Abstract
Background
Severe cases of coronavirus disease 2019 (COVID-19) have increased risk for acute kidney injury (AKI). The exacerbation of the immune response seems to contribute to AKI development, but the immunopathological process is not completely understood.
Objectives
To analyze levels of circulant immune mediators in COVID-19 patients evolving with or without AKI. We have also investigated possible associations of these mediators with viral load and clinical outcomes.
Methods
This is a longitudinal study performed with hospitalized patients with moderate to severe COVID-19. Serum levels of 27 immune mediators were measured by a multiplex immunoassay. Data were analyzed at two timepoints during the follow-up: within the first 13 days of the disease onset (early sample) and from the 14th day to death or hospital discharge (follow-up sample).
Results
We studied 82 COVID-19 patients (59.5 ± 17.5 years, 54.9% male). Of these, 34 (41.5%) developed AKI. These patients presented higher SARS-CoV-2 viral load (P = 0.03), higher frequency of diabetes (P = 0.01) and death (P = 0.0004). Overall, AKI patients presented significantly higher and sustained levels (P < 0.05) of CCL-2, CCL-3, CCL-4, CXCL-8, CXCL-10, IFN-γ, IL-2, IL-6, TNF-α, IL-1Ra, IL-10 and VEGF. Importantly, higher levels of CCL-2, CXCL-10, IL-2, TNF-α, IL-10, FGFb, and VEGF were observed in AKI patients independently of death. ROC curves demonstrated that early alterations in CCL-2, CXCL-8, CXCL-10, IFN-γ, IL-6, IL-1Ra and IL-10 show a good predictive value regarding AKI development. Lastly, immune mediators were significantly associated with each other and with SARS-CoV-2 viral load in AKI patients.
Conclusions
COVID-19 associated AKI is accompanied by substantial alterations in circulant levels of immune mediators, which could significantly contribute to the establishment of kidney injury.
Keywords: COVID-19, Acute kidney injury, Cytokines, Immune mediators
1. Introduction
Since December 2019, the new coronavirus (severe acute respiratory syndrome coronavirus 2 - SARS-CoV-2) has spread worldwide, causing the global outbreak of coronavirus disease 2019 (COVID-19) [1], [2]. The clinical spectrum resulting from SARS-CoV-2 infection is broad, ranging from asymptomatic to critical cases [2]. In severe cases, systemic involvement and multi-organ dysfunction are highly prevalent, especially for older patients and those presenting pre-existing diseases [3].
In this context, acute kidney injury (AKI) has been associated with severe COVID-19, with incidence rates varying from 0.5 to 29%, particularly among patients admitted at intensive care units (ICU) [4], [5], [6]. Biopsy findings show that patients can develop different types of lesions. Glomerular injury can course with collapsing segmental and focal glomerulosclerosis, tubular damage is often diagnosed as acute tubular necrosis and vascular lesions (e.g., thrombotic microangiopathy) are also present [7], [8]. Importantly, AKI associated with severe COVID-19 increases the risk for poor clinical outcomes, including the worsening of previous chronic kidney disease (CKD) and other comorbidities, in-hospital death, and post-COVID CKD development [9], [10], [11].
Although the pathophysiology of kidney injury in COVID-19 is diverse and multifactorial, comorbidities such as diabetes, hypertension, and coronary disease have been identified as risk factors for AKI development [4], [5], [10]. Nevertheless, several factors could be involved in the pathogenesis of kidney damage in SARS-CoV-2 infection, such as the hyper-activation of the angiotensin-converting enzyme 2 (ACE2) receptor, dysregulation of the renin-angiotensin-aldosterone system (RAAS), pro-coagulant stimuli, and the establishment of an exacerbated immune response, usually referred as “cytokine storm” [12], [13].
Altogether, even though various mechanisms have been proposed to elucidate the pathophysiology of AKI secondary to COVID-19, the immunopathological process is not yet fully understood. Thus, we aimed to analyze circulant levels of mediators such as anti- and pro-inflammatory cytokines, chemokines, and growth factors during the hospitalization period of moderate-to-severe COVID-19 in patients evolving with and without AKI. Moreover, we investigated the potential of immune mediators as predictive biomarkers for disease worsening.
2. Methods
2.1. Study design and data collection
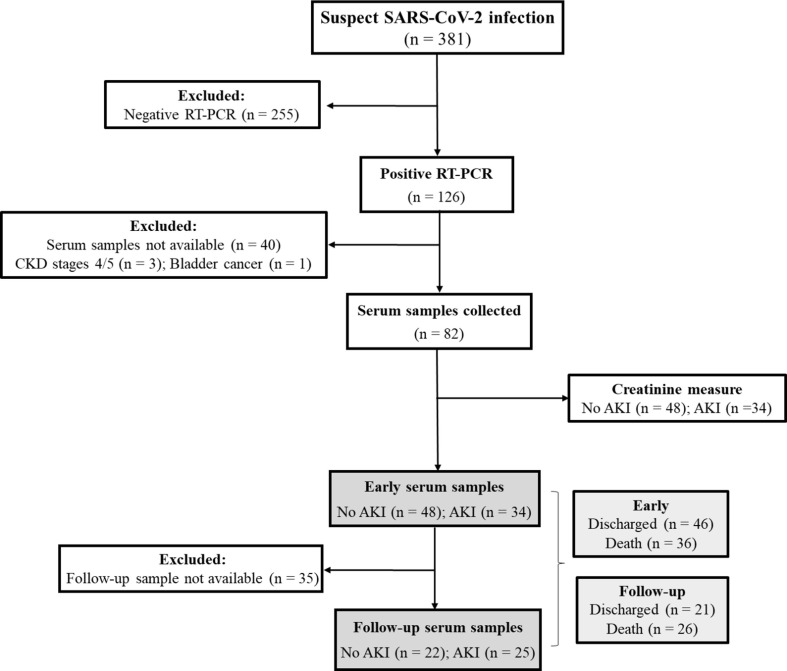
We conducted a longitudinal observational study, which was performed at the Hospital Universitário Antônio Pedro (HUAP - Niterói, Rio de Janeiro, Brazil) during the initial phase of the COVID-19 pandemic in Brazil (April to August 2020). This project was approved by the Research Ethics Committee of the Universidade Federal Fluminense (CAAE: 30623520.5.0000.5243). We included patients with a suspect diagnosis of SARS-CoV-2 infection admitted at HUAP (Fig. 1 ). Exclusion criteria was: negative RT-PCR result for SARS-CoV-2, CKD in advanced stages (4 or 5) or bladder cancer at the time of admission and unavailability of serum samples.
Fig. 1.
Flowchart demonstrating the inclusion of patients with suspect SARS-CoV-2 infection during April to August 2020.
HUAP attends high complexity cases (e.g., cancer, autoimmune disease, heart surgeries, kidney transplants) in the Metropolitan Region II of Rio de Janeiro State. During the initial phase of COVID-19 pandemics, was reference treatment center for moderate to severe cases. These cases were usually characterized by the presence of persistent fever and cough, dyspnea, and hypoxia in association with pulmonary involvement.
Patient data (e.g., gender, skin color, age, presence of comorbidities, and routine laboratory tests) were obtained from the patient's charts. Also, from medical records we identified the main clinical outcomes, development of AKI, and/or death, in addition to other variables such as intensive care unit admission, mechanical ventilation, and therapeutic schemes.
2.2. AKI definition
AKI cases were diagnosed using “The Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for Acute Kidney Injury”, which defines AKI as an increase in serum creatinine (SCr) by 0.3 mg/dL within 48 h or increase in SCr to 1.5 times in comparison to baseline. AKI stages were categorized according to the fold-increase in SCr during the hospitalization, as follows: stage 1 = 1.5–1.9 times baseline; stage 2 = 2.0–2.9 times baseline; and stage 3 = >3 times baseline or increase in SCr to 4.0 mg/dL or the initiation of renal replacement therapy [14].
2.3. Diagnosis of SARS-CoV-2 infection
COVID-19 cases were confirmed within the first week from the onset of symptoms (∼5 days) by RT-PCR, which was performed at the Multiuser Laboratory for Research Support in Nephrology and Medical Science (LAMAP/UFF). Briefly, nasopharyngeal or tracheal aspirate samples were collected by the medical team, and RNA extraction was performed using the QIAamp Viral RNA kit (Catalog no. 52906, QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Subsequently, RT-PCR was performed in three separate reactions per specimen for each target (N1, N2, and the internal control RP) using the 2019-nCOV RUO Kit (catalog no. 10006770, Integrated DNA Technologies, Inc - IDT, Iowa, USA) and the GoTaq® Probe 1-Step RT-qPCR (Catalog no. A6121, Promega Corporation, Wisconsin, USA). Amplification was performed using the 7500 System (Applied Biosystems, ThermoFisher Scientific, California, USA), and cycle threshold (Ct) values for N1, N2, and RP genes were analyzed.
For viral load determination, a quantitative RT-PCR (RT-qPCR) was performed using the Bio GeneCOVID-19 PCR Kit (catalog no. G089-3, lot. 0001, Bioclin/Quibasa, Minas Gerais, Brazil) targeting the SARS-CoV-2 E, N, and RdRp gene regions, following the manufacturer’s instructions. Data was reported as copies/mL, and the lower limit of detection was two copies/mL. Amplification was also performed using the 7500 System.
2.4. Blood sampling and laboratory tests
Peripheral blood samples were collected during the hospitalization period of COVID-19 patients. “Early samples” were collected during the first 13 days of the disease onset (8 ± 4 days) and “follow-up samples” were obtained from the 14th day of hospitalization to death or hospital discharge (15 ± 4 days). The decision to analyze the data at these timepoints was taken based on the mean length of hospitalization of our cohort, which was ∼ 25 days (between 4 and 5 weeks).
Serum levels of immune mediators were assessed through a high-performance microbead 27-plex assay (Cat no. M500KCAF0Y, Bio-Rad, Hercules, CA, USA). We analyzed circulating levels of (i) chemokines [chemokine C-C ligand (CCL)-2/monocyte chemoattractant protein 1/MCP-1, CCL-3/macrophage inflammatory protein 1α, CCL-4/macrophage inflammatory protein 1β, CCL-5/RANTES, CCL-11/eotaxin, interferon C-X-C motif chemokine ligand (CXCL)-8 and CXCL-10/gamma-induced protein 10]; (ii) cytokines [tumor necrosis factor-α/TNF-α, interferon γ/IFN-γ, interleukin 1 receptor antagonist/IL-1Ra; and interleukins (IL)-1β, −2, −4, −5, −6, −7, −9, −10, −12p70, −13, −15, and −17]; and (iii) growth factors (granulocyte–macrophage colony-stimulation factor/GM-CSF; granulocyte colony-stimulating factor/G-CSF; basic fibroblast growth factor/FGFb; platelet-derived growth factor BB/PDGF-BB, and vascular endothelial growth factor/VEGF). Serum samples were filtered using a 0.22 μm syringe filter with a minimum volume of 50 μL of per sample. Results were expressed as pg/mL, and samples were tested on a Bio-Plex 200 instrument (Bio-Rad, Hercules, CA, USA) according to manufacturer's instructions.
2.5. Statistical analysis
GraphPad Prism (GraphPad Software 8.0, San Diego, CA, USA) was used for the statistical analyses. Data were expressed as mean ± standard deviation (SD) or n (%). Differences between two independent groups were assessed by t-test or Mann-Whitney test according to the variable's distribution. Paired analysis of longitudinal data was performed using Repeated Measures ANOVA or Friedman test with their respective post hoc tests. Fisher’s exact test was used to analyze differences between proportions. The ability of circulant immune mediators to predict clinical outcomes was evaluated by the area under the curve (AUC) after performing the receiver operating characteristic curves (ROC) for these parameters. Spearman’s coefficients were assessed to investigate correlations between variables. P-values were considered significant when < 0.05.
The J48 method was used for developing best-fit trees aiming to select the minimal set of phenotypic features that efficiently segregated groups. For this, we considered 0.25 for pruning confidence (-C parameter) and 2 to minimum number of instances (-M parameter). The Leave-one-out cross validation (LOOCV) was calculated to estimate the accuracy of the generated model. These analyses were performed using the Weka software (Waikato Environment for Knowledge Analysis, version 3.6.11, University of Waikato, New Zealand). The heat map analysis was carried out using the heatmap.2 function in the R (Project for Statistical Computing Version 3.0.1) and gplots package.
3. Results
3.1. Profile of COVID-19 patients
From April to August 2020, we attended 381 patients suspected of SARS-CoV-2 infection. Of these, 126 had positive RT-PCR results. From 86 patients with serum samples, four patients were excluded: three had CKD in stages 4 or 5, and one patient had bladder cancer. Thus, for the analysis of circulant immune mediators, we evaluated 82 patients. Fig. 1 illustrates the inclusion of patients from the original cohort. Overall, patients presented a mean (±SD) age of 59.5 ± 17.5 years, and 54.9% (n = 45) were male. Patients presented various comorbidities such as diabetes (36.6%), cardiovascular disease (64.6%), onco-hematological disease (37.8%) and obesity (23.2%). Clinical and demographic characteristics of patients are presented on Table 1 .
Table 1.
General characteristics of COVID-19 hospitalized patients.
| Characteristics | Total (n = 82) | No AKI (n = 48) | AKI (n = 34) | P-value |
|---|---|---|---|---|
| Age (years, mean ± SD) | 59.5 ± 17.5 | 60.8 ± 18.5 | 59.5 ± 17.5 | 0.6 |
| Male, n (%) | 45 (54.9) | 24 (50) | 21 (61.8) | 0.4 |
| White (self-declared), n (%) | 35 (42.7) | 18 (35.3) | 19 (51.3) | 0.2 |
| Comorbidities, n (%) | ||||
| Diabetes | 30 (36.6) | 11 (22.9) | 19 (55.9) | 0.01 |
| CVD | 53 (64.6) | 29 (60.4) | 24 (70.6) | 0.4 |
| Immunosuppression | 19 (23.2) | 11 (22.9) | 8 (23.5) | 0.9 |
| Oncohematological disease | 31 (37.8) | 18 (37.5) | 13 (38.2) | 0.9 |
| Obesity | 19 (23.2) | 10 (20.8) | 9 (26.5) | 1.0 |
| Complications during hospitalization, n (%) | ||||
| Admission at the ICU | 49 (59.7) | 19 (39.6) | 30 (88.2) | <0.0001 |
| Mechanical ventilation | 38 (46.3) | 9 (18.7) | 29 (85.3) | <0.0001 |
| Renal replacement therapy, n (%) | – | 26 (76.5) | – | |
| Days on RRT (mean ± SD) | – | 8.4 ± 6.1 | – | |
| AKI staging, n (%) | ||||
| 1 | – | – | 3 (8.8) | – |
| 2 | – | – | 6 (17.6) | – |
| 3 | – | – | 25 (73.5) | – |
| Length of hospitalization (days), mean ± SD | 25.4 ± 21.3 | 23.5 ± 21.5 | 27.6 ± 21.3 | 0.4 |
| SARS-CoV-2 viral load (log10copies/mL) | 4.03 ± 1.4 | 2.74 ± 1.6 | 3.47 ± 1.6 | 0.03 |
| Treatment during hospitalization, n (%) | ||||
| Oseltamivir | 21 (25.6) | 5 (10.4) | 16 (47) | 0.0003 |
| Meropenem | 21 (25.6) | 5 (10.4) | 16 (47) | 0.0003 |
| Azitromycin | 25 (30.5) | 6 (12.5) | 19 (55.8) | <0.0001 |
| Ceftriaxone | 19 (23.2) | 5 (10.4) | 14 (41.2) | 0.003 |
| Polymyxin B | 16 (19.5) | 3 (6.2) | 13 (38.2) | 0.0005 |
| Tazocin | 15 (18.3) | 1 (2.1) | 14 (41.2) | <0.0001 |
| Vancomycin | 16 (19.5) | 1 (2.1) | 15 (44.1) | <0.0001 |
| Hydroxycloroquine | 6 (7.3) | – | 6 (17.6) | 0.004 |
| Fluconazole | 2 (2.4) | – | 2 (5.9) | 0.2 |
| Amines | 37 (45.1) | 9 (18.7) | 28 (82.3) | <0.0001 |
| Death, n (%) | 36 (43.9) | 12 (25) | 24 (70.6) | 0.0004 |
Data is expressed as mean ± SD or n (%). P-values were calculated using Mann-Whitney test or qui-square test and were considered statistically significant when < 0.05. CKD = chronic kidney disease, Ct = cycle threshold, CVD = cardiovascular disease, ICU = intensive care unit, RRT = renal replacement therapy (dialysis).
Importantly, the demographic and clinical profile of COVID-19 patients was also evaluated according to the development of AKI. In our cohort, 34 (41.5%) patients developed AKI during COVID-19 progression. Of those, three developed AKI stage 1 (8.8%), six AKI stage 2 (17.6%), and 25 AKI stage 3 (73.5%) requiring renal replacement therapy. We identified that patients with AKI were more frequently diabetic (P = 0.01) and, as expected, they also presented a higher frequency of complications during hospitalization, such as intensive care admission, invasive mechanical ventilation support (P < 0.0001), and use of medications such as antibiotics and amines. When evaluating the parameters obtained in the RT-qPCR, we observed that SARS-CoV-2 viral load was significantly higher in patients who developed AKI (P = 0.03). Lastly, AKI was associated with increased mortality (70.6% vs. 25%, P = 0.0004).
3.2. COVID-19 associated AKI is accompanied by significant alterations in circulant immune biomarkers during disease progression
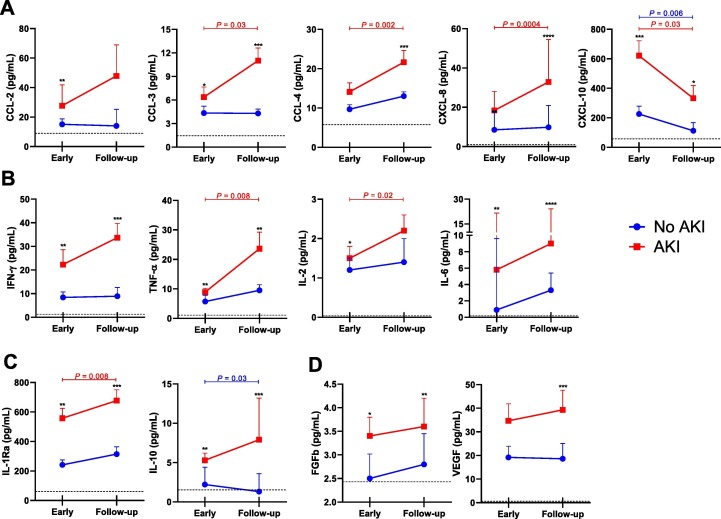
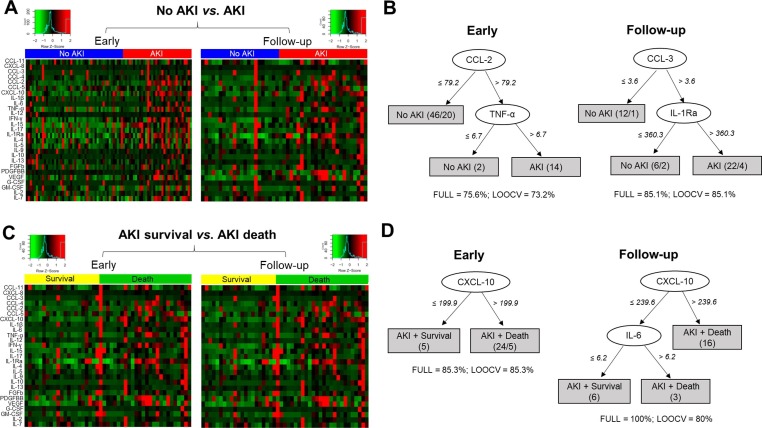
We evaluated serum levels of several immune mediators in both time points according to the development of AKI (Fig. 2 ). We identified that the early samples of AKI patients showed significantly higher levels of CCL-2 (P = 0.002), CCL-3 (P = 0.02), CXCL-8 (P = 0.005), CXCL-10 (P = 0.0002), IFN-γ (P = 0.001), IL-2 (P = 0.03), IL-6 (P = 0.005), TNF-α (P = 0.003), IL-1Ra (P = 0.001), IL-10 (P = 0.001), FGFb (P = 0.03). Except for CCL-2 and IL-2, these immune mediators were also significant higher in the follow-up samples of AKI patients when compared to “No AKI”. Moreover, the analysis of heatmaps also show the significant unbalance between circulating immune mediators in AKI patients in both timepoints (Fig. 3 A). Importantly, considering all mediators, the decision trees derived from the J48 method demonstrated that CCL-2 and TNF-α were the best mediators to discriminate AKI in the early timepoint and CCL-3 and IL-1Ra showed the best performance in the follow-up timepoint (Fig. 3 B)
Fig. 2.
Analysis of immune mediators of COVID-19 hospitalized patients evolving with and without AKI. Circulating levels of (A) chemokines, (B) pro-inflammatory cytokines, (c) anti-inflammatory cytokines and (D) growth factors were assessed by a multiplex assay. Data is shown as median ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 indicate comparison between groups (No AKI vs. AKI) in each time point (t test/Mann-Whiney test). P-values on horizontal bars indicate the comparison of time points (early vs. follow-up samples) for each group - blue lines for “No AKI” and red lines for “AKI” - (paired t test/Wilcoxon).
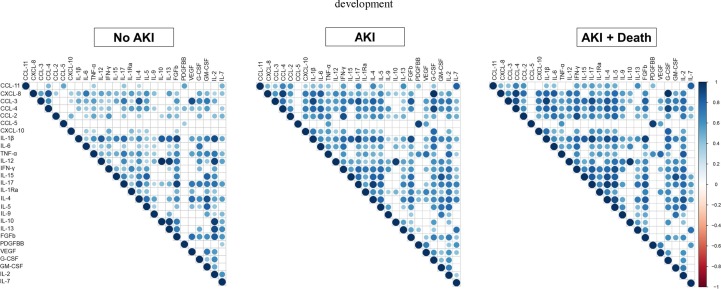
Fig. 3.
Exploring the profile of immune mediators in COVID-19 patients according to AKI development. Heatmaps show the landscape of immune mediators between “No AKI” and “AKI” (A) and “AKI + survival” and “AKI + death” (C). Best-fit decision trees identified the mediators that efficiently segregated “No AKI” from “AKI” (B) and “AKI + survival” from “AKI + death” (D). Levels were placed in the root of the tree according to the cytokine/chemokine value (pg/mL) that best divided groups. The total of classified registers (correct and incorrect) for each class are given in parentheses for each terminal node with the Full training (FULL) and Leave-one-out cross-validation (LOOCV) accuracies. If incorrectly classified registers exist, they will appear after slash “/”.
Subsequently, the paired analysis revealed that patients who developed AKI presented a significant increase of CCL-3 (P = 0.03), CCL-4 (P = 0.03), CXCL-8 (P = 0.0004), IL-2 (P = 0.02), TNF-α (P = 0.008) and IL-1Ra (P = 0.008) during COVID-19 progression; in addition to decreased CXCL-10 levels (P = 0.03). In the group that did not develop renal dysfunction, levels of CXCL-10 (P = 0.006) and IL-10 (P = 0.03) were significantly lower in the follow-up samples. These results are also demonstrated on Fig. 2.
Of note, other inflammatory parameters routinely assessed during hospitalization were also analyzed (Supplementary Table 1). We identified that C-reactive protein (CRP, P = 0.009) levels were significantly higher in AKI patients, but no differences were observed for ferritin (P = 0.5) and lactate dehydrogenase (LDH, P = 0.3). Differences were also not identified for hematological parameters, such as the absolute number of lymphocytes (P = 0.9).
3.3. Alterations in circulant immune mediators in COVID-19 associated AKI occur independently of death
Fig. 3C shows that COVID-19 patients who develop AKI and evolved to death present stronger alterations on serum levels of various immune mediators in comparison to AKI patients who survived. To discriminate AKI in association with death, decision trees highlights that CXCL-10 and the combination of CXCL-10 and IL-6 were the best mediators for early and follow-up timepoints, respectively (Fig. 3 D).
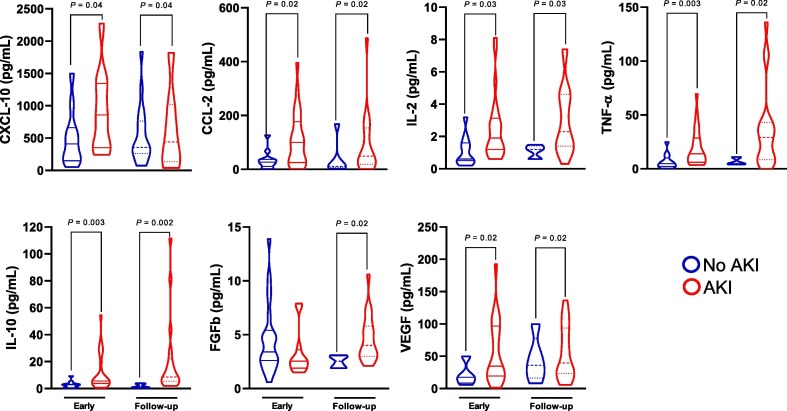
Despite that, we decided to investigate whether the changes in inflammatory mediators would be associated with AKI regardless of death. For this, serum levels of immune mediators were compared among all patients who died according to AKI development. Overall, we observed that AKI patients who died still present significantly higher concentrations of CCL-2 (P = 0.02), CXCL-10 (P = 0.04), IL-2 (P = 0.03), TNF-α (P = 0.02), IL-10 (P = 0.002), VEGF (P = 0.02), and FGFb (P = 0.02) when compared to COVID-19 patients who died with no significant alterations on kidney function. Except for FGFb, these differences were significant at both time points. These results are illustrated on Fig. 4 .
Fig. 4.
Analysis of immune mediators of COVID-19 hospitalized patients who died according to the development of AKI. Data is shown as violin plots. P-values indicates comparison between groups (No AKI vs. AKI; t test/Mann-Whitney).
3.4. Circulant immune biomarkers could be used as predictors of AKI in COVID-19 patients
In order to recognize possible biomarkers that could be useful as predictors of AKI in hospitalized COVID-19 patients, ROC curves were plotted for the levels of immune mediators obtained in early samples, considering “AKI” and “AKI + death” as outcomes (Table 2 ). Overall, we observed that CCL-2, CXCL-8, CXCL-10, IFN-γ, IL-6, IL-10, and IL-1Ra showed a predictive potential for AKI (P < 0.01); and, except for IL-6 and CXCL-8, serum levels of these immune mediators presented AUC values > 0.7. When analyzing “AKI + death” as the final outcome, CCL-2 and CXCL-10 showed the highest AUC values (AUC 0.879, P = 0.0006 and AUC 0.841, P = 0.002, respectively).
Table 2.
Parameters from ROC curves of circulant immune mediators as predictors of AKI or AKI + death in COVID-19 hospitalized patients at “Early” timepoint.
| Parameters | AUC | 95% CI | Cut-off | Sens (%) | Spec (%) | P-value |
|---|---|---|---|---|---|---|
| A) No AKI vs. AKI | ||||||
| CXCL-10 | 0.739 | 0.627–0.853 | >314.2 | 72.7 | 63.8 | 0.0003 |
| IFN-γ | 0.722 | 0.602–0.842 | >14.1 | 70.9 | 63.4 | 0.001 |
| IL-10 | 0.717 | 0.599–0.835 | >3.15 | 71.9 | 65.9 | 0.001 |
| IL-1Ra | 0.713 | 0.594–0.831 | >349 | 69.7 | 63.8 | 0.001 |
| CCL-2 | 0.703 | 0.584–0.822 | >22.0 | 63.6 | 61.7 | 0.002 |
| CXCL-8 | 0.682 | 0.563–0.802 | >17.9 | 54.5 | 80.5 | 0.006 |
| IL-6 | 0.681 | 0.560–0.802 | >1.75 | 63.6 | 65.9 | 0.006 |
| B) AKI + survival vs. AKI + death | ||||||
| CCL-2 | 0.879 | 0.764–0.994 | >21.5 | 83.3 | 80.0 | 0.0006 |
| CXCL-10 | 0.841 | 0.694–0.988 | >341.6 | 79.2 | 70.0 | 0.002 |
| IL-6 | 0.752 | 0.591–0.913 | >2.7 | 75.0 | 70.0 | 0.02 |
| CXCL-8 | 0.725 | 0.532–0.917 | >15.4 | 66.7 | 70.0 | 0.04 |
AUC = area under the curve; CI = confidence interval; sens = sensitivity; spec = specificity.
3.5. Immune mediators are associated with each other and with SARS-CoV-2 viral in AKI patients
We developed matrix correlations for all circulating levels of immune mediators in COVID-19 patients according to the development of AKI (Fig. 5 ). Overall, we identified that, in AKI patients at early timepoint, chemokines were significantly correlated with each other and with other anti- and pro-inflammatory cytokines, especially CCL-2, CCL-3, CCL-4 and CXCL-8. Moreover, IL-6 and IFN-γ also show different correlations with various immune mediators. Among growth factors, we highlight the associations between G-CSF and FGFb with other mediators; and IL-1Ra showed more associations when we evaluated anti-inflammatory cytokines. Importantly, when compared to patients who did not developed AKI, the strength of these associations were reduced or absent. At the same time, AKI patients who died showed slightly increased coefficients for some associations. Of note, similar patterns of matrix correlations for the follow-up timepoint are demonstrated on Supplementary Fig. 1.
Fig. 5.
Correlation matrix for comparing associations between immune mediators at early timepoint in COVID-19 patients according to AKI development. Spearman’s coefficients are represented in different colors, as demonstrated by the vertical bar on the right.
In COVID-19, as well as for other viral infections, it is suggested that higher viral load is associated with poor outcomes [16], [17], [18]. Thus, we investigated if SARS-CoV-2 viral load could be associated with serum levels of immune biomarkers in patients who developed AKI. We observed that SARS-CoV-2 viral load was significantly correlated with CCL-2, (P = 0.01) IFN-γ (P = 0.001), CXCL-8 (P = 0.03), IL-9 (P = 0.01) and IL-5 (P = 0.01). The best correlation coefficient was observed for IFN-γ (Spearman’s r = 0.786). These results are shown on Table 3 .
Table 3.
Correlation between immune mediators and SARS-CoV-2 viral load in COVID-19 patients with AKI.
| Parameters | SARS-CoV-2 Viral load |
|---|---|
| Chemokines | |
| CCL-2 | 0.637* |
| CXCL-8 | 0.588* |
| CXCL-10 | 0.379 |
| CCL-11 | 0.341 |
| CCL-5 | −0.324 |
| CCL-3 | 0.269 |
| CCL-4 | 0.011 |
| Pro-inflammatory cytokines | |
| IFN-γ | 0.786** |
| IL-9 | 0.663* |
| IL-6 | 0.495 |
| TNF-α | 0.352 |
| IL-7 | 0.297 |
| IL-12p70 | 0.278 |
| IL-15 | 0.201 |
| IL-2 | 0.198 |
| IL-1β | 0.135 |
| IL-17 | 0.110 |
| Anti-inflammatory cytokines | |
| IL-5 | 0.673* |
| IL-10 | 0.341 |
| IL-4 | 0.326 |
| IL-1Ra | 0.313 |
| IL-13 | −0.204 |
| Growth factors | |
| G-CSF | 0.544 |
| GM-CSF | 0.428 |
| FGFb | 0.171 |
| PDGFbb | 0.126 |
| VEGF | 0.118 |
Data is shown as Spearman’s coefficients. Significant correlations are shown in bold (*P < 0.05, **P < 0.01).
4. Discussion
SARS-CoV-2 is known not only to cause severe pulmonary impairment but also to affect multiple tissues and organs, such as the gastrointestinal tract, liver, and kidneys [19]. In this context, studies have been performed to investigate the prevalence, risk factors, and mechanisms of kidney dysfunction during COVID-19. Considering that an exacerbated immune response is regarded as a determinant factor for disease severity [20], [21], we aimed to assess serum levels of several immune mediators in hospitalized COVID-19 patients with moderate to severe disease evolving with and without AKI.
Overall, our results show that COVID-19 patients who develop AKI presented substantial alterations in serum levels of inflammatory mediators. Thus, higher concentrations of chemokines (CCL-2, CCL-3, CCL-4, CXCL-8 and CXCL-10), growth factors (FGFb and VEGF), and cytokines either pro-inflammatory (IFN-γ, IL-2, IL-6 and TNF-α) or anti-inflammatory (IL-1Ra and IL-10) were observed in the first weeks after the onset of symptoms, which significantly increased throughout the hospitalization.
Several studies have demonstrated that SARS-CoV-2 infection induces hypercytokinemia, which leads to a state of hyperinflammation in association with uncontrolled and sustained activation of T cells and macrophages, especially in patients with the severe forms of the disease [15], [20], [22], [23]. Thus, our results suggest that these dramatic changes in inflammatory mediators indicate that an unbalanced immune response can also be involved in the establishment of kidney injury associated with COVID-19. Moreover, one may suggest that alterations in anti-inflammatory mediators accompanies the excess of pro-inflammatory cytokines and chemokines in a probable attempt to suppress the inflammatory process. In this context, other studies reported that IL-10 is a cytokine that “should be watched” in COVID-19 [24], [25], [26]. Furthermore, Henry and colleagues (2020) demonstrated that IL-10 is a strong predictor of disease severity and AKI [26].
Anderberg and colleagues (2021) recently assessed the profile of circulant immune mediators in critically ill COVID-19 patients in a cross-sectional study. They observed that high serum levels of IL-1β, IL-1Ra, IL-2, IL-4, IL-6, IL-7, CXCL-8, IL-10, IL-13, IL-17a, G-CSF, IFN-γ, CXCL-10, CCL-2, and TNF-α were associated with AKI; however, they did not find correlations with CCL-3 and CCL-4 [27]. In fact, the dysregulation of the immune response significantly contributes to kidney disease. Besides the autoimmunity pathways, the loss of immunological homeostasis results in complement activation and glomerular injury with sustained activation of B and T cells leading to tissue damage and immune complex deposition on the glomerular basal membrane and blood vessels [28]. During AKI, damaged kidney cells induce the activation of resident macrophages and dendritic cells, which leads to the secretion of cytokines and chemokines, resulting in tissue inflammation [29].
In this study, we also observed that, when compared to COVID-19 patients who died with normal kidney function, AKI patients still showed higher levels CCL-2, CXCL-10, IL-2, TNF-α, IL-10, FGFb, and VEGF. As the frequency of death by COVID-19 was high in the group that developed AKI (70.6%), our objective was to evaluate whether changes in inflammatory mediators would be associated with AKI, regardless of death as the final event. Since the cytokine storm has been closely associated with severe COVID-19 and death [20], these results strengthen the hypothesis that a hyperinflammatory state also contributes to renal dysfunction in COVID-19. This could be a reflection of direct kidney injury promoted by SARS-CoV-2 infiltration [30], [31], with activation of the innate immune response [29] or reduction of plasma clearance of cytokines due to kidney function impairment [32].
We have also investigated the potential of circulating immune biomarkers in predicting AKI during COVID-19 progression. ROC curves demonstrated that alterations in CCL-2, CXCL-8, CXCL-10, IFN-γ, IL-6, IL-1Ra, and IL-10 during the early acute phase of the disease might have diagnostic value. Importantly, we observed that when using “AKI + death” as outcome, the predictive power was increased, especially CCL-2 and CXCL-10, which presented AUC values > 0.8. Previous studies have reported that cytokines can be used as predictive biomarkers for COVID-19 severity, especially IL-6 and IL-10, as demonstrated by a recent meta-analysis [24]. Interestingly, a combined analysis of different cytokines can help to predict COVID-19 prognosis [25]; however, a multivariate analysis was not performed in the present study. Considering the mediators which showed the best values, our results were, once again, similar to Anderberg and colleagues (2021), which reported that IL-1Ra, IL-6, CXCL-8, IL-17a, CXCL-10, and CCL-2 presented the strongest associations with AKI (Spearman coefficients > 0.7) [27].
In order to explore the hypothesis that high viral load is directly associated with exacerbation of the immune system, leading to increased disease severity and AKI, we sought to determine the associations between circulant levels of immune mediators, and SARS-CoV-2 viral load. Studies show that increased viral load is associated with COVID-19 severity [16], [17], [18]. Moreover, a recent study demonstrated that SARS-CoV-2 urinary viral load was significantly associated with AKI development [33]. Interestingly, Bermejo‑Martin and colleagues (2020) reported that SARS-CoV-2 viral load is correlated with several inflammatory mediators, in addition to coagulation factors and markers of endothelial dysfunction in critically ill patients; however, the frequency of AKI was not reported [34]. Here, we observed that serum levels of CCL-2, CXCL-8, IFN-γ, IL-9 and IL-5 are associated with higher SARS-CoV-2 viral load only in COVID-19 patients who developed AKI. The strongest correlation coefficient was observed for IFN-γ, which is a potent antiviral cytokine and a potential candidate for therapeutic strategies [35].
Besides the cytokine storm, other immunological events are pivotal for the establishment of the host’s response against the virus [22]. A significant increase in acute phase proteins is observed by different studies [36], [37]. In our study, patients who developed AKI also showed a significant increase in CRP levels, but differences were not observed for serum ferritin and LDH. Moreover, SARS-CoV-2-induced lymphocyte apoptosis also have a impact on COVID-19 immunopathogenesis [38], [39]. However, lymphopenia was not associated with renal dysfunction in the present study. Finally, an impaired type I IFN-response [40], [41], NLR3P inflammasome hyper-activation [42], decreased cytotoxic capacity of CD8+ lymphocytes, and natural killer cells [43], [44], [45] has also been associated with COVID-19 poor outcomes. In this regard, it would be interesting to investigate these parameters in COVID-19 patients who develop AKI.
Other mechanisms have been suggested as determinants for the establishment of kidney injury in COVID-19. Some factors such as hypoxia, sepsis, and septic shock (which implicates in hemodynamic alterations), in addition to baseline diseases such as hypertension and diabetes, were demonstrated to be closely associated with AKI development in COVID-19 patients [4], [5], [10]. In our study, diabetes was also associated with an increased risk for AKI. Moreover, as previously mentioned, kidney cells are rich in ACE2 receptors and the auxiliary protein trans membrane serine proteases, especially podocytes and proximal tubule cells, which are essential for viral cell entry [46]. Thus, SARS-CoV-2 is capable of infecting these cells and promoting a direct cytopathic effect [30], [31]. Interestingly, the recent study from Lite and colleagues (2021) showed that, by an interactome analysis, that ACE2 could mediate immunological interactions in different tissues [47].
Our study has some limitations. Besides the small number of patients, the analysis of serum levels of immune mediators could not be performed at daily or weekly basis to better understand the kinetics of these biomarkers. Studies have shown that early urinary alterations in dipstick tests can be detected in COVID-19 patients [48]; however, the majority of patients did not have results from analysis of urinary sediments in their charts. Importantly, the intrinsic conditions associated with severe COVID-19, such as the hemodynamic changes associated with the administration of several medications (e.g., vasoactive amines and antibiotics), can also affect renal function [8], [13]. In this study, as well as in other “real-life” reports focusing on the analysis of kidney injury in COVID-19, it was not possible to investigate AKI dissociated of secondary factors related to COVID-19 disease severity itself. Intensive care scores such as the Sequential Organ Failure Assessment (SOFA) could not be assessed. Finally, we could not compare levels of inflammatory mediators according to the degree of AKI since the majority of our patients were classified as KDIGO stage 3. Further studies should also consider to evaluate the profile of circulating immune mediators in AKI associated with COVID-19 in comparison to other ethiologies of kidney injury.
5. Conclusion
Altogether, our findings suggest that AKI associated with moderate-to-severe SARS-CoV-2 infection is accompanied by dramatic alterations in circulant levels of cytokines, chemokines, and growth factors. Moreover, this unbalance was associated with AKI independently of death and is significantly correlated with high viral load. This suggests that the loss of immunological homeostasis significantly contributes to the establishment of kidney injury in COVID-19. Lastly, early changes in these biomarkers could be considered as potential predictors of AKI and death in COVID-19 patients.
Funding
This study was supported by grants from Niterói Municipal Health Secretary (PDPA, #4423), Ministério da Educação e Cultura (MEC/Brazil), Brazilian National Research Council/CNPq (#406638/2021-7), Agency for Financing Studies and Projects ( FINEP/RTR/PRPq/REDECOVID-19, #27968*6), and by the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro/FAPERJ (#E-26/210.634/2019, #E-26/210.828/2021). This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior do Brasil/CAPES (finance code 001). TMwas a research fellow of the Brazilian National Research Council/CNPq. AAS is recognized with the grant Scientist of the State of Rio de Janeiro/FAPERJ (E-26/201.155/2021). The funders did not influence the study's conceptualization, data collection and analysis, publication decision, or manuscript preparation.
CRediT authorship contribution statement
Thalia Medeiros: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. Gabriel Macedo Costa Guimarães: Data curation, Investigation, Writing – review & editing. Fabiana Rabe Carvalho: Data curation, Investigation, Writing – review & editing. Lilian Santos Alves: Data curation, Investigation, Writing – review & editing. Ana Carolina Campi-Azevedo: Data curation, Investigation, Writing – review & editing. Andréa Teixeira-Carvalho: Data curation, Investigation, Writing – review & editing. Laurence Rodrigues do Amaral: Formal analysis, Software, Writing – review & editing. Olindo Assis Martins-Filho: Conceptualization, Funding acquisition, Formal analysis, Writing – review & editing. Jocemir Ronaldo Lugon: Conceptualization, Formal analysis, Writing - review & editing. Jorge Reis Almeida: Conceptualization, Funding acquisition, Formal analysis, Writing – review & editing. Andrea Alice Silva: Conceptualization, Supervision, Project administration, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to all professionals from Hospital Universitario Antônio Pedro who contributed to clinical follow-up, routine biochemical tests, and blood sampling. We would like to thank the team responsible for the routine of SARS-CoV-2 molecular testing at the Multiuser Laboratory for Research Support in Nephrology and Medical Science (LAMAP/UFF) and the Institute of Biology (UFF). Also, we thank the Medical Science and Pathology Graduate Programs. Lastly, we thank Dr. Luzia Maria de Oliveira Pinto, who contributed to the discussions concerning the immunological data obtained in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2022.155974.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Lu H., Stratton C.W., Tang Y. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020 Apr;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y.-C., Chen C.-S., Chan Y.-J. The outbreak of COVID-19: an overview. J. Chin. Med. Association. 2020 Mar;83(3):217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y., Ding M., Dong X., Zhang J., Kursat Azkur A., Azkur D., et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021 Feb;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 4.Durvasula R., Wellington T., McNamara E., Watnick S. COVID-19 and kidney failure in the acute care setting: our experience from seattle. Am. J. Kidney Dis. 2020 Jul;76(1):4–6. doi: 10.1053/j.ajkd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanelli V., Fiorentino M., Cantaluppi V., Gesualdo L., Stallone G., Ronco C., et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit. Care. 2020 Dec;24(1):155. doi: 10.1186/s13054-020-02872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pei G., Zhang Z., Peng J., Liu L., Zhang C., Yu C., et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. JASN. 2020 Jun;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudose S., Batal I., Santoriello D., Xu K., Barasch J., Peleg Y., et al. Kidney biopsy findings in patients with COVID-19. JASN. 2020 Sep;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Oliveira P., Cunha K., Neves P., Muniz M., Gatto G., Salgado Filho N., et al. Renal morphology in coronavirus disease: a literature review. Medicina. 2021 Mar 11;57(3):258. doi: 10.3390/medicina57030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020 Jul;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim J.-H., Park S.-H., Jeon Y., Cho J.-H., Jung H.-Y., Choi J.-Y., et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. JCM. 2020 Jun 3;9(6):1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, et al. Covid‐19 and kidney injury: Pathophysiology and molecular mechanisms. Rev Med Virol [Internet]. 2021 May [cited 2021 Sep 23];31(3). Available from: <https://onlinelibrary.wiley.com/doi/10.1002/rmv.2176>. [DOI] [PMC free article] [PubMed]
- 13.Smarz-Widelska I., Grywalska E., Morawska I., Forma A., Michalski A., Mertowski S., et al. Pathophysiology and clinical manifestations of COVID-19-related acute kidney injury—the current state of knowledge and future perspectives. IJMS. 2021 Jun 30;22(13):7082. doi: 10.3390/ijms22137082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Section 2: AKI Definition. Kidney International Supplements. 2012 Mar;2(1):19–36. [DOI] [PMC free article] [PubMed]
- 15.Gao Y.-M., Xu G., Wang B., Liu B.-C. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J. Intern. Med. 2021 Feb;289(2):147–161. doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Yan L.-M., Wan L., Xiang T.-X., Le A., Liu J.-M., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet. Infect. Dis. 2020 Jun;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao S.N., Manissero D., Steele V.R., Pareja J. A narrative systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020 Sep;9(3):573–586. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Massachusetts Consortium for Pathogen Readiness, Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020 Dec;11(1):5493. [DOI] [PMC free article] [PubMed]
- 19.Wadman M., Couzin-Frankel J., Kaiser J., Matacic C. A rampage through the body. Science. 2020 Apr 24;368(6489):356–360. doi: 10.1126/science.368.6489.356. [DOI] [PubMed] [Google Scholar]
- 20.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020 Jun;16(11):1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varchetta S, Mele D, Oliviero B, Mantovani S, Ludovisi S, Cerino A, et al. Unique immunological profile in patients with COVID-19. Cell Mol Immunol [Internet]. 2020 Oct 15 [cited 2020 Nov 5]; Available from: http://www.nature.com/articles/s41423-020-00557-9. [DOI] [PMC free article] [PubMed]
- 22.Bordallo B., Bellas M., Cortez A.F., Vieira M., Pinheiro M. Severe COVID-19: what have we learned with the immunopathogenesis? Adv Rheumatol. 2020 Dec;60(1):50. doi: 10.1186/s42358-020-00151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castelli V., Cimini A., Ferri C. Cytokine Storm in COVID-19: “When You Come Out of the Storm, You Won’t Be the Same Person Who Walked in”. Front. Immunol. 2020 Sep;2(11):2132. doi: 10.3389/fimmu.2020.02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine (CCLM). 2020 Jun 25;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 25.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes Infect. 2020 Jan 1;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry B.M., Benoit S.W., Vikse J., Berger B.A., Pulvino C., Hoehn J., et al. The anti-inflammatory cytokine response characterized by elevated interleukin-10 is a stronger predictor of severe disease and poor outcomes than the pro-inflammatory cytokine response in coronavirus disease 2019 (COVID-19) Clin. Chem. Laboratory Med. (CCLM). 2021 Feb 23;59(3):599–607. doi: 10.1515/cclm-2020-1284. [DOI] [PubMed] [Google Scholar]
- 27.Bülow Anderberg S., Luther T., Berglund M., Larsson R., Rubertsson S., Lipcsey M., et al. Increased levels of plasma cytokines and correlations to organ failure and 30-day mortality in critically ill Covid-19 patients. Cytokine. 2021 Feb;138 doi: 10.1016/j.cyto.2020.155389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tecklenborg J., Clayton D., Siebert S., Coley S.M. The role of the immune system in kidney disease: The immune system in kidney disease. Clin. Exp. Immunol. 2018 May;192(2):142–150. doi: 10.1111/cei.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yatim K.M., Lakkis F.G. A brief journey through the immune system. CJASN. 2015 Jul 7;10(7):1274–1281. doi: 10.2215/CJN.10031014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassler L, Reyes F, Sparks M, Welling P, Batlle D. Evidence For and Against Direct Kidney Infection by SARS-CoV-2 in Patients with COVID-19. CJASN. 2021 Jun 14;CJN.04560421. [DOI] [PMC free article] [PubMed]
- 31.Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. JASN. 2020 Aug;31(8):1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andres-Hernando A., Dursun B., Altmann C., Ahuja N., He Z., Bhargava R., et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol. Dial. Transplant. 2012 Dec 1;27(12):4339–4347. doi: 10.1093/ndt/gfs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caceres P, Savickas G, Murray S, Umanath K, Uduman J, Yee J, et al. High SARS-CoV-2 Viral Load in Urine Sediment Correlates with Acute Kidney Injury and Poor COVID-19 Outcome. JASN. 2021 Jun 4;ASN.2021010059. [DOI] [PMC free article] [PubMed]
- 34.Bermejo-Martin J.F., González-Rivera M., Almansa R., Micheloud D., Tedim A.P., Domínguez-Gil M., et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit. Care. 2020 Dec;24(1):691. doi: 10.1186/s13054-020-03398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020 Jun;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lino K., Guimarães G.M.C., Alves L.S., Oliveira A.C., Faustino R., Fernandes C.S., et al. Serum ferritin at admission in hospitalized COVID-19 patients as a predictor of mortality. Br. J. Infect. Dis. 2021 Mar;25(2) doi: 10.1016/j.bjid.2021.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L., Chen C. Contribution of acute-phase reaction proteins to the diagnosis and treatment of 2019 novel coronavirus disease (COVID-19) Epidemiol. Infect. 2020;148 doi: 10.1017/S095026882000165X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Li H, Luo M, Liu J, Wu L, Lin X, et al. Lymphopenia predicted illness severity and recovery in patients with COVID-19: A single-center, retrospective study. Adrish M, editor. PLoS ONE 2020 Nov 18;15(11):e0241659. [DOI] [PMC free article] [PubMed]
- 39.Cizmecioglu A., Akay Cizmecioglu H., Goktepe M.H., Emsen A., Korkmaz C., Esenkaya Tasbent F., et al. Apoptosis-induced T-cell lymphopenia is related to COVID-19 severity. J. Med. Virol. 2021 May;93(5):2867–2874. doi: 10.1002/jmv.26742. [DOI] [PubMed] [Google Scholar]
- 40.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020 Aug 7;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020 Dec;11(1):3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lara P.C., Macías-Verde D., Burgos-Burgos J. Age-induced NLRP3 inflammasome over-activation increases lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis. 2020;11(4):756. doi: 10.14336/AD.2020.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020 May;1(11):827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. Journal of Clinical Investigation. 2020 Aug 4;130(9):4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020 May;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan X., Xu D., Zhang H., Zhou W., Wang L., Cui X. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020 Jun;46(6):1114–1116. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.C. Lite, S.S.S.J. Ahmed, M. Juliet, A.J. Freddy, SARS-CoV-2/human interactome reveals ACE2 locus crosstalk with the immune regulatory network in the host. Pathogens Disease 2021 Feb 19;79(2):ftab005. [DOI] [PMC free article] [PubMed]
- 48.Liu R., Ma Q., Han H., Su H., Liu F., Wu K., et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin. Chem. Lab. Med. (CCLM). 2020 Jun 25;58(7):1121–1124. doi: 10.1515/cclm-2020-0220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.