE‐OM scores) Distress (PSYRATS – distress intensity subscore) Control over voices (PSYRATS – control subscore) Goals for CBTp (CHOICE score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "stratified block randomisation…" |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All post‐randomisation assessments were completed by research assistants from a different geographical centre who were blind to participant allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes seemed to be reported in full. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Daniels 1998.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single Duration: 8 weeks Design: parallel Setting: outpatients and day hospital Country: USA |
|
| Participants | Diagnosis: people who met DSM‐IV diagnostic criteria for a schizophrenia or schizoaffective disorder n = 20 Gender: 27 men, 30 women Age: mean 33.7 years; range 19–61 years History: mean age of illness onset 21.56 years, total number of hospitalisations 3.26. Unknown whether patients were taking concomitant psychotropic medication Exclusion criteria: taking medication non‐compliance as a current and clinically significant problem; history of alcohol/substance abuse or dependence in the preceding year; history of moderate‐to‐severe neurological impairment or mental retardation as documented in medical records as well; people with significantly psychiatrically unstable as defined by scores of 5 (maximums core per item = 7) or more in any of the following PANSS domains a. conceptual disorganisation b. hallucinatory behaviour, c. unusual thought content |
|
| Interventions | 1. Group CBTp (IBT), n = 10 IBT is an approach to SST with a combined focus on cognitive‐behavioural techniques (such as instruction, modelling and behavioural rehearsal) and group process strategies. Each IBT session is divided into 4 stages. In the Orientation and Cognitive Networking stage, the leaders encouraged and facilitated social interactions among group members. During the Warm‐up and Sharing phase, the second stage, there was a strong emphasis on self‐disclosure. During the 20‐minute Enactment phase of the group (third stage), the individual enacted an interpersonal situation that included group members as active participants. This stage involved 5 basic elements: 1. selecting a participant, 2. assigning an auxiliary, 3. using an interpersonal group process technique, 4. directing an encounter and 5. using cognitive‐behavioural strategies. The final Affirmation stage took place during the last 5 minutes of the group. The leaders and group members specifically identified and verbally reinforced socially competent behaviours displayed by individual members in the group. 16 sessions, 50 minutes per session. 2. Wait‐list group control, n = 10 |
|
| Outcomes | QoL (QLS score) Global State (GAF score) Negative Symptoms (SANS score) Overall Mental State (BPRS score) Not used: Social skills (BAT score) Global mental state (CGI score) Negative symptoms (SANS Asociality subscale subscore) |
|
| Notes | There was a discrepancy between the number of participants randomised and the number included in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…the 20 patients from each of the two sites were randomly assigned to a treatment group (n=10) or a waitlist group". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each participant was rated by the same trained single‐blind rater…" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is contradictory information on the number of participants, mentioning 40 on the "Subjects" section and 20 on the "Treatment" section. |
| Selective reporting (reporting bias) | High risk | Used PANSS as screening tool for positive symptoms but the author did not report the scores. |
| Other bias | Low risk | No evidence of any other source of bias. |
Deng 2014.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: unknown Duration: 5 weeks Design: parallel Setting: inpatients Country: China |
|
| Participants | Diagnosis: schizophrenia (no data on diagnostic criteria used) n = 118 Gender: 89 men, 29 women Age: range 21–58 years; mean not reported History: taking concomitant antipsychotic medications. No further data reported Exclusion criteria: no data reported |
|
| Interventions | 1. Group CBTp, n = 41 For each group session short games were prior to the session, points of activity. The sessions focused on: introduction of the basic model of CBT, emotion recognition and perception, diversity of thinking, automatic thinking, shift thinking, exploring self and finding own's automatic thinking. Sessions on learning styles and coping behaviours were also included. Sessions twice per week, for 1.5 hours per session 2. Standard care, n = 41 Routine recreational activities, mainly watching television, playing, taking medication, listening to music, learning to dance with TV, playing balls, reading newspapers or moving freely |
|
| Outcomes | Leaving study early Overall mental state (SCL‐90) Social anxiety (SAD) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other sources of bias |
Granholm 2007.
| Study characteristics | ||
| Methods | Allocation: randomised (stratified randomisation) Blinding: single Duration: 6 months Design: parallel Setting: outpatients Country: USA |
|
| Participants | Diagnosis: chronic schizophrenia, paranoid type, undifferentiated type, disorganised type, residual type, schizoaffective disorder according to DSM‐IV criteria n = 76 Gender: 56 men, 20 women Age: mean: group CBTp: 54.5 years; control: 53.1 years; range 42–74 years History: average illness duration: group CBTp: 30.1 years; control: 28.4 years Exclusion criteria: disabling medical problems that would interfere with testing, absence of medical records to inform diagnosis and diagnosis of dependence on substances other than nicotine or caffeine within past 6 months |
|
| Interventions | 1. Group CBTp (CBSST) + standard care, n = 37 CBSST is an intervention group integrating CBT and SST. Cognitive therapy is combined with role‐play practice of communication skills and problem‐solving training applied to everyday functioning activities. The CBT components of the manual were based on 1. a manual developed by Muñoz and Miranda (Muñoz 1993); 2. general CBT techniques; 3. CBT techniques developed specifically for people with schizophrenia and 4. techniques developed specifically for older patient populations. SST components were based on prepackaged SST interventions available from psychiatric rehabilitation consultants. Groups are limited to a maximum of 8 members. 24 weekly sessions, 2 hours per session 2. Standard care, n = 39 Defined by the author as: "whatever ongoing care they were receiving". |
|
| Outcomes | Leaving study early Overall mental state (PANSS total score) Positive symptoms (PANSS positive score) Negative symptoms (PANSS negative score) Depression (HAMD score) Social functioning (ILSS score) Not used: Social functioning (UPSA score) Insight (BCIS score) Social skills (CMT score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "Blind raters assessed social functioning, psychotic and depressive symptoms, cognitive insight, and skill mastery"; "assessors were independent of the therapists and blind to treatment‐group assignment". "The project coordinator assigned participants to treatments in the order that they consented, and the coordinator was the only staff person other than therapists with knowledge of group membership". "Assessment of the blind showed that raters were uncertain of group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | High risk | Insufficient information in terms of ILSS, UCSD Performance‐Based Skills Assessment, BCIS score, CMT as they reported them in unusable diagrams. |
| Other bias | Low risk | No evidence of any other source of bias. |
Granholm 2013.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single Duration: 9 months Design: parallel Setting: outpatient and inpatient Country: USA |
|
| Participants | Diagnosis: schizophrenia (paranoid type, undifferentiated type, disorganised type, residual type), schizoaffective disorder according to DSM‐IV at any stage n = 79 Gender: 43 men, 36 women Age: mean age 55 years; range 45–78 years History: sample had, on average, only moderate symptom severity (PANSS total mean 64.7, SD 19.1) Exclusion criteria: prior exposure to CBT during previous 5 years, level of care required at baseline that would interfere with outpatient therapy groups (e.g. hospitalisation for medical, psychiatric or substance abuse problems), or disabling medical problems that would interfere with therapy or testing |
|
| Interventions | 1. Group CBTp (CBSST): standard care + group CBTp, n = 41 CBSST is an intervention group integrating CBT and SST. Cognitive therapy is combined with role‐play practice of communication skills and problem‐solving training applied to everyday functioning activities. The CBT components of the manual were based on 1. a manual developed by Muñoz and Miranda (Muñoz 1993); general CBT techniques; 3. CBT techniques developed specifically for people with schizophrenia and 4. techniques developed specifically for older patient populations. SST components were based on prepackaged SST interventions available from psychiatric rehabilitation consultants. Groups were limited to a maximum of 8 members. 36 weekly sessions, 2 hours per session 2. GFSC, n = 38 GFSC is an active psychosocial control condition. The GFSC intervention was enhanced supportive contact that included a primary focus on setting and achieving functioning goals (e.g. living, learning, working and socialising). Sessions were semi‐structured and consisted of check‐in about symptoms and potential crisis management, followed by a flexible discussion about setting and working toward functioning goals. Sessions typically included components of psychoeducation; empathy; and non‐directive reinforcement of health, coping and symptom management behaviours, that grew out of group discussions, with minimal therapist guidance. 36 weekly sessions, 2 hours per session |
|
| Outcomes | Leaving study early Positive symptoms (PANSS positive score) Depression (BDI‐II score) Anxiety (BAI score) Social functioning (ILSS score) Life satisfaction (LSI score) Not used: Negative symptoms (SANS negative scales: diminished motivation and diminished expression subscores) Defeatist thoughts (DPAS score) Social skills (CMT score) Self‐esteem (SERS score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "participants were randomly assigned by an independent statistician to one of two treatment conditions: CBSST or GFSC". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "Blind raters assessed functioning…", "Assessors were blind to treatment allocation". "To assess the blind, raters guessed which treatment arm participants were in before completing assessments using a 7‐point scale (1=Definitely GFSC; 2=Most Likely GFSC; 3=Maybe GFSC; 4=Neutral/Unsure Either Way; 5=Maybe CBSST; 6=Most Likely CBSST; 7=Definitely CBSST). Only 6 end of treatment assessments and 8 6‐month follow‐up assessments were rated "most likely" or "definitely" CBSST or GFSC, and 6 of these 14 group membership guesses were wrong". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes data were reported. |
| Selective reporting (reporting bias) | High risk | PANSS negative outcome result were not reported. |
| Other bias | Low risk | No evidence of any other source of bias. |
Granholm 2014.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single Duration: 9 months Design: parallel Setting: outpatient and inpatient Country: USA |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder, according to SCID, 4th edition n = 149 Gender: 99 men, 50 women Age: ≥ 18 years; mean: group CBTp: 41.1 years; control: 41.6 years History: all but 4 participants were taking concomitant psychotropic medication. Duration of illness: mean: group CBTp: 21.3 years; control: 21.4 years Exclusion criteria: prior exposure to CBT or SST during previous 5 years; level of care required at baseline that would interfere with participation in outpatient therapy groups or assessments (e.g. disabling medical problems, or current hospitalisation for medical, psychiatric or substance abuse problems) |
|
| Interventions | 1. Group CBTp (CBSST): standard care + group CBTp, n = 73 CBSST is an intervention group integrating CBT and SST. Cognitive therapy is combined with role‐play practice of communication skills and problem‐solving training applied to everyday functioning activities. The CBT components of the manual were based on 1. a manual developed by Muñoz and Miranda (Muñoz 1993); 2. general CBT techniques; 3. CBT techniques developed specifically for people with schizophrenia and 4. techniques developed specifically for older patient populations. SST components were based on prepackaged SST interventions available from psychiatric rehabilitation consultants. Groups are limited to a maximum of 8 members. 24 weekly sessions, 2 hours per session. 2. GFSC, n = 76 GFSC is an active psychosocial control condition. The GFSC intervention was enhanced supportive contact that included a primary focus on setting and achieving functioning goals (e.g. living, learning, working and socialising). Sessions were semi‐structured and consisted of check‐in about symptoms and potential crisis management, followed by a flexible discussion about setting and working toward functioning goals. Sessions typically included components of psychoeducation; empathy; and non‐directive reinforcement of health, coping and symptom management behaviours, that grew out of group discussions, with minimal therapist guidance. 36 weekly sessions, 2 hours per session. |
|
| Outcomes | Leaving study early Social functioning (ILSS score) Positive symptoms (PANSS positive subscore) Depression (BDI‐II score) Not used: Social Competence (MASC score) Social skills (CMT score) Negative symptoms (SANS negative scales: diminished motivation and diminished expression subscores) Defeatist thoughts (DPAS score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible participants were randomly assigned to one of two treatment conditions". |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent statistician allocated participants to treatments according to a computer‐generated randomization list". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessors were blinded to treatment allocation, and therapists and the study coordinator, who were aware of treatment allocation, did not complete any outcome assessments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Assessors were blinded to treatment allocation". |
| Selective reporting (reporting bias) | Low risk | No evidence of incomplete outcome reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Granholm 2020.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single Duration: 6 months Design: parallel Setting: outpatient Country: USA |
|
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia or schizoaffective disorder, according to SCID, 4th edition n = 57 Gender: 47 men, 10 women Age: ≥ 45 years; mean not reported History: no data. Concomitant medication not clearly specified Exclusion criteria: prior exposure to CBT or SST during previous 5 years; level of care required at baseline that would interfere with participation in outpatient therapy groups or assessments (e.g. disabling medical problems; or current hospitalisation for medical, psychiatric or substance abuse problems) |
|
| Interventions | 1. Group CBTp (CBSST), n = 26 CBSST is an intervention group integrating CBT and SST. Cognitive therapy is combined with role‐play practice of communication skills and problem‐solving training applied to everyday functioning activities. The CBT components of the manual were based on 1. a manual developed by Muñoz and Miranda (Muñoz 1993); 2. general CBT techniques; 3. CBT techniques developed specifically for people with schizophrenia and 4. techniques developed specifically for older patient populations. SST components were based on prepackaged SST interventions available from psychiatric rehabilitation consultants. Groups are limited to a maximum of 8 members. 24 weekly sessions, 2 hours per session 2. Mobile‐assisted group CBTp (CBSST), n = 17 The same CBSST modules were presented in weekly 60‐ minute group therapy sessions over 24 weeks. This is a 50% reduction in provider contact relative to the full dose of CBSST. Handheld computers (Tungsten E2 Personal Digital Assistant) were used to supplement sessions throughout the entire 24‐week intervention phase, but not during follow‐up 3. Device contact only, n = 14 Handheld computer device (Tungsten E2 Personal Digital Assistant) contact and symptom‐monitoring but no CBSST skills training. No data on frequency of sessions |
|
| Outcomes | Social functioning (ILSS score) Positive symptoms (PANSS positive subscore) Not used: Social competence (MASC score) Social skills (CMT score) Negative symptoms (SANS negative scales: diminished motivation and diminished expression subscores) Defeatist thoughts (DPAS score) Insight (BCIS score) |
|
| Notes | Only 2 arms were compared: group CBTp and control (device contact only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participant responses were videotaped for subsequent coding by blinded raters on conversational content, non‐verbal content, and overall effectiveness, with the latter used in this study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Guan 2016.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: not reported Duration: 8 weeks Design: parallel Setting: inpatient Country: China |
|
| Participants | Diagnosis: schizophrenia n = 100 Gender: 56 men, 44 women for the entire sample Age: mean age 38.42 years; range 18–50 years for the entire sample History: duration of illness 2 months to 10 years, with a mean of 5.5 years for the entire sample. Both groups received concomitants psychotropic medication (olanzapine) Exclusion criteria: people with severe consciousness disorder, intellectual disorder and personality disorder; and people with brain organic diseases; heart, liver and kidney failure; pregnancy and lactation who could not co‐operate |
|
| Interventions | 1. Group CBTp, n = 50 Treatment divided into 3 stages. The first 2 sessions were the first stage, mainly to establish a therapeutic relationship, form a treatment group, let participants get to know each other, and enhance group cohesion and trust. The middle 5 sessions were the second stage. The psychotherapist fully introduced the methods of cognitive behavioural interactive group therapy, so that participants could recognise and accept their own diseases, and formulate treatment specifications with participants so that they could fully participate in the treatment, understand others, and strengthen the ability of interpersonal communication, so that they could recognise their own abilities, improve treatment compliance and the ability to recognise diseases, and allow people in the same team to communicate their problems with each other and seek solutions together. The last session was the third stage, where participants were assigned homework and summarised their group experience, expressed their feelings, planned their own future, formulated emergency response plans and consolidated treatment. Treatment once per week. 60 minutes per session. 2. Standard care, n = 50 Medication alone (olanzapine) |
|
| Outcomes | Overall mental state (PANSS total) Overall mental state (BPRS) Social functioning (PSP) Not used: Insight (Insight and Treatment Attitude Questionnaire) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random number table method was used to randomly divide into the study group of drugs combined with cognitive behavioral interaction group therapy". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Jones 2014.
| Study characteristics | ||
| Methods | Allocation: randomised (participants were randomised in a 2:1 ratio to either the group CBTp or a wait‐list control) Blinding: not blind Duration: 6 weeks Design: parallel Setting: outpatients Country: UK |
|
| Participants | Diagnosis: schizophrenia‐spectrum disorder (no details on criteria used) and ≥ 50% conviction in a distressing delusion n = 28 Gender: 12 men, 9 women (data only available for participants who completed the study) Age: mean: group CBTp: 44.43 years; control: 43.21 years; range: 18–65 years History: duration of illness mean: group CBTp: 8.21 years; control: 9.5 years. Most participants taking concomitant psychotropic medications Exclusion criteria: organic impairment, difficulty with spoken or written English, an intellectual disability or profound visual impairment, currently receiving CBT focusing on delusions |
|
| Interventions | 1. CBTp group, n = 13 (completed study) The group was adapted from the computerised Maudsley Training Programme (Waller 2011). The original programme was a combination of modules from the Maudsley Training Programme + additional slides from the Computerised Interventions for Thinking and Anxiety in Delusions (CITADEL) trial (Waller 2015). The content of the sessions was developed through a collaborative process, which involved drafting and amending in conjunction with the wider research team. It comprised 6 sessions, coping strategies, jumping to conclusion bias, relationship between mood and thinking, looking for alternative explanations. 1.5 hours per session 2. Wait list control, n = 6 (completed study) No further data reported |
|
| Outcomes | Positive symptoms (SAPS score) Depression (BDI‐II score) Anxiety (BAI score) Not used: Delusion conviction (visual analogue scale) Distress and preoccupation with belief (visual analogue scale) Paranoia (visual analogue scale) Reasoning (Probabilistic Reasoning Task) Delusions (MADS score) Beliefs about self or others (BCSS score) Functional outcome (TBM score) Intellectual functioning (WTAR score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants who agreed to participate were randomly allocated on a 2:1 ratio…". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "It was not possible for the experimenter to be blind to the treatment allocation…". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of incomplete outcome reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Li 2013a.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: not reported Duration: 9 months Design: parallel Setting: inpatient Country: China |
|
| Participants | Diagnosis: diagnosis of schizophrenia according to CCMD‐3 diagnostic criteria n = 120 Gender: 67 males, 36 females (data only available for treatment completers) Age: mean: group CBTp: 37.6 years; control: 38.3 years; range not reported History: duration of illness mean: group CBTp: 5.3 years; control: 5.4 years. All participants taking concomitant medication (risperidone) Exclusion criteria: not reported |
|
| Interventions | 1. Group CBTp, n = 48 The group focused on psychoeducation on schizophrenia, implementing CBT techniques, such as the application of self‐monitoring and response strategies, using a voice diary, allowing the participant to recognise any sound that may have appeared and existed, encouraging the analysis of experiences and suggestions of avoidance methods; later, the participants began to use these coping strategies when they heard the sound, and to develop awareness of the disease. Sessions ran once a week for the first 3 months, once every 2 weeks after 3 months, and once a month after 6 months; each session lasted 50–60 minutes 2. Standard care, n = 55 No further information |
|
| Outcomes | Leaving study early for any reason Overall mental state (PANSS total score) Hallucinations (AHRS score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Li 2013b.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: unknown Duration: 12 months Design: parallel Setting: inpatient Country: China |
|
| Participants | Diagnosis: schizophrenia according to CCMD‐3 criteria n = 120 Gender: group CBTp: 40 men, 20 women; control: 38 men, 22 women Age: group CBTp: mean 31.9 years, range 22–54 years; control: mean 32.6 years, range 23–55 years History: group CBTp: mean duration of illness 5.79 years, participants were taking concomitant risperidone as medication; control: mean duration of illness 5.23 years, participants were taking risperidone as medication Exclusion criteria: severe illness, impulsive, unco‐operative, mental retardation or other serious diseases |
|
| Interventions | 1. Group CBTp, n = 60 The implementation of behaviour therapy was conducted twice a week in the first month, mainly to establish trust with participants. CBT was performed once per week in the second and third months, except for cognition enhancement. The goal was to consolidate the treatment of the previous stage, mainly to help the participant to recognise the thinking activities and emotions, the relationship between behaviours helps participants recognise negative distorted or false perceptions and establish the correct understanding can help to change the negative and pessimistic way of thinking and establish a positive healthy way of thinking and content, so that they and their families can actively co‐operate with the treatment. After 3 months, treatment was conducted once every 2 weeks, mainly to use the support of family and society to strengthen what was learned before. After 6 months, treatment was conducted once per month, mainly for participants to practice some life behaviour skills training so that they could return to the family and society as soon as possible. Each session lasted 60 minutes. 2. Standard care, n = 60 Medication alone (risperidone) |
|
| Outcomes | Overall mental state (PANSS total score) Positive symptoms (PANSS positive subscore) Negative symptoms (PANSS negative subscore) Not used: Cognitive status (Trail Making Test A) Cognitive status (Trail Making Test B) Cognitive status (Wisconsin Card Sorting Test) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "120 patients were randomly divided into a control group and a treatment group, each with 60 cases…" |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Mortan Sevi 2020.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single blind Duration: 12 weeks Design: parallel Setting: outpatients Country: Turkey |
|
| Participants | Diagnosis: schizophrenia according to DSM‐IV criteria, currently experiencing auditory hallucinations or delusions (or both) (according to the psychiatric interview) n = 39 Gender: 74.4% men, 25.6% women (no data on actual numbers) Age: mean 36.87 years; range 18–65 years History: most participants were single (79%) and lived with their family of origins (79%). Only a minority (13%) was employed on a regular basis. All participants received concomitant psychotropic medication Exclusion criteria: alcohol/drug dependence, organic deficit, mental/physical disability, and significant cognitive impairments (were assessed by Verbal Memory Test, Trail Making Test and Digit Span Test) |
|
| Interventions | 1. CBTp + routine care, n = 10 Treatment employed coping techniques such as psychoeducation about the causes and symptoms of schizophrenia, coping strategy enhancement including focusing, distraction, relaxation, recognition of early warning signs and self‐confidence enhancement, review and relapse prevention. It also included monitoring the relationship between the situations and thoughts accompanying the symptoms, cognitive restructuring and imaginal exposure. 12 weekly sessions, 90 minutes per session 2. COPE‐CBT + routine care, n = 12 Treatment employed coping techniques only such as psychoeducation about the causes and symptoms of schizophrenia, coping strategy enhancement including focusing, distraction, relaxation, recognition of early warning signs and self‐confidence enhancement, review and relapse prevention. 12 weekly sessions, 90 minutes per session 3. Routine care. n = 10 Medication treatment. No further information |
|
| Outcomes | Leaving study early Hallucinations (PSYRATS voices subscore) Delusions (PSYRATS delusions subscore) Positive symptoms (SAPS score) Negative symptoms (SANS score) Depression (BDI‐II score) Depression (HAMD score) Global State (GAF score) QoL (QLS score) Not used: Emotional expression of caregiver (MFIPT score) Depression (CDS score) |
|
| Notes | We used data from 3 groups only: CBTp + routine care and routine care, as COPE‐CBT fully followed the CBTp model. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random group allocation". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Penn 2009.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single (assessors/raters were blind) Duration: 3 months Design: parallel Setting: outpatients Country: USA |
|
| Participants | Diagnosis: schizophrenia spectrum disorders and persistent hallucinations, schizophrenia or schizoaffective disorder according to DSM‐IV criteria n = 65 Gender: 33 men, 32 women Age: mean age: group CBTp: 41.7 years; control: 39.6 years; range 18–65 years History: average years of education: group CBTp: 12.8; control: 12.7; average IQ: group CBTp: 98.2; control: 89.0; average age of first hospitalisation: group CBTp 27.7 years; control: 22.8 years; average number of psychiatric hospitalisations: group CBTp: 7.6; control: 7.9 Exclusion criteria: mental retardation (based on both IQ and functional impairment criteria) or current substance dependence |
|
| Interventions | 1. Group CBT: group CBTp for auditory hallucinations, n = 32 The sessions covered the following themes: session 1: introduction to treatment, sessions 2 and 3: psychoeducation, sessions 4 and 5: content of auditory hallucinations (i.e. what was the theme of participant's voices, such as whether they were benevolent or malevolent), sessions 6 and 7: behavioural analysis of auditory hallucinations, sessions 8 and 9: increasing and decreasing strategies for auditory hallucinations (i.e. identifying situations that increase and decrease hallucination severity), sessions 10–12: coping strategies for auditory hallucinations. The intervention emphasised coping skills rather than cognitive restructuring and de‐emphasised self‐esteem work. 12 weekly sessions, 1 hour per session 2. Enhanced supportive therapy, n = 33 A 12‐week manual‐based group intervention which comprised emotional support and counselling of non‐symptom‐related problems, such as improving social relations with others. The primary goal of enhanced supportive care was to improve social integration into the community by providing a supportive environment for the client and helping the client become more satisfied with their level of social functioning and integration, 12 weekly sessions, 1 hour per session |
|
| Outcomes | Leaving study early Number of participants hospitalised during study Overall mental state (PANSS total score) Positive symptoms (PANSS positive score) Negative symptoms (PANSS negative score) Depression (BDI‐II score) Hallucinations (PSYRATS voices subscore) Delusions (PSYRATS delusions subscore) Social functioning (SFS score) Not used: Beliefs about voices (BAVQ‐R score) Insight (BCIS score) Self‐esteem (RSE score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants who completed the screening and baseline interviews were randomly assigned to one of two conditions that both lasted 12 weeks: 1) group CBT or 2) group ST…" "Randomization was stratified by gender to ensure equal numbers across groups using a computer randomization generator". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization to treatment condition (with condition being designated by a random number), was conducted by a RA blind to the correspondence between random number and treatment group". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The two raters, who were blind to treatment type". "Experimental blindness was maintained by asking participants not to talk to the RAs about their treatment. In addition, the RAs had minimal contact with the study therapists. Finally, RAs were kept blind to the coding system used to denote group membership". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Qi 2012.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: not reported Duration: 6 months Design: parallel Setting: inpatient Country: China |
|
| Participants | Diagnosis: schizophrenia (first episode) according to ICD‐10 criteria n = 60 Gender: not reported Age: mean age 23.47 years for the total sample History: mean duration of illness 3.57 months, mean hospital stay 89.67 days for the total sample. All participants were taking concomitant psychotropic medication Exclusion criteria: serious physical illness |
|
| Interventions | 1. Group CBTp, n = 30 Weekly group sessions. Treatment content included: evaluation and participation and establishment of collaborative relationships, sharing others' auditory hallucinations, delusions and patterns of mental symptoms; improve self‐confidence and help participants understand and improve strategies for coping with positive symptoms, and challenge the negative impact on mental disorders. Worked on bravely facing the disease, reducing stigma; clarified the core problem; direct intervention, reduced the occurrence of core problems or prevent their aggravation; prevent recurrence, maintain good attitude, including improving participants' self‐care skills and making full use of family and social support. Therapy was delivered twice a week in weeks 1–4, once a week in weeks 5–12 and once every 4 weeks in weeks 13–24. Each treatment session is 60 minutes. Total of 19 session in 6 months. 2. Standard care, n = 30 Medication alone (risperidone) |
|
| Outcomes | Overall mental state (PANSS total) Overall mental state (GAF) Positive symptoms (PANSS positive score) Negative symptoms (PANSS negative score) QoL (GQOLI‐74) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly divided into round group according to the order of visits". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Qu 2016.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Design: parallel Setting: inpatient Country: China |
|
| Participants | Diagnosis: schizophrenia (no criteria provided) n = 60 Gender: 33 males, 27 females Age: mean: group CBTp: 39 years; control: 39.7 years History: duration of illness mean: group CBTp: 15.4 years; control: 15.5 years. All participants received concomitant psychotropic medications. Exclusion criteria: severe physical diseases, mental illness in chronic recession |
|
| Interventions | 1. Group CBTp, n = 30 Group CBTp based on health education and education for participants, and increase participants' self‐discipline, knowledge of the disease itself, corrections to help participants establish a new and correct behaviour pattern, promote the improvement of participants' social adaptability. Twice a week, 30 minutes per session. 30 minutes. 2. Standard care, n = 30 No further information |
|
| Outcomes | Negative symptoms (SANS score) Overall mental state (BPRS score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Shen 2007.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: not reported Duration: 9 months Design: parallel Setting: outpatient Country: China |
|
| Participants | Diagnosis: schizophrenia according to CCMD‐3 criteria n = 56 Gender: group CBTp: 18 males, 10 females; control: 17 males, 11 females Age: range 16–50 years; mean age: group CBTp: 31.4 years; control: 30.8 years History: participants were taking concomitant psychotropic medications in both groups Exclusion criteria: serious physical illness or substance use; relocation plan within 2 years; severe physical disability; comorbid mental and physical illness; dementia and severe cognitive impairment; difficulties complying with long‐term medication |
|
| Interventions | 1. Group CBTp, n = 28 5 sessions. Sessions ran once a month for the first 3 months, every 3 months thereafter for a total of 5 times. Sessions were divided as follows: First session: self‐introduction to each other (including disease awareness). Explained the rules of treatment, introduced the content and method of physical therapy and cognitive behavioural basic theories and methods of treatment, which is to replace. Homework was assigned and cognition reviewed. Second session: a relatively brief self‐introduction. Reported the completion status of each homework, talked about events in life and work in the past month. Learned rationality and non‐rational thinking method: the problem of being loved and the end of pursuit beautiful. Assigned homework. Third session: reported the completion of the homework and talked about the past months in conjunction with the cognitive theory learned previously. Events in life and work. Learned new rational and irrational thinking methods. Treatment of unfairness and awareness of emotions. Assignment of homework. Learned the 4 rational and irrational thinking methods, and practical application in daily life. Fourth session: reviewed rational thinking and irrational thinking learned on the last 2 sessions. Talked about encounters in life and work during these 3 months. Discussion on how to resolve the difficulties. Fifth session: review of previous work. 50–60 minutes per session. 2. Standard care, n = 28 Medication alone |
|
| Outcomes | Overall mental state (PANSS total) Overall mental state (GAF) Positive symptoms (PANSS positive score) Negative symptoms (PANSS negative score) Depression (MADRS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Shi 2015.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: unknown Duration: 12 months Design: parallel Setting: inpatient Country: China |
|
| Participants | Diagnosis: schizophrenia, according to ICD‐10 criteria n = 120 Gender: group CBTp group, completers only: 26 males, 30 females; control completers only: 30 males, 28 females Age: mean: group CBTp: 28.54 years; control: 29.12 years History: group CBTp: mean course of illness 28.54 years, participants were taking concomitant medication. Type of atypical antipsychotics taken: clozapine, olanzapine, quetiapine, risperidone; control: mean course of illness 29.12 years, type of atypical antipsychotics taken: clozapine, olanzapine, quetiapine, risperidone, aripiprazole Exclusion criteria: comorbid mental disorders; dependent on alcohol or drugs; severe medical problems; seriously ill and could not co‐operate with treatment; illiterate, semi‐illiterate or unable to understand and undergo CBT treatment |
|
| Interventions | 1. Group CBTp, n = 60 Treatment divided into 3 stages. First phase of the CBT method (months 1–3) was based on forming a treatment alliance and provide psychoeducation on schizophrenia. Also, participants were encouraged to find out pathological beliefs and understand why they had emotional disorders, using the cognitive model. Homework required participants to do some exercises, practice observation and automatic thinking. The second phase (months 4–6 months) ran every 2 weeks. Goals were to strengthen the correct cognition and conduct behaviour training, including behaviour verification and behaviour planning. Depression and anxiety caused by sexual symptoms and participants' unreasonable beliefs were also discussed. Positive emotions, through the implementation of behaviour plans to change cognition were also promoted. The third phase (months 7–12) ran once a month and was aimed at skills training and learning behavioural skills training, and use the monthly collective discussions. 90 minutes per session. 2. Standard care, n = 60 Medication alone |
|
| Outcomes | Leaving study early Overall mental state (PANSS total score) Positive symptoms (PANSS positive subscore) Negative symptoms (PANSS negative subscore) Not used: Cognitive status (Wisconsin Card Sorting Test) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 120 subjects were enrolled and randomly divided into two groups according to the ward, so that the study group and the control group were in different wards to control the communication between the two groups". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Song 2012.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: not reported Duration: not reported Design: parallel Setting: inpatient Country: China |
|
| Participants | Diagnosis: diagnosis of schizophrenia according to CCMD‐3 diagnostic criteria n = 80 Gender: not reported Age: group CBTp: mean 37.3 years, range 20–53 years; control: mean 37.8 years, range 21–55 years History: duration of illness: group CBTp: mean 14.5 years; control: 14.2 years. Not known whether participants were taking concomitant medication Exclusion criteria: comorbid mental disorders; severe physical diseases |
|
| Interventions | 1. Group CBTp, n = 40 No information 2. Standard care, n = 40 No information |
|
| Outcomes | Negative symptoms (SANS total score) Social functioning (IPROS total score) Not used: Negative symptoms (SANS Anhedonia‐Asociality) Negative symptoms (SANS Avolition‐Apathy) Negative symptoms (SANS Affective Flattening) Negative symptoms (SANS Alogia) Social functioning (IPROS Functioning in Work Therapy score) Social functioning (IPROS Activities of Daily Living score) Social functioning (IPROS Social Functioning score) Social functioning (IPROS Personal Hygiene score) Social functioning (IPROS Interest in External Events score) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Song 2014.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: unknown Duration: 12 weeks Design: parallel Setting: inpatient and outpatient Country: China |
|
| Participants | Diagnosis: schizophrenia according to ICD‐10 diagnostic criteria n = 72 Gender: 29 males, 43 females Age: mean: group CBTp: 33.93 years; control: 34.83 years; range 16–60 years History: duration of illness mean: group CBTp: 5.56 years; control: 6.89 years. Participants were taking concomitant psychotropic medication Exclusion criteria: comorbidity with other mental disorders; severe physical diseases; received MECT in recent 1 month; undergoing other psychotherapy |
|
| Interventions | 1. Group CBTp, n = 36 Based on psychoeducation about voices and sharing voices experience, discussion of the modality of hallucinations, using coping strategies, examining factors related to sound intensity. 12 sessions of group CBTp for 12 weeks, once a week, for 12 consecutive sessions, 60 minutes per session. 2. Standard care, n = 36 Reported as "conventional treatment". No further details |
|
| Outcomes | Overall mental state (PANSS total score) Positive symptoms (PANSS positive score) Negative symptoms (PANSS negative score) QoL (WHOQOL‐BREF – Psychological domain subscore) Not used: Social function (PSP – Activities Beneficial to Society subscale scores) Social function (PSP – Self‐care subscale scores) Social function (PSP – Disturbance and Aggression subscale scores) QoL (WHOQOL‐BREF – Physical Health domain subscore) QoL (WHOQOL‐BREF – Social Relationships domain subscore) QoL (WHOQOL‐BREF – Environment domain subscore) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Use random number table method to divide into research group and control group". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of any other source of bias. |
Tao 2015.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: not reported Duration: 16 weeks Design: parallel Setting: outpatient Country: China |
|
| Participants | Diagnosis: schizophrenia according to ICD‐10 criteria n = 120 Gender: group CBTp: 28 males, 31 females; control: 29 males, 28 females Age: mean: group CBTp: 45.05 years; control: 49.81 years History: participants were taking concomitant psychotropic medication Exclusion criteria: severe physical illness |
|
| Interventions | 1. Group CBTp, n = 60 1 session every 2 weeks. Focused on introduction of common mental diseases, drug knowledge, symptoms, prevention of recurrence and behavioural countermeasures, community prevention and rehabilitation resources, policy introduction, skills training for participants on drug self‐management and symptom self‐monitoring; recognise emotions, identify existing problems, develop coping skills, and master relaxation skills; teach participants to recognise automatic thinking and core concepts, rebuild cognition, and solve problems; conduct family symposiums to express family emotions, increase communication between family members and promote recovery. Duration of each session 100–120 minutes, 8 sessions in total 2. Standard care, n = 60 Medication and general disease guidance and follow‐up |
|
| Outcomes | Leaving study early Overall mental state (PANSS total) Positive symptoms (PANSS positive score) Negative symptoms (PANSS negative score) Social functioning (SDSS) Not used: Insight (Insight and Treatment Attitude Scale) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data reporting. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Wykes 2005.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single blind Duration: 3 months Design: parallel Setting: inpatients and outpatients Country: UK |
|
| Participants | Diagnosis: participants with a diagnosis of schizophrenia based on DSM‐IV n = 85 Gender: 62 males, 23 females Age: mean 39.6 years; range 17–65 years History: relatively chronic schizophrenia. More than half had severe social behaviour problems and most had never married. The most prescribed medications were clozapine (28%) and olanzapine (35%). 13/85 participants were prescribed > 1 neuroleptic drug. In terms of their experience of voices, 79% heard voices at least daily and had little control over them and only 6 people reported that they had any successful way of coping with the voices. Exclusion criteria: substance abuse or medical disorder |
|
| Interventions | 1. Group CBTp: standard care + group CBTp, n = 43 Group CBT for the positive symptoms of psychosis provided the 4 key elements of CBT: engagement, collaborative discussion about an agreed model, cognitive restructuring of delusional beliefs and reducing negative self‐evaluation. Group CBT for voices was a manualised therapy each having a specific goal. The sessions were: engagement and sharing of information about the voices; exploring models of psychosis; exploring beliefs about hallucinations; developing effective coping strategies; how to improve self‐esteem; developing an overall model of coping with voices; follow‐up session. 7 sessions. No data on duration 2. Standard care, n = 42 No information |
|
| Outcomes | Leaving study early Overall mental state (PANSS total score) Positive symptoms (PANSS positive score) Negative symptoms (PANSS negative score) Overall psychotic symptoms (PSYRATS total scores) Social behaviour (SBS) Not used: Median number of coping strategies Self‐esteem (RSE) |
|
| Notes | Time points for assessment: baseline, week 10 (post‐therapy) and week 36 (follow‐up). Medication: 82% of the sample on atypical antipsychotic drugs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was carried out independently in blocks of typically 12 participants to ensure assignment to CBT groups at regular intervals". |
| Allocation concealment (selection bias) | Low risk | Quote: "After baseline assessment participants were randomly allocated by an independent statistician using a concealed randomisation method". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Symptoms were rated by a psychiatrist unaware of group allocation, who was based in a different building to the other researchers and the independent site of randomisation" … "Social behaviour data were collected from key‐worker or relative informants who were independent of the trial but not masked to group allocation". "Participants were informed that they should not reveal their group allocation prior to each assessment and none did so for the symptom assessment. Cognitive data were collected by independent assessors who, although initially masked to group allocation, were not unaware of all allocations since some participants revealed their randomisation group at the post‐treatment assessment point. However, as these data were collected either by computer or under clear guidance and instruction, the effect of the revealing of group allocation is unlikely to be significant". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "At all assessment times the main and secondary outcome measures were assessed and reported on the study". |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias. |
| Other bias | Low risk | No evidence of any other source of bias. |
Zhou 2015.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: no data Duration: 12 weeks Design: parallel Setting: inpatients Country: China |
|
| Participants | Diagnosis: schizophrenia according to ICD‐10 n = 60 Gender: 34 men, 26 women Age: mean 39.9 years; range 21–58 years History: duration of illness mean 175.6 months. Participants were taking concomitant medication Exclusion criteria: other mental disorders; mental symptoms unstable and unable to communicate effectively; received MECT in recent month; serious physical diseases |
|
| Interventions | 1. Group CBTp, n = 30 Based on establishing a treatment relationship with participants in the first to second interventions. Then it focused on psychoeducation, including disease management. It also focused on recognition of rationality, existing problems, recognition of automatic thinking and core beliefs, recognition of emotions, mastering relaxation techniques, arranged homework. Also the treatment discussed violent behaviour. Treatment continued with a consolidation phase. 2. Standard care, n = 30 Defined as standard nursing care |
|
| Outcomes | QoL (WHOQOL‐BREF – Psychological domain subscore) Not used: Social function (PSP – Personal and Social Relations subscale scores) Social function (PSP – Activities Beneficial to Society subscale scores) Social function (PSP – Self‐care subscale scores) Social function (PSP – Disturbance and Aggression subscale scores) QoL (WHOQOL‐BREF – Physical Health domain subscore) QoL (WHOQOL‐BREF – Social Relationships domain subscore) QoL (WHOQOL‐BREF – Environment domain subscore) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random number table method". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to achieve blinding of participants or personnel given the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
ABC: adversity or activating event, beliefs about the event, consequences; AHRS: Auditory Hallucinations Rating Scale; BAI: Beck Anxiety Inventory; BAT: Behavioral Assertiveness Test; BAVQ‐R: Beliefs About Voices Questionnaire – Revised; BCIS: Beck Cognitive Insight Scale; BCSS: Brief Core Schema Scale; BDI‐II: Beck Depression Inventory‐II; BHS: Beck Hopelessness Scale; BPRS: Brief Psychiatric Rating Scale; CBSST: cognitive behavioural social skills training; CBT: cognitive behavioural therapy; CBTp: cognitive behavioural therapy model targeting symptoms of psychosis; CCMD‐3: Chinese Classification of Mental Disorder Version 3; CDS: Cardiac Depression Scale; CGI: Clinical Global Impression; CHOICE: CHoice of Outcome In Cbt for psychosEs; CMT: Comprehensive Module Test; COPE‐CBT: Coping‐based group cognitive behavioural therapy; CORE‐OM: Clinical Outcomes in Routine Evaluation Outcome Measure; DPAS: Defeatist Performance Attitude Scale; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; GAF: Global Assessment of Functioning; GFSC: goal‐focused supportive contact; GQOLI‐74: General Quality of Life Inventory‐74; HADS: Hospital Anxiety and Depression Scale; HAMD: Hamilton Rating Scale for Depression; IBT: interactive behavioural training; ICD‐10: International Classification of Diseases 10th Revision; ILSS: Independent Living Skills Survey; IPROS: Inpatient Psychiatric Rehabilitation Outcome Scale; IQ: intelligence quotient; LSI: Life Satisfaction Index; MADRS: Montgomery‐Asberg Depression Rating Scale; MADS: Maudsley Assessment of Delusions Schedule; MASC: Measure of Adolescent Social Competence; MECT: modified electroconvulsive therapy; MFIPT: Marmara Family Interview for the Assessment of Psychiatric Treatment; MSQoL: Modular System for Quality of Life; n: number of participants; PANSS: Positive and Negative Syndrome Scale; PBCT: Person‐Based Cognitive Therapy; PSP: Personal and Social Performance Scale; PSYRATS: Psychotic Symptom Rating Scale; QLS: Quality of Life Scale; QoL: quality of life; RSE: Rosenberg Self‐Esteem; SADS: Social Avoidance and Distress Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms; SCID: Structured Clinical Interview for DSM‐IV; SCL‐90: Symptom Checklist‐90; SD: standard deviation; SERS: Self‐Esteem Rating Scale; SFS: Social Functioning Scale; SST: social skills training; TBM: Time Budget Measure; UPSA: UCSD Performance‐based Skills Assessment; WHOQOL‐BREF: World Health Organization Quality of Life Assessment Instrument; WTAR: Wechsler Test of Adult Reading.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ace 2004 | Allocation: randomised Participants: people with a first episode of psychosis Intervention: befriending condition or CBT intervention known as Active Cognitive Therapy for Early Psychosis |
| Ahuir 2018 | Allocation: randomised Participants: stable with a recent‐onset psychosis Interventions: MCT + psychoeducation vs psychoeducation + MCT |
| Allott 2011 | Allocation: randomised Participants: people with first‐episode psychosis Intervention: CBT (individual) vs befriending |
| Bruns 1992 | Allocation: randomised Participants: chronic outpatients with the diagnosis of schizophrenia Interventions: psychoeducational training programme for medication management with or without a cognitive therapy programme |
| Chen 2012 | Allocation: randomised Participants: people with chronic schizophrenia Intervention: individual CBT vs standard care |
| Collins 2007 | Allocation: randomised Participants: middle‐aged and older outpatients with chronic schizophrenia Interventions: CBSST vs standard care. |
| Freeman 1998 | Allocation: randomised Participants: people with drug‐resistant psychosis Intervention: CBT (individual) + standard care condition vs a standard care only control condition |
| Granholm 2011 | Allocation: randomised Participants: adults with schizophrenia or schizoaffective disorder Intervention: CBSST vs GFSC group therapy intervention |
| Hornung 1993 | Allocation: randomised Participants: adults with schizophrenia Interventions: psychoeducational medication training + leisure‐time group vs psychoeducational medication training + leisure‐time group + key person counselling vs cognitive psychotherapy + psychoeducational medication training vs cognitive psychotherapy + key‐person counselling + psychoeducational medication training |
| Hu 2016 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs individual supportive psychotherapy |
| Huang 2010 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Huang 2014 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs general psychotherapy |
| ISRCTN37178153 | Allocation: randomised Participants: adult outpatients with psychotic disorders and persistent positive psychotic symptoms Intervention: standard care + participation in Michael's game (targeting the ability to generate alternative hypotheses) vs standard care + being on a waiting list |
| ISRCTN50247539 | Allocation: randomised Participants: people with schizophrenia Interventions: REFLEX (a brief psychosocial intervention to improve insight in schizophrenia) vs active control condition, existing of cognitive remediation exercises in a group |
| ISRCTN95603741 | Allocation: randomised Participants: patients with schizophreniform disorder Intervention: individual CBT vs standard care |
| Jia 2005 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT nursing therapy vs standard care |
| Jiang 2005 | Allocation: randomised Participants: people with first‐episode schizophrenia Intervention: individual CBT vs standard care |
| Jiang 2008 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Johnson 2008 | Allocation: randomised Participants: people with DSM‐IV diagnosis of schizophrenia Interventions: group CBTp vs supportive therapy Outcome: no data on clinical variables: same sample as Penn 2009 |
| Kaizerman‐Dinerman 2018 | Allocation: randomised Participants: people with schizophrenia Interventions: metacognitive group intervention vs control group |
| Klingberg 2010 | Allocation: randomised Participants: people with schizophrenia Interventions: CBOS vs standard care |
| Landa 2018 | Allocation: pilot open trial + pilot randomised trial Participant: 19 people aged 12–25 years at high risk of developing psychosis and 20 family members Intervention: group and family‐based CBT |
| Langer 2012 | Allocation: randomisation Participants: people with psychosis Interventions: MBCT vs waiting list to receive MBCT therapy |
| Lecomte 2002 | Allocation: randomised Participants: people with first‐episode psychosis (affective and non‐affective) |
| Lecomte 2020 | Allocation: randomised Participants: people with a severe mental illness (schizophrenia, bipolar or major depression), being followed by a participating supported employment agency |
| Li 2005 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs individual support therapy |
| Li 2007 | Allocation: randomised Participants: people with schizophrenia Intervention: individual cognitive insight therapy vs standard care |
| Li 2009 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Li 2014a | Allocation: randomised Participants: people with chronic schizophrenia Intervention: cognitive remediation vs standard care |
| Li 2014b | Allocation: randomised Participants: people with first‐episode schizophrenia Intervention: individual CBT vs general health education |
| Li 2014c | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs medication only |
| Liang 2002 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs medication only |
| Lincoln 2011 | Allocation: randomised Participants: people with schizophrenia spectrum disorder Interventions: individual cognitive behavioural intervention vs wait‐list control |
| Liu 2012 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Liu 2015 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Long 2001 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Luo 2010 | Allocation: randomised Participants: people with first‐episode schizophrenia Intervention: individual CBT and general health education vs medication only and general health education |
| Ma 2007 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Ma 2012 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs individual supportive psychotherapy |
| Ma 2013 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs individual supportive psychotherapy |
| Mo 2015 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs control |
| NCT01704833 | Allocation: randomised Participants: adults with schizophrenia and schizoaffective disorder and drug‐refractory persecutory delusions Intervention: individual paranoia‐focused CBT or standard care |
| NCT02254733 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder Intervention: assertive community treatment alone or assertive community treatment + adapted cognitive behavioural social skills training |
| Nie 2016 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Owen 2015 | Allocation: randomised Participants: adults with psychotic illness: only 38/71 diagnosed with schizophrenia |
| Park 2013 | Allocation: randomisation Participants: middle‐ or older‐aged hospitalised people with schizophrenia Interventions: CBSST vs usual treatment |
| Pijnenborg 2013 | Allocation: randomised Participants: people diagnosed with schizophrenia with poor insight Interventions: a new intervention to improve insight in people with schizophrenia (REFLEX) vs an active control condition consisting of group‐wise drill and practice cognitive remediation training |
| Pu 2016 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs supportive psychotherapy |
| Puig‐Navarro 2020 | Allocation: randomised. Participants: adolescents (aged <18 years) with early‐onset psychosis Interventions: CBSST vs active control |
| Reddy 2019 | Allocation: randomised Participants: veterans with schizophrenia and moderate‐to‐high negative symptoms Interventions: group‐based motivational interviewing + CBT for negative symptoms or a mindfulness skills training group |
| Rees 2015 | Allocation: randomised Participants: people who self‐injured (not schizophrenia) |
| Roncone 2004 | Allocation: randomised Participants: people with schizophrenia Interventions: rehabilitation programme based on a metacognitive strategy vs control group |
| Tan 2015 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Tan 2016 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT nursing interventions vs routine care |
| Tang 2007 | Allocation: randomised Participants: people with first‐episode schizophrenia Intervention: individual CBT vs standard care |
| Tao 2016 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Wang 2003 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs supportive psychotherapy |
| Wang 2004 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Wang 2012 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs psychoeducation |
| Wang 2013 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs health education |
| Wang 2014 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Wu 2008 | Allocation: randomised Participants: people with chronic schizophrenia Intervention: individual CBT vs common psychotherapy |
| Wu 2013 | Allocation: randomised Participants: people with chronic schizophrenia Intervention: individual CBT vs standard care |
| Xia 2008 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs health education |
| Xie 2013 | Allocation: randomised Participants: people with schizophrenia Intervention: multimedia vs general health education |
| Yang 2010 | Allocation: randomised Participants: people with first‐episode schizophrenia Intervention: individual CBT vs medication only |
| Zha 2015 | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs standard care |
| Zhang 1999 | Allocation: randomised Participants: people with paranoid schizophrenia Intervention: individual CBT vs supportive psychotherapy |
| Zhang 2014 | Allocation: randomised Participants: homeless mental health patients (multiple diagnoses) Intervention: individual CBT vs standard care |
| Zhang 2015a | Allocation: randomised Participants: people with schizophrenia Intervention: individual CBT vs general work and recreational activity therapy |
| Zhang 2015b | Allocation: randomised Participants: people with schizophrenia Intervention: individual behaviour therapy vs supportive psychotherapy |
| Zhang 2015c | Allocation: randomised Participants: people with first‐episode schizophrenia Intervention: individual CBT vs standard care |
| Zhao 2014 | Allocation: randomised Participants: people with chronic schizophrenia Intervention: individual CBT vs standard care |
CBOS: comprehensive cognitive behaviourally oriented service; CBSST: cognitive behaviour social skills training; CBT: cognitive behavioural therapy; CBTp: cognitive behavioural therapy model targeting symptoms of psychosis; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; GFSC: goal‐focused supportive contact; MBCT: Mindfulness‐Based Cognitive Therapy; MCT: metacognitive training.
Characteristics of studies awaiting classification [ordered by study ID]
Brenner 1987.
| Methods | Unknown |
| Participants | People with schizophrenia |
| Interventions | Cognitive therapy |
| Outcomes | Unknown |
| Notes | Authors were not reachable or could not provide data |
Chung 2001.
| Methods | Randomised |
| Participants | People with schizophrenia, schizophreniform disorder and schizoaffective disorder |
| Interventions | 1. CBTp (unclear whether group or individual) 2. Supportive psychotherapy |
| Outcomes | Delusions Preoccupations on delusion Anxiety on delusions Model of explanation |
| Notes | Authors were not reachable or could not provide data |
Classen 1993.
| Methods | Unknown |
| Participants | People with schizophrenia |
| Interventions | Cognitive therapy |
| Outcomes | Unknown |
| Notes | Authors were not reachable or could not provide data |
Klingberg 2001.
| Methods | Randomised |
| Participants | People with schizophrenia and their relatives |
| Interventions | CBT |
| Outcomes | Unknown |
| Notes | Authors were not reachable or could not provide data |
Kraemer 1987.
| Methods | Unknown |
| Participants | People with schizophrenia |
| Interventions | CBTp |
| Outcomes | Unknown |
| Notes | Authors were not reachable or could not provide data |
McLeod 2007.
| Methods | Randomised |
| Participants | Adults with schizophrenia |
| Interventions | CBT (group) vs control |
| Outcomes | Unknown |
| Notes | Authors were not reachable or could not provide data |
Shafiei 2011.
| Methods | Unknown |
| Participants | People with schizophrenia |
| Interventions | Cognitive therapy |
| Outcomes | Unknown |
| Notes | Authors were not reachable or could not provide data |
CBT: cognitive behavioural therapy; CBTp: cognitive behavioural therapy model targeting symptoms of psychosis.
Characteristics of ongoing studies [ordered by study ID]
IRCT 20180817040818N.
| Study name | The effect of cognitive‐behavioral group training of self‐care skills on positive, negative symptoms, general psychopathology and self‐care in patients with schizophrenia |
| Methods | Randomised |
| Participants | People with schizophrenia |
| Interventions | 1. CBTp group 2. Standard care |
| Outcomes | Positive, negative symptoms and general psychopathology, self‐care |
| Starting date | 19 July 2020 |
| Contact information | Masoud Kashani Lotfabadi Islamic Azad university Torbat‐e Jam branch, Torbat‐e Jam Email: Kashanim1@mums.ac.ir |
| Notes |
NCT04144075.
| Study name | Application of a mindfulness and self‐compassion program in patients with schizophrenia. Randomized controlled trial |
| Methods | Randomised |
| Participants | Adult (aged 18–65 years) with schizophrenia |
| Interventions | 1. Experimental, mindfulness and self‐compassion + standard care 2. Active control, cognitive, behavioural and psychoeducational components + standard care 3. Passive control, which will receive standard care |
| Outcomes | 1. World Health Organization Quality of Life Instrument (WHOQOL‐BREF) 2. Self‐Compassion Scale |
| Starting date | Not yet recruiting |
| Contact information | María Elena F Gutiérrez‐Hernández Email: benchara@gmail.com |
| Notes |
CBTp: cognitive behavioural therapy model targeting symptoms of psychosis.
Differences between protocol and review
We have updated sections of the methods to latest changes in Cochrane Schizophrenia methods template. The changes are to wording of the text and additional text to clarify methodology, not changes to the methodology.
Since some of the studies included participants aged 16 years and over, we changed the inclusion criteria to aged 16 years and over.
We did not need to contact authors of included studies as all relevant data were available.
The protocol stated that a third review author would inspect a random 20% sample to ensure reliability in study selection. Since the study selection was performed independently by two review authors, this additional check was not performed and we thought this step added no value.
In the protocol, we planned that data extraction would be performed by one review author, and a random 10% of the studies would be extracted also by a second author. Instead, two review authors independently extracted data.
No study had more than 50% of lost participants in one arm, so we did not perform any additional analysis.
We did not divide outcomes into short‐term and long‐term as the length of treatment was very variable. Since each study varied in length, outcome measurement and definition of length of treatment, we preferred to focus on end of treatment outcomes to improve consistency across studies.
Very few studies reported data on occupational status so we did not extract it.
We moved the outcome 'death' to adverse effects/events and 'incidence of clinically important depression/anxiety and psychotic symptoms' to mental state. We also expanded outcomes to include 'any change' as well as clinically important change.
We have added anxiety as an outcome since a few studies included it.
We chose to modify some of the outcomes for the summary of findings table. We made this decision as we found that several studies reported rating scales scores (in particular PANSS scores). We considered that PANSS total scores were an appropriate proxy for clinically significant response in mental state – as defined by individual studies. We also added the functioning: overall: global assessment of functioning – average endpoint score – end of treatment (GAF).
Morelli AC and Chiodo D left the review team. Abbatecola M, Aali G, Tarantino F, Ebuenyi ID, Lucarini V, Li W, Zhang C and Pinto A joined the review team.
Contributions of authors
GG – devised the idea, contributed to the introduction and wrote the protocol, results and part of discussion section, helped with data extraction.
MA – extracted and inputted data.
GA – helped with data extraction, data entry, excluded and awaiting classification studies and proofread the manuscript.
FT – extracted and inputted data.
IE – helped with data extraction, data entry, excluded and awaiting classification studies.
VL – helped with data extraction, data entry, excluded and awaiting classification studies.
WL – extracted and inputted data for studies in Chinese language.
CZ – extracted and inputted data for studies in Chinese language.
AP – wrote part of the discussion and provided expert content review and supervision.
Sources of support
Internal sources
-
National Institute for Health and Care Research (NIHR), UK
provided funding for Cochrane Schizophrenia Group
External sources
-
None, Other
No source of support
Declarations of interest
GG – is an Editor at Cochrane Common Mental Disorder Group and he was not involved in any editorial process of this review.
MA – none.
GA – was Assistant Managing Editor at Cochrane Schizophrenia Group until April 2021 and is currently Managing Editor of Cochrane Gut group. She was excluded from any editorial process of this review.
FT – none.
IE – none.
VL – none.
WL – none.
CZ – none.
AP – is a practitioner and content expert of CBTp. No other conflict of interest declared.
New
References
References to studies included in this review
Barrowclough 2006 {published data only}Std10252
- Barrowclough C, Haddock G, Lobban F, Jones S, Siddle R, Roberts C, et al. Group cognitive behavioural therapy for schizophrenia – randomised controlled trial. British Journal of Psychiatry 2006;189:527-32. [CSZG: Ref14559] [DOI] [PubMed] [Google Scholar]
- Barrowclough C. An evaluation of the effectiveness of group cognitive therapy for people with recent onset schizophrenia. National Research Register 2001;1. [CSZG: Ref12948]
Bechdolf 2004 {published data only}Std7953
- Bechdolf A, Knost B, Kuntermann C, Schiller S, Hambrecht M, Klosterkotter J, et al. Coping-oriented versus psychoeducational group therapy for post acute patients with schizophrenia: results of a 6 month follow-up. Schizophrenia Research 2002;53(Suppl 1):263-4. [CSZG: Ref8053] [Google Scholar]
- Bechdolf A, Knost B, Kuntermann C, Schiller S, Klosterkotter J, Hambrecht M, et al. A randomized comparison of group cognitive behavioural therapy and group psychoeducation in patients with schizophrenia. Acta Psychiatrica Scandinavica 2004;110:21-8. [CSZG: Ref11268] [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Knost B, Nelson B, Schneider N, Veith V, Yung AR, et al. Randomized comparison of group cognitive behaviour therapy and group psychoeducation in acute patients with schizophrenia: effects on subjective quality of life. Australian and New Zealand Journal of Psychiatry 2010;44:144-50. [CSZG: Ref19694] [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Kohn D, Knost B, Pukrop R, Klosterkotter J. A randomized comparison of group cognitive-behavioural therapy and group psychoeducation in acute patients with schizophrenia: outcome at 24 months. Acta Psychiatrica Scandinavica 2005;112:173-9. [CSZG: Ref18491] [DOI] [PubMed] [Google Scholar]
- Bechdolf A. A randomized comparison of group cognitive-behavioural therapy and group psychoeducation in patients with schizophrenia: commentary reply. Acta Psychiatrica Scandinavica 2006;113:73-4. [CSZG: Ref18490] [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P. Erratum. Acta Psychiatrica Scandinavica 2004;110:483. [CSZG: Ref18577] [Google Scholar]
Chadwick 2016 {published data only}
- Chadwick P, Strauss C, Jones AM, Kingdon D, Ellett L, Dannahy L, et al. Group mindfulness-based intervention for distressing voices: a pragmatic randomised controlled trial. Schizophrenia Research 2016;175:168-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniels 1998 {published data only}
- Daniels L. A group cognitive-behavioural and process-oriented approach to treating the social impairment and negative symptoms associated with chronic mental illness. Journal of Psychotherapy Practice and Research 1998;7:167-76. [PMC free article] [PubMed] [Google Scholar]
Deng 2014 {published data only}
- Deng M, Ding N. Observation on the effect of implementing group CBT for patients with schizophrenia during rehabilitation [对精神分裂症康复期患者实施团体cbt的效果观察]. Contemporary Nurse 2014;10:116-8. [Google Scholar]
Granholm 2007 {published data only}Std10881
- Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. American Journal of Psychiatry 2005;162:520-9. [CSZG: Ref11453] [DOI] [PubMed] [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Link PC, Perivoliotis D, Gottlieb JD, et al. Randomized controlled trial of cognitive behavioral social skills training for older people with schizophrenia: 12-month follow-up. Journal of Clinical Psychiatry 2007;68:730-7. [CSZG: Ref14560] [DOI] [PubMed] [Google Scholar]
Granholm 2013 {published data only}Std10343
- Granholm E, Holden J, Link PC, McQuaid JR, Jeste DV. Randomized controlled trial of cognitive behavioral social skills training for older consumers with schizophrenia: defeatist performance attitudes and functional outcome. American Journal of Geriatric Psychiatry 2013;21(3):251-62. [CSZG: Ref28246] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00237796. Functional rehabilitation of older patients with schizophrenia. www.clinicaltrials.gov/ct2/show/NCT00237796 (first received 12 October 2005).
Granholm 2014 {published data only}
- Granholm E, Holden J, Link PC, McQuaid JR. Randomized clinical trial of cognitive behavioral social skills training for schizophrenia: improvement in functioning and experiential negative symptoms. Journal of Consulting and Clinical Psychology 2014;82:1173-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Holden J, Worley M. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: mediation by defeatist performance attitudes and asocial beliefs. Schizophrenia Bulletin 2018;44:653-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Granholm 2020 {published data only}
- Granholm E, Holden JL, Dwyer K, Link P. Mobile-assisted cognitive-behavioral social skills training in older adults with schizophrenia. Journal of Behavioral and Cognitive Therapy 2020;30:13-21. [Google Scholar]
Guan 2016 {published data only}
- Guan T, Yang D, Han P. The effect of olanzapine combined with group therapy on paranoid schizophrenia [奥氮平合并团体治疗对偏执型精神分裂症的影响]. Modern Chinese Doctor 2016;54:58-61. [Google Scholar]
Jones 2014 {published data only}
- Jones A. Targeting Reasoning Biases in Delusions; a Pilot Study of the 'Thinking Well Group' [Doctoral thesis]. London (UK): King's College, 2014. [Google Scholar]
Li 2013a {published data only}
- Li X, Guo Y, Fu C, Chen L, Zhang Z. Group cognitive behavioral therapy efficacy for refractory auditory hallucinations in schizophrenia [小组认 知行 为治疗对 精神分裂症顽 固性幻 听的疗效 观察]. Chinese Journal of Rehabilitation 2013;28:313-5. [Google Scholar]
Li 2013b {published data only}
- Li J, Deng X, Xie Y, Liang L. Effects on cognitive function in patients with chronic schizophrenia, cognitive behavioral therapy [认知行为治疗对慢性精神分裂症患者认知功能的影响]. Chinese and foreign medical research 2013;24:130-2. [Google Scholar]
Mortan Sevi 2020 {published data only}
- Mortan Sevi O, Tekinsav Sutcu S, Yesilyurt S, Turan Eroglu S, Gunes B. Comparison of the effectiveness of two cognitive-behavioral group therapy programs for schizophrenia: results of a short-term randomized control trial. Community Mental Health Journal 2020;56:222-8. [DOI] [PubMed] [Google Scholar]
Penn 2009 {published data only}Std13558
- Penn D. A 24-week investigation of group cognitive behavioral therapy (CBT) for medication-resistant auditory hallucinations in 60 patients. Stanley Foundation Research Programs 2004.
- Penn DL, Meyer PS, Evans E, Wirth RJ, Cai K, Burchinal M. A randomized controlled trial of group cognitive-behavioral therapy vs. enhanced supportive therapy for auditory hallucinations. Schizophrenia Research 2009;109:52-9. [CSZG: Ref18213] [DOI] [PubMed] [Google Scholar]
Qi 2012 {published data only}
- Qi H, Liu H. Studies on the efficacy of combining episode male schizophrenic cognitive behavioral therapy [结合认知行为治疗对首发男性精神分裂症的 疗效研究]. Hebei Medical Journal 2012;34:3229-31. [Google Scholar]
Qu 2016 {published data only}
- Qu W. Exploring the impact of cognitive psychotherapy applied to patients with schizophrenia on their cognitive function [探析认知心理治疗应用于精神分裂症患者中对其认知功能的影响]. Psychologist 2016;22:32-3. [Google Scholar]
Shen 2007 {published data only}
- Shen F, Yang YC, Deng H. The effect of group cognitive-behavioural therapy for schizophrenia after acute episode. West China Medical Journal 2007;22:236-7. [Google Scholar]
Shi 2015 {published data only}
- Shi Y, Jiao Y, Jiang L, Sun Y, Bai Z, Wu Q, et al. The effect of cognitive behavioral therapy on executive function of patients with chronic schizophrenia [认知行为治疗对慢性精神分裂症患者执行功能的影响]. Neurological diseases and mental health 2015;15:497-9. [Google Scholar]
Song 2012 {published data only}
- Song X, Lui Y, Zhang Y, Zhou L. Study on the effect of cognitive behavioral therapy on improving the negative symptoms of schizophrenia patients [认知行为治疗对改善精神分裂症病人阴性症状的效果研究]. Psychologist 2012;7:30-1. [Google Scholar]
Song 2014 {published data only}
- Song X, Li J, Zhang Y, Wang X, Wang E. Group CBT for patients with schizophrenia and auditory hallucinations: the impact of quality of life [团体cbt对精神分裂症幻听患者生存质量的影响]. International Medicine and Health Guidance News 2014;20:2572-4. [Google Scholar]
Tao 2015 {published data only}
- Tao C, Ding S, Zhu Y, Li X. Evaluation of comprehensive family knowledge, belief and behavior intervention on the rehabilitation efficacy of schizophrenia patients in the rehabilitation period [家庭综合知信行干预对康复期精神分裂症患者康复疗效的评价]. China Journal of Health Psychology 2015;23:970-2. [Google Scholar]
Wykes 2005 {published data only}Std10290
- Wykes T, Hayward P, Thomas N, Green N, Surguladze S, Fannon D, et al. What are the effects of group cognitive behaviour therapy for voices? A randomised control trial. Schizophrenia Research 2005;77:201-10. [CSZG: Ref17574] [DOI] [PubMed] [Google Scholar]
- Wykes T, Parr AM, Landau S. Group cognitive treatment for persistent auditory hallucinations: a controlled trial. Schizophrenia Research 1999;36:335. [Google Scholar]
- Wykes T, Thompson N, Green N, Surgaldze S, Hayward P. Changing voices: a randomised control trial of group cognitive behavioural treatment. Schizophrenia Research 2003;60:331. [Google Scholar]
- Wykes T, Thompson N, Hayward P. Changing voices: a randomised control trial of group cognitive treatment. Schizophrenia Research 2002;53(Suppl 1):12. [DOI] [PubMed] [Google Scholar]
Zhou 2015 {published data only}
- Zhou JJ. The effect of cognitive behavior therapy on the social function and life quality of schizophrenia patients [认知行为疗法对精神分裂症患者社会功能和生活质量的影响]. Health Research 2015;35:539-41. [Google Scholar]
References to studies excluded from this review
Ace 2004 {published data only}
- Allott K, Alvarez-Jimenez M, Killackey EJ, Bendall S, McGorry PD, Jackson HJ. Patient predictors of symptom and functional outcome following cognitive behaviour therapy or befriending in first-episode psychosis. Schizophrenia Research 2011;132(2-3):125-30. [DOI] [PubMed] [Google Scholar]
- Bendall S, Allott K, Jovev M, Marois MJ, Killackey EJ, Gleeson JF, et al. Therapy contamination as a measure of therapist treatment adherence in a trial of cognitive behaviour therapy versus befriending for psychosis. Behavioural and Cognitive Psychotherapy 2015;43(3):314-27. [DOI] [PubMed] [Google Scholar]
- Bendall S, Jackson HJ, Killackey E, Allott K, Johnson T, Harrigan S, et al. The credibility and acceptability of befriending as a control therapy in a randomized controlled trial of cognitive behaviour therapy for acute first episode psychosis. Behavioural and Cognitive Psychotherapy 2006;34(3):277-91. [Google Scholar]
- Bendall S. A comparison of the nonspecific effects of a control treatment with cognitive behavioural therapy in first episode psychosis. Schizophrenia Research 2004;70(1):71. [Google Scholar]
- Jackson H, McGorry P, Killackey E, Bendall S, Allott K, Johnston T, et al. The ACE project: a randomised controlled trial of CBT versus befriending: acute phase results. Schizophrenia Research 2004;70(1):56-7. [Google Scholar]
- Jackson HJ, Killackey E, Bendall S, Allott K, Dudgeon P, Harrigan S, et al. A randomised controlled trial of CBT for early psychosis with 1 year follow-up. Australian and New Zealand Journal of Psychiatry 2005;39(Suppl 2):A42. [Google Scholar]
- Jackson HJ, McGorry PD, Killackey E, Bendall S, Allott K, Dudgeon P, et al. Acute-phase and 1-year follow-up results of a randomized controlled trial of CBT versus befriending for first-episode psychosis: the ACE project. Psychological Medicine 2008;38(5):725-35. [DOI] [PubMed] [Google Scholar]
- Killackey E, Jackson H, Bendall S, Allott K, Harrigan S, Gleeson J, et al. An exploration of the relationship between dose and type of therapy and outcome in the ACE trial of CBT for first episode psychosis. Schizophrenia Research 2004;70(1):57. [Google Scholar]
- Killackey E, Jackson H, McGorry P, Bendall S, Allott K, Johnson T, et al. The ACE project: a randomised controlled trial of CBT versus befriending for acute first episode psychosis: acute phase results. Schizophrenia Bulletin 2005;31:526-7. [DOI] [PubMed] [Google Scholar]
Ahuir 2018 {published data only}
- Ahuir M, Cabezas A, Minano MJ, Algora MJ, Estrada F, Sole M, et al. Improvement in cognitive biases after group psychoeducation and metacognitive training in recent-onset psychosis: a randomized crossover clinical trial. Psychiatry Research 2018;270:720-3. [DOI] [PubMed] [Google Scholar]
Allott 2011 {published data only}
- Allott KA, Alvarez-Jimenez M, Bendall S, Killackey EJ, McGorry PD, Jackson HJ. Predictors of functional outcome following CBT in first-episode psychosis. Schizophrenia Bulletin 2011;37:258. [DOI] [PubMed] [Google Scholar]
Bruns 1992 {published data only}
- Bruns U, Franzen U, Hornung WP, Klingberg S, Wiesemann C. Effects of an ambulant group therapy program on psychosocial adaptation of chronic schizophrenic outpatients. Schizophrenia Research 1992;6(2):175. [Google Scholar]
Chen 2012 {published data only}
- Chen R. Analysis of the effect of cognitive behavioral psychological intervention on the rehabilitation of chronic schizophrenia [认知行为心理干预对慢性精神分裂症康复作用分析]. Guide of Chinese Medicine 2012;11:57-8. [Google Scholar]
Collins 2007 {published data only}
- Collins LM, Granholm E, McQuaid JR. Clinical insight moderates treatment outcome in cognitive behavioral social skills training for chronic schizophrenia. Schizophrenia Bulletin 2007;33:426. [Google Scholar]
Freeman 1998 {published data only}
- Freeman D, Garety P, Fowler D, Kuipers E, Dunn G, Bebbington P, et al. The London East Anglia randomized controlled trial of cognitive behaviour therapy for psychosis. IV: self esteem and persecutory delusions. British Journal of Clinical Psychology 1998;37(4):415-30. [DOI] [PubMed] [Google Scholar]
Granholm 2011 {published data only}
- Dwyer K, Holden J, Granholm E. Can individuals with neurocognitive impairment benefit from cognitive-behavioral social skills training? Schizophrenia Bulletin 2019;45:S218. [Google Scholar]
- Granholm E, Holden J, Link P. Randomized clinical trial of cognitive behavioral social skills training (CBSST) for older people with schizophrenia: replication and role of defeatist attitudes. Schizophrenia Bulletin 2011;37:304. [Google Scholar]
Hornung 1993 {published data only}
- Buchkremer G, Klingberg S, Holle R, Monking HS, Hornung WP. Psychoeducational psychotherapy for schizophrenic patients and their key relatives or care-givers: results of a 2-year follow-up. Acta Psychiatrica Scandinavica 1997;96:483-91. [DOI] [PubMed] [Google Scholar]
- Feldmann R, Buchkremer G, Hornung WP. Prognostic and therapeutic relevance of cognitive characteristics for the long-term course of schizophrenic illness following psychoeducational psychotherapeutic treatment [Prognostische und therapeutische relevanz kognitiver ressourcen fur den langzeitverlauf schizophrener erkrankung nach psychoedukativ-psychotherapeutischer behandlung]. Fortschritte der Neurologie und Psychiatrie 2000;68:54-60. [DOI] [PubMed] [Google Scholar]
- Feldmann R, Hornung WP, Buchkremer G, Arolt V. The influence of familial loading on the course of schizophrenic symptoms and the success of psychoeducational therapy. Psychopathology 2001;34(4):192-7. [DOI] [PubMed] [Google Scholar]
- Feldmann R, Hornung WP, Prein B, Buchkremer G, Arolt V. Timing of psychoeducational psychotherapeutic interventions in schizophrenic patients. European Archives of Psychiatry and Clinical Neuroscience 2002;252:115-9. [DOI] [PubMed] [Google Scholar]
- Hornung WP, Buchkremer G, Redbrake M, Klingberg S. Patient modified treatment. What are the effects of neuroleptic drugs on people with schizophrenia? [Patientmodifizierte Medikation: Wie gehen schizophrene Patienten mit ihren Neuroleptika um?]. Nervenarzt 1993;64:434-439. [PubMed] [Google Scholar]
- Hornung WP, Buchkremer G. Effective psycho-educative cognitive behavioural therapeutic interventions on chronic schizophrenic patients: Results of a prospective study. Verhaltenstherapie 1993;Suppl:A31. [Google Scholar]
- Hornung WP, Feldmann R, Klingberg S, Buchkremer G, Reker T. Long term effects of a psychoeducational psychotherapeutic intervention for schizophrenic outpatients and their key persons: results of a five year follow-up [Langzeitwirkungen einer psychoedukativen-psychotherapeutischen intervention bei schizophrenen ambulanten patienten und ihren bezugspersonen – ergebnisse einer fuenf-jahres-katamnese]. European Archives of Psychiatry and Clinical Neuroscience 1999;249:162-7. [DOI] [PubMed] [Google Scholar]
- Hornung WP, Feldmann R, Schonauer K, Schafer A, Monking HS, Klingberg S, et al. Psychoeducational psychotherapeutic treatment of schizophrenic patients and their caregivers. II. Supplementary findings at a 2 year follow up [Psychoedukativ-psychotherapeutische Behandlung von schizophrenen Patienten und ihren Bezugspersonen. II. Erganzende Befunde der 2-Jahres- Katamnese]. Nervenarzt 1999;70:444-9. [DOI] [PubMed] [Google Scholar]
- Hornung WP, Holle R, Schulze Monking H, Klingberg S, Buchkremer G. Psychoeducational-psychotherapeutic treatment of schizophrenic patients and their caregivers. Results of a 1-year catamnestic study [Psychoedukativ-psychotherapeutische Behandlung von schizophrenen Patienten und ihren Bezugspersonen. Ergebnisse einer 1-Jahres-Katamnese]. Nervenarzt 1995;66:828-34. [PubMed] [Google Scholar]
- Hornung WP, Kieserg A, Feldmann R, Buchkremer G. Psychoeducational training for schizophrenic patients: background, procedure and empirical findings. Patient Education and Counseling 1996;29:257-68. [DOI] [PubMed] [Google Scholar]
- Hornung WP, Klingberg S, Feldmann R, Schonauer K, Schulze Monking H. Collaboration with drug treatment by schizophrenic patients with and without psychoeducational training: results of a 1-year follow-up. Acta Psychiatrica Scandinavica 1998;97:213-9. [DOI] [PubMed] [Google Scholar]
- Hornung WP, Schonauer K, Feldmann R, Monking HS. Medication related attitudes of chronic schizophrenic patients. A follow up study after psycho educational intervention [Medikationsbezogene Einstellungen chronisch schizophrener Patienten. Eine Follow up Untersuchung 24 Monate nach psychoedukativer Intervention]. Psychiatrische Praxis 1998;25:25-8. [PubMed] [Google Scholar]
- Klingberg S, Buchkremer G, Holle R, Schulze-Monking H, Hornung WP. Differential therapy effects of psychoeducational psychotherapy for schizophrenic patients: results of a 2 year follow up. European Archives of Psychiatry and Clinical Neuroscience 1999;249:66-72. [DOI] [PubMed] [Google Scholar]
Hu 2016 {published data only}
- Hu X, Yan G, Chen J. The effect of cognitive behavioral therapy on improving the quality of life of patients with schizophrenia [认知行为治疗对改善精神分裂症患者生活质量的效果]. Laboratory Medicine and Clinical 2016;13:822-4. [Google Scholar]
Huang 2010 {published data only}
- Huang J, Guo J, Han S. Influence of cognitive behavior training on rehabilitation effect of chronic schizophrenia patients [认 知行 为训 练对 精 神分 裂症 病 人康复效 果影 响的研 究]. Chinese Nursing Research 2010;24:1260-1. [Google Scholar]
Huang 2014 {published data only}
- Huang S, Wang J. Application analysis of cognitive therapy in 120 patients with schizophrenia in remission [认知治疗在120例精神分裂症缓解期患者中的应用分析]. Guide of Chinese Medicine 2014;12:115-6. [Google Scholar]
ISRCTN37178153 {published data only}
- ISRCTN37178153. The effect of a cognitive behavioural program called "Michael's Game" on psychotic symptoms. www.isrctn.com/ISRCTN37178153 (first received 6 April 2013).
- Khazaal Y, Chatton A, Dieben K, Huguelet P, Boucherie M, Monney G, et al. Reducing delusional conviction through a cognitive-based group training game: a multicentre randomized controlled trial. Frontiers in Psychiatry 2015;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzenstadler L, Chatton A, Huguelet P, Lecardeur L, Bartolomei J, Brazo P, et al. Does change over time in delusional beliefs as measured with PDI predict change over time in belief flexibility measured with MADS? Psychiatric Quarterly 2010;90(4):693-702. [DOI] [PubMed] [Google Scholar]
- Penzenstadler L, Chatton A, Lecomte T, Huguelet P, Lecardeur L, Azoulay S, et al. Does the Beck Cognitive Insight Scale predict change in delusional beliefs? Psychology and Psychotherapy 2020;93(4):690-704. [DOI] [PubMed] [Google Scholar]
ISRCTN50247539 {published data only}
- ISRCTN50247539. Reflex. www.isrctn.com/ISRCTN50247539 (first received 4 May 2009).
- Pijnenborg GH, Vos AE, Timmerman ME, Gaag M, Sportel BE, Arends J, et al. Social cognitive group treatment for impaired insight in psychosis: a multicenter randomized controlled trial. Schizophrenia Research 2019;206:362-9. [DOI] [PubMed] [Google Scholar]
- Pijnenborg GH, Gaag M, Bockting CL, Meer L, Aleman A. Reflex, a social-cognitive group treatment to improve insight in schizophrenia: study protocol of a multi-center RCT. BMC Psychiatry 2011;11:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN95603741 {published data only}
- Feasibility study of culturally adapted cognitive behaviour therapy for psychosis for ethnic minority groups. www.isrctn.com/ISRCTN95603741 (first received 21 May 2010).
- Husain N, Mehmood N, Husain MO, Kiran T, Naeem F, Chaudhry IB. Feasibility study of culturally adapted cognitive behaviour therapy for psychosis in Pakistan. European Psychiatry 2016;33:S578-9. [Google Scholar]
- Phiri P. Adapting cognitive behaviour therapy for psychosis for black and minority ethnic communities. Dissertation Ann Arbor University of Southampton (United Kingdom) 2012.
Jia 2005 {published data only}
- Jia Y, Lou F, Feng M. Cognitive behavioral nursing therapy for patients with schizophrenia [精神分裂症 患者认 知行 为护理疗法探讨]. Journal of Nursing Science 2005;20:10-2. [Google Scholar]
Jiang 2005 {published data only}
- Jiang Y. Cognitive behavioral therapy term effect of the episode schizophrenia [认知行为治疗对首发精神分裂症近期疗效的影响]. In: Proceedings of the 2005 Zhejiang Psychiatry Annual Conference. 2005:284-6.
Jiang 2008 {published data only}
- Jiang JF, Wei JY, Bian MJ, Xu YF, Gu JY. Effect of cognitive behavioral intervention on recovery of social function of patients with schizophrenia [认知行为干预对精神分裂症患者恢复 社会功能 的作 用]. Heilongjiang Nursing Journal 2008;14:701-2. [Google Scholar]
Johnson 2008 {published data only}
- Johnson DP, Penn DL, Bauer DJ, Meyer P, Evans E. Predictors of the therapeutic alliance in group therapy for individuals with treatment-resistant auditory hallucinations. British Journal of Clinical Psychology 2008;47:171-83. [DOI] [PubMed] [Google Scholar]
Kaizerman‐Dinerman 2018 {published data only}
- Kaizerman-Dinerman A, Josman N, Roe D. The use of cognitive strategies among people with schizophrenia: a randomized comparative study. Open Journal of Occupational Therapy 2019;7:1-12. [Google Scholar]
- Kaizerman-Dinerman A, Roe D, Josman N. An efficacy study of a metacognitive group intervention for people with schizophrenia. Psychiatry Research 2018;270:1150-6. [DOI] [PubMed] [Google Scholar]
Klingberg 2010 {published data only}
- Klingberg S, Wittorf A, Fischer A, Jakob-Deters K, Buchkremer G, Wiedemann G. Evaluation of a cognitive behaviourally oriented service for relapse prevention in schizophrenia. Acta Psychiatrica Scandinavica 2010;121:340-50. [DOI] [PubMed] [Google Scholar]
Landa 2018 {published data only}
- Landa Y, Mueser K, Jacobs M, Jespersen R, Wyka K, Swiderski C, Cahalan C, et al. Group and family based cognitive behavioral therapy for youth at risk for psychosis: from research to real-word practice. Early Intervention in Psychiatry 2018;12(Suppl 1):183. [Google Scholar]
Langer 2012 {published data only}
- Langer AI, Cangas AJ, Salcedo E, Fuentes, B. Applying mindfulness therapy in a group of psychotic individuals: a controlled study. Behavioural and Cognitive Psychotherapy 2012;40(1):105-9. [DOI] [PubMed] [Google Scholar]
Lecomte 2002 {published data only}
- Lecomte T, Leclerc C, Corbiere M, Wykes T, Wallace CJ, Spidel A. Group cognitive behavior therapy or social skills training for individuals with a recent onset of psychosis? Results of a randomized controlled trial. Journal of Nervous and Mental Disease 2008;196:866-75. [DOI] [PubMed] [Google Scholar]
- Lecomte T, Leclerc C, Wykes T, Spidel A. Group CBT for early psychosis – are there still benefits 1 year later? Early Intervention in Psychiatry 2010;4(Suppl 1):150. [Google Scholar]
- Lecomte T, Leclerc C, Wykes T, Wallace CJ, Spidel A, Corbiere M. Group CBT versus skills training for first episodes of psychosis – results of a RCT. Schizophrenia Research 2006;86(Suppl 1):S45-6. [Google Scholar]
- Lecomte T, Leclerc C, Wykes T, Wallace CJ, Spidel A, Greaves C. Effectiveness of group cognitive-behaviour therapy for first episode psychosis – results of a randomized controlled trial. Schizophrenia Bulletin 2007;33:440. [Google Scholar]
- Lecomte T, Leclerc C, Wykes T, Wallace CJ. Group CBT versus group symptom management for treating psychotic symptoms of young individuals presenting a first episode of schizophrenia: preliminary results. In: Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25-28; Copenhagen, Denmark. 2002:88-9.
- Lecomte T, Leclerc C, Wykes T. Group CBT for early psychosis – are there still benefits one year later? International Journal of Group Psychotherapy 2012;62:309-21. [DOI] [PubMed] [Google Scholar]
Lecomte 2020 {published data only}
- Lecomte T, Corbiere M, Giguere CE, Titone D, Lysaker P. Group cognitive behaviour therapy for supported employment – results of a randomized controlled cohort trial. Schizophrenia Research 2020;215:126-33. [DOI] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li Y, Li Z, Xin L, Hou L, Zhao Y, Hou C. The efficacy of special cognitive-behavior approaches on schizophrenia during acute-phase [特 定 认 知 行 为 技 术 对 急 性 期 精 神 分 裂 症 的 干 预 效 果]. Hebei Medicine 2005;21:1018-20. [Google Scholar]
Li 2007 {published data only}
- Li S, Su M, Su T, Wang C, Ling H, Cheng G. The effect of cognition therapy on the insight and the compliance of schizophrenia patients [认知领 影响 悟治疗对精神分裂症患者 自知力及治疗依从性的]. International Medicine and Health Guidance News 2007;13:18-21. [Google Scholar]
Li 2009 {published data only}
- Li X. The impact of cognitive intervention on the rehabilitation of patients with split mental disorder [认 知 干 预 对 精 神 分 裂 症 患 者 康 复 的 影 响]. Journal of Qilu Nursing 2009;15:11-2. [Google Scholar]
Li 2014a {published data only}
- Li L, Hu L, Liang Z, Wen Z. The effect of cognitive intervention on the quality of life and related factors in patients with chronic schizophrenia [认知干预对慢性精神分裂症患者生活质量及相关因素的影响]. Clinical Medical Engineering 2014;21:1073-4. [Google Scholar]
Li 2014b {published data only}
- Li H. The effect of cognitive psychotherapy on the treatment compliance of patients with first episode schizophrenia [认知心理治疗对首发精神分裂症患者治疗依从性的影响]. Medical Information 2014;27:524. [Google Scholar]
Li 2014c {published data only}
- Li Y. The clinical effect of cognitive therapy in the treatment of schizophrenia and its influence on social function [认知疗法在精神分裂症患者治疗中的临床疗效及对社会功能的影响]. Medical Journal of Chinese People's Health 2014;26:38-9. [Google Scholar]
Liang 2002 {published data only}
- Liang Q, He Z, Deng L, Lu H. Cognitive therapy to improve the efficacy of schizophrenia insight analysis [认知治疗对改善精神分裂症患者自知力的疗效分析]. Modern Rehabilitation 2002;6:3234. [Google Scholar]
Lincoln 2011 {published data only}
- Lincoln T, Ziegler M, Mehl S, Luellmann E, Kesting ML, Westermann S, et al. Effectiveness of CBT in a German outpatient setting. Short and long term results of a controlled randomised trial. European Archives of Psychiatry and Clinical Neuroscience 2011;261:S18. [Google Scholar]
Liu 2012 {published data only}
- Liu G. The effect of cognitive psychotherapy on the quality of life and stigma of patients with schizophrenia during rehabilitation [认知心理治疗对精神分裂症康复期患者生活质量及病耻感的影响]. Psychologist 2012;8:71. [Google Scholar]
Liu 2015 {published data only}
- Liu J, Yong S, Xu X, Zhang J, Xie P, Xu C, et al. The effect of cognitive behavioral therapy on the rehabilitation of inpatients with schizophrenia [认知行为治疗对住院精神分裂症患者康复效果的影响]. Journal of Clinical Psychosomatic Diseases 2015;21:64-6. [Google Scholar]
Long 2001 {published data only}
- Long R, Li L. Cognitive behavioral therapy in the psychological care work (control analysis of 100 cases of schizophrenia) [认知行为疗法在 心理护理工作 中的应用 (附 100例住院精神分裂症对照分析]. Shandong Archives of Psychiatry 2001;14:261-3. [Google Scholar]
Luo 2010 {published data only}
- Luo H, Wu X. Episode schizophrenia patients with cognitive therapy follow-up study [首发精神分裂症患者认知治疗疗效随访研究]. Internal Medicine of China 2010;5:272-4. [Google Scholar]
Ma 2007 {published data only}
- Ma WH, Hu XF, Wang ZQ. A comparison of schizophrenic treated with method of corrected – behavior [精神分裂症认知行为疗法的对照研究]. Medical Journal of Chinese People's Health 2007;19:441, 443. [Google Scholar]
Ma 2012 {published data only}
- Ma Y, Li ZJ, Xu ZY, Gu ZH, Qu Y, Wang XQ, et al. Effects of cognitive behavioral therapy on quality of life in patients with schizophrenia: a single blind randomized controlled study [认知行为治疗改善精神分裂症患者 生活质量的随机单盲对照试验]. Chinese Mental Health Journal 2012;26:801-7. [Google Scholar]
Ma 2013 {published data only}
- Ma Y, Li Z, Xu Z, Guo Z, Qu Y, Wang X, et al. Effects of cognitive behavioral therapy on coping style for patients with schizophrenia [认知行为治疗对精神分裂症患者应对方式的影响]. Chinese Journal of Clinical Psychology 2013;21:455-7. [Google Scholar]
Mo 2015 {published data only}
- Mo Y, Li X, Fu C, He R, Cai Y, Mo T. The influence of cognitive behavior intervention on the mental state of vagrants with mental illness [认知行为干预对流浪精神疾病患者心理状态的影响]. Chinese Journal of Modern Drug Application 2015;9:201-2. [Google Scholar]
NCT01704833 {published data only}
- Landa Y, Chadwick P, Beck AT, Alexeenko L, Sheets M, Zhu Y, et al. Targeting information processing biases and social avoidance in group cognitive behavioral therapy for paranoia: a pilot randomized controlled clinical trial. Schizophrenia Bulletin 2011;37:271. [Google Scholar]
- Landa Y, Chadwick P, Stern E, Pan H, Alexeenko L, Zhu Y, et al. Cognitive behavioral therapy for paranoia: a pilot randomized controlled clinical trial and fMRI investigation of systems-level brain circuit modulation. Biological Psychiatry 2012;8(Suppl 1):65S-6S. [Google Scholar]
- NCT01704833. Cognitive behavioral therapy for paranoia in schizophrenia. clinicaltrials.gov/ct2/show/NCT01704833 (first received 11 October 2012).
NCT02254733 {published data only}
- Granholm E, Holden JL, Sommerfeld D, Rufener C, Perivoliotis D, Mueser K, et al. Enhancing assertive community treatment with cognitive behavioral social skills training for schizophrenia: study protocol for a randomized controlled trial. Trials 2015;16:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld DH, Aarons GA, Naqvi JB, Holden J, Perivoliotis D, Mueser KT, et al. Stakeholder perspectives on implementing cognitive behavioral social skills training on assertive community treatment teams. Administration and Policy in Mental Health 2019;46:188-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nie 2016 {published data only}
- Nie S, Deng L, Yang Y, Zhou Y. The effect of cognitive behavioral intervention on treatment compliance and quality of life of patients with schizophrenia in remission [认知行为干预对精神分裂症缓解期患者治疗依从性和生活质量的影响]. Chinese Clinical Nursing 2016;8:461-4. [Google Scholar]
Owen 2015 {published data only}
- Owen M, Sellwood W, Kan S, Murray J, Sarsam M. Group CBT for psychosis: a longitudinal, controlled trial with inpatients. Behaviour Research and Therapy 2015;65:76-85. [DOI] [PubMed] [Google Scholar]
Park 2013 {published data only}
- Park H. A randomized controlled pilot study of CBSST (cognitive behavioral social skills training) for middle-or older-aged patients with schizophrenia: a pilot study, revisited cognitively. International Psychogeriatrics 2013;25(Suppl 1):159. [Google Scholar]
Pijnenborg 2013 {published data only}
- Pijnenborg G, Vos A, Meer L, Sportel E, Bockting C, Gaag M, Aleman A. Results of a metacognitive group treatment to improve insight in psychosis (correct version of the abstract, this replaces the previously submitted abstract on reflex). Schizophrenia Research 2014;153(Suppl 1):S354. [Google Scholar]
- Pijnenborg G, Vos A, Meer L, Sportel E, Bockting C, Gaag M, et al. A metacognitive group treatment to improve insight in psychosis. Schizophrenia Research 2014;153(Suppl 1):S162. [Google Scholar]
- Pijnenborg M, Vos A, Meer L, Bockting C, Gaag M, Aleman A. A metacognitive group treatment to improve insight in psychosis and to reduce self-stigma. European Archives of Psychiatry and Clinical Neuroscience 2015;1:S72. [Google Scholar]
- Pijnenborg M, Meer L, Vos A, Bockting C, Gaag M, Aleman A. A metacognitive group treatment to improve insight in psychosis. Schizophrenia Bulletin 2013;39:S349. [Google Scholar]
Pu 2016 {published data only}
- Pu X, Wang Y. The effect of cognitive behavioral therapy on the coping style of patients with schizophrenia [认知行为治疗对精神分裂症患者应对方式的影响]. Health today 2016;15:87. [Google Scholar]
Puig‐Navarro 2020 {published data only}
- Puig-Navarro O, Badia F, Baeza I, Varela E, Sugranyes G, Garcia-Rizo C, et al. Efficacy of cognitive-behavioral social skills training improving symptoms and functioning in patients with early-onset psychosis: a randomized controlled trial. Schizophrenia Bulletin 2020;46:S39. [Google Scholar]
Reddy 2019 {published data only}
- Reddy F, Glynn S, Green M. Group-augmented motivational interviewing + CBT for negative symptoms. Schizophrenia Bulletin 2019;45:118-9. [Google Scholar]
Rees 2015 {published data only}
- Rees CS, Hasking P, Breen LJ, Lipp OV, Mamotte C. Group mindfulness based cognitive therapy vs group support for self-injury among young people: study protocol for a randomised controlled trial. BMC Psychiatry 2015;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roncone 2004 {published data only}
- Roncone R, Mazza M, Frangou S, Risio A, Ussorio D, Tozzini C, et al. Rehabilitation of theory of mind deficit in schizophrenia: a pilot study of metacognitive strategies in group treatment. Neuropsychological Rehabilitation 2004;14:421-35. [Google Scholar]
Tan 2015 {published data only}
- Tan C, Ye H, Huang M, Zhang Y, Zhong L. The effect of cognitive behavioral intervention on patients with first episode schizophrenia [认知行为干预对首发精神分裂症患者的影响]. Qilu Nursing Journal 2015;21:36-8. [Google Scholar]
Tan 2016 {published data only}
- Tan Y. The effect of cognitive behavioral nursing therapy on the insight of schizophrenia [认知行为护理疗法对精神分裂症自知力的影响]. China Health and Nutrition 2016;1:51. [Google Scholar]
Tang 2007 {published data only}
- Tang X, Zou J, Lu X. Effect of cognitive-behavioral therapy on therapeutic effect of the first-episode schizophrenic patients during recovery period [对首发精神分裂症康复期患者实施认知行为干预的效果观察]. China Journal of Health Psychology 2007;15:588-9. [Google Scholar]
Tao 2016 {published data only}
- Tao Y. The effect of cognitive behavioral therapy on the social function of patients with schizophrenia during rehabilitation [认知行为疗法对康复期精神分裂症患者社会功能的影响]. Modern Chinese Doctor 2016;54:16-9. [Google Scholar]
Wang 2003 {published data only}
- Wang Y. The role of cognitive therapy to improve the understanding of schizophrenia and some capacity [认知治疗改善精神分裂症患者认识及 部分行为能力的作用]. Modern Rehabilitation 2003;7:91. [Google Scholar]
Wang 2004 {published data only}
- Wang H. Controlled study of cognitive behavioral psychological care for schizophrenia [认知行为心理护理对精神分裂症疗效的对照研究]. Chinese Clinical Medicine and Nursing 2004;5:10-1. [Google Scholar]
Wang 2012 {published data only}
- Wang C, An Q. The effect of cognitive behavioral therapy on the compliance behavior of patients with schizophrenia [认知行为治疗对精神分裂症患者遵医行为的影响]. Seek Medical Advice 2012;7:651. [Google Scholar]
Wang 2013 {published data only}
- Wang H, Chen Y, Xie W, Xiao B, Qiu C, Xie S. The effect of cognitive therapy on treatment compliance of outpatients with schizophrenia [认知治疗对门诊精神分裂症患者治疗依从性的影响]. Medical Journal of Chinese People's Health 2013;25:16-8. [Google Scholar]
Wang 2014 {published data only}
- Wang Z, Guo Z, Han G, Wang J, Li Z. The effect of cognitive behavioral therapy on patients with schizophrenia in the community [认知行为治疗对社区精神分裂症患者的疗效]. China Journal of Health Psychology 2015;23:16-9. [Google Scholar]
- Wang Z, Guo Z, Wang J, Yan G, Han G, Li Z. The impact of standardized cognitive behavioral therapy on the quality of life and social function of patients with residual schizophrenia in the community [规范化认知行为治疗对社区残留型精神分裂症患者生活质量和社会功能的影响]. Neurological Diseases and Mental Health 2014;14:495-6. [Google Scholar]
Wu 2008 {published data only}
- Wu N, Wang Q, Kong L. A controlled study of cognitive behaviour therapy in chronic schizophrenia [认知行为治疗慢性精神分裂症对照研究]. Journal of Clinical Psychosomatic Diseases 2008;14:206-7. [Google Scholar]
Wu 2013 {published data only}
- Wu L. Psychological intervention in patients with chronic schizophrenia and negative symptoms [心 理 干 预 在 慢 性 精 神 分 裂 症 阴 性 症 状 患 者]. Medical Journal of Chinese People's Health 2013;25:81-2. [Google Scholar]
Xia 2008 {published data only}
- Xia JX, Gao SN, Qing GX. The effect of cognitive treatment on compliance of patients with schizophrenia [认知治疗对精神分裂症患者服药依从性及复发的影响]. Chinese Journal of Prevention and Control of Chronic Non Communicable Diseases 2008;16:41-2. [Google Scholar]
Xie 2013 {published data only}
- Xie W, Xie Y, Xiao Y, Wang H, Xiao B. Schizophrenia patients multimedia to enhance the effect of cognitive intervention study [精神分裂症患者应用多媒体加强认知干预的效果研究]. Journal of Nursing 2013;20:57-9. [Google Scholar]
Yang 2010 {published data only}
- Yang C. Systemic cognitive intervention for first-episode schizophrenia patients: clinical observation of rehabilitation effect [系统化认知干预对首发精神分裂症患者 康复效果的临床观察]. Hebei Medical Journal 2010;32:417-8. [Google Scholar]
Zha 2015 {published data only}
- Zha W. The effect of cognitive insight therapy on medication compliance of patients with schizophrenia [认知领悟治疗对精神分裂症患者服药依从性的影响]. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 2015;25:26-7. [Google Scholar]
Zhang 1999 {published data only}
- Zhang Z, Yao S. Controlled study on the role of cognitive psychotherapy in the remission of paranoid schizophrenia [认知治疗对偏执型精神分裂症患者]. Chinese Mental Health Journal 1999;13:174-5. [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y. The effect of cognitive behavioral intervention on medication compliance of hospitalized vagrant patients with mental illness during the recovery period [认知行为干预对恢复期住院流浪精神病患者服药依从性的影响]. Medical Journal of Chinese People's Health 2014;26:117-8. [Google Scholar]
Zhang 2015a {published data only}
- Zhang J. The clinical experience of cognitive correction in the treatment of schizophrenia [认知矫正治疗精神分裂症的临床体会]. Modern diagnosis and treatment 2015;26:2054-5. [Google Scholar]
Zhang 2015b {published data only}
- Zhang Y. Effect of cognitive behavior on coping style in patients with schizophrenia [认知 行为治疗对精神分裂症患者应对方式的影响]. World Latest Medicine Information 2015;13:63. [Google Scholar]
Zhang 2015c {published data only}
- Zhang H. Nursing observation on cognitive psychotherapy of psychotic patients in rehabilitation period [康复期精神病患者认知心理治疗的护理观察]. World Latest Medicine Information 2015;15:191-4. [Google Scholar]
Zhao 2014 {published data only}
- Zhao S, Wang J. A comparative study of cognitive behavioral therapy in the rehabilitation of patients with chronic schizophrenia [认知行为治疗对慢性精神分裂症患者康复疗效的对照研究]. Chinese Disability Medicine 2014;22:209-10. [Google Scholar]
References to studies awaiting assessment
Brenner 1987 {published data only}
- Brenner HD, Hodel B, Kube G, Roder V. Cognitive therapy with schizophrenics: analysis of the problem and experimental results. Nervenarzt 1987;58:72-83. [PubMed] [Google Scholar]
Chung 2001 {published data only}
- Chung YC, Kim JH, Eun HB, Hwang IK. Effect of cognitive-behavioral therapy on the delusion in schizophrenic patients. Journal of the Korean Neuropsychiatric Association 2001;40:63-71. [Google Scholar]
Classen 1993 {published data only}
- Classen W, Krajewski C, Boesken S. Cognitive and social processes during art (AT) or cognitive (CT) therapy and a simultaneous CT + AT therapy in schizophrenics. Verhaltenstherapie 1993;Suppl 1:A45. [Google Scholar]
- Classen W, Krajewski C, Boesken S. Comparison of art and cognitive therapy (IPT) with simultaneous cognitive and art therapy for schizophrenic patients regarding the change of cognitive processes. Pharmacopsychiatry 1993;26:171. [Google Scholar]
Klingberg 2001 {published data only}
- Klingberg S, Wiedemann G, Buchkremer G. Cognitive-behavioural therapy for schizophrenic patients – design and preliminary results of a randomised effectiveness trial. Zeitschrift Für Klinische Psychologie Und Psychotherapie 2001;30:259-67. [Google Scholar]
Kraemer 1987 {published data only}
- Kraemer S, Sulz KH, Schmid R, Lassle R. Cognitive therapy of schizophrenic patients: a control study. Nervenarzt 1987;58:84-90. [PubMed] [Google Scholar]
McLeod 2007 {published data only}
- McLeod T, Morris M, Birchwood M, Dovey A. Cognitive behavioural therapy group work with voice hearers. Part 1. British Journal of Nursing 2007;16:248-52. [DOI] [PubMed] [Google Scholar]
- McLeod T, Morris M, Birchwood M, Dovey A. Cognitive behavioural therapy group work with voice hearers. Part 2. British Journal of Nursing 2007;16:292-5. [DOI] [PubMed] [Google Scholar]
Shafiei 2011 {published data only}
- Shafiei Amiri M, Ahmadi Gatab T, Babakhani N. The efficacy of cognitive-behavior group therapy and social skill trainings on reduction of negative symptoms of schizophrenic patients. European Psychiatry 2011;26(Suppl 2):1327. [Google Scholar]
References to ongoing studies
IRCT 20180817040818N {published data only}
- IRCT 20180817040818N. The effect of cognitive-behavioral group training of self-care skills on positive, negative symptoms, general psychopathology and self-care in patients with schizophrenia. en.irct.ir/trial/47632 (first received 25 July 2020). [IRANIAN REGISTRY OF CLINICAL TRIAL: IRCT20180817040818N2]
NCT04144075 {unpublished data only}
- NCT04144075. Application of a mindfulness and self-compassion program in patients with schizophrenia. Randomized controlled trial (ACAMP2020). clinicaltrials.gov/ct2/show/NCT04144075 (first received 30 October 2019). [CLINICALTRIALS.GOV: NCT04144075]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1991
- Andreasen NC, Flaum M, Arndt S, Alliger R, Swayze VW. Positive and negative symptoms: assessment and validity. In: Marneros A, Andreasen NC, Tsuang MT, editors(s). Negative Versus Positive Schizophrenia. Berlin (Germany): Springer, 1991. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Press, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th – revised edition. Washington (DC): American Psychiatric Press, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Washington (DC): American Psychiatric Press, 2013. [Google Scholar]
Barraclough 2006
- Barrowclough C, Haddock G, Lobban F, Jones S, Siddle R, Roberts C, et al. Group cognitive-behavioural therapy for schizophrenia: randomised controlled trial. British Journal of Psychiatry 2006;189:527-32. [DOI] [PubMed] [Google Scholar]
Beck 1979
- Beck AT, Rush A, Shaw B, Emery G. Cognitive Therapy of Depression. New York (NY): Guilford Press, 1979. [Google Scholar]
Beck 1990
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio (TX): Psychological Corporation, 1990. [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio (TX): The Psychological Corporation, 1996. [Google Scholar]
Bighelli 2018
- Bighelli I, Salanti G, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry 2018;17:316-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birchwood 1990
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry 1990;157:853-9. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. Comparison of the indices and their use. Therapie 1999;54:405-11. [PubMed] [Google Scholar]
Burlingame 2020
- Burlingame GM, Svien H, Hoppe L, Hunt I, Rosendahl J. Group therapy for schizophrenia: a meta-analysis. Psychotherapy (Chicago, Ill.) 2020;57:219-36. [DOI] [PubMed] [Google Scholar]
Butler 2006
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioural therapy: a review of meta-analyses. Clinical Psychology Review 2006;26:17-31. [DOI] [PubMed] [Google Scholar]
Charlson 2018
- Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophrenia Bulletin 2018;44:1195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 2 March 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2000
- Deeks J. Issues in the selection for meta-analyses of binary data. In: Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25-28; Cape Town, South Africa. 2000.
Derogatis 1992
- Derogatis LR. SCL-90-R, Administration, Scoring and Procedures Manual-II for the R(evised) Version and Other Instruments of the Psychopathology Rating Scale Series. Townson (MD): Clinical Psychometric Research, 1992. [Google Scholar]
Dimeff 2007
- Dimeff LA, Koerner K. Dialectical Behavior Therapy in Clinical Practice: Applications Across Disorders and Settings. New York (NY): Guilford Press, 2007. [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623-9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971-80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichner 2016
- Eichner C, Berna F. Acceptance and efficacy of metacognitive training (MCT) on positive symptoms and delusions in patients with schizophrenia: a meta-analysis taking into account important moderators. Schizophrenia Bulletin 2016;42:952-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI] [PubMed] [Google Scholar]
Fordham 2021
- Fordham B, Sugavanam T, Edwards K, Hemming K, Howick J, Copsey B, et al. Cognitive-behavioural therapy for a variety of conditions: an overview of systematic reviews and panoramic meta-analysis. Health Technology Assessment 2021;25:1-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7-10. [DOI] [PubMed] [Google Scholar]
Gledhill 1998
- Gledhill A, Lobban F, Sellwood W. Group CBT for people with schizophrenia: a preliminary evaluation. Behavioural and Cognitive Psychotherapy 1998;26:63-76. [Google Scholar]
GRADE Profiler [Computer program]
- GRADE Profiler. Version 3.2. GRADE Working Group, 2004.
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876-83. [DOI] [PubMed] [Google Scholar]
Haddock 1999
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychological Medicine 1999;29:879-89. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50-5. [DOI] [PubMed] [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social Psychology 1967;6:278-96. [DOI] [PubMed] [Google Scholar]
Hazell 2016
- Hazell CM, Hayward M, Cavanagh K, Strauss C. A systematic review and meta-analysis of low intensity CBT for psychosis. Clinical Psychology Review 2016;45:183-92. [DOI] [PubMed] [Google Scholar]
Heinrichs 1984
- Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 1984;10:388-98. [DOI] [PubMed] [Google Scholar]
Hesdon 2004
- Hesdon B. Seeking Consensus on Adverse Effects on Cognitive Behaviour Therapy for Schizophrenia. Leeds (UK): University of Leeds, 2004. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hoffman 2003
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Archives of General Psychiatry 2003;60:49-56. [DOI] [PubMed] [Google Scholar]
Jauhar 2022
- Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet 2022;399:473-86. [DOI] [PubMed] [Google Scholar]
Jones 2004
- Jones C, Cormac I, Silveira da Mota Neto JI, Campbell C. Cognitive behaviour therapy for schizophrenia. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD000524. [DOI: 10.1002/14651858.CD000524.pub2] [DOI] [PubMed] [Google Scholar]
Jones 2010
- Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behaviour therapy versus psychosocial treatments for schizophrenia. Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No: CD008712. [DOI: 10.1002/14651858.CD008712] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2012
- Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD008712. [DOI: 10.1002/14651858.CD008712.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2018
- Jones C, Hacker D, Xia J, Meaden A, Irving CB, Zhao S, et al. Cognitive behavioural therapy plus standard care versus standard care for people with schizophrenia. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD007964. [DOI: 10.1002/14651858.CD007964.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi-Health Systems, 1986. [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The Positive And Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261-76. [DOI] [PubMed] [Google Scholar]
Kingdon 1998
- Kingdon D, Turkington D. Cognitive behaviour therapy of schizophrenia. In: Wykes T, Tarrier N, Lewis S, editors(s). Outcome and Innovation in Psychological Treatment of Schizophrenia. Bridgewater (NJ): Wiley, 1998. [Google Scholar]
Kingdon 2005
- Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. New York (NY): Guilford Press, 2005. [Google Scholar]
Kopelovich 2019
- Kopelovich SL, Strachan E, Sivec H, Kreider V. Stepped care as an implementation and service delivery model for cognitive behavioral therapy for psychosis. Community Mental Health Journal 2019;55:755-67. [DOI] [PubMed] [Google Scholar]
Kurtz 2016
- Kurtz MM, Gagen E, Rocha NB, Machado S, Penn DL. Comprehensive treatments for social cognitive deficits in schizophrenia: a critical review and effect-size analysis of controlled studies. Clinical Psychology Review 2016;43:80-9. [DOI] [PubMed] [Google Scholar]
Lawrence 2006
- Lawrence R, Bradshaw T. Group cognitive behavioral therapy for schizophrenia: a systematic review of the literature. Journal of Psychiatric and Mental Health Nursing 2006;13:673-81. [DOI] [PubMed] [Google Scholar]
Laws 2018
- Laws KR, Darlington N, Kondel TK, McKenna PJ, Jauhar S. Cognitive behavioural therapy for schizophrenia – outcomes for functioning, distress and quality of life: a meta-analysis. BMC Psychology 2018;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lecomte 2016
- Lecomte T, Leclerc C, Wykes T. Group CBT for psychosis. Oxford (UK): Oxford University Press, 2016. [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley and Sons, 2019:67-107. [DOI: 10.1002/9781119536604.ch4] [DOI] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366-71. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean? Schizophrenia Research 2005;79:231-8. [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF? Schizophrenia Bulletin 2007;33:183-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 1994
- Li G, Hu X, Jin D, Tian W, Phillips M. A rating instrument for the evaluation of in-patient rehabilitation programmes in China: results of reliability and validity testing. British Journal of Psychiatry 1994;165:58-65. [PubMed] [Google Scholar]
Liu 2005
- Liu Z, Gao B, Yuan S. The rating scale of social ability: development, and testing of reliability and validity. Chinese Journal of Clinical Psychology 2005;1:19-22. [Google Scholar]
Liu 2018
- Liu YC, Tang CC, Hung TT, Tsai PC, Lin MF. The efficacy of metacognitive training for delusions in patients with schizophrenia: a meta-analysis of randomized controlled trials informs evidence-based practice. Worldviews Evidence Based Nursing 2018;15:130-9. [DOI] [PubMed] [Google Scholar]
Lu 2009
- Lu W, Cui Y, Chen X, Zheng Y, Gu K, Cai H, et al. Changes in quality of life among breast cancer patients three years post-diagnosis. Breast Cancer Research and Therapy 2009;114:357-69. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams CE, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
McCutcheon 2020
- McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia – an overview. JAMA Psychiatry 2020;77:201-10. [DOI] [PubMed] [Google Scholar]
Mc Glanaghy 2021
- Mc Glanaghy E, Turner D, Davis GA, Sharpe H, Dougall N, Morris P, et al. A network meta-analysis of psychological interventions for schizophrenia and psychosis: impact on symptoms. Schizophrenia Research 2021;228:447-59. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Medical Research Methodology 2001;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [DOI] [PubMed] [Google Scholar]
Moritz 2007
- Moritz S, Woodward TS. Metacognitive training in schizophrenia: from basic research to knowledge translation and intervention. Current Opinion in Psychiatry 2007;20:619-25. [DOI] [PubMed] [Google Scholar]
Morosini 2000
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatrica Scandinavica 2000;101:323-9. [PubMed] [Google Scholar]
Morrison 2010
- Morrison AP, Barratt S. What are the components of CBT for psychosis? A Delphi study. Schizophrenia Bulletin 2010;1:136-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueser 2004
- Mueser KT, McGurk SR. Schizophrenia. Lancet 2004;362:2063-72. [DOI] [PubMed] [Google Scholar]
Muñoz 1993
- Muñoz RF, Miranda J . Group therapy manual for cognitive-behavioral treatment of depression. Revised. Unpublished manual, San Francisco General Hospital, Depression Oink, San Francisco 1993.
NICE 2014
- National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Adults: Treatment and Management. London (UK): National Institute for Health and Care Excellence, 2014. [Google Scholar]
Otto 2004
- Otto MW, Smits JA, Reese HE. Cognitive-behavioural therapy for the treatment of anxiety disorders. Journal of Clinical Psychiatry 2004;65:34-41. [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799-812. [Google Scholar]
Pinto 2008
- Pinto A, Turkington G, Kingdon D. Cognitive behaviour therapy for psychosis: enhancing the therapeutic relationship to improve the quality of life. In: Cognitive Behaviour Therapy: a Guide for the Practising Clinician, Volume 2. London (UK): Routledge, 2008. [Google Scholar]
Pukrop 2000
- Pukrop R, Moller HJ, Steinmeyer EM. Quality of life in psychiatry. A systematic contribution to construct validation and the development of the integrative assessment tool modular system of quality of life. European Archives of Psychiatry and Clinical Neuroscience 2000;250:120-32. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2021
- Roberts MT, Shokraneh F, Sun Y, Groom M, Adams CE. Classification of psychotherapy interventions for people with schizophrenia: development of the Nottingham Classification of Psychotherapies. Evidence-Based Mental Health 2021;24:62-9. [DOI: 10.1136/ebmental-2020-300151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available from handbook: training.cochrane.org/handbook/archive/v6.2.
Shokraneh 2017
- Shokraneh F, Adams CE. Study-based registers of randomized controlled trials: starting a systematic review with data extraction or meta-analysis. BioImpacts 2017;7(4):209-17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2019
- Shokraneh F, Adams CE. Study-based registers reduce waste in systematic reviewing: discussion and case report. Systematic Reviews 2019;8:129. [DOI: 10.1186/s13643-019-1035-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2020
- Shokraneh F, Adams CE. Cochrane schizophrenia group’s study-based register of randomized controlled trials: development and content analysis. Schizophrenia Bulletin Open 2020;1:sgaa061. [DOI: 10.1093/schizbullopen/sgaa061] [DOI] [Google Scholar]
Shokraneh 2021
- Shokraneh F, Adams CE. Classification of all pharmacological interventions tested in trials relevant to people with schizophrenia: a study-based analysis. Health Information and Libraries Journal 2021 Feb 18 [Epub ahead of print]. [DOI: 10.1111/hir.12366] [DOI] [PubMed] [Google Scholar]
Sitko 2020
- Sitko K, Bewick BM, Owens D, Masterson C. Meta-analysis and meta-regression of cognitive behavioral therapy for psychosis (CBTp) across time: the effectiveness of CBTp has improved for delusions. Schizophrenia Bulletin Open 2020;1:sgaa023. [Google Scholar]
Spencer 2020
- Spencer HM, Dudley R, Freeston MH, Turkington D. What are the essential ingredients of a CBT case conceptualization for voices and delusions in schizophrenia spectrum disorders? A study of expert consensus. Schizophrenia Research 2020;224:74-81. [DOI] [PubMed] [Google Scholar]
Stuart 1998
- Stuart GW. Cognitive behavioural therapy. In: Stuart GW, Sundeen SJ, editors(s). Principles and Practice of Psychiatric Nursing. 6th edition. London (UK): Mosby, 1998. [Google Scholar]
Tandon 2008
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophrenia Research 2008;102:1-18. [DOI] [PubMed] [Google Scholar]
Tarrier 1993
- Tarrier N, Beckett R, Harwood S, Baker A, Yusupoff L, Ugarteburu I. A trial of two cognitive-behavioural methods of treating drug-resistant residual psychotic symptoms in schizophrenic patients: I. Outcome. British Journal of Psychiatry 1993;162:524-32. [DOI] [PubMed] [Google Scholar]
Turkington 2006
- Turkington D, Dudley R, Warman DM, Beck AT. Cognitive-behavioural therapy for schizophrenia: a review. Journal of Lifelong Learning in Psychiatry 2006;2:223-33. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1-75. [PubMed] [Google Scholar]
van Oosterhout 2016
- Oosterhout B, Smit F, Krabbendam L, Castelein S, Staring AB, Gaag M. Metacognitive training for schizophrenia spectrum patients: a meta-analysis on outcome studies. Psychological Medicine 2016;46:47-57. [DOI] [PubMed] [Google Scholar]
van Os 2009
- Os J, Kapur S. Schizophrenia. Lancet 2009;374:635-45. [DOI] [PubMed] [Google Scholar]
Wallace 2000
- Wallace CJ, Liberman RP, Tauber R, Wallace J. The Independent Living Skills Survey: a comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophrenia Bulletin 2000;26:631-58. [DOI] [PubMed] [Google Scholar]
Waller 2011
- Waller H, Freeman D, Jolley S, Dunn G, Garety P. Targeting reasoning biases in delusions: a pilot study of the Maudsley Review Training Programme for individuals with persistent, high conviction delusions. Journal of Behavior Therapy and Experimental Psychiatry 2011;42:414-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waller 2015
- Waller H, Emsley R, Freeman D, Bebbington P, Dunn G, Fowler D, et al. Thinking Well: a randomised controlled feasibility study of a new CBT therapy targeting reasoning biases in people with distressing persecutory delusional beliefs. Journal of Behavior Therapy and Experimental Psychiatry 2015;48:82-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 1969
- Watson D, Friend R. Measurement of social-evaluative anxiety. Journal of Consulting and Clinical Psychology 1969;33:448-57. [DOI] [PubMed] [Google Scholar]
WHOQOL Group 1994
- The WHOQOL Group. The development of the World Health Organization Quality of Life Assessment Instrument (the WHOQOL). In: Orley J, Kuyken W, editors(s). Quality of Life Assessment: International Perspectives. Berlin (Germany): Springer, 1994. [Google Scholar]
WHOQOL Group 1996
- The WHQOL Group. WHOQOL-BREF. Introduction, administration, scoring and generic version of the assessment: field trial version. apps.who.int/iris/handle/10665/63529 (accessed prior to 7 June 2022).
Williams 2002
- Williams CJ, Garland E. A cognitive-behavioural therapy assessment model for use in everyday clinical practice. Advances in Psychiatric Treatment 2002;8:172-9. [Google Scholar]
Wykes 1986
- Wykes T, Sturt E. The measurement of social behaviour in psychiatric patients: an assessment of the reliability and validity of the SBS schedule. British Journal of Psychiatry 1986;148:1-11. [DOI] [PubMed] [Google Scholar]
Wykes 1999
- Wykes T, Parr AM, Landau S. Group treatment of auditory hallucinations: exploratory study of effectiveness. British Journal of Psychiatry 1999;175:180-5. [DOI] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254-7. [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Guaiana 2012
- Guaiana G, Morelli AC, Chiodo D. Cognitive behaviour therapy (group) for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD009608. [DOI: 10.1002/14651858.CD009608] [DOI] [PMC free article] [PubMed] [Google Scholar]


