Abstract
Pregnancy confers unique immune responses to infection and vaccination across gestation. To date, there are limited data comparing vaccine- and infection-induced neutralizing Abs (nAbs) against COVID-19 variants in mothers during pregnancy. We analyzed paired maternal and cord plasma samples from 60 pregnant individuals. Thirty women vaccinated with mRNA vaccines (from December 2020 through August 2021) were matched with 30 naturally infected women (from March 2020 through January 2021) by gestational age of exposure. Neutralization activity against the 5 SARS-CoV-2 spike sequences was measured by a SARS-CoV-2–pseudotyped spike virion assay. Effective nAbs against SARS-CoV-2 were present in maternal and cord plasma after both infection and vaccination. Compared with WT spike protein, these nAbs were less effective against the Delta and Mu spike variants. Vaccination during the third trimester induced higher cord-nAb levels at delivery than did infection during the third trimester. In contrast, vaccine-induced nAb levels were lower at the time of delivery compared with infection during the first trimester. The transfer ratio (cord nAb level divided by maternal nAb level) was greatest in mothers vaccinated in the second trimester. SARS-CoV-2 vaccination or infection in pregnancy elicits effective nAbs with differing neutralization kinetics that are influenced by gestational time of exposure.
Keywords: COVID-19, Infectious disease
Keywords: Adaptive immunity, Immunoglobulins
Introduction
Pregnant women are identified as an at-risk population for severe COVID-19, with increased rates of admission to the intensive care unit, invasive mechanical ventilation, and death (1–4). COVID-19 infection has exacerbated long-standing perinatal inequities among communities that already experienced higher rates of maternal mortality and morbidity and poor infant outcomes (5, 6). All leading professional and public health organizations have strongly recommended that pregnant individuals be vaccinated against SARS-CoV-2 (7). Although pregnant individuals were excluded from the first clinical trials of COVID-19 vaccines, a growing body of evidence indicates that fully vaccinated pregnant women have a significantly lower risk of SARS-CoV-2 infection, due to the generation of robust humoral and cellular immunity (8–11). Furthermore, maternal IgG Abs induced after vaccination and natural infection can be detected in umbilical cord blood of newborns at birth (10, 12). These Abs may protect newborns from SARS-CoV-2 infection in early life.
Two FDA-authorized mRNA vaccines that encode the SARS-CoV-2 spike protein, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), exhibit greater than 90% efficacy against cases of COVID-19 up to at least 4 months (13). However, genetic mutations in the spike protein change the transmissibility and sensitivity to neutralizing Abs (nAbs) elicited by the vaccines or natural infection. The WHO designates the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) variants as variants of concern to help track SARS-CoV-2 genetic lineages. The Delta variant is highly contagious and led to the 2021 resurgence of COVID-19 worldwide. Early data suggest sera from fully vaccinated or convalescent nonpregnant individuals have reduced neutralizing activity against the Delta variant compared with the Alpha variant (14). The Kappa variant (B.1.617.1) originated from the same lineage as the Delta variant and is categorized as a variant under monitoring, based on the epidemiological significance (15). The newly recognized Mu variant of interest (B.1.621) is described as 10.6-fold and 9.1-fold more resistant to sera from people convalescing from COVID or from those who received the BNT162b2 vaccine, respectively, compared with the ancestral WT virus (16).
A robust humoral response elicited by SARS-CoV-2 infection and vaccination, and efficient IgG transfer in pregnancy have been widely reported (10, 17–21). Previous studies indicate that the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein contains multiple conformational neutralizing epitopes (22). These include sites where single amino acid mutations including N501 (Alpha and Delta variants), L452 (Kappa and Delta variants), and E484 (Kappa and Mu variants) compromise Ab-mediated neutralization and/or transmission (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.157354DS1). The N501Y mutation is the only mutation in the interface between the RBD of the Alpha variant and does not have strong impact on the activity of most nAbs induced by natural infection and vaccination but improves viral transmission (23, 24). The Kappa and Delta variants possess the L452R mutation, which modestly affects nAb binding affinity (25). The Kappa variant possesses the E484Q mutation, which reduces sensitivity to vaccine-elicited nAbs, but the L452R and E484Q mutations are not synergistic for loss of sensitivity to nAbs (26). The Mu variant harbors the E484K mutation, which significantly reduces sensitivity to nAbs (27, 28). Furthermore, the Delta variant has the specific T478K mutation, which improves viral interaction with angiotensin-converting enzyme 2 (ACE2), but there is little knowledge about its potential role in resistance to neutralization by Abs (29). The Mu variant also has the specific R346K mutation, which may affect its sensitivity to nAbs (30).
Findings from previous studies indicated that maternal IgG production and maternal–fetal transfer might be influenced by timing of exposure, fetal sex, or Ab glycosylation profiles (11, 31, 32). However, the neutralizing activity against different SARS-CoV-2 variants during pregnancy and the transplacental transfer efficacy over gestation remains understudied. In this study, we assessed nAb activity against 5 strains of SARS-CoV-2, including the WT (Wuhan-Hu-1), Alpha, Kappa, Delta, and Mu variants in paired maternal and infant cord-blood plasma samples collected at the time of delivery. To understand the impact of timing during pregnancy on the development of maternal–fetal humoral immunity, we compared 2 cohorts that were matched by gestational age of exposure. Using samples from vaccinated and infected pregnant individuals, we compared and contrasted the nAbs elicited by mRNA vaccine with those elicited by natural infection, their efficacy against newly emerging variants, impact of the timing of vaccination or infection on maternal and neonatal protection, and clinical correlates of nAb production.
Results
Participant characteristics.
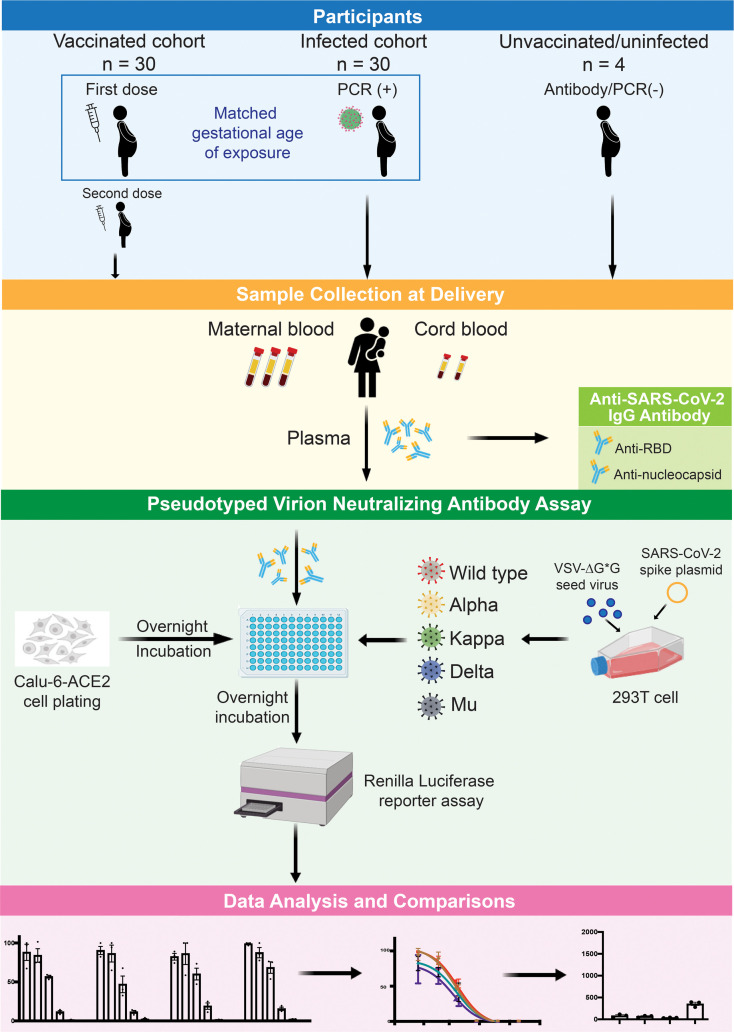
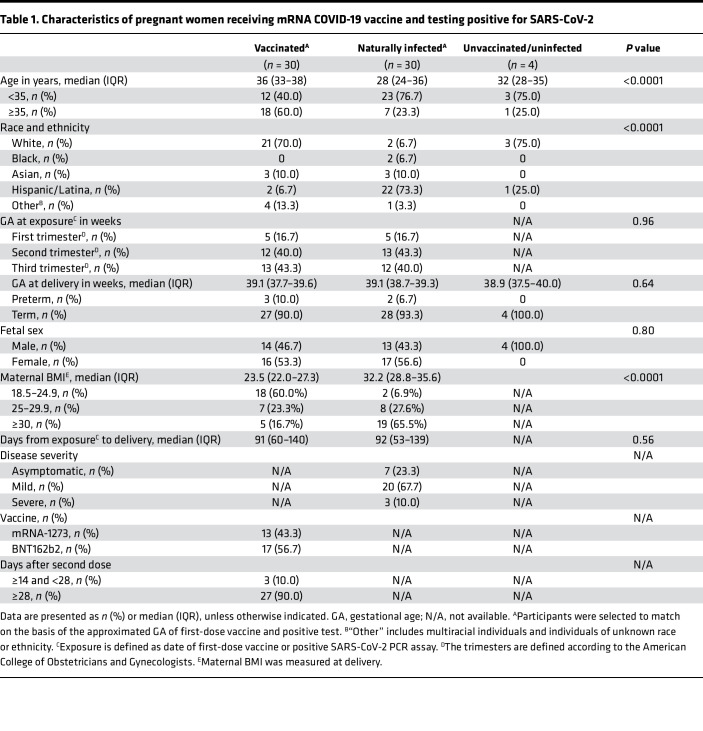
A total of 64 maternal–fetal dyads were included in this study (Table 1). Among the 30 vaccinated pregnant women, 5 (16.7%) received the first vaccine dose in the first trimester (from 0 to 13 weeks and 6 days), 12 (40.0%) in the second (from 14 weeks to 27 weeks and 6 days), and 13 (43.3%) in the third (from 28 weeks to 40 weeks and 6 days). All vaccinated individuals tested negative for anti-nucleocapsid IgG at the time of delivery when we collected the blood samples. In the 30 matched, naturally infected pregnant women, 5 (16.7%) had their first positive PCR result in the first trimester, 13 (43.3%) in the second, and 12 (40.0%) in the third. Maternal age was significantly older in the vaccinated group compared with the infected group (36 years vs. 28 years; P < 0.01). The participants were from all racial or ethnic groups; however, 70.0% (n = 21 of 30) vaccinated participants were White, and 73.3% (n = 22 of 30) of naturally infected participants were Hispanic or Latina (P < 0.01). Participants delivered at a median of 91 days (IQR, 60–140 days) after the first dose of vaccine from vaccinated mothers (n = 30 dyads), and 92 days (IQR, 53–139 days) after the earliest positive test from infected mothers (n = 30 dyads) (P = 0.56). Individuals with infection during pregnancy were more likely to be obese before delivery compared with the vaccinated group (median BMI, 32.2 vs. 23.5; P < 0.01). There were no differences between groups with respect to gestational age at delivery or fetal sex. Among participants infected with SARS-CoV-2, 7 (23.3%) were asymptomatic, 20 (67.7%) experienced mild disease, and 3 (10.0%) experienced severe disease (defined as having evidence of COVID-19 pneumonia, hypoxia (O2 saturation <94%), and/or need for intensive care). Four dyads who were unvaccinated and with no evidence of prior infection by serology recruited early in the pandemic served as negative controls.
Table 1. Characteristics of pregnant women receiving mRNA COVID-19 vaccine and testing positive for SARS-CoV-2.
Maternal and cord blood nAbs elicited by vaccination and infection.
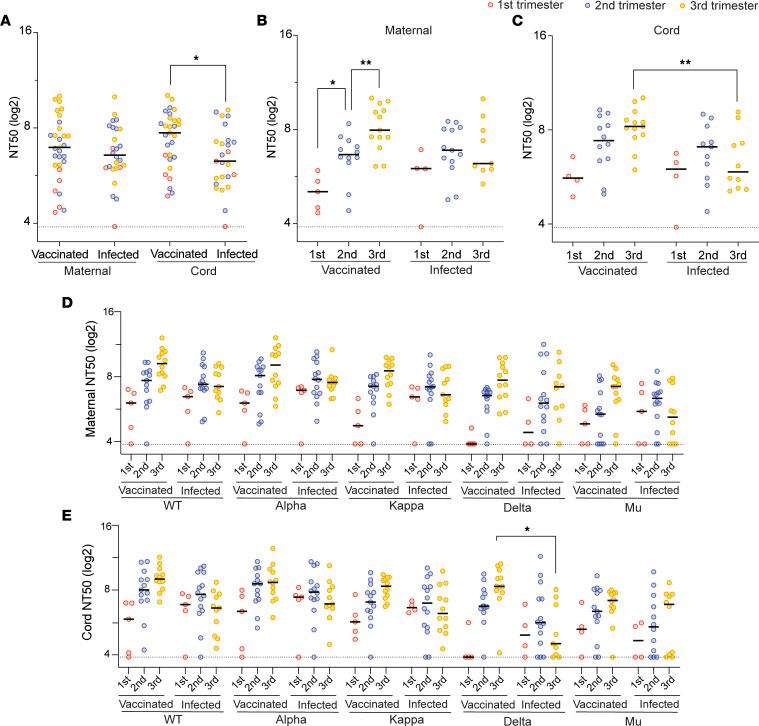
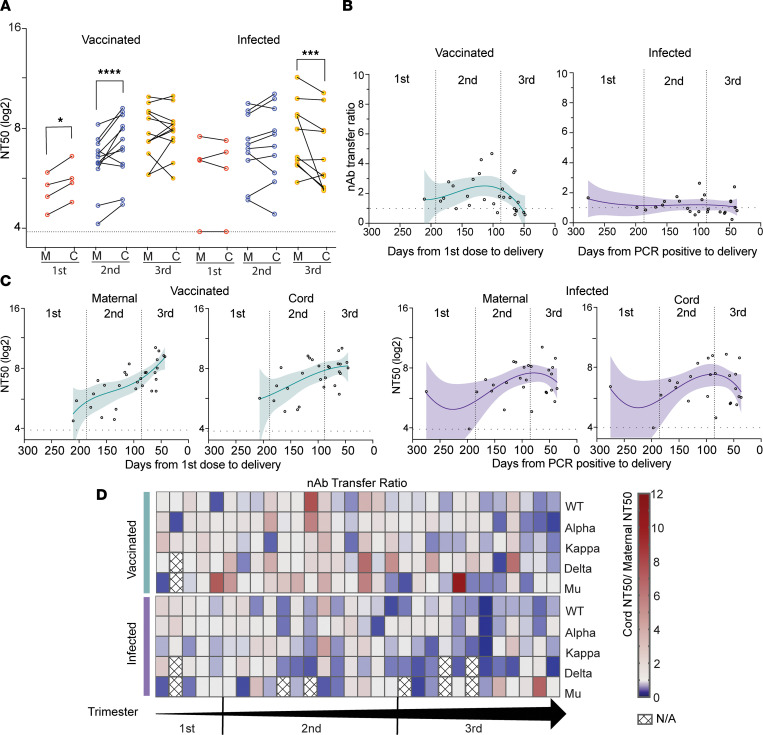
We assessed the ability of paired maternal and infant cord plasma to neutralize entry of SARS-CoV-2–pseudotyped virions into Calu-6-ACE2 target cells and compared the neutralization potency of plasma from the vaccinated and infected participants. No neutralizing activity was detected in the 4 unvaccinated, uninfected maternal and infant dyad samples (Supplemental Figure 1B). The composite of mean nAb titer at 50% inhibition (NT50) against all examined strains was higher in cord blood from vaccinated mothers than those from infected mothers (211 vs. 78; P = 0.03), but not in maternal blood (123 vs. 95; P = 0.51) (Figure 1A).
Figure 1. Neutralizing activity of maternal and cord plasma samples from the vaccination cohort and infection cohort.
(A) Comparison of the mean NT50 titers against all examined strains between the vaccinated (n = 30) and infected (n = 26) groups. The dot plots show mean NT50 values against variants of each patient in maternal plasma and cord plasma. Black bars represent the median of mean NT50 values. (B and C) Mean NT50 values of the maternal and cord blood were compared by 3 trimesters separately. The dot plots show mean NT50 values against all variants of each patient. Black bars represent the median of mean NT50 values. (D and E) Comparison of the NT50 values by trimester and by 5 strains in the maternal blood (D) and cord blood (E). The dot plots show NT50 values against each variant (vaccinated group: n = 30 for the WT strain and the Alpha, Kappa, Delta, and Mu variants; infected group: n = 30 for the WT strain and the Alpha and Kappa variants, n = 27 for the Delta variant, and n = 26 for the Mu variant). Black bars represent the median of NT50 values. The dotted lines indicate the cutoff threshold of this assay. Trimester of exposure is indicated by color (red, first trimester; blue, second trimester; yellow, third trimester). *P < 0.05; **P < 0.01; *** P < 0.001; ****P < 0.0001 by Mann-Whitney U test or Friedman’s test with Dunn’s multiple comparisons.
The total amount of neutralizing activity detected in maternal plasma collected at the time of delivery increased with vaccinations later in pregnancy, with the highest maternal NT50 values in women who were vaccinated in the third trimester (Figure 1B). A similar trend was observed in cord plasma, although this did not reach statistical significance (Figure 1C). In the infected cohort, there were no statistically significant differences in maternal (Figure 1B) and cord (Figure 1C) NT50 values when analyzed by trimester of infection. Cord blood nAb activity at delivery was higher after third trimester vaccination compared with third-trimester infection.
We then analyzed the NT50 values against each variant individually (Figure 1, D and E). The trend of higher maternal and cord blood nAb activity after vaccination compared with infection was seen in all 5 strains in the third trimester. These results suggest vaccination elicits higher titers of nAbs in pregnant individuals within the first few weeks than does infection, but the titer waned gradually throughout pregnancy. In contrast, infection-elicited Abs remained at a relatively stable level in maternal and cord blood until delivery.
Neutralizing activity against 4 variants in pregnancy.
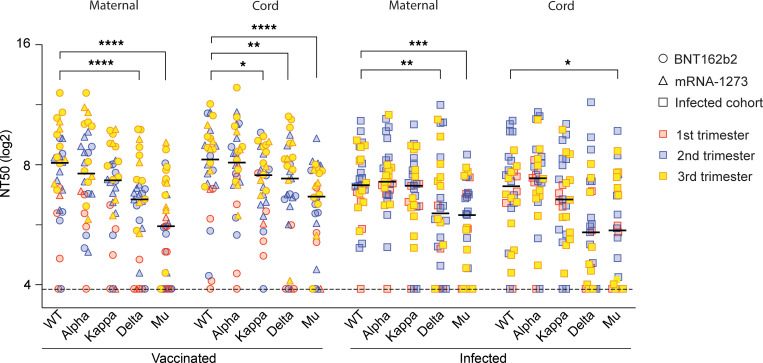
Emerging variants with mutations in the SARS-CoV-2 RBD raise concern about the efficacy of vaccine-induced and natural immunity, but the nAb activity against these variants in pregnancy is unknown. Here, we analyzed the nAb activity against the spike proteins from the Alpha, Kappa, Delta, and Mu variants in pregnant individuals. The NT50 values of each variant for all patients are provided in Supplemental Table 1. In the vaccinated cohort, maternal and cord blood nAbs against the Kappa spike variant were reduced by 1.7-fold (P = 0.50) and 1.7-fold (P = 0.05), respectively, compared with the WT strain. The anti-Delta variant was reduced by 2.9-fold (P < 0.01) and 1.8-fold (P < 0.01) in maternal and cord blood, respectively. The Mu variant showed the greatest resistance to nAb inhibition, with maternal and cord plasma having 4.9-fold (P < 0.01) and 3.0-fold (P < 0.01) reductions in NT50 values, respectively (Figure 2). nAb activity against the Alpha variant was not significantly different from that of the WT strain. Five of 17 women (29.4%) who received the BNT162b2 vaccine did not demonstrate nAb activity for at least 1 strain, and 3 women of 13 (23.1%) who received the mRNA-1273 vaccine did not have detectable nAb activity for at least 1 strain in maternal blood. There was no difference for the median NT50 values between the 2 vaccines in maternal or infant cord blood.
Figure 2. SARS-CoV-2 nAb activity in plasma from vaccinated and infected pregnant women and infant cord blood dyads.
Among a vaccinated cohort and an infected cohort, we matched the timing of the first vaccination to the timing of the infection. Neutralization assays were performed by exposing Calu-6-ACE2 cells with pseudotyped virions displaying the WT SARS-CoV-2 spike, or Alpha, Kappa, Delta, or Mu variants to serial dilutions of plasma. NT50 values of the plasma samples were defined as the sample dilution at which a 50% reduction (in RLUs) was observed relative to the average of the virus control wells. Triplicates were performed for each tested serum dilution. The dot plots represent NT50 values against each variant. Black bars represent the median NT50 values. Trimester of exposure is indicated by color (red, first trimester; blue, second trimester; yellow, third trimester). Type of exposure is indicated by different shapes (circle, BNT162b2; triangle, mRNA-1273; rectangle, infection). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 by Friedman’s test with Dunn’s multiple comparisons.
Among the infected cohort, maternal and cord blood nAbs against the Kappa variant were reduced by 1.1-fold (P > 0.99) and 1.5-fold (P > 0.99), respectively, compared with the WT strain. Activity against the Delta variant was reduced by 2.1-fold (P < 0.01) and 3.1-fold (P = 0.22), and that against the Mu variant was reduced by 2.5-fold (P < 0.01) and 3.0-fold (P = 0.03), in maternal and cord blood, respectively (Figure 2). Nine of 30 infected women (30.0%) did not exhibit nAb activity against at least 1 of the 5 strains tested.
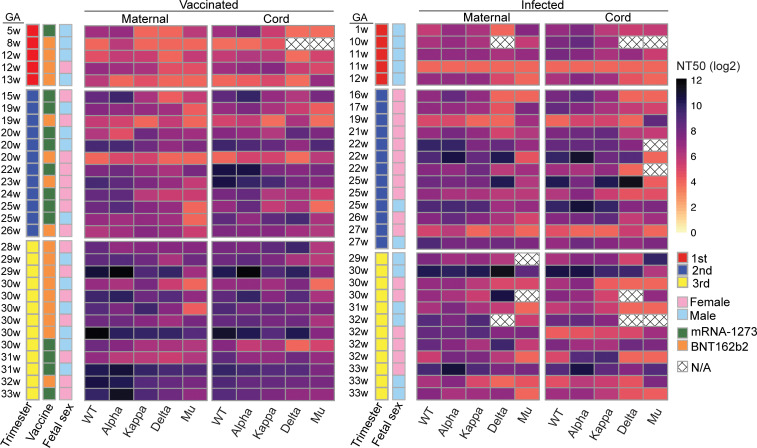
Next, we examined individual variability in nAb response across the 5 strains in maternal and cord blood in the context of exposure type (i.e., vaccination vs. infection) and trimesters of exposure (Figure 3). Participants were ordered by increasing gestational week and grouped by trimesters. Different levels of nAb responses to the variants were observed among different individuals. However, we did not observe a significant difference in the variance in nAb response on an individual level between vaccinated and infected cohorts in maternal (P = 0.81) or cord (P = 0.53) blood (Supplemental Figure 2, A and B), or across trimesters of both vaccination and infection cohorts (for maternal blood, P = 0.13; for cord blood, P = 0.37) (Supplemental Figure 2, C and D). In addition, we did not observe a significant interaction between vaccinated or infected cohort and trimester of exposure (for maternal blood, P = 0.17; for cord blood, P = 0.36; data not shown). Although each participant had variable nAb response to different SARS-CoV-2 strains, neither the vaccination nor the infection cohort nor trimester of exposure significantly contributed to this variance.
Figure 3. Variance in neutralizing activity in maternal and cord plasma from vaccination cohort and infection cohort.
Paired maternal and cord blood NT50 titers against each SARS-CoV-2 strain of each vaccinated patient and each infected patient were matched and displayed as a single row, ordered by increasing gestational age (GA), grouped by trimester. Variability of NT50 was compared using the Brown-Forsythe test. Patient characteristics are indicated by color (trimester of exposure: red, first trimester; blue, second trimester; yellow, third trimester. Vaccine type is indicated by color (green, mRNA-1273; orange, BNT162b2). Fetal sex is indicated by pink for female fetus; blue for male fetus). N/A, samples were not sufficient.
Transplacental transfer of maternal nAbs.
Reports have shown that maternal to fetal transfer of IgG Abs was effective and was affected by the timing of SARS-CoV-2 infection or vaccination (31–33), but the transfer efficiency of nAb induced from vaccination versus infection across gestation has not been well characterized. To address this question, we compared the cord and maternal blood nAbs across 3 trimesters to gain a deeper understanding of the degree of neonatal immunity conferred by maternal SARS-CoV-2 infection or vaccination.
In the vaccinated cohort, matched cord blood NT50 values were 1.6-fold higher (P = 0.02) compared with maternal blood NT50 values in mothers vaccinated during the first trimester and 1.7-fold higher (P < 0.01) during the second trimester, suggesting increased transfer efficiency for mothers vaccinated during this time (Figure 4A). In the third trimester, no significant difference was found between maternal and cord blood NT50 values. There were no detectable differences between transfer of neutralizing activity against the WT strain, and the Alpha, Delta, and Mu variants individually (Supplemental Figure 3A). In the infected cohort, cord blood NT50 values were not higher than maternal values in any trimester; in contrast, maternal blood NT50 values were 1.5-fold higher (P < 0.01) than cord blood NT50 values with third-trimester infections (Figure 4A), suggesting less efficient transfer of infection-elicited nAbs than vaccine-elicited nAbs. Similar to vaccinated mothers, no difference in transfer of neutralizing activity against the different strains was noted (Supplemental Figure 3B). There was no difference noted in transfer efficiency between the BNT162b2 and mRNA-1273 vaccines (data not shown).
Figure 4. Placental transfer of SARS-CoV-2 Abs.
(A) The effect of trimester on Ab transfer. The dot plots show NT50 titers for a cohort of vaccinated maternal–cord blood pairs. Three separate analyses were performed on the trimester at time of first exposure (red, first trimester; blue, second trimester; yellow, third trimester). Lines connect the maternal–cord blood dyads. Significance was determined by the Wilcoxon signed-rank test. (B) Maternal–cord blood TRs of nAbs were calculated by cord NT50 divided by maternal NT50 to assess efficiency of nAb transfer. nAb TRs are shown by timing of the first vaccine dose or the first positive PCR result. The dot plots represent mean TR of nAbs against variants. Correlations between the cord to maternal nAb ratio and days from events were analyzed using nonlinear regression analysis. (C) Maternal and cord blood NT50 values are shown by timing of the first vaccine dose or the first positive PCR result in the vaccinated or infected group. (D) Patient-based nAb TRs for each SARS-CoV-2 strain in both vaccinated and infected patients, showing the variance in nAb TR on an individual level. All patients were ordered according to increasing gestational age within their groups. *P < 0.05; ***P < 0.001; ****P < 0.0001 by Wilcoxon signed-rank test. C, cord blood; M, maternal blood; N/A, samples were not sufficient.
To further evaluate the correlation between in utero transfer efficiency of nAbs and the timing of exposure, we analyzed nAb transfer ratios (TRs) based on the days from exposure to delivery. TR is calculated by cord NT50 value divided by maternal NT50 value. Interestingly, the regression lines were distinct between the vaccinated and infected groups (Figure 4B), demonstrating different transfer kinetics of vaccination- and infection-elicited nAbs. We found that the TR was less than 1 when mothers were vaccinated in the third trimester and less than 60 days prior to delivery. When more than 60 days passed from first vaccination dose to delivery, 73% of the TRs were greater than 1. Peak TR in our vaccinated cohort was vaccination with the first dose at 114 days prior to delivery (TR = 2.5), which corresponded to 24 weeks’ gestational age. Compared with vaccination, infection-induced Ab transfer did not show significant change over gestation. When we analyzed the maternal and cord blood NT50 values based on the days from exposure to delivery, they were increased with the approach of delivery in the vaccinated group (Figure 4C).
To determine whether differences in nAb activity were explained by total IgG levels in patient samples, we analyzed the nAb titers relative to total anti–N and anti–RBD IgG Abs. Both maternal and cord blood WT NT50 titers were significantly correlated with anti–SARS-CoV-2 IgG titers (Supplemental Figure 3, C and D). However, greater variability was observed in the infected group, in which Pearson’s r was 0.51 (P < 0.01) for maternal plasma and 0.55 (P < 0.01) for cord plasma. In the vaccinated group, the correlation between NT50 and total IgG titers in maternal plasma was stronger than that in cord blood, with a Pearson’s r of 0.88 (P < 0.01) and 0.64 (P < 0.01), respectively. Furthermore, we plotted the correlation between TR of IgG Abs and the timing of exposure (Supplemental Figure 3E). The TR of nAbs across trimesters was consistent with the relatively higher correlation between total IgG titers and nAbs in vaccinated individuals, whereas no predictable correlation between total IgG and nAbs was observed in the infected group.
To obtain a deeper understanding about the transplacental transfer of maternal nAbs among SARS-CoV-2 strains, we calculated the TR of each strain and ordered the participants by gestational age (Figure 4D). In general, the TR was significantly higher in the vaccinated cohort compared with the infected cohort (P < 0.01) (Supplemental Figure 4A). TR was not significantly influenced by SARS-CoV-2 strains on its own, however, when a stratified analysis based on SARS-CoV-2 strains was conducted, the TR for nAbs against the Delta variant was significantly higher in the vaccinated cohort than in the infected cohort (P < 0.01) (Supplemental Figure 4B).
Impact of clinical factors on neutralizing activity.
We next explored the impact of clinical factors on maternal and cord blood nAb activity. First, we analyzed the effect of fetal sex, which has been reported to affect levels of total anti–SARS-CoV-2 IgG (11), on nAbs. However, nAb activity did not appear to differ with the sex of the fetuses, because no significant difference was observed after exposure in 3 trimesters (Supplemental Figure 5A). Of note, pregnant people in our cohort who were infected in the first trimester had male fetuses.
Because obesity is associated with chronic inflammation and dysfunctional immune responses, we next examined the relationship between maternal BMI and neutralizing activity, stratified by trimester. Vaccinated individuals with BMI between 25 and 29.9 at the time of delivery had higher NT50 values in both maternal and cord blood than did normal-weight individuals (BMI < 25) (for maternal blood, P < 0.01; for cord blood, P < 0.01) (Supplemental Figure 5B). There was not a significant difference based on maternal age (Supplemental Figure 5C). Then we analyzed the correlations between NT50 and maternal BMI and age with Spearman’s rank correlation in both groups, and no monotonic relationship was observed in maternal and cord plasma (P > 0.05) (Supplemental Figure 5, D and E).
Discussion
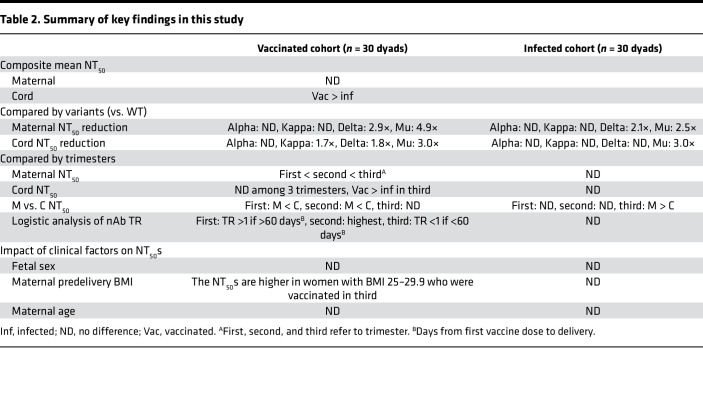
To our knowledge, this is the first study to directly compare nAb activity against 5 strains of SARS-CoV-2 in gestational age–matched vaccinated and infected maternal–fetal dyads across the entire course of gestation. Maternal vaccination and infection both produced effective nAbs against SARS-CoV-2, although activity against Delta and Mu variants was reduced compared with the WT strain. Overall, maternal nAb titers were comparable between vaccination and infection, whereas cord nAb titers were higher after vaccination than infection, supporting that vaccination during pregnancy benefits newborns as well as mothers. Mothers vaccinated in the third trimester had the highest level of nAbs at delivery in maternal and cord plasma. We found that the TR was maximal upon vaccination in the second trimester, whereas the TR of infection-induced nAbs was stable across gestation. However, vaccine-elicited nAbs waned gradually throughout pregnancy; both the titer and TR were lowest in mothers vaccinated in the first trimester. Maternal–fetal transfer efficiency was greater in vaccinated mothers than in infected mothers. Finally, our results demonstrate differences in the transfer of nAbs of differing specificity from mother to baby. These findings are summarized in Table 2.
Table 2. Summary of key findings in this study.
Our findings build upon those from a previous study with a cohort of 30 pregnant women vaccinated in the third trimester showing that mRNA vaccines were immunogenic in pregnancy but that neutralizing activity against the Alpha and Beta variants was reduced (34). Here, we provide data for all trimesters of pregnancy and report on nAb activity against the Delta variant, which remains predominant worldwide and accounts for greater than 99.5% of COVID cases during the fourth wave of the pandemic (35). One of the strengths of this study is the comparison of vaccine-induced versus natural immune responses to the Delta variant across pregnancy. We found reductions in nAb titers against the Delta variant in both vaccinated maternal blood and paired infant cord blood, a finding consistent with recent reports that the Delta variant is resistant to vaccines in the nonpregnant population as compared with the Alpha variant (14, 36). SARS-CoV-2 spike protein RBD is the major target of nAbs, and mutations in the RBD of the Delta variant reduce the neutralization sensitivity of plasma from vaccinated or infected individuals (14).
Furthermore, we have shown the activity of vaccinated and infection-induced Ab responses against the Mu variant in pregnancy. Our results confirm that in pregnancy Mu proves to be more resistant to neutralization than all other currently recognized variants (16, 37, 38). The Mu variant can cause cell-to-cell fusion, like the Delta variant, that facilitates escape from humoral immunity more efficiently than does the WT virus (37). The Mu variant also harbors 3 mutations in the RBD (R346K, E484K, and N501Y) that are not in the Delta variant (27). nAbs targeting the RBD have been divided into 4 classed based on structural analyses of whether antibody-bound RBD is oriented “up/open” conformation or “down/closed” conformation (39). “Up” conformation represents a receptor-accessible state, and “down” conformation represents a receptor-inaccessible state. Class 1 comprises nAbs that bind only to “up” RBDs; class 2 comprises ACE2-blocking nAbs that bind both “up” and “down” RBDs; class 3, nAbs that bind outside the ACE2 site and recognize both “up” and “down” RBDs; and class 4 comprises previously described Abs that do not block ACE2 and bind only to “up” RBDs (39). The E484K mutation can most strongly disrupt the binding of the class 2 Abs, whereas L452R is located in the class 3 site (40). In both vaccinated and convalescent populations, escape mutants targeting the class 2 Abs have the most critical impact on impaired neutralization (41). Developing vaccines that elicit polyclonal Abs with broader epitope specificities may improve the efficacy and longevity of current vaccines. Although the significant and sustained reduction in prevalence of the Mu variant indicates it is unlikely to be predominant relative to the Delta variant (42), there is a growing concern that current vaccines may need to be continually modified and updated against emerging variants with greater properties of immune escape and neutralizing resistance, especially for the vulnerable populations, such as pregnant individuals.
Interestingly, nAbs of different variant specificity have differing overall titers and maternal–fetal TRs, which also differ by type of exposure. In our pregnant cohorts, vaccination in the third trimester induced higher nAb levels at delivery, whereas NT50 values were lower with first-trimester vaccination compared with infection. The variation of nAb activity between trimesters was more significant in the vaccinated mothers. As reported in the nonpregnant individuals, the range of nAb activity elicited by vaccination is variable compared with that elicited by infection depending on the time after vaccination or infection (43). Consistent with prior studies in nonpregnant individuals (43), we found no differences in nAb specificity across variants between the 2 mRNA vaccines from the different manufacturers. More studies are needed to understand host factors that determine these variable maternal immune responses and transfer dynamics.
Neonates depend largely on passive immunity from maternal Abs due to functional immaturity of their immune system. Maternal–fetal transfer of IgG against SARS-CoV-2 after infection has been previously reported, with TR between 0.72 and 0.90 (20, 44, 45). However, greater than 85% of infected cases in these studies were diagnosed positive in the third trimester and close to delivery, which may not allow sufficient time for induction of maternal Ab responses and subsequent transfer. In our prior work with a cohort of pregnancies with natural infection, TRs of total IgG were significantly higher when the first maternal positive PCR was 60 to 180 days before delivery compared with less than 60 days (1.2 vs. 0.6; P < 0.0001) (33). Our results consistently demonstrated that TRs of total IgG were higher (>1.0) if the patients were vaccinated more than 60 days prior to delivery, and that the most efficient TRs of nAbs were found in the mothers who were vaccinated in the second and early third trimesters. By comparison, the TRs of nAbs were relatively stable after infection across all trimesters of pregnancy.
The differences between TRs of anti–SARS-CoV-2 total IgG Abs and nAbs suggest selective transfer of Abs across the placenta. The transport of IgG is thought to be carried out primarily by neonatal Fc receptor in the placental syncytiotrophoblast. Neonatal Fc receptor is an atypical Fc γ receptor that can bind the IgG Fc region and has variable affinity for different IgG subclasses (i.e., IgG1, IgG2, IgG3, and IgG4) regulated by many factors such as maternal IgG concentrations, disease states, infections, and timing of gestation (46–48). Also, Fc glycosylation states influence selective transfer, and reduced fucosylation was found on the Fc domain repertoire of IgG Abs produced by patients with COVID-19, resulting in enhanced interactions with the activating Fc γ receptor (49). It remains to be determined which subclass of IgG is the most effective in neutralizing SARS-CoV-2, and neonates might not be fully protected from SARS-CoV-2 infection even though amounts of Abs are detected in cord blood.
Ab transfer from mother to fetus depends on gestational age and maternal IgG levels. The transport happens in a linear fashion as the pregnancy progresses, with the largest amount transferred in the third trimester (46, 50, 51). Our results are consistent with these findings. Both maternal blood and cord blood have the highest amount of neutralizing activity in the third trimester. Interestingly, transfer efficiency appears to be greatest with second-trimester vaccination. This may be related to maximizing overall time available for maternal Ab production, as well as time for Abs to transfer and accumulate in the fetal circulation. As expected, the lowest amounts of neutralizing activity were noted at the time of delivery in maternal and cord blood after first-trimester vaccination and infection, consistent with the natural decline of overall Ab levels after initial exposure. On the whole, our results suggest that pregnant individuals should receive their first vaccine dose at any time during pregnancy to induce maternal protective responses, and that vaccination at least 60 days prior to their anticipated delivery date will also maximize transfer to the neonate. This may be important to some mothers because recent data have shown that maternal vaccination confers protection against neonatal hospitalization for COVID-19 up to 6 months of age (52).
As previously reported, effectiveness after only 1 dose of vaccine is much lower (30.7%–48.7%) among persons with Delta or Alpha variant infection (53). To gain the greatest protection for both mothers and neonates, pregnant people should also receive 2 vaccine doses before delivery. Because significantly decreased nAb was observed in the mothers vaccinated in the first trimester of pregnancy, a later booster dose would likely increase the levels of Abs transferred to the fetus for individuals who were vaccinated in early pregnancy. However, more studies are needed to evaluate the dynamics of Ab stimulation from boosters in pregnancy.
Of note, the patient demographics of the 2 cohorts were different in race and age. This reflects the population-based epidemiology of people who have highest rates of vaccine uptake and COVID-19 infection in the United States (54). This difference also speaks to the fact that there are structural factors (e.g., racism, inequitable access to COVID-19 personal protective equipment, testing, vaccines, quality health care) that place pregnant and postpartum women of color at greater risk for being exposed to COVID-19 (5, 6). Because vaccine efficacy was generally consistent across subgroups stratified by age, race, and ethnicity in the general population (55, 56), it is unlikely that these factors would affect the biologic immune response to an mRNA vaccine and SARS-CoV-2 in pregnancy. Maternal BMI at delivery was also significantly different between the vaccinated and infected cohorts. The majority (n = 27 of 29; 93%) of the mothers in the infected cohort were overweight, reflecting that obesity is a risk factor for disease severity in individuals with SARS-CoV-2 infection, because of reduced respiratory system compliance and impaired innate and adaptive immune responses (57–59). Also, communities of color are at higher risk of obesity because of food apartheid, limited access to healthy foods, food insecurity, and communities that do not have high walkability scores or green space. We found that the vaccinated individuals with BMI of 25–29.9 at delivery can produce the most nAb. Assuming that these patients were of normal BMI (18.5–24.9) prior to pregnancy, the result suggested that the vaccine response is most effective in this group compared with overweight or obese individuals.
A prior study showed that maternal SARS-CoV-2–specific IgG Ab production was reduced and placental Ab transfer was impaired significantly in pregnancies with a male fetus, in which all patients tested positive for SARS-CoV-2 upon admission for delivery (11), although in another study researchers did not observe a sex bias in placental Ab transfer after maternal SARS-CoV-2 exposure (60). Our study showed no significant difference in nAbs by fetal sex after exposure in either group. This may be explained by sex differences in the production of IgG Abs as a whole, but not the production or transfer of nAbs. More studies clearly are needed to understand this complex phenomenon.
There are several limitations and strengths of our study. First, our cohort is small, and we have few patients that were exposed to COVID-19 or vaccine in the first trimester. However, these elements are balanced against a significant strength: the participants were matched by timing of vaccine or live viral exposure during pregnancy, allowing direct comparison of vaccine-induced and natural immunity in pregnancy and different time points in pregnancy. Second, we do not have sequencing data for the natural infection cohort and thus do not know which strains caused the infection. However, all infected patients tested positive before January 2021, prior to broad circulation of the Alpha and Delta variants in the United States (61, 62). Presumably, individuals infected with the Delta variant would have higher specific nAb activity against this strain and may also have differing responses to the other variants. Future studies would be helpful to understand the immunogenicity evoked by the Delta, Mu, and other variants in pregnancy. Third, detailed Ab characterization, such as IgG subtyping or fucosylation state, is needed to understand the role of these factors in the dynamics of maternal–fetal Ab transfer. This should be addressed in future studies.
In conclusion, vaccination in pregnancy is highly effective in generating nAbs against all tested strains of SARS-CoV-2, although activity against the Delta and Mu variants is reduced. Vaccine-induced neutralizing activity is comparable to natural immunity; however, cord blood levels of nAb tend to be higher after vaccination, especially vaccination in the third trimester. Passive immunity to the neonate is maximized when vaccination was initiated at least 60 days prior to delivery. These results strengthen current recommendations to vaccinate all pregnant people against COVID-19.
Methods
Study design and sample collection.
Pregnant individuals who had a positive SARS-CoV-2 test by quantitative PCR were enrolled in our study from March 2020 through January 2021 from participating study sites. Pregnant individuals who received an mRNA-based COVID-19 vaccine were enrolled from December 2020 through August 2021 at UCSF. Thirty vaccinated and 30 COVID-19–infected pregnant women were chosen from these 2 cohorts to be matched on the approximate gestational age at first vaccine dose and the earliest confirmed SARS-CoV-2 test. Vaccinated participants were fully vaccinated (at least 14 days after the second dose) with the BNT162b2 (n = 17 of 30) or mRNA-1273 (n = 13 of 30) vaccine at the time of sample collection. Maternal blood and infant cord blood samples were collected at delivery in an EDTA collection tube and processed within 24 hours. Plasma was isolated from whole blood by centrifugation at 500g for 10 minutes, then aliquoted in the cryovial tubes and stored at –80°C until analysis. Clinical data were abstracted from the medical record.
Preparation of pseudotyped virions.
For the preparation of virions, 293T cells were transfected with the spike plasmid, followed by inoculation with a previously generated working stock of rVSVΔG-rLuc*G (G protein–deficient vesicular stomatitis virus) containing an integrated Renilla luciferase reporter gene) to generate the pseudotyped rVSVΔG-rLuc*SARS-CoV-2 (63). Pseudotyped virions were generated using spike plasmids harboring mutations found in the WT SARS-CoV-2 spike (Wuhan-Hu-1; GenBank accession number MN908947.3), the Alpha variant (H69 deletion, V70 deletion, Y144 deletion, N501Y, A570D, D614G, P681H, T716I, S982A, and D1118H), the Kappa variant (L452R, E484Q, D614G, P681R, and Q1071H), the Delta variant (T19R, G142D, E156 deletion, F157 deletion, R158G, L452R, T478K, D614G, P681R, and D950N), and the Mu variant (T95I, Y144T, Y145S, 146N insertion, R346K, E484K, N501Y, D614G, P681H, and D950N) (Supplemental Figure 1A). The 3 types of virions were titrated based on the TCID50 method and the infectivity titers were equalized.
Pseudotyped virion nAb assay.
To determine the neutralization activity of plasma, pseudotyped virion nAb experiments were performed with Calu-6 epithelial cells (ATCC HTB56) stably expressing human angiotensin-converting enzyme 2 (hACE2; OriGene, RC08442). Twenty-four hours before administration of virion, 2.5 × 104 Calu-6 hACE2 cells were plated per well of a 96-well plate in 200 μL of complete DMEM. The SARS-CoV-2 spike–pseudotyped virions harvested from the supernatant of the 293T cells were assayed for titration and then aliquots mixed for 30 minutes with heat-inactivated plasma samples. Plasma samples were diluted in calcium-free DMEM starting at a 1:15 dilution, and then at 3-fold serial dilutions for 6 final concentrations, in triplicate. The mixtures were then used to infect Calu-6-hACE2 cells and incubated at 37ºC and 5% CO2 for 24 hours. At 24 hours after infection, the cells were washed once with 1× PBS, then 20 μL of lysis buffer was added per well, followed by 100 μL of Renilla luciferase substrate/buffer (Promega, E2810) according to the manufacturer’s instructions. The plates were read on a luminometer. Results were analyzed by Prism software, version 9 (GraphPad Software). SARS-CoV-2 NT50 titers of the plasma samples were defined as the sample dilution at which a 50% reduction (in RLUs) was observed relative to the average of the virus control wells. All NT50 titers were calculated as an average of 3 independent experiments. The composite NT50 value was calculated by averaging the NT50 values of the WT strain and the 4 variants.
IgG Ab measurement.
Maternal and cord blood from both vaccinated and infected groups were measured for combined anti–RBD and anti–nucleocapsid IgG Abs using the Pylon 3D automated immunoassay system (ET Healthcare), as described previously (64). Maternal blood from vaccinated women at delivery was tested for anti–nucleocapsid IgG Abs to detect undiagnosed SARS-CoV-2 infection by human anti–N IgG ELISA kit (RayBiotech). All samples were run in technical replicates.
Statistics.
Differences between 3 SARS-CoV-2 strains using the same plasma samples were analyzed using the Wilcoxon signed-rank test. Differences between the vaccinated group and the infected group were analyzed using the Mann-Whitney U test. For comparisons of 3 or more groups, Friedman’s test with Dunn’s multiple comparisons was used. Correlations between NT50 titers and IgG Ab titers were analyzed using Pearson’s rank test. The variance of maternal NT50 titers among SARS-CoV-2 strains was calculated by deriving coefficient of variation values per participant and performing multiple linear regression to determine if the vaccinated or infected cohort or trimester of exposure was significantly associated with coefficient of variation values. To determine the trend of NT50 titers for maternal age and BMI, Spearman’s rank correlation was used. Maternal to cord blood nAb TRs were calculated by paired cord NT50/maternal NT50 titers to estimate transplacental transfer of nAbs. Multiple linear regression analysis was used to determine associations among TR and vaccination or infection cohorts or SARS-CoV-2 strains. Analyses were performed with GraphPad Prism. Heatmaps were created in R Studio and GraphPad Prism. P < 0.05 was considered statistically significant.
Study approval.
This study was approved by the IRB of UCSF (approval no. 20-32077), Santa Clara Valley Medical Center (approval no. 20-021), Oregon Health & Sciences University (approval no. STUDY00021569), and Marshall University (approval no. 1662248-1). Written informed consent was obtained from all participants. Demographic characteristics and clinical data were collected through questionnaires and medical record review.
Author contributions
YM, LL, WCG, and SLG designed the study. LL, MP, AGC, YG, VJG, CYL, UJ, MAC, LW, DS, PJ, DSR, BG, JN, MR, LM, IVA, and VJF recruited participants and collected samples. YM, LL, AGC, MM, TYT, MMK, BS, JMH, PC, GRK, and AHBW conducted experiments and acquired data. YM, LL, MP, NO, JRF, WCG and SLG analyzed data. YM, LL, NO, and SLG wrote the manuscript. MP, DS, PJ, DSR, IVA, VJF, YA, VLJ, APM, MO, WCG, and SLG provided funding. All authors assisted with editing the manuscript. WCG and SLG supervised the study. Authorship order for the co–first authors was determined by reverse alphabetical order.
Supplementary Material
Acknowledgments
We thank the mothers and infants who participated in this study. These studies were supported by the Bill and Melinda Gates Foundation (INV-017035 to SLG and VJF); the Marino Family Foundation (to MP); the NIH (National Institute of Allergy and Infectious Diseases grants K23AI127886 [to MP] and K08AI141728 [to SLG]); the Krzyzewski Family (to APM and SLG); UCSF National Center of Excellence in Women’s Health (to SLG, VJF, YA, and VLJ); the Roddenberry Foundation (YM and WCG); Centers for Disease Control and Prevention Foundation (to SLG, VJF, YA, and VLJ); the Valley Medical Center Foundation (to DS, PJ, and DSR), and individual donors who provided support through our crowdfunding websites: https://givingtogether.ucsf.edu/fundraiser/2718761 and https://spark.ucla.edu/project/20775.
Version 1. 05/17/2022
In-Press Preview
Version 2. 06/22/2022
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2022, Matsui et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2022;7(12):e157354.https://doi.org/10.1172/jci.insight.157354.
Contributor Information
Yusuke Matsui, Email: yusuke.matsui@gladstone.ucsf.edu.
Lin Li, Email: lin.li2@ucsf.edu.
Mary Prahl, Email: Mary.prahl@ucsf.edu.
Arianna G. Cassidy, Email: arianna.cassidy@ucsf.edu.
Nida Ozarslan, Email: Nida.Ozarslan@ucsf.edu.
Yarden Golan, Email: yarden.golanmaor@ucsf.edu.
Veronica J. Gonzalez, Email: veronica.gonzalez@ucsf.edu.
Christine Y. Lin, Email: christine.lin4@ucsf.edu.
Unurzul Jigmeddagva, Email: unurzul.jigmeddagva@ucsf.edu.
Megan A. Chidboy, Email: Megan.chidboy@ucsf.edu.
Mauricio Montano, Email: mauricio.montano@gladstone.ucsf.edu.
Taha Y. Taha, Email: taha.taha@gladstone.ucsf.edu.
Mir M. Khalid, Email: mir.khalid@gladstone.ucsf.edu.
Bharath Sreekumar, Email: bharath.sreekumar@gladstone.ucsf.edu.
Jennifer M. Hayashi, Email: jennifer.hayashi@gladstone.ucsf.edu.
Pei-Yi Chen, Email: Jesse.chen@gladstone.ucsf.edu.
G. Renuka Kumar, Email: renuka.kumar@gladstone.ucsf.edu.
Lakshmi Warrier, Email: lswarrier1@gmail.com.
Alan H.B. Wu, Email: alan.wu@ucsf.edu.
Dongli Song, Email: Dongli.Song@hhs.sccgov.org.
Priya Jegatheesan, Email: Priya.Jegatheesan@hhs.sccgov.org.
Daljeet S. Rai, Email: dsr@stanford.edu.
Balaji Govindaswami, Email: govindaswami@marshall.edu.
Monica Rincon, Email: rincon@ohsu.edu.
Leslie Myatt, Email: myattl@ohsu.edu.
Ifeyinwa V. Asiodu, Email: ifeyinwa.asiodu@ucsf.edu.
Valerie J. Flaherman, Email: valerie.flaherman@ucsf.edu.
Yalda Afshar, Email: yafshar@mednet.ucla.edu.
Vanessa L. Jacoby, Email: vanessa.jacoby@ucsf.edu.
Amy P. Murtha, Email: Amy.Murtha@ucsf.edu.
Joshua F. Robinson, Email: joshua.robinson@ucsf.edu.
Melanie Ott, Email: melanie.ott@gladstone.ucsf.edu.
Warner C. Greene, Email: Warner.Greene@gladstone.ucsf.edu.
Stephanie L. Gaw, Email: stephanie.gaw@ucsf.edu.
References
- 1. Centers for Disease Control and Prevention. Pregnant People At Increased Risk for Severe Illness from COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html Updated March 3, 2022. Accessed May 20, 2022.
- 2.Khan DSA, et al. The differences in clinical presentation, management, and prognosis of laboratory-confirmed COVID-19 between pregnant and non-pregnant women: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(11):5613. doi: 10.3390/ijerph18115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allotey J, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinn J, et al. Characteristics and outcomes of women with COVID-19 giving birth at US academic centers during the COVID-19 pandemic. JAMA Netw Open. 2021;4(8):e2120456. doi: 10.1001/jamanetworkopen.2021.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karasek D, et al. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Health Am. 2021;2:100027. doi: 10.1016/j.lana.2021.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The American College of Obstetricians and Gynecologists. Statement of Strong Medical Consensus for Vaccination of Pregnant Individuals Against COVID-19. https://www.acog.org/news/news-releases/2021/08/statement-of-strong-medical-consensus-for-vaccination-of-pregnant-individuals-against-covid-19 Updated September 14, 2021. Accessed May 20, 2022.
- 8.Goldshtein I, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theiler RN, et al. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray KJ, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bordt EA, et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci Transl Med. 2021;13(617):eabi7428. doi: 10.1126/scitranslmed.abi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhu M, et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol. 2021;138(2):278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. COVID-19 Vaccines and Vaccination. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html Updated September 15, 2021. Accessed May 20, 2022.
- 14.Planas D, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/? Updated May 18, 2022. Accessed May 20, 2022.
- 16.Uriu K, et al. Neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine serum. N Engl J Med. 2021;385(25):2397–2399. doi: 10.1056/NEJMc2114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zdanowski W, Wasniewski T. Evaluation of SARS-CoV-2 spike protein antibody titers in cord blood after COVID-19 vaccination during pregnancy in Polish healthcare workers: preliminary results. Vaccines (Basel) 2021;9(6):675. doi: 10.3390/vaccines9060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bwire GM, et al. Possible vertical transmission and antibodies against SARS-CoV-2 among infants born to mothers with COVID-19: a living systematic review. J Med Virol. 2021;93(3):1361–1369. doi: 10.1002/jmv.26622. [DOI] [PubMed] [Google Scholar]
- 19.Nir O, et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM. 2021;4(1):100492. doi: 10.1016/j.ajogmf.2021.100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flannery DD, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175(6):594–600. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon LC, et al. Relationship between viral load, infection-to-delivery interval and mother-to-child transfer of anti-SARS-CoV-2 antibodies. Ultrasound Obstet Gynecol. 2021;57(6):974–978. doi: 10.1002/uog.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greaney AJ, et al. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29(3):463–476. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng L, et al. Impact of the N501Y substitution of SARS-CoV-2 Spike on neutralizing monoclonal antibodies targeting diverse epitopes. Virol J. 2021;18(1):87. doi: 10.1186/s12985-021-01554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, et al. The N501Y spike substitution enhances SARS-CoV-2 transmission [preprint]. Posted on bioRxiv March 9, 2021. [DOI]
- 25.Li Q, et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182(5):1284–1294. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira I, et al. SARS-CoV-2 B.1.617 mutations L452R and E484Q are not synergistic for antibody evasion. J Infect Dis. 2021;224(6):989–994. doi: 10.1093/infdis/jiab368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 28. Collier DA, et al. SARS-CoV-2 B.1.1.7 sensitivity to mRNA vaccine-elicited, convalescent and monoclonal antibodies [preprint]. Posted on medRxiv February 15, 2021. [DOI]
- 29.Di Giacomo S, et al. Preliminary report on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike mutation T478K. J Med Virol. 2021;93(9):5638–5643. doi: 10.1002/jmv.27062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fratev F. The R346K mutation in the Mu variant of SARS-CoV-2 alter the interactions with monoclonal antibodies from class 2: a free energy of perturbation study. J Chem Inf Model. 2022;62(3):627–631. doi: 10.1021/acs.jcim.1c01243. [DOI] [PubMed] [Google Scholar]
- 31.Beharier O, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131(19):154834. doi: 10.1172/JCI154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atyeo C, et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184(3):628–642. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song D, et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: prospective cohort study. BMJ Open. 2021;11(7):e053036. doi: 10.1136/bmjopen-2021-053036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collier AY, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325(23):2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention. Monitoring Variant Proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed May 20, 2022.
- 36.Noori M, et al. Potency of BNT162b2 and mRNA-1273 vaccine-induced neutralizing antibodies against severe acute respiratory syndrome-CoV-2 variants of concern: a systematic review of in vitro studies. Rev Med Virol. 2022;32(2):e2277. doi: 10.1002/rmv.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miyakawa K, et al. Neutralizing efficacy of vaccines against the SARS-CoV-2 Mu variant [preprint]. Posted on medRxiv September 26, 2021. [DOI]
- 38.Messali S, et al. A cluster of the new SARS-CoV-2 B.1.621 lineage in Italy and sensitivity of the viral isolate to the BNT162b2 vaccine. J Med Virol. 2021;93(12):6468–6470. doi: 10.1002/jmv.27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes CO, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greaney AJ, et al. Mapping mutations to the SARS-CoV-2 RBD that escape binding by different classes of antibodies. Nat Commun. 2021;12(1):4196. doi: 10.1038/s41467-021-24435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greaney AJ, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13(600):eabi9915. doi: 10.1126/scitranslmed.abi9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html Updated April 26, 2022. Accessed May 20, 2022.
- 43.Wang Z, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph NT, et al. Maternal antibody response, neutralizing potency, and placental antibody transfer after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Obstet Gynecol. 2021;138(2):189–197. doi: 10.1097/AOG.0000000000004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edlow AG, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3(12):e2030455. doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmeira P, et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pyzik M, et al. The neonatal Fc receptor (fcrn): a misnomer? Front Immunol. 2019;10:1540. doi: 10.3389/fimmu.2019.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clements T, et al. Update on transplacental transfer of IgG subclasses: impact of maternal and fetal factors. Front Immunol. 2020;11:1920. doi: 10.3389/fimmu.2020.01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakraborty S, et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021;22(1):67–73. doi: 10.1038/s41590-020-00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saji F, et al. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod. 1999;4(2):81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 51.Malek A, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 52.Halasa NB, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 Months — 17 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez Bernal J, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Painter EM, et al. Demographic characteristics of persons vaccinated during the first month of the COVID-19 vaccination program — United States, December 14, 2020-January 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5):174–177. doi: 10.15585/mmwr.mm7005e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen CJ, et al. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura T, Namkoong H. Susceptibility of the obese population to COVID-19. Int J Infect Dis. 2020;101:380–381. doi: 10.1016/j.ijid.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med (Lond) 2020;20(4):e109–e113. doi: 10.7861/clinmed.2020-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gee S, et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat Immunol. 2021;22(12):1490–1502. doi: 10.1038/s41590-021-01049-2. [DOI] [PubMed] [Google Scholar]
- 61.Walensky RP, et al. SARS-CoV-2 variants of concern in the United States-challenges and opportunities. JAMA. 2021;325(11):1037–1038. doi: 10.1001/jama.2021.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borchering RK, et al. Modeling of future COVID-19 cases, hospitalizations, and deaths, by vaccination rates and nonpharmaceutical intervention scenarios — United States, April-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(19):719–724. doi: 10.15585/mmwr.mm7019e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Condor Capcha JM, et al. Generation of SARS-CoV-2 spike pseudotyped virus for viral entry and neutralization assays: a 1-week protocol. Front Cardiovasc Med. 2020;7:618651. doi: 10.3389/fcvm.2020.618651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch KL, et al. Magnitude and kinetics of anti-severe acute respiratory syndrome coronavirus 2 antibody responses and their relationship to disease severity. Clin Infect Dis. 2021;72(2):301–308. doi: 10.1093/cid/ciaa979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.