Abstract
As single cells, ciliates build, duplicate, and even regenerate complex cortical patterns by largely unknown mechanisms that precisely position organelles along two cell‐wide axes: anterior–posterior and circumferential (left–right). We review our current understanding of intracellular patterning along the anterior–posterior axis in ciliates, with emphasis on how the new pattern emerges during cell division. We focus on the recent progress at the molecular level that has been driven by the discovery of genes whose mutations cause organelle positioning defects in the model ciliate Tetrahymena thermophila. These investigations have revealed a network of highly conserved kinases that are confined to either anterior or posterior domains in the cell cortex. These pattern‐regulating kinases create zones of cortical inhibition that by exclusion determine the precise placement of organelles. We discuss observations and models derived from classical microsurgical experiments in large ciliates (including Stentor) and interpret them in light of recent molecular findings in Tetrahymena. In particular, we address the involvement of intracellular gradients as vehicles for positioning organelles along the anterior‐posterior axis.
Keywords: cell division, ciliates, cortical, gradients, kinase, morphogens, patterning, tetrahymena
THE COMPLEXITY OF A “SIMPLE” ORGANISM
Ciliates seem simple when viewed through the familiar lens of multicellularity (one cell = simple, many cells = complex). Upon closer examination, ciliates exhibit a bewildering and sophisticated organizational complexity, in a staggering diversity of forms (Figure 1).
FIGURE 1.
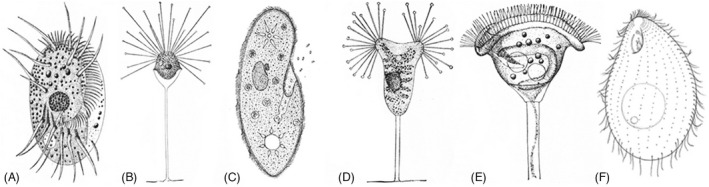
Ciliate diversity. (A) Diophrys. (B) Acineta divisa. (C) Paramecium spp. (D) Acineta tuberosa. (E) Vorticella patelline. (F) Tetrahymena thermophila. Images (A), (B), (D), (E) are from “The Project Gutenberg eBook,” Marine Protozoa from Woods Hole, by Gary N. Calkins. Image C is from D.G. McKean, (http://www.biology‐resources.com/drawing‐paramecium.html), and image F is from (Lynn, 2008)
When describing ciliates, we tend to draw on vocabulary from the more familiar metazoan reference despite entrance onto a unicellular theater. Conventionally, protozoologists assign anterior to the end of the cell that supports the mouth or oral apparatus (OA), while posterior refers to the region endowed with organs of excretion: the cytoproct (CYP) and contractile vacuole pores (CVPs), but these conventions quickly grow problematic. While serviceable for the Tetrahymena clade (Figures 1F and 2), in some ciliates such as Paramecium (Figure 1C), the OA is located roughly at midbody, and CVPs assemble at both ends of the cell. A better convention is to orient anterior and posterior with respect to the direction of locomotion as ciliates usually swim with oral structures facing forward. Even in species in which the OA is centrally located, forward swimming is the direction that propels food particles into the oral cavity. The side of the cell harboring the OA (often “face down” as in the hypotrich ciliates, Figure 1A), is assigned the term ventral while the side opposite is dorsal. From the cell's perspective, left and right also exhibit an asymmetry of organelle placement. In Tetrahymena, the CVPs are located to the cell's right of the oral meridian, along‐side ciliary rows 5,6 (Figure 2A).
FIGURE 2.
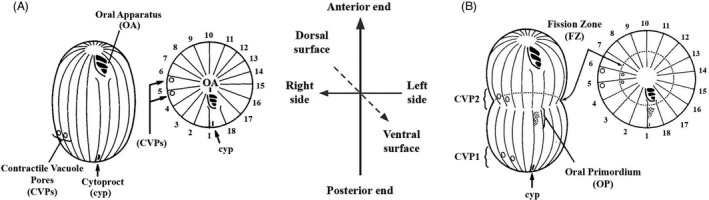
Tetrahymena cytogeometry and organelle nomenclature (Cole et al., 1987). (A) A ventral view and a polar projection of a nondividing cell. Ciliary rows are depicted as lines. The OA is positioned at the anterior and the cytoproct and contractile vacuole pores at the posterior. Two ciliary rows terminate just posterior to the OA. The left‐postoral row is often used as a spatial reference (row number 1), and serves as the “stomatogenic kinety” near which the new OA forms during cell division. (B) A ventral view and a polar projection of a dividing cell. A new oral primordium (OP) forms at mid‐body, just posterior to the fission zone (FZ), and a new pair of CVPs develops just anterior to the fission zone. Ciliary rows are numbered in the polar projection. The OP, CVPs, and FZ are our principle cortical landmarks
The complexity of ciliate architecture is seriously compounded when one confronts the problem of cell division often referred to as “tandem duplication.” In ciliates cytokinesis typically bisects the cell in a plane perpendicular to the long (anterior–posterior) axis, and at the cell's “equator.” The anterior daughter cell (conventionally named the “proter” in the older literature) inherits the preexisting anterior region with the old OA and assembles a new posterior region including a new CYP, and new CVPs (Figure 2B). The posterior daughter (or “opisthe”) inherits the preexisting posterior region (including the old CVPs and CYP), and assembles a new anterior region including a new apex and OA. What is remarkable is the fidelity with which ciliates assemble new structures at precise locations. The oral primordium (OP), the first new organelle to develop at the onset of cell division, assembles mid‐ventrally alongside the first postoral ciliary row, the so‐called “stomatogenic kinety” (Figure 2B, row # 1). Next, the circumferential fission zone (FZ) forms just anterior to the OP. Then, new CVPs and a CYP assemble at precise circumferential positions with reference to the stomatogenic kinety, and just anterior to the FZ. The new cell apex forms immediately posterior to the FZ. Clearly, precise mechanisms must be at work that define the positions of each cortical structure along both the anterior–posterior (A/P) axis and around the cell circumference (circumferential or C axis).
The goal of this review is to capture our current understanding of mechanisms that establish A/P organization. Though referencing a wealth of classical surgical experiments from the larger ciliates (Stentor and Blepharisma), most of this review will focus on recent genetic discoveries in the physically more modest species: Tetrahymena thermophila. Tetrahymena has proven an exceptional model for both forward and reverse genetic approaches (reviewed by Ruehle et al. (2016)). Much of the work we describe was inspired by the long and fruitful career of Joseph Frankel (University of Iowa), and his exquisitely characterized collection of Tetrahymena cortical pattern mutants (reviewed by Frankel (1989, 2008)).
THE PROBLEM OF PATTERN FORMATION
The paradigm of “pattern formation” comes to us from the study of development in metazoans, and protozoan pattern studies make frequent reference to it. In the metazoan embryo, a key role is played by “morphogens”: diffusible molecules that specify cell fates in a concentration‐dependent fashion (see Gilmour et al. (2017) and Kornberg (2014) for review). Morphogen concentrations are highest near their source, and diminish towards a “sink” (Figure 3A). They often manifest as dual, counter‐gradients. Cells in the developing metazoan respond to varying morphogen concentrations (or unique combinations of morphogens), by expressing specific gene products. Novel cell types driven by differential gene expression become the “read‐out” for patterning activity in the metazoan embryo, and together, morphogen driven differential gene expression has become the paradigm for studies into pattern formation. In this review we will evaluate how ciliates utilize intracellular gradients of cortical morphogens (frequently localized to the peri‐basal body spaces) as inputs to determine which cortical domains become committed to specific organelle assembly pathways (the output; Figure 3B).
FIGURE 3.
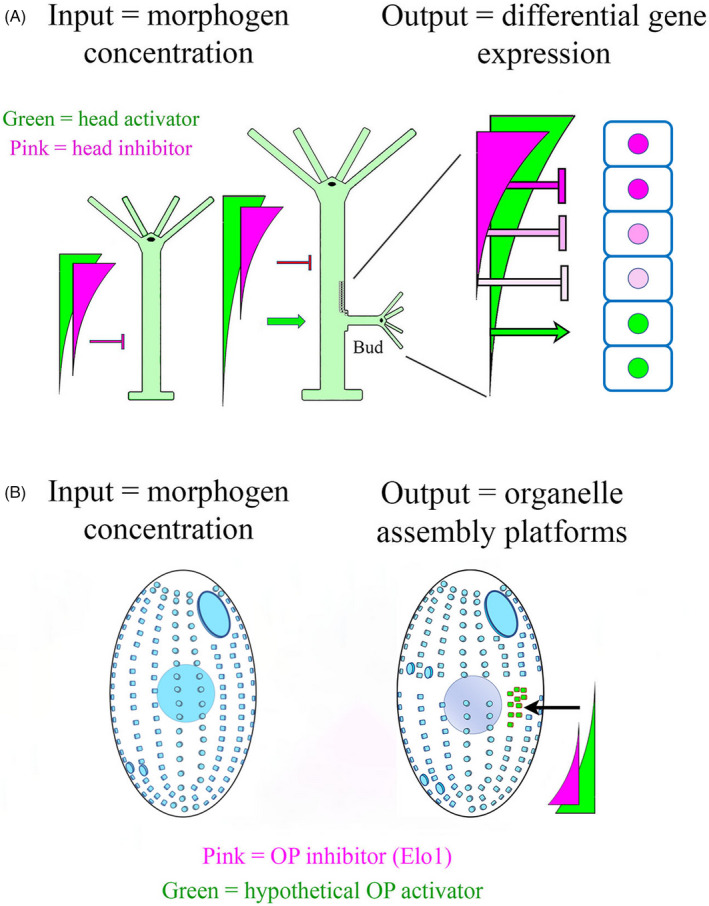
Morphogen gradients and their role in positional information in animals and ciliates. (A) In a simplified model of the freshwater Hydra, a gradient of “head activator” is established, with its high value at the head and low value at the base (green gradient). High levels of this rapidly‐diffusing morphogen lead to differentiation of tentacles, stinging cells and a mouth. Low levels are correlated with the expression of an adhesive disk at the base of the animal. An equally important morphogen gradient involves the head inhibitor (pink) that also emanates from the head region, but has a more limited ability to diffuse. The head inhibitor suppresses secondary head formation along the body axis. As the hydra grows in length, the head inhibitor gradient still covers the same limited physical range, so that at some point, tissues in the proximity of the base become exposed to supra‐threshold levels of activator, and sub‐threshold levels of inhibitor, and a new head forms (budding occurs) [after (Vogg et al., 2019; Webster & Wolpert, 1966); see Mercker et al. (2021) for recent review]. A more complete model would include the description of a “foot activator” and “foot inhibitor” emanating from the basal disk. (B) A simplified model of gradients involved in cortical patterning of Tetrahymena. A posterior‐high gradient of the oral inhibitor (pink = elo1) prevents initiation of oral assembly too close to the posterior. A purely hypothetical posterior‐high gradient of oral activator (green) stimulates oral development near mid‐body (arrow) as the cell launches predivision development
Hydra (a metazoan model) has several features relevant to our discussion. First, the concentration of a diffusible morphogen, specifies where the organism assembles specific structures such as the mouth and tentacles. An inhibitory morphogen (shown in pink) represses head formation. As cells far from the morphogen source escape the influence of this rapidly degraded inhibitor, genes are activated by the now, unconstrained and more stable “head activator” (shown in green). These gene products drive head and mouth formation and lateral budding occurs (Bode, 2009; MacWilliams, 1983a, 1983b). The features of this model that bear generalizing, are that a diffusible molecule establishes a concentration gradient across an organism, and different concentrations of this molecule trigger different morphogenetic “read‐outs.”
In the year of this writing, it is fitting to acknowledge the passing of Lewis Wolpert, and his enormous contribution to our understanding of pattern formation (see Tickle and Slack (2021)). Wolpert invented the language of positional information, capturing how simple, continuous gradients of diffusible molecules (the morphogens), could be interpreted across embryonic space. “There was evidence that the concentration at each point in the embryo was somehow interpreted to specify anatomy—and this indeed meant turning on different sets of genes at each level in the anteroposterior axis” (reviewed by Lawrence (2001)). Wolpert's ideas regarding positional information in the animal embryo (Wolpert, 1969, 1981), profoundly informed Frankel's approach as he took up the challenge of understanding patterning in ciliates. Indeed, much of Frankel's careful and painstaking analysis of cortical patterning was carried out in parallel with our vigorously expanding knowledge of metazoan development that took place in the 1980s and 1990s. In Drosophila these studies identified a dual‐gradient of transcription factors, which in a concentration‐dependent fashion define the insect's anterior–posterior segment identities (Driever & Nusslein‐Volhard, 1988; Wieschaus & Nusslein‐Volhard, 2016). In the developing chick limb, a gradient of the diffusible protein shh (sonic hedgehog), has been shown to define digit identity (Riddle et al., 1993). In the frog embryo, a gradient of TGF‐β, was shown to define mesodermal cell fates across the dorsal–ventral axis (Heasman, 1997). The stage was set to explore how single‐celled organisms create patterns of cortical architecture against the conceptual backdrop of patterning in the metazoan embryo.
INTRACELLULAR PROTEIN GRADIENTS
It is highly relevant to our discussion that molecular gradients can form inside cells (recently reviewed in Hubatsch and Goehring (2020)). In fact, the first well characterized morphogens in Drosophila (bicoid and nanos), form opposing antero‐posterior gradients during early stages of development when the embryo is effectively unicellular. The Drosophila egg is a large cell (~500 μm) in which morphogen gradients are established by transport of morphogen‐encoding mRNAs and their anchorage at the cell poles (localizing the source of their translation). However, stable protein gradients can form inside much smaller cells with few cytoskeletal structures, including bacteria. Generally, speaking, an intracellular protein concentration gradient can be sustained if a posttranslational modification changes the protein's diffusion coefficient, allowing for sorting of the fast and slow diffusing isoforms along the cell's axis (Lipkow & Odde, 2008; Wu et al., 2018). The formation of such a gradient requires spatially‐restricted enzymes that locally modify the protein to alter its diffusibility (e.g. by inducing oligomerization or anchoring to the plasma membrane or cytoskeleton). Typically, the switch that changes the protein's diffusion coefficient is based on either phosphorylation or nucleotide binding/hydrolysis. Importantly, such intracellular gradients can produce positional effects. Particularly relevant to this review are gradients that direct the site of cell division in bacteria and fungi. In the bacterium Caulobacter crescentus, an ATPase MipZ forms a bipolar gradient with the highest concentration at the cell poles. MipZ inhibits assembly of the FtsZ contractile ring thereby focusing cytokinesis at midbody (Kiekebusch & Thanbichler, 2014; Thanbichler & Shapiro, 2006). A conceptually similar mechanism operates in the fission yeast S. pombe, where a bipolar gradient of the Pom1 kinase prevents entry into cell division in cells that have not reached proper size (Gerganova et al., 2021; Martin & Berthelot‐Grosjean, 2009; Moseley et al., 2009; Wood & Nurse, 2015).
One way that protein‐modifying enzymes can partition across the cell is based on a “mutual cortical antagonism,” well documented during early development in C. elegans. The single cell embryo of C. elegans is among the best studied metazoan cell types that carry intracellular protein gradients (reviewed by Hoege and Hyman, (2013), Lang and Munro (2017) and Motegi and Seydoux (2013)) and this model may be particularly relevant when thinking about patterning in ciliates. Measuring 30 × 50 μm, C. elegans is well within the size range of many ciliates including Tetrahymena. During fertilization of the oocyte, the sperm‐derived centrosome polarizes the cytoskeleton to generate anterior and posterior cortical domains that accumulate distinct sets of plasma membrane‐bound Par proteins, including several highly conserved kinases (Hoege & Hyman, 2013; Motegi et al., 2011). The two Par complexes (anterior and posterior) engage in a mutual antagonism based on inhibitory cross‐phosphorylation, decreasing each other's ability to bind the plasma membrane, which results in a sharp boundary between their respective cortical domains. PAR‐1 kinase in the posterior Par complex phosphorylates an RNA‐binding protein MEX‐5, decreasing its affinity for cytoplasmic RNA and making it more mobile (Griffin et al., 2011; Lim et al., 2021). Because PAR1 is enriched in the posterior domain, this creates an anterior‐posterior gradient of MEX‐5. An opposing gradient of another RNA‐binding protein PIE‐1 is also established (Daniels et al., 2009). These gradients then specify the cell types that differentiate during subsequent embryonic development. As we will discuss later, a similar mechanism of sorting based on a mutual cortical antagonism (but with a different set of conserved kinases) appears to operate in the dividing Tetrahymena cell to position the division boundary, a critical step in the emergence of cortical pattern in the forming daughter cells (Jiang et al., 2020).
Hubatsch and Goehring (Hubatsch & Goehring, 2020) consider that the role of the known intracellular gradients may be limited to generation of two subcellular regions of contrasting properties, for example, the cell poles versus the midbody. Thus, it would be important to learn whether intracellular gradients could generate multiple positioning outcomes across a single cell, something that ciliates may utilize to position organelles with remarkable precision. Most if not all eukaryotic cells utilize sophisticated mechanisms for positioning organelles along both the antero‐posterior and even left‐right axis (reviewed in Inaki et al. (2016), Marshall (2020), and Spassky and Meunier (2017)). Examples include the placement of centrioles and cilia on the apical surface or the front of migrating cells (Albrecht‐Buehler, 1977; Guirao et al., 2010; Katsumoto et al., 1994; Ohata et al., 2014; Schneider et al., 2010; Wu & Mlodzik, 2017), and the alignment of stereocilia into triple rows of the inner hair cells within the inner ear (Barr‐Gillespie, 2015; Schwander et al., 2010). While pathways that polarize cells (including the Par and Wnt signaling networks) are important for organelle patterning, it is not well understood how polarity cues are translated into the precise positioning of organelles. With their elaborate cortical patterns, ciliates are particularly useful in exploring principles of intracellular patterning and specifically the role of intracellular gradients in organelle positioning. Finally, as unicellular organisms, ciliates focus our attention on intracellular mechanisms free of the layers of complexity brought by multicellularity.
THE CILIATE CELL CORTEX, A SELF‐ASSEMBLING ARCHITECTURE
There is little doubt that in ciliates, patterning mechanisms reside and operate within the cell cortex, the outer‐most layer of the cell that is rich in both cytoskeletal and membranous components (Figure 4). The cortex exhibits remarkable powers of self‐assembly, self‐propagation, healing, and regeneration (reviewed in Frankel (1989)). By way of example, the heterotrich ciliate, Stentor coeruleus, demonstrates impressive regenerative capabilities. An entire cell can regenerate from a surgically isolated cellular fragment only 1/64th the original size, providing it carries a portion of the macronucleus (Morgan, 1901). Stentor fragments can regenerate missing structures including all cortical organelles (Gruber, 1885; Tartar, 1960, 1961). However, regeneration only takes place if at least a small patch of cortex remains on the endoplasm surface (Tartar, 1956, 1961). Thus, pattern information appears to be contained within even minute cortical fragments. It is tempting to speculate that the minimal unit that can provide cortical patterning cues is the basal body (BB), a conserved centriole‐like structure that has intrinsic polarity (reviewed in Bornens (2019)). However, hypotrich ciliates resorb all BBs during encystment (induced by environmental stress or starvation) and yet the complete cortical pattern reforms when the cyst redevelops upon return to favorable growth conditions (Fryd‐Versavel et al., 2010; Grimes, 1973a, 1973b). Intriguingly, despite the lack of BBs, the hypotrich cysts retain a dense network of cortical microtubules albeit in a reorganized form (Fryd‐Versavel et al., 2010), suggesting that microtubules (themselves polar structures) may provide patterning cues upon excystment.
FIGURE 4.
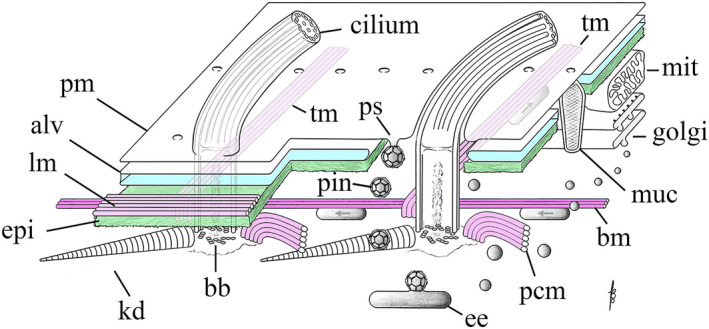
Organization of the cell cortex in Tetrahymena. A patch of cell cortex is depicted with anterior to the left, and posterior to the right. pm = plasma membrane; alv = Ca++ reservoirs known as alveolar sacs; lm = longitudinal band of microtubules lying just under the alveolar sacs and above the epiplasm; epi = a proteinaceous layer of nonmicrotubule based cytoskeleton resembling the spectrin layer in red blood cells; tm = transverse microtubules exposed to cytoplasm; kd = the kinetodesma (or striated fiber); bb = BB; pin = pinosomes; ps = parasomal sac, site of clathrin‐mediated pinocytosis; ee = early endosomes; pcm = postciliary microtubules (also exposed to cytoplasm) and the bm = basal microtubule: the one microtubule track that runs the length of the cell (anterior to posterior) that is exposed to the cytoplasm and hence available for A/P vesicle traffic; muc = mucocyst (dense‐core secretory granule); golgi = dictyosome; mit = mitochondrion
Another property of the cell cortex is the degree of autonomy of cortical structures and their ability to self‐propagate. Cortical abnormalities of all kinds (not caused by mutations) can be transmitted through multiple generations. Inversions of individual ciliary rows (Beisson & Sonneborn, 1965; Ng & Frankel, 1977), parabiotic doublets (“Siamese twins”; Nanney et al., 1975; Tartar, 1956) and even mirror‐image pattern reversals created by mechanical manipulations (Nelsen & Frankel, 1989; Nelsen et al., 1989a, 1989b) can be stably propagated over dozens of cell divisions, revealing a phenomenon described as “cortical inheritance” (Nelsen & Frankel, 1989; Nelsen et al., 1989a, 1989b; Sonneborn, 1963). Thus, there is a “structural memory” integrated within the cell cortex. Equally remarkable is the ability of cortical regions to sustain themselves even when moved to incorrect locations. For example, the dissected FZ of a dividing cell continues to constrict when grafted onto the wrong position of another Stentor as long as the recipient cell is also a divider (Tartar, 1968). Possibly, positive feedback loops sustain localized activities in the ciliate cortex, similar to how positive feedback drives polarization in the budding yeast (reviewed in Woods and Lew (2019)).
From outside in, there are four layers to the ciliate cortex. Outermost is the plasma membrane (Figure 4). Beneath this lies, in tight proximity, a network of flattened, membrane‐bound compartments, the alveolar sacs (turquoise in Figure 4) which may serve as calcium ion reservoirs (Hardt & Plattner, 2000; Lange et al., 1995; Plattner & Klauke, 2001; Plattner et al., 2012; Stelly et al., 1991, 1995). This creates a three‐membrane outer boundary and, one suspects, a barrier to vesicle trafficking between endo‐membrane compartments and the cell surface. Taxonomically, alveolar sacs also place ciliates among the “alveolates” (the clade of Alveolata) together with dinoflagellates and the parasitic apicomplexans (including Plasmodium and Toxoplasma). Alveolins are cytoskeletal proteins (containing charged repeat motifs) that are associated with alveoli and are conserved among alveolates (Gould et al., 2008). In Tetrahymena, a partial somatic knockout of Alv2 alveolin produces large multinucleated “monster” cells, suggesting that Alv2 is important for patterning during cell division, but its specific role or timing of action during cell division are unclear (El‐Haddad et al., 2013).
The triple‐membrane barrier at the cell surface is breached by BBs and by two sets of membrane‐exchange foci: the docking sites for dense‐core secretory granules or “mucocysts” (Briguglio et al., 2013; Sparvoli et al., 2020; Turkewitz, 2004), and rows of endocytic pits at the base of each cilium called “parasomal sacs” (Briguglio & Turkewitz, 2014; Elde et al., 2005; Figure 4). Mucocysts are engaged in stimulated secretion producing small, expanding packages of protein that may help in feeding (adhering to microbes and organic material that is later ingested) or may provide defense against predators. Parasomal sacs are located at the base of every cilium, and appear homologous to the flagellar or ciliary pockets found in other eukaryotes, that are known to be the sites of endocytosis (Ghossoub et al., 2011; Halliday et al., 2021). Parasomal sacs are active in both pinocytosis (clathrin‐mediated endocytosis (Elde et al., 2005)), and possibly in exocytosis (see Bayer‐Santos et al. (2013) and Flotenmeyer et al. (1999)). We draw attention to the parasomal sacs and their adjacent ciliated BBs, as potential players in the regulation of cortical pattern, possibly serving as sites of insertion for polarity factors within the plasma membrane.
Beneath the triple‐membrane of the plasma membrane and alveolar sacs, lies a layer of proteinaceous, nonmicrotubule cytoskeleton superficially resembling the spectrin layer in red blood cells. This is the “epiplasm” (Figure 4, green). One can think of the epiplasm as a cytoskeletal matrix surrounding the cell with numerous “caged” openings for organelles such as BB, mucocysts and parasomal sacs. Importantly, during cell division, the epiplasm temporarily clears from regions where new structures assemble (BBs, the OP, and the FZ). The major cytoskeletal proteins forming the epiplasm in Tetrahymena are EpA, EpB, and EpC1 (Honts & Williams, 2003; Williams et al., 1995). A knockout of EpC1 resulted in a rounded cell shape and mild disorganization of BBs in the ciliary rows and the OA. However, epiplasm is still present in the absence of EpC1, indicating that the remaining proteins are sufficient for epiplasm assembly (Williams, 2004). Thus, the overall role of epiplasm remains unclear.
Finally, stretching out across the cell, and lying both under and over the epiplasm, is the microtubular cytoskeleton with 750 BBs organized into 18–20 rows running parallel to the A/P axis [(Allen, 1967, 1969), reviewed in Wloga and Frankel (2012)]. Each BB has short rootlets or “appendages”: the transverse (TM) and postciliary microtubules (PC), and the well‐studied (nonmicrotubular) kinetodesmal fibers (KD). The polarity of the BB and its appendages within ciliary rows is uniform (e.g. all TMs extend to the cell's left side). TMs and PCs are most likely oriented with the plus ends of microtubules pointing outward, away from the BB (Thazhath et al., 2004). In addition, there are two sets of longitudinal microtubule bundles that are aligned parallel to the ciliary rows. The longitudinal microtubules (LMs) run along the cell's right of each ciliary row (as viewed from inside the cell looking out) and are sandwiched between the alveolar sacs and the epiplasm. The basal microtubules (BMs) run along the cell's left side of the BB rows (viewer's right). LMs are bundles of long, partly overlapping microtubules whose polarity is likely uniform: plus ends oriented towards the anterior end of the cell (Ng & Frankel, 1977). The polarity of the BMs is unknown, but, unlike the LMs, they are exposed to the endo‐cytoplasm where they could serve as anterior–posterior runways for the trafficking of vesicles and signaling components in ways that might be involved in cortical pattern. Several known pattern‐regulating proteins (described below) localize along the ciliary rows, suggesting an association with cortical microtubules. This raises the prospect that their distribution might involve transport along longitudinally oriented microtubules, a topic we will return to later on. Trafficking of vesicles from posterior to anterior along cortical runways has been observed in live cells, traveling at velocities consistent with microtubule‐mediated organelle transport (Zweifel et al., 2009). Conversely, phagosomes migrate from anterior to posterior, possibly along the BM microtubule tracks, or alternatively along the deep fiber bundle, a little studied microtubule track that runs deep within the cytoplasm from its origin at the OA and towards the cell posterior (Figure 5).
FIGURE 5.
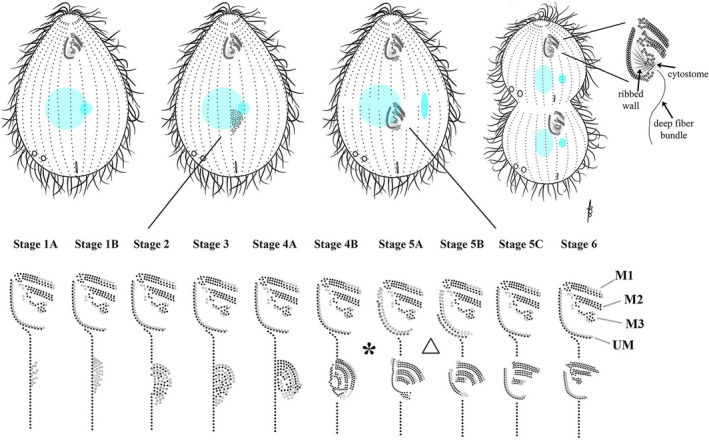
Oral assembly. Stages assigned to development of the oral primordium (lower Fig. from Lansing et al. [1985]). The asterisk (*) denotes the “physiological transition point” between stages 4B and 5A. In the lower panel the triangle indicates a stage at which the undulating membrane of the mature OA is remodeled, completing its reassembly in synchrony with that of the oral primordium. Also in the lower panel, ciliated and unciliated basal bodies are shown in black and grey respectively
CORTICAL DEVELOPMENT IN TETRAHYMENA
The cortex is primarily composed of small repeated units (BBs and their associated structures) that follow local rules of self‐assembly during vegetative cell growth. During interphase, ciliary rows typically grow through addition of new BBs anterior to the ciliated, pre‐existing BBs (Allen, 1969; Bayless et al., 2015). There are also major organelles that assemble during specific stages of the cell‐cycle and follow more global rules of patterning. These include, in order of appearance, the new OA, the FZ, the CVPs, and CYP. The first of these to assemble is the OA that functions as the cell's mouth (Figure 5). Its fundamental role is as the single site of phagocytosis. As such, it is a place of significant membrane turnover as food particles are continuously channeled into the buccal cavity and internalized within growing phagosomes. The most conspicuous components of the OA are the rows of ciliated BBs that form a set of four structures: three diagonally oriented “membranelles” (M1, M2, and M3) and a fourth “undulating membrane” (UM), hence, “Tetra”‐hymena (Figure 5). The three membranelles sweep food particles into the buccal cavity and into the cytostome (where food vacuoles form) while the UM is thought to reinforce the buccal cavity. The BBs of the OA are reinforced by microtubule architecture including the “ribbed wall” which is anchored to the UM and a “deep fiber” bundle that extends into the endoplasm (where it may provide a motility track for the newly formed phagosomes as they move inside the cell). In addition to tubulin, the OA is rich in organelle‐specific cytoskeletal proteins including “tetrins” (Honts & Williams, 2003).
Oral morphogenesis (stomatogenesis) is the first conspicuous step in the developmental program leading to tandem duplication, so we define the onset of cell division in Tetrahymena by the emergence of the OP. The OP appears within a mid‐body clearing of the epiplasm, just posterior to the equatorial position of the future FZ and alongside of the right postoral ciliary row (row 1 or the stomatogenic kinety, Figure 1, also see Figures 5, 6, 7; Bakowska, Frankel, et al., 1982; Bakowska, Nelsen, et al., 1982; Williams et al., 1987). Within this epiplasm clearing (or possibly concurrently) BBs proliferate in a manner not seen during interphase. Whole “streams” of BBs originate along the left side (cell's perspective) of the stomatogenic kinety, and migrate laterally into the inter‐row space. This creates an “anarchic field” of seemingly randomly oriented BBs in the space between the two postoral ciliary rows (Figure 5, stages 1A–1B). BBs then align into rows, the so‐called “pro‐membranelles” first visible as singlets and later as doublets (stages 2–3 in Figure 5). Next, the doublet rows are expanded by addition of a third row of BBs creating membranelles (Figure 5, stages 4A, B). The UM assembles to the cell's right of the three membranelles (stages 4B–5C). The period between stages 4A and 5B (asterisk in Figure 5) is noteworthy. Before this stage, environmental insults (heat shock or a variety of pharmacological inhibitors) can disrupt OP development and block cell division. After this point, oral development proceeds despite such treatments (Frankel, 1962; Williams, 1964). This has been termed the “physiological transition point.” It also marks the end of the temperature‐sensitive period for the cdaA‐1 mutant that is unable to form the FZ (Frankel et al., 1980a, 1980b). It is also noteworthy, that between stages 5A and 5B, the mature OA undergoes partial disassembly (including a loss of one row of BBs within the UM), and then re‐assembles in synchrony with the developing OP (Figure 5, triangle; Bakowska, Nelsen, et al., 1982).
FIGURE 6.
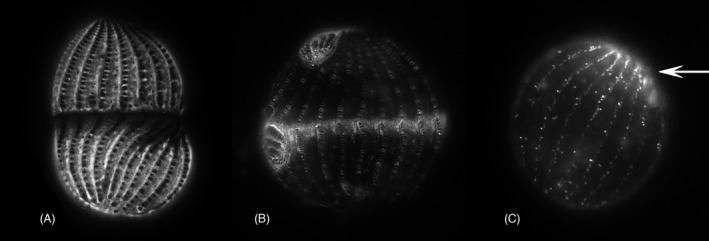
Localization of Tetrahymena proteins in the cell cortex. (A) GFP‐tagged Epc1 localization in a dividing cell, dorsal view (courtesy of Douglas Chalker). Note clearance around each BB and within the FZ. (B) GFP‐tagged fenestrin Fen1 localization in a dividing cell. Fenestrin appears everywhere the epiplasm is excluded including the developing FZ, a cage around each BB, the OA and the developing OP. (C) Cda12:GFP tagged vesicles (likely recycling endosomes) in a recently divided cell (After Zweifel et al., 2009). This is a posterior daughter cell and the putative endosomes appear more richly concentrated at the anterior (arrow), which recently was the fission zone at midbody prior to division
FIGURE 7.
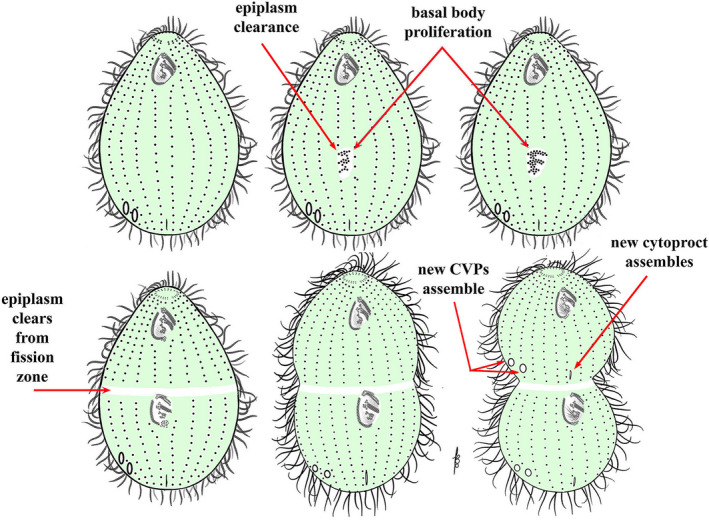
Sequence of cortical organelle dynamics leading to cell division. Development proceeds from left to right. In the first sign of prefission development, epiplasm proteins (green) disappear from the site of oral assembly (the OP). BBs proliferate within this clearing from the stomatogenic kinety. Note: it is not certain whether epiplasm clearance precedes or is concurrent with BB proliferation. BBs become organized into three transverse membranelles and the undulating membrane. The epiplasm clears from the future fission zone, and becomes free of BBs (there is now a midbody discontinuity in the ciliary rows known as the cortical subdivision). Cytokinesis is initiated, and the second cytoproct and CVP sets develop in the anterior daughter cell
The FZ starts to form after OP development is well under way (around stages 4B–5A). First a gap appears in the somatic ciliary rows, completely encircling the cell at its equator and generating what is known as the “cortical subdivision” (Frankel et al., 1981; Kaczanowska et al., 1999). The equatorial gap and the region immediately posterior undergo major molecular changes including clearance of epiplasm proteins (Kaczanowska et al., 1999) and infusion of the protein fenestrin Fen1 (Cole et al., 2008; Joachimiak et al., 2013; Kaczanowska et al., 2003; Nelsen et al., 1994; Yasuda et al., 1980; Figure 6). Fenestrin decorates the Tetrahymena cortex in a pattern that is almost the perfect complement to the epiplasm distribution. Namely, fenestrin decorates (and epiplasm is absent from) the cortical space around the somatic BB, the developing OP, and the FZ. During the final stages of cell division, a contractile ring forms within the FZ plane and cytokinesis is initiated (Jerka‐Dziadosz, 1981; Numata et al., 1995; Yasuda et al., 1980). The LM microtubule bundles undergo severing within the plane of constriction, due to localized activity of katanin that may be locally activated by posttranslational modifications of microtubules (Sharma et al., 2007; Thazhath et al., 2002; Waclawek et al., 2017).
From the perspective of global cell‐patterning, it is worth emphasizing that the FZ assembles immediately anterior to the developing OP. There are mutations that coordinately shift the position of both the OP and FZ, thus displacing the entire division plane (elo1‐1 and cdaI‐1, see below; Frankel, 2008; Jiang et al., 2017, 2019a). These phenotypes suggest that the positions of the two structures are coordinated (i.e. the FZ position either depends on the OP position, or both rely on a common patterning mechanism). However, certain mutant combinations and drug treatments that affect phosphorylation states shift the relative positions of the OP and FZ [(Jiang et al., 2020; Kaczanowska et al., 2012) and see below]. Furthermore, a removal of the OP by dissection [in Stentor (Tartar, 1966)] or UV microbeam irradiation [in Glaucoma (Frankel, 1961)] fails to block the subsequent development of the FZ (Frankel, 1961; Tartar, 1966). Thus, the mechanisms that position the OP and FZ show a degree of independence, suggesting that each of the two structures is positioned in reference to the whole cell axis and not to each other.
As the cell undergoes cytokinesis and as the plasma membrane constricts, a new apex forms in the posterior hemi‐cell (Jerka‐Dziadosz, 1981), and a new posterior end is remodeled anterior to the constriction zone in the anterior hemi‐cell. The cell apex is composed of pairs of BBs that represent the anterior‐most ends of a subset of posterior ciliary rows [reviewed in (Wloga & Frankel, 2012)]. New CVPs and CYP of the developing anterior daughter cell assemble just anterior to the developing FZ, in what will become the posterior end of the anterior hemi‐cell (Figure 7). Circumferentially, the site of cytoproct assembly also appears alongside the stomatogenic kinety, but the CVP domain is unique, assembling at a constant proportion of the cell circumference, approximately 83° to the cell's right (viewers’ left) of the stomatogenic kinety. This has been referred to as the “central angle” (Nanney, 1966), reviewed in (Frankel, 1989). Importantly, CVPs are positioned according to both global (cell‐wide) and local cues. As already mentioned, inverted ciliary rows can propagate through multiple cell divisions (Beisson & Sonneborn, 1965; Ng & Frankel, 1977). If an inverted row happens to be present within the exact area at which CVPs are expected to assemble, then new CVPs form correctly in respect to the A/P and C axes though locally they form on the reverse side of that inverted row (Ng & Frankel, 1977). This remarkable observation reinforces the idea that the positioning mechanisms integrate cues operating at multiple scales (global and local) and along multiple axes (A/P and C) at an organelle level. Likely the global positioning determines a coarse position while the local influences (such as the availability of binding sites in the cytoskeleton) define the fine position.
In summary, the major cortical organelles: the OA, cell apex, CVPs, and CYP appear at precise spatial locations and within a prescribed temporal sequence during prefission development. These are illustrated in Figure 7, and described in more detail in Table 1.
TABLE 1.
Steps in cell division in Tetrahymena
| Stages | Cortical events | Nuclear events | Molecular markers |
|---|---|---|---|
| 1 | Epiplasm proteins are cleared from the region of the future OP, and BB proliferation begins on the left side of the stomatogenic postoral row (anarchic field forms) |
Continuing from interphase, the Elo1 gradient marks the cortex posterior to the OP position CdaA streaks appear in the posterior hemi‐cell |
|
| 2 | In the OP, pro‐membranelles emerge as single rows | CdaA streaks cover the entire posterior hemi‐cell | |
| 3 | Pro‐membranelles form double rows. Two anterior pro‐membranelles (M1 and M2) are well differentiated | The micronucleus moves away from the macronucleus (Cole & Sugai, 2012; Kirk et al., 2008) | CdaI streaks appear in the anterior hemi‐cell, starting from the ventral side, posterior to the old OA |
| 4A | All three pro‐membranelles (M1, M2, and M3) organize as double rows | ||
| 4B | The UM begins to emerge as a single row. The pro‐membranelles gain a third row | The micronucleus elongates during its anaphase | |
| 5A−5B |
The third row is completed in membranelles. The new UM has a single row and a patch of randomly oriented BB at its posterior end The second row of BBs forms the new UM (stage 5B) The UM of the old OA begins to partially disassemble (BB of the outer row are resorbed) A gap starts to form at the equatorial region of BB rows (cortical subdivision) |
The micronucleus is in telophase (two division products are visible across the FZ) |
Epiplasm is cleared from the FZ region (and posterior to it), and fenestrin starts to decorate the same region CdaA and CdaI streaks form a sharp boundary at the margins of equatorial the cortical gap |
| 5C |
Membranelles of the OP shift to a more transverse position In the new UM, BB in the inner row lose cilia and BB of the outer row grow cilia In the old UM a new outer row of BB emerges and undergoes ciliation Ribbed wall microtubules grow out of the BB of the inner row of UM BB couplets appear at the anterior ends of ciliary rows of the posterior daughter cell and morphogenesis of the new apex is initiated |
The macronucleus assumes an elongated shape and moves to a position spanning the cell's equator |
CdaA appears in the anterior hemi‐cell as well p85 appears at the anterior ends of the posterior ciliary rows (Gonda et al., 2000; Gonda & Numata, 2002) |
| 6 and early cytokinesis |
Right ends of the membranelles undergo remodeling (trimming and rearrangement of the BB) A contractile ring forms within the FZ and cytokinesis is initiated. As the cell constricts, the LMs undergo severing. The new cell ends emerge including the new apex at the tip of the posterior daughter The new cell apex forms in the posterior daughter cell and a wave of ciliation of BB takes place posteriorly to the FZ New CVPs and new CYP form in the anterior hemi‐cell. A new apex forms in the posterior hemi‐cell |
The macronucleus constricts at the same time as the cell constricts and its division is driven by intranuclear polymerization that do not form a spindle (Fujiu & Numata, 1999, 2000) | Fimbrin, EF1a, actin, calmodulin co‐localize to the contractile ring (Shirayama & Numata, 2003; Gonda et al., 2000; Gonda & Numata, 2002) |
| Late cytokinesis | Cytokinesis progresses to a point when the daughters are connected by a thin cytoplasmic bridge. Rotokinesis takes place, a series of cilia‐driven circumferential rotations primarily of the posterior daughter that sever the cytoplasmic bridge releasing the two daughter cells (Brown, Hardin, et al., 1999) | The macronucleus completes its amitotic division | |
| Postdivision | The separated daughters continue to remodel their newly formed ends | Increased presence of the 12G9 epitope is observed at the new apical end of the posterior daughter (Jerka‐Dziadosz et al., 2001) |
ANTERIOR‐POSTERIOR GRADIENTS IN THE CILIATE CORTEX
Physiological and morphogenetic gradients
Having introduced the ciliate cell cortex as a dynamic theater for intracellular patterning, and as the site of assembly for the major organelles whose timing and spatial position take center stage, it is time to ask: do ciliates exhibit A/P gradients, and if so, do they use such gradients to establish cortical pattern?
Historically the first evidence pointing to cortical gradients in ciliates involved physiological approaches. Namely, in Paramecium, these studies uncovered an anterior–posterior decreasing gradient of plasma membrane conductivity attributed to mechanosensitive Ca2+ channels and an opposite posterior–anterior gradient of K+ conductivity (Ogura & Machemer, 1980). Additional A/P gradients attributable to either ion‐channels or chemoreceptors have been found in Paramecium and Stylonychia (Machemer & Deitmer, 1985; Oami, 1996; Peyer & Machemer, 1977; Preston & Van Houten, 1987).
Anterior–posterior and circumferential gradients of morphogenetic activities have also been described. As Tetrahymena grow during interphase, new BBs are inserted into the somatic ciliary rows, but the distribution of their insertion is not random. The region of greatest BB proliferation is centered ventrally along the stomatogenic row and its flanking ciliary rows, and equatorially at mid‐body. Curiously, the center of this area corresponds to the region in which BB assembly initiates OP formation in the early divider (Kaczanowski, 1978; Nanney, 1975).
During interphase in Tetrahymena, there is a significant delay between the time of assembly of new BBs and their ciliation (emergence of the cilium proper from the BB). Consequently, prior to cell division, many BBs are completely assembled and yet remain unciliated. Once the cell enters division, the posterior daughter cell undergoes an anterior‐to‐posterior wave of basal‐body ciliation (Frankel et al., 1981).
Wloga and colleagues noted that during interphase, there is an A/P gradient in the length of somatic cilia in Tetrahymena, with the shortest cilia at the anterior cell tip (Wloga et al., 2006). In the same study, several members of the NRK/NEK family of kinases were overproduced. NRK/NEK kinases have an evolutionarily conserved cilia‐shortening activity (Bradley & Quarmby, 2005; Mahjoub et al., 2004). Interestingly in Tetrahymena each of several overproduced NRKs caused preferential shortening of a different subset of cilia, in some cases producing gradients of ciliary resorption along either the A/P or circumferential axes (Wloga et al., 2006). Overall, these observations suggest that factors that control cilia length function as gradients.
Further evidence of anterior–posterior gradients comes from surgical manipulations of Stentor coeruleus [reviewed in Frankel (1989)]. Weisz (1951) and later Uhlig (1959) described ectopic tail formation (the “holdfast”; the posterior‐most structure that anchors Stentor to its substrate), in response to excision of a mid‐body patch of postoral cell cortex. Importantly, ectopic holdfasts were more readily formed the closer the excision was to the posterior end, possibly reflecting an underlying gradient of tail‐inducing capability.
When the anterior end of a Stentor cell carrying with it the long, spiral OA is surgically removed (Figure 8), a new OA starts to form as an OP appearing in the mid ventral region of the cell (Tartar, 1957). The OP forms as a long, linear structure, within the narrow region that can be described as a morphological seam: “the zone of stripe contrast,” the cell's meridian (likely analogous to the stomatogenic kinety in Tetrahymena). The elongated OP forms a buccal cavity (gullet) and cytostome (mouth) at its posterior‐most end, closest to the holdfast (Figure 8B, lower arrow). When the developing OP was surgically rotated 180°, two sets of buccal structures formed: one at the original site (now anterior‐most) and a second in the posterior (newly apposed to the posterior cell tail, Figure 8C). This suggests an influence from the cell's posterior region that induces construction of specific components within the developing OP. Moreover, when Uhlig (1959) and Tartar (1961) grafted an ectopic tail near the middle of an already developing OP, a second, partial set of buccal structures formed in the mid region of the OP as well as at the normal posterior, again demonstrating what appears to be a posterior inductive influence (Figure 8D). Frankel viewed these surgical data as evidence for a potential gradient of some oral development signal molecule emitted from the posterior region of the cell (Frankel, 1989). We will review evidence indicating that in Tetrahymena the posterior region also expresses an inhibitory influence that prevents the OP from developing too close to the posterior cell end (see below) and the gene products associated with this activity (Elo1 and Mob1) present in genuine decreasing posterior‐to‐anterior gradients (Jiang et al., 2019a; Tavares et al., 2012). Remarkably, these findings in both Tetrahymena and Stentor indicate the presence of two posterior‐to‐anterior gradients, one inhibitory and one stimulatory, in single celled organisms resembling those documented in the multicellular Hydra (Figure 3 ). To be accurate, these two posterior gradients affect different aspects of OP formation. The Elo1/Mob1 gradient in Tetrahymena specifies the initial position of the entire OP along the cell's A/P axis while the mouthpart‐inducing gradient of Stentor induces specific oral structures (the buccal cavity and cytostome) at the posterior end of Stentor's unusually elongate, ribbon‐like OP.
FIGURE 8.
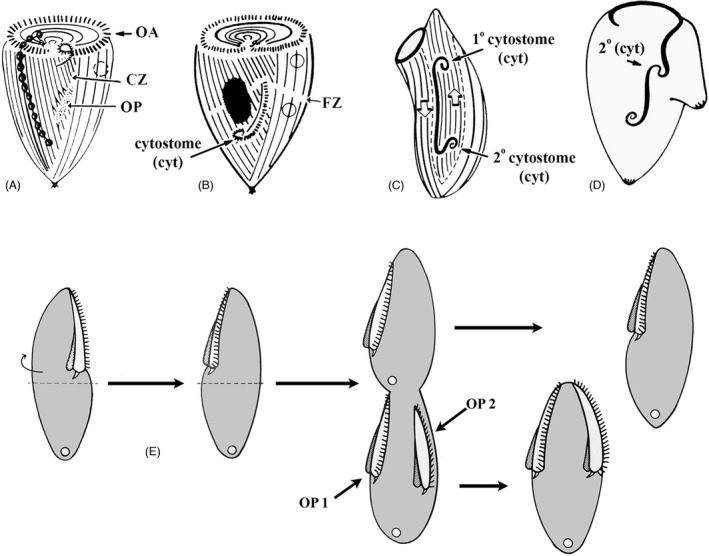
Anterior‐posterior morphogenetic gradients deduced from microsurgical experiments in Stentor [A–D, based on (Tartar, 1961)] and Blepharisma [E, modified from (Suzuki, 1957)]. Fig. A) diagrams a Stentor cell with a developing OP that assembles at the zone of stripe contrast (CZ = contrast zone). This region exhibits a visible “seam” between broad longitudinal stripes of pigment granules, and narrow stripes. (B) A dividing Stentor cell whose OP has begun to differentiate a “cytostome” at its posterior end (arrow). (C) A surgically manipulated Stentor in which the OP has been excised and rotated 180°. Its original cytostome is now positioned at the anterior end and a new, 2° cytostome has been induced at its new posterior. (D) A Stentor regenerating its OP, with a second posterior surgically grafted nearby. This has, apparently, induced formation of a 2° cytostome in the middle of the developing OP. (E) A classic surgical result from Blepharisma [modified from (Suzuki, 1957)]. In the first panel, the anterior hemicell of a nondividing cell is severed, rotated, and grafted back to the posterior hemicell. The right‐most panels depict development of two oral primordia, the first assembling just posterior to the mature OA (in a postequatorial region that would not normally form an OP). The second OP forms where it would normally have formed had there been no surgery. This highlights the ability of a mature OA to “induce” or at least specify where a novel OP will form
Gradients of protein phosphorylation
So far, we have reviewed evidence suggesting that ciliates exhibit cortical gradients in the form of physiological or morphogenetic activities. As we shall see, ciliates also exhibit gradients in protein “phospho‐states.” Kaczanowska and colleagues (Kaczanowska et al., 1999) probed Tetrahymena with the MPM‐2 monoclonal antibody originally raised against cell‐cycle‐specific mammalian phosphoproteins (Davis et al., 1983; Kuang et al., 1991). In dividing cells, and at early stages of FZ formation, MPM2 preferentially labeled BBs at both the (old) anterior end of the dividing cell, and just posterior to the FZ, in what will become the new anterior end of the posterior daughter. In cells with a fully developed FZ, the posterior daughter showed a strong MPM2 signal along the ciliary rows in the form of an A/P gradient, highest at the FZ (Kaczanowska et al., 1999). The area of most intense MPM2 signal corresponds to the area where epiplasm proteins are cleared and where fenestrin accumulates as the FZ develops (Cole et al., 2008; Joachimiak et al., 2013; Kaczanowska et al., 2003; Nelsen et al., 1994). It is tempting to theorize that gradients of kinase activity could position phosphorylation activities causing localized cortical remodeling. Future identification of cortical phosphorylated proteins that are recognized by MPM2 could be instructive.
Kaczanowska and colleagues used kinase inhibitors to explore the functional significance of the observed phosphorylated epitope gradients. In one study (Kaczanowska et al., 1999), Tetrahymena cells were treated with 6‐dimethylaminopurine (6‐DMAP): a protein kinase inhibitor that blocks the prophase‐metaphase transition in animal oocytes (Neant et al., 1989; Rime et al., 1989). 6‐DMAP prevented completion of Tetrahymena division, and in some cells, shifted the position of the OP and the FZ anteriorly (Figure 9B; Kaczanowska et al., 1999). These observations point to kinase activities defining the A/P location of the entire division plane. More recently, Kaczanowska et al. (2012), treated proliferating Tetrahymena cells with roscovitine, an inhibitor of cyclin‐dependent kinases (CDKs; De Azevedo et al., 1997). Remarkably, roscovitine displaced the FZ posteriorly, sometimes bisecting the developing OP, or localizing it posterior to the OP creating an anterior division product with two mouths (Figure 9C). Furthermore, in some roscovitine‐treated cells new CVPs formed posterior to the FZ (Kaczanowska et al., 1999). To summarize, Kaczanowska and colleagues’ pharmacological experiments indicate that protein phosphorylation (potentially produced by multiple kinase species), controls the A/P location of the FZ and new organelles. Importantly, these drug treatments produce phenotypes that resemble those in some single or double cell division/patterning mutants isolated by Frankel's laboratory (Frankel, 2008) and indeed the known loci encode kinases or kinase activators (see below). Namely, the end point produced by 6‐DMAP (an anteriorly shifted division plane) phenocopies cdaI‐1 and CdaI is a Hippo/Mst kinase (Jiang et al., 2017). Furthermore, the shifts in the relative positions of OP, FZ, and new CVPs were also observed in the double mutant cdaI‐1;cdaA‐1 (Jiang et al., 2020).
FIGURE 9.
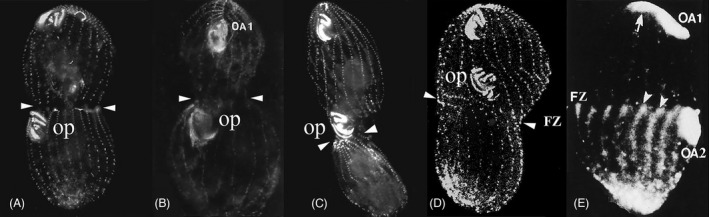
Role of protein phosphorylation in specification of the division plane position in Tetrahymena. (A) Control cell, BBs labeled with anticentrin (Kaczanowska et al., 2012). Arrows indicate FZ. (B) A 6‐DMAP‐treated cells showing anterior displacement of FZ and OP (note unequal size of daughter cells; Kaczanowska et al., 1999). (C) A roscovitine‐treated cells showing posterior displacement of FZ (but not the OP; Kaczanowska et al., 2012). (D) A double mutant (cdaA‐1/ cdaI‐1) grown at restrictive temperature (Jiang et al., 2020). OP is displaced anterior to the FZ. (E) MPM‐2 immunostaining of triton‐extracted T. pyriformis, a gradient of phosphorylated protein epitopes posterior to the FZ [from Kaczanowska et al. (1999)].
THE SEARCH FOR PATTERN‐REGULATOR GENES IN CILIATES
In the mid‐1970s, Peter Bruns (Cornell University) and Eduardo Orias (U.C. Santa Barbara) developed genetic techniques for making homozygous mutants in Tetrahymena thermophila (Bruns et al., 1976; Bruns & Sanford, 1978; Orias & Bruns, 1976). Shortly thereafter, Joseph Frankel (University of Iowa) began applying these methods to systematically prospect for Tetrahymena genes involved in pattern formation during cell division (Frankel et al., 1976a, 1976b, 1977). From the initial Cornell University collection, Frankel also characterized janA‐1 (janus A), a mutant that expresses a global, mirror‐image cortical pattern duplication (Frankel & Jenkins, 1979; Jerka‐Dziadosz & Frankel, 1979). These achievements encouraged Frankel to persevere in a career‐long search for mutations that influenced cortical pattern in Tetrahymena thermophila.
Following a strategy of nitrosoguanidine‐mutagenesis, homozygosis and clonal screening, the Frankel lab isolated over 40 recessive Tetrahymena pattern mutants (Frankel, 2008). Several mutant loci specifically affect A/P patterning during cell division: elo1, con1, cdaI, cdaH, cdaA, cdaK, and psm. The mutants identified in the Frankel lab hinted at a rich gallery of gene products that are involved in establishing and maintaining the A/P pattern in Tetrahymena, but for decades there was no means of identifying the causal mutations. Then, in 2006, the Tetrahymena macronuclear genome was published, fulfilling a long‐time goal for one of the pioneers of genome studies in Tetrahymena, Eduardo Orias (Eisen et al., 2006). Also, that same year, the first draft of the macronuclear genome of Paramecium tetraurelia was published (Aury et al., 2006).
These milestone achievements were followed by several “mapping by sequencing” studies that identified causal sequence variants in a number of mutants, first in Paramecium and later in Tetrahymena (Bhullar et al., 2018; Galati et al., 2014; Kontur et al., 2016; Marker et al., 2014). In both species, next‐generation sequencing (NGS) strategies, while differing in details, were based on the principle of “bulked segregant analysis.” This strategy examines pools of cross progeny to identify sequence variants that co‐segregate with the mutant phenotype (Birkeland et al., 2010; Michelmore et al., 1991). In 2014, Galati and colleagues (Galati et al., 2014) identified the first cortical pattern gene from Frankel's collection, disA‐1 (disorganized). disA‐1 mutants have highly fragmented and mispositioned ciliary rows but (surprisingly) manage to assemble the major structures (OP, FZ, CVPs) nearly correctly and divide (Jerka‐Dziadosz et al., 1995). DisA is a protein with weak homology to SF‐assemblin, a component of the BB‐associated striated fiber originally discovered in green algae (Lechtreck & Melkonian, 1998). DisA localizes to the striated fibers of Tetrahymena (also known as the kinetodesma or KD fibers; Figure 4; Galati et al., 2014). The disA‐1 phenotype is likely due to a loss of cohesion between the linearly adjacent BBs within a ciliary row that is normally provided by the kinetodesma (Galati et al., 2014; Iftode & Fleury‐Aubusson, 2003; Nabi et al., 2019; Soh et al., 2017, 2020). Because the A/P global pattern formation in disA‐1 is nearly unaffected (Jerka‐Dziadosz et al., 1995), DisA protein likely plays a role confined to local patterning. More recently, Jiang and colleagues used similar comparative NGS to identify CdaI, Elo1 and CdaA proteins that clearly play a role in global (cell‐wide) patterning along the A/P axis (Jiang et al., 2017, 2019a, 2020). In addition, reverse genetic approaches have implicated Mob1 (Tavares et al., 2012) and Sas4 (Ruehle et al., 2020) in the A/P patterning in Tetrahymena. The known gene products, and the yet‐to‐be‐identified loci involved in Tetrahymena A/P patterning are summarized in Table 2, with detailed descriptions to follow.
TABLE 2.
Gene products associated with A/P patterning in Tetrahymena
| Gene product | Gene ID | Early mutant phenotype | Localization | References |
|---|---|---|---|---|
| Elo1 (Lats kinase) | TTHERM_00035550 | Posterior shift of OP (elo1‐1) | Gradient decreasing from the posterior cell end | Jiang et al. (2019a) |
| Con1 | ? | Posterior shift of the OP (con1‐1) | ? | Doerder et al. (1975) |
| CdaI (Hippo/Mst kinase) | TTHERM_00971920 | Anterior shift of OP and FZ (cdaI‐1; cdaI‐3/39°C) | Anterior half of dividing cell before FZ formation | Jiang et al. (2017) |
| Mob1 (Mob adapter of Lats kinase) | TTHERM_00716080 | Anterior shift of OP and FZ (knockdown) | Gradient decreasing from the posterior cell end | Tavares et al. (2012) |
| CdaA (Cyc8; cyclin E domain protein) | TTHERM_00332170 | No FZ (cdaA‐1, cdaA‐4/39°C) | Posterior half of dividing cell before FZ formation | Jiang et al. (2020) |
| Sas4 | TTHERM_00382220 | Anterior shift of the FZ (knockout) | Basal bodies | Ruehle et al. (2020) |
| CdaH | ? | No FZ, anterior shift and partial degradation of OP (cdaH‐1/39°C), | ? | Frankel et al. (1980a), Frankel (2008) |
| CdaK | ? | Oblique FZ (cdaK‐1/39°C) | ? | Frankel (2008), Krzywicka et al. (1999) |
| PsmA | ? | OP spreads along the A/P axis (psmA‐3/39°C) | ? | Frankel et al. (1984) |
GENE PRODUCTS THAT CONTRIBUTE TO EARLY A/P PATTERNING (BEFORE FISSION ZONE INDUCTION)
We will divide the A/P polarity factors into two groups reflecting when the mutant phenotype first appears using the FZ as a landmark: those acting early (before the FZ formation) and those acting late (at the time of, or just after FZ formation). Mutant phenotypes are diagrammed in Figure 10.
FIGURE 10.
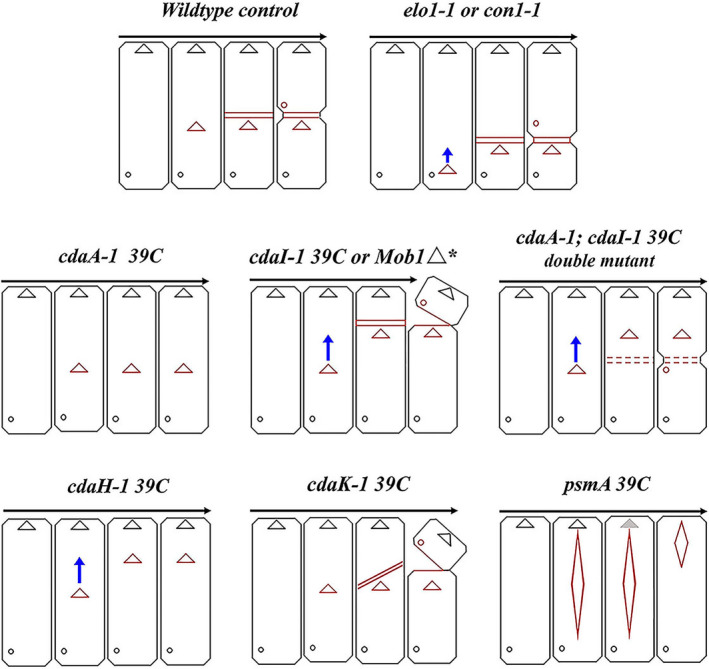
Diagram illustrating major features of cortical mutant phenotypes of Tetrahymena discussed. Red triangles and diamonds: developing OPs. Red cross‐bars: developing FZ. Small red circles: developing CVPs. Blue arrows indicate anterior cortical slippage of OP to more anterior location. Anterior hemi‐cells partially divided from posterior hemi‐cells indicate “hammerhead” phenotypes with incomplete fission.
The appearance of the OP marks the onset of the tandem duplication that culminates in cell division. Several cortical activities that control OP formation were first detected based on microsurgeries performed on larger ciliates. Studies in Stentor suggest that the cell has to achieve a certain minimal size to be able to activate the program of OP formation [oral/somatic ratio; (De Terra, 1969)]. The old OA may inhibit OP assembly in nondividing cells. For example, grafting an OA or its part onto a regenerating Stentor (from which OA was earlier excised), delays OP development or even triggers OP resorption (Hyvert et al., 1972; Tartar, 1958). On the other hand, the old OA may have an activating influence for OP development as well. In Blepharisma, when the anterior hemi‐cell (containing the mature OA) is rotated 180° and reattached to the posterior hemi‐cell, two OPs will form as the cell enters cell division: one along the longitude corresponding to the original OA position (suggesting residual OP‐determinants exist in the posterior hemicell) and another posterior to the newly positioned OA (suggesting that the grafted OA has transmitted OP‐determinants to the neighboring, posterior cortex (Frankel, 2008; Suzuki, 1957; Figure 8E). Again, as we pointed out for the posterior cell end, the anterior end may be the origin of both activator and repressor activities controlling OP formation. While the identity of oral activator and repressor activities are unknown, recent molecular studies of some of Frankel's mutants are beginning to shed light on how the precise position of the OP is determined (see below).
Elo1
In the elo1‐1 mutant there is a concerted posterior displacement of virtually all new cortical structures eventually leading to an unequal cell division producing a small posterior and a large anterior daughter cell, respectively (Frankel, 2008). Importantly, the earliest manifestation of the elo1‐1 defect is the formation of a structurally normal OP at a position that is excessively close to the posterior cell end (Figure 10). Interestingly, the initial extreme posterior OP location is partly normalized as division progresses, but at all times the OP remains excessively posterior (Frankel, 2008; Jiang et al., 2019a). Whether this correction is a result of anterior migration of the OP (cortical slippage) or differential growth of the cortex posterior to the OP is unclear. All subsequent new structures (FZ, new cell ends, and CVPs) form at posteriorly shifted positions but in local agreement with each other, and the daughter cells are structurally normal except for their size. Significantly, elo1‐1 shifts the positions of boundaries of cortical domains formed by polarity markers that appear at later stages and drive the FZ initiation and positioning (CdaI and CdaA, see below). The ELO1 gene (TTHERM_00035550) encodes a kinase orthologous to highly conserved Lats/Ndr kinases (Jiang et al., 2019a). In other species these are invariably part of the Hippo pathway. Hippo signaling was discovered in Drosophila as a pathway that controls the cell proliferation in the context of organ size control (Gokhale & Pfleger, 2019; Udan et al., 2003). Hippo signaling generates diverse outputs that contribute to the cell cycle, cell polarity, cell size regulation, mechano‐sensation and cell differentiation [reviewed in (Davis & Tapon, 2019; Misra & Irvine, 2018)]. The most conserved components of Hippo signaling are two kinases (Hippo/Mst and Lats/Ndr) and the Mob1 adapter that binds to Lats/Ndr. In metazoans, Hippo/Mst kinases are activated by diverse upstream signals including signaling complexes associated with the cell surface or cell junctions. Hippo/Mst then phosphorylates Lats/Ndr and Mob and the activated Lats/Ndr phosphorylates downstream targets. In animal embryos, the main targets of Lats/Ndr are transcription factors YAP/TAZ that are not conserved outside of the Metazoa. In fungi, Lats/Ndr kinases phosphorylate proteins involved in the exit from mitosis (Cdc14), cytokinesis (Cyk3, Hof1), and the differentiation of daughter cells [reviewed in (Hergovich & Hemmings, 2012)]. Elo1‐GFP (shown in green in Figure 11) localizes to the BBs in the posterior‐most region of the cell cortex both at interphase and during cell division (Jiang et al., 2019a). Importantly, Elo1‐GFP presents as a genuine gradient highest around the most posterior BBs. When the OP first emerges as an anarchic field, it assembles in the mid‐ventral region of the cell along the stomatogenic ciliary row, and just anterior to the low end of the Elo1 gradient [based on confocal and super‐resolution microscopy of Elo1 tagged at its own locus (Jiang et al., 2019a); depicted green in Figures 11, 12]. When the FZ forms, Elo1 makes a novel appearance at the newly formed posterior end of the anterior daughter cell (Figure 11).
FIGURE 11.
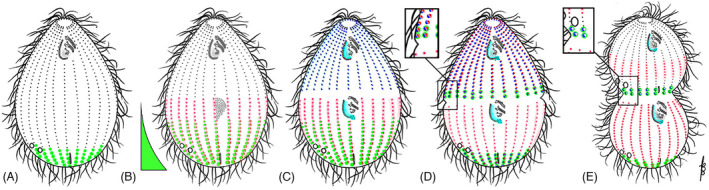
Localizations of proteins involved in A/P patterning in Tetrahymena. (A) interphase; (B) early oral development; (C) early cortical subdivision; (D) early cytokinesis/cell end emergence; (E) late cytokinesis. Green = Elol (Lats/Ndr Kinase) andMob1 (Lats/Ndr kinase adapter). Blue = CdaI (Hippo/Mst kinase). Red = CdaA (cyclin E). Elo1 forms a posterior‐high gradient present during the entire cell cycle including interphase (stage A). The OP forms anteriorly to the low end of the Elo1/Mob1 posterior gradient (B). Before the FZ induction, CdaA appears as streaks in the posterior hemi‐cell. With a slight delay, CdaI covers the anterior semi‐cell (B–C) The FZ first manifested as an equatorial cortical gap forms between the margins of CdaA and CdaI cortical zones (C). When the FZ is fully developed, CdaA also appears in the anterior hemi‐cell (D). After the FZ emergence, Elo1, Mob1 and CdaI accumulate at the new posterior cell end anteriorly to the FZ (D, E). Inserts highlight that, just anterior to the newly formed FZ, there is triple‐labeling around BBs including Elo1, CdaI, and CdaA. Later, as fission progresses, Elo1 and CdaI remain associated with the new posterior end. The CdaI signal disappears around the time of completion of cytokinesis (not shown). Thus, only Elo1 and Mob1 remain when the postdivider enter interphase
FIGURE 12.
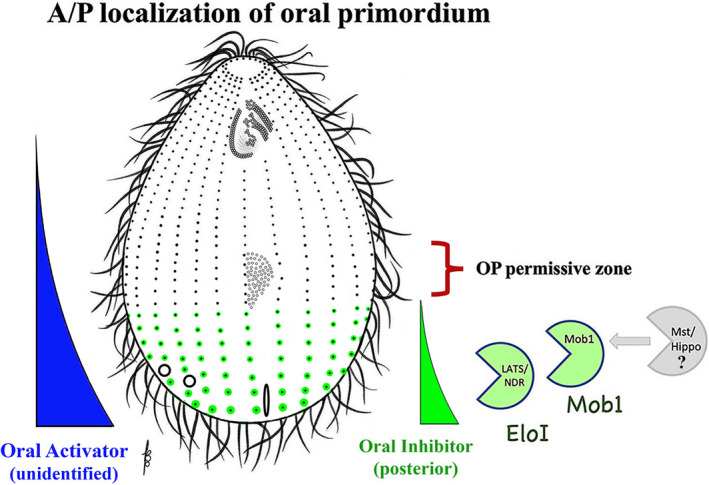
Early acting specification of the A/P position of OP assembly. The posterior early Hippo circuit (Elo1/ Mob1 driven), inhibits OP initiation within the most posterior cell region. On the left a hypothetical gradient of activity is depicted that originates from the posterior cell end and induces the formation of portions of the OA (based on the observations in Stentor). The molecular nature of this gradient is unknown
The simplest model that can account for both the Elo1 loss‐of‐function phenotype and its restricted localization is that Elo1 prevents the OP and all subsequent structures from forming within the (posterior) cortical domain that it occupies. This suggests that in the absence of Elo1, oral development (by default) initiates in a more posterior location along the stomatogenic kinety. These conclusions recall to mind the microsurgical experiments (in Stentor) indicating a cytostome‐inducing influence emanating from the posterior end of the cell (hypothetical blue gradient, Figure 12; Tartar, 1961; Uhlig, 1959; Weisz, 1951). Thus, both OP activating and inhibitory gradients may emanate from the posterior cell region.
Elo1 most likely operates as part of the conserved Hippo protein triad, activated by an unknown Hippo/Mst kinase and working in a complex with a Mob scaffolding protein. In addition to Hippo/Mst encoded by CdaI (that is known to act at the later stage to position the FZ), the genome of Tetrahymena encodes three unstudied Hippo/Mst kinases (TTHERM_00933100, TTHERM_01246760, and TTHERM_00580440). Thus, one or more could phosphorylate and activate Elo1. Based on a remarkable colocalization during interphase and cell division, Mob1 (described below) is the likely binding partner of Elo1/Lats (with the caveat that a knockdown of Mob1 phenocopies cdaI‐1 rather than elo1‐1, see below). Note that a second burst of Hippo signaling activity occurs at the time of FZ formation and cytokinesis and involves the CdaI Hippo/Mst kinase and most likely also Mob1 (see below). It appears that the two periods of Hippo activity (with Elo1 and CdaI) are consecutive. For example, a double mutant elo1‐1; cdaI‐1 initially has the phenotype of elo1‐1 alone (a posteriorly shifted OP) and later the OP undergoes an anterior migration characteristic of cdaI‐1 (see below). This goes beyond the anterior correction seen in the single elo1‐1 mutant. Consequently, in the double‐mutant the division plane is positioned correctly at the cell's equator (Jiang et al., 2019a). The signaling activity involving Elo1/Lats and likely Mob1 was named the “early Hippo circuit” while the subsequent activity with CdaI/Hippo/Mst and also Mob1 was named the “late Hippo circuit.”
Con1
Con1‐1 mutants also develop a posteriorized OP and divide unequally (Figure 10; Doerder et al., 1975; Frankel, 2008). The OP initially forms very close to the posterior cell end and later shifts a bit, but the division products remain of unequal size (like in elo1‐1). Unlike elo1‐1, the con1‐1 cells are also abnormally shaped. They are wider and the position of their maximal width is closer to the posterior end (in contrast to the wild type where the cell is widest midway between the two ends; Doerder et al., 1975; Lynn, 1977). However, another allele, con1‐2, displays the posteriorly shifted division plane while its overall shape is normal and resembles elo1‐1 more closely (Frankel, 2008). Thus, the abnormal shape is allele‐specific, and the defect shared by both alleles is the posterior shift of the division plane. The CON1 gene product remains unknown but based on the similarity of con phenotypes to elo1‐1, we speculate that Con1 may also be a protein that is part of the early Hippo circuit.
Mob1
Mob proteins are conserved components of Hippo signaling, functioning as activating adapters of Lats/Ndr kinases [reviewed in Delgado et al. (2020)]. Mob was discovered in yeast as a protein required for completion of M phase (Luca & Winey, 1998). Tavares and colleagues were first to implicate the Hippo pathway in the A/P positioning in Tetrahymena based on their studies of Mob1 (Tavares et al., 2012). The pattern of localization of Mob1 closely resembles that of Elo1‐GFP (Jiang et al., 2019a; Tavares et al., 2012). Both proteins exhibit a posterior to anterior gradient that is already present during interphase and is duplicated during a late stage of division coincident with the formation of the new posterior cell end in the anterior daughter cell (Figures 11 and 12, “green”). A homology‐based model of Elo1 reveals a high level of conservation with other Lats/Ndr kinases including the region known to bind Mob. Thus, it is likely that Elo1 works in a complex with a Mob, and likely this Mob is Mob1 based on the strict colocalization of the two proteins (Figure 12). However, Tetrahymena possess a second unstudied Mob, Mob4 (TTHERM_001262898). Because the knockdown of Mob1 phenocopies cdaI‐1 (a component of the late Hippo circuit, see below), and not elo1‐1 (Jiang et al., 2017), Mob4 may be redundant with Mob1 in the early Hippo circuit. Alternatively, as suggested, a knockdown of Mob1 does not affect the early Hippo circuit because Mob1 is already present in the cell cortex in interphase, but does affect the late Hippo circuit because it may require newly synthesized Mob1 to be deposited at the forming posterior cell end (Jiang et al., 2017).
The presence of both Elo1 and Mob1 gradients during interphase raises a question about whether they contribute to organelle positioning during interphase prior to their role in positioning of the OP. A clue that there could be an interphase function for Hippo signaling came from manipulations of Mob1 in Stentor (Slabodnick et al., 2014). In this giant ciliate, Mob1 also occupies the posterior cell end and forms a clear gradient decreasing toward the cell's anterior end. Like in Tetrahymena, in the dividing Stentor Mob1 appears at the developing posterior end of the anterior daughter cell. Mob1 depletion by RNAi severely disturbs Stentor's shape. Importantly, in dissected Stentors that regenerate either the anterior or posterior cell region, Mob1 depletion blocks or slows down regeneration and some cells produce lateral projections that look like ectopic tails (Slabodnick et al., 2014). These data suggest that Mob1 has a role outside of cell division, in the maintenance of the A/P polarity axis. Further investigation of the potential roles of Mob1 and Elo1 during interphase is therefore of great interest and would benefit from development of additional genetic tools to rapidly inactivate these proteins, such as fast‐acting conditional alleles [such alleles have been reported for Mob1 in the original study in budding yeast (Luca & Winey, 1998)]. Also, a null allele for Elo1 is needed to determine whether the early Hippo signaling has additional roles beyond acting as a posterior “exclusion ruler” for the OP. Because only one allele is available (elo1‐1) and it is not clear whether the mutation causes a complete loss of function, the early Hippo signaling may also play a role in the induction of the OP, not just its posterior positioning. Such an inductive role was shown for the late Hippo circuit in the case of the FZ (see below).
Psm gene products
Pseudomacrostome mutants (psmA, psmB, and psmC) occupy an uncertain place in the gallery of A/P pattern mutants (Frankel, 2008; Frankel et al., 1984). psmA‐3 is a particularly useful temperature‐sensitive allele that grows well at 30°C and fails to undergo cell divisions at 39°C (Frankel, 2008). We are considering the Psm gene products among the participants of the early stage of cell division (prior to the FZ formation) because the first sign of deviation from normal development in psm mutants is that the OP at the stage of anarchic field abnormally expands (on the A/P axis) to include cortical territories both anterior and posterior to the normal mid‐body position. In psmA‐3/39°C mutants the shift in the anterior direction is particularly impressive as the BBs of the anarchic field reach the vicinity of the old OA. The BBs then gradually organize into a more condensed nearly normal‐looking (yet enlarged) OP, but cells fail to divide (no FZ forms). At this point, normal cortical development is aborted. The mature OA is resorbed, and the enlarged “macrostome” simply replaces it at the anterior end of the undivided cell. This strongly resembles an alternative developmental program called “oral replacement” that occurs in starving Tetrahymena (Frankel, 1969; Kaczanowski, 1976). We can propose two alternative models to explain the psm phenotype. In one, Psm gene products restrict BB proliferation in the developing OP. The subsequent abnormal “replacement” of the mature OA by the OP would then be an odd amendment to the juxtaposition of the OP next to the old OA. If this scenario is correct, the Psm gene products may interact with Elo1 and Con1 based on the shared effects on initial OP localization. Alternatively, the psm mutations may trigger Tetrahymena’s alternate developmental program of oral replacement rather than midbody oral development leading to fission.
GENE PRODUCTS THAT CONTRIBUTE TO THE LATE STAGES OF CELL DIVISION STARTING WITH THE FISSION ZONE INDUCTION
During the early stages of cell division discussed above, the developing OP is the most conspicuous indicator that a cell has initiated “tandem duplication.” The subsequent stages of cell division bring a dramatic reorganization of the cell cortex around the cell's equator and just anterior to the developing OP. This generates the FZ, sculpts the newly emerging cell ends and importantly seeds the components of the early Hippo circuit (Elo1 and Mob1) at the new posterior cell end. The FZ normally forms in close proximity to the anterior edge of the OP. Both Stentor and Blepharisma can divide after a surgical removal of the OP (Suzuki, 1957; Tartar, 1966). Thus, while the FZ is precisely positioned in spatial relation to the OP, it is not dependent on the OP. In Frankel's collection, mutations in three gene products specifically affect either FZ initiation, its positioning, or both: CdaI, CdaA, and CdaH. All three mutant classes initiate OP development at normal locations (and therefore are not affected in what we define as the early stages of cell division), but in two of them (cdaH‐1 and cdaI alleles), there is a subsequent “cortical slippage,” as the OP shifts to a more anterior destination. Furthermore, in cdaH‐1, cdaA‐1 (and cdaA‐4), and cdaI‐3 mutants, the FZ fails to develop and cytokinesis is not initiated. In cdaI‐1 the FZ develops at an abnormal, anterior location but there is incomplete cytokinesis creating the iconic “hammerhead” pattern phenotype, as well as defects in nuclear divisions. The partial overlap in phenotypes among the mutants affecting the three loci suggest that the gene products interact with each other and this has indeed been shown for CdaA and CdaI (see below). Furthermore, reverse genetics has also revealed roles for Mob1 and Sas4 in late divisional events based on their loss of function phenotypes. These activities are summarized in Figure 13.
FIGURE 13.
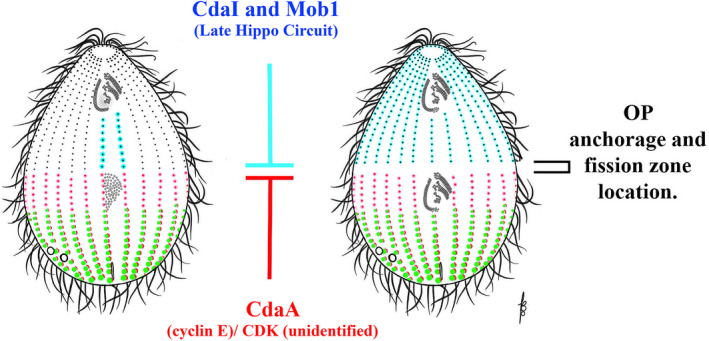
Late‐acting specification of the fission zone. This model is informed by mutant (loss‐of‐function) and GFP‐localization data. Prior to FZ induction (left panel) the posterior CdaA‐Cyclin E/CDK circuit (CDK not yet identified), represses fission zone formation (red). Later, the anterior late Hippo‐circuit (CdaI‐driven) is expressed, and it inhibits FZ assembly within the anterior hemi‐cell (blue). The CdaA and CdaI zones exclude each other. The FZ assembles at the boundary between the CdaI and the CdaA‐domains. Later, the CdaA/CdaI boundary may also contribute to assembly of the early Hippo circuit components just anterior to the fission zone which may in turn promote establishment of CdaA in the anterior hemicell during the next generation
CdaI
cdaI‐1 is a temperature‐sensitive allele. At 39°C, the cdaI‐1 mutants initially form their OP at a correct sub‐equatorial location but later the OP shifts in the anterior direction (cortical slippage), followed by the formation of the FZ at the anteriorly displaced location (Figure 10; Frankel, 2008; Jiang et al., 2017). The portions of postoral ciliary rows that are located between the old OA and new OA shorten as the OP shifts suggesting that the displacement is not merely a physical phenomenon and that some degree of cortical remodeling along the path of displacement takes place (Jiang et al., 2017). While the FZ forms at an anteriorly shifted position it is in a local agreement with the OP position (anteriorly to the OP). The new CVPs also form correctly in a local reference, close to the posterior end of the anterior daughter (Jiang et al., 2017). Thus, cdaI‐1 is defective in both the maintenance of the OP position (anchoring?) and the A/P placement of structures that form later. The degree of completion of cytokinesis arrest and nuclear division varies. Often, despite the formation of the FZ, cytokinesis is not completed and the anterior daughter tilts resulting in the “hammerhead” shape of the division‐arrested cell. While some cdaI‐1 mutants divide completely, there is frequent mis‐segregation of both the micronuclei and macronuclei and the smallest anterior daughter can lack one or both nuclei (Jiang et al., 2017). cdaI‐1 is likely a hypomorphic allele, as another allele cdaI‐3, shows a stronger defect in the FZ formation and cell division fails more often (Frankel, 2008). It is not clear whether the defects in cytokinesis and nuclear divisions reflect the continuing late role of CdaI beyond the initial positioning of the division plane or are indirect consequences of the mispositioned (anteriorly shifted) division plane.
CdaI (TTHERM_00971920) is a Hippo/Mst kinase (Jiang et al., 2017) that is proposed to be the part of the late Hippo circuit. The cdaI‐1 mutation is a P216S substitution in the kinase substrate binding region, indicating that the mutant protein has reduced enzymatic activity. Unlike Elo1 and Mob1, CdaI is undetectable in the cortex of interphase cells and first appears sometime after the emergence of the OP but (importantly) before the emergence of the FZ. CdaI‐GFP is localized in longitudinal streaks, coincident with ciliary rows within the future anterior daughter cell (blue in Figures 11 and 13). Intriguingly, the streaks start to appear on the ventral, postoral side suggesting an involvement of the old OA (or stomatogenic kinety) in initiating signaling. The postoral localization could be significant in light of Tartar's observation that a loss of the OA in the dividing cell causes an anterior shift of the division plane (Tartar, 1961), an apparent phenocopy of cdaI‐1. Possibly the old OA provides a signal for cortical loading of CdaI and its loss reduces the levels of active CdaI. In addition to the streaks along the ciliary rows, CdaI‐GFP also marked both the old and new OA. Importantly, these streaks appear before the FZ assembly. When the FZ appears as the cortical subdivision, it forms immediately outside of the posterior‐most ends of the CdaI streaks. Because the FZ moves anteriorly when CdaI is mutated, CdaI acts as an anterior cortical “exclusion ruler” for the FZ, in the manner analogous to how Elo1 act earlier on the position of the OP in the posterior region. However, as mentioned earlier, Elo1 and CdaI act consecutively and are unlikely to oppose each other in a direct way (Jiang et al., 2017).
As cell division progresses, and coincident with the formation of the FZ, CdaI‐GFP becomes more concentrated in the proximity of the FZ and persists at the newly formed posterior cell end until the end of cytokinesis. The most likely enzymatic function of CdaI (as a Hippo/Mst kinase) is to phosphorylate an unknown Lats/Ndr kinase (possibly one or more of the two unstudied Lats/Ndr kinases other than Elo1: TTHERM_00151590 and TTHERM_00283290). Mob1 is likely acting in a complex with that kinase and is activated by CdaI phosphorylation (see below and Tavares et al. (2012)).
We note that CdaI generally remains restricted to the cortex of the anterior hemi‐cell, with two exceptions. The first one is the OP that is positive for CdaI (and Mob1 (Tavares et al., 2012)) even before FZ formation. When the positions of CdaI streaks are traced for each row, they all end anterior to the FZ. This is true even for the two postoral rows of the anterior daughter. Thus, the OP signal of CdaI represents an isolated enclave of expression inside the posterior domain that generally lacks CdaI (but is occupied by CdaA, see below). The second exception is the presence of a weak signal of CdaI‐GFP at the old posterior cell end that appears exactly at the time when CdaI‐GFP signal becomes enhanced at the FZ (Jiang et al., 2017). This observation suggests that following the FZ formation (and the onset of emergence of new cell ends) there is metamerization of not only cortical structures but also molecular markers. Because CdaI‐GFP becomes undetectable during interphase, the presence of CdaI‐GFP at the old posterior end in dividers is transient. There are two explanations that we can think of. One is that the old posterior end is morphogenetically active even without visible signs of organelle assembly. Another simpler explanation is that once the new posterior cell end emerges, CdaI cannot distinguish between the old and new posterior cell region due to the presence of its binding sites at both locations. Generally, the studied polarity factors tend to be present throughout the cell as documented most clearly for CdaA using super‐resolution microscopy (Jiang et al., 2020) and therefore their enrichment to specific cortical locations is not caused by diffusion barriers but rather by selective retention or biased transport.
Mob1
In Tetrahymena, a knockdown of Mob1 produces an anterior displacement of the entire division plane (OP and FZ), defects in nuclear segregation and cytokinesis, an apparent exact phenocopy of cdaI‐1. With regard to the anterior‐displacement of the OP, Tavares and colleagues (Tavares et al., 2012) did not report whether the OP initially assembled in an abnormally anterior location, or whether it assembled normally and later underwent cortical slippage as is the case of cdaI‐1 (Frankel, 2008; Jiang et al., 2017). However, the frequent end‐point of the Mob1 knockdown is the hammerhead configuration, strongly suggesting that the Mob1 knockdown phenocopies cdaI‐1. This suspicion of course is strongly reinforced by the fact that both proteins are orthologs of the components of the highly conserved “triad” of Hippo signaling (Hippo/Mst‐Mob‐Lats/Ndr). Most likely CdaI phosphorylates and activates Mob1 and unknown Lats/Ndr kinase that is a binding partner of Mob1 and these events drive the formation of the FZ and possibly later cortical events. Mob1p makes a fresh appearance at the new posterior cell end during the FZ formation (Tavares et al., 2012) at the time corresponding to the polarization of CdaI (accumulation within the FZ region). This positions Mob1p at the right time and place to respond to the anterior‐localized CdaI (Hippo/Mst) and helps to form and position the FZ.
CdaA
Like the cdaI‐1 mutant, the cdaA‐1 mutant phenotype initially exhibits a normal placement of the OP, but later fails to develop FZ, and any indication of the new cell ends including a failure to develop new CVPs and CYP (Figure 10). However, unlike in the cdaI‐1 mutant, the OP usually stays roughly at its original position following FZ failure (Frankel et al., 1976a, 1976b, 1977, 1980a). The new apex, CVPs, and CYP do not appear (Gonda, Katoh, et al., 1999; Joachimiak et al., 2004; Kaczanowska et al., 1992, 1993, 1999). CDAA [TTHERM_00332170 also known as CYC8 (Stover & Rice, 2011)], encodes a protein CdaA with a cyclin E domain (Jiang et al., 2020). cdaA‐1 and the less studied cdaA‐4 alleles both carry substitutions in the cyclin E domain that are predicted to affect its binding to a currently unknown CDK partner (Jiang et al., 2020). That CdaA/cyclin E activates an unknown CDK is supported by an observation that inhibition of phosphatases (with okadaic acid) partially rescues the cell division arrest in cdaA‐1 mutants (Buzanska & Wheatley, 1994).
In dividing cells, CdaA initially appears as streaks in the posterior cortex at about the same time as CdaI (or perhaps a bit earlier), after the emergence of the OP but prior to the FZ formation as linear streaks along the ciliary rows (Jiang et al., 2020). When fully developed, the streaks of CdaA and those of CdaI are strictly complementary, forming a sharp boundary precisely at the cell's equator and importantly before any sign of the FZ formation. FZ assembly is initiated by “cortical subdivision,” as a narrow equatorial gap devoid of BBs, which forms in all ciliary rows (Frankel et al., 1981). Remarkably, the equatorial gap forms within the narrow area between the BBs representing the margins of CdaA and CdaI cortical domains (Jiang et al., 2020). The formation of the cortical subdivision also occurs at the exact stage that is blocked in the cdaA‐1 mutant phenotype (Frankel et al., 1980a, 1980b; Joachimiak et al., 2004). The localization of CdaA immediately prior to the formation of subdivision suggests that CdaA has a dual role: in both positioning (as a posterior “exclusion ruler”) and in inducing the formation of the subdivision (or more broadly the FZ). It should be noted that after the emergence of the FZ, CdaA appears in streaks within the anterior hemi‐cell as well. Likely this “symmetrization” of CdaA corresponds to the time at which the single A/P polarity axis is duplicated, corresponding to the emergence of daughter cells (see below). The symmetrization of CdaA is reminiscent of the transient presence of CdaI at both the new and old posterior cell end at about the same stage (see above). We also note that at about the same time major morphogenetic activities occur in sync in both the emerging anterior and posterior daughters. Following earlier partial disassembly of the old OA, the UM is being rebuilt at the same time in both daughters (Bakowska, Nelsen, et al., 1982). Thus, the symmetrization of the polarity markers correlates with the synchronization of cortical activities during oral morphogenesis.
The pattern of CdaA and CdaI prior to FZ emergence and in complementary cortical domains, raises the possibility that CdaA and CdaI antagonize one another other, in an analogy to how anterior and posterior Par complexes exclude each other in the C. elegans embryo [reviewed in (Lang & Munro, 2017; Motegi & Seydoux, 2013)]. Jiang and colleagues (Jiang et al., 2020) showed that CdaA and CdaI are indeed engaged in a mutual antagonism that controls their respective cortical boundaries while inducing the formation of the FZ. These studies revealed a role for CdaA in both localization and induction of the FZ, something that could not be anticipated based on the phenotype of the cdaA‐1 mutant alone that indicated a role solely in the FZ induction. That CdaA and CdaI control each other's cortical boundaries along the equator is indicated by the abnormal leakage of the CdaI streaks into the posterior hemi‐cell in the cdaA‐1 mutant, and a reciprocal, and complementary effect on the CdaA streaks in the cdaI‐1 mutant (at the restrictive temperatures). Furthermore, the double mutant, cdaA‐1; cdaI‐1 showed partial recovery of the FZ failure that occurs in the single cdaA‐1 mutant. Because cdaA‐1 and cdaI‐1 are both predicted to be loss‐of‐function alleles, likely CdaI activity inhibits CdaA in regard to FZ formation. That CdaA activity also inhibits CdaI is based on the observation that in the double mutant, cdaA‐1 rescues the anterior shift of the FZ conferred by cdaI‐1 alone. Interestingly, the anterior shift of the OP that occurs in the cdaI‐1 mutant is not rescued by cdaA‐1 and as a result, the FZ is positioned posteriorly to the OP in the double mutant. Thus, it appears that CdaA and CdaI act antagonistically to induce the FZ but CdaI has an independent role in controlling the position of the OP. We can speculate that CdaA and CdaI interact along the FZ but the separate pool of CdaI that localizes to the OP (where it may interact with CdaH, see below) may be involved in maintaining the subequatorial position of the OP.
Further support for a model of mutual antagonism between CdaA and CdaI in regard to the FZ positioning comes from observations of double mutants cdaA‐1;cdaI‐1 grown at the intermediate temperature 32°C. At this temperature the single mutant cdaA‐1 does not divide, while the double mutant does. In the dividing double‐mutant the position of the FZ is often abnormal in relation to the OP and as a result the FZ cuts through the OP suggesting that under these conditions the two activities are not perfectly balanced (Jiang et al., 2020). This is reminiscent of the phenotype observed in cells treated with roscovitine, a CDK inhibitor (Kaczanowska et al., 2012). We note however in the double mutant cdaA‐1;cdaI‐1, the FZ tends to be either equatorial or anteriorly shifted while in the roscovitine‐treated cells the FZ seems to be shifted posteriorly. Possibly multiple CDKs control the positioning of new structures including the postulated CDK activated by CdaA/cyclin E and another one that is inhibited by roscovitine. The genome of Tetrahymena encodes a total of 20 CDKs, most of them unstudied (Stover & Rice, 2011; Yan, Dang, et al., 2016; Yan, Zhang, et al., 2016).
To summarize, both initiation and A/P positioning of the FZ are controlled by the anteriorly restricted late Hippo circuit (with CdaI and a subpool of Mob1) that antagonizes the posterior activity containing CdaA. One fundamental role for the CdaI/CdaA antagonism is to enable the cortical loading of components of the early Hippo circuit (Elo1 and a subpool of Mob1). Then, in the next generation the early Hippo circuit positions the boundary between CdaI and CdaA.
In addition to CdaI, Mob1, and CdaA the following gene products may also be associated with activities during the late stages of cell division.
CdaH
This temperature‐sensitive mutant (Frankel, 2008; Frankel et al., 1980a) phenotype exhibits normal initial placement of the OP, but fails to form a FZ, new CVP set and CYP (Figure 10). Later the OP is partially resorbed and its residue migrates anteriorly ending up in the close proximity of the old OA. The CDAH gene remains unknown. The FZ failure resembles the phenotype of cdaA‐1 while the anterior migration of the OP resembles the phenotype of cdaI‐1 (except that in cdaI‐1 cells the OP remains intact). That cdaH‐1 manifests a combination of the phenotypes attributed to losses of either CdaA or CdaI suggests that CdaH interacts with both CdaA and CdaI. One possibility is that CdaH activity is an outcome of the CdaA/CdaI mutual antagonism and takes part in induction of the FZ. Furthermore, because CdaI and CdaH alleles cause anterior migration of the OP, CdaH, and CdaI may interact to anchor the OP position.
CdaK
In the conditional cdaK‐1 mutant, at the restrictive temperature, the OP forms correctly. However, the FZ forms in a slanted fashion with the gap positions progressively shifted to the anterior starting to the cell's right side of the postoral region (Figure 10). Some rows fail to develop gaps and cytokinesis is incomplete resulting in a hammerhead (Krzywicka et al., 1999). Note: while the end‐point of cdaK‐1 is similar to that of cdaI‐1, the way in which the defects develop in these mutants are fundamentally different (Frankel, 2008). The as yet‐unknown CdaK gene product is of high interest as a rare mutation in which the A/P defect is dependent on the cortical position on the C axis. Thus, CdaK could be a part of the mechanism that coordinates cues from the two global polarity axes (A/P and C).
Sas4
SAS‐4 protein is a highly conserved and essential component of centrioles as well as closely structurally related BBs (Gogendeau et al., 2011; Gopalakrishnan et al., 2011; Kirkham et al., 2003; Leidel & Gonczy, 2003). In Tetrahymena, a knockout of SAS4 led to a complete loss of BBs (Ruehle et al., 2020). Intriguingly, the SAS‐4 knockout cells eventually arrest in cell division with the hammerhead shape that resembles the terminal phenotype of cdaI‐1 and Mob1 knockdown cells. In the SAS‐4 knockout cells, GFP‐Mob1 is lost from the cell cortex (Ruehle et al., 2020). Thus, Mob1, a critical component of the late Hippo circuit, may require SAS4 for its binding to the posterior BBs and this may be important for the functionality of Hippo signaling. It should be noted that the same study reports that Mob1 forms a proper posterior gradient in disA‐1 mutants that have highly mispositioned BBs (Galati et al., 2014; Jerka‐Dziadosz et al., 1995; Ruehle et al., 2020) indicating that the precise alignment of BB into longitudinal rows is not required for the formation of posterior gradients by Hippo components and for proper Hippo signaling.
TERMINATION OF CELL DIVISION: CYTOKINESIS AND COMPLETION OF NEW CELL ENDS
Following the emergence of cortical subdivision, the cell constricts, new cell ends develop, and the macronucleus divides by amitosis. The contractile ring forms along the FZ plane, which contains a divergent actin (Hirono et al., 1987; Sehring et al., 2007), possibly a divergent myosin (Sugita et al., 2011), EF1α translation initiation factor (Numata et al., 2000), and several actin‐binding proteins (Edamatsu et al., 1992; Shirayama & Numata, 2003; Wilkes & Otto, 2003). Cda12, a small protein not conserved outside of the phylum of ciliates, is also required for cytokinesis. This conclusion was based on depletion of Cda12 expression by an antisense rRNA that produced large multinucleate “monsters” composed of multiple “cell units” likely resulting from cytokinesis failures (Zweifel et al., 2009). The cortical boundaries between the individual “sub‐cells” were present suggesting that the FZ forms at least to some extent and that the arrest does not affect patterning, but occurs during cytokinesis itself. Cda12 is associated with vesicles that travel along ciliary rows from posterior to anterior along the length of the cell, occasionally docking at the cell cortex. GFP‐Cda12 vesicles were also seen traveling circumferentially for short stretches, from cell right to left, possibly along the TMs. The motility speeds were similar to those reported for microtubule‐dependent motors, kinesins. In the dividing cells that have begun to furrow, GFP‐Cda12 becomes concentrated in the area just posterior to the FZ (Figure 6C; Zweifel et al., 2009). Zweifel and colleagues proposed that Cda12p associates with the recycling endosomes. In other species, recycling endosomes have been implicated in supplying membrane for the ingressing cleavage furrow during cell division (reviewed in Fraschini (2020) and Fremont and Echard (2018)).
Several mutants isolated by Frankel and colleagues also affect the terminal stages of cell division and arrest at the stage of partial constriction, possibly during cytokinesis. The genes carrying these mutations (cdaC, cdaE, cdaF, cdaG, and cdaJ alleles) remain unknown (Frankel, 2008).
At the time of emergence of the cortical subdivision, the terminal BBs in the posterior daughter cell form pairs (or couplets). These couplets appear at the anterior ends of rows 5 to n‐2 (Jerka‐Dziadosz, 1981; Wloga & Frankel, 2012). As the cell constricts, the couplets coalesce to form the “apical crown,” the cell's apex of the developing posterior daughter cell. In the mature apical crown only the posterior BBs of each pair are ciliated and the base of the apical crown is associated with a filamentous ring (the apical band; Jerka‐Dziadosz, 1981; McCoy, 1974). Cmb1/p85, a Ca2+/calmodulin‐binding protein co‐localizes with the couplets at the time of subdivision and remains associated with the apical crown in the posterior daughter (Gonda, Katoh, et al., 1999; Gonda & Numata, 2002; Numata et al., 1995). The gel mobility of Cmb1/p85 changes in cdaA‐1 cells at the restrictive temperature (Ohba et al., 1986), indicating that Cmb1/p85 is modified posttranslationally downstream of phosphorylation mediated by CdaA‐cyclin E/CDK. The function of Cmb1/p85 has not been reported.
Not much is known about how the new posterior cell end forms. New CVP and CYP assemble in the proximity of the new posterior end, just anterior to the FZ. The timing of CVP assembly with respect to oral development or FZ assembly has not been carefully documented. The CVP assembly likely requires the establishment of the FZ based on the lack of these structures in the mutants that fail to assemble the FZ (cdaA‐1, cdaH‐1; Frankel et al., 1980a, 1981; Gonda, Nishibori, et al., 1999; Kaczanowska et al., 1992, 1993, 1999). Possibly these organelles are induced and positioned only after the A/P axis is duplicated as division progresses (see below). Earlier displacement of the FZ either towards the anterior or posterior of the cell equator, typically displaces the CVP positions as well, preserving their spatial relationship (e.g. elo1‐1 and cdaI‐1). There are several notable exceptions to this. In the double mutant (cdaI‐1; cdaA‐1) raised at the restrictive temperature, CVPs sometimes develop posterior to the FZ (while the OP is positioned anterior to the FZ; Jiang et al., 2020). A hypomorphic single mutant cdaA‐2 occasionally assembles CVPs posterior to the FZ, as well (Frankel, 1979). Furthermore, in the roscovitine‐treated Tetrahymena cells new CVPs form posteriorly to the FZ (Kaczanowska et al., 2012). These observations indicate that the mechanisms that position the three developing structures (OP, FZ, and CVPs) respond to the same signaling components (Elo1, CdaA, and CdaI). However, the differential effects on positions of specific structures caused by double mutations or roscovitine indicate the mechanisms for positioning of each organelle are unique in some ways, for example, by incorporating organelle‐specific components. Overall, a complex picture emerges where the positioning of multiple structures is regulated by both the late Hippo circuit (CdaI and Mob1) and possibly multiple CDKs one of which is activated by CdaA/cyclin E. CdaI has a uniform effect on positioning of all new structures so its loss causes their shift but does not change their relative positions. The contribution of CDKs [including the undetermined CDK(s) that are activated by CdaA/cyclin E] is more structure‐specific.
At the very end of cytokinesis, when the new cell ends are fully developed, the two daughter cells remain connected by a very thin cytoplasmic bridge. The final separation of the two daughters involves a rupture of that bridge that occurs during so called “rotokinesis” (Brown, Hardin, et al., 1999). The posterior daughter undergoes a series of rotations around its long axis, while the anterior daughter remains stationary. This generates a tension on the connecting cytoplasmic bridge that is severed by several posterior movements of the two daughters. The rotations and pulling require ciliary motility, and not surprisingly, mutants lacking or having paralyzed cilia fail to separate and the two daughters gradually integrate into a compound cell (Brown et al., 2003; Brown, Marsala, et al., 1999; Williams et al., 2006). Rotokinesis is profoundly asymmetric on the A/P axis, because the posterior daughter rotates while the anterior does not. One could take this asymmetry as evidence that despite the emergence of the nearly complete daughters, some differences between the anterior and posterior division product continue to exist. In fact, the anterior and posterior daughter remain nonequivalent at least until the cytokinetic bridge is finally ruptured and the membrane breakage sites are healed at the two new cell ends. Moreover, this developmental asymmetry likely continues for some time after the scission of the division products. For example, the posterior division product shows increased presence of epitopes of the 12G9 antibody at the (new) cell apex (Jerka‐Dziadosz et al., 2001).
SUMMARY AND OVERVIEW
An important conclusion that Vance Tartar arrived at, based on his elegant surgical experiments on Stentor, was that at some point early in the cell cycle, the ciliate cortex is subdivided into future territories for the anterior and posterior daughter, and that this prepattern (or in Tartar's own words “blocking out” of the cortical domains) is established long before the first signs of fission. As Frankel states: “A central fact of ciliate life is that cortical organization is duplicated before the ciliate begins its actual constriction into two cells” (Frankel, 1989). Recent studies reviewed here, have begun to identify the signaling proteins that form this cortical blueprint, namely the Hippo signaling proteins (Elo1, CdaI, and Mob1) and CdaA/cyclin E (Jiang et al., 2017, 2019a, 2020; Tavares et al., 2012). These proteins mark cortical territories early in the cell cycle (Elo1 and Mob1) or shortly after the OP emergence but before the formation of the FZ (CdaI and CdaA). Moreover, the two Hippo proteins function as cortical “exclusion rulers” preventing structures from forming in the cortical domains that these proteins occupy (Elo1 and CdaI). Thus, Tartar's “blocking out” appears to be based on zones of cortical inhibition, such that cortical structures only form in areas that escape these inhibitory influences.
One way to achieve the needed precision in organelle localization is to invoke two opposing inhibitory zones, emanating from both the anterior and posterior ends of the cell. It appears that such a “double‐exclusion” mechanism positions the FZ between the margins of the anterior CdaI and posterior CdaA expression domains (Jiang et al., 2020). CdaI and CdaA are engaged in a mutual antagonism that both induces FZ assembly and positions it at the cell's equator. Thus, CdaA and CdaI act in a striking analogy to how the Par domains operate in the C. elegans embryo (Motegi & Seydoux, 2013). In animal cells some structures form precisely at the boundary between opposing polarity (Par) domains, including the cytokinetic ring during the first division of the C. elegans embryo, or the adherens junctions in animal epithelial cells (Bonello et al., 2021; Bruser & Bogdan, 2017; Davies et al., 2016; Jordan et al., 2016). Further investigations in ciliates could provide broader insights into the role of cortical exclusion in organelle positioning.
We speculate that the principle of “double exclusion” may contribute to the positioning of other structures that appear either before or after the FZ, namely the OP and new cell‐end associated organelles (including CVP and CYP). For example, during the early stages of cortical development, the OP forms outside of a “posterior exclusion zone” established by the early Hippo circuit (operating with Elo1 and likely Mob1). Another (yet‐to‐be‐identified) OP exclusion activity may be located in the anterior cell region, acting antagonistically to the posterior early‐Hippo‐circuit and the sum of the two activities may precisely position the OP.
It is intriguing however, that based on surgical experiments in Stentor (Tartar, 1961; Uhlig, 1959; Weisz, 1951) and Blepharisma (Suzuki, 1957), cell ends also possess positive or activating influences, including an anterior OP inducing activity and a posterior gradient that induces buccal cavity and cytostome formation in the developing OP. Possibly, the induction and positioning of organelles involves a combination of activating and inhibitory influences originating from both ends, analogous to the Hydra model of head activator/inhibitor described earlier.
In search of the primary polarity generators
As described in Tetrahymena, multiple conserved signaling proteins show dramatic asymmetric localization along the A/P axis and some (Elo1 and Mob1) form gradients. It is not yet known whether these proteins themselves generate distinct cortical domains (anterior, posterior, and mid‐body), or whether their spatial asymmetry is downstream of some, as yet unknown “primary” polarity generators. Based on studies in both Metazoa and yeast, Hippo signaling is usually not regarded as a primary cell polarization mechanism but rather as a relay responding to cues from upstream polarity factors, the Par complexes for example (reviewed in Ajduk and Zernicka‐Goetz (2016)). Among the highly conserved proteins that are known to be critical for the emergence of cell polarization, two have orthologs in ciliates. The first one is TTHERM_00267910, an ortholog of the GTPase CDC42 (Kaczanowska et al., 2008). In the budding yeast, CDC42‐GTP forms a micron‐scale patch at the plasma membrane that controls the bud emergence (Adams et al., 1990; Woods et al., 2015). As already discussed, cortical domains of ciliates are highly autonomous as indicated by their ability to sustain activities after grafting to new (and sometimes bizarre) positions (Tartar, 1968). It may be relevant that in yeast, cortical CDC42‐GTP amplifies itself in a positive feedback loop that recruits its own positive regulator CDC24 GEF (reviewed in Miller et al. (2020)). Another potential candidate for a polarity domain generator is TTHERM_00046140, a DIX domain protein (van Dop et al., 2020). DIX domains mediate self‐polymerization that forms cortical domains of polarity proteins including Dishevelled, a key component of Wnt signaling in animals, and Soseki, a polarity protein in land plants (van Dop et al., 2020). To our knowledge, the function of both proteins in ciliates have not been investigated at the time of this writing.
The role of cortical cytoskeleton in the polarized morphogen distribution
There is a singular feature of the intracellular morphogen gradients observed in ciliates so far. That is the restricted localization of pattern‐gene products to linear rows of punctae associated (either directly or indirectly) with the BBs. Elo1, Mob1, CdaA, and CdaI, while restricted to their respective anterior or posterior global domains, are all aligned along ciliary rows in intimate association with the basal bodies. Elo1 and Mob1 are actually bound to the BBs. CdaA and CdaI, while concentrated around the basal bodies, also appear to spread as streaks into the linear spaces between them. BBs seem to have emerged as important spatial foci for the distribution of many or most prepattern factors. Preliminary observations on circumferential pattern gene products, and a variety of other cortical kinases reinforce this impression, (Cole, Chalker, and Gaertig, unpublished observations).
There is a curious “disconnect” here that bears examination. BBs may well be serving as destination foci for the distribution of cortical‐pattern‐gene products. From punctate anchorages, these agents (most involved in regulating the phospho‐states of as yet unidentified target proteins), may influence their neighboring cortical architecture (reorganizing the epiplasm and defining organelle‐assembly‐domains).
That said, there are at least two lines of evidence suggesting that rather profound disruptions in BB organization have little effect on global, cortical patterning. As already mentioned, hypotrich ciliates resorb their BBs during encystment, and yet the cortical pattern redevelops upon excystment (Fryd‐Versavel et al., 2010; Grimes, 1973a, 1973b). Also, the phenotype of the disA‐1 (disorganized) Tetrahymena mutant further argues against a role connecting BBs to the more global A/P pattern. In the disA‐1 mutant cells short fragments of BB rows are dispersed and oriented randomly, yet the OP and the FZ assemble at correct A/P positions during cell division. Furthermore, the posterior gradient of Mob1 is also preserved in disA‐1 mutants (Ruehle et al., 2020). The picture that is emerging, is that delivery of pattern gene products (or “prepattern factors”) to their respective global domains (anterior vs. posterior) occurs via a mechanism that is indifferent to the condition of local BB organization.
An attractive set of candidates for this delivery system are the bundles of longitudinal microtubules that remain reasonably well preserved and oriented along the A/P axis (Jerka‐Dziadosz et al., 1995) even within the disA1 mutant phenotype. Furthermore, in the hypotrich cyst, an extensive network of cortical microtubules persist through both encystment/excystment (Fryd‐Versavel et al., 2010). We hypothesize that polarity factors may be distributed by trafficking along the longitudinally oriented bundles of cortical microtubules (LMs or the more exposed BMs) and as this material is off‐loaded, it docks and is delivered to whatever peri‐basal body spaces are in the vicinity (organized or disorganized). This focuses attention on the role of intact microtubule tracks in establishing and maintaining A/P domains and gradients within the ciliate cell cortex.
The Tetrahymena genome encodes a remarkably large number of kinesin microtubule motors [a total of 77 (Wickstead & Gull, 2006)]. So far only a handful have been studied revealing conserved functions in cilia and meiosis and are not important for patterning (Brown, Marsala, et al., 1999; Kushida et al., 2017; Vasudevan et al., 2015). Exploration of the potential pattern‐forming activities of unstudied kinesins is an important future goal that can be realized by a method that allows for high throughput generation of gene knockouts using the endogenous DNA rearrangement machinery (co‐deletion; Hayashi & Mochizuki, 2015).
Duplication of an A/P cortical gradient
The A/P axis appears to be duplicated at the time of FZ formation as a result of mutual antagonism between CdaA and CdaI across the division plane. This antagonism produces the new ends for the gradients of early Hippo activity, resulting in the gradient (and A/P pattern) duplication. These observations are reminiscent of how the cortical Par antagonism produces MEX‐5 and PIE‐1 gradients in C. elegans (Daniels et al., 2009; Griffin et al., 2011). One fundamental difference is that in ciliates the cortical antagonism at the time of the FZ formation produces not one cell‐wide A/P gradient but two gradients arranged in tandem with the high and low gradient ends forming an adjacency at the FZ. A persistent question is how the A/P pattern (dueling gradients of kinase activity) undergoes tandem duplication. For discussion let's assume that the entire A/P pattern is governed by a pair of opposing, continuous cortical gradients (Figure 14). At some point during cell division and before emergence of the daughter cells, the gradient has to be duplicated, a process that we will call “intracellular metamerism.” Put plainly, if one establishes a high‐point in a diffusible molecule, it should diffuse symmetrically and in all directions. If the "high point" is located at one end of a closed system (the anterior or posterior end of an elongate cell for example) one creates a continuous, monotonic gradient. Problems arise when that cell divides. As one cell reorganizes into two, the new posterior of what will become the anterior daughter cell, will emerge adjacent to, and contiguous with the anterior end of the newly formed posterior daughter. If one re‐establishes a “high‐point” of some diffusible morphogen at mid‐body, it should diffuse symmetrically, into both posterior and anterior division products. How can the diffusion (or delivery) of an intracellular morphogen, become highly enriched at midbody, and yet be constrained, thereby establishing tandem, sawtooth monotonic A/P gradients?
FIGURE 14.
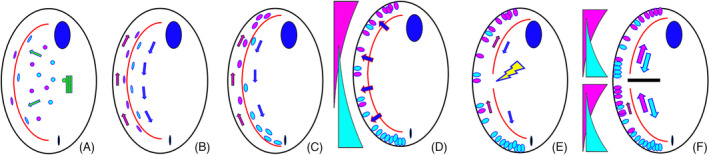
A hypothetical model depicting establishment and tandem duplication of anterior–posterior gradients of positional information in the ciliate cell cortex. (A) Hypothetical packages of “cortical morphogen” (CMPs) are synthesized and diffuse out to the cell cortex. (B) CMPs are loaded onto the exposed basal microtubule tracks, where plus‐end or minus‐end directed motors transport them towards the cell's anterior or posterior poles respectively. (C) CMPs become more concentrated in the anterior and posterior poles of the cell depending on their motor‐protein orientations. (D) If such vesicles up‐load and off‐load stochastically, and dock to deliver their contents at the cell surface (where lateral diffusion is limited), one would expect monotonic gradients of anterior morphogen (pink), highest at the cell anterior, and posterior‐morphogen (turquoise) highest at the cell posterior. (E, F) Disrupting morphogen transport at midbody would create two “high‐point” destinations for each class of CMP, one at the cell's original pole, and a second at midbody
A purely hypothetical solution is illustrated in Figure 14. In this model, cortical pattern determinants are synthesized uniformly within the cell's cytoplasm and endo‐membrane system (even this assumption is not a given). From this dispersed source of synthesis, packages diffuse to the cell cortex where they upload as cargo onto longitudinal microtubule tracks (these would most likely need to be the “basal microtubules” in this model, as they are the ones exposed and accessible to the cytoplasm). If these packages are marked with “plus‐end” directed motor proteins (pink), they migrate anteriorly (assuming the BM’s show plus ends oriented in this way as they are in the LMs). Similarly, different cargo tagged with minus‐end directed motors (turquoise), would progress to the posterior. If we imagine stochastic offloading dynamics, a gradient of cargo will be established highest in the anterior (in our plus‐end directed model). Off‐loading would (in our model) be coupled with cortical docking at the most accessible nearby sites: the peri‐basal body space.
In order to drive intracellular metamerism, one simply needs to interrupt traffic at midbody. The cell equator then becomes a new and secondary “off‐loading” depot, and the formerly continuous, monotonic gradient becomes a saw‐tooth gradient with new high/low points established at the cell‐equator or fission zone. Several published observations support at least the broadest strokes of this model. Cda12:GFP‐decorated endosomal vesicles have been observed traveling in anterior‐directed bursts along cortical tracks using live cell, confocal fluorescent imaging (Zweifel, et al., 2008). Furthermore, as cytokinesis proceeds, these Cda12:GFP‐decorated vesicles (resembling recycling endosomes) become concentrated just posterior to the developing FZ, and subsequently are enriched at the anterior end of the newly formed posterior daughter cells following cell division (Figure 6C). This supports the idea that cortical trafficking can create an anterior‐high distribution of membrane‐cargo, and that cytokinesis can lead to a secondary high‐point located just posterior to the division furrow (the “new” anterior). On a side note, CDC42 (mentioned earlier as a candidate for a “primary generator” of cortical pattern domains), has been implicated in recycling‐endosome trafficking in metazoan cells in the context of establishing cellular polarity (Harris & Tepass, 2010).
Surgical experiments on Stentor suggest further support for this model. Weisz demonstrated that removal of a cortical strip just posterior to the OP, or severing the cell at mid‐body (and rotating) lead to the emergence of a new, posterior holdfast anterior to the cortical break (Figure 15; Weisz, 1951). These results are consistent with a model in which tail‐inducing (minus‐end directed) cargo accumulates at the newly created terminus or discontinuity as recently suggested by Marshall (Marshall, 2021). It should be noted that an ectopic anterior end was never observed following these surgical interventions.
FIGURE 15.
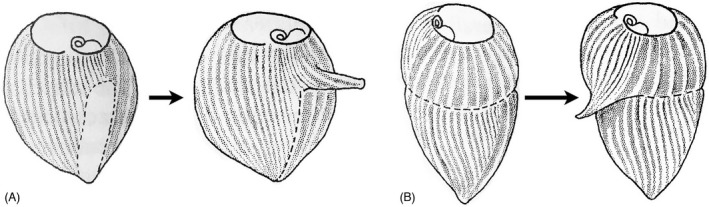
Surgical experiments by Weisz (1951) depicted by Tartar (1961). (A) Weisz removed a strip of cortex along the stomatogenic “seam” resulting in transient expression of a tail and holdfast anterior to the cut. (B) Weisz severed and rotated the anterior hemi‐cell with respect to its posterior half, provoking, again, a temporary tail and holdfast anterior to the cut. In both cases, (and especially the latter), a discontinuity is created in the cell cortex, provoking tail formation anterior to the discontinuity. This is consistent with a model in which the posterior transport of cortical determinants is interrupted, causing accumulation of posterior determinants in the more anterior location and provoking ectopic tail formation
There is precedent for developmentally‐induced disruption of the cytoskeleton at midbody during cytokinesis. At the onset of cytokinesis, the cell starts to constrict and the LM microtubule bundles undergo severing (depolymerization) within the FZ plane due to localized activity of katanin (Sharma et al., 2007; Waclawek et al., 2017). Because LMs are uniformly oriented (with plus ends closer to the anterior cell end), their severing at the FZ would create a new set of microtubule termini (minus ends in the anterior daughter cell and plus ends in the posterior daughter) for off‐loading of cargoes near forming cell ends. This, in turn, could drive the formation of new midbody highpoints within A/P directed gradients. Though establishing precedent for equatorial disruption of the longitudinal microtubule tracks that resembles our model, it must be noted that this example (at least) occurs too late in prefission development for the kind of remodeling that redistributes most of the gene products described earlier in this review (the “blocking out” that occurs long before cytokinesis). In fact, eliminating Tetrahymena's katanin “severing‐activity,” while blocking cytokinesis, in no way disrupts A/P cortical patterning (Sharma et al., 2007). Any midbody disruption of A/P trafficking (if the model holds) must involve some other mechanism.
EPILOG
Long after the phenomena of cortical patterning were first observed and described in ciliates, the molecules driving them have begun to emerge. On the A/P axis, patterning mechanisms appear to involve networks of spatially restricted and highly conserved protein kinases and kinase regulators. These players are likely serving as intermediates between signals from unknown primary polarity generators acting upstream, and the equally obscure effectors that execute cortical remodeling and organelle assembly downstream. The emerging operational principle of A/P patterning in ciliates appears to be “cortical exclusion.” The boundaries of cortical exclusion zones may be determined by specific locations within signaling gradients. This recent progress has been driven by application of modern gene mapping techniques to classical pattern mutants. Many of the gene products involved may be scarce so biochemical approaches could be challenging [but may be feasible with synchronized cell populations (Liu et al., 2021)]. That said, some of the existing pattern mutants provide selectable phenotypes (failure to divide at restrictive temperatures, or conjugal blocks) and therefore might serve as a starting point for identification of gene interactors using second‐site suppressor screens. To this end, there has already been recent success in identifying a suppressor mutation using comparative NGS (Jiang et al., 2019b). This review clearly marks the threshold of what promises to be a rich future full of new discoveries. Curiously, the words of Carl Linnaeus, the 18th century naturalist, still hold true when describing the “Chaos Infusoria” (microscopic life‐forms later known as the protozoa): “obscurae etiamnum latent plurimae moleculae vivae, quae forte ad hanc familiam spectant, posteris relinquendae” (…a great many living molecules belonging to this family remain obscure, left as puzzles for our descendants… (Linnaeus, 1767).
ACKNOWLEDGMENTS
This paper is a tribute to the pioneering work of Joseph Frankel on the mechanisms of intracellular pattern formation. The ideas, models and tools developed by Joseph Frankel will continue to provide the needed direction for future explorations of patterning mechanisms in ciliates. Research in the authors’ laboratories was supported by grants: NSF #1947608 (ESC) and NIH R01GM135444 (JG). The authors thank Joseph Frankel, Andrzej Kaczanowski, Maria Jerka‐Dziadosz, Helmut Plattner and Dorota Wloga for their careful reading of the manuscript and numerous suggestions. Our thanks to the St. Olaf College classics department for translation from the Latin of Linnaeus' quote.
Cole, E. & Gaertig, J. (2022) Anterior–posterior pattern formation in ciliates. Journal of Eukaryotic Microbiology, 69,e12890. 10.1111/jeu.12890
REFERENCES
- Adams, A.E. , Johnson, D.I. , Longnecker, R.M. , Sloat, B.F. & Pringle, J.R. (1990) CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae . Journal of Cell Biology, 111, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajduk, A. & Zernicka‐Goetz, M. (2016) Polarity and cell division orientation in the cleavage embryo: from worm to human. Molecular Human Reproduction, 22, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht‐Buehler, G. (1977) Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell, 12, 333–339. [DOI] [PubMed] [Google Scholar]
- Allen, R.D. (1967) Fine structure, reconstruction and possible functions of components of the cortex of Tetrahymena pyriformis. The Journal of Protozoology, 14, 553–565. [DOI] [PubMed] [Google Scholar]
- Allen, R.D. (1969) The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. Journal of Cell Biology, 40, 716–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aury, J.M. , Jaillon, O. , Duret, L. , Noel, B. , Jubin, C. , Porcel, B.M. et al. (2006) Global trends of whole‐genome duplications revealed by the ciliate Paramecium tetraurelia . Nature, 444, 171–178. [DOI] [PubMed] [Google Scholar]
- Bakowska, J. , Frankel, J. & Nelsen, E.M. (1982) Regulation of the pattern of basal bodies within the oral‐apparatus of tetrahymena‐thermophila. Journal of Embryology and Experimental Morphology, 69, 83–105. [PubMed] [Google Scholar]
- Bakowska, J. , Nelsen, E.M. & Frankel, J. (1982) Development of the ciliary pattern of the oral apparatus of Tetrahymena thermophila. The Journal of Protozoology, 29, 366–382. [Google Scholar]
- Barr‐Gillespie, P.G. (2015) Assembly of hair bundles, an amazing problem for cell biology. Molecular Biology of the Cell, 26, 2727–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer‐Santos, E. , Cunha‐e‐Silva, N.L. , Yoshida, N. & Franco da Silveira, J. (2013) Expression and cellular trafficking of GP82 and GP90 glycoproteins during Trypanosoma cruzi metacyclogenesis. Parasites & Vectors, 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless, B.A. , Galati, D.F. & Pearson, C.G. (2015) Tetrahymena basal bodies. Cilia, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson, J. & Sonneborn, T.M. (1965) Cytoplasmic inheritance of the organization of the cell cortex in paramecium Aurelia. Proceedings of the National Academy of Sciences of the United States of America, 53, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar, S. , Wilkes, C.D. , Arnaiz, O. , Nowacki, M. , Sperling, L. & Meyer, E. (2018) A mating‐type mutagenesis screen identifies a zinc‐finger protein required for specific DNA excision events in Paramecium. Nucleic Acids Research, 46, 9550–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland, S.R. , Jin, N. , Ozdemir, A.C. , Lyons, R.H. Jr. , Weisman, L.S. & Wilson, T.E. (2010) Discovery of mutations in Saccharomyces cerevisiae by pooled linkage analysis and whole‐genome sequencing. Genetics, 186, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, H.R. (2009) Axial patterning in hydra. Cold Spring Harbor Perspectives in Biology, 1, a000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonello, T. , Aguilar‐Aragon, M. , Tournier, A. , Thompson, B.J. & Campanale, J.P. (2021) A picket fence function for adherens junctions in epithelial cell polarity. Cells & Development, 203719. [DOI] [PubMed] [Google Scholar]
- Bornens, M. (2019) Cell polarity and the innovation of the primary cilium/centrosome organ in Metazoa. Medical Sciences, 35, 452–461. [DOI] [PubMed] [Google Scholar]
- Bradley, B.A. & Quarmby, L.M. (2005) A NIMA‐related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. Journal of Cell Science, 118, 3317–3326. [DOI] [PubMed] [Google Scholar]
- Briguglio, J.S. , Kumar, S. & Turkewitz, A.P. (2013) Lysosomal sorting receptors are essential for secretory granule biogenesis in Tetrahymena. Journal of Cell Biology, 203, 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briguglio, J.S. & Turkewitz, A.P. (2014) Tetrahymena thermophila: a divergent perspective on membrane traffic. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 322, 500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.M. , Fine, N.A. , Pandiyan, G. , Thazhath, R. & Gaertig, J. (2003) Hypoxia regulates assembly of cilia in suppressors of Tetrahymena lacking an intraflagellar transport subunit gene. Molecular Biology of the Cell, 14, 3192–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.M. , Hardin, C. & Gaertig, J. (1999) Rotokinesis, a novel phenomenon of cell locomotion‐assisted cytokinesis in the ciliate Tetrahymena thermophila. Cell Biology International, 23, 841–848. [DOI] [PubMed] [Google Scholar]
- Brown, J.M. , Marsala, C. , Kosoy, R. & Gaertig, J. (1999) Kinesin‐II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Molecular Biology of the Cell, 10, 3081–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns, P.J. , Brussard, T.B. & Kavka, A.B. (1976) Isolation of homozygous mutants after induced self‐fertilization in Tetrahymena. Proceedings of the National Academy of Sciences of the United States of America, 73, 3243–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns, P.J. & Sanford, Y.M. (1978) Mass isolation and fertility testing of temperature‐sensitive mutants in Tetrahymena. Proceedings of the National Academy of Sciences of the United States of America, 75, 3355–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruser, L. & Bogdan, S. (2017) Adherens junctions on the move‐membrane trafficking of E‐cadherin. Cold Spring Harbor Perspectives in Biology, 9, a029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzanska, L. & Wheatley, D.N. (1994) Okadaic acid promotes cell‐division in synchronized tetrahymena‐pyriformis and in the cell division‐arrested (Cdaa1) temperature‐sensitive mutant of T‐thermophila. European Journal of Cell Biology, 63, 149–158. [PubMed] [Google Scholar]
- Cole, E.S. , Anderson, P.C. , Fulton, R.B. , Majerus, M.E. , Rooney, M.G. , Savage, J.M. et al. (2008) A proteomics approach to cloning fenestrin from the nuclear exchange junction of tetrahymena. Journal of Eukaryotic Microbiology, 55, 245–256. [DOI] [PubMed] [Google Scholar]
- Cole, E.S. , Frankel, J. & Jenkins, L.M. (1987) bcd: a mutation affecting the width of organelle domains in the cortex of Tetrahymena thermophila. Roux's Archives of Developmental Biology, 196, 421–433. [DOI] [PubMed] [Google Scholar]
- Cole, E. & Sugai, T. (2012) Developmental progression of Tetrahymena through the cell cycle and conjugation. Methods in Cell Biology, 109, 177–236. [DOI] [PubMed] [Google Scholar]
- Daniels, B.R. , Perkins, E.M. , Dobrowsky, T.M. , Sun, S.X. & Wirtz, D. (2009) Asymmetric enrichment of PIE‐1 in the Caenorhabditis elegans zygote mediated by binary counterdiffusion. Journal of Cell Biology, 184, 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, T. , Jordan, S.N. & Canman, J.C. (2016) Cell polarity is on PAR with cytokinesis. Cell Cycle, 15, 1307–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, F.M. , Tsao, T.Y. , Fowler, S.K. & Rao, P.N. (1983) Monoclonal antibodies to mitotic cells. Proceedings of the National Academy of Sciences of the United States of America, 80, 2926–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J.R. & Tapon, N. (2019) Hippo signalling during development. Development, 146, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Azevedo, W.F. , Leclerc, S. , Meijer, L. , Havlicek, L. , Strnad, M. & Kim, S.H. (1997) Inhibition of cyclin‐dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. European Journal of Biochemistry, 243, 518–526. [DOI] [PubMed] [Google Scholar]
- De Terra, N. (1969) Differential growth in the cortical fibrillar system as the trigger for oral differentiation and cell division in Stentor. Experimental Cell Research, 56, 142–153. [DOI] [PubMed] [Google Scholar]
- Delgado, I.L.S. , Carmona, B. , Nolasco, S. , Santos, D. , Leitao, A. & Soares, H. (2020) MOB: pivotal conserved proteins in cytokinesis, cell architecture and tissue homeostasis. Biology, 9, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder, F.P. , Frankel, J. , Jenkins, L.M. & Debault, L.E. (1975) Form and pattern in ciliated protozoa—analysis of a genic mutant with altered cell‐shape in tetrahymena‐pyriformis, syngen‐1. Journal of Experimental Zoology, 192, 237–258. [DOI] [PubMed] [Google Scholar]
- van Dop, M. , Fiedler, M. , Mutte, S. , de Keijzer, J. , Olijslager, L. , Albrecht, C. et al. (2020) DIX domain polymerization drives assembly of plant cell polarity complexes. Cell, 180, 427–439 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever, W. & Nusslein‐Volhard, C. (1988) The bicoid protein determines position in the Drosophila embryo in a concentration‐dependent manner. Cell, 54, 95–104. [DOI] [PubMed] [Google Scholar]
- Edamatsu, M. , Hirono, M. & Watanabe, Y. (1992) Tetrahymena profilin is localized in the division furrow. Journal of Biochemistry, 112, 637–642. [DOI] [PubMed] [Google Scholar]
- Eisen, J.A. , Coyne, R.S. , Wu, M. , Wu, D. , Thiagarajan, M. , Wortman, J.R. et al. (2006) Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biology, 4, e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elde, N.C. , Morgan, G. , Winey, M. , Sperling, L. & Turkewitz, A.P. (2005) Elucidation of clathrin‐mediated endocytosis in tetrahymena reveals an evolutionarily convergent recruitment of dynamin. PLoS Genetics, 1, e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Haddad, H. , Przyborski, J.M. , Kraft, L.G. , McFadden, G.I. , Waller, R.F. & Gould, S.B. (2013) Characterization of TtALV2, an essential charged repeat motif protein of the Tetrahymena thermophila membrane skeleton. Eukaryotic Cell, 12, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotenmeyer, M. , Momayezi, M. & Plattner, H. (1999) Immunolabeling analysis of biosynthetic and degradative pathways of cell surface components (glycocalyx) in Paramecium cells. European Journal of Cell Biology, 78, 67–77. [DOI] [PubMed] [Google Scholar]
- Frankel, J. (1961) Spontaneous astomy—loss of oral areas in glaucoma chattoni. Journal of Protozoology, 8, 250. [Google Scholar]
- Frankel, J. (1962) The effects of heat, cold, and para‐fluorophenylalanine on morphogenesis in synchronized tetrahymena‐pyriformis Gl. Comptes Rendus Des Travaux Du Laboratoire Carlsberg, 33, 1–000. [Google Scholar]
- Frankel, J. (1969) Participation of the undulating membrane in the formation of oral replacement primordia in Tetrahymena pyriformis. The Journal of Protozoology, 16, 26–35. [DOI] [PubMed] [Google Scholar]
- Frankel, J. (1979) An analysis of cell‐surface patterning in tetrahymena. In: Subtelny, S. (Ed.) Determinants of spatial organization. Academic Press, pp. 215–245. [Google Scholar]
- Frankel, J. (1989) Pattern formation. Ciliate studies and models. New York: Oxford University Press. [Google Scholar]
- Frankel, J. (2008) What do genic mutations tell us about the structural patterning of a complex single‐celled organism? Eukaryotic Cell, 7, 1617–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel, J. , Jenkins, L.M. & DeBault, L.E. (1976a) Causal relations among cell cycle processes in Tetrahymena pyriformis. An analysis employing temperature‐sensitive mutants. Journal of Cell Biology, 71, 242–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel, J. , Jenkins, L.M. , Doerder, F.P. & Nelsen, E.M. (1976b) Mutations affecting cell division in Tetrahymena pyriformis. I. Selection and genetic analysis. Genetics, 83, 489–506. [PMC free article] [PubMed] [Google Scholar]
- Frankel, J. & Jenkins, L.M. (1979) A mutant of Tetrahymena thermophila with a partial mirror‐image duplication of cell surface pattern. II. Nature of genic control. Journal of Embryology and Experimental Morphology, 49, 203–227. [PubMed] [Google Scholar]
- Frankel, J. , Jenkins, L.M. , Bakowska, J. & Nelsen, E.M. (1984) Mutational analysis of patterning of oral structures in Tetrahymena. I. Effects of increased size on organization. Journal of Embryology and Experimental Morphology, 82, 41–66. [PubMed] [Google Scholar]
- Frankel, J. , Mohler, J. & Frankel, A.K. (1980a) Temperature‐sensitive periods of mutations affecting cell division in Tetrahymena thermophila. Journal of Cell Science, 43, 59–74. [DOI] [PubMed] [Google Scholar]
- Frankel, J. , Mohler, J. & Frankel, A.K. (1980b) The relationship between the excess‐delay phenomenon and temperature‐sensitive periods in Tetrahymena thermophila. Journal of Cell Science, 43, 75–91. [DOI] [PubMed] [Google Scholar]
- Frankel, J. , Nelsen, E.M. & Jenkins, L.M. (1977) Mutations affecting cell division in Tetrahymena pyriformis, syngen 1. II. Phenotypes of single and double homozygotes. Developmental Biology, 58, 255–275. [DOI] [PubMed] [Google Scholar]
- Frankel, J. , Nelsen, E.M. & Martel, E. (1981) Development of the ciliature of tetrahymena‐thermophila. 2. Spatial subdivision prior to cytokinesis. Developmental Biology, 88, 39–54. [DOI] [PubMed] [Google Scholar]
- Fraschini, R. (2020) Cytokinesis in eukaryotic cells: the furrow complexity at a glance. Cells, 9, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont, S. & Echard, A. (2018) Membrane traffic in the late steps of cytokinesis. Current Biology, 28, R458–R470. [DOI] [PubMed] [Google Scholar]
- Fryd‐Versavel, G. , Lemullois, M. & Aubusson‐Fleury, A. (2010) Maintaining cell polarity through vegetative cell pattern dedifferentiation: cytoskeleton and morphogenesis in the hypotrich ciliate Sterkiella histriomuscorum. Protist, 161, 222–236. [DOI] [PubMed] [Google Scholar]
- Fujiu, K. & Numata, O. (1999) Localization of microtubules during macronuclear division in Tetrahymena and possible involvement of macronuclear microtubules in ‘amitotic’ chromatin distribution. Cell Structure and Function, 24, 401–404. [DOI] [PubMed] [Google Scholar]
- Fujiu, K. & Numata, O. (2000) Reorganization of microtubules in the amitotically dividing macronucleus of tetrahymena. Cell Motility and the Cytoskeleton, 46, 17–27. [DOI] [PubMed] [Google Scholar]
- Galati, D.F. , Bonney, S. , Kronenberg, Z. , Clarissa, C. , Yandell, M. , Elde, N.C. et al. (2014) DisAp‐dependent striated fiber elongation is required to organize ciliary arrays. Journal of Cell Biology, 207, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerganova, V. , Bhatia, P. , Vincenzetti, V. & Martin, S.G. (2021) Direct and indirect regulation of Pom1 cell size pathway by the protein phosphatase 2C Ptc1. Molecular Biology of the Cell, 32, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghossoub, R. , Molla‐Herman, A. , Bastin, P. & Benmerah, A. (2011) The ciliary pocket: a once‐forgotten membrane domain at the base of cilia. Biology of the Cell, 103, 131–144. [DOI] [PubMed] [Google Scholar]
- Gilmour, D. , Rembold, M. & Leptin, M. (2017) From morphogen to morphogenesis and back. Nature, 541, 311–320. [DOI] [PubMed] [Google Scholar]
- Gogendeau, D. , Hurbain, I. , Raposo, G. , Cohen, J. , Koll, F. & Basto, R. (2011) Sas‐4 proteins are required during basal body duplication in Paramecium. Molecular Biology of the Cell, 22, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale, R. & Pfleger, C.M. (2019) The power of drosophila genetics: the discovery of the hippo pathway. Methods in Molecular Biology, 1893, 3–26. [DOI] [PubMed] [Google Scholar]
- Gonda, K. , Katoh, M. , Hanyu, K. , Watanabe, Y. & Numata, O. (1999) Ca(2+)/calmodulin and p85 cooperatively regulate an initiation of cytokinesis in Tetrahymena. Journal of Cell Science, 112(Pt 21), 3619–3626. [DOI] [PubMed] [Google Scholar]
- Gonda, K. , Komatsu, M. & Numata, O. (2000) Calmodulin and Ca2+/calmodulin‐binding proteins are involved in Tetrahymena thermophila phagocytosis. Cell Structure and Function, 25, 243–251. [DOI] [PubMed] [Google Scholar]
- Gonda, K. , Nishibori, K. , Ohba, H. , Watanabe, A. & Numata, O. (1999) Molecular cloning of the gene for p85 that regulates the initiation of cytokinesis in Tetrahymena. Biochemical and Biophysical Research Communications, 264, 112–118. [DOI] [PubMed] [Google Scholar]
- Gonda, K. & Numata, O. (2002) p85 binds to G‐actin in a Ca(2+)/calmodulin‐dependent manner, thus regulating the initiation of cytokinesis in tetrahymena. Biochemical and Biophysical Research Communications, 292, 1098–1103. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan, J. , Mennella, V. , Blachon, S. , Zhai, B. , Smith, A.H. , Megraw, T.L. et al. (2011) Sas‐4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nature Communications, 2, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, S.B. , Tham, W.H. , Cowman, A.F. , McFadden, G.I. & Waller, R.F. (2008) Alveolins, a new family of cortical proteins that define the protist infrakingdom Alveolata. Molecular Biology and Evolution, 25, 1219–1230. [DOI] [PubMed] [Google Scholar]
- Griffin, E.E. , Odde, D.J. & Seydoux, G. (2011) Regulation of the MEX‐5 gradient by a spatially segregated kinase/phosphatase cycle. Cell, 146, 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes, G.W. (1973a) Differentiation during encystment and excystment in Oxytricha fallax. The Journal of Protozoology, 20, 92–104. [DOI] [PubMed] [Google Scholar]
- Grimes, G.W. (1973b) Morphological discontinuity of kinetosomes during the life cycle of Oxytricha fallax. Journal of Cell Biology, 57, 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, A. (1885) Ueber kiinstliche Teilung bei Infusorien. Biologisches Zentralblatt, 3, 580–582. [Google Scholar]
- Guirao, B. , Meunier, A. , Mortaud, S. , Aguilar, A. , Corsi, J.M. , Strehl, L. et al. (2010) Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia (vol 12, pg 341, 2010). Nature Cell Biology, 12, 520. [DOI] [PubMed] [Google Scholar]
- Halliday, C. , de Castro‐Neto, A. , Alcantara, C.L. , Cunha, E.S.N.L. , Vaughan, S. & Sunter, J.D. (2021) Trypanosomatid flagellar pocket from structure to function. Trends in Parasitology, 37, 317–329. [DOI] [PubMed] [Google Scholar]
- Hardt, M. & Plattner, H. (2000) Sub‐second quenched‐flow/X‐ray microanalysis shows rapid Ca2+ mobilization from cortical stores paralleled by Ca2+ influx during synchronous exocytosis in Paramecium cells. European Journal of Cell Biology, 79, 642–652. [DOI] [PubMed] [Google Scholar]
- Harris, K.P. & Tepass, U. (2010) Cdc42 and vesicle trafficking in polarized cells. Traffic, 11, 1272–1279. [DOI] [PubMed] [Google Scholar]
- Hayashi, A. & Mochizuki, K. (2015) Targeted gene disruption by ectopic induction of DNA elimination in tetrahymena. Genetics, 201, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman, J. (1997) Patterning the Xenopus blastula. Development, 124, 4179–4191. [DOI] [PubMed] [Google Scholar]
- Hergovich, A. & Hemmings, B.A. (2012) Hippo signalling in the G2/M cell cycle phase: lessons learned from the yeast MEN and SIN pathways. Seminars in Cell & Developmental Biology, 23, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono, M. , Nakamura, M. , Tsunemoto, M. , Yasuda, T. , Ohba, H. , Numata, O. et al. (1987) Tetrahymena actin: localization and possible biological roles of actin in tetrahymena cells1. The Journal of Biochemistry, 102, 537–545. [DOI] [PubMed] [Google Scholar]
- Hoege, C. & Hyman, A.A. (2013) Principles of PAR polarity in Caenorhabditis elegans embryos. Nature Reviews Molecular Cell Biology, 14, 315–322. [DOI] [PubMed] [Google Scholar]
- Honts, J.E. & Williams, N.E. (2003) Novel cytoskeletal proteins in the cortex of Tetrahymena. Journal of Eukaryotic Microbiology, 50, 9–14. [DOI] [PubMed] [Google Scholar]
- Hubatsch, L. & Goehring, N.W. (2020) Intracellular morphogens: specifying patterns at the subcellular scale. Current Topics in Developmental Biology, 137, 247–278. [DOI] [PubMed] [Google Scholar]
- Hyvert, N. , Pelvat, B. & De Haller, G. (1972) Morphogenèse expérimentale chez les Ciliés: IV. Sur le rôle de la Zone de Membranelles Adorales dans la régénération chez Stentor coeruleus. Revue Suisse De Zoologie, 79, 1060–1068. [Google Scholar]
- Iftode, F. & Fleury‐Aubusson, A. (2003) Structural inheritance in Paramecium: ultrastructural evidence for basal body and associated rootlets polarity transmission through binary fission. Biology of the Cell, 95, 39–51. [DOI] [PubMed] [Google Scholar]
- Inaki, M. , Liu, J. & Matsuno, K. (2016) Cell chirality: its origin and roles in left‐right asymmetric development. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371, 20150403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerka‐Dziadosz, M. (1981) Cytoskeleton‐related structures in tetrahymena thermophila: microfilaments at the apical and division‐furrow rings. Journal of Cell Science, 51, 241–253. [DOI] [PubMed] [Google Scholar]
- Jerka‐Dziadosz, M. & Frankel, J. (1979) A mutant of Tetrahymena thermophila with a partial mirror‐image duplication of cell surface pattern. I. Analysis of the phenotype. Journal of Embryology and Experimental Morphology, 49, 167–202. [PubMed] [Google Scholar]
- Jerka‐Dziadosz, M. , Jenkins, L.M. , Nelsen, E.M. , Williams, N.E. , Jaeckelwilliams, R. & Frankel, J. (1995) Cellular polarity in ciliates ‐ persistence of global polarity in a disorganized mutant of tetrahymena‐thermophila that disrupts cytoskeletal organization. Developmental Biology, 169, 644–661. [DOI] [PubMed] [Google Scholar]
- Jerka‐Dziadosz, M. , Strzyzewska‐Jowko, I. , Wojsa‐Lugowska, U. , Krawczynska, W. & Krzywicka, A. (2001) The dynamics of filamentous structures in the apical band, oral crescent, fission line and the postoral meridional filament in Tetrahymena thermophila revealed by monoclonal antibody 12G9. Protist, 152, 53–67. [DOI] [PubMed] [Google Scholar]
- Jiang, Y.Y. , Maier, W. , Baumeister, R. , Minevich, G. , Joachimiak, E. , Ruan, Z. et al. (2017) The hippo pathway maintains the equatorial division plane in the ciliate tetrahymena. Genetics, 206, 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y.Y. , Maier, W. , Baumeister, R. , Joachimiak, E. , Ruan, Z. , Kannan, N. et al. (2019a) Two antagonistic hippo signaling circuits set the division plane at the medial position in the ciliate tetrahymena. Genetics, 211, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y.Y. , Maier, W. , Baumeister, R. , Minevich, G. , Joachimiak, E. , Wloga, D. et al. (2019b) LF4/MOK and a CDK‐related kinase regulate the number and length of cilia in Tetrahymena. PLoS Genetics, 15, e1008099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y.Y. , Maier, W. , Chukka, U.N. , Choromanski, M. , Lee, C. , Joachimiak, E. et al. (2020) Mutual antagonism between Hippo signaling and cyclin E drives intracellular pattern formation. Journal of Cell Biology, 219, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachimiak, E. , Kaczanowska, J. , Kiersnowska, M. & Kaczanowski, A. (2004) Syndrome of the failure to turn off mitotic activity in Tetrahymena thermophila: in cdaA1 phenotypes. Acta Protozoologica, 43, 291–301. [Google Scholar]
- Joachimiak, E. , Kiersnowska, M. , Jedynak, K. , Majewska, M. , Fabczak, H. & Fabczak, S. (2013) Cell cycle‐dependent modulations of fenestrin expression in Tetrahymena pyriformis. European Journal of Protistology, 49, 564–574. [DOI] [PubMed] [Google Scholar]
- Jordan, S.N. , Davies, T. , Zhuravlev, Y. , Dumont, J. , Shirasu‐Hiza, M. & Canman, J.C. (2016) Cortical PAR polarity proteins promote robust cytokinesis during asymmetric cell division. Journal of Cell Biology, 212, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczanowska, J. , Buzanska, L. & Frontczak, M. (1992) The influence of fission line expression on the number and positioning of oral primordia in the cdaA1 mutant of Tetrahymena thermophila. Developmental Genetics, 13, 216–222. [DOI] [PubMed] [Google Scholar]
- Kaczanowska, J. , Buzanska, L. & Ostrowski, M. (1993) Relationship between spatial pattern of basal bodies and membrane skeleton (epiplasm) during the cell cycle of Tetrahymena: cdaA mutant and anti‐membrane skeleton immunostaining. Journal of Eukaryotic Microbiology, 40, 747–754. [DOI] [PubMed] [Google Scholar]
- Kaczanowska, J. , Joachimiak, E. , Buzanska, L. , Krawczynska, W. , Wheatley, D.N. & Kaczanowski, A. (1999) Molecular subdivision of the cortex of dividing Tetrahymena is coupled with the formation of the fission zone. Developmental Biology, 212, 150–164. [DOI] [PubMed] [Google Scholar]
- Kaczanowska, J. , Joachimiak, E. , Kiersnowska, M. , Krzywicka, A. , Golinska, K. & Kaczanowski, A. (2003) The fenestrin antigen in submembrane skeleton of the ciliate Tetrahymena thermophila is proposed as a marker of cell polarity during cell division and in oral replacement. Protist, 154, 251–264. [DOI] [PubMed] [Google Scholar]
- Kaczanowska, J. , Kaczanowski, S. , Kiersnowska, M. , Fabczak, H. , Tulodziecka, K. & Kaczanowski, A. (2008) Acquisition of cell polarity during cell cycle and oral replacement in Tetrahymena. International Journal of Developmental Biology, 52, 249–258. [DOI] [PubMed] [Google Scholar]
- Kaczanowska, J. , Kiersnowska, M. , Fabczak, H. , Kaczanowski, S. & Kaczanowski, A. (2012) Effects of roscovitine on schedule of divisional morphogenesis, basal bodies proliferation and cell divisions in Tetrahymena thermophila. Acta Protozoologica, 51, 91–111. [Google Scholar]
- Kaczanowski, A. (1976) An analysis of MP gene affected morphogenesis in Tetrahymena pyriformis, syngen 1, (Species 1) ciliates. Journal of Experimental Zoology, 196, 215–230. [DOI] [PubMed] [Google Scholar]
- Kaczanowski, A. (1978) Gradients of proliferation of ciliary basal bodies and the determination of the position of the oral primordium in Tetrahymena. Journal of Experimental Zoology, 204, 417–430. [DOI] [PubMed] [Google Scholar]
- Katsumoto, T. , Higaki, K. , Ohno, K. & Onodera, K. (1994) The orientation of primary cilia during the wound response in 3Y1 cells. Biology of the Cell, 81, 17–21. [DOI] [PubMed] [Google Scholar]
- Kiekebusch, D. & Thanbichler, M. (2014) Spatiotemporal organization of microbial cells by protein concentration gradients. Trends in Microbiology, 22, 65–73. [DOI] [PubMed] [Google Scholar]
- Kirk, K.E. , Christ, C. , McGuire, J.M. , Paul, A.G. , Vahedi, M. , Stuart, K.R. et al. (2008) Abnormal micronuclear telomeres lead to an unusual cell cycle checkpoint and defects in Tetrahymena oral morphogenesis. Eukaryotic Cell, 10, 1712–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham, M. , Muller‐Reichert, T. , Oegema, K. , Grill, S. & Hyman, A.A. (2003) SAS‐4 is a C. elegans centriolar protein that controls centrosome size. Cell, 112, 575–587. [DOI] [PubMed] [Google Scholar]
- Kontur, C. , Kumar, S. , Lan, X. , Pritchard, J.K. & Turkewitz, A.P. (2016) Whole genome sequencing identifies a novel factor required for secretory granule maturation in Tetrahymena thermophila. G3, 6, 2505–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg, T.B. (2014) Cytonemes and the dispersion of morphogens. Wiley Interdisciplinary Reviews: Developmental Biology, 3, 445–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywicka, A. , Kiersnowska, M. , Wloga, D. & Kaczanowska, J. (1999) Analysis of the effects of the cdaK1 mutation of Tetrahymena thermophila on the morphogenesis of the fission line. European Journal of Protistology, 35, 342–352. [Google Scholar]
- Kuang, J. , Penkala, J.E. , Wright, D.A. , Saunders, G.F. & Rao, P.N. (1991) A novel M phase‐specific H1 kinase recognized by the mitosis‐specific monoclonal antibody MPM‐2. Developmental Biology, 144, 54–64. [DOI] [PubMed] [Google Scholar]
- Kushida, Y. , Takaine, M. , Nakano, K. , Sugai, T. , Vasudevan, K.K. , Guha, M. et al. (2017) Kinesin‐14 is important for chromosome segregation during mitosis and meiosis in the ciliate Tetrahymena thermophila. Journal of Eukaryotic Microbiology, 64, 293–307. [DOI] [PubMed] [Google Scholar]
- Lang, C.F. & Munro, E. (2017) The PAR proteins: from molecular circuits to dynamic self‐stabilizing cell polarity. Development, 144, 3405–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, S. , Klauke, N. & Plattner, H. (1995) Subplasmalemmal Ca2+ stores of probable relevance for exocytosis in Paramecium. Alveolar sacs share some but not all characteristics with sarcoplasmic reticulum. Cell Calcium, 17, 335–344. [DOI] [PubMed] [Google Scholar]
- Lansing, T.J. , Frankel, J. & Jenkins, L.M. (1985) Oral ultrastructure and oral development of the misaligned undulating membrane mutant of Tetrahymena thermophila. The Journal of Protozoology, 32, 126–139. [DOI] [PubMed] [Google Scholar]
- Lawrence, P.A. (2001) Morphogens: how big is the big picture? Nature Cell Biology, 3, E151–E154. [DOI] [PubMed] [Google Scholar]
- Lechtreck, K.F. & Melkonian, M. (1998) SF‐assemblin, striated fibers, and segmented coiled coil proteins. Cell Motility and the Cytoskeleton, 41, 289–296. [DOI] [PubMed] [Google Scholar]
- Leidel, S. & Gonczy, P. (2003) SAS‐4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Developmental Cell, 4, 431–439. [DOI] [PubMed] [Google Scholar]
- Lim, Y.W. , Wen, F.L. , Shankar, P. , Shibata, T. & Motegi, F. (2021) A balance between antagonizing PAR proteins specifies the pattern of asymmetric and symmetric divisions in C. elegans embryogenesis. Cell Reports, 36, 109326. [DOI] [PubMed] [Google Scholar]
- Linnaeus, C. (1767) Systema naturae, per regna tria naturae: secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Vienna: Vindobonae Typic Ioannis Thomae. [Google Scholar]
- Lipkow, K. & Odde, D.J. (2008) Model for protein concentration gradients in the cytoplasm. Cellular and Molecular Bioengineering, 1, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Nan, B. , Niu, J. , Kapler, G.M. & Gao, S. (2021) An optimized and versatile counter‐flow centrifugal elutriation workflow to obtain synchronized eukaryotic cells. Frontiers in Cell and Developmental Biology, 9, 664418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca, F.C. & Winey, M. (1998) MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Molecular Biology of the Cell, 9, 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn, D.H. (1977) Proportional control of organelle position by a mechanism which similarly monitors cell‐size of wild‐type and conical form‐mutant tetrahymena. Journal of Embryology and Experimental Morphology, 42, 261–274. [Google Scholar]
- Lynn, D.H. (2008) Ciliated protozoa. New York: Pergamon Press. [Google Scholar]
- Machemer, H. & Deitmer, J.W. (1985) Mechanoreception in Ciliates. In: Autrum, H. , Ottoson, D. , Perl, E.R. , Schmidt, R.F. , Shimazu, H. & Willis, W.D. (Eds.) Progress in sensory physiology. Berlin Heidelberg: Springer, pp. 81–118. [Google Scholar]
- MacWilliams, H.K. (1983a) Hydra transplantation phenomena and the mechanism of Hydra head regeneration. II. Properties of the head activation. Developmental Biology, 96, 239–257. [DOI] [PubMed] [Google Scholar]
- MacWilliams, H.K. (1983b) Hydra transplantation phenomena and the mechanism of Hydra head regeneration. I. Properties of the head inhibition. Developmental Biology, 96, 217–238. [DOI] [PubMed] [Google Scholar]
- Mahjoub, M.R. , Qasim Rasi, M. & Quarmby, L.M. (2004) A NIMA‐related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Molecular Biology of the Cell, 15, 5172–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker, S. , Carradec, Q. , Tanty, V. , Arnaiz, O. & Meyer, E. (2014) A forward genetic screen reveals essential and non‐essential RNAi factors in Paramecium tetraurelia . Nucleic Acids Research, 42, 7268–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, W.F. (2020) Pattern formation and complexity in single cells. Current Biology, 30, R544–R552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, W.F. (2021) Regeneration in Stentor coeruleus. Frontiers in Cell and Developmental Biology, 9, 753625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S.G. & Berthelot‐Grosjean, M. (2009) Polar gradients of the DYRK‐family kinase Pom1 couple cell length with the cell cycle. Nature, 459, 852–856. [DOI] [PubMed] [Google Scholar]
- McCoy, J.W. (1974) New features of the Tetrahymenid cortex revealed by protargol stainning. Acta Protozoologica, 13, 155–160. [Google Scholar]
- Mercker, M. , Kazarnikov, A. , Tursch, A. , Hoger, S. , Lengfeld, T. , Ozbek, S. et al. (2021) β‐catenin and canonical Wnts in Hydra pattern formation: insights from mathematical modelling into two distinct systems. bioRxiv. 10.1101/2021.02.05.429954 [DOI] [Google Scholar]
- Michelmore, R.W. , Paran, I. & Kesseli, R.V. (1991) Identification of markers linked to disease‐resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proceedings of the National Academy of Sciences of the United States of America, 88, 9828–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K.E. , Kang, P.J. & Park, H.O. (2020) Regulation of Cdc42 for polarized growth in budding yeast. Microbial Cell, 7, 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra, J.R. & Irvine, K.D. (2018) The Hippo signaling network and its biological functions. Annual Review of Genetics, 52, 65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, T.H. (1901) Regeneration of proportionate structures in Stentor. Biological Bulletin, 2, 311–328. [Google Scholar]
- Moseley, J.B. , Mayeux, A. , Paoletti, A. & Nurse, P. (2009) A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature, 459, 857–860. [DOI] [PubMed] [Google Scholar]
- Motegi, F. & Seydoux, G. (2013) The PAR network: redundancy and robustness in a symmetry‐breaking system. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368, 20130010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi, F. , Zonies, S. , Hao, Y. , Cuenca, A.A. , Griffin, E. & Seydoux, G. (2011) Microtubules induce self‐organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nature Cell Biology, 13, 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi, A. , Yano, J. , Valentine, M.S. , Picariello, T. & Van Houten, J.L. (2019) SF‐Assemblin genes in Paramecium: phylogeny and phenotypes of RNAi silencing on the ciliary‐striated rootlets and surface organization. Cilia, 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney, D.L. (1966) Cortical integration in Tetrahymena: an exercise in cytogeometry. Journal of Experimental Zoology, 161, 307–317. [DOI] [PubMed] [Google Scholar]
- Nanney, D.L. (1975) Patterns of basal body addition in ciliary rows in Tetrahymena. Journal of Cell Biology, 65, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney, D.L. , Chow, M. & Wozencraft, B. (1975) Considerations of symmetry in cortical integration of Tetrahymena doublets. Journal of Experimental Zoology, 193, 1–13. [DOI] [PubMed] [Google Scholar]
- Neant, I. , Charbonneau, M. & Guerrier, P. (1989) A requirement for protein phosphorylation in regulating the meiotic and mitotic cell cycles in echinoderms. Developmental Biology, 132, 304–314. [DOI] [PubMed] [Google Scholar]
- Nelsen, E.M. & Frankel, J. (1989) Maintenance and regulation of cellular handedness in Tetrahymena. Development, 105, 457–471. [DOI] [PubMed] [Google Scholar]
- Nelsen, E.M. , Frankel, J. & Jenkins, L.M. (1989a) Non‐genic inheritance of cellular handedness. Development, 105, 447–456. [DOI] [PubMed] [Google Scholar]
- Nelsen, E.M. , Frankel, J. & Williams, N.E. (1989b) Oral assembly in left‐handed Tetrahymena thermophila. The Journal of Protozoology, 36, 582–596. [DOI] [PubMed] [Google Scholar]
- Nelsen, E.M. , Williams, N.E. , Yi, H. , Knaak, J. & Frankel, J. (1994) Fenestrin and conjugation in tetrahymena‐thermophila. Journal of Eukaryotic Microbiology, 41, 483–495. [DOI] [PubMed] [Google Scholar]
- Ng, S.F. & Frankel, J. (1977) 180 degrees rotation of ciliary rows and its morphogenetic implications in Tetrahymena pyriformis. Proceedings of the National Academy of Sciences of the United States of America, 74, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata, O. , Kurasawa, Y. , Gonda, K. & Watanabe, Y. (2000) Tetrahymena elongation factor‐1 alpha is localized with calmodulin in the division furrow. Journal of Biochemistry, 127, 51–56. [DOI] [PubMed] [Google Scholar]
- Numata, O. , Suzuki, H. , Ohba, H. & Watanabe, Y. (1995) The mutant gene product of a Tetrahymena cell‐division‐arrest mutant cdaA is localized in the accessory structure of specialized basal body close to the division furrow. Zoological Science, 12, 133–135. [DOI] [PubMed] [Google Scholar]
- Oami, K. (1996) Distribution of chemoreceptors to quinine on the cell surface of Paramecium caudatum. Journal of Comparative Physiology a‐Neuroethology Sensory Neural and Behavioral Physiology, 179, 345–352. [Google Scholar]
- Ogura, A. & Machemer, H. (1980) Distribution of mechanoreceptor channels in the paramecium surface‐membrane. Journal of Comparative Physiology, 135, 233–242. [Google Scholar]
- Ohata, S. , Nakatani, J. , Herranz‐Perez, V. , Cheng, J. , Belinson, H. , Inubushi, T. et al. (2014) Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron, 83, 558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba, H. , Ohmori, I. , Numata, O. & Watanabe, Y. (1986) Purification and immunofluorescence localization of the mutant‐gene product of a tetrahymena‐cdaal mutant affecting cell‐division. Journal of Biochemistry, 100, 797–808. [DOI] [PubMed] [Google Scholar]
- Orias, E. & Bruns, P.J. (1976) Induction and isolation of mutants in Tetrahymena. Methods in Cell Biology, 13, 247–282. [PubMed] [Google Scholar]
- Peyer, J.E.D. & Machemer, H. (1977) Membrane excitability in stylonychia ‐ properties of 2‐peak regenerative Ca‐response. Journal of Comparative Physiology, 121, 15–32. [Google Scholar]
- Plattner, H. & Klauke, N. (2001) Calcium in ciliated protozoa: sources, regulation, and calcium‐regulated cell functions. International Review of Cytology ‐ a Survey of Cell Biology, 201, 115–208. [DOI] [PubMed] [Google Scholar]
- Plattner, H. , Sehring, I.M. , Mohamed, I.K. , Miranda, K. , De Souza, W. , Billington, R. et al. (2012) Calcium signaling in closely related protozoan groups (Alveolata): non‐parasitic ciliates (Paramecium, Tetrahymena) vs. parasitic Apicomplexa (Plasmodium, Toxoplasma). Cell Calcium, 51, 351–382. [DOI] [PubMed] [Google Scholar]
- Preston, R.R. & Van Houten, J.L. (1987) Localization of the chemoreceptive properties of the surface membrane of Paramecium tetraurelia . Journal of Comparative Physiology A, 160, 537–541. [DOI] [PubMed] [Google Scholar]
- Riddle, R.D. , Johnson, R.L. , Laufer, E. & Tabin, C. (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell, 75, 1401–1416. [DOI] [PubMed] [Google Scholar]
- Rime, H. , Neant, I. , Guerrier, P. & Ozon, R. (1989) 6‐Dimethylaminopurine (6‐DMAP), a reversible inhibitor of the transition to metaphase during the first meiotic cell division of the mouse oocyte. Developmental Biology, 133, 169–179. [DOI] [PubMed] [Google Scholar]
- Ruehle, M.D. , Orias, E. & Pearson, C.G. (2016) Tetrahymena as a unicellular model eukaryote: genetic and genomic tools. Genetics, 203, 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehle, M.D. , Stemm‐Wolf, A.J. & Pearson, C.G. (2020) Sas4 links basal bodies to cell division via Hippo signaling. Journal of Cell Biology, 219, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, L. , Cammer, M. , Lehman, J. , Nielsen, S.K. , Guerra, C.F. , Veland, I.R. et al. (2010) Directional cell migration and chemotaxis in wound healing response to PDGF‐AA are coordinated by the primary cilium in fibroblasts. Cellular Physiology and Biochemistry, 25, 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander, M. , Kachar, B. & Muller, U. (2010) Review series: the cell biology of hearing. Journal of Cell Biology, 190, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehring, I.M. , Reiner, C. , Mansfeld, J. , Plattner, H. & Kissmehl, R. (2007) A broad spectrum of actin paralogs in Paramecium tetraurelia cells display differential localization and function. Journal of Cell Science, 120, 177–190. [DOI] [PubMed] [Google Scholar]
- Sharma, N. , Bryant, J. , Wloga, D. , Donaldson, R. , Davis, R.C. , Jerka‐Dziadosz, M. et al. (2007) Katanin regulates dynamics of microtubules and biogenesis of motile cilia. Journal of Cell Biology, 178, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama, S. & Numata, O. (2003) Tetrahymena fimbrin localized in the division furrow bundles actin filaments in a calcium‐independent manner. Journal of Biochemistry, 134, 591–598. [DOI] [PubMed] [Google Scholar]
- Slabodnick, M.M. , Ruby, J.G. , Dunn, J.G. , Feldman, J.L. , DeRisi, J.L. & Marshall, W.F. (2014) The kinase regulator mob1 acts as a patterning protein for stentor morphogenesis. PLoS Biology, 12, e1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh, A.W.J. , van Dam, T.J.P. , Stemm‐Wolf, A.J. , Pham, A.T. , Morgan, G.P. , O'Toole, E.T. et al. (2020) Ciliary force‐responsive striated fibers promote basal body connections and cortical interactions. Journal of Cell Biology, 219, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh, W. , van Dam, J. , Stemm‐Wolf, A. & Pearson, C.G. (2017) Basal body associated striated fibers control their length to organize ciliary arrays. Molecular Biology of the Cell, 28. [Google Scholar]
- Sonneborn, T.M. (1963) Does preformed cell structure play an essential role in cell heredity? The nature of biological diversity. New York: McGraw‐Hill. [Google Scholar]
- Sparvoli, D. , Zoltner, M. , Cheng, C.Y. , Field, M.C. & Turkewitz, A.P. (2020) Diversification of CORVET tethers facilitates transport complexity in Tetrahymena thermophila. Journal of Cell Science, 133, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky, N. & Meunier, A. (2017) The development and functions of multiciliated epithelia. Nature Reviews Molecular Cell Biology, 18, 423–436. [DOI] [PubMed] [Google Scholar]
- Stelly, N. , Halpern, S. , Nicolas, G. , Fragu, P. & Adoutte, A. (1995) Direct visualization of a vast cortical calcium compartment in Paramecium by secondary ion mass spectrometry (SIMS) microscopy: possible involvement in exocytosis. Journal of Cell Science, 108(Pt 5), 1895–1909. [DOI] [PubMed] [Google Scholar]
- Stelly, N. , Mauger, J.P. , Claret, M. & Adoutte, A. (1991) Cortical alveoli of Paramecium: a vast submembranous calcium storage compartment. Journal of Cell Biology, 113, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover, N.A. & Rice, J.D. (2011) Distinct cyclin genes define each stage of ciliate conjugation. Cell Cycle, 10, 1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita, M. , Iwataki, Y. , Nakano, K. & Numata, O. (2011) Unique sequences and predicted functions of myosins in Tetrahymena thermophila. Gene, 480, 10–20. [DOI] [PubMed] [Google Scholar]
- Suzuki, H. (1957) Morphogenesis in the regeneration of Blepharisma undulans. Bulletin of the Yamagata University. Natural Science, 4, 85–192. [Google Scholar]
- Tartar, V. (1956) Pattern and substance in Stentor. In: Rudnick, D. (Ed.) Cellular mechanisms in differentiation and growth. Princeton University Press. [Google Scholar]
- Tartar, V. (1957) Deletion experiments on the oral primordium of Stentor coeruleus. Journal of Experimental Zoology, 136, 53–74. [DOI] [PubMed] [Google Scholar]
- Tartar, V. (1958) Induced resorption of oral primordia in regenerating Stentor coeruleus. Journal of Experimental Zoology, 139, 1–31. [DOI] [PubMed] [Google Scholar]
- Tartar, V. (1960) Reconstitution of minced Stentor coeruleus. Journal of Experimental Zoology, 144, 187–207. [DOI] [PubMed] [Google Scholar]
- Tartar, V. (1961) The sentor. Elmsford, NY: Pergamon Oress. [Google Scholar]
- Tartar, V. (1966) Fission after division primordium removal in the ciliate Stentor coeruleus and comparable experiments on reorganizers. Experimental Cell Research, 42, 357–370. [DOI] [PubMed] [Google Scholar]
- Tartar, V. (1968) Micrurgical experiments on cytokinesis in Stentor coeruleus. Journal of Experimental Zoology, 167, 21–36. [DOI] [PubMed] [Google Scholar]
- Tavares, A. , Goncalves, J. , Florindo, C. , Tavares, A.A. & Soares, H. (2012) Mob1: defining cell polarity for proper cell division. Journal of Cell Science, 125, 516–527. [DOI] [PubMed] [Google Scholar]
- Thanbichler, M. & Shapiro, L. (2006) MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell, 126, 147–162. [DOI] [PubMed] [Google Scholar]
- Thazhath, R. , Jerka‐Dziadosz, M. , Duan, J. , Wloga, D. , Gorovsky, M.A. , Frankel, J. et al. (2004) Cell context‐specific effects of the beta‐tubulin glycylation domain on assembly and size of microtubular organelles. Molecular Biology of the Cell, 15, 4136–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazhath, R. , Liu, C.B. & Gaertig, J. (2002) Polyglycylation domain of beta‐tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nature Cell Biology, 4, 256–259. [DOI] [PubMed] [Google Scholar]
- Tickle, C. & Slack, J. (2021) Lewis Wolpert (1929–2021). Science, 371, 1208. [DOI] [PubMed] [Google Scholar]
- Turkewitz, A.P. (2004) Out with a bang! Tetrahymena as a model system to study secretory granule biogenesis. Traffic, 5, 63–68. [DOI] [PubMed] [Google Scholar]
- Udan, R.S. , Kango‐Singh, M. , Nolo, R. , Tao, C. & Halder, G. (2003) Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nature Cell Biology, 5, 914–920. [DOI] [PubMed] [Google Scholar]
- Uhlig, G. (1959) Polaritatsabhangige Anlagenentwicklung Bei Stentor‐Coeruleus. Zeitschrift Fur Naturforschung Part B‐Chemie Biochemie Biophysik Biologie Und Verwandten Gebiete, 14, 353–354. [Google Scholar]
- Vasudevan, K.K. , Jiang, Y.Y. , Lechtreck, K.F. , Kushida, Y. , Alford, L.M. , Sale, W.S. et al. (2015) Kinesin‐13 regulates the quantity and quality of tubulin inside cilia. Molecular Biology of the Cell, 26, 478–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogg, M.C. , Beccari, L. , Iglesias Olle, L. , Rampon, C. , Vriz, S. , Perruchoud, C. et al. (2019) An evolutionarily‐conserved Wnt3/beta‐catenin/Sp5 feedback loop restricts head organizer activity in Hydra. Nature Communications, 10, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclawek, E. , Joachimiak, E. , Hall, M.H. , Fabczak, H. & Wloga, D. (2017) Regulation of katanin activity in the ciliate Tetrahymena thermophila. Molecular Microbiology, 103, 134–150. [DOI] [PubMed] [Google Scholar]
- Webster, G. & Wolpert, L. (1966) Studies on pattern regulation in hydra. I. Regional differences in time required for hypostome determination. Journal of Embryology and Experimental Morphology, 16, 91–104. [PubMed] [Google Scholar]
- Weisz, P.B. (1951) An experimental analysis of morphogenesis in stentor coeruleus. Journal of Experimental Zoology, 116, 231–257. [DOI] [PubMed] [Google Scholar]
- Wickstead, B. & Gull, K. (2006) A “holistic” kinesin phylogeny reveals new kinesin families and predicts protein functions. Molecular Biology of the Cell, 17, 1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus, E. & Nusslein‐Volhard, C. (2016) The heidelberg screen for pattern mutants of drosophila: a personal account. Annual Review of Cell and Developmental Biology, 32, 1–46. [DOI] [PubMed] [Google Scholar]
- Wilkes, D.E. & Otto, J.J. (2003) Profilin functions in cytokinesis, nuclear positioning, and stomatogenesis in Tetrahymena thermophila. Journal of Eukaryotic Microbiology, 50, 252–262. [DOI] [PubMed] [Google Scholar]
- Williams, N.E. (1964) Relations between temperature sensitivity + morphogenesis in tetrahymena pyriformis Gl. Journal of Protozoology, 11, 566. [DOI] [PubMed] [Google Scholar]
- Williams, N.E. (2004) The epiplasm gene EPC1 influences cell shape and cortical pattern in Tetrahymena thermophila. Journal of Eukaryotic Microbiology, 51, 201–206. [DOI] [PubMed] [Google Scholar]
- Williams, N.E. , Honts, J.E. & Jaeckel‐Williams, R.F. (1987) Regional differentiation of the membrane skeleton in Tetrahymena. Journal of Cell Science, 87(Pt 3), 457–463. [DOI] [PubMed] [Google Scholar]
- Williams, N.E. , Honts, J.E. , Dress, V.M. , Nelsen, E.M. & Frankel, J. (1995) Monoclonal‐antibodies reveal complex structure in the membrane skeleton of tetrahymena. Journal of Eukaryotic Microbiology, 42, 422–427. [DOI] [PubMed] [Google Scholar]
- Williams, N.E. , Tsao, C.C. , Bowen, J. , Hehman, G.L. , Williams, R.J. & Frankel, J. (2006) The actin gene ACT1 is required for phagocytosis, motility, and cell separation of Tetrahymena thermophila. Eukaryotic Cell, 5, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga, D. , Camba, A. , Rogowski, K. , Manning, G. , Jerka‐Dziadosz, M. & Gaertig, J. (2006) Members of the NIMA‐related kinase family promote disassembly of cilia by multiple mechanisms. Molecular Biology of the Cell, 17, 2799–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga, D. & Frankel, J. (2012) From molecules to morphology: cellular organization of Tetrahymena thermophila. Methods in Cell Biology, 109, 83–140. [DOI] [PubMed] [Google Scholar]
- Wolpert, L. (1969) Positional information and the spatial pattern of cellular differentiation. Journal of Theoretical Biology, 25, 1–47. [DOI] [PubMed] [Google Scholar]
- Wolpert, L. (1981) Positional information and pattern formation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 295, 441–450. [DOI] [PubMed] [Google Scholar]
- Wood, E. & Nurse, P. (2015) Sizing up to divide: mitotic cell‐size control in fission yeast. Annual Review of Cell and Developmental Biology, 31, 11–29. [DOI] [PubMed] [Google Scholar]
- Woods, B. , Kuo, C.C. , Wu, C.F. , Zyla, T.R. & Lew, D.J. (2015) Polarity establishment requires localized activation of Cdc42. Journal of Cell Biology, 211, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, B. & Lew, D.J. (2019) Polarity establishment by Cdc42: key roles for positive feedback and differential mobility. Small GTPases, 10, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. & Mlodzik, M. (2017) Wnt/PCP instructions for cilia in left‐right asymmetry. Developmental Cell, 40, 423–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Han, B. , Li, Y. , Munro, E. , Odde, D.J. & Griffin, E.E. (2018) Rapid diffusion‐state switching underlies stable cytoplasmic gradients in the Caenorhabditis elegans zygote. Proceedings of the National Academy of Sciences of the United States of America, 115, E8440–E8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, G.X. , Dang, H. , Tian, M. , Zhang, J. , Shodhan, A. , Ning, Y.Z. et al. (2016) Cyc17, a meiosis‐specific cyclin, is essential for anaphase initiation and chromosome segregation in Tetrahymena thermophila. Cell Cycle, 15, 1855–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, G.X. , Zhang, J. , Shodhan, A. , Tian, M. & Miao, W. (2016) Cdk3, a conjugation‐specific cyclin‐dependent kinase, is essential for the initiation of meiosis in Tetrahymena thermophila. Cell Cycle, 15, 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, T. , Numata, O. , Ohnishi, K. & Watanabe, Y. (1980) A contractile ring and cortical changes found in the dividing Tetrahymena pyriformis. Experimental Cell Research, 128, 407–417. [DOI] [PubMed] [Google Scholar]
- Zweifel, E. , Smith, J. , Romero, D. , Giddings, T.H. Jr. , Winey, M. , Honts, J. et al. (2009) Nested genes CDA12 and CDA13 encode proteins associated with membrane trafficking in the ciliate Tetrahymena thermophila. Eukaryotic Cell, 8, 899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]


