Abstract
Background/Objectives:
Older children with atopic dermatitis (AD) suffer from poor sleep and attention problems. However, until recently, the dearth of developmentally sensitive assessment tools impeded characterization in younger children. We aimed to characterize sleep and attention problems in young children with AD and identify modifiable factors.
Methods:
A cross-sectional study of children with AD aged 1–4 years was stratified by disease severity (Patient-Oriented Eczema Measure), age, and racial/ethnic groups. Developmentally sensitive surveys assessed attention (Multidimensional Assessment Profile of Attention Regulation), sleep, and itch (Patient-Reported Outcomes Measurement Information System). Linear regression models identified predictors of sleep health and attention dysregulation.
Results:
Parents (n = 60) of children aged 2.78 ± 0.98 years with severe (n = 25), moderate (n = 25), or mild (n = 10) AD were recruited across the United States. Significantly reduced sleep health (T-score ≥ 60) was reported in 86% of children with moderate/severe disease (n = 43), and 50% had ≥5 nights of disturbed sleep per week. A suboptimal sleep environment was identified with 32% of children with too much light, noise, or electronic device usage. With regard to attention regulation, in children with severe AD, 80% had trouble sitting still and 72% of children had trouble paying attention no matter their surroundings. In fully adjusted models, AD severity was a significant predictor of poor sleep health (B = 0.79 [0.31–1.28], p < .01) and attention dysregulation (B = 1.22 [0.51–1.93], p < .01).
Conclusions:
More severe AD correlates with poor sleep health and attention dysregulation. In addition to aggressive treatment of AD, clinicians should advise on modifiable sleep hygiene practices and consider screening for attention dysregulation in young children.
Keywords: atopic dermatitis, attention regulation, early childhood, eczema, sleep
1 ∣. INTRODUCTION
Atopic dermatitis (AD) afflicts 10–20% of US children, with 90% diagnosed by 5 years of age.1,2 Sleep disturbance (SDi) is common in AD,3-7 with findings from the few studies available in children <5 years suggesting 68% parent-reported prevalence of children having SDi8 and frequent nighttime awakenings.4,9 These studies used varied assessment tools. Fortunately, newer measures of sleep are available for this age group, Patient-Reported Outcomes Measurement Information System (PROMIS®) Early Childhood Parent Report—Sleep Health10 Measure, and the Pediatric Sleep Practices Questionnaire (PSPQ).11
In older children with AD, SDi appears to independently increase the risk for daytime distraction and impaired attention.12 Chronic SDi is associated with problems in discipline, daytime behavior, and attention regulation (including attention-deficit/hyperactivity disorder [ADHD]).13-15 Even resolved early SDis can translate to deficiencies by school age.16 Older children and adults with AD are particularly vulnerable to these effects of SDi. When comparing poor sleepers with no AD vs. severe AD, the odds of attention problems (including ADHD) were 1.83 (1.47–2.26) vs. 16.83 (7.02–40.33), respectively.17
Although AD disproportionately affects infants and preschool-aged children, limited research exists on the impact of SDi6 and inattention in this age group. To our knowledge, no studies exist on attention regulation and AD in children <5 years old, beyond a single question about a diagnosis of ADHD.17,18 This is likely due to the challenges of developmentally sensitive measurement of these domains during this period of rapid change and high rates of normative variability.19-21 To this end, we drew on the new developmentally sensitive tool characterizing attention dysregulation, Multidimensional Assessment Profile of Attention Regulation (MAPS-AR), adapted from well-validated early childhood multidimensional measures.22 (Nili, manuscript in preparation).
We hypothesized that in young children, more severe AD is associated with poorer sleep health and greater attention dysregulation (AdR). Our objectives were to: 1) characterize sleep and AdR in young children with AD using developmentally sensitive measures, 2) identify targetable features of sleep health and attention for treatment, and 3) explore the association with disease severity.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Study design
We designed a cross-sectional study to test our hypothesis in a national sample of school-aged children23 and 60 infants and preschool-aged children with AD stratified by Patient-Oriented Eczema Measure (POEM) parent report of child race/ethnicity (White, African American or Black, and Other), and age, through the panel company OP4G and the National Eczema Association (Figure S1). Subjects were included if they were between 1 and 4 years of age and had AD (parent reported “yes” to the question, “Has a doctor or another health care provider told you that your child has eczema?”). Our primary measure of AD severity was the POEM and has been used in our previous studies.24,25 Adjusted POEM scores (without itch and sleep question) were calculated into a composite score for statistical analysis in a linear regression model to isolate the contribution of itch and sleep health as predictor variables in the model (Additional methods in the Supplemental methods).
2.2 ∣. Assessment of quality of life and itch, sleep health, and attention dysregulation
Quality of life was assessed by the Infant Dermatitis Quality of Life Index (IDQoL).26 Itch severity was measured by the question, “In the past 7 days…How bad was your child's itch on average?” with responses ranging from 0 (no itch) to 10 (worst imaginable itch). In addition, the 6-item PROMIS Itch Questionnaire—Child (PIQ-C) scale measured how itch impacts quality of life, and a T-score was computed.27
The PROMIS EC Sleep Health Measure (PROMIS EC Sleep) includes SDi and sleep-related impairment items, and a T-score was computed based on a custom set of questions (mea n = 50, standard deviation = 10) (Table S1).11 Characterization of sleep habits (not included in regression modeling) was assessed using an adapted Pediatric Sleep Practices Questionnaire (PSPQ) for 1-to 5-year-olds.11
The MAPS-AR is a developmentally sensitive measure to capture early behavioral expressions of inattention, hyperactivity, and impulsivity and their contexts in age-appropriate terms as the expression of ADHD-related patterns in young children. It is a component scale of the MAPS measures, which characterize narrowband dimensions of clinical phenotype within developmental context (Wakschlag et al., unpublished data, 2020).22 Respondents endorsed the frequency of a given behavior, which was dichotomized into groups of: never, rarely (<1/week), some days (1–3/week), most days (4–6/week), and daily. Higher scores in MAPS-AR indicate greater AdR, meaning more frequent problems, potentially occurring in developmentally atypical contexts.
2.3 ∣. Covariates
Covariates were selected based on previous studies of what influences attention in patients with AD, itch, sex, age, race/ethnicity, and socioeconomic status (parental highest educational level and household income based on the parent filling out the survey).17,28-30
2.4 ∣. Analytical strategy
All analyses were conducted using SPSS version 16. Baseline characteristics by AD severity strata were compared using ANOVA and chi-square tests. Bivariate and multivariate frequencies and prevalence among different severity groups were assessed for each measure. The associations between continuous variables were assessed with Pearson’s correlation coefficients and an equal variance t test. An assessment of potential confounding factors between AdR or sleep health and age, race/ethnicity, gender, socioeconomic status, itch severity, and PIQ-C scores was undertaken prior to analyses.
Our base linear regression model was developed to determine the association of the co-primary outcomes of MAPS-AR and PROMIS EC Sleep with the primary determinate of AD disease severity. All significant covariates identified as potential confounders were included in the subsequent models. The final multivariate models included AD severity by POEM, PROMIS EC Sleep, itch severity, maternal income, and the dummy race/ethnicity variable for African American or Black.
3 ∣. RESULTS
3.1 ∣. Participant characteristics
A total of 60 patients were planned for recruitment from targeted strata (Figure S1). Characteristics of the study population are shown in Table 1. By design, disease severity groups were similar in age, sex, and race/ethnicity.
TABLE 1.
Patient characteristics stratified by Patient-Oriented Eczema Measure severity
| Disease severity by Patient-Oriented Eczema Measure (POEM) | |||||
|---|---|---|---|---|---|
| Total (n = 60) | Mild AD (n = 10) |
Moderate AD (n = 25) |
Severe AD (n = 25) |
p-Value | |
| Age, mean (SD) | 2.78 (0.98) | 2.21 (0.99) | 3.00 (0.93) | 2.80 (0.97) | .10 |
| Male, n (%) | 33 (5S) | 7 (70) | 16 (64) | 10 (40) | .14 |
| Race/Ethnicity | .92 | ||||
| Caucasian, n (%) | 21 (35) | 3 (30) | 9 (36) | 9 (36) | |
| Black, n (%) | 19 (32) | 3 (30) | 8 (36) | 8 (32) | |
| Other, n (%) | 20 (33) | 4 (30) | 8 (28) | 8 (32) | |
| Parental education levels | .53 | ||||
| High school graduate(%) | 2 (3) | 0 | 0 | 2 (8) | |
| Some college or BA (%) | 40 (67) | 7 (70) | 18 (72) | 15 (60) | |
| Higher degree (PhD., master's)(%) | 18 (30) | 3 (30) | 7 (28) | 8 (32) | |
| Household income in past 12 months | <.01 | ||||
| <$30,000 (%) | 6 (10) | 0 | 2 (8) | 4 (16) | |
| $30,000–$74,999 (%) | 32 (53) | 3 (30) | 9 (36) | 20 (80) | |
| >$75,000 (%) | 19 (32) | 7 (70) | 11 (44) | 1 (4) | |
| Omitted (%) | 1 (4) | 0 | 1 (4) | 0 | |
| Sleep disturbance (nights of disturbed sleep) | <.01 | ||||
| 1–2 days, n (%) | 16 (27) | 6 (60) | 8 (32) | 2 (8) | |
| 3–4 days, n (%) | 14 (23) | 0 | 11 (44) | 3 (12) | |
| 5–6 days, n (%) | 13 (22) | 0 | 6 (24) | 7 (28) | |
| Every day, n (%) | 12 (20) | 0 | 0 | 12 (48) | |
| No day, n (%) | 5 (8) | 4 (40) | 0 | 1(4) | |
| Skin treatment (yes) | 52 (87) | 6 (60) | 22 (88) | 24 (96) | .02 |
| POEM (SD) | 15.25 (6.30) | 5.60 (1.65) | 13.00 (2.22) | 21.36 (2.96) | <.01 |
| PROMIS Early Childhood Sleep Health T-score (SD) | 65.16 (8.87) | 53.60 (8.03) | 64.83 (5.99) | 70.11 (7.22) | <.01 |
| PROMIS EC Sleep Health score ≥60, n (%) | 44 (73) | 1 (10) | 20 (80) | 23 (92) | <.01 |
| IDQoL (SD) | 10.92 (5.29) | 4.30 (1.95) | 10.08 (3.49) | 14.40 (4.85) | <.01 |
| PIQ-C T-score (SD) | 51.28 (9.47) | 38.78 (6.81) | 51.39 (7.68) | 56.17 (9.47) | <.01 |
| Itch Severity Numeric Rating Scale (SD) | 5.93 (2.04) | 3.60 (1.78) | 5.40 (1.47) | 7.40 (0.44) | <.01 |
| MAPS-AR Composite Score (SD) | 45.55 (12.50) | 34.20 (8.40) | 43.52 (12.03) | 52.12 (10.47) | <.01 |
Note: Bold = statistically significant, p < .05.
Abbreviations: IDQoL, Infant Dermatitis Quality of Life Index; MAPS-AR, Multidimensional Assessment Profile of Attention Regulation; PIQ-C, Patient-Reported Outcomes Measurement Information System Itch Questionnaire—Child; POEM, Patient-Oriented Eczema Measure; PROMIS EC, Patient-Reported Outcomes Measurement Information System Early Childhood.
3.2 ∣. Association between AD severity and sleep health
Sleep disturbance due to AD was reported on at least five nights per week in 76% of children with severe AD, 24% of children with moderate AD, and in no patients with mild AD (p = .01). The average PROMIS EC Sleep T-scores were more than 1 SD larger than would be expected in a general pediatric population in moderate (64.83 ± 5.99) and severe patients (65.16 ± 8.87). In fact, 86% of children with moderate/severe AD (n = 43) in our cohort had a PROMIS EC Sleep T-score ≥60. Table 2 compares responses to PROMIS EC Sleep questions by the disease severity group. Poor sleep resulted in significant mood impairment in children with moderate/severe AD compared to children with mild AD, with 36% having trouble getting along with other children and 68% crying easily because of poor sleep. Table 2 also highlights sleep habits and routines adapted from the PSPQ. Children with moderate-to-severe AD had more environmental interruptions at night, 32% who had too much light or noise in their room or electronic devices (34%) before bed.
TABLE 2.
Sleep-related impairment and habits (PROMIS EC Sleep and adapted from PSPQ)
| Almost always/Always responses by POEM disease severity | ||||
|---|---|---|---|---|
| Item, N (%) | Mild AD (n = 10) |
Moderate AD (n = 25) |
Severe AD (n = 25) |
p-Value |
| When my child didn't sleep well, it was hard for him/her to play | 0 | 4 (16) | 15 (60) | <.01 |
| When my child didn't sleep well, he/she had problems getting along w/ other children during the day | 0 | 4 (16) | 14 (56) | <.01 |
| My child's daytime activities or routines were disturbed by poor sleep | 1 (10) | 12 (48) | 16 (64) | .02 |
| When my child didn't sleep well, he/she got mad easily | 1 (10) | 12 (48) | 15 (60) | .03 |
| When my child didn't sleep well, he/she had more temper tantrums than usual | 3 (30) | 17 (68) | 16 (64) | .10 |
| When my child didn't sleep well, he/she cried easily | 2 (20) | 17 (68) | 17 (68) | .02 |
| My child slept in my bed at some point during the night | 3 (30) | 11 (44) | 15 (60) | .24 |
| My child followed a bedtime routine before falling asleep | 9 (90) | 17 (68) | 22 (88) | .14 |
| My child woke up at about the same time every morning | 4 (16) | 13 (52) | 15 (60) | .55 |
| My child tried to fall asleep at about the same time every night | 7 (70) | 20 (80) | 15 (60) | .30 |
| My child watched TV shows or videos just before falling asleep | 2 (20) | 12 (48) | 15(60) | .10 |
| My child played videos or video games just before falling asleep | 0 | 1 (4) | 11 (44) | <.01 |
| My child used a phone, computer, tablet or electronic device just before falling asleep | 0 | 6 (24) | 11 (44) | .03 |
| My child had trouble falling asleep b/c their room was too noisy | 0 | 5 (20) | 11 (44) | .02 |
| My child had problems falling asleep b/c there was too much light in their room | 0 | 6 (24) | 10 (40) | .05 |
| My child needed someone w/ him or her to fall asleep | 2 (20) | 13 (52) | 17 (68) | .04 |
Note: Bold = statistically significant, p < .05.
Abbreviations: POEM, Patient-Oriented Eczema Measure; PROMIS EC, Patient-Reported Outcomes Measurement Information System Early Childhood; PSPQ, Pediatric Sleep Practices Questionnaire.
3.3 ∣. Association between AD severity and attention dysregulation
Children with more severe AD had greater AdR as indicated by higher MAPS-AR scores (r = .65, p < .01). Table 3 outlines specific MAPS-AR items and responses by the disease severity group to illustrate behaviors most strongly associated with AD severity. Items that were developmentally possible, but atypical if frequent, were common in patients with more severe diseases, such as “trouble paying attention… no matter what is going on around him/her,” and occurred in 72% of children with severe AD, 40% of children with moderate AD, and 20% of children with mild AD (p = .01).
TABLE 3.
Attention regulation: Inattention and hyperactivity/impulsivity (MAPS-AR)
| Number of sometimes, almost always, and always responses by POEM disease severity |
||||
|---|---|---|---|---|
| Item, n (%) | Mild AD (n = 10) |
Moderate AD (n = 25) |
Severe AD (n = 25) |
p-Value |
| Inattention | ||||
| Seem easily distracted by things that were happening around him/her | 1 (10) | 13 (52) | 15 (60) | .03 |
| Jump quickly from one toy or activity to another without really playing with toys | 3 (30) | 8 (32) | 19 (72) | <.01 |
| Have trouble paying attention to a simple book or story for 5 mins or longer | 0 | 11 (44) | 17 (68) | <.01 |
| Hyperactivity/Impulsivity | ||||
| Demand your attention w/o being able to wait | 5 (50) | 18 (72) | 22 (88) | .06 |
| Have trouble sitting still even for a few minutes | 1 (10) | 12 (48) | 20 (80) | <.01 |
| Run of climb so dangerously that you couldn’t take your eyes off him/her | 1 (10) | 11 (44) | 17 (68) | <.01 |
| Fidget restlessly during feedings or mealtime | 3 (30) | 17 (68) | 20 (80) | .02 |
| Context | ||||
| Have trouble paying attention, keeping still or waiting during daily routines, that is, meal time, bedtime, getting dressed | 3 (30) | 15 (60) | 19 (56) | .04 |
| Have trouble paying attention, keeping still, or waiting no matter what is going around him/her | 2 (20) | 10 (40) | 18 (72) | <.01 |
| Have trouble paying attention, keeping still or waiting when excited | 2 (20) | 13 (52) | 16 (64) | .06 |
| Have trouble paying attention, keeping still, or waiting when frustrated, angry or upset | 2 (20) | 14 (56) | 22 (88) | <.01 |
| Have trouble paying attention, keeping still, or waiting when tired, hungry or sick | 4 (40) | 17 (68) | 19 (72) | .12 |
Note: Bold = statistically significant, p < .05.
Abbreviations: MAPS-AR, Multidimensional Assessment Profile of Attention Regulation; POEM, Patient-Oriented Eczema Measure.
3.4 ∣. Predictors of problems with sleep or attention dysregulation in children with AD
We analyzed the co-primary outcomes of PROMIS EC Sleep and MAPS-AR scores in Table 4. Our sleep health model revealed that in univariate analysis, AD severity was associated with worse sleep health (B = 1.22 (0.88–1.56), p < .01), a finding that persists when adding itch severity into the model (B = 1.01 (0.56–1.45), p < .01). In fully adjusted models for significant demographic variables, AD severity remained a significant predictor of poor sleep health (B = 0.79 (0.31–1.28), p < .01) and being African American or Black (B = 3.89 (0.49–7.28), p = .03).
TABLE 4.
Linear regression of PROMIS EC sleep health (Sleep Disturbance) and MAPS-AR (Attention Dysregulation)
| PROMIS EC Sleep (Sleep Disturbance) |
MAPS-AR (Attention Dysregulation) |
||||
|---|---|---|---|---|---|
| Variable | Unstandardized β value (95% CI) |
p-Value | Variable | Unstandardized β value (95% CI) |
p-Value |
| Model 1 | Model 1 | ||||
| POEM w/o itch/sleep | 1.22 [0.88 to 1.56] | <.01 | POEM w/o itch/sleep | 1.72 [1.24 to 2.203] | <.01 |
| Model 2 | Model 2 | ||||
| POEM w/o itch/sleep | 1.01 [0.56 to 1.45] | <.01 | POEM w/o itch/sleep | 1.21 [0.57 to 1.85] | <.01 |
| Itch severity | 0.80 [−0.29 to 1.88] | .15 | PROMIS EC Sleep | 0.42 [−0.06 to −0.78] | .02 |
| Model 3 | Model 3 | ||||
| POEM w/o itch/sleep | 0.79 [0.31 to 1.28] | <.01 | POEM w/o itch/sleep | 1.04 [0.33 to 1.75] | <.01 |
| Itch severity | 0.85 [−0.19 to 1.88] | .31 | PROMIS EC Sleep Health | 0.38 [0.20 to 0.75] | .04 |
| Black | 3.89 [0.49 to 7.28] | .03 | Itch severity | 0.80 [−0.71 to 2.31] | .29 |
| Parental income | −1.05 [−2.20 to 0.10] | .07 | Model 4 | ||
| POEM w/o itch/sleep | 1.22 [0.51 to 1.93] | <.01 | |||
| Itch severity | 0.86 [−0.56 to 2.29] | .23 | |||
| PROMIS EC Sleep | 0.15 [−0.22 to 0.52] | .41 | |||
| Black | 7.79 [3.01 to 12.58] | <.01 | |||
| Parental Income | −0.30 [−1.89 to 1.30] | .71 | |||
Note: Bold = statistically significant, p < .05.
Abbreviations: MAPS-AR, Multidimensional Assessment Profile of Attention Regulation; POEM, Patient-Oriented Eczema Measure; PROMIS, Patient-Reported Outcomes Measurement Information System.
Our AdR model revealed that in univariate analysis, AD severity was associated with increased AdR (B = 1.72 (1.24–2.20), p < .01). This association of AD severity on problems with attention regulation persisted with both the addition of sleep health alone in model 2 (B = 1.21 (0.57–1.85), p < .01) and sleep health and itch severity in model 3 (B = 1.04 (0.33–1.75), p < .01). In the fully adjusted model, AD severity remained a significant predictor of AdR (B = 1.22 (0.51–1.93), p < .01). Being African American or Black was also another significant predictor of overall problems with AdR (B = 7.79 (3.01–12.58), p < .01).
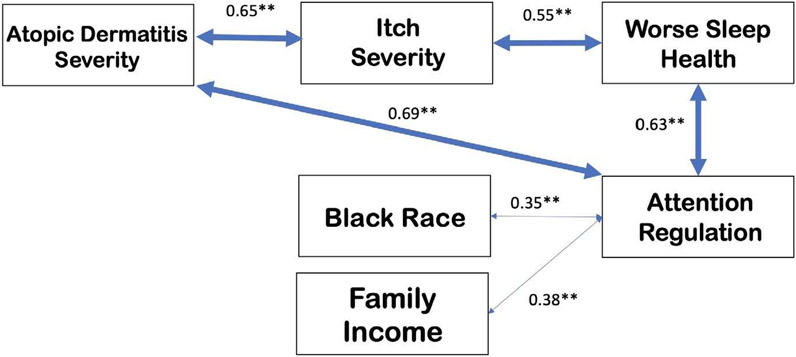
Figure 1 displays the hypothesized interrelationship of AD and AdR with additional associations with itch, sleep health, African American or Black race, and family income.
FIGURE 1.
Proposed model for hypothesized relationship between AD, itch, sleep disturbance, and attention regulation with demographic modulators. Bidirectional arrows represent the effect of one variable on another variable, with values above representing the correlation coefficient. Several other covariates are also associated with atopic dermatitis and attention regulation such as sex at birth, parental smoking or drug exposure, prematurity, obesity, and family history; however, they were not tested or not significant in our study. **p < .01
4 ∣. DISCUSSION
Our study highlights that children <5 years with more severe AD are at greater risk by parent report of poor sleep health and AdR. With regard to sleep, 86% of children with moderate/severe disease had significantly poor sleep health (T-score ≥60 on PROMIS EC Sleep). In fact, 48% of children with severe AD experienced nightly SDi, with most parents reporting their child gets mad easily (60%) and has problems getting along with other children (56%). With regard to AdR, difficultly sitting still was reported in 80% of children with severe AD in comparison with moderate (48%) and mild AD (10%). AdR, as measured by the MAPS-AR composite score, indicated more dysregulated patterns and greater occurrence in developmentally unexpected contexts in severe (52.12 ± 10.47) vs. moderate (43.52 ± 12.03) vs. mild AD groups (34.20 ± 8.40).
The nocturnal flare of AD is in part responsible for this SDi, with inflammatory upregulation and worsening of skin barrier function,31-33 all of which are exacerbated by circadian disrupters noted frequently in our study, such as light in the bedroom at night and pre-bed screen time.34 We identified these potentially modifiable sleep habits that clinicians could discuss with their patients, such as limiting lights, minimizing noise, and restricting pre-bedtime usage of electronic devices.
To our knowledge, the present study is the first to characterize patterns of AdR in very young children with AD employing a measure designed to differentiate normative variation from clinical risk in early development.23 The link between inattention and AD might be in part due to heightened sensory afferent signals from the skin in AD,35 resulting in chronic brain stimulation with shared pathways to ADHD, such as the prefrontal cortex.36,37
Importantly, burgeoning research highlights the predictive utility of early indicators of attention problems: Infants diagnosed with AD were more likely to demonstrate inattention/hyperactivity symptoms later in childhood, at 10 years of age.38 From our work, children with more severe AD were found to exhibit more inattention symptoms in atypical contexts (eg, when minimal cognitive demands are found) rather than contexts where most young children have attention dysregulation. This suggests that even in early childhood, AD inattention symptoms can be screened and addressed. Future work will need to address whether early screening/treatment of AD inattention can prevent ADHD or other neurocognitive dysfunction.17 Of racial groups studied, we found African American or Black children with AD were at higher risk for SDi and inattention even when controlling for SES status and AD severity. Future studies with larger sample sizes should be performed in order to establish directional and cumulative effects using structural equation modeling to elucidate the relationship between AD, sleep, AdR, race, and SES status.
4.1 ∣. Limitations
Several limitations should be considered, such as the small cohort of patients. Selection bias may have been introduced through non-random sampling such as participants who did not complete the study or were not included due to being over quota in a priori determined strata. In addition, this study relied on parent report of their child's disease severity, sleep problems, and AdR symptoms, and findings may reflect parental biases.39 However, parent reports for inattention and hyperactivity symptoms are significantly predictive of overall ADHD severity.40,41 The cross-sectional study design inhibits the ability to determine causal sequences, limiting our hypothesized model (Figure 1) to correlational relationships. Future longitudinal studies beginning early in life are needed to address causation and should include objective data in conjunction with parent self-report data.
We suggest clinicians monitor sleep and attention in younger children with AD, particularly those with moderate/severe disease, and can use the assessments in this manuscript (see the Supplement). Clinicians might also address maladapted sleep hygiene practices, such as screen time before bed in light-filled, noisy bedrooms. Although devices help redirect children's attention away from itch,42,43 we ask to limit this before bed. We suggest parents and clinicians focus on implementing healthy sleep hygiene habits in children with AD. Because AD has implications not only for present neurocognitive behaviors in early childhood but also for school performance even up to 10 years old,38,14 AdR is important to screen with referrals to a psychologist if needed. Clinicians should be cognizant of monitoring AD severity and aggressively treating flares. Sleep health and AdR in young children with AD, especially those from minoritized backgrounds, should be studied to better address the impact of sleep quality and daytime function.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Beaumont for her contributions to the statistical analysis.
Funding Information
This study was supported by the Ann & Robert H. Lurie Children’s Hospital of Chicago and the Agency for Healthcare Research and Quality (grant number K12HS023011 to AF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The development of the attention regulation measure was supported in part via a diversity supplement to Amanda Nili (R01MH10765-S1, PI Wakschlag).
Footnotes
CONFLICT OF INTEREST
None relevant to this manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011;131(1):67–73. doi: 10.1038/jid.2010.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30(1):35–39. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer LJ, Flewelling KD, Jump S, Gyorkos E, White M, Hauk PJ. Impact of atopic dermatitis treatment on child and parent sleep, daytime functioning, and quality of life. Ann Allergy Asthma Immunol. 2020;124(4):385–392. doi: 10.1016/j.anai.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 4.Chang YS, Chou YT, Lee JH, et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics. 2014;134(2):e397–e405. doi: 10.1542/peds.2014-0376 [DOI] [PubMed] [Google Scholar]
- 5.Hon KL, Lam MC, Leung TF, et al. Nocturnal wrist movements are correlated with objective clinical scores and plasma chemokine levels in children with atopic dermatitis. Br J Dermatol. 2006;154(4):629–635. doi: 10.1111/j.1365-2133.2006.07213.x [DOI] [PubMed] [Google Scholar]
- 6.Bringhurst C, Waterston K, Schofield O, Benjamin K, Rees JL. Measurement of itch using actigraphy in pediatric and adult populations. J Am Acad Dermatol. 2004;51(6):893–898. doi: 10.1016/j.jaad.2004.05.039 [DOI] [PubMed] [Google Scholar]
- 7.Chang YS, Lin MH, Lee JH, et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: a randomized clinical trial. JAMA Pediatr. 2016;170(1):35–42. doi: 10.1001/jamapediatrics.2015.3092 [DOI] [PubMed] [Google Scholar]
- 8.Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159(8):745–750. doi: 10.1001/archpedi.159.8.745 [DOI] [PubMed] [Google Scholar]
- 9.Dogan DG, Canaloglu SK, Kivilcim M, Kum YE, Topal E, Catal F. Sleep patterns of young children with newly diagnosed atopic dermatitis. Postepy Dermatol Alergol. 2017;34(2):143–147. doi: 10.5114/ada.2017.67080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackwell CK, Wakschlag LS, Krogh-Jespersen S, et al. Pragmatic health assessment in early childhood: the PROMIS® of developmentally based measurement for pediatric psychology. J Pediatr Psychol. 2020;45(3):311–318. doi: 10.1093/jpepsy/jsz094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meltzer LJ, Forrest CB, de la Motte A, Mindell JA, Bevans KB. Development and validation of the pediatric sleep practices questionnaire: a self-report measure for youth ages 8–17 years. Behav Sleep Med. 2021;19(1):126–143. doi: 10.1080/15402002.2020.1714625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camfferman D, Kennedy JD, Gold M, Martin AJ, Winwood P, Lushington K. Eczema, sleep, and behavior in children. J Clin Sleep Med. 2010;6(6):581–588. [PMC free article] [PubMed] [Google Scholar]
- 13.Paller AS, Mcalister RO, Doyle JJ, Jackson A. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41(5):323–332. doi: 10.1177/000992280204100505 [DOI] [PubMed] [Google Scholar]
- 14.Sivertsen B, Harvey AG, Reichborn-Kjennerud T, Torgersen L, Ystrom E, Hysing M. Later emotional and behavioral problems associated with sleep problems in toddlers: a longitudinal study. JAMA Pediatr. 2015;169(6):575–582. doi: 10.1001/jamapediatrics.2015.0187 [DOI] [PubMed] [Google Scholar]
- 15.Shur-Fen GS. Prevalence of sleep problems and their association with inattention/hyperactivity among children aged 6–15 in Taiwan. J Sleep Res. 2006;15(4):403–414. doi: 10.1111/j.1365-2869.2006.00552.x [DOI] [PubMed] [Google Scholar]
- 16.Quach JL, Nguyen CD, Williams KE, Sciberras E. Bidirectional associations between child sleep problems and internalizing and externalizing difficulties from preschool to early adolescence. JAMA Pediatr. 2018;172(2):e174363. doi: 10.1001/jamapediatrics.2017.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strom MA, Fishbein AB, Paller AS, Silverberg JI. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175(5):920–929. doi: 10.1111/bjd.14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atefi N, Rohaninasab M, Shooshtari M, et al. The association between attention-deficit/hyperactivity disorder and atopic dermatitis: a study among Iranian. Children. 2019;64(6):451–455. doi: 10.4103/ijd.IJD_458_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chacko A, Wakschlag LS, Hill C, Danis B, Espy K. Viewing preschool disruptive behavior disorders and ADHD through a developmental lens: what do we know and what do we need to know? Child Adolesc Psychiatr Clin N Am. 2009;18(3):627–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magee CA, Gordon R, Caputi P. Distinct developmental trends in sleep duration during early childhood. Pediatrics. 2014;133(6):e1561–1567. doi: 10.1542/peds.2013-3806 [DOI] [PubMed] [Google Scholar]
- 21.Wakschlag LS, Tolan PH, Leventhal BL. Research review: ‘Ain’t misbehavin’: towards a developmentally-specified nosology for preschool disruptive behavior. J Child Psychol Psychiatr. 2010;51(1):3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakschlag LS, Briggs-Gowan MJ, Choi SW, et al. Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior. J Am Acad Child Adolesc Psychiatry. 2014;53(1):82–96.e3. doi: 10.1016/j.jaac.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishbein AB, Cheng BT, Tilley C, et al. Sleep disturbance in school-aged children with atopic dermatitis: prevalence and severity in a representative US sample. J Allergy Clin Immunol Pract. 2021;9(8):3120–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519. doi: 10.1001/archderm.140.12.1513 [DOI] [PubMed] [Google Scholar]
- 25.Fishbein AB, Lor J, Penedo FJ, Forrest CB, Griffith JW, Paller AS. Patient-reported outcomes for measuring sleep disturbance in pediatric atopic dermatitis: cross sectional study of PROMIS pediatric sleep measures and actigraphy. J Am Acad Dermatol. 2020:S0190-9622(20)31015-X. doi: 10.1016/j.jaad.2020.05.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis-Jones MS, Finlayv AY, Dykes PJ. The infants’ dermatitis quality of life index. Br J Dermatol. 2001;144(1):104–110. doi: 10.1046/j.1365-2133.2001.03960.x [DOI] [PubMed] [Google Scholar]
- 27.Paller AS, Shei J, Rangel S, et al. 509 PIQ-C, a new PROMIS® tool, measures intensity and impact of itch on children with atopic dermatitis. J Invest Dermatol. 2020;140(7 Suppl):S69. doi: 10.1016/j.jid.2020.03.518 [DOI] [Google Scholar]
- 28.Kruse L, Cices A, Fishbein AB, Paller AS. Neurocognitive function in moderate-severe pediatric atopic dermatitis: a case-control study. Pediatric Dermatol. 2019;36(1):110–114. doi: 10.1111/pde.13710 [DOI] [PubMed] [Google Scholar]
- 29.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in atopic dermatitis. J Allergy Clin Immunol. 2013;131(2):428–433. doi: 10.1016/j.jaci.2012.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanos M, Gerlach M, Warnke A, Schmitt J. Association of attention-deficit/hyperactivity disorder and atopic eczema modified by sleep disturbance in a large population-based sample. J Epidemiol Community Health. 2010;64(3):269–273. doi: 10.1136/jech.2009.093534 [DOI] [PubMed] [Google Scholar]
- 31.Bender BG, Ballard R, Canono B, Murphy JR, Leung DY. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol. 2008;58(3):415–420. doi: 10.1016/j.jaad.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 32.Lavery MJ, Stull C, Kinney MO, Yosipovitch G. Nocturnal pruritus: the battle for a peaceful night's sleep. Int J Mol Sci. 2016;17(3):425. doi: 10.3390/ijms17030425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yosipovitch G, Xiong GL, Haus E, Sackett-Lundeen L, Ashkenazi I, Maibach HI. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J Invest Dermatol. 1998;110(1):20–23. doi: 10.1046/j.1523-1747.1998.00069.x [DOI] [PubMed] [Google Scholar]
- 34.Fishbein AB, Vitaterna O, Haugh IM, et al. Nocturnal eczema: review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol. 2015;136(5):1170–1177. doi: 10.1016/j.jaci.2015.08.028 [DOI] [PubMed] [Google Scholar]
- 35.Treister AD, Stefek H, Grimaldi D, et al. Sleep and limb movement characteristics of children with atopic dermatitis coincidentally undergoing clinical polysomnography. J Clin Sleep Med. 2019;15(8):1107–1113. doi: 10.5664/jcsm.7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol. 2009;161(5):1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen C, Luo Q, Chamberlain SR, et al. What is the link between attention-deficit/hyperactivity disorder and sleep disturbance? A multimodal examination of longitudinal relationships and brain structure using large-scale population-based cohorts. Biol Psychiat. 2020;88(6):459–469. doi: 10.1016/j.biopsych.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt J, Chen CM, Apfelbacher C, et al. Infant eczema, infant sleeping problems, and mental health at 10 years of age: the prospective birth cohort study LISAplus. Allergy. 2011;66(3):404–411. doi: 10.1111/j.1398-9995.2010.02487.x [DOI] [PubMed] [Google Scholar]
- 39.Reljic V, Gazibara T, Nikolic M, Zaric M, Maksimovic N. Parental knowledge, attitude, and behavior toward children with atopic dermatitis. Int J Dermatol. 2017;56(3):314–323. doi: 10.1111/ijd.13529 [DOI] [PubMed] [Google Scholar]
- 40.Power TJ, Doherty BJ, Panichelli-Mindel SM, et al. The predictive validity of parent and teacher reports of ADHD symptoms. J Psychopathol Behav Assess. 1998;20:57–81. doi: 10.1023/A:1023035426642 [DOI] [Google Scholar]
- 41.Powell K, Le Roux E, Banks J, Ridd MJ. GP and parent dissonance about the assessment and treatment of childhood eczema in primary care: a qualitative study. BMJ Open. 2018;8(2):e019633. doi: 10.1136/bmjopen-2017-019633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capozza K, Redding M. A call for a compassionate approach to itch-related scratching in children with eczema. Dermatologist, 2020;28(1). https://www.hmpgloballearningnetwork.com/site/thederm/article/call-compassionate-approach-itch-related-scratching-children-eczema Accessed November 20, 2021 [Google Scholar]
- 43.Leibovici V, Magora F, Cohen S, Ingber A. Effects of virtual reality immersion and audiovisual distraction techniques for patients with pruritus. Pain Res Manag. 2009;14(4):283–286. doi: 10.1155/2009/178751 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.