Abstract
Introduction:
Heart rate variability is a measure of autonomic activity which is growing in popularity as a research outcome. However, despite its increased use, the known effects of respiration on heart rate variability measures are rarely accounted for in rehabilitation medicine research, leading to potential misinterpretation.
Objective:
Describe the impact that unpaced and paced breathing introduces to heart rate variability measures in a rehabilitation medicine relevant example of individuals with spinal cord injury.
Design:
Cross sectional comparison of heart rate variability during unpaced and paced breathing (0.25Hz, 15 breaths per minute) within the same individuals during the same lab session.
Setting:
Academic autonomic physiology laboratory
Interventions:
Not applicable
Main Outcome Measures:
Mean low frequency (LF) and high frequency (HF) heart rate variability power, percentage of total power derived from the LF spectrum, LF:HF ratio.
Results:
Fifty-nine individuals with spinal cord injury completed laboratory assessments using standardized protocols (NCT02139436). In repeated measures within individuals, mean LF power was significantly higher in unpaced breathing compared to paced breathing (1292 vs. 573 ms2, p<0.001). A Bland-Altman plot demonstrated significant positive proportional bias for LF power when comparing unpaced and paced breathing conditions (R2=0.39). Mean HF power was similar between unpaced and paced breathing conditions, though demonstrated wide positive and negative differences between measures, leading to notable uncertainty when respiratory confounders were not accounted for. The percentage of total power derived from the LF spectrum and the mean LF:HF ratio were both significantly higher for unpaced breathing compared to paced breathing (64 vs. 42%, p<0.001; and 3.2 vs. 1.1, p<0.001, respectively).
Conclusion:
Respiration has a significant impact on heart rate variability following spinal cord injury, and not accounting for this has serious consequences for accurate interpretation of unpaced data. Future studies of heart rate variability in rehabilitation medicine should accordingly consider paced breathing.
Keywords: Neurophysiology, outcomes assessment/measurement, spinal cord injury
Introduction:
Heart rate variability is commonly seen as an appealing research tool to analyze autonomic function across a host of medical conditions pertinent to Physical Medicine & Rehabilitation. These measurements are derived from standard resting EKG recordings and have known clinical correlates; reduced variability is associated with increased risk of cardiac complications (1,2). Prior to the 1990s, manually extracting the necessary data and performing the associated complicated mathematical calculations prevented most researchers and clinicians from using heart rate variability methods (3,4). More recently, digitized data capture and programs for automated processing and analysis have greatly lowered barriers to use, with an explosion of literature using heart rate variability in rehabilitation medicine, from brain injury to distance runners (5–7). However, with this improved accessibility outside of dedicated autonomic physiology labs, tightly controlled approaches in recording methods are not always observed. This can lead to introduction of confounders, perhaps none as important as uncontrolled breathing. The resultant errors in heart rate variability estimates that stem from these confounders may distort our collective understanding of important clinical problems that our patients experience.
As a window into autonomic function, analysis of both low and high frequency heart rate variability (termed LF and HF) has been proposed by some to provide quantified metrics of sympathetic and parasympathetic activity. To obtain these measures, time intervals between detected R-waves from the EKG are quantified (8), and fluctuations in the time series are statistically transformed into frequencies using fast-Fourier transforms. These frequencies are grossly grouped into LF or HF bands. The magnitude of variability (power) in each frequency band is typically averaged to provide a proposed quantitative window into cardiac sympathetic (LF) and parasympathetic (HF) activity.
Physiologically, heart rate variability is well known to be impacted by changes with inspiration and expiration (9). Respiratory frequency and depth directly influence the magnitude of both variability (10,11) and vagal nerve outflow to the sinoatrial node (12). Due to this observed vagal influence, HF heart rate variability power has been thought to be representational of cardiovagal tone. These HF variations change in well-characterized patterns, likely representing phasic vagal modulation proportional to prevailing vagal outflow (13,14).
Given that low levels of HF heart rate variability power are associated with increased risk of cardiac death (2), disagreement among studies is clinically worrisome. For example, studies of individuals with spinal cord injury (SCI) have suggested that HF heart rate variability power is either the same (15–18) or significantly decreased (18–20) compared to uninjured controls. Insight to these disparate results may be found within the differing study methods. Often, inclusion criteria in these studies crosses multiple confounders and importantly, none of the studies accounted for the effects of respiration. Either characterizing spontaneous respiratory patterns or standardizing them with paced breathing, are especially important because respiratory patterns may change unpredictably throughout the recording session, shifting among frequencies and depths. The scope of this problem may be compounded in individuals with SCI, because altered respiratory mechanics from paralysis/spasticity can change respiratory characteristics (21). To highlight this issue in rehabilitation, we aimed to describe the impact of unpaced and paced breathing on heart rate variability in individuals with SCI. As a growing number of rehabilitation medicine publications utilize heart rate variability, our goal was to improve both the rigor of future Physiatric research and the critical reading skills of PM&R physicians while reviewing heart rate variability literature.
Methods:
Data were derived from a larger study (NCT02139436), assessing exercise benefits for individuals with SCI. Individuals with SCI were included in this analysis if they 1) had SCI with neurological levels of injury between C1 and T12 (22), confirmed by a trained physician; and 2) had recorded continual heart rates during both unpaced (spontaneous) and paced breathing during a single testing session. All individuals were at least three months post-SCI and had been discharged to the community following inpatient rehabilitation. All recordings were completed prior to the parent study’s longitudinal exercise intervention. To further minimize potential confounding effects, all individuals were free from clinically diagnosed cardiovascular disease, hypertension, or diabetes, and no participant was using any tobacco product or taking any cardioactive medication. Age was further restricted to individuals ≤ 40 years old, due to known declines in heart rate variability with age (10,23).
Prior to testing, participants were instructed to refrain from vigorous exercise, caffeine, or alcohol for 24 hours prior, since all influence heart rate variability and could act as potential confounders. Laboratory testing occurred between 8:00 and 10:00 AM to minimize known circadian variations in autonomic control and testing was completed following a 12-hour overnight fast (as oral intake is known to impact heart rate variability, 24). Participants were instrumented with a 5-lead EKG and nasal canula for respiratory monitoring. R-R interval was recorded from lead II of the EKG, with end tidal CO2 monitoring capturing respiratory frequency. Prior to recording, each participant rested in a semi-supine position for at least 15 minutes in a quiet room with the lights dimmed (25) to establish a steady baseline. Participants then had five minutes of quiet, resting heart rate recorded with unpaced, spontaneous breathing. Subsequently, in response to auditory cues for inspiration and expiration, participants had five minutes of data recorded while they paced their breathing at a frequency of 15 breaths per minute (0.25 Hz).
Data Analysis
Data were digitized and stored at a sampling rate of 1000 Hz (PowerLab, ADInstruments), with respiratory cycles analyzed during paced breathing to ensure adherence to the 0.25 Hz target. Beat-by-beat time series of the R-R intervals were extracted using custom peak detection algorithms (MATLAB, The MathWorks. Inc) with visual inspection for artifact or error. Time series were then interpolated to 5 Hz and linearly detrended for subsequent spectral analysis. Power spectral estimates were calculated using a Welch’s modified periodogram algorithm (26), wherein each 5-minute time series was divided into five equal windows, overlapping by 50% and smoothed via a Hanning window before being fast-Fourier transformed. To match commercially available heart rate variability programs, spectral densities were integrated over LF (0.04–0.15 Hz) and HF (0.15–0.40 Hz) ranges to provide mean powers. To further illustrate the relationship between respiration and heart rate variability, respiration time series were similarly fast-Fourier transformed and analyzed, with cross spectral relationships between R-R intervals and respiration calculated.
Statistical Analysis
Descriptive statistics were calculated for group level demographics (Table 1). To compare heart rate variability for unpaced and paced breathing recordings, LF and HF powers were log-transformed (to establish normal distribution) and these values were analyzed with paired t-tests. Non-log transformed power values are reported per convention. Total power was calculated over the full 0.04–0.40 Hz range for each condition. For further comparison, percentage of total power that was derived from the LF spectrum (non-log transformed) and LF:HF ratio were also calculated for both unpaced and paced breathing and similarly compared (27). To further look for potential bias that unpaced breathing may introduce (compared to paced breathing), Bland-Altman plots were created to compare LF and HF measures between breathing conditions (28). These standardized plots allow assessment of agreement between two measures (unpaced and paced breathing) across individuals by plotting the difference in measures vs the mean of measures (29). Per convention, least product linear regressions were calculated to assess potential bias between measurements, with an associated line fit to this plot. Any non-zero slope of this regression line indicates a proportional bias, wherein the measurements become increasingly (or decreasingly) inaccurate as the values increase. 95% confidence intervals of the slope were calculated to serve as ranges of this bias between heart rate variability measures under unpaced and paced breathing conditions. Percentage of total respiratory variability in the LF frequency band and coherence between LF heart rate variability and respiratory variability were also calculated for each breathing condition. Statistics were calculated in RStudio (RStudio Inc. v1.1.463) with a p-value of <0.05 considered statistically significant. All values are expressed as mean ± standard deviation.
Table 1.
Study participant demographics.
| Sex | 51 M/8 F | |
| Age | 28.8 ± 5.3 years old | |
| AIS* | A | 35 (60%) |
| B | 12 (21%) | |
| C | 10 (17%) | |
| D | 1 (2%) | |
| NLI* | C1-C8 | 29 (50%) |
| T1-T6 | 20 (34%) | |
| T7-T10 | 9 (16%) | |
| Time since Injury | 2.6 ± 3.7 years | |
AIS= American Spinal Injury Association Impairment Scale; NLI= Neurological Level of Injury.
Out of n=58 where full AIS and NLI were recorded.
Results:
Sixty-eight total individuals were enrolled and assessed for eligibility. Of this, 59 individuals with SCI met inclusion criteria and had unpaced and paced breathing time series analyzed for heart rate variability per our controlled protocol. Demographics for this group appear in Table 1. The mean unpaced respiratory frequency was 12.3 (± 4.1) breaths per minute, which accordingly increased to 15.0 (± 0.4) breaths per minute for paced breathing.
Mean power of LF heart rate variability was significantly higher during unpaced breathing compared to paced breathing (Table 2). Interestingly, there was no significant difference in mean power of HF heart rate variability. The percentage of total power in the LF spectrum was significantly higher for unpaced breathing compared for paced breathing (64 ± 17% vs. 42 ± 20%, p<0.001). In total, 22 of 59 (37%) individuals had differences in percentage of LF power greater than 30% between unpaced and paced breathing conditions. LF:HF power ratio was significantly higher for unpaced breathing than paced breathing within the same individuals. Standard deviation of normal sinus intervals (SDNN, a proposed measure of sympathetic and parasympathetic activity) was significantly higher for unpaced breathing. Root Mean Square of Successive Differences between beats (RMSSD, which is proposed to reflect vagally-mediated changes in heart rate variability) was similar between unpaced and paced breathing within the same individuals.
Table 2.
Mean (± standard deviation) heart rate variability components for unpaced and paced breathing following spinal cord injury.
| Heart Rate Variability Component | Unpaced Breathing | Paced Breathing | P-value |
|---|---|---|---|
| LF power (ms2) | 1292 ± 1328 | 573 ± 741 | p<0.001 |
| HF power (ms2) | 849 ± 1218 | 989 ± 1454 | p=0.53 |
| LF:HF ratio | 3.2 ± 5.1 | 1.1 ± 1.6 | p<0.001 |
| SDNN (ms) | 58.2 ± 27.4 | 54.3 ± 28.9 | p=0.04 |
| RMSSD (ms) | 50.2 ± 35.3 | 53.1 ± 40.5 | p=0.83 |
LF= Low Frequency; HF= High Frequency; SDNN=Standard Deviation of NN intervals; RMSSD=Root Mean Square of Successive Differences between beats.
Bland-Altman plots (Figure 1A–C) demonstrate a positive proportional bias for LF heart rate variability, increasing in magnitude as LF power increases. This positive proportional bias was robust, with R2=0.39. In the HF band of heart rate variability, an unclear fixed or proportional bias was present, with wider variance as HF power increases (R2=0.04, Figure 1B). While lower HF power demonstrated closer absolute agreement between unpaced and paced breathing measures, there was still wide relative disagreement, with mean HF power values of <1000 ms2 deviating 59% on average between measures (Figure 1C). The differences between measures ranged equally across both negative and positive disagreement.
Figure 1:
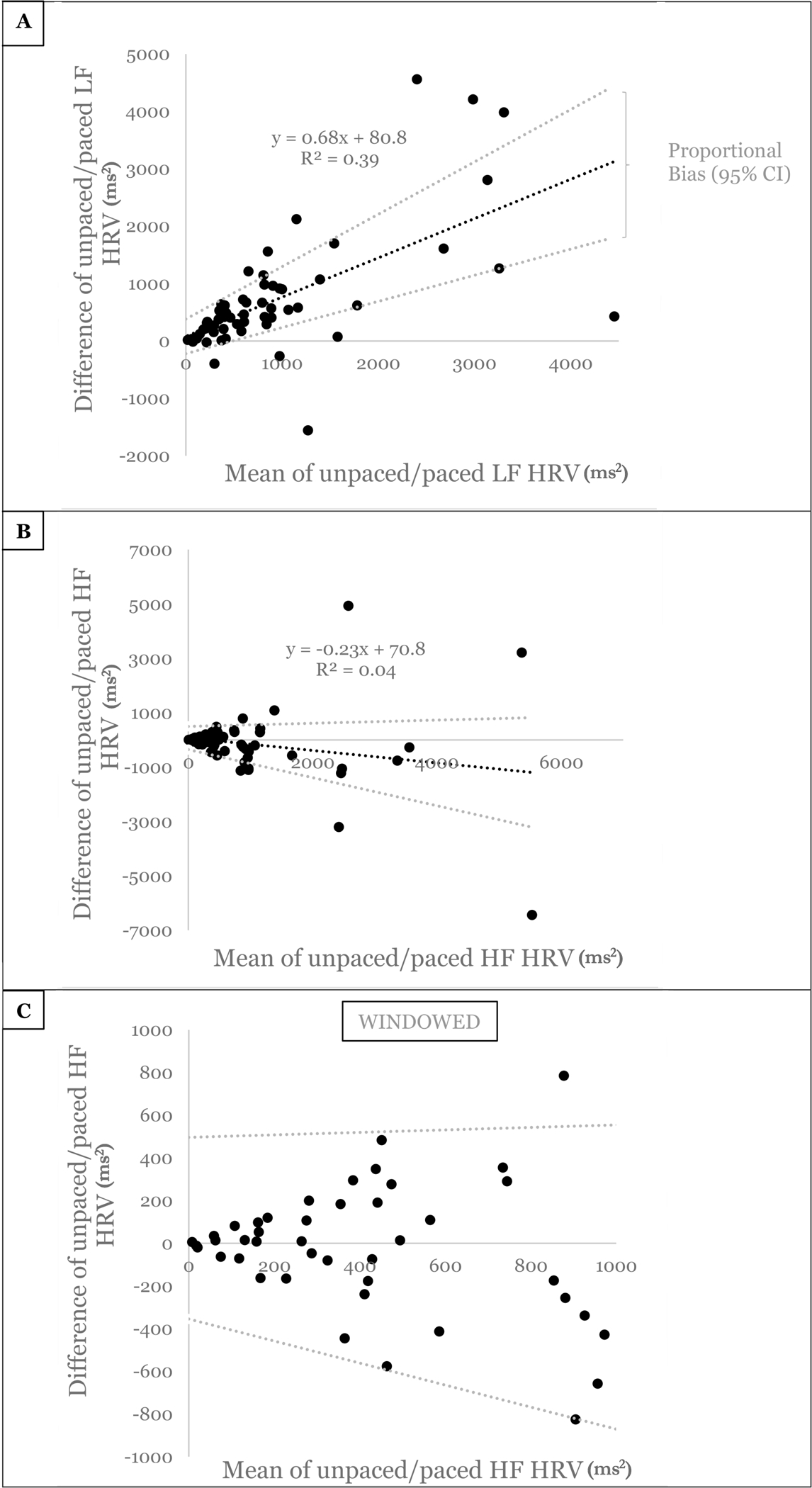
Bland-Altman plots of A) Low frequency (LF) and B) High frequency (HF) heart rate variability (HRV) powers comparing unpaced vs paced breathing within the same individuals with spinal cord injuries. C) Windowed areas zoomed in around lower values of HF HRV Bland-Altman to demonstrate similar patterns at low mean HF power. Least product linear regression indicating proportional bias is estimated with the black dashed line, with accompanying 95% confidence intervals (CI, gray dashed lines). Of note, that these 95% confidence intervals remain above zero difference in A indicates a positive proportional bias. Plainly, this means that as values of LF HRV increase, unpaced breathing becomes less accurate compared to paced breathing. In panels B/C, that these 95% confidence intervals encompass zero difference indicates that a bias may exist, but it may be a fixed bias with slope of zero, or a negative or positive proportional bias. The sizable width of these 95% confidence intervals for HF HRV means that a generous amount of uncertainty exists when unpaced breathing is being used, relative to controlled, paced breathing. For those interested, further walk throughs on Bland-Altman plots are available (30).
To illustrate how unpaced breathing can impact heart rate variability, one representative individual is shown as an example. This individual was a 32-year-old male with T3 American Spinal Injury Association Impairment Scale A paraplegia (22) suffered 9.4 months prior to testing. Heart rate variability mean power demonstrated notable differences between unpaced and paced breathing (LF 689 ms2 vs 47 ms2, HF 269 ms2 vs 259 ms2, LF:HF ratio 2.6 vs 0.2). Utilizing the data from unpaced breathing alone, as is commonly done, would suggest a strong LF power component in heart rate variability. However, when breathing is paced, this LF power is markedly lower. This individual’s respiratory frequency during unpaced breathing (Figure 2A) demonstrated a sizable portion in the LF power range, leading to marked confounding of heart rate variability at these frequencies (Figure 2B). With paced breathing, LF power is no longer confounded by respiration and accordingly decreases. Importantly, unpaced breathing leads to similar HF power compared to paced breathing in this example. However, this occurs only because unpaced breathing respiratory frequencies extend beyond 0.15 Hz into the HF band (Figure 2A). This means that HF power in this unpaced breathing example is derived from a minority of relatively shorter spontaneous breathes (which may or may not occur in other individuals). In the paced breathing condition, power is centered at the prescribed respiratory frequency of 0.25 Hz. Analyzing LF:HF ratio alone for this individual using unpaced breathing, results appear near the normal values for uninjured controls. However, when paced breathing eliminates the respiratory frequency confounder, LF:HF ratio falls significantly below these normal values.
Figure 2:
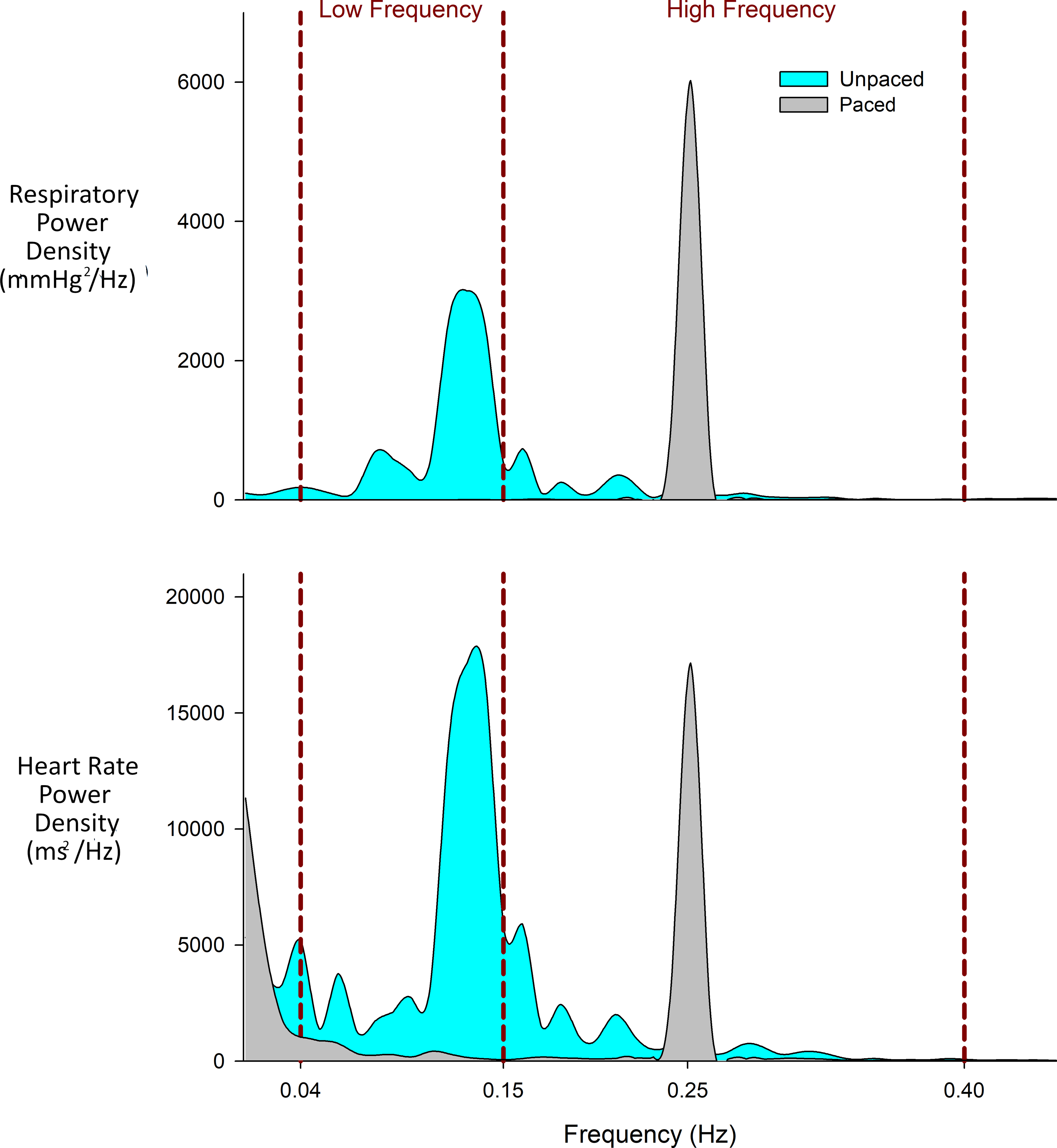
Example of one individual during unpaced and paced breathing. A) Respiratory power spectral density showing respiratory frequencies over the five minutes of recording. B) Heart rate power spectral density. Of note, there is importantly little overlap in individual power spectrums within the same individual during unpaced and paced breathing.
Corroborating this example, significant respiration in unpaced breathing occurred in the LF range within the entire study population; percentage of total respiratory variability contained in the LF band was significantly higher in unpaced breathing than paced breathing (22 ± 25% vs. 3 ± 5%, p<0.001). This increase in LF respiratory power corresponded to an increase in LF heart rate variability as indicated by significantly higher LF cross-spectral coherence during unpaced breathing (0.41 ± 0.16 vs. 0.30 ± 0.09 for paced breathing, p=0.02).
Discussion
Respiratory frequency has a significant impact on heart rate variability and not accounting for respiration has serious consequences for accurate interpretation of these data. Our results demonstrate the potential scope of misestimation for common measures of heart rate variability that may markedly differ with unpaced versus paced breathing in those with SCI. However, beyond SCI, the established physiology that underlies these findings is translatable to many other rehabilitation medicine-centric conditions. Even with strict inclusion criteria, standardized study protocols, and avoidance of confounders (e.g., prior exercise, caffeine/dietary intake, circadian rhythms, and restricted age), not accounting for respiratory frequency significantly skewed LF power, the percentage of total power in the LF range, the LF:HF ratio, and SDNN. This resulted in a proportional bias in LF measurements, magnifying the error in these recordings as power increased. Moreover, the effect was not limited to LF power; HF power across the entire range of values had unacceptable limits of agreement when uncontrolled is compared to controlled respiration.
Our findings on the role of respiration in altering heart rate variability are consistent with the previous physiology literature in individuals without SCI. In these individuals, respiratory-induced variance in the power spectra can have as much as a 10-fold effect (31). Respiration exerts its effect on heart rate variability via the arterial baroreceptors, the chemoreceptors, the pulmonary/thoracic stretch receptors, as well as central respiratory gating of vagal outflow (32,33). Although some have suggested that unpaced breathing is preferable for heart rate variability measurements following SCI (34), the underlying physiology does not agree with this.
Within heart rate variability, HF fluctuations are of especial importance as a marker of cardiovagal tone and as an indicator of cardiovascular risk. Although our findings in individuals with SCI corroborate past results (10) that there was not a significant difference in mean HF power between unpaced and paced breathing, caution is warranted from interpreting this to say that HF heart rate variability power is equivalent between unpaced and paced breathing. This is not the case. The Bland-Altman analysis demonstrated wide disagreement in measures that ranged both positive and negative, with a standard deviation over ten times the mean difference. This significant uncertainty would mean that, for example, an HF power of 500 ms2 during unpaced breathing may correspond to a true (paced) value as high as 1,000 ms2 or as low as ~0 ms2.
In reviewing the Rehabilitation Medicine literature for this study, we found that respiratory monitoring or paced breathing were rarely included in heart rate variability analyses. Additionally, other confounds were routinely present: wide range of age, lack of fasting, or insufficient restrictions on exercise. Standard guidelines exist for evaluating heart rate variability (8) and to ensure reproducibility and minimize confounders, we would urge future investigators to draw from these well-accepted methods.
While this study illustrates how unpaced breathing may confound heart rate variability results, it should be noted that heart rate variability does not reflect a single simple mechanism or correlate linearly with autonomic outflow (35). These are qualitative measures which provide general information on the autonomic nervous system and are not as simple as “sympathetic/parasympathetic balance”. Even the best of these measurements, HF variability, demonstrates relationships between measured parasympathetic vagal activity only at low to moderate vagal outflow and only after eliminating sympathetic effects (14). Finally, we would be remiss if we did not mention that while this study highlights the commonly used LF percentage of total power and LF:HF ratio, these normalized units have little grounding in actual physiology (35,36). In fact, for these ratios to reflect changes in “sympathovagal balance” as has been claimed, total spectral power must remain constant. In our results, total spectral power changes with respiratory frequency alone, raising questions about any conclusions regarding autonomic control that are contingent on these ratios.
Limitations
Respiration is an established driver of heart rate variability in short-term recordings. Yet within respiration, both respiratory frequency and tidal volume are known to exert influence (10,32). Cooke et al. demonstrated that stringent tidal volume control attenuated LF variability, likely by influencing CO2-sensitive chemoreceptor stimulation (32). Nasal canula CO2 monitoring which we recorded did not allow accurate tidal volume estimates, however future studies would be well served to additionally consider this variable. Further, the order of unpaced vs paced breathing was fixed throughout these experiments. Randomization of these orders would dispel any potential temporal effects, though we feel these are likely minimal given unpaced breathing is the natural state.
Conclusions
Respiratory frequency has a significant impact on heart rate variability in those with spinal cord injury, and not accounting for this potential confounder skews results. Future studies of heart rate variability in the SCI population and in other rehabilitation medicine patients should accordingly consider paced breathing to minimize uncontrolled error.
Acknowledgments:
With a goal of clear science communication to clinicians, the authors vetted this manuscript with a group of Rehab Medicine Physicians prior to submission. This group’s comments and guidance to improve broad readability were indispensable. We would accordingly like to thank Drs. Margaret Jones, MD, Andrew Park MD, Nicholas Race, MD, PhD, Brendan McNeish MD, and Julie K Silver, MD.
Disclosures: Dr. Daghici reports a grant from the Paralyzed Veterans of America Research Foundation, unrelated to the current work
Funding details:
Dr. Solinsky’s time was protected by a career development award from the National Institute of Health, NICHD, 1K23HD102663-01. All data were derived from NIH R01 HL117037 (J.A.T.).
References
- 1.Hamaad A, Lip GY, MacFadyen RJ. Heart rate variability estimates of autonomic tone: relationship to mapping pathological and procedural stress responses in coronary disease. Ann Med 2004;36: 448–61. [DOI] [PubMed] [Google Scholar]
- 2.LaRovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998;351:478–84. [DOI] [PubMed] [Google Scholar]
- 3.Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol 1989; 14: 1139–48. [DOI] [PubMed] [Google Scholar]
- 4.Hayano J, Taylor JA, Mukai S, Okada A, Watanabe Y, Takata K, et al. Assessment of frequency shifts in R-R interval variability and respiration with complex demodulation. J Appl Physiol 1994;77(6):2879–88. [DOI] [PubMed] [Google Scholar]
- 5.Keren O, Yupatov S, Radai MM, Elad-Yarum R, Faraggi D, Abboud S, et al. Heart rate variability (HRV) of patients with traumatic brain injury (TBI) during the post-insult sub-acute period. Brain Injury 2005;19(8):605–11. [DOI] [PubMed] [Google Scholar]
- 6.Arad M, Abboud S, Radai MM, Adunsky A. Heart rate variability parameters correlate with functional independence measures in ischemic stroke patients. J Electrocardiol 2002;35:243. [DOI] [PubMed] [Google Scholar]
- 7.Pichot V, Roche F, Gaspoz JM, Enjolras F, Antoniadis A, Minini P, et al. Relation between heart rate variability and training load in middle-distance runners. Med Sci Sports Exerc 2000;32(10):1729–36. [DOI] [PubMed] [Google Scholar]
- 8.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996;17: 354–81. [PubMed] [Google Scholar]
- 9.Davies CTM, Neilson JMM. Sinus arrythmia in man at rest. J Appl Physiol 1967; 22:947–55. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol-Heart C 1981;241(4):H620–9. [DOI] [PubMed] [Google Scholar]
- 11.Angelone A, Coulter NA. Respiratory sinus arrythmia: a frequency dependent phenomenon. J Appl Physiol 1964; 19:479–82. [DOI] [PubMed] [Google Scholar]
- 12.Hayano JS, Mukai M, Sakakibara A, Okada A, Takata K, Fujinami T. Effects of respiratory interval on vagal modulation of heart rate. Am J Physiol-Heart C 1994b; 36:33–40. [DOI] [PubMed] [Google Scholar]
- 13.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–55. [DOI] [PubMed] [Google Scholar]
- 14.Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, et al. Accuracy of assessment of cardiac vagal tone by heart variability in normal subjects. Am J Cardiol. 1991;67(2):199–204. [DOI] [PubMed] [Google Scholar]
- 15.El-Kotob R, Craven BC, Mathur S, Ditor DS, Oh P, Miyatani M, et al. Assessing heart rate variability as a surrogate measure of cardiac autonomic function in chronic traumatic spinal cord injury. Top Spinal Cord Inj Rehabil 2018;24(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues D, Tran Y, Guest R, Middleton J, Craig A. Influence of neurological lesion level on heart rate variability and fatigue in adults with spinal cord injury. Spinal Cord 2016;54(4):292–7. [DOI] [PubMed] [Google Scholar]
- 17.Serra-Añó P, Montesinos LL, Morales J, López-Bueno L, Gomis M, García-Massó X, et al. Heart rate variability in individuals with thoracic spinal cord injury. Spinal Cord 2015;53(1):59–63. [DOI] [PubMed] [Google Scholar]
- 18.Claydon VE, Krassioukov AV. Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. Am J Physiol Heart Circ 2008;294(2):H668–78. [DOI] [PubMed] [Google Scholar]
- 19.Karri J, Zhang L, Li S, Chen YT, Stampas A, Li S. Heart rate variability: a novel modality for diagnosing neuropathic pain after spinal cord injury. Front Phsyiol 2017;8:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyriakides A, Poulikakos D, Galata A, Konstantinou D, Panagiotopoulos E, Chroni E. The effect of level of injury and physical activity on heart rate variability following spinal cord injury. J Spinal Cord Med 2019;42(2):212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 2003;82(10):803–14. [DOI] [PubMed] [Google Scholar]
- 22.Kirshblum S, Waring W 3rd Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabil Clin N Am 2014;25(3):505–17. [DOI] [PubMed] [Google Scholar]
- 23.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997; 20:1561–8. [DOI] [PubMed] [Google Scholar]
- 24.Mazurak N, Günther A, Grau FS, Muth ER, Pustovoyt M, Bischoff SC, et al. Effects of a 48-h fast on heart rate variability and cortisol levels in healthy female subjects. Eur J Clin Nutr 2013;67(4):401–6. [DOI] [PubMed] [Google Scholar]
- 25.Freeman R Assessment of cardiovascular autonomic function. Clin Neurophysiol 2006;117(4):716–30. [DOI] [PubMed] [Google Scholar]
- 26.Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroaccoust 1967; 15:70–3. [Google Scholar]
- 27.Berger RD, Saul JP, and Cohen RJ. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am J Physiol Heart-C 1989;256: H142–52. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 29.Lipman RD, Salisbury JK, Taylor JA. Spontaneous Indices Are Inconsistent With Arterial Baroreflex Gain. Hypertension. 2003; 42:481–7. [DOI] [PubMed] [Google Scholar]
- 30.Giavarina D Understanding bland altman analysis. Biochemia medica. 2015;25(2):141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol 1993;75: 2310–7. [DOI] [PubMed] [Google Scholar]
- 32.Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, Ames IV JE, et al. Controlled breathing protocols probe human autonomic cardiovascular rhythms. Am J Physiol Heart-C 1998;274(2):H709–18. [DOI] [PubMed] [Google Scholar]
- 33.Eckberg DL. Topical Review: The human respiratory gate. J Physiol 2003;548(2):339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucci VE, Inskip JA, McGrath MS, Ruiz I, Lee R, Kwon BK, et al. Longitudinal Assessment of Autonomic Function during the Acute Phase of Spinal Cord Injury: Use of Low-Frequency Blood Pressure Variability as a Quantitative Measure of Autonomic Function. J Neurotrauma 2021;38(3):309–21. [DOI] [PubMed] [Google Scholar]
- 35.Taylor JA, Studinger P. Final words on debate “point: counterpoint cardiovascular variability is/is not an index of autonomic control of circulation” J Appl Physiol 2006 [DOI] [PubMed] [Google Scholar]
- 36.Parati G, Mancia G, Rienzo MD, Castiglioni P, Taylor JA, Studinger P. Cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol 2006; 101(2):690–1. [DOI] [PubMed] [Google Scholar]


