Abstract
Introduction
Type 1 diabetes is associated with an increased risk of vascular complications. We aimed to investigate the association between serum and tissue advanced glycation end-products (AGEs) and micro- and macrovascular complications in type 1 diabetes (T1D).
Methods
We conducted a cross-sectional study on 196 adults with T1D (mean age 44.53 ± 16, mean duration of diabetes 22 ± 12 years, mean HbA1c 8 ± 1.2%). AGEs were measured in blood serum (i.e., carboxymethyllysine (CML), methylglyoxal-hydroimidazolone-1 (MGH1), and pentosidine) and by measurement of skin autofluorescence (SAF). Associations between AGEs levels and vascular complications were analyzed using binary logistic regression. Correlations between AGEs and pulse wave velocity (PWV) were also assessed by linear regressions. Significant differences were set for p values less than 0.05.
Results
We found positive associations between different AGEs and vascular complications. SAF was associated with both microangiopathy (retinopathy: OR = 1.92, p = 0.011; neuropathy: OR = 2.02, p = 0.04; any microangiopathy: OR = 2.83, p < 0.0001) and macroangiopathy (coronaropathy: OR = 3.11, p = 0.009; any macroangiopathy: OR = 2.78, p = 0.003). For circulating AGEs, pentosidine was significantly associated with coronaropathy (OR = 1.61, p = 0.01) and any macroangiopathy (OR = 1.52, p = 0.005) while MGH1 was associated with nephropathy (OR 1.72, p = 0.03). Furthermore, a significant linear correlation was found between PWV and SAF (r = 0.43, p < 0.001), pentosidine (r = 0.28, p < 0.001), and MGH1 (r = 0.16, p = 0.031), but not for CML (r = 0.03, p = 0.598).
Conclusions
Skin autofluorescence appears to be a useful marker for investigating both micro- and macrovascular complications in T1D. In this study, pentosidine was associated with macroangiopathy and MGH1 with nephropathy among the circulating AGEs. Furthermore, the correlations between PWV and AGEs may suggest their value in early prediction of vascular complications in T1D.
Keywords: Type 1 diabetes, Skin autofluorescence, Advanced glycation end-products, Pentosidine, Carboxymethyllysine, Methylglyoxal, Vascular complications, Microangiopathy, Macroangiopathy
Key Summary Points
| Type 1 diabetes (T1D) is marked by debilitating degenerative complications on the long run due to chronic hyperglycemia. |
| Advanced glycation end-products seem to play a key role as potential biomarkers of vascular complications in diabetes. |
| We explored in a cross-sectional study the association between the circulating (i.e., carboxymethyllysine (CML), pentosidine, methylglyoxal–hydroimidazolone-1 (MGH1)) and tissue (i.e., skin autofluorescence (SAF)) advanced glycation end–products (AGEs) and the micro and macro vascular complications in 196 patients with T1D. |
| SAF and circulating AGEs display significant associations with micro and macrovascular complications in T1D. |
| SAF and circulating AGEs have potential therapeutic implications in T1D by identifying sub-populations of T1D requiring more aggressive treatment and monitoring to prevent vascular events. |
Introduction
The incidence of type 1 diabetes (T1D) is rising with an earlier age of diagnosis, leading to an increased risk of vascular complications and mortality among young adults [1–5]. Since the earliness and severity of diabetes complications are directly related to the quality of diabetes control as demonstrated by the DCCT-EDIC studies, T1D appears as a model of chronic hyperglycemia consequences on the vascular system [6, 7]. Indeed, both the DCCT and EDIC studies showed that an improvement in hemoglobin A1c (HbA1c) control is associated with a decrease in the incidence of micro and macrovascular complications [6, 7].
The pathophysiology of diabetes complications involves several biological changes occurring within the vascular wall. Among those mechanisms, the accumulation of advanced glycation end-products (AGEs) due to non-enzymatic glycation seems to play a key role by promoting inflammation and endothelial dysfunction [8–10]. Indeed, AGEs are involved in the aging of proteins and can be measured in excess in several diseases including cardiovascular disease, chronic kidney disease, and diabetes [9]. While skin autofluorescence (SAF), which reflects AGE tissular content, has been studied as a surrogate marker of vascular AGE impregnation in both micro and macrovascular complications in several studies, circulating AGEs seem less studied, with disparate results among authors [11–14]. In addition, to our best knowledge, circulating and tissue AGEs have not been explored in the same population to assess their potential concordance regarding their association with diabetes complications.
We aimed to investigate, in a cross-sectional study, whether AGEs measured in blood serum (i.e., carboxymethyllysine (CML), methylglyoxal-hydroimidazolone-1 (MGH1), and pentosidine) and tissues by the assessment of SAF, are associated with micro and/or macrovascular complications of T1D.
Methods
Study Design
Type of Study and Population
We conducted a cross-sectional study on 196 adult patients (over 18 years of age) with type 1 diabetes with more than 10 years of diabetes evolution, recruited in the Adult Endocrine, Diabetes, and Nutrition Department of the Reims University Hospital, in France.
Description of the DIABAGE Study Population and Ethical Considerations
The DIABAGE (DIABetes Advanced Glycation End products) study is one of the tasks of the university research project VIVA (VIeillissement VAsculaire et protéique-« Vascular and protein aging») which aims to study the links between protein molecular aging and vascular complications in different populations of elderly or accelerated-aging patients. A total of 196 patients were identified from the CARéDIAB (Champagne Ardenne Réseau DIABète) database described in a previous study [15]. All patients were included in the study by their endocrinologist and then admitted to the hospital for a day to undergo a complete clinical and biological diabetes examination.
Formal consent was obtained from all patients included in the study and statistical analyses were performed anonymously. The study was conducted in accordance with the Declaration of Helsinki on medical research involving human subjects. CARéDIAB database is approved by the National Committee on data processing and liberties (“CNIL- Comité National Informatique et Libertés”)-Approval number 1434306.
Inclusion and Exclusion Criteria
Patients were consecutively included in the study since they had T1D with a minimum of 10 years of evolution; 518 patients with more than 10 years of diabetes evolution were identified from the CARéDIAB database, which included 1987 T1D patients. Among those patients, 196 were consecutively included in the study on a regular basis during their diabetes check-ups. Patients with acute illness and inflammatory syndrome at the time of investigation were excluded.
Exposure and Outcome Variables
Definitions of T1D and Diabetes Vascular Complications
T1D was defined according to the World Health Organization criteria (WHO) based on the 1997’s American Diabetes Association (ADA) recommendations [16]. In this study, T1D was assessed by the presence of hyperglycemia requiring insulin therapy with one or more positive specific antibodies among the following: anti-Islet cells antibodies (ICA), anti-glutamic acid decarboxylase (GAD), tyrosine kinase, anti-ZnT8, anti-insulin). All patients selected in our study received diabetes typing by determination of specific antibodies. Each complication was assessed by the medical records (history of complications reported in the medical files) and completed by the following exams:
Electrocardiography for coronaropathy complemented, if abnormal, by cardiological exploration performed by a cardiologist.
Ankle Brachial Pressure index for arteriopathy of the lower limbs (less than 0.9) or documented history of arteriopathy with revascularization or not.
Stroke was assessed on the medical history of patients who experienced either transient ischemic attack or constituted stroke.
Pulse wave velocity (PWV) was performed to assess the arterial stiffness using the SphygmoCor device. Carotid-femoral pulse wave velocity (cfPWV), the gold-standard non-invasive tool for evaluating arterial stiffness as recommended by the American Heart Association, was applied in this study [17]. The PWV was determined over the carotid-femoral region by measuring the propagation time of the pressure pulse from the carotid to femoral arteries using an arterial tonometer for recording pressure waveforms. To determine the cfPWV, the traveled path length was measured as the arterial length from the ascending aorta to the measurement point at the femoral artery minus the length from the ascending aorta to the measurement point at the carotid artery. The PWV was expressed in meters per second (m/s).
Direct examination of the retinal fundus by an ophthalmologist.
Urine albumin excretion: normal: < 30 mg/24 h; microalbuminuria: 30–300 mg/24 h; macroalbuminuria > 300 mg/24 h. Nephropathy is defined by a 24-h microalbuminuria ≥ 30 mg/24 h or urine albumin/urine creatinine ratio ≥ 3 mg/mmol.
Polyneuropathy by monofilament test using the standardized technique recommended by the International Working Group on the Diabetic Foot (IWGDF) [18] or presence of painful neuropathy evaluated by DN4 questionnaire [19].
Description of the Variables Studied
Exposure Variables
Measurement of Circulating AGEs
Three protein-bound AGEs (carboxymethyllysine (CML), pentosidine, and methylglyoxal-hydroimidazolone (MGH1)) were measured by liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) as previously described [20, 21]. Serum CML and pentosidine were quantified by LC–MS/MS (API4000 system ABSciex, Les Ulis, France) in dialyzed (24 h at 4 °C against 150 mM NaCl) serum samples. For MG-H1 measurement, 35 μl of dialyzed serum spiked with 20 μM d3-MG-H1, used as internal standard, were hydrolyzed (6 M HCl, 110 °C, 18 h, final volume: 1 ml). Hydrolysates were then evaporated to dryness under nitrogen stream and dried hydrolyzates were resuspended in 100 μl of 125 mM ammonium formate, before being filtered using Uptidisc PTFE filters (4 mm, 0.45 μm, Interchim, France) prior to LC–MS/MS analysis. The chromatographic separation was performed using a Kinetex PFP column (100 × 4.6 mm, 2.6 μm – Phenomenex) with a gradient program composed of 5 mM ammonium formate (pH 2.9) as mobile phase A and 100% acetonitrile as mobile phase B. The flow rate was constant at 0.7 ml/min during all separation steps. The injection volume was 4 μl and oven temperature was set at 30 °C. Detection was performed using an API4000 system in positive-ion mode with an electrospray ionization (ESI) source. Multiple reaction monitoring (MRM) transitions were as follows: 229.2 > 166.1 and 229.2 > 212.2 for the quantification and confirmation transitions, whereas 232.2 > 169.1 was used for the internal standard.
Measurement of AGEs by Skin Auto Fluorescence (SAF)
Tissue AGEs were evaluated non-invasively by measuring SAF using the AGE-Reader® device (Diagnostics Technologies B.V., Groningen, The Netherlands) [22]. The autofluorescence reader illuminates a skin area of about 1 cm2, protected from surrounding light, with an excitation light source between 300 and 420 nm. The emission light and the reflected excitation light of the skin are measured with a spectrometer in the range of 300–600 nm, using 200 μm of fiberglass. The measurements were performed at ambient temperature at the flying side of the arm about 10 cm under the bend, while the patients were seated. Since skin pigmentation can influence autofluorescence by light absorption, autofluorescence was calculated by dividing the average light intensity emitted by nanomole in the range of 420 to 600 nm by the average excited light intensity per nanometer in the 300–420 nm range. The autofluorescence was expressed in arbitrary units (AU) and multiplied by 100. The reflection of the skin was calculated in the range of 300–420 nm by dividing the average intensity reflected by the skin by the average intensity reflected by a white Teflon block (assuming a 100% reflection). Intra-series and between-series coefficients of variation for SAF were 4.4 and 6.4%, respectively, as previously reported in our center [20, 21]. The value used for statistical analysis was the average of three successive measurements.
Other Variables Collected
Several variables were selected as covariates: age at examination, gender, duration of diabetes, body mass index, smoking, systolic blood pressure, diastolic blood pressure, HbA1c, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and glomerular filtration rate (GFR) by CKD-EPI method. Smokers were classified into two groups: current smokers (active smokers or smoking cessation less than 3 years) and former smokers (smoking cessation for 3 years or more). We included the current smokers as a covariate in the analyses.
Dependent Variables
We selected six vascular complications as dependent variables: retinopathy, nephropathy, neuropathy, coronaropathy, arteriopathy of lower limbs, and stroke. These six outcomes were subdivided into two groups: microangiopathy defined by the presence of at least one microvascular complication (i.e., retinopathy, nephropathy, and neuropathy) and macroangiopathy defined by the presence of at least one macrovascular complication (i.e., coronaropathy, arteriopathy of lower limbs, and stroke).
Statistical Analysis
For the descriptive analysis, continuous variables were expressed as mean and standard deviation if the distribution was normal, otherwise as median and interquartile range (Q1–Q3). Patients with any of the complications were compared to patients without complications for each variable using Student’s t test or Mann–Whitney test, accordingly. Categorical variables were presented as numbers and percentages and comparisons were performed by Chi-square or Fisher’s exact test, accordingly. Associations between different types of AGEs (i.e., SAF and circulating pentosidine, CML, and MGH1) and complications defined as dependent variables were analyzed in a binary logistic regression model and corresponding odd ratios (OR) identified in univariate and multivariate analysis adjusting for potential confounders (age, gender, duration of diabetes, glycated hemoglobin, body mass index, hypertension, dyslipidemia, GFR: glomerular filtration rate by CKD-EPI; smoking status). A linear regression was performed to analyze the correlation between the PWV and different AGEs listed above. Analyses were performed with the software SPSS 17.0. All tests were considered significant at p values less than 0.05.
Results
Description of the Study Population
The characteristics of the study population are summarized in Table 1. The mean age at inclusion was 44.53 ± 16 years and 53.6% of patients were women. The duration of diabetes was 26.06 ± 12.19 years. In terms of microvascular complications, 66 patients (33.7%) had retinopathy, 36 (18.4%) had nephropathy, and 39 (19.9%) had neuropathy. With regards to macrovascular complications, 13 patients (6.5%) had coronaropathy, 10 (5.10%) had a history of stroke, and 32 (16.32%) patients had arteriopathy of lower limbs defined by either documented arteriopathy or Ankle Brachial Pressure index less than 0.9. The mean SAF level was 2.29 ± 0.6 arbitrary units (AU), the pentosidine concentration was 2.04 ± 1.41 mmol/mol lysine, the CML concentration was 348.24 ± 82.25 µml/mol lysine, and the concentration of MGH1 was 3.21 ± 0.73 mmol/mol lysine.
Table 1.
Baseline characteristics of patients included in the study (n = 196)
| Characteristics | Total n = 196 | No complications (n = 94) | Microangiopathy only (n = 51) | Macroangiopathy only (n = 9) | Microangiopathy and macroangiopathy (n = 42) | P** |
|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 44.5 (16) | 39.1 (14.4) | 44.68 (15.3) | 41.6 (14.3) | 55.7 (12.5) | < 0.0001 |
| Duration of diabetes (years), mean (SD) | 26.1 (12.2) | 21.4 (9.3) | 27.74 (12.8) | 22.4 (9.8) | 34.7 (12.4) | < 0.0001 |
| Female, n (%) | 105 (53.6) | 53 (56.4) | 28 (54.9) | 4 (44.4) | 19 (45.2) | 0.31 |
| Treatment (pump*), n (%) | 113 (57.65) | 52 (55.3) | 31 (60.8) | 5 (55.5) | 25 (59.5) | 0.78 |
| HbA1c (%/mmol/ml), mean (SD) | 8.1 (1.4) /65.5 (11.1) | 8.1(1.2) /64.8(9.3) | 8.4 (1.9) /68.4 (15.3) | 8.0 (1.1) /63.9 (8.6) | 8 (1.2) /63.6 (9.5) | 0.61 |
| BMI (kg/m2), mean ± SD | 25.78 (4.5) | 25.3 (3.7) | 24.2 (2.2) | 26.2 (5.3) | 26.5 (5.1) | 0.17 |
| Hypertension, n (%) | 71 (36.6) | 16 (17) | 20 (39.2) | 7 (77.8) | 34 (81) | < 0.0001 |
| SBP, mean ± SD | 125(13.9) | 122(12) | 126 (15) | 123(12.7) | 130 (14.7) | 0.001 |
| DBP, mean ± SD | 74(9.6) | 71(8.0) | 74(11.6) | 73(8.5) | 76(9.8) | 0.27 |
| Dyslipidemia, n (%) | 78 (39.6) | 20 (21.3) | 26 (51) | 6 (66.7) | 29 (69) | < 0.0001 |
| Total cholesterol (mmol/l), mean (SD) | 4.9 (0.9) | 4.93 (1) | 4.89 (0.9) | 5 (1.1) | 4.7 (0.9) | 0.14 |
| LDL cholesterol (mmol/l), mean (SD) | 2.63 (0.8) | 2.70 (0.9) | 2.65 (0.7) | 2.8 (0.8) | 2.4 (0.6) | 0.05 |
| Triglycerides (mmol/l), mean (SD) | 1.75 (0.5) | 1.74 (0.5) | 1.83 (0.6) | 1.7 (0.5) | 1.7 (0.5) | 0.74 |
| HDL cholesterol (mmol/l), mean (SD) | 1.00 (0.5) | 0.97 (0.5) | 0.99 (0.5) | 0.9 (0.3) | 1.1 (0.5) | 0.09 |
| GFR (< 60 ml/min/1.73 m2), n (%) | 12 (6.1) | 1 (1.1) | 3 (5.9) | 0 (0.0) | 8 (19) | 0.001 |
| Current smokers, n (%) | 45 (22.9) | 20 (21.3) | 13 (25.5) | 3 (33.3) | 11 (26.2) | 0.68 |
| Current and former smokers, n (%) | 51 (26.3) | 21 (22.3) | 15 (29.4) | 4 (44.4) | 13 (31) | 0.39 |
HbA1c glycated hemoglobin, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, LDL low-density lipoprotein, HDL high-density lipoprotein, GFR glomerular filtration rate by CKD-EPI
aTreatment by insulin pump versus multiple injection
bComparison between patients without complication (column 3) and those with both micro and macroangiopathy (column 6)
AGEs and Macroangiopathy
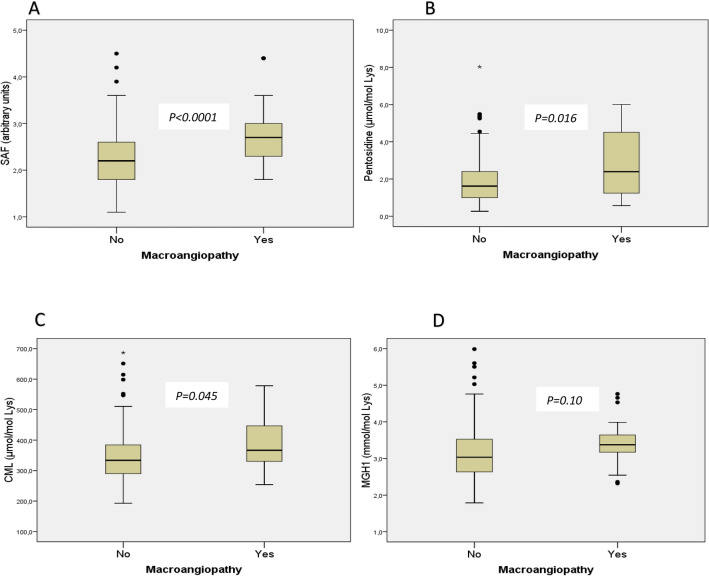
SAF, pentosidine, and CML were significantly higher in patients with composite criterion of macroangiopathy, whereas no difference was found for MGH1 (Fig. 1). Univariate and multivariate analyses for the association between macroangiopathy and the AGEs are presented in Table 2.
Fig. 1.
Comparison of tissue (evaluated by SAF) and circulating (i.e., CML, pentosidine, MGH1) AGEs between patients with macroangiopathy and those without macroangiopathy. Data are presented in box plots comparing patients with macroangiopathy and those without macroangiopathy. Skin autofluorescence (SAF), which reflects the tissue content of advanced glycation end-products, is represented in box plot A and circulating AGE are represented in box plots B for pentosidine, C for carboxymethylation, and D for methylglyoxal, respectively
Table 2.
Univariate and multivariate logistic regression of the association between AGEs and macroangiopathy
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Coronaropathy (n = 13) | ||||
| SAF (AU) | 3.40 (1.56, 7.43) | 0.002 | 3.11 (1.32, 7.33) | 0.009 |
| CML (100 µmol/mol lysine)a | 1.36 (0.74, 2.50) | 0.31 | 1.22 (0.63, 2.34) | 0.55 |
| Pentosidine (µmol/mol lysine) | 1.61 (1.15, 2.26) | 0.005 | 1.61 (1.12, 2.33) | 0.01 |
| MGH1 (µmol/mol lysine) | 1.06 (0.49, 2.27) | 0.88 | 1.05 (0.46, 2.45) | 0.89 |
| Arteriopathy of lower limbs (n = 32) | ||||
| SAF (AU) | 2.13 (1.11, 4.08) | 0.02 | 1.84 (0.93, 3.64) | 0.08 |
| CML (100 µmol/mol lysine)a | 1.46 (0.89, 2.38) | 0.12 | 1.37 (0.83, 2.27) | 0.22 |
| Pentosidine (µmol/mol lysine) | 1.20 (0.89, 1.62) | 0.22 | 1.15 (0.84, 1.57) | 0.37 |
| MGH1 (mmol/mol lysine) | 0.70 (0.298, 1.655) | 0.419 | 0.77 (0.18, 3.27) | 0.77 |
| History of stroke (n = 10) | ||||
| SAF (AU) | 2.07 (0.87, 4,95) | 0.09 | 2.24 (0.95, 5,30) | 0.06 |
| CML (100 µmol/mol lysine)a | 1.56 (0.81, 2.97) | 0.17 | 1.62 (0.83, 3.14) | 0.15 |
| Pentosidine (µmol/mol lysine) | 1.31 (0.89, 1.94) | 0.16 | 1.32 (0.89, 1.95) | 0.17 |
| MGH1 (mmol/mol lysine) | 1.18 (0.46, 3.03) | 0.732 | 2.03 (0.97, 4.28) | 0.06 |
| Composite criteria of macroangiopathy (n = 51) | ||||
| SAF (AU) | 3.07 (1.61, 5.83) | 0.001 | 2.78 (1.42, 5.41) | 0.003 |
| CML (100 µmol/mol lysine)a | 1.59 (1.00, 2.52) | 0.05 | 1.49 (0.92, 2.43) | 0.10 |
| Pentosidine (µmol/mol lysine) | 1.53 (1.16, 2.02) | 0.002 | 1.52 (1.13, 2.04) | 0.005 |
| MGH1 (mmol/mol lysine) | 1.56 (0.91, 2.68) | 0.11 | 1.59 (0.90, 2.81) | 0.10 |
In multivariate analysis, AGEs were adjusted to potential confounders (i.e., age, sex, duration of diabetes, hypertension, dyslipidemia, BMI, smoking status, and glomerular filtration rate by CKD-EPI)
SAF skin autofluorescence, CML carboxymethyllysine, MGH1 methylglyoxal-hydroimidazolone-1, AU arbitrary units
aIn the regression models, CML was divided by 100 to obtain a clinically meaningful OR. Therefore, the reported odds ratio corresponds to the increase or decrease of 100 µmol/mol lysine), as appropriate
Coronaropathy
In multivariate analysis, there was a significant positive association between the presence of coronaropathy and SAF (OR 3.11; CI 1.32–7.33) as well as for the pentosidine concentration (OR 1.61; CI 1.12–1.63). There were no statistically significant associations between coronaropathy and other circulating AGEs (i.e., CML and MGH1).
Stroke
A trend towards positive associations with borderline significance were found between stroke and both SAF (OR 2.24, p = 0.06) and circulating MGH1 (OR 2.03, p = 0.06).
Arteriopathy of Lower Limbs
SAF was statistically associated with arteriopathy in univariate analysis (OR 2.13, CI 1.11–4.08) with a trend towards association in multivariate analysis (OR 1.84, CI 0.93–3.64). Circulating CML and pentosidine had a positive but not statistically significant association with arteriopathy.
Composite Criteria of Macroangiopathy
In univariate analysis, there was a significant positive association between the presence of macroangiopathy and SAF (OR 3.07, CI 1.61–5.83) as well as with pentosidine (OR 1.53, CI 1.16–2.02). A trend towards positive association was found for CML (OR 1.52, CI 1.00–2.52) and MGH1 (OR 1.56, CI 0.91–2.68). In multivariate analysis, significant association remained for SAF (OR 2.78, CI 1.42–5.41) and pentosidine (OR 1.52, CI 1.13–2.04).
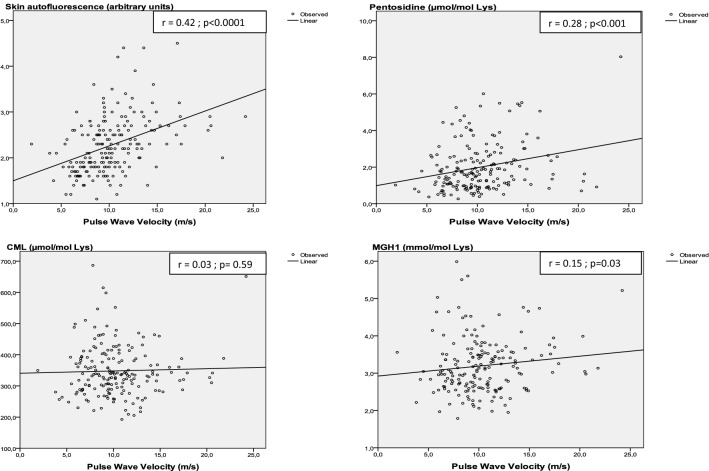
Correlation Between Pulse Wave Velocity and AGEs
In linear regression (Fig. 2), there was a positive and significant correlation between the PWV and SAF (r = 0.42; p < 0.001), pentosidine (r = 0.28; p < 0.001), and MGH1 (r = 0.15; p = 0.03) but not with CML (r = 0.03, p = 0.59).
Fig. 2.
Linear correlation between the pulse wave velocity and tissue (evaluated by SAF) and circulating AGEs (i.e., pentosidine, CML, and MGH1)
AGEs and Microangiopathy
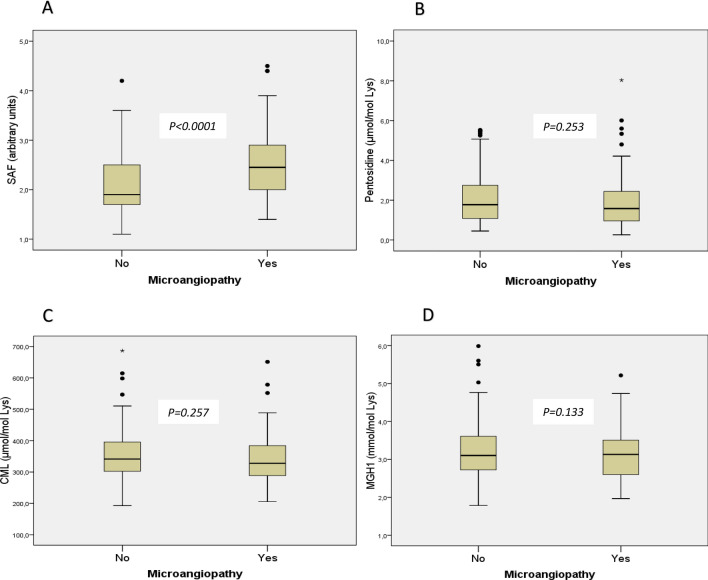
SAF was significantly higher in patients with composite criterion of microangiopathy, but no difference was found for CML, pentosidine, and MGH1 (Fig. 3). Univariate and multivariate analyses for the association between the microangiopathy and the AGEs are presented in Table 3.
Fig. 3.
Comparison of tissue (evaluated by SAF) and circulating (i.e., CML, pentosidine, MGH1) AGEs between patients with microangiopathy and those without microangiopathy. Data are presented in box plots comparing patients with microangiopathy and those without microangiopathy. Skin autofluorescence (SAF), which reflects the tissue content of advanced glycation end-products, is represented in box plot A and circulating AGE are represented in box plots B for pentosidine, C for carboxymethyllisine, and D for methylglyoxal, respectively
Table 3.
Univariate and multivariate logistic regression of the association between AGEs and microangiopathy
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Retinopathy (n = 66) | ||||
| SAF (AU) | 2.12 (1.30, 3.47) | 0.002 | 1.92 (1.16, 3.18) | 0.011 |
| CML (100 µmol/mol lysine)a | 0.99 (0.95, 1.03) | 0.66 | 0.98 (0.94, 1.02) | 0.39 |
| Pentosidine (µmol/mol lysine) | 0.97 (0.77, 1.21) | 0.80 | 0.97 (0.77, 1.23) | 0.33 |
| MGH1 (mmol/mol lysine) | 0.81 (0.52, 1.24) | 0.34 | 0.75 (0.48, 1.17) | 0.22 |
| Nephropathy (n = 36) | ||||
| SAF (AU) | 1.96 (1.13, 3.39) | 0.016 | 1.63 (0.89, 2.97) | 0.10 |
| CML (100 µmol/mol lysine)a | 1.03 (0.99, 1.07) | 0.158 | 1.003 (0.999, 1.008) | 0.18 |
| Pentosidine (mmol/mol lysine) | 1.09 (0.84, 1.41) | 0.50 | 1.14 (0.86, 1.51) | 0.34 |
| MGH1 (µmol/mol lysine) | 1.51 (0.94, 2.43) | 0.08 | 1.72 (1.02, 2.87) | 0.03 |
| Neuropathy (n = 39) | ||||
| SAF | 3.63 (1.99, 6.62) | < 0.0001 | 2.02 (1.00, 4.07) | 0.04 |
| CML (100 µmol/mol lysine)a | 0.99 (0.95, 1,04) | 0.86 | 0.99 (0.94, 1.04) | 0.82 |
| Pentosidine (µmol/mol lysine) | 1.16 (0.90, 1.48) | 0.24 | 1.17 (0.90, 1.51) | 0.24 |
| MGH1 (mmol/mol lysine) | 1.00 (0.61, 1.64) | 0.98 | 0.92 (0.54, 1.56) | 0.77 |
| Composite criteria of microangiopathy (n = 93) | ||||
| SAF | 3.26 (1.90, 5.60) | < 0.0001 | 2.83 (1.63, 4.93) | < 0.0001 |
| CML (100 µmol/mol lysine)a | 0.98 (0.94, 1.01) | 0.25 | 0.97 (0.93, 1.01) | 0.11 |
| Pentosidine (µmol/mol lysine) | 0.88 (0.71, 1.09) | 0.25 | 0.90 (0.72, 1.23) | 0.36 |
| MGH1 (mmol/mol lysine) | 0.73 (0.49, 1.10) | 0.13 | 0.71 (0.47, 1.09) | 0.11 |
In multivariate analysis, AGEs were adjusted to potential confounders (i.e., age, sex, duration of diabetes, hypertension, dyslipidemia, BMI, smoking status, and glomerular filtration rate by CKD-EPI)
SAF skin autofluorescence, CML carboxymethyllysine, MGH1 methylglyoxal-hydroimidazolone-1, AU arbitrary units
aIn the regression models, CML was divided by 100 to obtain a clinically meaningful OR. Therefore, the reported odds ratio corresponds to the increase or decrease of 100 µmol/mol lysine), as appropriate
Diabetic Retinopathy
SAF was significantly associated with retinopathy in univariate (OR 2.12, CI 1.30–3.47) and multivariate analysis (OR 1.92, CI 1.16–3.18) while none of the circulating AGEs was associated with the presence of retinopathy.
Nephropathy
MGH1 was associated with nephropathy in multivariate analysis (OR 1.72, CI 1.02–2.87). There was no significant association between nephropathy and SAF, CML, and pentosidine.
Neuropathy
Neuropathy was significantly associated with SAF in univariate regression (OR 3.63, CI 1.99–6.62). This association persisted in the multivariate model (OR 2.02, CI 1.00–4.07). There was no association between neuropathy and circulating AGEs (i.e., pentosidine, CML, MGH1).
Composite Criteria of Microangiopathy
SAF was significantly associated with any microangiopathy in univariate analysis (OR 3.26, CI 1.90–5.60), this association persisted in multivariate analysis (OR 2.83, CI 1.63–4.93). Circulating AGEs were not associated with the composite criteria of microangiopathy.
Discussion
Advanced glycation end-products have been associated with the pathophysiology of aging process, cardiovascular and chronic kidney diseases, and vascular complications of diabetes [9, 23–25]. We conducted an original study in which we measured both SAF and circulating AGE in a population of T1D with 26 years of disease evolution, representing a model of correlation between the metabolic memory and the vascular complications of diabetes [26, 27]. Indeed, except for the EDIC (post-DCCT) cohort [6, 7], the population included in this study had a longer history of glycemic load when compared to the existing literature on T1D and AGEs, making them more prone to chronic complications. In this study, we found significant associations for both tissue and some circulating AGEs with micro- and macrovascular complications.
SAF, Circulating AGEs, and Macroangiopathy
Positive associations were found between different types of AGEs and macrovascular outcomes, especially between coronary artery disease and both SAF and plasma pentosidine concentrations. The association between SAF and macroangiopathy, focusing on coronary artery disease, was reported in several previous studies, displaying a significant association between increased levels of SAF and macrovascular complications in type 1 diabetes [28–32]. A previous study reported a correlation between SAF and coronary artery disease defined by the calcium score or abnormal coronarography [30]. Van Eupen et al. also showed a significant association between coronary artery disease and pentosidine plasma concentration but not with the other AGEs such as CML [30]. To the best of our knowledge, this is the first study to investigate the potential link between AGEs and stroke as well as peripheral artery disease in T1D patients. It displays a significant association between the peripheral artery disease and the SAF but not with the circulating AGEs. Regarding stroke, a borderline association was found with SAF and MGH1.
The linear correlation found between the PWV, a marker of arterial stiffness, and different types of AGEs, especially the SAF, pentosidine, and MGH1 suggests that AGEs could play a role in the modifications of the arterial wall. Hence, AGEs could be interesting surrogate markers for “early” prediction of macrovascular complications in T1D.
The association observed between the AGEs and the macrovascular complications in this population of T1D patients could be explained by two mechanisms. On one hand, some AGEs such as pentosidine can create cross-links at the level of collagen and elastin fibers, resulting in loss of elasticity and occurrence of arterial stiffness [9, 10, 33, 34]. The association we found between pentosidine and CAD suggests that cross-links may be an important mechanism in the development of this complication. On the other hand, AGEs can interact with specific receptors such as receptor of advanced glycated end-products (RAGE), which triggers several signaling pathways leading to the activation of nuclear transcription factors and the synthesis of pro-inflammatory cytokines and vascular adhesion molecules. This sustained oxidative stress could lead to macrovascular complications of diabetes [9, 10].
One limitation of this study was the relatively low rate of macrovascular complications, which may have led to a lack of power, as a result of the strict criteria used to define macrovascular complications in this population, especially for coronaropathy and cerebral arteriopathy where events were defined by documented myocardial infarction and stroke, respectively. Hence, there was a probable underestimation of the frequency of macrovascular complications.
SAF, Circulating AGEs, and Microangiopathy
With regards to the microvascular complications, increased SAF was significantly associated with retinopathy, neuropathy, and composite criteria of microangiopathy in this study. Results reported in the literature are controversial. Two studies by Araszkiewicz et al. found significant associations for all microvascular complications (retinopathy, nephropathy, and neuropathy) among 71 subjects with T1D in 2015 while this association was found only for nephropathy among 144 T1D patients in a previous study conducted in the same center in 2011 [14, 35]. Rajaobelina et al. found a significant predictive value of increased SAF in peripheral neuropathy over 4 years prospective follow-up of a cohort of T1D patients [36]. In addition to these results, the same team reported SAF as a marker of cardiorenal outcomes in T1D [12]. Likewise, a previous study reported a positive correlation between albumin–creatinine ratio and SAF levels in patients with T1D [37].
Previous studies have also shown significant associations between SAF and microvascular complications using invasive methods such as skin biopsy [38]. However, other studies have reported a non-significant association between SAF and retinopathy [13, 14]. For circulating AGEs, only MGH1 was statistically associated with nephropathy, but not with other microvascular complications (i.e., retinopathy and neuropathy), nor with the composite criteria of microangiopathy.
Overall, SAF seems more consistently associated with the occurrence of both macro and microangiopathy. One of the possible explanations is that the renewal of tissue AGEs expressed by the SAF is much lower than that of circulating AGEs and could be a better marker of glycemic load and “metabolic memory”.
Conclusions
In conclusion, we found significant associations between increased circulating and tissue (i.e., SAF) AGEs and vascular complications in T1D patients. SAF is associated with both micro- and macrovascular complications. For circulating AGEs, pentosidine is significantly associated with macrovascular complications, especially coronaropathy, while circulating MGH1 is associated with nephropathy only among the microvascular complications. Also, PWV is correlated with different AGEs, suggesting their role in the early modifications of the arterial wall. Overall, increased SAF appears to be most consistently associated with micro- and macrovascular complications in T1D. Further studies are needed to confirm our findings in general, and a longitudinal study could be interesting to assess the predictive role of circulating AGE and SAF in micro- and macrovascular complications. These results could have therapeutic implications by identifying sub-populations of T1D requiring more aggressive treatment and monitoring in order to prevent vascular events.
Acknowledgements
We thank Tatiana DE CAMPOS DOS SANTOS and Isabelle FLANDRE for performing skin autofluorescence and pulse wave velocity analyses, Edith KASSA for data entry in the CARéDIAB database, Matthieu BIREBENT for performing database extraction, Rebecca REDDY for English proofreading, and the participants of the study.
Funding
The study was conducted as part of the University Research Project VIVA (VIeillissement VAsculaire et protéique-« Vascular and protein aging») with the institutional support of the University of Reims Champagne-Ardenne and the University Hospital of Reims. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Alpha M. Diallo performed patients’ clinical examination, analyzed, interpreted the patients’ data, and wrote the manuscript. Stéphane Jaisson performed biological analyses, interpreted the patients’ data, and was a major contributor in writing the manuscript. Romain Barriquand performed patients’ clinical examination and was a major contributor in writing the manuscript. Céline Lukas contributed to the study design, included patients, supervised the patients’ clinical examination, interpreted patients’ data, and contributed to the manuscript writing. Sara Barraud, Bénédicte Decoudier, Maud François, and Sang Ly included patients and contributed to the manuscript writing. Carl Arndt organized and validated the retinal examination seeking retinopathy and reviewed the manuscript. Rachid Mahmoudi reviewed the manuscript and gave methodological and statistical advice. Pierre Nazeyrollas validated cardiovascular examination, gave methodological and statistical advice, and reviewed the manuscript. Philippe Gillery obtained the funding for the vascular and protein aging (“PHU VIVA”) project at the University of Reims Champagne-Ardenne and coordinated the whole project, validated biological analyses, interpreted patients’ data, and was a major contributor in writing the manuscript. Brigitte Delemer validated the DIABAGE study protocol, supervised the study, included patients, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Prior Presentation
The main results of this study have been presented as an e-poster during the virtual International Diabetes Federation (IDF) Virtual Congress held in December 2021.
Disclosures
Brigitte Delemer declares speaker’s honoraria advisory board membership and congress invitations from Boehringer Ingelheim, Eli Lilly, Sanofi Aventis, Novo Nordisk, Abbott, Medtronic and Insulet. Alpha Diallo, Romain Barriquand, Sara Barraud, Céline Lukas, Maud François, Bénédicte Decoudier, Sang Ly were invited to conferences by Novo Nordisk, Sanofi, and Lilly. Pierre Nazeyrollas declares speaker’s honoraria advisory board membership and congress invitations from Boehringer Ingelheim, Eli Lilly, Sanofi, Novo Nordisk, Bayer, Pfizer, Novartis, Amgen, MSD, Astra Zeneca, and Vifor. Stéphane Jaisson, Rachid Mahmoudi, Carl Arndt, and Philippe Gillery have nothing to disclose.
Compliance with Ethic Guidelines
The study was conducted in accordance with the Declaration of Helsinki on medical research involving human subjects. Formal consent was obtained from all patients included in the study and statistical analyses were performed anonymously. The data collection and publication for this study is approved by the National Committee on data processing and liberties (“CNIL- Comité National Informatique et Libertés”)-Approval number 1434306.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Piffaretti C, Mandereau-Bruno L, Guilmin-Crepon S, Choleau C, Coutant R, Fosse-Edorh S. Trends in childhood type 1 diabetes incidence in France, 2010–2015. Diabetes Res Clin Pract. 2019;149:200–207. doi: 10.1016/j.diabres.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 3.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 4.Carstensen B, Rønn PF, Jørgensen ME. Lifetime risk and years lost to type 1 and type 2 diabetes in Denmark, 1996–2016. BMJ Open Diabetes Res Care. 2021;9(1):e001065. doi: 10.1136/bmjdrc-2019-001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial (DCCT) Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.Lachin JM, Orchard TJ, Nathan DM, DCCT/EDIC Research Group Update on cardiovascular outcomes at 30 years of the diabetes complications and control trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):39–43. doi: 10.2337/dc13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons TJ, Basu A. Biomarkers in diabetes: haemoglobin A1c, vascular and tissue markers. Transl Res. 2012;159(4):303–312. doi: 10.1016/j.trsl.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stiban A, Gawlowski T, Roden M. Vascular effects of advanced glycation end products: Clinical effects and molecular mechanisms. Mol Metab. 2013;3(2):94–108. doi: 10.1016/j.molmet.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 11.Genuth S, Sun W, Cleary P, Gao X, Sell DR, Lachin J, The DCCT/EDIC Research Group. Monnier VM. Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes. 2015;64:266–278. doi: 10.2337/db14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vélayoudom-Céphise FL, Rajaobelina K, Helmer C, Nov S, Pupier E, Blanco L, et al. Skin autofluorescence predicts cardio-renal outcome in type 1 diabetes: a longitudinal study. Cardiovasc Diabetol. 2016;15:127. doi: 10.1186/s12933-016-0448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabroux S, Canoui-Poitrine F, Reffet S, et al. Advanced glycation end products assessed by skin autofluorescence in type 1 diabetics are associated with nephropathy, but not retinopathy. Diabetes Metab. 2010;36:152–157. doi: 10.1016/j.diabet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Araszkiewicz A, Naskret D, Niedzwiecki P, Samborski P, Wierusz-Wysocka B, Zozulinska-Ziolkiewicz D. Increased accumulation of skin advanced glycation end products is associated with microvascular complications in Type 1 Diabetes. Diabetes Technol Ther. 2011;13:837–842. doi: 10.1089/dia.2011.0043. [DOI] [PubMed] [Google Scholar]
- 15.Diallo AM, Novella JL, Lukas C, Souchon PF, Dramé M, François M, Decoudier B, Barraud S, Salmon AS, Ancelle D, Arndt C, Delemer B. Early predictors of diabetic retinopathy in type 1 diabetes: the Retinopathy Champagne Ardenne Diabète (ReCAD) study. J Diabetes Complicat. 2018;32(8):753–758. doi: 10.1016/j.jdiacomp.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 16.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 17.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apelqvist J, Bakker K, van Houtum WH, Schaper NC, International Working Group on the Diabetic Foot (IWGDF) Editorial Board Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) Prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2008;24(Suppl 1):S181–S187. doi: 10.1002/dmrr.848. [DOI] [PubMed] [Google Scholar]
- 19.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoudi R, Jaisson S, Badr S, Jaidi Y, Bertholon LA, Novella JL, Gillery P. Post-translational modification-derived products are associated with frailty status in elderly subjects. Clin Chem Lab Med. 2019;57(8):1153–1161. doi: 10.1515/cclm-2018-1322. [DOI] [PubMed] [Google Scholar]
- 21.Jaisson S, et al. Early formation of serum advanced glycation end-products in children with type 1 diabetes mellitus: relationship with glycemic control. J Pediatr. 2016;172:56–62. doi: 10.1016/j.jpeds.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 22.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jaqer JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 23.Del Turco S, Basta G. An update on advanced glycation endproducts and atherosclerosis. BioFactors. 2012;38(4):266–274. doi: 10.1002/biof.1018. [DOI] [PubMed] [Google Scholar]
- 24.Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46(4):217–224. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93(4):803–813. doi: 10.1016/j.kint.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Drzewoski J, Kasznicki J, Trojanowski Z. The role of “metabolic memory” in the natural history of diabetes mellitus. Pol Arch Med Wewn. 2009;119(7–8):493–500. [PubMed] [Google Scholar]
- 27.Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The “metabolic memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients. 2017;9(5):437. doi: 10.3390/nu9050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bos DC, de Ranitz-Greven WL, de Valk HW. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther. 2011;13(7):773–779. doi: 10.1089/dia.2011.0034. [DOI] [PubMed] [Google Scholar]
- 29.Blanc-Bisson C, Velayoudom-Cephise FL, Cougnard-Gregoire A, Helmer C, Rajaobelina K, Delcourt C, Alexandre L, Blanco L, Mohammedi K, Monlun M, Rigalleau V. Skin autofluorescence predicts major adverse cardiovascular events in patients with type 1 diabetes: a 7-year follow-up study. Cardiovasc Diabetol. 2018;17(1):82. doi: 10.1186/s12933-018-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway BH, Aroda VR, Maynard JD, Matter N, Fernandez S, Ratner RE, et al. Skin intrinsic fluorescence is associated with coronary artery disease in individuals with long duration of type 1 diabetes. Diabetes Care. 2012;35:2331–2336. doi: 10.2337/dc12-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Eupen MG, Schram MT, Colhoun HM, Scheijen JL, Stehouwer CD, Schalkwijk CG. Plasma levels of advanced glycation endproducts are associated with type 1 diabetes and coronary artery calcification. Cardiovasc Diabetol. 2013;17(12):149. doi: 10.1186/1475-2840-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Eupen MG, Schram MT, van Sloten TT, Scheijen J, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, Henry RM, Kroon AA, Smit AJ, Stehouwer CD, Schalkwijk CG. Skin autofluorescence and pentosidine are associated with aortic stiffening: The Maastricht Study. Hypertension. 2016;68(4):956–963. doi: 10.1161/HYPERTENSIONAHA.116.07446. [DOI] [PubMed] [Google Scholar]
- 33.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264(36):21597–21602. doi: 10.1016/S0021-9258(20)88225-8. [DOI] [PubMed] [Google Scholar]
- 34.Chellan P, Nagaraj RH. Early glycation products produce pentosidine cross-links on native proteins. Novel mechanism of pentosidine formation and propagation of glycation. J Biol Chem. 2001;276(6):3895–3903. doi: 10.1074/jbc.M008626200. [DOI] [PubMed] [Google Scholar]
- 35.Araszkiewicz A, Naskret D, Zozulinska-Ziolkiewicz D, Pilacinski S, Uruska A, Grzelka A, et al. Skin autofluorescence is associated with carotid intima-media thickness, diabetic microangiopathy, and long-lasting metabolic control in type 1 diabetic patients. Results from Poznan prospective study. Microvasc Res. 2015;98:62–67. doi: 10.1016/j.mvr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Rajaobelina K, Farges B, Nov S, Maury E, Cephise-Velayoudom FL, Gin H, et al. Skin autofluorescence and peripheral neuropathy four years later in type 1 diabetes. Diabetes Metab Res Rev. 2017;33(2):e2832. doi: 10.1002/dmrr.2832. [DOI] [PubMed] [Google Scholar]
- 37.Skrha J, Jr, Soupal J, Loni Ekali G, Prázný M, Kalousová M, Kvasnička J, Landová L, Zima T, Skrha J. Skin autofluorescence relates to soluble receptor for advanced glycation end-products and albuminuria in diabetes mellitus. J Diabetes Res. 2013;2013:650694. doi: 10.1155/2013/650694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314:403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.