Abstract
Hyaluronan (HA) is a glycosaminoglycan that consists of single‐chain polymers of disaccharide units of glucuronic acid and N‐acetylglucosamine. It is a chief constituent of the extracellular matrix. About 27% of the total HA in the body is expressed in the skeleton and connective tissue, while 8% is expressed in muscles. In physiological conditions, HA functions as a lubricant and viscoelastic shock absorber. Additionally, HA is part of complex cellular signaling which modulates nociception and inflammation. This study aims to understand the role that HA plays in the musculoskeletal system, specifically in muscles and the surrounding fascia. This review is also intended to further understand HA homeostasis and the process of its synthesis, degradation, and clearance from the local tissue. The authors examined muscle pain and stiffness as pathological conditions associated with HA accumulation.
INTRODUCTION
Hyaluronan (HA) is a glycosaminoglycan (GAG) consisting of single‐chain polymers of disaccharide units of glucuronic acid and N‐acetylglucosamine. It is expressed by most cells in the body and is a chief constituent of the extracellular matrix (ECM) in all tissues. 1 The structure of HA is unique compared to other GAGs in the ECM due to its long unbranched chains and absence of sulfate groups. In physiologic quantities, HA functions as a lubricant and viscoelastic shock absorber. 2 Muscle tissue consists of 8% to 10% of the HA in the body; it is located in the endomysium, perimysium, and epimysium, and it plays an important role in lubrication and lateral force transmission during muscle contraction. 3 , 4 In addition, HA of high molecular mass can neutralize free radicals and reduce inflammation and pain. 5 HA homeostasis is of critical importance in its physiological properties. Reduced and excessive levels of HA can have profound effects on the musculoskeletal system and results in pathology. This review will specifically shed light on the evolving role of HA in pain and muscle stiffness, in the setting of disrupted HA homeostasis.
METHODS
In this narrative review, a literature search using Cochrane Library, PubMed, Google Scholar, and SCOPUS databases was performed. Keywords for the initial screen included hyaluronan, ischemic myalgia, myofascial pain, stiffness, spasticity, and hyaluronidase. The search filter was set to cover studies published between 1940 and 2021. The keywords were applied in both the titles and abstracts of the searched articles. The inclusion criteria for this review were articles with a combination of the keyword “hyaluronan” and any or all the following keywords: “ischemic myalgia,” “myofascial pain,” “stiffness,” “spasticity,” “hyaluronidase.” The exclusion criteria for this study were articles published in non‐English languages. Approximately 100 articles were reviewed by the authors, and 59 met the search criteria above and were included in this review.
HYALURONAN SYNTHESIS, DISTRIBUTION, AND HOMEOSTASIS
HA synthesis is carried out by three HA synthases isomers (HAS 1, 2, 3) in response to mechanical stimuli such as strain and movement 6 or chemical stimuli including inflammatory mediators and hyperglycemia. 7 HA synthases are located on the plasma membrane. 8 HA polymer size can range between 105 Dalton and 107 Dalton, with a half‐life ranging from 0.5 to 3 days. 2 Once HA is synthesized, it is either transported to the ECM or remains associated with the cell membrane via interactions with HA synthase and cell membrane receptors like CD44. 9
In mammals, 27% of the total HA is expressed in the skeleton and connective tissue, whereas 8% to 10% of it is expressed in muscles. 2 Morphological studies showed that HA is concentrated in the joint synovial fluid, skin, and muscle connective tissue, that is, the endomysium and perimysium surrounding the perivascular and perineural areas 10 and the fascia or loose connective tissue. 11 The distribution of HA can be explained from an evolutionary lens as mobile structures such as joints, skin, and muscle and sensitive neurovascular structures need an added layer of cushioning to protect them from external forces. 12 The average amount of HA at different anatomic sites varies according to the sliding properties of fascia required in the specific region. 13
HA degradation is carried by hyaluronidase (HYAL). There are two degrading enzymes, HYAL1 and HYAL2. HYAL1 is lysosomal enzyme that hydrolyzes small fragments of HA into monosaccharides. 14 HYAL 2 is a glycosylphosphatidylinositol‐anchored protein in the cell membrane that cleaves high‐molecular‐weight (HMW) HA into smaller fragments of molecular size of about 20 kDa. 15 After initial cleavage by HYAL2, the small HA fragments undergo receptor‐mediated endocytosis locally or after being transported by the lymphatics and bloodstream. 3 Once in the circulation, HA has a quick turnover with a half‐life of 2 to 5 minutes and a turnover rate of 15% to 35% per minute. 2 , 9 Studies have shown that only between 10% and 15% of HA is catabolized locally in the tissue per day, whereas the major part of HA is cleared by the lymph and blood circulation for subsequent degradation in the local lymph nodes and the liver. 16 The rate of clearance and elimination of HA from tissues into the circulation is dependent on lymphatic and blood flow; hence there is a positive correlation between general circulation flow and serum levels of HA. 17 , 18 It is estimated that about 25% of the total HA in tissues is unbound and constitutes a “free pool” that can be turned over at a quicker rate than the rest of the HA. 18 HA homeostasis could shift toward pathological excess in conditions where the general circulation of blood and lymph in tissues is impaired due to reduced clearance.
HYALURONAN SIGNALING AND MECHANICAL PROPERTIES
HA is involved in a complex intracellular signal transduction. 19 , 20 It interacts with multiple membrane receptors, including TRPV1 and CD44 receptors, which are associated with inflammation and nociception. 21 , 22 CD44 is the main receptor for HA, and the downstream effect of HA‐CD44 interaction can result in either modulation or stimulation of the expression of pro‐inflammatory cytokines and chemokines. 23 The properties of HA in regulating nociceptive signals and inflammation depend on its molecular size, as sufficient evidence supports that HMW HA is associated with anti‐inflammatory and pain‐modulating effects, whereas low‐molecular‐weight (LMW) HA is associated with pro‐inflammatory and nociceptive signal transduction. 19 , 24 , 25 HA has a nociceptive and pro‐inflammatory effect when its molecular mass ranges between that of a hexasaccharide and 500 kDa. 26 , 27 LMW HA injection induced hyperalgesia in rats similar to the hyperalgesia induced by injection of A6, a CD44 receptor agonist. However, with HMW HA injection, the hyperalgesic effect was attenuated, mimicking the effect of A5G27, a CD44 antagonist. 23 , 28 HMW HA also inhibits the inflammatory pain caused by carrageenan, an inflammatory agent acting on CD44. 28 LMW HA produced a significant increase in excitability of small‐diameter dorsal root ganglion (DRG) neurons in vitro by decreasing their action potential threshold, confirming that it has a direct pro‐nociceptive effect. HMW HA, however, did not produce a statistically significant decrease in the excitability of cultured DRG neurons. 23
The chemical structure of HA, in particular the presence of −OH groups, makes it highly hydrophilic. Under physiological conditions, HA assumes an expanded random coil structure surrounded by water molecules, which occupies a very large volume. 29 Most of the volume of HMW HA is water, which contributes to the effective size of each HA molecule because of its frictional interaction with closely spaced polymer segments. The time‐average shape of HA can be described as a sphere, with greatest density of chain segments near the center. As the molecular weight of HA increases, the volume of the HA chain increases exponentially due to binding to more water molecules. This also leads to an exponential increase in HA viscosity due to the macromolecular crowding of the polymer. 30 The viscosity of HA solutions affects the lubrication of tissues. For example, at the cartilage‐cartilage interface, the relative effectiveness of friction reduction (especially static friction or the resistance to start up motion) is dependent on the molecular weight of HA; the higher the molecular weight, the lower the friction. 31 This is thought to be due to a “viscous boundary layer” of HA at the surface of cartilage that facilitates low velocity/high load movements. 32 In contrast, for high velocity/low load movements, such as in muscle and fascia, the thickness of the HA‐containing boundary is large compared with the diameter of the molecules. 33 Here friction would increase with increased HA concentration and viscosity, negatively affecting lubrication. 30
HYALURONAN AND PAIN
Low HA concentrations in the joint space can result in pain. Physiologically occurring HA in synovial fluid is of high molecular mass. 5 In osteoarthritis (OA), both the molecular weight and concentration of HA is decreased compared to the synovium of a healthy joint. 3 , 34 In addition to the pro‐nociceptive and pro‐inflammatory environment created by the disproportionally elevated LMW HA, decreased HA concentration leads to loss of the joint's viscoelasticity. Subsequently, the cartilage will be exposed to inflammatory cytokines, which result in a greater rate of cartilage degradation. 35 There is an extensive body of research about the efficacy of hyaluronic acid injections, as they work to restore the physiological joint environment and provide pain relief and functional improvement. 36 , 37
In addition to its complex cellular signaling abilities, HA can have a direct mechanical effect on the sensitive neurovascular structures it surrounds. When HA levels are pathologically elevated, the excessive tissue swelling caused by HA increases the interstitial pressure compressing neurovascular structures. This compressive effect is well demonstrated by the correlation between HA content in solid tumors and their resistance to chemotherapy. The accumulation of HA in the interstitium collapses the tumor vasculature, which precludes therapeutic agents from reaching deep into the tumor microenvironment. 1
Another condition that highlights the possible ischemic effect of HA accumulation in muscles is eosinophilia‐myalgia syndrome (EMS), which was an epidemic disease in the United States in 1989, caused by the ingestion of supplemental l‐tryptophan. Clinical features of EMS included severe myalgia, joint and skin contracture, and multisystemic involvement. Chronic myalgia persisted in up to 91% of patients. 38 Although the pathogenesis of this disease is still poorly understood, cutaneous and muscle biopsies of patients with EMS showed accumulation of acid mucopolysaccharide deposits in the fascial and perimysial regions, specifically around the arterioles and small arteries, without evidence of arteritis. 39 , 40 Perineural fibrosis and loss of microvasculature were noted on histopathological studies of EMS patients. 39 Focal perifascicular atrophy was also reported in the progression of the disease. This finding is suggestive of ischemia. 41 Another histochemical study showed that the acid mucopolysaccharide deposits in EMS patients were almost exclusively made of HA. 42 Biopsies from EMS did not show myonecrosis or vessel destruction, and creatinine kinase levels were also normal. 39 , 41 Taken together, these findings suggest that the severe myalgia associated with EMS was not due to tissue necrosis but could potentially be caused by ischemia associated with the accumulation of HA.
HA accumulation has also been associated with myofascial pain (MFP). 43 The formation of the taut bands that constitute trigger points is attributed to an increased ECM viscosity from focal accumulation of HMW chains of HA (macromolecular crowding). 44 This can result in dysfunctional deep fascial planes gliding over each other and over muscle, which predicts the basis of MFP. 11 In a randomized, double‐blind study following 61 adults with MFP of trapezius muscles for 14 days post trigger point injection (TPI), it was found that adults who received TPI with hyaluronidase/lidocaine had better post TPI soreness than those who only had lidocaine TPI. 45 Another randomized single‐blind study of patients with trapezius MFP showed that hyaluronidase TPI was superior to bicarbonate and lidocaine in reducing visual analogue scale 4 weeks after the injections. 46
HYALURONAN AND MUSCLE STIFFNESS
As alluded to earlier, excessive accumulation of HA in the ECM of muscle can dramatically increase its viscosity and alter its lubricating properties. Viscosity of the ECM has not been traditionally considered to contribute to passive resistance in muscles. Muscle overactivity due to spasticity has been associated with hyper‐viscous ECM. The resulting increase of passive resistance to movement and reduction in force transmission can lead to muscle stiffness. 47 , 48 That also results in reversal of its lubricating function in the ECM and can result in stiffness. In a controlled clinical study, 3D‐T1P magnetic resonance imaging was used to compare HA quantity in muscles of five healthy participants to that of five post‐stroke patients with stiffness. It was found that HA concentration in patients with post‐stroke muscle stiffness is higher compared to controls, as shown in Figure 1. 49 , 50 Other small clinical trials showed that after treating patients with post‐stroke muscle stiffness with intramuscular hyaluronidase injection, there was a significant improvement in stiffness and increase in passive and active movement, as shown in Figure 2. 49 , 50 , 51 , 52 Joint contracture was a common finding in EMS. It persisted in the intermediate and chronic phases of the diseases. 53 It remains a possibility that HA accumulation plays a role in these findings (Figure 3).
FIGURE 1.
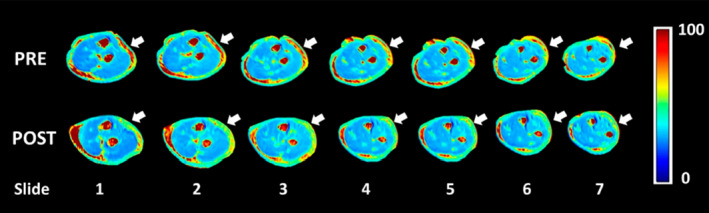
Quantification of glycosaminoglycan using T1rho (T1ρ) magnetic resonance imaging in myofascial pain. Note increased T1ρ relaxation times (red) in the extra‐muscular fascia in patients with lateral elbow pain (pre, arrows) and its reduction after fascial manipulation (post, arrows). Source: From Menon R (2020), with permission
FIGURE 2.
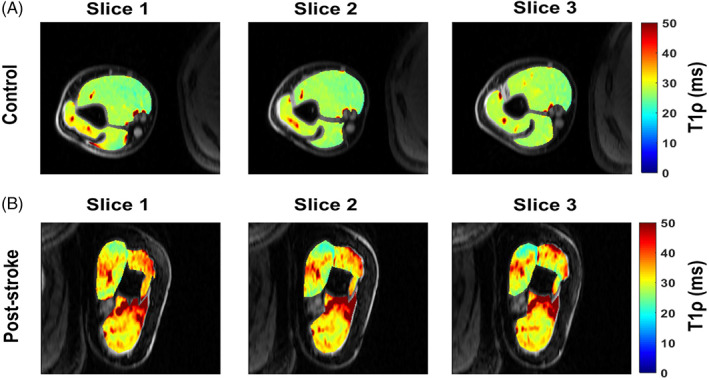
Quantification of glycosaminoglycan in muscle using T1rho (T1ρ) magnetic resonance imaging. Note increased intramuscular T1ρ relaxation times in patients with post‐stroke muscle stiffness compared to controls. Source: From Menon R (2019), with permission
FIGURE 3.
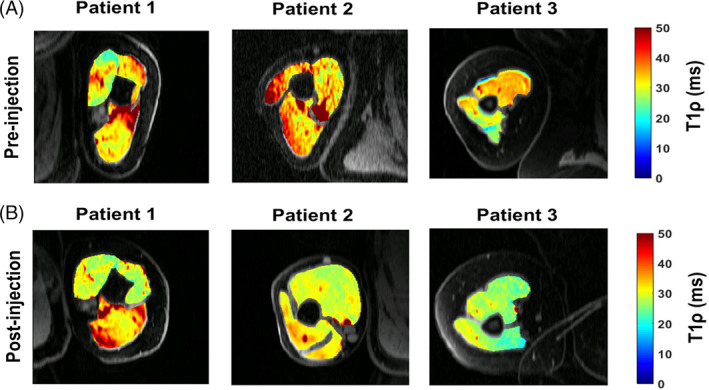
T1rho (T1ρ) magnetic resonance imaging maps before and after hyaluronidase injection treatment of three patients with post‐stroke muscle stiffness. Note the change in relaxation times and muscle shape. Source: From Menon R (2019) with permission
Serum HA is elevated in patients with inflammatory arthropathies such as rheumatoid arthritis, scleroderma, and psoriatic arthritis. 54 , 55 The synovial fluid of RA joints showed increased levels of HA but the ratio of LMW HA to that of HMW HA was increased. 56 Serum HA in patients with rheumatoid arthritis dramatically rises during the first hour that patients are out of bed in the morning. 57 It has been shown that morning stiffness in these patients results from HA accumulation in the joints and muscles overnight, resulting in stiffness, and that stiffness improves as HA is mechanically driven out into the circulation by physical activity. 58 Exercise in healthy individuals has been shown to increase serum HA significantly, which decreases rapidly to lower than resting levels by 30 minutes post exercise, 59 suggesting that it is plausible that the morning stiffness is due to overnight HA accumulation.
CONCLUSION
HA is an integral molecule of the ECM with complex signaling properties. Yet its mechanical effect on the musculoskeletal and neurovasculature can be as important clinically, especially when it accumulates in muscles, joints, and surrounding matrix. It is important to note that preclinical data indicate dual functions for HA depending on MW in relation to nociception 23 , 28 whereas clinical data suggest that overproduction of HA can result in muscle stiffness. 49 , 51 It will be important in the future to uncover the basis for these discrepancies by further assessing how varying levels of HA in the muscles differentially contribute to pain development or muscle stiffness at both the preclinical and clinical levels. Double‐blind controlled clinical trials are needed to investigate whether intramuscular hyaluronidase injections is an effective treatment to decrease symptoms of pain, stiffness, and spasticity in select patients with conditions resulting from accumulation of HA secondary to overproduction or under‐metabolization of HA.
DISCLOSURE
Drs Stecco and Raghavan are founders of Movease, Inc., and they report patents issued and filed for use of Hyaluronidase for Muscle Stiffness. Dr Stecco is the president of the Fascial Manipulation Association. Dr Jankowski reports the following grants paid to institution unrelated to the current work: NIH/NINDS: R01NS105715, NIH/NIAMS: R61AR078060
DoD/HJF: W81XWH‐20‐C‐0031
ACKNOWLEDGMENTS
This work was supported by grants to MPJ from the NIH/NINDS (R01NS113965 to MPJ) and the Sheikh Khalifa Stroke Institute (PR). Open access funding enabled and organized by Projekt DEAL.
Amir A, Kim S, Stecco A, Jankowski MP, Raghavan P. Hyaluronan homeostasis and its role in pain and muscle stiffness. PM&R. 2022;14(12):1490‐1496. doi: 10.1002/pmrj.12771
Funding information NIH/NINDS, Grant/Award Number: R01NS113965; Sheikh Khalifa Stroke Institute
REFERENCES
- 1. Infante JR, Korn RL, Rosen LS, et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br J Cancer. 2018;118(2):153‐161. doi: 10.1038/bjc.2017.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27‐33. doi: 10.1046/j.1365-2796.1997.00170.x [DOI] [PubMed] [Google Scholar]
- 3. Laurent TC, ed. The Chemistry, Biology, and Medical Applications of Hyaluronan and its Derivatives. London: Portland Press; 1998. [Google Scholar]
- 4. Purslow PP. Muscle fascia and force transmission. J Bodyw Mov Ther. 2010;14(4):411‐417. doi: 10.1016/j.jbmt.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 5. Salwowska NM, Bebenek KA, Żądło DA, Wcisło‐Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol. 2016;15(4):520‐526. doi: 10.1111/jocd.12237 [DOI] [PubMed] [Google Scholar]
- 6. Dowthwaite GP, Ward AC, Flannely J, et al. The effect of mechanical strain on hyaluronan metabolism in embryonic fibrocartilage cells. Matrix Biol. 1999;18(6):523‐532. doi: 10.1016/s0945-053x(99)00044-x [DOI] [PubMed] [Google Scholar]
- 7. Wang A, Hascall VC. Hyperglycemia, intracellular hyaluronan synthesis, cyclin D3 and autophagy. Autophagy. 2009;5(6):864‐865. doi: 10.4161/auto.9041 [DOI] [PubMed] [Google Scholar]
- 8. Wilkinson TS, Bressler SL, Evanko SP, Braun KR, Wight TN. Overexpression of hyaluronan synthases alters vascular smooth muscle cell phenotype and promotes monocyte adhesion. J Cell Physiol. 2006;206(2):378‐385. doi: 10.1002/jcp.20468 [DOI] [PubMed] [Google Scholar]
- 9. Manou D, Caon I, Bouris P, et al. The complex interplay between extracellular matrix and cells in tissues. Methods Mol Biol. 2019;1952:1‐20. doi: 10.1007/978-1-4939-9133-4_1 [DOI] [PubMed] [Google Scholar]
- 10. Laurent C, Johnson‐Wells G, Hellström S, Engström‐Laurent A, Wells AF. Localization of hyaluronan in various muscular tissues. A morphological study in the rat. Cell Tissue Res. 1991;263(2):201‐205. doi: 10.1007/bf00318761 [DOI] [PubMed] [Google Scholar]
- 11. Stecco C, Stern R, Porzionato A, et al. Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat. 2011;33(10):891‐896. doi: 10.1007/s00276-011-0876-9 [DOI] [PubMed] [Google Scholar]
- 12. Hellström M, Engström‐Laurent A, Hellström S. Expression of the CD44 receptor in the blood vessel system: an experimental study in rat. Cells Tissues Organs. 2005;179(3):102‐108. doi: 10.1159/000085001 [DOI] [PubMed] [Google Scholar]
- 13. Fede C, Angelini A, Stern R, et al. Quantification of hyaluronan in human fasciae: variations with function and anatomical site. J Anat. 2018;233(4):552‐556. doi: 10.1111/joa.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jadin L, Bookbinder LH, Frost GI. A comprehensive model of hyaluronan turnover in the mouse. Matrix Biol. 2012;31(2):81‐89. doi: 10.1016/j.matbio.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 15. Heldin P, Basu K, Olofsson B, Porsch H, Kozlova I, Kahata K. Deregulation of hyaluronan synthesis, degradation and binding promotes breast cancer. J Biochem. 2013;154(5):395‐408. doi: 10.1093/jb/mvt085 [DOI] [PubMed] [Google Scholar]
- 16. Laurent UB, Dahl LB, Reed RK. Catabolism of hyaluronan in rabbit skin takes place locally, in lymph nodes and liver. Exp Physiol. 1991;76(5):695‐703. doi: 10.1113/expphysiol.1991.sp003536 [DOI] [PubMed] [Google Scholar]
- 17. Lebel L, Smith L, Risberg B, Gerdin B, Laurent TC. Effect of increased hydrostatic pressure on lymphatic elimination of hyaluronan from sheep lung. J Appl Physiol (1985). 1988;64(4):1327‐1332. doi: 10.1152/jappl.1988.64.4.1327 [DOI] [PubMed] [Google Scholar]
- 18. Reed RK, Laurent TC, Taylor AE. Hyaluronan in prenodal lymph from skin: changes with lymph flow. Am J Physiol. 1990;259(4 Pt 2):H1097‐H1100. doi: 10.1152/ajpheart.1990.259.4.H1097 [DOI] [PubMed] [Google Scholar]
- 19. Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21(1):25‐29. doi: 10.1016/s0945-053x(01)00184-6 [DOI] [PubMed] [Google Scholar]
- 20. Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589‐4592. doi: 10.1074/jbc.R100038200 [DOI] [PubMed] [Google Scholar]
- 21. Lesley J, Hyman R. CD44 structure and function. Front Biosci. 1998;3:d616‐d630. doi: 10.2741/a306 [DOI] [PubMed] [Google Scholar]
- 22. Caires R, Luis E, Taberner FJ, et al. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat Commun. 2015;6:8095. doi: 10.1038/ncomms9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrari LF, Khomula EV, Araldi D, Levine JD. CD44 signaling mediates high molecular weight hyaluronan‐induced Antihyperalgesia. J Neurosci. 2018;38(2):308‐321. doi: 10.1523/jneurosci.2695-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonet IJM, Araldi D, Khomula EV, Bogen O, Green PG, Levine JD. Mechanisms mediating high‐molecular‐weight hyaluronan‐induced Antihyperalgesia. J Neurosci. 2020;40(34):6477‐6488. doi: 10.1523/jneurosci.0166-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pahwa R, Nallasamy P, Jialal I. Toll‐like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J Diabetes Complications. 2016;30(4):563‐572. doi: 10.1016/j.jdiacomp.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 26. McKee CM, Penno MB, Cowman M, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98(10):2403‐2413. doi: 10.1172/jci119054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knudson W, Knudson CB. Assembly of a chondrocyte‐like pericellular matrix on non‐chondrogenic cells. Role of the cell surface hyaluronan receptors in the assembly of a pericellular matrix. J Cell Sci. 1991;99(Pt 2):227‐235. [DOI] [PubMed] [Google Scholar]
- 28. Ferrari LF, Araldi D, Bogen O, Levine JD. Extracellular matrix hyaluronan signals via its CD44 receptor in the increased responsiveness to mechanical stimulation. Neuroscience. 2016;324:390‐398. doi: 10.1016/j.neuroscience.2016.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cowman MK, Lee HG, Schwertfeger KL, McCarthy JB, Turley EA. The content and size of hyaluronan in biological fluids and tissues. Front Immunol. 2015;6:261. doi: 10.3389/fimmu.2015.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cowman MK, Schmidt TA, Raghavan P, Stecco A. Viscoelastic properties of hyaluronan in physiological conditions. F1000Res. 2015;4:622. doi: 10.12688/f1000research.6885.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwiecinski JJ, Dorosz SG, Ludwig TE, Abubacker S, Cowman MK, Schmidt TA. The effect of molecular weight on hyaluronan's cartilage boundary lubricating ability‐‐alone and in combination with proteoglycan 4. Osteoarthr Cartil. 2011;19(11):1356‐1362. doi: 10.1016/j.joca.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 32. Yakubov GE, McColl J, Bongaerts JH, Ramsden JJ. Viscous boundary lubrication of hydrophobic surfaces by mucin. Langmuir. 2009;25(4):2313‐2321. doi: 10.1021/la8018666 [DOI] [PubMed] [Google Scholar]
- 33. Stecco A, Gesi M, Stecco C, Stern R. Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep. 2013;17(8):352. doi: 10.1007/s11916-013-0352-9 [DOI] [PubMed] [Google Scholar]
- 34. Balazs EA, Denlinger JL. Sodium hyaluronate and joint function. J Equine Vet. 1985;5(4):217‐228. doi: 10.1016/S0737-0806(85)80102-7 [DOI] [Google Scholar]
- 35. Moreland LW. Intra‐articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5(2):54‐67. doi: 10.1186/ar623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bashaireh K, Naser Z, Hawadya KA, Sorour S, Al‐Khateeb RN. Efficacy and safety of cross‐linked hyaluronic acid single injection on osteoarthritis of the knee: a post‐marketing phase IV study. Drug des Devel Ther. 2015;9:2063‐2072. doi: 10.2147/dddt.s81524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miltner O, Schneider U, Siebert CH, Niedhart C, Niethard FU. Efficacy of intraarticular hyaluronic acid in patients with osteoarthritis‐‐a prospective clinical trial. Osteoarthr Cartil. 2002;10(9):680‐686. doi: 10.1053/joca.2002.0815 [DOI] [PubMed] [Google Scholar]
- 38. Sack KE, Criswell LA. Eosinophilia‐myalgia syndrome: the aftermath. South Med J. 1992;85(9):878‐882. doi: 10.1097/00007611-199209000-00005 [DOI] [PubMed] [Google Scholar]
- 39. Bulpitt KJ, Verity MA, Clements PJ, Paulus HE. Association of L‐tryptophan and an illness resembling eosinophilic fasciitis. Clinical and histopathologic findings in four patients with eosinophilia‐myalgia syndrome. Arthritis Rheum. 1990;33(7):918‐929. doi: 10.1002/art.1780330702 [DOI] [PubMed] [Google Scholar]
- 40. Verity MA, Bulpitt KJ, Paulus HE. Neuromuscular manifestations of L‐tryptophan‐associated eosinophilia‐myalgia syndrome: a histomorphologic analysis of 14 patients. Hum Pathol. 1991;22(1):3‐11. doi: 10.1016/0046-8177(91)90054-s [DOI] [PubMed] [Google Scholar]
- 41. Herrick MK, Chang Y, Horoupian DS, Lombard CM, Adornato BT. L‐tryptophan and the eosinophilia‐myalgia syndrome: pathologic findings in eight patients. Hum Pathol. 1991;22(1):12‐21. doi: 10.1016/0046-8177(91)90055-t [DOI] [PubMed] [Google Scholar]
- 42. Valicenti JM, Fleming MG, Pearson RW, Budz JP, Gendleman MD. Papular mucinosis in L‐tryptophan‐induced eosinophilia‐myalgia syndrome. J Am Acad Dermatol. 1991;25(1 Pt 1):54‐58. doi: 10.1016/0190-9622(91)70174-z [DOI] [PubMed] [Google Scholar]
- 43. Pratt RL. Hyaluronan and the fascial frontier. Int J Mol Sci. 2021;22(13). doi: 10.3390/ijms22136845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stecco A, Pirri C, Caro R, Raghavan P. Stiffness and echogenicity: development of a stiffness‐echogenicity matrix for clinical problem solving. Eur J Transl Myol. 2019;29(3):8476. doi: 10.4081/ejtm.2019.8476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi JW, Lee CJ, Lee SM, Shin BS, Jun B, Sim WS. Effect of hyaluronidase addition to lidocaine for trigger point injection in myofascial pain syndrome. Pain Pract. 2016;16(8):1019‐1026. doi: 10.1111/papr.12362 [DOI] [PubMed] [Google Scholar]
- 46. Ghasemi M, Mosaffa F, Hoseini B, Behnaz F. Comparison of the effect of bicarbonate, hyaluronidase, and lidocaine injection on myofascial pain syndrome. Anesth Pain Med. 2020;10(3):e101037. doi: 10.5812/aapm.101037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rasool G, Wang AB, Rymer WZ, Lee SSM. Shear waves reveal viscoelastic changes in skeletal muscles after hemispheric stroke. IEEE Trans Neural Syst Rehabil Eng. 2018;26(10):2006‐2014. doi: 10.1109/tnsre.2018.2870155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang AB, Perreault EJ, Royston TJ, Lee SSM. Changes in shear wave propagation within skeletal muscle during active and passive force generation. J Biomech. 2019;94:115‐122. doi: 10.1016/j.jbiomech.2019.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Menon RG, Raghavan P, Regatte RR. Quantifying muscle glycosaminoglycan levels in patients with post‐stroke muscle stiffness using T(1ρ) MRI. Sci Rep. 2019;9(1):14513. doi: 10.1038/s41598-019-50715-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Menon RG, Oswald SF, Raghavan P, Regatte RR, Stecco A. T1ρ‐mapping for musculoskeletal pain diagnosis: case series of variation of water bound glycosaminoglycans quantification before and after fascial manipulation® in subjects with elbow pain. Int J Environ Res Public Health. 2020;17(3):708. doi: 10.3390/ijerph17030708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raghavan P, Lu Y, Mirchandani M, Stecco A. Human recombinant hyaluronidase injections for upper limb muscle stiffness in individuals with cerebral injury: a case series. EBioMedicine. 2016;9:306‐313. doi: 10.1016/j.ebiom.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raghavan P. Emerging therapies for spastic movement disorders. Phys Med Rehabil Clin N Am. 2018;29(3):633‐644. doi: 10.1016/j.pmr.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kilbourne EM, Posada de la Paz M, Abaitua Borda I, Diez Ruiz‐Navarro M, Philen RM, Falk H. Toxic oil syndrome: a current clinical and epidemiologic summary, including comparisons with the eosinophilia‐myalgia syndrome. J Am Coll Cardiol. 1991;18(3):711‐717. doi: 10.1016/0735-1097(91)90794-a [DOI] [PubMed] [Google Scholar]
- 54. Engström‐Laurent A, Hällgren R. Circulating hyaluronic acid levels vary with physical activity in healthy subjects and in rheumatoid arthritis patients. Relationship to synovitis mass and morning stiffness. Arthritis Rheum. 1987;30(12):1333‐1338. doi: 10.1002/art.1780301203 [DOI] [PubMed] [Google Scholar]
- 55. Engström‐Laurent A. Changes in hyaluronan concentration in tissues and body fluids in disease states. Ciba Found Symp. 1989;143:233‐240; discussion 240‐7, 281‐5. doi: 10.1002/9780470513774.ch14 [DOI] [PubMed] [Google Scholar]
- 56. Ragan C, Meyer K. The hyaluronic acid of synovial fluid in rheumatoid arthritis. J Clin Invest. 1949;28(1):56‐59. doi: 10.1172/jci102053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paimela L, Heiskanen A, Kurki P, Helve T, Leirisalo‐Repo M. Serum hyaluronate level as a predictor of radiologic progression in early rheumatoid arthritis. Arthritis Rheum. 1991;34(7):815‐821. doi: 10.1002/art.1780340706 [DOI] [PubMed] [Google Scholar]
- 58. Laurent TC, Laurent UB, Fraser JR. Serum hyaluronan as a disease marker. Ann Med. 1996;28(3):241‐253. doi: 10.3109/07853899609033126 [DOI] [PubMed] [Google Scholar]
- 59. Piehl‐Aulin K, Laurent C, Engström‐Laurent A, Hellström S, Henriksson J. Hyaluronan in human skeletal muscle of lower extremity: concentration, distribution, and effect of exercise. J Appl Physiol (1985). 1991;71(6):2493‐2498. doi: 10.1152/jappl.1991.71.6.2493 [DOI] [PubMed] [Google Scholar]


