Abstract
The global health burden of diabetes is on the rise and has affected more than half a billion people worldwide, particularly in Southeast Asia, North Africa, Africa, and the Western Pacific, Middle East, and South and Central America regions of the International Diabetes Federation (IDF). Despite many new treatments being available for the management of diabetes, glycemic control remains suboptimal in Asia, compared to the rest of the world. Delay in timely insulin initiation and inadequate titration of insulin are regarded to be some of the important reasons for inadequate glycemic control. Additionally, Asian populations have a distinct phenotype, including a younger age of onset and higher glycemic excursions, suggestive of a lower beta-cell function, as compared to non-Asians. Although there are multiple local and international guidelines on insulin initiation and titration, some of these guidelines can be complex. There is an unmet need for guideline recommendations on basal insulin initiation and titration to be simplified and customized for the Asian population with type 2 diabetes mellitus (T2DM). A unified approach would increase adoption of basal insulin initiation by primary care and family medicine physicians, which in turn would help reduce the inertia to insulin initiation. With this background, a consensus-seeking meeting was conducted with 14 experts from seven Asian countries to delineate appropriate practices for insulin initiation and titration in the Asian context. The key objective was to propose a simple insulin titration algorithm, specific for the Asian population, to improve glycemic control and optimize therapeutic outcomes of people with T2DM on basal insulin. Following a detailed review of literature and current guidelines, and potential barriers to insulin initiation and titration, the experts proposed a simplified insulin titration algorithm based on both physician- and patient-led components. The consensus recommendations of the experts related to basal insulin initiation and titration have been summarized in this article, along with the proposed titration algorithm for optimizing glycemic control in the Asian population with T2DM.
Keywords: Diabetes mellitus, Basal insulin, Insulin titration, Initiation, Self-monitoring of blood glucose, Titration algorithm
Key Summary Points
| It is recommended to start insulin therapy if glycemic goals are not met on oral antidiabetic drugs alone, and a “basal first” approach is recommended in most cases. |
| Despite having a higher rate of increase in the prevalence of diabetes over the past few years, the use of insulin in Asia has not increased to a large extent, and nearly half of the insulin users from Asia fail to achieve glycemic targets. |
| Although guideline recommendations for insulin initiation and titration exist, clinically, physicians and patients do not employ the guideline-recommended titration algorithms because they consider them to be very burdensome. |
| The current article summarizes the consensus recommendations related to insulin initiation and titration, along with the proposed titration algorithm for optimizing glycemic control in the Asian population with type 2 diabetes mellitus. |
| A practical and actionable consensus would facilitate insulin initiation and optimization in Asia. |
Introduction
Diabetes is a global health problem, with more than half a billion people being affected worldwide [1]. The worldwide prevalence of diabetes, especially type 2 diabetes mellitus (T2DM), has increased drastically over the past three decades [2]. More than 60% of the global population with diabetes live in Asia and the Western Pacific region [2]. Notably, while the global prevalence of diabetes is estimated to surge by 46% in 2045, the prevalence in Southeast Asia alone is expected to increase by 68% within the same period [1].
T2DM in Asians is characterized by a young age of onset, predisposition to β-cell failure, and visceral adiposity [2]. Furthermore, Asian subjects have higher glucose excursions, as compared to non-Asians [3]. Another important feature of Asians with diabetes is reduced insulin secretory function relative to increased insulin resistance [4]. As compared to Caucasians, the Asian population with diabetes is at a higher risk of albuminuria and microvascular complications [4]. As compared to non-Asians, Asians have different responses to antidiabetic medications [4], and the requirement of lower mean doses of basal insulin therapy has been documented among Asians [5]. Owing to the innate biological susceptibility toward developing diabetes coupled with the high glycemic index diet of Asians, there is a large scope for early and aggressive management efforts for tackling the incessantly increasing burden of diabetes in Asia [6].
As compared to their Western counterparts, the Asian population with T2DM undergoes a more rapid decline in β-cell function and requires an early initiation of insulin therapy. Despite having a higher rate of increase in the prevalence of diabetes over the past few years, the use of insulin in Asia has not increased to a large extent, and nearly half of the insulin users from Asia fail to achieve glycemic targets [1, 7]. Real-world evidence from the International Diabetes Management Practices Study (IDMPS), which included multiple Asian countries, reported suboptimal glycemic control in people with T2DM over a span of 12 years [8]. Further poor persistence of insulin therapy was observed in people with T2DM, thereby emphasizing the need for patient education on self-monitoring of blood glucose (SMBG), ensuring ease of insulin usage and access to insulin [9]. The study identified the lack of insulin titration as an important cause of poor glycemic control in these countries [10]. Only one-third of people with diabetes achieve glycemic targets in Indonesia, which is largely attributable to poor adherence [11]. A real-world study from Thailand highlighted suboptimal titration of insulin and reported that a very low proportion of people with T2DM achieved glycemic targets (glycated hemoglobin [HbA1c ≤ 7%]) [12]. A study involving five Asian countries, Malaysia, Hong Kong, Thailand, Philippines, and Taiwan, reported high baseline HbA1c levels (≥ 9%) in more than half of the study population at the time of insulin initiation, which suggested a delay in insulin initiation in these regions [13]. Poor glycemic control in the majority of Asian individuals with T2DM has also been reported by the DiabCare study from Indonesia [14]. Overall, delayed initiation of insulin and insufficient titration are the key challenges in Asia in the context of insulin therapy [15].
Despite insulin therapy, poor glycemic control strongly indicates inadequate dose titration [15]. Both early initiation and appropriate dose titration of insulin therapy are crucial for achieving glycemic targets [16]. Currently, the International Diabetes Federation (IDF) recommends the initiation of insulin at 10 U or 0.2 U/kg and titrating the dose once or twice weekly at 1–2 U each time till fasting plasma glucose [FPG] is 70–130 mg/dL [17]. Despite these recommendations, the overall glycemic control in the Asian population is generally suboptimal [12].
Although guideline recommendations for insulin initiation and titration exist, clinically, physicians and patients do not employ the guideline-recommended titration algorithms because they consider them to be very burdensome [16, 18]. The IDMPS-Indonesia study identified a gap between clinical practices and recommendations from the most recent guidelines, which contributed to high mean HbA1c levels and poor glycemic control [19]. Recognizing the common issue of delay in insulin initiation and inadequate insulin titration pertaining to poor glycemic control and taking into account the unique requirements of the Asian population with diabetes such as severe insulin deficiency, a consensus-seeking meeting was organized. Leading experts from several Asian countries participated in this meeting and delineated appropriate practices for insulin initiation and titration in the Asian context. The objectives of the consensus meeting were as follows:
To understand the experts’ approach to their clinical practice
To assess what experts would give as guidance for primary care physicians
Development of practical and actionable consensus to facilitate insulin initiation and optimization in Asia
Methods
Fourteen diabetologists and endocrinologists from Vietnam, Pakistan, Thailand, Indonesia, Malaysia, Philippines, and Singapore, who were members of the Asia Insulin Steering and Advocacy Committee (AISAC), came together for virtual meetings in April and May 2021. A premeeting discussion was conducted to set the objectives, initiate review and discussion, and understand the experts’ approach in their clinical practice. During the premeeting, the topics relevant to key challenges in insulin initiation and titration were selected. The meeting was initiated by reviewing the current practices in insulin initiation and titration in different Asian countries. After much deliberation, resource challenges to be adopted in resource-constrained conditions, including challenges in implementation and adaptation of local and international guidelines, were decided. The steering committee meeting aimed to develop a customized insulin titration algorithm for the Asian population. The modified Delphi method [20] was used to arrive at a consensus, as the opinions of several experts were reported on different topics. During the steering meeting, opinions and views of the experts were assessed on the topics pertaining to the control of diabetes, such as SMBG, choice of insulin for initiation, dosing, and titration. During the meeting, there was a detailed panel discussion on the selected topics, in addition to seeking expert opinions on questions related to prespecified topics. The statements were assessed, and the consensus was set a priori at 80% or higher agreement. If no consensus was reached, e-voting was done following another round of discussion. Thereafter, practical and actionable consensus statements were developed to facilitate insulin initiation and optimization in Asian countries. This article is based on previously conducted studies and does not contain any new data collected from human participants or animals.
Results
A consensus was reached (at least 80% agreed) on the initiation of SMBG for people with T2DM at diagnosis as it can improve compliance and provide the basis for dose adjustment of medications, particularly insulin. The experts were not aligned on recommending a minimum of 2–3 SMBG readings/week during insulin initiation (50% agreed) and regarding the use of FPG (2–3 times/week) as the preferred guide for titration of basal insulin (57% agreed). The majority of the panelists advocated basal insulin as the preferred insulin for initiation in insulin-naïve people with T2DM. Most of the experts agreed that younger age and high HbA1c should trigger early insulin initiation in the Asian population. However, the fear of hypoglycemia and lack of education are the main barriers to insulin initiation in the region. The experts remained divided in their opinion regarding the use of a simple starting dose of insulin for physicians who are less used to initiating basal insulin (57% agreed) and considering both body weight and HbA1c for determining the initial dose of insulin (68% agreed). During the revoting phase, all the experts agreed that a dose of 10 U/day as an initiation dose should be recommended for the majority of Asian patients in the primary care setting. However, individualization is needed in certain cases (100% agreed). In the repolling session, individualization of initiation dose and taking body weight (0.1–0.3 U/kg/day) and glycemic status into consideration should be recommended, wherever feasible (100% agreed).
For insulin titration, a consensus was reached on the statement that titration should be accomplished within the first 16 weeks after insulin initiation (at least 80% agreed). The experts were discordant on their opinions regarding starting titration at the time of initiation (60% agreed), keeping the active titration period as 8–12 weeks, with the total daily dose going up to 0.5–0.7 U/kg (usually not exceeding 60 U/day) as the maximum dose of basal insulin (61% agreed). During the repolling session, all the experts agreed (100%) that titration should be ideally started at the same time or within 2 weeks of insulin initiation (the 2-week window is to assess patient comfort and familiarize with insulin initiation), and 86% of the experts agreed that in primary care settings, titration should continue until FPG goals are met. The period of such active titration should ideally be 12 weeks and may go up to 16 weeks in the real-world setting.
Regarding the insulin titration algorithm, a consensus was reached (at least 80% agreed) on the statement that having a basic simple titration regimen will make it easier to follow and comply, for both healthcare providers (HCPs) and patients. The experts remained divided in their opinion on allowing some flexibility in titration using a simple algorithm of adjusting 2 U every 3–4 days until FPG goals are met (61% agreed). In the revoting session, a consensus was reached on the recommendation of titrating basal insulin once or twice a week (in conjunction with the SMBG readings) in the primary care physician setting (92% agreed). Additionally, a total of 83% of experts agreed that a simple up–down titration algorithm should be recommended in primary care settings, with a dose escalation of 2–4 U/week.
In the context of physician- vs. patient-led titration, the experts reached a consensus on recommending that patient-led titration is the preferred choice in the Asian region and every effort must be made to achieve this goal (at least 80% agreed). The experts advocated the need for robust patient awareness initiatives and an increase in outreach programs involving diabetes educators who can help in empowering patients to titrate insulin (at least 80% agreed). Less than half of the experts agreed that at the primary care physician level, patients and caregivers should lead titration efforts (46% agreed). During the revoting session, all the experts (100%) agreed that in primary care settings, both physicians and patients should drive insulin titration with more emphasis on patient-led titration.
The expert recommendations and the available clinical evidence on the topics pertaining to SMBG, choice of insulin for initiation, dosing, and titration, as discussed in the meeting, are presented in the following section.
Discussion
Self-Monitoring of Blood Glucose
The American Diabetes Association (ADA)-recommended glycemic target for adults (excluding pregnant and older adults) is HbA1c < 7% (53 mmol/mol) without significant hypoglycemia [21]. Both SMBG and HbA1c help optimize glycemic control in people with diabetes [22]. However, the usage of HbA1c as a metric for glycemic control has certain limitations, such as it cannot reveal ephemeral changes in glycemic levels, whether postprandial hyperglycemia or severe hypoglycemia, which may require short-term therapeutic readjustments [22]. In this context, SMBG has been very beneficial in the control of glycemia in people with diabetes on insulin therapy. People with T2DM who use SMBG regularly have shown improved glycemic control [16]. Although SMBG has some limitations (e.g., it provides limited or sporadic glucose data, sometimes considered inconvenient and painful, has an impact on long-term adherence, and is subject to incorrectly recorded data or user errors) [23, 24], the use of SMBG is supported by guidelines and clinical studies and is recommended to be implemented in a structured approach [23].
Although newer techniques, such as continuous glucose monitoring (CGM), provide frequent and automated readings of glucose levels and can provide a higher resolution daily glucose profile than SMBG, its use is currently limited in low resource settings, such as the Asia-Pacific region. The major limitation of this technique is the high cost [25]. As compared to CGM, SMBG devices are more preferred by populations with diabetes in the Asia-Pacific region, owing to economic affordability, lower sophistication, and being more user-friendly. Currently, SMBG devices hold 75% of the blood glucose monitoring market in the Asia-Pacific region [26]. Furthermore, given the diverse socioeconomic and clinical settings in the Asia-Pacific region, and acknowledging the different lifestyle and dietary habits of people in this region, a structured SMBG method is still the most preferred and recommended method of glucose monitoring in the region [25]. However, in Asian countries, approximately 40% of people with diabetes who monitor blood glucose levels do not practice SMBG routinely [10].
After considering existing literature on the usage and benefits of SMBG, the experts agreed on the need to initiate SMBG early from the time of diagnosis, since it can help improve compliance and aid in dose adjustments of oral antidiabetic drugs (OADs) and insulin. Ideally, SMBG should be practiced once daily. However, in a resource-constrained setting, routine SMBG could be further simplified to 1–3 times per week. The consensus recommendations of the experts on SMBG are presented in Box 1.
Box 1 Consensus Statements on SMBG.
Initiation of SMBG should be considered as soon as T2DM has been diagnosed as it can improve compliance and help in dose adjustments of antidiabetic medications, especially insulin.
SMBG is generally recommended while initiating insulin. The frequency and type of the glucose monitoring device depend on the patient’s convenience and physician’s advice.
For basal insulin initiation/titration, a daily fasting glucose measurement is the optimal recommendation.
In resource-constrained settings, simplified SMBG could be FPG measurement 1–3 times/week.
Delay in Insulin Initiation
Clinical inertia related to insulin therapy, typically manifested as a delay in insulin initiation and titration, is a well-acknowledged concern in routine clinical practice [27]. Commonly insulin initiation is delayed because both the physicians and patients are subject to misconceptions and fears about the progression of diabetes and the role of insulin [28]. Patient-related factors responsible for insulin therapy include lack of awareness about the benefits of insulin, fear of perceived complications of insulin therapy, hypoglycemia, and other myths regarding insulin therapy [15, 28]. Resistance to insulin therapy is more prevalent in the less-educated Asian population, as observed by a study conducted on a cohort of insulin-naïve Asian people in whom a majority of people did not have tertiary education (88.5%). Seven of every 10 people with diabetes did not approve of initiating insulin therapy [15, 29]. Physician-related factors that delay insulin therapy include concerns over the effectiveness, safety, and flexibility of insulin therapy; lack of knowledge about diabetes management guidelines; inadequate infrastructure, such as shortage of staff, diabetes care team, and medications, particularly in rural areas; and lack of confidence in patients’ capability of self-management [15, 28].
Current Options for Insulin Initiation and Dosing
Effective communication with the patient allows for collaborative decision-making, which enables the patient to understand the key advantages of insulin initiation and facilitates their decision to initiate and continue insulin therapy. Furthermore, discussing insulin therapy with patients helps establish appropriate goals and titration methods [30, 31]. Basal insulin is suggested to be the optimal choice for insulin initiation in people with T2DM as it offers significant improvement in glycemic control, with lower incidence of hypoglycemia, lesser risk of weight gain, increased treatment satisfaction, good treatment adherence, and better treatment persistence [32–35]. In people with T2DM, nearly 76% of treatment adherence and retention in patients was found at 6 months of basal insulin therapy initiation [35]. Basal insulin is often the preferred initial insulin for people with T2DM because it has a lower risk of inducing hypoglycemia and causes less weight gain compared with premixed insulin or basal–bolus insulin therapy. The development and introduction of first-generation basal insulin analogues in clinical practice (insulin glargine 100 U/mL [Gla-100; Sanofi] and insulin detemir [IDet; Novo Nordisk]) resulted in a significant reduction in hypoglycemia compared to human basal insulins. The second-generation basal insulin analogues, insulin glargine 300 U/mL (Gla-300; Sanofi) and insulin degludec (IDeg; Novo Nordisk), are proven to have an extended duration of action, less glycemic variability, and reduced events of hypoglycemia compared to first-generation basal insulin analogues [36, 37].
Various international guidelines, such as the ADA and the IDF, recommend the use of basal insulin for initiating insulin therapy [17, 21]. The guidelines on insulin therapy as recommended by the international guidelines and guidelines of Asian countries are depicted in Table 1.
Table 1.
Asian and international guidelines on insulin therapy for the management of T2DM
| International guidelines | Guideline recommendations |
|---|---|
| ADA [21] |
The early introduction of insulin should be considered if there is evidence of ongoing catabolism (weight loss), if symptoms of hyperglycemia are present, or when HbA1c levels (> 10% [86 mmol/mol]) or blood glucose levels (≥ 300 mg/dL [16.7 mmol/L]) are very high Basal insulin alone is the most convenient initial insulin regimen and can be added to metformin and other oral agents. Starting doses can be estimated on the basis of the body weight (0.1–0.2 U/kg/day) and the degree of hyperglycemia, with individualized titration over days to weeks as needed Glucagon-like peptide 1 receptor agonists with or without metformin based on glycemic needs are the first-line injectable therapy for individuals with type 2 diabetes with or at high risk for atherosclerotic cardiovascular disease, heart failure, and/or chronic kidney disease |
| IDF [17] |
Consider starting insulin alone or in combination with other glucose-lowering drugs when people with T2DM are unstable, with symptoms and signs of acute decompensation Basal insulin should be preferred, and it can be temporary |
| Local Asian guidelines | Guideline recommendations |
|---|---|
| Vietnam [38] | The first Vietnam national guidelines for type 2 diabetes diagnosis and treatment were issued by the Ministry of Health in 2017 and the second one in 2020 (available only in Vietnamese). Initiation of basal insulin or premixed insulin (once or twice daily) is recommended by the current guidelines. However, glucagon-like peptide 1 receptor agonists are preferred to insulin in these cases. The early introduction of insulin should be considered if there is evidence of ongoing catabolism (weight loss), if symptoms of hyperglycemia are present, or when HbA1c levels (≥ 9%) or blood glucose levels (≥ 300 mg/dL [16.7 mmol/L]) are very high |
| Pakistan [39] | If metformin is contraindicated, sulfonylureas, dipeptidyl peptidase IV inhibitors, or insulin can be used as an alternative |
| Thailand [40, 41] |
Thailand has its own guidelines for diabetes treatment (available only in Thai), endorsed by the Thai Diabetes Association, the Endocrine Society of Thailand, and the Ministry of Public Health. The guidelines are updated every 3–4 years and the latest version was published in 2020 The essential diabetes drug list includes insulin, metformin, and other glucose-lowering drugs |
| Philippines [42] | People with T2DM who are on noninsulin regimens should be initiated only with basal insulin, which can be combined with meal-time rapid-acting insulin injections |
| Malaysia [43] | Insulin/OAD in combination with metformin if HbA1c ≥ 7.5. Basal insulin/premixed insulin + combination therapy or intensive insulin therapy + OAD if HbA1c > 10.0 |
| Indonesia [44] |
Basal insulin can be initiated in combination with dual/triple OADs if HbA1c is ≥ 7.5% to < 9% with long-standing diabetes. Start basal insulin with 10 U/day or 0.2 U/kg/day. Basal insulin dose titration is needed to achieve FPG 80–130 mg/dL: > 180 mg/dL: increase 4 U 130–180 mg/dL: increase 2 U < 130 mg/dL: maintain the dose |
| Singapore [45] | Insulin should be initiated if glycemic goals are not met with OADs alone. Basal insulin such as intermediate- or long-acting insulin should be used for initiation. The use of concomitant OADs should be reviewed while initiating insulin therapy, and metformin ± SGLT2 inhibitors to be continued, if appropriate. Insulin is to be initiated at 0.1–0.2 U/kg/day depending on age, comorbidities, and blood glucose levels. Dosage should be adjusted by 2–4 U once or twice weekly, or as clinically indicated, until the FPG target is reached |
| RSSDI [46] | Basal insulin dosage is estimated on the basis of weight and is normally initiated at 10 U or 0.1–0.2 U/kg/day and then uptitrated on the basis of glycemic value, with typical doses ranging from 0.2 to 1.0 U/kg/day |
ADA American Diabetes Association, FPG fasting plasma glucose, HbA1c glycated hemoglobin, IDF International Diabetes Federation, OAD oral antidiabetic drug, SGLT2 sodium-glucose cotransporter 2, T2DM type 2 diabetes mellitus, RSSDI Research Society for the Study of Diabetes in India
Different insulins have different initiation doses based on the local labels. The starting dose of insulin for people with T2DM is usually 10 U/day or 0.1–0.2 U/kg/day [31]. According to the recommendations from the ADA, Standards of Medical Care in Diabetes—2022, the estimation of starting doses of basal insulin should be based on the degree of hyperglycemia and body weight (0.1–0.2 U/kg/day), and titration should be individualized over days to weeks as needed [21]. The American Association of Clinical Endocrinology (AACE) suggests initiating basal insulin on the basis of HbA1c levels. For HbA1c < 8%, the total daily doses (TDD) of 0.1–0.2 U/kg/day is suggested, whereas in case of HbA1c > 8%, the TDD of 0.2–0.3 U/kg/day is recommended [47]. However, as per the BEYOND VIII trial, a higher insulin starting dose of 0.3 U/kg/day was equivalent, in terms of safety, to the standard starting dose of 0.2 U/kg/day among overweight/obese people with T2DM, and self-monitored FBG goals were attained earlier with the higher dose vs. the standard dose [48].
Even at similar doses of basal insulin, the overall glycemic control is worse in the Asian population as compared to that of non-Asians, which calls for a higher daily dose of insulin or suggests further addition of antidiabetic drugs for achieving adequate glycemic control [49]. Moreover, as compared to non-Asians, fewer Asian individuals with T2DM achieve glycemic targets even with a similar reduction in FPG levels. This suggests that the prandial glycemic needs should be addressed among some Asians who are on basal insulin therapy [5]. In this regard, fixed-ratio combinations (FRCs) of basal insulin and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) provide a novel alternative. The FRCs—insulin degludec/liraglutide and insulin glargine/lixisenatide—have a more physiological mode of action, as they target both postprandial and prandial glucose levels [50–52]. The FRCs might be an option for initiation in individuals with high postprandial glycemic surges, which are typically observed in the Asian population [30, 51, 52]. A recently published European Expert Opinion also deliberated on and recommended that OAD failure in patients with T2DM and HbA1c > 9%, obesity, high risk for hypoglycemia, high postprandial glycemic levels, or gastrointestinal adverse events during the previous GLP-1 RA treatment may potentially benefit more from initiation with an FRC; with titration following the similar concept of basal insulin titration, based on fasting SMBG levels [53].
After a careful assessment of the local and international guidelines on diabetes management with insulin therapy, and in view of the unique requirements of the Asian population with diabetes, the panel recommendations on insulin initiation and dosing are mentioned in Box 2. The experts also opined that when initiating insulin, a review of the concomitant glucose-lowering agents is advisable. In general, metformin and sodium-glucose cotransporter 2 inhibitor may be continued, while sulfonylureas and dipeptidyl peptidase 4 inhibitor (DPP4i) may need re-evaluation. If an FRC is opted for, then the DPP4i should be stopped, since they act on the same incretin pathway. DPP4 inhibitors can potentially be used in combination with insulin treatment. Several studies have shown a decrease in insulin dosage and hypoglycemic episodes. However, it is strongly advised not to combine DPP4 inhibitors with GLP-1 receptor agonists, and DPP4 inhibitor treatment should be discontinued when treatment is intensified with an injectable therapy later in the process with a GLP-1 receptor agonist [54]. Although the combination of glitazones and insulin is intriguing since it allows for better glycemic control while lowering daily insulin requirements, it is associated with hypoglycemic episodes and may favor weight gain due to increased adipogenesis and fluid retention. Fluid retention can aggravate or even trigger congestive heart failure, which typically needs medication withdrawal [55].
Box 2: Consensus Statements on Insulin Initiation and Dosing.
Younger age and high HbA1c should trigger early insulin initiation in the Asian population, but fear of hypoglycemia and lack of education are the main barriers to insulin initiation in the region.
Basal insulin is the preferred insulin for initiation.
A dose of 10 U/day as an initiation dose should be recommended for the majority of patients in the primary care setting. However, individualization is needed in certain cases, taking body weight and glycemic status into consideration, wherever feasible (0.1–0.3 U/kg/day).
Insulin Optimization and Titration
Suboptimal dose titration is acknowledged to be the major cause of poor glycemic control in the Asian population with diabetes [15]. In the FINE-Asia registry study, a minimal insulin dose titration was documented in several Asian countries [56, 57]. Similarly, the A1chieve study reported limited dose titration of basal insulin in China, East Asia, and South Asia [58]. Local clinical practice guidelines in several Asian countries, such as Singapore, Taiwan, the Philippines, and Hong Kong, do not specify any standardized insulin titration algorithm, and the titration is based on the judgment of individual physicians on their patients’ conditions [15]. The Vietnamese guidelines recommend a simple basal insulin titration algorithm, wherein the insulin dose should be increased by 10–15% or 2–4 U once or twice a week, until the FBG target is achieved [38]. Inadequate titration observed in Asian countries is also attributable to the conservative approach of physicians toward insulin titration [15]. While people with diabetes can be trained on self-titration, the majority of patients in Asian countries are either unwilling or unable to self-titrate. These patients are heavily dependent on physicians for insulin titration, and infrequent clinic visits lead to suboptimal titration and inadequate glycemic control [15].
Pre-titration Requirements
The insulin regimen should be selected as per the patients’ requirements, apprehensions, and requests. Glycemic targets, such as postprandial glucose, fasting glucose, and HbA1c targets, should be cross-checked after insulin initiation in people with T2DM [28]. People with diabetes should be educated and trained on how and when to practice SMBG. Additionally, patients should be advised to continue the initial dose of insulin, unless instructed by the physician to start titration [27].
Clinical Evidence on Titration Algorithms
Several studies have been conducted to gather clinical evidence on titration algorithms [59–63]. The AT-LANTUS (A Trial comparing LANTUS® Algorithms to achieve Normal blood glucose Targets in subjects with Uncontrolled Blood Sugar) study by Davies et al. compared two treatment algorithms for insulin glargine initiation and titration. The study recommends 2 U of dose adaptation every 3 days [59]. The LANMET (insulin glargine or NPH combined with metformin in type 2 diabetes) study and INITIATE (INITiate Insulin by Aggressive Titration and Education) study by Yki-Järvinen et al. compared the combination therapy of insulin glargine plus metformin (G + MET) with NPH insulin plus metformin (NPH + MET). These studies recommended 2–4 U of dose adaptation every 3 days [60, 61]. The PREDICTIVE™ 303 (Predictable Results and Experience in Diabetes through Intensification and Control to Target: An International Variability Evaluation 303) study done by Meneghini et al., which evaluated the effectiveness and safety of insulin detemir in controlling glucose levels in people with T2DM, recommend 3 U of dose adaptation every 3 days [62].
Recommended Titration Methods
The dose of insulin should be increased or decreased by 2 U when the current FPG level is below or above the goals, respectively, and should remain unchanged if the target is achieved. Titration of both insulins should be customized on the basis of individualized patient requirements [63]. Gla-300 has a proven efficacy and safety in simple two- to four-step algorithms. Similarly, IDeg, when titrated, either using a simple titration schedule (single pre-breakfast blood glucose reading) with 4 U dosage adjustments or an average of daily readings with 2 U dose adjustments (stepwise weekly titration schedules), has been proven to be effective and well tolerated [63, 64]. For Gla-300, a patient-driven titration algorithm of 1 U/day is reported to be effective [65]. The BRIGHT (Efficacy and Safety of Toujeo® Versus Tresiba® in Insulin-Naive Patients With Type 2 Diabetes Mellitus Inadequately Controlled With Oral Antihyperglycemic Drug(s) ± GLP-1 Receptor Agonist) study suggested a 12-week titration period for second-generation basal insulin analogues Gla-300 and IDeg, which should be initiated soon after insulin initiation [66, 67].
However, insulin titration is a largely neglected therapeutic area in the management of T2DM. Thus, there is an unmet need for guideline recommendations on insulin titration, specifically for the Asian population. Therefore, the present consensus statements delineate expert recommendations on insulin titration in the Asian context. They suggested that, ideally, titration should be initiated at the time of insulin initiation, or within 2 weeks from initiation. Basal insulin should be titrated once or twice a week, and the active titration period should be within the first 12 weeks following insulin initiation. The panel recommendations on insulin titration are mentioned in Box 3.
Box 3: Consensus Statements on Insulin Titration.
Titration should be ideally started at the same time or within 2 weeks of insulin initiation (the 2-week window is to assess patient comfort and allow familiarization with insulin initiation).
In primary care settings, titration should continue till FPG goals are met. The period of such active titration should ideally be 12 weeks and may go up to 16 weeks in the real-world setting.
Having a simple titration regimen will make it easier to follow and comply for both HCPs and patients.
In primary care physician’s settings, basal insulin should be titrated once or twice a week (in conjunction with the SMBG readings).
A simple up–down titration algorithm should be recommended in primary care settings, with a dose escalation of 2–4 U/week.
Physician- vs. Patient-Led Titration
Insulin titration can be managed either by the physician or HCPs or by the patient under the guidance of the physician. Evidence suggests that when guided appropriately by their clinicians, patients can titrate effectively, similar to that of clinicians. However, the determining factor for this is the patient’s ability and preparedness for managing titration [16]. Numerous treat-to-target trials have evaluated the efficacy of patient-led titration algorithms and have reported that it is more effective in achieving near-target glycemic levels. Among these, AT-LANTUS, LANMET, INITIATE, and PREDICTIVE 303 trials have validated simple titration algorithms. These trials observed the association of a simple patient-administered titration algorithm and significant improvement in glycemic control, with a low incidence of severe hypoglycemia [16, 59, 60]. The ATLAS study revealed that patient-driven insulin titration attained desired blood glucose levels in people with uncontrolled T2DM in Asia who were on two oral glucose-lowering drugs. This study reported adequate self-titration by Asian patients when guided [68]. Arnolds et al. evaluated 22 clinical trials that investigated the outcomes of basal insulin therapy with physician-led and patient-empowered titration. Improvement in HbA1c and FPG was reported in all these trials, along with a low incidence of hypoglycemia. The factors that influenced basal insulin titration included daily blood glucose variability, insulin dose, and complexity of the titration algorithm [69]. In the REWARDS study, titration was physician-driven in 58.2% of patients and patient-driven in 41.8% of patients [12]. The experts agreed that there is an urgent need to actively involve both the physicians and patients in the insulin titration panel and develop a set of recommendations on physician- vs. patient-led titration as mentioned in Box 4. The experts agreed that patient-led titration is the best choice, though both physicians and patients should drive patient-led titration. To ensure the same, every effort should be made to increase patient awareness and education to empower patients to self-titrate insulin. Similar recommendations were provided in a Southeastern European consensus [27].
Box 4: Consensus Statements on Titration Algorithm.
Patient-led titration is the preferred method of titration, and every effort must be made to achieve this goal.
Robust patient awareness initiatives and an increase in outreach programs involving diabetes educators can help in empowering patients to titrate insulin.
In primary care settings, both physicians and patients should drive insulin titration with more emphasis on patient-led titration.
Recommended Insulin Titration Algorithm for the Asian Population with T2DM
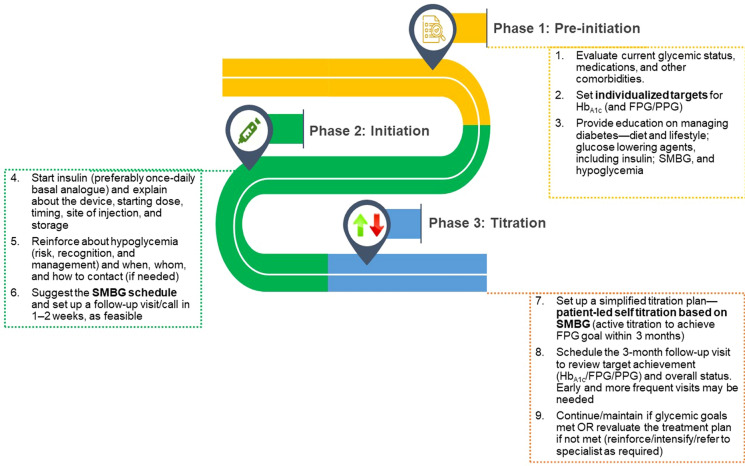
Since patient involvement is well recognized as a key factor in glycemic management, the experts proposed a customizable stepwise insulin titration algorithm to guide the patient. The patient-related values should be provided by the physician [24, 27]. The simplified stepwise insulin titration algorithm recommended by experts for physicians and patients is presented in Fig. 1 and Table 2, respectively.
Fig. 1.
Physician pathway and checklist for insulinization. FPG fasting plasma glucose, HbA1c glycated hemoglobin, PPG postprandial glucose, SMBG self-monitoring of blood glucose
Table 2.
Patient’s insulin order for insulin initiation
| Insulin order for ____________________________ (Patient name/age/gender) Date:__/__/____ | |
|---|---|
| Glycemic status (current) |
HbA1c…………% FPG …. mmol/L (or mg/dL) PPG …. mmol/L (or mg/dL) |
| Glycemic goals (target) |
Target HbA1c < ………% Target FPG range …. to …. mmol/L (or mg/dL) Target PPG range …. to …. mmol/L (or mg/dL) |
| Insulin (type/name, starting dose and timing) |
……………….. ……(type/name) ……Units (U) ……am/pm everyday For one week□/ two weeks□ (select one) |
| SMBG schedule (morning fasting) |
Mon □ Tue□ Wed□ Thu□ Fri□ Sat□ Sun□ (select all that apply) |
| Insulin dose-adjustment (self-titration, based on average FPG, SMBG) |
Increase daily dose by …U if FPG > …. mmol/L (or mg/dL) Decrease daily dose by …U if FPG < …. mmol/L (or mg/dL) Every Mon □ Tue□ Wed□ Thu□ Fri□ Sat□ Sun□ (Titrate insulin dose once or twice a week) |
| Additional instructions/remarks |
Follow-up visit on ______________(date)b Free text box (other medications, special instructions, initial call or visit to set up patient led self-titration, etc.)a Contact me/nurse at __________(phone/e-mail) |
| In case of emergency/hypoglycemia | In case of any medical emergency/hypoglycemia (or if blood glucose < …. mmol/L [or mg/dL]), contact the nearest hospital/ clinic for medical assistance |
FPG fasting plasma glucose, HbA1c glycated hemoglobin, PPG postprandial glucose, SMBG self-monitoring of blood glucose
aEnsure regular diabetes education on managing diabetes—diet and lifestyle, glucose-lowering agents including insulin, SMBG, and hypoglycemia
bTypically review with lab reports in 3 months (may schedule first review call or visit in 1–2 weeks after initiation, as needed)
If the target goals are reached after the first 12–16 weeks of active titration, insulin should be continued at the stable insulin dose to maintain FPG within the recommended range. The physician should continue communication with the patient to ensure persistence and successful management of glycemic levels with insulin [30], and titration should be done as and when required. The HbA1c level should be rechecked after 3–6 months. If target HbA1c is not reached despite using 0.5–0.7 U/kg body weight/day insulin doses [21], the physician should consider factors such as compliance, other medications, diet and lifestyle, and then consider therapy intensification or follow-up with a specialist, if required.
Summary of Recommendations
Preinitiation
Regular SMBG should be recommended after diagnosis.
Glycemic targets should be individualized, and in general, the following may be considered: HbA1c < 7%; FPG, 90–110 mg/dL (5–6 mmol/L); PPG, 160–180 mg/dL (9–10 mmol/L).
Patient education on diabetes self-management and conversation about insulin should be started shortly after diagnosis to address common myths and set a positive context for insulin therapy.
-
2.
Initiation
Start insulin therapy if glycemic goals are not met on OADs alone, and a “basal first” approach is recommended in most cases.
An initiation dose of 10 U/day is recommended for the majority of patients in the primary care setting. Individualization is needed in certain cases, taking body weight and glycemic status into consideration, wherever feasible (0.1–0.3 U/kg/day).
Ideally, a daily fasting glucose measurement is the optimal recommendation following insulin initiation. In resource-constrained settings, SMBG could be considered 1–3 times/week.
-
3.
Titration
Titration should be started simultaneously or within 2 weeks of insulin initiation (the 2-week window is to assess patient comfort and familiarize with insulin initiation) and should continue until FPG goals are attained. The period of such active titration should ideally be 12 weeks (up to 16 weeks in real-world settings).
A simple up–down titration algorithm is recommended with dose escalation of 2–4 U/week. Basal insulin should be titrated once or twice a week (in conjunction with the SMBG readings).
Both physicians and patients should drive insulin titration with more emphasis on patient-led titration. App-based titration aids could be a useful tool for primary care physicians.
Future Perspectives
A study reported that although knowledge on T2DM guidelines is high among primary care physicians, adherence to these guidelines is low in clinical practice, largely because of their attitude and beliefs and access to these guidelines [70, 71]. There is poor awareness among Asian people with T2DM regarding SMBG and hypoglycemia, which reiterates the need for patient education [71]. In the Asian context, implementation of an ideal physician education program can potentially improve glycemic control, with better management of complications and comorbidities [72]. Therefore, in addition to proposing the recommendations and simplified algorithms to direct patient- and physician-led titration, the experts moved a step further to ensure adoption and active implementation of these recommendations, especially by the primary care physicians in Asia. Following the consensus meeting, a process of continual medical education on these guidelines regarding insulin initiation and adequate titration has been initiated for more than 5000 HCPs across Asia. There is an ongoing process of gaining endorsements from societies of different Asian countries for the adoption of the simplified recommendations in different local Asian guidelines including Malaysia, Singapore, Pakistan, Thailand, Vietnam, Philippines, and Indonesia. Furthermore, an online portal is being developed (www.insulintoolkit.com) to reach out to a maximum number of HCPs from this region of the world to facilitate clinical decision-making on optimum insulin initiation and titration. Successful implementation of these steps, and widespread adoption of the recommendations by the HCPs, especially in primary care settings in Asia, might aid in the effective management of diabetes with insulin therapy.
Limitations
Because the consensus meeting only featured 14 experts from seven countries that were members of the AISAC, the consensus does not reflect the views of experts from around Asia, as countries with high diabetes burden such as India, China, Japan, Sri Lanka, and Bangladesh were not represented. Although Indian experts were not involved in this initiative, collaboration with Indian and other Asian peers may be considered in future editions.
Conclusions
Although basal insulin is recommended by guidelines for achieving glycemic control in people with T2DM, delay in insulin initiation and inadequate titration are recognized to be key elements that contribute to poor glycemic control, especially in the Asian population. Several local Asian and international guidelines have delineated insulin initiation and titration algorithms, but they are not routinely employed in clinical practice, which is a potential contributing factor to suboptimal glycemic control. After a thorough review of literature and guidelines, the experts proposed consensus recommendations and algorithms specific for the Asian population with T2DM. These are aimed at helping both the physicians and patients to initiate and titrate insulin therapy following simple and practical steps. Effective implementation of these steps may potentially help in achieving effective glycemic control in people with T2DM in Asia.
Acknowledgements
Funding
This initiative and Rapid Service Fee was supported by Sanofi. Medical writing related charges were paid for by Sanofi. The authors received no honoraria from Sanofi directly or indirectly related to the development of this publication.
Medical Writing and Editorial Assistance
We would like to acknowledge Dr Rajshri Mallabadi and Dr Poonam Bohra from BioQuest Solutions Sdn Bhd, Kuala Lumpur, for providing medical writing and editorial support in the preparation of this manuscript paid for by Sanofi. We would also like to thank Dr Amal Mathew (Medical Lead; Sanofi Thailand, Malaysia and Singapore) for his assistance in author liaising and project/process oversight during the planning and development of this review article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Prof. Chan Siew Pheng (Corresponding author), Prof. Dr. A.H. Aamir Assoc., Prof. Bee Yong Mong, Prof. Chaicharn Deerochanawong, Dr. Elizabeth Paz-Pacheco, Dr. Fatma Tiu, Dr. Foo Siew Hui, Dr. Kevin Tan, Dr. Le Quang Toan, Dr. Made Ratna Saraswati, Asst. Prof. Pongamorn Bunnag, Dr. Roy Panusunan Sibarani, Dr. Syed Abbas Raza, Dr. Tran Quang Nam all contributed to concept, design and reviewing the manuscript and providing guidance where necessary.
Disclosures
Chan Siew Pheng, A.H. Aamir, Bee Yong Mong, Chaicharn Deerochanawong, Elizabeth Paz-Pacheco, Fatma Tul, Foo Siew Hui, Kevin EK Tan, Le Quang Toan, Made Ratna Saraswati, Pongamorn Bunnag, Roy Panusunan, Syed Abbas Raza and Tran Quang Nam declare no conflicts of interest. All authors had full access to the articles reviewed in this manuscript, have read and reviewed the final draft of this manuscript and take complete responsibility for the integrity and accuracy of this manuscript. The content published herein solely represents the views and opinions of the authors. The details published herein are intended for informational, educational, academic and/or research purposes and are not intended to substitute for professional medical advice, diagnosis or treatment.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new data collected from human participants or animals.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.International Diabetes Federation. Diabetes Atlas 2021. 2021. https://diabetesatlas.org/atlas/tenth-edition/. Accessed 08 Dec 2021. [PubMed]
- 2.Hussain A. Diabetes in Asia: special challenges and solutions. J Diabetol. 2018;9:69–72. doi: 10.4103/jod.jod_22_18. [DOI] [Google Scholar]
- 3.Chan JC, Yeung R, Luk A. The Asian diabetes phenotypes: challenges and opportunities. Diabetes Res Clin Pract. 2014;105(1):135–139. doi: 10.1016/j.diabres.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul) 2015;30(3):263–269. doi: 10.3803/EnM.2015.30.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JCN, Bunnag P, Chan SP, et al. Glycaemic responses in Asian and non-Asian people with type 2 diabetes initiating insulin glargine 100 units/mL: a patient-level pooled analysis of 16 randomised controlled trials. Diabetes Res Clin Pract. 2018;135:199–205. doi: 10.1016/j.diabres.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013;1281(1):51–63. doi: 10.1111/j.1749-6632.2012.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen FS, Hwu CM. Challenges of optimizing insulin therapy for patients with type 2 diabetes mellitus. J Diabetes Investig. 2021;12(9):1523–1525. doi: 10.1111/jdi.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschner P, Gagliardino JJ, Ilkova H, et al. Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS) Diabetologia. 2020;63(4):711–721. doi: 10.1007/s00125-019-05078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JCN, Gagliardino JJ, Ilkova H, et al. One in seven insulin-treated patients in developing countries reported poor persistence with insulin therapy: real world evidence from the cross-sectional International Diabetes Management Practices Study (IDMPS) Adv Ther. 2021;38(6):3281–3298. doi: 10.1007/s12325-021-01736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagliardino JJ, Chan JC, Ilkova H, et al. Management of glycaemic control in people with type 1 diabetes in low-/middle-income countries: Wave 8 of the International Diabetes Management Practices Study (IDMPS). EASD Virtual Meeting, 24 September 2020, Abstract #649. https://www.easd.org/virtualmeeting/home.html%20#!resources/management-of-glycaemic-control-in-people-with-type-1-diabetes-in-low-middle-income-countries-wave-8-of-the-international-diabetes-management-practices-study-idmps-1aad4a05-65b6-4762-94ef-68020ba0f285. Accessed 08 Dec 2021.
- 11.Suastika K. The challenges of metabolic disorders in Indonesia: focus on metabolic syndrome, prediabetes, and diabetes. Med J Indones. 2020;29(4):350–353. doi: 10.13181/mji.com.205108. [DOI] [Google Scholar]
- 12.Deerochanawong C, Leelawattana R, Kosachunhanun N, Tantiwong P. Basal insulin dose titration for glycemic control in patients with type 2 diabetes mellitus in Thailand: results of the REWARDS real-world Study. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420935930. doi: 10.1177/1179551420935930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbar A, Mohamed WMIBW, Ozaki R, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Western Pacific region. Curr Med Res Opin. 2018;34(9):1653–1662. doi: 10.1080/03007995.2018.1484712. [DOI] [PubMed] [Google Scholar]
- 14.Soewondo P, Soegondo S, Suastika K, Pranoto A, Soeatmadji DW, Tjokroprawiro A. The DiabCare Asia 2008 study: outcomes on control and complications of type 2 diabetic patients in Indonesia. Med J Indones. 2010;19(4):235–244. doi: 10.13181/mji.v19i4.412. [DOI] [Google Scholar]
- 15.Chan WB, Chen JF, Goh SY, et al. Challenges and unmet needs in basal insulin therapy: lessons from the Asian experience. Diabetes Metab Syndr Obes. 2017;10:521–532. doi: 10.2147/DMSO.S143046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain SM, Seshadri K, Unnikrishnan AG, et al. Best practices and tools for titrating basal insulins: expert opinion from an Indian panel via the modified Delphi consensus method. Diabetes Ther. 2020;11(3):621–632. doi: 10.1007/s13300-020-00770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Diabetes Federation. Recommendations for managing type 2 diabetes in primary care. 2017. https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html Accessed 24 Nov 2021.
- 18.Deerochanawong C, Bajpai S, Dwipayana IMP, Hussein Z, et al. Optimizing glycemic control through titration of insulin glargine 100 U/mL. A review of current and future approaches with a focus on Asian populations. Diabetes Ther. 2017;8:1197–1214. doi: 10.1007/s13300-017-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soewondo P. Current practice in the management of type 2 diabetes in Indonesia: results from the International Diabetes Management Practices Study (IDMPS) J Indon Med Assoc. 2011;61(12):474–481. [Google Scholar]
- 20.Linstone HA, Turoff M. The Delphi method: techniques and applications. 2002. http://is.njit.edu/pubs/delphibook/delphibook.pdf. Accessed 24 Nov 2021.
- 21.American Diabetes Association Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S1–S264. doi: 10.2337/dc22-Sint. [DOI] [PubMed] [Google Scholar]
- 22.Bergenstal RM, Gavin JR, Global Consensus Conference on Glucose Monitoring Panel The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med. 2005;118(Suppl 9A):1S–6S. doi: 10.1016/j.amjmed.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 23.Erbach M, Freckmann G, Hinzmann R, et al. Interferences and limitations in blood glucose self-testing: an overview of the current knowledge. J Diabetes Sci Technol. 2016;10(5):1161–1168. doi: 10.1177/1932296816641433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong WM, Chua SS, Ng CJ. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adher. 2014;8:237–246. doi: 10.2147/PPA.S57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury S, Ji L, Suwanwalaikorn S, Yu NC, Tan EK. Practical approaches for self-monitoring of blood glucose: an Asia-Pacific perspective. Curr Med Res Opin. 2015;31(3):461–476. doi: 10.1185/03007995.2015.1005832. [DOI] [PubMed] [Google Scholar]
- 26.Mordor Intelligence. Asia-Pacific blood glucose monitoring market - growth, trends, COVID-19 impact, and forecasts (2022–2027). 2022. https://www.mordorintelligence.com/industry-reports/asia-pacific-blood-glucose-monitoring-market-industry. Accessed 11 Jan 2022.
- 27.Hancu N, Janez A, Lalic N, et al. Expert opinion: a call for basal insulin titration in patients with type 2 diabetes in daily practice: southeast European perspective. Diabetes Ther. 2021;12(5):1575–1589. doi: 10.1007/s13300-021-01037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253–261. doi: 10.2147/DMSO.S14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong S, Lee J, Ko Y, Chong MF, Lam CK, Tang WE. Perceptions of insulin therapy amongst Asian patients with diabetes in Singapore. Diabet Med. 2011;28(2):206–211. doi: 10.1111/j.1464-5491.2010.03195.x. [DOI] [PubMed] [Google Scholar]
- 30.Chun J, Strong J, Urquhart S. Insulin initiation and titration in patients with type 2 diabetes. Diabetes Spectr. 2019;32(2):104–111. doi: 10.2337/ds18-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta R, Goldenberg R, Katselnik D, Kuritzky L. Practical guidance on the initiation, titration, and switching of basal insulins: a narrative review for primary care. Ann Med. 2021;53(1):998–1009. doi: 10.1080/07853890.2021.1925148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalra S, Gupta Y. Clinical use of insulin degludec: practical experience and pragmatic suggestions. North Am J Med Sci. 2015;7:81–85. doi: 10.4103/1947-2714.153918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baser O, Tangirala K, Wei W, Xie L. Real-world outcomes of initiating insulin glargine-based treatment versus premixed analog insulins among US patients with type 2 diabetes failing oral antidiabetic drugs. Clinicoecon Outcomes Res. 2013;5:497–505. doi: 10.2147/CEOR.S49279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Xie YJ, Meng DD, Zhang HH, Chen H, Liu E. Clinical study of treatment switching from premixed insulin to basal insulin combined with oral hypoglycemic drugs in patients with type 2 diabetes. Diabetol Metab Syndr. 2014;6(1):37. doi: 10.1186/1758-5996-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji L, Zhang P, Zhu D, et al. Observational Registry of Basal Insulin Treatment (ORBIT) in patients with type 2 diabetes uncontrolled with oral antihyperglycaemic drugs: real-life use of basal insulin in China. Diabetes Obes Metab. 2017;19(6):822–830. doi: 10.1111/dom.12886. [DOI] [PubMed] [Google Scholar]
- 36.Cheng AYY, Wong J, Freemantle N, Acharya SH, Ekinci E. The safety and efficacy of second-generation basal insulin analogues in adults with type 2 diabetes at risk of hypoglycemia and use in other special populations: a narrative review. Diabetes Ther. 2020;11(11):2555–2593. doi: 10.1007/s13300-020-00925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauricio D, Hramiak I. Second-generation insulin analogues-a review of recent real-world data and forthcoming head-to-head comparisons. Eur Endocrinol. 2018;14(Suppl1):2–9. doi: 10.17925/EE.2018.14supp1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vietnam Ministry of Health. Guidelines for type 2 diabetes diagnosis and treatment. 2020. https://www.moh.gov.vn/en_US/web/ministry-of-health. Accessed 11 Jan 2022.
- 39.Shera AS, Basit A, Prompt Team Pakistan's recommendations for optimal management of diabetes from primary to tertiary care level (PROMPT) Pak J Med Sci. 2017;33(5):1279–1283. doi: 10.12669/pjms.335.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deerochanawong C, Ferrario A. Diabetes management in Thailand: a literature review of the burden, costs, and outcomes. Global Health. 2013;9:11. doi: 10.1186/1744-8603-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diabetes Association of Thailand. Clinical practice guideline for diabetes 2020. 2022. http://www.tdaconference.com/event/file_pdf/day1/file4.pdf. Accessed 11 Jan 2022.
- 42.Philippine Center for Diabetes Education Foundation. Philippine practice guidelines on the diagnosis and management of diabetes mellitus. 2021. http://www.pcdef.org/philippine-clinical-practice-guidelines-for-diabetes. Accessed 12 Oct 2021.
- 43.Ministry of Health Malaysia. Clinical practice guidelines. Management of type 2 diabetes mellitus, 6th edition. 2021. https://www.moh.gov.my/moh/resources/Penerbitan/CPG/Endocrine/QR_T2DM_6th_Edition_QR_Guide_Digital.pdf. Accessed 12 Oct 2021.
- 44.PERKENI. Guidelines for the management and prevention of type 2 diabetes mellitus in Indonesia 2021. 2021. https://pbperkeni.or.id/unduhan. Accessed 08 Dec 2021.
- 45.Agency for Care Effectiveness (ACE). Singapore, ACE guidelines. Initiating basal insulin in type 2 diabetes mellitus. November, 2017. 2021. https://www.ace-hta.gov.sg/healthcare-professionals/ace-clinical-guidances-(acgs)/details/initiating-basal-insulin-in-type-2-diabetes-mellitus. Accessed 08 Dec 2021.
- 46.Chawla R, Makkar BM, Aggarwal S, et al. RSSDI consensus recommendations on insulin therapy in the management of diabetes. Int J Diabetes Dev Ctries. 2019;39(2):1–52. doi: 10.1007/s13410-018-0687-1. [DOI] [Google Scholar]
- 47.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26(1):107–139. doi: 10.4158/CS-2019-0472. [DOI] [PubMed] [Google Scholar]
- 48.Ji L, Wan H, Wen B, et al. Higher versus standard starting dose of insulin glargine 100 U/mL in overweight or obese Chinese patients with type 2 diabetes: results of a multicentre, open-label, randomized controlled trial (BEYOND VII) Diabetes Obes Metab. 2020;22(5):838–846. doi: 10.1111/dom.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai ST, Chan J, Bunnag P, et al. Comparison of glycemic control in Asian and non-Asian T2D patients initiating insulin glargine 100 U/mL as add-on therapy to OADs. Diabetes Res Clin Pract. 2016;120:S114–S115. doi: 10.1016/S0168-8227(16)31207-4. [DOI] [Google Scholar]
- 50.Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026–2035. doi: 10.2337/dc16-0917. [DOI] [PubMed] [Google Scholar]
- 51.Gomez-Peralta F, Al-Ozairi E, Jude EB, Li X, Rosenstock J. Titratable fixed-ratio combination of basal insulin plus a glucagon-like peptide-1 receptor agonist: a novel, simplified alternative to premix insulin for type 2 diabetes. Diabetes Obes Metab. 2021;23(7):1445–1452. doi: 10.1111/dom.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skolnik N, Hinnen D, Kiriakov Y, Magwire ML, White JR., Jr Initiating titratable fixed-ratio combinations of basal insulin analogs and glucagon-like peptide-1 receptor agonists: what you need to know. Clin Diabetes. 2018;36(2):174–182. doi: 10.2337/cd17-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haluzík M, Flekač M, Lengyel C, et al. Expert opinion on the therapeutic use of the fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide: a central/eastern European perspective. Diabetes Ther. 2020;11(4):1029–1043. doi: 10.1007/s13300-020-00777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallwitz B. Clinical Use of DPP-4 inhibitors. Front Endocrinol (Lausanne) 2019;10:389. doi: 10.3389/fendo.2019.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheen AJ. Combined thiazolidinedione-insulin therapy: should we be concerned about safety? Drug Saf. 2004;27(12):841–856. doi: 10.2165/00002018-200427120-00002. [DOI] [PubMed] [Google Scholar]
- 56.Tsai ST, Pathan F, Ji L, et al. First insulinization with basal insulin in patients with type 2 diabetes in a real-world setting in Asia. J Diabetes. 2011;3(3):208–216. doi: 10.1111/j.1753-0407.2011.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji L, Tsai ST, Lin J, Bhambani S. National variations in comorbidities, glycosylated hemoglobin reduction, and insulin dosage in Asian patients with type 2 diabetes: the FINE-Asia registry. Diabetes Ther. 2015;6(4):519–530. doi: 10.1007/s13300-015-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Home P, Naggar NE, Khamseh M, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94(3):352–363. doi: 10.1016/j.diabres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 59.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R, ATLANTUS Study Group Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28(6):1282–1288. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- 60.Yki-Järvinen H, Kauppinen-Makelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49(3):442–451. doi: 10.1007/s00125-005-0132-0. [DOI] [PubMed] [Google Scholar]
- 61.Yki-Järvinen H, Juurinen L, Alvarsson M, et al. INITIATE (Initiate Insulin by Aggressive Titration and Education): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groups. Diabetes Care. 2007;30(6):1364–1369. doi: 10.2337/dc06-1357. [DOI] [PubMed] [Google Scholar]
- 62.Meneghini L, Koenen C, Weng W, Selam JL. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes–results of the randomized, controlled PREDICTIVE 303 study. Diabetes Obes Metab. 2007;9(6):902–913. doi: 10.1111/j.1463-1326.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 63.Cheng AYY, Patel DK, Reid TS, Wyne K. Differentiating basal insulin preparations: understanding how they work explains why they are different. Adv Ther. 2019;36(5):1018–1030. doi: 10.1007/s12325-019-00925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philis-Tsimikas A, Brod M, Niemeyer M, Ocampo Francisco AM, Rothman J. Insulin degludec oncedaily in type 2 diabetes: simple or step-wise titration (BEGIN: once simple use) Adv Ther. 2013;30:607–622. doi: 10.1007/s12325-013-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yale JF, Berard L, Groleau M, Javadi P, Stewart J, Harris SB. TITRATION: a randomized study to assess 2 treatment algorithms with new insulin glargine 300 units/mL. Can J Diabetes. 2017;41(5):478–484. doi: 10.1016/j.jcjd.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 Units/mL versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41(10):2147–2154. doi: 10.2337/dc18-0559. [DOI] [PubMed] [Google Scholar]
- 67.Cheng A, Harris S, Giorgino F, et al. Similar glycaemic control and less hypoglycaemia during active titration after insulin initiation with glargine 300 units/mL and degludec 100 units/mL: a subanalysis of the BRIGHT study. Diabetes Obes Metab. 2020;22(3):346–354. doi: 10.1111/dom.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garg SK, Admane K, Freemantle N, et al. Patient-led versus physician-led titration of insulin glargine in patients with uncontrolled type 2 diabetes: a randomized multinational ATLAS study. Endocr Pract. 2015;21(2):143–157. doi: 10.4158/EP14079.OR. [DOI] [PubMed] [Google Scholar]
- 69.Arnolds S, Heise T, Flacke F, Sieber J. Common standards of basal insulin titration in type 2 diabetes. J Diabetes Sci Technol. 2013;7(3):771–788. doi: 10.1177/193229681300700323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Widyahening IS, van der Graaf Y, Soewondo P, Glasziou P, van der Heijden GJM. Awareness, agreement, adoption and adherence to type 2 diabetes mellitus guidelines: a survey of Indonesian primary care physicians. BMC Fam Pract. 2014;15:72. doi: 10.1186/1471-2296-15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rudijanto A, Saraswati MR, Yunir E, Kumala P, Puteri HH, Mandang VV. Indonesia cohort of IO HAT study to evaluate diabetes management, control, and complications in retrospective and prospective periods among insulin-treated patients with type 1 and type 2 diabetes. Acta Med Indones. 2018;50(1):26–37. [PubMed] [Google Scholar]
- 72.Yunir E, Soewondo P, Soelistijo SA, Rudijanto A. Knowledge and behavior changes in clinician after training of partnership for Diabetes Control in Indonesia. Diabetes Metab Syndr. 2021;15(3):719–724. doi: 10.1016/j.dsx.2021.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.