Abstract
The developmental origins of psychopathology begin before birth and perhaps even prior to conception. Understanding the intergenerational transmission of psychopathological risk is critical to identify sensitive windows for prevention and early intervention. Prior research demonstrates that maternal trauma history, typically assessed retrospectively, has adverse consequences for child socioemotional development. However, very few prospective studies of preconception trauma exist, and the role of preconception symptoms of posttraumatic stress disorder (PTSD) remains unknown. The current study prospectively evaluates whether maternal preconception PTSD symptoms predict early childhood negative affectivity, a key dimension of temperament and predictor of later psychopathology. One hundred and eighteen women were recruited following a birth and prior to conception of the study child and were followed until the study child was 3 to 5 years old. Higher maternal PTSD symptoms prior to conception predicted greater child negative affectivity, adjusting for concurrent maternal depressive symptoms and sociodemographic covariates. In exploratory analyses, we found that neither maternal prenatal nor postpartum depressive symptoms or perceived stress mediated this association. These findings add to a limited prospective literature, highlighting the importance of assessing the mental health of women prior to conception and providing interventions that can disrupt the intergenerational sequalae of trauma.
Keywords: Preconception, Trauma, PTSD, Intergenerational transmission, Negative affectivity
Maternal mental health history is strongly implicated in the developmental trajectory of child emotional development and psychopathology risk (Goodman & Gotlib, 1999; Bijl, Cuijpers, & Smit, 2002; Swales et al., 2020). This intergenerational link between maternal and child psychological wellbeing is especially evident among mothers who have experienced trauma and subsequent symptoms of posttraumatic stress (Lambert, Holzer, & Hasbun, 2014). Almost 7 out of every 10 adult women will be exposed to a traumatic event in their lifetime (Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993) and approximately 4 to 6% will go on to meet criteria for Posttraumatic Stress Disorder (PTSD) (National Comorbidity Survey, 2017). Notably, PTSD rates are even higher among women living in poverty (Seng, Low, Sperlich, Ronis, & Liberzon, 2009). Maternal trauma and subsequent PTSD symptoms have been shown to have intergenerational consequences, including deleterious effects on child socioemotional development and mental health outcomes, such as elevated risk for anxiety, depression, and behavior problems (Lambert et al., 2014).
Consistent with a Developmental Origins of Health and Disease (DOHaD) framework, maternal perinatal PTSD symptoms are associated with their children’s vulnerability to psychopathology via biological mechanisms of fetal programing as well as postnatal environmental cues (e.g., parenting behaviors, quality of parent-child attachments) (Doyle & Cicchetti, 2018). However, a growing literature suggests that ontogenetic pathways of risk and resilience for the development of psychopathology do not begin at conception (Keenan, Hipwell, Class, & Mbayiwa, 2018). Rather maternal mental health prior to conception, including PTSD symptoms, may also relate to developmental trajectories of mental health in the next generation via alterations in prenatal stress physiology, epigenetic pathways, and other peripartum mechanisms (Bale, 2014; Bowers & Yehuda, 2016; Buss et al., 2017; Keenan et al., 2018; Scorza et al., 2019; Swales et al., 2018). The current study expands the DOHaD framework to include preconception influences, utilizing a prospective, longitudinal design to examine how maternal PTSD symptoms prior to conception may relate to outcomes in the next generation, such as negative affectivity, which contribute to subsequent vulnerability to later psychopathology (e.g., Clark et al., 1994; Derryberry & Rothbart, 1997; Lonigan, Vasey, Phillips, & Hazen, 2004; Muris & Ollendick, 2005; Nigg, 2006).
Intergenerational Consequences of Preconception Stress
Experimental research with animals provides compelling evidence that maternal stress exposure prior to conception exerts lasting intergenerational consequences (see Klengel, Dias, & Ressler, 2016 for review). Maternal stress exposure (e.g., chronic and unpredictable stress, overcrowding, temperature) that is limited to the preconception period adversely impacts rat offspring brain development including anatomical changes in the medial prefrontal cortex (Bock et al., 2016; Harker, Raza, Williamson, Kolb, & Gibb, 2015; Huang et al., 2010), altered stress physiology (Zaidan, Leshem, & Gaisler-Salomon, 2013), and increased anxious and anhedonic behavior (Bock et al., 2016; Li et al., 2010; Zaidan, Leshem, & Gaisler-Salomon, 2013). These experimental studies provide important evidence that severe stress exposure limited to the preconception period has intergenerational consequences.
In humans, evidence for the intergenerational transmission of trauma has been supported by epidemiological research as well as studies of collective trauma (e.g., parental exposure to genocide prior to conception) (Class et al., 2014; Class et al., 2015; Keenan et al., 2018; Power et al., 2007; Flory, Bierer, & Yehuda, 2011; Gangi, Talamo, & Ferracuti, 2009; Perroud et al., 2014; Solomon, Kitler, & Mikulincer, 1988; Yehuda, Bell, Bierer, & Schmeidler, 2008; Yehuda, Halligan, & Grossman, 2001). Additionally, maternal retrospective report of traumatic and stressful life experiences, such as history of childhood maltreatment (e.g., Adverse Childhood Events Scale, ACES, Felitti et al., 1998; and Childhood Trauma Questionnaires, CTQ, Bernstein et al., 1994), show that maternal trauma can have intergenerational effects. Children of women with trauma histories, assessed by maternal recall of trauma in her own childhood, are more likely to experience dysregulated temperament in infancy, including heightened negative affectivity (Bosquet Enlow et al., 2017; Lang et al., 2010) and poor emotional and behavioral outcomes in childhood (Bosquet Enlow, Englund, & Egeland, 2018; Khan & Renk, 2019; Plant, Jones, Pariante, & Pawlby, 2017; Plant, Pawlby, Pariante, & Jones, 2018). These studies provide epidemiological and retrospective evidence that maternal history of traumatic and potentially traumatic experiences prior to conception predict poor emotional outcomes for the next generation. However, additional prospective research is needed to address limitations inherent to such study designs. First, retrospective recall of adverse experiences is only moderately associated with prospectively collected measures (see Baldwin, Reuben, Newbury, & Danese, 2019 for meta-analysis and systematic review). Second, retrospective recall of PTSD symptoms is even more susceptible to recall biases than recall of past trauma exposure (Baldwin et al., 2019; Hardt & Rutter, 2004; Moffitt et al., 2010; Newbury et al., 2018; Reuben et al., 2016).
Prospective Assessment of Preconception Trauma
Given the inherent limitations of retrospective studies, there has been increasing recognition of the need for prospective, longitudinal studies of preconception stress (Bowers & Yehuda, 2016; Keenen et al., 2018). However, a dearth of prospective research has examined women’s mental health prior to pregnancy and child outcomes due to the logistical challenges of recruiting women before conception and following them through their pregnancies. A few published prospective studies show that elevated preconception stress and exposure to stressful life experiences relate to adverse birth outcomes (i.e., shorter gestation and lower birthweight) (Harville, Boynton-Jarrett, Power, & Hyppönen, 2010; Mahrer, Guardino, Hobel, Dunkel Schetter et al., 2020) and poor sleep quality in infancy (Baird, Hill, Kendrick, & Inskip, 2009). A large prospective study of women in Australia (n = 756) reported that maternal preconception symptoms of depression and anxiety predicted heightened emotional reactivity in infancy (Spry, Moreno-Betancur, Becker, & Romaniuk, 2020). Another study conducted by Hipwell and colleagues (2019) prospectively assessed maternal childhood trauma exposure prior to conception via standardized interviews and items from the Parent-Child Conflict Tactics Scale and found that infants of mothers exposed to childhood emotional abuse were more likely to demonstrate low emotional reactivity to the still face procedure, whereas infants of mothers exposed to childhood emotional neglect demonstrated heightened emotional reactivity, even after controlling for postpartum depressive symptoms. Collectively, these few, prospective studies are important as they reduce the influence of recall bias and affirm the need to expand research on preconception maternal trauma and mental health and child emotion regulation outcomes.
A key gap in this literature is that additional studies are needed to prospectively assess whether preconception symptoms of posttraumatic stress predict outcomes for the next generation. It is important to distinguish maternal exposure to trauma or a potentially traumatic event from posttraumatic stress symptoms because not all women who are exposed to a potentially traumatic event will develop subsequent PTSD symptoms. Rather mediating pathways of risk and resilience have been shown to amplify or disrupt the emergence of subsequent psychopathology following trauma exposure (McLaughlin & Lambert, 2017). Identifying whether preconception maternal PTSD symptoms relate to child outcomes is therefore important next step in exploring whether maternal mental health prior to conception confers vulnerability for subsequent psychopathology in the next generation.
Additional work is also needed to explore potential pathways that may underly this hypothesized link between preconception PTSD symptoms and child psychopathological risk. Perinatal mental health, especially maternal depression, is a plausible candidate pathway because prior work suggests that offspring may be more vulnerable to the biopsychosocial sequalae of maternal trauma during the fetal period of development (Bouvette-Turcot et al., 2020; Bowers & Yehuda, 2016; Buss et al., 2017; Davis & Narayan, 2020; Keenan et al., 2018; Swales et al., 2018; Yehuda & Meaney, 2018). Past studies demonstrate that retrospective recall of trauma history is associated with elevated internalizing symptoms (e.g., depression, anxiety, and PTSD) during pregnancy (Alvarez-Sengura et al., 2014; Atzl, Narayan, Rivera, & Liberman, 2019; River, Narayan, Atzl, Rivera, & Lieberman, 2019), and prenatal maternal mental health predicts heightened negative emotionality in offspring during childhood (Blair, Glynn, Sandman, & Davis, 2011; Davis et al., 2007; Erickson, Gartstein, & Dotson, 2017; Glynn et al., 2018; Werner et al., 2007). Prenatal depression is a potential pathway of particular interest because PTSD and depression diagnoses are highly comorbid (O’Donnell, Creamer, & Pattison, 2004) and depressive symptoms are commonly screened for in clinical settings and thus offer implications for clinical practice. The current study provides a unique opportunity to investigate these exploratory pathways.
The Current Study
The current study prospectively evaluates the association between maternal preconception symptoms of posttraumatic stress and child negative affectivity. Mothers were from a socioeconomically, racially, and ethnically diverse larger cohort of women who were studied following a birth. This subset was then followed when mothers became pregnant again with the study child and until the study child was 3 to 5 years of age. Preconception is defined in the current study as prior to conception of the study child (which for all participants in the study sample is also an interconception period between consecutive births). As the primary aim of the current analyses, we evaluated whether preconception maternal PTSD symptoms predict child negative affectivity after accounting for sociodemographic covariates and concurrent maternal depressive symptoms (an indicator of concurrent maternal psychopathology and reporter bias). In exploratory post-hoc analyses, we tested maternal prenatal and postpartum symptoms of depression as potential mediators in the relation between preconception symptoms of PTSD and child negative affectivity. Prenatal and postpartum levels of perceived stress were additionally considered as mediators in secondary, exploratory analyses.
Method
Participants
Participants were 118 mother-child dyads. Women were recruited from three study sites in the Community Child Health Network (CCHN) (i.e., North Carolina, Washington, DC, and Lake County, IL). CCHN is a multi-site research network designed to investigate disparities in maternal and child mental health (see Dunkel Schetter et al., 2013; O’Campo et al., 2016; and Ramey et al., 2015 for overview of CCHN recruitment procedures, study design, data collection methods, and cohort demographics). Two hundred and forty-five women from the 3 selected CCHN study sites who became pregnant again between 2009 and 2013 and were assessed prospectively from preconception (i.e., between births and prior to conception of the study child) through their pregnancy and into the postpartum period. One hundred and twenty-seven women then went on to participate in a follow up assessment when the study child was 3 to 5 years of age.
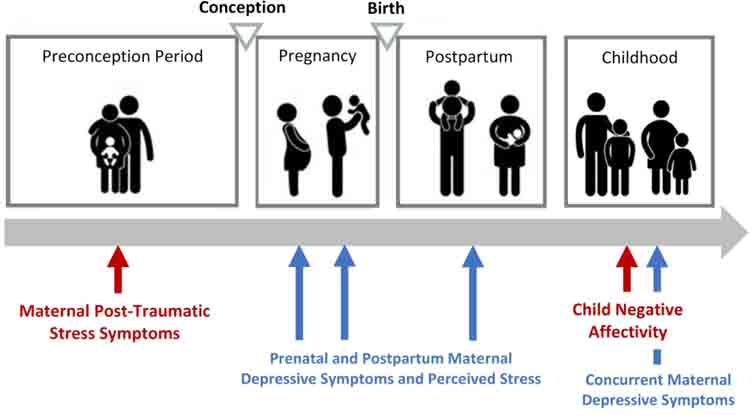
Additional inclusion criteria for the current analyses were mothers who provided: a) ratings of preconception PTSD or completed at least one prenatal study visit (i.e., provided at least one rating of mood or stress during pregnancy), and b) ratings of child negative affectivity in early childhood. One hundred and nineteen women met these criteria. One dyad was excluded from analyses due to preterm birth less than 34 weeks’ gestation. See Figure 1 for overview of study design.
Figure 1:
Overview of study measures from preconception to early childhood
The 118 women in the current study analyses were more likely to be older in age (t = 4.1, p < .001), have a longer interpregnancy interval (t = 2.6, p = .002), have a lower adjusted household income (t = −2.0, p = .049), be Latina/Hispanic (X2 = 8.6, p = .003), and be born outside of the United States (X2 = 8.6, p = .003), in comparison to women who became pregnant during the eligibility window but were not included in the present analyses (either because they did not participate in the follow up assessment in early childhood and/or were missing preconception or prenatal study measures). Sample attrition was not associated with maternal education (t = −1.1, p = .275), concurrent depressive symptoms (t = 0.7, p = .484), study site (X2 = 0.8, p = .655), cohabitation status (X2 = 2.4, p = .124), or PTSD symptoms (t = 1.0, p = .326).
Mothers included in the current study sample were an average of 33 years of age at the time of child assessment (SD = 5.5 years) and 39% identified as Latina/Hispanic, 32% non-Latina/Hispanic Black, and 29% non-Latina/Hispanic White (see Supplemental Table S1 for descriptive information on study measures stratified by maternal race/ethnicity). Additionally, 40% of women were born outside of the United States (46 out of the 47 of whom also identified as Latina or Hispanic). Further, 38% lived below the federal poverty line (FPL), and 21% lived near poverty at 100% to 200% FPL. Children were on average 3.8 years of age at the time of assessment (SD = 0.4 years) and 58% were female (see Table 1). All study procedures were approved by the Institutional Review Board for the protection of human subjects at the relevant institutions, and each mother provided written and informed consent for herself and her child.
Table 1.
Sample characteristics
| Sample Characteristics (n = 118) | |
|
| |
| Study site (N; %) | |
| Chicago, Illinois | 74; 62.7 |
| Rural eastern North Carolina | 17; 14.4 |
| District of Columbia | 27; 22.8 |
| Time from preconception assessment to conception (in weeks) | 52.9 ± 47.3; 2–175 |
|
| |
| Maternal Characteristics | |
|
| |
| Maternal age (in years; M ± SD; Range) | 33.4 ± 5.5; 24.1–44.2 |
| Cohabitating with partner (N; %) | 77; 65.3 |
| Race/Ethnicity (N; %) | |
| Latina/Hispanic | 46; 39.3 |
| Non Latina/Hispanic Black | 38; 32.0 |
| Non-Latina/Hispanic White | 34; 28.7 |
| Nativity status (N; % born outside of the U.S.) | 47; 39.8 |
| Federal poverty line (FPL) (N; %) | |
| Poverty (<100% FPL) | 45; 38.1 |
| Near poverty (100–200% FPL) | 25; 21.1 |
| Above poverty (>200% FPL) | 48; 40.7 |
| Per capita household income adjusted for cost of living (M ± SD; Range) | 13,757.0 ± 7,232.4; 0–89,222.0 |
| Years of education (M ± SD; Range) | 12.6 ± 2.9; 5–21 |
| Parity (N; %) | |
| 1 | 62; 52.5 |
| 2 | 38; 32.2 |
| 3 | 17; 14.4 |
|
| |
| Child Characteristics | |
|
| |
| Child age at assessment (in years; M± SD; Range) | 3.8 ± .4; 3.4–5.5 |
| Biological sex at birth (N; % female) | 68; 57.7 |
| Gestational age at birth (in weeks) (M ± SD; Range) | 38.8 ± 2.1; 32.6–42 |
Procedures
All self-report data were collected through semi-structured interviews by trained interviewers in participants’ homes (see Dunkel Schetter et al. 2013). Maternal ratings of posttraumatic stress symptoms and demographic data were collected before women became pregnant with the study child. For present purposes, the preconception assessment occurred an average of 52.9 weeks prior to conception of the study child, although time from assessment to conception varied (SD = 47.3, Range: 2.0–175.0 weeks). The beginning of the pregnancy was determined using the first day of the last menstrual period and/or ultrasound examinations, obtained via medical record abstraction. Standardized measures of maternal depression symptoms and perceived stress were administered twice during pregnancy in second and third trimesters, on average at 20.2 weeks gestation (SD = 5.0) and 32.7 weeks gestation (SD = 3.8). Maternal depression symptoms were assessed once during the postpartum period on average at 12.5 weeks after birth (SD = 4.7). Additional perinatal and birth outcomes data were extracted from neonatal records. Mother-child dyads participated in an early childhood study visit when the child was 3 to 5 years of age, at which time child negative affectivity was assessed. Early childhood visits were conducted in participants’ homes, in the mother’s preferred language (English or Spanish).
Measures
Maternal Measures
Sociodemographic and Pregnancy Factors.
Sociodemographic characteristics, including maternal age, years of education, per capita household income adjusted for cost of living, cohabitation status (i.e., living with partner), parity, foreign birth (i.e., whether mother was born in a country outside of the United States), and race/ethnicity, were assessed during interviews. An outlier for adjusted household income (i.e., >8 SD above the mean) was replaced with a value 3 SD above the mean, retaining its rank as the highest value. Maternal education and per capita household income adjusted for cost of living were standardized and averaged together to create a composite of socioeconomic status (Cohen, Doyle, & Baum, 2006). Maternal early life adversity was assessed by the 10-item Risky Families Questionnaire (Taylor et al., 2004), a measure of family stressors during the mother’s childhood (e.g., childhood abuse, parental warmth). Mothers completed the measure during the early childhood visit, rating each item on a 5-point Likert scale, with a range of 1 (not at all) to 5 (very often). Final sum scores could range from 10 to 50, with higher scores indicating greater early life adversity. Gestational age at birth was determined from medical record abstraction.
Maternal Posttraumatic Stress Symptoms.
Maternal symptoms of posttraumatic stress were assessed prior to conception using the Posttraumatic Stress Disorder (PTSD) Checklist – Civilian Version (PCL-C; Blanchard, Jones-Alexander, Buckley, & Forneris, 1996). Participants provided ratings of how often they have been bothered by various symptoms of PTSD in the past month, on a 5-point Likert scale with a range of 1 (not at all) to 5 (extremely). Final sum scores could range from 17 to 85, with higher scores indicating more PTSD symptoms (see Table 2). A cut-off score of 30 was used to indicate clinically elevated symptoms and a probable diagnosis of PTSD. This threshold and scoring approach were selected because prior research demonstrates that utilizing a cut-off score of 30 maximizes the sensitivity and specificity of accurately detecting a PTSD diagnosis amongst civilian women (McDonald & Calhoun, 2010; Walker et al., 2002). The PCL-C is a reliable and well validated measure of PTSD symptoms (Ruggiero, Del Ben, Scotti, & Rabalais, 2003), and has been previously utilized in studies of women across pregnancy and the postpartum period (e.g. Huth-Bocks et al., 2013; Thomas, Cleveland, Pietrzak, Dunkel Schetter, & Sumner, 2021; Thomas, Carter, Dunkel Schetter, & Sumner, 2021). Within the current study sample, the PCL-C demonstrated excellent internal consistency (α = .92) and 18 participants (21%) reported scores above the clinical threshold. Notably, the rate of probable PTSD diagnosis was higher in this sample than in the general population (Breslau, 2009), likely reflecting the converging risk factors for trauma and PTSD symptoms experienced by many of the women in the study (e.g., poverty, low education, acculturative stress) (Breslau, Davis, & Andreski, 1995; Pole, Gone, & Kulkarni, 2008; Trickey et al., 2012). Indeed, women who experienced elevated symptoms of PTSD in the study sample were more likely to be born outside of the United States (t = −2.62, p = .012), to have completed fewer years of education (r = −.367, p < .001), and to have a lower per capita income adjusted for cost of living (r = −.31, p = .004). PCL-C scores were not associated with race/ethnicity or cohabitation status.
Table 2.
Descriptive statistics for maternal and child measures across all timepoints.
| Maternal and Child Measures | M ± SD; Range; Skewness |
|---|---|
|
| |
| Maternal Preconception PTSD Symptoms (PCL-C) | |
| Preconception PCL-C | 25.1 ± 10.2; 17–63; 1.98 |
| Maternal Depressive Symptoms (CESD-D) | |
| Prenatal CES-D (20 weeks’ GA) | 8.9 ± 3.5; 2–20; 1.28 |
| Prenatal CES-D (33 weeks’ GA) | 10.0 ± 3.4; 4–18; 1.36 |
| Postpartum CES-D (13 weeks postpartum) | 9.4 ± 3.4; 4–21; 1.61 |
| Concurrent CES-D (at the time of child assessment) | 5.0 ± 4.7; 0–24; 1.56 |
| Perceived Stress Symptoms (PSS) | |
| Prenatal PSS (20 weeks’ GA) | 13.8 ± 6.2; 0–29; 0.00 |
| Prenatal PSS (33 weeks’ GA) | 13.9 ± 6.0; 1–33; 0.77 |
| Postpartum PSS (13 weeks postpartum) | 13.0 ± 6.2; 3–35; 0.86 |
| Children’s Behavior Questionnaire (CBQ) | |
| Negative Affectivity | 4.2 ± 0.8; 2.2–6.5; 0.21 |
Maternal Depressive Symptoms.
Maternal depressive symptoms were assessed using the 9-item short form of the Center for Epidemiological Studies Depression Inventory (CES-D-9; Santor & Coyne, 1997). The CES-D is a reliable and widely used self-report measure of depressive symptoms. Participants rated the frequency at which each depressive symptom occurred during the past week on a four-point Likert scale, ranging from 0 (rarely or none of the time [less than 1 day]) to 3 (most of the time [5–7 days]). Final sum scores could range from 0 to 27 (see Table 2). The CES-D scores utilized in the current analyses were assessed twice during pregnancy, once during the postpartum period, and once at the time of child assessment. CES-D scores across both prenatal timepoints were averaged to create a composite score of prenatal depressive symptoms. The CES-D-9 demonstrated fair to good internal consistency across study time points (α’s ranging from .76 to .85). The CES-D-9 has also been shown in prior work to be a well validated measure of depression in adults (Corcoran & Fisher, 1987; Santor & Coyne, 1997), including pregnant and postpartum women (Mosack & Shore, 2006).
Maternal Perceived Stress.
Maternal perceived stress symptoms were evaluated using the 10-item version of Cohen’s Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983). The PSS is a self-report measure of generalized or non-specific stress, which evaluates participants’ feelings about how they were able to handle day-to-day problems and hassles, how often they felt nervous and stressed, and how often they felt things were going well during the past week. Responses were made on a 5-point Likert scale, ranging from 0 (never) to 4 (almost always), with a final sum score which could range from 0 to 40 and higher scores indicating greater impairment (see Table 2). The PSS scores utilized in the current analyses were assessed twice during pregnancy, once during the postpartum period, and once at the time of child assessment. PSS scores across both prenatal timepoints were averaged to create a composite score of prenatal levels of perceived stress. The PSS demonstrated good internal consistency across study time points (α’s ranging from .84 to .87). The PSS has been widely used to evaluate perceived stress symptoms in women during prenatal and postpartum periods (Nast, Bolten, Meinlschmidt, & Hellhammer, 2013).
Child Negative Affectivity
Child negative affectivity was assessed using the very short form of the Children’s Behavior Questionnaire (CBQ-VSF), a parent report measure of child temperament (Putnam & Rothbart 2006; Rothbart, Ahadi, Hershey, & Fisher, 2001). The very short form of the CBQ is a 36-item questionnaire, yielding three factor scores, of which Negative Affectivity was used in the current analyses. The 12-item Negative Affectivity scale reflects mood instability and tendency to experience dysregulated negative emotions. When completing the scale, mothers were asked the degree to which each statement (e.g., “gets quite frustrated when prevented from doing something s/he wants to do”) described their child’s behavior over the past six months on a 7-point Likert scale, ranging from 1 (extremely untrue of your child) to 7 (extremely true of your child) (see Table 2). An average rating was calculated for the negative affectivity scale. The CBQ-VSF has been shown to demonstrate adequate internal consistency and substantial inter-rater reliability (Putnam & Rothbart, 2006; Rothbart et al., 2001), and has been widely used in studies of child temperament and negative affectivity. Further, the CBQ-VSF takes advantage of mother’s ability to observe her child across a wide range of contexts and relies on maternal observation of concrete behaviors rather than abstract judgements of temperament to reduce the potential influence of maternal bias in reporting. Within the study sample, the CBQ-VSF demonstrated good internal consistency on the Negative Affectivity subscale (α = .84).
Statistical Analysis
Identification of Covariates
Spearman’s rank correlations (utilized to address skewness in tested covariates), t-tests, and ANOVAs were used to identify maternal (socioeconomic status composite score, age, cohabitation status, race/ethnicity, foreign-born, concurrent depressive symptoms, early life adversity, parity) and child factors (gestational age at birth, sex, age at early childhood assessment) that might influence child negative affectivity. Study site and time from preconception assessment to conception also were considered as potential covariates. Variables associated with child negative affectivity at p < 0.10 level of significance were included as a covariate in all subsequent regression analyses. Only maternal age and the socioeconomic status (SES) composite score met criterion for inclusion as a covariate (see Table 3).
Table 3.
Associations between participant characteristics and child negative affectivity
| Participant Characteristics | Test Statistic | p-value |
|---|---|---|
|
| ||
| Maternal socioeconomic status composite | −.17a^ | .06 |
| Maternal age | −.21b* | .02 |
| Cohabitation status | 1.73b | .32 |
| Maternal race/ethnicity | 1.46c | .24 |
| Foreign-born | 3.10b | .71 |
| Maternal early life adversity | .001 | .99 |
| Parity | .46c | .63 |
| Time from preconception assessment to conception | −.16a | .11 |
| Gestational age at birth | .11a | .25 |
| Child age | .14a | .17 |
| Child sex | .01b | .71 |
| Concurrent maternal depressive symptoms | .14a | .13 |
| Study site | .60c | .55 |
Note: Reported tests statistics are
Spearman’s rho
t, or
F.
Maternal childhood adversity was assessed via the Risk Families Questionnaire. Concurrent maternal depressive symptoms were assessed via the Center for Epidemiological Studies Depression Inventory (CES-D).
p < .1.
p < .05.
p < .01.
Bivariate Correlations
Preliminary analyses were conducted using bivariate Pearson correlation coefficients, assessing the relations between preconception PTSD symptoms, prenatal and postpartum depressive symptoms and perceived stress, and child negative affectivity. Pearson correlations were utilized because tested variables were normally distributed (i.e., Skewness > −2 and < 2; see Table 2; George & Mallery, 2010). Spearman’s rank correlations are also available in the Supplement (see Table S2).
Data Imputation
To address data missingness, missing preconception PTSD data was imputed. Eighty-seven women provided PTSD symptom ratings prior to conception (as well as ratings of child negative affectivity) and 31 participants completed a prenatal mood or stress measure and completed the child negative affectivity measure but were missing PTSD ratings prior to conception (26% rate of missingness). Multiple imputations of missing data were generated using MPlus (Muthén & Muthén, 1998–2017; Asparouhov & Muthén, 2010), generating 20 imputed data sets. When running subsequent analyses, parameter estimates were computed and averaged over the 20 sets. Sensitivity tests were performed to assess the influence of imputation on study findings by repeating all regression and mediational models analyses including only participants with complete data.
Testing of Study Aims
Preconception Maternal PTSD Symptoms and Child Negative Affectivity.
Linear regression was run in Mplus to test the primary hypothesis that preconception maternal symptoms of posttraumatic stress predict child negative affectivity after inclusion of identified covariates. Concurrent maternal depressive symptoms were then added as a covariate to evaluate whether any effect of preconception maternal PTSD symptoms on child negative affectivity remains after accounting for maternal mental health at the time of child assessment.
Assessment of Prenatal and Postpartum Depressive Symptoms and Perceived Stress as Mediating Pathways.
Finally, mediational pathways were added in exploratory analyses to examine whether prenatal and/or postpartum maternal depression symptoms may mediate the relation between maternal preconception PTSD symptoms and child negative affectivity. Prenatal and postpartum levels of perceived stress were also tested as mediators in secondary, exploratory analyses.
Results
Bivariate Correlations
Preliminary bivariate correlations between maternal preconception PTSD, maternal prenatal and postpartum depressive symptoms, and child negative affectivity are presented in Table 4. Maternal preconception PTSD symptoms were positively associated with child negative affectivity on the CBQ-VSF (r = .26, p = .014). PTSD symptoms also were associated with higher prenatal and postpartum depressive symptoms (r = .46, p < .001; and r = .26, p = .020 respectively) and prenatal and postpartum perceived stress (r = .59, p <.001; and r = .48, p <.001 respectively). Prenatal and postpartum depressive symptoms in turn were associated with higher child negative affectivity scores (r = .20, p = .043; and r = .20, p = .048 respectively). Postpartum but not prenatal levels of perceived stress positively correlated with elevated child negative affectivity (r = .21, p = .037; and r = .12, p = .210 respectively).
Table 4.
Pearson correlations between preconception PTSD symptoms, prenatal and postpartum depressive symptoms and perceived stress, and child negative affectivity.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
|
| ||||||
| 1. Preconception PTSD | -- | .46*** | .26* | .59*** | .48*** | .26* |
| 2. Prenatal CES-D | -- | -- | .40*** | .55*** | .31** | .20* |
| 3. Postpartum CES-D | -- | -- | -- | .39*** | .65*** | .20* |
| 4. Prenatal PSS | -- | -- | -- | -- | .44*** | .12 |
| 5. Postpartum PSS | -- | -- | -- | -- | -- | .21* |
| 6. CBQ Negative Affectivity | -- | -- | -- | -- | -- | -- |
Note.
p < .05.
p < .01.
p < .001.
Testing of Study Aims
Preconception Maternal PTSD Symptoms and Child Negative Affectivity
Regression analyses testing the primary hypothesis revealed that greater maternal symptoms of PTSD measured prior to conception predicted heightened child negative affectivity in early childhood (β = 0.20, SE = 0.09, p = .022) even after inclusion of sociodemographic covariates (R2 = .093; see Table 5, Model 1). Further, this association remained after covarying concurrent symptoms of maternal depression (β = 0.19, SE = 0.10, p = .045; R2 = .096; R2 change = .003 see Table 5, Model 2). Sensitivity analyses were performed including only participants with complete data, and as shown in Supplemental Table S3, the effect size and significance of this finding remained.
Table 5.
Regression models of preconception maternal PTSD symptoms and child negative affectivity.
| Model | Predictors | β | SE | p |
|---|---|---|---|---|
|
| ||||
| Model 1: Regression model with identified covariates | ||||
|
| ||||
| Preconception PTSD | .201 | .087 | .022* | |
| SES | −.083 | .095 | .383 | |
| Maternal age | −.157 | .096 | .100 | |
|
| ||||
| Model 2: Regression model with identified covariates and concurrent maternal depressive symptoms | ||||
|
| ||||
| Preconception PTSD | .187 | .094 | .045* | |
| SES | −.081 | .095 | .395 | |
| Maternal age | −.153 | .097 | .113 | |
| Concurrent maternal depressive symptoms | .050 | .087 | .566 | |
Note. Missing preconception PTSD data was imputed (n = 118). SES = socioeconomic status (composite of maternal education and per capita household income adjusted for cost of living).
p < .05.
Assessment of Prenatal and Postpartum Depressive Symptoms and Perceived Stress as Mediating Pathways
Exploratory, post-hoc analyses evaluated maternal prenatal and postpartum depressive symptoms as possible mediational pathways. This mediational model is summarized in Supplementary Figure S1. The total effect of the model was significant (β = 0.21, SE = 0.09, p = .022), however prenatal and postpartum depressive symptoms were not significant mediators (total indirect effect: β = 0.04, SE = 0.04, p = .289; specific indirect effect of prenatal depressive symptoms: β = 0.02, SE = 0.03, p = .511; specific indirect effect of postpartum depressive symptoms: β = 0.02, SE = 0.03, p = .506). Next, prenatal and postpartum levels of perceived stress were considered as mediational pathways. This mediational model is summarized in Supplementary Figure S2. The total effect of the model was significant (β = 0.20, SE = 0.09, p = .022) although neither prenatal nor postpartum levels of perceived mediated the relation between preconception PTSD symptoms and child negative affectivity (total indirect effect: β = 0.04, SE = 0.06, p = .448; specific indirect effect of prenatal levels of perceived stress: β = −0.02, SE = 0.04, p = .653; specific indirect effect of postpartum levels of perceived stress: β = 0.06, SE = 0.05, p = .250).
Discussion
The current study advances our understanding of the intergenerational impact of PTSD by prospectively evaluating maternal posttraumatic stress symptoms prior to conception and negative affectivity in the next generation. Using this prospective, longitudinal approach within a socioeconomically diverse sample of women and children, we found that more severe maternal symptoms of PTSD prior to conception predicted higher negative affectivity in their children during early childhood. Notably, the relation between maternal preconception PTSD symptoms and child negative affectivity persisted when covarying socioeconomic status and concurrent maternal depressive symptoms, suggesting that this association is likely not fully accounted for by postnatal shared environmental circumstances or current maternal mental health. Further, maternal early life adversity was not associated with child negative affectivity and thus did not meet covariate criteria, suggesting that maternal mental health symptoms prior to conception may be a more robust predictor of child development than history of adverse experiences. Collectively, these results build upon our understanding of the enduring effects of PTSD on development in the next generation, suggesting that elevated PTSD symptoms prior to conception is an indicator of risk for heightened negative affectivity in the offspring. Findings also underscore the crucial importance of considering the mental health of women prior to pregnancy in the intergenerational sequalae of trauma.
The current study directly builds upon the retrospective and epidemiological literature, linking maternal recall of preconception traumatic events to poor emotional outcomes in children (Briggs et al., 2014; Bosquet Enlow, Englund, & Egeland, 2018; McDonnel & Valentino, 2016) and adds to a very small number of prospective studies exploring the impact of preconception stress on emotional outcomes for the next generation (Hipwell et al., 2019; Spry et al., 2020). Current findings extend the work of Hipwell and colleagues (2019), who reported that in a prospective cohort, children of women exposed to traumatic events in their own childhoods were more likely to exhibit emotion dysregulation in infancy. With our direct evaluation of PTSD symptoms (rather than exposure to traumatic events), we provide novel evidence of an intergenerational association between posttraumatic stress symptoms and child negative affectivity outcomes. This finding provides evidence that PTSD symptoms are likely part of a pathway by which maternal trauma history may have a dysregulating impact on child negative affectivity; and that PTSD symptoms measured prior to conception may be a useful indicator of intergenerational risk.
Potential Mediating Pathways Underlying the Intergenerational Impact of Trauma
Preconception PTSD symptoms were positively associated with symptoms of depression during pregnancy. These findings align with retrospective studies linking maternal recall of trauma history to her mental health during pregnancy (Alvarez-Sengura et al., 2014; Atzl et al., 2019; River et al., 2019). Further, prenatal maternal depressive symptoms were correlated with child negative affectivity, consistent with a vast fetal programming literature prospectively documenting the influence of prenatal depression on the processes of fetal development and subsequent child socioemotional outcomes (Blair et al., 2011; Davis et al., 2007; Erickson et al., 2017; Glynn et al., 2018; Letourneau et al., 2019; Werner et al., 2007). We did not find evidence that maternal depressive symptoms or perceived stress during pregnancy or the postpartum period mediated the relation between preconception PTSD and child emotional outcomes, nor did the direct effect of prenatal depressive symptoms on child negative affectivity reach statistical significance in this mediational model. It is plausible that the current study was underpowered to detect the role of perinatal depression symptoms or perceived stress in mediating this relation. Given the relatively small sample size, mediational analyses should be interpreted with caution. It is also possible that other candidate pathways may be involved in this intergenerational association. For example, other domains of maternal mental health may be more directly implicated in the intergenerational impact of PTSD, including persistent perinatal PTSD symptoms. Although depression and PTSD are often comorbid (Shalev et al., 1998), the unique features of PTSD symptomatology such as heightened vigilance may have a more potent impact on fetal development. Multiple perinatal biological mechanisms, including stress physiology (e.g., perinatal maternal and placental HPA axis activity), epigenetic mechanisms (beginning even prior to conception), immune functioning and gut microbiota, have also been implicated in models of intergenerational stress (Bowers & Yehuda, 2016; Bouvette-Turcot et al., 2020; Bowers & Yehuda, 2016; Buss et al., 2017; Davis & Narayan, 2020; Keenan et al., 2018; Scorza et al., 2019, Swales et al., 2018; Yehuda & Meaney, 2018). Additionally, maternal parenting (such as maternal warmth and sensitivity) and the security of attachment relationships have been frequently implicated in the intergenerational sequela PTSD, as trauma history has been shown to be a risk factor for negative parent-child interactions (Cohen, Hien, & Batchelder, 2008; Savage et al., 2019). It is important that future studies continue to explore possible mediating pathways and broaden the scope of candidate mechanisms considered.
Strengths, Limitations, and Future Directions
The current study has several key strengths. Due to the challenges of recruiting and assessing women prior to pregnancy, few prospective studies of preconception mental health exist. Further, the multifaceted diversity of the study sample is a notable strength. The women in the study sample came from racial, ethnic, geographical, and socioeconomic backgrounds which are underrepresented in maternal and child health research (e.g., over a third of women identified as Latina or Hispanic and approximately a third of women identified as African American or Black) (Conradt, Carter, & Crowell, 2020). Another key advantage of the current study is the use of multiple assessment timepoints. Although PTSD symptoms were only measured once in the preconception period, maternal depressive symptoms and levels of perceived stress were evaluated twice during pregnancy, once postpartum, and once during the time of childhood assessment. This approach allowed for continued evaluation of maternal mental health across the peripartum period and into early childhood.
Despite these strengths, there also are also several limitations and key identified areas for future research. First, the sample includes only mothers who had at least one prior pregnancy at the time of recruitment. There was also a high rate of attrition amongst eligible participants (48%), reflecting the usual challenges of recruiting participants prior to conception and retaining them until 3 to 5 years postpartum. Participants included in the current analyses were more likely to be older, Latina, and born outside of the United States, and had a longer interpregnancy interval than those who were did not participate in the present study, and notably PTSD symptoms did not differ between the two groups. These demographic predictors of attrition should be considered in interpreting study findings. Additionally, maternal PTSD symptoms were only assessed at one time point and not during pregnancy or at any postnatal time points. Future studies should evaluate maternal PTSD symptoms over time. We also evaluated PTSD symptoms dimensionally within an at-risk community sample, with 21% of participants surpassing the clinical threshold for likely PTSD diagnosis. Future work would also benefit from exploring these associations within samples of participants recruited from clinical settings and including full diagnostic assessment of PTSD symptoms. Another limitation is that child negative affectivity was assessed via maternal report of child behavior. The likelihood of bias is reduced by the CBQ measure design which asks about behavior in concrete situations. Further, we covaried maternal depressive symptoms at the time of reporting to partially account for the influence of concurrent mental health on reporting biases; however, future studies should also seek to obtain ratings from other caregivers and through direct observations of child temperament. Finally, future studies should consider additional processes of risk and resilience that may impact the intergenerational transmission of maternal stress, such as cultural factors (considering the role of enculturation, acculturation, acculturative stress and discrimination, and community and identity based protective factors) as well as benevolent childhood experiences and other maternal characteristics which may buffer against the impact of preconception PTSD symptoms.
Conclusion
The current findings have important implications for understanding the intergenerational impact of PTSD and stress. This study supports the continued expansion of the developmental origins of health and disease model to consider how experiences prior to pregnancy may have lasting intergenerational consequences. Specifically, these findings carry important clinical significance, as heightened negative affectivity in early childhood is a key predictor of later psychopathology (Nigg, 2006), suggesting that elevated preconception PTSD symptoms may indicate risk for later mental health problems in the child. Findings underscore the critical importance of early identification of trauma and PTSD symptoms, and subsequent implementation of effective therapeutic interventions. Such screenings and therapeutic supports should be targeted not only during pregnancy and for new mothers, but also before women enter motherhood. This is important as targeted preconception interventions may confer a two-generational benefit, supporting the mental health of trauma survivors as well as disrupting intergenerational risk pathways to foster positive emotional development in children.
Supplementary Material
Funding Statement:
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD072021; Dunkel Schetter, PI) and earlier work by the Child Community Health Network (CCHN), supported through cooperative agreements with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; U HD44207, U HD44219, U HD44226, U HD44245, U HD44253, U HD54791, U HD54019, U HD44226-05S1, U HD44245-06S1, R03 HD59584) and the National Institute for Nursing Research (U NR008929). Author N.E.M. received support from the National Institute of Mental Health (T32 MH015750).
Footnotes
Conflicts of Interest: None
References
- Alvarez-Segura M, Garcia-Esteve L, Torres A, Plaza A, Imaz ML, Hermida-Barros L, … & Burtchen N (2014). Are women with a history of abuse more vulnerable to perinatal depressive symptoms? A systematic review. Archives of Women’s Mental Health, 17(5), 343–357. doi: 10.1007/s00737-014-0440-9 [DOI] [PubMed] [Google Scholar]
- Atzl VM, Narayan AJ, Rivera LM, & Lieberman AF (2019). Adverse childhood experiences and prenatal mental health: Type of ACEs and age of maltreatment onset. Journal of Family Psychology, 33(3), 304–314. doi: 10.1080/10926771.2018.1524806 [DOI] [PubMed] [Google Scholar]
- Asparouhov T, & Muthén B (2010). Multiple imputation with Mplus. MPlus Web Notes. https://www.statmodel.com/download/Imputations7.pdf
- Baird J, Hill CM, Kendrick T, Inskip HM, & SWS Study Group. (2009). Infant sleep disturbance is associated with preconceptional psychological distress: Findings from the Southampton Women’s Survey. Sleep, 32(4), 566–568. doi: 10.5665/sleep/32.4.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, & Danese A (2019). Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry, 76, 584–93. doi: 10.1001/jamapsychiatry.2019.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL (2014). Lifetime stress experience: Transgenerational epigenetics and germ cell programming. Dialogues in Clinical Neuroscience, 16(3), 297–305. doi: 10.31887/dcns.2014.16.3/tbale [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, … Ruggiero J (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry, 151(8), 1132–1136. doi: 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- Bierer LM, Bader HN, Daskalakis NP, Lehrner AL, Makotkine I, Seckl JR, & Yehuda R (2014). Elevation of 11β-hydroxysteroid dehydrogenase type 2 activity in Holocaust survivor offspring: Evidence for an intergenerational effect of maternal trauma exposure. Psychoneuroendocrinology, 48, 1–10. doi: 10.1016/j.psyneuen.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl RV, Cuijpers P, & Smit F (2002). Psychiatric disorders in adult children of parents with a history of psychopathology. Social Psychiatry and Psychiatric Epidemiology, 37(1), 7–12. 10.1007/s127-002-8208-8 [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, & Forneris CA (1996). Psychometric properties of the PTSD Checklist (PCL). Behaviour Research and Therapy, 34(8), 669–673. doi: 10.1016/0005-7967(96)00033-2 [DOI] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, & Davis EP (2011). Prenatal maternal anxiety and early childhood temperament. Stress, 14(6), 644–651. doi: 10.3109/10253890.2011.594121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Poeschel J, Schindler J, Börner F, Shachar-Dadon A, Ferdman N, … Poeggel G (2016). Transgenerational sex-specific impact of preconception stress on the development of dendritic spines and dendritic length in the medial prefrontal cortex. Brain Structure and Function, 221(2), 855–863. doi: 10.1007/s00429-014-0940-4 [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, Devick KL, Brunst KJ, Lipton LR, Coull BA, & Wright RJ (2017). Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy, 22(4), 492–513. doi: 10.1111/infa.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Englund MM, & Egeland B (2018). Maternal childhood maltreatment history and child mental health: Mechanisms in intergenerational effects. Journal of Clinical Child & Adolescent Psychology, 47(sup1), S47–S62. doi: 10.1080/15374416.2016.1144189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvette-Turcot AA, Fleming AS, Unternaehrer E, Gonzalez A, Atkinson L, Gaudreau H, … & Meaney MJ (2020). Maternal symptoms of depression and sensitivity mediate the relation between maternal history of early adversity and her child temperament: The inheritance of circumstance. Development and Psychopathology, 32(2), 605–613. doi: 10.1017/s0954579419000488 [DOI] [PubMed] [Google Scholar]
- Bowers ME, & Yehuda R (2016). Intergenerational transmission of stress in humans. Neuropsychopharmacology, 41(1), 232–244. doi: 10.1038/npp.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, & Andreski P (1995). Risk factors for PTSD-related traumatic events: A prospective analysis. The American Journal of Psychiatry, 152(4), 529–535. doi: 10.1176/ajp.152.4.529 [DOI] [PubMed] [Google Scholar]
- Breslau N (2009). The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, Violence, & Abuse, 10(3), 198–210. doi: 10.1177/1524838009334448 [DOI] [PubMed] [Google Scholar]
- Briggs RD, Silver EJ, Krug LM, Mason ZS, Schrag RD, Chinitz S, & Racine AD (2014). Healthy steps as a moderator: The impact of maternal trauma on child social-emotional development. Clinical Practice in Pediatric Psychology, 2(2), 166–175. doi: 10.1037/cpp0000060 [DOI] [Google Scholar]
- Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, … Wadhwa PD (2017). Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. Journal of the American Academy of Child & Adolescent Psychiatry, 56(5), 373–382. doi: 10.1016/j.jaac.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Carter SE, & Crowell SE (2020). Biological embedding of chronic stress across two generations within marginalized communities. Child Development Perspectives, 14(4), 208–214. doi: 10.1111/cdep.12382 [DOI] [Google Scholar]
- Clark LA, Watson D, & Mineka S (1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103(1), 103–116. doi: 10.1037/0021-843x.103.1.103 [DOI] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, … D’Onofrio BM (2014). Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychological Medicine, 44(1), 71–84. doi: 10.1017/s0033291713000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Mortensen PB, Henriksen TB, Dalman C, D’Onofrio BM, & Khashan AS (2015). Preconception maternal bereavement and infant and childhood mortality: a Danish population-based study. Psychosomatic Medicine, 77(8), 863–869. doi: 10.1097/psy.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, & Baum A (2006). Socioeconomic status is associated with stress hormones. Psychosomatic Medicine, 68(3), 414–420. doi: 10.1097/01.psy.0000221236.37158.b9 [DOI] [PubMed] [Google Scholar]
- Cohen LR, Hien DA, & Batchelder S (2008). The impact of cumulative maternal trauma and diagnosis on parenting behavior. Child Maltreatment, 13(1), 27–38. doi: 10.1177/1077559507310045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Corcoran K, & Fisher J (1987). Measures for clinical practice: A source book. New York: Free Press. [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46(6), 737–746. doi: 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, & Narayan AJ (2020). Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Development and Psychopathology, 32(5), 1625–1639. doi: 10.1017/s0954579420001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, & Rothbart MK (1997). Reactive and effortful processes in the organization of temperament. Development and Psychopathology, 9(4), 633–652. doi: 10.1017/s0954579497001375 [DOI] [PubMed] [Google Scholar]
- Doyle C, & Cicchetti D (2018). Future directions in prenatal stress research: Challenges and opportunities related to advancing our understanding of prenatal developmental origins of risk for psychopathology. Development and Psychopathology, 30(3), 721–724. doi: 10.1017/s095457941800069x [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Schafer P, Lanzi RG, Clark-Kauffman E, Raju TN, Hillemeier MM, & Community Child Health Network. (2013). Shedding light on the mechanisms underlying health disparities through community participatory methods: The stress pathway. Perspectives on Psychological Science, 8(6), 613–633. doi: 10.1177/1745691613506016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson NL, Gartstein MA, & Dotson JAW (2017). Review of prenatal maternal mental health and the development of infant temperament. Journal of Obstetric, Gynecologic & Neonatal Nursing, 46(4), 588–600. doi: 10.1016/j.jogn.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS (1998). Household dysfunction to many of the leading causes of death in adults the Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. doi: 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Flory JD, Bierer LM, & Yehuda R (2011). Maternal exposure to the holocaust and health complaints in offspring. Disease Markers, 30(2–3), 133–139. doi: 10.1155/2011/250470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangi S, Talamo A, & Ferracuti S (2009). The long-term effects of extreme war-related trauma on the second generation of Holocaust survivors. Violence and Victims, 24, 687–687–701. doi: 10.1891/0886-6708.24.5.687 [DOI] [PubMed] [Google Scholar]
- George D, & Mallery P (2010). SPSS for Windows step by step. A simple study guide and reference, 17.0 update (10a ed.). Pearson Education, Inc. [Google Scholar]
- Glynn LM, Howland MA, Sandman CA, Davis EP, Phelan M, Baram TZ, & Stern HS (2018). Prenatal maternal mood patterns predict child temperament and adolescent mental health. Journal of Affective Disorders, 228, 83–90. doi: 10.1016/j.jad.2017.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, & Gotlib IH (1999). Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review, 106(3), 458–490. doi: 10.1037/0033-295x.106.3.458 [DOI] [PubMed] [Google Scholar]
- Hardt J, & Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry, 45(2), 260–273. doi: 10.1111/j.1469-7610.2004.00218.x [DOI] [PubMed] [Google Scholar]
- Harker A, Raza S, Williamson K, Kolb B, & Gibb R (2015). Preconception paternal stress in rats alters dendritic morphology and connectivity in the brain of developing male and female offspring. Neuroscience, 303, 200–210. doi: 10.1016/j.neuroscience.2015.06.058 [DOI] [PubMed] [Google Scholar]
- Harville EW, Boynton-Jarrett R, Power C, & Hyppönen E (2010). Childhood hardship, maternal smoking, and birth outcomes: a prospective cohort study. Archives of Pediatrics & Adolescent Medicine, 164(6), 533–539. doi: 10.1001/archpediatrics.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Tung I, Northrup J, & Keenan K (2019). Transgenerational associations between maternal childhood stress exposure and profiles of infant emotional reactivity. Development and Psychopathology, 31(3), 887–898. doi: 10.1017/s0954579419000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Shi X, Xu H, Yang H, Chen T, Chen S, & Chen X (2010). Chronic unpredictable stress before pregnancy reduce the expression of brain-derived neurotrophic factor and N-methyl-D-aspartate receptor in hippocampus of offspring rats associated with impairment of memory. Neurochemical Research, 35(7), 1038–1049. doi: 10.1007/s11064-010-0152-0 [DOI] [PubMed] [Google Scholar]
- Huth-Bocks AC, Krause K, Ahlfs-Dunn S, Gallagher E, & Scott S (2013). Relational trauma and posttraumatic stress symptoms among pregnant women. Psychodynamic Psychiatry, 41(2), 277–301. doi: 10.1521/pdps.2013.41.2.277 [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE, Class QA, & Mbayiwa K (2018). Extending the developmental origins of disease model: Impact of preconception stress exposure on offspring neurodevelopment. Developmental Psychobiology, 60(7), 753–764. doi: 10.1002/dev.21773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, & Renk K (2019). Mothers’ adverse childhood experiences, depressive symptoms, parenting, and attachment as predictors of young children’s problems. Journal of Child Custody, 16(3), 268–290. doi: 10.1080/15379418.2019.1575318 [DOI] [Google Scholar]
- Klengel T, Dias BG, & Ressler KJ (2016). Models of intergenerational and transgenerational transmission of risk for psychopathology in mice. Neuropsychopharmacology, 41(1), 219–231. doi: 10.1038/npp.2015.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JE, Holzer J, & Hasbun A (2014). Association between parents’ PTSD severity and children’s psychological distress: A meta-analysis. Journal of Traumatic Stress, 27(1), 9–17. doi: 10.1002/jts.21891 [DOI] [PubMed] [Google Scholar]
- Lang AJ, Gartstein MA, Rodgers CS, & Lebeck MM (2010). The impact of maternal childhood abuse on parenting and infant temperament. Journal of Child and Adolescent Psychiatric Nursing, 23(2), 100–110. 10.1111/j.1744-6171.2010.0022 [DOI] [PubMed] [Google Scholar]
- Letourneau N, Dewey D, Kaplan BJ, Ntanda H, Novick J, Thomas JC, … & APrON Study Team. (2019). Intergenerational transmission of adverse childhood experiences via maternal depression and anxiety and moderation by child sex. Journal of Developmental Origins of Health and Disease, 10(1), 88–99. doi: 10.1017/s2040174418000648 [DOI] [PubMed] [Google Scholar]
- Li H, Zhang L, Fang Z, Lin L, Wu C, & Huang Q (2010). Behavioral and neurobiological studies on the male progeny of maternal rats exposed to chronic unpredictable stress before pregnancy. Neuroscience Letters, 469(2), 278–282. doi: 10.1016/j.neulet.2009.12.017 [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Vasey MW, Phillips BM, & Hazen RA (2004). Temperament, anxiety, and the processing of threat-relevant stimuli. Journal of Clinical Child and Adolescent Psychology, 33(1), 8–20. doi: 10.1207/s15374424jccp3301_2 [DOI] [PubMed] [Google Scholar]
- Mahrer NE, Guardino CM, Hobel C, & Dunkel Schetter C (2020). Maternal stress before conception is associated with shorter gestation. Annals of Behavioral Medicine, 55(3), 242–252. doi: 10.1093/abm/kaaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SD, & Calhoun PS (2010). The diagnostic accuracy of the PTSD checklist: A critical review. Clinical Psychology Review, 30(8), 976–987. doi: 10.1016/j.cpr.2010.06.012 [DOI] [PubMed] [Google Scholar]
- McDonnell CG, & Valentino K (2016). Intergenerational effects of childhood trauma: evaluating pathways among maternal ACEs, perinatal depressive symptoms, and infant outcomes. Child Maltreatment, 21(4), 317–326. doi: 10.1177/1077559516659556 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, & Lambert HK (2017). Child trauma exposure and psychopathology: Mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. doi: 10.1016/j.copsyc.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, & Poulton R (2010). How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine, 40(6), 899–909. doi: 10.1017/s0033291709991036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosack V, & Shore ER (2006). Screening for depression among pregnant and postpartum women. Journal of Community Health Nursing, 23(1), 37–47. doi: 10.1207/s15327655jchn2301_4 [DOI] [PubMed] [Google Scholar]
- Muris P, & Ollendick TH (2005). The role of temperament in the etiology of child psychopathology. Clinical Child and Family Psychology Review, 8(4), 271–289. doi: 10.1007/s10567-005-8809-y [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus User’s Guide. Eighth Edition. Muthén & Muthén. [Google Scholar]
- Narayan A, Lieberman AF, Masten AS (2021). Intergenerational transmission and prevention of adverse childhood experiences (ACEs). Clinical Psychology Review, 101997. doi: 10.1016/j.cpr.2021.101997 [DOI] [PubMed] [Google Scholar]
- Nast I, Bolten M, Meinlschmidt G, & Hellhammer DH (2013). How to measure prenatal stress? A systematic review of psychometric instruments to assess psychosocial stress during pregnancy. Paediatric and Perinatal Epidemiology, 27(4), 313–322. doi: 10.1111/ppe.12051 [DOI] [PubMed] [Google Scholar]
- National Comorbidity Survey (NCS). (2017). Retrieved from https://www.hcp.med.harvard.edu/ncs/index.php. Data Table 2: 12-month prevalence DSM-IV/WMH-CIDI disorders by sex and cohort.
- Newbury JB, Arseneault L, Moffitt TE, Caspi A, Danese A, Baldwin JR, & Fisher HL (2018). Measuring childhood maltreatment to predict early-adult psychopathology: Comparison of prospective informant-reports and retrospective self-reports. Journal of Psychiatric Research, 96, 57–64. doi: 10.1016/j.jpsychires.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47(3–4), 395–422. doi: 10.1111/j.1469-7610.2006.01612.x [DOI] [PubMed] [Google Scholar]
- O’Campo P, Schetter CD, Guardino CM, Vance MR, Hobel CJ, Ramey SL, … & Network CCH (2016). Explaining racial and ethnic inequalities in postpartum allostatic load: Results from a multisite study of low to middle income woment. SSM-Population Health, 2, 850–858. doi: 10.1016/j.ssmph.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell ML, Creamer M, & Pattison P (2004). Posttraumatic stress disorder and depression following trauma: Understanding comorbidity. American Journal of Psychiatry, 161(8), 1390–1396. 10.1176/appi.ajp.161.8.1390 [DOI] [PubMed] [Google Scholar]
- Pole N, Gone JP, & Kulkarni M (2008). Posttraumatic stress disorder among ethnoracial minorities in the United States. Clinical Psychology: Science and Practice, 15(1), 35–61. doi: 10.1111/j.1468-2850.2008.00109.x [DOI] [Google Scholar]
- Perroud N, Rutembesa E, Paoloni-Giacobino A, Mutabaruka J, Mutesa L, Stenz L, … Karege F (2014). The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. The World Journal of Biological Psychiatry, 15(4), 334–345. doi: 10.3109/15622975.2013.866693 [DOI] [PubMed] [Google Scholar]
- Plant DT, Jones FW, Pariante CM, & Pawlby S (2017). Association between maternal childhood trauma and offspring childhood psychopathology: Mediation analysis from the ALSPAC cohort. The British Journal of Psychiatry, 211(3), 144–150. doi: 10.1192/bjp.bp.117.198721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant DT, Pawlby S, Pariante CM, & Jones FW (2018). When one childhood meets another–maternal childhood trauma and offspring child psychopathology: A systematic review. Clinical Child Psychology and Psychiatry, 23(3), 483–500. doi: 10.1177/1359104517742186 [DOI] [PubMed] [Google Scholar]
- Power C, Atherton K, Strachan DP, Shepherd P, Fuller E, Davis A, … Macfarlane GJ (2007). Life-course influences on health in British adults: effects of socio-economic position in childhood and adulthood. International Journal of Epidemiology, 36(3), 532–539. doi: 10.1093/ije/dyl310 [DOI] [PubMed] [Google Scholar]
- Putnam SP, & Rothbart MK (2006). Development of short and very short forms of the Children’s Behavior Questionnaire. Journal of Personality Assessment, 87(1), 102–112. doi: 10.1207/s15327752jpa8701_09 [DOI] [PubMed] [Google Scholar]
- Ramey SL, Schafer P, DeClerque JL, Lanzi RG, Hobel C, Shalowitz M, … & Raju TN (2015). The preconception stress and resiliency pathways model: A multi-level framework on maternal, paternal, and child health disparities derived by community-based participatory research. Maternal and Child Health Journal, 19(4), 707–719. doi: 10.1007/s10995-014-1581-1 [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, & Best CL (1993). Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology, 61(6), 984–991. doi: 10.1037/0022-006x.61.6.984 [DOI] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, … Danese A (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57(10), 1103–1112. doi: 10.1111/jcpp.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- River LM, Narayan AJ, Atzl VM, Rivera LM, & Lieberman AF (2019). Past made present: The legacy of childhood maltreatment for romantic relationship quality and psychopathology during pregnancy. Psychology of Violence, 10(3), 324–333. doi: 10.1037/vio0000273 [DOI] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, & Fisher P (2001). Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Development, 72(5), 1394–1408. doi: 10.1111/1467-8624.00355 [DOI] [PubMed] [Google Scholar]
- Ruggiero KJ, Del Ben K, Scotti JR, & Rabalais AE (2003). Psychometric properties of the PTSD Checklist—Civilian version. Journal of Traumatic Stress, 16(5), 495–502. doi: 10.1023/a:1025714729117 [DOI] [PubMed] [Google Scholar]
- Santor DA, & Coyne JC (1997). Shortening the CES–D to improve its ability to detect cases of depression. Psychological Assessment, 9(3), 233–243. doi: 10.1037/1040-3590.9.3.233 [DOI] [Google Scholar]
- Savage LÉ, Tarabulsy GM, Pearson J, Collin-Vézina D, & Gagné LM (2019). Maternal history of childhood maltreatment and later parenting behavior: A meta-analysis. Development and Psychopathology, 31(1), 9–21. doi: 10.1017/s0954579418001542 [DOI] [PubMed] [Google Scholar]
- Scorza P, Duarte CS, Hipwell AE, Posner J, Ortin A, Canino G, … & Program Collaborators for Environmental influences on Child Health Outcomes. (2019). Research review: Intergenerational transmission of disadvantage: epigenetics and parents’ childhoods as the first exposure. Journal of Child Psychology and Psychiatry, 60(2), 119–132. doi: 10.1111/jcpp.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Low LMK, Sperlich M, Ronis DL, & Liberzon I (2009). Prevalence, trauma history, and risk for posttraumatic stress disorder among nulliparous women in maternity care. Obstetrics and Gynecology, 114(4), 839–847. doi: 10.1097/aog.0b013e3181b8f8a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, & Pitman RK (1998). Prospective study of posttraumatic stress disorder and depression following trauma. American Journal of Psychiatry, 155(5), 630–637. doi: 10.1176/ajp.155.5.630 [DOI] [PubMed] [Google Scholar]
- Solomon Z, Kotler M, & Mikulincer M (1988). Combat-related posttraumatic stress disorder among second-generation Holocaust survivors: preliminary findings. The American Journal of Psychiatry, 145(7), 865–868. doi: 10.1176/ajp.145.7.865 [DOI] [PubMed] [Google Scholar]
- Spry E, Moreno-Betancur M, Becker D, Romaniuk H, Carlin JB, Molyneaux E, … & Macdonald JA (2020). Maternal mental health and infant emotional reactivity: A 20-year two-cohort study of preconception and perinatal exposures. Psychological Medicine, 50(5), 827–837. doi: 10.1017/s0033291719000709 [DOI] [PubMed] [Google Scholar]
- Swales DA, Snyder HR, Hankin BL, Sandman CA, Glynn LM, & Davis EP (2020). Maternal depressive symptoms predict general liability in child psychopathology. Journal of Clinical Child & Adolescent Psychology, 1–12. doi: 10.1080/15374416.2020.1723598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales DA, Stout-Oswald SA, Glynn LM, Sandman C, Wing DA, & Davis EP (2018). Exposure to traumatic events in childhood predicts cortisol production among high risk pregnant women. Biological Psychology, 139, 186–192. doi: 10.1016/j.biopsycho.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, & Seeman TE (2004). Early environment, emotions, responses to stress, and health. Journal of Personality, 72(6), 1365–1394. doi: 10.1111/j.1467-6494.2004.00300.x [DOI] [PubMed] [Google Scholar]
- Thomas JL, Cleveland S, Pietrzak RH, Dunkel Schetter C, & Sumner JA (2021). Elucidating posttraumatic stress symptom dimensions and health correlates among postpartum women. Journal of Affective Disorders, 294, 314–321. doi: 10.1016/j.jad.2021.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Carter SE, Dunkel Schetter C, & Sumner JA (2021). Racial and ethnic disparities in posttraumatic psychopathology among postpartum women. Journal of Psychiatric Research, 137, 36–40. doi: 10.1016/j.jpsychires.2021.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey D, Siddaway AP, Meiser-Stedman R, Serpell L, & Field AP (2012). A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clinical Psychology Review, 32(2), 122–138. doi: 10.1016/j.cpr.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Walker EA, Newman E, Dobie DJ, Ciechanowski P, & Katon W (2002). Validation of the PTSD checklist in an HMO sample of women. General Hospital Psychiatry, 24(6), 375–380. 10.1016/s0163-8343(02)00203-7 [DOI] [PubMed] [Google Scholar]
- Werner EA, Myers MM, Fifer WP, Cheng B, Fang Y, Allen R, & Monk C (2007). Prenatal predictors of infant temperament. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 49(5), 474–484. doi: 10.1002/dev.20232 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bell A, Bierer LM, & Schmeidler J (2008). Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of Psychiatric Research, 42(13), 1104–1111. doi: 10.1016/j.jpsychires.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, & Grossman R (2001). Childhood trauma and risk for PTSD: Relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development and Psychopathology, 13(3), 733–753. doi: 10.1017/s0954579401003170 [DOI] [PubMed] [Google Scholar]
- Yehuda R, & Meaney MJ (2018). Relevance of psychological symptoms in pregnancy to intergenerational effects of preconception trauma. Biological Psychiatry, 83(2), 94–96. doi: 10.1016/j.biopsych.2017.10.027 [DOI] [PubMed] [Google Scholar]
- Zaidan H, Leshem M, & Gaisler-Salomon I (2013). Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biological Psychiatry, 74(9), 680–687. doi: 10.1016/j.biopsych.2013.04.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.