Dear Editor,
We read with interest the paper by Menzies-Gow et al. [1] aimed at assessing the impact of benralizumab on clinical remission in patients suffering from severe asthma. The study [1] was a post hoc analysis including selected pooled patients from the SIROCCO [2], CALIMA [3], and ZONDA [4] trials. The analysis was performed by applying a composite definition of severe asthma clinical remission adapted from Delphi documents [5, 6] and results were reported in a descriptive manner with no statistical analysis.
Post hoc analyses are characterized by intrinsic weaknesses such as type I error and the possibility of being misinterpreted [7]; furthermore, Delphi methods are limited by the fact that they are subjective by nature and based on opinions of people who should be expert in a specific field [8]. The misuse of these techniques may result in biased clinical evidence.
However, when conducted properly, both post hoc analyses and Delphi surveys may lead to clinically valuable insights and recommendations [7, 9, 10]. In fact, high-quality evidence may originate from prespecified post hoc analyses [11] and by strictly applying the criteria resulting from consensus frameworks [5, 6], conditions not fully met in the study by Menzies-Gow et al. [1]. As a matter of fact, the authors performed an unplanned (non-prespecified) pooled post hoc analysis by applying, in an arbitrary way, a combination of criteria for the definition of severe asthma clinical remission derived from two different Delphi processes [5, 6]. The four-item composite definition of clinical remission used in the pooled post hoc analysis [1] was not fully consistent with the criteria proposed by the Delphi studies [5, 6], as shown in Table 1.
Table 1.
Criteria for defining the composite definition of severe asthma clinical remission used in the pooled post hoc analysis by Menzies-Gow et al. [1] and comparison with the reference Delphi documents [5, 6]
| Items | Composite definition of severe asthma clinical remission used by Menzies-Gow et al. (all criteria needed) [1] | Severe asthma remission and/or super-responder criteria from Delphi processes [5, 6] | Consistency |
|---|---|---|---|
| Daily symptoms | ACQ score ≤ 0.75 at 6 and 12 months | ACQ score ≤ 0.75 or < 1.0 for ≥ 12 months | Yes at 12 months, no at 6 months |
| Exacerbation | Zero exacerbations at 6 and 12 months | Exacerbation elimination for ≥ 12 months | Yes at 12 months, no at 6 months |
| OCS | Zero OCS use at 6 and 12 months | Cessation of maintenance OCS for ≥ 12 months | Yes at 12 months, no at 6 months |
| Lung function | Pre-BD FEV1 increase ≥ 100 mL at 6 and 12 months | ≥ 500 mL improvement in FEV1 for ≥ 12 months | No |
ACQ Asthma Control Questionnaire, BD bronchodilator, FEV1 forced expiratory volume in 1 s, OCS oral corticosteroids
Looking at one of the primary Delphi publications [6], only zero exacerbation and zero corticosteroids (OCS) use can be considered major criteria of clinical remission; conversely, the Asthma Control Questionnaire (ACQ) and forced expiratory volume in 1 s (FEV1) identified minor criteria. Moreover, regardless of the weight of criteria, both the Delphi documents [5, 6] required that the improvements in each item should be assessed for at least 12 months. Thus, since the ZONDA [4] study lasted 28 weeks, this trial should not have been included in the post hoc analysis [1]; nor should the data at 6 months for the SIROCCO [2] and CALIMA [3] studies. Menzies-Gow et al. [1] correctly reported that all the four items were concurrently necessary to define clinical remission in severe asthma. This approach was consistent to the framework for the clinical remission in asthma and/or the assessment of criteria for defining super-responders [5, 6], but the increase in FEV1 should have been defined as ≥ 500 mL [6] and not as ≥ 100 mL as set by Menzies-Gow et al. [1].
Overall, across all the results reported by the authors [1], only those shown in Fig. 4B and Supplementary Tables 1 and 2 (clinical remission at 12 months) may be suitable for an appropriate post hoc analysis. However, since Menzies-Gow et al. [1] reported in the methods that only patients from SIROCCO [2] and CALIMA [3] studies not receiving OCS were included in the pooled post hoc analysis and that patients who were receiving OCS at baseline were excluded, it is unclear why the item “No OCS” was considered in these figures and tables.
In any case, if one ignores the unclear point concerning the OCS use in the SIROCCO [2] and CALIMA [3] studies and assuming an increase in FEV1 ≥ 100 mL as a valid remission criterion, the data reported in Fig. 4B and Supplementary Tables 1 and 2 remain useful for an independent statistical analysis and clinical interpretation. In this respect, the Fisher’s exact test and the relative risk (RR) analysis showed that, when compared to placebo, benralizumab 30 mg every 8 weeks (Q8W, first three doses 4 weeks apart) elicited significant severe asthma clinical remission in the overall pooled population from SIROCCO [2] and CALIMA [3] studies (Fig. 1a; RR 1.87, 95% confidence interval [CI] 1.34–2.62, P < 0.001). Similar results were found also in the patient subgroup with blood eosinophil count ≥ 150 cells/μL (Fig. 1b; RR 1.74, 95% CI 1.23–2.45, P < 0.01) and ≥ 300 cells/μL (Fig. 1c; RR 1.67, 95% CI 1.17–2.40, P < 0.01).
Fig. 1.
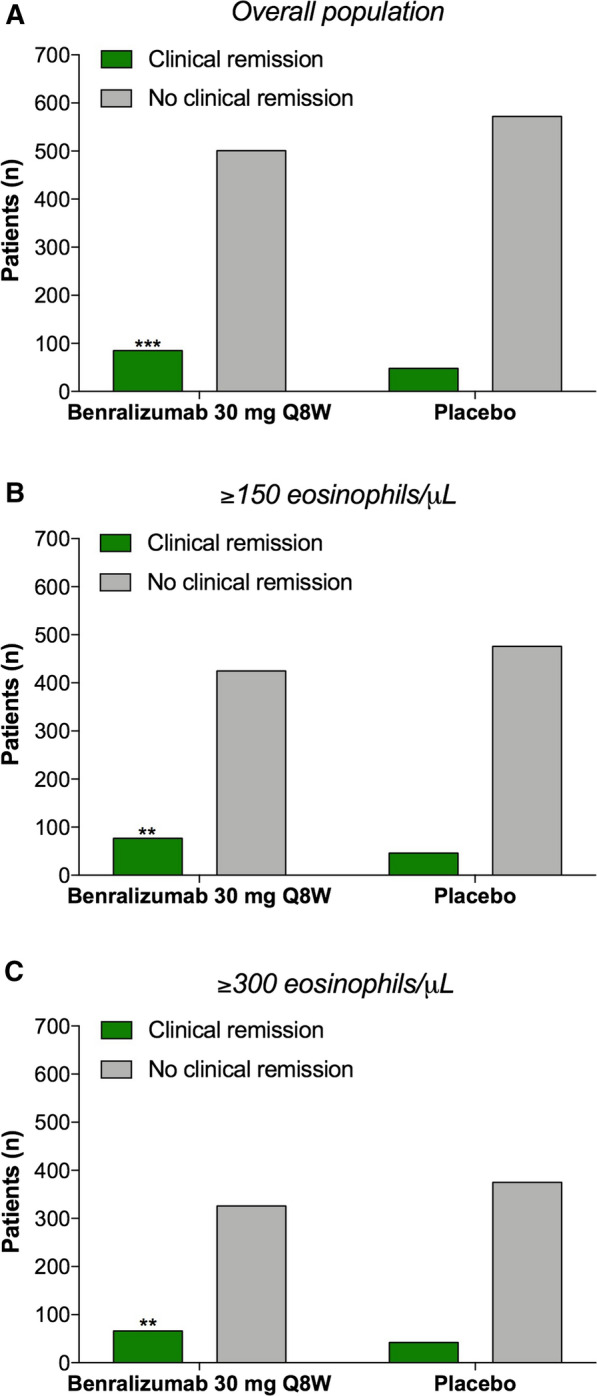
Fisher’s exact test comparing the number of severe asthmatic patients treated for 12 months with benralizumab 30 mg Q8W or placebo achieving the clinical remission according to the four-item composite definition proposed by Menzies-Gow et al. [1] (zero exacerbations and zero OCS use and ACQ-6 ≤ 0.75 and pre-BD FEV1 increase ≥ 100 mL). The analysis was performed on the overall population from SIROCCO [2] and CALIMA [3] studies (a) and in subgroup populations with blood eosinophil count ≥ 150 cells/μL (b) and ≥ 300 cells/μL (c). **P < 0.01 and ***P < 0.001. ACQ Asthma Control Questionnaire, BD bronchodilator, FEV1 forced expiratory volume in 1 s, OCS oral corticosteroids, Q8W every 8 weeks, first three doses 4 weeks apart
Interestingly, these statistically significant results are worth of an objective clinical interpretation via the analysis of the number need to treat (NNT), an absolute measure providing the clinically beneficial effect of a medical intervention in a specific population [12, 13]. It resulted that 15 (95% CI 10–32) patients had to be treated with benralizumab 30 mg Q8W to have one patient achieve clinical remission compared to placebo over 12 months, with no significant difference according to the levels of blood eosinophil count (≥ 150 eosinophils/μL: NNT 15, 95% CI 9–42; ≥ 300 eosinophils/μL: NNT 15, 95% CI 9–53).
Post hoc analyses are neither necessary nor forbidden, but if carried out they should fulfill strict criteria and be built on solid scientific background [7]. Here we have provided evidence that a well-performed pooled post hoc analysis of SIROCCO [2] and CALIMA [3] studies may provide scientific findings useful from both a statistical and clinical point of view. Although the concept and definition of clinical remission in asthma have yet to be refined and validated through studies designed ad hoc, generally they include the absence of significant symptoms, no exacerbations, no use of OCS, and large improvement in lung function after at least 12 months of treatment [5, 6]. In this context, benralizumab 30 mg Q8W was effective in eliciting clinical remission of severe asthma in one patient out of 15, at least according to the four-item composite definition proposed by Menzies-Gow et al. [1]. Certainly, airway inflammation and remodelling may be present also during clinical remission of atopic asthma [14]; however, such an NNT value looks of remarkable clinical impact since it resulted from the concurrent presence of four different remission criteria in the same patient.
Concluding, the data resulting from our independent post hoc analysis further supports the clinical benefit of benralizumab 30 mg Q8W after 12 months of treatment in severe asthmatic patients, but the obtained NNT value should be applied with caution to real-life clinical settings because it originated from the pooled analysis of randomized controlled trials. In all likelihood, post-marketing trials reporting open patient-level data available for independent research could definitively assess, in an unbiased manner, the real percentage of severe asthmatic patients treated with benralizumab achieving clinical remission.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Both the authors (LC and PR) equally contributed in concept and design, statistical analysis, drafting and writing the manuscript.
Disclosures
Luigino Calzetta has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis, received non-financial support from AstraZeneca, a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall, and is or has been a consultant to ABC Farmaceutici, Edmond Pharma, Zambon, Verona Pharma, and Ockham Biotech. His department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, Zambon. Paola Rogliani has participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis. Her department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici Novartis, and Zambon.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Menzies-Gow A, Hoyte FL, Price DB, et al. Clinical remission in severe asthma: a pooled post hoc analysis of the patient journey with benralizumab. Adv Ther. 2022 doi: 10.1007/S12325-022-02098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016 doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 4.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/nejmoa1703501. [DOI] [PubMed] [Google Scholar]
- 5.Menzies-Gow A, Bafadhel M, Busse WW, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Upham JW, Le Lievre C, Jackson DJ, et al. Defining a severe asthma super-responder: findings from a Delphi process. J Allergy Clin Immunol Pract. 2021 doi: 10.1016/j.jaip.2021.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas TR, Ho B, Kang J, Kaplan B. Post hoc analyses: after the facts. Transplantation. 2015;99:17–20. doi: 10.1097/TP.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 8.Baker J, Lovell K, Harris N. How expert are the experts? An exploration of the concept of “expert” within Delphi panel techniques. Nurse Res. 2006 doi: 10.7748/nr2006.10.14.1.59.c6010. [DOI] [PubMed] [Google Scholar]
- 9.Mullen PM. Delphi: myths and reality. J Health Organ Manag. 2003;17:37–52. doi: 10.1108/14777260310469319. [DOI] [PubMed] [Google Scholar]
- 10.Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8:1000393. doi: 10.1371/JOURNAL.PMED.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran-Everett D, Milgrom H. Post-hoc data analysis: benefits and limitations. Curr Opin Allergy Clin Immunol. 2013 doi: 10.1097/ACI.0b013e3283609831. [DOI] [PubMed] [Google Scholar]
- 12.Mendes D, Alves C, Batel-Marques F. Number needed to treat (NNT) in clinical literature: an appraisal. BMC Med. 2017 doi: 10.1186/s12916-017-0875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calzetta L, Aiello M, Frizzelli A, Rogliani P, Chetta A. Dexamethasone in patients hospitalized with COVID-19: whether, when and to whom. J Clin Med. 2021;10:1607. doi: 10.3390/jcm10081607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Den Toorn LM, Overbeek SE, De Jongste JC, et al. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001 doi: 10.1164/ajrccm.164.11.2006165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


