Abstract
Alzheimer's disease (AD) and other dementias are a global challenge. Early diagnosis is important to manage the disease. However, there are barriers to diagnosis that differ by region. Researchers from Brazil, China, Nigeria, Spain, and Sweden have identified key barriers to AD diagnosis in their countries. In Brazil, socioeconomic inequalities and poor recognition of dementia by physicians can prevent diagnosis. In China, a very large population and lack of physician training in dementia make diagnosis problematic. In Nigeria, socioeconomic inequalities and cultural stigma can stand in the way of diagnosis. In Spain, patient hesitancy and an overloaded health‐care system are barriers to diagnosis. In Sweden, inconsistent use of biomarkers is a prominent barrier to diagnosis of AD. To support diagnosis, more focus is needed on education of patients and physicians, increased use of support services, and improved access to biomarkers to accurately diagnose AD.
Keywords: Alzheimer's disease, biomarkers, Brazil, China, Nigeria, socioeconomic inequalities, Spain, Sweden
1. INTRODUCTION
Alzheimer's disease (AD) represents a global challenge and is the leading cause of dementia. 1 It is estimated that approximately 50 million people are living with dementia, including AD dementia, a figure which could rise to more than 150 million by 2050. 2 With the prevalence of AD and dementia projected to increase by 68% in low‐ and middle‐income countries by 2050, several barriers may arise in access to diagnosis and treatments. 1 There is no single test for the diagnosis of AD; currently in clinical practice, AD is typically diagnosed by a multi‐disciplinary workup based on patient history; clinical symptoms; a variety of neuropsychiatric, physical, and functional assessments; and, if available, imaging (computed tomography, magnetic resonance imaging [MRI], or positron emission tomography [PET]) assessments and blood tests, which are particularly important to rule out certain other causes of dementia. Additionally, many European countries use cerebrospinal fluid (CSF) biomarkers in the clinical evaluation of AD. 1 , 3
Earlier diagnosis of AD is important to enable use of symptomatic therapies, treat behavioral symptoms, and adopt lifestyle changes that are used more commonly worldwide to reduce the risk of developing dementia, and eventually, to slow disease progression. 1 , 4 Inherent global challenges to timely diagnosis of AD and its subsequent management are further complicated by regionally specific challenges. 5 In June 2021, aducanumab received accelerated approval from the United States Food and Drug Administration, making it the first available disease‐modifying treatment (DMT) to address AD pathology by targeting amyloid beta (Aβ) plaques. However, the drug is not globally accessible, did not receive approval by the European Medicines Agency, and the AD community is awaiting further trial data to support its approval. 6
Lifestyle factors are important for prevention of dementia. The Lancet Commission on dementia has defined 12 potential modifiable risk factors contributing to 40% of worldwide dementia: low education, hearing impairment, physical inactivity, obesity, hypertension, diabetes, smoking, depression, low social contact, air pollution, excessive alcohol consumption, and traumatic brain injury. 4 Taking specific actions such as maintaining physical activity in mid‐ and later life, reducing exposure to air pollution, and providing all children with primary and secondary education can prevent or delay dementia. 4
In an effort to better understand practical challenges and health‐care disparities experienced by AD and dementia practitioners worldwide, a panel of clinicians and researchers from four continents convened at the 15th International Conference on Alzheimer's and Parkinson's Diseases and related neurological disorders (AD/PD™), held virtually in 2021. This panel engaged researchers and clinicians in cross‐regional learning from diverse health‐care systems located in Brazil, China, Nigeria, Spain, and Sweden (see Table 1 for country‐specific demographics). Drawing upon these individual country insights, a variety of common themes and challenges to AD diagnosis and management were identified, as well as regionally specific hurdles. Globally, biomarkers were identified as integral to the accurate differential diagnosis of AD; however, the global availability of biomarkers differs significantly in each country (Table 2). 3 Specific socioeconomic factors were recognized as major hurdles for access to AD diagnosis and adequate AD care in low‐ and middle‐income countries as well as rural regions. Improved education and enhanced outreach were highlighted as a need to improve AD diagnosis and care worldwide, with regionally specific communication goals to reduce cultural stigmas that impact perceptions around AD and the readiness of patients to seek treatment. Finally, dedicated training of primary care physicians (PCPs) for rural and low‐income areas that lack specialists and educating health‐care professionals around the appropriate usage of biomarkers were also identified as areas for growth. These factors are challenges to AD diagnosis and management in all the aforementioned countries, as well as many other nations, to varying degrees.
TABLE 1.
Country demographics
| Brazil | China | Nigeria | Spain | Sweden | |
|---|---|---|---|---|---|
| Total population (thousands) 50 | 212,559 | 1,402,112 | 206,140 | 47,352 | 10,353 |
|
Population 65+ (%) 51 |
10 | 12 | 3 | 20 | 20 |
| Life expectancy at birth (years) 52 | 76 | 77 | 55 | 83 | 83 |
| Primary school enrollment (% of projected age group) a , 53 | 132 | 102 | 85 | 102 | 129 |
| Secondary school enrollment (% of projected age group) a , 54 | 95 | 88 | 42 | 126 | 152 |
| Tertiary school enrollment (% of projected age group) a , 55 | 43 | 54 | 10 | 91 | 72 |
| GDP per capita (USD) 56 | 6796.8 | 10,500.4 | 2097.1 | 27,057.2 | 51,925.7 |
| Gini index 57 | 53.4 | 38.5 | 35.1 | 34.7 | 30.0 |
| Hospital beds (per 1000 people) 58 | 2.1 | 4.3 | 0.5 | 3.0 | 2.1 |
Abbreviations: GDP, gross domestic product; USD, United States dollar.
High percentages, particularly percentages above 100, indicate more children outside of the expected age range attending school possibly due to repetition or late entry.
TABLE 2.
Available biomarkers in public and private health‐care systems
| Countries | Public health‐care biomarkers | Private health‐care biomarkers |
|---|---|---|
| Brazil |
|
|
| China |
|
|
| Nigeria* |
|
|
| Spain |
|
|
| Sweden |
|
|
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; CT, computed tomography; FDG‐PET, fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging; PET, positron emission tomography
HIGHLIGHTS
Prominent barriers to the diagnosis of Alzheimer's disease differ by region.
Issues include socioeconomic inequalities and poor recognition of dementia.
Stigma, patient hesitancy, and inconsistent use of biomarkers are also problems.
More focus is needed on education of patients and physicians.
Other support services and increased access to biomarkers are also needed.
2. ENABLING EARLY AD DIAGNOSIS IN SPAIN VIA SCREENING
In Spain, the prevalence of dementia in people 65 and older ranges from 4% to 9%, in which AD is the most common cause of dementia. 7 Incidence rates of dementia are similar to the rates of dementia in other European countries, exhibiting the commonly described pattern of age‐dependent increases. 7 In Spain, as observed globally, a large proportion of people with dementia remain undiagnosed. Hesitancy to seek a formal diagnosis, due to the lack of disease awareness, at the patient and family level (sometimes due to stigma), as well as overload of the public health system, low availability of adequate testing among PCPs and lack of a DMT, are commonly cited factors for lack of diagnosis. 7 As has been described anecdotally elsewhere in Europe, such as in France, 8 in Spain, many younger patients (younger than 65 years old) with cognitive complaints are seldom referred by PCPs to specialty clinics. 9 , 10 However, a validated diagnosis at earlier stages of AD (associated with younger ages) is important for any decisions to initiate non‐pharmacological or symptomatic interventions and to facilitate life planning. 7 Early diagnosis will likely play an integral role in the potential use of DMTs, as agents in development within this class are focused on the earliest stages of AD. 11 , 12
Recognizing this issue for younger people in Spain, Ace Alzheimer Center Barcelona initiated an Open House Initiative (OHI) program providing cognitive screenings for free to anyone over 50 years old, without requiring a referral. 13 As well as identifying those with cognitive impairments to improve access to clinical care for patients with AD since 2008, this initiative has enabled connections to be forged between more than 3000 cognitively unimpaired individuals and the clinic. 13 , 14 Of the patients treated at Ace Alzheimer Center Barcelona, 12% entered directly from the OHI rather than via the conventionally described Spanish patient pathway (Figure 1). Recently, Ace Alzheimer Center Barcelona has also implemented a self‐administered computerized test in the OHI program, to facilitate the cognitive screening of the population with a test design to detect memory deficits related to AD. 15 By removing initial socioeconomic barriers and expanding access to AD care across local communities, more patients may be assessed for AD and perhaps eventually become eligible for treatment with a DMT. The Models of Patient Engagement for Alzheimer's Disease (MOPEAD) project evaluated the OHI, as well as three other similar pre‐screening initiatives to manage early AD. 16 Compared to two other pre‐screening initiatives, individuals pre‐screened in the OHI had higher Mini‐Mental State Examination (MMSE) scores and a large proportion of individuals with subjective cognitive decline. 16 Aside from allowing for an early diagnosis among pre‐screened individuals, the OHI was found to raise public awareness of the importance of early AD diagnosis. Overall, all four pre‐screening initiatives were useful for identifying individuals at high risk of having early AD. 16 Another study made a cost‐consequence analysis of MOPEAD in which the primary health‐economic outcome was the cost per true‐positive case of AD from the screened population. 17 The cost per true‐positive case was €2722 for the OHI, which was expensive. Among other factors, the high costs can mainly be attributed to the varied staff types (physicians, nurses, neuropsychologists) in the pre‐screening stage. 17 A potential solution to lower these costs is to use only specially trained nurses. 17 In addition, during the pandemic, Ace Alzheimer Center Barcelona launched telemedicine evaluation to ensure access to medical care for patients with cognitive impairment. 18
FIGURE 1.
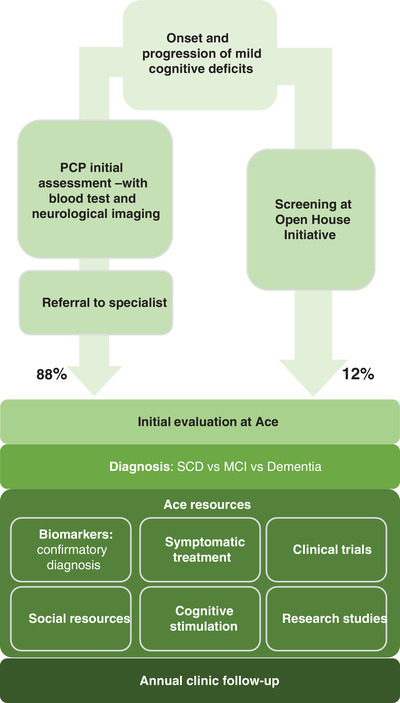
Patient flow at Ace Alzheimer Center Barcelona. MCI, mild cognitive impairment; PCP, primary care physician; SCD, subjective cognitive decline
For example, a 60‐year‐old patient with a family history of dementia presented at the OHI having experienced a 4‐year history of memory complaints, including a difficulty with naming things. At presentation, cognitive assessments were normal, as were neuroimaging results. The patient agreed to participate in an observational study and was identified as apolipoprotein E ε4 homozygous. The patient remained cognitively normal reporting subjective cognitive decline after 4‐year follow‐up. Then, the patient was able to undergo biomarker assessment with MRI and lumbar puncture as part of an AD biomarker study. The MRI neuroimaging showed atrophy in the medio‐temporal and parietal lobes, and the AD CSF biomarker results demonstrated low levels of Aβ42, an abnormal Aβ42/Aβ40 ratio, and normal levels of total tau (t‐tau) and phosphorylated tau (p‐tau), indicating preclinical pathological changes associated with AD. A year later, upon presenting with amnestic mild cognitive impairment (MCI), the patient accepted an offer to be enrolled in a clinical trial. The battery of biomarker assessments allowed selection of this patient as a suitable candidate for a DMT.
While biomarkers, in this case, confirmed AD pathology, they were assessed due to his participation in a research study, following the recommendations of local guidelines for the use of biomarkers in cognitively normal individuals, 19 and considering the ethical implications of the results. This policy aligns with the recent International Working Group (IWG) recommendations for the diagnosis of AD in clinical practice, in which cognitively unimpaired people are not recommended for biomarker analysis in the absence of a DMT. 20 These guidelines require clinical evidence of AD and a positive biomarker assessment for a diagnosis of AD. If those who are asymptomatic or have subjective memory complaints are tested and have biomarker evidence of AD pathology, they are categorized as at risk for AD dementia rather than diagnosed with preclinical AD. 20
In addition to screening programs at memory centers to identify patients at risk of dementia in Spain, it would be helpful to better train PCPs for early detection and use of associated diagnostic tools. 7 , 13 Additionally, there is a need to further educate patients and their caregivers about AD, and to consider the wider implications of diagnosis and potential intervention for the patient and family members. 7 Educating the population to raise awareness of AD and reduce stigma may increase participation in clinical trials, especially among cognitively unimpaired or at‐risk individuals. 13 Establishing registries of individuals with various biomarker statuses may facilitate well‐designed clinical trials. 13
In October 2019, Spain announced that it had adopted a national strategy to tackle AD and other dementias. Part of their focus in this plan was to improve the diagnostic capacities of their health system and progress the ability to diagnose patients earlier and select the most appropriate treatments. 21
3. BUILDING CONFIDENCE IN EARLY AD DIAGNOSIS IN SWEDEN
The differential diagnosis of AD from other dementias is possible through the identification of AD pathology using biomarkers (Figure 2). However, biomarkers have not yet been adopted in large‐scale practice globally. In Sweden, national guidelines have been developed to improve the diagnosis of dementias 22 that allow for the use of biomarkers to support clinical diagnosis of AD in difficult cases, including in complex cases with potentially confounding comorbidities, earlier disease stages, or in younger patients. Despite no general recommendation, CSF biomarkers are being increasingly used by many clinicians. 23 , 24 According to the Swedish Dementia Registry, CSF biomarker analysis is performed in ≈50% of the patients seeking medical advice in memory clinics but varies across the country from 20% in some parts of northern Sweden to 90% in some districts in southern Sweden. This suggests further work is required to mitigate health inequity and provide a more equal implementation of diagnostic algorithms for the evaluation of dementia‐causing diseases. Breakthroughs in fluid‐based biomarkers, 12 , 23 such as plasma neurofilament light chain (NfL), have accelerated their more routine use in Sweden and are attracting interest in other European countries. 25 Amyloid or tau PET scans are used in Sweden only in exceptional cases in clinical practice. 22 Changes in biomarkers can occur many years before symptoms are apparent and CSF (and potentially plasma biomarkers) are useful tools to predict which patients with mild symptoms may convert to AD.
FIGURE 2.
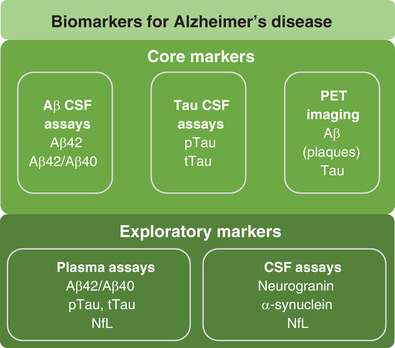
Core and exploratory biomarkers for Alzheimer's disease. Aβ, beta‐amyloid; CSF, cerebrospinal fluid; NfL, neurofilament light chain; PET, positron emission tomography; pTau, phosphorylated tau; tTau, total tau
As AD biomarkers become more widely adopted by both specialists and general practitioners, careful education on the use and interpretation of biomarkers will be essential to patient treatment. This is highlighted in the published case of a 66‐year‐old retired man who was diagnosed with mild AD dementia according to clinical criteria. 26 Several years prior to diagnosis, the patient had started to complain of cognitive decline, which progressed over the years, and he developed orientation difficulties and a tendency to misplace belongings. Upon physical examination, there was no atrophy or white matter changes on MRI, and CSF analyses showed slightly elevated CSF/serum albumin ratio (a sign of an impaired blood–brain barrier), normal Aβ42, and elevated t‐tau. The patient started symptomatic AD treatment with the acetylcholinesterase inhibitor galantamine.
Four years after AD diagnosis, the patient was screened for participation in a clinical trial. 26 The patient had stable cognition over 4 years of treatment (MMSE score decrease from 28 to 25) and normal somatic status, with no symptoms of infection or neurological complaints. MRI showed slight atrophy with mild white matter changes and core AD CSF biomarkers consistent with AD. In addition to this evidence of AD progression, CSF screening showed a mildly elevated albumin ratio, immunoglobulin M index, and CSF monocytes, indicative of active inflammation. Upon further examination, while serum was anti‐Borrelia antibody negative, CSF was anti‐Borrelia antibody positive, indicating that the patient had contracted Lyme neuroborreliosis.
This case study highlights the complexity of biomarker assessment and laboratory analyses for potential clinical trial enrollment, as well as evaluations in relation to patient treatment. If the patient had been included in a clinical trial, the Lyme disease infection of the nervous system could have been misinterpreted as a side effect of study treatment. If undiscovered and untreated, the neuroborreliosis might also have led to deteriorating brain functions in addition to AD pathological processes. While CSF AD biomarkers may assist in defining inclusion for clinical trials, a thorough biomarker assessment should be conducted to help exclude patients with underlying conditions that could mimic or aggravate symptoms of AD. Consequently, physicians will need adequate training on the use of AD biomarkers to ensure holistic patient treatment considers other comorbidities despite evidence of AD pathology.
Fluid biomarkers have the potential to enable more effective clinical trials and establish a more personalized approach to AD diagnosis and treatment. However, there remains an unmet need to develop standardized pre‐analytical protocols for measuring fluid markers. 23 Universal access to the tools needed to assess biomarkers will be critical for their wider adoption in clinical practice. As biomarkers may be increasingly adopted with breakthroughs in fluid‐based biomarkers, it will be integral to understand when to test and how to interpret results regarding AD pathogenesis. Effective communication of biomarker results to the patients will be vital, particularly if blood tests are used more in general practice, as well as discussing with patients how they might wish to communicate any results to family and friends.
In May 2018, the Swedish government launched a national dementia strategy that focuses on several improvements including collaboration between health and social care, staffing, knowledge and skills, monitoring and evaluation, family and friends, society, and digital and assistive technologies. 27
4. BARRIERS TO AD DIAGNOSIS IN BRAZIL
Latin America is a vast region comprised of a diverse population of approximately 645 million people, with prominent socioeconomic inequalities. 28 Of the 40 nations with the worst deviations of individual income distributions from perfectly equal within an economy (Gini index), 15 are located in Latin America. 28 The prevalence of dementia in Latin America is higher than in most world regions. 29 Of note, there is a higher prevalence among younger age groups. In people with dementia aged 65 to 69 years old, the prevalence of dementia is 2.4% (95% confidence interval [CI], 2.11–2.72) compared to prevalence of 1.2% (95% CI, 0.8–1.5) in European countries. 30 This earlier emergence of dementia may be due to low education and poor control of cardiovascular risk factors, which are associated with higher rates of dementia and are considered modifiable risk factors. 4 , 30 , 31
Patients with cognitive impairment are often not recognized in Latin America, as demonstrated in a study conducted in a public university hospital in São Paulo, Brazil. Out of 248 outpatient older adults randomly selected, 43 were found to have cognitive impairment or dementia but only seven (16.3%) of these patients had this information recorded previously in their clinical files. 32 Across Latin America, the diagnosis of MCI or dementia is usually made by specialists and only sporadically by a PCP. 31 This is in stark contrast to many European countries, where most patients with cognitive impairment or dementia are diagnosed at the primary care level. 31 Also, in contrast to more well‐financed health‐care systems elsewhere in the world, confirmation of AD diagnosis using biomarkers is problematic in Brazil and across Latin America, as access to facilities and care is frequently limited. In Latin America, formal neuropsychological assessments for dementia diagnosis are mainly offered at private institutions, being available only in some public academic centers, highlighting disparate access to diagnostics and health care in public versus private institutions. 31 For biomarker confirmation of AD pathology, there are very limited PET facilities, 33 which are mostly located at research institutions or in large cities. Similarly, CSF biomarker testing is only available in large cities and is not covered by the public health system. Blood‐based biomarker testing is currently unavailable. To improve early disease detection, there is a critical need for new low‐cost biomarkers.
In the absence of more affordable biomarkers, Latin American investigators have developed various cognitive assessments to test for AD in those with limited education. 34 For illustration, a clinical vignette is presented below.
A 75‐year‐old Brazilian woman with 4 years of education complained of short‐term memory decline over the past 12 months. She had maintained independence in basic and instrumental activities of daily living and did not report mood symptoms. She had dyslipidemia regularly treated with simvastatin and she was also taking calcium supplements. Physical and neurological examination were unremarkable, as were her blood tests; she had an MMSE score of 27 with a Functional Activities Questionnaire (FAQ) score of 3. MRI showed slight globally diffused reduced brain volume, including mild atrophy of the left hippocampus. No other abnormalities or significant vascular lesions were noted.
A formal neuropsychological assessment would be necessary for confirmation of MCI due to AD, which was unavailable. To assess the patient, she was given the Brief Cognitive Screening Battery (BCSB); this instrument was designed for the diagnosis of dementia in populations with heterogeneous levels of education and has been reported to show similar performance across subjects regardless of literacy level. 35 The BCSB is a 7‐ to 10‐minute process and includes a figure memory test (with 10 items) to assess incidental memory, immediate memory, and learning. 35 , 36 Then category fluency (animals/minute) and clock drawing tests are administered as interference tasks, followed by delayed recall and recognition of the figures. 35 , 36 The patient had a total BCSB score of 50 with a delayed recall of 4 out of 10, suggestive of AD dementia. Biomarker analyses were not feasible to confirm the diagnosis, highlighting the importance for validation of cognitive assessments.
In a recent study, the diagnostic accuracy of the BCSB was assessed in patients with MCI or mild dementia due to AD confirmed by CSF biomarkers. The BCSB total score discriminated AD cases from cognitively healthy controls with 80% sensitivity and 91% specificity, while the BCSB total score and the delayed recall of the figure memory test showed 80% sensitivity and 81.5% specificity, respectively. 37
Validation of the BCSB and similar scales is essential to reach those who do not have access to specialty centers. Learning how to perform adjustments for education and how to use scales in patients who have very low education levels is also helpful. Digital biomarkers may be one approach to streamlining AD management in low‐ and middle‐income countries. New lower‐cost digital biomarkers that use wearable and mobile technologies may assist in detection of sensory or motor changes that occur in the earliest stages of AD, and may be especially helpful in the absence of easy access to traditional biomarker assessments. 38 Cognitive testing is time‐intensive, can be limited by cultural bias and is rater‐dependent. Digital biomarkers, conversely, do not have these limitations and have the potential to accelerate an AD diagnosis and reduce financial burden on the health‐care system by allowing the patient to be treated on an as‐needed basis. 38 Notably, the development of national dementia plans in Latin America has the potential to raise awareness and knowledge, overcome barriers to proper management, and promote establishment of specialized diagnostic and care centers. 31 The Alzheimer's Disease International's aim to galvanize governments to develop and implement national dementia plans in their countries led to the Brazilian senate passing the National Law of Care of People with Alzheimer's and Other Dementia. This law hopes to improve the lives of those living with dementia and their carers. 39
5. CULTURAL AND SOCIOECONOMIC CHALLENGES TO EARLY DIAGNOSIS IN NIGERIA
As in Latin America, socioeconomic inequalities in Nigeria and other countries in sub‐Saharan Africa result in limited access to AD diagnostics, therapies, and screening tools. The number of patients with dementia in low‐ and middle‐income countries continues to grow at a faster rate than in high‐income countries. 4 As the world's most rapidly aging population is located in sub‐Saharan Africa, people there will be especially at risk for dementia. 40 As in Latin America, digital biomarkers may address issues around access to resources and ultimately improve care. There is also a similar need to expand available cognitive assessments for those with low education levels.
A widely reported challenge to dementia diagnosis in sub‐Saharan Africa is socio‐cultural stigma tied to traditional spiritual beliefs that draw a link between unusual behavior and witchcraft. 40 Accusation of witchcraft, especially for older women, has not only negative connotations but can also lead to social and physical isolation. 40 Individuals in the early stages of dementia may not seek health care in an effort to avoid this social stigma and accusations of witchcraft, delaying patient presentation. 40 In some regions of sub‐Saharan Africa, symptoms of dementia are considered normal parts of aging and are associated with a loss of authority and child‐like behavior. 41 When people do seek medical treatment, it is often in combination with traditional or faith‐healing practices, and patients may switch between treatments based on perceived effects. 41
To face this challenge, awareness and education are needed throughout the community from health‐care professionals and leaders in the faith community. 40 These educational resources should communicate the differences between dementia and normal aging to the community in a culturally sensitive manner, especially in the absence of effective DMTs for dementia and AD. 41 Non‐specialists need to be empowered to conduct comprehensive assessments and increase capacity in primary care to assist in the diagnosis and management of comorbidities, especially in rural areas that lack memory centers. 41
When patients and their families do come forward to seek health care for symptoms of dementia, this care is largely self‐funded, which is a barrier to treatment. In one case, a 65‐year‐old legal practitioner was brought to a neurology clinic at the University College Hospital in Ibadan, Nigeria, reporting intermittent memory deterioration that had affected his job for 3 years. The patient appeared unkempt and had impairment in three‐item recall, calculation, and abstraction. Physical assessments were normal except for high blood pressure and irregular pulse; he had a history of hypertension and was receiving anti‐hypertensive medications. No biomarkers were assessed due to financial concerns. One month after this initial visit, the patient developed urinary incontinence, regression of social functions, and impaired recall of past events. Over the next 6 years, the patient's cognitive function deteriorated, manifested by neighborhood wandering, apathy, and difficulties in dressing and walking despite normal motor power in the legs.
This Nigerian case presents several key features that illustrate the difficulties with diagnosis and management of AD that are also reported in other areas of sub‐Saharan Africa. The patient had a chronic and progressive illness, with significant cognitive and functional impairment along with vascular risk factors. The advanced dementia with apathy could be associated with AD or mixed dementia; however, without laboratory investigations or neuroimaging, differential diagnosis capabilities were limited as were treatment options. Adequate access to resources, such as diagnostic tools or neuroimaging, to provide a dementia diagnosis is an unmet need across sub‐Saharan Africa. There is a need to improve awareness around the use of diagnostic and management tools in countries of the region; tackling these issues will require the establishment of multidisciplinary teams of general practitioners, neurologists, nurses, social workers, and other patient advocates, as well as centers of excellence.
6. DIFFICULTIES DUE TO A LARGE POPULATION AND COMORBIDITIES IN CHINA
It is estimated that China may have 21.1 million people with AD by 2050. 42 The challenges of AD care in China are considerable: not only are there many patients, but a large incidence of vascular complications. Those who suffer transient ischemic attack (TIA) and stroke have substantially higher risk of developing dementia. In the year after a major stroke, incidence of dementia was almost 50 times greater than in the general population in the Oxford Vascular Study, a community‐based study in Oxfordshire, UK. 43 China has a younger age of onset of stroke than Europe, and approximately 1 in 4 people with stroke show cognitive impairment within 1 year. 43 Mechanistically, the pathophysiology of stroke may exacerbate amyloid production. Chronic cerebral hypoperfusion, a result of persistent atrial fibrillation, may cause the upregulation of Aβ‐producing enzymes while simultaneously lowering Aβ clearance efficiency. Hypoperfusion may also exacerbate tau pathology through the upregulation of tau‐phosphorylating enzymes and indirectly via the amyloid cascade. 44 In addition to vascular complication, a study comparing the prevalence of MCI in people aged 65 or older in urban and rural areas of China, reported that the prevalence of MCI in rural areas of China is twice that of urban areas, suggesting a pressing need to improve AD management in rural China. 45
The overlap of stroke and early AD in China complicates clinical diagnosis, especially given the large population. For example, a 75‐year‐old retired engineer first presented in 2014 with memory loss, reporting that he had forgotten the names of people he had known for years. Otherwise, he was doing well and had a good social network with family, friends, and people in the neighborhood. The patient had previously suffered a TIA in 1994, and had diabetes, hypertension, and hyperlipidemia. Due to a family history of AD dementia, the patient's PCP prescribed donepezil. The patient's physical and neurological exams were unremarkable and brain MRI showed very mild atrophy and mild vascular ischemic changes. The next year (March 2015), the patient had several suspected TIAs with confusion, clumsiness, and visual changes that spontaneously resolved. During October 2015, the patient had multiple incidents while driving and often forgot to take medication despite using a pillbox. By February 2016, the patient was moved into an assisted‐living facility where he was agitated and argued with other residents. Because of this relatively rapid progression, a brain MRI was conducted that showed more small vessel ischemic changes around the ventricle area in the corona radiata, and increased atrophy, especially in the left temporal lobe. There was evidence of amyloid pathology on a PET scan. This case illustrates the comorbidities of AD dementia and vascular etiology that makes an early diagnosis difficult.
Definitive diagnosis of AD requires a multidisciplinary approach, but access to multidisciplinary teams and therapeutics can be limited, especially in rural areas. Because the disease is progressive in nature, long‐term follow‐ups are key, but this presents a significant financial burden on the health‐care system in large populations. Neurology departments at general hospitals are not equipped with enough dementia doctors or memory clinics to promptly diagnose and treat the growing population of people with AD in China. 46 An additional challenge is the wide range of dementia diagnoses in China, depending on the tier of health care. Dementia diagnosis can be made at well‐staffed academic hospitals by specialists who diagnose AD according to internationally accepted criteria or at county hospitals with non‐specialist staff resulting in a high incidence of misdiagnosis or missed diagnosis altogether. 47 Doctors without specialized dementia training are often unaware of the symptomatic therapies available, and up to 80% of Chinese people with known dementia are not treated with any drug. 46 Recently, the Chinese government has approved plans for the Professional Improvement Committee of the Neurology Branch of the Chinese Medical Doctor Association to conduct a program that prepares and trains dementia specialists. As part of this program, doctors will be trained in dementia centers at academic hospitals nationwide, and graduate with certificates. A national dementia plan for China was launched as part of China's Action Plan for Healthy China 2030. The plan's scope includes key action areas such as risk reduction and slowing of cognitive decline, providing family respite, and encouraging social‐psychological service systems. 48
While biomarkers may help identify patients at risk for AD, in China some practical problems are observed. CSF markers have poor compliance and acceptance, and PET imaging is expensive. A definitive diagnosis of AD is limited in rural areas, where multidisciplinary teams are not available. The majority of people with dementia in China are cared for at home, normally by a family caregiver. 46
7. DISCUSSION
From these individual reports by clinicians and researchers from different countries and regions, some common themes emerge that illustrate the practical challenges for improving care of patients with AD and potential ways to address them (Figure 3, Table 3). These challenges are multifaceted and have varying degrees of impact on different countries and regions. To address dementia‐related issues in a way that is tailored to each country, Brazil, China, Spain, and Sweden have developed national dementia plans (Table 4). National dementia plans can be capable of addressing the needs of people with dementia, promoting public awareness of dementia, and improving the quality of health care and social services for patients and their families. Stigma is a substantial barrier hindering people from seeking care and preventing an early diagnosis of AD. The lack of treatment options for people experiencing dementia and supportive services for care providers can greatly impact quality of life. There is a general need to improve education around AD and dementia to reduce fear and cultural stigma, and to enable access to care and earlier diagnosis.
FIGURE 3.
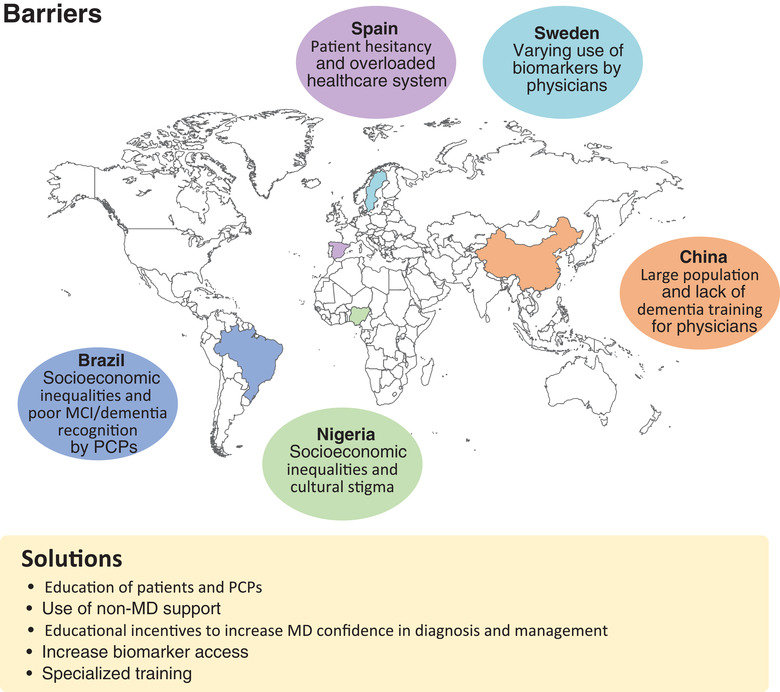
Barriers and solutions to AD diagnosis. AD, Alzheimer's disease; DMT, disease‐modifying treatment; PCP, primary care physician; MCI, mild cognitive impairment; MD, medical doctor.
TABLE 3.
Challenges and solutions to AD management in each country
| Countries | Challenges in AD management | Proposed solutions |
|---|---|---|
| Brazil |
|
|
| China |
|
|
| Nigeria |
|
|
| Spain |
|
|
| Sweden |
|
|
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; OHI, Open House Initiative; PCP, primary care physician; TIA, transient ischemic attack.
TABLE 4.
Status of national dementia plans in each country
| Countries | National dementia plan status | Gross national income category |
|---|---|---|
| Brazil |
|
Upper‐middle‐income |
| China |
|
Middle‐income |
| Nigeria |
|
Lower‐middle‐income |
| Spain |
|
High‐income |
| Sweden |
|
High‐income |
Outreach to non‐specialist physicians, including training for PCPs and digital support platforms could help reduce disparities (income‐ and geography‐based). Regions that have populations with low education need tailored cognitive assessment that include education‐adjusted scoring. As demonstrated in Spain, free cognitive testing and the use of telemedicine can help reduce economic barriers to diagnosis.
There is a need for less expensive and less invasive biomarkers that will enable wide access and usage in different regions. Clinicians who lack adequate access to biomarkers and diagnostic tools may have issues with providing patients with an accurate diagnosis; this in turn perpetuates health inequity. Additionally, biomarker use requires adequate training of health‐care professionals to interpret results and then communicate them to the patients and their families while still considering other comorbidities. It is important to stratify patients for biomarker testing to identify those who are more likely to have AD based on other assessments. As with other AD clinical trials, many biomarker studies predominantly recruit White participants and their validity in other populations, with different ethnic and genetic backgrounds, requires verification. Caution is needed in extrapolating population‐level risk to individual risk.
The view among this panel is that early diagnosis will be important for patient selection for DMTs. Affordability of therapeutics will be very important in low‐ and middle‐income countries, specifically out‐of‐pocket contributions. Symptomatic therapies will still be important due to the high incidence of mixed dementia. There will be an increasing burden to society as new therapies are available and patients live longer; we need to consider the implications of this on how we manage patients with AD and other dementias.
In low‐ and middle‐income countries control of cardiovascular risk factors is still poor. However, it has been suggested that the potential for dementia prevention is greater than in high‐income countries because of a higher prevalence of these modifiable risk factors. 49 Addressing inequalities in access to resources, including education and access to health care, will be critical to help reduce these risk factors worldwide.
CONFLICTS OF INTEREST
Diana Kerwin is an advisory board member for Biogen, AbbVie, Eisai, and Roche, and has received research funding from AbbVie, Roche, Genentech, Inc., Global Alzheimer's Platform (GAP), Green Valley, Eisai, Eli Lilly, and the National Institute on Aging (NIA). Paulo Caramelli prepared material for Continuing Medical Education (CME) and participated as a speaker in symposia sponsored by Aché, Libbs, Nutricia, Roche, Sandoz, Torrent, and Zodiac laboratories; participates on advisory boards for Aché, Biogen, EMS, Nutricia, and Roche; has participated as principal investigator in a clinical trial from Roche; receives research funding from CNPq, Brazil. Jiong Shi is a scientific board member for Roche and Biogen. Henrik Zetterberg has served on scientific advisory boards and/or as a consultant for AbbVie, Alector, Eisai, Denali, Roche, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx, and Red Abbey Labs; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Carla Abdelnour has received honoraria from Zambon, F. Hoffmann‐La Roche Ltd, and Schwabe Farma Ibérica S.A.U. in the last 3 years. Martin Traber is a full‐time employee of F. Hoffmann‐La Roche Ltd. Adesola Ogunniyi has no competing interests to declare.
ACKNOWLEDGMENTS
The authors would like to thank Rufus Akinyemi for useful suggestions. Medical writing support for the development of the manuscript was provided by Sarah Engelberth, PhD, of Medical Expressions, Chicago, IL, and Laura Geuss, PhD, of Prime Global. Funding for the symposium and manuscript was provided by F. Hoffmann‐La Roche Ltd.
Kerwin D, Abdelnour C, Caramelli P, et al. Alzheimer's disease diagnosis and management: Perspectives from around the world. Alzheimer's Dement. 2022;14:e12334. 10.1002/dad2.12334
REFERENCES
- 1. Alzheimer's Association , 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327‐406. [DOI] [PubMed] [Google Scholar]
- 2. Alzheimer's Disease International (ADI) . World Alzheimer Report 2019: Attitudes to dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf. Accessed November 2019.
- 3. Zetterberg H, Bendlin BB, Biomarkers for Alzheimer's disease—preparing for a new era of disease‐modifying therapies. Mol Psychiatry. 2021;26(1):296‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Livingston G, Huntley J, Sommerlad A, et al., Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parra MA, Butler S, McGeown WJ, et al., Globalising strategies to meet global challenges: The case of ageing and dementia. J Glob Health. 2019;9(2):020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biogen Inc . ADUHELM™ (aducanumab‐avwa). Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761178s003lbl.pdf. Accessed August 2021.
- 7. Galende AV, Ortiz ME, Velasco SL, et al., Report by the Spanish foundation of the brain on the social impact of Alzheimer disease and other types of dementia. Neurologia (Engl Ed). 2019;36(1):39‐49. [DOI] [PubMed] [Google Scholar]
- 8. Alzheimer F. Personnes malades jeunes: Les oubliées d'Alzheimer. https://www.francealzheimer.org/personnes‐malades‐jeunes‐les‐oubliees‐dalzheimer/. Accessed 20 May 2021.
- 9. Graff‐Radford J, Yong KXX, Apostolova LG, et al., New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salem LC, Andersen BB, Nielsen TR, et al., Inadequate diagnostic evaluation in young patients registered with a diagnosis of dementia: a nationwide register‐based study. Dement Geriatr Cogn Dis Extra. 2014;4(1):31‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Hlávka J, Hillestad R, et al., Assessing the preparedness of the US health care system infrastructure for an Alzheimer's treatment. RAND Corp. 2017;Online only1‐16. [Google Scholar]
- 12. Lewczuk P, Riederer P, O'Bryant SE, et al., Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the consensus of the task force on biological markers in psychiatry of the world federation of societies of biological psychiatry. World J Biol Psychiatry. 2018;19(4):244‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boada M, Santos‐Santos MA, Rodríguez‐Gómez O, et al., Patient engagement: the Fundació ACE framework for improving recruitment and retention in Alzheimer's disease research. J Alzheimers Dis. 2018;62(3):1079‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ACE Alzheimer Center Barcelona . Qué hacemos: Jornada de puertas abiertas. https://www.fundacioace.com/es/jornada‐de‐puertas‐abiertas.html. Accessed 20 May 2021.
- 15. Alegret M, Muñoz N, Roberto N, et al., A computerized version of the Short Form of the Face‐Name Associative Memory Exam (FACEmemory®) for the early detection of Alzheimer's disease. Alzheimers Res Ther. 2020;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boada M, Rodrigo A, Jessen F, et al., Complementary pre‐screening strategies to uncover hidden prodromal and mild Alzheimer's disease: Results from the MOPEAD project. Alzheimers Dement. ;n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wimo A, Belger M, Bon J, et al, A cost‐consequence analysis of different screening procedures in Alzheimer's disease: results from the MOPEAD project. J Alzheimers Dis. 2021;83(3):1149‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alegret M, Espinosa A, Ortega G, et al., From face‐to‐face to home‐to‐home: Validity of a teleneuropsychological battery. J Alzheimers Dis. 2021;81(4):1541‐1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balasa M, Ripoll GP, Guix JLM, et al. Guies clíniques d´us de biomarcadors de malaltia d´Alzheimer en el líquid cefalorraquidi en l´avaluació de pacients amb deteriorament cognitiu. https://www.academia.cat/files/204‐3749‐FITXER/GUIESCLNIQUESUSBIOMARCADORSJULIOL2015DEFINITIVA.pdf. Accessed 22 September 2021.
- 20. Dubois B, Villain N, Frisoni GB, et al., Clinical diagnosis of Alzheimer's disease: recommendations of the International Working Group. Lancet Neurol. 2021;20(6):484‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. A.s.D.I. (ADI) . Spanish government launches national plan to tackle dementia. https://www.alzint.org/news‐events/news/spanish‐government‐launches‐national‐plan‐to‐tackle‐dementia/#:~:text=Type%3A%20News‐,The%20Spanish%20Ministry%20of%20Health%2C%20Consumption%20and%20Social%20Welfare%20has,now%20and%20in%20the%20future. Accessed 15 October 2019.
- 22. National Board of Health and Welfare (NBHW) . Nationella riktlinjer för vård och omsorg vid demenssjukdom. https://www.socialstyrelsen.se/regler‐och‐riktlinjer/nationella‐riktlinjer/riktlinjer‐och‐utvarderingar/demens/. Accessed August 2021.
- 23. Hansson O, Rutz S, Zetterberg H, et al., Pre‐analytical protocol for measuring Alzheimer's disease biomarkers in fresh CSF. Alzheimers Dement (Amst). 2020;12(1):e12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. SveDem . Swedish registry for cognitive/dementia disorders, SveDem. https://www.ucr.uu.se/svedem/in‐english. Accessed August 2021.
- 25. Zetterberg H, Blennow K, Moving fluid biomarkers for Alzheimer's disease from research tools to routine clinical diagnostics. Mol Neurodegener. 2021;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andreasen N, Blennow K, Zetterberg H, Neuroinflammation screening in immunotherapy trials against Alzheimer's disease. Int J Alzheimers Dis. 2010;2010638379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. M.o.H.a.S. Affairs . Dementia strategy focusing on care. https://www.government.se/articles/2018/07/dementia‐strategy‐focusing‐on‐care/. Accessed 13 March 2022.
- 28. Nitrini R, Barbosa MT, Dozzi Brucki SM, et al., Current trends and challenges on dementia management and research in Latin America. J Glob Health. 2020;10(1):010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prince M, Bryce R, Albanese E, et al., The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63‐75.e62. [DOI] [PubMed] [Google Scholar]
- 30. Nitrini R, Bottino CM, Albala C, et al., Prevalence of dementia in Latin America: a collaborative study of population‐based cohorts. Int Psychogeriatr. 2009;21(4):622‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baez S, Ibáñez A, Dementia in Latin America: an emergent silent tsunami. Front Aging Neurosci. 2016;8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacinto AF, Brucki S, Porto CS, et al., Detection of cognitive impairment in the elderly by general internists in Brazil. Clinics (Sao Paulo). 2011;66(8):1379‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parra MA, Baez S, Sedeño L, et al., Dementia in Latin America: paving the way toward a regional action plan. Alzheimers Dement. 2021;17(2):295‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parra MA, Baez S, Allegri R, et al., Dementia in Latin America: assessing the present and envisioning the future. Neurology. 2018;90(5):222‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nitrini R, Caramelli P, Herrera E, Jr. , et al., Incidence of dementia in a community‐dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2004;18(4):241‐246. [PubMed] [Google Scholar]
- 36. Nitrini R, Bucki SMD, Yassuda MS, et al., The Figure Memory Test: diagnosis of memory impairment in populations with heterogeneous educational background. Dement Neuropsychol. 2021;15(2):173‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peles P, Salvador L, de Souza L, et al., Accuracy of the brief cognitive screening battery in the diagnosis of Alzheimer's disease defined by cerebrospinal fluid biomarkers and AT(N) classification: a case‐control study. Arq Neuropsiquiatr.. 2022;80(1):23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kourtis LC, Regele OB, Wright JM, et al., Digital biomarkers for Alzheimer's disease: the mobile/wearable devices opportunity. NPJ Digit Med. 2019;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. A.s.D.I. (ADI) . #WhatsYourPlan https://www.alzint.org/news‐events/news/whatsyourplan/. Accessed 13 March 2022.
- 40. Brooke J, Ojo O, Contemporary views on dementia as witchcraft in sub‐Saharan Africa: a systematic literature review. J Clin Nurs. 2020;29(1‐2):20‐30. [DOI] [PubMed] [Google Scholar]
- 41. Guerchet MM, Mayston R, Lloyd‐Sherlock P, et al. Dementia in sub‐Saharan Africa: challenges and opportunities. https://www.alzint.org/resource/dementia‐in‐sub‐saharan‐africa/. Accessed August 2021.
- 42. Feng L, Li J, Yu J‐T, et al., Prevention of Alzheimer's disease in Chinese populations: status, challenges and directions. J Prev Alzheimers Dis. 2018;5(2):90‐94. [DOI] [PubMed] [Google Scholar]
- 43. Pendlebury ST, Rothwell PM, Oxford vascular study, Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population‐based Oxford Vascular Study. Lancet Neurol. 2019;18(3):248‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ihara M, Washida K, Linking atrial fibrillation with Alzheimer's disease: epidemiological, pathological, and mechanistic evidence. J Alzheimers Dis. 2018;62(1):61‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu D, Li L, An L, et al., Urban–rural disparities in mild cognitive impairment and its functional subtypes among community‐dwelling older residents in central China. Gen Psychiatry. 2021;34(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jia J, Zuo X, Jia XF, et al., Diagnosis and treatment of dementia in neurology outpatient departments of general hospitals in China. Alzheimers Dement. 2016;12(4):446‐453. [DOI] [PubMed] [Google Scholar]
- 47. Chen S, Boyle LL, Conwell Y, et al., Dementia care in rural China. Ment Health Fam Med. 2013;10(3):133‐141. [PMC free article] [PubMed] [Google Scholar]
- 48. A.s.D.I. (ADI) . China adopts a national dementia plan. https://www.alzint.org/news‐events/news/china‐adopts‐a‐national‐dementia‐plan/. Accessed 28 March 2022.
- 49. Mukadam N, Sommerlad A, Huntley J, et al., Population attributable fractions for risk factors for dementia in low‐income and middle‐income countries: an analysis using cross‐sectional survey data. Lancet Glob Health. 2019;7(5):e596‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. The World Bank . Population, total. https://data.worldbank.org/indicator/SP.POP.TOTL. Accessed 2 July 2021.
- 51. The World Bank . Population ages 65 and above (% of total population). https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS?view=chart. Accessed 6 July 2021.
- 52. The World Bank . Life expectancy at birth, total (years). https://data.worldbank.org/indicator/SP.DYN.LE00.IN?view=chart. Accessed 2 July 2021.
- 53. The World Bank . School enrollment, primary (% gross). https://data.worldbank.org/indicator/SE.PRM.ENRR?view=chart. Accessed 6 July 2021.
- 54. The World Bank . School enrollment, secondary (% gross). https://data.worldbank.org/indicator/SE.SEC.ENRR?view=chart. Accessed 6 July 2021.
- 55. The World Bank . School enrollment, tertiary (% gross). https://data.worldbank.org/indicator/SE.TER.ENRR?view=chart. Accessed 6 July 2021.
- 56. The World Bank . GDP per capita (current US$). https://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 2 July 2021.
- 57. The World Bank . Gini index (World Bank estimate). https://data.worldbank.org/indicator/SI.POV.GINI?view=chart. Accessed 6 July 2021.
- 58. The World Bank . Hospital beds (per 1,000 people). https://data.worldbank.org/indicator/SH.MED.BEDS.ZS?view=chart. Accessed 6 July 2021.


