Abstract
Background
Prophylactic anticoagulation is commonly used following operative treatment of spinal fractures to prevent Venous Thromboembolism (VTE) but carries a risk of bleeding complications. The purpose of the study was to compare VTE and bleeding complications for MID (≤72h) versus LATE (>72h) chemoprophylaxis timing after spinal fracture operative intervention.
Methods
This is a retrospective review of patients treated for spinal fractures that received anticoagulation chemoprophylaxis between May 2015 and June 2019. Chemoprophylaxis initiation timing (MID vs. LATE) was the primary grouping variable. Patients with traumatic brain injury or evidence of intracranial or intraspinal bleed were excluded. Demographics, injury mechanisms, operative procedures, timing of administration of VTE prophylaxis, Injury Severity Score (ISS) and Spine Abbreviated Injury Scale (AIS), and complications including VTE and bleeding complications were collected. Predictors of VTE were identified using a binary logistic regression.
Results
Eighty-eight patients (65M, 23F) met inclusion criteria. The median age was 55 years, and median Injury Severity Score (ISS) was 14. MID had 68 patients and LATE had 20. Nine patients developed VTE (6 LATE, 3 MID, p<0.01). Three patients developed bleeding complications, and all occurred in the LATE group (p=0.01). ISS (p<0.01) and GCS (p<0.01) also correlated with an increased VTE rate.
Conclusions
Chemoprophylactic anticoagulation at 72 hours in surgically treated spinal fracture patients demonstrates a lower VTE rate without increasing complications. VTE prophylaxis can be initiated at 72 hours following spine fixation to decrease postinjury morbidity and mortality in this high-risk patient population.
Keywords: Venous Thromboembolism, Spinal Fracture, Prophylactic Anticoagulation, Intervention, Bleeding Complications, Epidural Hematoma, Deep Venous Thrombosis
Introduction
Traumatic spine injury is one of the leading causes of death and disability in adults as well as children [1]. Although injury prevention programs have been established, the United States is still at the top of the list with the highest overall incidence rate of spine injuries in the world [2]. Spinal injuries are associated with high mortality rates, ranging from 22% to 32% within one year of admission [3]. Reports showed that surgically treated spinal fractures are associated with lower mortality than that treated nonoperatively [3,4]. However, operatively managed spinal fractures carry a risk of morbidity and mortality following surgery [5].
Patients sustaining traumatic accidents commonly transition to a hypercoagulable state during the post-injury period [6], specifically those with spinal trauma who have a high risk of developing venous thromboembolism (VTE) due to limited mobility following surgical fracture fixation [7].
Venous thromboembolism (VTE) can lead to significant morbidity and mortality among certain patient populations. In Europe, VTE is responsible for an estimated 540,000 deaths each year [8] and is the third most common cause of death, along with hemorrhage in trauma patients. It is estimated that VTE incidence exceeds 50% in patients who were not treated with anticoagulation prophylaxis and sustained severe trauma, such as motor-vehicle collision (MVC) and gunshot wounds (GSW), resulting in spinal fractures.
Prophylactic anticoagulation is commonly used following spinal fracture surgqical treatment; however, bleeding complication risks exist. Previous studies demonstrate that preoperative anticoagulation does not increase bleeding complications in patients with spinal fractures and may reduce the risk of VTE [9]. However, few studies have discussed the impact of postoperative anticoagulation on VTE and bleeding complications following operative treatment of spinal fractures [10], [11], [12], [13].
Trauma centers in the United States implement different practice management guidelines (PMG) for VTE prophylaxis. Some recommend chemoprophylaxis initiation within 72 hours of spinal fracture operative fixation, while others recommend initiation after 72 hours [14], [15], [16]. Currently, there is no consensus for optimal postoperative VTE chemoprophylactic anticoagulation timing (4).
Prior to 2017, patients with operative spinal fractures admitted to the trauma service at the University of Kansas Medical Center received VTE prophylaxis two weeks post-operatively. In June 2017, a new protocol was implemented, initiating chemoprophylaxis 72 hours post-operatively in patients who have undergone a spinal operation for a spinal fracture.
The purpose of this study was to evaluate the efficacy and safety of the PMG for early VTE prophylaxis in patients treated surgically for traumatic spinal fractures. A retrospective chart review was conducted to assess the relative efficacy and safety of the new PMG for early VTE prophylaxis in patients with operative spinal fractures. This chart compares the outcomes in patients admitted within two years before protocol implementation (May 1, 2015 to May 31, 2017) and two years after implementation (June 1, 2017 to June 30, 2019).
Methods
This is a 4-year (2015-2019) retrospective analysis of our institutional trauma registry (American College of Surgeons-verified level I trauma center). Consecutive patients treated for operative fixation of spinal fractures at our institution between May 2015 and June 2019 were identified. This study was approved by the Institutional Review Board.
Inclusion Criteria
Patients who were 18 years or older, admitted with a spinal fracture requiring surgical management and who received postoperative VTE chemoprophylaxis were included.
Exclusion Criteria
Patients younger than 18 years, with a traumatic brain injury, or intracranial or intraspinal bleeding on admission were excluded.
Patient Stratification
Patients were stratified into two groups based on the timing of initiation of VTE chemoprohylaxis: MID vs LATE. MID initiation was defined as chemoprophylaxis administered within 72 hours. Late initiation was defined as chemoprophylaxis administered after the first 72 hours of surgery.
Data Collection
An electronic chart review was performed. Age, sex, injury mechanisms, transfers, Glasgow Coma Scale (GCS), Injury Severity Score (ISS), Abbreviated Injury Scale (AIS) for the spine, upper and lower extremities were collected. Spinal fractures were described using the fracture level (cervical, thoracic, lumbar, or sacral), imaging reports, and American Spinal Injury Association (ASIA) Impairment Score. Admission hematologic labs, preoperative and postoperative VTE prophylaxis, time of initiation of postoperative VTE prophylaxis, including type and dose, were recorded.
Operative data points included operative procedure, timing, length, estimated blood loss, and surgical service (Orthopedic or Neurosurgery). Complications including VTE (Deep vein thrombosis (DVT) and/or pulmonary embolism (PE)) and bleeding (epidural, subdural, intraabdominal, intrathoracic hematoma or subarachnoid/intramedullary hemorrhage) were recorded. Data was entered into a REDCap database.
VTE Protocol
The institutional protocol implemented for patients surgically treated for spinal fractures included VTE chemoprophylaxis re-initiated 72 hours post-operatively with low molecular weight heparin (LMWH; enoxaparin) 30mg subcutaneously every 12 hours, based on previous reports [14,15]. Patients with clinical symptoms of DVT were screened by venous duplex ultrasonography.
PE was diagnosed based on computed tomographic angiography or ventilation-perfusion scans in symptomatic patients. Computed tomography (CT) reports were reviewed for the presence of intraspinal hematoma (epidural or subdural hematoma, subarachnoid or intramedullary hemorrhage) or extraspinal hematoma (intraabdominal or intrathoracic hematoma) in symptomatic patients. Intraspinal or extraspinal hematoma was defined as any new bleeding on subsequent imaging.
MID VTE prophylaxis was LMWH within 72 hours post-operatively. LATE VTE prophylaxis was defined as greater than 72 hours after surgery. Timepoints were divided into three subgroups, MID (< 24hr; 24-48hr; 48-72hr) and LATE (72h – 1 week, 1-2 weeks, >2weeks) for additional sub-analyses.
Statistical Analysis
Analyses were conducted using statistical software (SPSS, IBM Corp., Version 27). Data are reported as mean ± standard deviation or median (range) for continuous variables. Frequency rates (percentage) were reported for categorical variables. Data were tested for normality. Differences between non-normally distributed continuous variables were analyzed with Mann-Whitney U test. Categorical variables were analyzed with Fisher's Exact test. A binary logistic regression was performed to determine which variables were predictive of VTE occurrence.
Results
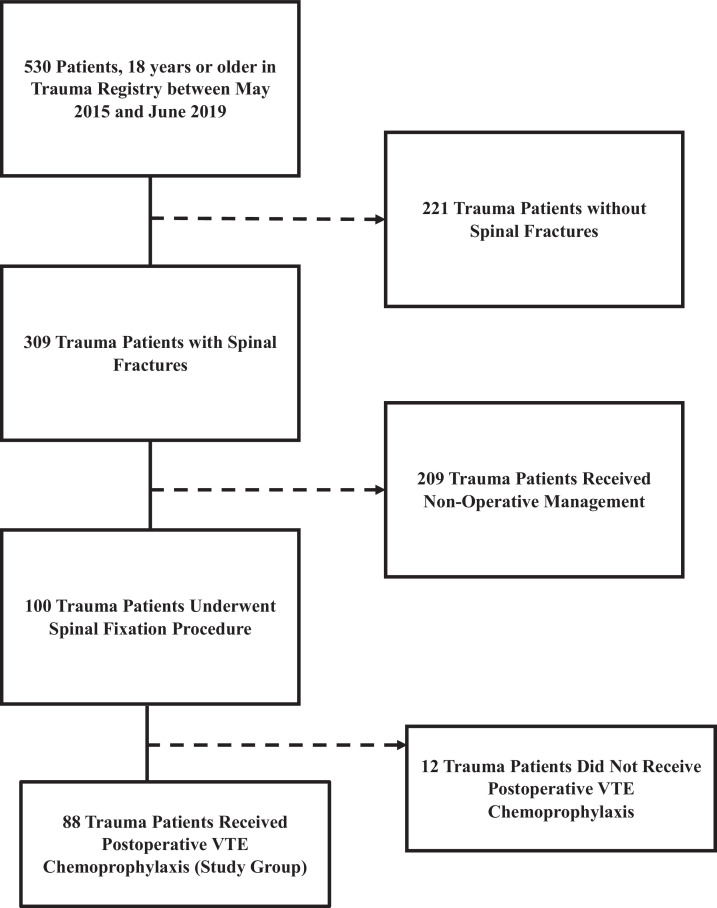
During the study period, 530 patients were identified with spinal traumas. Eighty-eight patients qualified for study inclusion. The median age was 55 years, and 74% were male (Table 1). Fifty-one patients were transferred from an outside facility. Median GCS and ISS were 15 and14, respectively. The median spine abbreviated injury scale was 4.
Table 1.
Characteristics of Study Patients.
| Characteristics of Study Patients | |
|---|---|
| N= 88 (%) | |
| Gender | |
| Male | 65 (74%) |
| Female | 23 (26%) |
| Agea | 55 [18 - 89] |
| Mechanism of Injury | |
| Fall | 46 (52.3) |
| MVC | 25 (28.4) |
| MCC | 7 (8) |
| MVC vs Pedestrian | 6 (6.8) |
| Blunt Assault | 3 (3.4) |
| Unknown | 1 (1.1) |
| Patient Transfer | 51 (58) |
| Surgical Service | |
| Orthopedic Surgery | 34 (38.6) |
| Neurological Surgery | 54 (61.4) |
| Glascow Coma Scalea | 15 [3 – 15] |
| Injury Severity Scorea | 14 [4 – 75] |
| AIS Spinea | 4 [2 – 7] |
| Spine Fracture Level | |
| Cervical | 38 (43.2) |
| Thoracic | 39 (44.3) |
| Lumbar | 35 (39.8) |
| Sacral | 10 (11.4) |
| Coccygeal | 1 (1.1) |
| Preoperative VTE Prophylaxis Administration | 29 (33) |
| Duration of Operationb | 3.5 ± 1.5 |
| Estimated Blood Loss During Operationa | 250 [0 – 3200] |
| Postoperative VTE Prophylaxis Administration | |
| EARLY (≤72h) | 68 (77.3) |
| LATE (>72h) | 20 (22.7) |
| Subjects with VTE Complication | 9 (10.2) |
| Deep Vein Thrombosis (DVT) | 8 (9.1) |
| Pulmonary Embolism (PE) | 2 (2.3) |
| Other | 1 (1.1) |
| Subjects with Bleeding Complication | 3 (3.4) |
| Epidural Hematoma | 1 (1.1) |
| Other | 2 (2.3) |
*p < 0.05, statistically significant.
Two subjects had 2 documented VTE complications each.
Median [range]
Mean ± SD
With regards to the level of spine fracture, there was a predominance of thoracic fractures (44.3%) followed by Cervical (43.2%), Lumbar (39.8%), Sacral (11.4%), and Coccygeal (1.1%). Twenty-nine (33%) of our patients received preoperative VTE prophylaxis. The mean operative duration was 3.5 hours with a median estimated blood loss of 250 ml.
Sixty-eight patients (77.3%) were in MID, and 20 (22.7%) were in the LATE group (Table 1). There was a significant difference in number of transfers (p = 0.018), surgical service (p = 0.008), GCS (p = 0.03), VTE (p < 0.01) and bleeding (p = 0.01) complication rates between patients in MID vs LATE groups. There were no significant differences between MID vs LATE for age (53 vs. 56), sex, mechanism of injury, Injury Severity Score, AIS spine, level of spinal fracture, preoperative VTE prophylaxis administration, duration of operation, or estimated blood loss. (All p>0.05) (Table 2).
Table 2.
Characteristics of Timepoint Groups.
| Characteristics of Timepoint Groups | |||
|---|---|---|---|
| MID N= 68 (%) | LATE N= 20 (%) | P value | |
| Male | 48 | 17 | 0.159 |
| Female | 20 | 3 | |
| Agea | 53 [18 – 87] | 56 [23 – 89] | 0.40 |
| Mechanism of Injury | |||
| Fall | 37 (54.4) | 9 (45) | 0.70 |
| MVC | 19 (27.9) | 6 (30) | |
| MCC | 4 (5.9) | 3 (15) | |
| MVC vs Pedestrian | 5 (7.4) | 1 (5) | |
| Blunt Assault | 2 (2.9) | 1 (5) | |
| Unknown | 1 (1.5) | 0 (0) | |
| Patient Transfer | 42 (61.8) | 9 (45) | 0.018* |
| Surgical Service | |||
| Orthopedic Surgery | 21 (30.9) | 13 (65) | 0.008* |
| Neurological Surgery | 47 (69.1) | 7 (35) | |
| Glasgow Coma Scalea | 15 [3 – 15] | 15 [3 – 15] | 0.03* |
| Injury Severity Scorea | 13 [4 – 75] | 17 [14 – 26] | 0.06 |
| AIS Spinea | 3 [2 – 7] | 4 [3 – 6] | 0.10 |
| Spine Fracture Level | |||
| Cervical | 27 (39.7) | 11 (55) | >0.05 |
| Thoracic | 28 (41.2) | 11 (55) | |
| Lumbar | 26 (38.2) | 9 (45) | |
| Sacral | 7 (10.3) | 3 (15) | |
| Coccygeal | 1 (1.5) | 0 (0) | |
| Preoperative VTE Prophylaxis Administration | 24 (35.3) | 5 (25) | 0.43 |
| Duration of Operation b | 3.5 ± 1.5 | 4 ± 2 | 0.26 |
| Estimated Blood Loss During Operationa | 200 [100 – 400] | 400 [188 – 600] | 0.02* |
| Subjects with VTE Complication | 3 (4.4) | 6 (30) | <0.01* |
| Subjects with Bleeding Complication | 0 (0) | 3 (15) | 0.01* |
Median [range]
Mean ± SD
p < 0.05, statistically significant
In the MID group sub-analyses, six patients (6.8%) received LMWH within 24 hours post-operatively; 37 (42%) received the initial dose between 24-48 hours, and 25 (28.4%) received the initial dose between 48-72 hours. LATE group sub-analyses showed that nine patients (10.2%) received postop prophylaxis 72h – 1 week after surgery, 7 (8%) received their initial dose 1 – 2 weeks, and 4 (4.5%) received it more than two weeks after surgery.
Overall, the type and dose of postoperative VTE prophylaxis were similar between groups, in which subcutaneous Enoxaparin 30 mg was administered twice daily. Nine patients (10.2 %) developed VTE following spinal fracture operative fixation (Table 1). There was a lower frequency of VTE when comparing MID (3) and LATE (6) anticoagulation groups (P=0.004). There were more bleeding complications in the LATE (3) compared to MID (0) (P=0.01). (Table 2)
Fig. 1.
Flow Diagram of Study Patient Inclusion Criteria.
Table 3.
Characteristics of VTE Groups.
| Characteristics of VTE Groups | |||
|---|---|---|---|
| VTE N= 9 (%) | No VTE N= 79 (%) | P value | |
| Male | 7 | 58 | ns |
| Female | 2 | 21 | |
| Agea | 64 [24 - 82] | 53 [18 – 89] | ns |
| Mechanism of Injury | |||
| Fall | 5 (55.6) | 41 (51.9) | ns |
| MVC | 4 (44.4) | 21 (26.6) | |
| MCC | 0 (0) | 7 (8.9) | |
| MVC vs Pedestrian | 0 (0) | 6 (7.6) | |
| Blunt Assault | 0 (0) | 3 (3.8) | |
| Unknown | 0 (0) | 1 (1.3) | |
| Patient Transfer | 4 (44.4) | 47 (59.5) | ns |
| Surgical Service | |||
| Orthopedic Surgery | 5 (55.6) | 29 (36.7) | ns |
| Neurological Surgery | 4 (44.4) | 50 (63.3) | |
| Glasgow Coma Scalea | 14 [3 – 15] | 15 [3 – 15] | <0.001* |
| Injury Severity Scorea | 27 [13 – 38] | 14 [4 – 75] | 0.006* |
| AIS Spinea | 4 [3 – 6] | 4 [2 – 7] | ns |
| Spine Fracture Level | |||
| Cervical | 4 (44.4) | 34 (43) | ns |
| Thoracic | 7 (77.8) | 32 (40.5) | |
| Lumbar | 4 (44.4) | 31 (39.2) | |
| Sacral | 0 (0) | 10 (12.7) | |
| Coccygeal | 0 (0) | 1 (1.3) | |
| Preoperative VTE Prophylaxis Administration | 3 (33.3) | 26 (32.9) | ns |
| Duration of Operation b | 3 ± 1 | 4 ± 1.5 | ns |
| Estimated Blood Loss During Operationa | 200 [0 – 600] | 250 [0 – 3200] | ns |
| Time of Administration of VTE Prophylaxis | |||
| MID (≤72h) | 3 (33.3) | 65 (82.3) | 0.004* |
| LATE (> 72h) | 6 (66.6) | 14 (17.7) | |
Median [range]
Mean ± SD
p < 0.05, statistically significant
ns = p > 0.05
Binary logistic regression identified Age (p=0.039) and GCS (p=0.041) as significant predictor variables of the VTE outcome variable (R-square 0.541, 89.8% predictive accuracy). Gender, ISS, AIS spine, operative time, EBL and timing of anticoagulation were not significant in the model (p>0.05).
Discussion
Venous thromboembolism (VTE) is a common cause of morbidity and mortality following operative fixation of traumatic spinal injuries [17]. According to the National Quality Forum, VTE contributes to the death of 300,000 people in the United States each year [18]. Different practice management guidelines for VTE prevention exist, and the use of anticoagulation after surgery has been extensively studied in the literature. However, widely accepted guidelines for VTE prophylaxis, and particularly the timing of postoperative chemoprophylaxis following operative fixation of spinal fractures, do not exist [19,20].
Furthermore, the risk of developing VTE or bleeding, specifically epidural hematoma, following spinal surgery is unclear. Glotzbecker et al. reported a scarcity of evidence concerning VTE and bleeding complications following spinal surgeries in addition to a deficit in data supporting the efficacy and safety of VTE prophylaxis protocols [21].
To address these issues as a participant in the American College of Surgeons Trauma Quality Improvement Program (TQIP), we standardized our approach to VTE prophylaxis in patients with operative spinal fractures. Our institutional protocol involved chemoprophylaxis with Enoxaparin 30 mg subcutaneously every 12 hours initiated 72 hours post-operatively. Enoxaparin is only held if there is evidence of bleeding or another contraindication. This protocol aimed to identify patients at risk for venous thromboembolism and implement appropriate interventions to minimize VTE occurrence.
Regarding the differences in injury scales between the patients receiving early vs. late VTE prophylaxis, Kim DY et al. reported that ISS, GCS, and head AIS were higher in the late VTE prophylaxis. Still, their study included patients with traumatic brain injuries. On the contrary, in our study, only GCS was higher in the LATE group. This indicates that the patient's injury may be more likely to have an impact on VTE and bleeding complications following spinal surgeries than on the timing of VTE prophylaxis administration.
We further investigated the mechanism of injury, location of spinal fracture, duration of the operation, and estimated blood loss intraoperatively in our patient population. Most of our patients had a fall mechanism. Spinal fractures were mainly located at the thoracic level followed by the cervical level, consistent with the literature [15].
Rates of thromboembolic disease following operative fixation of spinal fractures have been reported by several studies varying between 0.3% and 31% [18]. Discrepancies in the rate of VTE are explained by several factors, such as genetic diversity between the patient populations, variability of screening protocols, surgeons’ approach, types and timing of VTE prophylaxis, and patient's compliance with prophylactic medications. The overall rate of VTE in our study aligns with the rates in the literature, where recently published studies reported overall VTE rates ranging between 1% and 12% [[14], [15], [16],22] comparable to the 10.2 % overall rate observed in our study.
Our objective was to examine the influence of VTE prophylaxis on the outcomes of VTE versus bleeding in patients with operative spinal fractures. Early administration of VTE prophylaxis (≤ 72 hours) post-operatively correlated with a decreased risk of VTE (4.4%) compared to late VTE prophylaxis (30%). Tracy BM et al. [23] reported a significant increase in the rate of VTE in patients with delayed administration of VTE prophylaxis. Similarly, early initiation of VTE prophylaxis was associated with a decreased incidence of VTE, as reported in a study conducted by Chang R. et al. [14].
The prevalence of epidural hematoma in patients with operative spinal fractures is less than 1%, as reported in the literature [21,24,25]. Dhillon E.S. et al. [16] reported a 0.19% overall rate of epidural hematoma in their patient population. A systematic review of 25 articles conducted by Glotzbecker et al. [21] demonstrated an overall rate of 0% - 1% for the development of an epidural hematoma. Although our study population is small, only one patient who received late VTE prophylaxis developed epidural hematoma with an overall rate of 1.1%, consistent with the existing literature.
It is widely acknowledged that an increased risk of bleeding accompanies the initiation of VTE prophylaxis in patients with traumatic spinal injuries. Most studies in the literature focused only on epidural hematomas as the primary outcome for bleeding and did not assess other bleeding complications [22]. In our study, bleeding complications were not restricted to epidural hematomas only, but we also captured any bleeding complications following spinal surgery.
As a result, we found that two patients who received late VTE prophylaxis developed an intrathoracic hematoma. This is a small sample size. It is likely that these new findings on follow-up CT imaging were not the result of chemoprophylaxis administration but more likely related to the magnitude of the injury itself.
The primary weakness of our study is its retrospective design and small sample size. Our sample sizes resulted in a statistical power below the standard target of greater than 80%. Although this questions the certainty and generalizability of our findings, we believe there is value in reporting them nonetheless due the rarity of VTEs among this patient population and the need for improved clinical data reporting [11,26,27].
Additionally, patients were not all followed after hospital discharge. Some patients may have developed asymptomatic epidural hematoma after discharge or may have been treated at another hospital. However, it is less likely to develop chemoprophylaxis-related bleeding complications after discharge.
Most anticoagulants were stopped prior to hospital discharge unless the patient had a known DVT or PE. Moreover, we did not collect data concerning the use of other VTE prophylactic measures, such as mechanical prophylaxis and mobilization, that are known for their role in minimizing the risk of VTE in trauma patients.
Ultimately, as the incidence of epidural hematoma in this population is extremely low, our small sample size precludes a definitive judgment concerning the safety of early VTE prophylaxis with respect to bleeding. Despite the limitations, our study is one of the few studies in the trauma literature investigating the timing of administration of VTE prophylaxis following operative spine fixation.
Conclusions
Routine anticoagulation by 72 hours in patients who have undergone fixation for spinal fractures is associated with a lower rate of VTE without increasing bleeding or wound complications. Large, multicenter prospective studies are required to define further the efficacy and safety of an early pharmacological VTE prophylaxis strategy in this high-risk patient population.
Funding Disclosure
No funds were received in support of this study. None of the authors have conflicts of interest relative to the subject of this study.
Conflicts of Interest
None.
Acknowledgement
This study was approved by the University of Kansas IRB STUDY00145102. KU Trauma Registry database was used for ICD-9 and ICD-10 code search in patient ascertainment. Data was recorded using secured access to REDCap software.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2022.100141.
Appendix. Supplementary materials
References
- 1.New P.W., et al. Estimating the incidence and prevalence of traumatic spinal cord injury in Australia. Archives of physical medicine and rehabilitation. 2015;96(1):76–83. doi: 10.1016/j.apmr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Jain N.B., et al. Traumatic spinal cord injury in the United States, 1993-2012. Jama. 2015;313(22):2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson A.M., et al. C2 vertebral fractures in the Medicare population: incidence, outcomes, and costs. JBJS. 2016;98(6):449–456. doi: 10.2106/JBJS.O.00468. [DOI] [PubMed] [Google Scholar]
- 4.Tator C., et al. Comparison of surgical and conservative management in 208 patients with acute spinal cord injury. Canadian journal of neurological sciences. 1987;14:60–69. doi: 10.1017/s0317167100026858. S1. [DOI] [PubMed] [Google Scholar]
- 5.Donovan W. Operative and nonoperative management of spinal cord injury. A review. Spinal Cord. 1994;32(6):375–388. doi: 10.1038/sc.1994.64. [DOI] [PubMed] [Google Scholar]
- 6.Sumislawski J.J., et al. Dynamic coagulability after injury: Is delaying venous thromboembolism chemoprophylaxis worth the wait? The journal of trauma and acute care surgery. 2018;85(5):907. doi: 10.1097/TA.0000000000002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shackford S.R., et al. Venous thromboembolism in patients with major trauma. The American Journal of Surgery. 1990;159(4):365–369. doi: 10.1016/s0002-9610(05)81272-3. [DOI] [PubMed] [Google Scholar]
- 8.Turpie A.G. Thromboprophylaxis After Major Orthopaedic Surgery: State of the Art. European Instructional Lectures. 2009:29–38. [Google Scholar]
- 9.Sharpe J.P., et al. Impact of venous thromboembolism chemoprophylaxis on postoperative hemorrhage following operative stabilization of spine fractures. Journal of Trauma and Acute Care Surgery. 2017;83(6):1108–1113. doi: 10.1097/TA.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 10.Zeeshan M., et al. Optimal timing of initiation of thromboprophylaxis in spine trauma managed operatively: A nationwide propensity-matched analysis of trauma quality improvement program. Journal of Trauma and Acute Care Surgery. 2018;85(2):387–392. doi: 10.1097/TA.0000000000001916. [DOI] [PubMed] [Google Scholar]
- 11.Hamidi M., et al. Early Thromboprophylaxis in Operative Spinal Trauma Does Not Increase Risk of Bleeding Complications. Journal of Surgical Research. 2021;258:119–124. doi: 10.1016/j.jss.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Hecht J.P., et al. Association of timing of initiation of pharmacologic venous thromboembolism prophylaxis with outcomes in trauma patients. Journal of Trauma and Acute Care Surgery. 2021;90(1):54–63. doi: 10.1097/TA.0000000000002912. [DOI] [PubMed] [Google Scholar]
- 13.Ahlquist S., et al. Venous thromboembolism chemoprophylaxis within 24 hours of surgery for spinal cord injury: is it safe and effective? Neurospine. 2020;17(2):407. doi: 10.14245/ns.1938420.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang R., et al. Early chemoprophylaxis is associated with decreased venous thromboembolism risk without concomitant increase in intraspinal hematoma expansion after traumatic spinal cord injury. The journal of trauma and acute care surgery. 2017;83(6):1088. doi: 10.1097/TA.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D.Y., et al. Early pharmacological venous thromboembolism prophylaxis is safe after operative fixation of traumatic spine fractures. Spine. 2015;40(5):299–304. doi: 10.1097/BRS.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon E.S., et al. Timing and risks of chemoprophylaxis after spinal surgery: a single-center experience with 6869 consecutive patients. Journal of Neurosurgery: Spine. 2017;27(6):681–693. doi: 10.3171/2017.3.SPINE161076. [DOI] [PubMed] [Google Scholar]
- 17.Bryson D.J., Uzoigwe C.E., Braybrooke J. Thromboprophylaxis in spinal surgery: a survey. Journal of orthopaedic surgery and research. 2012;7(1):1–8. doi: 10.1186/1749-799X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forum N.Q. National Quality Forum Washington, DC; 2006. National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, preferred practices, and initial performance measures. [Google Scholar]
- 19.Clagett G.P., et al. Prevention of venous thromboembolism. Chest. 1998;114(5):531S–560S. doi: 10.1378/chest.114.5_supplement.531s. [DOI] [PubMed] [Google Scholar]
- 20.Glotzbecker M.P., et al. Surgeon practices regarding postoperative thromboembolic prophylaxis after high-risk spinal surgery. Spine. 2008;33(26):2915–2921. doi: 10.1097/BRS.0b013e318190702a. [DOI] [PubMed] [Google Scholar]
- 21.Glotzbecker M.P., et al. Thromboembolic disease in spinal surgery: a systematic review. Spine. 2009;34(3):291–303. doi: 10.1097/BRS.0b013e318195601d. [DOI] [PubMed] [Google Scholar]
- 22.Cox J.B., et al. Decreased incidence of venous thromboembolism after spine surgery with early multimodal prophylaxis. Journal of Neurosurgery: Spine. 2014;21(4):677–684. doi: 10.3171/2014.6.SPINE13447. [DOI] [PubMed] [Google Scholar]
- 23.Tracy B.M., et al. Venous thromboembolism prophylaxis in neurosurgical trauma patients. Journal of Surgical Research. 2016;205(1):221–227. doi: 10.1016/j.jss.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 24.Gerlach R., et al. Postoperative nadroparin administration for prophylaxis of thromboembolic events is not associated with an increased risk of hemorrhage after spinal surgery. European Spine Journal. 2004;13(1):9–13. doi: 10.1007/s00586-003-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs L.J., et al. Safety of thromboembolic chemoprophylaxis in spinal trauma patients requiring surgical stabilization. Spine. 2013;38(16):E1041–E1047. doi: 10.1097/BRS.0b013e31829879cc. [DOI] [PubMed] [Google Scholar]
- 26.Intiyanaravut T., et al. Enoxaparin versus no anticoagulation prophylaxis after total knee arthroplasty in Thai patients: a randomized controlled trial. J Med Assoc Thai. 2017;100(01):42–49. [PubMed] [Google Scholar]
- 27.Casella I.B., Puech-Leão P. Generic versus branded enoxaparin in prophylaxis and treatment of vein thrombosis. Revista da Associação Médica Brasileira. 2015;61:44–50. doi: 10.1590/1806-9282.61.01.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.