Abstract
Copper (Cu), an essential micronutrient, plays an essential role in several physiological processes, including cell proliferation and angiogenesis; however, its dysregulation induces oxidative stress and inflammatory responses. Significant Cu accumulation is observed in several tumor tissues. The bioavailability of intracellular Cu is tightly controlled by Cu transporters, including Cu transporter 1 (CTR1) and Cu-transporting P-type ATPase α and β (ATP7A and ATP7B), and Cu chaperones, including Cu chaperone for superoxide dismutase 1 (CCS) and antioxidant-1 (Atox-1). In several tumor tissues, these abnormalities that induce intracellular Cu accumulation are involved in tumor progression. In addition, functional disturbance in Cu-containing secretory enzymes, such as superoxide dismutase 3 (SOD3), and lysyl oxidase enzymes (LOX and LOXL1–4) with abnormal Cu dynamics plays a key role in tumor metastasis. For example, the loss of SOD3 in tumor tissues induces oxidative stress, which promotes neovascularization and epithelial-to-mesenchymal transition (EMT). LOX promotes collagen crosslinking, which functions in the metastatic niche formation. Accordingly, restricted Cu regulation may be a novel strategy for the inhibition of tumor metastasis. However, it is unclear how these Cu disturbances occur in tumor tissues and the exact molecular mechanisms underlying Cu secretory enzymes. In this review article, I discuss the role of Cu transporters, Cu chaperones, and Cu-containing secretory enzymes in tumor progression to better understand the role of Cu homeostasis in tumor tissues.
Keywords: copper, copper transporters, copper chaperone, copper-containing secretory enzymes, tumor metastasis
Introduction
Essential micronutrients play an essential role in development, replication, and differentiation. Copper (Cu) is one essential micronutrient that is important in human physiology.(1–3) Defective Cu acquisition is involved in neurological, cardiac, and connective diseases. On the other hand, excess Cu accumulation in the tissues facilitates reactive oxygen species (ROS) generation, and is closely associated with Wilson’s disease and neurodegeneration.(4,5) Recent studies suggested that Cu levels increase in several tumor tissues and angiogenic lesions, and promote neovascularization.(6) Accordingly, Cu chelators, such as bathocuproinedisulfonic acid, tetrathiomolybdate, and penicillamine, may function as anti-tumor agents to prevent Cu-related tumorigenesis.(7)
To maintain Cu homeostasis, the bioavailability of Cu is strictly controlled by Cu transporters, including Cu transporter 1 (CTR1), Cu chaperone for superoxide dismutase 1 (CCS), antioxidant-1 (Atox-1), and Cu-transporting P-type ATPase α and β (ATP7A and ATP7B).(8,9) These Cu transporters deliver Cu ions to Cu-containing enzymes, which are involved in a variety of metabolic processes, including aerobic respiration, superoxide dismutation, and synthesis of extracellular matrix. The two oxidation states of Cu, Cu (I), and Cu (II), are both necessary. For example, Cu-containing enzymes, such as mitochondrial cytochrome c oxidase (CCO), Cu,Zn-superoxide dismutase (SOD1 and SOD3), and lysyl oxidase enzymes (LOX and LOXL1–4), require Cu (I) as a cofactor. Accordingly, disturbances in Cu transporters induce Cu trafficking and abnormalities of Cu-containing enzymes, all of which are involved in tumor progression. However, it remains unclear how these Cu disturbances occur in tumor tissues and the exact molecular mechanisms underlying Cu secretory enzymes. In this review article, I first focus on the role of Cu transporters, and then discuss the role of Cu-containing enzymes secreted into the extracellular spaces in tumor progression.
Cu Transporters in the Tumor Microenvironment
Cu importer CTR1
Among the essential micronutrients, Cu functions as a cofactor of oxidoreductases, which play an important role in energy production, superoxide dismutation, and formation of extracellular matrix. On the other hand, excess Cu induces pathological processes through the induction of oxidative stress, which results in the oxidative degradation of cellular components. Accordingly, the intracellular Cu concentrations are strictly controlled by Cu transporters. The current view of Cu trafficking is illustrated in Fig. 1.
Fig. 1.
Proposed models of intracellular Cu-trafficking. STEAPs participate in extracellular Cu (II) reduction, and then reduced Cu (I) is taken up into the cells through membrane-bound CTR1. CTR1 delivers Cu (I) to Cu chaperone CCS, COX17, and Atox-1. These chaperones transfer Cu (I) to SOD1, CCO, and ATP7A, respectively. Atox-1 also translocates into the nucleus, binds to Atox-1 response element (GAAAGA), and functions as a Cu-dependent transcription factor for cyclin D1, NADPH p47phox, and SOD3. ATP7A delivers Cu (I) to Cu-containing secretory enzymes, including SOD3, and functions in the Cu egress to maintain the level of intracellular Cu (I).
Cellular Cu uptake is accomplished through the Cu importer CTR1 (SLC31A1).(10) The CTR1 family comprises evolutionarily conserved transporters that are present in yeast, plants, and mammals. All CTR1 transporters have three transmembrane domains, and their N-terminal methionine-rich domain is essential for Cu importation. CTR1-mediated Cu uptake plays an essential role in cellular homeostasis because gene deletion of CTR1 is embryonic lethal.(11) CTR1-mediated reduced Cu (I) transfers Cu to Cu chaperones such as CCS, COX17, and Atox-1. The plasma membrane-bound six-transmembrane epithelial antigen of the prostate (STEAP) family proteins (STEAP1–6) are considered to function in the extracellular Cu (II) reduction.(12) STEAPs have a similar structure composed of transmembrane domains, N-terminal, and C-terminal domains and exhibit metal reductase activity. As the overexpression of STEAPs is observed in several types of cancers, including prostate, colon, pancreas, and breast, these observations are consistent with CTR1 being overexpressed in cancer.(13) Accordingly, STEAP/CTR1-mediated Cu uptake is considered to facilitate tumor progression.
Cu chaperones CCS, COX17, and Atox-1
CCS and COX17 deliver Cu to SOD1 and CCO, respectively. CCS plays an essential role in oxidative metabolism.(14,15) Inhibition of CCS function increases the ROS level due to the significant accumulation of Cu and the suppression of SOD1 activity. On the other hand, high CCS expression is observed in invasive breast cancer cells and gene silencing of CCS suppresses tumor cell growth through the activation of ROS-mediated MEK/ERK pathways, suggesting that overexpressed CCS functions as a tumor promoter.(16) CCO, a mitochondrial respiratory chain enzyme, is involved in the synthesis of ATP.(17) COX17 has been suggested to function in the Cu-trafficking pathway of CCO.(18) As tumor cells have a high ATP requirement, COX17 is frequently overexpressed in clinical tumor tissues and tumor cell lines. Thus, the inhibition of COX17 may lead to the reduction of mitochondrial ATP levels and tumor cell death.
Atox-1, a Cu chaperone, contains a single N-terminal CXXC Cu-binding motif and plays a key role in Cu homeostasis. Atox-1 regulates the intracellular Cu concentrations by transferring Cu to ATP7A at the trans-Golgi network.(19) Atox-1-deficient mice fail to thrive immediately after birth, with 45% of pups dying before weaning.(20) Moreover, surviving animals exhibit growth failure, skin laxity, and seizures. On the other hand, Atox-1 expression is upregulated in several tumors, including breast, colorectal, uterus, and liver.(21) Immunofluorescence studies revealed Atox-1 expression at the leading edge, where it facilitates tumor cell mobility.(22) A recent study demonstrated that gene deletion of Atox-1 significantly reduces single-cell migration velocity and directionality, which may involve Cu delivery to ATP7A.(23) Due to the pro-tumor activity of Atox-1, it may be a novel biomarker for breast cancer metastasis.(24)
In addition to being a Cu chaperone, Atox-1 functions as a Cu-dependent transcription factor. Of note, Atox-1 has a conserved lysine-rich domain (KKTGK) at its C-terminal region, which may function in its nuclear localization. Atox-1 was reported to induce the expression of cyclin D1 and NADPH oxidase p47phox, which leads to increased cell proliferation and ROS generation.(25,26) Recent studies revealed that Atox-1 is expressed in both the cytoplasm and nucleus, and its nuclear localization is highly observed in metastatic tumor cells.(27) Furthermore, our study suggested that nuclear Atox-1 binds to the proximal SOD3 promoter region and induces its expression in THP-1 cell-derived macrophages.(28) It is unknown whether increased SOD3 in macrophages is involved in tumor progression, and the functional role of SOD3 in macrophages needs to be clarified.
Cu exporter ATP7A
ATP7A is a Cu-exporting P-type ATPase that regulates cytoplasmic Cu concentrations. The biological function of intracellular ATP7A is to deliver Cu to secretory enzymes such as SOD3 and LOXs. This function of ATP7A was identified by its gene deletion, which resulted in the reduction of SOD3 and LOX activities.(29) ATP7A is also involved in Cu egress when excess Cu is accumulated in cells. Excess Cu generates oxidative stress, which induces cell death, but in some tumor cells, increased ATP7A promotes Cu efflux and protects the tumor cells from the damaging effects of Cu-mediated oxidative stress.(30) Based on our previous report, phorbol ester increases ATP7A expression in human leukemic THP-1 cells,(28) which may function in Cu egress and deliver Cu to secretory enzymes such as LOX enzymes.
Overall, the significant induction of Cu transporters is involved in tumor progression through the production of ATP, the acquisition of resistance to oxidative stress, and the formation of extracellular matrix, which is suitable for tumor progression.
Cu transporters in tumor therapy
Platinum (Pt)-mediated chemotherapy is the standard-of-care for several cancers. Cisplatin, a widely used Pt-mediated agent, crosslinks DNA, interfering with RNA transcription and DNA replication activities.(31) However, resistance to anti-tumor drugs is a major limitation in the clinical application of cisplatin. Mechanisms involved in drug resistance are complicated such as decreased drug uptake and increased drug efflux. Previous studies revealed that CTR1 plays a key role in the uptake of cisplatin. Overexpression of CTR1 sensitizes cells to Pt-mediated drugs by facilitating drug uptake.(32,33) On the other hand, gene silencing of CTR1 causes resistance to these drugs. In addition, cisplatin induces proteasomal degradation of CTR1 protein. Of note, Atox-1 plays a key role in cisplatin-mediated CTR1 degradation.(34) Exploratory studies of natural compounds that regulate CTR1 expression are also underway, and it has been reported that (−)-epigallocatechin-3-gallate (EGCG) increases cisplatin sensitivity by inducing CTR1 expression, caspase activation, and cell cycle arrest, which all ultimately lead to apoptosis.(35) However, as mentioned above, CTR1 is essential for Cu uptake in both malignant and non-malignant cells; therefore, it is necessary to selectively suppress CTR1 expression and/or its function in tumor cells.
ATP7A facilitates the export of Pt-mediated drugs to the extracellular space. Indeed, increased ATP7A is closely associated with poorer outcomes in patients with ovarian cancer treated with Pt-mediated drugs.(36) Accordingly, the reduction of ATP7A is considered to increase the cisplatin sensitivity in target tumor cells. Regarding the regulation of ATP7A, we previously reported that oxidative stress induced by 6-hydroxydopamine (6-OHDA) reduces the level of ATP7A in neuroblastoma cells.(37) In this context, it is possible that autophagic degradation of ATP7A facilitates Cu-mediated cell death. ATP7A has several autophagic domains called the KFERQ motif. Although no effects of 6-OHDA on the expression of ATP7A in breast cancer MDA-MB-231 cells were observed, our study provided novel insight into the degradation of ATP7A under specific conditions. Exosomes, which are extracellular vesicles, from tumor cells contain ATP7A(38) and those ranging in size of 50–150 nm in diameter are composed of numerous proteins, mRNAs, miRNAs, and lipids.(39) Accordingly, exosomes function as a carrier to target cells,(40,41) suggesting that exosomal secretion of ATP7A facilitates the efflux of Pt-mediated drugs. Previous studies provide new insights into the functional role of ATP7A in Pt-mediated cell toxicity.
Role of Cu-Containing Secretory Enzymes in Tumor Progression
SOD3 in tumor metastasis
ROS are generated by activated endothelial cells, macrophages, and tumor cells, and induce oxidative stress, which has been implicated in many pathological processes. To protect the cells and tissues from oxidative stress, mammals have several anti-oxidative enzymes, including SOD, catalase, and glutathione peroxidase.(42,43) Among them, SOD protects the cells from the damaging effects of superoxide by accelerating the dismutation reaction of superoxide. There are three SOD isozymes, SOD1, SOD2, and SOD3, and all require a redox-active transition metal in the active site to carry out the dismutation reaction. SOD3 is extracellularly localized and distributed mainly in blood vessel walls, binding to the heparan sulfate proteoglycan on the cell surface.(44,45) The SOD3 level is relatively low compared with those of SOD1 and SOD2, whereas the presence of SOD3 throughout the vessel plays a key role in the regulation of redox homeostasis.(46,47) Furthermore, SOD3 functions in the regulation of nitric oxide-derived vasodilation by preventing the generation of peroxynitrite, which has potent oxidative activity, and mediates the oxidation of both nonprotein and protein sulfhydryls.(48,49) Accordingly, maintaining high SOD3 expression aids in the inhibition of oxidative stress-mediated diseases, including cancer, atherosclerosis, and diabetes. However, the molecular mechanisms underlying SOD3 expression have not been elucidated.
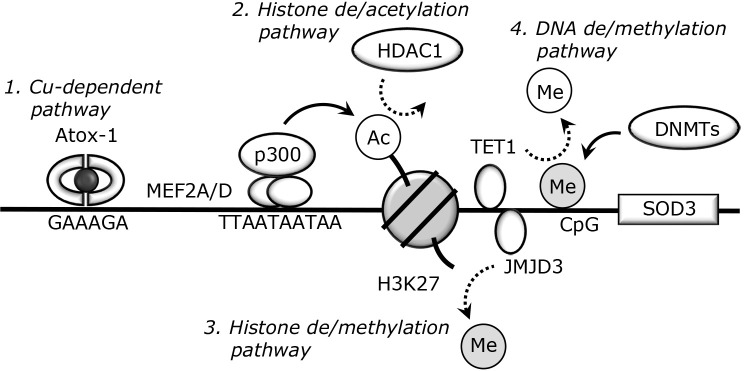
Recent studies revealed the significant reduction of SOD3 in breast, spleen, and liver tumor tissues.(50–52) This suggests that the loss of SOD3 facilitates oxidative stress and tumor progression, thus SOD3 can act as an anti-tumor enzyme. Indeed, endogenous administration of recombinant SOD3 or transient induction of SOD3 suppresses tumor cell metastasis.(53) Regarding SOD3 reduction in tumor tissues, epigenetic changes, such as histone acetylation and DNA methylation, are involved. Epigenetics is usually referred to as mitotically heritable changes in gene expression that do not involve changes in the DNA sequence.(54) DNA methylation is a major epigenetic factor and occurs at the 5' position of cytosine within CpG. Hypermethylation within gene promoters is involved in tissue- and cell-specific gene silencing.(55) Indeed, complex DNA methylation patterns have been observed in tumor cells and function in the regulation of pro- and anti-tumor genes.(56,57) The molecular mechanisms underlying Cu-dependent and epigenetic SOD3 expression are shown in Fig. 2. SOD3 expression in human lung cancer A549 cells is silenced through DNA methylation,(58,59) which is accompanied by the significant reduction of DNA demethylase ten-eleven-translocation 1 (TET1). TET1 requires oxygen for DNA demethylation processes; therefore, DNA demethylation is considered to be dysregulated in hypoxic tumor tissues.
Fig. 2.
Cu-dependent and epigenetic SOD3 regulation. 1. Cu-dependent pathway. The Cu chaperone Atox-1 is expressed in the nucleus under several conditions and binds to the proximal promoter region of SOD3. The transcription activity of Atox-1 has been confirmed in several cells such as smooth muscle cells and monocytes/macrophages. 2. Histone de/acetylation pathway. The de/acetylation within the SOD3 promoter is involved in its regulation. Our previous studies suggested that MEF2 proteins bind to MEF2 response motif (TTAATAATAA) and function as scaffold proteins that interact with histone acetyltransferase p300. On the other hand, HDAC1-mediated histone deacetylation plays a key role in SOD3 silencing. The lined or dotted arrows indicate the addition or removal of histone or DNA marks, respectively. 3. Histone de/methylation pathway. Trimethylated-H3K27 is well recognized as the marker of gene reduction. We demonstrated that JMJD3, a histone demethylase, reduces the level of H3K27me3 within the SOD3 promoter region and is involved in its induction. 4. DNA de/methylation pathway. DNMTs transfer methyl residues to cytosines within CpG sequences and reduce target gene expression. TET1, a DNA demethylase, plays a key role in the removal of methyl residues from the SOD3 promoter region, which induces its expression.
Like CpG methylation, histone modifications of the N-terminal tail, including acetylation and methylation at lysine or arginine residues, are involved in gene regulation. Chromatin may exist in two different states, open and closed configurations, and these states are associated with the acetylation or deacetylation of histone tails, respectively.(60,61) Our previous study suggested that histone acetylation plays an essential role in the regulation of SOD3 in phorbol ester-treated THP-1 cells.(62) Analysis of the molecular mechanisms involved in SOD3 regulation revealed that the dissociation of histone deacetylase 1 (HDAC1) from the SOD3 promoter region and the binding of histone acetylase p300 are involved in its induction.(63) In addition, we suggested that myocyte enhancer factor 2 (MEF2), a transcription factor that regulates myogenesis, functions as a scaffold protein that interacts with HDAC1 or p300 and regulates SOD3 expression. This is consistent with SOD3 being abundantly expressed in smooth muscle cells in which MEF2 proteins participate in cell growth and migration. We also demonstrated that natural product-derived 4-hydroperoxy-2-decenoic acid ethyl ester and exenatide significantly induce SOD3 expression through histone acetylation and DNA demethylation, respectively.(64–66) Based on our studies, these compounds may control redox homeostasis, which ultimately inhibits tumor progression.
On the other hand, we confirmed that SOD3 expression in metastatic MDA-MB-231 cells is higher than in non-metastatic MCF7 cells.(67) This suggests that SOD3 is involved in breast cancer cell progression. Moderately sustained overexpression of SOD3 facilitates tumor progression in aggressive anaplastic cancer cells. Moreover, it activates small GTPase signaling involved in cell proliferation.(53,68) Therefore, SOD3 has both anti-tumor and pro-tumor properties. However, our studies revealed only part of the regulatory mechanism of SOD3 expression, and further studies are required to clarify the exact molecular mechanisms underlying SOD3 expression and its pro-metastatic properties.
LOXs in tumor metastasis
It is well known that the cause of mortality of tumors is metastasis. Recent studies revealed that LOXs, including LOX and LOXL1–4, play an essential role in tumor metastasis. As shown in Fig. 3.1., LOXs catalyze lysine oxidation within collagen and maintain extracellular stiffness. On the other hand, there is evidence to suggest that LOXs function in tumor metastasis through the production of hydrogen peroxide (H2O2), which leads to the activation of Src/FAK signaling and the epithelial-to-mesenchymal transition (EMT) processes (Fig. 3.2.).(69,70) The pro-tumor effects of ATP7A are closely related to the activation of LOX enzymes. High ATP7A expression correlates with decreased survival in patients with estrogen receptor (ER)-negative invasive breast cancer cells, which highly express LOX enzymes.(29) Our recent study revealed that LOX expression is significantly induced in tumor-associated macrophages and promotes ER-negative MDA-MB-231 cell migration.(71)
Fig. 3.
The functional role of LOXs in tumor progression. 1. Collagen crosslinking. LOXs catalyze the conversion of lysine residue to aldehyde allysine, and spontaneous reaction with other lysine and allysine residues creates crosslinks in collagen. 2. EMT induction. During the crosslinking of collagen, LOXs produce H2O2 and NH3 as byproducts. The generated H2O2 activates several signal pathways, including FAK/Src signaling, which facilitates EMT processes. 3. Exosomal secretion. Recent studies revealed that LOXs are extracellularly secreted through exosomes. Exosomal secretion of LOXs generates H2O2 in recipient cells and functions in the acquisition of metastatic properties. 4. Gene regulation. There is accumulating evidence supporting the involvement of LOXs in the deamination of lysine residues in trimethylated H3K4, a transcription marker. At present, LOXs are considered to catalyze deamination in H3K4 within the CDH1 promoter and reduce its expression.
β-Aminopropionitrile, a LOX inhibitor, has widespread applications in LOX-related biological studies; however, the lack of amenable sites for chemical modification has prevented its development into a clinically optimal drug.(72) Accordingly, the molecular mechanisms underlying LOX regulation are under investigation. Regarding the induction of LOX in tumor-associated macrophages, we previously reported that JMJD3-mediated histone H3K27 demethylation plays a key role in its expression.(71) Histone lysine demethylases, including JMJD3, are upregulated in several tumors.(73,74) In addition, macrophage differentiation into tumor-associated M2 macrophages is regulated by JMJD3-mediated H3K27 demethylation.(75) Our studies may help clarify the molecular mechanisms governing epigenetic LOX induction in the tumor microenvironment. We also demonstrated that hypoxia-inducible factor 1α (HIF1α), a well-known transcription factor induced in tumor tissues, is involved in LOX expression in tumor-associated macrophages.(76) This is consistent with LOX expression increasing in the tumor microenvironment under hypoxic conditions.
It was recently reported that LOXL2 and LOXL4 are secreted into the extracellular space through exosomes (Fig. 3.3.).(77,78) LOXL2 and LOXL4, which are loaded into exosomes, exert pro-metastatic effects, suggesting that exosomes are required for LOX-mediated tumor progression. We also confirmed that the N-glycosylation of LOXL2 is required for its secretion and that it is involved in kidney fibrosis, leading to kidney tumor progression (unpublished data). As complex N-glycosylation is observed in tumor tissues, it is necessary to identify the glycans involved in LOXL2 secretion and function.(79) LOXL2 is also expressed in the nucleus where it oxidizes trimethylated H3K4 within the cadherin 1 (CDH1) gene, which leads to its reduction (Figure 3.4.).(80) As the loss of CDH1 expression is associated with the induction of EMT, LOXL2 functions as an EMT promoter by repressing CDH1 expression. On the other hand, there are conflicting observations regarding the functional role of LOXL4 in cancers. LOXL4 in bladder cancer suppresses tumor progression by inhibiting RAS- and/or ERK-mediated oncogenic signaling pathways.(81) However, gene deletion of LOXL4 promotes MDA-MB-231 cell migration through the induction of collagen I and IV expression.(82) These conflicting observations may be explained by differences in the context among cancers. However, the exact mechanisms by which LOXL4 functions as a tumor promoter or a tumor suppressor remain largely unclear.
Conclusion
Cu dysregulation is involved in tumor progression through the upregulation of Cu transporters and Cu-containing pro-metastatic enzymes. However, it remains largely unknown how Cu-containing enzymes are involved in tumor progression. Future studies must systematically analyze the role of these enzymes based on molecular, cellular, and clinical data, which will aid in the development of a novel anti-tumor therapy.
Acknowledgments
This study was supported in part by grants from JSPS KAKENHI Grant Numbers 23790190, 26460090, and 17K08277, a Sasakawa Scientific Research Grant from The Japan Science Society (2018-4007), a Koshiyama Research Grant, Suzuken Memorial Foundation, and a grant for the encouragement of young scientists from Gifu Pharmaceutical University.
Abbreviations
- Atox-1
antioxidant-1
- ATP7A
copper-transporting P-type ATPase α
- ATP7B
copper-transporting P-type ATPase β
- CCO
cytochrome c oxidase
- CCS
copper chaperone for superoxide dismutase 1
- CDH1
cadherin 1
- CTR1
copper transporter 1
- Cu
copper
- EMT
epithelial-to-mesenchymal transition
- ER
estrogen receptor
- HDAC1
histone deacetylase 1
- HIF1α
hypoxia-inducible factor 1α
- H2O2
hydrogen peroxide
- LOX
lysyl oxidase
- MEF2
myocyte enhancer factor 2
- 6-OHDA
6-hydroxydopamine
- Pt
platinum
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STEAP
plasma membrane-bound six-transmembrane epithelial antigen of the prostate
- TET1
ten-eleven-translocation 1
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Harris ED. Copper transport: an overview. Proc Soc Exp Biol Med 1991; 196: 130–140. [DOI] [PubMed] [Google Scholar]
- 2.Bull PC, Cox DW. Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet 1994; 10: 246–252. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez JP, Rios S, Gonzalez M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J Cell Biochem 2002; 85: 92–100. [PubMed] [Google Scholar]
- 4.Oe S, Miyagawa K, Honma Y, Harada M. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp Cell Res 2016; 347: 192–200. [DOI] [PubMed] [Google Scholar]
- 5.Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 2019; 24: 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lelièvre P, Sancey L, Coll JL, Deniaud A, Busser B. The multifaceted roles of copper in cancer: a trace metal element with dysregulated metabolism, but also a target or a bullet for therapy. Cancers (Basal) 2020; 12: 3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldari S, Dí Rocco G, Toietta G. Current biomedical use of copper chelation therapy. Int J Mol Sci 2020; 21: 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev 2007; 87: 1011–1046. [DOI] [PubMed] [Google Scholar]
- 9.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 2008; 4: 176–185. [DOI] [PubMed] [Google Scholar]
- 10.Scheiber I, Dringen R, Mercer JF. Copper: effects of deficiency and overload. Met Ions Life Sci 2013; 13: 359–387. [DOI] [PubMed] [Google Scholar]
- 11.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci U S A 2001; 98: 6836–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes IM, Maia CJ, Santos CR. STEAP proteins: from structure to applications in cancer therapy. Mol Cancer Res 2012; 10: 573–587. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Liu L, Bai Q, et al. Prognostic value of copper transporter 1 expression in patients with clear cell renal cell carcinoma. Oncol Lett 2017; 14: 5791–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki Y, Ali M, Fischer M, Riemer J. Human copper chaperone for superoxide dismutase 1 mediates its own oxidation-dependent import into mitochondria. Nat Commun 2013; 4: 2430. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Luo C, Shan C, et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat Chem 2015; 7: 968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Liang R, Zhang X, et al. Copper chaperone for superoxide dismutase promotes breast cancer cell proliferation and migration via ROS-mediated MAPK/ERK Signaling. Front Pharmacol 2019; 10: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson-Miller S, Babcock GT. Heme/copper terminal oxidases. Chem Rev 1996; 96: 2889–2908. [DOI] [PubMed] [Google Scholar]
- 18.Palumaa P, Kangur L, Voronova A, Sillard R. Metal-binding mechanism of Cox17, a copper chaperone for cytochrome c oxidase. Biochem J 2004; 382 (Pt 1): 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatori Y, Lutsenko S. The role of copper chaperone Atox1 in coupling redox homeostasis to intracellular copper distribution. Antioxidants (Basal) 2016; 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamza I, Faisst A, Prohaska J, Chen J, Gruss P, Gitlin JD. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc Natl Acad Sci U S A 2001; 98: 6848–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blockhuys S, Celauro E, Hildesjö C, et al. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics 2017; 9: 112–123. [DOI] [PubMed] [Google Scholar]
- 22.Blockhuys S, Zhang X, Wittung-Stafshede P. Copper chaperone Atox1 plays role in breast cancer cell migration. Biochem Biophys Res Commun 2017; 483: 301–304. [DOI] [PubMed] [Google Scholar]
- 23.Blockhuys S, Zhang X, Wittung-Stafshede P. Single-cell tracking demonstrates copper chaperone Atox1 to be required for breast cancer cell migration. Proc Natl Acad Sci U S A 2020; 117: 2014–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blockhuys S, Brady DC, Wittung-Stafshede P. Evaluation of copper chaperone ATOX1 as prognostic biomarker in breast cancer. Breast Cancer 2020; 27: 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh S, Kim HW, Nakagawa O, et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem 2008; 283: 9157–9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen GF, Sudhahar V, Youn SW, et al. Copper transport protein antioxidant-1 promotes inflammatory neovascularization via chaperone and transcription factor function. Sci Rep 2015; 5: 14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jana A, Das A, Krett NL, et al. Nuclear translocation of Atox1 potentiates activin A-induced cell migration and colony formation in colon cancer. PLoS One 2020; 15: e0227916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamiya T, Takeuchi K, Fukudome S, Hara H, Adachi T. Copper chaperone antioxidant-1, Atox-1, is involved in the induction of SOD3 in THP-1 cells. Biometals 2018; 31: 61–68. [DOI] [PubMed] [Google Scholar]
- 29.Shanbhag V, Jasmer-McDonald K, Zhu S, et al. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc Natl Acad Sci U S A 2019; 116: 6836–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S, Shanbhag V, Wang Y, Lee J, Petris M. A Role for The ATP7A copper transporter in tumorigenesis and cisplatin resistance. J Cancer 2017; 8: 1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA 2008; 299: 672–684. [DOI] [PubMed] [Google Scholar]
- 32.Holzer AK, Samimi G, Katano K, et al. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol 2004; 66: 817–823. [DOI] [PubMed] [Google Scholar]
- 33.Noordhuis P, Laan AC, van de Born K, Losekoot N, Kathmann I, Peters GJ. Oxaliplatin activity in selected and unselected human ovarian and colorectal cancer cell lines. Biochem Pharmacol 2008; 76: 53–61. [DOI] [PubMed] [Google Scholar]
- 34.Safaei R, Maktabi MH, Blair BG, Larson CA, Howell SB. Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J Inorg Biochem 2009; 103: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Jiang P, Wang P, Yang CS, Wang X, Feng Q. EGCG enhances cisplatin sensitivity by regulating expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. PLoS One 2015; 10: e0125402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samimi G, Safaei R, Katano K, et al. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res 2004; 10: 4661–4669. [DOI] [PubMed] [Google Scholar]
- 37.Kondo M, Hara H, Kamijo F, Kamiya T, Adachi T. 6-Hydroxydopamine disrupts cellular copper homeostasis in human neuroblastoma SH-SY5Y cells. Metallomics 2021; 13: mfab041. [DOI] [PubMed] [Google Scholar]
- 38.Lukanović D, Herzog M, Kobal B, Černe K. The contribution of copper efflux transporters ATP7A and ATP7B to chemoresistance and personalized medicine in ovarian cancer. Biomed Pharmacother 2020; 129: 110401. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 2019; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579. [DOI] [PubMed] [Google Scholar]
- 41.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 42.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 2006; 55: 928–934. [DOI] [PubMed] [Google Scholar]
- 44.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A 1982; 79: 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ookawara T, Imazeki N, Matsubara O, et al. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol 1998; 275: C840–C847. [DOI] [PubMed] [Google Scholar]
- 46.Svensk AM, Soini Y, Pääkkö P, Hiravikoski P, Kinnula VL. Differential expression of superoxide dismutases in lung cancer. Am J Clin Pathol 2004; 122: 395–404. [DOI] [PubMed] [Google Scholar]
- 47.Teoh ML, Fitzgerald MP, Oberley LW, Domann FE. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res 2009; 69: 6355–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 1990; 87: 1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 1991; 266: 4244–4250. [PubMed] [Google Scholar]
- 50.Singh B, Bhat HK. Superoxide dismutase 3 is induced by antioxidants, inhibits oxidative DNA damage and is associated with inhibition of estrogen-induced breast cancer. Carcinogenesis 2012; 33: 2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Leary BR, Fath MA, Bellizzi AM, et al. Loss of SOD3 (EcSOD) expression promotes an aggressive phenotype in human pancreatic ductal adenocarcinoma. Clin Cancer Res 2015; 21: 1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teoh-Fitzgerald MLT, Fitzgerald MP, Jensen TJ, Futscher BW, Domann FE. Genetic and epigenetic inactivation of extracellular superoxide dismutase promotes an invasive phenotype in human lung cancer by disrupting ECM homeostasis. Mol Cancer Res 2012; 10: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laukkanen MO. Extracellular superoxide dismutase: growth promoter or tumor suppressor? Oxid Med Cell Longev 2016; 2016: 3612589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kristensen LS, Nielsen HM, Hansen LL. Epigenetics and cancer treatment. Eur J Pharmacol 2009; 625: 131–142. [DOI] [PubMed] [Google Scholar]
- 55.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 2007; 447: 425–432. [DOI] [PubMed] [Google Scholar]
- 56.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science 2003; 300: 455. [DOI] [PubMed] [Google Scholar]
- 57.Nishigaki M, Aoyagi K, Danjoh I, et al. Discovery of aberrant expression of R-RAS by cancer-linked DNA hypomethylation in gastric cancer using microarrays. Cancer Res 2005; 65: 2115–2124. [DOI] [PubMed] [Google Scholar]
- 58.Zelko IN, Mueller MR, Folz RJ. CpG methylation attenuates Sp1 and Sp3 binding to the human extracellular superoxide dismutase promoter and regulates its cell-specific expression. Free Radic Biol Med 2010; 48: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamiya T, Nakahara R, Mori N, Hara H, Adachi T. Ten-eleven translocation 1 functions as a mediator of SOD3 expression in human lung cancer A549 cells. Free Radic Res 2017; 51: 329–336. [DOI] [PubMed] [Google Scholar]
- 60.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell 2007; 128: 669–681. [DOI] [PubMed] [Google Scholar]
- 61.Esteller M. Epigenetics in cancer. N Engl J Med 2008; 358: 1148–1159. [DOI] [PubMed] [Google Scholar]
- 62.Kamiya T, Machiura M, Makino J, Hara H, Hozumi I, Adachi T. Epigenetic regulation of extracellular-superoxide dismutase in human monocytes. Free Radic Biol Med 2013; 61: 197–205. [DOI] [PubMed] [Google Scholar]
- 63.Ichihara M, Kamiya T, Hara H, Adachi T. The MEF2A and MEF2D function as scaffold proteins that interact with HDAC1 or p300 in SOD3 expression in THP-1 cells. Free Radic Res 2018; 52: 799–807. [DOI] [PubMed] [Google Scholar]
- 64.Makino J, Ogasawara R, Kamiya T, et al. Royal jelly constituents increase the expression of extracellular superoxide dismutase through histone acetylation in monocytic THP-1 cells. J Nat Prod 2016; 79: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 65.Yasuda H, Mizukami K, Hayashi M, Kamiya T, Hara H, Adachi T. Exendin-4 promotes extracellular-superoxide dismutase expression in A549 cells through DNA demethylation. J Clin Biochem Nutr 2016; 58: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yasuda H, Ohashi A, Nishida S, et al. Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells. J Clin Biochem Nutr 2016; 59: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamiya T, Yamaguchi Y, Oka M, Hara H. Combined action of FOXO1 and superoxide dismutase 3 promotes MDA-MB-231 cell migration. Free Radic Res 2022; 56: 106–114. [DOI] [PubMed] [Google Scholar]
- 68.Laukkanen MO, Cammarota F, Esposito T, Salvatore M, Castellone MD. Extracellular superoxide dismutase regulates the expression of small GTPase regulatory proteins GEFs, GAPs, and GDI. PLoS One 2015; 10: e0121441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laczko R, Szauter KM, Jansen MK, et al. Active lysyl oxidase (LOX) correlates with focal adhesion kinase (FAK)/paxillin activation and migration in invasive astrocytes. Neuropathol Appl Neurobiol 2007; 33: 631–643. [DOI] [PubMed] [Google Scholar]
- 70.Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 2013; 32: 1863–1868. [DOI] [PubMed] [Google Scholar]
- 71.Takemoto R, Kamiya T, Hara H, Adachi T. Lysyl oxidase expression is regulated by the H3K27 demethylase Jmjd3 in tumor-associated M2-like macrophages. J Clin Biochem Nutr 2020; 66: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leung L, Niculescu-Duvaz D, Smithen D, et al. Anti-metastatic inhibitors of lysyl oxidase (LOX): design and structure-activity relationships. J Med Chem 2019; 62: 5863–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 2003; 22: 5323–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD. JMJD3 is a histone H3K27 demethylase. Cell Res 2007; 17: 850–857. [DOI] [PubMed] [Google Scholar]
- 75.Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 2010; 11: 936–944. [DOI] [PubMed] [Google Scholar]
- 76.Takemoto R, Kamiya T, Atobe T, Hara H, Adachi T. Regulation of lysyl oxidase expression in THP-1 cell-derived M2-like macrophages. J Cell Biochem 2021; 122: 777–786. [DOI] [PubMed] [Google Scholar]
- 77.de Jong OG, van Balkom BW, Gremmels H, Verhaar MC. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J Cell Mol Med 2016; 20: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li R, Wang Y, Zhang X, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer 2019; 18: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 2015; 15: 540–555. [DOI] [PubMed] [Google Scholar]
- 80.Herranz N, Dave N, Millanes-Romero A, et al. Lysyl oxidase-like 2 (LOXL2) oxidizes trimethylated lysine 4 in histone H3. FEBS J 2016; 283: 4263–4273. [DOI] [PubMed] [Google Scholar]
- 81.Wu G, Guo Z, Chang X, et al. LOXL1 and LOXL4 are epigenetically silenced and can inhibit ras/extracellular signal-regulated kinase signaling pathway in human bladder cancer. Cancer Res 2007; 67: 4123–4129. [DOI] [PubMed] [Google Scholar]
- 82.Choi SK, Kim HS, Jin T, Moon WK. LOXL4 knockdown enhances tumor growth and lung metastasis through collagen-dependent extracellular matrix changes in triple-negative breast cancer. Oncotarget 2017; 8: 11977–11989. [DOI] [PMC free article] [PubMed] [Google Scholar]