Abstract
STUDY QUESTION
Are there differences in thyroid function between adolescents and young adults conceived with and without ART?
SUMMARY ANSWER
This study demonstrated no evidence of clinically relevant differences in thyroid function between adolescents and young adults conceived with and without ART.
WHAT IS KNOWN ALREADY
Studies to date have reported an increase in subclinical hypothyroidism in offspring conceived after ART. It has been suggested that the increase in maternal estrogen (E2) after fresh embryo transfers could affect thyroid function of the offspring. Suboptimal thyroid function at a young age can cause irreversible damage to the central nervous system, which makes early detection and correct treatment essential.
STUDY DESIGN, SIZE, DURATION
The Growing Up Healthy Study (GUHS) is a prospective cohort study, which aimed to recruit all adolescents born after conception with ART between 1991 and 2001 in the study area. The included participants (n = 303, aged 13–20 years) completed various health assessments. Depending on the age at enrolment, participants completed thyroid assessments at the 14- or 20-year follow-up. The outcomes of these replicated thyroid assessments were compared to those of participants conceived without ART from the Raine Study Generation 2 (Gen2). The Gen2 participants (n = 2868) were born between 1989 and 1992 and have been recognized to be representative of the local population.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Thyroid function assessments were compared between n = 134 GUHS and n = 1359 Gen2 adolescents at age 14 years and between n = 47 GUHS and n = 914 Gen2 young adults at age 20 years. The following mean thyroid hormone concentrations were compared between the cohorts: thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), free thyroxine (fT4) and thyroid peroxidase antibodies (TPOAb). The prevalence of the following thyroid hormone profiles, based on individual thyroid hormone concentrations, was compared: euthyroidism, subclinical and overt hypo- and hyperthyroidism and thyroid autoimmunity. Outcomes were compared between the cohorts, and univariately between fresh embryo transfers (ET) and frozen ET (FET) within the GUHS. The correlation between maternal peak E2 concentrations (pE2) and fT4 was assessed within the GUHS.
MAIN RESULTS AND THE ROLE OF CHANCE
All mean thyroid function outcomes fell within the normal range. At both ages, we report no differences in TSH concentrations. At age 14 years, lower fT3 concentrations (4.80 versus 5.35 pmol/L, P < 0.001) and higher fT4 concentrations (12.76 versus 12.19 pmol/L, P < 0.001) were detected in the GUHS adolescents compared to Gen2 adolescents. At age 20 years, higher fT3 and fT4 concentrations were reported in GUHS adolescents (4.91 versus 4.63 pmol/L, P = 0.012; 13.43 versus 12.45 pmol/L, P < 0.001, respectively) compared to Gen2 participants. No differences in the prevalence of subclinical and overt hypo- and hyperthyroidism or thyroid autoimmunity were demonstrated between the cohorts at age 14 and 20 years. Thyroid function did not differ between ET and FET, and no correlation between pE2 and fT4 was reported.
LIMITATIONS, REASONS FOR CAUTION
The observational nature of the study limits the ability to prove causation. Furthermore, the comparison of ET and FET offspring at age 20 years may be lacking power. We were unable to differentiate between different types of ART (e.g. IVF versus ICSI) owing to the low number of ICSI cycles at the time of study. As ART laboratory and clinic data were collected contemporaneously with the time of treatment, no other data pertaining to the ART cycles were sought retrospectively; hence, some factors could not be accounted for.
WIDER IMPLICATIONS OF THE FINDINGS
This study does not support previous findings of clinically relevant differences in thyroid function when comparing a cohort of adolescents conceived after ART to counterparts conceived without ART. The minor differences detected in fT3 and fT4 were considered not biologically relevant. Although these findings appear reassuring, they warrant reinvestigation in adulthood.
STUDY FUNDING/COMPETING INTERESTS
This project was funded by an NHMRC Grant (Hart et al., ID 1042269). R.J.H. is the Medical Director of Fertility Specialists of Western Australia and a shareholder in Western IVF. He has received educational sponsorship from MSD, Merck-Serono and Ferring Pharmaceuticals. P.B. is the Scientific Director of Concept Fertility Centre, Subiaco, Western Australia. J.L.Y. is the Medical Director and a shareholder of PIVET Medical Centre, Perth, Western Australia.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: ART, IVF, ICSI, thyroid function, long-term outcomes, ART outcomes, adolescents, young adults
Introduction
ART, such as IVF and ICSI, have been used since 1978 to assist infertile couples to conceive. In Australia, currently, 1 in 20 children is born after assisted conception, while worldwide over 8 million children have been born after ART (Fauser, 2019; Newman et al., 2020). Short-term health outcomes of offspring conceived after ART have been studied extensively and indicate an increased risk of preterm birth (Declercq et al., 2015), low birthweight (LBW) (Schieve et al., 2002), congenital malformations (Pandey et al., 2012; Qin et al., 2015) and imprinting disorders (Vermeiden and Bernardus, 2013). Increasingly, studies are now investigating longer term health outcomes in adolescents conceived after ART. One of the outcomes of particular interest is thyroid function, as this may be influenced by the large fluctuations in maternal estrogen (E2) concentrations around conception. Studies investigating thyroid function in offspring conceived after ART are relatively limited. However, all studies to date report evidence of differences in thyroid function between offspring conceived with and without ART. Studies have reported an increase in subclinical hypothyroidism (SCH) (Sakka et al., 2009; Onal et al., 2012), and an increase in free triiodothyronine (fT3) (Gkourogianni et al., 2014), free thyroxine (fT4) and thyroid-stimulating hormone (TSH) concentrations in ART-conceived offspring (Lv et al., 2014). It has been suggested that an increase in maternal E2 after fresh embryo transfers (ET) could affect thyroid function of the offspring through direct effects on the thyrocytes (thyroid epithelial cells) and through epigenetic alterations (Manole et al., 2001; Banu et al., 2002; Ho et al., 2006). A Greek study, suggested that the reported increase in TSH in ART-conceived children aged 4–14 years may be related to an epigenetic alteration in the set point of TSH, resulting in mild TSH resistance (Sakka et al., 2009). This is in line with previous studies showing that ART may induce epigenetic alterations around the susceptible window of conception, which can lead to the development of various disorders, including thyroid dysfunction, later in life (Fleming et al., 2018).
Thyroid dysfunction at a young age can cause irreversible damage to the central nervous system, with subsequent mental impairment. Quality of life, anxiety, symptoms of depression, cognitive function and memory can be altered in patients with SCH, although studies are conflicting (Baldini et al., 1997; Canaris et al., 2000; Jorde et al., 2006; Cooper and Biondi, 2012). In addition, changes in thyroid function can alter metabolic parameters such as lipid profiles, which, if left untreated, can lead to cardiovascular disease later in life (Duntas, 2002; Rodondi et al., 2010; Delitala et al., 2017). As most of these unfavourable effects appear to be reversible with the levothyroxine replacement therapy, early detection of thyroid dysfunction is essential for correct treatment (Serter et al., 2004; Razvi et al., 2007). If there is a significant association between ART conception and thyroid dysfunction, this opens possibilities for routine screening and subsequent treatment for ART-conceived offspring.
The aim of this study was to compare the thyroid function of a cohort of adolescents and young adults conceived after ART to that of a well established and representative cohort of similar age and sex, conceived without ART.
Materials and methods
Thyroid function was compared between a total of 181 adolescents conceived after ART (n = 134 at age 14 and n = 47 at age 20 years) and 1359 adolescents conceived without ART (n = 1359 at age 14 and n = 914 at age 20 years).
Study populations
ART cohort
The Growing Up Healthy Study (GUHS) is a prospective cohort study investigating the long-term health of adolescents and young adults conceived after ART. The study aimed to recruit all adolescents conceived after ART in the two fertility clinics operating at the time (1991–2001): PIVET Medical Centre and Concept Fertility Centre, both in Perth, Western Australia, Australia. Four hundred and four families were recruited through the fertility clinics and a final number of 303 eligible adolescents participated in the study. Included adolescents completed various health assessments at ages 14, 17 and 20 years, which included thyroid function assessments at ages 14 and 20 years. Assessments were conducted between 2013 and 2017, at which time point n = 152 were 14 years of age and n = 66 were 20 years of age. Of these, n = 134 and n = 60 underwent thyroid function assessments. Thirteen offspring in the 20-year follow-up were conceived through gamete intra-fallopian transfer (GIFT) and were respectively excluded from the thyroid function analyses. This resulted in thyroid function assessment results of n = 134 at age 14 years, and n = 47 at age 20 years, used in this study. Numbers at the 20-year follow-up are lower owing to the infrequent use of ART in the early 1990s. The full recruitment process can be viewed in Fig. 1. Included participants were conceived through IVF (n = 125) and ICSI (n = 40), of whom n = 100 were fresh ETs and n = 65 frozen ETs (FET). Sixteen participants had a confirmed ART status but unconfirmed type of ART. owing to age ineligibility, none of the included GUHS participants completed thyroid assessments at both follow-ups. GUHS participants replicated assessments previously completed by adolescents of similar age and sex, conceived without ART, from the Raine Study. Outcomes of these health assessments were then compared between the cohorts.
Figure 1.
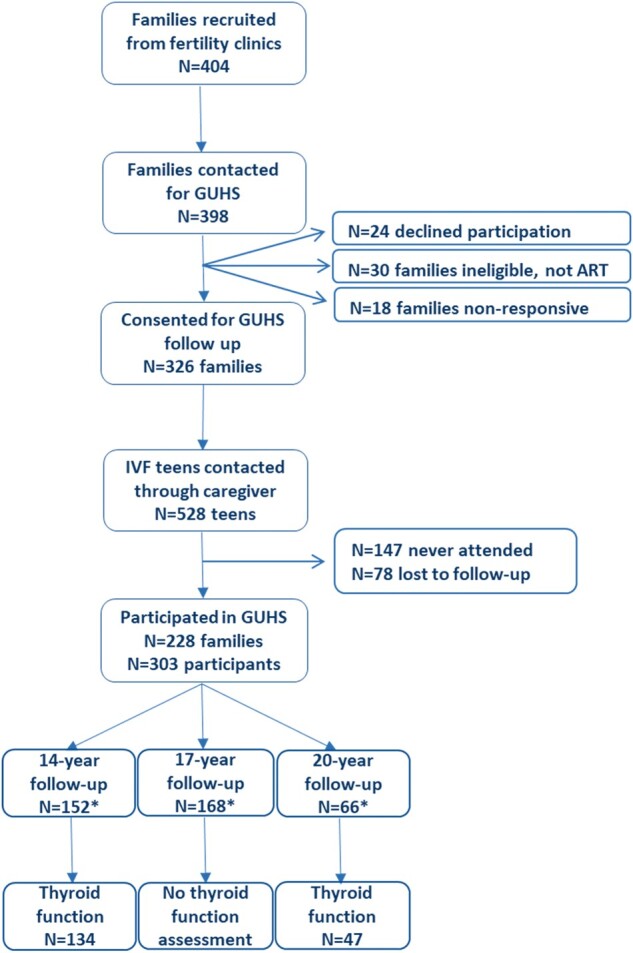
Flowchart of study recruitment process of the Growing Up Healthy Study. *Numbers do not total N = 303, as some participants completed >1 follow-up (depending on age at enrolment).
Non-ART cohort
The Raine Study is a cohort study, which recruited 2900 pregnant women in Western Australia between 1989 and 1991 to investigate the safety and effects of ultrasound on the foetus (https://www.rainestudy.org.au) (Newnham et al., 1991). A total of 2868 children born to 2804 mothers formed Raine Study Generation 2 (Gen2). The Gen2 participants have completed assessments at various follow-ups throughout their lives, to investigate the effect of perinatal health on subsequent childhood and adult health (Straker et al., 2017). Assessments were completed at birth, at ages 1, 2, 3, 5, 8 and 10 years and then at ages 14, 17, 18, 20, 22, 27 and 28 years. The cohort (aged 30–31 years) has a current follow-up rate of 70%, including 1800 participants. The Raine study adolescents are representative of the Western Australian adolescent population at the time of assessments (Robinson et al., 2010). The 14- and 20-year follow-ups, including thyroid function assessments, were completed by 1369 Gen2 participants (in 2003–2005 and 2009–2011, respectively). Overall, results from n = 1359 Gen2 participants conceived without ART (age 14 years n = 1359, and age 20 years n = 914) were included in this study, after the exclusion of five participants conceived after IVF and five participants conceived after GIFT.
Data collection
Laboratory and thyroid function assessments
At both follow-ups, fasting blood samples for biochemical analyses were taken and plasma was frozen until TSH, fT3, fT4 and thyroid peroxidase antibodies (TPOAb) concentrations were measured by automated immunoassay using Abbott ARCHITECT analyser (Abbott Diagnostic, IL, USA). Mean TSH, fT3, fT4 and TPOAb concentrations were compared between the cohorts. Reference ranges, derived by local consensus and based on local population-based studies and laboratory data (O'Leary et al., 2006; Panicker et al., 2010), are: TSH 0.4–4.0 mU/L, fT3 3.0–6.5 pmol/L (age 11–14 years), 3.0–5.0 pmol/L (age 15–18 years), 3.0–5.5 pmol/L (age ≥19 years) and fT4 9–19 pmol/L. TPOAb positivity is defined as TPOAb >6 kU/mL (according to the manufacturer’s recommendation) at all ages. Thyroid hormone profiles were compared based on individual concentrations of thyroid function assessments: euthyroidism (TSH: 0.4–4.0 mU/L, fT4: 9–19 pmol/L, fT3 age 14 years: 3–6.5 pmol/L, fT3 age 19–20 years: 3–5.5 pmol/L), subclinical hypothyroidism (↑TSH, ↔fT4), hypothyroidism (↑TSH, ↓fT4), subclinical hyperthyroidism (↓TSH, ↔fT4), hyperthyroidism (↓TSH, ↑fT4), and autoimmune thyroid disorder (TPOAb >6 kU/L). Participants were classified as having autoimmune thyroid disorder if they had positive antibodies (TPOAb >6 kU/L) and did not fall into another category. The effect of maternal peak circulating peri-ovulatory estrogen (pE2) was examined by comparing thyroid function assessments of ETs and FETs separately to Gen2 and to each other within the GUHS. It was further examined whether there was a correlation between maternal pE2 and fT4 within the GUHS cohort (n = 160).
Anthropometric assessments
At both follow-ups, standing height was measured using a stadiometer (to the nearest 0.1 cm) and weight was measured seated, with participants lightly clothed, using an electronic scale (to the nearest 0.1 kg). BMI was calculated (kg/m2).
Questionnaires
Detailed questionnaires containing health and demographic information were used for information regarding smoking and use of medications in the past 6 months. At age 14 years, questionnaires were completed by the participant and the primary caregiver, and at age 20 years, the questionnaires were completed by the participant. Furthermore, for Gen2 participants information regarding the type of conception and pregnancy outcomes [i.e. gestational age (GA), birthweight (BW) and multiplicity] was collected via questionnaires.
Fertility clinic records
For all GUHS participants, information regarding cause of infertility, previous IVF cycles, IVF cycle of index pregnancy, medication scheme, day of ET, donor use, maternal peak circulating pE2 (in pmol/L) and pregnancy outcomes, was collected from the clinical records at PIVET Medical Centre and Concept Fertility Centre.
Midwives notification system
Additional information regarding pregnancy outcomes for GUHS pregnancies was collected from the Midwives Notification System from the Western Australian Department of Health. This was primarily used in the case of missing information from fertility clinics, as these clinics did not always hold information regarding pregnancy outcomes.
Ethical approval
The study received ethical approval for both contacting the participants as well as collection of data from the University of Western Australia (RA/4/1/5860) and from the Human Research Ethics Committee of the Western Australian Department of Health (project number 2013/25). Informed and written consent for the study and for every follow-up was obtained from the parents or guardians for participants who were underage, or from the participants themselves if they were over the age of 18 years.
Statistical analysis
Differences in demographic characteristics between groups were evaluated by Student’s t-test, Mann–Whitney U test, Chi2 test or Fisher’s exact test. For continuous outcomes univariate cohort, differences were examined using Mann–Whitney U tests and for categorical outcomes Chi2 and Fisher’s exact tests were used. Adjusted analyses were performed using generalized estimating equations that modelled siblings as a random factor. In addition to adjustment for sex, current age and BMI (Gkourogianni et al., 2014; Lv et al., 2014), further covariates were added to account for cohort differences that may also influence thyroid function (i.e. non-singleton, BW z-score, GA and use of anticonvulsants). Outcomes at both ages were adjusted for: non-singleton, BW z-score, GA, sex and age of participant. At age 20 years, analyses were additionally adjusted for BMI and use of anticonvulsants in the past 6 months, owing to differences between the cohorts on these covariates at age 20 years. Log transformations of thyroid hormone concentrations were conducted when normality assumption was not met, and the estimated back-transformed means and their 95%CI were reported. Effects of ART in the categorical models were summarised using adjusted odds ratios and their 95% CI. Stratifying analyses by sex did not alter the results, and to maintain statistical power, analyses for both sexes were combined. Univariate subgroup analyses were performed by comparing outcomes between ET and FET (age 14 years: n = 70 versus 53; age 20 years: n = 30 versus 12), and by comparing ET and FET separately to Gen2 participants. Spearman correlation was used to assess an association between pE2 and fT4 (n = 160). All analyses were performed in IBM SPSS Statistics version 25.0 (IBM Corp, Armonk, NY, USA). P < 0.05 were considered significant.
Results
Demographics
Table I gives an overview of demographic characteristics at ages 14 and 20 years. The GUHS cohort demonstrated a significantly lower mean birthweight (age 14 years: P < 0.001; age 20 years: P = 0.042), lower GA (age 14 and 20 years: P < 0.001), as well as significantly more multiple births at both ages (age 14 years: P < 0.001; age 20 years: P = 0.029) than the Gen2 participants. The GUHS cohort was significantly older at both follow-ups (age 14 years and age 20 years: P < 0.001). At age 20 years, GUHS adolescents had a significantly lower BMI (P = 0.017) and an increased use of anticonvulsants in the past 6 months, compared to Gen2 (P = 0.001). Smoking and use of other medication did not differ between the cohorts at age 20 years. BMI and the use of medication did not differ at age 14 years. Sex distribution did not differ statistically at either age, although apparently fewer GUHS males participated at age 20 years.
Table I.
Demographic characteristics for the ART and non-ART cohorts at ages 14 and 20 years.
| Demographics | Age 14 years |
P-value | Age 20 years |
P-value | ||
|---|---|---|---|---|---|---|
| *ART | **Non-ART | *ART | **Non-ART | |||
| (N = 134) | (N = 1359) | (N = 47) | (N = 914) | |||
| Age (years) | 14.9 (14.3 to 15.6) | 14.1 (14.0 to 14.2) | <0.001 | 20.4 (20.1 to 20.9) | 19.9 (19.7 to 20.3) | <0.001 |
| [13.1 to 16.9] | [13.0 to 15.1] | [18.0 to 23.0] | [19.1 to 22.1] | |||
| Sex | ||||||
| Male | 68 (50.7%) | 705 (51.9%) | 0.796 | 19 (40.4%) | 487 (53.3%) | 0.085 |
| Birthweight (g) | 3175 (2794 to 3616) | 3375 (3020 to 3678) | 0.001 | 3120 (2829 to 3555) | 3375 (3033 to 3660) | 0.042 |
| [970 to 4470] | [760 to 5550] | [1580 to 4360] | [845 to 5185] | |||
| Birthweight (z-score) | −0.098 (−0.825 to 0.541) | −.076 (−0.527 to 0.590) | 0.181 | −0.256 (−0.634 to 0.430) | 0.036 (−0.515 to 0.550) | 0.176 |
| [−3.057 to 8.392] | [−2.454 to 3.741] | [−1.618 to 2.046] | [−2.294 to 3.396] | |||
| Birthweight (g) | ||||||
| <2500 | 20 (14.9%) | 115 (8.5%) | 0.034 | 7 (15.9%) | 69 (7.6%) | 0.133 |
| 2500–3999 | 99 (73.9%) | 1109 (81.7%) | 33 (75.0%) | 761 (83.4%) | ||
| >4000 | 15 (11.2%) | 133 (9.8%) | 4 (9.1%) | 83 (9.1%) | ||
| Birth length (cm) | 49 (47 to 51) | 49 (48 to 51) | 0.709 | 49 (48 to 51) | 49 (48 to 51) | 0.681 |
| [34 to 56] | [33 to 57] | [41 to 53] | [35 to 57] | |||
| Gestational age (weeks) | 38.4 (37.4 to 39.4) | 39.7 (38.3 to 40.6) | <0.001 | 38.7 (37.5 to 39.8) | 39.4 (38.3 to 40.6) | <0.001 |
| [26.9 to 42.0] | [24.6 to 42.9] | [32.4 to 41.0] | [24.6 to 42.9] | |||
| Gestational age (weeks) | ||||||
| <37 weeks | 28 (22.8%) | 139 (10.3%) | <0.001 | 9 (21.4%) | 92 (10.1%) | 0.048 |
| 37–42 weeks | 95 (77.2%) | 1192 (87.9%) | 33 (78.6%) | 804 (88.1%) | ||
| >42 weeks | 0 (0.0%) | 25 (1.8%) | 0 (0.0%) | 17 (1.9%) | ||
| Plurality | ||||||
| Singleton | 97 (72.4%) | 1299 (95.7%) | < 0.001 | 41 (87.2%) | 873 (95.5%) | 0.029 |
| Twin | 34 (25.4%) | 56 (4.1%) | 6 (12.8%) | 38 (4.2%) | ||
| Triplet | 3 (2.2%) | 3 (0.2%) | 0 (0.0%) | 3 (0.3%) | ||
| BMI (kg/m2) | 20.3 (18.5 to 22.7) | 20.4 (18.6 to 23.2) | 0.451 | 21.6 (19.7 to 25.2) | 23.3 (21.3 to 26.2) | 0.017 |
| [14.7 to 33.3] | [14.0 to 43.8] | [18.0 to 34.9] | [15.5 to 51.7] | |||
| Use of anticonvulsants in past 6 months | 1 (0.7%) | 8 (0.6%) | 0.469 | 4 (8.5%) | 6 (0.7%) | 0.001 |
| Use of oral steroids in past 6 months | 0 (0%) | 4 (0.3%) | 1.000 | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Use of OCP females in past 6 months | 2 (3.1%) | 16 (2.5%) | 0.679 | 7 (28.0%) | 210 (47.3%) | 0.060 |
| Smoking | ||||||
| Yes | – | – | 5 (10.6%) | 109 (11.9%) | 0.435 | |
OCP, oral contraceptive pill. Data are presented as median (25th–75th percentile) [range] and as N (%). Differences in demographic characteristics between groups were evaluated by Student’s t-test, Mann–Whitney U test, Chi2 test or Fisher’s exact test. Bold text indicates statistical significance.
The Growing Up Healthy Study is a prospective cohort study, which aimed to recruit all adolescents born after conception with ART between 1991 and 2001 in the study area.
Participants conceived without ART from the Raine Study Generation 2 (Gen2). The Gen2 participants were born between 1989 and 1992 and have been recognised to be representative of the local population.
Thyroid function
14-Year follow-up
At age 14 years, thyroid function was compared between n = 134 GUHS adolescents and n = 1359 Gen2 adolescents (Table II). Mean TSH concentrations did not differ between GUHS and Gen2 participants (P = 0.401) and dividing TSH into quartiles showed no difference (P = 0.532). Mean fT3 concentration was significantly lower in GUHS versus Gen2 participants in univariate and adjusted analyses (P < 0.001), while mean fT4 concentration was significantly higher in GUHS versus Gen2 participants in both univariate and adjusted analyses (P < 0.001). The proportion of participants with positive TPO antibodies did not differ between GUHS versus Gen2 participants (4.5% versus 4.7%, P = 0.927) (data not shown). The prevalence of the thyroid hormone profiles did not differ between the cohorts (e.g. euthyroidism, subclinical and overt hypothyroidism, subclinical and overt hyperthyroidism, and autoimmune thyroid disorder) (P = 0.374) (Supplementary Table SI). Comparing these parameters separately between ET, FET and offspring conceived without ART did not alter the results (P = 0.874) (data not shown). Mean pE2 concentrations were significantly higher in pregnancies after ET versus FET (6561.4 versus 1538.7 pmol/L, P < 0.001). Comparison of thyroid function between ET (n = 53) versus FET (n = 70) showed no differences in mean concentrations of TSH (P = 0.513), fT3 (P = 0.400) and fT4 (P = 0.171) (Supplementary Table SII). No correlation between pE2 and fT4 concentrations was detected (correlation coefficient = 0.106, P = 0.243). Comparing ET and FET separately to Gen2 showed the same trends as comparing the entire GUHS cohort to Gen2 (Supplementary Table SIII). Stratification by sex did not alter the results when comparing GUHS versus Gen2 (data not shown).
Table II.
Estimated mean thyroid hormone concentrations (95% CI) at ages 14 and 20 years.
| Thyroid function | Age 14 years |
Age 20 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *ART | **Non-ART | P-value univariate | ART | Non-ART | P-value adjusted1 | *ART | **Non-ART | P-value univariate | ART | Non-ART | P-value adjusted2 | |
| TSH mU/L | 2.22 (2.05–2.40) | 2.07 (2.02–2.13) | 0.115 | 2.03 (1.82–2.26) | 1.94 (1.82–2.05) | 0.401 | 1.89 (1.63–2.16) | 2.34 (2.06–2.62) | 0.185 | 1.84 (1.58–2.15) | 1.91 (1.77–2.07) | 0.581 |
| fT3 pmol/L | 5.12 (5.02–5.22) | 5.48 (5.44–5.51) | <0.001 | 4.80 (4.61–4.98) | 5.35 (5.27–5.42) | <0.001 | 4.89 (4.70–5.07) | 4.68 (4.65–4.71) | 0.003 | 4.91 (4.69–5.14) | 4.63 (4.55–4.71) | 0.012 |
| fT4 pmol/L | 13.10 (12.86–13.33) | 12.30 (12.22–12.37) | <0.001 | 12.76 (12.50–13.06) | 12.19 (12.02–12.36) | <0.001 | 13.53 (13.15–13.92) | 12.59 (12.50–12.67) | <0.001 | 13.43 (12.97–13.93) | 12.45 (12.27–12.65) | <0.001 |
fT3, free triiodothyronine; fT4, free thyroxine; TSH, thyroid-stimulating hormone. Comparison between ART versus non-ART: age 14 univariate: TSH n = 134 versus 1337, fT3 n = 134 versus 1352, fT4 n = 134 versus 1348, age 14 adjusted: TSH n = 123 versus 1333, fT3 n = 123 versus 1348, fT4 n = 123 versus 1344; age 20 univariate: TSH n = 47 versus 905, fT3 n = 47 versus 914, fT4 n = 47 versus 914, age 20 adjusted: TSH n = 38 versus 870, fT3 n = 38 versus 878, fT4 n = 38 versus 838. For continuous outcomes, univariate cohort differences were examined using Mann–Whitney U test and for categorical outcomes Chi2 and Fisher’s exact tests were used. Adjusted analyses were performed using generalized estimating equations that modelled siblings as a random factor. Bold text indicates statistical significance.
The Growing Up Healthy Study is a prospective cohort study, which aimed to recruit all adolescents born after conception with ART between 1991 and 2001 in the study area.
Participants conceived without ART from the Raine Study Generation 2 (Gen2). The Gen2 participants were born between 1989 and 1992 and have been recognised to be representative of the local population.
Adjusted for: non-singleton, birthweight z-score, gestational age, sex and age of participant.
Adjusted for: non-singleton, birthweight z-score, gestational age, sex, age and BMI of participant and use of anticonvulsants in the past 6 months.
20-Year follow-up
At age 20 years, thyroid function was compared between n = 47 GUHS young adults and n = 914 Gen2 young adults (Table II). Mean TSH concentrations did not differ between the cohorts (P = 0.581) and dividing TSH into quartiles did not show any differences (P = 0.457). Mean fT3 and fT4 concentrations were significantly higher in GUHS versus Gen2 participants (P = 0.012 and P < 0.001, respectively). The proportion of participants with positive TPO antibodies did not significantly differ between the cohorts (P = 0.203) (data not shown). The prevalence of the thyroid hormone profiles did not differ between the cohorts (e.g. euthyroidism, subclinical and overt hypothyroidism, subclinical and overt hyperthyroidism and autoimmune thyroid disorder) (P = 0.565) (Supplementary Table SI). Comparing these parameters separately between ET, FET and offspring conceived without ART did not alter the results (P = 0.985) (data not shown). Mean pE2 concentrations were significantly higher in pregnancies after ET versus FET (8388.3 versus 1762.9 pmol/L, P < 0.001). Comparison of thyroid function between ET (n = 12) versus FET (n = 30) showed no differences in mean concentrations of TSH (P = 0.989), fT3 (P = 0.288) and fT4 (P = 0.536) (Supplementary Table SII). No correlation between fT4 and pE2 was detected (correlation coefficient: 0.025, P = 0.883). Comparing ET and FET separately to Gen2, showed the same trends as comparing the entire GUHS cohort to Gen2 (Supplementary Table SIII). Stratification by sex did not alter the results when comparing GUHS versus Gen2 (data not shown).
Discussion
In the present study of 14- and 20-year-old participants, we report no evidence of suboptimal thyroid function in those who were conceived after ART, when compared to those conceived without ART. All mean thyroid function assessments fell within the normal range at both ages and no differences in the percentage of participants with thyroid disorders were detected. However, fT3 and fT4 concentrations differed statistically at both follow-ups. As the absolute differences are small and fall within the normal range, they are not clinically significant. These findings are reassuring, although require replication and follow-up. A study by Sakka et al. (2009) suggests that the reported increase in SCH in 106 ART newborns could potentially be explained by epigenetic alterations in the set point of TSH sensitivity, resulting in a mild TSH resistance. In our current study, at age 20 years, TSH concentrations were comparable between the cohorts (minimally lower in the GUHS versus Gen2 participants, albeit non-significant) and fT3 and fT4 concentrations were significantly higher in the ART cohort, which could potentially indicate early differences in thyroid function towards SCH, and should be followed up in later adulthood.
Similar to the findings by Sakka et al. a Turkish study of ART newborns also reported an increase in TSH concentrations (n = 98) (Onal et al., 2012). The authors suggest that this could be related to an increase in HCG concentrations in ART pregnancies (Heinonen et al., 1996). HCG is a thyrotrophic hormone, meaning that it has a direct influence on the secretory activity of the thyroid gland. Studies have shown that thyroid function in women after repeated ART cycles was normal, but anti-TPO concentrations were high, potentially affecting foetal thyroid development (Yoshimura and Hershman, 1995; Bussen et al., 2000). In our study, information on HCG concentrations was not available; however, we demonstrated no differences in thyroid function when comparing fresh ET with FET.
Studies have suggested an association between increased maternal E2 concentrations in fresh ET pregnancies and altered thyroid function, such as an increase in thyroid hormone concentrations, in the offspring (Mintziori et al., 2011; Poppe et al., 2011; Santin and Furlanetto, 2011; Gracia et al., 2012). Suggested underlying mechanisms are alterations in foetal DNA methylation after E2 exposure, as well as a direct effect of E2 on thyrocytes (Manole et al., 2001; Banu et al., 2002; Ho et al., 2006). In a 2014 study, researchers reported significantly increased concentrations of T4, fT4 and TSH in 3–10-year-old singletons born after fresh ET compared to offspring conceived without ART (total n = 949), as well as in a separate set of newborns (n = 183) (Lv et al., 2014). A correlation between concentrations of fT4 in the ART newborns and maternal serum concentrations of E2 during early pregnancy was demonstrated. Thyroid function of children born after FET was closer to that of offspring conceived without ART. Interestingly, the authors demonstrated that E2 concentrations were significantly increased after fresh ET throughout the entire first trimester, and not just around time of implantation (Lv et al., 2014).This is important, because the most critical period of foetal thyroid development is the first trimester of pregnancy. In the present study, no differences were detected in concentrations of TSH, fT3 and fT4 when comparing ET with FET although, particularly at the 20-year follow-up, our analyses were lacking statistical power due to low numbers. However, these findings are supported by the lack of a correlation between maximum maternal pE2 and fT4 concentrations in the GUHS offspring.
LBW, among other features, has been shown to characterize women who develop spontaneous hypothyroidism in adult life (Kajantie et al., 2006). The increased risk of LBW in ART pregnancies might explain the increase in (subclinical) hypothyroidism reported by some studies. In our study, the ART cohort was born at a significantly lower birthweight and earlier gestation, which could mediate a difference in thyroid function, however adjusting the analyses for birthweight z-scores and GA did not alter the results. Studies have furthermore demonstrated an association between multiple gestations and congenital hypothyroidism, however whether this association also exists for acquired SCH is unclear (Olivieri et al., 2007). In our study the ART cohort contained significantly more multiples, although adjusting the analyses for ‘non-singletons’ did not alter the results. A Greek study comparing thyroid function between 42 ICSI-conceived children and 42 controls (mean age 6.80 years), reported a significant increase in fT3 concentrations in ICSI offspring, with no differences in fT4 and TSH concentrations (Gkourogianni et al., 2014). An increase in fT3 concentrations could be translated to an increased peripheral conversion of fT4 to fT3, which can be seen in obese patients (Ortega et al., 2012). In the present study, fT3 concentrations were significantly increased in ART offspring at age 20 years, whereas BMI was significantly decreased, and adjustment for BMI did not alter the results. The numbers in our study did not allow for separate investigation of ICSI-conceived offspring. Lastly, iodine deficiency can alter thyroid function; however, at present, the Western Australian population appears to have a sufficient iodine status. In a 2003–2004 study, West Australian children were found to be iodine replete and, as of 2009, mandatory iodine fortification of bread was introduced. Therefore, we believe it can be assumed that the study participants were iodine sufficient, as all assessments in both cohorts were conducted after 2003 (Li et al., 2006). Furthermore, as ART pregnancies are always planned, most women may be expected to have an adequate vitamin intake during early pregnancy.
Detection of suboptimal thyroid function is important, as untreated it can lead to serious and irreversible damage to the central nervous system and long-term unfavourable cardiovascular parameters (Lv et al., 2014). Most unfavourable effects are reversible with correct treatment, which makes early detection essential (Serter et al., 2004; Razvi et al., 2007). Despite other studies reporting evidence of a suboptimal thyroid function in ART offspring, our study does not support these findings. This is the first study investigating thyroid function beyond adolescence. Potentially early life differences in thyroid function, caused by epigenetic alterations, are mitigated later in life and therefore no longer present during adolescence and beyond. A recent study demonstrated no differences in methylation profiles between the GUHS and Gen2 offspring (Penova-Veselinovic et al., 2021). However, as mean concentrations of fT3 are significantly lower at age 14 years and higher at age 20 years, and mean fT4 concentrations are significantly higher at both ages in the ART cohort, albeit within the normal range but potentially indicating early signs of altered thyroid function, verification of our findings later in life, as well as in larger cohorts, is of great importance.
Strengths and limitations
The main strength of this study is the study design, where the adolescents conceived after ART underwent the same assessments as previously completed by their representative counterparts conceived without ART. In combination with the relatively static population of Western Australia, this is a particular study design that would be difficult to replicate elsewhere in Australia or internationally. In addition, as it was attempted to approach all adolescents conceived after ART in Western Australia during a 10-year period, through the two operational fertility clinics at the time of study, selection bias is reduced. To our knowledge, this is the first study investigating thyroid function in ART-conceived offspring beyond childhood. The study also has various limitations: study size is limited, and therefore the results need to be interpreted with caution and replicated in independent cohorts. Furthermore, sample size did not allow for comparison of different types of ART (i.e. IVF and ICSI) or an adequately powered comparison of ET and FET at age 20 years. To maintain power the presented analyses are not stratified by sex. However, when running analyses differentiated by sex, the results were similar without any suggestion of a sex-specific effect. Furthermore, the study adjusted for the use of anticonvulsants at age 20 years, as this significantly differed between the cohorts. However, information regarding the specific type of anticonvulsant was not available and could not be accounted for. Similarly, it was not possible to adjust for use of thyroid hormone, growth hormone and aspirin. Prospective recruitment of the ART cohort during adolescence and young adulthood prevented the ability to adjust for maternal health and pregnancy complication data. No information regarding maternal thyroid disorders during pregnancy was available. Lastly, having adjusted for BW and GA may bias the total effect of ART on thyroid function, although this effect is likely minimal as adjusting for these factors did not alter the results.
Conclusion
Our study does not support the previous findings (reported in 2012–2014) of clinically significant altered thyroid function in ART adolescents. The differences detected in fT3 and fT4 concentrations at both ages (14 and 20 years) are not clinically relevant at this age, but should be reinvestigated in adulthood. Our findings are reassuring although they require replication in independent, and preferably larger, cohorts. Knowledge about long-term health outcomes in ART offspring, such as thyroid function, is important to provide informed advice on any risks and guidance on the appropriate screening and treatment options.
Data availability
The data underlying this article cannot be shared publicly for ethical reasons and privacy protection of the individuals who participated in the study. The data will be shared upon reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
We would like to acknowledge the GUHS participants, family and staff for their cooperation in the study; the NHMRC for their grant support for the GUHS (Hart et al., 1042269); PIVET Medical Centre and Concept Fertility Centre for providing ART data; the department of Health Western Australia, Midwives Notification System for data regarding pregnancy; and PathWest Laboratory Medicine—Perth, Western Australia for fasting biochemistry sample collection. We would like to acknowledge the Raine Study participants and their families for their ongoing participation in the study and the Raine Study team for study co-ordination and data collection. We also thank the NHMRC for their long-term contribution to funding the study over the last 30 years. The core management of the Raine Study is funded by The University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia, and the Raine Medical Research Foundation. The 14-year follow-up from the Raine Study was funded by NHMRC grant: Sly et al., ID 211912; NHMRC Program Grant: Stanley et al., ID 003209; and The Raine Medical Research Foundation. The 14-year biological specimen’s collection was funded by NHMRC grant: Beilin et al., ID 403981; thyroid function data: Abbott Diagnostics Australia for donating immunoassay reagents for the analysis of thyroid function. This article was presented at the ‘American Society for Reproductive Medicine’ Scientific Congress and Expo 2021 (P431, DOI: https://doi.org/10.1016/j.fertnstert.2021.07.775).
Authors’ roles
L.A.W. performed data clean up, data analyses and interpretation of results and wrote the draft manuscript. D.A.D. designed and assisted with statistical analyses. J.A.K., V.P., P.B., and J.L.Y. provided interpretation of results and expert opinion. R.J.H. played a vital role in setting up the study, as well as the study design, interpretation of results and critical discussion. All authors reviewed and agreed to the final version of the manuscript.
Funding
This project was funded by an NHMRC Grant (Hart et al., ID 1042269). Data analysis was carried out as part of L.A.W.’s PhD, conducted at the University of Western Australia, which was supported by an Australian Government Research Training Program Scholarship.
Conflict of interest
R.J.H. is the Medical Director of Fertility Specialists of Western Australia and a shareholder in Western IVF. He has received educational sponsorship from MSD, Merck-Serono and Ferring Pharmaceuticals. P.B. is the Scientific Director of Concept Fertility Centre, Subiaco, Western Australia. J.L.Y. is the Medical Director and a shareholder of PIVET Medical Centre, Perth, Western Australia.
Contributor Information
L A Wijs, Division of Obstetrics and Gynaecology, University of Western Australia, Perth, WA, Australia.
D A Doherty, Division of Obstetrics and Gynaecology, University of Western Australia, Perth, WA, Australia; Women and Infants Research Foundation, Perth, WA, Australia.
J A Keelan, Division of Obstetrics and Gynaecology, University of Western Australia, Perth, WA, Australia; Women and Infants Research Foundation, Perth, WA, Australia; University of Western Australia, School of Biomedical Sciences, Perth, WA, Australia.
V Panicker, Department of Endocrinology, Sir Charles Gairdner Hospital, Perth, WA, Australia; Division of Internal Medicine, University of Western Australia, Perth, WA, Australia.
P Burton, School of Medical and Health Sciences, Edith Cowan University, Perth, WA, Australia; Concept Fertility Centre, Perth, WA, Australia.
J L Yovich, School of Pharmacy and Biomedical Sciences, Curtin University, Perth, WA, Australia; PIVET Medical Centre, Perth, WA, Australia.
R J Hart, Division of Obstetrics and Gynaecology, University of Western Australia, Perth, WA, Australia; Fertility Specialists of Western Australia, Perth, WA, Australia.
References
- Baldini IM, Vita A, Mauri MC, Amodei V, Carrisi M, Bravin S, Cantalamessa L.. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997;21:925–935. [DOI] [PubMed] [Google Scholar]
- Banu SK, Govindarajulu P, Aruldhas MM.. Testosterone and estradiol differentially regulate TSH-induced thyrocyte proliferation in immature and adult rats. Steroids 2002;67:573–579. [DOI] [PubMed] [Google Scholar]
- Bussen S, Steck T, Dietl J.. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Hum Reprod 2000;15:545–548. [DOI] [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC.. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160:526–534. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Biondi B.. Subclinical thyroid disease. The Lancet 2012;379:1142–1154. [DOI] [PubMed] [Google Scholar]
- Declercq E, Luke B, Belanoff C, Cabral H, Diop H, Gopal D, Hoang L, Kotelchuck M, Stern JE, Hornstein MD.. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Fertil Steril 2015;103:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delitala AP, Fanciulli G, Maioli M, Delitala G.. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. Eur J Intern Med 2017;38:17–24. [DOI] [PubMed] [Google Scholar]
- Duntas LH. Thyroid disease and lipids. Thyroid 2002;12:287–293. [DOI] [PubMed] [Google Scholar]
- Fauser BC. Towards the global coverage of a unified registry of IVF outcomes. Reprod Biomed Online 2019;38:133–137. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, Yajnik CS, Eckert JJ. et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018;391:1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkourogianni A, Kosteria I, Telonis AG, Margeli A, Mantzou E, Konsta M, Loutradis D, Mastorakos G, Papassotiriou I, Klapa MI. et al. Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI. PLoS One 2014;9:e94001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia CR, Morse CB, Chan G, Schilling S, Prewitt M, Sammel MD, Mandel SJ.. Thyroid function during controlled ovarian hyperstimulation as part of in vitro fertilization. Fertil Steril 2012;97:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S, Ryynänen M, Kirkinen P, Hippeläinen M, Saarikoski S.. Effect of in vitro fertilization on human chorionic gonadotropin serum concentrations and Down’s syndrome screening. Fertil Steril 1996;66:398–403. [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS.. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase. Cancer Res 2006;66:5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG.. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006;91:145–153. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI, Osmond C, Barker DJ, Forsen T, Eriksson JG.. Spontaneous hypothyroidism in adult women is predicted by small body size at birth and during childhood. J Clin Endocrinol Metab 2006;91:4953–4956. [DOI] [PubMed] [Google Scholar]
- Li M, Eastman CJ, Waite KV, Ma G, Byth K, Zacharin MR, Topliss DJ, Harding PE, Walsh JP, Ward LC. et al. Are Australian children iodine deficient?: results of the Australian National Iodine Nutrition Study. Med J Aust 2006;184:165–169. [DOI] [PubMed] [Google Scholar]
- Lv P, Meng Y, Lv M, Feng C, Liu Y, Li JY, Yu DQ, Shen Y, Hu XL, Gao Q. et al. Altered thyroid hormone profile in offspring after exposure to high estradiol environment during the first trimester of pregnancy: a cross-sectional study. BMC Med 2014;12:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M.. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab 2001;86:1072–1077. [DOI] [PubMed] [Google Scholar]
- Mintziori G, Goulis DG, Toulis KA, Venetis CA, Kolibianakis EM, Tarlatzis BC.. Thyroid function during ovarian stimulation: a systematic review. Fertil Steril 2011;96:780–785. [DOI] [PubMed] [Google Scholar]
- Newman JE, Paul RC, Chambers GM.. Assisted Reproductive Technology in Australia and New Zealand 2018. Sydney, Australia: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales, 2020.
- Newnham JP, O'Dea MRA, Reid KP, Diepeveen DA.. Doppler flow velocity waveform analysis in high risk pregnancies: a randomized controlled trial. Br J Obstet Gynaecol 1991;98:956–963. [DOI] [PubMed] [Google Scholar]
- O'Leary PC, Feddema PH, Michelangeli VP, Leedman PJ, Chew GT, Knuiman M, Kaye J, Walsh JP.. Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. Clin Endocrinol 2006;64:97–104. [DOI] [PubMed] [Google Scholar]
- Olivieri A, Medda E, De Angelis S, Valensise H, De Felice M, Fazzini C, Cascino I, Cordeddu V, Sorcini M, Stazi MA; Study Group for Congenital Hypothyroidism. High risk of congenital hypothyroidism in multiple pregnancies. J Clin Endocrinol Metab 2007;92:3141–3147. [DOI] [PubMed] [Google Scholar]
- Onal H, Ercan O, Adal E, Ersen A, Onal Z.. Subclinical hypothyroidism in in vitro fertilization babies. Acta Paediatr 2012;101:e248–252. [DOI] [PubMed] [Google Scholar]
- Ortega FJ, Jilkova ZM, Moreno-Navarrete JM, Pavelka S, Rodriguez-Hermosa JI, Kopeck Ygrave J, Fernandez-Real JM.. Type I iodothyronine 5′-deiodinase mRNA and activity is increased in adipose tissue of obese subjects. Int J Obes (Lond) 2012;36:320–324. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A.. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- Panicker V, Wilson SG, Walsh JP, Richards JB, Brown SJ, Beilby JP, Bremner AP, Surdulescu GL, Qweitin E, Gillham-Nasenya I. et al. A locus on chromosome 1p36 is associated with thyrotropin and thyroid function as identified by genome-wide association study. Am J Hum Genet 2010;87:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penova-Veselinovic B, Melton PE, Huang RC, Yovich JL, Burton P, Wijs LA, Hart RJ.. DNA methylation patterns within whole blood of adolescents born from Assisted Reproductive Technology are not different from adolescents born from natural conception. Hum Reprod 2021;36:2035–2049. [DOI] [PubMed] [Google Scholar]
- Poppe K, Unuane D, D'Haeseleer M, Tournaye H, Schiettecatte J, Haentjens P, Velkeniers B.. Thyroid function after controlled ovarian hyperstimulation in women with and without the hyperstimulation syndrome. Fertil Steril 2011;96:241–245. [DOI] [PubMed] [Google Scholar]
- Qin J, Sheng X, Wang H, Liang D, Tan H, Xia J.. Assisted reproductive technology and risk of congenital malformations: a meta-analysis based on cohort studies. Arch Gynecol Obstet 2015;292:777–798. [DOI] [PubMed] [Google Scholar]
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU.. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007;92:1715–1723. [DOI] [PubMed] [Google Scholar]
- Robinson M, Oddy WH, McLean NJ, Jacoby P, Pennell CE, de Klerk NH, Zubrick SR, Stanley FJ, Newnham JP.. Low-moderate prenatal alcohol exposure and risk to child behavioural development: a prospective cohort study. BJOG 2010;117:1139–1150. [DOI] [PubMed] [Google Scholar]
- Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH. et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010;304:1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakka SD, Malamitsi-Puchner A, Loutradis D, Chrousos GP, Kanaka-Gantenbein C.. Euthyroid hyperthyrotropinemia in children born after in vitro fertilization. J Clin Endocrinol Metab 2009;94:1338–1341. [DOI] [PubMed] [Google Scholar]
- Santin AP, Furlanetto TW.. Role of estrogen in thyroid function and growth regulation. J Thyroid Res 2011;2011:875125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS.. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med 2002;346:731–737. [DOI] [PubMed] [Google Scholar]
- Serter R, Demirbas B, Korukluoglu B, Culha C, Cakal E, Aral Y.. The effect of L-thyroxine replacement therapy on lipid based cardiovascular risk in subclinical hypothyroidism. J Endocrinol Invest 2004;27:897–903. [DOI] [PubMed] [Google Scholar]
- Straker L, Mountain J, Jacques A, White S, Smith A, Landau L, Stanley F, Newnham J, Pennell C, Eastwood P.. Cohort profile: the Western Australian Pregnancy Cohort (Raine) Study-Generation 2. Int J Epidemiol 2017;46:1384–1385j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeiden JP, Bernardus RE.. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil Steril 2013;99:642–651. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Hershman JM.. Thyrotropic action of human chorionic gonadotropin. Thyroid 1995;5:425–434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly for ethical reasons and privacy protection of the individuals who participated in the study. The data will be shared upon reasonable request to the corresponding author.


