Abstract
Introduction
There is little evidence on the relationship between achieved low-density lipoprotein cholesterol (LDL-C) levels and costs in patients on lipid-lowering therapy (LLT). We described healthcare resource use and costs (direct and indirect) by achieved LDL-C in patients receiving LLT after a recent myocardial infarction (MI) in Spain.
Methods
This was a retrospective observational study of anonymized electronic medical records from seven regions in Spain (BIG-PAC® database; n = 1.9 million). Eligible patients were adults (≥ 18 years) hospitalized for an MI between January 2015 and December 2017, treated with a statin and/or ezetimibe, and having recorded LDL-C values at baseline and during follow-up. Healthcare resource use and direct and indirect costs (in 2018, €) were described by achieved LDL-C levels during a follow-up of 18 months.
Results
Of 6025 patients (mean age, 69.7 years; 77% male), only 11% achieved LDL-C goals as defined in the 2016 ESC/EAS guidelines (< 70 mg/dL), and just 1% reached the lower target (< 55 mg/dL) in the current 2019 guidelines. Achieving lower LDL-C levels translated to lower healthcare resource use and costs. Mean total (direct and indirect) costs ranged from €5044 for patients with LDL-C < 55 mg/dL to €7567 for patients with LDL-C ≥ 130 mg/dL.
Conclusion
Very few patients achieved recommended LDL-C goals despite using LLT. Achieving lower LDL-C levels after an MI might be associated with lower healthcare resource use and costs. Use of more intensive LLT, leading to greater reductions in LDL-C, could therefore be beneficial both from a clinical and an economic perspective.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02187-1.
Keywords: Cardiovascular diseases, Cholesterol, Costs and cost analysis, Ezetimibe, Health resources, Lipids, Statins
Key Summary Points
| Why carry out this study? |
| There is little evidence on the relationship between achieved low-density lipoprotein cholesterol (LDL-C) levels and resource use and costs in patients on lipid-lowering therapy (LLT) after a recent myocardial infarction (MI). |
| In Spain, there is a substantial economic burden (direct and indirect costs) associated with patients experiencing a recent MI. |
| In this retrospective observational study, electronic medical records of 6025 patients, hospitalized for an MI and on LLT, were described, including achieved LDL-C levels, healthcare resource use, and costs. |
| What did the study ask? What was the hypothesis of the study? |
| This study aimed to determine the effectiveness of LLT in reducing LDL-C levels in real-world clinical practice, and the subsequent impact on healthcare resource use and related direct and indirect costs. |
| What was learned from the study? |
| Achieving lower LDL-C levels in patients treated with LLT who had a recent MI might be associated with lower healthcare resource use and costs. |
| What were the study outcomes/conclusions? |
| Very few patients achieved recommended LDL-C levels following a recent MI, with only 11% of patients achieving the recommended goal of < 70 mg/dL at the time of study, and 1% of patients achieving the goal of < 55 mg/dL in the updated 2019 ESC/EAS guidelines. |
| Lower achieved LDL-C levels and higher intensity of LLT both appeared to be associated with lower healthcare resource use and costs. |
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality throughout industrialized countries [1–3], and it is the most common cause of death in Spain [4]. An elevated low-density lipoprotein cholesterol (LDL-C) level is one of the most important modifiable causal risk factors for CVD [5]. The management of patients with elevated LDL-C levels involves lipid-lowering therapy (LLT) with statins, ezetimibe [3], proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (evolocumab and alirocumab), as well as novel LLT, such as bempedoic acid and inclisiran. Studies in patients receiving LLT have shown that the lower the achieved LDL-C level, the lower the risk of future cardiovascular events [3]. Many patients, however, fail to achieve LDL-C targets despite maximally tolerated LLT [6–8].
In Spain, over half of all cardiovascular morbidity is the result of myocardial infarction (MI) and stroke [9]. Of individuals who experience a non-fatal MI or stroke, 10% will experience a subsequent event within 1 year, and 20% will experience a subsequent event within 4 years [10–12]. Importantly, recurrent events tend to be more severe than the initial ones, and thus have a greater impact on the patient and are associated with higher healthcare costs [13, 14]. In Spain, annual direct costs associated with MI and stroke are approximately €2.8 billion [9]. In addition, there is a substantial economic impact of CVD as a result of productivity loss, amounting to 25% of annual work days following an MI or stroke [15]. To our knowledge, however, the relationship between direct and indirect costs and achieved LDL-C levels in patients with a recent MI receiving LLT has not been described.
A retrospective, observational study using data from electronic medical records in Spain was undertaken to describe the characteristics of patients receiving LLT after an MI. In this population, the effectiveness of LLT in reducing LDL-C levels in real-world clinical practice, and the subsequent impact on healthcare resource use and related direct and indirect costs were described.
Methods
Study Design and Patient Population
This was a multicenter, longitudinal, retrospective observational study using the BIG-PAC® administrative database (Atrys Health-RLD; https://www.encepp.eu/encepp=/viewResource.htm?id=29236), which includes anonymized electronic medical records (EMR) from 1.9 million inhabitants from seven regions of Spain [16]. The primary data was exported from the computerized medical records of publicly funded integrated health areas of public health, including primary care centers and hospitals, from seven Spanish regions. Prior to export to BIG-PAC®, EMRs underwent rigorous anonymization at the centers/hospitals of origin, in compliance with Organic Law 3/2018 on the Protection of Personal Data and Guarantee of Digital Rights (https://www.boe.es/eli/es/lo/2018/12/05/3). Atrys Health-RLD had no access to primary data sources. Study approval was obtained from the Regional Ethics Research Committee, and all study procedures were conducted in accordance with the Declaration of Helsinki.
Eligible patients were adults (aged ≥ 18 years) hospitalized for a first MI (index event) between January 2015 and December 2017 (index date), who had received at least two LLT prescriptions (i.e., statins and/or ezetimibe) in the 4 weeks after the event (Supplementary Material; Fig. S1). Patients were required to have LDL-C levels recorded at baseline (defined as 1 year before the index date and during hospitalization for the index event) and at least one LDL-C level recorded in the year after the index event (≥ 8 weeks after the index event). Patients were excluded if they were transferred to another center or region during the study period, were permanently institutionalized, had a terminal disease, or were receiving dialysis.
Outcomes
Demographic and clinical characteristics were collected during the baseline period. Data on LDL-C levels and LLT use were collected at baseline and during the 18 months of follow-up. Intensity of LLT was defined according to the Anatomical Therapeutic Chemical Classification System [17], based on the expected LDL-C reduction as follows: ezetimibe monotherapy (approx. 20% reduction); low-intensity statin (≤ 30%); moderate-intensity statin (31–40%); high-intensity statin (> 40% reduction); and ezetimibe with a statin (variable reduction).
Data on healthcare resource use (i.e., medical visits, days of hospitalization, emergencies, diagnostic or therapeutic tests, and pharmaceutical prescriptions) and unit costs (Supplementary Material; Table S1) were extracted from the BIG-PAC® database and proprietary analytical accounting of Atrys Health-RLD. To estimate indirect costs, days of sick leave were extracted from the database and the associated costs were calculated using the average interprofessional salary in Spain as a proxy [18].
Statistical Analysis
Data were summarized both by achieved LDL-C levels, defined as the last on-treatment LDL-C level recorded during follow-up (≥ 8 weeks after the index date), and intensity of LLT. Achieved LDL-C levels were categorized according to the 2016 and 2019 European Society for Cardiology (ESC)/European Atherosclerotic Society (EAS) guidelines for the management of dyslipidemias: < 55 mg/dL, 55–69 mg/dL, 70–99 mg/dL, 100–129 mg/dL, and ≥ 130 mg/dL [3, 19]. Healthcare resource use and costs were assessed using baseline and follow-up values. Costs were adjusted by age, sex, time from diagnosis, CVD history, and comorbidities (Charlson comorbidity index [20]) using analysis of covariance (ANCOVA) with bootstrapping and Poisson regression models. Statistical analyses were conducted using IBM SPSS 27.
Results
Patient Population and LDL-C Levels
In total, 6025 patients were included in the analysis (Supplementary Material; Fig. S1), with a mean (standard deviation) age of 69.7 (12.2) years (Table 1). Overall, 77% of patients were male, and the most commonly reported comorbidities included hypertension (61.8%), diabetes (29.9%), and obesity (22.3%). Approximately half of patients (51.6%) had a Charlson comorbidity index of 1, with 26.6% and 21.2%, respectively, having a Charlson comorbidity index of 2 or 3. Overall, 63.7% of patients were receiving high-intensity statin treatment, with a further 4.7% receiving ezetimibe with a statin. On average, patients were receiving more than three concomitant medications, the most common drug classes being antiplatelet agents (92.9% of patients), beta-blockers (80.0%), and antithrombotics (74.1%).
Table 1.
Baseline characteristics, categorized by intensity of LLT
| Ezetimibe monotherapy | Statin therapy | Ezetimibe with a statin | Overall | |||
|---|---|---|---|---|---|---|
| Low | Moderate | High | ||||
| Patients, n (%) | 148 (2.5) | 304 (5.0) | 1453 (24.1) | 3836 (63.7) | 284 (4.7) | 6025 (100) |
| Age (years), mean (SD) | 69.0 (10.8) | 71.4 (12.8) | 72.6 (12.0) | 68.6 (12.2) | 68.5 (10.7) | 69.7 (12.2) |
| Sex (Male), % | 82.4 | 74.3 | 73.6 | 78.1 | 79.6 | 77.0 |
| BMI (kg/m2), mean (SD) | 28.8 (3.3) | 28.2 (3.8) | 29.0 (4.2) | 29.0 (4.1) | 29.3 (4.0) | 28.9 (4.1) |
| Charlson comorbidity index, % | ||||||
| 1 | 54.7 | 48.0 | 48.5 | 53.2 | 48.2 | 51.6 |
| 2 | 18.2 | 25.0 | 28.9 | 26.1 | 28.5 | 26.6 |
| 3+ | 27.0 | 27.0 | 22.6 | 20.8 | 23.2 | 21.8 |
| Comorbidities, % | ||||||
| Cardiovascular history (%) | ||||||
| Hypertension | 62.8 | 62.8 | 64.6 | 60.5 | 64.8 | 61.8 |
| Atrial fibrillation | 8.1 | 11.5 | 10.4 | 10.3 | 8.8 | 10.3 |
| Heart failure | 9.5 | 12.8 | 10.8 | 8.7 | 9.5 | 9.5 |
| Stroke | 7.4 | 9.5 | 7.6 | 8.6 | 9.2 | 8.4 |
| Other comorbidities, % | ||||||
| Diabetes | 31.8 | 28.6 | 32.8 | 28.7 | 31.7 | 29.9 |
| Obesity | 19.6 | 20.4 | 20.2 | 23.4 | 21.8 | 22.3 |
| Depression | 10.8 | 9.9 | 11.4 | 9.2 | 10.6 | 9.8 |
| Neoplasia | 10.8 | 11.5 | 9.4 | 8.5 | 9.2 | 8.9 |
| Active smoking | 7.4 | 7.2 | 6.1 | 9.7 | 7.7 | 8.6 |
| Renal failure | 10.8 | 10.5 | 7.8 | 8.1 | 8.5 | 8.2 |
| Statin intolerance | 10.8 | 3.6 | 2.8 | 2.2 | 2.8 | 2.7 |
| Alcoholism | 0.7 | 0.7 | 0.6 | 0.8 | 0.7 | 0.7 |
| Concomitant medications, mean (SD) | 3.5 (1.1) | 3.5 (1.1) | 3.5 (1.2) | 3.5 (1.1) | 3.5 (1.2) | 3.5 (1.1) |
| Concomitant medications, % | ||||||
| Antiplatelet agents | 93.9 | 91.8 | 90.9 | 93.5 | 95.8 | 92.9 |
| Beta-blockers | 76.4 | 78.0 | 77.1 | 81.6 | 77.8 | 80.0 |
| Antithrombotics | 77.7 | 76.0 | 76.2 | 72.8 | 77.1 | 74.1 |
| ARBs | 68.2 | 69.1 | 68.5 | 67.2 | 70.1 | 67.7 |
| Diuretics | 27.7 | 31.6 | 31.3 | 26.8 | 28.9 | 28.2 |
| Antihypertensives | 3.4 | 4.3 | 4.1 | 3.0 | 4.2 | 3.4 |
ARB angiotensin II receptor blockers, BMI body mass index, LLT lipid-lowering therapy, SD standard deviation
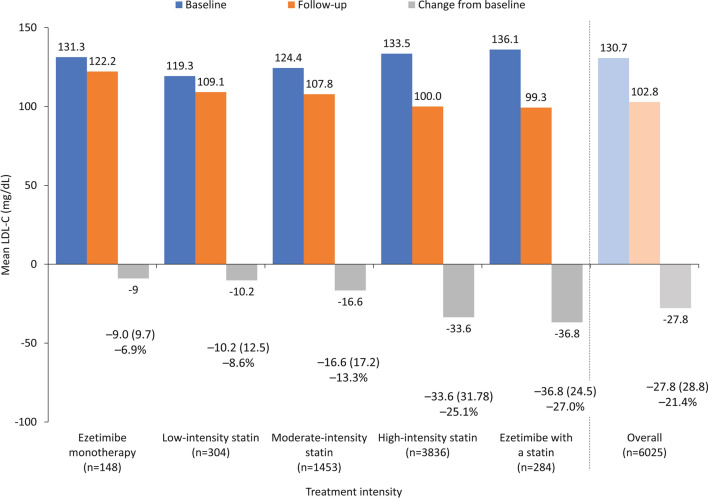
LDL-C was reduced to a greater extent with more intensive therapy compared with less intensive therapy, with a reduction of 27.0% in patients receiving ezetimibe with a statin compared with 25.1%, 13.3%, and 8.6% with high-, medium-, and low-intensity statins, respectively, and 6.9% with ezetimibe monotherapy (Fig. 1). Only 641 patients (10.6%) achieved LDL-C levels < 70 mg/dL, as recommended in the 2016 ESC/EAS guidelines for very high-risk patients [19]. Furthermore, only 68 patients (1.1%) achieved the more ambitious LDL-C goal (< 55 mg/dL) recommended in the current 2019 ESC/EAS guidelines [3]. Of the remaining patients, 2213 (36.7%) had LDL-C 70–99 mg/dL, 1995 (33.1%) had LDL-C 100–129 mg/dL, and 1176 (19.5%) had LDL-C ≥ 130 mg/dL.
Fig. 1.
Mean LDL-C levels (baseline, follow-up, and change from baseline), according to intensity of LLT. Data expressed as mean (SD). LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapy, SD standard deviation
Healthcare Resource Use
Overall, healthcare resource use tended to increase with increasing achieved LDL-C levels, although the relative contribution of the different components to the total varied with LDL-C threshold (Table 2). Primary care visits were the largest component of resource use (mean, 29.9 visits per patient), increasing with achieved LDL-C from a mean of 27.6 visits per patient with achieved LDL-C < 55 mg/dL to 31.0 visits in patients with achieved LDL-C ≥ 130 mg/dL. Laboratory tests were the second largest component of resource use (mean, 3.3 per patient), and were highest in patients with achieved LDL-C < 55 mg/dL (mean 4.0 per patient) or ≥ 130 mg/dL (mean 3.7 per patient). Mean days in hospital increased from 0.1 day per patient with achieved LDL-C < 55 mg/dL to 2.0 days per patient in those with achieved LDL-C ≥ 130 mg/dL.
Table 2.
Healthcare resource use during the 18 months after an MI, according to achieved LDL-C level
| Achieved LDL-C (mg/dL) | Overall (n = 6025) | |||||
|---|---|---|---|---|---|---|
| < 55 (n = 68) | 55–69 (n = 573) | 70–99 (n = 2213) | 100–129 (n = 1995) | ≥ 130 (n = 1176) | ||
| Healthcare resource use | ||||||
| Primary care visits | 27.6 (20.7) | 29.9 (27.1) | 29.5 (24.9) | 29.7 (24.2) | 31.0 (26.1) | 29.9 (25.1) |
| Laboratory requests | 4.0 (5.1) | 3.4 (3.6) | 3.2 (3.2) | 3.2 (3.1) | 3.7 (1.9) | 3.3 (3.0) |
| Specialized visits | 2.5 (1.0) | 2.8 (1.1) | 2.7 (1.1) | 3.2 (1.3) | 3.2 (1.2) | 3.0 (1.2) |
| Other tests | 1.3 (0.5) | 1.5 (0.6) | 1.6 (0.6) | 1.7 (0.7) | 1.7 (0.8) | 1.6 (0.7) |
| Days in hospital | 0.1 (0.8) | 0.8 (2.4) | 1.1 (3.4) | 1.7 (4.3) | 2.0 (4.7) | 1.4 (3.9) |
| Radiology requests | 0.7 (1.0) | 0.9 (1.3) | 0.9 (1.2) | 0.9 (1.1) | 0.9 (1.2) | 0.9 (1.2) |
| Emergency room visits | 0.3 (0.5) | 0.5 (1.8) | 0.5 (0.9) | 0.7 (1.2) | 0.9 (1.4) | 0.6 (1.2) |
| Productivity loss | ||||||
| Days of sick leave | 34.6 (61.5) | 35.7 (69.3) | 41.6 (69.6) | 41.1 (79.7) | 41.3 (78.6) | 40.8 (74.8) |
Data expressed as mean (SD)
LDL-C low-density lipoprotein cholesterol, MI myocardial infarction, SD standard deviation
Productivity Loss
Productivity loss generally increased with increasing LDL-C levels. The mean number of sick leave days per patient in the 18-month follow-up period was 40.8 overall, ranging from 34.6 days in patients with achieved LDL-C < 55 mg/dL and 35.7 days in those with achieved LDL-C 55–69 mg/dL, to 41.6, 41.1, and 41.3 days in patients with achieved LDL-C 70–99 mg/dL, 100–129 mg/dL, and ≥ 130 mg/dL, respectively.
Direct and Indirect Costs
Descriptive statistics of direct and indirect costs, by achieved LDL-C levels and LLT intensity, are presented in Tables S2 and S3 in the Supplementary Material, respectively.
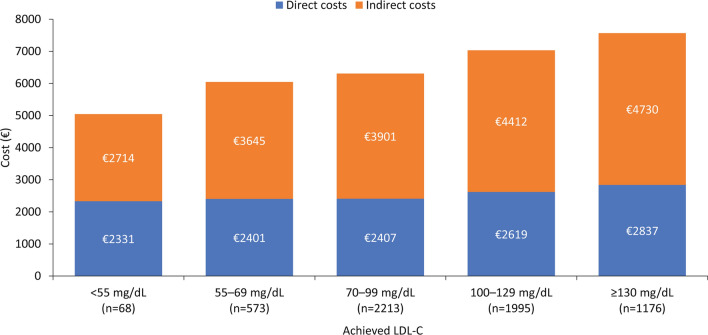
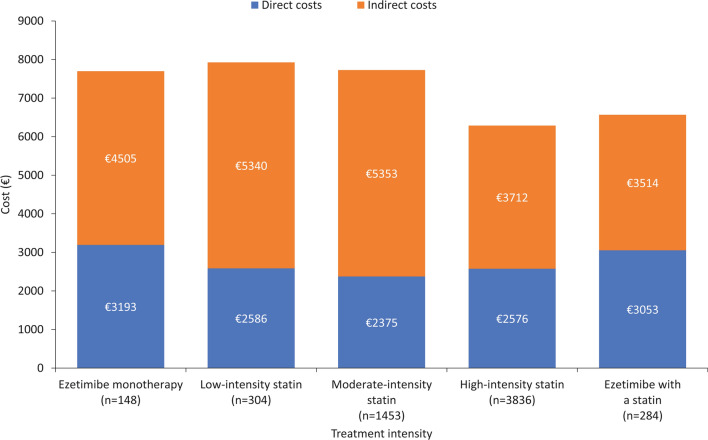
On the basis of the ANCOVA with bootstrapping model, both direct and indirect costs decreased with decreasing achieved LDL-C levels (Fig. 2). Mean direct costs during the 18 months after an MI were €2714 in patients with LDL-C < 55 mg/dL, €3645 in those with LDL-C 55–69 mg/dL, €3901 in those with LDL-C 70–99 mg/dL, €4412 in those with LDL-C 100–129 mg/dL, and €4730 in those with LDL-C ≥ 130 mg/dL. Similarly, indirect costs were €2331, €2401, €2407, €2619, and €2837, respectively. In general, when patients were categorized according to intensity of LLT, total costs (direct and indirect) tended to decrease with intensity of LLT treatment (Fig. 3).
Fig. 2.
Mean costs during the 18 months post-MI, according to achieved LDL-C level (ANCOVA with bootstrapping model). Costs are corrected by ANCOVA based on pairwise comparisons between the estimated marginal averages. ANCOVA analysis of covariance, LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapy, MI myocardial infarction
Fig. 3.
Mean costs during the 18 months post-MI, according to intensity of LLT (ANCOVA with bootstrapping model). Costs are corrected by ANCOVA based on pairwise comparisons between the estimated marginal averages. ANCOVA analysis of covariance, LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapy, MI myocardial infarction
The ANCOVA with bootstrapping and the Poisson regression models provided very consistent results (Supplementary Material; Tables S4 and S5).
Discussion
Results from this analysis of electronic medical records in Spain showed that, despite the use of statin and/or ezetimibe, very few patients achieved recommended LDL-C levels after experiencing an MI. Indeed, only 11% of patients achieved the goal of < 70 mg/dL, which was the recommended LDL-C level reported in the ESC/EAS guidelines at the time the study was conducted [19]. Furthermore, just 1% achieved the goal of < 55 mg/dL, which was the updated recommendation in the current 2019 ESC/EAS guidelines [3]. This is despite 64% of patients receiving high-intensity statin therapy and a further 5% receiving ezetimibe with a statin. For all treatment intensities, however, the LDL-C decrease observed was generally lower than reported in clinical trials [21–24]. In a simulation study using the Swedish nationwide SWEDEHEART register, including patients who had attended a follow-up visit 6–10 weeks after an MI, 82.9% of patients would be eligible for intensified lipid-lowering therapy according to the 2019 ESC/EAS guidelines [25]. In addition, 84.3% of patients were receiving monotherapy with high-intensity statins, and the use of ezetimibe, as either monotherapy or in combination, was very low. The 2019 ESC/EAS guidelines recommend the use of high-intensity statins, and the addition of ezetimibe and PCSK9 inhibitors if LDL-C goals are not met. In the SWEDEHEART study, when the use of high-intensity statins followed by add-on LLT with ezetimibe was simulated, LDL-C goals were met by almost 50% of patients. Furthermore, when use of alirocumab or evolocumab was simulated in those patients eligible for a PCSK9 inhibitor, around 90% of patients attained their LDL-C goal [25].
Overall, total (direct and indirect) costs remained high, rising to more than €7500 in the 18 months after an MI for patients with the highest LDL-C levels (≥ 130 mg/dL) and for those receiving low-to-moderate-intensity LLT. The largest cost categories were hospitalizations (up to 30% of direct costs) and primary care visits (up to 28%). Lower achieved LDL-C levels and higher intensity of LLT both appeared to be associated with lower healthcare resource use and costs. The exceptions to this trend were the two subgroups of patients receiving ezetimibe, but as only 148 patients (2%) received ezetimibe monotherapy and 284 patients (5%) received ezetimibe with a statin, this may be an artifact of the small sample sizes.
The results of this analysis are broadly consistent with previous analyses of healthcare resource use in patients with atherosclerotic CVD. For example, a study of productivity loss and indirect costs associated with acute coronary syndrome and stroke in Spain showed that total indirect costs were €11,366 in the year after the event [15]. Although the sample size in this study was limited, these Spanish patients were part of a larger European study, which reported a mean indirect cost in the year after the event of €13,953 [26]. In an analysis by the European Heart Network, the direct costs associated with ischemic heart disease in Spain in 2015 were €1.5 billion, while the direct costs associated with cerebrovascular disease were €1.2 billion [9]. Indirect costs associated with the two conditions were €1.9 billion and €0.9 billion, respectively.
The main strength of the study is the use of the BIG-PAC® database, which is highly representative of the general Spanish population. The results of our analysis can be therefore broadly generalized to the Spanish population, although the analysis does not account for potential differences in clinical practice, demographics, and socioeconomic conditions between different regions.
A potential limitation is selection bias, as older and more frail patients are likely to have worse outcomes, because of their overall condition and because they are more likely to receive lower-intensity LLT. While there were no obvious differences in demographics or clinical characteristics across patient groups in our study, it is possible that there are other potential confounding factors that were not accounted for. In addition, there may be overrepresentation of patients with high LDL-C levels in the BIG-PAC® database, as patients with well-controlled lipid levels are less likely to seek care and have their LDL-C levels monitored frequently than those who are not well controlled. It should also be noted that the calculations of cost in the study do not include “out-of-pocket” costs incurred directly by patients or costs related to caregivers. In addition, as discussed earlier, the LDL-C percentage reduction observed in this study was generally lower than reported in clinical trials. This could be potentially explained by poor adherence to and persistence with oral LLT. Adherence to and persistence with LLT were not accounted for in our study, which constitutes a potential limitation. Finally, this study was descriptive in nature and no formal statistical testing was performed.
Conclusions
This analysis of real-world data from a medical records database in Spain showed that very few patients achieved recommended LDL-C levels after experiencing a recent MI, despite receiving LLT (i.e., a statin and/or ezetimibe). Achieving lower LDL-C levels after a recent MI might be associated with lower healthcare resource use and costs, and thus use of more intense LLT, leading to a greater reduction in LDL-C, might be beneficial from both a clinical and an economic perspective.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work was funded by Amgen (Europe) GmbH, which has also funded the journal's Rapid Service and Open Access fees.
Medical Writing Assistance
Medical writing assistance was provided by Dan Booth PhD and Tina Tremaine PhD (Bioscript Medical Communications, Macclesfield, UK) and funded by Amgen (Europe) GmbH.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author’s Contributions
Carlos Escobar-Cervantes, Guillermo Villa, Ignasi Campos-Tapias, Francesc Sorio-Vilela, Javier Lozano, Doreen A Kahangire, Miriam Fernandez-Delgado, Aram Sicras-Navarro, and Antoni Sicras-Mainar were involved in the design of the study. Aram Sicras-Navarro and Antoni Sicras-Mainar were responsible for data analysis. All authors were involved in the interpretation of the results, and the drafting, revision and approval of the manuscript.
Prior Presentation
Results from this analysis were presented at the European Society of Cardiology Online Congress; 29 August–1 September 2020.
Disclosures
Carlos Escobar-Cervantes has received honoraria from Amgen for lectures and as an advisor. Guillermo Villa, Ignasi Campos-Tapias, Francesc Sorio-Vilela, Javier Lozano, Doreen A Kahangire, and Miriam Fernández-Delgado are Amgen employees and stockholders. Antoni Sicras-Mainar and Aram Sicras-Navarro are employees of Atrys Health, which was contracted and paid by Amgen to conduct the study.
Compliance with Ethics Guidelines
Ethical approval was granted by the Regional Ethics Committee of Consorci Sanitari de Terrassa on September 16, 2019. No consent to participate was required for this retrospective database analysis. BIG-PAC® database contains secondary information sent anonymously from primary and secondary care centers in Spain, which collect primary information from patients (requiring consent). As such, according to the Spanish legislation, patients do not need to provide explicit consent for their information to be included in the BIG-PAC® database. Ethical approval and consent waiver was in any case granted by Consorci Sanitari de Terrassa.
Data Availability
The data underlying this article are available in the article and in its online Supplementary Material.
References
- 1.Halcox JP, Banegas JR, Roy C, et al. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc Disord. 2017;17(1):160. doi: 10.1186/s12872-017-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 3.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 4.Fundación Española del Corazón. Mortalidad cardiovascular en España en 2018. https://fundaciondelcorazon.com/corazon-facil/recursos-didacticos/infografias.html?download=63:la-mortalidad-cardiovascular-en-2018. Accessed 20 Feb 2022.
- 5.Roth GA, Fihn SD, Mokdad AH, Aekplakorn W, Hasegawa T, Lim SS. High total serum cholesterol, medication coverage and therapeutic control: an analysis of national health examination survey data from eight countries. Bull World Health Organ. 2011;89(2):92–101. doi: 10.2471/BLT.10.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiner Ž, De Backer G, Fras Z, et al. Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries—findings from the EUROASPIRE IV survey. Atherosclerosis. 2016;246:243–250. doi: 10.1016/j.atherosclerosis.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Hartgers ML, Ray KK, Hovingh GK. New approaches in detection and treatment of familial hypercholesterolemia. Curr Cardiol Rep. 2015;17(12):109. doi: 10.1007/s11886-015-0665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray KK, Molemans B, Schoonen WM, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2021;28(11):1279–89. [DOI] [PubMed]
- 9.Wilkins EWL, Wickramasinghe K, Bhatnagar P, et al. European cardiovascular disease statistics 2017. Brussels, European Heart Network AISBL; 2017. https://ehnheart.org/component/attachments/attachments.html?task=attachment&id=3115. Accessed 20 Feb 2022.
- 10.Sulo E, Vollset SE, Nygård O, et al. Trends in 28-day and 1-year mortality rates in patients hospitalized for a first acute myocardial infarction in Norway during 2001–2009: a "Cardiovascular disease in Norway" (CVDNOR) project. J Intern Med. 2015;277(3):353–361. doi: 10.1111/joim.12266. [DOI] [PubMed] [Google Scholar]
- 11.Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 13.Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532–540. doi: 10.1161/CIRCOUTCOMES.111.964700. [DOI] [PubMed] [Google Scholar]
- 14.Danese MD, Gleeson M, Kutikova L, et al. Estimating the economic burden of cardiovascular events in patients receiving lipid-modifying therapy in the UK. BMJ Open. 2016;6(8):e011805. doi: 10.1136/bmjopen-2016-011805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escobar Cervantes C, Gomez-Ulloa D, Elorriaga A, et al. PCV61—productivity loss and local indirect costs associated with acute coronary syndrome and stroke in Spain. Value Health. 2018;21:S102. doi: 10.1016/j.jval.2018.09.608. [DOI] [Google Scholar]
- 16.European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Data source. Big-Pac. http://www.encepp.eu/encepp/viewResource.htm?id=29236. Accessed 10 Feb 2022.
- 17.World Health Organization (WHO). The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). http://www.who.int/classifications/atcddd/en/. Accessed 10 Feb 2022.
- 18.Instituto Nacional de Estadística 2017. Ganancia media laboral por edad y sexo. https://www.ine.es/dynt3/inebase/index.htm?padre=4563&capsel=4563.%20Consultado:%2006/11/2020. Accessed 10 Mar 2022.
- 19.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568–580. doi: 10.1111/j.1365-2796.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 22.Ara R, Tumur I, Pandor A, et al. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess. 2008;12(21):iii. doi: 10.3310/hta12210. [DOI] [PubMed] [Google Scholar]
- 23.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence (NICE). Cardiovascular disease: risk assessment and reduction, including lipid modification. Clinical guideline [CG181]. https://www.nice.org.uk/guidance/cg181. Accessed 01 Mar 2022. [PubMed]
- 25.Allahyari A, Jernberg T, Hagstrom E, Leosdottir M, Lundman P, Ueda P. Application of the 2019 ESC/EAS dyslipidaemia guidelines to nationwide data of patients with a recent myocardial infarction: a simulation study. Eur Heart J. 2020;41(40):3900–3909. doi: 10.1093/eurheartj/ehaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotseva K, Gerlier L, Sidelnikov E, et al. Patient and caregiver productivity loss and indirect costs associated with cardiovascular events in Europe. Eur J Prev Cardiol. 2019;26(11):1150–1157. doi: 10.1177/2047487319834770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Material.