Abstract
Glutathione (GSH) is synthesized from three amino acids and the overall process is highly dependent on the availability of l-cysteine (l-Cys). GSH serves as an essential cofactor for glutathione peroxidase 4 (Gpx4), which reduces phospholipid hydroperoxides. The inactivation of Gpx4 or an insufficient supply of l-Cys results in the accumulation of lipid hydroperoxides, eventually leading to iron-dependent cell death, ferroptosis. In this study, we investigated the anti-ferroptotic properties of d-cysteine (d-Cys) under conditions of dysfunction in cystine transporter, xCT. l-Cys supplementation completely rescued ferroptosis that had been induced by the erastin-mediated inhibition of xCT in Hepa 1-6 cells. Upon d-Cys supplementation, the erastin-treated cells remained completely viable for periods of up to 24 h but eventually died after 48 h. d-Cys supplementation suppressed the production of lipid peroxides, thereby ferroptosis. The addition of d-Cys sustained intracellular Cys and GSH levels to a certain extent. When Hepa 1-6 cells were treated with a combination of buthionine sulfoximine and erastin, the anti-ferroptotic effect of d-Cys was diminished. These collective results indicate that, although d-Cys is not the direct source of GSH, d-Cys supplementation protects cells from ferroptosis in a manner that is dependent on GSH synthesis via stimulating the uptake of l-Cys.
Keywords: ferroptosis, glutathione, cystine, l-cysteine, d-cysteine
Introduction
Ferroptosis is a recently characterized form of cell death that is dependent on intracellular ferrous iron.(1,2) The hydroxyl radicals produced in the Fenton reaction, which involve iron, are highly reactive and can cause an increase in the generation of lipid peroxides in membrane phospholipids that, in turn, cause ferroptosis by disturbing the integrity of the plasma membrane. The enzyme glutathione peroxidase 4 (Gpx4) uses glutathione (GSH), a major cellular antioxidant, to scavenge such lipid peroxides, and appears to play a pivotal role in protection against ferroptosis.(3) It has been reported that ferroptosis is associated with various pathological conditions, including cancer, neurodegenerative diseases, ischemic diseases, and inflammatory diseases. Because of this, the regulation of ferroptosis is expected to lead to novel therapeutic and preventive methods for the treatment of these conditions.(4)
Cysteine (Cys) is an amino acid that contains a sulfhydryl group (−SH), and its availability restricts the synthesis of GSH. Accordingly, Cys uptake is specifically required by many cells to prevent the onset of ferroptosis.(1,2) There is some evidence to show that Cys is directly taken up by cells from the environment, possibly via SLC1A4/5 (ASCT1/2) amino acid transporters.(5) However, in the oxidizing environment outside the cell, it is thought that free Cys is largely present in the form of cystine, a dimeric form of Cys linked by disulfide bond.(6) Cystine can be imported into cells via the amino acid transporter system xc−, a heterodimer of solute carrier family 7 member 11 (SLC7A11, xCT) and solute carrier family 3 member 2 (SLC3A2, 4F2hc),(6) on the cell membrane. When the xc− system is inhibited by erastin, or when extracellular Cys/cystine is degraded using an engineered enzyme, cyst(e)inase, ferroptosis is induced in diverse cancer cell lines and model animals.(7–9)
In a previous study, we reported that the inhibition of GSH synthesis alone by buthionine sulfoximine (BSO) is not sufficient to induce ferroptosis in some types of cells,(10) suggesting that free l-Cys functions independently in protecting against ferroptosis. It has been reported that d-Cys is not a biosynthetic precursor of GSH but it has also been shown that hydrogen sulfide (H2S) and its storage form (bound sulfur) are synthesized from d-Cys as well as from l-Cys in vivo.(11–13) Both d-Cys and l-Cys contain free −SH groups, but it has not been established that d-Cys functions in antioxidant defense against ferroptosis. In the current study, the anti-ferroptotic properties of d-Cys were examined under conditions of xCT dysfunction. The findings show that d-Cys supplementation partially suppresses ferroptosis by promoting the cellular uptake of l-Cys and the subsequent synthesis of GSH.
Materials and Methods
Cell culture and chemicals
Hepa 1-6 cells, a mouse hepatoma-derived cell line, were obtained from the RIKEN Bioresource Centre (Tsukuba, Japan). All cells were maintained in high-glucose Dulbecco’s Modified Eagle’s Medium (FUJIFILM Wako Pure Chemical, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS; Biowest, Riverside, MO) and a penicillin-streptomycin solution (FUJIFILM Wako Pure Chemical) at 37°C in a 5% CO2 incubator. For cystine deprivation, cystine-free medium was prepared by using DMEM/high glucose/no glutamine/no methionine/no cystine (Thermo Fisher Scientific, Waltham, MA) supplemented back with 10% FBS, a penicillin-streptomycin solution, 4 mM l-glutamine (FUJIFILM Wako Pure Chemical), 1 mM sodium pyruvate (FUJIFILM Wako Pure Chemical), and 0.2 mM l-methionine (PEPTIDE INSTITUTE, Osaka, Japan). Mouse embryonic fibroblasts (MEFs) were isolated from xCT-knockout (xCT-KO) mice, as previously described.(14) xCT-KO MEFs were maintained in DMEM supplemented with 10% FBS, a penicillin-streptomycin solution, and 50 μM β-mercaptoethanol (β-ME; FUJIFILM Wako Pure Chemical). Where indicated, cells were treated with dimethyl sulfoxide (DMSO; FUJIFILM Wako Pure Chemical) as a vehicle control, BSO (FUJIFILM Wako Pure Chemical), or erastin (Cayman Chemical, Ann Arbor, MI).
Evaluation of the cytotoxicity of cells
Cells were seeded at a density of 1 × 105 cells/ml of medium in 35-mm tissue culture dishes (2 ml/dish) and incubated overnight. Cytotoxicity was determined by means of a lactate dehydrogenase (LDH) assay, as described previously.(15) The reaction mixture contained 20 μl of culture medium, 0.3 mM NADH, 1 mM sodium pyruvate, and 200 mM sodium phosphate buffer, at pH 7.4 in a total volume of 100 μl. Initial activities were calculated from the rate of disappearance of NADH during the starting linear phase of the reaction by monitoring the absorbance of the medium at 340 nm.
Evaluation of the viability of cells
Cells were seeded at an initial density of (1.0 × 105/ml). Cell viability was determined using a CellTiter-Blue® Cell Viability Assay (Promega, Madison, WI) according to the manufacturer’s instructions. Fluorescence intensity was measured using a microplate reader Varioskan Flash (Thermo Fisher Scientific) with an excitation wavelength of 560 nm and an emission wavelength of 590 nm. Fluorescence values were then normalized with respect to cells and are considered to be 100%.
Measurements of cystine, GSH, and Cys levels by liquid chromatography-mass spectrometry analyses
Sample preparation and liquid chromatography (LC)-mass spectrometry (MS) were performed by previously reported methods.(16) For measurement of extracellular cystine levels, equal volumes of conditioned medium and 50 mM ammonium bicarbonate containing 20 mM N-ethylmaleimide (NEM), pH 8.0, was mixed, boiled for 3 min and then incubated at room temperature for 10 min. The reactants were mixed with an equal volume of methanol and an equal volume of methanol containing a 10 μM internal standard. After incubation on ice for 2 h, the mixture was centrifuged at 15,000 rpm for 15 min at 4°C and the resulting supernatant was used for LC-MS analysis. For the measurement of intracellular GSH and Cys, cells were lysed by treatment with 50 mM ammonium bicarbonate containing 20 mM NEM, pH 8.0, boiled for 3 min and then incubated at room temperature for 10 min. The resultant lysate was mixed well with an equal volume of methanol and an equal volume of methanol containing a 10 μM internal standard. A two-fold volume of chloroform was added to the mixture with vigorous mixing. After centrifugation at 15,000 rpm for 15 min at 4°C, the upper phase was used for the LC-MS analysis. The samples were applied to a Sequant ZICpHILIC (5 μm, 2.1 × 150 mm; Merck, Darmstadt, Germany), using an Ultimate 3000 LC system (Dionex, Sunnyvale, CA), equilibrated with 10% mobile phase A (20 mM ammonium bicarbonate, pH 9.8) and 90% mobile phase B (100% acetonitrile), and eluted at a flow rate of 0.15 ml/min with a gradient of 90–42% mobile phase B in 20 min and then maintained at 42% B in 10 min. The LC system was composed by a WPS-3000 TRS autosampler, a TCC-3000 RS column oven, and an HPG-3400RS quaternary pump (Dionex, Sunnyvale, CA) and connected to a quadrupole Orbitrap (Q-Exactive, Thermo Scientific) mass spectrometer equipped with a heated electro-spray ionization source. Standard curves for cystine, GSH-NEM, and Cys-NEM were generated in the ranges of 0.1–3,000 μM, 0.01–300 μM, and 0.01–300 μM, respectively. The resulting curves showed linearity in these ranges for the authentic compound level. System control, data acquisition, and quantitative analysis were performed with the Xcalibur 2.2 software (Thermo Fisher Scientific).
Flow cytometry
For lipid peroxidation assays, cells were incubated with 10 μM C11-BODIPY581/591 (Thermo Fisher Scientific) for 30 min following the manufacturer’s instructions and then washed with PBS. After trypsinization, the cells were collected and subjected to flow cytometry (FACSCantoTM II, BD Biosciences, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using the GraphPad Prism ver. 6.0 for Mac (GraphPad Software, San Diego, CA). A p value of less than 0.05 was considered to be significant.
Results
d-Cys supplementation protects against ferroptosis induced by xCT inhibition in Hepa 1-6 cells
To examine the issue of whether d-Cys has ability to suppress ferroptosis, we examined effect of inhibiting xCT by adding erastin to the medium. The erastin treatment resulted in a decreased cell viability in Hepa 1-6 cells (Fig. 1A). When l-Cys was added to the medium, the cell viability was improved and this improvement was concentration-dependent. Thus, an insufficient supply of Cys to the cells was found to be responsible for the erastin-induced cell death. As we expected, d-Cys also improved cell viability to a certain extent, although this was less effective than l-Cys.
Fig. 1.
Effects of d-Cys supplementation on ferroptosis induced by elastin treatment. (A) Viability of Hepa 1-6 cells were determined by using a CellTiter-Blue® assay after 24 h of 10 μM erastin-treatment in the presence or absence of l-Cys or d-Cys at the indicated concentrations. Data represent the mean ± SEM (n = 3). *p<0.05, ***p<0.001. ns, not significant. Both l-Cys and d-Cys significantly improved the survival rate at 0.1 mM or higher. (B) Cytotoxicity of cells, as assessed by measuring released LDH activity. Hepa 1-6 cells were treated with DMSO (control) or 10 μM erastin in the presence or absence of 1 mM l-Cys or 1 mM d-Cys for 24 or 48 h. Data represent the mean ± SEM (n = 3). (C) Representative phase-contrast images of cells. Hepa 1-6 cells were treated under the same conditions as in (B). (D) Lipid peroxide production, as assessed by flow cytometry using C11-BODIPY581/591. Hepa 1-6 cells were treated with DMSO (control) or 10 μM erastin in the presence or absence of 1 mM l-Cys or 1 mM d-Cys for 24 h, treated with C11-BODIPY581/591, and then examined by flow cytometry. Values for the fluorescence relative to cells cultured in control medium are shown (n = 3). Different letters indicate statistically significant differences between groups (p<0.05, Tukey’s test).
We next measured LDH activity, a hallmark of necrotic cell death, in the culture supernatant to evaluate the properties of this cell death (Fig. 1B). While the treatment of the cells with erastin elevated the LDH activity in the medium, erastin-induced LDH release was completely suppressed by l-Cys supplementation during the 48 h culture period. When d-Cys was added, the Hepa 1-6 cells remained completely alive for periods of up to 24 h, which was also confirmed by phase-contrast microscopy (Fig. 1C), but the cells eventually died 48 h later. Thus, d-Cys was protective for limited periods under conditions of xCT inhibition.
We next evaluated the levels of lipid peroxidation products, which are considered to be a characteristic of ferroptosis among the various types of cell death, using C11-BODIPY581/591, a sensitive fluorescent probe for the detection of lipid peroxidation products. At the 24-h time point, the fluorescent intensity was elevated in the cells that had been treated with erastin, indicating the augmentation of lipid peroxidation in the cells. Consistent with the results of the cell death assay, the increased fluorescent intensity was also completely suppressed by the addition of d-Cys as well as l-Cys at this time point (Fig. 1D).
We also examined the effect of d-Cys against ferroptosis induced by cystine-free medium. As a result, LDH activity in the medium increased when Hepa 1-6 cells were cultured in cystine-free medium, whereas the addition of l-Cys completely suppressed ferroptosis during the 48 h culture period (Fig. 2A and B). On the other hand, the addition of d-Cys was able to somewhat inhibit the cell death of Hepa 1-6 cells up to 24 h, but the cells eventually died 48 h later. Furthermore, the increase in lipid peroxidation caused by cystine deprivation was completely inhibited by l-Cys supplementation, but the protective effect of d-Cys was less pronounced (Fig. 2C). These results indicate that the protection by d-Cys against ferroptosis was less effective in a Cys/cystine-deficient environment compared to that under xCT inhibition by erastin.
Fig. 2.
Effects of d-Cys supplementation on ferroptosis induced by cystine deprivation. (A) Cytotoxicity of cells, as assessed by measuring released LDH activity. Hepa 1-6 cells were incubated in conventional or cystine-free medium in the presence or absence of 1 mM l-Cys or 1 mM d-Cys for 24 or 48 h. Data represent the mean ± SEM (n = 3). (B) Representative phase-contrast images of cells. Hepa 1-6 cells were treated under the same conditions as in (A). (C) Lipid peroxide production, as assessed by flow cytometry using C11-BODIPY581/591. Hepa 1-6 cells were incubated in conventional or cystine-free medium in the presence or absence of 1 mM l-Cys or 1 mM d-Cys for 24 h, treated with C11-BODIPY581/591, and then examined by flow cytometry. Values for the fluorescence relative to cells cultured in control medium are shown (n = 3). Different letters indicate statistically significant differences between groups (p<0.05, Tukey’s test).
To gain insights into the molecular mechanism responsible for this, we then measured the intracellular levels of total Cys, which were the sum of the levels of l-Cys and d-Cys, and GSH (Fig. 3), which were produced from l-Cys only,(11,12) by means of LC-MS. Their concentrations were markedly decreased after 12 h of the erastin treatment while l-Cys supplementation drastically restored them (Fig. 3A and B). Under xCT inhibition by erastin, the addition of d-Cys improved intracellular levels of Cys to a certain extent and also intracellular GSH levels were maintained. The addition of l-Cys and d-Cys to the cystine-deprived medium both increased the amount of intracellular Cys (Fig. 3C) but the amount of intracellular GSH increased only when l-Cys was added (Fig. 3D). The levels of extracellular cystine, the sum of the l-Cys dimer, the d-Cys dimer, and chimera of l-Cys and d-Cys, were comparable between the l-Cys- and d-Cys-supplemented medium (Fig. 3E and F). Collectively, these results indicate that intracellular levels of Cys and GSH were maintained until a certain time even by d-Cys supplementation, leading to protection against ferroptosis.
Fig. 3.
Effects of d-Cys supplementation on intracellular Cys and GSH levels during ferroptosis. (A, B) Intracellular concentrations of (A) Cys and (B) GSH in Hepa 1-6 cells 12 h after the treatment with 10 μM erastin in the presence or absence of 1 mM l-Cys or 1 mM d-Cys. (C, D) Intracellular concentrations of (D) Cys and (E) GSH in Hepa 1-6 cells 6 h after the incubation in cystine-free medium in the presence or absence of 1 mM l-Cys or 1 mM d-Cys. (E, F) Extracellular concentrations of cystine in cultivation medium of Hepa 1-6 cells (E) 12 h after the treatment with 10 μM erastin or (F) 6 h after the incubation in cystine-free medium in the presence or absence of 1 mM l-Cys or 1 mM d-Cys. Data are presented as the mean ± SEM (n = 3). Different letters indicate statistically significant differences between groups (p<0.05, Tukey’s test).
d-Cys protects xCT-KO MEFs from death induced by the withdrawal of β-ME from the culture medium
To further confirm the anti-ferroptotic action of d-Cys, we used embryonic fibroblasts established from xCT-knockout mice (xCT-KO MEFs). Since xCT-KO MEFs do not produce xCT, the cells die when cultured in standard culture medium without supplementation with β-ME, which can rescue the death of xCT-KO MEFs.(14) After removing β-ME, cell death occurred, as evidenced by an increase in LDH levels in the culture medium (Fig. 4A). When l-Cys was added instead of β-ME, cell death was completely suppressed during the 48 h treatment, suggesting that the l-Cys is taken up via corresponding amino acid transporters other than xCT. On the other hand, d-Cys suppressed cell death until 24 h, but the protective effect was lost after 48 h, indicating that d-Cys rescued xCT-KO MEFs from ferroptosis but only at an earlier period of incubation. We next determined the intracellular levels of Cys and GSH in xCT-MEFs and observed significant decreases in their levels under β-ME-free conditions (Fig. 4B and C). Supplementation with l-Cys or d-Cys significantly restored both Cys and GSH levels to similar extents between the two groups of cells.
Fig. 4.
Effects of d-Cys supplementation on the survival of xCT-KO MEFs under β-ME free conditions. (A) xCT-KO MEF were first cultured in the presence of 50 μM β-ME and then cultured in β-ME-removed medium in the presence or absence of 1 mM l-Cys or 1 mM d-Cys for 24 or 48 h. Data represent the mean ± SEM (n = 3). (B, C) Intracellular concentration of (B) Cys and (C) GSH in xCT-KO MEF cells 6 h after culture in complete (control) or β-ME-free medium supplemented with or without 1 mM l-Cys or 1 mM d-Cys. Data are presented as the mean ± SEM (n = 3). Different letters indicate statistically significant differences between groups (p<0.05, Tukey’s test).
d-Cys supplementation protects cells from ferroptosis in a GSH synthesis-dependent manner
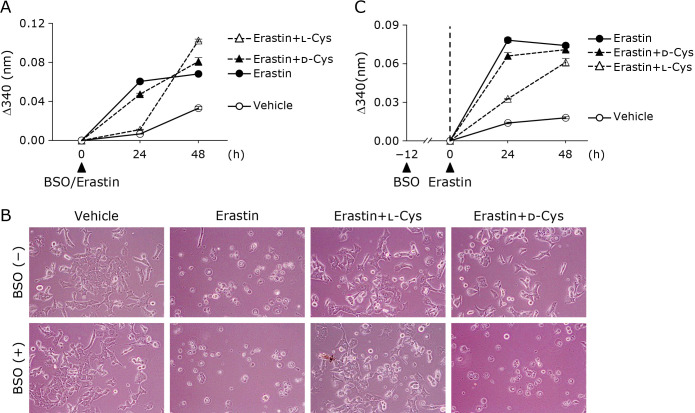
Finally, to examine the protective mechanism of d-Cys, we treated Hepa 1-6 cells with a combination of erastin and BSO, a compound that inhibits the action of γ-glutamylcysteineligase (GCL), the rate-limiting enzyme for GSH synthesis (Fig. 5). Consistent with our previous report,(10) the BSO treatment alone was not sufficient to result in lethality in Hepa 1-6 cells, as evidenced by an LDH assay. We found that the simultaneous treatment with BSO and erastin (Fig. 5A and B) or the pretreatment with BSO followed by treatment with erastin (Fig. 5C) abolished the protective effect of d-Cys against erastin-induced ferroptosis immediately after the start of the erastin treatment. Because the BSO treatment inhibited GSH synthesis, these data suggest that the rescuing action of d-Cys required the synthesis of GSH. Collectively, these results suggest that d-Cys supplementation protects cells from ferroptosis in a GSH synthesis-dependent manner, even though d-Cys cannot be used for the GSH synthesis.
Fig. 5.
Effects of d-Cys supplementation on erastin-induced ferroptosis under conditions of the inhibition of GSH synthesis. (A) Cytotoxicity of cells assessed by measuring released LDH activity. Hepa 1-6 cells were treated with DMSO (control) or 10 μM erastin simultaneously with 0.5 mM BSO in the presence or absence of 1 mM l-Cys or 1 mM d-Cys for 24 or 48 h. Data represent the mean ± SEM (n = 3). (B) Representative phase-contrast images of cells. Hepa 1-6 cells were treated under the same conditions as in (A). (C) Effects of pretreatment with BSO on the cytoprotective action of Cys supplementation. Cytotoxicity of cells was assessed by measuring released LDH activity. Hepa 1-6 cells were pre-treated with 0.5 mM BSO for 12 h and then further treated with DMSO (vehicle) or 10 μM erastin in the presence or absence of 1 mM l-Cys or 1 mM d-Cys for 24 or 48 h. Data represent the mean ± SEM (n = 3).
Discussion
In the current study, we found that ferroptosis that is induced by the inhibition of xCT by erastin was suppressed not only by the addition of l-Cys but also by the addition of d-Cys (Fig. 1). Although d-Cys is not a direct source of GSH synthesis, d-Cys supplementation resulted in both intracellular Cys and GSH levels being temporarily maintained under xCT dysfunction (Fig. 3). On the other hand, under extracellular cystine deprivation, d-Cys supplementation did not increase the intracellular GSH level, and the protection by d-Cys against ferroptosis was less effective (Fig. 2). These observations confirmed pivotal action of preserved GSH, which was only temporarily performed by d-Cys, for the cytoprotective action. We also found that d-Cys suppressed ferroptosis in xCT-KO MEFs after removing β-ME from the medium (Fig. 4), which eliminates the possible involvement of xCT in the d-Cys-mediated protection in erastin-induced ferroptosis. Finally, we found that GSH synthesis was essential in this d-Cys-mediated suppression of ferroptosis (Fig. 5). These collective data clearly indicate that the Cys-GSH axis is involved in the d-Cys-mediated protective action against ferroptosis that is induced by xCT dysfunction.
Potential mechanisms that explain the protective action of d-Cys against ferroptosis caused by xCT dysfunction can be grouped into two categories; one of which is related to the reducing power of d-Cys and the other is attributable to the function of sulfur. Disulfide exchange reactions occur spontaneously under physiological conditions. Reactions between d-Cys and l-cystine, which is included in the standard culture medium, can result in the formation of mixed disulfides between d-Cys and l-Cys and the simultaneous release of one l-Cys. The resulting released l-Cys is transported into cells via a neutral amino acid transporter.(6) In fact, this disulfide exchange reaction is partly responsible for the protective role of β-ME in maintaining xCT-deficient cells.(14,17,18) β-ME reacts with l-cystine to produce a mixed disulfide between l-Cys and β-ME via thiol-disulfide interchange reactions, which results in one l-Cys being released. Both the released l-Cys and a mixed disulfide linking l-Cys and β-ME can be transported into the cell via membrane transporters for neutral amino acids. The mixed disulfide is then reduced back to l-Cys and β-ME after entering the cells. β-ME is exported out of cells and reacts with l-cystine in the extracellular milieu again. This β-ME-mediated cyclic action allows cells to maintain sufficient levels of l-Cys and consequent GSH levels under culture conditions even in the case of xCT-deficient cells.(18) However, because the measurement method used in our study does not distinguish between Cys isomers, it is not possible to conclude that the chimeric cystine consisting of Cys isomers is transported into the cells. In the case where the chimeric cystine is not incorporated into cells, the d-Cys-mediated l-Cys supply to the cells would be less efficient compared to the β-ME-mediated one. Our results show that rescuing the action of d-Cys was effective but only within 24 h, and suggest that Hepa 1-6 cells were unable to incorporate the chimeric cystine.
On the other hand, we cannot eliminate other possibilities that involve the function of sulfur derived from d-Cys because the inhibition of GSH synthesis alone does not cause ferroptosis in some cells, including the Hepa 1-6 cells used in this study.(10) For example, both l-Cys and d-Cys can serve as a precursor for the biosynthesis of hydrogen sulfide (H2S),(13) which has ability to reduce the disulfide bonds of l-cystine, resulting in the formation of two free l-Cys molecules.(19) d-Cys is first converted to 3-mercaptopyurvate by d-amino acid oxidase and then to H2S by the action of 3-mercaptopyrvate sulfurtransferase in some organs. Although H2S was not measured in our study, we consider that this mechanism is unlikely here because the enzymes responsible for the conversion of d-Cys to H2S are localized in only limited tissues. l-Cys is a precursor for synthesizing not only proteins, GSH, and H2S but also some primary compounds, as for example, sulfur-iron complex,(20) coenzyme A (CoA),(8,21,22) and Cys persulfides.(23) Whether d-Cys is utilized in their syntheses will require further investigation and there remain unanticipated mechanisms for the suppression of ferroptosis by d-Cys.
In this study, d-Cys was found to partially protect cells against ferroptosis that is induced by xCT inhibition or the ablation of the xCT gene. The protective mechanism appears to be attributable to the l-Cys that is released through the replacement of one l-Cys in l-cystine with d-Cys by a disulfide exchange reaction. In fact, in the absence of extracellular l-cystine, d-Cys supplementation did not increase intracellular GSH levels, and the anti-ferroptotic action was limited. The preserved l-Cys is then incorporated into cells via neutral amino acid transporters and is used to synthesize GSH, leading to protection against ferroptosis. Regarding the mechanism responsible for this process, the chimeric cystine composed of Cys isomers will need to be identified.
Acknowledgments
This work was partly supported by the YU-COE (C) (C31-3) program of Yamagata University.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012; 149: 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017; 171: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014; 156: 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii J, Homma T, Kobayashi S. Ferroptosis caused by cysteine insufficiency and oxidative insult. Free Radic Res 2020; 54: 969–980. [DOI] [PubMed] [Google Scholar]
- 5.Freidman N, Chen I, Wu Q, et al. Amino acid transporters and exchangers from the SLC1A family: structure, mechanism and roles in physiology and cancer. Neurochem Res 2020; 45: 1268–1286. [DOI] [PubMed] [Google Scholar]
- 6.Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system xc−: cystine supplier and beyond. Amino Acids 2012; 42: 231–246. [DOI] [PubMed] [Google Scholar]
- 7.Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 2014; 3: e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badgley MA, Kremer DM, Maurer HC, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020; 368: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer SL, Saha A, Liu J, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med 2017; 23: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homma T, Kobayashi S, Fujii J. Cysteine preservation confers resistance to glutathione-depleted cells against ferroptosis via CDGSH iron sulphur domain-containing proteins (CISDs). Free Radic Res 2020; 54: 397–407. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Khan S, O'Brien PJ. The glutathione dependence of inorganic sulfate formation from L- or D-cysteine in isolated rat hepatocytes. Chem Biol Interact 1998; 110: 189–202. [DOI] [PubMed] [Google Scholar]
- 12.Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-l-cysteine in vivo: studies with N-acetyl-d-cysteine in mice. J Pharmacol Exp Ther. 1986;238:54–61. [PubMed] [Google Scholar]
- 13.Shibuya N, Koike S, Tanaka M, et al. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun 2013; 4: 1–7. [DOI] [PubMed] [Google Scholar]
- 14.Sato H, Shiiya A, Kimata M, et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem 2005; 280: 37423–37429. [DOI] [PubMed] [Google Scholar]
- 15.Homma T, Kobayashi S, Sato H, Fujii J. Edaravone, a free radical scavenger, protects against ferroptotic cell death in vitro. Exp Cell Res 2019; 384: 111592. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Tokairin Y, Miyakoshi T, et al. Quantitative analysis of γ-glutamylpeptides by liquid chromatography-mass spectrometry and application for γ-glutamyltransferase assays. Anal Biochem 2019; 578: 13–22. [DOI] [PubMed] [Google Scholar]
- 17.Ishii T, Bannai S, Sugita Y. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J Biol Chem 1981; 256: 12387–12392. [PubMed] [Google Scholar]
- 18.Ishii T, Mann GE. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol 2014; 2: 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy B, Bhattacharya R, Mukherjee P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J 2019; 33: 13098–13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez SW, Sviderskiy VO, Terzi EM, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 2017; 551: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leu JI, Murphy ME, George DL. Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proc Natl Acad Sci U S A 2019; 116: 8390–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leu JI, Murphy ME, George DL. Functional interplay among thiol-based redox signaling, metabolism, and ferroptosis unveiled by a genetic variant of TP53. Proc Natl Acad Sci U S A 2020; 117: 26804–26811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Tsutsuki H, Ono K, Akaike T, Sawa T. Antioxidative and anti-inflammatory actions of reactive cysteine persulfides. J Clin Biochem Nutr 2021; 68: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]